- 1Department of Chemistry, University of Lucknow, Lucknow, India
- 2Department of Chemical Engineering, Yeungnam University, Gyeongsan, Republic of Korea
Rhodium(III) catalysis has been used for C-H activation of N-nitrosoanilines with substituted allyl alcohols. This method provides an efficient synthesis of the functional N-nitroso ortho β-aryl aldehydes and ketones with low catalyst loading, high functional group tolerance, and superior reactivity of allyl alcohols toward N-nitrosoanilines. We demonstrated that reaction also proceeds through the one-pot synthesis of N-nitrosoaniline, followed by subsequent, C-H activation. The protocol was also feasible with acyrlaldehyde and methyl vinyl ketone which furnished the same oxidative N-nitroso coupling product.
Introduction
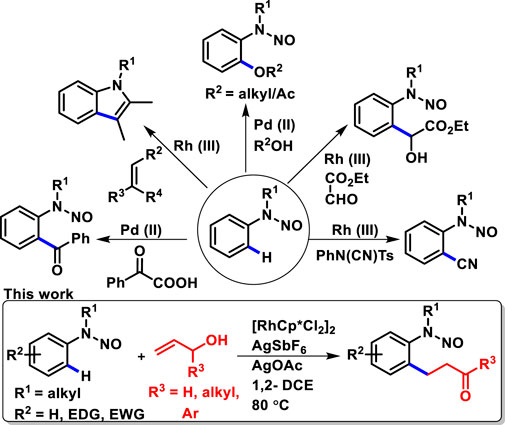
Transition metal-catalyzed reactions have a great impact on the synthesis of natural products and in medicinal chemistry (Crawley and Trost, 2011; Du Bois, 2011; Hayler et al., 2019; Brandsma et al., 1999). They have been proven to be the promising and key-driven strategy for the C-H activation of functionalized arenes. In this context, traditional palladium-catalyzed C-H olefination (the oxidative Heck reaction or Fujiwara–Moritani reaction) has been known for generating a new C-C bond (Oestreich, 2009; Moritani and Fujiwara, 1967; Fujiwara et al., 1969). Later, in 2000, Matsumoto disclosed the rhodium-catalyzed oxidative Heck reaction (Matsumoto and Yoshida, 2000; Matsumoto et al., 2002). Furthermore, various research groups have significantly contributed toward rhodium-catalyzed C-H functionalization (Colby et al., 2009; Tian and Loh, 2015; Huang et al., 2013; Li B. et al., 2015; Deng et al., 2016; Kim et al., 2017; Font et al., 2018; Wu et al., 2019; Shi et al., 2020). In this respect, transitory directing groups have reached a remarkable milestone. Indeed, among the various nitrogen-based directing groups (amide, amine, imine, pyridine, pyrimidine, and pyrazole), we focused on N-nitrosoanilines with all the key features, which can be transformed to various prevalent structural motifs with significant synthetic and biological importance.
N-nitrosamines are chemical compounds that are extensively found in naturally active molecules and also in a range of food and cosmetic products (Loeppky and Outram, 1982; Loeppky and Michejda, 1994; Wang et al., 2005). The chemistry of N-nitrosamines has gained interest in organic and medicinal chemists. In this respect, N-nitrosoanilines are found to be a very attractive directing group as they can be easily removed and installed (Chaudhary et al., 2016a; Chaudhary et al., 2018). N-nitrosamines belong to a versatile class of compounds as they serve as important building blocks. They have become valuable intermediates, which are generally used as an anchor for further transformation, such as reduction to hydrazines (Hartman and Roll, 1933; Chaudhary et al., 2016b) and amines (Chaudhary et al., 2018), synthesis of mesoionic–heterocyclic compound sydnones (Stewart, 1964; Browne and Harrity, 2010) and aryl C-nitroso compounds through Fischer–Hepp rearrangement (Williams, 1975; Cikotiene et al., 2013), and, also, derivatization at the α-carbon of N-nitrosamines (Seebach and Enders, 1975). In addition to these, recently, N-nitrosoanilines have emerged as a traceless directing group since the nitroso functionality possesses the lone pair which can coordinate with the transition metal for the activation of the inert C-H bond (Lee et al., 2002). Several research groups have developed metal-catalyzed ortho-functionalization of N-nitrosoanilines, for example, alkenylation, acylation, alkoxylation, acyloxylation, and cyanation (Scheme 1) (Liu B. et al., 2013; Wu et al., 2016; Gao and Sun, 2014; Li D.-D. et al., 2015; Dong et al., 2015; Huang et al., 2016; Xiong et al., 2023). Despite this recent progress in the C-H functionalization of N-nitrosoanilines, to the best of our knowledge, the oxidative alkylation of N-nitrosoanilines with allyl alcohols has not been reported. Allyl alcohols serve as immensely important building blocks in organic synthesis and have been explored as a reaction partner for C-H functionalization (Wang et al., 2018; Chen et al., 2012; Wang et al., 2019; Ouyang et al., 2022). Herein, we report rhodium-catalyzed regioselective ortho C-H oxidative alkylation of N-nitrosoanilines with various substituted allyl alcohols to provide valuable functional N-nitroso ortho β-aryl aldehydes and ketones (Scheme 1).
