- 1State Key Laboratory of Southwestern Chinese Medicine Resources, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 3Institute of Innovative Medicine Ingredients of Southwest Specialty Medicinal Materials, Chengdu University of Traditional Chinese Medicine, Chengdu, China
Two new sesquiterpenoids, curcumanes E (1) and F (2), were isolated from the rhizome of Curcuma longa, and their structures and absolute configurations were examined using extensive spectroscopic analyses and ECD calculations. Interestingly, compounds 1 and 2 are diastereoisomers possessing a rare sesquiterpenoid skeleton that has been reported only once before. Both curcumanes E and F exhibit significant vasorelaxant effects against KCl-induced contraction of rat aortic rings, with EC50 values of 5.10 ± 0.79 and 5.58 ± 1.77 μM, respectively. These findings enrich the data concerning this rare type of sesquiterpenoids and further indicate that these rare sesquiterpenoids can effectively reduce blood pressure.
Introduction
Sesquiterpenoids are representative terpenoid molecules that are widely distributed in plants, microbes, and microorganisms. Over 300 natural sesquiterpenoid skeletons have been reported so far (Cane, 1999; Liu et al., 2012), and novel skeletons are being discovered on a regular basis. The reported compounds have been found to have extensive bioactivities, such as anti-inflammatory (Chang et al., 2020), lipid regulatory (Zhu et al., 2020; Yin et al., 2021), antiviral (Liu et al., 2021), anti-proliferative (Wu et al., 2021), and proangiogenic (Han et al., 2022) activities. Thus, their intriguing structures and impressive bioactivities have attracted the attention of many chemists and pharmacologists.
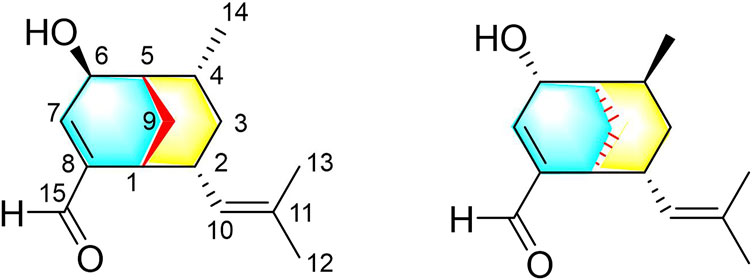
Curcuma longa L. (Zingiberaceae) is used as a traditional Chinese medicine (TCM) to promote blood circulation and remove blood stasis, and it is often added to food as a coloring and flavoring agent (Chen et al., 2010; Sun et al., 2017; Yuan et al., 2018). So far, more than 200 chemical components have been isolated from C. longa, including curcuminoids, sesquiterpenoids, monoterpenoids, and alkaloids (Singh et al., 2010; Xiao et al., 2011; Lin et al., 2012; Prete et al., 2016; Sun et al., 2017). These components exhibit a variety of pharmacological effects, including anti-inflammatory (Ti et al., 2021), antibacterial (Moghadamtousi et al., 2014), anticancer (Chen et al., 2014), and antioxidant (Llano et al., 2019) effects. As part of our long-term project to explore active natural compounds in blood-activating TCMs, we have continuously investigated the extract of the C. longa rhizome, and we have successfully isolated several novel sesquiterpenoids with significant vasorelaxant activity (Liu et al., 2019; Qiao et al., 2019; Chen et al., 2022). In particular, two bicyclic sesquiterpenoids (curcumanes A and B) possessing unprecedented skeletons with a dicyclo [3.2.1]octane or a dicyclo [3.3.1]nonane moiety have been isolated and identified (Liu et al., 2019). In addition, an unusual seco-cadinane sesquiterpenoid (curcumane C) and a pair of unusual nor-bisabolene enantiomers (curcumane D) with significant vasorelaxant activity have been isolated from C. longa (Qiao et al., 2019). To explore whether other rare sesquiterpenoids play a role in the vasorelaxant effect of C. longa, two curcumane B analogues (1 and 2) featuring a dicyclo [3.3.1]nonane moiety were isolated and characterized in this study (Figure 1). The isolation, structure elucidation, absolute configuration, and vasorelaxant activities of 1 and 2 are detailed hereafter.
