
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
HYPOTHESIS AND THEORY article
Front. Comput. Sci., 09 January 2023
Sec. Theoretical Computer Science
Volume 4 - 2022 | https://doi.org/10.3389/fcomp.2022.976801
This article is part of the Research TopicCurrent Thoughts on the Brain-Computer Analogy - All Metaphors Are Wrong, But Some Are UsefulView all 14 articles
Philosophers have long recognized the value of metaphor as a tool that opens new avenues of investigation. By seeing brains as having the goal of representation, the computer metaphor in its various guises has helped systems neuroscience approach a wide array of neuronal behaviors at small and large scales. Here I advocate a complementary metaphor, the internet. Adopting this metaphor shifts our focus from computing to communication, and from seeing neuronal signals as localized representational elements to seeing neuronal signals as traveling messages. In doing so, we can take advantage of a comparison with the internet's robust and efficient routing strategies to understand how the brain might meet the challenges of network communication. I lay out nine engineering strategies that help the internet solve routing challenges similar to those faced by brain networks. The internet metaphor helps us by reframing neuronal activity across the brain as, in part, a manifestation of routing, which may, in different parts of the system, resemble the internet more, less, or not at all. I describe suggestive evidence consistent with the brain's use of internet-like routing strategies and conclude that, even if empirical data do not directly implicate internet-like routing, the metaphor is valuable as a reference point for those investigating the difficult problem of network communication in the brain and in particular the problem of routing.
Metaphor consists in giving the thing a name which belongs to something else.
Aristotle, Poetics xxi, tr. Bywater
Mathematics is the art of giving the same name to different things.
Henri Poincaré, The Future of Mathematics, 1908
Philosophers have long recognized that the development of a new metaphor can encourage researchers to take unorthodox ideas seriously (Bartha, 2022). In the sciences, new metaphor can spur theorists to build classes of models different from those that already exist. Each new metaphor succeeds not by capturing the exact workings of the analogized system but rather by giving us a new vision of some otherwise unapproachable entity. Theory in the physical sciences has been especially reliant on insights from a succession of metaphors, each an improvement on its predecessor: the container space metaphor for the physical universe gives way to Einstein's fabric of space time.
Metaphor is just as important if not more so to biological theory. Its foundational idea, Darwinian evolution, was crystalized in the metaphor of a tree. Darwin's tree of life was not literally a tree—all life does not spring forth from a single plant. Instead, the metaphor brings together several key properties of the system: rootedness, or the idea that the base of the system of living organisms on earth has one or a small number of main roots; divergence, or the idea that branches spread out and bifurcate, but rarely inosculate (rejoin); and relatedness, or the historical dependence and elaboration of distal twigs on proximal branches. Though graphical depictions of various proposals for the chain of life preceded Darwin, no one before him had seen the problem in this way. The metaphor has proven transformative. It remains in common use today even as knowledge of phylogenetic complexity unknown to Darwin has accumulated (Quammen, 2018).
As attested by the present Research Topic articles—and indeed most issues of any research journal in the neurosciences—researchers rely on the computer metaphor when studying the brain, even if they disagree about its formulation and in what way it is useful (e.g., Richards and Lillicrap, 2022). Historically, McCulloch and Pfeiffer (1949) saw single neurons as a transistor in a “multi-gridded” brain. Most prominently today, the metaphor inheres when neuronal “representation” is seen as having the effect of generating elements of Turing machine symbols and operations (Richards and Lillicrap, 2022), or when neuronal tuning properties are seen to serve as elements in a particular code (e.g., Olshausen and Field, 1996). One thing different instantiations of the computer metaphor seem to have in common is that they see things from the point of view representational elements (see also Poldrack, 2021; Anderson and Champion, 2022; Brette, 2022; Hipólito, 2022; John, 2022). In this view, activity in a given neuron embodies an act of representation in one form or another (see e.g., Baker et al., 2022). A given pattern of activity in neurons and/or across neuronal populations is seen to indicate the brain's invocation of a particular coding element (e.g., for visual data, as a basis function, or as the features in some layer of a convolutional neural network).
The “brain-as-representation machine” metaphor is also made visible in works such as Gidon et al. (2022). These authors propose a thought experiment regarding the nature of consciousness and ask whether “replay” of neuronal signals via external means is equivalent to an identical endogenous experience. Whatever one thinks about the thought experiment, it assumes brain function consists only of representational processes, to the point where the authors illustrate the procedure of the thought experiment with cartoon “play” and “record” icons.
This view concretizes a particular understanding of the brain's goals, and facilitates the importation of ready insights, tools, and methods from other fields, especially mathematics, to attack the difficult problem of understanding the purpose and meaning of neuronal signals. This effort has propelled the field through a period of rapid advances in the 20th and 21st centuries (Cobb, 2020; Lindsay, 2021). The metaphor helped identify a problem to be solved, and offered a range of more and less literal implementations to consider. Even as the limits of the metaphor are probed, it retains value as an impetus and sometimes a foundation for more precise understanding.
As useful as the representational metaphor is, it cannot capture all system goals when the system is as complex as the brain. Brains instantiate many goals. For this reason, not all signals extracted from the brain should necessarily be seen to serve the goal of representation. Here I argue for another class of metaphors that we can invoke in addition to other metaphors: the internet (Danilova and Mollon, 2003; Graham and Rockmore, 2011; Oka et al., 2015; Graham, 2021).
The internet and the brain are clearly different, just as physical computers, and indeed Turing machines, are different from the brain. But, taking inspiration from the history of computational neuroscience and its metaphorical framework, we can profit from considering the conceptual infrastructure for communication on the internet as a point of reference to the problem space of network communication in the brain. This can help us determine which neuronal signals relate to communication and which to representation, and in what way representation and communication relate to each other.
If neurons compute, there is of course a superficial correspondence between the brain as a whole and the internet, since both systems involve the networked linkage of many localized computational units. But one can't simply wire computers together and expect them to communicate reliably. Even the simplest computer networks of the Web 1.0 era required “phenomenally complex” network engineering (Meyers, 2004). A comprehensive and cohesive conceptual framework is needed to make it work.
In adopting the internet metaphor, we attempt to see the brain from the point of view of messages, rather than representational elements. In neuronal terms, this shift implies a consideration not only of how neurons relate to environmental inputs and behavioral outputs—“outside-in neuroscience”—but especially a consideration of how neurons relate to each other—an “inside out” approach (Buzsáki, 2019; Fields et al., 2022; Mayner et al., 2022). More specifically, the goal is to understand how the brain's vast and interconnected network of elements organizes message passing within itself by examining a variety of possible schemes for communication (Graham et al., 2020). This approach is consonant with other integrative conceptions of brain function such as neural re-use (Steriade, 2004; Anderson, 2010), neuronal recycling (Dehaene, 2005), computational flexibility (Pessoa et al., 2019), and emergence (e.g., Varley and Hoel, 2022), among others, and can be seen as a way to bring these related proposals together under a common and more concrete framework.
Historically, the goal of understanding network communication was an initial impetus for the cybernetics movement and has antecedents going back at least to Spencer (1896). Pavlov (1927) and Sherrington (1947) highlighted the problem as well, in part by making a comparison with telephone and rail networks. But the advent of the modern internet in the second half of the twentieth century, based on the conceptual underpinning of packet-switched networking, transformed understanding of distributed network communication. This development had ramifications far and wide, and brain science soon took notice. Just ahead of the launch of NSFNet, Poggio (1984) had begun to sketch out a fundamental role for routing in the brain, using the existing ARPANET's packet-switched routing system as an analogy. Since that time, others have built models of routing on brain networks, though such ideas do not always explicitly reference the internet. These include the dynamic routing model (Olshausen et al., 1993) and the notion of routing by synchrony (also called communication through coherence: Fries, 2005; Mishra et al., 2006; Nádasdy, 2010), with additional routing-based insights being offered by Wolfrum (2010) and Navlakha et al. (2018), among others. The present work is an attempt to unify and advance these investigations via a more systematic examination of the characteristics of effective routing, and to point out some of the challenges inherent in network communication. Of particular importance is how the internet flexibly deals with interacting signals that make use of shared resources.1
Routing systems govern how messages travel among nodes that are connected by links. Internet protocol embodies one routing strategy, while other strategies include those underlying postal and traditional telephone systems. Routing is necessary when communicating nodes are separated in space by distances much larger than the size of a node, and when nodes are not all directly connected to one another. As such, routing requires a degree of mutual trust among nodes and a preparedness for faults and errors.
Though it is implemented locally, routing allows nodes across the network to select different targets across the network at will (Graham, 2014). Routing presumes that some nodes can receive messages over multiple incoming edges and transmit them over multiple outgoing edges, based on some rules or algorithms. In the case of converging inputs, routing rules arbitrate among messages arriving on different incoming edges. When outgoing edges diverge, routing serves to direct messages on outgoing edge(s). Routing thus serves to manage congestion and enable flexibility in message passing.
Routing strategies become irrelevant if the number of incoming and outgoing edges at all nodes is the same and messages arriving at a node on edge a always leave on edge b. However, the term “node” in this case loses its meaning. Each path of incoming and outgoing edges through the node can be simplified in a network description as a single edge, and the node and indeed the network as such disappears.
While brains can manage message flow by reorganizing connectivity (e.g., Fauth and Tetzlaff, 2016), this process is too slow to direct neuronal signals over millisecond, second, and minute timescales. Even if changes in network structure do alter message flow, routing processes are still necessary to achieve reliable, selective communication. Thus, if it is at all sensible to describe brains as networks composed of nodes and edges, then we need to consider how to find and execute paths on an essentially fixed network, and how signal interactions might be managed in brains like ours that have no central controller.
What evidence is there that brains need to perform routing of the kind described here? Though direct measurement of signal flow over structurally identified neuronal networks is not yet robustly achievable, there are many levels of organization where the need for routing is apparent, and where suggestive evidence of routing processes has been found.
In terms of brain region structural connectivity, there is clearly the possibility of routing, even if it is not normally described in this way. Treating regions as nodes, with messages incoming and outgoing along white matter tracts, signals arising in a given region—say V1—can be sent via axons originating in layer 2/3 or 4 directly to other regions (say V2), or along projections via the thalamus to other cortical regions (with potential for modulation of these signals by the cortical target), or to thalamus and back to a different part of V1, or to other cortical areas, and then on to propagate to other destinations (see e.g., Reichova and Sherman, 2004; Anderson and Martin, 2016). Routing in this core of the network can happen very quickly: signals can be relayed on round-trips between thalamus and cortex in as little as 9 milliseconds (Briggs and Usrey, 2007). Though white matter signals may arrive in structurally segregated parts of a region, they stand a good chance of interaction given the high interconnectivity within regions, and therefore appear subject to some system of routing.
Brain imaging studies have given functional indications of routing at the regional level. In humans, Cole et al. (2013), found evidence that frontal and parietal areas flexibly communicate with different modalities as well as other systems (e.g., motor) at different times. Gerraty et al. (2018) found evidence that striatal nuclei can selectively engage different cortical targets in different behavioral contexts. Mechanisms that allow this kind of routing may involve synchronization of subthreshold oscillations between or among areas (Singer, 1999; Fries, 2005; Womelsdorf et al., 2007; Nádasdy, 2010; Gisiger and Boukadoum, 2011; Palmigiano et al., 2017; Javadzadeh and Hofer, 2021; Boroujeni and Womelsdorf, 2022; Sakalar et al., 2022). Oscillatory mechanisms may also contribute to routing functions within regions (e.g., communication between subpopulations in V1; Gray et al., 1989).