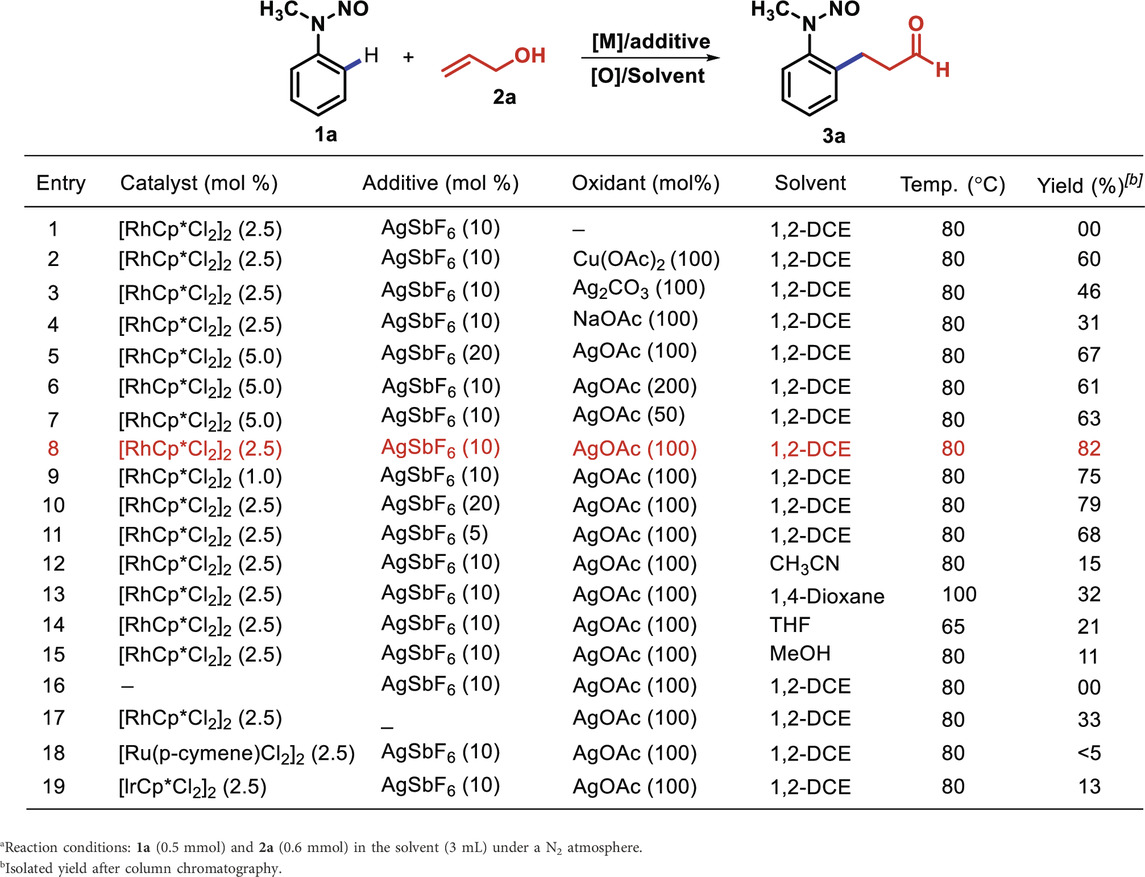
Our investigation to optimize reaction conditions began with the reaction of N-methyl N-nitrosoaniline (1a) and allyl alcohol (2a) under different catalysts, additives, and solvents (Table 1). Initially, we studied that the reaction of 1a with 2a in the presence of [RhCp*Cl2]2 (5 mol%) and AgSbF6 (10 mol%) in 1,2-dichloroethane (DCE) at 80°C for 12 h did not provide any product. (Table 1, entry 1). Therefore, the role of additives was examined for any improvement in the reaction. The employment of 100 mol% of Cu(OAc)2, Ag2CO3, NaOAc, and AgOAc in dichloroethane at 80°C for 12 h afforded 3a with 60, 46, 31% and 67% yields, respectively (Table 1, entries 2–5). An increase or decrease in the amount of AgOAc from 200 mol% to 50 mol% did not affect the yield of the desired product 3a (Table 1, entries 6 and 7). However, decline in the loading of the [RhCp*Cl2]2 catalyst to 2.5 mol% along with AgSbF6 (10 mol%) and 100 mol% of AgOAc in DCE at 80°C for 12 h provided a high yield of 3a (82%) (Table 1, entry 8). The product 3a was obtained as syn- and anti-isomers in 1: 0.13 ratio. Furthermore, the decrease in the amount of the [RhCp*Cl2]2 catalyst to 1 mol% was experimented for 12 h, which lowered the yield of 3a to 75% (Table 1, entry 9). However, any increase or decrease in the amount of AgSbF6 from 20 mol% to 5 mol% did not enhance the yield (Table 1, entries 10 and 11). Later, the effect of the solvents was explored. In non-polar and polar solvents such as CH3CN, 1,4-dioxane, THF, and MeOH, 3a was obtained in 15, 32, 21%, and 11% yields, respectively (Table 1, entries 12–15).
In the absence of the [RhCp*Cl2]2 catalyst, no product was detected with AgSbF6 and AgOAc in 1,2-DCE (Table 1, entry 16), whereas the absence of AgSbF6 leads to a lower yield of 3a (33%) (Table 1, entry 17) in the presence of 2.5% [RhCp*Cl2]2. Furthermore, other catalysts such as [Ru (p-cymene)Cl2]2 and [IrCp*Cl2]2 were found to be less efficient compared to [RhCp*Cl2]2 for the transformation (Table 1, entries 18 and 19). The 1H NMR spectrum of 3a showed distinctive signals for adjacent methylene (–CH2-CH2-) protons to aldehyde (δ 2.76 and δ 2.86 ppm as a triplet, J = 7.4 Hz), and the aldehyde singlet proton was observed at δ 9.75 ppm. The 13C NMR spectrum of 3a showed a representative signal for the adjacent methylene carbon to aldehyde, which was observed at 44.7 ppm, and the next methylene carbon to it was observed at 23.8 ppm; the carbonyl group of the aldehyde was observed at 200.5 ppm. 3a was obtained as a mixture of syn and anti at a ratio of approximately 1: 0.13 (determined using the 1H NMR spectrum) with 82% yield (Figure 1).
