- 1Department of Medical Microbiology and Immunology, University of Nairobi, Hospital Road, Kenyatta National Hospital, Nairobi, Kenya
- 2Department of Tropical and Infectious Diseases, Kenya Institute of Primate Research, Nairobi, Kenya
- 3Weldon School of Biomedical Engineering, Purdue University, West Lafayette, IN, United States
- 4Division of Experimental Medicine, Department of Medicine, University of California, San Francisco, San Francisco, CA, United States
Despite decades of control efforts, the prevalence of schistosomiasis remains high in many endemic regions, posing significant challenges to global health. One of the key factors contributing to the persistence of the disease is the complex life cycle of the Schistosoma parasite, the causative agent, which involves multiple stages of development and intricate interactions with its mammalian hosts and snails. Among the various stages of the parasite lifecycle, the deposition of eggs and their migration through host tissues is significant, as they initiate the onset of the disease pathology by inducing inflammatory reactions and tissue damage. However, our understanding of the mechanisms underlying Schistosoma egg extravasation remains limited, hindering efforts to develop effective interventions. Microphysiological systems, particularly organ-on-a-chip systems, offer a promising approach to study this phenomenon in a controlled experimental setting because they allow the replication of physiological microenvironments in vitro. This review provides an overview of schistosomiasis, introduces the concept of organ-on-a-chip technology, and discusses its potential applications in the field of schistosomiasis research.
Introduction
Schistosomiasis, commonly referred to as bilharziasis, is a neglected tropical disease caused by parasitic trematodes of the genus Schistosoma which affects more than 251.4 million people worldwide, particularly in regions with limited resources including poor access to clean water (World Health Organization, 2023). The disease is endemic in tropical and subtropical regions, where it poses a significant burden on public health and socioeconomic development (Colley et al., 2014). Human schistosomiasis is caused by several species of Schistosoma parasites, the most predominant being Schistosoma haematobium, Schistosoma mansoni, and Schistosoma japonicum, each of which has a unique geographical distribution and clinical manifestation (Ross et al., 2002) along with other less prevalent species such as Schistosoma mekongi, Schistosoma guineensis, and Schistosoma intercalatum.
The life cycle of Schistosoma parasites involves two hosts: a definitive mammalian host and an intermediate host, usually a freshwater snail (McManus et al., 2018). Infection occurs when cercariae, the larval stage of the parasite, penetrates the human skin during contact with contaminated water. Once inside the human host, the cercariae metamorphose into a schistosomulum, and develop into adult worms that pair up as male and female couples that reside in the blood vessels surrounding the intestines (S. mansoni and S. japonicum) or bladder (S. haematobium). Female worms produce hundreds of eggs per day, which are then released into the bloodstream or lymphatic system (Gryseels et al., 2006).
Central to the pathogenesis of schistosomiasis is the process of egg deposition and extravasation within the host’s tissues. The eggs must cross the endothelial barrier of blood vessels to reach the lumen of the intestine or bladder, where they are excreted in stool or urine, respectively (Chitsulo et al., 2000). However, some eggs become trapped in tissues, leading to the formation of granulomas and fibrosis, which are hallmarks of chronic schistosomiasis. These pathological changes can result in severe morbidity, including liver and spleen enlargement, bladder cancer, and neurological complications (Chitsulo et al., 2000).
Despite decades of research, the molecular and cellular mechanisms underlying Schistosoma egg extravasation are incompletely understood. In part, this can be attributed to overreliance on animal models, which, due to their nature of complexity, do not allow the high-resolution studies necessary to decipher molecular mechanisms. Although animal experiments are indispensable, especially for preclinical screening in the drug discovery process, various issues, such as ethical considerations and species differences, remain (Kimura et al., 2018). To circumvent these challenges, cell-based assays using human-derived cells have been actively pursued. Furthermore, in recent years, there has been a growing interest in leveraging advanced biomedical technologies to overcome these limitations and unravel the “mysteries” of schistosome egg extravasation. To this end, organ-on-a-chip (OOC) platforms have emerged as powerful tools for recapitulating the physiological microenvironments of human organs in vitro (Bhatia and Ingber, 2014). OOC models offer unique advantages by providing dynamic multicellular systems that mimic the structural and functional complexity of human tissues. These in vitro models can provide valuable information on the pathogenesis of schistosomiasis, as they offer the simplicity of high-resolution imaging, allowing studying the molecular mechanisms of the disease in detail (Yeh et al., 2022; Yeh et al., 2024). Furthermore, OOCs are devoid of ethical and practical considerations that often restrict the use of human participants and animal models in the research of schistosomiasis.
This review explores the utility of OOC systems in unraveling the process of extravasation of S. mansoni eggs in humans. By systematically analyzing the existing literature, we identify key findings, knowledge gaps, and future research directions in this rapidly evolving field. Through a comprehensive examination of OOC-based studies, our objective is to elucidate the potential of this innovative technology to advance our understanding of the pathogenesis of schistosomiasis focusing on the role of immune cells in granuloma formation that could facilitate the development of novel therapeutic strategies.
Schistosomiasis life cycle
Schistosoma species have intricate life cycles involving both a freshwater snail as an intermediate host and a mammalian host, such as humans, as the definitive host as illustrated in Figure 1. S. mansoni eggs are expelled through feces, while S. haematobium eggs exit via urine. Upon reaching water body, these eggs hatch under favorable conditions such as temperature, pH and turbidity. In snails (e.g., Biomphalaria for S. mansoni), asexual reproduction produces large numbers of free-swimming cercariae, which actively seek out and penetrate the skin of a mammalian host, developing into schistosomula. Initially residing in the skin, schistosomula then migrate to the bloodstream and, within 5-7 days, reach the lungs. After circulating for about two weeks, they settle in the hepatoportal system for S. mansoni and veins of the bladder and the pelvic organs for S. haematobium, where they sexually mature.
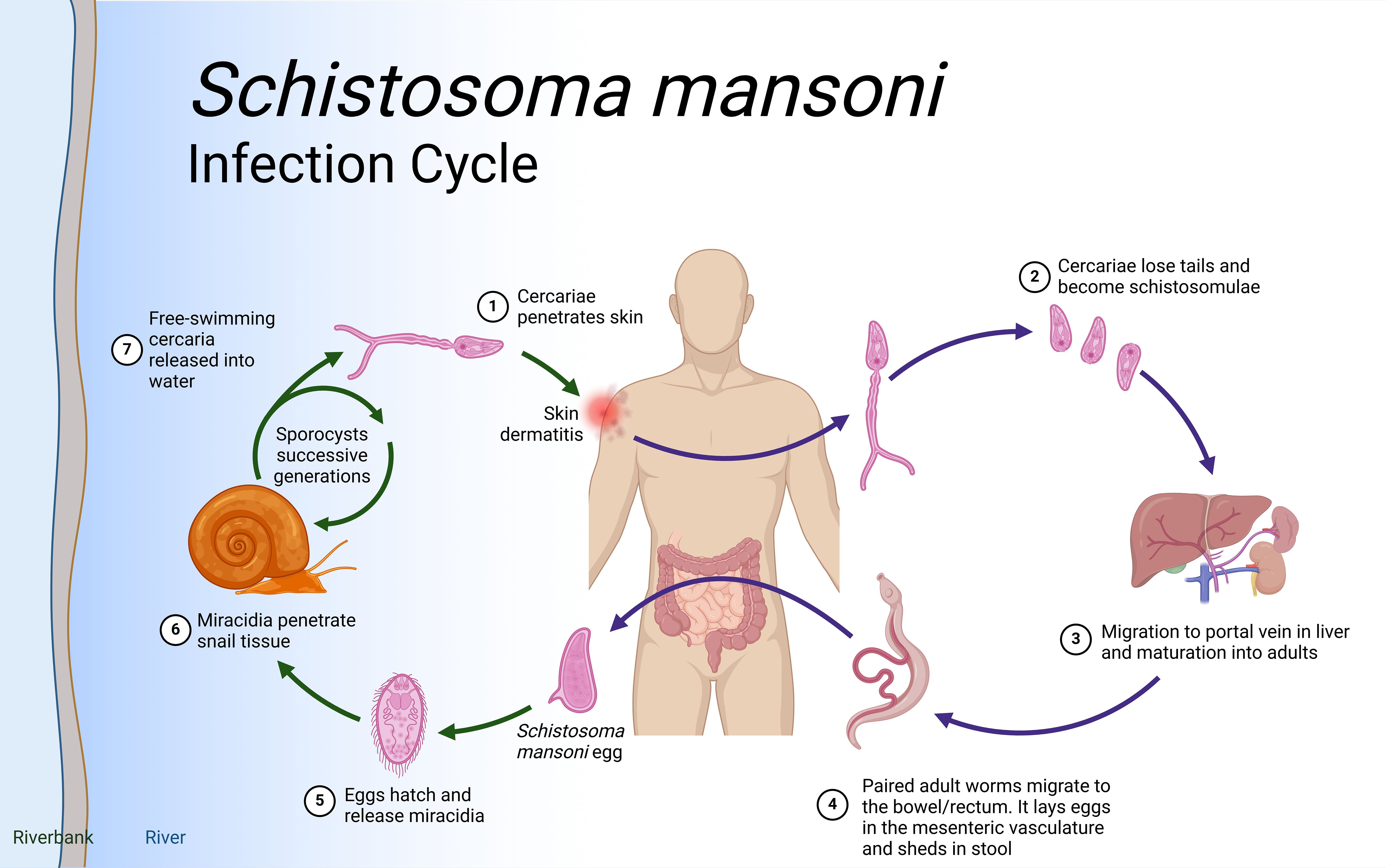
Figure 1. Schistosoma life cycle. Infection begins when (Step 1) free-living cercariae penetrate human skin and (Step 2) cercariae lose tails and become schistosomula, followed by (Step 3) migration through the portal vein in the liver and maturation to adult schistosomes. Once in the liver, the female and male adults pair up (Step 4) and migrate to the intestines where the females release eggs. Interaction with the microbiome, epithelial cell death and remodeling lead to the active release of eggs, which are then released to the environment with host feces. (Step 5). Lastly, when in water eggs hatch into miracidia (step 6) which eventually penetrate the tissue of Biomphalaria spp snails to continue the cycle (step 7). Eggs that do not extravasate are encapsulated by vascular endothelia cells that trigger intravascular host-immune responses to induce VEC inflammation, proliferation, and migration. This then leads to the formation of granuloma in the liver that results in liver fibrosis after about 8 weeks. Created in BioRender.com.
Mature worm pairs migrate to the mesenteric veins, with adult S. mansoni worms primarily residing in the inferior mesenteric veins surrounding the colon and caecum. Female worms lay eggs in capillary walls, where they either enter the bloodstream or move through the intestinal epithelium into the lumen. These metabolically active and immunogenic eggs provoke an inflammatory response, resulting in granuloma formation that aids their passage through intestinal tissues. The life cycle is completed when eggs are excreted in feces. In the case of S. haematobium, once matured, the female worm lays eggs in the blood vessels. The eggs move through the bladder wall and are expelled with urine, continuing the cycle. Some eggs, however, become trapped in surrounding tissues, causing inflammation and leading to urogenital pathology such as bladder fibrosis, calcification, and, over time, an increased risk of bladder cancer.
Acute schistosomiasis may present as a mild skin rash (swimmer’s itch) and systemic symptoms like fever, fatigue, and cough (Katayama fever). The pathological hallmark of schistosomiasis is the immunopathology associated with egg deposition in host tissues and their subsequent excretion. Vascular endothelial cells (VECs) encapsulate these eggs, triggering an inflammatory granulomatous response that facilitates egg extravasation (Yeh et al., 2022; Yeh et al., 2024). Some eggs fail to reach the intestines or bladder, becoming trapped in host tissues and forming granulomas, which consist of immune cells, fibroblasts, and collagen. These granulomas are characteristic of chronic schistosomiasis, contributing to tissue damage, fibrosis, and organ dysfunction (Pearce and MacDonald, 2002). This can cause portal hypertension, gastrointestinal bleeding, hepatic encephalopathy, and liver failure.
Gaps in our understanding of extravasation of S. mansoni eggs
The S. mansoni egg proteome has been characterized in many studies but the pathogenic consequences of the interaction of egg molecules with the host immune system, as well as the processes that underlie the passage of eggs through tissues and their subsequent release with the feces, are still poorly understood (Schwartz and Fallon, 2018). Mechanisms by which eggs travel through tissues of the intestinal wall are believed to include modulation of local immune responses that favor their migration to the intestinal lumen. This mechanism by which schistosome eggs extravasate from the bloodstream into host tissues is complex and involves multiple steps as proposed in Figure 2 (Schwartz and Fallon, 2018). When reaching the small blood vessels of the liver or other organs, the eggs lodge in the endothelium (Wynn and Cheever, 1995). This triggers a cascade of inflammatory responses characterized by the recruitment of immune cells and the release of cytokines and chemokines (Chiaramonte et al., 1999). Macrophages, neutrophils, eosinophils, and other immune cells are recruited to the site of deposition, where they interact with the endothelium and surrounding tissues (Colley and Secor, 2014). The production of pro-inflammatory cytokines, such as interleukin-1β (IL-1β), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6), promotes endothelial activation and permeability, facilitating egg extravasation (Mpotje, 2017).
