
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol., 24 January 2025
Sec. Parasite and Host
Volume 15 - 2025 | https://doi.org/10.3389/fcimb.2025.1529770
 Di Zhang1†
Di Zhang1† Yan Zhao1†
Yan Zhao1† Dongyan Liu2†
Dongyan Liu2† Fei Liu1
Fei Liu1 Pengbo Liu1
Pengbo Liu1 Biying Zhang1
Biying Zhang1 Zifang Wu1
Zifang Wu1 Wanlapa Roobsoong3
Wanlapa Roobsoong3 Sirasate Bantuchai3
Sirasate Bantuchai3 Sataporn Thongpoon3
Sataporn Thongpoon3 Piyarat Sripoorote3
Piyarat Sripoorote3 Meilian Wang4*
Meilian Wang4* Liwang Cui5*
Liwang Cui5* Yaming Cao1*
Yaming Cao1*Background: Plasmodium vivax is a major cause of malaria, particularly outside Africa, necessitating effective strategies for public health management. Transmission-blocking vaccines (TBVs) have shown the potential to inhibit malaria transmission by targeting antigens expressed in sexual-stage parasites. Pbg37, a conserved protein expressed in sexual stages from gametocyte to ookinete in the rodent parasite P. berghei, is a viable target for TBV development.
Methods and findings: In this study, we constructed a transgenic strain, TrPvg37Pb, expressing Pvg37 using the P. berghei ΔPbg37 strain. Initial findings demonstrated that the replacement of Pbg37 with the exogenous Pvg37 did not impact parasite growth or development. Notably, Pvg37 was expressed during the gametocyte to ookinete development and was associated with the plasmic membrane, similar to Pbg37. To evaluate the potential of Pvg37 as a TBV candidate, we synthesized two Pvg37 polypeptides and immunized rabbits to generate antibodies. In vitro experiments demonstrated that anti-Pvg37-P2 antibodies significantly inhibited the formation of male gametes and ookinetes in the transgenic TrPvg37Pb parasite. Additionally, in mosquito feeding assays, mosquitos feeding on TrPvg37Pb-infected mice passively transferred with anti-Pvg37-P2 antibodies showed a significant 80.2% decrease in oocyst density compared to the control group. Furthermore, in direct membrane feeding experiments using four clinical P. vivax isolates, the anti-Pvg37 antibodies significantly reduced oocyst density by 28.6–50.4%.
Conclusion: Pvg37 is a promising candidate for P. vivax TBV development, deserving further research and optimization to enhance its immunogenicity and transmission-blocking activity.
Malaria is a severe parasitic disease caused by Plasmodium parasites. According to the World Health Organization’s World Malaria Report 2023, there were 249 million cases worldwide in 2022, an increase of 5 million cases compared with 2021 (WHO, 2023). Plasmodium vivax is a major cause of malaria outside of Africa and accounts for about 72% of all cases in Southeast Asia and the Americas (Flannery et al., 2019). Managing and treating P. vivax malaria is more challenging than P. falciparum malaria, as it produces dormant hypnozoites in the liver that are responsible for relapse (Adams and Mueller, 2017; Flannery et al., 2022). Currently, primaquine and tafenoquine are utilized to clear hypnozoites, but they are contraindicated in individuals with glucose-6-phosphate dehydrogenase (G6PD) deficiency due to the risk of hemolysis (Lacerda et al., 2019; Llanos-Cuentas et al., 2019; Thriemer et al., 2021). The Malaria Eradication Research Agenda (MalERA) considers that interrupting malaria transmission is a key measure for malaria elimination, with transmission-blocking vaccines (TBVs) uniquely suited for this task.
In membrane-feeding assays, antibodies targeting antigens consumed in a blood meal can suppress the growth of parasites within mosquito vectors. TBV candidate antigens are primarily expressed on the surface of the mosquito-stage malaria parasite (Miura et al., 2019). Thus, they are less susceptible to selective pressure from the vertebrate immune system and display lower levels of polymorphism. According to their expression patterns, TBV antigens fall into two categories. Pre-fertilization antigens such as P48/45 and P230 are expressed in gametocytes and gametes, while post-fertilization antigens such as P25 and P28 are expressed on the surfaces of zygotes and the maturing ookinetes (Hisaeda et al., 2001; van Dijk et al., 2001; Doi et al., 2011; Arévalo-Herrera et al., 2015). Although TBV research has received considerable attention, only a limited number of candidate antigens have been identified (de Jong et al., 2020), especially for P. vivax. These include pre-fertilization antigens Pvs230, Pvs48/45, and PvHAP2, post-fertilization antigens Pvs25 and Pvs28, and the mosquito midgut antigen AgAPN1 (Malkin et al., 2005; Tachibana et al., 2012, 2015; Tentokam et al., 2019). Recombinant Pvs25H expressed in Saccharomyces cerevisiae has been evaluated in Phase I clinical trials with alum or Montanide ISA51 as an adjuvant (Malkin et al., 2005; Wu et al., 2008). It has been shown that >25% of endemic populations showed natural antibody responses to the Pvs230 domain 1 (Tentokam et al., 2019). In P. falciparum, Pfs230 regarded as a homologue of Pvs230 is considered a more promising TBV candidate (Healy et al., 2021). A vaccine targeting the first domain of Pfs230 has demonstrated a stronger TBA than the comparable Pfs25 vaccine and is currently in Phase II field trials in Mali (Duffy, 2022). Another pre-fertilization antigen, PvHAP2, showed transmission-reducing activity (TRA) of 40.3–89.7% in a direct membrane feeding assay (DMFA) (Qiu et al., 2020). Therefore, there is a clear priority in TBV antigen discovery for P. vivax.
We have identified Pbg37 as a conserved sexual-stage antigen across the genus Plasmodium. It was expressed intracellularly in gametocytes, but the protein became membrane-associated during gametogenesis and zygote-ookinete development (Liu et al., 2018). Pbg37 is essential for sexual development, as its deletion led to a significant reduction in gametocytemia and oocyst numbers in mosquitoes. Direct feeding of mosquitoes on mice immunized with recombinant Pbg37 resulted in a 49.1% reduction in oocyst density. This TRA and the conservation of Pbg37 in Plasmodium prompted us to investigate the TB potential of its ortholog in P. vivax, Pvg37. By replacing Pbg37 with Pvg37, we generated a transgenic P. berghei parasite line expressing Pvg37. Using this transgenic parasite and clinical P. vivax isolates, we conducted mosquito-feeding assays and demonstrated that antibodies against Pvg37 also possessed substantial TRA.
Female BALB/c mice and New Zealand white rabbits were purchased from the Beijing Animal Institute. The P. berghei ANKA strain 2.34 was maintained by serial passage and used for challenge infection as described previously (Bai et al., 2023). The Δpbg37 parasite used for generating a transgenic parasite expressing Pvg37 was from an earlier study (Liu et al., 2018). Adult (3-5 days old) Anopheles stephensi and An. dirus mosquitoes were fed on a 10% (w/v) glucose solution and kept in an insectary under 25°C ± 1°C and 50 – 80% relative humidity, with a 12 h light and dark cycle. All animal procedures were carried out per the welfare and ethical review standards of China Medical University.
The TrPv37Pb line was generated by inserting the complete Pvg37 open reading frame (ORF, 1053 bp) tagged with 3×HA at its C-terminus into the pL0034 vector at the ApaI and XhoI sites. The Pvg37 ORF was flanked by the 5’ UTR and 3’ UTR of the Pbg37 gene. Ten micrograms of the plasmid were digested with ApaI and XhoI and then electroporated into purified Δpbg37 schizonts using a Nucleofector system. Subsequently, the parasites were injected intraperitoneally into two mice. After 24 h, the TrPv37Pb line was selected through gavage in mice using 5-fluorocytosine (20 mg/mL in water). To confirm the proper integration of the Pvg37 gene at the Pbg37 locus in the P. berghei genome, PCR analysis was performed using specific primers (Supplementary Table S1). The TrPv37Pb parasites were then cloned using a limiting dilution technique.
To investigate the impact of Pvg37 on parasite development, we compared the development among the (wild-type) WT P. berghei, TrPvg37Pb, and Δpbg37 lines. Three groups of BALB/c mice (3 mice per group) were intraperitoneally injected with 1×106 infected red blood cells (iRBCs) of the respective parasite clones. Asexual parasitemia levels were monitored on days 3, 5, 7, 9, and 11 post-infections by examining Giemsa-stained thin blood smears. The number of mature gametocytes per 100 parasites was determined during the parasitemia range of 10–20%. In each mouse, 100 mature gametocytes were differentiated into male and female gametocytes based on morphological characteristics to establish the gametocyte sex ratio. Following induction for gametogenesis at 25°C for 15 min, the culture was transferred onto a coverslip, and exflagellation centers were counted under a phase-contrast microscope at 400× magnification. To observe ookinete formation, 10 μL of infected blood containing equal gametocyte counts were mixed with the ookinete culture medium in a total volume of 50 μL and maintained at 19°C for 24 h. The gametocyte counts were normalized according to gametocytemia. The ookinete number in 0.5 μL of culture was counted using an IFA, with ookinetes stained with a monoclonal antibody (mAb) against Pbs21.
The Pvg37 protein fragments spanning amino acids 25 to 38 and 55 to 68 were synthesized as polypeptides (Genescipt, China), namely Pvg37-P1 and Pvg37-P2, respectively, which were conjugated to keyhole limpet hemocyanin (KLH) for immunization. Three rabbits were subcutaneously immunized with 500 μg of Pvg37 peptides emulsified in Freund’s complete adjuvant. Three booster immunizations were performed at weeks 2, 5, and 8 after emulsification with 250 μg of Pvg37 peptides and incomplete Freund’s adjuvant. The immune serum was collected 10 days after the last immunization. IgGs were purified from Pvg37 immune and pre-immnue sera, respectively, using Protein A columns. The concentrations of anti-Pvg37 and pre-immune control antibodies were determined using the BCA Protein Assay kit.