Experimental
General experimental procedures
IR spectra and optical rotations were measured using an Agilent cary 600 FT-IR microscope (Agilent Technologies Inc., CA, United States) and an Anton Paar MCP 200 automatic polarimeter (Anton Paar GmbH, Austria), respectively. ECD spectra were recorded on an Applied photophysics Chirascan and Chirascan-plus circular dichroism spectrometer (Applied Photophysics Ltd., Leatherhead, England), while NMR spectra were recorded on a Bruker Avance III 600 NMR spectrometer (Bruker Corporation, Billerica, MA, United States) with solvent peaks as internal standards. HRESIMS measurements were carried out using a Q Exactive instrument (Thermo Scientific™, MA, United States), and TLC experiments were performed using glass plates precoated with silica gel (GF254, Qingdao Marine Chemical Inc., Qingdao, China). Silica gel (200–300 mesh, Yantai Institute of Chemical Technology, Yantai, China) and Sephadex LH-20 (Amersham Pharmacia Biotech AB, Uppsala, Sweden) were used for column chromatography. HPLC separations were achieved using an Agilent 1100 instrument (Agilent Technologies Inc., CA, United States) equipped with a Zorbax SB-C18 (250 × 9.4 mm2, 5 μm) semipreparative column. Vasorelaxant activity assays were conducted using a PL3508B6/C-V Panlab 8 Chamber Organ Bath System (including stimulating electrodes, Panlab eight-chamber organ baths, organ chambers, tissue hooks, and Labchart Pro software).
Plant material
The rhizome of Curcuma longa L. (Zingiberaceae) was purchased from Sichuan Neautus Traditional Chinese Medicine Co., Ltd. (Chengdu, China) and identified by Prof. Min Li of Chengdu University of Traditional Chinese Medicine (Chengdu, China). A voucher specimen (CL-20160803) was deposited at the Institute of Innovative Medicine Ingredients of Southwest Specialty Medicinal Materials at Chengdu University of Traditional Chinese Medicine.
Extraction and isolation
The dried rhizome of C. longa (50 kg) were extracted three times with 95% EtOH under reflux. The durations of the first, second, and third extractions were 3, 2, and 1.5 h, respectively. The yellow residue (7 kg) obtained by evaporating the EtOH extract under reduced pressure was dispersed in H2O and partitioned sequentially with petroleum ether and EtOAc. The EtOAc extract (3 kg) was separated on a silica gel column, using gradient elution with petroleum ether–EtOAc (1:0, 7:3, and 4:6) and EtOAc–MeOH (1:0, 1:1, and 0:1) to yield six fractions (A–F). Fraction B was separated further on a silica gel column using CH2Cl2–EtOAc (1:0–0:1) as eluent to yield 16 fractions (F1–F16). Subfractions F6-1–F6-12 were obtained from fraction F6 via RP-MPLC with gradient elution using a solution of MeOH in H2O (30–100%) as mobile phase. The F6-7 subfraction was further fractionated on a Sephadex LH-20 column (petroleum ether–CH2Cl2–MeOH, 5:5:1) to obtain subfractions F6-7-1 and F6-7-2. Finally, purification of F6-7-1 by preparative TLC (CH2Cl2–EtOAc, 15:1) and RP semipreparative HPLC (69% MeOH in H2O) afforded 1 (4.8 mg) and 2 (3.7 mg).
Spectroscopic data
Curcumane E (1): colorless oil;
Curcumane F (2): colorless oil;
Effects of compounds 1 and 2 on the KCl-induced contractions of rat aortic rings
Male Sprague-Dawley rats (180–220 g) were purchased from Da Shuo Biotechnology Co., Ltd (Chengdu, Sichuan, China). All of the rats were housed under standard environmental conditions at a temperature between 25 ± 1°C, humidity between 50 ± 5%, and food and water were provided ad-libitum during the study period. All of the experimental procedures and protocols were approved by the Committee on the Ethics of Animal Experiments of Chengdu University of Traditional Chinese Medicine (Approval No. 2020–04) and followed the guidelines of the Management Committee for Experimental Animals, China.