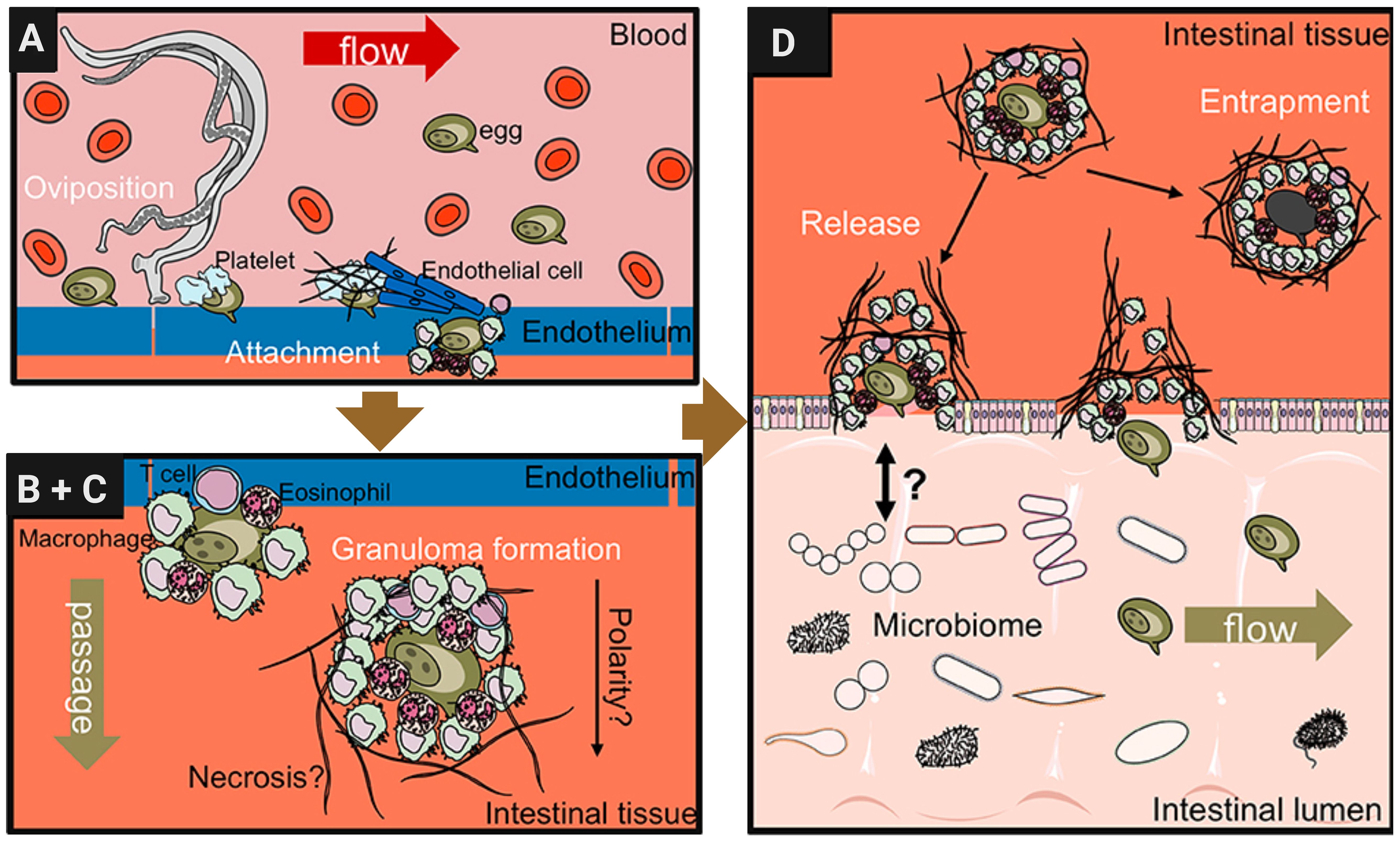
Figure 2. Schistosome egg transition through gastrointestinal tissues. Modified from Schwartz and Fallon (2018) with further editing in Biorender.com. (A) Adult female schistosomes deposit eggs (about 300 eggs per day for S. mansoni) into the vasculature close to the lamina propria. Platelets and fibrinogen adhere to the eggs and activate the endothelium. Endothelial cells actively grow over the egg supporting its extravasation. Eggs that do not cross the endothelial border are disseminated by the blood flow and become trapped mostly in the liver portal system. (B, C) Immune cells, such as macrophages, T cells and eosinophils start to encapsulate the egg. Granuloma formation occurs around the egg and together with other processes, such as fibrinolysis, egg secretions-induced necrosis, leading to the passage of the egg toward the intestinal lumen. (D) Entrapped eggs become fibrotic and calcified in the liver during chronic schistosomiasis infection.
In addition, basophils can be activated directly by Immunomodulatory protein secreted by schistosomes alpha 1 (IPSE/α1) to release Interleukin (IL-4) that is present in intestinal granulomas. Recent studies have uncovered a novel molecular mechanism in which IPSE, a member of the βγ-crystallin superfamily, binds to Immunoglobulin E (IgE) via its crystallin fold. This interaction activates basophils independently of traditional IgE cross-linking (Cass et al., 2007). Once outside the bloodstream, eggs interact with host cells and components of the extracellular matrix (ECM), triggering additional inflammatory and fibrotic responses (Costain et al., 2018). Immune cells, particularly macrophages and eosinophils, recognize and phagocytose eggs, releasing cytokines and chemokines that recruit additional immune cells to the site of deposition (Pearce and MacDonald, 2002). Fibroblasts are activated to produce collagen and other ECM proteins, to complete the formation of granulomas (Colley and Secor, 2014). The size and composition of the granulomas vary depending on the number and location of the trapped eggs and the immune response of the host (Cass et al., 2007).
In vitro immunological studies in schistosomiasis
One of the most intriguing aspects of schistosomiasis is its ability to evade the host immune system. Various studies have used in vitro models to investigate how Schistosoma spp. manipulates host immune responses. In a notable study, Kazura et al., 1981 used an in vitro approach to demonstrate that S. mansoni schistosomula releases excretory-secretory (ES) products that inhibit the production of reactive oxygen species (ROS) by human neutrophils. ROS are typically a key part of the immune response to pathogens, and the ability of the parasite to suppress ROS production aids in evasion of immune defenses. This study laid the foundation for further research into the role of ES products in immune evasion (Kazura et al., 1981). More recently, in vitro assays have shown that the extracellular vesicles (EVs) secreted by schistosomula play an essential role in immune modulation. These studies have demonstrated that EVs carry microRNAs (miRNAs) capable of altering host gene expression and effectively dampening pro-inflammatory responses. Their findings suggested that schistosomula-derived EVs can downregulate the expression of genes involved in innate immune signaling, helping the parasite establish an infection (Wu et al., 2019; Kuipers et al., 2020; Keshtkar et al., 2022).
Schistosome eggs are highly immunogenic and play a critical role in shaping immune responses during infection. An earlier study by Linder (2017) established that perioval granuloma formation is driven by the host immune responses where immune cells form protective structures around the eggs and this shields tissues from damage. Also, this process leads to significant fibrosis and pathology. The immune system responds to egg antigens by activating cells like macrophages and eosinophils, which initiate inflammation and help push the eggs through tissue barriers. The study also stated that mechanical forces associated with muscle contraction aid in egg movement to the perivascular space. Coagulation and fibrinolysis are also essential, as balanced clot formation and breakdown facilitate egg movement without excessive clotting or bleeding. The study distinguished between “successful” eggs, which reach the gut or bladder for excretion, and “unsuccessful” eggs, which become trapped in tissues, causing chronic inflammation and fibrosis (Linder, 2017).
Many studies have focused on understanding the immunomodulatory properties of egg-secreted proteins. For example, Pearce et al., 2005 identified specific egg antigens, including omega-1 (ω1), which are responsible for driving the T helper type 2 (Th2) immune response, a hallmark of chronic schistosomiasis. Using in vitro assays with dendritic cells, they showed that ω1, a glycoprotein secreted by schistosome eggs, induces the production of interleukin-4 (IL-4) by T helper cells. This discovery highlights the central role of egg antigens in skewing the immune response toward a Th2 phenotype, which is associated with granuloma formation and tissue fibrosis (Pearce, 2005). In this study, it was also found that egg antigens induce dendritic cells to produce thymic stromal lymphopoietin (TSLP), a cytokine that primes dendritic cells to support the development of Th2 responses. This provides additional mechanistic insight into how schistosome eggs drive chronic immune activation (Pearce, 2005).
The immune response to schistosome infection is tightly regulated to balance effective defense mechanisms and prevent excessive tissue damage. Regulatory T cells (Tregs) play a pivotal role in modulating immune responses during schistosome infections. Layland et al., 2007 conducted in vitro and ex vivo experiments to investigate the role of Tregs in schistosomiasis. They showed that during chronic infection, Tregs expand in response to schistosome egg antigens and are essential for controlling the size of granulomas. In vitro assays demonstrated that granuloma formation became more pronounced when Tregs were depleted, leading to increased tissue damage. This study highlights the importance of Tregs in preventing excessive immunopathology during schistosomiasis (Layland et al., 2007). More recently, studies have shown use of in vitro co-culture systems to investigate the effects of schistosome-derived products on the differentiation and function of Tregs. These findings revealed that schistosome eggs secrete molecules that directly promote the expansion of Tregs, thereby modulating the host immune environment to favor parasite survival, while limiting inflammation and pathology (Mu et al., 2021).
Granuloma formation around the schistosome eggs is a key pathological feature of chronic schistosomiasis. In vitro models have been developed to study cellular and molecular processes underlying granuloma formation. In an important study Zouain et al., 2004 used an in vitro granuloma model to investigate how S mansoni PIII antigen influences granuloma size and composition. They found that granulomas formed around schistosome eggs in vitro are dependent on the presence of IL-13, a cytokine that promotes fibrosis. This study provides direct evidence linking IL-13 levels to the fibrotic pathology observed in schistosomiasis (Zouain et al., 2004). Furthermore, studies have shown that schistosome egg antigens drive macrophages toward an alternatively activated (M2) phenotype, which is associated with tissue repair and fibrosis in vitro. These findings help explain the dual role of macrophages in controlling infection and contributing to pathology (Pearce and MacDonald, 2002).
In addition to immune modulation, schistosomes release proteolytic enzymes, which facilitate tissue invasion and egg translocation. Several in vitro assays have been used to explore the role of these proteases in schistosome biology. Felleisen et al., 1990 conducted in vitro assays using recombinant schistosome proteases to study their role in degrading host extracellular matrix proteins. They showed that the protease cathepsin B, secreted by schistosomula, is crucial for the ability of the parasite to enter the circulatory system (blood vessels or lymphatics) and are carried passively by the bloodstream to establish infection. This study highlights the importance of proteolytic enzymes in the early stages of infection (Felleisen and Klinkert, 1990).
Animal models in schistosomiasis research
Human studies on schistosomiasis are typically conducted in endemic regions, and often focus on observations before and after treatment. While analyzing human samples, such as blood, tissue, and stool, is essential for translating animal research into human systems (Colley and Secor, 2014), these samples have limitations, including variability in genetic background, medical history, co-infections, and environmental factors. Moreover, ongoing studies using controlled human infection with S. mansoni promise to shed new light on many aspects of human infection (Janse et al., 2018). However, ethically studying the egg excretion process in humans poses challenges, particularly when considering deliberate chronic infections involving male and female worms, which can lead to egg production, tissue damage, and significant health risks. Longitudinal studies in endemic areas could be an alternative, although they present logistical difficulties, such as the need for colonoscopy, to examine the intestinal epithelium.
Animal models have played a critical role in the investigation of schistosomiasis, facilitating the study of parasite biology, host immune responses, disease pathogenesis, drug efficacy, and vaccine development. Various animal species have been used to model distinct stages of Schistosoma infection, each offering unique advantages in understanding the disease, as shown in Table 1. Baboons and chimpanzees are the most accurate models, replicating key features of human schistosomiasis, such as peri-portal fibrosis and intestinal lesions (Sadun et al., 1966; Abe et al., 1993; Yole, 1996; Nyindo and Farah, 1999; Farah et al., 2000) but these large animal models are costly with stricter ethical considerations. Despite some differences, particularly in hepatic fibrosis and pathology, which are more closely linked to the granulomatous response to trapped parasite eggs in the liver and intestine in mice (Fallon, 2000), the formation of granulomas in mice is still a valuable reflection of human disease. However, despite decades of research, the immunological and molecular mechanisms underlying the extravasation of schistosome eggs remain poorly understood.
OOC systems for modeling human physiology
OOC systems as summarized in Table 2 represents a revolutionary approach to modeling human physiology in vitro by recreating the structural and functional complexity of human organs and tissues (Huh et al., 2013). These microfluidic devices, also known as 3D microphysiological systems (3D MPS), offer a unique platform for studying biological processes in a controlled environment that closely mimics the in vivo microenvironment (Bhatia and Ingber, 2014). OOC systems revolves around the creation of microscale devices that replicate the essential features of specific organs or tissues (Esch et al., 2014). These systems typically consist of microfluidic channels lined with living cells that simulate the structure and function of the target tissue (Bein et al., 2018). By culturing cells in a 3D microenvironment, OOC models provide more physiologically relevant conditions than traditional two-dimensional (2D) cell culture systems (Jiang et al., 2019).
