- 1TCM and Ethnomedicine Innovation and Development International Laboratory, Innovative Materia Medica Research Institute, School of Pharmacy, Hunan University of Chinese Medicine, Changsha, China
- 2Department of Chemistry, Karakoram International University, Gilgit, Pakistan
- 3Hunan Province Key Laboratory of Plant Functional Genomics and Developmental Regulation, College of Biology, Hunan University, Changsha, China
- 4Clinic Experimental Research Center, The First People’s Hospital of Huaihua, Huaihua, China
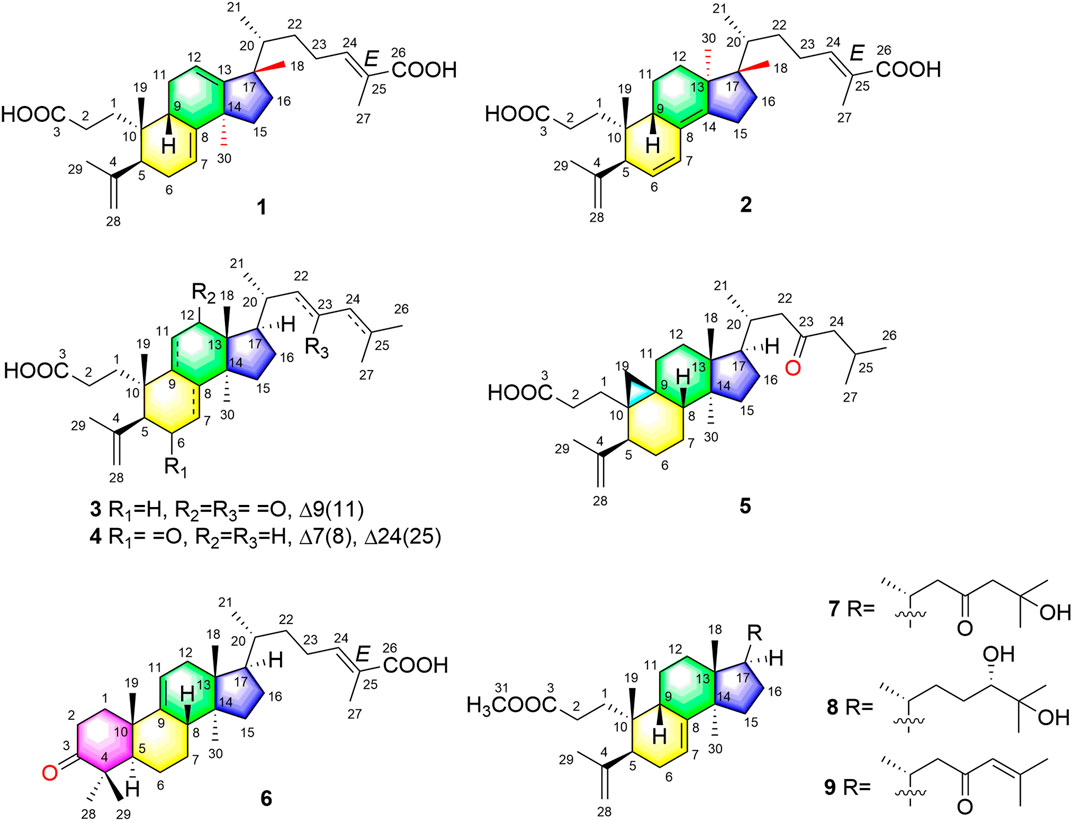
One new 3,4-seco-17,13-friedo-lanostane triterpenoid heilaohuacid A (1), one new 3,4-seco-17,14-friedo-lanostane triterpenoid heilaohuacid B (2), five new 3,4-seco-lanostane triterpenoids heilaohuacids C-D (3–4) and heilaohumethylesters A-C (7–9), one new 3,4-seco-cycloartane triterpenoid heilaohuacid E (5), and one new intact-lanostane triterpenoid heilaohuacid F (6), together with twenty-two known analogues (10–31), were isolated from heilaohu. Their structures were determined using HR-ESI-MS data, 1D and 2D NMR spectra, 13C NMR calculations, and electronic circular dichroism (ECD) calculations. Heilaohuacids A and B (1 and 2) contain a 3,4-seco ring A and unprecedented migration of Me-18 from C-13 to C-17 or C-14 to C-18. This type of lanostane triterpenoid derivatives was rarely reported so far. More importantly, all compounds against inflammatory cytokines IL-6 and TNF-α levels on LPS-induced RAW 264.7 macrophages were evaluated, and compounds 4 and 31 significantly inhibited the release level of IL-6 with IC50 values of 8.15 and 9.86 μM, respectively. Meanwhile, compounds 17, 18, and 31 significantly inhibited proliferation of rheumatoid arthritis-fibroblastoid synovial (RA-FLS) cells in vitro with IC50 values of 7.52, 8.85, and 7.97 μM, respectively.
Introduction
Schisandraceae is a famous medicinal plant family, comprising only two genera Kadsura and Schisandra. This family of medicinal plants are enriched with lanostane, cycloartane, and schinortriterpenoid (SNT) triterpenoids (Shi et al., 2015), which possesses remarkable anti-inflammation (Yu H.-H. et al., 2019), cytotoxicity (Gao et al., 2008), and anti-HIV activities (Yang et al., 2010). The dried roots of Kadsura coccinea called “heilaohu” in Chinese have been used in Tujia ethnomedicine to treat rheumatic arthritis (RA), gastric and duodenal ulcers, etc. (Xu et al., 2019). In the past decade, tremendous development has been made on the chemistry and biological properties of K. coccinea, which have yielded a number of dibenzocyclooctadiene lignans (Liu et al., 2014) and lanostane triterpenoids (Hu et al., 2016). In our early research studies, we have reported the isolation and structural elucidation of several new triterpenoids, sesquiterpenoids, and lignans from K. coccinea and other species of the same genus (Liu et al., 2018; Cao et al., 2019; Shehla et al., 2020). Furthermore, Kadsura heteroclita (Roxb.) Craib. in the same genus Kadsura displayed good anti-rheumatoid arthritis, anti-inflammatory, and analgesic effects (Yu H.-H. et al., 2019; Yu H. et al., 2019). Tujia ethnomedicine heilaohu also possess anti-RA agents. Herein, the phytochemistry, anti-RA-FLS cells, and anti-inflammation activity investigations on structurally interesting triterpenoids from the roots of K. coccinea were carried out. One new 3,4-seco-17,13-friedo-lanostane triterpenoid heilaohuacid A (1), one new 3,4-seco-17,14-friedo-lanostane triterpenoid heilaohuacid B (2), five new 3,4-seco-lanostane triterpenoids heilaohuacids C-D (3–4) and heilaohumethylesters A-C (7–9), one new 3,4-seco-cycloartane triterpenoid heilaohuacid E (5), and one new intact-lanostane triterpenoid heilaohuacid F (6) (Figure 1), together with twenty-two known analogues (10–31), were isolated from heilaohu (Supplementary Figure S1). Their structures were determined by various chromatographic and spectroscopic techniques. All compounds were evaluated for their anti-inflammatory effects and inhibited proliferation of RA-FLS cell activity. Herein, the isolation, structural elucidation of new compounds 1–9, along with in vitro anti-inflammatory and inhibited proliferation of RA-FLS cell activity screening will be reported.