The thoracic aorta of SD rats was carefully dissected and immediately immersed in 4°C oxygenated Krebs-Henseleit (K-H) solution [composition (mM): NaCl, 120; KCl, 4.6; KH2PO4, 1.2; MgSO4, 1.2; NaHCO3, 25; glucose, 10; CaCl2, 2.5]. Subsequently, 3–5-mm-long rings were prepared by cleaning the fat and connective tissues surrounding the aorta then cutting it. Before starting the experiment, the aortic rings were equilibrated in a 20 ml K-H solution (constant temperature of 37°C; bubbled with a gas mixture of 95% O2 and 5% CO2) for 1 h under 1 g initial tension. The aortic rings were stably pre-contracted by induction with 60 mM KCl solution, then cumulative concentrations of the test compounds (0.25, 0.75, 2.5, 7.5, and 25 μM) were added to the organ bath. All of the data were recorded using a computerized system, and Labchart Pro software was used to measure the tension of the prepared samples. Methoxyverapamil was used as a positive control (Xiong et al., 2015; Hu et al., 2018). The EC50 and Emax (maximal vasorelaxation) values of the test compounds and the positive drug were calculated based on the cumulative concentration–tension curves, and the relaxant responses of KCl-induced maximal contractile tension were considered 100%. Statistical analysis was performed using Student′s t-test, and p < 0.05 signified a statistically significant difference. All of the values are expressed as mean ± SEM.
Results and discussion
Structure elucidation of compounds
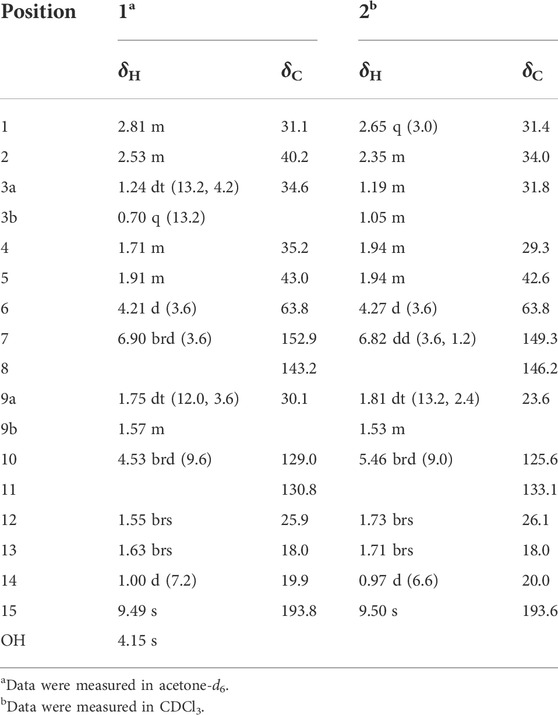
Compound 1 was obtained as a colorless oil, and based on HRESIMS analysis, its molecular formula is C15H22O2 (m/z 257.1504 [M + Na]+; calcd 257.1512), which signifies that it has five degrees of unsaturation. The IR spectrum of 1 exhibits absorption bands corresponding to hydroxy (3389 cm−1), aldehyde (2865, 2720, and 1686 cm−1), and olefinic (1523 cm−1) groups. The resonance peaks observed in its 1H NMR spectrum may be attributed to three methyl groups [δH 1.00 (d, J = 7.2 Hz), 1.55 (brs), and 1.63 (brs)], two olefinic methines [δH 4.53 (brd, J = 9.6 Hz) and 6.90 (brd, J = 3.6 Hz)], an oxymethine [δH 4.21 (d, J = 3.6 Hz)], an aldehyde group [δH 9.49 (s)], and several