The key advantage of OOC system is its ability to simulate dynamic microenvironments, including fluid flow, mechanical forces, and cell-cell interactions (Huh et al., 2010). The microfluidic channels embedded within the device allow precise control over the delivery of nutrients, oxygen, and signaling molecules to cultured cells, mimicking the physiological conditions found in vivo (Esch et al., 2015). OOC systems are suitable for studying complex biological processes such as host-pathogen interactions. Unlike animal models, OOC systems allow the use of human cells, reducing the need for animal experimentation and improving the relevance of the results to human physiology (Bhatia and Ingber, 2014). Furthermore, OOC systems can be customized to mimic specific disease conditions, allowing researchers to study the mechanisms of disease and test potential therapeutics in a high-throughput manner (Esch et al., 2011).
These systems have been utilized in the studies of other infectious diseases, including malaria, tuberculosis, and influenza (Esch et al., 2014). These models recapitulate key aspects of host-pathogen interactions, such as the interaction between immune cells and pathogens, the dynamics of infection within host tissues, and the efficacy of antimicrobial drugs. For example, lung-on-chip systems have revealed important insights into pathogenesis of respiratory infections, including the recruitment of immune cells to the site of infection and the inflammatory response induced by the pathogen (Huh et al., 2011). Similarly, gut-on-a-chip models have uncovered the mechanisms of microbial colonization and host immune response to gut microbiota and enteric pathogens (Lu et al., 2018). Therefore, OOC systems represent a groundbreaking leap in biomedical research, offering unparalleled advantages by recreating human tissue environments with remarkable fidelity. This makes them particularly valuable in studying complex diseases such as schistosomiasis, where the lifecycle of the parasite and its effects on human tissues such as the liver and vasculature, are difficult to fully capture in existing models.
Application of OOC systems in schistosomiasis research
In the context of schistosomiasis research, OOC technology offers a powerful platform for studying the mechanisms underlying the extravasation of schistosome eggs (Lu et al., 2018). By replicating the physiological conditions of the host vasculature and surrounding tissues, OOC models can provide insights into the interactions between schistosome eggs and the host immune system, as well as the factors that regulate egg extravasation (Costain et al., 2018). Furthermore, OOC platforms allow real-time imaging and analysis of cell responses, allowing for visualization and quantifying the dynamics of egg migration in a controlled experimental setting (Wu et al., 2020). This technology holds a great promise in advancing our understanding of schistosomiasis by providing a physiologically relevant platform to study the mechanisms underlying extravasation of Schistosoma eggs. Yeh et al., 2022, have demonstrated the utility of organ-on-chip models in elucidating the complex interactions between Schistosoma eggs and host tissues, as well as the factors that regulate egg extravasation (Yeh et al., 2022). By culturing endothelial cells in microfluidic channels and exposing them to schistosome eggs, we can simulate the initial stages of egg extravasation and investigate the mechanisms by which eggs breach the endothelial barrier. Past studies have revealed that schistosome eggs induce endothelial activation and permeability through the release of pro-inflammatory cytokines and chemokines, leading to the recruitment of immune cells to the site of infection (Angeles et al., 2020).
For example, a microfluidic system was developed by Girod et al., to mimic flow conditions in the human blood system that allowed the evaluation of drug effects on worm attachment and viability under dynamic conditions (Girod et al., 2022). The system demonstrated that healthy worms remained attached to the walls and resisted flow, while damaged worms were eliminated. This study highlights the importance of developing novel screening systems for the identification of schistosomicidal drugs contributing to the advancement of drug screening methodologies and provides a promising platform to evaluate the efficacy of other potential treatments against these parasites. Future advancements in this technology could implement automated worm counting for real-time analysis. OOC systems also enable real-time imaging and analysis of cellular responses, allowing researchers to visualize the dynamics of egg migration and granuloma formation in a controlled experimental setting. By incorporating advanced imaging techniques, such as confocal microscopy and live cell imaging, we can monitor the interactions between schistosome eggs and host cells in real time, providing insight into the spatiotemporal dynamics of egg extravasation (Singh et al., 2022). Furthermore, computational modeling approaches can be used to simulate the biophysical forces that regulate egg migration and predict optimal conditions for the formation of granulomas (King et al., 2021).
In addition to studying the mechanisms of egg extravasation, OOC models can be used to screen potential therapeutics targeting Schistosoma infection (Hong et al., 2024). By culturing immune cells and endothelial cells in microfluidic devices and exposing them to Schistosoma eggs, it is possible to evaluate the efficacy of anti-inflammatory drugs, immune modulators, and antiparasitic agents in preventing egg extravasation and granuloma formation (Mannino et al., 2018). These studies have identified several promising candidates for further preclinical testing, including small-molecule inhibitors of cytokine signaling pathways and monoclonal antibodies targeting immune cell receptors (Wynn and Cheever, 1995). Furthermore, OOC platforms can be used to study the long-term effects of Schistosoma infection on host tissues and organs (Colley et al., 2014). Culturing of cells from different organs, such as the liver, intestine, and bladder, in interconnected microfluidic devices, can model the systemic effects of chronic schistosomiasis and investigate the mechanisms underlying disease progression (Colley and Secor, 2014). These studies can uncover novel therapeutic targets to prevent or mitigate long-term complications of schistosomiasis, such as liver fibrosis and bladder cancer (Pearce and MacDonald, 2002).
Using OOC systems to decipher the forces driving schistosome egg migration
Since schistosome eggs do not possess known motility mechanism, the puzzling and yet scientifically interesting question is how the eggs transit through layers of host tissues, including endothelial and epithelial barriers to reach the intestinal lumen and be excreted to continue the life cycle. As earlier stated, there is a better understanding of host immune interaction with the parasites, including characterization of proteins and enzymes such as matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) which are key regulators of extracellular matrix (ECM) turnover and remodeling in schistosomiasis infection (Page-McCaw et al., 2007). These play a critical role in both host defense and tissue pathology released by the parasites to aid in host invasion and immunomodulation for survival inside the host (Pearce and MacDonald, 2002).
The formation of granuloma is a complex process which involve infiltrations of alternatively activated macrophages, eosinophils, Th2 cells, fibroblast proliferation (Hams et al., 2013), angiogenesis, endothelial activation, and the release of blood clotting factors (Shariati et al., 2011; Mebius et al., 2013). As such, both in vitro and in vivo studies have reported that the timing and formation of granuloma are crucial to the successful translocation of schistosome eggs (Hams et al., 2013; Linder, 2017; Schwartz and Fallon, 2018; Takaki et al., 2021). These stromal cells play key roles in tissue repair, and become activated following tissue damage, as would happen during egg deposition (Gabbiani, 2003).
However, despite intense studies, precisely how the eggs transit through the complex granuloma ecosystem to reach the gut lumen remains largely unknown. Equally unclarified is the role of fibroblasts in granuloma formation and egg propulsion since fibroblasts are the most abundant cells in the stroma where eggs are laid. In normal development and physiology, fibroblasts are the major producers of the ECM. These stromal cells play key roles in tissue repair and become activated following tissue damage, as would happen during egg deposition (Gabbiani, 2003). During wound healing, these cells can produce transforming growth factor-β (TGFβ) and acquire a highly contractile phenotype associated with the expression of alpha smooth muscle antigen (αSMA) (Rockey et al., 2013). In this state, fibroblasts are termed ‘myofibroblasts’. Both in normal homeostasis and following injury they participate in crosstalk with adjacent epithelia, and their ability to influence local epithelial stem cell behavior has been documented (Brizzi et al., 2012; Le Guen et al., 2015). In addition, fibroblasts can also promote angiogenesis via the production of vascular endothelial growth factor A (VEGFA) and coordinate the function of the immune system via the production of chemokines and cytokines (Kraman et al., 2010; Buechler and Turley, 2018). Given the myriad roles of fibroblasts in homeostasis and being the most contractile among the cells within the granuloma microenvironment, it is likely that these cells provide thrust to the eggs. In support of this, earlier studies have pointed to the role of fibroblasts in granuloma formation in tuberculosis (TB) where they, confoundingly, provide both protection to the host as well as facilitate bacteria dissemination (Evans et al., 2019). Cancer associated fibroblasts (CAFs) are also suspected to produce contractile forces which drive cancer metastasis (reviewed in (Sahai et al., 2020)). Could a similar mechanism exist in schistosome egg dissemination, and if so, what is the exact role of fibroblasts in this process?
OOC can be employed to clarify the migratory mechanism of schistosome eggs within gastrointestinal tissues from the perspective of tissue mechanics. Given the role of fibroblasts in remodeling the extracellular matrix (ECM) and generating contractile forces, we propose that egg-induced immune stimulation and/or ECM alterations—such as collagen and fibrin deposition and degradation—within the granuloma microenvironment may activate fibroblasts into a highly contractile myofibroblast phenotype. This activation could produce sufficient contractile forces to facilitate the migration of schistosome eggs. Thus, using in vitro studies utilizing microphysiological systems (MPS) as illustrated in Figure 3, which are now widely accepted as animal alternatives (Wikswo, 2014), we can take a tissue mechanistic approach to quantitatively map and dissect the nature and origin of forces that propel schistosome egg migration. Considering the roles of fibroblasts in matrix deposition and contractile force generation, we can use in vitro studies employing 3D MPS to determine the role of fibroblast-generated contractile forces in egg propulsion. Because cell contractility is closely associated with matrix stiffness such that on a stiffer matrix where cells can establish stronger focal adhesions, stronger contractile forces are generated compared with a soft matrix.
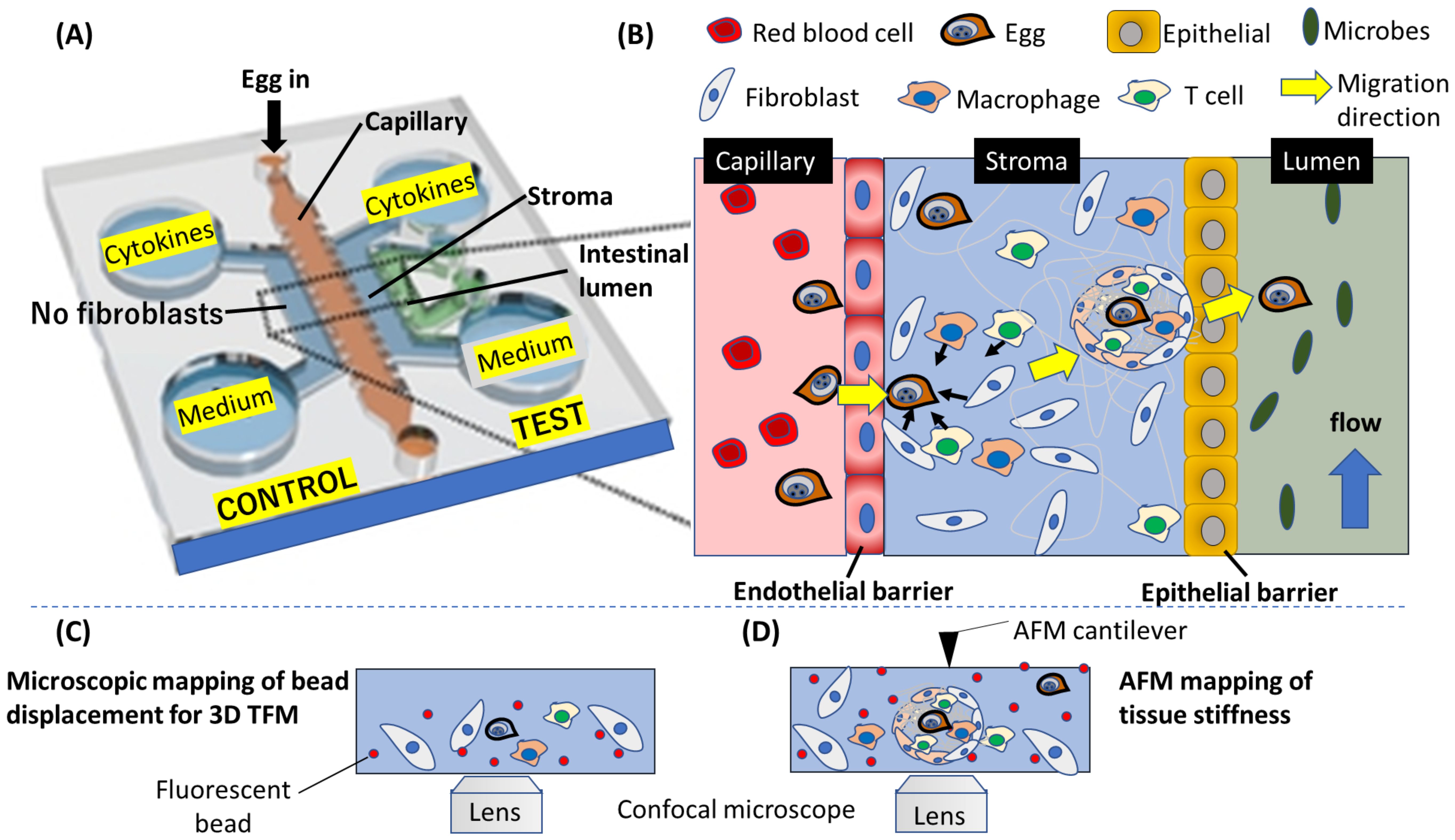
Figure 3. A conceptualized potential application of OOC to mimic schistosome egg migration through the gastrointestinal tissues. (A) A layout of the OOC showing the various components for tracking egg migration. (B) An enlarged diagram depicting the migration process on the OOC. The complex process involved in the egg migration, including the penetration of endothelial barrier wall, egg interaction with fibroblast and immune cells leading to the formation of granuloma in the stroma, and finally, egg transition through epithelial barrier into the intestinal lumen are depicted. (C) Fluorescence microscopy can be applied to track fluorescent beads embedded in the gel mimicking the stroma, and (D) force characterization using atomic force microscope (AFM) can yield quantitative information about the forces produced by host cells to propel Schistosome egg during the egg migration process. (A) is modified from Lee et al., 2019 (Lee et al., 2019).