ELISA was utilized to determine antibody titers of sera. A 96-well plate was coated with polypeptides Pvg37-P1 and Pvg37-P2 in 0.05 M sodium carbonate buffer (pH 9.6) and incubated overnight at 4°C. The samples were then washed three times with PBST (0.05% Tween-20, 0.1 M PBS) and incubated with 1% BSA (Sigma) for 1 h at 37°C. Following another round of washing with PBST, the anti-Pvg37 peptide sera and negative control sera were diluted in PBS containing 1% BSA at multiple proportions ranging from 1:1000 to 1:512000. The samples were incubated at 37°C for 2 h and washed three times with PBST. HRP-labeled sheep anti-rabbit IgG, diluted in 3% BSA (1:5000), was added to the 96-well plates, and the samples were incubated at 37°C for 1 h. Subsequently, the plates were washed five times, and tetramethyl-benzidine was added for color development in the dark for 10 min. The reaction was stopped by adding 2 mM H2SO4, and the absorbance value at 490 nm was measured. The final dilution value was considered to be higher than the mean + 3 × standard deviation (cut-off value) of the pre-immune control sera.
IFA was performed on gametocytes, gametes, zygotes, retorts, and ookinetes. TrPvg37Pb parasites were fixed with 4% paraformaldehyde and 0.0075% glutaraldehyde in PBS for 30 min at room temperature. Then, the parasites were washed twice with PBS. After being permeabilized with 0.1% Triton X-100, parasites were blocked with PBS containing 3% BSA for 1 h at 37°C. The rabbit anti-Pvg37-P2 sera (1:200) in PBS containing 3% BSA were added into parasites for 1 h at 37°C. All parasites were co-incubated with mouse antisera against PbMSP1 (1:500), Pbs47 (1:500), Pbα-tubulin (1:500), and Pbs21 (1:500) as specific markers for schizonts, female gametocytes/gametes, male gametocytes/gametes, and zygotes/ookinetes, respectively. These antisera were self-made and have been previously reported (Zheng et al., 2024). After washing the slides with PBS, Alexa Fluor 488-conjugated anti-rabbit IgG secondary antibodies (1: 500, Invitrogen) and Alexa-555 conjugated goat anti-mouse IgG secondary antibodies (1: 500, Abcam) were added into the parasites for 1 h at 37°C. WT ookinetes were used as the negative control. Images were acquired using a Leica SP8 confocal laser scanning microscope. For comparison, mouse anti-HA mAb (1:500, abclone) was also used to probe parasites to determine the expression stage of Pvg37. Furthermore, the localization of Pvg37 protein on the P. vivax gametocytes was confirmed using anti-Pvg37-P2 IgGs and isolating gametocytes from clinical samples of P. vivax.
Different gradient Nycodenz was used to isolate and purify TrPvg37Pb parasites at various stages. When the parasitemia reached 3-5%, mouse blood was collected and mixed with schizont culture medium (RPMI 1640, 50 mg/L penicillin, 50 mg/L streptomycin, 100 mg/L neomycin, 25% fetal bovine serum, and 6 U/mL heparin). The mixture was cultured at 37°C for 20 h. Schizonts were subsequently isolated and purified on a 56% (v/v) Nycodenz gradient. For purifying sexual stages, mice were treated with sulfadiazine (Sigma, Burlington, USA, 20 mg/L) for 2 days to eliminate asexual blood stages when the parasitemia ranged between 10 and 20%. When parasites reached the gametocyte stage, blood was collected and mixed with PBS at 4°C to prevent gametocyte activation. Gametocytes were then isolated and purified on a 48% (v/v) Nycodenz gradient. To obtain ookinetes, 1 mL of blood was mixed with 9 mL of ookinete medium (50 mg/L penicillin, 50 mg/L streptomycin, 100 mg/L neomycin, 20% fetal bovine serum, 1 mg/L heparin, pH 8.3) and cultured at 19°C for 24 h. Ookinetes were then isolated and purified using a 62% (v/v) Nycodenz gradient. Finally, purified parasites from each stage mentioned above were washed twice with PBS.
To determine the expression of Pvg37 at different stages, purified schizonts, gametocytes, and ookinetes were treated with 0.2% saponin to lyse erythrocytes. After three washings with PBS, parasites were treated with RIPA lysis buffer containing phenylmethylsulfonyl fluoride three times to extract total proteins. Protein concentrations were determined using the BCA Protein Assay kit. Equal parasite proteins (20 μg/lane) were separated by 10% SDS-PAGE and transferred to a 0.22 μm PVDF membrane. Then, the PVDF membrane was blocked with TBST containing 5% skim milk for 1 h at 37°C. After blocking, the PVDF membrane was probed with anti-Pvg37 rabbit immune sera (1:200) and anti-rHSP70 sera (1: 1000), and after three washing with TBST, HRP-conjugated goat anti-rabbit IgG antibodies (1: 10000, Invitrogen) as the secondary antibodies were added. The blots were then detected by an ECL Western Blotting Kit (Beyotime).
The TB potential of Pvg37 was evaluated using two assays: an in vitro ookinete formation assay and a direct mosquito feeding assay. The in vitro assay employed various dilutions of the immune sera. Groups of BALB/c mice (three mice per group) were injected intraperitoneally with 1×106 iRBCs of the TrPv37Pb line. The exflagellation of male gametocytes was quantified using the method mentioned above. Purified pre-immune IgGs or anti-Pvg37-P2 IgGs were added into the ookinete culture medium at a final concentration of 0.2, 1.0, and 2.0 µg/µL and mixed with 10 μL of blood from mice infected with the TrPvg37Pb parasites, resulting in a total volume of 50 μL. The number of exflagellation centers was observed in each microscope field at 400x magnification. Ookinete cultures were incubated at 19°C for 24 h, and mature ookinetes in 0.5 μL of culture were counted using a fluorescence microscope (100× oil objective). For antibody transfer experiments, 150 µL of purified anti-Pvg37-P2 IgGs were injected into the tail veins of the mice one hour prior to An. stephensi mosquito direct feeding. Twelve days after feeding, mosquitoes were dissected to assess the number of oocysts using a compound microscope at 200× magnification.
We conducted DMFA using blood samples from volunteers infected with P. vivax. Prior to participation, informed written consent was obtained from four volunteers. Parasitemia was estimated using Giemsa-stained films. The anti-Pvg37-P2 antibody and the negative control antibody were diluted 1:1 with 90 μl of heat-inactivated (complement negative) healthy human AB+ serum, resulting in a total volume of 180 μl. The diluted antibodies were then mixed with RBCs collected from P. vivax malaria patients in a 1:1 ratio. Pooled blood samples were incubated at 37°C for 15 min and then introduced into a membrane feeder. Approximately 100 An. dirus mosquitoes (starved for 12 h before the experiment) were fed with the blood samples for 30 min using the membrane feeder maintained at 37°C. Unfed mosquitoes were subsequently removed, and fed mosquitoes were allowed to feed on cotton pads dipped in a 10% sucrose solution at 27°C and 80% relative humidity for one week. For each group, 20 mosquitoes were dissected to count oocysts.
Genomic DNA from the four P. vivax isolates used in the DMFA was extracted using a QIAamp DNA Blood Mini kit (Qiagen, Germany). The pvg37 DNA fragment encoding aa 1–182 was amplified by PCR with primers designed based on the Sal-I sequence (Supplementary Table S1). The purified PCR products were sequenced using the ABI Prism BigDye™ cycle sequencing kit (Applied Biosystems, Thermo Fisher Scientific). The sequences were aligned using ClustalW in MEGA7.0.26.
Statistical analyses were conducted using SPSS software version X (SPSS Inc., USA). Ordinary one-way ANOVA was used to compare the groups in terms of asexual parasitemia, gametocytes, sex ratio, exflagellation, and ookinete numbers. Mann-Whitney U test was employed to analyze oocyst density (oocyst number per midgut), while Fisher’s exact test was used to analyze infection prevalence. The results are presented as the mean ± SD. A significance level of 0.05 was considered statistically significant.
The TrPvg37Pb transgenic strain was constructed using the Gene Insertion and Marker Out (GIMO) technique. This approach involves replacing the drug resistance gene in the obtained Δpbg37 strain with the pvg37 gene, which is tagged with a 3×HA tag, under negative selection with 5-fluorocytosine (Figure 1A). The genotypes of WT, Δpbg37, and TrPvg37Pb were identified by PCR amplification using primers 1 and 2, 1 and 3, and 1 and 4, respectively (Figure 1A). Diagnostic PCR results confirmed successful recombination of the pvg37 gene as specific bands were only amplified from corresponding parasite gDNA samples (Figure 1B). Additionally, Pvg37-HA protein expression in transgenic P. berghei parasite lines was confirmed by Western blotting using the anti-HA mAb. A protein band of approximately 39 kDa was detected in the TrPvg37Pb parasites but not found in WT parasites (Figure 1C).