aliphatic methylenes and methines between δH 0.70 and 2.81 (Table 1). The 13C NMR and DEPT data of 1 reveal the presence of one carbonylic carbon (δC 193.8), one oxygenated carbon [δC 63.8 (CH)], and four olefinic carbons [δC 129.0 (CH), 130.8 (C), 143.2 (C), 152.9 (CH)] (Table 1). Overall, the spectroscopic data indicate that compound 1 is a bicyclic sesquiterpenoid possessing an oxymethine group, an aldehyde group, and two trisubstituted double bonds. Based on the 1H−1H COSY correlations of H-1/H-2/H2-3/H-4/H-5/H2-9/H-1, H-2/H-10, and H-4/H3-14, as well as the HMBC correlations of H3-14 with C-3, C-4, and C-5; H2-3 with C-1, C-2, C-4, C-10, and C-14; H2-9 with C-1, C-2, C-4, and C-5; H-10 with C-1, C-2, C-3, C-12, and C-13; and H3-12 and H3-13 with C-10 and C-11 (Figure 2), compound 1 comprises a six-membered ring A with an isobutenyl unit at C-2 and a methyl group at C-4. The elucidation of the other six-membered ring B possessing OH-6 and CHO-8 substituents is based on the HMBC correlations of OH-6 with C-5, C-6, and C-7; H-15 with C-1, C-7, and C-8; H2-9 with C-6 and C-8; H-2 with C-8; and H-7 with C-1, C-5, and C-15. Thus, the planar structure of 1 is determined.
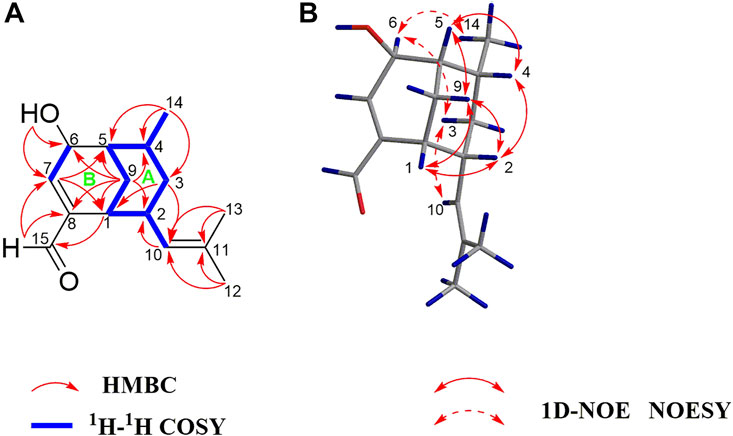
FIGURE 2. (A) Key 1H–1H COSY and HMBC correlations of 1; (B) Key 1D-NOE and NOESY correlations of 1.
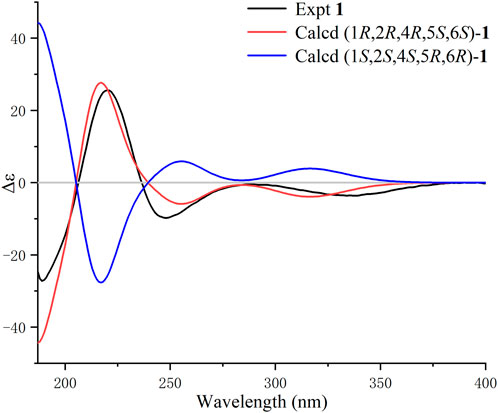
The NOE difference spectrum of 1 shows that the H-2 and H-9b signals are enhanced when H-1 is irradiated; and the H-4 and H-9b signals are enhanced when H-5 is irradiated (Figure 2). This indicates that H-2 and H-4 have the same orientation as the methano bridge (C-1−C-9−C-5), which is consistent with the NOESY correlations of H-2 with H-4 and H-9b. However, the correlations of H3-14/H-6, H-3b/H-6, and H-3b/H-10 indicate that these protons are oriented in the opposite direction of the methano bridge. Based on the comparison of calculated and experimental ECD data, the absolute configuration of 1 is 1R,2R,4R,5S, 6S (Figure 3). Interestingly, compound 1 is an analogue of curcumane B, which was reported as a sesquiterpenoid with an unprecedented skeleton in 2019 (Liu et al., 2019). Compound 1 is labelled curcumane E.