By adjusting the microenvironment in an OOC system, we can further investigate the conditions that promote or inhibit egg migration, distinguishing between “successful” (excreted) and “unsuccessful” (trapped) eggs. This allows for the identification of molecular and mechanical cues that influence egg migration outcomes. The OOC system can also mimic coagulation pathways by incorporating blood vessel-like structures that simulate vascular responses to egg transit. Introducing blood flow and clotting factors enables real-time observation of how coagulation and fibrinolysis are regulated during egg migration. Additionally, microfluidic channels can simulate blood vessels to explore clot formation, and how the fibrinolytic system clears clots to facilitate egg movement (Linder, 2017).
Thus, we hypothesize that when egg-induced matrix degradation occurs due to egg-secreted matrix metalloproteinase (MMP) (Singh et al., 2004; Singh et al., 2006), fibroblasts on the degraded part of the granuloma can exert less contractility compared with those on the unaffected part, creating an anisotropic contraction which may push the eggs out of the granuloma towards the blood vessels as shown in Figure 4. To clarify this, in 3D MPS, we can deploy high resolution live cell tracking, 3D force mapping techniques, and tissue mechanics, coupled with immunological characterization and genetic profiling to characterize fibroblast-egg interaction right from the initiation of granuloma formation to the egg migration stage. 3D MPS can recapitulate the gastrointestinal microenvironment where we could deploy it to spatiotemporally and quantitatively study the role of fibroblasts in the formation of granuloma and, eventually, egg migration. Leveraging on tissue mechanics, we can map forces within the granuloma microenvironment, using quantitative approaches integrating 3D traction force microscopy (3D TFM) and atomic force microscopy (AFM) measurements. From cellular migration and tissue deformation analyses, we could generate a force map to reveal the force vector dictating the trajectory of egg migration within the gastrointestinal ecosystem as depicted in Figure 5. 3D MPS can therefore be used to map the nature and origin of the forces that propel egg migration, with focus on fibroblasts.
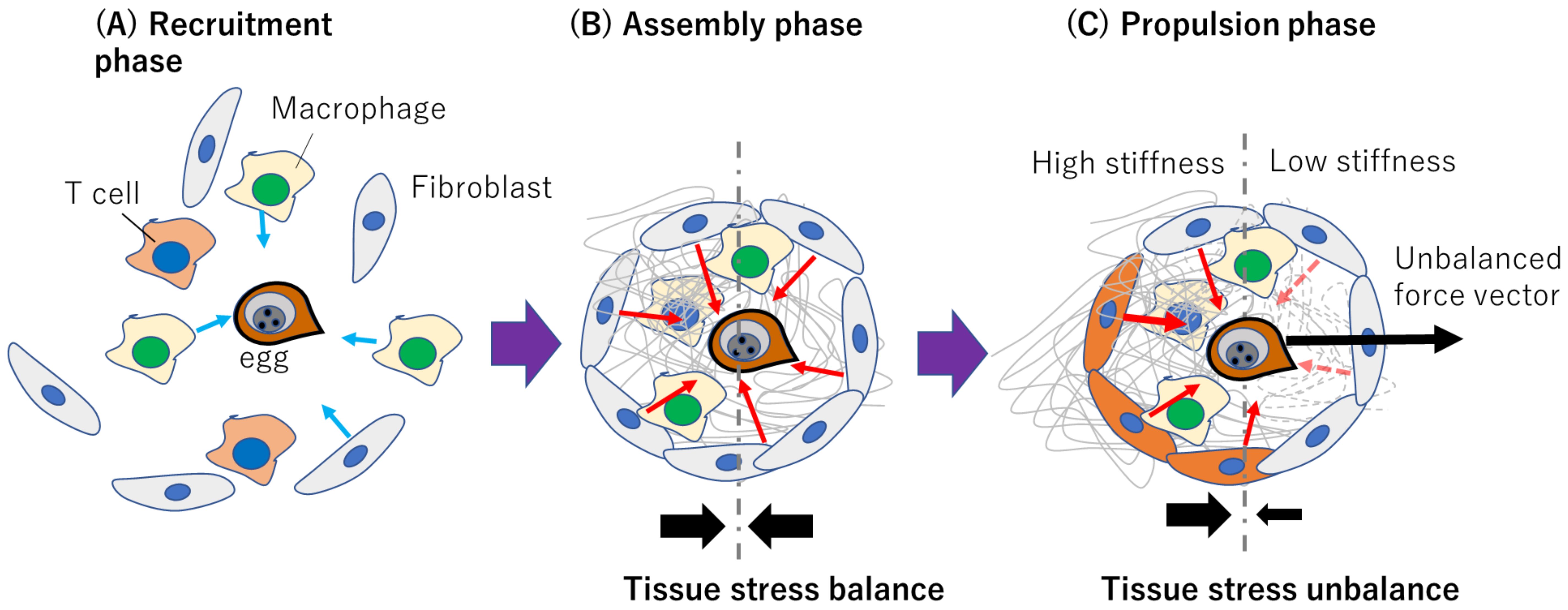
Figure 4. Conceptualized representation of how fibroblast-dependent tissue mechanics may drive schistosome egg migration. (A) Recruitment phase where immune cells and fibroblasts respond to egg released immunogenically. Light blue arrows indicate migration direction. (B) Assembly phase involving ECM deposition and isotropic contraction without a resultant force due to tissue stress balance. Red arrows indicate hypothesized fibroblast-generated contractile forces within the granuloma (C) Propulsion phase where polarized ECM degradation or myofibroblast differentiation contributes to anisotropic contraction and egg propulsion. The red dashed arrows indicate diminishing forces due to matrix degradation. The solid black arrows in (B, C) represent the tissue stress balance or unbalance.
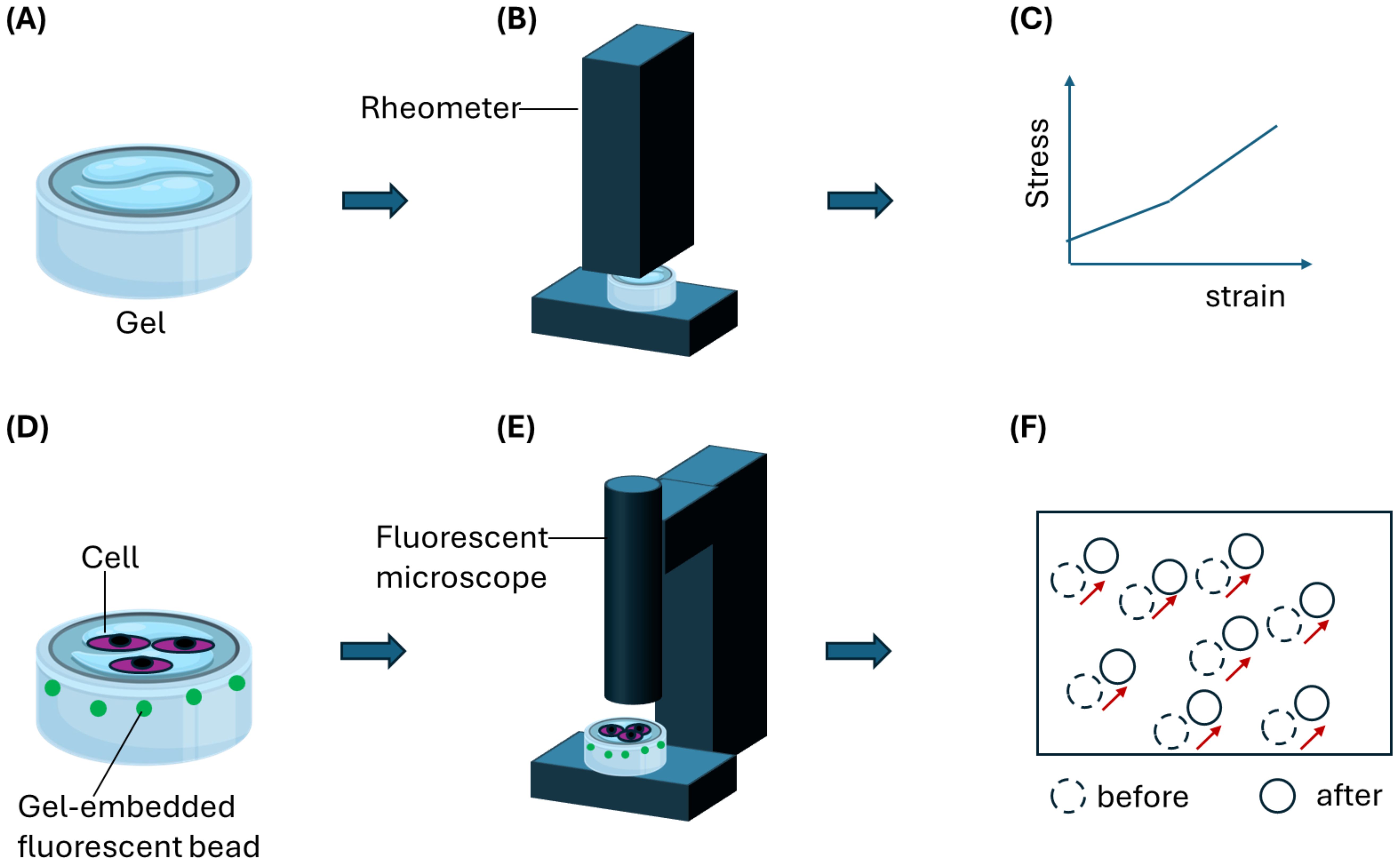
Figure 5. An illustration of a gel-based assay to decipher forces produced by host cells such as contractile fibroblasts to aid Schistosoma eggs migration. (A) A hydrogel is first characterized using (B) stiffness measurement equipment such as AFM or rheometer. (C) A stress-strain relationship obtained from the characterization is then used to derive quantitative stiffness information such as Young’s modulus. (D) Gel samples can be embedded with fluorescence particles such as beads for optical tracking and mapping of gel deformation during cell migration on or through the gel. Schistosoma eggs can be co-cultured on the gel surface in contact with host cells such as fibroblasts. (E) Tracking bead displacement using a fluorescent microscope would yield (F) a displacement field resulting from gel deformation. By applying particle image velocimetry (PIV) analysis, migration forces produced by host cells can be determined quantitatively.
Conclusion
Over the past few decades, research has revealed many fascinating aspects of S. mansoni biology and the host’s immune response to both the worms and their eggs. The immunology of granuloma formation around the eggs has been a major focus, given its central role in the immunopathology of schistosomiasis infections. Despite significant advances, many unknown aspects remain to be identified and experimentally validated. One such area is the process by which granulomas evolve to facilitate egg migration through the intestinal wall, liver, spleen and other organs. Thus, a 3D MPS can recapitulate the gastrointestinal microenvironment where we could deploy it to spatiotemporally and quantitatively study the role of immune cells and fibroblasts in the formation of granuloma and, eventually, egg migration. Leveraging on tissue mechanics, we can map forces within the granuloma microenvironment, using quantitative approaches integrating 3D TFM and AFM measurements. From cellular migration and tissue deformation analyses, we could generate a force map to reveal the force vector dictating the trajectory of egg migration within the gastrointestinal ecosystem. 3D MPS can therefore be used to map the nature and origin of the forces that propel egg migration, with focus on immune cells and fibroblasts. This cutting-edge platform, which mimics the structure and function of human organs, could allow for detailed investigation of egg transit in a controlled environment.
Author contributions
MA: Conceptualization, Writing – original draft, Writing – review & editing. LO: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing. KO: Conceptualization, Writing – review & editing, Funding acquisition, Supervision. EB: Writing – review & editing. PO: Writing – review & editing, Conceptualization, Supervision. DO: Supervision, Writing – review & editing. KN: Supervision, Writing – review & editing. JR: Supervision, Writing – review & editing, Resources.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Human Frontier Science Program (HFSP) reference number: RGP020/2023: DOI https://doi.org/10.52044/HFSP.RGP0202023.pc.gr.168605.