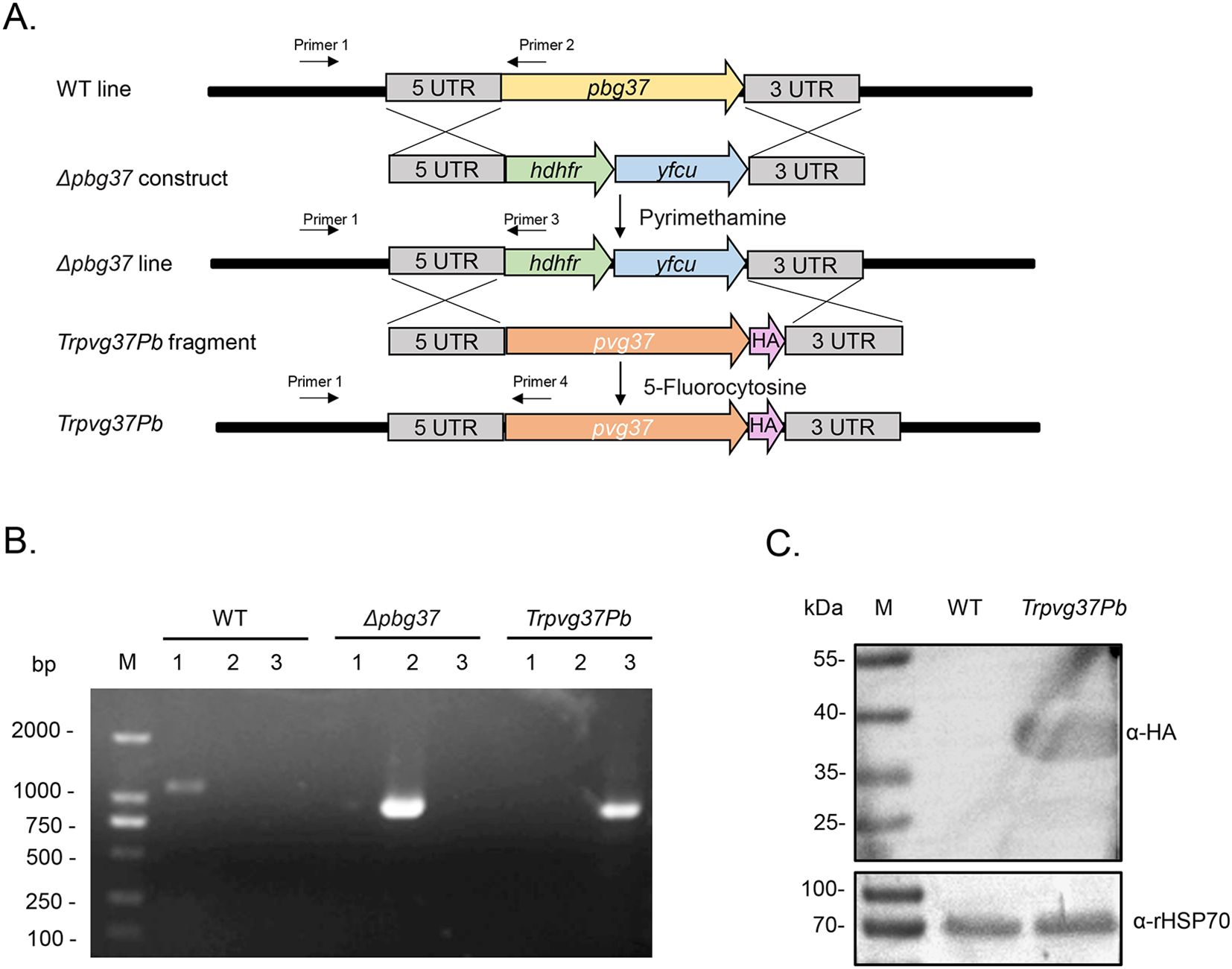
Figure 1. Generation of the TrPvg37Pb parasites. (A) Schematic diagram illustrating the construction of the TrPvg37Pb transgenic parasite through double homologous recombination, wherein the hdhfr::yfcu selection cassette in Δpbg37 parasites is replaced with the pvg37 gene. Primers 1 – 4 used for diagnostic PCR are depicted. (B) PCR identification of the TrPvg37Pb transgenic line. Primers 1 + 2 were employed to confirm wild-type (WT) locus, primers 1 + 3 were used to identify pbg37 deletion, while primers 1 + 4 were utilized to confirm successful replacement of pvg37 gene in P. berghei line. The resulting PCR products showed distinct band sizes: lanes 1, PCR with primers 1 + 2 (1104 bp); lanes 2, PCR with primers 1 + 3 (868 bp); lanes 3, PCR with primers 1 + 4 (796 bp). (C) Western blot analysis for identifying WT and TrPvg37Pb parasites using anti-HA monoclonal antibody (top), while HSP70 was employed as a protein loading control (bottom).
The impact of complementing Pvg37 on the growth and development of P. beighei was assessed through phenotype analysis performed on TrPvg37Pb, Δpbg37, and WT strains. Equal numbers of parasite-infected RBCs for each strain were injected into BALB/c mice via the tail vein. The results showed no significant difference in asexual-stage parasitemia among TrPvg37Pb, Δpbg37, and WT parasites (Figure 2A). However, the Δpbg37 strain exhibited significantly lower gametocytemia and a higher female/male gametocyte ratio (Figures 2B, C). Furthermore, compared to WT parasites, the Δpbg37 strain displayed a significant reduction of 27.8% in exflagellation centers and 34.4% in ookinete formation (Figures 2D, E), consistent with previously published data (Liu et al., 2018). Notably, no significant differences were observed between the TrPvg37Pb strain and WT strain in terms of gametocyte formation, exflagellation centers, or ookinete production (Figures 2B-E). These findings indicate that substituting Pbg37 with Pvg37 does not affect the development of both asexual and sexual stages of P. berghei. Moreover, Pvg37 compensates for the abnormal phenotype caused by pbg37 deletion, suggesting a functional equivalence between Pvg37 and Pbg37.
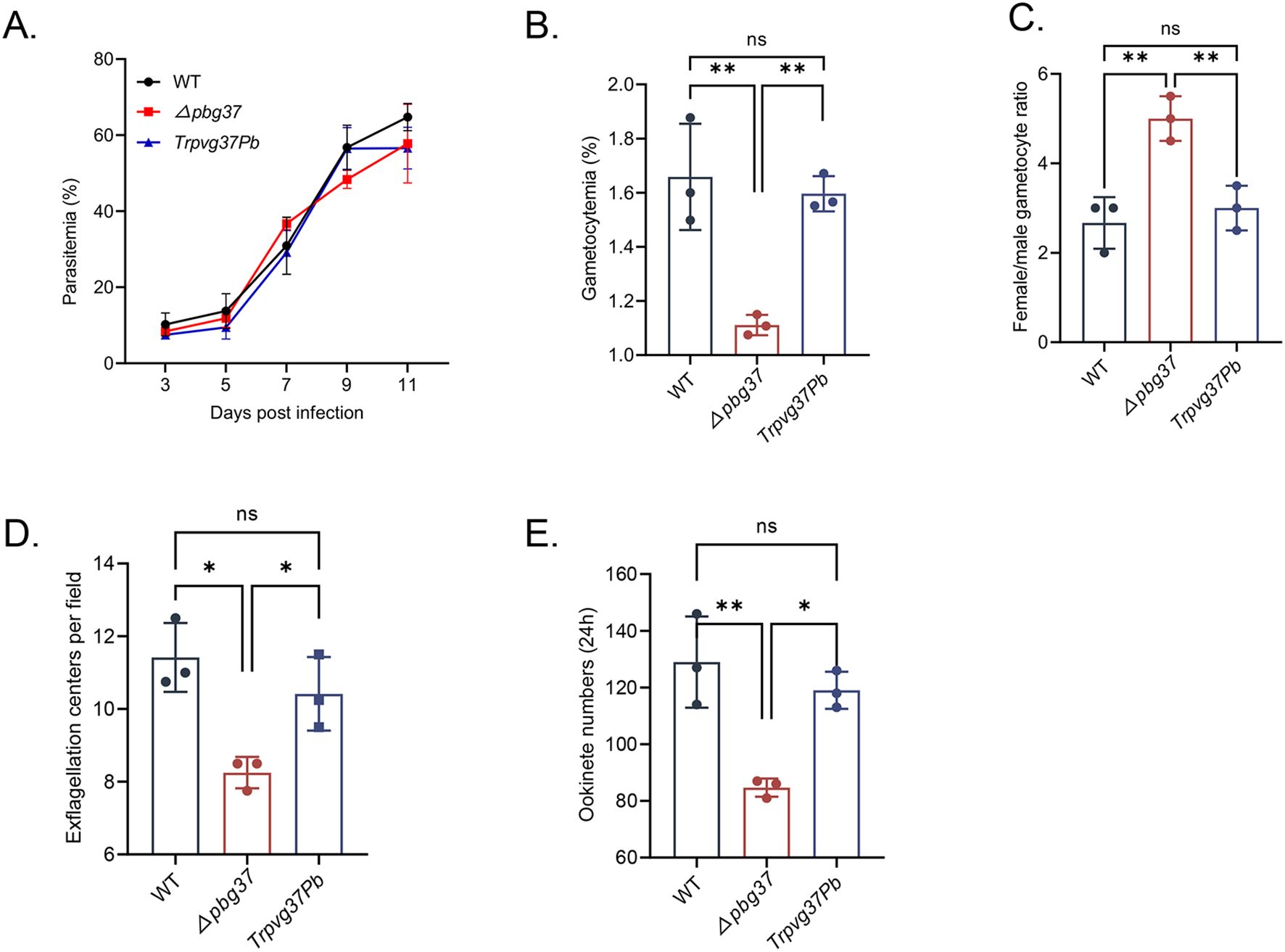
Figure 2. Phenotypic analysis of TrPvg37Pb parasites. (A) Parasitemia of mice infected with WT, Δpbg37, or TrPvg37Pb parasites. (B) Gametocytemia (percentage of gametocyte in 100 RBC). (C) Female/male gametocyte ratios. (D) Number of exflagellation centers per field at 400× magnification. (E) Quantification of ookinete numbers in 0.5 μl of in vitro culture through immunostaining with anti-Pbs21 mAb. All experiments were performed in triplicate. Error bars indicate mean ± SD. *P < 0.05, **P < 0.01 (one way ANOVA). "ns" denotes no significance.
The Pvg37 protein possesses seven transmembrane domains similar to Pbg37, and multiple sequence alignment revealed a high degree of conservation among Plasmodium species. Two highly conserved regions in Pvg37 were identified at amino acids 25-38 and 55-68, which showed abundant B-cell antigenic epitopes (Liu et al., 2018). Therefore, we opted to utilize these two highly conserved regions, namely Pvg37-P1 and Pvg37-P2, for peptide synthesis. Polyclonal antibodies against Pvg37-P1 and Pvg37-P2 were generated by immunizing rabbits separately. ELISA showed that the final antibody titers for Pvg37-P1 and Pvg37-P2 reached 1:8000 and 1: 128000, respectively (Figure 3A). Remarkably, the anti-Pvg37-P2 sera exhibited significantly higher antibody titers than the anti-Pvg37-P1 sera. Thus, we selected the Pvg37-P2 antibodies for subsequent experiments. IgG concentrations purified from the Pvg37-P2 immunized serum and pre-immunized serum were 11.667 and 14.168 μg/μL, respectively, which were adjusted to 10 mg/mL with PBS for transmission-blocking assessment.