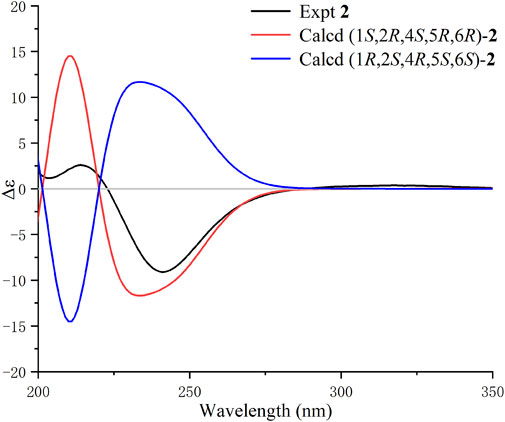
The UV, IR, HRESIMS, and NMR data of compound 2 suggest that it is an isomer of compound 1. Analysis of the 2D NMR (1H−1H COSY and HMBC) data of 2 (Figure 4) confirms that this compound has the same planar structure as 1. However, the H-3b, H-4, H-10, and H-12 resonances in the 1H NMR spectrum of 2 are deshielded by ΔδH +0.35, +0.23, +0.93, and 0.18 ppm, respectively, compared to the same resonances in the spectrum of 1. Meanwhile, the H-1 and H-2 resonances are shielded by ΔδH −0.16 and −0.18 ppm, respectively. The 13C chemical shifts of C-2, C-3, C-4, C-7, C-8, C-9, and C-10 in 2 are also different from those of the same carbon atoms in 1. Therefore, compounds 2 and 1 are a pair of diastereomers. The NOESY spectrum of 2 exhibits correlations of H-6 with H3-14 and H-3b; and H-10 with H-9a and H-3a, which reveals that the isobutenyl-2, H-4, and OH-6 moieties are close to the methano bridges (C-1–C-9–C-5), while H-2, Me-4, and H-6 are oriented in the opposite direction of this bridge (Figure 4). Finally, the absolute configuration of 2 is elucidated as 1S,2R,4S,5R,6R, based on the comparison of the calculated and experimental ECD data (Figure 5). Compound 2 is labelled curcumane F.
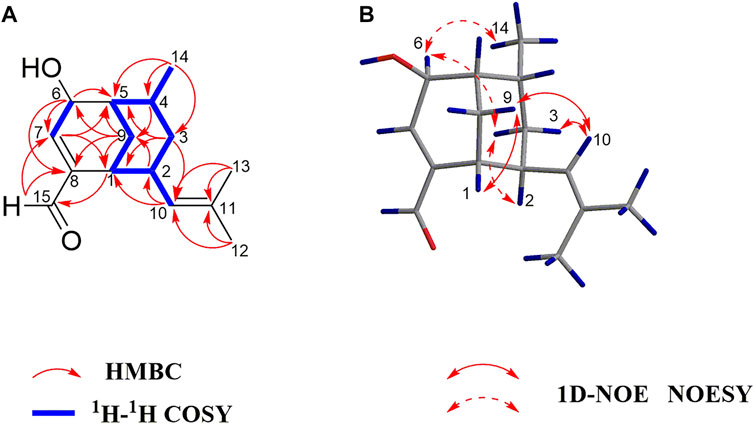
FIGURE 4. (A) Key 1H–1H COSY and HMBC correlations of 2; (B) Key 1D-NOE and NOESY correlations of 2.