Results and Discussion
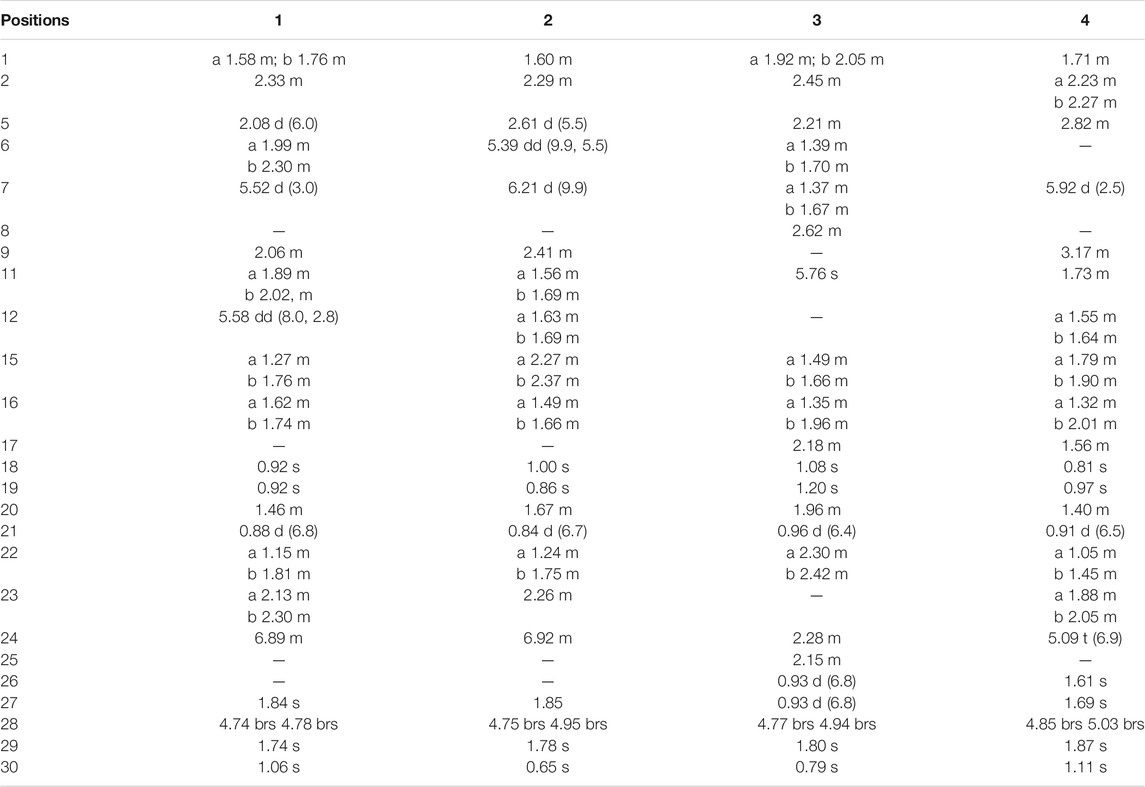
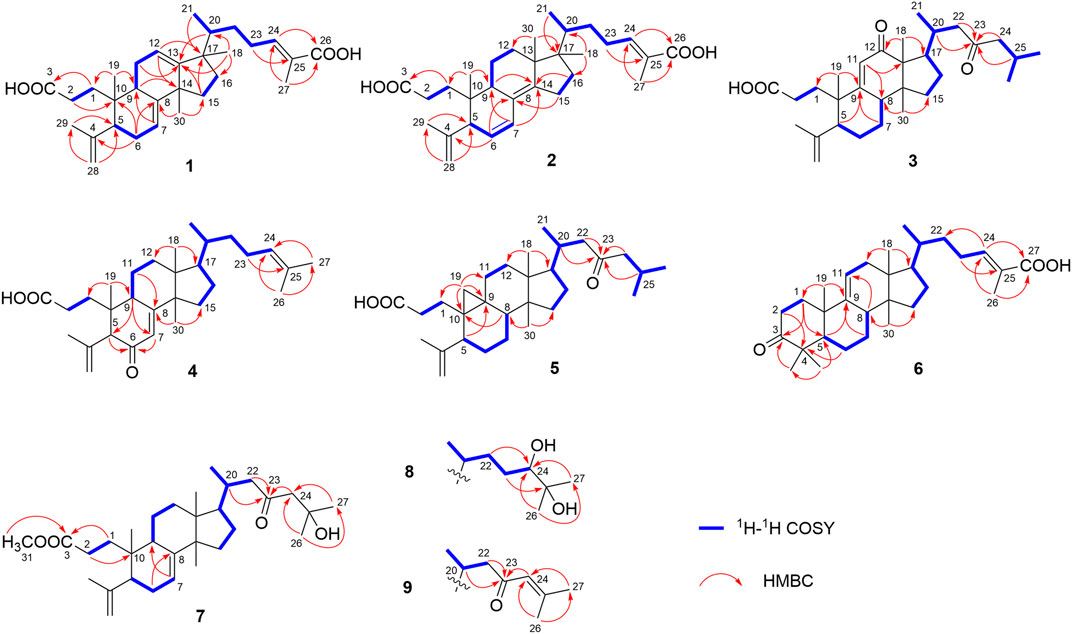
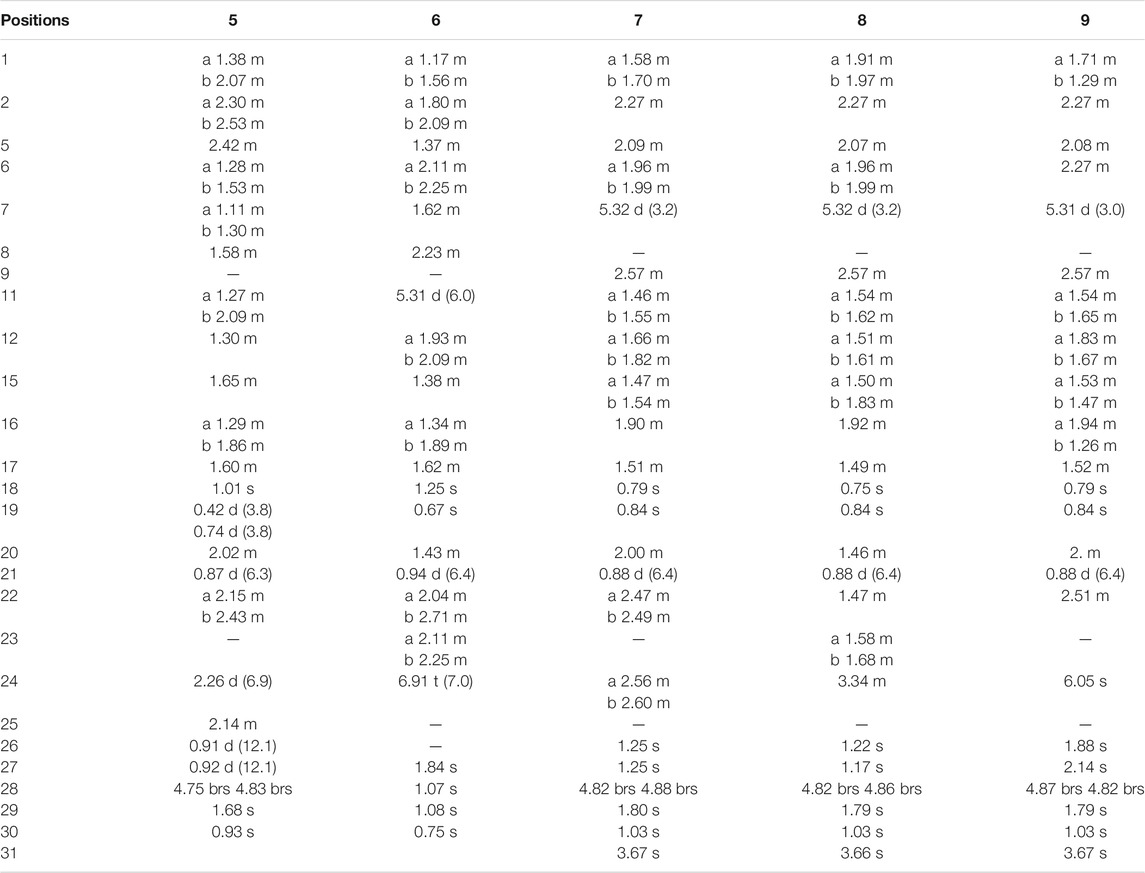
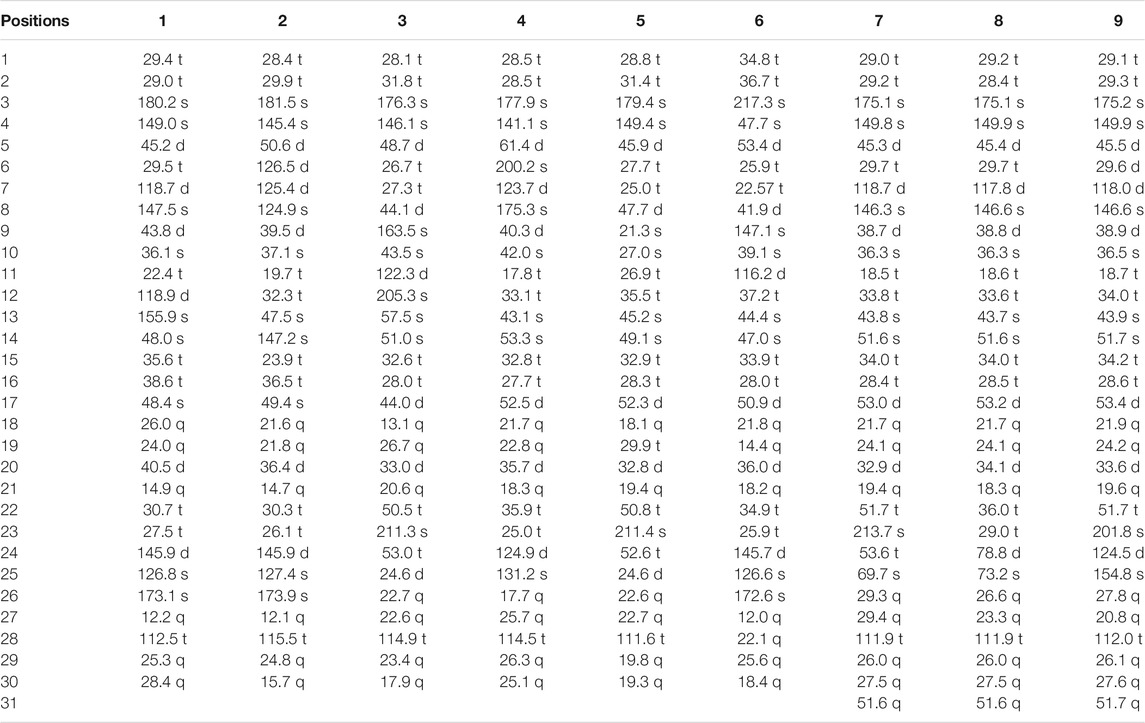
Compound 1 was derived as a white amorphous powder, and the molecular formula C30H44O4, with 9 degrees of unsaturation, was deduced from the HR-ESI-MS at 491.3143 [M + Na]+ (calcd. for 491.3137, C30H44O4Na) and its 13C NMR data. The 1H NMR data showed typical resonances for five tertiary methyls (δH 0.92, 0.92, 1.84, 1.74, and 1.06, each 3H, s), one double methyl (δH 0.88, d, J = 6.8 Hz), and five olefinic protons (δH 5.52, 5.58, 6.89, 4.74, and 4.78, each 1H). The 13C NMR data with the aid of DEPT and HSQC spectra revealed the presence of six methyls, nine methylenes, six methines (three tri-substituted double bond), and nine quaternary carbons (two carboxyl groups). Detailed analyses of 1H NMR, 13C NMR, DEPT, and HSQC spectra enabled all proton resonances of 1 to be attributed to their respective carbons (Tables 1, 3). The planar structure of 1 was elucidated by interpretation of HMBC and 1H-1H COSY spectra. The 1H-1H COSY spectrum of 1 revealed the presence of five independent spin systems (H2-1/H2-2, H-5/H-6/H-7, H-9/H2-11/H-12, H2-15/H2-16, and H3-21/H-20/H2-22/H2-23/H-24). The HMBC cross-peaks (Figure 2) of H-1 with C-3, H2-28 with C-5 and C-29, H2-6 and H3-30 with C-8, H2-11 with C-13, H-12 with C-13, C-14, and C-17, H2-23 and H-24 with C-24, C-25, and C-27, and H3-27 with C-24 and C-26 constructed that the ring A was seco between C-3 and C-4, as well as the presence of a carboxylic acid at C-26, and three double bone groups at C-7/C-8, C-12/C-13, and C-24/C-25, respectively. Importantly, the HMBC correlations from H3-18 to C-17, C-16, and C-20 indicated that an unprecedented