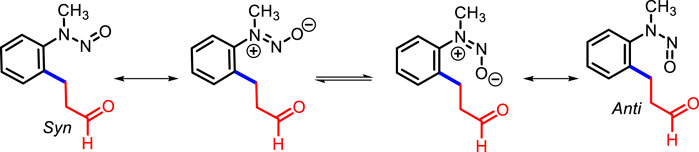
Figure 1. Syn and anti orientation of 3a (Liu B. et al., 2013).
Having established the optimized condition, the reaction of substituted N-nitroso N-alkyl anilines was investigated with allyl alcohol. Treatment of 2a with several substituted N-nitroso N-alkyl anilines, namely, 1b–1m, bearing electron-donating and electron-deficient groups, was observed (Table 2). The p-substituted electron-donating N-nitroso N-methyl anilines (methyl and isopropyl) were converted to the corresponding products 3b and 3c with good yields (72% and 75%, respectively). Similarly, the substrates bearing halide groups such as Br, Cl, and F at the para position under optimized conditions provided the desired products 3d–3f with good yields (60%–73%). It is noteworthy that other p-substituted functionalities that have strong electron-withdrawing tendencies, such as trifluoromethyl, cyano, nitro, ester, and acetyl groups, provided the expected products 3g–3k with 62%–77% yields in 12 h.
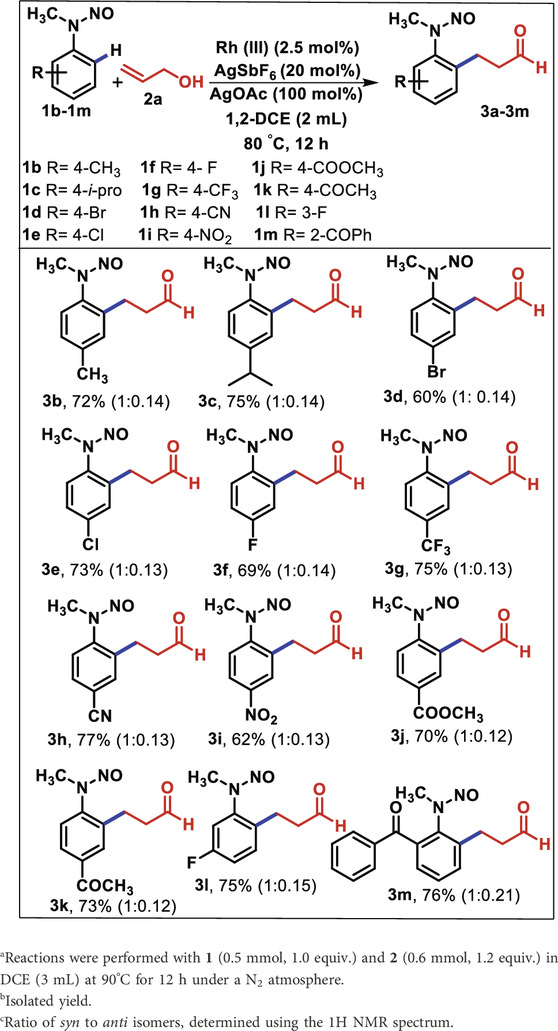
Table 2. Substrate scope of various N-nitroso N-alkyl nitrosoanilines 1b–1m and allyl alcohol 2aa,b,c.
The oxidative alkylation of m-fluoro N-methyl N-nitrosoaniline also yielded the expected product 3l away from the sterically hindered position with 75% yield. Later, the attempted reaction of sterically hindered 1m with 2a regioselectively afforded the ortho-substituted (2-benzoyl-6-(3-oxopropyl) phenyl)-N-methyl N-nitrosoaniline (3m) smoothly with 76% yield. Overall, the simple allyl alcohol underwent C-C bond formation effectively with a range of N-nitrosoanilines of varying electronic and steric factors.
