- 1Department of Clinical Pharmacy, Xiangtan Central Hospital, Xiangtan, China
- 2Institute of Pharmacy and Pharmacology, School of Pharmaceutical Sciences, University of South China, Hengyang, China
Necrostatin-1, a small molecular alkaloid, was identified as an inhibitor of necroptosis in 2005. Investigating the fundamental mechanism of Necrostatin-1 and its role in various diseases is of great significance for scientific and clinical research. Accumulating evidence suggests that Necrostatin-1 plays a crucial role in numerous neurological disorders. This review aims to provide a comprehensive overview of the potential functions of Necrostatin-1 in various neurological disorders, offering valuable insights for future research.
1 Introduction
In recent years, exploring the mechanisms of cell death has been a hot topic in medicine, cytology, and biology. Cell death can occur through various pathways, such as necrosis, apoptosis, necroptosis, pyroptosis, and ferroptosis (Yin et al., 2015). Necroptosis, a form of programmed cell death, has been shown to play a crucial role in immune regulation, tissue damage, and tumorigenesis (Gong et al., 2019; Gao W. et al., 2022) (Figure 1). Morphologically, necroptosis shares similarities with necrosis, characterized by cell swelling, organelle swelling, cell lysis, and the release of cellular debris (Davidovich et al., 2014). Necrostatins are a class of compounds that prevent necroptosis, including Necrostatin-1, necrostatin-2, necrostatin-5, and necrostatin-7 (Degterev et al., 2008). Since its discovery in 2005, Necrostatin-1 has become the most widely used necroptotic inhibitor (Degterev et al., 2005). Further studies have revealed that Necrostatin-1 specifically inhibits receptor-interacting protein 1 (RIP1). Geng et al. (2017) investigated the pharmacokinetics and bioavailability of Necrostatin-1 using an LC–MS/MS method, reporting an absolute bioavailability of 54.8%. Elucidating the fundamental mechanism of Necrostatin-1 and its role in various diseases is of great importance for both scientific and clinical research. Emerging evidence suggests that Necrostatin-1 possesses numerous pharmacological activities, including anti-cancer (Liu et al., 2015; Polito et al., 2016), anti-osteoporosis (Feng et al., 2018; Chen et al., 2018b; Feng et al., 2023), anti-glaucoma (Dong et al., 2012; Liu M. et al., 2022), anti-periodontitis (Yan et al., 2018; Tan et al., 2023), anti-osteoarthritis (Liang et al., 2018), and protective effects on the kidneys (Linkermann et al., 2013; Dong et al., 2018; Shen et al., 2019), lungs (Guan et al., 2017; Mou and Mou, 2020), liver (Zhou et al., 2013; Kim and Lee, 2017; Xie and Huang, 2019), heart (Carbone et al., 2016; Qiao et al., 2021; Erdogmus Ozgen et al., 2022), and nervous system and so on. Currently, increasing studies are exploring the neuroprotective role of Necrostatin-1 in neurological disorders. Therefore, the published work in this topic should not be neglected. Compared with other review papers (Zhang et al., 2017; Liao et al., 2020; Yu et al., 2021), this paper reviews the latest research of Necrostatin-1 in neurological disorders. Meanwhlie, this paper also introduces the “Toxicity of Necrostatin-1 in nervous system” and “Necrostatin-1 plays a neuroprotective role via other cell death pathways.” These findings will offer valuable insights for future research.
Necroptosis plays an important role in various organs, such as the bone, brain, heart, kidney, skin, lungs, colon and so on.
2 Signaling pathway of Necrostatin-1
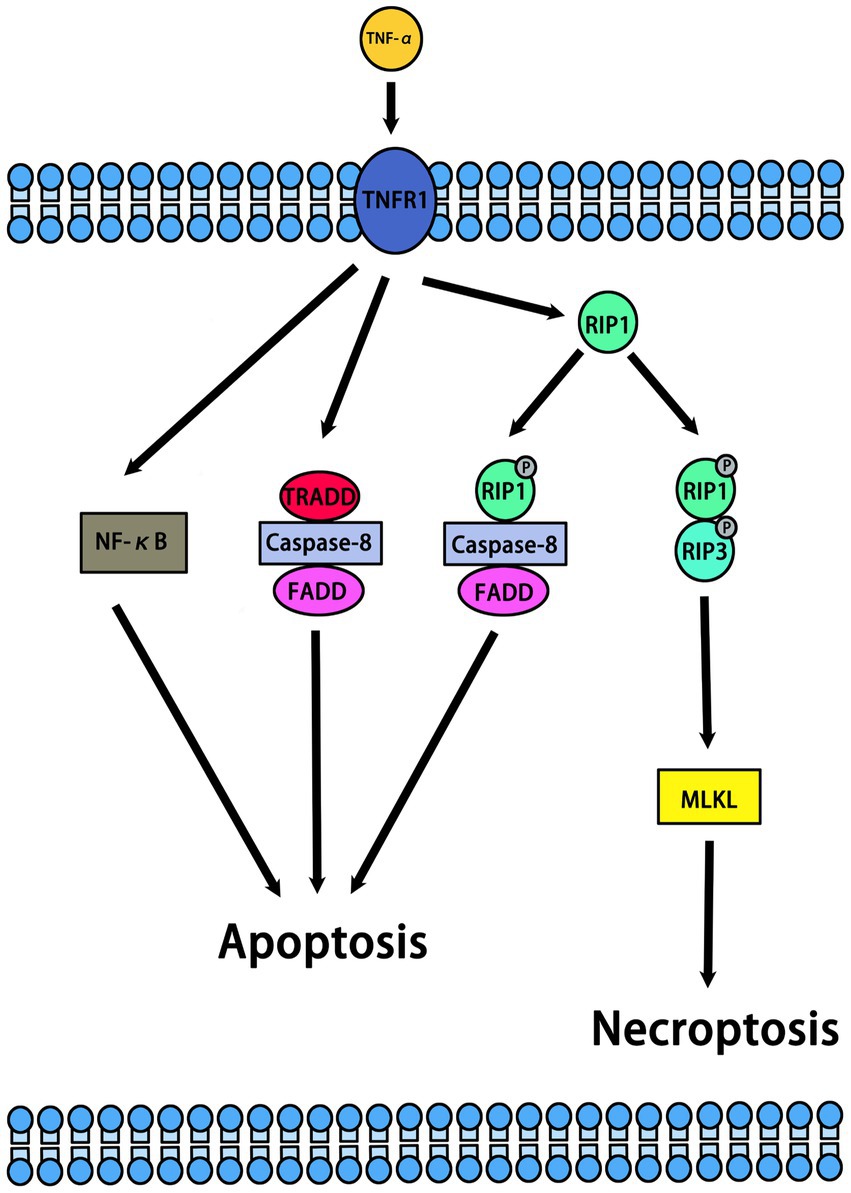
Necroptosis is a form of programmed necrosis that is independent of caspase regulation. When caspase is inhibited or not activated, necroptosis is activated (Zanetti and Weinlich, 2021). Previous studies have indicated that necrostatins are a class of compounds that inhibit RIP1. In normal and pathological conditions, necrostatins play an important role by inhibiting necroptosis or other pathways. In cells, necroptosis can be initiated by multiple upstream regulators, including TNF-α, FASL, APO-1 L, TRAIL, and IFN-α/β. Among them, TNF-α is the most important upstream regulator of necroptosis (Kearney et al., 2015; Pinci et al., 2022) (Figure 2). The binding of TNF-α to TNFR1 on the cell membrane stimulates different signaling pathways, including necroptosis, RIP1-dependent apoptosis (RDA), RIP1-independent apoptosis (RIA), and nuclear factor kappa B (NF-κB). Meanwhile, RIPK1, RIPK3, and MLKL are important downstream regulators of necroptosis. The mechanism of necroptosis is related to the activation of RIP1, RIP3 and MLKL (Cao and Mu, 2021) (Figure 3). By interacting with the T-loop, necrostatins can potently inhibit RIP1 autophosphorylation. RIP1 phosphorylation leads to the recruitment of RIP3 to RIP1 and subsequent formation of RIP1-RIP3 complex. This complex induces the phosphorylation of MLKL, which forms small holes in the plasma membrane. Eventually, disruptions of the plasma membrane lead to cell death (Cao and Mu, 2021). Therefore, necrostatins efficiently blocks RIP1/RIP3/MLKL signal transduction by inhibiting RIP1 phosphorylation. Interestingly, Necrostatin-1 has no direct inhibitory effect on RIP3 and does not block its autophosphorylation. In addition, necrostatins may be involved in hair cycle regulation under normal physiological conditions. Mechanistically, necrostatins upregulated Wnt3a and Wnt5b mRNA expression and increased the translocalization of β-catenin into the nucleus by stimulating β-catenin promoter binding activity (Zheng et al., 2020).