migration of Me-18 from C-13 to C-17 occurred. Accordingly, the planar structure of 1 was determined as a novel 3,4-seco-17,13-friedo-lanostane triterpenoid derivative with a tricyclic skeleton. To the best of our knowledge, this type of lanostane triterpenoid with A ring 3,4-seco along with Me-18 migration from C-13 to C-17 was rarely reported before.
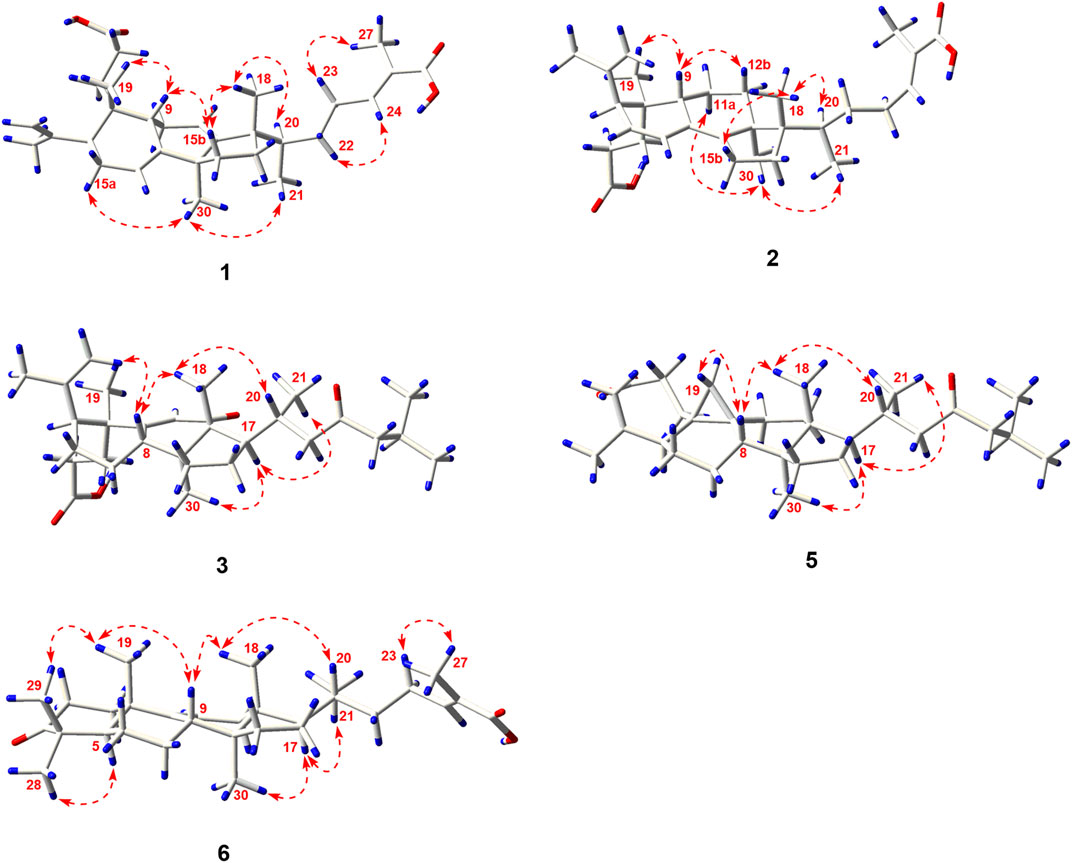
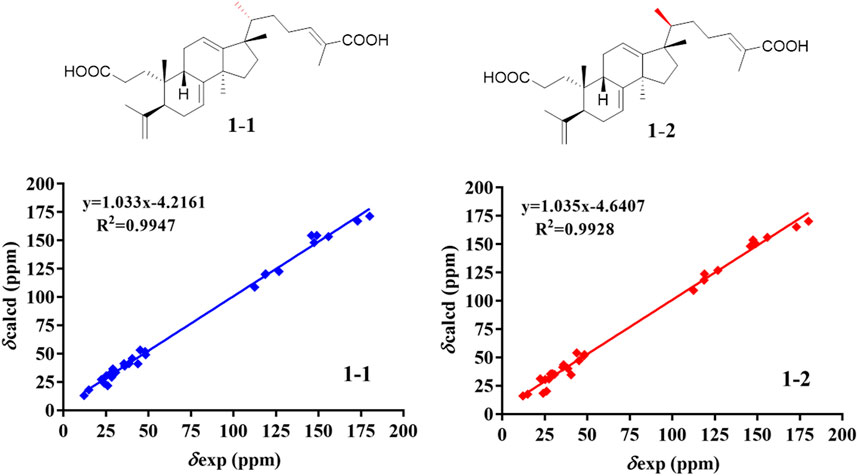
The relative configuration of 1 was ascertained through interpretation of its ROESY spectrum. H3-19 was assigned a β-orientation. The NOE correlations (Figure 3) of H3-19 with H-9 and H-15b, H3-18 with H-15b and H-20, and H-15a with H3-30; in addition, there are no NOE correlations between H-9 and H3-30, suggesting that H-9 and H3-18 were β-oriented, and H3-30 was a-oriented. Lack of NOE correlations of H3-27 with H-24 indicated that the double bond between C-24 and C-25 has a trans-configuration. Additionally, the relative configuration of C-20 was investigated by the TDDFT to calculate the 13C NMR data for 1-1 and 1-2. As shown in Figure 4, the 13C NMR chemical shifts of isomers were calculated at the mPW1PW91/6-31+G** level. The calculation result of 1-1 (R2 = 0.9947) matched the experimental data better than 1-2 (R2 = 0.9928), which indicated that H3-21 has an a-orientation. To further elucidate its absolute configuration, the electronic circular dichroism (ECD) spectrum of 1 was recorded in MeOH, and it showed a good agreement with the calculated ECD spectrum of the (5, 9, 10, 14, 17S, and 20R) model (Figure 5), which supported the absolute configuration of 1 should be identical to 5, 9, 10, 14, 17S, and 20R. Thus, compound 1 was elucidated as a novel 3,4-seco-17,13-friedo-lanostane triterpenoid and named heilaohuacid A, accordingly.