Acknowledgments
We would like to express our gratitude to Professor Abdussamad Muhammad Abdussamad for his invaluable contributions in reviewing multiple drafts of this manuscript. We also extend our sincere thanks to the reviewers for their insightful input during the review process.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Generative AI was used Paperpal.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdulla, M. H., Ruelas, D. S., Wolff, B., Snedecor, J., Lim, K. C., Xu, F., et al. (2009). Drug discovery for schistosomiasis: Hit and lead compounds identified in a library of known drugs by medium-throughput phenotypic screening. PloS Negl. Trop. Dis. 3. doi: 10.1371/journal.pntd.0000478
Abe, K., Kagei, N., Teramura, Y., Ejima, H. (1993). Hepatocellular carcinoma associated with chronic Schistosoma mansoni infection in a chimpanzee. J. Med. Primatol. 22. doi: 10.1111/j.1600-0684.1993.tb00662.x
Abla, N., Keiser, J., Vargas, M., Reimers, N., Haas, H., Spangenberg, T. (2017). Evaluation of the pharmacokinetic-pharmacodynamic relationship of praziquantel in the Schistosoma mansoni mouse model. PloS Negl. Trop. Dis. 11. doi: 10.1371/journal.pntd.0005942
Abouel-Nour, M. F., Lotfy, M., El-Kady, I., El-Shahat, M., Doughty, B. L. (2005). Localization of leucine aminopeptidase in the Schistosoma mansoni eggs and in liver tissue from infected mice. J. Egypt Soc. Parasitol. 35.
Abou Rashed, A., Senna, F. M., Sabry, A. H., Morsy, T. A. (1997). Studies on granuloma formation in Syrian hamsters experimentally infected with Schistosoma mansoni. J. Egypt Soc. Parasitol. 27.
Ahmad, G., Zhang, W., Torben, W., Ahrorov, A., Damian, R. T., Wolf, R. F., et al. (2011). Preclinical prophylactic efficacy testing of Sm-p80-based vaccine in a nonhuman primate model of schistosoma mansoni infection and immunoglobulin G and e responses to Sm-p80 in human serum samples from an area where schistosomiasis is endemic. J. Infect. Dis. 204. doi: 10.1093/infdis/jir545
Alghamdi, T., Salem, D. A., El-Refaei, M. F. (2023). Anti-angiogenic and anti-proliferative activity of ziziphus leaf extract as a novel potential therapeutic agent for reducing hepatic injury in experimental hamster schistosomiasis. PloS Negl. Trop. Dis. 17. doi: 10.1371/journal.pntd.0011426
Allan, L. A., Kutima, H. L., Muya, S., Ayonga, D., Yole, D. (2014). The efficacy of a herbal drug, schitozim over praziquantel in the management of Schistosoma mansoni infection in BALB/c mice. J. Biol. Agric. Healthc. 4.
Andrade, Z. A. (2004). Schistosomal hepatopathy. Memorias do Instituto Oswaldo Cruz. doi: 10.1590/S0074-02762004000900009
Andrade, Z. A., Santana, T. S. (2010). Angiogenesis and schistosomiasis. Mem Inst Oswaldo Cruz. 105. doi: 10.1590/S0074-02762010000400013
Angeles, J. M. M., Mercado, V. J. P., Rivera, P. T. (2020). Behind enemy lines: immunomodulatory armamentarium of the schistosome parasite. Front. Immunol. 11. doi: 10.3389/fimmu.2020.01018
Bartley, P., Ramm, G., Jones, M., Ruddell, R., Li, Y., Mcmanus, D. (2006). A contributory role for activated hepatic stellate cells in the dynamics of Schistosoma japonicum egg-induced fibrosis. Int. J. Parasitol. 36, 993–1001. doi: 10.1016/j.ijpara.2006.04.015
Beaurivage, C., Naumovska, E., Chang, Y. X., Elstak, E. D., Nicolas, A., Wouters, H., et al. (2019). Development of a gut-on-a-chip model for high throughput disease modeling and drug discovery. Int. J. Mol. Sci. 20. doi: 10.3390/ijms20225661
Bein, A., Shin, W., Jalili-Firoozinezhad, S., Park, M. H., Sontheimer-Phelps, A., Tovaglieri, A., et al. (2018). Microfluidic organ-on-a-chip models of human intestine. CMGH 5. doi: 10.1016/j.jcmgh.2017.12.010
Bhatia, S. N., Ingber, D. E. (2014). Microfluidic organs-on-chips. Nat. Biotechnol. 32. doi: 10.1038/nbt.2989
Blundell, C., Tess, E. R., Schanzer, A. S. R., Coutifaris, C., Su, E. J., Parry, S., et al. (2016). A microphysiological model of the human placental barrier. Lab. Chip 16. doi: 10.1039/C6LC00259E
Blundell, C., Yi, Y. S., Ma, L., Tess, E. R., Farrell, M. J., Georgescu, A., et al. (2018). Placental drug transport-on-a-chip: A microengineered in vitro model of transporter-mediated drug efflux in the human placental barrier. Adv. Healthc Mater 7. doi: 10.1002/adhm.201700786
Botros, S., El-Lakkany, N., Seif-el-Din, S. H., Sabra, A. N., Ibrahim, M. (2011). Comparative efficacy and bioavailability of different praziquantel brands. Exp. Parasitol. 127. doi: 10.1016/j.exppara.2010.10.019
Brizzi, M. F., Tarone, G., Defilippi, P. (2012). Extracellular matrix, integrins, and growth factors as tailors of the stem cell niche. Curr. Opin. Cell Biol. 24. doi: 10.1016/j.ceb.2012.07.001
Buechler, M. B., Turley, S. J. (2018). A short field guide to fibroblast function in immunity. Semin. Immunol. 35. doi: 10.1016/j.smim.2017.11.001
Burke, M. L., McManus, D. P., Ramm, G. A., Duke, M., Li, Y., Jones, M. K., et al. (2010). Temporal expression of chemokines dictates the hepatic inflammatory infiltrate in a murine model of schistosomiasis. PloS Negl. Trop. Dis. 4. doi: 10.1371/journal.pntd.0000598
Carvalho-Queiroz, C., Nyakundi, R., Ogongo, P., Rikoi, H., Egilmez, N. K., Farah, I. O., et al. (2015). Protective potential of antioxidant enzymes as vaccines for schistosomiasis in a non-human primate model. Front. Immunol. 6. doi: 10.3389/fimmu.2015.00273
Cass, C. L., Johnson, J. R., Califf, L. L., Xu, T., Hernandez, H. J., Stadecker, M. J., et al. (2007). Proteomic analysis of Schistosoma mansoni egg secretions. Mol. Biochem. Parasitol. 155. doi: 10.1016/j.molbiopara.2007.06.002
Chambers, B. E., Weaver, N. E., Wingert, R. A. (2023). The “3Ds” of growing kidney organoids: advances in nephron development, disease modeling, and drug screening. Cells 12. doi: 10.3390/cells12040549
Chen, L., Liu, W., Lei, J.h., Guan, F., Li, M.j., Song, W.j., et al. (2012). Chronic Schistosoma japonicum Infection Reduces Immune Response to Vaccine against Hepatitis B in Mice. PloS One 7. doi: 10.1371/journal.pone.0051512
Chiaramonte, M. G., Donaldson, D. D., Cheever, A. W., Wynn, T. A. (1999). An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2–dominated inflammatory response. J. Clin. Invest. 104, 777–785. doi: 10.1172/JCI7325
Chitsulo, L., Engels, D., Montresor, A., Savioli, L. (2000). The global status of schistosomiasis and its control. Acta Trop. 77, 41–51. doi: 10.1016/s0001-706x(00)00122-4
Chiu, B. C., Chensue, S. W. (2002). Chemokine responses in schistosomal antigen-elicited granuloma formation. Parasite Immunol. doi: 10.1046/j.1365-3024.2002.00466.x
Cho, S. W., Malick, H., Kim, S. J., Grattoni, A. (2024). Advances in skin-on-a-chip technologies for dermatological disease modeling. J. Invest. Dermatol. 144. doi: 10.1016/j.jid.2024.01.031
Colley, D. G., Bustinduy, A. L., Secor, W. E., King, C. H. (2014). Human schistosomiasis. Lancet 383, 2253–2264. doi: 10.1016/S0140-6736(13)61949-2
Colley, D. G., Secor, W. E. (2014). Immunology of human schistosomiasis. Parasite Immunol. 36. doi: 10.1111/pim.2014.36.issue-8
Costain, A. H., MacDonald, A. S., Smits, H. H. (2018). Schistosome egg migration: mechanisms, pathogenesis and host immune responses. Front. Immunol. 9. doi: 10.3389/fimmu.2018.03042
Dean, D. A., Wistar, R., Chen, P. (1975). Immune response of Guinea pigs to Schistosoma mansoni. I. In vitro effects of antibody and neutrophils, eosinophils and macrophages on schistosomula. Am. J. Trop. Med. Hygiene 24. doi: 10.4269/ajtmh.1975.24.74
de Oliveira Santos Bernardes, W. P., Dutra, I. T. X., da-Silva-Pereira, R. A., Mourão, M. M., Fonseca, C. T. (2022). SmTAL-9, a member of the schistosoma mansoni tegument allergen-like family, is important for parasite survival and a putative target for drug/vaccine development. Front. Immunol. 13.
de Rus Jacquet, A., Alpaugh, M., Denis, H. L., Tancredi, J. L., Boutin, M., Decaestecker, J., et al. (2023). The contribution of inflammatory astrocytes to BBB impairments in a brain-chip model of Parkinson’s disease. Nat. Commun. 14. doi: 10.1038/s41467-023-39038-8
Elguindy, D. A. S., Ashour, D. S., Elmarhoumy, S. M., El-Guindy, D. M., Ismail, H. I. H. (2024). The efficacy of cercarial antigen loaded on nanoparticles as a potential vaccine candidate in Schistosoma mansoni-infected mice. J. Parasitic Diseases. 48, 381–399. doi: 10.1007/s12639-024-01677-z
El Ridi, R., Tallima, H. (2009). Schistosoma mansoni ex vivo lung-stage larvae excretory-secretory antigens as vaccine candidates against schistosomiasis. Vaccine 27.
el-Sebai, M. M., Kamal, A. M., Shahin, M. A. (1989). Hepatic fibrosis in hamsters experimentally infected with S. mansoni. J. Egypt Soc. Parasitol. 19.
Esch, E. W., Bahinski, A., Huh, D. (2015). Organs-on-chips at the frontiers of drug discovery. Nat. Rev. Drug Discovery 14. doi: 10.1038/nrd4539
Esch, M. B., Mahler, G. J., Stokol, T., Shuler, M. L. (2014). Body-on-a-chip simulation with gastrointestinal tract and liver tissues suggests that ingested nanoparticles have the potential to cause liver injury. Lab. Chip. 14, 3081. doi: 10.1039/C4LC00371C
Esch, M. B., Post, D. J., Shuler, M. L., Stokol, T. (2011). Characterization of in vitro endothelial linings grown within microfluidic channels. Tissue Eng. Part A. 17, 2965. doi: 10.1089/ten.tea.2010.0371
Essaouiba, A., Okitsu, T., Jellali, R., Shinohara, M., Danoy, M., Tauran, Y., et al. (2020a). Microwell-based pancreas-on-chip model enhances genes expression and functionality of rat islets of Langerhans. Mol. Cell Endocrinol. 514. doi: 10.1016/j.mce.2020.110892
Essaouiba, A., Okitsu, T., Kinoshita, R., Jellali, R., Shinohara, M., Danoy, M., et al. (2020b). Development of a pancreas-liver organ-on-chip coculture model for organ-to-organ interaction studies. Biochem. Eng. J. 164. doi: 10.1016/j.bej.2020.107783
Etxeberria, L., Messelmani, T., Badiola, J. H., Llobera, A., Fernandez, L., Vilas-Vilela, J. L., et al. (2022). Validation of hepG2/C3A cell cultures in cyclic olefin copolymer based microfluidic bioreactors. Polymers (Basel) 14. doi: 10.3390/polym14214478
Evans, S., Wong, E. A., Flynn, J. L., Mattila, J. T., Kirschner, D. (2019). Unraveling the role of fibrosis in the TB Granuloma. J. Immunol. 202. doi: 10.4049/jimmunol.202.Supp.182.86
Ewing, L. E., Skinner, C. M., McGill, M. R., Kennon-McGill, S., Clement, K., Quick, C. M., et al. (2024). Human quad liver-on-chip system as a tool toward bridging the gap between animals and humans regarding toxicology and pharmacology of a cannabidiol-rich cannabis extract. Drug Chem. Toxicol., 1–8. doi: 10.1080/01480545.2024.2388292
Fallon, P. G. (2000). Immunopathology of schistosomiasis: A cautionary tale of mice and men. Immunol. Today 21. doi: 10.1016/S0167-5699(99)01551-0
Farah, I. O., Mola, P. W., Kariuki, T. M., Nyindo, M., Blanton, R. E., King, C. L. (2000). Repeated exposure induces periportal fibrosis in schistosoma mansoni- infected baboons: role of TGF-β and IL-4. J. Immunol. 164.