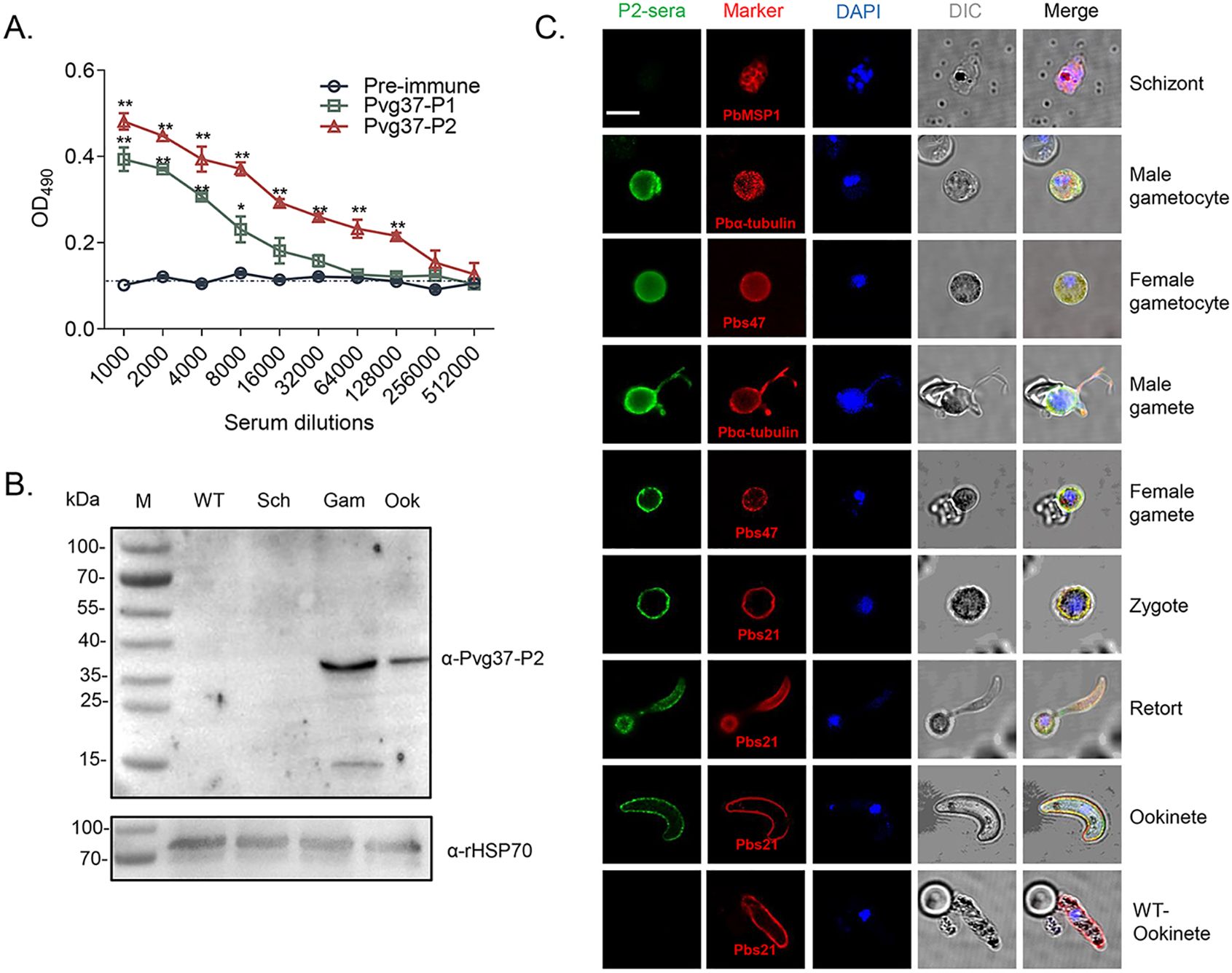
Figure 3. Pvg37 expression and localization in TrPvg37Pb parasites. (A) The total antibody titer of anti-Pvg37-P1 and anti-Pvg37-P2 sera at 10 days after the final immunization was analyzed by ELISA. Mean of control pre-immune sera + 3 × SD is shown by the broken lines. IgG titers were determined as the highest dilution of anti-Pvg37 sera where OD490 values were above the cut-off values. Cut-off value was defined as that of the pooled sera from control mice. The error bar shows mean ± SD. *P < 0.05, **P < 0.01 (Student’s t-test). (B) Western blot analysis was performed on lysates containing 20 μg of protein per lane derived from the control WT parasites, as well as purified schizonts (Sch), gametocytes (Gam), and ookinetes (Ook) of the TrPvg37Pb parasites. The proteins were probed with anti-Pvg37-P2 IgGs (top), while HSP70 served as a loading control for protein quantification (bottom). (C) Immunofluorescence assays were conducted on TrPvg37Pb parasites at various stages using anti-Pvg37-P2 IgGs. The WT ookinete was used as control. The scale bar represents 5µm.
To confirm the expression and localization of the Pvg37 protein in transgenic parasites, we purified TrPvg37Pb parasites at different stages, including schizonts, gametocytes, gametes, and ookinetes. Western blot using the rabbit anti-Pvg37-P2 sera detected a ~37 kDa protein band in TrPvg37Pb gametocytes and ookinetes but not schizonts. The expression level in gametocytes appeared higher than in ookinetes (Figure 3B).
We examined the subcellular localization of Pvg37 protein in TrPvg37Pb parasites by IFA with rabbit anti-Pvg37-P2 IgGs. Fluorescent signals were observed in the cytosol and at the plasma membrane of gametocytes (Figure 3C). During the gametogenesis of microgametocytes, signals were prominently observed along the flagellas. In subsequent development, Pvg37 was specifically associated with the plasma membranes of gametes, zygotes, retorts, and ookinetes (Figure 3C). No signal indicating Pvg37 expression was detected in TrPvg37Pb schizonts or WT ookinetes. Since the Pvg37 protein was C-terminally tagged with a 3×HA tag in the TrPvg37Pb parasite, we also performed IFA with a mouse anti-HA mAb and obtained similar results as with anti-Pvg37-P2 IgGs (Supplementary Figure S1). Overall, these findings demonstrate that Pvg37 exhibited a plasma membrane localization pattern during gamete–ookinete transition, similar to Pbg37.
Initially, we investigated the inhibitory activity of anti-Pvg37 IgGs on the formation of exflagellation centers and ookinetes using in vitro assays. The inhibitory effects of the anti-Pvg37-P2 IgGs were concentration-dependent. Compared with control IgGs, anti-Pvg37-P2 IgGs at 0.2 μg/μL had no inhibitory effect on exflagellation or ookinete formation (Figures 4A, B). However, at 1.0 and 2.0 μg/μL concentrations, anti-Pvg37-P2 IgGs inhibited the number of exflagellation centers by 28.6% and 60.0%, respectively (Figure 4A). Similarly, these concentrations of the anti-Pvg37-P2 IgGs reduced the number of ookinetes by 43. 7% and 69.7%, respectively (Figure 4B).
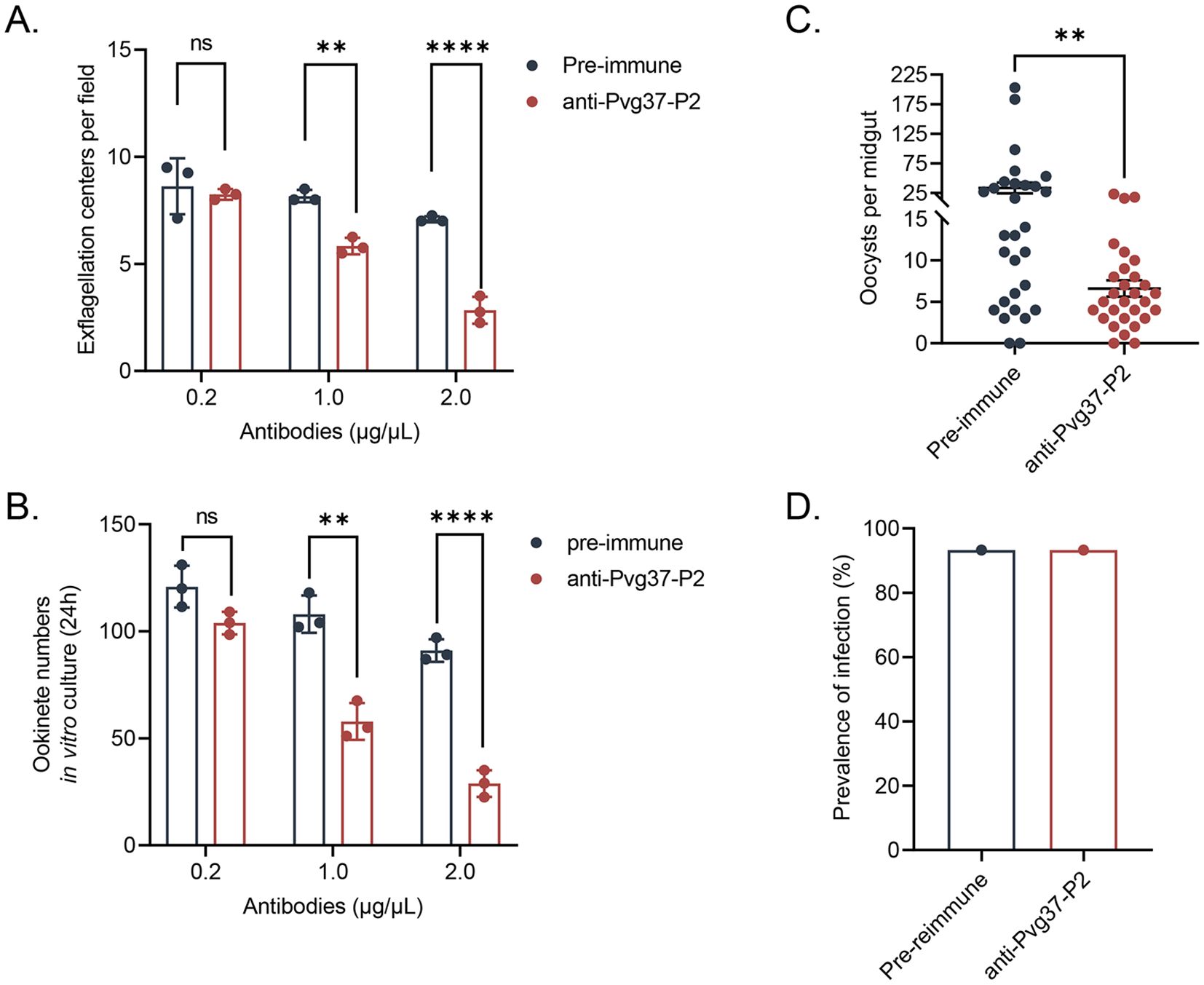
Figure 4. Evaluation of the transmission blocking effect of anti-Pvg37-P2 IgGs on transgenic parasites. The inhibition of the anti-Pvg37-P2 IgGs on (A) male gametocyte exflagellation and (B) ookinete formation was assessed by in vitro assays. The purified anti-Pvg37-P2 IgGs and pre-immune IgGs were added at concentrations of 0.2, 1.0, and 2.0 μg/μL in culture medium, respectively, incubated with the TrPvg37Pb parasites. Data were representative of three separate experiments. The error bar shows mean ± SD. ****P < 0.0001, **P < 0.01, ns, no significance (Student’s t-test). (C) The number of oocysts per midgut in mosquitos after 10 days of feeding. N=29, error bar represents mean ± SEM, ** P < 0.01 (Student’s t-test). (D) Mosquito infection rate (oocyst-infected mosquitoes/dissected mosquitoes).