At the level of single neurons, the ability to route or “steer” messages on different paths has long been posited for single neurons (Waxman, 1972; Scott, 1977). Several single neuron-level cortical mechanisms have recently been observed that could dynamically manage incoming messages. For example, input selection may be partly shaped by exclusive-or (XOR) gating at dendrites (Gidon et al., 2020) or via other dendritic gating mechanisms (Steriade and Paré, 2007; Gollisch and Meister, 2010; Oz et al., 2021). Steering via axon gating is also possible given considerable axonal branching in cortex (Winnubst et al., 2019) which has long been suspected to allow transmission control at branch points. Axonal mechanisms of routing could also involve axon-axon interactions (e.g., Epsztein et al., 2010). Other mechanisms that could perform routing at the single neuron level have been suggested such as ephaptic interactions (Sheheitli and Jirsa, 2020); glia-mediated synapses (Möller et al., 2007); and local spreading of neuroendocrine molecules (Bargmann and Marder, 2013). Probabilistic modeling of signal transmission among four neurons in hippocampus provides suggestive evidence consistent of a highly flexible capacity for routing in the brain (Nádasdy et al., 1999), though this study's results can be interpreted in other ways; see Section Introduction and Box 1 for a detailed discussion of this study.
Box 1. Evidence for flexible routing in hippocampal circuits?
In Nádasdy et al. (1999), extracellular tetrode recordings were obtained from hippocampal CA1 pyramidal layer neurons in 18 rats during sleep and conditioned wheel running. Clustering was performed on multi-channel signals to identify four individual neurons. The researchers then used Monte Carlo models to track temporal patterns of spikes as they appeared to propagate between four hippocampal neurons. In particular, they used shuffling of spike train patterns to identify patterns of spike timing in different neurons that could not be reasonably explained as chance occurrences. They interpreted these spike trains as messages passed from neuron to neuron, which allows one to trace their putative paths of propagation, bearing in mind that ground-truth connectivity was not measured. Some of the results obtained from this analysis are shown in Figure 2.
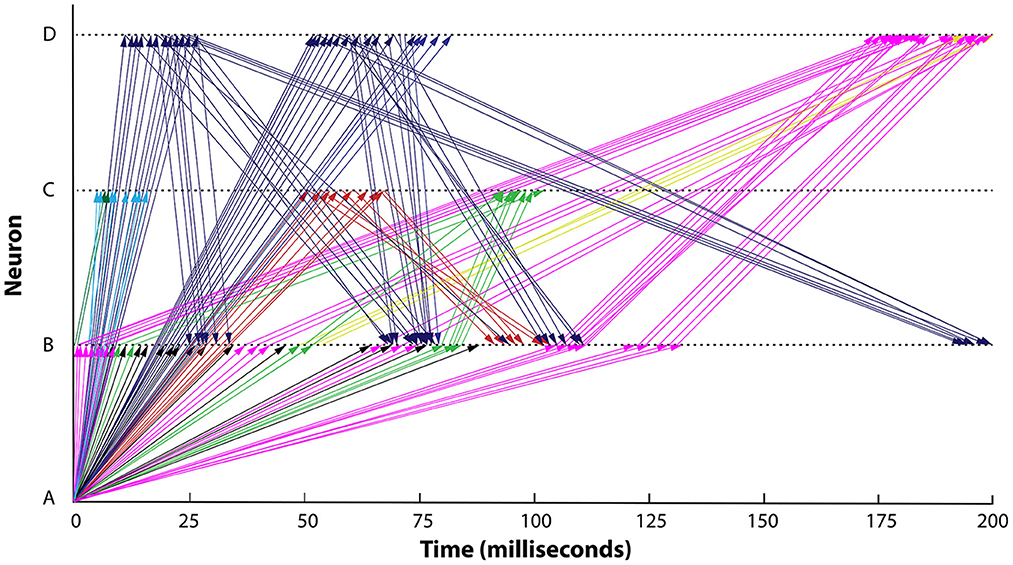
Figure 2. Exchange of spike train messages among four neurons over time in the rat hippocampus during wheel running. Messages are seen to pass among four neurons, labeled A–D. Colors indicate messages traveling on the same path. Horizontal axis indicates time (0–200 ms). Data from Nádasdy et al. (1999), figure redrawn from Buzsáki (2004).
Interpreting these signal transmissions as messages, as the authors do, single neurons appear to have the ability to direct signals on different paths to the same or different targets. These patterns of message flow also vary systematically between behavioral conditions (see Figure 4 in Nádasdy et al., 1999). Arrival times of spike train messages show both short and long delays (latencies), indicating that messages may travel over one or more intermediaries when traveling between the measured neurons (some delays were over 100 milliseconds). It is important to note that each of the message paths suggested here is not a one-off, but is rather a path observed at least a dozen times, which suggests that polysynaptic (multi-hop) transmission is reliable. In sum, one interpretation of these data is that the routing protocol that controls this subnetwork allows all of the following flexible behaviors:
• Sending messages to different destinations.
• Sending messages on different paths to the same destination.
• Sending messages on a given route with a small or large variation in timing.
• All of the above on polysynaptic paths.
• Flexibly changing routing in different behavioral contexts.
As noted above, connectivity was not measured in this study. One certainly cannot rule out the possibility that the observed patterns are artifacts of analysis, or epiphenomena. It could certainly be the case that cells not recorded from are driving the four cells studied. For example, a given “control” cell could produce a particular spiking pattern, which could be relayed by four sets of intermediaries that provide different delays such that that pattern appears at its observed targets at corresponding times. Yet this interpretation would not necessarily invalidate the view that the system is demonstrating flexible routing. Intermediaries would need to faithfully transmit the control message of the spike train in mostly unaltered form and they would need to “protect” these messages from interference from other incoming signals, all while being able to change control patterns reliably both within and across behavioral states. For a common control cell to generate different patterns of delay in the four observed neurons, the intermediaries would need to dynamically change their latencies, and/or selectively direct messages on different intermediary paths.
Nevertheless, the results of Nádasdy et al. (1999) are ambiguous. More than two decades after this study, it remains very difficult to trace signals as they traverse multiple nodes of known connectivity in a brain network (see van der Meij and Voytek, 2018; Hodassman et al., 2022). Models that rely on inferring causality linking separate measurements of structure and activation (e.g., Javadzadeh and Hofer, 2021) can be misleading (see, e.g., Mehler and Kording, 2018; Brette, 2019; Bruineberg et al., 2021).
But though tracing signal propagation faces great procedural challenges, part of the reason why studies that directly trace signal propagation remain rare may be that we have not yet fully appreciated the challenges of flexible routing. As a result, we have limited expectations about what neuronal signatures to expect. Often, we see a neuron's “job” as participating in a computation or representation, where correlations between predicted patterns of activity and observed activity in a given context are seen as sufficient evidence that the brain is carrying out the proposed computation. Approaches like Nádasdy et al. (1999), on the other hand, see spike trains as indications that there are messages to be propagated (see also Luczak et al., 2013; discussed in Insight 4; Grosmark and Buzsáki, 2016). In this view, some aspect of a spike-based message passed between neurons maintains coherence as it propagates, though its structure may be subject to new transformations as it travels—analogously to the way an internet data packet is wrapped in different containers at different points in its journey (e.g., frames and flows). Approaches that build on this insight may lead to advances in our understanding.
To integrate and understand how these kinds of neurobiological mechanisms may be deployed to perform routing, it is helpful to consider the strategies and goals that led to the construction of the modern internet. I offer nine insights that helped make the modern internet possible and begin to apply these ideas to the brain. The goal is to move toward more concrete models and hypotheses, though these are yet to be developed.
Internet routing protocol specifies communication procedures and standards across essentially all modern computer and mobile device networks. However, the internet is defined not so much by its physical implementation in linkages among devices but rather as a set of rules governing the treatment of messages. The core framework for internet communications is the open systems interconnection (OSI) model. The OSI model is not a theory rooted in basic mathematics or physics. Rather, it comprises two broad branches: (1) a conceptual architecture for overall engineering design to route messages successfully and (2) a hierarchy of protocol standards. The simplified “layers” of the OSI protocol model are briefly summarized in Figure 1.
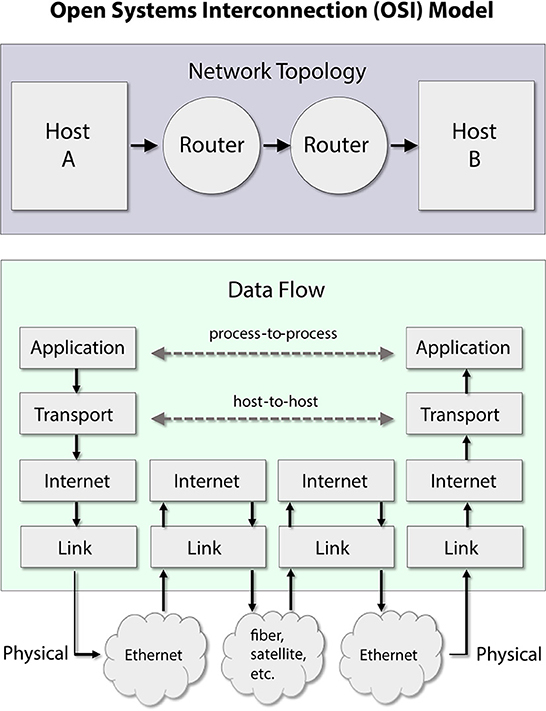
Figure 1. A simplified conceptual design of the internet protocol stack, based on the open systems interconnection (OSI) model. Network topology determines the passage of messages across the network between hosts. The flow of data across the network is organized into conceptual layers. A message originates in the application layer and descends by way of the transport layer and internet layer to a physical layer link (e.g., wire or fiber-optic cable). At intermediary routers, messages ascend only to the internet layer, which plans out the message's forward route. The message then returns to the physical layer for onward travel. This diagram omits the presentation layer and the session layer, which are less relevant for our purposes, and can be seen as part of the application layer.
The design goals of the OSI model and their implementation on the modern internet are especially relevant for the study of processes of routing in the brain, or what might be called “communicatory neuroscience.” The following sections highlight strands of neurobiological evidence that are suggestive of—but do not verify—neuronal implementations of sophisticated routing strategies in the mammal brain; two lines of evidence are examined in more detail and framed as interrogatives in Boxes 1, 2.
Box 2. Could thalamo-cortical loops deliver message acknowledgments (ACKs)?
The thalamus lies near the center of the human brain, and appears to play the role of network backbone (see Hilgetag et al., 2016). Under the “higher-order relays” picture of connections between thalamus and cortex (Sherman and Guillery, 1998, 2001, 2002; see Figure 3), the thalamus contains first-order relays (e.g., lateral geniculate), which receive inputs from the sensorium (e.g., retina). First-order relays pass those inputs on to first-order cortical targets (e.g., V1), which reciprocate back to the same area of the thalamus. The thalamus also contains higher-order relays such as the pulvinar, which receive input from first-order cortical territories, and have connections back to those first-order areas, as well as connections to “higher-order” cortical areas (e.g., V2). In this way, information can travel widely in the cortex in just a few hops via thalamic relays.