Farah, I., Ngotho, M., Kagira, J., Kariuki, T., Jeneby, M., Maina, N., et al. (2013). “Animal models for tropical parasitic diseases,” in Handbook of Laboratory Animal Science, Volume III, Third Edition: Animal Models. (Boca Raton: CRC Press) pp. 43–105. doi: 10.1201/b16317
Felleisen, R., Klinkert, M. Q. (1990). In vitro translation and processing of cathepsin B of Schistosoma mansoni. EMBO J. 9. doi: 10.1002/j.1460-2075.1990.tb08120.x
Fernández-Costa, J. M., Ortega, M. A., Rodríguez-Comas, J., Lopez-Muñoz, G., Yeste, J., Mangas-Florencio, L., et al. (2023). Training-on-a-chip: A multi-organ device to study the effect of muscle exercise on insulin secretion in vitro. Adv. Mater Technol. 8.
Frantz, F. G., Ito, T., Cavassani, K. A., Hogaboam, C. M., Lopes Silva, C., Kunkel, S. L., et al. (2011). Therapeutic DNA vaccine reduces Schistosoma mansoniinduced tissue damage through cytokine balance and decreased migration of myofibroblasts. Am. J. Pathol. 179.
Gabbiani, G. (2003). The myofibroblast in wound healing and fibrocontractive diseases. J. Pathol. 200. doi: 10.1002/path.v200:4
Gent, V., Waihenya, R., Kamau, L., Nyakundi, R., Ambala, P., Kariuki, T., et al. (2019). An investigation into the role of chronic Schistosoma mansoni infection on Human Papillomavirus (HPV) vaccine induced protective responses. PloS Negl. Trop. Dis. 13. doi: 10.1371/journal.pntd.0007704
Girod, V., Houssier, R., Sahmer, K., Ghoris, M. J., Caby, S., Melnyk, O., et al. (2022). A self-purifying microfluidic system for identifying drugs acting against adult schistosomes. R Soc. Open Sci. 9. doi: 10.1098/rsos.220648
Gordon, J. R., McLaren, D. J. (1988). Immunity to Schistosoma mansoni in Guinea-pigs vaccinated with radiation-attenuated cercariae. T-cell activation of macrophages for larval killing. Immunology 63.
Greigert, V., Brion, N., Lang, C., Regnard, P., Pfaff, A. W., Abou-Bacar, A., et al. (2019). Cestode infections in non-human primates suggest the existence of zoonotic cycles in the area surrounding the Strasbourg primatology center. Parasite 26. doi: 10.1051/parasite/2019025
Gryseels, B., Polman, K., Clerinx, J., Kestens, L. (2006). Human schistosomiasis. Lancet 368, 1106–1118. doi: 10.1016/S0140-6736(06)69440-3
Guo, H., Deng, N., Dou, L., Ding, H., Criswell, T., Atala, A., et al. (2020). 3-D human renal tubular organoids generated from urine-derived stem cells for nephrotoxicity screening. ACS Biomater Sci. Eng. 6. doi: 10.1021/acsbiomaterials.0c01468
Hall, V., Bendtsen, K. M. S. (2023). Getting closer to modeling the gut-brain axis using induced pluripotent stem cells. Front. Cell Dev. Biol. 11. doi: 10.3389/fcell.2023.1146062
Hambrook, J. R., Kaboré, A. L., Pila, E. A., Hanington, P. C. (2018). A metalloprotease produced by larval Schistosoma mansoni facilitates infection establishment and maintenance in the snail host by interfering with immune cell function. PloS Pathog. 14. doi: 10.1371/journal.ppat.1007393
Hams, E., Aviello, G., Fallon, P. G. (2013). The Schistosoma granuloma: Friend or foe? Front. Immunol. 4.
Han, H., Peng, J., Hong, Y., Zhang, M., Han, Y., Fu, Z., et al. (2013). Comparison of the differential expression miRNAs in Wistar rats before and 10 days after S.japonicum infection. Parasit Vectors 6.
Hassan, A. S., Houle, S., Labrie, L., Perera, D. J., Dozois, C. M., Ward, B. J., et al. (2023). Salmonella Typhimurium expressing chromosomally integrated Schistosoma mansoni Cathepsin B protects against schistosomiasis in mice. NPJ Vaccines 8. doi: 10.1038/s41541-023-00599-w
Helmy, A. H. I., Abdel-Hady, A. A., el-Shanawany, F., Hammam, O., Abdel-Hady, A. (2005). The pharmacological approach to reverse portal hypertention and hepatic schistosomal fibrosis in Egypt, control experimental study. J. Egypt Soc. Parasitol. 35.
Hong, A., Umar, A., Chen, H., Yu, Z., Huang, J. (2024). Advances in the study of the interaction between schistosome infections and the host’s intestinal microorganisms. Parasit Vectors. 17, 185. doi: 10.1186/s13071-024-06245-1
Hu, Y., Chen, J., Xu, Y., Zhou, H., Huang, P., Ma, Y., et al. (2020). Alterations of gut microbiome and metabolite profiling in mice infected by schistosoma japonicum. Front. Immunol. 11. doi: 10.3389/fimmu.2020.569727
Huang, Q., Gurarie, D., Ndeffo-Mbah, M., Li, E., King, C. H. (2022). Schistosoma transmission in a dynamic seasonal environment and its impact on the effectiveness of disease control. J. Infect. Dis. 225.
Huang, H., Hu, D., Chen, Z., Xu, J., Xu, R., Gong, Y., et al. (2022). Immunotherapy for type 1 diabetes mellitus by adjuvant-free Schistosoma japonicum-egg tip-loaded asymmetric microneedle patch (STAMP). J. Nanobiotechnol. 20. doi: 10.1186/s12951-022-01581-9
Huh, D.(. (2015). A human breathing lung-on-a-chip. Ann. Am. Thorac. Soc. 12, S42–S44. doi: 10.1513/AnnalsATS.201410-442MG
Huh, D., Hamilton, G. A., Ingber, D. E. (2011). From 3D cell culture to organs-on-chips. Trends Cell Biol. 21. doi: 10.1016/j.tcb.2011.09.005
Huh, D., Kim, H. J., Fraser, J. P., Shea, D. E., Khan, M., Bahinski, A., et al. (2013). Microfabrication of human organs-on-chips. Nat. Protoc. 8, 2135–2157. doi: 10.1038/nprot.2013.137
Huh, D., Matthews, B. D., Mammoto, A., Montoya-Zavala, M., Hsin, H. Y., Ingber, D. E. (2010). Reconstituting organ-level lung functions on a chip. Sci. (1979) 328, 1662. doi: 10.1126/science.1188302
Janse, J. J., Langenberg, M. C. C., Van Oosterhoud, J. K., Ozir-Fazalalikhan, A., Brienen, E. A. T., Winkel, B. M. F., et al. (2018). Establishing the production of male schistosoma mansoni cercariae for a controlled human infection model. J. Infect. Dis. 218. doi: 10.1093/infdis/jiy275
Jiang, K., Chaimov, D., Patel, S., Liang, J. P., Wiggins, S., Samojlik, M., et al. (2019). 3-D physiomimetic extracellular matrix hydrogels provide a supportive microenvironment for rodent and human islet culture. Biomaterials 198, 37. doi: 10.1016/j.biomaterials.2018.08.057
Joyce, K. L., Morgan, W., Greenberg, R., Nair, M. G. (2012). Using eggs from Schistosoma mansoni as an in vivo model of helminth-induced lung inflammation. J. Visualized Experiments 64).
Kanabekova, P., Kadyrova, A., Kulsharova, G. (2022). Microfluidic organ-on-a-chip devices for liver disease modeling in vitro. Micromachines 13. doi: 10.3390/mi13030428
Kariuki, T. M., Farah, I. O., Yole, D. S., Mwenda, J. M., Van Dam, G. J., Deelder, A. M., et al. (2004). Parameters of the attenuated schistosome vaccine evaluated in the olive baboon. Infect. Immun. 72. doi: 10.1128/IAI.72.9.5526-5529.2004
Kazura, J. W., Fanning, M. M., Blumer, J. L., Mahmoud, A. A. F. (1981). Role of cell-generated hydrogen peroxide in granulocyte-mediated killing of schistosomula of Schistosoma mansoni in vitro. J. Clin. Invest. 67. doi: 10.1172/JCI110037
Kefallinou, D., Grigoriou, M., Boumpas, D. T., Gogolides, E., Tserepi, A. (2020). Fabrication of a 3D microfluidic cell culture device for bone marrow-on-a-chip. Micro Nano Eng. 9. doi: 10.1016/j.mne.2020.100075
Keshtkar, S., Soleimanian, S., Kaviani, M., Sarvestani, F. S., Azarpira, N., Asvar, Z., et al. (2022). Immune cell-derived extracellular vesicles in the face of pathogenic infections. Front. Immunol. 13. doi: 10.3389/fimmu.2022.906078
Kim, J. J., Park, J. Y., Nguyen, V. V. T., Bae, M., Kim, M., Jang, J., et al. (2023). Pathophysiological reconstruction of a tissue-specific multiple-organ on-A-chip for type 2 diabetes emulation using 3D cell printing. Adv. Funct. Mater 33. doi: 10.1002/adfm.202213649
Kimura, H., Sakai, Y., Fujii, T. (2018). Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metab. Pharmacokinet. 33. doi: 10.1016/j.dmpk.2017.11.003
King, M., Carson, J., Stewart, M. T., Gobert, G. N. (2021). Revisiting the Schistosoma japonicum life cycle transcriptome for new insights into lung schistosomula development. Exp. Parasitol. 223. doi: 10.1016/j.exppara.2021.108080
Kraman, M., Bambrough, P. J., Arnold, J. N., Roberts, E. W., Magiera, L., Jones, J. O., et al. (2010). Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-α. Sci. (1979) 330. doi: 10.1126/science.1195300
Krautz-Peterson, G., Debatis, M., Tremblay, J. M., Oliveira, S. C., Da’dara, A. A., Skelly, P. J., et al. (2017). Schistosoma mansoni infection of mice, rats and humans elicits a strong antibody response to a limited number of reduction-sensitive epitopes on five major tegumental membrane proteins. PloS Negl. Trop. Dis. 11. doi: 10.1371/journal.pntd.0005306
Kuipers, M. E., Nolte-’t-Hoen, E. N. M., van der Ham, A. J., Ozir-Fazalalikhan, A., Nguyen, D. L., de Korne, C. M., et al. (2020). DC-SIGN mediated internalisation of glycosylated extracellular vesicles from Schistosoma mansoni increases activation of monocyte-derived dendritic cells. J. Extracell Vesicles 9. doi: 10.1080/20013078.2020.1753420
Lam, H. Y. P., Huang, S. P., Liang, T. R., Wu, W. J., Cheng, P. C., Chang, K. C., et al. (2022). Increased immunogenicity and protection of recombinant Sm14 antigens by heat-killed Cutibacterium acnes in BALB/c mice infected with Schistosoma mansoni. Parasitol. Int. 86. doi: 10.1016/j.parint.2021.102446
Lambertucci, J. R., Fidelis, T. A., Pereira, T. A., Coelho, P. M. Z., Araujo, N., de Souza, M. M., et al. (2014). Brain schistosomiasis in mice experimentally infected with Schistosoma mansoni. Rev. Soc. Bras. Med. Trop. 47. doi: 10.1590/0037-8682-0083-2013
Layland, L. E., Rad, R., Wagner, H., Prazeres da Costa, C. U. (2007). Immunopathology in schistosomiasis is controlled by antigen-specific regulatory T cells primed in the presence of TLR2. Eur. J. Immunol. 37. doi: 10.1002/eji.200737063
Lee, H., Chae, S., Kim, J. Y., Han, W., Kim, J., Choi, Y., et al. (2019). Cell-printed 3D liver-on-a-chip possessing a liver microenvironment and biliary system. Biofabrication 11. doi: 10.1088/1758-5090/aaf9fa
Le Guen, L., Marchal, S., Faure, S., De Santa Barbara, P. (2015). Mesenchymal-epithelial interactions during digestive tract development and epithelial stem cell regeneration. Cell. Mol. Life Sci. 72.
Lenoir, S., Lahaye, R. A., Vitet, H., Scaramuzzino, C., Virlogeux, A., Capellano, L., et al. (2022). Pridopidine rescues BDNF/TrkB trafficking dynamics and synapse homeostasis in a Huntington disease brain-on-a-chip model. Neurobiol. Dis. 173. doi: 10.1016/j.nbd.2022.105857
Lombardo, F. C., Pasche, V., Panic, G., Endriss, Y., Keiser, J. (2019). Life cycle maintenance and drug-sensitivity assays for early drug discovery in Schistosoma mansoni. Nat. Protoc. 14. doi: 10.1038/s41596-018-0101-y
Lu, S., Cuzzucoli, F., Jiang, J., Liang, L. G., Wang, Y., Kong, M., et al. (2018). Development of a biomimetic liver tumor-on-a-chip model based on decellularized liver matrix for toxicity testing. Lab. Chip. 18, 3379. doi: 10.1039/C8LC00852C
Ma, C., Witkowski, M. T., Harris, J., Dolgalev, I., Sreeram, S., Qian, W., et al. (2020). Leukemia-on-a-chip: Dissecting the chemoresistance mechanisms in B cell acute lymphoblastic leukemia bone marrow niche. Sci. Adv. 6.