Heilaohuacid B (2) had the same molecular formula of C30H44O4 as compound 1, based on the HR-ESI-MS at m/z 467.3105 [M-H]− (calcd. for 467.3161, C30H43O4). Its 1H NMR spectrum displayed signals characteristic of five olefinic protons at δH 6.92 (m, H-24), 6.21 (d, J = 9.9 Hz, H-7), 5.39 (dd, J = 9.9, 5.5 Hz, H-6), 4.95 (br s, Hb-28), and 4.75 (br s, Ha-28). The 1H NMR spectrum also displayed one methyl doublet at δH 0.84 (d, J = 6.7 Hz, H3-21) and five methyl groups at δH 1.85 (H3-27), 1.78 (H3-29), 1.00 (H3-18), 0.86 (H3-19), and 0.65 (H3-30). The 13C NMR and DEPT spectra data (Table 3) of 2 highlighted the presence of 30 carbon signals, including six methyls, nine methylenes, six methines, and nine quaternary carbons. This confirmed that compound 2 was a lanostane triterpenoid derivative with tricyclic skeleton. The HMBC cross-peaks (Figure 2) of H-1 with C-3, H2-28 with C-5 and C-29, H-6 with C-4 and C-8, H-7 with C-9 and C-14, H-9 and H2-15 with C-8 and C-14, H2-23 and H-24 with C-24, C-25, and C-26, and H3-27 with C-24 and C-26 constructed that the ring A was seco between C-3 and C-4, as well as the presence of a carboxylic acid at C-26, and three double bone groups at C-6/C-7, C-8/C-14, and C-24/C-25, respectively. Additionally, the HMBC correlations from H3-18 to C-17, C-16, and C-20, and from H3-30 to C-13, C-12, and C-17 indicated that interesting migrations of Me-18 from C-13 to C-17 and Me-30 from C-14 to C-13 took place. To date, this type of lanostane triterpenoid derivative was rarely reported (Lavoie et al., 2013). Accordingly, the planar structure of 2 was determined as a 3,4-seco-17,14-friedo-lanostane triterpenoid.
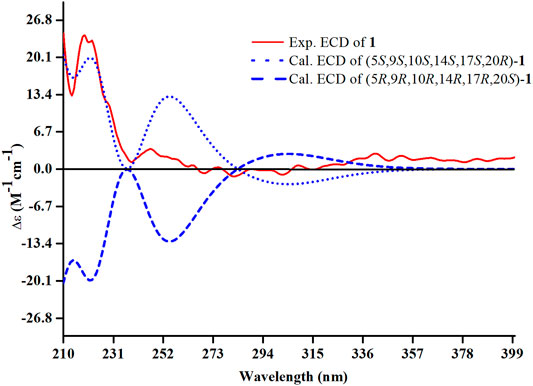
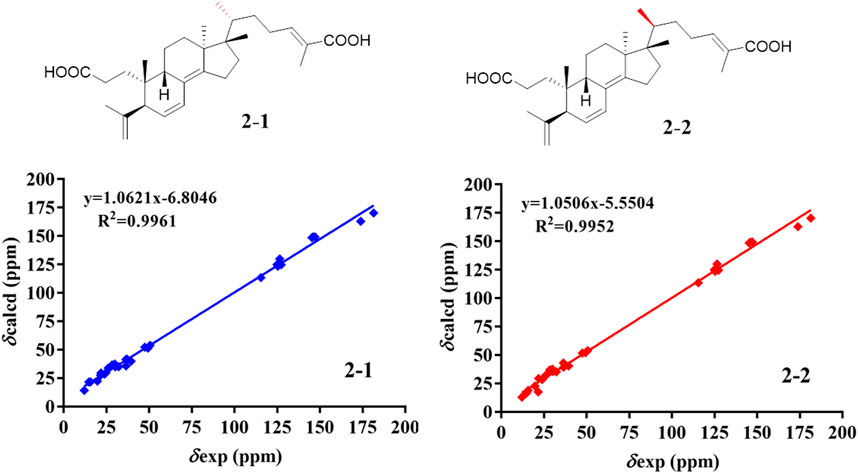
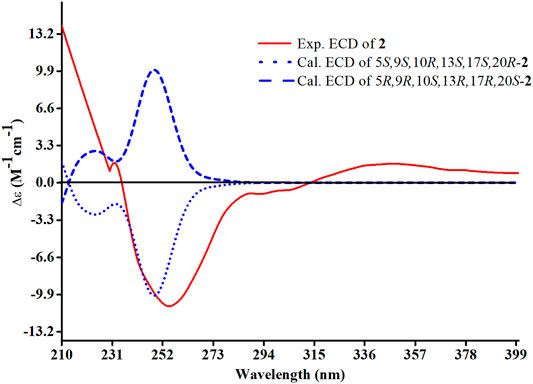
The relative configuration of 2 was determined following the NOE effects, which showed correlations (Figure 3) of H3-19 with H-9 and H-12b, H3-18 with H-15b and H-20, and H3-30 with H-11a and H3-21, suggesting that H-9 and H3-18 were β-oriented, and H3-21 and H3-30 were a-oriented. Moreover, the no NOE correlations of H3-27 with H-24 were found, suggesting that the double bond between C-24 and C-25 has a trans-configuration. Additionally, the relative configuration of C-20 was investigated by the TDDFT to calculate the 13C NMR data for 2-1 and 2-2. As shown in Figure 6, the 13C NMR chemical shifts of isomers were calculated at the mPW1PW91/6-31+G** level. The calculation result of 2–1 (R2 = 0.9961) matched the experimental data better than 2-2 (R2 = 0.9952), which indicated that H3-21 has an a-orientation. The absolute configuration of 2 was determined by ECD data. The experimental ECD spectrum of 2 exhibited a negative cotton effect around 250 nm, which was consistent with the calculated ECD data of the (5S,9S,10R,13S,17S,20R) model (Figure 7). Thus, heilaohuacid B (2) was elucidated as a 5S, 9S, 10R, 13S, 17S, and 20R absolute configuration.
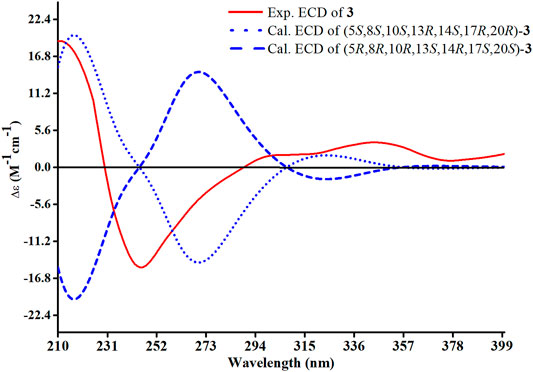
Compound 3 had a molecular formula of C30H46O4, requiring eight indices of hydrogen deficiency by analyzing the HR-ESI-MS at m/z 469.3352 [M-H]− (calcd. for 469.3328, C30H45O4). Comprehensive analysis of the 1D and 2D NMR data revealed it to be the derivative of seco-coccinic acid K (Wang et al., 2012). The differences were that a methylene group at C-12 was replaced by a conjugated ketone group (δC 205.3, C-12) and the absence of a methoxy group at C-31 in 3, which were confirmed by the HMBC correlations of H-11 with C-12, C-9, and C-13, H3-18 with C-12, and H2-1 and H2-2 with C-3. The relative configuration of H-8 was determined to be β-oriented, through the NOE correlations of H3-18 with H-8. The 5, 8, 10S, 13R, 14S, 17R, and 20R absolute configuration of 3 was determined by comparing the experimental and calculated ECD spectra (Figure 8). Accordingly, the structure of compound 3 was deduced as shown and given the trivial name heilaohuacid C.
Compound 4 was derived as a white amorphous powder with a molecular formula of C30H46O3. The molecular formula of compound 4 was determined by analyzing the HR-ESI-MS at m/z 453.3406 [M-H]− (calcd. for 453.3374, C30H45O4). Comprehensive analyses of its NMR data (Tables 1, 3) suggested 4 to be a structural analogue of 3 as both shared the same 3,4-seco-lanostane triterpenoid skeleton. However, the obvious differences were that an α,β-conjugated ketone group (δC 200.2, C-6; δC 123.7, C-7; δC 175.3, C-8) shifted from ring C to ring B, a double bond at C-24/C-25 was present, but a ketone group was absent at C-23 in 4, as supported using the HMBC spectral analyses. The relative configuration was confirmed by ROESY spectral analyses. Based on the NOE correlation of H3-19 with H-9, the H-9 was classified as β-oriented. Thus, the structure of 4 was assigned as shown in Figure 1, and named heilaohuacid D.