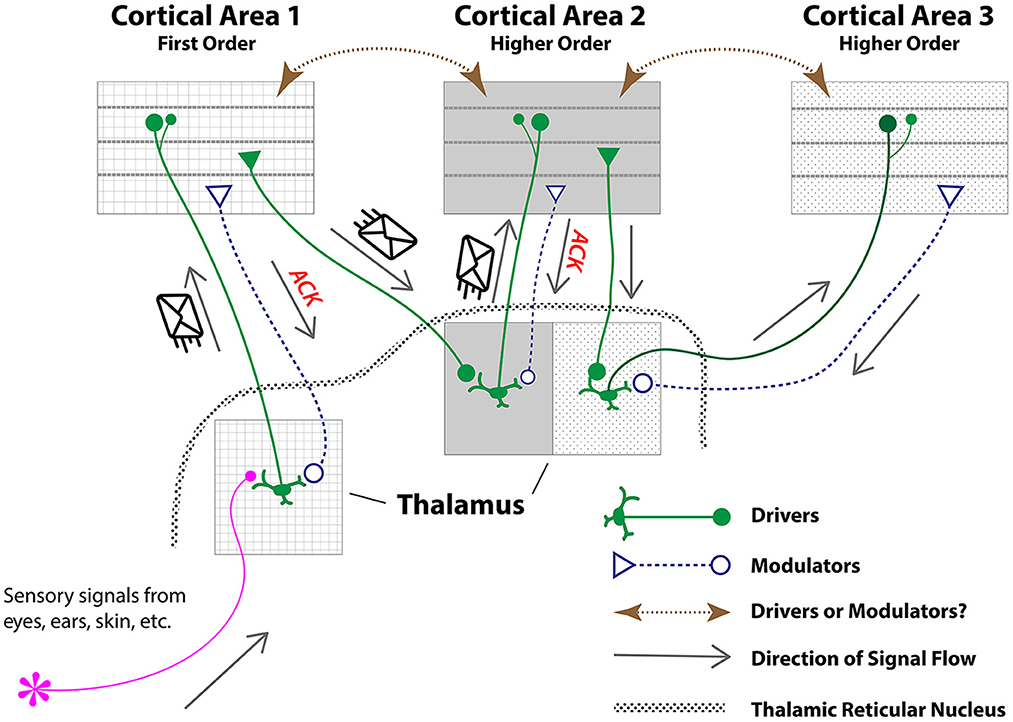
Figure 3. A schematic, hypothetical model under which thalamic relays provide ACKs over the “higher-order relays” organization of thalamo-cortical circuits (Sherman and Guillery, 1998, 2001, 2002). Under the scheme proposed in the current paper, messages containing “content” are sent by “driver” neurons, while “modulators” return ACK-like messages back to thalamic senders, either directly or by way of the thalamic reticular nucleus. If the driver's message is delivered successfully, modulator ACK messages would prevent resending. If a timely ACK is not received, a driver in thalamus could be triggered to resend the missing message. Note that an ACK sent from cortex to thalamus confirming receipt implicitly confirms that the message successfully traveled on an earlier leg from cortex to thalamus (since thalamic excitations are seen as signals relayed from elsewhere), perhaps obviating the need for ACKs on the earlier leg. Figure adapted from Reichova and Sherman (2004).
Given the comparatively large distances traveled between cortex and thalamus and the possibility of spike failure (which is more common in long axons), as well as other types of message loss or corruption, some system of delivery verification would seem appropriate for this core of the brain network. The conventional idea that descending connections serve to “adjust the weights” of incoming signals (e.g., as a way to modulate attention) does not explain why long loops to the thalamus would be required—weights could in principle be adjusted by local circuits in cortex itself, without the cost, delay, and risk of making a long projection back to the thalamus. Instead, this architecture appears better suited to flexible and verifiable message passing among cortical areas via the thalamus.
Cortico-thalamo-cortical communication in the Sherman and Guillery picture is thought to be mediated by two parallel links that go in opposite directions: (1) “driver” connections originate in higher-order thalamic nuclei, traveling to higher-order cortical areas (drivers are also considered to include projections from first-order thalamic relays, such as LGN, to first-order cortical areas, such as V1, and projections from layer 5 of first-order cortical areas to higher-order thalamic relays, such as pulvinar); (2) “modulator” projections originate in layer 6 of the areas targeted by higher-order thalamic relay drivers, and descend to the thalamus to synapse onto the dendritic arbors of drivers (Sherman and Guillery, 1998, 2001, 2002).3 Drivers form a minority of inputs but are seen to deliver primary messages. Modulators are much more numerous, and can affect the likelihood of transmission of driver signals but do not seem to alter the content of those signals (e.g., they do not change receptive field properties of first-order thalamic relays from LGN to V1; Reichova and Sherman, 2004). Modulators also connect to the thalamic reticular nucleus, which can exert inhibitory influence on most connections between the thalamus and cortex.
By thinking of the brain in terms of messages and routing, we can sketch a scheme by which thalamic relays could provide ACKs. Messages containing “content” are sent by drivers, while modulators return ACK-like messages back to thalamic senders, either directly or by way of the thalamic reticular nucleus. If the message is delivered successfully, modulator ACK messages would prevent resending via inhibition. If a timely ACK is not received, a driver in thalamus could be triggered to resend the missing message. In this scheme, ACKs are not performed to confirm receipt of driver messages sent from cortex to thalamus. This may be a sensible strategy. An ACK sent from cortex to thalamus confirming receipt implicitly confirms that the message successfully traveled on an earlier leg from cortex to thalamus (since thalamic excitations are seen as signals relayed from elsewhere). Too many ACKs can clog a system so providing ACKs on only half of each loop could make better use of bandwidth. One would predict that a capacity exists in the thalamus (possibly in the reticular nucleus) for buffering in case ACKs are not received in the thalamus and driver messages need to be resent.4
Matsuyama and Tanaka (2021) have recently found in vivo electrophysiological evidence of “switch-type” neurons in higher-order thalamic nuclei in primates that produce strong bursts after initial visual-auditory stimulus presentation (flash and tone), but become suppressive with repetition (see also Guo et al., 2017; Sieveritz and Raghavan, 2021). This kind of behavior could serve as a building block for a system of ACKs like that described here (see also Crabtree, 2018). However, more detailed models than can be offered here are needed. Indeed, the message acknowledgment scheme proposed here is merely a first step toward a model under a reconceptualization of brain networks as communication systems, which co-exist with computational architectures. The scheme is not a model in and of itself. It should also be emphasized that a solution like ACKs might make sense in cortical-subcortical loops but would not make sense in, for example, spinal reflex arcs, where motor fibers need not receive neural feedback from muscles, but can rather rely on sensory feedback directly.
The internet's flexibility is its central goal. In terms of function, any sender and receiver, no matter their degree of separation on the vast network, can communicate at will with each other, as long as a limited set of protocol is followed. Crucially, flexible communication is delivered over shared resources. As Danilova and Mollon (2003) observe, “The essential feature of the Internet is that it eliminates the need for a dedicated cable between any particular pair of computers that need to communicate.” Flexibility is needed not only in who communicates with whom, but also what path messages take once targets are chosen (see Insight 2) and what kinds of information nodes exchange (Insight 5). Achieving the overarching goal of flexibility shapes all other features, and these features are described in the remaining sections.
Flexibility is similarly fundamental to the brain. Full interconnectivity is impossible: in the human brain, it would require a 20 km-wide head (Nelson and Bower, 1990). Moreover, the behaviors and tasks brains need to accomplish in the world and the brain's network infrastructure strongly suggest flexible control of information flow (Kreiter, 2020; Safron et al., 2022). The most well-developed models of neuronal mechanisms for this kind of flexibility relate to perceptual invariances (Olshausen et al., 1993; see also Wiskott, 2006) and attention (e.g., Mishra et al., 2006) but other “outside-in” functions like flavor perception, decision making, reasoning, problem-solving, sociality, planning, language, creativity, and many others also plainly require flexible management of information flow. The brain's routing strategies must support the accomplishment of highly varied tasks based on highly varied inputs, and do so on a network structure that is fixed in the short term. To take one example, it is possible for the brain to extract different information from the same scene or context depending on one's goal (Günseli and Aly, 2020). Likewise, decision making, whether modeled as evidence accumulation in frontal neurons (Gold and Shadlen, 2007), or as some other “choosing” process, must include delivery of chosen outputs to distinct neuronal subsystems along paths that were equally viable before the decision was “made.” Flexibility may also help the brain to reroute signals around focal lesions without growing new connections (Zalesky et al., 2007; Fornito et al., 2015).
From an “inside-out” perspective, flexibility is suggested by the evidence noted above of selective targeting at the regional level and by the fact that there are numerous short paths between most pairs of regions (considered further in the next section). At the single cell level, suggestive but not conclusive evidence for flexible steering of signals comes from the in vivo electrophysiological study of Nádasdy et al. (1999) discussed in Box 1. However, tracing signal propagation across neuronal networks with known connectivity, which could provide more conclusive evidence of flexible routing, remains an unsolved problem in neuroscience (see Box 1).
The founders of the modern internet saw that network topology and the design of routing protocol were inextricably linked. The two key innovations that led to the internet— distributed network architecture and packet-based protocol—were conceived in tandem by Baran (1964); see also Boehm and Baran (1964).2 I will deal with the effects of network architecture first, and consider its packeted nature in the next section.
Baran realized that a distributed network—one that compromised between a star-shaped network and a lattice—would allow short paths between almost any pair of nodes, but without central switchboards. Short characteristic path length (i.e., low average shortest path length) would later be recognized as a defining property of “small-world” networks, along with high clustering (Watts and Strogatz, 1998). Routing design can take advantage of networks with short paths between nodes. On the modern internet, this is achieved through backbone nodes and peering, i.e., building short cuts between subnetworks to achieve robust interconnection of diverse entities spread across large distances. Not only are paths short on a distributed network, Baran realized, there are usually multiple short paths available, allowing compensation for lost nodes and links, as well as for changes in traffic volume. The system is designed specifically so that, as new conditions arise, new routes are chosen, even as network structure remains the same. This has been termed “robust yet fragile” behavior (Li et al., 2004; Doyle et al., 2005; Sneppen et al., 2005).
In the brain, the connectomics movement has shown that network architecture is also characterized by short average path lengths between nodes (see, e.g., Sporns, 2012). Cortical areas of the macaque monkey are on average about 1.5 hops from each other, and in the mouse the value is closer to 1 (e.g., Knoblauch et al., 2016; Gǎmǎnut et al., 2018). The value for the entire primate connectome is not known but I predict it is around 3 or 4 for most pairs of neurons (see also Parsons et al., 2022). This implies that a given brain component can and does interact with most other components via redundant short paths.
Moreover, connectivity in the brain is redundant at multiple levels. Populations of e.g., neurons tuned to the same feature such as orientation columns, are usually connected to common target populations. At the level of brain regions, the network statistic of “communication efficiency” (Latora and Marchiori, 2001) gauges the number of parallel short paths between a given pair of nodes. This and related measures [“search information” based on the measure of Rosvall et al. (2005)] are found to be accentuated in brain networks, and conducive to effective communication, in comparison to randomly rewired networks of the same degree sequence (see e.g., Avena-Koenigsberger et al., 2017; Seguin et al., 2018).
However, in the brain, the existence of short paths implies that signals passed between components stand a good chance of interacting with each other en route, potentially in deleterious ways. This problem necessitates systematic routing strategies. The likelihood of signal interactions on networks is greatly reduced if the network has a different architecture, such as a lattice or a tree, but this would engender longer paths (but note that some network architectures that differ from that of the internet and that of the connectome, such as random Erdos-Renyi graphs, also have short characteristic path lengths). Shortest path measures are often used in network neuroscience to evaluate the ways that network architecture affects communication among nodes. However, shortest paths are only short if there is no possibility of message interaction, and therefore of errors, congestion, and delay. As Seguin et al. (2019) have argued, it is implausible that the brain has global awareness of network structure necessary for finding all shortest paths (but see Mišić et al., 2015). But it is less plausible still that the brain can always use shortest paths without running into congestion. Instead, in the following sections, I consider local, protocol-based approaches to management of short (but not necessarily shortest) paths and specifically how the internet packages messages and shares communication links. These strategies can serve as potential points of reference for how the brain achieves parsimonious and reliable movement of messages.