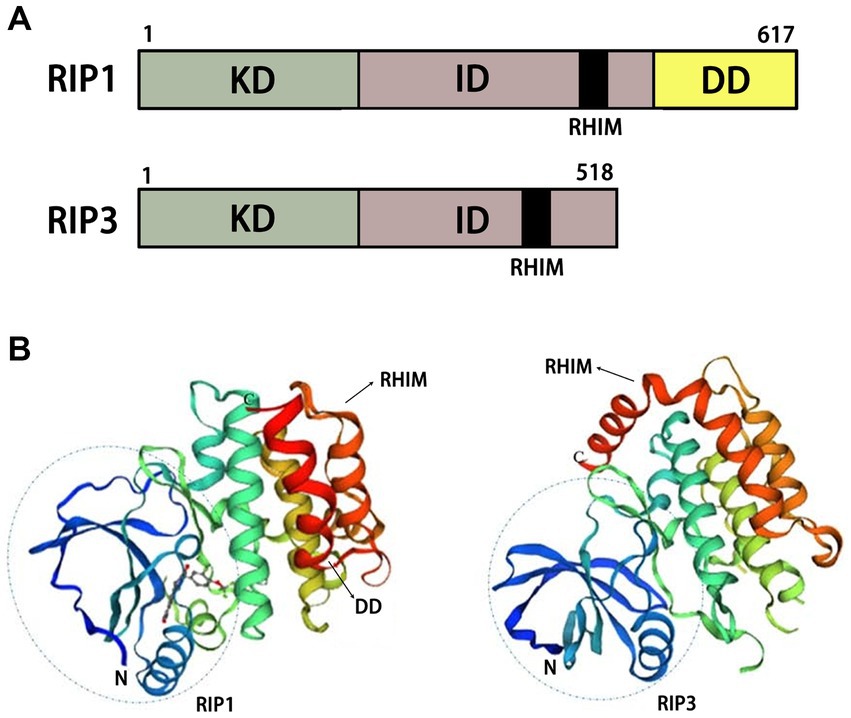
Figure 3. Structural diagrams of RIP1 and RIP3. (A) Schematic of functional domains of RIP1 and RIP3. (B) Protein tertiary structures of RIP1 and RIP3. KD, kinase domain; ID, intermediate domain; RHIM, RIP homotypic interaction motif; DD, death domain.
The combination of TNF-α and TNFR1 on the cell membrane stimulates different signaling pathways, including necroptosis, RIP1-dependent apoptosis (RDA), RIP1-independent apoptosis (RIA), nuclear factor kappa B (NF-κB). The RIP1 autophosphorylation sites include Ser14/15, Ser20, Ser161, and Ser166.
3 Necrostatin-1 and other cell death pathways
Increasing studies show that Necrostatin-1 not only suppresses necroptosis but also inhibits other cell death pathways (ferroptosis, apoptosis, pyroptosis). Ferroptosis is caused by the iron-mediated accumulation of lipid peroxidation, which is distinct from apoptosis and necroptosis (Newton et al., 2024). Necrostatin-1 not only perform a critical role in necroptosis but also in ferroptosis and maintain significant cellular mechanism. Yuk et al. (2021) demonstrated that Necrostatin-1 blocked ferroptosis through a mechanism independent from RIP1 and IDO inhibition in Huh7 and SK-HEP-1 cells. Caspase-8 is an executor of apoptosis. The aggregation of caspase-8 can lead to self-activation and activation of exogenous apoptotic pathways. Meanwhile, they promote the degradation of RIP1/RIP3 and lead to the closure of necroptosis signaling pathways (Fritsch et al., 2019). Some studies have explored the role of Necrostatin-1 on brain injury and its relationship with cell death pathways. They found that Necrostatin-1 not only blocked the occurrence of necroptosis but also significantly inhibited the expression of caspase-3 (an apoptosis-associated protein) and beclin-1 (an autophagy-associated protein) (Wang et al., 2012). In addition, Necrostatin-1 attenuates caspase-1-dependent pyroptosis induced by the RIP1/ZBP1 pathway in ventilator-induced lung injury (Shao et al., 2022).
4 Toxicity of Necrostatin-1
Although numerous studies have shown that Necrostatin-1 plays a neuroprotective role, there is evidence to support that Necrostatin-1 may damage the nervous system. In rotenone-induced PD model, Necrostatin-1 abolished necroptosis but did not prevent toxicity (Ye et al., 2023). Most likely, Necrostatin-1 activates a switch between cell death pathways. We think that Necrostatin-1 induces apoptosis and necroptosis by inhibiting mitophagy and promoting the accumulation of mitochondrial damage. Autophagy and necroptosis play an important role in most neurodegenerative diseases. Goodall et al. described a strong interaction between necrosome components and autophagy-related proteins. The knockdown of Necrostatin-1 abrogates this interaction and promotes apoptosis (Goodall et al., 2016). The inhibitory effect of Necrostatin-1 on autophagy has been reported in 6-hydroxydopamine treated neurons (Wu et al., 2015). Additionally, RIP1 knockdown upregulated autophagy, while Necrostatin-1 was shown to downregulate autophagy (Yonekawa et al., 2015). By inhibiting mitophagy, Necrostatin-1 affects mitochondrial morphology and mitochondrial clearance, which could enhance the effect of any Parkinsonian toxin (Alegre-Cortés et al., 2020). These different research results indicate that the underlying mechanism among Necrostatin-1, necroptosis and apoptosis is a complicated network, which is why Necrostatin-1 exhibits different effects in the nervous system.
5 Necrostatin-1 and inflammation
Neurodegenerative diseases are a large group of neurological disorders characterized by neuronal loss, including Alzheimer’s disease (AD), Parkinson’s disease (PD), and others (Dugger and Dickson, 2017). Although these neurodegenerative diseases have different pathogenetic mechanisms, inflammation plays a crucial role in their progression. Inflammation is the body’s defensive response to stimuli, and there is a mutually reinforcing effect between necroptosis and inflammation (Pasparakis and Vandenabeele, 2015). Necroptosis eventually leads to the release of cellular contents, causing an inflammatory response. Simultaneously, inflammation induces necroptosis via pro-inflammatory mediators (Kearney et al., 2015). Therefore, inhibiting necroptosis has great potential for treating neurodegenerative diseases by reducing inflammation. RIP1, a key target of necroptosis, promotes inflammatory responses via necroptotic cell death. In addition to inducing necroptotic cell death, RIP1 can also directly induce inflammation by producing pro-inflammatory cytokines, independent of cell death (Ofengeim and Yuan, 2013). As an inhibitor of RIP1, Necrostatin-1 exhibits significant anti-inflammatory effects in various inflammatory diseases, including hepatitis, pneumonia, and arthritis (Zhou et al., 2013; Jhun et al., 2019). Apoptosis of neutrophils is necessary for the resolution of inflammation. Necrostatin-1 is not only an inhibitor of necroptosis but also a promoter of neutrophil apoptosis, inhibiting the development of inflammation (Jie et al., 2016). Indoleamine 2,3-dioxygenase (IDO), a rate-limiting enzyme of tryptophan catabolism, plays a crucial role in inflammation. Necrostatin-1 is also an inhibitor of IDO (Vandenabeele et al., 2013), suppressing inflammation through this mechanism in addition to necroptosis inhibition. Neuroinflammation is responsible for generating and sustaining the sensitization of nociceptive neurons that lead to chronic pain. Liang et al. found that Necrostatin-1 ameliorates neuropathic pain by inhibiting neuroinflammation (Liang et al., 2019).