MacDonald, A. S., Pearce, E. J. (2002). Cutting edge: polarized th cell response induction by transferred antigen-pulsed dendritic cells is dependent on IL-4 or IL-12 production by recipient cells. J. Immunol. 168. doi: 10.4049/jimmunol.168.7.3127
Maciel, L. F., Morales-Vicente, D. A., Silveira, G. O., Ribeiro, R. O., Olberg, G. G. O., Pires, D. S., et al. (2019). Weighted Gene Co-Expression Analyses Point to Long Non-Coding RNA Hub Genes at Different Schistosoma mansoni Life-Cycle Stages. Front. Genet. 10. doi: 10.3389/fgene.2019.00823
Mannino, R. G., Qiu, Y., Lam, W. A. (2018). Endothelial cell culture in microfluidic devices for investigating microvascular processes. Biomicrofluidics 12. doi: 10.1063/1.5024901
McManus, D. P., Dunne, D. W., Sacko, M., Utzinger, J., Vennervald, B. J., Zhou, X. N. (2018). Schistosomiasis. Nat. Rev. Dis. Primers 4, 13. doi: 10.1038/s41572-018-0013-8
Mebius, M. M., van Genderen, P. J. J., Urbanus, R. T., Tielens, A. G. M., de Groot, P. G., van Hellemond, J. J. (2013). Interference with the host haemostatic system by schistosomes. PloS Pathog. 9. doi: 10.1371/journal.ppat.1003781
Melkus, M. W., Le, L., Siddiqui, A. J., Molehin, A. J., Zhang, W., Lazarus, S., et al. (2020). Elucidation of cellular responses in non-human primates with chronic schistosomiasis followed by praziquantel treatment. Front. Cell Infect. Microbiol. 10. doi: 10.3389/fcimb.2020.00057
Molehin, A. J., Gray, S. A., Turner, C., Davis, J., Zhang, W., Khatoon, S., et al. (2021). Process development of sj-p80: A low-cost transmission-blocking veterinary vaccine for asiatic schistosomiasis. Front. Immunol. 11. doi: 10.3389/fimmu.2020.578715
Morimoto, Y., Mori, S., Sakai, F., Takeuchi, S. (2016). Human induced pluripotent stem cell-derived fiber-shaped cardiac tissue on a chip. Lab. Chip. 16. doi: 10.1039/C6LC00422A
Mossallam, S. F., Abou-El-naga, I. F., Bary, A. A., Elmorsy, E. A., Diab, R. G. (2021). Schistosoma mansoni egg-derived extracellular vesicles: A promising vaccine candidate against murine schistosomiasis. PloS Negl. Trop. Dis. 15. doi: 10.1371/journal.pntd.0009866
Moustafa, F. E., Hamouda, M. M., el-Shazly, A. M., Sheha, G., Hamid, S., el-Naggar, H. M., et al. (1996). Effect of anti-schistosomal treatment on schistosomal hepatic pathology: an experimental study in Syrian golden hamsters. J. Egypt Soc. Parasitol. 26.
Mpotje, T. (2017). The role of BATF2 during of experimental murine Schistosomiasis. Available online at: https://api.semanticscholar.org/CorpusID:91810696 (Accessed February 06, 2025).
Mu, Y., McManus, D. P., Hou, N., Cai, P. (2021). Schistosome infection and schistosome-derived products as modulators for the prevention and alleviation of immunological disorders. Front. Immunol. 12. doi: 10.3389/fimmu.2021.619776
Muchirah, P. N. (2012). Determination of effective praziquantel dose in different mouse strains: BALB/c and Swiss mice in treatment of Schistosoma mansoni. J. Clin. Immunol. Immunopathol. Res. 4.
Müller, B., Sulzer, P., Walch, M., Zirath, H., Buryška, T., Rothbauer, M., et al. (2021). Measurement of respiration and acidification rates of mammalian cells in thermoplastic microfluidic devices. Sens Actuators B Chem. 334. doi: 10.1016/j.snb.2021.129664
Mutuku, M. W., Lu, L., Otiato, F. O., Mwangi, I. N., Kinuthia, J. M., Maina, G. M., et al. (2017). A Comparison of Kenyan Biomphalaria pfeifferi and B. Sudanica as Vectors for Schistosoma mansoni, Including a Discussion of the Need to Better Understand the Effects of Snail Breeding Systems on Transmission. J. Parasitol. 103.
Nasello, G., Cóndor, M., Vaughan, T., Schiavi, J. (2021). Designing hydrogel-based bone-on-chips for personalized medicine. Appl. Sci. (Switzerland) 11. doi: 10.3390/app11104495
Nelson, M. R., Ghoshal, D., Mejías, J. C., Rubio, D. F., Keith, E., Roy, K. (2021). A multi-niche microvascularized human bone marrow (hBM) on-a-chip elucidates key roles of the endosteal niche in hBM physiology. Biomaterials 270. doi: 10.1016/j.biomaterials.2021.120683
Nguyen, K. H., Boersch-Supan, P. H., Hartman, R. B., Mendiola, S. Y., Harwood, V. J., Civitello, D. J., et al. (2021). Interventions can shift the thermal optimum for parasitic disease transmission. Proc. Natl. Acad. Sci. U.S.A. 118. doi: 10.1073/pnas.2017537118
Nono, J. K., Fu, K., Mpotje, T., Varrone, G., Aziz, N. A., Mosala, P., et al. (2020). Investigating the antifibrotic effect of the antiparasitic drug Praziquantel in in vitro and in vivo preclinical models. Sci. Rep. 10.
Nyakundi, R. K., Hau, J., Ogongo, P., Nyamongo, O., Jeneby, M., Akinyi, M., et al. (2022). Acquired clinical immunity to malaria in nonhuman primates coinfected with schistosoma and plasmodium parasites. Infect. Immun. 90. doi: 10.1128/iai.00464-21
Nyakundi, R. K., Nyamongo, O., Maamun, J., Akinyi, M., Mulei, I., Farah, I. O., et al. (2016). Protective effect of chronic schistosomiasis in baboons coinfected with Schistosoma mansoni and Plasmodium knowlesi. Infect. Immun. 84. doi: 10.1128/IAI.00490-15
Nyindo, M., Farah, I. O. (1999). The baboon as a non-human primate model of human schistosome infection. Parasitol. Today 15. doi: 10.1016/S0169-4758(99)01569-0
Oloris, S. C. S., Mesnil, M., de Souza Reis, V. N., Sakai, M., Matsuzaki, P., de Souza Monteiro Fonseca, E., et al. (2007). Hepatic granulomas induced by Schistosoma mansoni in mice deficient for connexin 43 present lower cell proliferation and higher collagen content. Life Sci. 80, 1228–1235. doi: 10.1016/j.lfs.2006.12.030
Padalino, G., El-Sakkary, N., Liu, L. J., Liu, C., Harte, D. S. G., Barnes, R. E., et al. (2021). Anti-schistosomal activities of quinoxaline-containing compounds: From hit identification to lead optimisation. Eur. J. Med. Chem. 226. doi: 10.1016/j.ejmech.2021.113823
Page-McCaw, A., Ewald, A. J., Werb, Z. (2007). Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 8, 221–233. doi: 10.1038/nrm2125
Pearce, E. J. (2005). Priming of the immune response by schistosome eggs. Parasite Immunol. 27. doi: 10.1111/j.1365-3024.2005.00765.x
Pearce, E. J., MacDonald, A. S. (2002). The immunobiology of schistosomiasis. Nat. Rev. Immunol. 2. doi: 10.1038/nri843
Pearce, E. J., McLaren, D. J. (1983). Schistosoma mansoni: in vivo and in vitro studies of immunity using the Guinea-pig model. Parasitology 87. doi: 10.1017/S0031182000082998
Pearson, M. S., Becker, L., Driguez, P., Young, N. D., Gaze, S., Mendes, T., et al. (2015). Of monkeys and men: Immunomic profiling of sera from humans and non-human primates resistant to schistosomiasis reveals novel potential vaccine candidates. Front. Immunol. 6. doi: 10.3389/fimmu.2015.00213
Pereira, R. V., Gomes, M. D. S., Olmo, R. P., Souza, D. M., Cabral, F. J., Jannotti-Passos, L. K., et al. (2015). Ubiquitin-specific proteases are differentially expressed throughout the Schistosoma mansoni life cycle. Parasit Vectors 8. doi: 10.1186/s13071-015-0957-4
Phan, P., Liang, D., Zhao, M., Wyeth, R. C., Fogarty, C., Duke, M. G., et al. (2022). Analysis of rhodopsin G protein-coupled receptor orthologs reveals semiochemical peptides for parasite (Schistosoma mansoni) and host (Biomphalaria glabrata) interplay. Sci. Rep. 12.
Pica-Mattoccia, L., Cioli, D. (2004). Sex- and stage-related sensitivity of Schistosoma mansoni to in vivo and in vitro praziquantel treatment. Int. J. Parasitol. 34. doi: 10.1016/j.ijpara.2003.12.003
Pinto, H. A., Assis, J. C. A., Silva, B. C. M., Gonçalves, N. Q., Melo, A. L. (2019). Zygocotyle lunata as a model for in vivo screening of anthelmintic activity against paramphistomes: Evaluation of efficacy of praziquantel, albendazole and closantel in experimentally infected mice. Exp. Parasitol. 199. doi: 10.1016/j.exppara.2019.02.007
Quan, Q., Weng, D., Li, X., An, Q., Yang, Y., Yu, B., et al. (2022). Analysis of drug efficacy for inflammatory skin on an organ-chip system. Front. Bioeng. Biotechnol. 10. doi: 10.3389/fbioe.2022.939629
Queiroz, F. R., Silva, L. M., De Jesus Jeremias, W., Hideo Babá, É., Caldeira, R. L., Coelho, P. M. Z., et al. (2017). Differential expression of small RNA pathway genes associated with the Biomphalaria glabrata/Schistosoma mansoni interaction. PloS One 12. doi: 10.1371/journal.pone.0181483
Rahman, S., Kwee, B., Li, M., Chidambaram, M., He, X., Bryant, M., et al. (2024). Evaluation of a microphysiological human placental barrier model for studying placental drug transfer. Reprod. Toxicol. 123. doi: 10.1016/j.reprotox.2023.108523
Reinholdt, C., Winkelmann, F., Koslowski, N., Reisinger, E. C., Sombetzki, M. (2023). Unisexual infection with Schistosoma mansoni in mice has the potential to boost the immune response against eggs after challenge infection. Front. Immunol. 14. doi: 10.3389/fimmu.2023.1125912
Rockey, D. C., Weymouth, N., Shi, Z. (2013). Smooth muscle α actin (Acta2) and myofibroblast function during hepatic wound healing. PloS One 8. doi: 10.1371/journal.pone.0077166
Ross, A. G. P., Bartley, P. B., Sleigh, A. C., Olds, G. R., Li, Y., Williams, G. M., et al. (2002). Schistosomiasis. N Engl. J. Med. 346, 1212–1220. doi: 10.1056/NEJMra012396
Rumbley, C. A., Zekavat, S. A., Sugaya, H., Perrin, P. J., Ramadan, M. A., Phillips, S. M. (1998). The schistosome granuloma: characterization of lymphocyte migration, activation, and cytokine production. J. Immunol. 161. doi: 10.4049/jimmunol.161.8.4129
Sadun, E. H., Von Lichtenberg, F., Hickman, R. L., Bruce, J. I., Smith, J. H., Schoenbechler, M. J. (1966). Schistosomiasis mansoni in the chimpanzee: parasitologic, clinical, serologic, pathologic and radiologic observations. Am. J. Trop. Med. Hyg. 15. doi: 10.4269/ajtmh.1966.15.496
Sahai, E., Astsaturov, I., Cukierman, E., DeNardo, D. G., Egeblad, M., Evans, R. M., et al. (2020). A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 20. doi: 10.1038/s41568-019-0238-1
Salama, W. M., El-Naggar, S. A., Harras, S. F., El-Said, K. S. (2021). An adjuvant effect of metformin as an anti-fibrotic agent when administered with the anti-schistosomal praziquantel in schistosoma mansoni infected mice. Trop. Biomed. 38.