The internet has additional architectural motifs such as scale-free architecture (Barabási and Albert, 1999; Caldarelli et al., 2000) and rich clubs (Zhou and Mondragón, 2004; Colizza et al., 2006). Brains show some of these motifs (e.g., Van den Heuvel and Sporns, 2011). However, wiring patterns in the brain are diverse. We should expect that specialized motifs will shape the design of the brain's routing strategies. But these motifs should co-exist with global rules and the network-wide phenomenon of short average path lengths. In this context message interactions must be managed. Because they produce nonlinear effects, principled numerical simulations of routing protocols on brain networks may help us uncover novel relationships between network structure and message interactions on networks (see Hao and Graham, 2020).
The existence of routing presupposes that one has specified the nature of a message. Paul Baran's second insight related to the structure of messages. He realized that message components need not be sent in contiguous units of arbitrary size, the way a phone conversation or a postal letter is. Instead, messages can be divided up into equal-sized chunks—packets—and spread through the network dynamically. This approach was married to a strategy of sharing resources and treating everyone's packets as interchangeable. Sharing in this way requires a leap of faith that “my” message parts won't get lost among those belonging to everyone else as they travel across the network, since no one has exclusive access to intermediary links. Baran and others deliberately imbued each part of the network with sharing and with trust in the wider network—this is the “openness” of the OSI model. Organizing the use of shared resources over an open, small-world network is accomplished by a collection of communication engineering tricks, which are described in the remaining sections.
If communication resources in the brain are shared, as connectome structure described in Insight 2 implies, the system might need to employ solutions like those of the internet. It is worth considering if a shared resources strategy is consistent with the finding of “non-necessary” neurons in the frontal lobe, whose activity correlates with task performance, but which can be lesioned without noticeable effect on task performance (Tremblay et al., 2022). This result does not necessarily make sense from the point of view of optimal representation/computation or information theoretic efficiency. But it could fit into a routing framework. These neurons could be providing shared paths for relevant signals to traverse. In a distributed routing system with shared resources, no single router is strictly necessary, since signals can be actively rerouted. Removing one or several “non-necessary” nodes performing routing may not lead to a visible effect. In Tremblay et al. (2022) data, task performance-related activity peaks at different latencies in the “non-necessary” areas compared to “necessary” areas. This is consistent with a picture where different parts of the network are capable of flexibly transmitting the same messages over different paths.
Of course, much caution is due here. Without knowing network structure, results like Tremblay et al. (2022) can't on their own provide direct evidence for shared resources. Sharing may in fact be more important in the resource-limited and metabolically costly cortical white matter networks (Mollon and Danilova, 2019; Mollon et al., 2022) than in local cortical circuits. Despite the difficulty of recordings from intracortical white matter links at present (e.g., Li et al., 2016), a recognition of the importance of these signals as potentially evidence for shared resources could spur innovation in recording methods. In any case, evidence from human brain imaging of neural re-use (Anderson, 2010) and from neuroanatomy indicating computational flexibility (Pessoa et al., 2019) seems consistent with some level of shared resources in cortex.
If signals have the ability to interfere with each other in a communication network that shares resources, each node would do well to exploit knowledge of its network environment to plan out a good route for messages it transmits. The system as a whole requires a kind of self-awareness—an on-going process for tracking network conditions and message deliveries. Internet routers monitor local network status to ensure they and their neighbors are aware of the existence of paths on shared links and current traffic load over those links. All devices wishing to join the network must support these core mechanisms of network monitoring. Mechanisms include keep alives which are regular heartbeat-like messages sent out by a router to all of its network neighbors to let them know the router is in service. There are also echo requests, which are small probe messages sent to a specific address, which must be reciprocated by the receiver, with all intermediaries reporting transit times for each leg of the journey. Perhaps most important of all are acknowledgments or ACKs, which are small return-receipt messages sent in retrograde fashion after a tranche of packets is successfully received. Note that these mechanisms are superfluous in computers: schematically, connectivity—e.g., two-way buses between processors and memory banks—is simple and highly reliable. Consider messages from processors to memory requesting stored data: the delivery of the data itself serves as confirmation that the request was received. Consequently, stand-alone computers do not generally require components to monitor each other or confirm signal receipt.
The brain, however, would seem to require systems for monitoring the operation of its communication network. Like the internet, such mechanisms would need to operate in distributed fashion over a network whose components are separated by comparatively long distances, suffer some degree of errors, and must trust each other.
Subnetworks in the brain could use spontaneous activity as a kind of keep alive-like message. In this scheme, spontaneous firing facilitates message passing along the same routes as those traveled by evoked signals. There is suggestive evidence of this. Mohajerani et al. (2013) used voltage sensitive dyes in the exposed cortex of mice, combined with prior connectomic maps, to show that both spontaneous and stimulus-evoked activity produced similar motifs of signal transmission. Mohajerani et al. (2013) call this pattern of spontaneous activity a “reverberation” of sensory signals, but perhaps it is better conceived as a preparation for transmitting such signals in the future. Spontaneous signals in this view serve as network status messages. Complementing these results is a microelectrode study in rat auditory cortex, Luczak et al. (2013), investigating what they called “packetization.” Packets as defined in the study were repeating sequences of spike trains in different recorded neurons, much like the putative trajectories of Nádasdy et al. (1999), but rather unlike internet protocol packets. Luczak et al. (2013) found that spontaneous and stimulus evoked packets were similar in structure (see Luczak et al., 2015). This finding is consistent with the idea that neurons exchange content-bearing messages and network status messages on the same footing and over the same conduits. However, these findings are merely suggestive and do not serve as direct evidence of a keep-alive scheme. One intriguing avenue would be for experimentalists to test whether individual neurons or groups of neurons reliably pass signals on polysynaptic paths in ways that can be predicted based on prior patterns of spontaneous activity from the target of the path.
If patterns of transmission treat different types of messages (i.e., stimulus-evoked “content” and spontaneous network status signals) in similar ways, cortico-thalamic connection architecture would seem to naturally possess properties appropriate for providing delivery verification. Core brain networks would also appear to have a need for such a function. See Box 2 for a discussion of possible neuronal substrates in the thalamo-cortical networks that could support ACKs.
These hypothetical mechanisms for network status monitoring and delivery verification do not exactly mirror those used on the internet. Nevertheless, the metaphorical framework of the internet spurs us to conceptualize and investigate the brain in new and potentially transformative ways, which could help explain other puzzling problems at the core of brain organization. For example, the notion of a self-aware system of distributed, communicating elements offers a novel way to approach processes of allostasis in the brain (e.g., Sterling, 2012; Katsumi et al., 2022): the brain may need updates not only about the nature of planned or performed action but also knowledge of the network's readiness to carry out such actions.
Packaging all data into a standard size and structure, i.e., packets, not only allows sharing of resources, it also allows signals of different kinds—including messages with representational “content,” as well as signals related to network status monitoring, and other kinds of messages—to travel together on the same network, all directed by the same routing rules. The potential for any imaginable data to be put into a packet was a basic part of ARPANET design, even though only two functions, remote login and file transfer, were possible on the original network, and indeed for decades afterward. Today this vision has been realized.
In the brain, we know there is a fundamental interoperability among cortical territories: for example, in sighted subjects, primary visual areas begin processing tactile stimuli within hours or days during blindfolding (Pascual-Leone and Hamilton, 2001), and this activity supports enhanced tactile sensitivity. This is not enough time to build extensive new connectivity—nor, presumably, to change the system's basic routing strategy. The influences of the messages of touch and their routing in vision processing systems were there all along and appear interoperable with vision-related signals in this part of the brain's communication network. Indeed, practically any real-world cognitive task requires integrating memories or knowledge from different domains (see e.g., Zeki, 2020). The requirement of interoperability applies not only between systems that deal in messages of different functional kinds but between systems of distinct phylogenetic ages, origins, and structure, such as cortical regions with six cell layers (isocortex/neocortex) and those with three or four layers (e.g., paleocortex).
Interoperability can be achieved in part by obviating the need to inspect or decode messages at most nodes. A router doesn't need to know what a packet contains. This is part of the cleverness of the internet: content is dealt with by senders and receivers, not by processing intermediaries. Could the same be true for neurons of the cortex? Consider that a “visual” neuron in V1 encoding an edge doesn't “know” about edges. Instead, it is responding based on inputs that traverse a particular network of connections before arriving at that neuron. However, its pattern of activity is often seen, under the computing/representational metaphor, as evidence that visual neurons do in fact “know” something about edges or faces or motion, because their spikes can be predicted fairly well from, e.g., deep learning models of visual representation (Yamins et al., 2014). We can draw inspiration from the design of internet routing to help us move beyond this kind of thinking. To complement the computer metaphor-based framework, I argue that we should start to consider things from the message's point of view: where a message originates, how it propagates and is transformed, how routing mechanisms deal with it and ensure it takes an efficient, reliable path, and where and when it is “delivered”.
The principles that govern internet routing are fully scalable: new links and nodes can be added gracefully, with modest cost to network operation. Network communication systems with topology and routing protocol that differ from those of the internet have less graceful scaling. Circuit-switched systems running on star-shaped networks, for example, risk overload without carefully planned growth: intuitively, if your neighbor adds a landline on a traditional telephone network, it will not affect communication over your landline. However, if too many new lines are added, switching stations risk running out of lines, preventing anyone not already using a line from starting a call. In contrast, the internet was specifically designed to scale up with modest cost and without central planning. Thanks to this design insight, most facets of internet routing strategy have required little fundamental modification even with rapid increases in nodes, links, and traffic.
The brain also undergoes upscaling in both ontogeny and phylogeny (though brains additionally experience network downscaling in the form of pruning and cell death). We should therefore expect a routing system in the brain that allows graceful scaling of message-passing; the system should by its nature avoid sharp discontinuities or precipitous changes, much as the internet does. It also must deal with the costs to network communication as it scales up.
Comparative investigations across mammals of different brain sizes could provide evidence of the costs of scaling up brain communication networks and could indicate a likely underlying strategy, just as can be done with black-box routing systems. As they scale up, brain divisions show consistent relationships between regional volume and overall volume (Finlay and Darlington, 1995), which are mediated by shifts in neurodevelopmental timelines. Neuron numbers, neuron size, neuron density, synapse numbers, and network topology scale together in more complex ways. However, the net effect of these relationships may have a global signature that reflects the brain's fundamental routing scheme. In particular, one could examine costs related to transmission of intrabrain signals. If these costs scale up monotonically but gradually in brains of increasing size, this would suggest an internet-like system that shares resources. In contrast, a routing system without shared resources—analogous to circuit-switched telephone networks—would show no increase in cost as the network grows since links are exclusive. However, such a system would be at risk of overload and could not scale organically. Cost in terms of metabolism may be difficult to define and measure but may be reflected in proxy measures. For example, the maximum speed of transmission of messages over multi-hop paths (normalized for distance traveled) could be such a proxy. All else being equal, maximum speed under an internet-like routing scheme would be hypothesized to slowly decrease (i.e., cost slowly increases) in bigger brains. If we see no slow down with brain size, this would be more consistent with a circuit-switched system. Other proxies such as sparseness may offer purchase on this question (see Graham, 2021). A detailed set of predictions about interrelationships among brain scaling, routing strategy and cost is outside the scope of the present paper but is under development.
The internet would not have grown so gracefully if its basic operation had been too energetically expensive. It continues to succeed today despite massive network growth in part because message transport over optical fibers is very efficient (IEA, 2022). But beyond message transport, routing strategies implemented at nodes can also grant efficiency, sometimes in surprising ways.