6 Necrostatin-1 and reactive oxygen species
Reactive oxygen species (ROS), highly reactive chemical substances, have long been studied in nervous system diseases (Singh et al., 2019). ROS, as regulators of mitochondrial dynamics, regulate neuronal development and function. However, a dramatic increase in ROS levels leads to cell structure damage under harmful conditions (Singh et al., 2019). Relevant studies indicate that the generation of ROS is probably RIP1-dependent (Jantas and Lasoń, 2021). ROS can increase the expression of RIP1/RIP3 and improve the stability of the RIP1-RIP3 complex (Chauhan et al., 2017). Glutamate, an important neurotransmitter, plays a crucial role in various neurological diseases. In HT-22 cells, Necrostatin-1 inhibits glutamate-induced oxytosis by increasing cellular glutathione (GSH) and reducing ROS (Xu et al., 2007). Additionally, Necrostatin-1 suppresses the phosphorylation of ERK1 and ERK2 after glutamate treatment (Zhang et al., 2013). CoCl2-induced neurotoxicity is associated with ERK1/2 phosphorylation and ROS production, which inhibit cell differentiation and lead to cell death. Chen R. et al. (2018) found that Necrostatin-1 inhibits CoCl2-induced neurotoxicity by decreasing ROS production and ERK1/2 phosphorylation. In H2O2-induced SH-SY5Y cell lines, Necrostatin-1 reduces oxidative stress-induced cell damage by inhibiting cathepsin D (Jantas et al., 2020). In peripheral nerve injury (PNI) and spinal cord injury (SCI) rat models, Necrostatin-1 can reduce ROS and inflammation (Yu et al., 2023). Further studies indicate that Necrostatin-1 not only inhibits necrosis by inhibiting RIP1/RIP3/MLKL but also inhibits apoptosis by activating Bcl-2 (Wang et al., 2014).
7 Necrostatin-1 and neurological disorders
7.1 Ischemic stroke and ischemia/reperfusion
Ischemic stroke (IS) often results in injury to oligodendroglia. Oligodendrocyte precursor cells (OPCs) are more vulnerable to cerebral ischemia than other mature oligodendroglia. Necrostatin-1 significantly promotes oligodendrocyte precursor cell survival and reduces white matter damage after cerebral ischemia (Chen et al., 2018a) through the RIPK1/RIPK3/MLKL signaling pathway (Deng et al., 2019). Necrostatin-1 also provides neuroprotection in neonatal hypoxia-ischemia (HI) by preserving mitochondrial function (Chavez-Valdez et al., 2012). Cerebral ischemia/reperfusion (I/R) induces selective neuronal injury in the CA1 region of the hippocampus. In cerebral I/R rats, Necrostatin-1 improves locomotive ability and relieves anxious behavior while decreasing the death rate of neurons through the RIP3/DAXX signaling pathway (Yang et al., 2017). Traumatic brain injury (TBI) is a leading cause of cerebral I/R injury. In a TBI mouse model, You et al. found that Necrostatin-1 has anti-inflammatory effects (You et al., 2008), while Wang et al. found that Necrostatin-1 inhibits autophagy and apoptosis (Wang et al., 2012). These results suggest that Necrostatin-1 may have therapeutic potential for IS and cerebral I/R.
7.2 Parkinson’s disease
PD is a neurodegenerative disorder characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta. Several types of cell death, including apoptosis, autophagy-induced cell death, and necrosis, have been implicated in PD progression. In PD models, Necrostatin-1 prevents rotenone-induced necroptosis by affecting mitochondrial morphology (Alegre-Cortés et al., 2020) and exerts a protective effect on dopaminergic neurons by decreasing the expression of cathepsin B and increasing the expression of Bcl-2 (Wu et al., 2015; Jantas and Lasoń, 2022).
7.3 Epilepsy
Epilepsy is a common, highly debilitating neurological disease characterized by the abnormal discharge of brain neurons. Necrosis and apoptosis are the major forms of neuronal death post-epilepsy. In an epileptic mouse model, Necrostatin-1 significantly decreases damage to hippocampal tissue and downregulates apoptosis/necroptosis-related proteins such as cleaved-caspase-3, Bax, RIP1, RIP3, and MLKL (Lin et al., 2020). A 40 μM concentration of Necrostatin-1 has an optimal effect (Lin et al., 2020), and inhibition of necroptosis may prolong seizure latency (Guan et al., 2021).
7.4 Alzheimer’s disease
Aluminum (Al) is a risk factor for AD. In the Al-induced AD model, Necrostatin-1 enhances acetylcholine (ACh) levels and downregulates the expression of AD-related genes and proteins (Gao X. et al., 2022). Furthermore, Necrostatin-1 inhibits neural cell degeneration and alleviates learning and memory deficits (Qinli et al., 2013; Jantas and Lasoń, 2022). Postoperative cognitive dysfunction (POCD) has become a prevalent complication in the elderly population. It is particularly concerning that persistent POCD is likely to progress into AD. In POCD patients, sevoflurane stimulates calcium overload and neurotoxicity (Yin et al., 2022). Necrostatin-1 attenuated sevoflurane-induced cognitive impairment via brain-derived neurotrophic factor (BDNF)-tyrosine receptor kinase B (TrkB) signaling (Yin et al., 2022). Additionally, Necrostatin-1 mitigated cognitive dysfunction in prediabetic rats (Jinawong et al., 2020).
7.5 Subarachnoid hemorrhage
Cerebral vasospasm, cerebral edema, and blood–brain barrier disruption are pathogenic factors in subarachnoid hemorrhage (SAH). Relevant studies indicate that inflammation plays a crucial role in cerebral vasospasm. Sahin et al. found that Necrostatin-1 ameliorates SAH-induced vasospasm in a rat model (Sahin et al., 2021). Liu C. et al. (2022) discovered that Necrostatin-1 decreases inflammatory markers after SAH. In SAH rats, Necrostatin-1 also exerts a neuroprotective effect by attenuating blood–brain barrier disruption and brain edema (Su et al., 2015; Chen et al., 2019). Mechanistically, necroptosis is a significant cause of cell death after SAH. Necrostatin-1 attenuates early brain injury after SAH by inhibiting necroptosis (Chen et al., 2017; Jantas and Lasoń, 2022). Another study suggested that Necrostatin-1 plays a neuroprotective role by inhibiting apoptosis and autophagy pathways in the SAH model (Chang et al., 2014).
7.6 Spinal cord injury
SCI is a severe nerve injury. Endoplasmic reticulum stress (ERS) is a critical pathological consequence of SCI. Necrostatin-1 has a protective effect on the endoplasmic reticulum by inhibiting the expression of ERS-related genes and proteins, such as C/EBP homologous protein (CHOP), immunoglobulin-binding protein (BiP/GRP78), and X-box-binding protein-1 (XBP-1) (Wang et al., 2017). Moreover, Necrostatin-1 improves mitochondrial functions in SCI (Jantas and Lasoń, 2022). It decreases Ca2+ concentration, increases adenosine triphosphate (ATP) generation, inhibits cytochrome c release, and preserves the mitochondrial membrane potential (MMP) level (Wang et al., 2015). In SCI mice, Necrostatin-1 significantly promotes locomotor function recovery by inhibiting the M1 polarization of microglia/macrophages (Tang et al., 2021). Necrostatin-1 also attenuates experimental autoimmune encephalomyelitis (EAE) and delayed paraplegia after SCI (Wang et al., 2019; Nishijima et al., 2023).
