- 1Department of Periodontics, Stomatological Hospital, School of Stomatology, Southern Medical University, Guangzhou, China
- 2Department of Periodontics, Shenzhen Longgang Otolaryngology hospital, Shenzhen, China
- 3Department of Orthodontics, Stomatological Hospital, School of Stomatology, Southern Medical University, Guangzhou, China
- 4Department of Stomatology, The First People’s Hospital of Kashi, Kashi, China
Many systemic diseases, including Alzheimer disease (AD), diabetes mellitus (DM) and cardiovascular disease, are associated with microbiota dysbiosis. The oral and intestinal microbiota are directly connected anatomically, and communicate with each other through the oral-gut microbiome axis to establish and maintain host microbial homeostasis. In addition to directly, periodontal bacteria may also be indirectly involved in the regulation of systemic health and disease through the disturbed gut. This paper provides evidence for the role of periodontal bacteria in systemic diseases via the oral-gut axis and the far-reaching implications of maintaining periodontal health in reducing the risk of many intestinal and parenteral diseases. This may provide insight into the underlying pathogenesis of many systemic diseases and the search for new preventive and therapeutic strategies.
1 Introduction
As the largest microbial community in the human body, the gut microbiota plays a crucial role in establishing and maintaining host physiological homeostasis. A variety of human diseases are known to be associated with dysbiosis of the gut microbiota, such as obesity, cardiovascular disease and neurological disorders (Jin et al., 2019; Chen et al., 2021). The proposed theories of gut-liver axis, gut-brain axis, gut-lung axis, and gut-bone axis also fully illustrate the close relationship between the gut microbiota and various organs and systems in the human body (Cryan et al., 2019; Dang and Marsland, 2019; Albillos et al., 2020; Tu et al., 2021). The oral cavity is the second largest microbial habitat in the human body after the gastrointestinal tract (GIT). Studies have shown significant disease-specific patterns in the composition of the salivary microbiota of periodontitis patients (Lundmark et al., 2019). The dominant phyla included Firmicutes, Fusobacteria, Actinobacteria, Synergistes, Spirochetes, Proteobacteria, Saccharibacteria (TM7), and Bacteroidetes are highly associated with periodontal disease, contributing in part to the diversity and functional differences in the oral microbial community (Pei et al., 2020). The oral microbiota can be modified by systemic diseases with increased inflammation, such as diabetes mellitus (DM) and rheumatoid arthritis (RA), which consequently increase bacterial pathogenicity and susceptibility to periodontitis (Graves et al., 2019). Conversely, oral bacteria can impact systemic disease through bacteremia and has been linked to worsening of Alzheimer disease (AD), DM, cardiovascular disease and RA (Hajishengallis et al., 2022; Brewer et al., 2023). More interestingly, oral bacteria may indirectly affect these diseases by influencing the composition of the gut microbiota (Mesa et al., 2019; Sureda et al., 2020; Park et al., 2021; Barutta et al., 2022).
The oral cavity is directly connected to the GIT, and the progression of the ecological niche from the oral cavity to the gut has been defined as the “oral-gut microbiome axis” (hereafter referred to as the “oral-gut axis”). In addition to oral diseases, the oral microbiota is also involved in the regulation of extra-oral diseases through the oral-gut axis. The oral cavity is a microbial reservoir that constantly replenishes the gut microbiota (Schmidt et al., 2019). One study using 16S ribosomal RNA analysis provides evidence of widespread translocation of oral bacteria to the gut. After analyzing 144 pairs of saliva and stool samples, it was found that shared amplicon sequence variants between the salivary and gut microbiota were present in 72.9% of subjects, and that their total relative abundance in the gut was significantly higher in older subjects or those with dental plaque accumulation (Kurushima et al., 2023). Another large-scale controlled study of saliva and fecal samples taken separately from periodontally diseased/healthy subjects confirmed that periodontal status may indeed drive variations in the salivary and gut microbiota (Kurushima et al., 2023). Bao et al. further verified that periodontitis can induce intestinal dysbiosis and inflammation through the influx of salivary microbes by transplanting saliva from patients with periodontitis into mice via oral gavage (Bao et al., 2022). Furthermore, periodontal treatment may improve systemic health through the oral-gut axis further supports this concept and suggests target that periodontal therapy may be systemically useful by reducing the impact of the oral microbiota on intestinal bacteria. Analysis of stool and saliva samples from periodontitis patients using 16S ribosomal RNA gene amplicon sequencing confirmed that periodontal treatment both mitigated oral dysbiosis and altered gut microbial composition (Baima et al., 2024). Other evidence comes from studies examining a positive effect of periodontal therapy on the development of liver disease in cirrhotic patients by reducing bacterial dysbiosis in the feces (Bajaj et al., 2015; Bajaj et al., 2018). The need for a periodontal examination prior to liver transplantation has also been noted (Guggenheimer et al., 2007; Raghava et al., 2013).
How the oral and gut microbiomes interdependently regulate physiological functions to impact systemic health has not been fully investigated. In this paper, we provide potential mechanisms by which periodontal bacteria regulate extra-oral diseases via the oral-gut axis, and the far-reaching implications of maintaining periodontal health in reducing the risk of many intestinal and parenteral diseases.
2 Occurrence of periodontal pathogenic bacteria
Host–microbiome homeostasis in the oral cavity can be seen as an ‘armed peace’ that maintains a controlled state of inflammation. The transition from health to disease requires a susceptible host and a dysregulated oral microbiota. For susceptible host, as shown in diabetic mice, diabetes-enhanced IL-17 alters the oral microbiota and renders it more pathogenic (Xiao et al., 2017). On the contrary, local factors, such as poor oral hygiene, may induce oral microbial dysbiosis (e.g. altered proportions of coccobacilli), which ultimately leads to inflammation of the periodontal tissues (Lin et al., 2021). The ensuing increased flow of gingival crevicular fluid not only introduces a component of the host’s defenses, but also provides the substrate necessary for the growth of many ‘inflammophilic’ bacteria (i.e. those that thrive within an inflammatory environment, e.g. Porphyromonas gingivalis) (Hajishengallis, 2014). Inflammophilic bacteria actually gain a growth advantage over commensal microbiome in response to inflammation. With the aim of self-feeding, Inflammophilic bacteria develop a range of pathogenic strategies (e.g. affecting neutrophil functions) that gradually disrupt the ecological balance of the existing subgingival microbial community, leading to a more intense host response (Higashi et al., 2024).