Effects of compounds 1 and 2 on the KCl-induced contractions of rat aortic rings
Previous studies show that the sesquiterpenoids isolated from C. longa possess endothelium-dependent vasorelaxant activity, endothelium-independent vasorelaxant activity, or both (Liu et al., 2019; Qiao et al., 2019; Chen et al., 2022). Therefore, the vasorelaxant effects of compounds 1 and 2 on pre-contracted rat aorta rings were investigated in this study, using methoxyverapamil as the positive control. As shown in Figure 6, compounds 1 and 2 exhibit a concentration-dependent relaxation effect on the KCl-induced contraction of rat aortic rings, with EC50 values of 5.10 ± 0.79 and 5.58 ± 1.77 μM, respectively (EC50 = 0.50 ± 0.05 μM for methoxyverapamil). The Emax values corresponding to the activities of 1, 2, and methoxyverapamil against KCl-induced contractions are 82.87 ± 5.36%, 83.44 ± 5.24%, and 100.00%, respectively. Unfortunately, no follow-up mechanism research was carried out due to the limited amounts of 1 and 2. Notably, the vasorelaxant activities of 1 and 2 are similar, which suggests that this activity is not significantly affected by stereochemistry. However, a comparison of the EC50 values of compound 1 and curcumane B (Liu et al., 2019) indicates that the substituents at C-3 and C-8 play an important role in vasorelaxation.
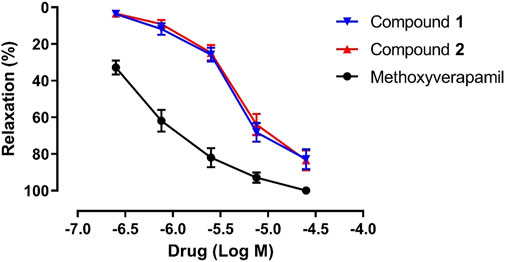
FIGURE 6. Vasorelaxant effects of compounds 1, 2, and methoxyverapamil on rat aortic rings pre-contracted with KCl (n = 5).
Conclusion
In summary, two unusual sesquiterpenoids with a dicyclo [3.3.1]nonane moiety (curcumanes E and F) were isolated from the rhizome of C. longa. The sesquiterpenoid skeleton characterized herein has been reported only once before (Liu et al., 2019). Moreover, curcumanes E and F are a pair of diastereomers that have similar vasorelaxant effects on the contracted rat aortic rings induced by KCl. Collectively, this study and our previous studies (Liu et al., 2019; Qiao et al., 2019) reveal that the rare sesquiterpenoids extracted from the rhizome of C. longa are considerably effective substances, even though they are not the main types of sesquiterpenoids in C. longa.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by the Committee on the Ethics of Animal Experiments of Chengdu University of Traditional Chinese Medicine (Approval No. 2020–04).
Author contributions
JL and M-MQ performed most of the phytochemical experiments and wrote the manuscript, both authors contributed equally to this work. H-ZS performed most of the pharmacological experiments. C-WM helped with phytochemical experiments. CP supervised the work. LX and FL designed the research and revised the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 82022072, 81903777, and 82104371), the Fok Ying Tung Education Foundation (Grant No. 171037), and the Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (Grant No. ZYYCXTD-D-202209).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.995950/full#supplementary-material
References
Cane, D. E. (1999). Sesquiterpene biosynthesis: Cyclization mechanisms. Compr. Nat. Prod. Chem. 2, 155–200. doi:10.1016/B978-0-08-091283-7.00039-4