8 Other RIP inhibitors in neurological disorders
Increasing evidence suggest that RIP inhibitors play an important role in neurological pathologies. Necroptosis-associated RIP inhibitors include RIP1 inhibitors and RIP3 inhibitors (Figure 4 and Table 1). Besides the Necrostatin-1, Necrostatin-1 s is another important RIP1 inhibitor. Preeti et al. (2023) want to evaluate the neuroprotective effect of Necrostatin-1 s in the type-2 diabetes mellitus model. They found that Necrostatin-1 s mitigates cognitive decrement. Further, Necrostatin-1 s reduced tau and amyloid oligomer load. In the periventricular leukomalacia model, the expression level of RIP1 was drastically increased. Necrostatin-1 s greatly ameliorated cerebral ischemic injury and long-term neurobehavioral abnormalities, exhibiting a reduction of cerebral infarct size and neuronal loss (Sun et al., 2024). In addition, Kartik et al. (2023) found that Necrostatin-1 s significantly improve the survival of dopaminergic neurons in the PD mouse model. Other RIP1 inhibitors such as GSK772, PK68, GSK095, and GSK547 were not reported to improve nerve damage. GSK872 is a widely used RIPK3 inhibitor. Similar to Necrostatin-1 s, GSK872 improves various nerve damage such as retinal neuroinflammation, neurodegeneration, SCI, hydrocephalus and so on (He et al., 2021; Liu et al., 2021; Huang et al., 2023). Necrosulfonamide is a specific MLKL inhibitor. In a transient middle cerebral artery occlusion (tMCAO) rat model, necrosulfonamide reduces infarction volume and improves neurological deficits (Zhou et al., 2023). Besides the neuroprotective effects of tMCAO, necrosulfonamide also ameliorates SCI and intracerebral hemorrhage injury (Wang et al., 2018; Zhang et al., 2022). Interestingly, necrosulfonamide increased cleaved PARP-1 levels, indicating the protective effects of necrosulfonamide is not related to apoptosis (Zhou et al., 2017).
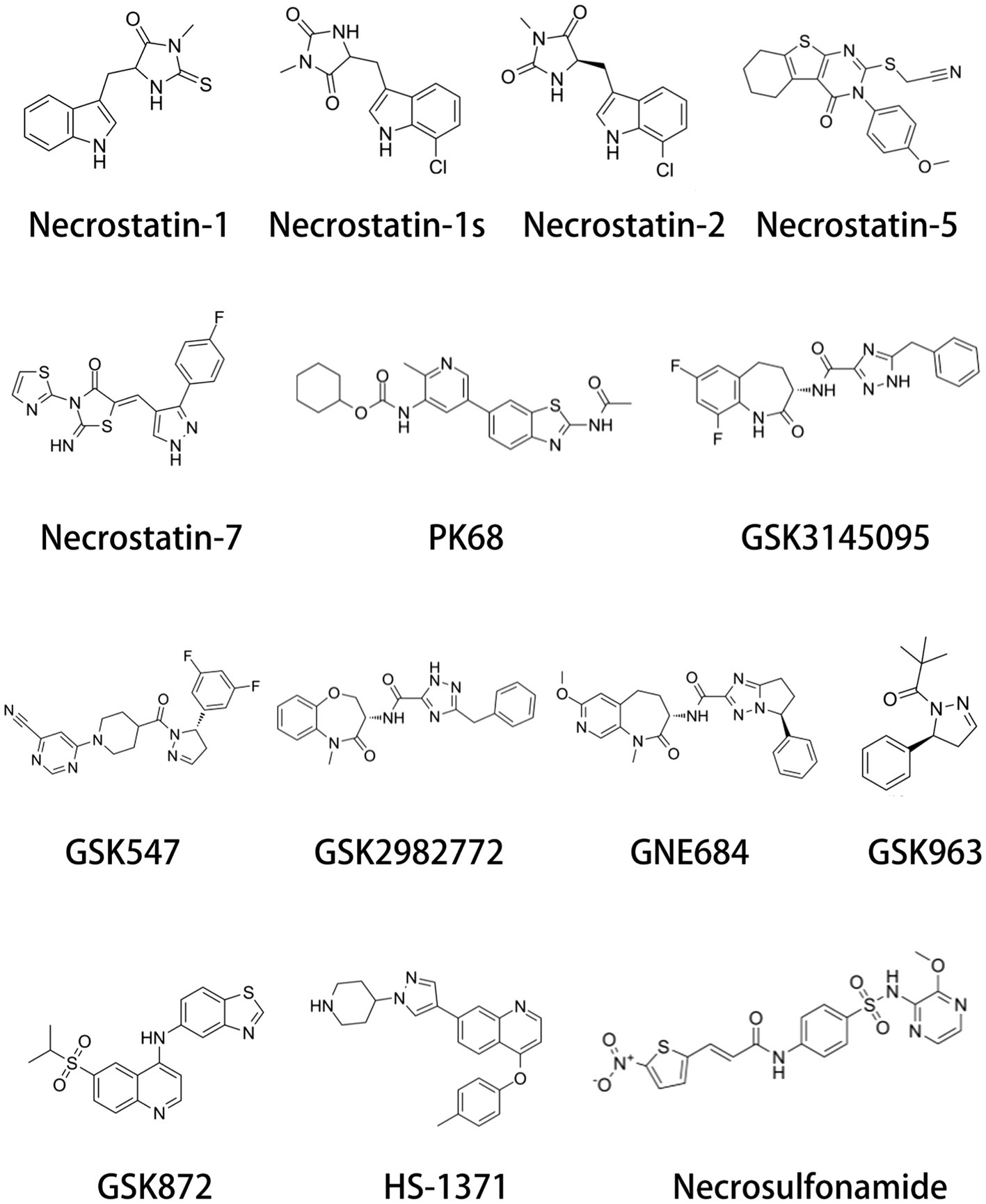
Figure 4. Chemical structure of necroptosis inhibitors. Necroptosis inhibitors include RIP1 inhibitors, RIP3 inhibitors, and MLKL inhibitors.
9 Application of Necrostatin-1
Beyond treating various diseases, Necrostatin-1 plays a crucial role in plastic surgery, preservation, transplantation, and inhibition of drug toxicity. Plastic surgery failure is a challenge for the medical cosmetology industry. Increasing research shows that Necrostatin-1 can treat various I/R injuries, such as those affecting the heart, lung, kidney, and skeletal muscle. In flap surgery, I/R injury is considered the primary problem. Liu et al. (2019) found that Necrostatin-1 has a protective effect against I/R injury in a skin flap model. These results suggest that Necrostatin-1 could be a promising novel strategy in plastic surgery. Cryopreservation of spermatogonial stem cells (SSCs) is important for preserving the lineages of valuable livestock and producing transgenic animals. As a potential cryoprotectant, Necrostatin-1 improves the cryopreservation efficiency of SSCs (Jung et al., 2020). Jo et al. (2015) also found that Necrostatin-1 improves the survival of mouse oocytes. Numerous studies show that Necrostatin-1 promotes the maturation, development, and graft function of neonatal porcine islets (Lau et al., 2020a,b, 2021), providing an effective strategy for the future application of islet grafts (Qin et al., 2022). Emerging evidence suggests that Necrostatin-1 has potential radical scavenging activities (Ushijima and Monzaki, 2023). Ning et al. found that Necrostatin-1 can decrease cisplatin-induced nephrotoxicity by inhibiting oxidative stress (Ning et al., 2018). Takemoto et al. discovered that Necrostatin-1 ameliorates acetaminophen-induced hepatotoxicity by inhibiting ROS (Takemoto et al., 2014). These results suggest that Necrostatin-1 has some benefit in alleviating drug toxicity. Interestingly, Necrostatin-1 can mitigate and treat radiation-induced damage in mice (Huang et al., 2016).
10 Discussion
In this review, we explored the mechanisms and roles of Necrostatin-1 in various neurological disorders (Table 2). Meanwhile, we propose that Necrostatin-1 has great clinical potential in the treatment of these disorders. In addition to treating various diseases, Necrostatin-1 plays an important role in plastic surgery, preservation, transplantation, and inhibition of drug toxicity. Nevertheless, there are still many questions regarding Necrostatin-1 that need to be addressed. First, Necrostatin-1 has a short half-life, which may affect its application. Second, it remains unclear whether Necrostatin-1 can affect one or multiple RIP1-dependent pathways in various neurological disorders. These findings suggest that the mechanism of Necrostatin-1 in disease is quite complex. In the future, it is necessary for scientists to further explore Necrostatin-1.