The emergence of periodontal bacterial pathogenicity may be related to plaque biofilms attached to the surfaces of teeth, interdental spaces or restorations. Dental plaque represents the microbial community whose collective properties differ significantly from planktonic bacteria, which enhance the survival, metabolism and pathogenesis of oral microorganisms (Freire et al., 2021). Periodontitis-specific pathogenic bacteria, such as P.gingivalis, Treponema denticola, Filifactor alocis, Tannerella forsythia and Aggregatibacter actinomycetemcomitans, colonize and proliferate predominantly within the host periodontal pocket (Darveau et al., 2012). Three key factors may be involved in the induction of inflammation by periodontal pathogenic bacteria to promote periodontal tissue damage, including oral mucosal inflammation and alveolar bone destruction. The first is a change in the relative abundance of dominant species (e.g., inflammophilic bacteria). High levels of the genera Porphyromonas (32.2%), Fretibacterium (10.4%), Rothia (5.3%), and Filifactor (3.1%) were observed in periodontitis (Abusleme et al., 2021). Studies have shown that a very small proportion of Porphyromonas gingivalis in the community can orchestrate the normal benign microbiota into a dysbiotic community structure (Darveau et al., 2012). The second is related to the location of the plaque biofilm. In line with this view, Carrouel et al. noted that even in periodontally healthy young adults, the interdental space favors the development of periodontal disease as an ecological niche where microbial communities congregate (Carrouel et al., 2016). Compared to supragingival biofilms of other oral mucosa, the interdental biofilm is located between two teeth and the gingiva, where bacteria are in a more anaerobic environment. Due to its unusual anatomy, the body has few or no alternative defenses against the interdental space, and traditional methods of daily control (e.g. toothbrushing and saliva) are inadequate for biofilm disruption at this anatomical location (Carrouel et al., 2016). Quantitative real-time PCR assays have been applied to reflect microbial succession events in developing interdental biofilms, strongly suggesting that oral microbial dysbiosis is associated with the risk of periodontal and related diseases (Carrouel et al., 2016). The final factor is bacterial virulence. For example, gingipains are critical virulence factors for Porphyromonas gingivalis to colonize and proliferate in the gingival crevice and to invade the periodontium (Silva and Cascales, 2021). F. nucleatum mediates important biofilm-organizing behaviour and interactions with host cells through the expression of numerous adhesins (e.g. RadD) (Brennan and Garrett, 2019).
Because of the deleterious effects of periodontal pathogenic bacteria, there is increasing interest in whether daily antimicrobial strategies can prevent or reverse oral dysbiosis by altering the structure and function of the plaque biofilm, including healthy diet, oral hygiene, and the use of antimicrobials and probiotics. There have been studies showing changes in the oral microbiota associated with different dietary patterns, such as the amount of fermentable carbohydrates, fats, and anti-inflammatory/pro-inflammatory components, the degree of processing, and supplementation with nitrate (Anderson et al., 2020; Stanisic et al., 2021). An oral health optimized diet (low in carbohydrates, rich in Omega-3 fatty acids, and rich in vitamins C and D, antioxidants and fiber) has been found to reduce the load of potential cariogenic and periodontal bacterial species in the plaque biofilm, and even to reduce gingival and periodontal inflammation in humans (Tennert et al., 2020). However, some human studies have observed no clear relationship between diet and the composition of oral bacterial communities (Santonocito et al., 2022). Scholars holding this view believe that food is actually present in the mouth for a limited period of time and that the primary nutritional sources for oral bacteria appears to be saliva and gingival crevicular fluid. It has also been suggested that some dietary effects on the oral microbiota may occur indirectly by altering the gut microbiota. In conclusion, more research is still needed to elucidate whether healthy dietary patterns can prevent/reverse oral dysbiosis by altering the composition of oral microbiota/plaque biofilms.