Chang, Y. C., Chiang, C. C., Chang, Y. S., Chen, J. J., Wang, W. H., Fang, L. S., et al. (2020). Novel caryophyllane-related sesquiterpenoids with anti-inflammatory activity from Rumphella antipathes (linnaeus, 1758). Mar. Drugs 18 (11), 554. doi:10.3390/md18110554
Chen, B., Zhang, Y., Wang, Y., Rao, J., Jiang, X., and Xu, Z. (2014). Curcumin inhibits proliferation of breast cancer cells through nrf2-mediated down-regulation of Fen1 expression. J. Steroid Biochem. Mol. Biol. 143, 11–18. doi:10.1016/j.jsbmb.2014.01.009
Chen, J. F., Liu, F., Qiao, M. M., Shu, H. Z., Li, X. C., Peng, C., et al. (2022). Vasorelaxant effect of curcubisabolanin A isolated from Curcuma longa through the PI3K/Akt/eNOS signaling pathway. J. Ethnopharmacol. 294, 115332. doi:10.1016/j.jep.2022.115332
Chen, J. J., Tsai, C. S., Hwang, T. L., Shieh, P. C., Chen, J. F., and Sung, P. J. (2010). Sesquiterpenes from the rhizome of Curcuma longa with inhibitory activity on superoxide generation and elastase release by neutrophils. Food Chem. x. 119 (3), 974–980. doi:10.1016/j.foodchem.2009.07.060
Han, X., Wang, Q., Luo, X., Tang, X., Wang, Z., Zhang, D., et al. (2022). Lemnalemnanes A-C, three rare rearranged sesquiterpenoids from the soft corals Paralemnalia thyrsoides and lemnalia sp. Org. Lett. 24 (1), 11–15. doi:10.1021/acs.orglett.1c03386
Hu, G. Y., Peng, C., Xie, X. F., Xiong, L., Zhang, S. Y., and Cao, X. Y. (2018). Patchouli alcohol isolated from Pogostemon cablin mediates endothelium-independent vasorelaxation by blockade of Ca2+ channels in rat isolated thoracic aorta. J. Ethnopharmacol. 220, 188–196. doi:10.1016/j.jep.2017.09.036
Lin, X., Ji, S., Li, R., Dong, Y., Qiao, X., Hu, H., et al. (2012). Terpecurcumins A-I from the rhizomes of Curcuma longa: Absolute configuration and cytotoxic activity. J. Nat. Prod. 75 (12), 2121–2131. doi:10.1021/np300551g
Liu, J. Q., Wang, C. F., Li, Y., Luo, H. R., and Qiu, M. H. (2012). Isolation and bioactivity evaluation of terpenoids from the medicinal fungus Ganoderma sinense. Planta Med. 78 (4), 368–376. doi:10.1055/s-0031-1280441
Liu, M., Li, P., Tang, X., Luo, X., Liu, K., Zhang, Y., et al. (2021). Lemnardosinanes A-I: New bioactive sesquiterpenoids from soft coral lemnalia sp.J. Org. Chem. 86 (1), 970–979. doi:10.1021/acs.joc.0c02463
Liu, Y., Liu, F., Qiao, M. M., Guo, L., Chen, M. H., Peng, C., et al. (2019). Curcumanes A and B, two bicyclic sesquiterpenoids with significant vasorelaxant activity from Curcuma longa. Org. Lett. 21 (4), 1197–1201. doi:10.1021/acs.orglett.9b00149
Llano, S., Gómez, S., Londoño, J., and Restrepo, A. (2019). Antioxidant activity of curcuminoids. Phys. Chem. Chem. Phys. 21 (7), 3752–3760. doi:10.1039/c8cp06708b
Moghadamtousi, S. Z., Kadir, H. A., Hassandarvish, P., Tajik, H., Abubakar, S., and Zandi, K. (2014). A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed. Res. Int. 2014, 1–12. doi:10.1155/2014/186864
Prete, D. D., Millán, E., Pollastro, F., Chianese, G., Luciano, P., Collado, J. A., et al. (2016). Turmeric sesquiterpenoids: Expeditious resolution, comparative bioactivity, and a new bicyclic turmeronoid. J. Nat. Prod. 79 (2), 267–273. doi:10.1021/acs.jnatprod.5b00637