Consider the back-off algorithm, a core tool found throughout internet-like networks. These algorithms deal with an ever-present problem: what to do when two messages “collide” i.e., attempt to occupy the same frame or clock tick at the input of, for example, an Ethernet router. When this happens, both messages are destroyed. For each destroyed message the router then essentially flips a coin—heads, a copy of the message cached at its sender is allowed pass, tails, it has to wait for the next tick. If a message collides on further attempts and draws tails, it has to wait up to 2 ticks, then up to 4 ticks, then up to 8 ticks. This algorithm is termed binary exponential back-off. It results in an exponential distribution of delays. The basic design principle of imposing randomized wait times for colliding messages has been in place since the earliest days of internet-based communication systems, starting with the ALOHA packet-switched radio network in Hawaii (Kleinrock, 1976). Routinely injecting timing noise into message passing systems remains a cornerstone of routing efficiency across the internet. Notice that this is an example of an engineering insight in communication that differs greatly from insights exploited in the computer metaphor: adding timing noise at all nodes would not have an obvious benefit for representational systems (though deep learning systems do employ “drop-out” for somewhat related purposes) but is demonstrably successful when communication is the goal.
The possibility of something like exponential back-off in the brain is worth consideration. The ubiquity of Poisson-like spike generation in the mammalian cerebral cortex (see, e.g., Averbeck, 2009) produces exponentially-distributed interspike intervals (ISIs). If we see ISIs as delays, this behavior is consistent with the brain performing exponential back-off as part of its network communication strategy. If the brain uses similar routing strategies as those described in this paper, exponentially-distributed ISIs could serve to minimize the effects of collisions. Though many processes generate exponential distributions, this distribution is a hallmark of internet dynamics, so it is curious that a similar distribution is found also throughout mammal cortex. Exponentially-distributed ISIs are observed also for spontaneous firing in cortex (Mazzoni et al., 2007) suggesting that they are not due only to dynamics of stimulus experience but also due to intrinsic factors. However, back-off algorithms, like ACKs, require node buffers to allow resending, which remain hypothetical in the brain (see Box 2).
We can take a wider view of efficiency. Progress in understanding representational aspects of brain function has been aided by efficiency arguments (e.g., Doi and Lewicki, 2014), so a similar approach may be profitable in terms of communication. It has long been clear that transmitting signals down axons is very costly, leading to wiring minimization models (see e.g., Cherniak et al., 2002; Chklovskii and Koulakov, 2004). A recent estimate suggests neuronal communication is in fact far more costly than neuronal computation (Balasubramanian, 2021; Levy and Calvert, 2021). With transmission already expensive, routing strategies in the brain—whose costs were not considered in the estimate of Levy and Calvert (2021)—must be shaped to a significant degree by efficiency concerns. Sparse activity in time and space also contributes to efficiency both on the internet and in the brain; (see Graham, 2021; Graham and Rockmore, 2011) for further discussion of the role of sparseness.
However, gauging the large-scale efficiency of routing in the brain will be a major challenge because we lack a mathematical formalism for describing information theoretic limits on network communication. Shannon's information theory, which is widely invoked in studies of efficiency in neuronal representations (e.g., Wainwright, 1999) applies only to the case of “two-port” communication (Cover and Thomas, 1991), i.e., point encoding and decoding with channel noise. New approaches to the study of efficiency in network communication broadly construed may be needed [see the “network information theory” of El Gamal and Kim (2011); see also Pastor-Satorras and Vespignani (2004), Sun et al. (2015); and Amico et al. (2021)]. The problem in the network case is that resources are shared. One needs to balance cost and reliability when ongoing signal generation can influence individual routing actions in very complex ways. The efficient solutions the brain employs—if they can be determined, and if they are in some sense optimal—may in fact help us understand more fundamental principles of network information theory. However, the internet's demonstrable efficiency suggests that basic principles of efficient network communication may already be instantiated in its array of solutions.
Distributed strategies have advantages but also impose constraints: the internet, for example, is fundamentally an asynchronous communication system: senders and receivers generally cannot establish complete, on-going circuits; full “communion” is unachievable. However, because path lengths are short and because delays on the network are miniscule by human standards, a simulation of synchrony is possible. Human senders and receivers readily perceive its real-time functions (e.g., video chat) as synchronous and simultaneous.5
In the brain, anatomical and network distances are long enough and propagation of neuronal excitations slow enough that the system as a whole functions asynchronously, even if our conscious experience makes it feel as if there is a fully synchronous, delay-free “now” (see, e.g., Zeki and Bartels, 1998; Hogendoorn, 2021). However, functions like object perception may achieve short intervals of synchrony on subnetworks, allowing faster and more coordinated action among dispersed brain elements (Gray et al., 1989; Fries, 2005; Vezoli et al., 2021; Uran et al., 2022).6 Elaborate systems of precisely-timed delays are also a basic feature of cortical signaling, which helps coordinate activity of asynchronous elements (Innocenti et al., 2016).
Seemingly, routing in the brain, as on the internet, is fundamentally asynchronous, but is capable of simulating synchrony over short time scales among subgroups of nodes. The internet's solutions, such as content delivery networks used by Netflix and others, which store multiple copies of a given resource at different points on the network, are worth considering in reference to brain networks.
Despite being composed of billions of dispersed elements, the internet is a unified entity. However, unity is not imposed from a central controller. Instead, a common set of routing rules is implemented locally, and the rules can be locally modified to some extent as well. For example, subnetworks can prioritize some packets over others, and novel devices and protocol can be added so long as basic protocol is followed. A few network services on the internet are organized centrally (e.g., the domain name server) but this is largely a convenience for human end-users. Basic operations of message transmission require no central entity (nor human intervention: connectable devices can now be found and added to the network automatically; Mišić and Mišić, 2014). Even key services like time keeping, which is performed centrally in a computer, are decentralized on the internet using network time protocol. The internet does include modular elements (e.g., autonomous systems), which can exert specialized, central control over a domain (e.g., firewalls). But each module must ultimately be compatible with the wider network by way of common routers. And in the operation of an autonomous system (AS), most of the same “tricks” found in parts of the network outside the AS are employed internally as well.
Protocol in the brain may likewise be a global phenomenon where a relatively small set of rules apply equally throughout, but can be modified. It is reasonable to first consider if there is a single protocol for the whole mammal or vertebrate brain. A patchwork of multiple, non-overlapping networks, each with its own protocol, seems more characteristic of nerve nets (Dupre and Yuste, 2017) than of highly structured and interconnected brains like those of mammals, birds, and especially humans (Hofman, 1988). But we should not rule out the possibility of overlapping neural systems running on different protocol that achieve widespread influence on the network but with limited functionality, such as the fast emergency alert system centered on brainstem nuclei.
Unified protocol in the brain could tolerate considerable local variation and tuning in different regions. Different species may also show specialization in routing. Local modification of global routing rules may influence brain organization within a species. The cortex is typified by “unity and diversity” of structure, shown in, for example, its laminar and columnar architecture (Schüz and Miller, 2002). In different brain regions, variations in a set of conserved genes could shape overall routing strategies. Through the effect of interactions of these genes, small tweaks in units controlling how routing “protocol” is implemented during neurodevelopment could generate significant changes in brain dynamics and function, just as small tweaks to cell growth can affect brain size (e.g., “late equals large” Finlay et al., 2010). With local alteration in routing could come a diversity of function. Through such mechanisms, distributed specializations of protocol in different brain systems could be engineered without sacrificing global integration. The same basic process may help shape phylogenetic variation.
These insights allow us to imagine a rich problem space that we can consider in relation to the study of the brain's strategy for network communication. Internet engineering provides a collection of effective strategies that may be similar to those the brain uses. However, a full description of the neuronal toolkit that could implement the above functions is needed. Testable hypotheses will need to be developed, and these will require more precise models of possible neuronal substrates than I have offered here.
A good metaphor in scientific theory indicates the span and orientation of a problem to be solved. Like a microscope or an electrode, metaphor is a tool, one used in service of theory, rather than experiment. Metaphor is not sufficient for theory, but can be its precursor. Metaphor can help us get to a place where we can specify quantities of interest and understand why measuring those quantities and not others will be meaningful. Technological metaphor, because it refers to engineered systems with goals, is of special potential use since biological organisms and their brains are shaped by evolutionary “engineering.” We can ask, “what would be a good way to design a neuronal system that must operate under certain conditions, such as those that permit flexible exchange of signals across a densely connected network?” The routing strategies of the internet, a technological system that was specifically designed to solve such problems, are worth consideration in relation to this question.
Yet if we grant that the brain must perform flexible routing, the endeavor to understand its strategies in light of the internet still faces major difficulties. One is addressing, a key feature of any routing scheme, which shapes all other features. Selective communication presupposes the existence of an addressing system, though explicit address “headers” may not be required in the brain. Schemes that invoke synchronous oscillations (e.g., Fries, 2005; Nádasdy, 2010) seem to obviate the need for “headers” that travel with a message, but such schemes have not yet dealt with how targets are selectively chosen, nor how congestion could be managed. Indeed, these problems have not been recognized. If headers are needed, spike timing could conceivably carry this information: most paths are likely to be only a few hops in length, so header information could be small. However, detailed models of this kind have not been elucidated let alone tested.
Metaphors can be misleading, especially if taken too literally. This is true not least for the computer metaphor. A physical computer, unlike the brain, has a clock that strictly synchronizes all operations, while a Turing machine requires infinite “tape” on which to order symbols. The brain is obviously not a literal internet either.
Nor can we say that the internet is the only good solution to the problem of dynamic network communication. There exist an unknown number of possible schemes. One can imagine communication systems that include multiple senders and/or receivers where a “multi-message” of distributed chunks travels on parallel paths; along similar lines, it may be better to think in terms of “sources” and “sinks” of signal flow (Mohajerani et al., 2013) rather than single copies of messages with a single path. Part of the problem here is that we lack a grounding of network communication theory in terms of basic mathematics. This is a contrast with the view of brains as computers and representation machines, where we understand the fundamental limitations and possibilities thanks to the well-understood underlying theories of functions and computability.
But the internet's demonstrable success—through pandemic, war, and malicious attack—suggests it embodies basic insights regarding the organization and integration of flexible message flow on large, complex, growing networks. Ultimately, a turn toward the internet metaphor accords with the longstanding desire to understand the integration of computational functions in the brain, and how distributed signals are related and bound to one another (e.g., Popper and Eccles, 1977). The internet metaphor offers more precise language and deeper analogies compared to earlier analogies of brain integration, such as “workspaces” (Dehaene and Changeux, 2005; Baldauf and Deubel, 2010), “bulletin boards,” (Baars, 1997; Goyal et al., 2021), “puzzle pieces” (Chater, 2018; John et al., 2022), or reactions involving “catalysts” and “bonding” (Varela et al., 1991). Brain science stands to profit from considering the internet's strategies and solutions and from asking how the brain might solve similar problems. An understanding of routing in the brain has the potential to illuminate many aspects of brains, not least the decipherment of neural codes, but also evolutionary and developmental patterns, functional differentiation, neurological conditions affecting large-scale brain intra-communication (e.g., multiple sclerosis and epilepsy), as well as intelligence and consciousness.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The author confirms being the sole contributor of this work and has approved it for publication.
I would like to thanks David Field, Barbara Finlay, Don Spector, Yan Hao, Marc-Thorsten Hütt, Kevin Tang, and Niko Grupen for helpful discussions.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. ^Others have noted additional metaphorical links to how applications on the internet, such as the World Wide Web, organize distributed information (Varela et al., 2001; Griffiths et al., 2007). However, these applications do not relate to routing per se, which is the focus of the current paper.