In oral hygiene, the use of toothbrushes, interdental brushes, chewing gum, and even anti-plaque agents (e.g. chlorhexidine) are effective in reducing plaque or interrupting plaque maturation, but none of them seem to be able to influence the structure of the bacterial community in plaque. The main reason for this is the blocking effect of biofilms (Rosier et al., 2018). For example, although an anti-plaque agent blocks the formation of plaque, it has little activity against established plaque. The use of probiotics has the potential to prevent and/or treat oral and related diseases by reversing oral dysbiosis. Studies have shown that the addition of probiotics (e.g. Lactobacillus rhamnosus) does result in significant changes in the composition of oral bacterial communities, including an increase in microbial diversity and a decrease in the relative abundance of opportunistic pathogens (Di Stefano et al., 2023). It should be noted that this effect may be reversed after a period of cessation of treatment.
3 Possible pathogenic mechanisms of periodontal bacteria through the oral-gut axis
Microbial transmission between the oral cavity and the gut can shape and/or reshape the microbial ecosystem in both habitats, which in turn affects the overall microbial community of the organism. Multiple changes in the gut microbiota, intestinal barrier function and immune system induced by periodontal bacteria can lead to an increased risk of systemic diseases by promoting low-grade inflammation (Figure 1). Therefore, exploring the pathogenic role of periodontal bacteria through the oral-gut axis may help us to understand one of several avenues by which periodontitis may affect systemic diseases.
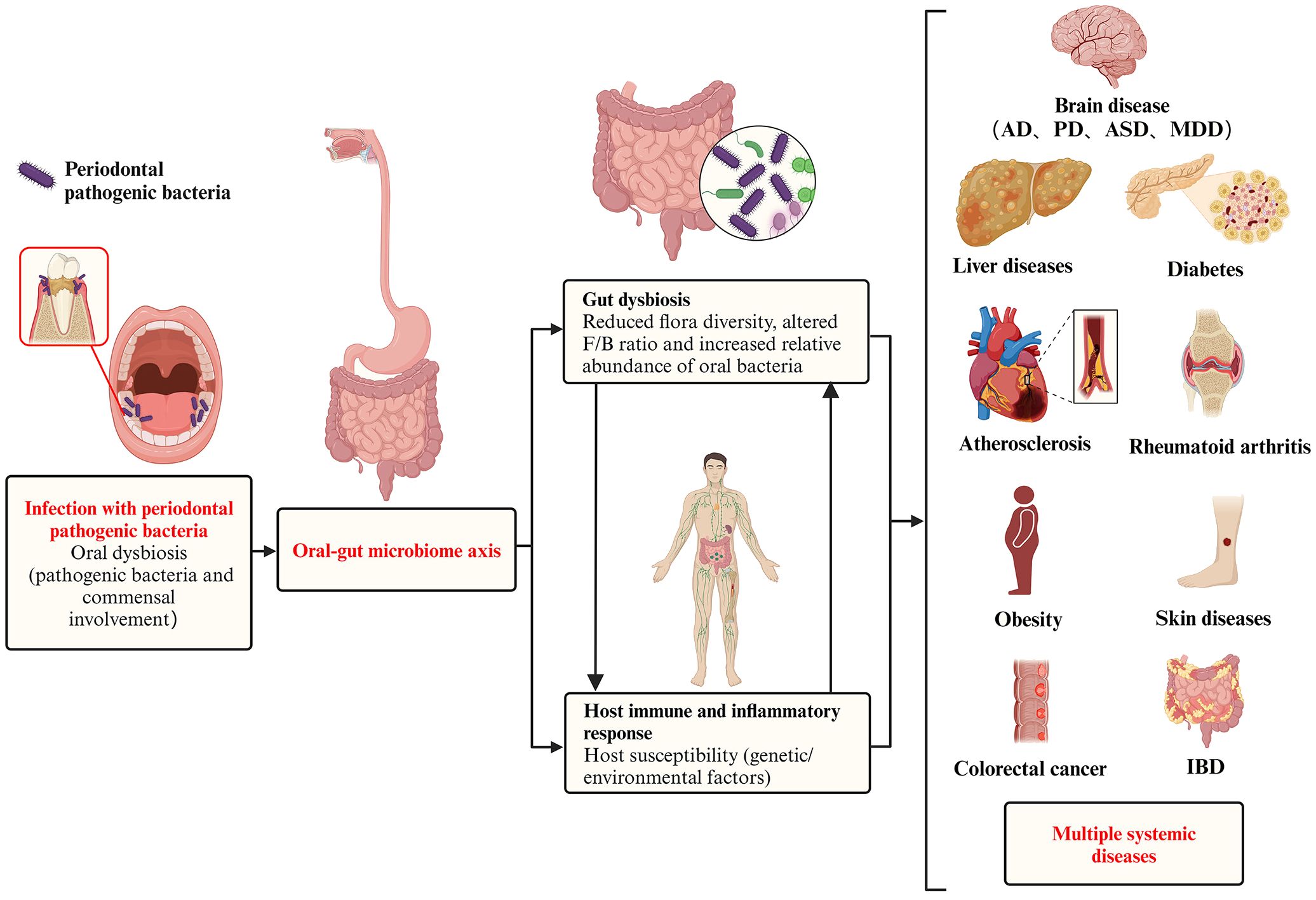
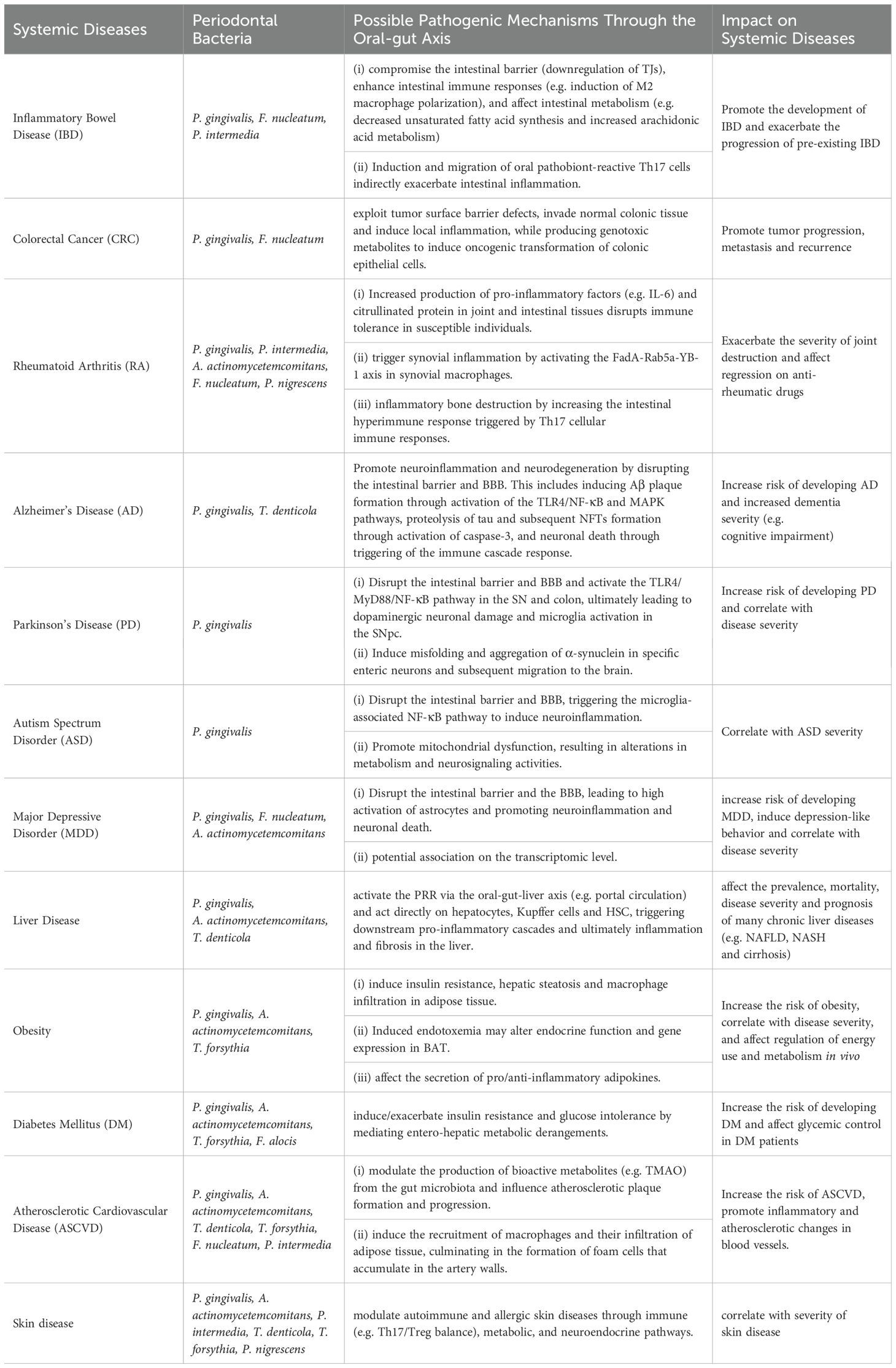
Figure 1. Pathogenic effect of periodontal pathogenic bacteria on systemic diseases via the oral-gut axis. In a pathogenic environment with excessive plaque accumulation, the dynamic equilibrium between microbial invasion and host defense is disrupted, and the periodontal pathogenic bacteria and commensal bacteria together give rise to oral dysbiosis and disease states. The oral cavity is directly connected to the gastrointestinal tract, and the progression of the ecological niche from the oral cavity to the gut has been defined as the ‘oral-gut microbiome axes. These periodontal pathogenic bacteria and their virulence products then breakthrough the barrier between the oral cavity and the gut and translocate in large numbers into the gut, causing gut dysbiosis, which is characterized by (i) a reduction in overall microbial community diversity, (ii) an alteration of the Firmicutes/Bacteroidetes (F/B) ratio, and (iii) as well as a reduction in probiotics and a corresponding increase in opportunistic pathogens. In addition to microbiota dysbiosis, the transition from health to disease requires a susceptible host (including genetic/environmental factors), accompanied by a complex set of interacting mechanisms that ultimately trigger destructive immune and inflammatory responses in the host. In addition to oral diseases, systemic diseases in which the oral microbiota may be involved in regulation via the oral-gut axis include intestinal diseases (e.g. inflammatory bowel disease and colorectal cancer), rheumatoid arthritis, brain diseases (e.g. Alzheimer’s disease, Parkinson’s disease, autism spectrum disorders, and major depressive disorder), liver diseases (e.g. non-alcoholic fatty liver disease, cirrhosis, and hepatocellular carcinoma), obesity, diabetes, atherosclerosis, and skin diseases.
The nature of the dysbiotic change that induces periodontitis has not been well defined and it has been difficult to distinguish between pathogenic and commensal bacteria. However, in the gut, dysbiosis has been identified as a reduction in alpha and beta diversity and an increase in bacterial pathogens, including a decrease in microbial population and functional diversity and stability (e.g. specific alterations in Peptococcaceae and Prevotellaceae), altered Firmicutes/Bacteroidetes (F/B) ratios, decreased abundance of beneficial SCFA-producing bacteria (e.g. Blautia, Roseburia, and Lachnospiraceae), and a corresponding increase in opportunistic pathogens (e.g. Megasphaera, Enterobacter, and Desulfovibrio) (Sultan et al., 2021; Chidambaram et al., 2022).