Author contributions
K-qC: Writing – original draft. S-zW: Writing – review & editing, Investigation. H-bL: Writing – review & editing, Formal analysis. XL: Writing – review & editing, Data curation.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alegre-Cortés, E., Muriel-González, A., Canales-Cortés, S., Uribe-Carretero, E., Martínez-Chacón, G., Aiastui, A., et al. (2020). Toxicity of Necrostatin-1 in Parkinson's disease models. Antioxidants 9:524. doi: 10.3390/antiox9060524
Cao, L., and Mu, W. (2021). Necrostatin-1 and necroptosis inhibition: pathophysiology and therapeutic implications. Pharmacol. Res. 163:105297. doi: 10.1016/j.phrs.2020.105297
Carbone, F., Oliveira, P. J., and Montecucco, F. (2016). Protective role of necrostatin-1 in acute myocardial infarction. Eur. J. Clin. Investig. 46, 99–100. doi: 10.1111/eci.12568
Chang, P., Dong, W., Zhang, M., Wang, Z., Wang, Y., Wang, T., et al. (2014). Anti-necroptosis chemical necrostatin-1 can also suppress apoptotic and autophagic pathway to exert neuroprotective effect in mice intracerebral hemorrhage model. J. Mol. Neurosci. 52, 242–249. doi: 10.1007/s12031-013-0132-3
Chauhan, A. K., Min, K. J., and Kwon, T. K. (2017). Rip1-dependent reactive oxygen species production executes artesunate-induced cell death in renal carcinoma Caki cells. Mol. Cell. Biochem. 435, 15–24. doi: 10.1007/s11010-017-3052-7
Chavez-Valdez, R., Martin, L. J., Flock, D. L., and Northington, F. J. (2012). Necrostatin-1 attenuates mitochondrial dysfunction in neurons and astrocytes following neonatal hypoxia-ischemia. Neuroscience 219, 192–203. doi: 10.1016/j.neuroscience.2012.05.002
Chen, J., Jin, H., Xu, H., Peng, Y., Jie, L., Xu, D., et al. (2019). The neuroprotective effects of Necrostatin-1 on subarachnoid hemorrhage in rats are possibly mediated by preventing blood-brain barrier disruption and Rip3-mediated necroptosis. Cell Transplant. 28, 1358–1372. doi: 10.1177/0963689719867285
Chen, F., Su, X., Lin, Z., Lin, Y., Yu, L., Cai, J., et al. (2017). Necrostatin-1 attenuates early brain injury after subarachnoid hemorrhage in rats by inhibiting necroptosis. Neuropsychiatr. Dis. Treat. 13, 1771–1782. doi: 10.2147/NDT.S140801
Chen, R., Xu, J., She, Y., Jiang, T., Zhou, S., Shi, H., et al. (2018). Necrostatin-1 protects C2C12 myotubes from CoCl2-induced hypoxia. Int. J. Mol. Med. 41, 2565–2572. doi: 10.3892/ijmm.2018.3466
Chen, Y., Zhang, L., Yu, H., Song, K., Shi, J., Chen, L., et al. (2018a). Necrostatin-1 improves long-term functional recovery through protecting oligodendrocyte precursor cells after transient focal cerebral ischemia in mice. Neuroscience 371, 229–241. doi: 10.1016/j.neuroscience.2017.12.007
Chen, Y., Zhu, C. J., Zhu, F., Dai, B. B., Song, S. J., Wang, Z. Q., et al. (2018b). Necrostatin-1 ameliorates adjuvant arthritis rat articular chondrocyte injury via inhibiting Asic1a-mediated necroptosis. Biochem. Biophys. Res. Commun. 504, 843–850. doi: 10.1016/j.bbrc.2018.09.031
Davidovich, P., Kearney, C. J., and Martin, S. J. (2014). Inflammatory outcomes of apoptosis, necrosis and necroptosis. Biol. Chem. 395, 1163–1171. doi: 10.1515/hsz-2014-0164
Degterev, A., Hitomi, J., Germscheid, M., Ch'en, I. L., Korkina, O., Teng, X., et al. (2008). Identification of Rip1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 4, 313–321. doi: 10.1038/nchembio.83
Degterev, A., Huang, Z., Boyce, M., Li, Y., Jagtap, P., Mizushima, N., et al. (2005). Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 1, 112–119. doi: 10.1038/nchembio711
Deng, X. X., Li, S. S., and Sun, F. Y. (2019). Necrostatin-1 prevents necroptosis in brains after ischemic stroke via inhibition of Ripk1-mediated Ripk3/Mlkl signaling. Aging Dis. 10, 807–817. doi: 10.14336/AD.2018.0728
Dong, W., Li, Z., Chen, Y., Zhang, L., Ye, Z., Liang, H., et al. (2018). Necrostatin-1 attenuates sepsis-associated acute kidney injury by promoting autophagosome elimination in renal tubular epithelial cells. Mol. Med. Rep. 17, 3194–3199. doi: 10.3892/mmr.2017.8214
Dong, K., Zhu, H., Song, Z., Gong, Y., Wang, F., Wang, W., et al. (2012). Necrostatin-1 protects photoreceptors from cell death and improves functional outcome after experimental retinal detachment. Am. J. Pathol. 181, 1634–1641. doi: 10.1016/j.ajpath.2012.07.029
Dugger, B. N., and Dickson, D. W. (2017). Pathology of neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 9:a028035. doi: 10.1101/cshperspect.a028035
Erdogmus Ozgen, Z., Erdinc, M., Kelle, İ., Erdinc, L., and Nergiz, Y. (2022). Protective effects of necrostatin-1 on doxorubicin-induced cardiotoxicity in rat heart. Hum. Exp. Toxicol. 41:096032712110660. doi: 10.1177/09603271211066066
Feng, M., Qiang, H., Zhang, R. R., Wang, K. Z., Wang, C. S., and Yang, P. (2018). Necrostatin-1 inhibits the cell death of osteoblasts induced by glucocorticoid. Int. J. Clin. Exp. Pathol. 11, 675–684
Feng, M., Zhang, R., Zhang, M., Chen, M., Ji, L., Duan, D., et al. (2023). Administration of necrostatin-1 ameliorates glucocorticoid-induced osteonecrosis of the femoral head in rats. J. Mol. Histol. 54, 207–216. doi: 10.1007/s10735-023-10124-x
Fritsch, M., Günther, S. D., Schwarzer, R., Albert, M. C., Schorn, F., Werthenbach, J. P., et al. (2019). Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 575, 683–687. doi: 10.1038/s41586-019-1770-6
Gao, W., Wang, X., Zhou, Y., Wang, X., and Yu, Y. (2022). Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct. Target. Ther. 7:196. doi: 10.1038/s41392-022-01046-3
Gao, X., Zhang, P., Chen, J., Zhang, L., Shang, N., Chen, J., et al. (2022). Necrostatin-1 relieves learning and memory deficits in a zebrafish model of Alzheimer's disease induced by aluminum. Neurotox. Res. 40, 198–214. doi: 10.1007/s12640-021-00463-6
Geng, F., Yin, H., Li, Z., Li, Q., He, C., Wang, Z., et al. (2017). Quantitative analysis of necrostatin-1, a necroptosis inhibitor by Lc-Ms/Ms and the study of its pharmacokinetics and bioavailability. Biomed. Pharmacother. 95, 1479–1485. doi: 10.1016/j.biopha.2017.09.063
Gong, Y., Fan, Z., Luo, G., Yang, C., Huang, Q., Fan, K., et al. (2019). The role of necroptosis in cancer biology and therapy. Mol. Cancer 18:100. doi: 10.1186/s12943-019-1029-8
Goodall, M. L., Fitzwalter, B. E., Zahedi, S., Wu, M., Rodriguez, D., Mulcahy-Levy, J. M., et al. (2016). The autophagy machinery controls cell death switching between apoptosis and necroptosis. Dev. Cell 37, 337–349. doi: 10.1016/j.devcel.2016.04.018
Guan, E., Wang, Y., Wang, C., Zhang, R., Zhao, Y., and Hong, J. (2017). Necrostatin-1 attenuates lipopolysaccharide-induced acute lung injury in mice. Exp. Lung Res. 43, 378–387. doi: 10.1080/01902148.2017.1384083
Guan, Z. B., Zhou, Y. Y., Cen, Y., Feng, H. D., Liu, W. W., Yi, H. J., et al. (2021). Necrostatin-1 prolongs latency to convulsion in mice exposed to high oxygen partial pressure. Diving Hyperb. Med. 51, 134–139. doi: 10.28920/dhm51.2.134-139
He, N., Qu, Y. J., Li, D. Y., and Yue, S. W. (2021). Rip3 inhibition ameliorates chronic constriction injury-induced neuropathic pain by suppressing Jnk signaling. Aging 13, 24417–24431. doi: 10.18632/aging.203691
Huang, Z., Epperly, M., Watkins, S. C., Greenberger, J. S., Kagan, V. E., and Bayır, H. (2016). Necrostatin-1 rescues mice from lethal irradiation. Biochim. Biophys. Acta 1862, 850–856. doi: 10.1016/j.bbadis.2016.01.014
Huang, Z., Liang, J., Chen, S., Ng, T. K., Brelén, M. E., Liu, Q., et al. (2023). Rip3-mediated microglial necroptosis promotes neuroinflammation and neurodegeneration in the early stages of diabetic retinopathy. Cell Death Dis. 14:227. doi: 10.1038/s41419-023-05660-z
Jantas, D., Chwastek, J., Grygier, B., and Lasoń, W. (2020). Neuroprotective effects of Necrostatin-1 against oxidative stress-induced cell damage: an involvement of Cathepsin D inhibition. Neurotox. Res. 37, 525–542. doi: 10.1007/s12640-020-00164-6
Jantas, D., and Lasoń, W. (2021). Preclinical evidence for the interplay between oxidative stress and Rip1-dependent cell death in neurodegeneration: state of the art and possible therapeutic implications. Antioxidants 10:1518. doi: 10.3390/antiox10101518
Jantas, D., and Lasoń, W. (2022). “Necrostatin-1 as a Neuroprotectant” in Handbook of neurotoxicity. ed. R. M. Kostrzewa (Cham: Springer International Publishing), 123–155.