The molecular formula of compound 5 was C30H48O3, as determined by the HR-ESI-MS ion at m/z 455.3532 [M-H]− (calcd. for 455.3525, C30H47O3), suggesting 7° of unsaturation. The 1H and 13C NMR spectra showed typical resonances for 3,4-seco ring A δH 4.75, 4.83 (each 1H, br s, H2-28), 1.68 (3H, s, H3-29) and δC 179.4 (C-3), 149.4 (C-4), 111.6 (C-28), and a pair of methylene doublets at δH 0.42 (J = 3.8 Hz) and 0.74 (J = 3.8 Hz), characteristics of the C-19 protons and carbon of the cyclopropane ring, suggesting that six was a 3,4-seco-cycloartane triterpenoid (Yang et al., 2015). Analysis of the 1D NMR data (Tables 2, 3) revealed that the structure of 5 was very similar to nigranoic acid (25) (Sun et al., 1996). The obvious differences were the presence of a ketone group at C-23, and the absence of double bond at C-24/C-25 and carboxyl groups at C-27 in 5. The HMBC cross-peaks of H2-22 (δH 2.15, 2.43) and H2-24 (δH 2.26) with C-23 (δC 211.4) supported the ketone group locate at C-23. In a ROESY experiment, H-8 (δH 1.58) showed correlations with H3-18 (δH 1.01), which indicated that H-8 was β-oriented. Hence, compound 5 was elucidated as shown and named heilaohuacid E.
Compound 6 was obtained as a white amorphous powder and its molecular formula was deduced to be C30H46O3 based on the HR-ESI-MS showing molecular ion at m/z 453.3364 [M-H]− (calcd. for 453.3374, C30H45O3) with 8° of unsaturation. The 1H NMR data (Table 2) showed the characteristic signals attributable to one methyl doublet at δH 0.94 (d, J = 6.4 Hz, H3-21), six methyl singlet protons at δH 1.25 (H3-18), 0.67 (H3-19), 1.84 (H3-27), 1.07 (H3-28), 1.08 (H3-29), and 0.75 (H3-30), and two olefinic protons at δH 5.31 (d, J = 6.0 Hz, H-11) and 6.91 (t, J = 7.0 Hz, H-24). Analyses of the 13C NMR and DEPT data (Table 3) showed that compound 6 contained seven methyls, nine methylenes, six methines (two olefinics), and eight quaternary carbons (two carboxyl group). These evidences indicated that compound 6 was an intact lanostane-type triterpenoid, whose 1H and 13C NMR spectroscopic data were very similar to those of coccinic acid (Li and Xue, 1986). The only difference was in the geometry of the double bond between C-24 and C-25. Because the carbon chemical shift of C-27 was shifted upfield by 8.0 ppm, compared with coccinic acid, this showed the presence of a double bond between C-24 and C-25 in 6, which was different with that of coccinic acid. Additionally, the configuration of 6 was determined using the ROESY spectrum (Supplementary Figure S42), in which H-27 showed correlation with H-23 but no NOE correlation was observed between H-27 and H-24, demonstrating that the geometry of the double bond must be E configuration (Figure 3). Therefore, the structure of 6 was elucidated as shown and assigned name heilaohuacid F.
Heilaohumethylester A (7) was assigned a molecular formula of C31H50O4 based on the HR-ESI-MS spectra and NMR data analysis (Tables 2, 3), suggesting that 7 was a methylated analogue of seco-coccinic acid C (16) (Wang et al., 2008). The presence of a methoxy group (δC 51.6, δH 3.67) was located at C-31, confirmed by the HMBC correlations of H3-31 with the carbonyl (δC 175.1) at C-3. The similar chemical shifts, coupling constants, and NOE correlations with 16 determined the relative configurations of 7. Therefore, the structure of 7 was elucidated as shown.
Compound 8 was deduced to have the molecular formula of C31H52O4 from the molecular ion at m/z 511.3734 [M + Na]+ (calcd. for C31H52O4Na, 511.3758) in the HR-ESI-MS data. The NMR data of 8 were highly similar to those of 7; HMBC spectral analysis showed that the obvious differences were absence of a ketone group at C-23 and a hydroxyl group was present at C-24 in 8. Comparison of the NMR data of 8 with those of a pair of 24-epimers (Hong et al., 2013) with the OH at C-24 possessed different orientations, 24(S)-24,25-dihydroxytirucall-7-en-3-one (δC 78.6, δH 3.32) and (24R)-24,25-dihydroxytirucall-7-en-3-one (δC 79.5, δH 3.29). This indicated that the NMR data of 8 (δC 78.8, δH 3.34) were almost similar to the corresponding 24(S)-24,25-dihydroxytirucall-7-en-3-one, suggesting that the C-24 stereochemistry should be assigned as 24S in 8. According to analysis of the NOE effect, and the ROESY cross-peaks of H3-19 with H-9, the H-9 was classified as β-oriented. Finally, the structure of 8 was identified, and named heilaohumethylester B accordingly.
Compound 9 was obtained as a white amorphous powder, and its molecular formula was found to be C31H48O3 deduced from HR-ESI-MS, indicating quasi-molecular ion peak at m/z 491.3499 [M + Na]+ (calcd. for 491.3501, C31H48O3Na). The 1H and 13C NMR data (Tables 2, 3) resembled that of 7. However, a proton signal for OH-25 was absent with the presence of a double bond between C-24 and C-25. This was supported by the carbon chemical shifts of C-25 at δC 154.8, and verified by the HMBC correlations. The relative configurations of all the stereo-genic centers were assigned to be the same as 7. Hence, the structure of 9 was deduced as shown and named heilaohumethylester C.
Twenty-two known analogues were identified as masticadienoic acid (10) (Jain et al., 1995), abiesatrine D (11) (Yang et al., 2010), 24(E)-3,4-seco-9βH-lanosta-4 (28),7,24-triene-3,26-dioic acid (12) (Benosman et al., 1994), (24Z)-3,4-seco-tirucalla-4(28),7,24-triene-3,26-dioic acid (13) (Kim et al., 2004), seco-coccinic acids A–C, F, and G (14-18) (Wang et al., 2008; Ban et al., 2009; Wang et al., 2012), kadsuracoccinic acids A and C (19 and 20) (Li et al., 2008), micranoic acid A (21) (Li et al., 2003), schiglausin H (22) (Zou et al., 2012), manwuweizic acid (23) (Liu et al., 1988), 3-monomethyl ester leucophyllic acid (24) (Abdelilah et al., 1994), nigranoic acid (25) (Sun et al., 1996), abiesatrine J (26) (Yang et al., 2010), changnanic acid (27) (Wang et al., 2006), schiglausins T (28) (Yu et al., 2016), schisandronic acid (29) (Wang et al., 2006), kadsulactone (30) (Tan et al., 1991), and schisanlactone B (31) (Wang et al., 2006), by comparison of their reported NMR spectroscopic data with those of corresponding published compounds.
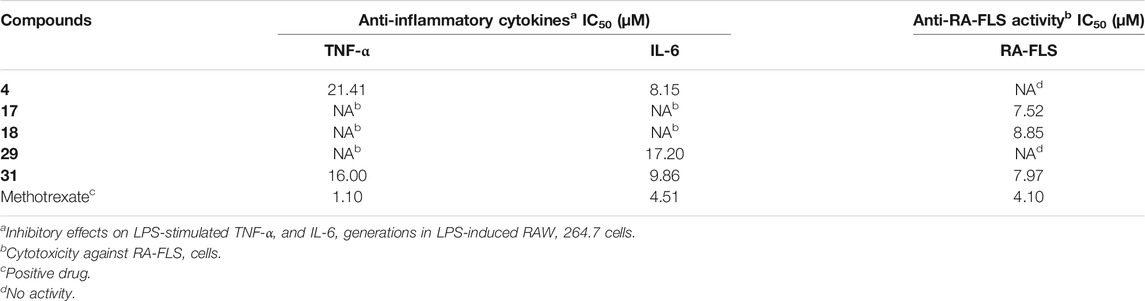
The inhibited proliferation activity in RA-FLS cells of the isolated compounds (1–31) were evaluated using the MTT method, and methotrexate was used as the positive control (IC50 4.10 µM). The results (Table 4) indicated that compounds 17, 18, and 31 exhibited good inhibition activities against RA-FLS cells with IC50 values of 7.52, 8.85, and 7.97 µM, respectively. Furthermore, all isolated compounds were evaluated for their inflammatory activity on inflammatory cytokines (IL-6 and TNF-α) released by LPS-induced RAW 264.7 cells. The inflammatory activity of the isolated compounds was determined using ELISA kits, with methotrexate as positive control. The results (Table 4) showed that compounds 4 and 31 suppressed the TNF-a expression in cell supernatant with IC50 values of 21.41 and 16.00 μM, respectively. Compounds 4, 29, and 31 suppressed IL-6 generation with IC50 values of 8.15, 17.20, and 9.86 μM, respectively.