The gut originally is colonized by bacteria from the oral cavity or transit through the oral cavity. For example, Streptococcus salivarius and Streptococcus parasanguinis localized in the oral cavity most often colonize the intestinal niche (Kageyama et al., 2023). In addition, oral bacteria can alter the composition of the gut bacteria either directly or indirectly. In the intestine they may alter commensal bacteria through bacteria-bacteria interactions. For example, potential pathogens entering the gut can signal through quorum sensing to commensals and trigger the expression of toxins, virulence factors, and biofilm formation (Coquant et al., 2021). Periodontal bacteria (e.g. P. gingivalis) have been found to express a interspecies quorum sensing signal known as autoinducer-2 (AI-2), which modulates gut microbiota composition by enhancing Firmicutes growth and increasing the F/B ratio (Thompson et al., 2015; du Teil Espina et al., 2019).
A recent mouse study not only demonstrated that oral administration of P. gingivalis induced intestinal dysbiosis, reduced intestinal barrier function, and intestinal inflammation, but also further elucidated the pathological mechanisms behind the disruption of intestinal homeostasis by P. gingivalis through gut microbiota transplantation (Sohn et al., 2022). This study collected the gut microbiota of normal mice after oral administration of P. gingivalis and transferred it to genetically susceptible mice. The same intestinal inflammation was eventually observed in the recipient mice, verifying that P. gingivalis-induced intestinal dysbiosis is itself sufficient to promote intestinal inflammation (Figure 2).
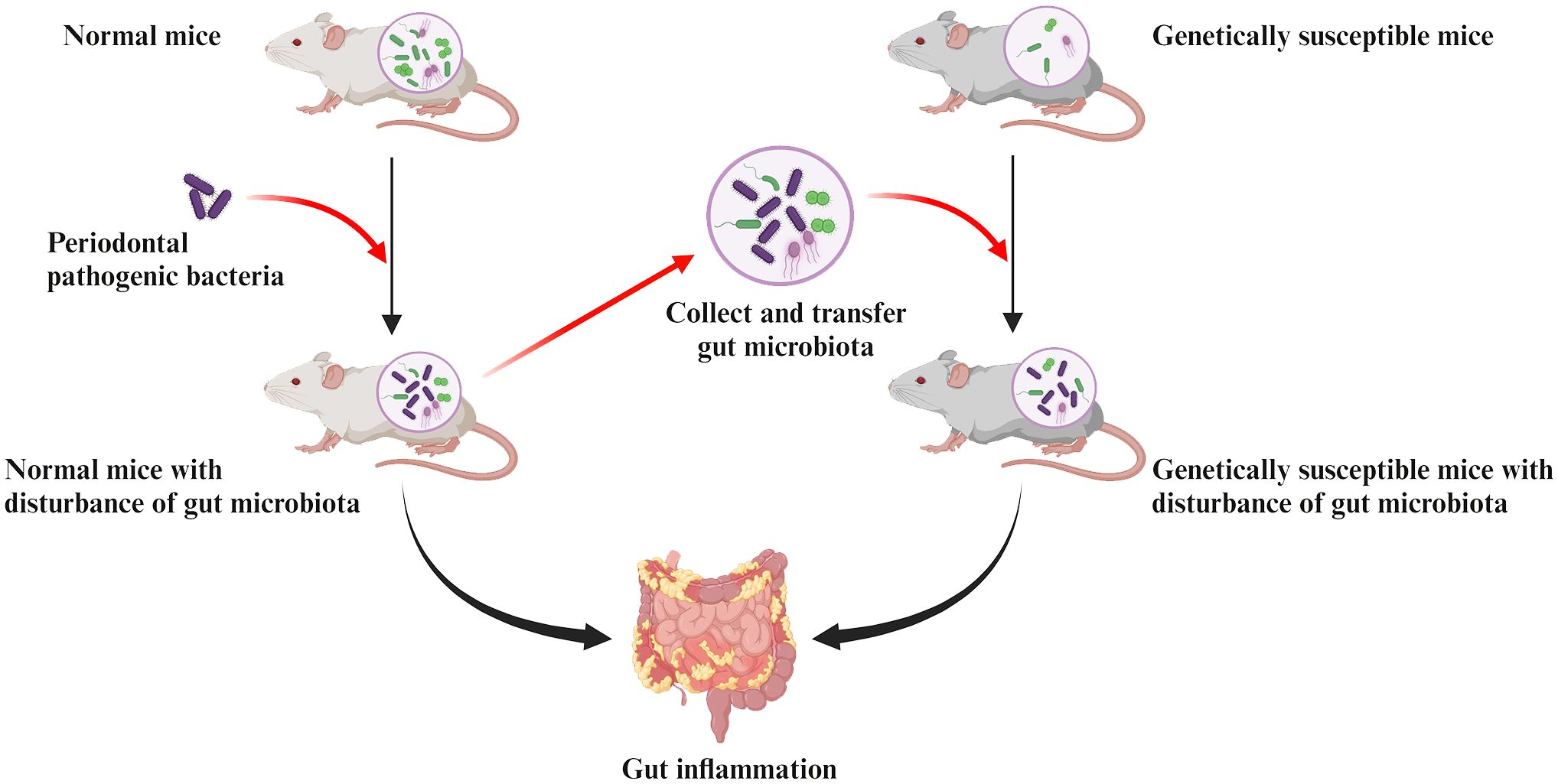
Figure 2. Pathologic mechanisms of intestinal inflammation induced by periodontal pathogenic bacteria are associated with the gut dysbiosis. Collecting the gut microbiota of normal mice after oral administration of periodontal pathogens and transferring it to genetically susceptible mice can observe the same intestinal inflammation in recipient mice. This confirms that the disruption of gut microbiota induced by periodontal pathogens is sufficient to cause their own intestinal inflammation.
3.1 Pathways of translocation of oral bacteria to the gut
More than half of the bacterial species in the gastrointestinal system undergo oral-gut translocation (Schmidt et al., 2019). First, the oral cavity and gut are inherently linked through saliva. Studies have demonstrated that periodontal bacteria migrating via enteral dissemination (salivary pathway) are able to colonize and survive in the gut for at least 24 hours (Bao et al., 2022). Second, the rich blood circulation in the oral cavity and the ulcerated surface of the lining of periodontal pockets in patients with periodontitis also allow the oral microbiota to colonize the gut through hematogenous dissemination. A study comparing colorectal cancer (CRC) colonization by gavage vs. intravenous inoculated Fusobacterium nucleatum in a mouse model and found that hematogenous fusobacteria were more successful in CRC colonization than gavaged ones. This may indicate that the circulatory system appears to be theefficient route for some periodontal bacteria to reach the gut. However, more evidence is still needed to support this.