On account of these results, under the assistance of N-nitroso as a directing group, we attempted to explore the reactivity of substituted allyl alcohol 2b–2d toward different N-alkyl N-nitrosoanilines (Table 3). The unsubstituted nitrosoaniline reacted with 1-methyl and 1-ethyl, which yielded the corresponding products 4a and 4b in 72% and 70%, respectively. Similarly, the oxidative alkylation of the p-substituted electron-donating substrate (-Me and -iPr) provided the corresponding β-aryl ketones 4c and 4d in good yields (78% and 79%). In addition, aryl halides such as bromide, chloride, and fluoride at the p-position were found to be stable during the reaction conditions and produced the products 4e, 4f, and 4g with 80%, 83%, and 81% yield, respectively. Furthermore, the sensitive functionalities, such as 4-CF3, 4-CN, 4-COOCH3, and 4-COCH3, on the benzene ring of N- nitrosoanilines were allowed to react with 2b, as a result of which 4h–4l were successfully isolated with 73%–80% yield. Moreover, the reaction of 2b with m-substituents such as methyl and fluoro nitrosoanilines afforded the corresponding products 4m and 4n with 71% and 70% yield, respectively. C-H activation occurred toward a less sterically hindered position of nitrosoaniline. The variation in N-alkyl substitution of 2-methyl N-nitrosoaniline from methyl 1o’ to ethyl 1p’ was found to have well-participated under the standard reaction conditions, and products 4o and 4p were obtained in good yields (70%–72%). The other N-methyl nitrosoanilines containing o-substituents such as -Ph and -COPh provided the products 4q and 4r in 75% and 74% yields, respectively. This indicates that the protocol has the least influence of steric encumbrance of ortho-substituents. Intriguingly, we investigated that the reaction of 1a’ with 1-phenyl allyl alcohol (2d) yielded the products 4s and 4t in 32% and 17% yields, respectively. Moreover, the combination of 1a’ with pent-3-en-2-ol (2e) was also inspected, but no product was observed. This may be due to the steric influence of the methyl group of 2e, which hindered the oxidative coupling to N-methyl N-nitrosoaniline (1a’).
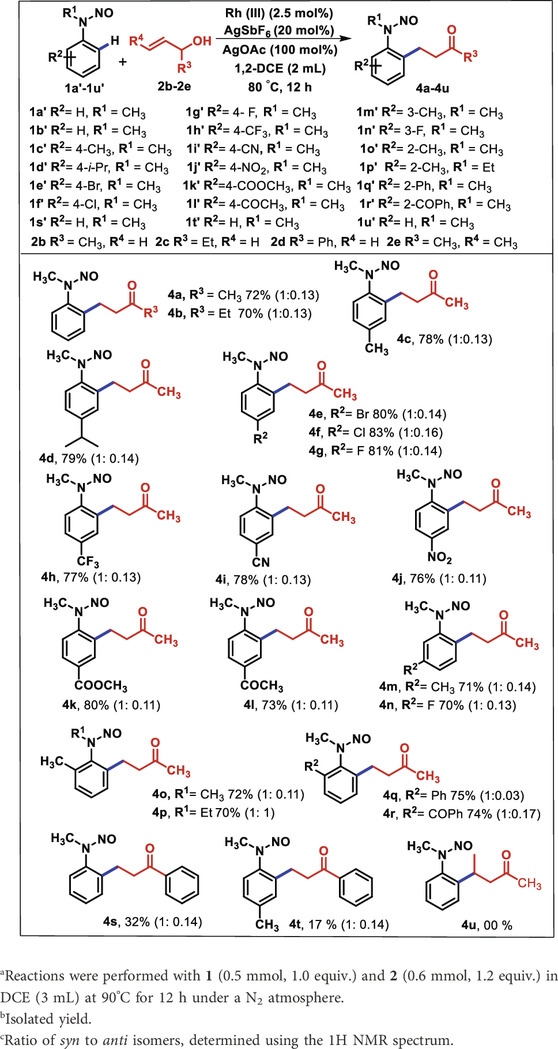
Table 3. Substrate scope of various N-nitroso N-alkyl nitrosoanilines 1a’-1u’ and substituted allyl alcohol 2b-2ea,b,c.
Regarding Csp2-Csp2 bond formation, we attempted one-pot synthesis (Hayashi, 2016), where the addition of the nitroso group (Chaudhary et al., 2018) to N-methyl aniline (1aa) leads to the corresponding nitrosoaniline, and fortunately, we obtained the oxidative coupled product (3a) under the standard reaction conditions with 51% yield (Scheme 2A).
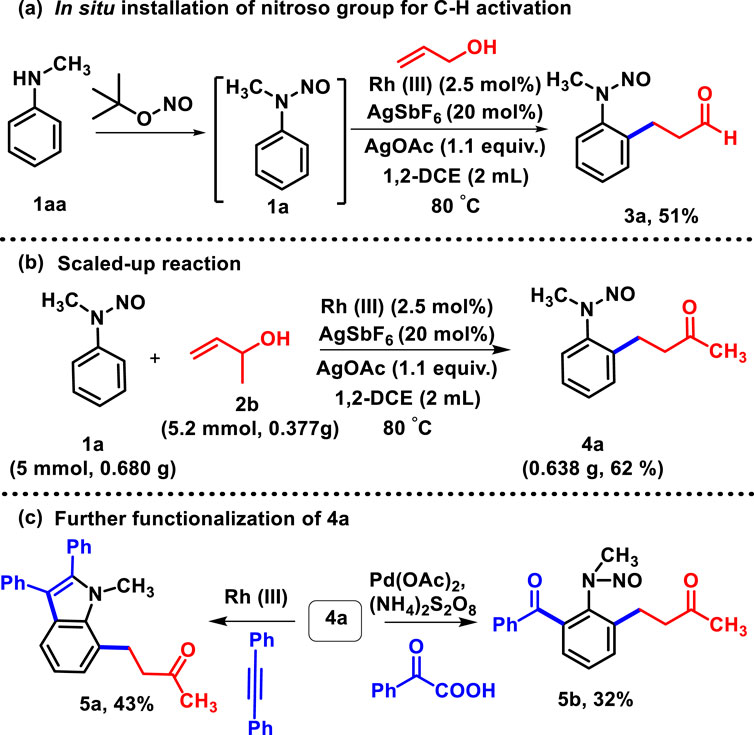
Scheme 2. (A) In situ addition of the N-nitroso group to N-methyl aniline for C-H activation. (B) Scaled-up reaction. (C) Further transformation of 4a.