Qiao, M. M., Liu, F., Liu, Y., Guo, L., Zhou, Q. M., Peng, C., et al. (2019). Curcumane C and (±)-Curcumane D, an unusual seco-cadinane sesquiterpenoid and A pair of unusual nor-bisabolane enantiomers with significant vasorelaxant activity from Curcuma longa. Bioorg. Chem. 92, 103275. doi:10.1016/j.bioorg.2019.103275
Singh, G., Kapoor, I. P. S., Singh, P., de Heluani, C. S., de Lampasona, M. P., and Catalan, C. A. N. (2010). Comparative study of chemical composition and antioxidant activity of fresh and dry rhizomes of turmeric (Curcuma longa linn.). Food Chem. Toxicol. 48 (4), 1026–1031. doi:10.1016/j.fct.2010.01.015
Sun, W., Wang, S., Zhao, W., Wu, C., Guo, S., Gao, H., et al. (2017). Chemical constituents and biological research on plants in the genus Curcuma. Crit. Rev. Food Sci. Nutr. 57 (7), 1451–1523. doi:10.1080/10408398.2016.1176554
Ti, H., Mai, Z., Wang, Z., Zhang, W., Xiao, M., Yang, Z., et al. (2021). Bisabolane-type sesquiterpenoids from Curcuma longa L. Exert anti-influenza and anti-inflammatory activities through NF-κB/MAPK and RIG-1/STAT1/2 signaling pathways. Food Funct. 12 (15), 6697–6711. doi:10.1039/d1fo01212f
Wu, J. W., Tang, C. P., Yao, S., Ke, C. Q., and Ye, Y. (2021). Three new carabrane sesquiterpenoid derivatives from the whole plant of Carpesium abrotanoides L. Chin. J. Nat. Med. 19 (11), 868–873. doi:10.1016/S1875-5364(21)60091-2
Xiao, Y. C., Xie, J., Yu, M., Liu, M., Ran, J., Xi, Z., et al. (2011). Bisabocurcumin, A new skeleton curcuminoid from the rhizomes of Curcuma longa L. Chin. Chem. Lett. 22 (12), 1457–1460. doi:10.1016/j.cclet.2011.09.002
Xiong, L., Zhou, Q. M., Zou, Y., Chen, M. H., Guo, L., Hu, G. Y., et al. (2015). Leonuketal, a spiroketal diterpenoid from Leonurus japonicus. Org. Lett. 17 (24), 6238–6241. doi:10.1021/acs.orglett.5b03227
Yin, G. P., Gong, M., Xue, G. M., Gong, T., Cao, X., Wang, W., et al. (2021). Penispidins A-C, aromatic sesquiterpenoids from Penicillium virgatum and their inhibitory effects on hepatic lipid accumulation. J. Nat. Prod. 84 (10), 2623–2629. doi:10.1021/acs.jnatprod.1c00185
Yuan, T., Zhang, C., Qiu, C., Xia, G., Wang, F., Lin, B., et al. (2018). Chemical constituents from Curcuma longa L. And their inhibitory effects of nitric oxide production. Nat. Prod. Res. 32 (16), 1887–1892. doi:10.1080/14786419.2017.1354185
Keywords: Curcuma longa, Zingiberaceae, sesquiterpenoids, absolute configuration, vasorelaxant activity
Citation: Liu J, Qiao M-M, Peng C, Shu H-Z, Meng C-W, Liu F and Xiong L (2022) Curcumanes E and F, two rare sesquiterpenoids with a dicyclo[3.3.1]nonane moiety, from Curcuma longa and their vasorelaxant activities. Front. Chem. 10:995950. doi: 10.3389/fchem.2022.995950
Received: 16 July 2022; Accepted: 05 August 2022;
Published: 02 September 2022.
Edited by:
Giselle Zenker Justo, Universidade Federal de Sao Paulo, BrazilReviewed by:
Hiroshi Noguchi, Nihon Pharmaceutical University, JapanLv Tian Ming, Shenyang Pharmaceutical University, China
Copyright © 2022 Liu, Qiao, Peng, Shu, Meng, Liu and Xiong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheng Peng, cGVuZ2NoZW5nQGNkdXRjbS5lZHUuY24=; Fei Liu, ZmVpZmVpZmx5NTU1QDEyNi5jb20=; Liang Xiong, eGlsaW5nQGNkdXRjbS5lZHUuY24=
†These authors have contributed equally to this work
 Juan Liu1,2,3†
Juan Liu1,2,3† Liang Xiong
Liang Xiong