2. ^British researcher Donald Davies made essentially the same two proposals, also in 1964.
3. ^Signals also travel via far more numerous cortico-cortical connections within gray matter. These connections seem to be classifiable as drivers or modulators (Sherman and Guillery, 2011) and could conceivably support an acknowledgment system that runs in parallel to the postulated thalamocortical system. However, such direct, short-range cortico-cortical connections may be reliable enough to not require ACKs.
4. ^On the internet, nodes use buffers to perform queueing, or lining up incoming messages in a small memory allocation based on when they arrived, and directing them on the proper outgoing path one-by-one. In the brain, hypothetical buffers might only need to store a single message, and for only a brief period. Delay circuit-like mechanisms for such buffers have been proposed (Goldman-Rakic, 1996; Funahashi, 2015), and some models of connectome dynamics include node buffers (Mišić et al., 2014; Fukushima and Leibnitz, 2022). Buffers remain hypothetical but with the impetus of the internet metaphor, they invite further investigation.
5. ^However, even the short time delays of the internet can become noticeable. In a Zoom meeting, try this experiment: One person starts to clap at a slow tempo. Then others try to match the beat. Participants often become 180 degrees out of phase with the reference clap.
6. ^The idea of synchrony in digital computation and communication systems is not entirely equivalent to synchrony in dynamical systems of the kind described by Vezoli et al. (2021) and others. In dynamical systems, synchrony usually implies periodicity and occurs when oscillators coordinate the timing of their actions. In digital computing, synchrony means that one system can interfere with the concurrent operation of another system, but there is not necessarily periodicity.
Amico, E., Abbas, K., Duong-Tran, D. A., Tipnis, U., Rajapandian, M., Chumin, E., et al. (2021). Toward an information theoretical description of communication in brain networks. Netw. Neurosci. 5, 646–665. doi: 10.1162/netn_a_00185
Anderson, J. C., and Martin, K. A. C. (2016). “Interareal connections of the macaque cortex: how neocortex talks to itself,” in Axons and brain architecture (Academic Press), 117–134. doi: 10.1016/B978-0-12-801393-9.00006-2
Anderson, M. L. (2010). Neural reuse: A fundamental organizational principle of the brain. Behav. Brain Sci. 33, 245–266. doi: 10.1017/S0140525X10000853
Anderson, M. L., and Champion, H. (2022). Some dilemmas for an account of neural representation: A reply to Poldrack. Synthese. 200, 1–13. doi: 10.1007/s11229-022-03505-4
Avena-Koenigsberger, A., Miši,ć, B., Hawkins, R. X., Griffa, A., Hagmann, P., Goñi, J., et al. (2017). Path ensembles and a tradeoff between communication efficiency and resilience in the human connectome. Brain Struct. Funct. 222, 603–618. doi: 10.1007/s00429-016-1238-5
Averbeck, B. B. (2009). Poisson or not poisson: differences in spike train statistics between parietal cortical areas. Neuron 62, 310–311. doi: 10.1016/j.neuron.2009.04.021
Baars, B. J. (1997). In the Theater of Consciousness: The Workspace of the Mind. USA: Oxford University Press. doi: 10.1093/acprof:oso/9780195102659.001.1
Baker, B., Lansdell, A., and Kording, K. (2022). Three aspects of representation in neuroscience. Trends Cognit. Sci. 26, 942–958. doi: 10.1016/j.tics.2022.08.014
Balasubramanian, V. (2021). Brain power. Proc. Nat. Acad. Sci. 118, e2107022118. doi: 10.1073/pnas.2107022118
Baldauf, D., and Deubel, H. (2010). Attentional landscapes in reaching and grasping. Vision research 50, 999–1013. doi: 10.1016/j.visres.2010.02.008
Barabási, A. L., and Albert, R. (1999). Emergence of scaling in random networks. science 286, 509–512. doi: 10.1126/science.286.5439.509
Baran, P. (1964). On distributed communications networks. IEEE transactions on Communications Systems 12, 1–9. doi: 10.1109/TCOM.1964.1088883
Bargmann, C. I., and Marder, E. (2013). From the connectome to brain function. Nature methods 10, 483–490. doi: 10.1038/nmeth.2451
Bartha, P. (2022). “Analogy and Analogical Reasoning”, The Stanford Encyclopedia of Philosophy (Summer 2022 Edition), Edward N. Zalta (ed.), forthcoming URL = < https://plato.stanford.edu/archives/sum2022/entries/reasoning-analogy/>.
Boehm, S. P., and Baran, P. (1964). On distributed communications: II. Digital simulation of hot-potato routing in a broadband distributed communications network. Memorandum of the RAND corporation prepared for United States Air Force.
Boroujeni, K. B., and Womelsdorf, T. (2022). Routing States Transition During Oscillatory Bursts and Attention States bioRxiv 2022.10.29.514374.
Brette, R. (2019). Is coding a relevant metaphor for the brain?. Behavioral and Brain Sciences 42. doi: 10.1017/S0140525X19000049
Brette, R. (2022). Brains as computers: metaphor, analogy, theory or fact? Frontiers in Ecology and Evolution 10, 878729. doi: 10.3389/fevo.2022.878729
Briggs, F., and Usrey, W. M. (2007). A Fast, Reciprocal Pathway Between the Lateral Geniculate Nucleus and Visual Cortex in the Macaque Monkey. Journal of Neuroscience 27, 20: 5431–5436. doi: 10.1523/JNEUROSCI.1035-07.2007
Bruineberg, J., Dolega, K., Dewhurst, J., and Baltieri, M. (2021). The Emperor's New Markov Blankets. Behavioral and Brain Sciences, 1-63. doi: 10.1017/S0140525X21002351
Buzsáki, G. (2004). Large-scale recording of neuronal ensembles. Nature neuroscience 7, 446–451. doi: 10.1038/nn1233
Buzsáki, G. (2019). The brain from inside out. Oxford University Press. doi: 10.1093/oso/9780190905385.001.0001
Caldarelli, G., Marchetti, R., and Pietronero, L. (2000). The fractal properties of Internet. EPL (Europhysics Letters) 52, 386. doi: 10.1209/epl/i2000-00450-8
Chater, N. (2018). The mind is flat: The remarkable shallowness of the improvising brain. Yale University Press. doi: 10.12987/9780300240610
Cherniak, C., Mokhtarzada, Z., and Nodelman, U. (2002). Optimal-wiring models of neuroanatomy. Computational neuroanatomy 71–82. doi: 10.1385/1-59259-275-9:71
Chklovskii, D. B., and Koulakov, A. A. (2004). Maps in the brain: what can we learn from them?. Annual review of neuroscience 27, 369–392. doi: 10.1146/annurev.neuro.27.070203.144226
Cole, M. W., Reynolds, J. R., Power, J. D., Repovs, G., Anticevic, A., and Braver, T. S. (2013). Multi-task connectivity reveals flexible hubs for adaptive task control. Nature neuroscience 16, 1348–1355. doi: 10.1038/nn.3470
Colizza, V., Flammini, A., Serrano, M. A., and Vespignani, A. (2006). Detecting rich-club ordering in complex networks. Nature physics 2, 110–115. doi: 10.1038/nphys209
Cover, T., and Thomas, J. (1991). Elements of Information Theory. New York, NY: Wiley. doi: 10.1002/0471200611
Crabtree, J. W. (2018). Functional Diversity of Thalamic Reticular Subnetworks. Front Syst Neurosci. 12:41. doi: 10.3389/fnsys.2018.00041
Danilova, M. V., and Mollon, J. D. (2003). Comparison at a distance. Perception 32, 395–414. doi: 10.1068/p3393
Dehaene, S. (2005). Evolution of human cortical circuits for reading and arithmetic: The “neuronal recycling” hypothesis. From monkey brain to human brain 133–157. doi: 10.7551/mitpress/3136.003.0012
Dehaene, S., and Changeux, J. P. (2005). Ongoing spontaneous activity controls access to consciousness: a neuronal model for inattentional blindness. PLoS Biol. 3, e141. doi: 10.1371/journal.pbio.0030141
Doi, E., and Lewicki, M. S. (2014). A simple model of optimal population coding for sensory systems. PLoS computational biology 10, e1003761. doi: 10.1371/journal.pcbi.1003761
Doyle, J. C., Alderson, D. L., Li, L., Low, S., Roughan, M., Shalunov, S., et al. (2005). The “robust yet fragile” nature of the Internet. Proc. Nat. Acad. Sci. 102, 14497–14502. doi: 10.1073/pnas.0501426102
Dupre, C., and Yuste, R. (2017). Non-overlapping neural networks in Hydra vulgaris. Curr. Biol. 27, 1085–1097. doi: 10.1016/j.cub.2017.02.049
El Gamal, A., and Kim, Y. H. (2011). Network Information Theory. USA: Cambridge University Press. doi: 10.1017/CBO9781139030687
Epsztein, J., Lee, A. K., Chorev, E., and Brecht, M. (2010). Impact of spikelets on hippocampal CA1 pyramidal cell activity during spatial exploration. Science 327, 474–477. doi: 10.1126/science.1182773
Fauth, M., and Tetzlaff, C. (2016). Opposing effects of neuronal activity on structural plasticity. Front. Neuroanat. 10, 75. doi: 10.3389/fnana.2016.00075
Fields, C., Glazebrook, J. F., and Levin, M. (2022). Neurons as hierarchies of quantum reference frames. Biosystems. 129, 104714. doi: 10.1016/j.biosystems.2022.104714
Finlay, B. L., Clancy, B., and Darlington, R. B. (2010). Late still equals large. Brain, Behav. Evolut. 75, 4. doi: 10.1159/000295350
Finlay, B. L., and Darlington, R. B. (1995). Linked regularities in the development and evolution of mammalian brains. Science 268, 1578–1584. doi: 10.1126/science.7777856
Fornito, A., Zalesky, A., and Breakspear, M. (2015). The connectomics of brain disorders. Nat. Rev. Neurosci. 16, 159–172. doi: 10.1038/nrn3901
Fries, P. (2005). A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cognit. Sci. 9, 11. doi: 10.1016/j.tics.2005.08.011
Fukushima, M., and Leibnitz, K. (2022). Packetization improves communication efficiency in brain networks with rapid and cost-effective propagation strategies. bioRxiv. doi: 10.1101/2022.06.30.498099
Funahashi, S. (2015). Functions of delay-period activity in the prefrontal cortex and mnemonic scotomas revisited. Front. Syst. Neurosci. 9, 2. doi: 10.3389/fnsys.2015.00002
Gǎmǎnut, R., Kennedy, H., Toroczkai, Z., Ercsey-Ravasz, M., Van Essen, D. C., Knoblauch, K., et al. (2018). The mouse cortical connectome, characterized by an ultra-dense cortical graph, maintains specificity by distinct connectivity profiles. Neuron 97, 698–715. doi: 10.1016/j.neuron.2017.12.037
Gerraty, R. T., Davidow, J. Y., Foerde, K., Galvan, A., Bassett, D. S., and Shohamy, D. (2018). Dynamic flexibility in striatal-cortical circuits supports reinforcement learning. J. Neurosci. 38, 2442–2453. doi: 10.1523/JNEUROSCI.2084-17.2018
Gidon, A., Aru, J., and Larkum, M. (2022). Does brain activity cause consciousness? A thought experiment. PLoS Biol. 20, e3001651. doi: 10.1371/journal.pbio.3001651
Gidon, A., Zolnik, T. A., Fidzinski, P., Bolduan, F., Papoutsi, A., Poirazi, P., et al. (2020). Dendritic action potentials and computation in human layer 2/3 cortical neurons. Science 367, 83–87. doi: 10.1126/science.aax6239
Gisiger, T., and Boukadoum, M. (2011). Mechanisms gating the flow of information in the cortex: what they might look like and what their uses may be. Front. Comput. Neurosci. 5, 1. doi: 10.3389/fncom.2011.00001
Gold, J. I., and Shadlen, M. N. (2007). The neural basis of decision making. Ann. Rev. Neurosci. 30, 535–574. doi: 10.1146/annurev.neuro.29.051605.113038
Goldman-Rakic, P. S. (1996). Regional and cellular fractionation of working memory. Proc. Nat. Acad. Sci. 93, 13473–13480. doi: 10.1073/pnas.93.24.13473
Gollisch, T., and Meister, M. (2010). Eye smarter than scientists believed: neural computations in circuits of the retina. Neuron 65, 150–164. doi: 10.1016/j.neuron.2009.12.009
Goyal, A., Didolkar, A., Lamb, A., Badola, K., Ke, N. R., Rahaman, N., et al. (2021). Coordination among neural modules through a shared global workspace. arXiv preprint arXiv:2103.01197.