The TB potential of anti-Pvg37 IgGs was further assessed through mosquito feeding assays. In a passive antibody transfer experiment, anti-Pvg37-P2 IgGs significantly reduced the number of oocysts per midgut in mosquitoes by 80.2% compared to the control group (Figure 4C), although we did not observe noticeable TBA for the anti-Pvg37-P2 IgGs (Figure 4D).
We studied Pvg37 expression and localization in clinical P. vivax samples. In both male and female gametocytes, Pvg37 exhibited cytoplasmic localization and a punctate distribution along the plasma membrane. In contrast, the control IgGs did not show any staining in P. vivax gametocytes. These findings demonstrate that anti-Pvg37-P2 IgGs specifically reacted with P. vivax gametocytes (Figure 5A).
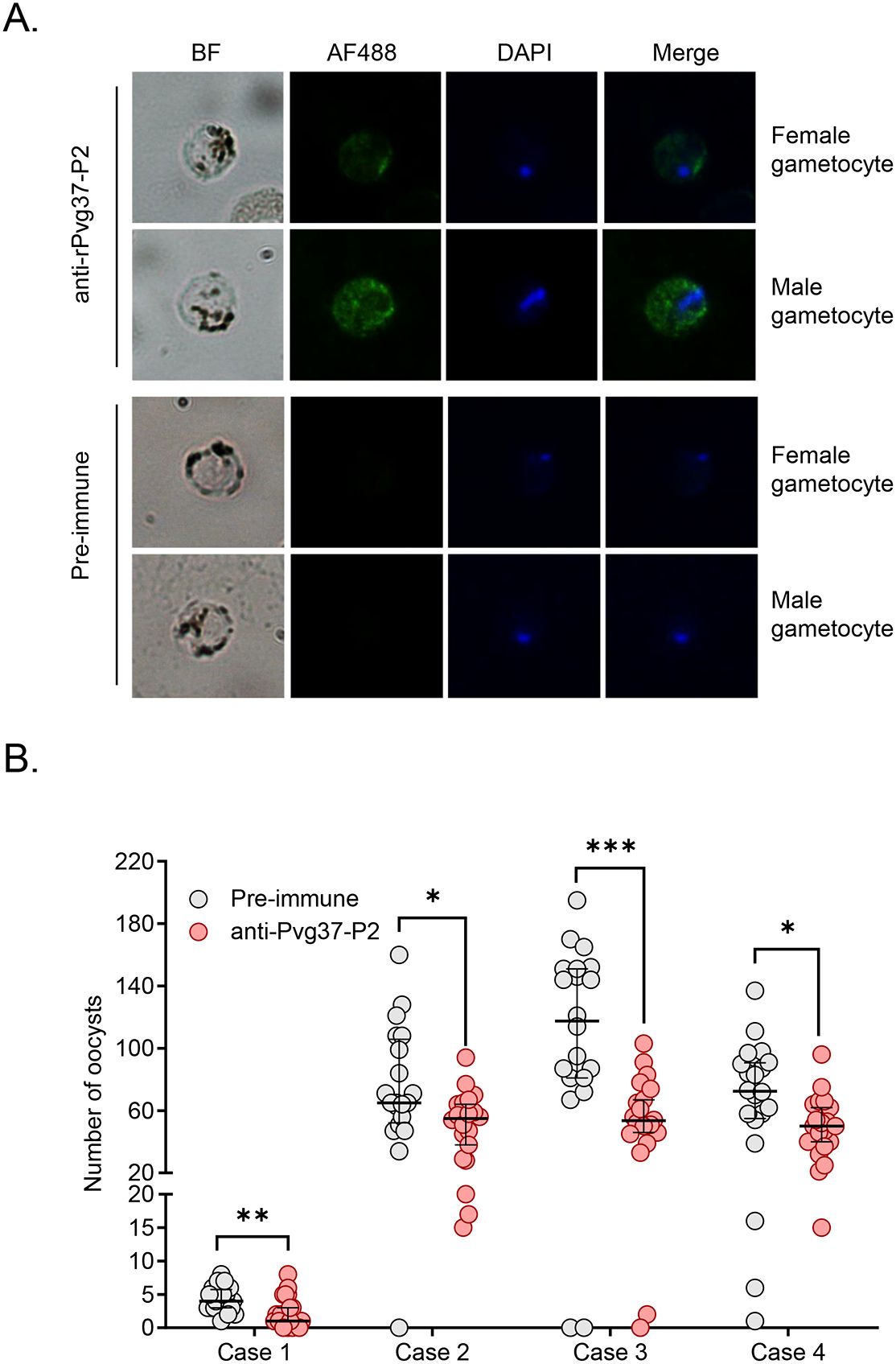
Figure 5. Detection of Pvg37 expression in P. vivax gametocytes and TRA of antibody against Pvg37-P2 in DMFA. (A) Gametocytes were stained with the purified anti-Pvg37-P2 IgGs and Alexa Fluor 488-conjugated anti-rabbit IgG antibodies. Pre-immune IgGs was used as the negative control. Nuclei were stained with DAPI. BF, bright field; AF488, Alexa Fluor 488; Merge, AF488 + DAPI. (B) DMFA was performed using four P. vivax isolates with purified IgGs mixed with heat-inactivated (complement minus) AB+ human serum in the ratio of 1:1. Numbers of oocysts in mosquito midguts were shown as scatter dot plots. The black horizontal bar indicates the mean number of oocysts in each group. Statistical difference in the mean number of oocysts between the pre-immune and Pvg37 groups was analyzed by the Mann-Whitney U test (*P < 0.05, **P < 0.01, ***P < 0.001).
To investigate the efficacy of anti-Pvg37-P2 IgGs in blocking P. vivax transmission, DMFA was performed using P. vivax samples collected from four Thai patients with P. vivax mono-infection, as confirmed by PCR analysis targeting the 18S rRNA using species-specific primers. In DMFA, An. dirus mosquitoes feeding on all four blood samples mixed with pre-immune antibodies displayed a mean midgut oocyst intensity of 4.3, 75.4, 111.2, and 69.4, respectively (Table 1). In comparison, mosquitoes feeding on the same blood samples mixed with the anti-Pvg37-P2 IgGs at a 2.5 μg/μL concentration showed a mean infection intensity of 2.3, 50.6, 55.2, and 49.6 oocysts per midgut, corresponding to a respective reduction in oocyst density by ~5.9%, 32.9%, 50.4%, and 28.6% (P < 0.05, Figure 5B; Table 1). However, compared to the control antibodies, the anti-Pvg37-P2 IgGs did not show a significant reduction in infection prevalence (Figure 5B; Table 1).
The genetic diversity of malaria vaccine candidates in endemic parasites poses a challenge to vaccine development (Takala and Plowe, 2009). To investigate whether the variability of TRA among the different isolates might be attributed to genetic polymorphisms of the pvg37 gene, we sequenced the pvg37 gene fragments from the four P. vivax isolates used in DMFA. Our results showed that these samples had identical amino acid sequences of Pvg37 with the Sal-I strain (Supplementary Figure S2).
TBV candidates identified by the MalERA as potential tools for malaria eradication exhibit lower genetic diversity compared to blood or pre-erythrocytic stage antigens, likely due to reduced exposure to human immunity (Alonso et al., 2011; Lopez et al., 2017). However, efforts towards TBV development against P. vivax, the second major cause of malaria morbidity, significantly lag behind those targeting P. falciparum. All current P. vivax TBV candidates (Pvs25, Pvs28, Pvs47, Pvs48/45, Pvs230, and PvHAP2) are orthologs of known P. falciparum candidates (Kaslow et al., 1988; Hisaeda et al., 2000; Tachibana et al., 2012, 2015; Qiu et al., 2020). Given the substantial differences in biological characteristics and epidemiology between these two species of Plasmodium parasites, methodologies developed for P. falciparum TBVs may not always be directly applicable for combating P. vivax infection, including the utilization of orthologous vaccine antigens (Mueller et al., 2009). Therefore, it is imperative to identify novel candidate antigens targeted to P. vivax to expedite research and development efforts toward an effective TBV.