Finally, after invasion of host cells, periodontal pathogenic bacteria (e.g. P. gingivalis and F. nucleatum) may survive within them and subsequently disseminate to the gut (Xue et al., 2018; Kitamoto and Kamada, 2022a). For example, periodontal bacteria with virulence factors that inhibit phagolysosome formation are able to survive within the host cell and migrate intracellularly via Trojan horse mode or vesicular trafficking (Yilmaz et al., 2004; Sansores-España et al., 2021). P. gingivalis has been shown to survive within macrophages, epithelial cells, endothelial cells and smooth muscle cells and to spread from one cell to another (Wang et al., 2007; Li et al., 2008; Carrion et al., 2012). Thus, theoretically, periodontal bacteria may hijack these cells as a vehicle for migration to the gut (Kitamoto et al., 2020; Hajishengallis and Chavakis, 2021). However, more evidence is still required to support that periodontal bacteria translocated via this pathway are of sufficient pathogenic significance in the oral-gut axis mediating systemic disease progression. In conclusion, enteral dissemination (the main pathway), hematogenous dissemination, and host cell hijacking are all possible ways in which the oral microbiota can translocate to the gut, resulting in a complex association of the oral-gut axis based on microbial crosstalk (Figure 3).
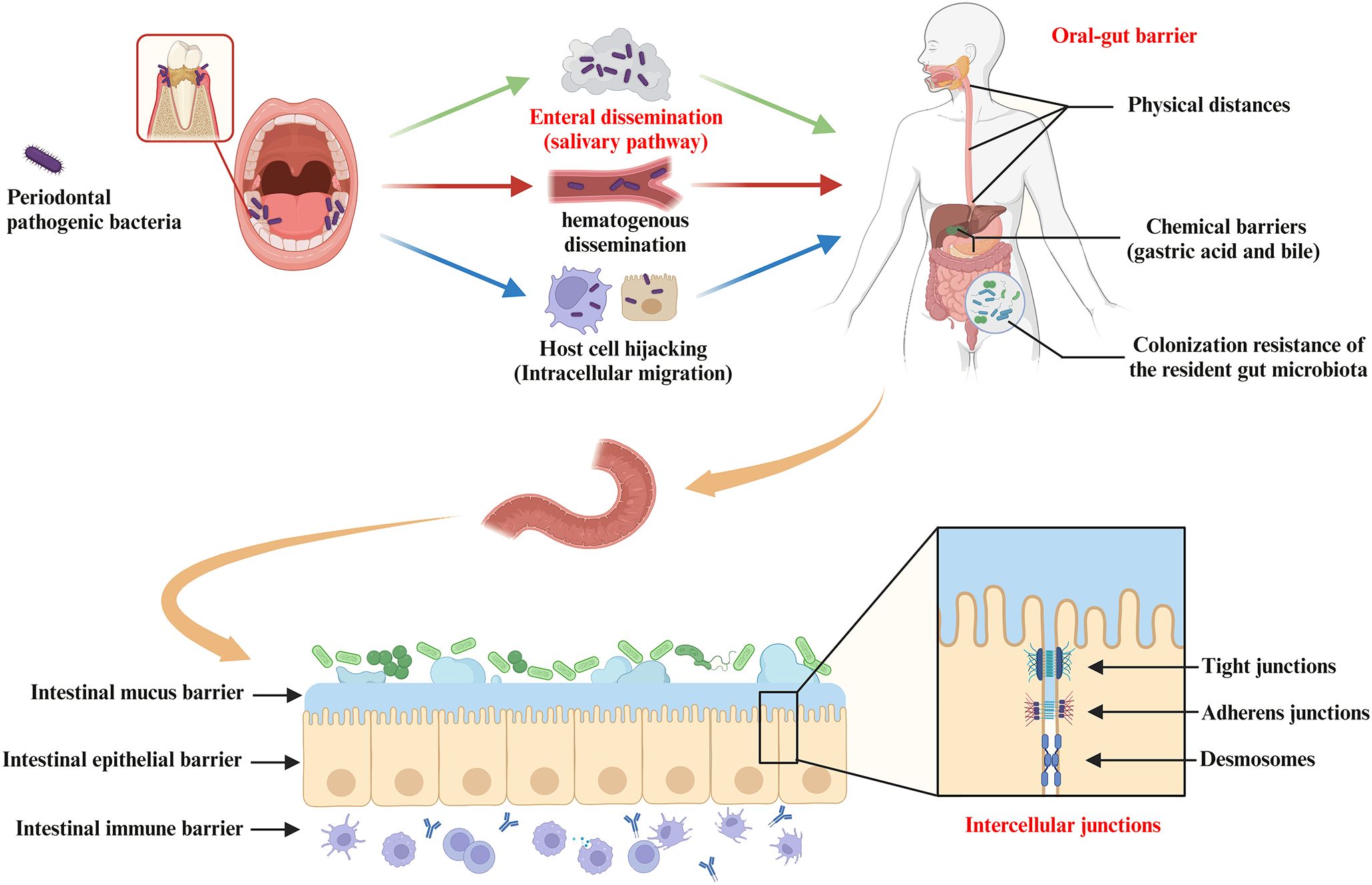
Figure 3. Pathways of oral bacterial translocation to the gut and host barrier systems limiting oral-gut microbial translocation. Intestinal translocation of periodontal pathogenic bacteria is pathogenically important in the progression of systemic diseases mediated by the oral-gut axis. Possible routes of oral microbiota translocation to the gut include (i) migration by enteral dissemination (salivary route), (ii) colonization of the gut by hematogenous dissemination, and (iii) hijacking of host cells as a vehicle for migration to the gut. First, the oral-gut barrier is one of the important strategies for the host to maintain microbial homeostasis in order to limit microbial translocation between the oral cavity and the gut. The oral-gut barrier includes physical distances and chemical barriers (e.g. gastric acid and bile), as well as colonization resistance of the resident gut microbiota to the intestinal migration of oral microbes with pathogenic potential. If oral bacteria break through the oral-gut barrier to reach the lumen, the barrier function of the intestinal wall is still able to separate the host from adjacent microbiota. The intestinal barrier consists of (i) the intestinal mucus barrier composed of mucin and antimicrobial peptides (AMPs) overlying IEC, (ii) the intestinal epithelial barrier composed of IEC and intercellular junctions (e.g. tight junctions, adherens junctions and desmosomes), and (iii) the intestinal immune barrier composed of innate and adaptive immune cells (gut-associated lymphoid tissue).
3.2 Breach of the oral-gut barrier by periodontal bacteria
The oral-gut barrier is one of the important strategies for the host to maintain microbial homeostasis in order to limit microbial translocation between the oral cavity and the gut. The oral-gut barrier includes physical distances and chemical barriers (e.g. gastric acid and bile), as well as colonization resistance of the resident gut microbiota to the intestinal migration of oral microbes with pathogenic potential (Figure 3). Intestinal commensal bacteria can play an important role in regulating intestinal mucosal homeostasis and inhibiting colonization of potential oral pathogens via distinct mechanisms (Suárez et al., 2021). For example, intestinal commensal bacteria can compete for nutrients and produce antimicrobial peptides and metabolites that affect the colonization and virulence of oral pathogens. Intestinal commensal bacteria also promote the induction of effector T and B cells locally and systemically in response to pathogens (Suárez et al., 2021). However, this protective mechanism of colonization resistance may be disturbed by microbiota imbalance or local changes in host response, leading to disruption of host-microbiome homeostasis.