Materials and Methods
General Experimental Procedures
Optical rotations of compounds were determined by a Rudolph Research Analytical Autopol Ⅲ automatic polarimeter. UV analysis of compounds was performed on a Shimadzu 2450 UV-vis spectrometer. An Applied Photophysics Chirascan plus CD spectrometer was used to determine ECD spectrum. An Agilent Technologies Cary 630 FTIR spectrometer was used to determine IR spectra of compounds. 1H, 13C, 1H-1H COSY, HSQC, and HMBC spectra of compounds were determined by a Bruker AV-600 spectrometer with a single NMR probe at 600 MHz for 1H and 150 MHz for 13C in CDCl3. HR-ESI-MS experiments were performed using Waters UHPLC-H-CLASS/XEVO G2-XS Q-tof and Agilent 6,530 Accurate-Mass Q-TOF LC/MS. Column chromatographic silica gel was purchased from Qingdao Marine Chemical Inc., P. R. China. Semi-preparative HPLC was performed using an Agilent 1,260 Infinity Ⅱ liquid chromatograph with Agilent C18 (34 mm × 25 cm) column. Extract fractions were analyzed using TLC, and spots were visualized by heating silica gel plates sprayed with 5% H2SO4 in Vanillin solution. Petroleum ether (PE), hexane, ethyl acetate (EtOAc), ethanol, n-butanol (n-BuOH), methanol (MeOH), and dichloromethane (CH2Cl2) were purchased from Shanghai Titan Scientific Co. Ltd. Acetonitrile and methanol (HPLC grade) were purchased from Merck KGaA, 64,271 Darmstadt, Germany.
Plant Material
The dried roots of K. coccinea were collected from Huaihua, Hunan Province, People’s Republic of China, in July 2015. Plant material was identified by one of the co-authors (WW). A voucher specimen (2,015,071,501) was deposited at TCM and Ethnomedicine Innovation and Development International Laboratory, Innovative Materia Medica Research Institute, School of Pharmacy, Hunan University of Chinese Medicine, Changsha, Hunan, People’s Republic of China.
Extraction and Isolation
The dried roots of K. coccinea (100 kg) were extracted twice with 80% ethanol for 2 h each time under reflux and filtered. All extract solvents were evaporated under vacuum to obtain crude EtOH extract (3 kg). Half of the whole ethanol extract (1.5 kg) was suspended in H2O and successively partitioned with PE, CH2Cl2, EtOAc, and n-BuOH to give a PE-soluble fraction (182 g), CH2Cl2-soluble fraction (545 g), EtOAc-soluble fraction (330 g), n-BuOH-soluble portion (173 g), and H2O layer. The CH2Cl2-soluble fraction (545 g) was subjected to silica gel column chromatography (CC) eluted with hexane-EtOAc (80:1-0:1) to afford twelve fractions (C1–C12). Fractions C2 (13.1 g), C4 (45.2 g), and C6 (35.6 g) were separated by silica gel CC with cyclohexane-EtOAc (80:1-0:1) to yield 17 (12.0 g) and 13 (600 mg), respectively. Fraction C3 (29.5 g) was chromatographed on a silica gel column eluted with cyclohexane-EtOAc (200:1-0:1) gradients further to give 13 fractions (C3-1–13) and 12 (300 mg). Subfraction C3-5 (6 g) was separated on silica gel CC with hexane-EtOAc (100:1-20:1) to give 18 (2.0 g). Subfraction C3-7 (50 mg) was purified using semipreparative HPLC (99% MeOH in H2O) to yield compound 19 (tR 28.3 min, 5 mg). Fraction C5 (49.5 g) was chromatographed on a silica gel column eluted with cyclohexane-CH2Cl2-EtOAc (80:1:0-200:1-0:5:1) gradients to give 12 further fractions (C5-1∼12). Subfraction C5-8 (5.0 g) was purified by using repeated silica gel CC eluted with hexane-EtOAc (40:1-0:1) and then by semipreparative HPLC with the mobile phase (93% MeOH/H2O) to obtain 30 (1 mg, tR 18.3 min) and 8 (5.2 mg, tR 26.8 min). Subfraction C5-9 (5.0 g) was further purified by using silica gel CC with hexane- EtOAc (100:1-0:1) to obtain 14 further fractions (C5-9-1–14), 10 (15 mg) was isolated from subfraction C5-9-12 by using silica gel CC eluted with hexane-CHCl3 (20:1-0:1), and subfraction C5-9-11 (128 mg) was purified by using semipreparative HPLC with the mobile phase (72% MeOH/H2O) to yield 14 (6 mg, tR 20.2 min) and 29 (15 mg, tR 23.8 min). Subfraction C5-10 (5.3 g) was chromatographed on a silica gel CC and eluted with hexane-EtOAc (40:1-0:1) gradients to give 15 (1.0 g). C5-9-12 (229.2 mg) was purified by semipreparative HPLC with the mobile phase (91% MeOH/H2O) obtained 22 (2.0 mg, tR 22.1 min), 11 (4.5 mg, tR 23.1 min), 7 (8.6 mg, tR 24.5 min), and 24 (4.1 mg, tR 31.7 min). Fraction C8 (15.5 g) was chromatographed on a silica gel column while eluted with PE-acetone (40:1-0:1) gradients to obtain 12 fractions (C8-1–12), subfraction C8-8 (15.5 g) was subjected to silica gel CC eluted with PE-acetone (40:1-0:1) to afford twelve subfractions (C8-8-1–12), 20 (3 mg, tR 25.6 min) was isolated from the subfraction C8-8-9 (52.5 mg) by semipreparative HPLC with the mobile phase (93% MeOH/H2O), and 28 (3 mg, tR 30.5 min) was isolated from the subfraction C8-8-8 (30.0 mg) by semipreparative HPLC with the mobile phase (90% MeOH/H2O). Fraction C9 (53.9 g) was chromatographed on a silica gel column eluted with PE-EtOAc (10:1-0:1) gradients to give 16 fractions (C9-1–16), subfraction C9-8 (5.3 g) was further subjected to silica gel CC with hexane-acetone (40:1-0:1) as mobile phase to obtain 17 further fractions (C9-8-1–17), and 1 (5 mg, tR 64.2 min) and 26 (5 mg, tR 79.1 min) were isolated from the subfraction C9-8-16 (120 mg) by semipreparative HPLC with the mobile phase (73% MeOH/H2O, 0–30 min →88% MeOH/H2O, 31–81 min →100% MeOH, 82–120 min). Compound 2 (3 mg, tR 24.3 min) was isolated by semipreparative HPLC with the mobile phase (90% MeOH/H2O) from subfraction C9-8-15 (50 mg), subfraction C9-8-13 (68.7 mg) was purified by semipreparative HPLC with the mobile phase (70% MeOH/H2O) to obtain 4 (5 mg, tR 38.3 min), subfraction C9-8-14 (135 mg) was purified by semipreparative HPLC with the mobile phase (57% MeOH/H2O, 0–17 min→85% MeOH/H2O, 18–30 min→90% MeOH/H2O, 31–60 min) to obtain 25 (12 mg, tR 57.5 min), and 27 (5 mg, tR 55.7 min), subfraction C9-8-15 (181.7 mg) was separated on C18 column eluted with 30%–100% MeOH-H2O to yield 21 (4 mg). Subfraction C9-10 (8.0 g) was subjected to silica gel CC eluted with hexane-acetone (40:1-0:1) gradients to 16 (1.0 g). Subfraction C9-12 (3.6 g) was repeatedly purified by silica gel CC with CHCl3-EtOAc (40:1-0:1) to obtain 12 further fractions (C9-12-1–12), and subfraction C9-12-2 (89.8 mg) was purified by semipreparative HPLC with the mobile phase (76% MeOH) to yield 31 (8 mg, tR 32.9 min). C9-12–5 (22.9 mg) was purified by semipreparative HPLC with the mobile phase (90% MeOH/H2O) to yield 23 (14.1 mg, tR 23.0 min) and 3 (11.6 mg, tR 25.8 min). C9-12–6 (50.0 mg) was purified by semipreparative HPLC with the mobile phase (92% MeOH/H2O) to yield 6 (7.6 mg, tR 15.8 min) and 5 (1.3 mg, tR 17.4 min).
The EtOAc-soluble fraction (330 g) was applied to silica gel column chromatography, eluted with PE-EtOAc (100:0-0:100), to give twelve fractions (Fr. E1–E12). Fr. E5 (90 g) was separated by silica gel column chromatography using Hexane-EtOAc (100%-0) to afford eight subfractions (Fr. E5-1–8). Fr. E5-3 was purified by semipreparative HPLC (80% MeOH, 20 min) to obtain compound 9 (18.2 mg).
Spectroscopic Data
Heilaohuacid A (1)
White amorphous powder [α]25 D - 11 (c 0.01 CHCl3); UV (CHCl3) λmax (log ε) 219 (4.2) nm, 248 (1.8) nm; IR (KBr) νmax 2,926, 2,853, 1,718, 1,696, 1,656, 1,561, 1,457, 1,372, 1,260, 962, and 900 cm−1; 1H- and 13C NMR data, see Tables 1, 3; HR-ESI-MS m/z 491.3143 [M + Na]+ (calcd. for C30H44O4Na, 491.3137).