TBVs elicit antibodies that neutralize the sexual stages of the parasite in blood meals ingested by the Anopheles mosquitos, disrupting parasite development in the mosquito and preventing transmission. Upon ingesting the parasite and antibodies, certain antibodies recognize pre-fertilization antigens on the gametocytes/gametes, while others target post-fertilization antigens on zygotes/ookinetes. In malaria-endemic areas, natural antibodies against pre-fertilization antigens exist within populations, providing an immune advantage; however, antibodies targeting post-fertilization antigens may prolong antibody blockade duration (Jones et al., 2015; Dinko et al., 2016; Muthui et al., 2019). Therefore, simultaneous antigens expression during pre- and post-fertilization stages can elicit more significant transmission blockade responses. Our previous study demonstrated that Pbg37 is expressed on the surface of both pre-fertilization (gametes) and post-fertilization (zygotes and ookinetes) stages, suggesting its potential as a candidate antigen for a TBV (Liu et al., 2018). Through functional studies, we determined the importance of Pbg37 during gametocytogenesis, particularly in male gametocyte development. Furthermore, we showed that antiserum against a small 63-amino-acid Pbg37 polypeptide was able to induce moderate TB activity in a mosquito-feeding assay (Liu et al., 2018). Although Pvg37 shares 59% sequence identity with Pbg37 at the amino acid level, it remains unclear if their functional characteristics and expression patterns are consistent across different species.
The biggest challenge facing vaccine development for vivax malaria is the inability to establish long-term in vitro cultures of P. vivax (da Veiga et al., 2022). However, transgenic rodent malaria parasites expressing a P. vivax TBV candidate gene in place of their native genes offer a promising alternative assay system (Ramjanee et al., 2007; Cao et al., 2018). It utilizes the principle that the target cell genome can undergo homologous recombination with the homologous sequences of exogenous DNA to perform precise gene editing or modification, thereby achieving precise manipulation of the target gene (Rocha-Martins et al., 2015). In the current study, we used the transgenic rodent malaria parasites to assess the Pvg37 gene function. We found that Pvg37 was expressed similarly to Pbg37, mainly on the surface of both pre-fertilization (gametes) and post-fertilization (zygotes and ookinetes) stages. The phenotype of TrPvg37Pb was similar to the WT line, indicating that Pvg37 fully restored the defects of ΔPbg37 parasites during sexual development, demonstrating the potential of this parasite line for evaluating the TB capability of Pvg37.
Recently, peptide-based vaccines have become an attractive alternative approach. These vaccines utilize short protein fragments to induce immune responses against malaria parasites (Skwarczynski et al., 2020). To further elucidate the TB effect of the Pvg37 antibodies, we synthesized two highly conserved and B-cell epitope-rich peptides of Pvg37 to mitigate challenges associated with protein folding, aiming to optimize the functional potential of the protein. Enhanced antibody titers have been shown to correlate with improved TB effects against malaria, particularly in the context of P. falciparum (Tachibana et al., 2011, 2012). Studies on antibodies targeting the ookinete surface protein Pfs25 have demonstrated a strong association between high titers and effective TBA, indicating that elevated antibody levels can persist for months while maintaining their blocking efficacy (Kubler-Kielb et al., 2007). In this study, we selected anti-Pvg37-P2 antibodies with higher antibody titers for validation of TRA and TBA. Using the transgenic parasite line, we observed that the anti-Pvg37-P2 IgGs significantly reduced exflagellation and ookinete conversion in vitro. Furthermore, an antibody transfer experiment revealed that anti-Pvg37-P2 IgGs led to an 80.2% reduction in oocyst density in mosquitoes. These findings expand upon the TB potential of Pvg37 and highlight the utility of transgenic rodent parasites for evaluating vaccine candidates against P. vivax.
The standard membrane feeding assay (SMFA) is currently considered the in vivo “gold standard” (Churcher et al., 2012). The DMFA follows a similar design as the SMFA but uses freshly collected gametocyte-infected blood from infected individuals instead of cultured gametocytes to feed and infect mosquitoes (Duffy, 2021). DMFA offers the advantage of testing multiple experimental conditions on a single blood sample, thereby reducing uncontrolled variability. Due to the inability to culture gametocytes for P. vivax, DMFA remains the most appropriate method available for this species (Miura et al., 2020). In this study, TRA and TBA for IgGs against Pvg37-P2 were evaluated using DMFA with four clinical P. vivax isolates. In DMFA, the transmission reduction rate of anti-Pvg37-P2 IgGs against four clinical P. vivax parasites in midgut oocyst density ranged from 28.6% to 50.4%, which is lower than that observed on transgenic strain (80.2%). These findings are consistent with the previous studies (Zhang et al., 2024; Zheng et al., 2024). This disparity may be attributed to various uncontrollable factors of DMFA using field parasite isolates, such as gametocyte density, the proportion of mature gametocytes, the male/female gametocyte ratio, and fertilization pattern among field isolates (Kiattibutr et al., 2017; Ouattara et al., 2024). Certainly, variations in antibody concentrations in the blood meal cannot be overlooked. However, the estimated concentration of purified antibodies in DMFA (~2.5 µg/µL) was higher than that in passively transferred mice (~1.3 µg/µL), suggesting that this difference may not be the primary reason. Additionally, complement might play a role, as passive transfer was performed in mice with complement, while DMFA used purified IgG and inactivated serum. Previous studies show that human complement enhances the TB activity of antibodies against P. falciparum and P. vivax (Mendis et al., 1987; Quakyi et al., 1987; Healer et al., 1997). Unfortunately, we lacked a positive control for complement in our DMFA and could not directly confirm whether the TB activity of these antibodies depends on complement. The anti-Pvg37 antibody generally elicits a lower TRA compared to existing TBV antigens for P. vivax (Qiu et al., 2020; Zheng et al., 2024). However, direct comparison is not appropriate because the proteins used for immunization are expressed using different systems, which affect their immunogenicity and the observed antibody response.
Altogether, both in vivo studies with the transgenic parasites in mice and in vitro DMFA using clinical P. vivax isolates corroborate the TB potential of Pvg37. While our findings suggest that infection prevalence does not significantly decrease, the reduction in oocyst density is crucial. Research has demonstrated that lower oocyst densities hinder the development of P. falciparum in mosquitoes, thereby reducing the number of infectious bites transmitted to humans (Guissou et al., 2023). With fewer oocysts, the likelihood of mosquitoes becoming infective is diminished, ultimately lowering the risk of human infection. Further experimental validation is still required to enhance the TRA and TBA of Pvg37. Peptide-based vaccines often suffer from low immunogenicity, which can be mitigated by developing more advanced adjuvant-based delivery systems. RTS, S/AS02 demonstrated increased antibody titers and augmented cell-mediated immune responses through the utilization of a novel adjuvant (AS02), comprising an oil-in-water formulation containing MPL (a non-toxic derivative of lipopolysaccharide) and QS21 (Garcon et al., 2003). Matrix-M is a promising vaccine adjuvant based on Quillaja saponins, which has demonstrated acceptable safety and the ability to enhance both cellular and humoral immune responses of vaccines (Bengtsson et al., 2013, 2016). Nanoparticle-based platforms, including liposomes, hydrogels, and nanocapsules, can be functionalized for targeted delivery of vaccines (Ouji et al., 2018; Shakeel et al., 2019; Kekani and Witika, 2023; Zhuo et al., 2024). Furthermore, incorporating modified antigens into virus-like particles (VLPs) may augment immunogenicity (Jelínková et al., 2023; Yao et al., 2023). Carrier proteins like Exoprotein A (EPA) have already been successfully employed to elicit enhanced immune responses against TBV candidates (Rausch et al., 2023; Sagara et al., 2023). Additionally, synergistic effects can be achieved by combining multiple stages and antigens in combination vaccines (Sherrard-Smith et al., 2018; Yang et al., 2021). Finally, mRNA vaccines have also exhibited the capacity to induce high levels of antibodies, as evidenced by their impact on Pvs25 (Kunkeaw et al., 2023).
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The animal study was approved by Laboratory Animal Welfare and Ethics Committee of China Medical University. The study involving the use of human blood for membrane feeding of mosquitoes will be approved by the Institutional IRB of China Medical University and conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from all participants prior to their involvement in this study.
DZ: Investigation, Validation, Visualization, Writing – original draft. YZ: Investigation, Methodology, Validation, Visualization, Writing – original draft. DL: Investigation, Validation, Writing – original draft. FL: Methodology, Writing – original draft. PL: Methodology, Writing – original draft. BZ: Methodology, Writing – original draft. ZW: Methodology, Writing – original draft. WR: Investigation, Methodology, Writing – review & editing. SB: Methodology, Writing – review & editing. ST: Investigation, Methodology, Writing – review & editing. PS: Methodology, Writing – review & editing. MW: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. LC: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. YC: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Institute of Allergy and Infectious Diseases (grants R01AI150533 and U19AI089672).
We appreciate all the participants for their willingness to take part in this study. We are indebted to the technicians, nurses, and other workers from local hospitals for their assistance in completing the sample collection. We also want to express our gratitude to Mrs. Jun Liu for her assistance with mosquito feeding.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1529770/full#supplementary-material
TBVs, transmission-blocking vaccines; G6PD, glucose-6-phosphate dehydrogenase; TBA, transmission-blocking activity; TRA, transmission-reducing activity; DMFA, direct membrane feeding assay; iRBCs, infected red blood cells; WT, wild-type; mAb, monoclonal antibody; KLH, keyhole limpet hemocyanin; ELISA, enzyme-linked immunosorbent assay; IFA, indirect immunofluorescence assay; GIMO, Gene insertion and marker out; SMFA, standard membrane feeding assay; VLPs, virus-like particles; EPA, exoprotein A.