Jhun, J., Lee, S. H., Kim, S. Y., Ryu, J., Kwon, J. Y., Na, H. S., et al. (2019). Ripk1 inhibition attenuates experimental autoimmune arthritis via suppression of osteoclastogenesis. J. Transl. Med. 17:84. doi: 10.1186/s12967-019-1809-3
Jie, H., He, Y., Huang, X., Zhou, Q., Han, Y., Li, X., et al. (2016). Necrostatin-1 enhances the resolution of inflammation by specifically inducing neutrophil apoptosis. Oncotarget 7, 19367–19381. doi: 10.18632/oncotarget.8346
Jinawong, K., Apaijai, N., Wongsuchai, S., Pratchayasakul, W., Chattipakorn, N., and Chattipakorn, S. C. (2020). Necrostatin-1 mitigates cognitive dysfunction in Prediabetic rats with no alteration in insulin sensitivity. Diabetes 69, 1411–1423. doi: 10.2337/db19-1128
Jo, J. W., Lee, J. R., Jee, B. C., Suh, C. S., and Kim, S. H. (2015). Exposing mouse oocytes to necrostatin 1 during in vitro maturation improves maturation, survival after vitrification, mitochondrial preservation, and developmental competence. Reproduct. Sci. 22, 615–625. doi: 10.1177/1933719114556482
Jung, S. E., Ahn, J. S., Kim, Y. H., Oh, H. J., Kim, B. J., and Ryu, B. Y. (2020). Necrostatin-1 improves the cryopreservation efficiency of murine spermatogonial stem cells via suppression of necroptosis and apoptosis. Theriogenology 158, 445–453. doi: 10.1016/j.theriogenology.2020.10.004
Kartik, S., Pal, R., Chaudhary, M. J., Tiwari, P. C., Nath, R., and Kumar, M. (2023). Anti-oxidative and anti-neuroinflammatory role of Necrostatin-1s and docosahexaenoic acid in rip-1-mediated neurotoxicity in Mptp-induced Parkinson's disease model. Fundam. Clin. Pharmacol. 37, 794–806. doi: 10.1111/fcp.12881
Kearney, C. J., Cullen, S. P., Tynan, G. A., Henry, C. M., Clancy, D., Lavelle, E. C., et al. (2015). Necroptosis suppresses inflammation via termination of Tnf- or Lps-induced cytokine and chemokine production. Cell Death Differ. 22, 1313–1327. doi: 10.1038/cdd.2014.222
Kim, S. J., and Lee, S. M. (2017). Necrostatin-1 protects against D-Galactosamine and lipopolysaccharide-induced hepatic injury by preventing Tlr4 and rage signaling. Inflammation 40, 1912–1923. doi: 10.1007/s10753-017-0632-3
Lau, H., Corrales, N., Alexander, M., Mohammadi, M. R., Li, S., Smink, A. M., et al. (2020a). Necrostatin-1 supplementation enhances young porcine islet maturation and in vitro function. Xenotransplantation 27:e12555. doi: 10.1111/xen.12555
Lau, H., Corrales, N., Rodriguez, S., Luong, C., Mohammadi, M., Khosrawipour, V., et al. (2020b). Dose-dependent effects of necrostatin-1 supplementation to tissue culture media of young porcine islets. PLoS One 15:e0243506. doi: 10.1371/journal.pone.0243506
Lau, H., Corrales, N., Rodriguez, S., Park, S., Mohammadi, M., Li, S., et al. (2021). The effects of necrostatin-1 on the in vitro development and function of young porcine islets over 14-day prolonged tissue culture. Xenotransplantation 28:e12667. doi: 10.1111/xen.12667
Liang, S., Lv, Z. T., Zhang, J. M., Wang, Y. T., Dong, Y. H., Wang, Z. G., et al. (2018). Necrostatin-1 attenuates trauma-induced mouse osteoarthritis and Il-1β induced apoptosis via Hmgb1/Tlr4/Sdf-1 in primary mouse chondrocytes. Front. Pharmacol. 9:1378. doi: 10.3389/fphar.2018.01378
Liang, Y. X., Wang, N. N., Zhang, Z. Y., Juan, Z. D., and Zhang, C. (2019). Necrostatin-1 ameliorates peripheral nerve injury-induced neuropathic pain by inhibiting the Rip1/Rip3 pathway. Front. Cell. Neurosci. 13:211. doi: 10.3389/fncel.2019.00211
Liao, S., Apaijai, N., Chattipakorn, N., and Chattipakorn, S. C. (2020). The possible roles of necroptosis during cerebral ischemia and ischemia / reperfusion injury. Arch. Biochem. Biophys. 695:108629. doi: 10.1016/j.abb.2020.108629
Lin, D. Q., Cai, X. Y., Wang, C. H., Yang, B., and Liang, R. S. (2020). Optimal concentration of necrostatin-1 for protecting against hippocampal neuronal damage in mice with status epilepticus. Neural Regen. Res. 15, 936–943. doi: 10.4103/1673-5374.268903
Linkermann, A., Heller, J. O., Prókai, A., Weinberg, J. M., de Zen, F., Himmerkus, N., et al. (2013). The Rip1-kinase inhibitor necrostatin-1 prevents osmotic nephrosis and contrast-induced Aki in mice. J. Am. Soc. Nephrol. 24, 1545–1557. doi: 10.1681/ASN.2012121169
Liu, C., Cao, Y., Wang, H. X., Zhao, L., Chen, Y. X., Zhong, K. H., et al. (2022). Necrostatin-1 decreases necroptosis and inflammatory markers after intraventricular hemorrhage in mice. Neural Regen. Res. 17, 2710–2716. doi: 10.4103/1673-5374.339488
Liu, C., Chen, Y., Cui, W., Cao, Y., Zhao, L., Wang, H., et al. (2021). Inhibition of neuronal necroptosis mediated by Rip1/Rip3/Mlkl provides neuroprotective effects on kaolin-induced hydrocephalus in mice. Cell Prolif. 54:e13108. doi: 10.1111/cpr.13108
Liu, M., Li, H., Yang, R., Ji, D., and Xia, X. (2022). Gsk872 and necrostatin-1 protect retinal ganglion cells against necroptosis through inhibition of Rip1/Rip3/Mlkl pathway in glutamate-induced retinal excitotoxic model of glaucoma. J. Neuroinflammation 19:262. doi: 10.1186/s12974-022-02626-4
Liu, Z. Y., Wu, B., Guo, Y. S., Zhou, Y. H., Fu, Z. G., Xu, B. Q., et al. (2015). Necrostatin-1 reduces intestinal inflammation and colitis-associated tumorigenesis in mice. Am. J. Cancer Res. 5, 3174–3185
Liu, H., Zhang, M., Dong, X., Liu, Y., Hao, Y., and Wang, Y. (2019). Necrostatin-1 protects against ischemia/reperfusion injury by inhibiting receptor-interacting protein 1 in a rat flap model. J. Plast. Reconstr. Aesthet. Surg. 72, 194–202. doi: 10.1016/j.bjps.2018.10.019
Mou, F., and Mou, C. (2020). Necrostatin-1 alleviates bleomycin-induced pulmonary fibrosis and extracellular matrix expression in interstitial pulmonary fibrosis. Med. Sci. Monit. 26:e919739. doi: 10.12659/MSM.919739
Newton, K., Strasser, A., Kayagaki, N., and Dixit, V. M. (2024). Cell death. Cell 187, 235–256. doi: 10.1016/j.cell.2023.11.044
Ning, Y., Shi, Y., Chen, J., Song, N., Cai, J., Fang, Y., et al. (2018). Necrostatin-1 attenuates cisplatin-induced nephrotoxicity through suppression of apoptosis and oxidative stress and retains klotho expression. Front. Pharmacol. 9:384. doi: 10.3389/fphar.2018.00384