Heilaohuacid B (2)
White amorphous powder [α]25 D - 39 (c 0.02 MeOH); UV (MeOH) λmax (log ε) 212 (6.7) nm, 252 (6.52) nm; IR (KBr) νmax 3,525, 2,972, 2,866, 1,791, 1,696, 1,652, 1,569, 1,472, 1,394, 1,056, 1,032, and 746 cm−1; 1H- and 13C NMR data, see Tables 1, 3; HR-ESI-MS m/z 467.3105 [M-H]− (calcd. for 467.3161, C30H43O4).
Heilaohuacid C (3)
White amorphous powder [α]25 D - 67 (c 0.07 MeOH); UV (MeOH) λmax (log ε) 204 (3.5) nm, 250 (2.8) nm; IR (KBr) νmax 3,414, 1,699, 1,652, 1,457, 1,372, 1,260, 962, 900, and 668 cm−1; 1H- and 13C NMR data, see Tables 1, 3; HR-ESI-MS m/z 469.3352 [M-H]− (calcd. for C30H45O4, 469.3328).
Heilaohuacid D (4)
White amorphous powder [α]25 D - 38 (c 0.04 MeOH); UV (MeOH) λmax (log ε) 206 (6.4) nm, 245 (6.3) nm; IR (KBr) νmax 3,628, 2,950, 1,771, 1,558, 1,436, 1,374, 1,260, 1,032, 667, and 646 cm−1; 1H- and 13C NMR data, see Tables 1, 3; HR-ESI-MS m/z 453.3406 [M-H]− (calcd. for 453.3374, C30H45O4).
Heilaohuacid E (5)
White amorphous powder [α]25 D + 19 (c 0.02 MeOH); UV (MeOH) λmax (log ε) 204 (6.3) nm; IR (KBr) νmax 3,401, 3,223, 2,972, 2,856, 1,749, 1,699, 1,558, 1,460, 1,246, 1,056, and 643 cm−1; 1H- and 13C NMR data, see Tables 2, 3; HR-ESI-MS m/z 455.3532 [M-H]− (calcd. for 455.3525, C30H47O3).
Heilaohuacid F (6)
White amorphous powder [α]25 D - 83 (c 0.06 MeOH); UV (MeOH) λmax (log ε) 204 (3.6) nm, 240 (2.8) nm; IR (KBr) νmax 2,944, 2,899, 1,715, 1,652, 1,448, 1,402, 1,372, 1,280, 959, and 900 cm−1; 1H- and 13C NMR data, see Tables 2, 3; HR-ESI-MS m/z 453.3364 [M-H]− (calcd. for C30H45O3, 453.3374).
Heilaohumethylester A (7)
White amorphous powder [α]25 D - 72 (c 0.02 MeOH); UV (MeOH) λmax (log ε) 205 (6.6) nm; IR (KBr) νmax 3,369, 2,946, 2,833, 1,699, 1,635, 1,506, 1,456, 1,372, 1,035, and 667 cm−1; 1H- and 13C NMR data, see Tables 2, 3; HR-ESI-MS m/z 509.3561 [M + Na]+ (calcd. for 509.3601, C31H50O4Na).
Heilaohumethylester B (8)
White amorphous powder [α]25 D - 88 (c 0.03 MeOH); UV (MeOH) λmax (log ε) 205 (6.5) nm; IR (KBr) νmax 3,518, 3,199, 2,963, 1,718, 1,653, 1,558, 1,260, 1,014, 804, and 650 cm−1; 1H- and 13C NMR data, see Tables 2, 3; HR-ESI-MS m/z 511.3734 [M + Na]+ (calcd. for C31H52O4Na, 511.3758).
Heilaohumethylester C (9)
White amorphous powder [α]25 D - 55 (c 0.05 CHCl3); UV (CHCl3) λmax (log ε) 240 (3.4) nm; IR (KBr) νmax 2,929, 2,856, 1,757, 1,704, 1,558, 1,460, 1,246, and 1,024 cm−1; 1H- and 13C NMR data, see Tables 2, 3; HR ESI MS m/z 491.3499 [M + Na]+ (calcd. for C31H48O3Na, 491.3501).
ECD Calculations
Methods of quantum chemical ECD calculations for compounds 1–3 are described in the Supporting Information (Supplementary Figure S1).
NMR Calculations
Methods of 13C NMR calculations for compounds 1 and 2 are described in the Supporting Information (Supplementary Figure S1).
Cell Culture
Human RA-FLS cell line was purchased from Fenghui Biological Technology Co., Ltd. (Changsha, China). RAW264.7 cell line was purchased from Fuheng Biological Technology Co., Ltd. (Shanghai, China). Human RA-FLS and RAW264.7 cells were cultured in DMEM/F12 with 10% FBS and DMEM with 10% FBS in 5% CO2 at 37°C, respectively.
Anti-Inflammatory Bioassay
Inhibition effects of all compounds (1–31) on release of inflammatory cytokines (IL-6 and TNF-α) in the supernatants on LPS-induced RAW264.7 cells were determined using ELISA kits (BOSTER Biological Technology Co. Ltd., Wuhan, China) following the manufacturer’s instructions. Methotrexate was used as a positive control.
Inhibited Proliferation Activity Against RA-FLS Bioassay
Inhibited proliferation activity against RA-FLS cells was determined by the standard MTT assay methods as described previously. RA-FLS cells were seeded into 96-well plates and treated with different concentrations of all compounds for 48 h. Ten microliters of MTT (5 mg/ml) was then added to each well and incubated for 4 h. The supernatants were retrieved, and 100 μl of DMSO was added to each well and mixed by shaking for 5 min. Optical density values at 490 nm were measured using a microplate reader.
Conclusion
The roots of K. coccinea, as a Tujia ethnomedicine, have been used to the treat rheumatoid arthritis for a long time in China. The present study has reported that nine new triterpenoids (1–9), along with 22 known analogues (10–31), were isolated from the roots of K. coccinea. Heilaohuacids A and B (1 and 2) contain a 3,4-seco ring A and unprecedented migration of Me-18 from C-13 to C-17 or C-14 to C-18; their relative and absolute configurations were determined by 13C NMR calculations and ECD data analysis. To the best of our knowledge, this type of lanostane triterpenoid derivative was rarely reported so far, which enriched the structural types of lanostane triterpenoids in K. coccinea. Additionally, compounds 4, 17, 18, 29, and 31 showed good anti-RA and/or anti-inflammatory activities. These findings suggest that lanostane triterpenoids from K. coccinea might serve as therapeutic agents for RA treatment.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author Contributions
Y-PY and Y-QJ conducted the chemical experiments and the pharmacological experiments, and wrote the original manuscript. Y-BL, MI, and Q-LX assisted the chemical experiments and analyzed the NMR data. H-HY and BW conducted the pharmacological experiments and analyzed the corresponding data. BL, R-YM, and BL assisted in revising the manuscript. C-YP and WW designed and guided all the chemical experiments, analyzed the data, and rewrote and revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (82174078, 81803708, 81874369, and 82074122) and Changjiang Scholars Program in Ministry Education, People’s Republic of China (T2019133).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2021.808870/full#supplementary-material
References
Abdelilah, B., Pascal, R., Thierry, S., Hamid, A. H. A., and Jean, B. (1994). Secotirucallane Triterpenes from the Stem Bark of Agalia Leucophylla. Phytochemistry 37, 1143–1145. doi:10.1016/S0031-9422(00)89545-X
Ban, N., Thanh, B., Kiem, P., Minh, C., Cuong, N., Nhiem, N., et al. (2009). Dibenzocyclooctadiene Lignans and Lanostane Derivatives from the Roots ofKadsura Coccineaand Their Protective Effects on Primary Rat Hepatocyte Injury Induced Byt-Butyl Hydroperoxide. Planta Med. 75, 1253–1257. doi:10.1055/s-0029-1185537
Benosman, A., Richomme, P., Sevenet, T., Hamid, A., Hadi, A., and Bruneton, J. (1994). Secotirucallane Triterpenes from the Stem Bark of Aglaia Leucophylla. Phytochemistry 37, 1143–1145. doi:10.1016/S0031-9422(00)89545-X
Cao, L., Shehla, N., Tasneem, S., Cao, M., Sheng, W., Jian, Y., et al. (2019). New Cadinane Sesquiterpenes from the Stems of Kadsura Heteroclita. Molecules 24, 1664. doi:10.3390/molecules24091664