Graham, D. (2021). An Internet in Your Head: A New Paradigm for How the Brain Works. New York, NY: Columbia University Press. doi: 10.7312/grah19604
Graham, D., Avena-Koenigsberger, A., and Mišić, B. (2020). Network communication in the brain. Netw. Neurosci. 4, 976–979. doi: 10.1162/netn_e_00167
Graham, D., and Rockmore, D. (2011). The packet switching brain. J. Cognit. Neurosci. 23, 267–276. doi: 10.1162/jocn.2010.21477
Graham, D. J. (2014). Routing in the brain. Front. Comput. Neurosci. 8, 44. doi: 10.3389/fncom.2014.00044
Gray, C. M., König, P., Engel, A. K., and Singer, W. (1989). Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature 338, 334–337. doi: 10.1038/338334a0
Griffiths, T. L., Steyvers, M., and Firl, A. (2007). Google and the mind: Predicting fluency with PageRank. Psychol. Sci. 18, 1069–1076. doi: 10.1111/j.1467-9280.2007.02027.x
Grosmark, A. D., and Buzsáki, G. (2016). Diversity in neural firing dynamics supports both rigid and learned hippocampal sequences. Science 351, 1440–1443. doi: 10.1126/science.aad1935
Günseli, E., and Aly, M. (2020). Preparation for upcoming attentional states in the hippocampus and medial prefrontal cortex. Elife 9, e53191. doi: 10.7554/eLife.53191.sa2
Guo, Z. V., Inagaki, H. K., Daie, K., Druckmann, S., Gerfen, C. R., and Svoboda, K. (2017). Maintenance of persistent activity in a frontal thalamocortical loop. Nature 545, 181–186. doi: 10.1038/nature22324
Hao, Y., and Graham, D. (2020). Creative destruction: Sparse activity emerges on the mammal connectome under a simulated communication strategy with collisions and redundancy. Netw. Neurosci. 4, 1055–1071. doi: 10.1162/netn_a_00165
Hilgetag, C. C., Medalla, M., Beul, S. F., and Barbas, H. (2016). The primate connectome in context: principles of connections of the cortical visual system. NeuroImage 134, 685–702. doi: 10.1016/j.neuroimage.2016.04.017
Hipólito, I. (2022). Cognition without neural representation: dynamics of a complex system. Front. Psychology 5472. doi: 10.3389/fpsyg.2021.643276
Hodassman, S., Vardi, R., Tugendhaft, Y., Goldental, A., and Kanter, I. (2022). Efficient dendritic learning as an alternative to synaptic plasticity hypothesis. Scientific Rep. 12, 1–12. doi: 10.1038/s41598-022-10466-8
Hofman, M. A. (1988). Size and shape of the cerebral cortex in mammals. Brain, Behav. Evolut. 32, 17–26. doi: 10.1159/000116529
Hogendoorn, H. (2021). Perception in real-time: predicting the present, reconstructing the past. Trends Cogn. Sci. 26, 128–141. doi: 10.1016/j.tics.2021.11.003
IEA (2022). Data Centres and Data Transmission Networks, IEA, Paris. License: CC BY 4.0. Available online at: https://www.iea.org/reports/data-centres-and-data-transmission-networks (accessed December 21, 2022).
Innocenti, G. M., Carlén, M., and Dyrby, T. B. (2016). “The Diameters of Cortical Axons and Their Relevance to Neural Computing in Axons and Brain Architecture,” in Axons and Brain Architecture, ed. R. Kathleen (New York, NY: Academic Press) 317–355. doi: 10.1016/B978-0-12-801393-9.00015-3
Javadzadeh, M., and Hofer, S. B. (2021). Dynamic causal communication channels between neocortical areas. Neuron 110, 1–14. doi: 10.1101/2021.06.28.449892
John, Y. (2022). ‘Representing' means exactly what you think it means. Available online at: https://yohanjohn.com/neurologism/representing-means-exactly-what-you-think-it-means/ (accessed October 7, 2022).
John, Y. J., Sawyer, K. S., Srinivasan, K., Müller, E. J., Munn, B. R., and Shine, J. M. (2022). It's about time: Linking dynamical systems with human neuroimaging to understand the brain. Netw. Neurosci. 6, 960–979. doi: 10.1162/netn_a_00230
Katsumi, Y., Theriault, J. E., Quigley, K. S., and Barrett, L. F. (2022). Allostasis as a core feature of hierarchical gradients in the human brain. Netw. Neurosci. 6, 1010–1031. doi: 10.1162/netn_a_00240
Knoblauch, K., Ercsey-Ravasz, M., Kennedy, H., and Toroczkai, Z. (2016). “The brain in space,” in Micro-, meso-and macro-connectomics of the Brain, 45–74. doi: 10.1007/978-3-319-27777-6_5
Kreiter, A. K. (2020). Synchrony, flexible network configuration, and linking neural events to behavior. Curr. Opin. Physiol. 16, 98–108. doi: 10.1016/j.cophys.2020.08.008
Latora, V., and Marchiori, M. (2001). Efficient behavior of small-world networks. Phys. Rev. Lett. 87, 198701. doi: 10.1103/PhysRevLett.87.198701
Levy, W. B., and Calvert, V. G. (2021). Communication consumes 35 times more energy than computation in the human cortex, but both costs are needed to predict synapse number. Proc. Nat. Acad. Sci. 118, 173118. doi: 10.1073/pnas.2008173118
Li, L., Alderson, D., Willinger, W., and Doyle, J. (2004). A first-principles approach to understanding the internet's router-level topology. ACM SIGCOMM Comput. Commun. Rev. 34, 3–14. doi: 10.1145/1030194.1015470
Li, L., Velumian, A. A., Samoilova, M., and Fehlings, M. G. (2016). A novel approach for studying the physiology and pathophysiology of myelinated and non-myelinated axons in the CNS white matter. PLoS ONE 11, e0165637. doi: 10.1371/journal.pone.0165637
Lindsay, G. (2021). Models of the Mind: How Physics, Engineering and Mathematics Have Shaped Our Understanding of the Brain. London: Bloomsbury Publishing. doi: 10.5040/9781472966445
Luczak, A., Bartho, P., and Harris, K. D. (2013). Gating of sensory input by spontaneous cortical activity. J. Neurosci. 33, 1684–1695. doi: 10.1523/JNEUROSCI.2928-12.2013
Luczak, A., McNaughton, B. L., and Harris, K. D. (2015). Packet-based communication in the cortex. Nat. Rev. Neurosci. 16, 745–755. doi: 10.1038/nrn4026
Matsuyama, K., and Tanaka, M. (2021). Temporal prediction signals for periodic sensory events in the primate central thalamus. J. Neurosci. 41, 1917–1927. doi: 10.1523/JNEUROSCI.2151-20.2021
Mayner, W. G., Marshall, W., Billeh, Y. N., Gandhi, S. R., Caldejon, S., Cho, A., et al. (2022). Measuring stimulus-evoked neurophysiological differentiation in distinct populations of neurons in mouse visual cortex. Eneuro. 9, 280. doi: 10.1523/ENEURO.0280-21.2021
Mazzoni, A., Broccard, F. D., Garcia-Perez, E., Bonifazi, P., Ruaro, M. E., and Torre, V. (2007). On the dynamics of the spontaneous activity in neuronal networks. PLoS ONE 2, e439. doi: 10.1371/journal.pone.0000439
McCulloch, W. S., and Pfeiffer, J. (1949). Of digital computers called brains. Scientific Monthly 69, 368–376.
Mehler, D. M. A., and Kording, K. P. (2018). The lure of misleading causal statements in functional connectivity research. arXiv preprint arXiv:1812.03363.
Mishra, J., Fellous, J. M., and Sejnowski, T. J. (2006). Selective attention through phase relationship of excitatory and inhibitory input synchrony in a model cortical neuron. Neural Netw. 19, 1329–1346. doi: 10.1016/j.neunet.2006.08.005
Mišić, B., Betzel, R. F., Nematzadeh, A., Goi, J., Griffa, A., Hagmann, P., et al. (2015). Cooperative and competitive spreading dynamics on the human connectome. Neuron 86, 1518–1529. doi: 10.1016/j.neuron.2015.05.035
Mišić, B., Sporns, O., and McIntosh, A. R. (2014). Communication efficiency and congestion of signal traffic in large-scale brain networks. PLoS Comput. Biol. 10, e1003427. doi: 10.1371/journal.pcbi.1003427
Mišić, V. B., and Mišić, J. (2014). Machine-to-Machine Communications: Architectures, Technology, Standards, and Applications. Boca Raton, FL: CRC Press.
Mohajerani, M. H., Chan, A. W., Mohsenvand, M., LeDue, J., Liu, R., McVea, D. A., et al. (2013). Spontaneous cortical activity alternates between motifs defined by regional axonal projections. Nat. Neurosci. 16, 1426–1435. doi: 10.1038/nn.3499
Möller, C., Lücke, J., Zhu, J., Faustmann, P. M., and Von Der Malsburg, C. (2007). Glial cells for information routing? Cogn. Syst. Res. 8, 28–35. doi: 10.1016/j.cogsys.2006.07.001
Mollon, J., and Danilova, M. (2019). Cortical communication and the comparison of colors. Curr. Opin. Behav. Sci. 30, 203–209. doi: 10.1016/j.cobeha.2019.10.002
Mollon, J. D., Takahashi, C., and Danilova, M. V. (2022). What kind of network is the brain? Trends Cogn. Sci. 26, 312–324. doi: 10.1016/j.tics.2022.01.007
Nádasdy, Z. (2010). Binding by asynchrony: the neuronal phase code. Front. Neurosci. 4, 51. doi: 10.3389/fnins.2010.00051
Nádasdy, Z., Hirase, H., Czurkó, A., Csicsvari, J., and Buzsáki, G. (1999). Replay and time compression of recurring spike sequences in the hippocampus. J. Neurosci. 19, 9497–9507. doi: 10.1523/JNEUROSCI.19-21-09497.1999
Navlakha, S., Bar-Joseph, Z., and Barth, A. L. (2018). Network design and the brain. Trends Cogn. Sci. 22, 64–78. doi: 10.1016/j.tics.2017.09.012
Nelson, M. E., and Bower, J. M. (1990). Brain maps and parallel computers. Trends Neurosci. 13, 403–408. doi: 10.1016/0166-2236(90)90119-U
Oka, M., Abe, H., and Ikegami, T. (2015). Dynamic homeostasis in packet switching networks. Adapt. Behav. 23, 50–63. doi: 10.1177/1059712314556369
Olshausen, B. A., Anderson, C. H., and Van Essen, D. C. (1993). A neurobiological model of visual attention and invariant pattern recognition based on dynamic routing of information. J. Neurosci. 13, 4700–4719. doi: 10.1523/JNEUROSCI.13-11-04700.1993
Olshausen, B. A., and Field, D. J. (1996). Emergence of simple-cell receptive field properties by learning a sparse code for natural images. Nature 381, 607–609. doi: 10.1038/381607a0
Oz, O., Matityahu, L., Mizrahi-Kliger, A., Kaplan, A., Berkowitz, N., Tiroshi, L., et al. (2021). Non-uniform distribution of dendritic nonlinearities differentially engages thalamostriatal and corticostriatal inputs onto cholinergic interneurons. bioRxiv. doi: 10.1101/2021.11.29.470423
Palmigiano, A., Geisel, T., Wolf, F., and Battaglia, D. (2017). Flexible information routing by transient synchrony. Nat. Neurosci. 20, 1014–1022. doi: 10.1038/nn.4569
Parsons, N., Ugon, J., Morgan, K., Shelyag, S., Hocking, A., Chan, S. Y., et al. (2022). Structural-functional connectivity bandwidth of the human brain. NeuroImage 263, 119659. doi: 10.1016/j.neuroimage.2022.119659
Pascual-Leone, A., and Hamilton, R. (2001). The metamodal organization of the brain. Progr. Brain Res. 134, 427–445. doi: 10.1016/S0079-6123(01)34028-1
Pastor-Satorras, R., and Vespignani, A. (2004). Evolution and Structure of the Internet: A Statistical Physics Approach. Cambridge: Cambridge University Press. doi: 10.1017/CBO9780511610905
Pavlov, I. P. (1927). Conditioned Reflexes: An Investigation of the Physiological Activity of the Cerebral Cortex, trans. G. V. Anrep Oxford: Oxford University Press.