Henceforth, the protocol exemplifies that implementation of a nitroso unit and subsequent oxidative C-H activation with allyl alcohol reduces time and labor, which, obviously, displays its advantages. To show the synthetic utility of the protocol, the gram-scale synthesis of 4a had been performed (Scheme 2B) and the product was obtained with 62% yield, which was utilized for different transformation processes (Scheme 2C). Versatile N-nitroso directing allowed further catalyzation of C-H activation of 4b at the other ortho-position with rhodium (Liu B. Q. et al., 2013) and palladium (Wu et al., 2016). The activation with diphenyl acetylene and phenyl glyoxylic acid yielded the desired products 5a and 5b with 43% and 32% yields, respectively. In the course of our study, we performed the reaction of N-nitrosoaniline under the same reaction conditions with acrylaldehyde and methyl vinyl ketone. To the best of our knowledge, only β-hydride-eliminated products were obtained such as 3b and 4a; no olefinated or protonolysis product was observed (Scheme 3) (Sun et al., 2010; Peng et al., 2012).
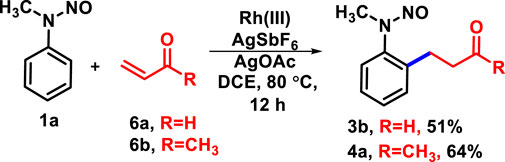
Scheme 3. C-H activation of N-nitrosoaniline with α,β-unsaturated aldehyde and ketone under standard conditions.
The chemoselectivity of two different N-methyl N-nitrosoanilines toward allyl alcohol (2a) was examined under the standard reaction conditions (Scheme 4A). First, the reaction of the unsubstituted (1a) and 4-methyl N-methyl N-nitrosoaniline (1b) subjected to react with allyl alcohol (2a) for 12 h led to the respective products 3a and 3b with 25% and 39% yields, respectively (Scheme 4; Scheme 4A). Similarly, treatment of unsubstituted (1a) and 4-fluoro N-methyl N-nitrosoaniline (1f) with 2a afforded the products 3a and 3f with 41% and 22% yields, respectively (Scheme 4B). The results indicated that the N-nitrosoaniline bearing the electron-donating group is more chemoselective than the unsubstituted and electron-withdrawing bearing substrate. Even the chemoselectivity of 4-methyl N-methyl N-nitrosoaniline (1b) toward allyl alcohol (2a) and 1-methyl allyl alcohol (2b) was also investigated, which demonstrated the formation of 3a:4b in the ratio of 1:6 at the same refractive index (Rf) (Scheme 4C). These intermolecular competitive reactions indicate the simultaneous formation of rhodium carbon and the cleavage of the C-H bond probably by a concerted metalation mechanism (CMD) (Lapointe and Fagnou, 2010).
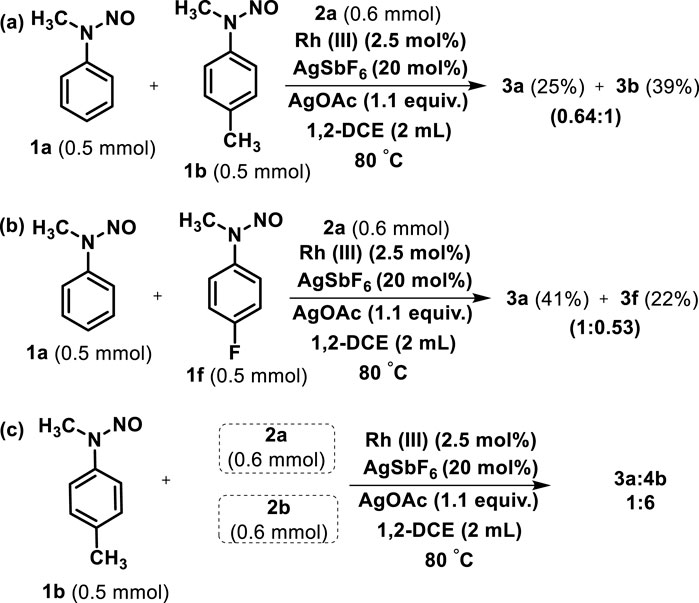
Scheme 4. (A) Chemoselectivity of between N-methyl N-nitrosoanilines 1a and 1b with allyl alcohol (2a). (B) Chemoselectivity of between N-methyl N-nitrosoanilines 1a and 1f with allyl alcohol (2a). (C) Chemoselectivity of 4-methyl N-methyl N-nitrosoaniline (1b) with allyl alcohol (2a) and 1-methyl allyl alcohol (2b).
To gain mechanistic insights, we conducted deuterium labeling experiments, as shown in Scheme 5. The H/D exchange experiment was carried out with 2.5 mol% Rh(III), AgSbF6 (10 mol%), and AgOAc (1 equiv.) to yield 1a (Scheme 5A). The incorporation of 37% deuterium was observed at ortho positions of 1a, which revealed that the C-H activation step is reversible. The parallel kinetic isotopic effect (KIE) was evaluated through an experiment with D5-1a and 1a and was found to be KD/KH = 4.1. This interprets that the C-H bond activation step probably is the rate-determining step (Scheme 5B).