3.3 Effect of periodontal bacteria on intestinal barrier function
The intestinal barrier has several physical and chemical barriers separating the host from the adjacent microbiota (Figure 3). However, when the intestinal barrier function is pathologically altered, increased intestinal permeability and inflammatory response may allow the gut to “leak”, i.e. allowing pathogenic substances to cross the intestinal wall and spread systemically with pathological consequences (Hollander and Kaunitz, 2020). Prolonged translocation of periodontal bacteria to the gut may further trigger and exacerbate the putative disease “leaky gut syndrome” by affecting gut microbiota homeostasis and barrier function, thus participating in the pathogenesis of various gastrointestinal and systemic diseases (Kinashi and Hase, 2021) (Figure 4). This will be discussed in more detail below.
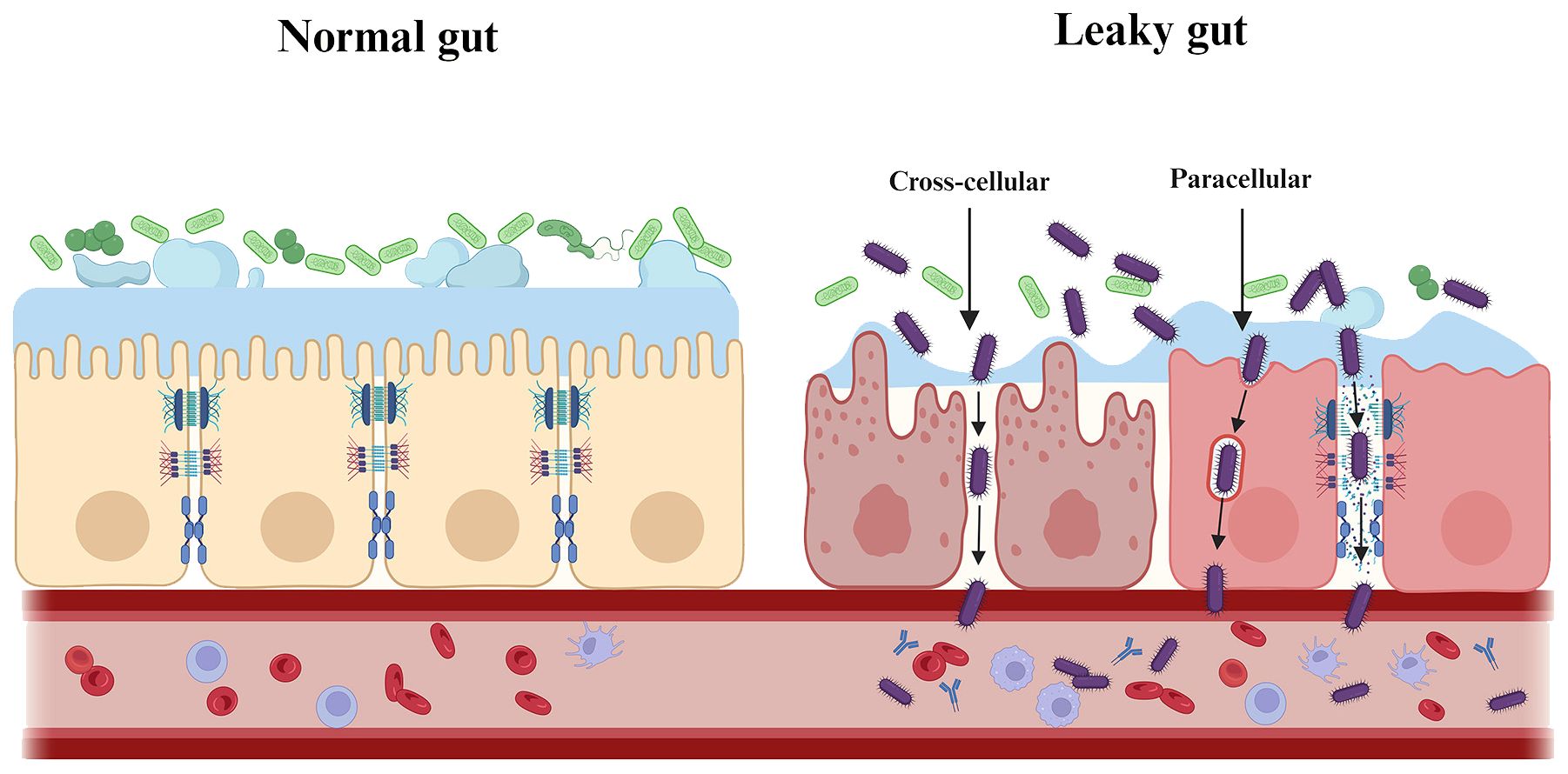
Figure 4. Periodontal pathogenic bacteria trigger and exacerbate “leaky gut syndrome”. Prolonged translocation of periodontal bacteria to the gut may further trigger and exacerbate the putative disease “leaky gut syndrome” by affecting gut microbiota homeostasis and barrier function. As shown, periodontal pathogenic bacteria are involved in the pathogenesis of various systemic diseases by either directly/indirectly reducing intestinal epithelial intercellular junctions (paracellular) or by invading intestinal epithelial cells (transcellular), which ultimately affects and breaches the intestinal barrier.
3.3.1 Intestinal mucus barrier
A gel-like sieve structure formed by mucin overlying intestinal epithelial cells (IECs) is the first physical barrier that separates bacteria in the lumen from the IEC (Pelaseyed et al., 2014). At the same time, antimicrobial peptides (AMPs) (e.g. alpha-defensins, lysozyme C and C-type lectins), which are components of the intestinal mucus, form a chemical barrier that prevents and clears intestinal pathogens and protects intestinal cells from external factors (Birchenough et al., 2015; Liu et al., 2021b).
Periodontal bacteria are capable of disrupting the integrity and function of the intestinal mucus barrier. For example, P. gingivalis secretes Gingipain B (RgpB) that cleaves mucin 2 (MUC2) to disrupt its polymerization (van der Post et al., 2013). P. gingivalis can also circumvent or manipulate AMP to disrupt intestinal homeostasis, further exacerbating intestinal ‘leakiness’ (Ji et al., 2007; Hussain et al., 2015).
3.3.2 Intestinal epithelial barrier
The intestinal epithelial barrier is provided by IEC that form multiple types of junctions, including tight junctions (TJs), adherens junctions and desmosomes (Figure 3). The composition and abundance of the different components of intercellular junctions are decisive for intestinal permeability and, together with IEC, maintain an important mechanical barrier preventing the transit (cross-cellular/paracellular) of pathogenic substances such as toxins and bacteria from the lumen to other parts of the body. In addition, IEC can regulate the persistence of antigens on the surface through epithelial shedding (Suárez et al., 2021).
In affecting and breaching the intestinal epithelial barrier, periodontal pathogenic bacteria can directly/indirectly reduce inter-epithelial adhesions, or invade and transit apically through epithelial cells in several ways. 1) Downregulation of adhesion molecule expression. Pathogenic bacteria (e.g. P. gingivalis, A. actinomycetemcomitans and T. denticola) reduce intestinal epithelial surface expression of E-cadherin, resulting in the destruction of adherens junctions and allowing transmigration (Devaux et al., 2019). In animal studies, a downregulation of tight junctions at the mRNA level was also observed in the gut of mice following oral administration of periodontal pathogenic bacteria (e.g. P. gingivalis and F. nucleatum) (Meilian et al., 2016; Olsen and Yamazaki, 2019; Cao et al., 2020). 2) Degradation of adhesion molecules. For example, P. gingivalis uses gingival proteases to directly degrade tight junctions (e.g. Occludin) and adherens junctions (E-cadherin) (Takahashi et al., 2019; Tsuzuno et al., 2021). 3) Cause re-distribution of E-cadherin from membrane to cytoplasm. Substantial remodeling of cell junctions may attenuate the integrity of the intestinal epithelial barrier. The recombinant cyto-lethal distending toxin (Cdt), a putative virulence factor of A. actinomycetemcomitans, affects epithelial barrier function by altering E-cadherin’s cytosolic distribution (Takahashi et al., 2019). 4) Excessive cell death may lead to barrier dysfunction and translocation of pathogenic bacteria (Crawford et al., 2022). F. nucleatum and its LPS have been shown to promote cell apoptosis and pro-inflammatory cytokine production in gut by activating autophagy pathway in IEC in vivo and in vitro (Su et al., 2020).