Pessoa, L., Medina, L., Hof, P. R., and Desfilis, E. (2019). Neural architecture of the vertebrate brain: implications for the interaction between emotion and cognition. Neurosci. Biobehav. Rev. 107, 296–312. doi: 10.1016/j.neubiorev.2019.09.021
Poggio, T. (1984). Routing thoughts. Massachusetts Institute of Technology Artificial Intelligence Laboratory Working Paper 258.
Poldrack, R. A. (2021). The physics of representation. Synthese 199, 1307–1325. doi: 10.1007/s11229-020-02793-y
Popper, K. R., and Eccles, J. C. (1977). The Self and its Brain. Berlin: Springer International. doi: 10.1007/978-3-642-61891-8
Quammen, D. (2018). The Tangled Tree: A Radical New History of Life. New York, NY: Simon and Schuster.
Reichova, I., and Sherman, S. M. (2004). Somatosensory corticothalamic projections: Distinguishing drivers from modulators. J. Neurophysiol. 92, 2185–2197. doi: 10.1152/jn.00322.2004
Richards, B. A., and Lillicrap, T. P. (2022). The brain-computer metaphor debate is useless: A matter of semantics. Front. Comput. Sci. 4, 810358. doi: 10.3389/fcomp.2022.810358
Rosvall, M., Trusina, A., Minnhagen, P., and Sneppen, K. (2005). Networks and cities: An information perspective. Phys. Rev. Lett. 94, 028701. doi: 10.1103/PhysRevLett.94.028701
Safron, A., Klimaj, V., and Hipólito, I. (2022). On the importance of being flexible: dynamic brain networks and their potential functional significances. Front. Syst. Neurosci. 149, 688424. doi: 10.3389/fnsys.2021.688424
Sakalar, E., Klausberger, T., and Lasztóczi, B. (2022). Neurogliaform cells dynamically decouple neuronal synchrony between brain areas. Science 377, 324–328. doi: 10.1126/science.abo3355
Seguin, C., Razi, A., and Zalesky, A. (2019). Inferring neural signalling directionality from undirected structural connectomes. Nat. Commun. 10, 1–13. doi: 10.1038/s41467-019-12201-w
Seguin, C., Van Den Heuvel, M. P., and Zalesky, A. (2018). Navigation of brain networks. Proc. Nat. Acad. Sci. 115, 6297–6302. doi: 10.1073/pnas.1801351115
Sheheitli, H., and Jirsa, V. K. (2020). A mathematical model of ephaptic interactions in neuronal fiber pathways: Could there be more than transmission along the tracts? Netw. Neurosci. 4, 595–610. doi: 10.1162/netn_a_00134
Sherman, S. M., and Guillery, R. W. (1998). On the actions that one nerve cell can have on another: distinguishing “drivers” from “modulators”. Proc. Nat. Acad. Sci. 95, 7121–7126. doi: 10.1073/pnas.95.12.7121
Sherman, S. M., and Guillery, R. W. (2002). The role of the thalamus in the flow of information to the cortex. Philosoph. Trans. R. Soc. London. 357, 1695–1708. doi: 10.1098/rstb.2002.1161
Sherman, S. M., and Guillery, R. W. (2011). Distinct functions for direct and transthalamic corticocortical connections. J. Neurophysiol. 106, 1068–1077. doi: 10.1152/jn.00429.2011
Sherrington, C. (1947). Integrative Action of the Nervous System. Cambridge: Cambridge University Press.
Sieveritz, B., and Raghavan, R. T. (2021). The central thalamus: gatekeeper or processing hub? J. Neurosci. 41, 4954–4956. doi: 10.1523/JNEUROSCI.0573-21.2021
Singer, W. (1999). Neuronal synchrony: a versatile code for the definition of relations? Neuron 24, 49–65. doi: 10.1016/S0896-6273(00)80821-1
Sneppen, K., Trusina, A., and Rosvall, M. (2005). Hide-and-seek on complex networks. EPL (Europhysics Letters) 69, 853. doi: 10.1209/epl/i2004-10422-0
Sporns, O. (2012). Discovering the Human Connectome. Cambridge, MA: MIT press. doi: 10.7551/mitpress/9266.001.0001
Steriade, M. (2004). Neocortical cell classes are flexible entities. Nat. Rev. Neurosci. 5, 121–134. doi: 10.1038/nrn1325
Steriade, M., and Paré, D. (2007). Gating in Cerebral Networks. Cambridge: Cambridge University Press. doi: 10.1017/CBO9780511541735
Sterling, P. (2012). Allostasis: a model of predictive regulation. Physiol. Behav. 106, 5–15. doi: 10.1016/j.physbeh.2011.06.004
Sun, J., Taylor, D., and Bollt, E. M. (2015). Causal network inference by optimal causation entropy. SIAM J. Appl. Dyn. Syst. 14, 73–106. doi: 10.1137/140956166
Tremblay, S., Testard, C., Inchauspe, J., and Petrides, M. (2022). Non-necessary neural activity in the primate cortex. bioRxiv. doi: 10.1101/2022.09.12.506984
Uran, C., Peter, A., Lazar, A., Barnes, W., Klon-Lipok, J., Shapcott, K. A., et al. (2022). Predictive coding of natural images by V1 firing rates and rhythmic synchronization. Neuron 110, 1240–1257. doi: 10.1016/j.neuron.2022.01.002
Van den Heuvel, M. P., and Sporns, O. (2011). Rich-club organization of the human connectome. J. Neurosci. 31, 15775–15786. doi: 10.1523/JNEUROSCI.3539-11.2011
van der Meij, R., and Voytek, B. (2018). Uncovering neuronal networks defined by consistent between-neuron spike timing from neuronal spike recordings. Eneuro 5, 379. doi: 10.1523/ENEURO.0379-17.2018
Varela, F., Lachaux, J. P., Rodriguez, E., and Martinerie, J. (2001). The Brainweb: phase synchronization and large-scale integration. Nat. Rev. Neurosci. 2, 229–239. doi: 10.1038/35067550
Varela, F. G., Maturana, H. R., and Uribe, R. (1991). “Autopoiesis: The organization of living systems, its characterization and a model,” in Facets of Systems Science (Boston, MA: Springer), 559–569. doi: 10.1007/978-1-4899-0718-9_40
Varley, T. F., and Hoel, E. (2022). Emergence as the conversion of information: a unifying theory. Philosop. Trans. R. Soc. A 380, 20210150. doi: 10.1098/rsta.2021.0150
Vezoli, J., Vinck, M., Bosman, C. A., Bastos, A. M., Lewis, C. M., Kennedy, H., et al. (2021). Brain rhythms define distinct interaction networks with differential dependence on anatomy. Neuron 109, 3862–3878. doi: 10.1016/j.neuron.2021.09.052
Wainwright, M. J. (1999). Visual adaptation as optimal information transmission. Vision Res. 39, 3960–3974. doi: 10.1016/S0042-6989(99)00101-7
Watts, D. J., and Strogatz, S. H. (1998). Collective dynamics of ‘small-world' networks. Nature 393, 440–442. doi: 10.1038/30918
Waxman, S. G. (1972). Regional differentiation of the axon: a review with special reference to the concept of the multiplex neuron. Brain Res. 47, 269–288. doi: 10.1016/0006-8993(72)90639-7
Winnubst, J., Bas, E., Ferreira, T. A., Wu, Z., Economo, M. N., Edson, P., et al. (2019). Reconstruction of 1, 000 projection neurons reveals new cell types and organization of long-range con- nectivity in the mouse brain. Cell 179, 268–281.e13. doi: 10.1016/j.cell.2019.07.042
Wiskott, L. (2006). How does our visual system achieve shift and size invariance? Problems Syst. Neurosci. 26, 322–340. doi: 10.1093/acprof:oso/9780195148220.003.0016
Wolfrum, P. (2010). “Switchyards-Routing Structures in the Brain,” in Information Routing, Correspondence Finding, and Object Recognition in the Brain (Berlin, Heidelberg: Springer), 69–89. doi: 10.1007/978-3-642-15254-2_4
Womelsdorf, T., Schoffelen, J. M., Oostenveld, R., Singer, W., Desimone, R., Engel, A. K., et al. (2007). Modulation of neuronal interactions through neuronal synchronization. Science 316, 1609–1612. doi: 10.1126/science.1139597
Yamins, D. L., Hong, H., Cadieu, C. F., Solomon, E. A., Seibert, D., and DiCarlo, J. J. (2014). Performance-optimized hierarchical models predict neural responses in higher visual cortex. Proc. Nat. Acad. Sci. 111, 8619–8624. doi: 10.1073/pnas.1403112111
Zalesky, A., Vu, H. L., Rosberg, Z., Wong, E. W. M., and Zukerman, M. (2007). OBS contention resolution performance. Perform. Eval. 64, 357–373. doi: 10.1016/j.peva.2006.06.002
Zeki, S. (2020). “Multiplexing” cells of the visual cortex and the timing enigma of the binding problem. Eur. J. Neurosci. 52, 4684–4694. doi: 10.1111/ejn.14921
Zeki, S., and Bartels, A. (1998). The asynchrony of consciousness. Proc. R. Soc.London. 265, 1583–1585. doi: 10.1098/rspb.1998.0475
Keywords: computer metaphor, internet metaphor, systems neuroscience, theoretical neuroscience, packet switching, brain dynamics, network communication, computational neuroscience
Citation: Graham DJ (2023) Nine insights from internet engineering that help us understand brain network communication. Front. Comput. Sci. 4:976801. doi: 10.3389/fcomp.2022.976801
Received: 05 July 2022; Accepted: 12 December 2022;
Published: 09 January 2023.
Edited by:
Pedro Martinez, University of Barcelona, SpainReviewed by:
John Mollon, Cambridge University, United KingdomCopyright © 2023 Graham. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel J. Graham,  Z3JhaGFtQGh3cy5lZHU=
Z3JhaGFtQGh3cy5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.