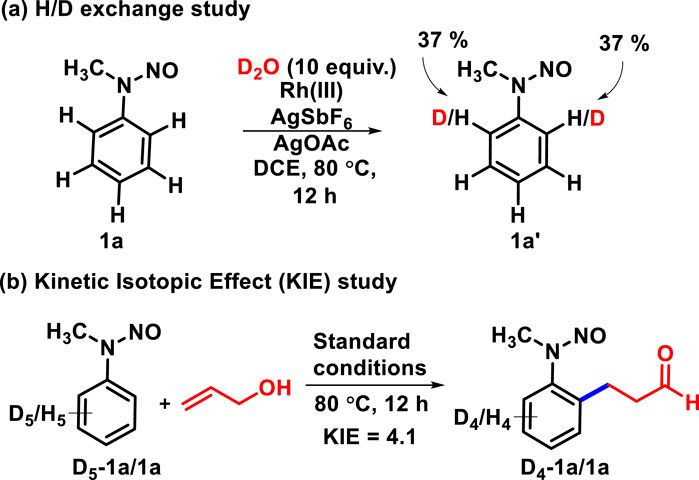
Scheme 5. Preliminary mechanistic studies. (A) H/D Exchange Study. (B) Kinetic Isotopic Effect (KIE) Study.
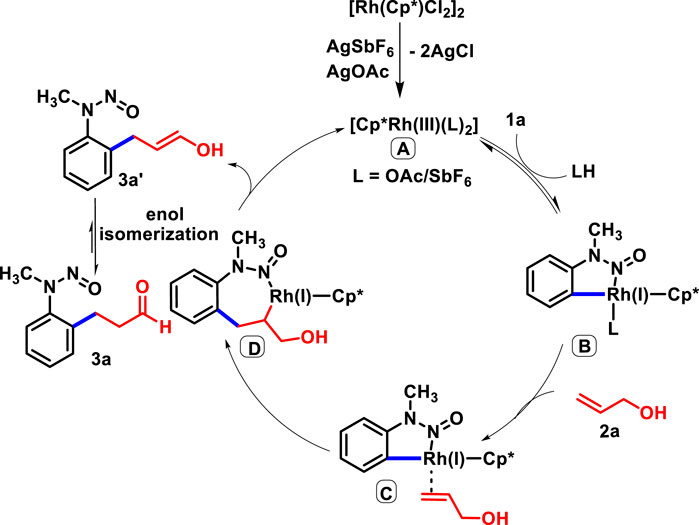
Based on the deuterium labeling mechanistic studies and available literature, we depicted the catalytic cycle. The foremost step involves the ortho C-H activation of 1a with the active rhodium catalyst A, which yields five-membered rodacycle B. Subsequently, the co-ordination of 2a leads to C, which undergoes migratory insertion to generate seven-membered rodacycle D. The β-hydride elimination of D produces 3a’, which undergoes enol isomerization to yield the desired product 3a with concomitant regeneration of the Rh(III) catalyst for the next catalytic cycle (Scheme 6).
In conclusion, we have developed efficient Rh(III)-catalyzed C-H functionalization of N-nitrosoanilines using substituted allyl alcohols. The protocol was applied to a wide range of substrates which gave good yields of products with high functional group tolerance. The protocol provides rapid access for one-pot C-H activation and, also, feasible C-H activation of N-nitrosoanilines with α,β-unsaturated carbonyls. Low catalyst loading, great functional group tolerance, and superior reactivity of N-nitrosoanilines with substituted allyl alcohols are some of the key features of this protocol.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author contributions
PC: conceptualization, formal analysis, investigation, methodology, project administration, resources, validation, writing–original draft, and writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
PC would like to thank Yeungnam University for providing research laboratory and chemical facility for performing the experiments. PC would also like to thank the University of Lucknow for providing basic facilities for manuscript preparation.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2024.1506493/full#supplementary-material
References
Brandsma, L., Verkruijsse, H. D., and Vasilevsky, S. F. (1999). Application of transition metal catalysts in organic synthesis. Springer Desktop Ed. Chem. Vol. 1. doi:10.1007/978-3-642-60328-0
Browne, D. L., and Harrity, J. P. A. (2010). Recent developments in the chemistry of sydnones. Tetrahedron 66, 553–568. doi:10.1016/j.tet.2009.10.085
Chaudhary, P., Gupta, S., Muniyappan, N., Sabiah, S., and Kandasamy, J. (2016a). An efficient synthesis of N-nitrosamines under solvent, metal and acid free conditions using tert-butyl nitrite. Green. Chem. 18, 2323–2330. doi:10.1039/c5gc02880a
Chaudhary, P., Gupta, S., Sureshbabu, P., Sabiah, S., and Kandasamy, J. (2016b). A metal free reduction of aryl-N-nitrosamines to the corresponding hydrazines using a sustainable reductant thiourea dioxide. Green Chem. 18, 6215–6221. doi:10.1039/c6gc02444k
Chaudhary, P., Korde, R., Gupta, S., Sureshbabu, P., Sabiah, S., and Kandasamy, J. (2018). An efficient metal-free method for the denitrosation of aryl N-nitrosamines at room temperature. Adv. Synth. Catal. 360, 556–561. doi:10.1002/adsc.201701047
Chen, M., Wang, J., Chai, Z., You, C., and Leia, A. (2012). C-X (X=Br, I) bond-tolerant aerobic oxidative cross- coupling: a strategy to selectively construct β-aryl ketones and aldehydes. Adv. Synth. Catal. 354, 341–346. doi:10.1002/adsc.201100782
Cikotiene, I., Jonusis, M., and Jakubkiene, V. (2013). The first example of the Fischer–Hepp type rearrangement in pyrimidines. Beilstein J. Org. Chem. 9, 1819–1825. doi:10.3762/bjoc.9.212
Colby, D. A., Bergman, R. G., and Ellman, J. A. (2009). Rhodium-catalyzed C–C bond formation via heteroatom-directed C–H bond activation. Chem. Rev. 110, 624–755. doi:10.1021/cr900005n
Crawley, M. L., and Trost, B. M. (2011). Applications of transition metal catalysis in drug discovery and development: an industrial perspective. New York: Wiley.