Finally, periodontal pathogenic bacteria indirectly reduce inter-epithelial adhesion by stimulation of inflammation. Mechanistically, F. nucleatum targets caspase activation and caspase recruitment domain 3 (CARD3) to activate the endoplasmic reticulum stress (ERS) pathway or the IL-17F/NF-κB pathway, mediating damage to the intestinal epithelial barrier (Cao et al., 2020; Chen et al., 2020a). F. nucleatum can also increase inflammatory genes (e.g. NF-κB) through its FadA adhesin binding to E-cadherin (Pignatelli et al., 2023). In addition, studies have shown that even a single oral dose of P. gingivalis can cause the prevalence of inflammatory microbiota in the gut (Nakajima et al., 2015). The increase in the proportion of intestinal pathogenic bacteria leads to the enrichment of LPS in the lumen. LPS is an important stimulus for impaired intestinal epithelial barrier function via an intracellular mechanism involving TLR-4-dependent up-regulation of CD14 membrane expression (Ciesielska et al., 2021).
3.3.3 Intestinal immune barrier
As the largest immune organ in the body, the intestinal immune barrier consists of innate and adaptive immune cells (gut-associated lymphoid tissue). Disruption of the homeostasis of the gut microbiota and immune barrier may trigger an excessive intestinal immune response, leading to the intestinal barrier damage and ultimately inducing systemic diseases associated with altered intestinal immune responses (Liu et al., 2021b).
Studies have observed an upregulation of pro-inflammatory cytokines in the gut by periodontal pathogenic bacteria. Colonization by P. gingivalis drives an increase in pro-inflammatory factors such as IL-6 and TNF-α in the gut (Lee et al., 2022). P. gingivalis also induces an increase in intestinal-derived IL-17 in peripheral blood (leaky gut) and has been shown to be associated with increased LPS in the gut (Arimatsu et al., 2014; Sato et al., 2017). The exact mechanism of action currently requires further research to elucidate.
Disruption of the Th17/Treg balance and increased secretion of pro-inflammatory cytokines in the body are important ways in which periodontal pathogenic bacteria can trigger systemic disease by affecting immune homeostasis in the gut. Periodontal pathogenic bacteria in the gut have been shown to be involved in the pathogenesis of diseases including inflammatory bowel disease (IBD), rheumatoid arthritis (RA), Parkinson’s disease (PD), psoriasis, and liver disease through activation of Th17-related pathways (Vernal et al., 2014; Bunte and Beikler, 2019; Feng et al., 2020; Kitamoto et al., 2020; Liu et al., 2021a). It has also been found that recovery of the Th17/Treg balance in periodontitis by the local injection of 3D-exos (a mesenchymal stem cell-derived exosomes, MSC-exos) attenuated experimental colitis. However, further human studies to validate the efficacy and feasibility of Th17-targeted therapies are still lacking.
4 Impact of periodontal bacteria on systemic diseases via the oral-gut axis
4.1 Intestinal diseases
4.1.1 Inflammatory bowel disease
IBD is a chronic, recurrent inflammatory disease of the GIT. Periodontitis, also a chronic inflammatory disease, significantly increase the risk of IBD and its disease severity (Vavricka et al., 2013; Lin et al., 2018). Increased periodontal pathogenic bacteria in the gut of IBD patients is positively correlated with the severity of IBD (Lucas López et al., 2017; Schirmer et al., 2018). Animal studies suggest that colitis may be worsened by gut microbial disturbances, which was promoted by gavage of periodontitis salivary microbiota or by infection with periodontal pathogenic bacteria (e.g. P. gingivalis and F. nucleatum) (Cao et al., 2020; Tsuzuno et al., 2021). Successful intestinal colonization of those inoculated oral pathobionts serves as an important trigger to exacerbate IBD. Further study on intestinal colonization, Kitamoto et al. found that a significant level of gut colonization by oral pathobionts was only observed in mice with both oral and gut dysbiosis, rather than only one of the two (Kitamoto and Kamada, 2022b). This implied that periodontal bacteria are pathogenic only to susceptible hosts or promote the progression of pre-existing IBD. Possible pathogenic mechanisms for IBD after intestinal colonization include the ability of periodontal bacteria to compromise the intestinal barrier (downregulation of TJs), enhance intestinal immune responses (e.g. induction of M2 macrophage polarization), and affect intestinal metabolism (e.g. decreased unsaturated fatty acid synthesis and increased arachidonic acid metabolism). Recent evidence suggested that P. gingivalis administration aggravates IBD via a gut microbiota-metabolite linoleic acid (LA)-Th17/Treg cell balance axis. Mechanistically, under Th17-polarizing culture conditions, LA, by specifically binding to AHR as an antagonist, drive Stat1 phosphorylation at Ser727, which in turn represses IL-17 while enhancing Foxp3 expression. Further studies revealed that LA supplementation alleviates P. gingivalis-induced colitis and exacerbation of Th17/Treg cell imbalance (Jia et al., 2024).
4.1.2 Colorectal cancer
CRC has the third highest incidence of all cancers (Bray et al., 2018). The gut microbiota of CRC patients is significantly altered compared to that of the healthy population (Chen et al., 2012). Patients with more severe periodontitis have a higher number of oral-derived microorganisms in their gut microbiota and a higher risk of developing CRC, as well as a worse prognosis (Momen-Heravi et al., 2017; Negrut et al., 2023).
In addition to the increased number of oral pathobionts, analysis using 16S rRNA gene sequencing revealed that some of the periodontal bacteria (e.g. F. nucleatum, P. gingivalis, T. denticola, and P. intermedia) were significantly associated with the progression of CRC (Negrut et al., 2023). Animal studies further transplanted the fecal microbiota of periodontitis patients into CRC mice and the same tumor-promoting effect was observed, suggesting that periodontal bacteria may promote CRC by remodelling the oral and gut microbiota (Shi et al., 2023). The current mechanistic study found that in a dysregulated intestinal environment mediated by periodontal bacteria, opportunistic pathogens are able to exploit tumor surface barrier defects, invade normal colonic tissue and induce local inflammation, while producing genotoxic metabolites to induce oncogenic transformation of colonic epithelial cells (Chen et al., 2017).