Nishijima, T., Fujita, S., Harada, T., Uchiyama, H., Matsuda, K., Mitsuo, H., et al. (2023). Necrostatin-1 attenuates delayed paraplegia after transient spinal cord ischemia in rabbits by inhibiting the upregulation of receptor-interacting protein kinase 1 and 3. Ann. Vasc. Surg. 96, 382–392. doi: 10.1016/j.avsg.2023.05.011
Ofengeim, D., and Yuan, J. (2013). Regulation of Rip1 kinase signalling at the crossroads of inflammation and cell death. Nat. Rev. Mol. Cell Biol. 14, 727–736. doi: 10.1038/nrm3683
Pasparakis, M., and Vandenabeele, P. (2015). Necroptosis and its role in inflammation. Nature 517, 311–320. doi: 10.1038/nature14191
Pinci, F., Gaidt, M. M., Jung, C., Nagl, D., Kuut, G., and Hornung, V. (2022). Tumor necrosis factor is a necroptosis-associated alarmin. Front. Immunol. 13:1074440. doi: 10.3389/fimmu.2022.1074440
Polito, L., Bortolotti, M., Pedrazzi, M., Mercatelli, D., Battelli, M. G., and Bolognesi, A. (2016). Apoptosis and necroptosis induced by stenodactylin in neuroblastoma cells can be completely prevented through caspase inhibition plus catalase or necrostatin-1. Phytomedicine 23, 32–41. doi: 10.1016/j.phymed.2015.11.006
Preeti, K., Fernandes, V., Sood, A., Khan, I., Khatri, D. K., and Singh, S. B. (2023). Necrostatin-1S mitigates type-2 diabetes-associated cognitive decrement and lipotoxicity-induced neuro-microglia changes through p-Ripk-Ripk3-p-Mlkl axis. Metab. Brain Dis. 38, 1581–1612. doi: 10.1007/s11011-023-01185-8
Qiao, S., Zhao, W. J., Li, H. Q., Ao, G. Z., An, J. Z., Wang, C., et al. (2021). Necrostatin-1 analog Dimo exerts Cardioprotective effect against ischemia reperfusion injury by suppressing necroptosis via Autophagic pathway in rats. Pharmacology 106, 189–201. doi: 10.1159/000510864
Qin, T., Hu, S., Smink, A. M., de Haan, B. J., Silva-Lagos, L. A., Lakey, J. R. T., et al. (2022). Inclusion of extracellular matrix molecules and necrostatin-1 in the intracapsular environment of alginate-based microcapsules synergistically protects pancreatic β cells against cytokine-induced inflammatory stress. Acta Biomater. 146, 434–449. doi: 10.1016/j.actbio.2022.04.042
Qinli, Z., Meiqing, L., Xia, J., Li, X., Weili, G., Xiuliang, J., et al. (2013). Necrostatin-1 inhibits the degeneration of neural cells induced by aluminum exposure. Restor. Neurol. Neurosci. 31, 543–555. doi: 10.3233/RNN-120304
Sahin, M. H., Akyuz, E., and Kadioglu, H. H. (2021). The effects of Necrostatin-1 on cerebral vasospasm-induced subarachnoid hemorrhage. Turk. Neurosurg. doi: 10.5137/1019-5149.JTN.35167-21.4
Shao, R. G., Xie, Q. W., Pan, L. H., Lin, F., Qin, K., Ming, S. P., et al. (2022). Necrostatin-1 attenuates Caspase-1-dependent pyroptosis induced by the Ripk1/Zbp1 pathway in ventilator-induced lung injury. Cytokine 157:155950. doi: 10.1016/j.cyto.2022.155950
Shen, B., Mei, M., Pu, Y., Zhang, H., Liu, H., Tang, M., et al. (2019). Necrostatin-1 attenuates renal ischemia and reperfusion injury via meditation of Hif-1α/mir-26a/Trpc6/Parp1 signaling. Mol. Therapy Nucleic Acids 17, 701–713. doi: 10.1016/j.omtn.2019.06.025
Singh, A., Kukreti, R., Saso, L., and Kukreti, S. (2019). Oxidative stress: a key modulator in neurodegenerative diseases. Molecules 24:1583. doi: 10.3390/molecules24081583
Su, X., Wang, H., Kang, D., Zhu, J., Sun, Q., Li, T., et al. (2015). Necrostatin-1 ameliorates intracerebral hemorrhage-induced brain injury in mice through inhibiting Rip1/Rip3 pathway. Neurochem. Res. 40, 643–650. doi: 10.1007/s11064-014-1510-0
Sun, J., Wang, W., Ma, Q., Pan, X., Zhai, H., Wang, J., et al. (2024). Necrostatin-1s suppresses Ripk1-driven necroptosis and inflammation in periventricular leukomalacia neonatal mice. Neurochem. Res. 49, 129–141. doi: 10.1007/s11064-023-04013-8
Takemoto, K., Hatano, E., Iwaisako, K., Takeiri, M., Noma, N., Ohmae, S., et al. (2014). Necrostatin-1 protects against reactive oxygen species (Ros)-induced hepatotoxicity in acetaminophen-induced acute liver failure. Febs Open Bio 4, 777–787. doi: 10.1016/j.fob.2014.08.007
Tan, L., Chan, W., Zhang, J., Wang, J., Wang, Z., Liu, J., et al. (2023). Regulation of Rip1-mediated necroptosis via necrostatin-1 in periodontitis. J. Periodontal Res. 58, 919–931. doi: 10.1111/jre.13150
Tang, H., Song, Y., Li, T., Zheng, J., Jiang, P., Zhao, L., et al. (2021). Necrostatin-1 promotes locomotor recovery after spinal cord injury through inhibiting apoptosis and M1 polarization of microglia/macrophage in mice. Chin. J. Cell. Mol. Immunol. 37, 775–780
Ushijima, H., and Monzaki, R. (2023). An in vitro evaluation of the antioxidant activities of necroptosis and apoptosis inhibitors: the potential of necrostatin-1 and necrostatin-1i to have radical scavenging activities. Pharmacol. Rep. 75, 490–497. doi: 10.1007/s43440-023-00450-y
Vandenabeele, P., Grootjans, S., Callewaert, N., and Takahashi, N. (2013). Necrostatin-1 blocks both Ripk1 and Ido: consequences for the study of cell death in experimental disease models. Cell Death Differ. 20, 185–187. doi: 10.1038/cdd.2012.151
Wang, Y., Guo, L., Wang, J., Shi, W., Xia, Z., and Li, B. (2019). Necrostatin-1 ameliorates the pathogenesis of experimental autoimmune encephalomyelitis by suppressing apoptosis and necroptosis of oligodendrocyte precursor cells. Exp. Ther. Med. 18, 4113–4119. doi: 10.3892/etm.2019.8005
Wang, Y., Wang, H., Tao, Y., Zhang, S., Wang, J., and Feng, X. (2014). Necroptosis inhibitor necrostatin-1 promotes cell protection and physiological function in traumatic spinal cord injury. Neuroscience 266, 91–101. doi: 10.1016/j.neuroscience.2014.02.007
Wang, Y., Wang, J., Wang, H., Feng, X., Tao, Y., Yang, J., et al. (2018). Necrosulfonamide attenuates spinal cord injury via necroptosis inhibition. World Neurosurg. 114, e1186–e1191. doi: 10.1016/j.wneu.2018.03.174
Wang, Y., Wang, J., Yang, H., Zhou, J., Feng, X., Wang, H., et al. (2015). Necrostatin-1 mitigates mitochondrial dysfunction post-spinal cord injury. Neuroscience 289, 224–232. doi: 10.1016/j.neuroscience.2014.12.061