Deng, H., Li, H., and Wang, L. (2016). ortho-Heteroarylation of azobenzenes by Rh-catalyzed cross-dehydrogenative coupling: an approach to conjugated biaryls. Org. Lett. 18, 3110–3113. doi:10.1021/acs.orglett.6b01277
Dong, J., Wu, Z., Liu, Z., Liu, P., and Sun, P. (2015). Rhodium(III)-Catalyzed direct cyanation of aromatic C–H bond to form 2-(alkylamino)benzonitriles using N-nitroso as directing group. J. Org. Chem. 80, 12588–12593. doi:10.1021/acs.joc.5b01666
Du Bois, J. (2011). Rhodium-catalyzed C–H amination. An enabling method for chemical synthesis. Org. Process Res. Dev. 15, 758–762. doi:10.1021/op200046v
Font, M., Cendón, B., Seoane, A., Mascareñas, J. L., and Gulías, M. (2018). Rhodium(III)-Catalyzed annulation of 2-alkenyl anilides with alkynes through C−H activation: direct access to 2-substituted indolines. Angew. Chem. Int. Ed. 57, 8255–8259. doi:10.1002/anie.201802830
Fujiwara, Y., Moritani, I., Danno, S., Asano, R., and Teranishi, S. (1969). Aromatic substitution of olefins. VI. Arylation of olefins with palladium(II) acetate. J. Am. Chem. Soc. 91, 7166–7169. doi:10.1021/ja01053a047
Gao, T., and Sun, P. (2014). Palladium-catalyzed N-nitroso-directed C–H alkoxylation of arenes and subsequent formation of 2-alkoxy-N-alkylarylamines. J. Org. Chem. 79, 9888–9893. doi:10.1021/jo501902d
Hartman, W. W., and Roll, L. J. (1933). N-nitrosomethylaniline [aniline, N-methyl-N-nitroso-]. Org. Synth. 13, 66–67. doi:10.15227/orgsyn.013.0082
Hayashi, Y. (2016). Pot economy and one-pot synthesis. Chem. Sci. 7, 866–880. doi:10.1039/c5sc02913a
Hayler, J. D., Leahy, D. K., and Simmons, E. M. (2019). A pharmaceutical industry perspective on sustainable metal catalysis. Organometallics 38, 36–46. doi:10.1021/acs.organomet.8b00566
Huang, J., Chen, Z., Yuan, J., and Peng, Y. (2016). Recent advances in highly selective applications of nitroso compounds. Asian J. Org. Chem. 5 (8), 951–960. doi:10.1002/ajoc.201600242
Huang, L., Wang, Q., Qi, J., Wu, X., Huan, K., and Jiang, H. (2013). Rh(iii)-catalyzed ortho-oxidative alkylation of unactivated arenes with allylic alcohols. Chem. Sci. 4, 2665–2669. doi:10.1039/c3sc50630d
Kim, S., Han, S., Park, J., Sharma, S., Mishra, N. K., Oh, H., et al. (2017). Cp*Rh(iii)-catalyzed C(sp3)–H alkylation of 8-methylquinolines in aqueous media. Chem. Commun. 53, 3006–3009. doi:10.1039/c6cc09830d
Lapointe, D., and Fagnou, K. (2010). Overview of the mechanistic work on the concerted metallation–deprotonation pathway. Chem. Lett. 39, 1118–1126. doi:10.1246/cl.2010.1118
Lee, J., Chen, L., West, A. H., and Richter-Addo, G. B. (2002). Interactions of organic nitroso compounds with metals. Chem. Rev. 102, 1019–1065. doi:10.1021/cr0000731
Li, B., Lan, J., Wu, D., and You, J. (2015a). Rhodium(III)-Catalyzed ortho-heteroarylation of phenols through internal oxidative C-H activation: rapid screening of single-molecular white-light-emitting materials. Angew. Chem. Int. Ed. 54, 14008–14012. doi:10.1002/anie.201507272
Li, D. D., Cao, Y. X., and Wang, G. W. (2015). Palladium-catalyzed ortho-acyloxylation of N-nitrosoanilines via direct sp2 C–H bond activation. Org. Biomol. Chem. 25, 6958–6964. doi:10.1039/C5OB00691K
Liu, B., Fan, Y., Gao, Y., Sun, C., and Xu, C. (2013a). Rhodium(III)-Catalyzed N-Nitroso-Directed C–H olefination of arenes. High-yield, versatile coupling under mild conditions. J. Am. Chem. Soc. 135 (1), 468–473. doi:10.1021/ja3099245
Liu, B. Q., Song, C., Sun, C., Zhou, S. G., and Zhu, J. (2013b). Rhodium(III)-Catalyzed indole synthesis using N–N bond as an internal oxidant. J. Am. Chem. Soc. 135, 16625–16631. doi:10.1021/ja408541c
Loeppky, R. N., and Michejda, C. J. (1994). “Nitrosamines and related N-nitroso compounds: chemistry and biochemistry, ACS,” in Symposium series. Washington, DC: American Chemical Society.