Adams, J. H., Mueller, I. (2017). The biology of plasmodium vivax. Cold Spring Harb. Perspect. Med. 7 (9), a025585. doi: 10.1101/cshperspect.a025585
Alonso, P. L., Brown, G., Arevalo-Herrera, M., Binka, F., Chitnis, C., Collins, F., et al. (2011). A research agenda to underpin malaria eradication. PloS Med. 8, e1000406. doi: 10.1371/journal.pmed.1000406
Arévalo-Herrera, M., Vallejo, A. F., Rubiano, K., Solarte, Y., Marin, C., Castellanos, A., et al. (2015). Recombinant Pvs48/45 antigen expressed in E. coli generates antibodies that block malaria transmission in Anopheles albimanus mosquitoes. PloS One 10, e0119335. doi: 10.1371/journal.pone.0119335
Bai, J., Liu, F., Yang, F., Zhao, Y., Jia, X., Thongpoon, S., et al. (2023). Evaluation of transmission-blocking potential of Pv22 using clinical Plasmodium vivax infections and transgenic Plasmodium berghei. Vaccine 41, 555–563. doi: 10.1016/j.vaccine.2022.11.058
Bengtsson, K. L., Karlsson, K. H., Magnusson, S. E., Reimer, J. M., Stertman, L. (2013). Matrix-M adjuvant: enhancing immune responses by ‘setting the stage’ for the antigen. Expert Rev. Vaccines 12, 821–823. doi: 10.1586/14760584.2013.814822
Bengtsson, K. L., Song, H., Stertman, L., Liu, Y., Flyer, D. C., Massare, M. J., et al. (2016). Matrix-M adjuvant enhances antibody, cellular and protective immune responses of a Zaire Ebola/Makona virus glycoprotein (GP) nanoparticle vaccine in mice. Vaccine 34, 1927–1935. doi: 10.1016/j.vaccine.2016.02.033
Cao, Y., Hart, R. J., Bansal, G. P., Kumar, N. (2018). Functional Conservation of P48/45 Proteins in the Transmission Stages of Plasmodium vivax (Human Malaria Parasite) and P. berghei (Murine Malaria Parasite). mBio 9 (5), e01627-18. doi: 10.1128/mBio.01627-18
Churcher, T. S., Blagborough, A. M., Delves, M., Ramakrishnan, C., Kapulu, M. C., Williams, A. R., et al. (2012). Measuring the blockade of malaria transmission–an analysis of the Standard Membrane Feeding Assay. Int. J. Parasitol. 42, 1037–1044. doi: 10.1016/j.ijpara.2012.09.002
da Veiga, G. T. S., Moriggi, M. R., Vettorazzi, J. F., Müller-Santos, M., Albrecht, L. (2022). Plasmodium vivax vaccine: What is the best way to go? Front. Immunol. 13. doi: 10.3389/fimmu.2022.910236
de Jong, R. M., Tebeje, S. K., Meerstein-Kessel, L., Tadesse, F. G., Jore, M. M., Stone, W., et al. (2020). Immunity against sexual stage Plasmodium falciparum and Plasmodium vivax parasites. Immunol. Rev. 293, 190–215. doi: 10.1111/imr.12828
Dinko, B., King, E., Targett, G. A., Sutherland, C. J. (2016). Antibody responses to surface antigens of Plasmodium falciparum gametocyte-infected erythrocytes and their relation to gametocytaemia. Parasite Immunol. 38, 352–364. doi: 10.1111/pim.12323
Doi, M., Tanabe, K., Tachibana, S., Hamai, M., Tachibana, M., Mita, T., et al. (2011). Worldwide sequence conservation of transmission-blocking vaccine candidate Pvs230 in Plasmodium vivax. Vaccine 29, 4308–4315. doi: 10.1016/j.vaccine.2011.04.028
Duffy, P. E. (2021). Transmission-blocking vaccines: harnessing herd immunity for malaria elimination. Expert Rev. Vaccines 20, 185–198. doi: 10.1080/14760584.2021.1878028
Duffy, P. E. (2022). The virtues and vices of pfs230: from vaccine concept to vaccine candidate. Am. J. Trop. Med. Hyg., tpmd211337. doi: 10.4269/ajtmh.21-1337
Flannery, E. L., Kangwanrangsan, N., Chuenchob, V., Roobsoong, W., Fishbaugher, M., Zhou, K., et al. (2022). Plasmodium vivax latent liver infection is characterized by persistent hypnozoites, hypnozoite-derived schizonts, and time-dependent efficacy of primaquine. Mol. Ther. Methods Clin. Dev. 26, 427–440. doi: 10.1016/j.omtm.2022.07.016
Flannery, E. L., Markus, M. B., Vaughan, A. M. (2019). Plasmodium vivax. Trends Parasitol. 35, 583–584. doi: 10.1016/j.pt.2019.04.005
Garcon, N., Heppner, D. G., Cohen, J. (2003). Development of RTS,S/AS02: a purified subunit-based malaria vaccine candidate formulated with a novel adjuvant. Expert Rev. Vaccines 2, 231–238. doi: 10.1586/14760584.2.2.231
Guissou, E., Da, D. F., Hien, D. F. S., Yameogo, K. B., Yerbanga, S. R., Ouédraogo, G. A., et al. (2023). Intervention reducing malaria parasite load in vector mosquitoes: No impact on Plasmodium falciparum extrinsic incubation period and the survival of Anopheles Gambiae. PloS Pathog. 19, e1011084. doi: 10.1371/journal.ppat.1011084
Healer, J., McGuinness, D., Hopcroft, P., Haley, S., Carter, R., Riley, E. (1997). Complement-mediated lysis of Plasmodium falciparum gametes by malaria-immune human sera is associated with antibodies to the gamete surface antigen Pfs230. Infect. Immun. 65, 3017–3023. doi: 10.1128/iai.65.8.3017-3023.1997
Healy, S. A., Anderson, C., Swihart, B. J., Mwakingwe, A., Gabriel, E. E., Decederfelt, H., et al. (2021). Pfs230 yields higher malaria transmission-blocking vaccine activity than Pfs25 in humans but not mice. J. Clin. Invest. 131 (7), e146221. doi: 10.1172/jci146221
Hisaeda, H., Collins, W. E., Saul, A., Stowers, A. W. (2001). Antibodies to Plasmodium vivax transmission-blocking vaccine candidate antigens Pvs25 and Pvs28 do not show synergism. Vaccine 20, 763–770. doi: 10.1016/s0264-410x(01)00402-9
Hisaeda, H., Stowers, A. W., Tsuboi, T., Collins, W. E., Sattabongkot, J. S., Suwanabun, N., et al. (2000). Antibodies to malaria vaccine candidates Pvs25 and Pvs28 completely block the ability of Plasmodium vivax to infect mosquitoes. Infect. Immun. 68, 6618–6623. doi: 10.1128/IAI.68.12.6618-6623.2000
Jelínková, L., Roberts, B., Ajayi, D. T., Peabody, D. S., Chackerian, B. (2023). The immunogenicity of a VLP-based malaria vaccine targeting CSP in pregnant and neonatal mice. Biomolecules 13 (2), 202. doi: 10.3390/biom13020202
Jones, S., Grignard, L., Nebie, I., Chilongola, J., Dodoo, D., Sauerwein, R., et al. (2015). Naturally acquired antibody responses to recombinant Pfs230 and Pfs48/45 transmission blocking vaccine candidates. J. Infect. 71, 117–127. doi: 10.1016/j.jinf.2015.03.007
Kaslow, D. C., Quakyi, I. A., Syin, C., Raum, M. G., Keister, D. B., Coligan, J. E., et al. (1988). A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature 333, 74–76. doi: 10.1038/333074a0
Kekani, L. N., Witika, B. A. (2023). Current advances in nanodrug delivery systems for malaria prevention and treatment. Discovery Nano 18, 66. doi: 10.1186/s11671-023-03849-x
Kiattibutr, K., Roobsoong, W., Sriwichai, P., Saeseu, T., Rachaphaew, N., Suansomjit, C., et al. (2017). Infectivity of symptomatic and asymptomatic Plasmodium vivax infections to a Southeast Asian vector, Anopheles dirus. Int. J. Parasitol. 47, 163–170. doi: 10.1016/j.ijpara.2016.10.006
Kubler-Kielb, J., Majadly, F., Wu, Y., Narum, D. L., Guo, C., Miller, L. H., et al. (2007). Long-lasting and transmission-blocking activity of antibodies to Plasmodium falciparum elicited in mice by protein conjugates of Pfs25. Proc. Natl. Acad. Sci. U.S.A. 104, 293–298. doi: 10.1073/pnas.0609885104
Kunkeaw, N., Nguitragool, W., Takashima, E., Kangwanrangsan, N., Muramatsu, H., Tachibana, M., et al. (2023). A Pvs25 mRNA vaccine induces complete and durable transmission-blocking immunity to Plasmodium vivax. NPJ Vaccines 8, 187. doi: 10.1038/s41541-023-00786-9
Lacerda, M. V. G., Llanos-Cuentas, A., Krudsood, S., Lon, C., Saunders, D. L., Mohammed, R., et al. (2019). Single-dose tafenoquine to prevent relapse of plasmodium vivax malaria. N Engl. J. Med. 380, 215–228. doi: 10.1056/NEJMoa1710775
Liu, F., Li, L., Zheng, W., He, Y., Wang, Y., Zhu, X., et al. (2018). Characterization of plasmodium berghei pbg37 as both a pre- and postfertilization antigen with transmission-blocking potential. Infect. Immun. 86 (8), e00785-17. doi: 10.1128/iai.00785-17
Llanos-Cuentas, A., Lacerda, M. V. G., Hien, T. T., Vélez, I. D., Namaik-Larp, C., Chu, C. S., et al. (2019). Tafenoquine versus Primaquine to Prevent Relapse of Plasmodium vivax Malaria. N Engl. J. Med. 380, 229–241. doi: 10.1056/NEJMoa1802537
Lopez, C., Yepes-Perez, Y., Hincapie-Escobar, N., Diaz-Arevalo, D., Patarroyo, M. A. (2017). What Is Known about the Immune Response Induced by Plasmodium vivax Malaria Vaccine Candidates? Front. Immunol. 8. doi: 10.3389/fimmu.2017.00126
Malkin, E. M., Durbin, A. P., Diemert, D. J., Sattabongkot, J., Wu, Y., Miura, K., et al. (2005). Phase 1 vaccine trial of Pvs25H: a transmission blocking vaccine for Plasmodium vivax malaria. Vaccine 23, 3131–3138. doi: 10.1016/j.vaccine.2004.12.019