Wang, Y. Q., Wang, L., Zhang, M. Y., Wang, T., Bao, H. J., Liu, W. L., et al. (2012). Necrostatin-1 suppresses autophagy and apoptosis in mice traumatic brain injury model. Neurochem. Res. 37, 1849–1858. doi: 10.1007/s11064-012-0791-4
Wang, S., Wu, J., Zeng, Y. Z., Wu, S. S., Deng, G. R., Chen, Z. D., et al. (2017). Necrostatin-1 mitigates endoplasmic reticulum stress after spinal cord injury. Neurochem. Res. 42, 3548–3558. doi: 10.1007/s11064-017-2402-x
Wu, J. R., Wang, J., Zhou, S. K., Yang, L., Yin, J. L., Cao, J. P., et al. (2015). Necrostatin-1 protection of dopaminergic neurons. Neural Regen. Res. 10, 1120–1124. doi: 10.4103/1673-5374.160108
Xie, L., and Huang, Y. (2019). Antagonism of Rip1 using necrostatin-1 (Nec-1) ameliorated damage and inflammation of Hbv X protein (Hbx) in human normal hepatocytes. Artif. Cells Nanomed. Biotechnol. 47, 1194–1199. doi: 10.1080/21691401.2019.1575231
Xu, X., Chua, C. C., Kong, J., Kostrzewa, R. M., Kumaraguru, U., Hamdy, R. C., et al. (2007). Necrostatin-1 protects against glutamate-induced glutathione depletion and caspase-independent cell death in Ht-22 cells. J. Neurochem. 103, 2004–2014. doi: 10.1111/j.1471-4159.2007.04884.x
Yan, B., Zhang, H., Dai, T., Gu, Y., Qiu, X., Hu, C., et al. (2018). Necrostatin-1 promotes ectopic periodontal tissue like structure regeneration in Lps-treated Pdlscs. PLoS One 13:e0207760. doi: 10.1371/journal.pone.0207760
Yang, R., Hu, K., Chen, J., Zhu, S., Li, L., Lu, H., et al. (2017). Necrostatin-1 protects hippocampal neurons against ischemia/reperfusion injury via the Rip3/Daxx signaling pathway in rats. Neurosci. Lett. 651, 207–215. doi: 10.1016/j.neulet.2017.05.016
Ye, K., Chen, Z., and Xu, Y. (2023). The double-edged functions of necroptosis. Cell Death Dis. 14:163. doi: 10.1038/s41419-023-05691-6
Yin, B., Xu, Y., Wei, R. L., He, F., Luo, B. Y., and Wang, J. Y. (2015). Inhibition of receptor-interacting protein 3 upregulation and nuclear translocation involved in Necrostatin-1 protection against hippocampal neuronal programmed necrosis induced by ischemia/reperfusion injury. Brain Res. 1609, 63–71. doi: 10.1016/j.brainres.2015.03.024
Yin, C., Zhang, Q., Zhao, J., Li, Y., Yu, J., Li, W., et al. (2022). Necrostatin-1 against sevoflurane-induced cognitive dysfunction involves activation of Bdnf/TrkB pathway and inhibition of necroptosis in aged rats. Neurochem. Res. 47, 1060–1072. doi: 10.1007/s11064-021-03505-9
Yonekawa, T., Gamez, G., Kim, J., Tan, A. C., Thorburn, J., Gump, J., et al. (2015). Rip1 negatively regulates basal autophagic flux through Tfeb to control sensitivity to apoptosis. EMBO Rep. 16, 700–708. doi: 10.15252/embr.201439496
You, Z., Savitz, S. I., Yang, J., Degterev, A., Yuan, J., Cuny, G. D., et al. (2008). Necrostatin-1 reduces histopathology and improves functional outcome after controlled cortical impact in mice. J. Cereb. Blood Flow Metab. 28, 1564–1573. doi: 10.1038/jcbfm.2008.44
Yu, Z., Jiang, N., Su, W., and Zhuo, Y. (2021). Necroptosis: a novel pathway in Neuroinflammation. Front. Pharmacol. 12:701564. doi: 10.3389/fphar.2021.701564
Yu, C., Wang, X., and Qin, J. (2023). Effect of necrostatin-1 on sciatic nerve crush injury in rat models. J. Orthop. Surg. Res. 18:74. doi: 10.1186/s13018-023-03565-3
Yuk, H., Abdullah, M., Kim, D. H., Lee, H., and Lee, S. J. (2021). Necrostatin-1 prevents Ferroptosis in a Ripk1- and Ido-independent manner in hepatocellular carcinoma. Antioxidants 10:1374. doi: 10.3390/antiox10091347
Zanetti, L. C., and Weinlich, R. (2021). Necroptosis, the other Main caspase-independent cell death. Adv. Exp. Med. Biol. 1301, 123–138. doi: 10.1007/978-3-030-62026-4_7
Zhang, M., Li, J., Geng, R., Ge, W., Zhou, Y., Zhang, C., et al. (2013). The inhibition of Erk activation mediates the protection of necrostatin-1 on glutamate toxicity in Ht-22 cells. Neurotox. Res. 24, 64–70. doi: 10.1007/s12640-012-9361-4
Zhang, S., Tang, M. B., Luo, H. Y., Shi, C. H., and Xu, Y. M. (2017). Necroptosis in neurodegenerative diseases: a potential therapeutic target. Cell Death Dis. 8:e2905. doi: 10.1038/cddis.2017.286
Zhang, X., Zhang, Y., Wang, F., Liu, Y., Yong, V. W., and Xue, M. (2022). Necrosulfonamide alleviates acute brain injury of intracerebral hemorrhage via inhibiting inflammation and necroptosis. Front. Mol. Neurosci. 15:916249. doi: 10.3389/fnmol.2022.916249
Zheng, M., Choi, N., Jang, Y., Kwak, D. E., Kim, Y. S., Kim, W. S., et al. (2020). Hair growth promotion by necrostatin-1s. Sci. Rep. 10:17622. doi: 10.1038/s41598-020-74796-1
Zhou, Y., Dai, W., Lin, C., Wang, F., He, L., Shen, M., et al. (2013). Protective effects of necrostatin-1 against concanavalin A-induced acute hepatic injury in mice. Mediat. Inflamm. 2013:706156, 1–15. doi: 10.1155/2013/706156
Zhou, X. Y., Lin, B., Chen, W., Cao, R. Q., Guo, Y., Said, A., et al. (2023). The brain protection of Mlkl inhibitor necrosulfonamide against focal ischemia/reperfusion injury associating with blocking the nucleus and nuclear envelope translocation of Mlkl and Rip3K. Front. Pharmacol. 14:1157054. doi: 10.3389/fphar.2023.1157054
Zhou, Y., Zhou, B., Tu, H., Tang, Y., Xu, C., Chen, Y., et al. (2017). The degradation of mixed lineage kinase domain-like protein promotes neuroprotection after ischemic brain injury. Oncotarget 8, 68393–68401. doi: 10.18632/oncotarget.19416
Glossary
Keywords: necroptosis, necrostatin-1, neurological disorders, apoptosis, necrostatins
Citation: Chen K-q, Wang S-z, Lei H-b and Liu X (2024) Necrostatin-1: a promising compound for neurological disorders. Front. Cell. Neurosci. 18:1408364. doi: 10.3389/fncel.2024.1408364
Edited by:
Walace Gomes-Leal, Federal University of Western Pará, BrazilReviewed by:
Mohammad Hasanain, University of Miami Health System, United StatesMarco Aurelio M. Freire, Federal University of Sergipe, Brazil
Danuta Jantas, Polish Academy of Sciences, Poland
Copyright © 2024 Chen, Wang, Lei and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shu-zhi Wang, c2h1LXpoaS53YW5nQHVzYy5lZHUuY24=; Hai-bo Lei, Mjg2MjAwNTcxQHFxLmNvbQ==; Xiang Liu, TFgxOTg5MEAxNjMuY29t
 Ke-qian Chen
Ke-qian Chen Shu-zhi Wang2*
Shu-zhi Wang2*