Gao, X.-M., Pu, J.-X., Huang, S.-X., Lu, Y., Lou, L.-G., Li, R.-T., et al. (2008). Kadcoccilactones A−J, Triterpenoids from Kadsura Coccinea. J. Nat. Prod. 71, 1182–1188. doi:10.1021/np800078x
Hong, Z.-L., Xiong, J., Wu, S.-B., Zhu, J.-J., Hong, J.-L., Zhao, Y., et al. (2013). Tetracyclic Triterpenoids and Terpenylated Coumarins from the Bark of Ailanthus Altissima ("Tree of Heaven"). Phytochemistry 86, 159–167. doi:10.1016/j.phytochem.2012.10.008
Hu, Z.-X., Hu, K., Shi, Y.-M., Wang, W.-G., Du, X., Li, Y., et al. (2016). Rearranged 6/6/5/6-fused Triterpenoid Acids from the Stems of Kadsura Coccinea. J. Nat. Prod. 79, 2590–2598. doi:10.1021/acs.jnatprod.6b00508
Jain, M. K., Bao-Zhu Yu, B.-Z., Rogers, J. M., Smith, A. E., Boger, E. T. A., Ostrander, R. L., et al. (1995). Specific Competitive Inhibitor of Secreted Phospholipase A2 from Berries of Schinus Terebinthifolius. Phytochemistry 39, 537–547. doi:10.1016/0031-9422(94)00960-2
Kim, H. J., Choi, E. H., and Lee, I.-S. (2004). Two Lanostane Triterpenoids from Abies Koreana. Phytochemistry 65, 2545–2549. doi:10.1016/j.phytochem.2004.07.007
Lavoie, S., Gauthier, C., Legault, J., Mercier, S., Mshvildadze, V., and Pichette, A. (2013). Lanostane- and Cycloartane-type Triterpenoids from Abies Balsamea Oleoresin. Beilstein J. Org. Chem. 9, 1333–1339. doi:10.3762/bjoc.9.150
Li, H., Wang, L., Miyata, S., and Kitanaka, S. (2008). Kadsuracoccinic Acids A−C, Ring-A Seco-Lanostane Triterpenes from Kadsura Coccinea and Their Effects on Embryonic Cell Division of Xenopus laevis. J. Nat. Prod. 71, 739–741. doi:10.1021/np700739t
Li, R.-T., Han, Q.-B., Zhao, A.-H., and Sun, H.-D. (2003). Micranoic Acids A and B: Two New Octanortriterpenoids from Schisandra Micrantha. Chem. Pharm. Bull. 51, 1174–1176. doi:10.1248/cpb.51.1174
Lian-niang, L., and Hong, X. (1986). Triterpenoids from Roots and Stems ofKadsura Coccinea. Planta Med. 52, 492–493. doi:10.1055/s-2007-969264
Liu, J.-S., Huang, M.-F., and Tao, Y. (1988). Anwuweizonic Acid and Manwuweizic Acid, the Putative Anticancer Active Principle of Schisandra Propinqua. Can. J. Chem. 66, 414–415. doi:10.1139/v88-072
Liu, J., Qi, Y., Lai, H., Zhang, J., Jia, X., Liu, H., et al. (2014). Genus Kadsura, a Good Source with Considerable Characteristic Chemical Constituents and Potential Bioactivities. Phytomedicine 21, 1092–1097. doi:10.1016/j.phymed.2014.01.015
Liu, Y., Yang, Y., Tasneem, S., Hussain, N., Daniyal, M., Yuan, H., et al. (2018). Lignans from Tujia Ethnomedicine Heilaohu: Chemical Characterization and Evaluation of Their Cytotoxicity and Antioxidant Activities. Molecules 23, 2147. doi:10.3390/molecules23092147
Shehla, N., Li, B., Cao, L., Zhao, J., Jian, Y., Daniyal, M., et al. (2020). Xuetonglactones A-F: Highly Oxidized Lanostane and Cycloartane Triterpenoids from Kadsura Heteroclita Roxb. Craib. Front. Chem. 7, 935. doi:10.3389/fchem.2019.00935
Shi, Y.-M., Xiao, W.-L., Pu, J.-X., and Sun, H.-D. (2015). Triterpenoids from the Schisandraceae Family: an Update. Nat. Prod. Rep. 32, 367–410. doi:10.1039/c4np00117f
Sun, H.-D., Qiu, S.-X., Lin, L.-Z., Wang, Z.-Y., Lin, Z.-W., Pengsuparp, T., et al. (1996). Nigranoic Acid, a Triterpenoid from Schisandra Sphaerandra that Inhibits HIV-1 Reverse Transcriptase. J. Nat. Prod. 59, 525–527. doi:10.1021/np960149h
Tan, R., Xue, H., and Li, L.-N. (1991). Kadsulactone and Kadsudilactone, Two New Triterpenoid Lactones fromKadsuraSpecies. Planta Med. 57, 87–88. doi:10.1055/s-2006-960031
Wang, N., Li, Z.-L., Song, D.-D., Li, W., Pei, Y.-H., Jing, Y.-k., et al. (2012). Five New 3,4-seco-lanostane-type Triterpenoids with Antiproliferative Activity in Human Leukemia Cells Isolated from the Roots of Kadsura Coccinea. Planta Med. 78, 1661–1666. doi:10.1055/s-0032-1315260
Wang, N., Li, Z., Song, D., Li, W., Fu, H., Koike, K., et al. (2008). Lanostane-type Triterpenoids from the Roots of Kadsura Coccinea. J. Nat. Prod. 71, 990–994. doi:10.1021/np7007522
Wang, W., Liu, J., Han, J., Xu, Z., Liu, R., Liu, P., et al. (2006). New Triterpenoids fromKadsura Heteroclitaand Their Cytotoxic Activity. Planta Med. 72, 450–457. doi:10.1055/s-2005-916263
Xu, H.-C., Hu, K., Shi, X.-H., Tang, J.-W., Li, X.-N., Sun, H.-D., et al. (2019). Synergistic Use of NMR Computation and Quantitative Interproton Distance Analysis in the Structural Determination of Neokadcoccitane A, a Rearranged Triterpenoid Featuring an Aromatic Ring D from Kadsura Coccinea. Org. Chem. Front. 6, 1619–1626. doi:10.1039/C9QO00281B
Yang, G.-Y., Li, Y.-K., Wang, R.-R., Li, X.-N., Xiao, W.-L., Yang, L.-M., et al. (2010). Dibenzocyclooctadiene Lignans from Schisandra Wilsoniana and Their Anti-HIV-1 Activities. J. Nat. Prod. 73, 915–919. doi:10.1021/np100067w
Yang, J.-H., Pu, J.-X., Wen, J., Li, X.-N., He, F., Su, J., et al. (2015). Unusual Cycloartane Triterpenoids from Kadsura Ananosma. Phytochemistry 109, 36–42. doi:10.1016/j.phytochem10.1016/j.phytochem.2014.10.014
Yang, X.-W., Li, S.-M., Wu, L., Li, Y.-L., Feng, L., Shen, Y.-H., et al. (2010). Abiesatrines A-J: Anti-inflammatory and Antitumor Triterpenoids from Abies Georgei Orr. Org. Biomol. Chem. 8, 2609–2616. doi:10.1039/c001885f
Yu, H.-H., Lin, Y., Zeng, R., Li, X., Zhang, T., Tasneem, S., et al. (2019a). Analgesic and Anti-inflammatory Effects and Molecular Mechanisms of Kadsura Heteroclita Stems, an Anti-arthritic Chinese Tujia Ethnomedicinal Herb. J. Ethnopharmacology 238, 111902. doi:10.1016/j.jep.2019.111902
Yu, H.-Y., Li, J., Liu, Y., Wu, W.-M., and Ruan, H.-L. (2016). Triterpenoids from the Fruit of Schisandra Glaucescens. Fitoterapia 113, 64–68. doi:10.1016/j.fitote.2016.07.005
Yu, H., Zeng, R., Lin, Y., Li, X., Tasneem, S., Yang, Z., et al. (2019b). Kadsura Heteroclita Stem Suppresses the Onset and Progression of Adjuvant-Induced Arthritis in Rats. Phytomedicine 58, 152876. doi:10.1016/j.phymed.2019.152876
Keywords: schisandraceae, Kadsura coccinea, heilaohu, triterpenoids, anti-inflammatory, Tujia ethnomedicine
Citation: Yang Y-p, Jian Y-q, Liu Y-b, Ismail M, Xie Q-l, Yu H-h, Wang B, Li B, Peng C-y, Liu B, Man R-y and Wang W (2021) Triterpenoids From Kadsura coccinea With Their Anti-inflammatory and Inhibited Proliferation of Rheumatoid Arthritis-Fibroblastoid Synovial Cells Activities. Front. Chem. 9:808870. doi: 10.3389/fchem.2021.808870
Received: 04 November 2021; Accepted: 15 November 2021;
Published: 09 December 2021.
Edited by:
Jianrong Steve Zhou, Peking University, ChinaReviewed by:
Bin Du, Hebei Normal University of Science and Technology, ChinaXurong Qin, Southwest University, China
Copyright © 2021 Yang, Jian, Liu, Ismail, Xie, Yu, Wang, Li, Peng, Liu, Man and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cai-yun Peng, cGF1ZHlAMTI2LmNvbQ==; Wei Wang, d2FuZ3dlaTQwMkBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Yu-pei Yang1†
Yu-pei Yang1† Wei Wang
Wei Wang