Mendis, K. N., Munesinghe, Y. D., de Silva, Y. N., Keragalla, I., Carter, R. (1987). Malaria transmission-blocking immunity induced by natural infections of Plasmodium vivax in humans. Infect. Immun. 55, 369–372. doi: 10.1128/iai.55.2.369-372.1987
Miura, K., Swihart, B. J., Fay, M. P., Kumpitak, C., Kiattibutr, K., Sattabongkot, J., et al. (2020). Evaluation and modeling of direct membrane-feeding assay with Plasmodium vivax to support development of transmission blocking vaccines. Sci. Rep. 10, 12569. doi: 10.1038/s41598-020-69513-x
Miura, K., Tachibana, M., Takashima, E., Morita, M., Kanoi, B. N., Nagaoka, H., et al. (2019). Malaria transmission-blocking vaccines: wheat germ cell-free technology can accelerate vaccine development. Expert Rev. Vaccines 18, 1017–1027. doi: 10.1080/14760584.2019.1674145
Mueller, I., Galinski, M. R., Baird, J. K., Carlton, J. M., Kochar, D. K., Alonso, P. L., et al. (2009). Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect. Dis. 9, 555–566. doi: 10.1016/S1473-3099(09)70177-X
Muthui, M. K., Kamau, A., Bousema, T., Blagborough, A. M., Bejon, P., Kapulu, M. C. (2019). Immune responses to gametocyte antigens in a malaria endemic population-the African falciparum context: A systematic review and meta-analysis. Front. Immunol. 10. doi: 10.3389/fimmu.2019.02480
Ouattara, S. B., Hien, D., Nao, E. T., Paré, P. S. L., Guissou, E., Cohuet, A., et al. (2024). A simple, field-applicable method to increase the infectivity of wild isolates of Plasmodium falciparum to mosquito vectors. Malar. J. 23, 135. doi: 10.1186/s12936-024-04969-0
Ouji, M., Augereau, J. M., Paloque, L., Benoit-Vical, F. (2018). Plasmodium falciparum resistance to artemisinin-based combination therapies: A sword of Damocles in the path toward malaria elimination. Parasite 25, 24. doi: 10.1051/parasite/2018021
Qiu, Y., Zhao, Y., Liu, F., Ye, B., Zhao, Z., Thongpoon, S., et al. (2020). Evaluation of Plasmodium vivax HAP2 as a transmission-blocking vaccine candidate. Vaccine 38, 2841–2848. doi: 10.1016/j.vaccine.2020.02.011
Quakyi, I. A., Carter, R., Rener, J., Kumar, N., Good, M. F., Miller, L. H. (1987). The 230-kDa gamete surface protein of Plasmodium falciparum is also a target for transmission-blocking antibodies. J. Immunol. 139, 4213–4217. doi: 10.4049/jimmunol.139.12.4213
Ramjanee, S., Robertson, J. S., Franke-Fayard, B., Sinha, R., Waters, A. P., Janse, C. J., et al. (2007). The use of transgenic Plasmodium berghei expressing the Plasmodium vivax antigen P25 to determine the transmission-blocking activity of sera from malaria vaccine trials. Vaccine 25, 886–894. doi: 10.1016/j.vaccine.2006.09.035
Rausch, K. M., Barnafo, E. K., Lambert, L. E., Muratova, O., Gorres, J. P., Anderson, C., et al. (2023). Preclinical evaluations of Pfs25-EPA and Pfs230D1-EPA in AS01 for a vaccine to reduce malaria transmission. iScience 26, 107192. doi: 10.1016/j.isci.2023.107192
Rocha-Martins, M., Cavalheiro, G. R., Matos-Rodrigues, G. E., Martins, R. A. (2015). From Gene Targeting to Genome Editing: Transgenic animals applications and beyond. Acad. Bras. Cienc 87, 1323–1348. doi: 10.1590/0001-3765201520140710
Sagara, I., Healy, S. A., Assadou, M. H., Kone, M., Swihart, B. J., Kwan, J. L., et al. (2023). Malaria transmission-blocking vaccines Pfs230D1-EPA and Pfs25-EPA in Alhydrogel in healthy Malian adults; a phase 1, randomised, controlled trial. Lancet Infect. Dis. 23, 1266–1279. doi: 10.1016/s1473-3099(23)00276-1
Shakeel, K., Raisuddin, S., Ali, S., Imam, S. S., Rahman, M. A., Jain, G. K., et al. (2019). Development and in vitro/in vivo evaluation of artemether and lumefantrine co-loaded nanoliposomes for parenteral delivery. J. Liposome Res. 29, 35–43. doi: 10.1080/08982104.2017.1410173
Sherrard-Smith, E., Sala, K. A., Betancourt, M., Upton, L. M., Angrisano, F., Morin, M. J., et al. (2018). Synergy in anti-malarial pre-erythrocytic and transmission-blocking antibodies is achieved by reducing parasite density. Elife 7, e35213. doi: 10.7554/eLife.35213
Skwarczynski, M., Chandrudu, S., Rigau-Planella, B., Islam, M. T., Cheong, Y. S., Liu, G., et al. (2020). Progress in the development of subunit vaccines against malaria. Vaccines (Basel) 8 (3), 373. doi: 10.3390/vaccines8030373
Tachibana, M., Sato, C., Otsuki, H., Sattabongkot, J., Kaneko, O., Torii, M., et al. (2012). Plasmodium vivax gametocyte protein Pvs230 is a transmission-blocking vaccine candidate. Vaccine 30, 1807–1812. doi: 10.1016/j.vaccine.2012.01.003
Tachibana, M., Suwanabun, N., Kaneko, O., Iriko, H., Otsuki, H., Sattabongkot, J., et al. (2015). Plasmodium vivax gametocyte proteins, Pvs48/45 and Pvs47, induce transmission-reducing antibodies by DNA immunization. Vaccine 33, 1901–1908. doi: 10.1016/j.vaccine.2015.03.008
Tachibana, M., Wu, Y., Iriko, H., Muratova, O., MacDonald, N. J., Sattabongkot, J., et al. (2011). N-terminal prodomain of Pfs230 synthesized using a cell-free system is sufficient to induce complement-dependent malaria transmission-blocking activity. Clin. Vaccine Immunol. 18, 1343–1350. doi: 10.1128/CVI.05104-11
Takala, S. L., Plowe, C. V. (2009). Genetic diversity and malaria vaccine design, testing and efficacy: preventing and overcoming ‘vaccine resistant malaria’. Parasite Immunol. 31, 560–573. doi: 10.1111/j.1365-3024.2009.01138.x
Tentokam, B. C. N., Amaratunga, C., Alani, N. A. H., MacDonald, N. J., Narum, D. L., Salinas, N. D., et al. (2019). Naturally acquired antibody response to malaria transmission blocking vaccine candidate pvs230 domain 1. Front. Immunol. 10. doi: 10.3389/fimmu.2019.02295
Thriemer, K., Ley, B., von Seidlein, L. (2021). Towards the elimination of Plasmodium vivax malaria: Implementing the radical cure. PloS Med. 18, e1003494. doi: 10.1371/journal.pmed.1003494
van Dijk, M. R., Janse, C. J., Thompson, J., Waters, A. P., Braks, J. A., Dodemont, H. J., et al. (2001). A central role for P48/45 in malaria parasite male gamete fertility. Cell 104, 153–164. doi: 10.1016/s0092-8674(01)00199-4
WHO (2023). World Malaria Report 2023 (Geneva, Switzerland: World Health Organization). Available at: https://www.who.int/publications/i/item/9789240086173.
Wu, Y., Ellis, R. D., Shaffer, D., Fontes, E., Malkin, E. M., Mahanty, S., et al. (2008). Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PloS One 3, e2636. doi: 10.1371/journal.pone.0002636
Yang, F., Liu, F., Yu, X., Zheng, W., Wu, Y., Qiu, Y., et al. (2021). Evaluation of two sexual-stage antigens as bivalent transmission-blocking vaccines in rodent malaria. Parasit Vectors 14, 241. doi: 10.1186/s13071-021-04743-0
Yao, G., Min, H., Yu, X., Liu, F., Cui, L., Cao, Y. (2023). A nanoparticle vaccine displaying the ookinete PSOP25 antigen elicits transmission-blocking antibody response against Plasmodium berghei. Parasit Vectors 16, 403. doi: 10.1186/s13071-023-06020-8
Zhang, B., Feng, H., Zhao, Y., Zhang, D., Yu, X., Li, Y., et al. (2024). Evaluation of transmission-blocking potential of PvPSOP25 using transgenic murine malaria parasite and clinical isolates. PloS Negl. Trop. Dis. 18, e0012231. doi: 10.1371/journal.pntd.0012231
Zheng, W., Cheng, S., Liu, F., Yu, X., Zhao, Y., Yang, F., et al. (2024). Immunogenicity and transmission-blocking potential of quiescin sulfhydryl oxidase in Plasmodium vivax. Front. Cell Infect. Microbiol. 14. doi: 10.3389/fcimb.2024.1451063
Keywords: Plasmodium vivax, transmission-blocking vaccine, polypeptide, transgenic parasite, gametocyte
Citation: Zhang D, Zhao Y, Liu D, Liu F, Liu P, Zhang B, Wu Z, Roobsoong W, Bantuchai S, Thongpoon S, Sripoorote P, Wang M, Cui L and Cao Y (2025) Evaluation of the transmission-blocking potential of Plasmodium vivax antigen Pvg37 using transgenic rodent parasites and clinical isolates. Front. Cell. Infect. Microbiol. 15:1529770. doi: 10.3389/fcimb.2025.1529770
Received: 17 November 2024; Accepted: 02 January 2025;
Published: 24 January 2025.
Edited by:
Kai Matuschewski, Humboldt University of Berlin, GermanyReviewed by:
Yunuen Avalos-Padilla, Institute for Bioengineering of Catalonia (IBEC), SpainCopyright © 2025 Zhang, Zhao, Liu, Liu, Liu, Zhang, Wu, Roobsoong, Bantuchai, Thongpoon, Sripoorote, Wang, Cui and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meilian Wang, d2FuZ21laWxpYW5fbUBob3RtYWlsLmNvbQ==; Liwang Cui, bGl3YW5nY3VpQHVzZi5lZHU=; Yaming Cao, eW1jYW9AY211LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.