Sanches, R. C. O., Souza, C., Marinho, F. V., Mambelli, F. S., Morais, S. B., Guimarães, E. S., et al. (2020). NLRP6 plays an important role in early hepatic immunopathology caused by schistosoma mansoni infection. Front. Immunol. 11. doi: 10.3389/fimmu.2020.00795
Sarin, S. K., Mosca, P., Sabbà, C., Groszmann, R. J. (1991). Hyperdynamic circulation in a chronic murine schistosomiasis model of portal hypertension. Hepatology 13. doi: 10.1002/hep.1840130329
Schneider, O., Moruzzi, A., Fuchs, S., Grobel, A., Schulze, H. S., Mayr, T., et al. (2022). Fusing spheroids to aligned μ-tissues in a heart-on-chip featuring oxygen sensing and electrical pacing capabilities. Mater Today Bio 15. doi: 10.1016/j.mtbio.2022.100280
Schwartz, C., Fallon, P. G. (2018). Schistosoma “Eggs-iting” the host: Granuloma formation and egg excretion. Front. Immunol. 9. doi: 10.3389/fimmu.2018.02492
Sengupta, M. E., Hellström, M., Kariuki, H. C., Olsen, A., Thomsen, P. F., Mejer, H., et al. (2019). Environmental DNA for improved detection and environmental surveillance of schistosomiasis. Proc. Natl. Acad. Sci. U.S.A. 116. doi: 10.1073/pnas.1815046116
Shah, D., Dave, B., Chorawala, M. R., Prajapati, B. G., Singh, S., Elossaily, G. M., et al. (2024). An insight on microfluidic organ-on-a-chip models for PM2.5-induced pulmonary complications. ACS Omega 9.
Shah, U. K., de Oliveira Mallia, J., Singh, N., Chapman, K. E., Doak, S. H., Jenkins, G. J. S. (2018). A three-dimensional in vitro HepG2 cells liver spheroid model for genotoxicity studies. Mutat. Res. Genet. Toxicol. Environ. Mutagen 825. doi: 10.1016/j.mrgentox.2017.12.005
Shariati, F., Pérez-Arellano, J. L., Carranza, C., López-Abán, J., Vicente, B., Arefi, M., et al. (2011). Evaluation of the role of angiogenic factors in the pathogenesis of schistosomiasis. Exp. Parasitol. 128. doi: 10.1016/j.exppara.2011.01.016
Shen, J., Yu, S. F., Peng, M., Lai, D. H., Hide, G., Wu, Z. D., et al. (2022). iNOS is essential to maintain a protective Th1/Th2 response and the production of cytokines/chemokines against Schistosoma japonicum infection in rats. PloS Negl. Trop. Dis. 16. doi: 10.1371/journal.pntd.0010403
Singh, K. P., Gerard, H. C., Hudson, A. P., Boros, D. L. (2004). Dynamics of collagen, MMP and TIMP gene expression during the granulomatous, fibrotic process induced by Schistosoma mansoni eggs. Ann. Trop. Med. Parasitol. 98.
Singh, K. P., Gerard, H. C., Hudson, A. P., Boros, D. L. (2006). Differential expression of collagen, MMP, TIMP and fibrogenic-cytokine genes in the granulomatous colon of Schistosoma mansoni-infected mice. Ann. Trop. Med. Parasitol. 100, 611–620. doi: 10.1179/136485906X118530
Singh, D., Mathur, A., Arora, S., Roy, S., Mahindroo, N. (2022). Journey of organ on a chip technology and its role in future healthcare scenario. Appl. Surface Sci. Advances 9. doi: 10.1016/j.apsadv.2022.100246
Sulbarán, G., Noya, O., Brito, B., Ballén, D. E., Cesari, I. M. (2013). Immunoprotection of mice against schistosomiasis mansoni using solubilized membrane antigens. PloS Negl. Trop. Dis. 7. doi: 10.1371/journal.pntd.0002254
Sun, G., Ding, B., Wan, M., Chen, L., Jackson, J., Atala, A. (2020). Formation and optimization of three-dimensional organoids generated from urine-derived stem cells for renal function in vitro. Stem Cell Res. Ther. 11. doi: 10.1186/s13287-020-01822-4
Sun, S., Jin, L., Zheng, Y., Zhu, J. (2022). Modeling human HSV infection via a vascularized immune-competent skin-on-chip platform. Nat. Commun. 13. doi: 10.1038/s41467-022-33114-1
Taavitsainen, S., Juuti-Uusitalo, K., Kurppa, K., Lindfors, K., Kallio, P., Kellomäki, M. (2024). Gut-on-chip devices as intestinal inflammation models and their future for studying multifactorial diseases. Front. Lab. Chip Technol. 2. doi: 10.3389/frlct.2023.1337945
Takaki, K. K., Rinaldi, G., Berriman, M., Pagán, A. J., Ramakrishnan, L. (2021). Schistosoma mansoni eggs modulate the timing of granuloma formation to promote transmission. Cell Host Microbe 29. doi: 10.1016/j.chom.2020.10.002
Tanaka, T. (2012). Development of an inflammation-associated colorectal cancer model and its application for research on carcinogenesis and chemoprevention. Int. J. Inflammation 2012. doi: 10.1155/2012/658786
Tang, X., Karuna, I., Quack, T., Grevelding, C., Kadesch, P., Roderfeld, M., et al. (2020). Schistosoma mansoni eggs modulate lipid metabolism in hepatocytes. 36 Jahrestagung der Deutschen Arbeitsgemeinschaft zum Studium der Leber.
Tedla, M. G., Every, A. L., Scheerlinck, J. P. Y. (2022). Measuring the manipulation of T helper immune responses by schistosoma mansoni. Int. J. Mol. Sci. 23. doi: 10.3390/ijms23031462
Teixeira de Melo, T., Araujo, J. M., Campos de Sena, I., Carvalho Alves, C., Araujo, N., Toscano Fonseca, C. (2013). Evaluation of the protective immune response induced in mice by immunization with Schistosoma mansoni schistosomula tegument (Smteg) in association with CpG-ODN. Microbes Infect. 15.
Tiggelman, A. M., Boers, W., Moorman, A. F., de Boer, P. A. J., van der Loos, C. M., Rotmans, J. P., et al. (1996). Localization of alpha 2-macroglobulin protein and messenger RNA in rat liver fibrosis: Evidence for the synthesis of alpha 2-macroglobulin withinSchistosoma mansoni egg granulomas. Hepatology 23, 1260–1267.
Torisawa, Y. S., Spina, C. S., Mammoto, T., Mammoto, A., Weaver, J. C., Tat, T., et al. (2014). Bone marrow-on-a-chip replicates hematopoietic niche physiology in vitro. Nat. Methods 11. doi: 10.1038/nmeth.2938
van der Helm, M. W., van der Meer, A. D., Eijkel, J. C. T., van den Berg, A., Segerink, L. I. (2016). Microfluidic organ-on-chip technology for blood-brain barrier research. Tissue Barriers 4. doi: 10.1080/21688370.2016.1142493
Wang, B., Lee, J., Li, P., Saberi, A., Yang, H., Liu, C., et al. (2018). Stem cell heterogeneity drives the parasitic life cycle of Schistosoma Mansoni. Elife 7. doi: 10.7554/eLife.35449
Wang, T., Zhao, M., Rotgans, B. A., Strong, A., Liang, D., Ni, G., et al. (2016). Proteomic analysis of the schistosoma mansoni miracidium. PloS One 11. doi: 10.1371/journal.pone.0147247
Warren, K. S. (1966). The pathogenesis of “clay-pipe stem cirrhosis” in mice with chronic schistosomiasis mansoni, with a note on the longevity of the schistosomes. Am. J. Pathol. 49.
Wikswo, J. P. (2014). The relevance and potential roles of microphysiological systems in biology and medicine. Exp. Biol. Med. 239. doi: 10.1177/1535370214542068
World Health Organization (2023). “Schistosomiasis,” in Schistosoma: Biology, Pathology and Control.
Wu, Q., Liu, J., Wang, X., Feng, L., Wu, J., Zhu, X., et al. (2020). Organ-on-a-chip: recent breakthroughs and future prospects. BioMed. Eng. Online 19, 9. doi: 10.1186/s12938-020-0752-0
Wu, Z., Wang, L., Li, J., Wang, L., Wu, Z., Sun, X. (2019). Extracellular vesicle-mediated communication within host-parasite interactions. Front. Immunol. 10. doi: 10.3389/fimmu.2018.03066
Wufuer, M., Lee, G., Hur, W., Jeon, B., Kim, B. J., Choi, T. H., et al. (2016). Skin-on-a-chip model simulating inflammation, edema and drug-based treatment. Sci. Rep. 6, 37471. doi: 10.1038/srep37471
Wynn, T. A., Cheever, A. W. (1995). Cytokine regulation of granuloma formation in schistosomiasis. Curr. Opin. Immunol. 7. doi: 10.1016/0952-7915(95)80095-6
Wynn, T. A., Thompson, R. W., Cheever, A. W., Mentink-Kane, M. M. (2004). Immunopathogenesis of schistosomiasis. Immunol. Rev. 201. doi: 10.1111/j.0105-2896.2004.00176.x
Xian, C., Zhang, J., Zhao, S., Li, X. G. (2023). Gut-on-a-chip for disease models. J. Tissue Eng. 14. doi: 10.1177/20417314221149882
Yang, J., Hirai, Y., Iida, K., Ito, S., Trumm, M., Terada, S., et al. (2023). Integrated-gut-liver-on-a-chip platform as an in vitro human model of non-alcoholic fatty liver disease. Commun. Biol. 6. doi: 10.1038/s42003-023-04710-8
Yeh, Y. T., del Álamo, J. C., Caffrey, C. R. (2024). Biomechanics of parasite migration within hosts. Trends Parasitol. 40. doi: 10.1016/j.pt.2023.12.001
Yeh, Y. T., Skinner, D. E., Criado-Hidalgo, E., Chen, N. S., De Herreros, A. G., El-Sakkary, N., et al. (2022). Biomechanical interactions of Schistosoma mansoni eggs with vascular endothelial cells facilitate egg extravasation. PloS Pathog. 18. doi: 10.1371/journal.ppat.1010309
Yin, F., Zhu, Y., Zhang, M., Yu, H., Chen, W., Qin, J. (2019). A 3D human placenta-on-a-chip model to probe nanoparticle exposure at the placental barrier. Toxicol. Vitro 54. doi: 10.1016/j.tiv.2018.08.014
Yole, D. S. (1996). Protective immunity to Schistosoma mansoni induced in the olive baboon Papio anubis by the irradiated cercaria vaccine. Parasitology 112. doi: 10.1017/S0031182000065057
Yu, Y., Deng, W., Lei, J. (2015). Interleukin-33 promotes Th2 immune responses in infected mice with Schistosoma japonicum. Parasitol. Res. 114. doi: 10.1007/s00436-015-4492-1
Zandi Shafagh, R., Youhanna, S., Keulen, J., Shen, J. X., Taebnia, N., Preiss, L. C., et al. (2022). Bioengineered pancreas–liver crosstalk in a microfluidic coculture chip identifies human metabolic response signatures in prediabetic hyperglycemia. Advanced Sci. 9.
Zhang, W., Ahmad, G., Molehin, A. J., Torben, W., Le, L., Kim, E., et al. (2018). Schistosoma mansoni antigen Sm-p80: Prophylactic efficacy using TLR4 agonist vaccine adjuvant glucopyranosyl lipid A-Alum in murine and non-human primate models. J. Invest. Med. 66. doi: 10.1136/jim-2018-000786
Zhang, F., Qu, K. Y., Zhou, B., Luo, Y., Zhu, Z., Pan, D. J., et al. (2021). Design and fabrication of an integrated heart-on-a-chip platform for construction of cardiac tissue from human iPSC-derived cardiomyocytes and in situ evaluation of physiological function. Biosens. Bioelectron. 179. doi: 10.1016/j.bios.2021.113080
Zhang, W., Zhu, J., Song, X., Xu, Z., Xue, X., Chen, X., et al. (2015). An association of Aquaporin-4 with the immunoregulation of liver pathology in mice infected with Schistosoma japonicum. Parasit Vectors 8. doi: 10.1186/s13071-015-0650-7
Zhu, X., Chen, L., Wu, J., Tang, H., Wang, Y. (2017). Salmonella typhimurium Infection Reduces Schistosoma japonicum Worm Burden in Mice. Sci. Rep. 7.
Keywords: schistosomiasis, egg extravasation, granuloma, organ-on-a-chip (OOC), 3D microphysiological systems (3D MPS), animal models
Citation: Alfred MO, Ochola L, Okeyo K, Bae E, Ogongo P, Odongo D, Njaanake K and Robinson JP (2025) Application of microphysiological systems to unravel the mechanisms of schistosomiasis egg extravasation. Front. Cell. Infect. Microbiol. 15:1521265. doi: 10.3389/fcimb.2025.1521265
Received: 01 November 2024; Accepted: 23 January 2025;
Published: 18 February 2025.
Edited by:
Sudhir Kumar, Iowa State University, United StatesReviewed by:
Alexander James Webb, Imperial College London, United KingdomEwert Linder, Karolinska Institutet (KI), Sweden
Copyright © 2025 Alfred, Ochola, Okeyo, Bae, Ogongo, Odongo, Njaanake and Robinson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martin Omondi Alfred, bWF0b2FsZnJlZEBnbWFpbC5jb20=; b21vbmRpbUBwcmltYXRlcmVzZWFyY2gub3Jn
 Martin Omondi Alfred
Martin Omondi Alfred Lucy Ochola
Lucy Ochola Kennedy Okeyo
Kennedy Okeyo Euiwon Bae
Euiwon Bae Paul Ogongo
Paul Ogongo David Odongo
David Odongo Kariuki Njaanake1
Kariuki Njaanake1 J. Paul Robinson
J. Paul Robinson