Loeppky, R. N., and Outram, J. R. (1982). “N-nitroso compounds: occurrence and biological effects,”. Lyon: IARC Scientific Publishers.
Matsumoto, T., Periana, R. A., Taube, D. J., and Yoshida, H. (2002). Direct synthesis of styrene by rhodium-catalyzed oxidative arylation of ethylene with benzene. J. Catal., 2, 272–280. doi:10.1006/jcat.2001.3471
Matsumoto, T., and Yoshida, H. (2000). Oxidative arylation of ethylene with benzene to produce styrene. Chem. Lett. 29, 1064–1065. doi:10.1246/cl.2000.1064
Moritani, I., and Fujiwara, Y. (1967). Aromatic substitution of styrene-palladium chloride complex. Tetrahedron Lett. 8, 1119–1122. doi:10.1016/s0040-4039(00)90648-8
Ouyang, W., Liu, B., He, Y., Wen, Y., Gao, Y., Huo, Y., et al. (2022). Modular construction of functionalized anilines via switchable C–H and N-alkylations of traceless N-nitroso anilines with olefins. Org. Chem. Front. 9, 2746–2752. doi:10.1039/d2qo00389a
Peng, Q., Yan, H., Zhang, X., and Wu, Y.-D. (2012). Conjugate addition vs Heck reaction: a theoretical study on competitive coupling catalyzed by isoelectronic metal (Pd(II) and Rh(I)). J. Org. Chem. 77, 7487–7490. doi:10.1021/jo301319j
Seebach, D., and Enders, D. (1975). Umpolung of amine reactivity. Nucleophilic α-(secondary amino)-alkylation via metalated nitrosamines. Angew. Chem. Int. Ed. Engl. 14, 15–32. doi:10.1002/anie.197500151
Shi, Y., Xing, H., Huang, T., Liu, X., Chen, J., Guo, X., et al. (2020). Divergent C–H activation synthesis of chalcones, quinolones and indoles. Chem. Commun. 56, 1585–1588. doi:10.1039/c9cc08926h
Stewart, F. H. C. (1964). The chemistry of the sydnones. Chem. Rev. 64, 129–147. doi:10.1021/cr60228a004
Sun, Z.-M., Zhang, J., Manan, R. S., and Zhao, P. (2010). Rh(I)-Catalyzed olefin hydroarylation with electron-deficient perfluoroarenes. J. Am. Chem. Soc. 132, 6935–6937. doi:10.1021/ja102575d
Tian, P., and Loh, T. P. (2015). Rhodium-catalysed C(sp2)–C(sp2) bond formation via C–H/C–F activation. Nat. Commun. 6, 7472–7478. doi:10.1038/ncomms8472
Wang, P. G., Cai, T. B., and Taniguchi, N. (2005). “Nitric oxide donor, for pharmaceutical and biological applications,” in WILEY-VCH verlag GmbH and Co. Weinheim: KGaA.
Wang, Q., Tian, S., Zhang, C., Li, J., Wang, Z., Du, Y., et al. (2019). Rh-catalyzed regioselective dialkylation of cage B–H bonds in o-carboranes: oxidative Heck reactions via an enol isomerization. Org. Lett. 21, 8018–8021. doi:10.1021/acs.orglett.9b03009
Wang, Y., Chen, Y., Yang, Y., and Zhou, B. (2018). A Rh(iii)-catalyzed redox-neutral C–H alkylation reaction with allylic alcohols by using a traceless oxidizing directing group. Org. Chem. Front. 5, 1844–1847. doi:10.1039/c8qo00265g
Williams, D. L. H. (1975). The mechanism of the Fischer-Hepp rearrangement of aromatic N-nitroso-amines. Tetrahedron 31, 1343–1349. doi:10.1016/0040-4020(75)80181-5
Wu, X., Xiao, Y., Sun, S., Yu, J.-T., and Cheng, J. (2019). Rhodium-catalyzed reaction of sulfoxonium ylides and anthranils toward indoloindolones via a (4 + 1) annulation. Org. Lett. 21, 6653–6657. doi:10.1021/acs.orglett.9b02249
Wu, Y., Sun, L., Chen, Y., Zhou, Q., Huang, J.-W., Miao, H., et al. (2016). Palladium-catalyzed decarboxylative acylation of N-nitrosoanilines with α-oxocarboxylic acids. J. Org. Chem. 81, 1244–1250. doi:10.1021/acs.joc.5b02535
Keywords: N-nitrosamines, oxidative coupling, allyl alcohols, rhodium (III) catalysis, C-H activation and functionalization
Citation: Chaudhary P (2024) Oxidative coupling of N-nitrosoanilines with substituted allyl alcohols under rhodium (III) catalysis. Front. Chem. 12:1506493. doi: 10.3389/fchem.2024.1506493
Received: 05 October 2024; Accepted: 11 November 2024;
Published: 17 December 2024.
Edited by:
Indubhusan Deb, Indian Institute of Chemical Biology (CSIR), IndiaReviewed by:
Zhiyuan Chen, Hangzhou City University, ChinaRamesh Mamidala, Cambrex Corporation, a Pharmaceutical Company, United States
Laksmikanta Adak, Indian Institute of Engineering Science and Technology, India
Copyright © 2024 Chaudhary. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Priyanka Chaudhary, cHJpeWFua2FjaWl0Ymh1QGdtYWlsLmNvbQ==
†ORCID: Priyanka Chaudhary, orcid.org/0000-0002-9861-0610
 Priyanka Chaudhary
Priyanka Chaudhary