Many periodontal bacteria are associated with the development of gastrointestinal cancers, with F. nucleatum being most closely associated with CRC (Fan et al., 2018; Brennan and Garrett, 2019). F. nucleatum is usually detected in cancer tissue of CRC patients as well as in secondary distal metastases (e.g. liver and lung) (Flanagan et al., 2014; Abed et al., 2016; Mima et al., 2016; Bullman et al., 2017). Moreover, patients with higher abundance of F. nucleatum in the cancer tissue usually have a shorter survival time and are more likely to recur (Wang et al., 2021). F. nucleatum can be involved in influencing the various stages of CRC development through a variety of mechanisms.
At the stage of tumor progression, F. nucleatum acts to induce a pro-cancer immune microenvironment and assists tumor immune evasion by inhibiting anti-tumor cells as well as increasing the number and function of immunosuppressive cells. For example, F. nucleatum binds and activates the human inhibitory receptors TIGIT and CEACAM1, thereby inhibiting T and NK cells and suppressing anti-tumor immunity (Gur et al., 2015). F. nucleatum also suppresses immunity and increases tumor multiplicity by selectively recruiting tumor-infiltrating myeloid cells (Kostic et al., 2013). In addition to modulating immune cells, F. nucleatum can target tumor cells themselves. F. nucleatum can increase the expression of inflammatory genes (e.g. NF-κB) and oncogenes (e.g. Myc and Cyclin D1) via the FadA/E-cadherin/β-catenin pathway (Rubinstein et al., 2013). This active pathway also leads to F. nucleatum-induced over-expression of chk2, which facilitates DNA damage and tumor growth (Guo et al., 2020). Furthermore, LPS produced by F. nucleatum can upregulate microRNA-21 expression via the TLR4/Myd88/NF-κB pathway, thereby activating the MAPK pathway and enhancing cancer cell proliferation (Yang et al., 2017). In promoting tumor metastasis, F. nucleatum can activate autophagy signaling via the upregulation of CARD3 expression, regulate epithelial-mesenchymal transition (EMT), activate the NF-κB pathway and transmit exosomes (Chen et al., 2020b; Chen S. et al., 2020; Guo et al., 2021; Kong et al., 2021).
During the tumor treatment phase, F. nucleatum increases chemotherapy resistance in CRC patients, ultimately leading to tumor recurrence. F. nucleatum reduces the responsiveness of CRC cells to chemotherapeutic agents (e.g. oxaliplatin and 5-fluorouracil) by upregulating BIRC3 via the TLR4/NF-κB pathway or by inhibiting specific miRNAs involved in autophagy (Yu et al., 2017; Zhang et al., 2019). Therefore, F. nucleatum has been used as a non-invasive biomarker for CRC screening and assessment of prognosis.
Finally, F. nucleatum-based bacteriotherapy may be a potential therapeutic target for CRC. F. nucleatum expresses a variety of virulence factors associated with adhesion (e.g. RadD, Aid1 and Fap2) and invasion (e.g. FadA) of host cells, thereby exerting its pathogenicity through colonization, dissemination, evasion of host defenses and induction of host responses. Some of the F. nucleatum virulence factors have been shown to be closely associated with CRC. For example, FadA promotes the growth of CRC cells, while Fap2 enhances CRC progression by suppressing immune cell activity (Shang and Liu, 2018). An F. nucleatum-specific bacteriophage, FNU1, was found to kill cells and eradicate onco-bacterium from tumor tissue (Wang et al., 2024). In addition, antibiotic inoculation with F. nucleatum could eliminate F. nucleatum from breast cancer and further suppressed F. nucleatum-induced tumor growth (Parhi et al., 2020). Therefore, F. nucleatum (or other oral bacteria)-mediated therapies for CRC may be worth exploring further.
4.2 Rheumatoid arthritis
RA is a chronic inflammatory autoimmune disease characterized by bone destruction in multiple joints throughout the body. RA patients often have significant alterations in their oral and gut microbiota that correlate with the severity of joint destruction and the efficacy of antirheumatic treatment in RA patients (Phillips, 2015; Zhang X. et al., 2015; Drago et al., 2019; Möller et al., 2020). Several periodontal bacteria (e.g. P. gingivalis and Prevotella intermedia) are detected in the gut, serum and synovial fluid of RA patients (Martinez-Martinez et al., 2009; Pianta et al., 2017; Drago et al., 2019). RA may be a reactive arthritis aggravated by the repeated translocation of these periodontal bacteria to the joints. Whether oral pathobionts reach the joints through the oral-gut axis or through hematogenous dissemination, most studies suggest that both routes are possible, but more research is needed to determine which route is predominant or prior.
As the most important diagnostic biomarker for RA, serum levels of anti-citrullinated protein antibodies (ACPA) positively correlate with the severity of both RA and periodontitis (Lappin et al., 2013; Bellando-Randone et al., 2021). P. gingivalis can convert arginine residues in proteins to citrulline and increases serum levels of ACPA (Möller et al., 2020). A. actinomycetemcomitans can also indirectly increase protein citrullination through the pathway of leukotoxin (LtxA)-induced peptidyl-arginine deiminase (PAD) dysregulation, thereby increasing ACPA levels (Konig et al., 2016). Thus, one of the important mechanisms by which periodontal bacteria promote RA via the oral-gut axis is chronic exposure to citrullinated protein (CP) triggers the expression of ACPA in joint synovium throughout the body.
In an arthritis mouse model, oral administration of P. gingivalis induced dysbiosis of the gut microbiota, increased IL-6 and CP production in serum, joint and intestinal tissues, and exacerbated joint destruction (Hamamoto et al., 2020). Further, fecal microbiota transplantation (FMT) from P. gingivali-inoculated experimental arthritis mice reproduced donor gut microbiota and resulted in severe joint destruction with increased IL-6 and CP production in joint and intestinal tissues. This suggests that the gut dysbiosis induced by P. gingivalis oral infection may be sufficient to trigger or exacerbate arthritis.
F. nucleatum is also enriched in the gut of RA patients and positively correlate with RA severity (Hong et al., 2023). Mechanistically, F. nucleatum delivers FadA to the joints via outer membrane vesicles (OMVs) and triggers synovial inflammation by activating the Rab5a-YB-1 axis in synovial macrophages. In addition, some studies have treated with the zonulin antagonist larazotide acetate, which specifically increases intestinal barrier integrity (modulating TJs), effectively reduces arthritis onset (Tajik et al., 2020).
The mechanism by which periodontal bacteria promote RA may also be related to the activation of Th17-related pathways. Furthermore, periodontal bacteria (e.g. P. gingivalis and Prevotella nigrescens) are involved in inflammatory bone destruction in RA by increasing the intestinal hyperimmune response triggered by Th17 cellular immune responses, which can induce higher levels of RANKL and TRAP+ osteoclasts (Vernal et al., 2014). P. nigrescens also can reduce the osteoprotective effects of Th2 (de Aquino et al., 2014). All of this evidence demonstrates that the oral-gut axis plays an important role in the influence of periodontal bacteria on RA.
4.3 Brain diseases
Periodontal bacteria may be indirectly associated with brain diseases through the gut pathway. In addition to ischemic (e.g. stroke) and autoimmune (e.g. multiple sclerosis) brain diseases, evasion strategies of specific pathogens may also alter the function of the blood-brain barrier (BBB) (Daneman and Rescigno, 2009). Here we focus on the pathogenic mechanisms of the oral-gut-brain axis in brain disease, i.e., periodontal bacteria disrupt the gut barrier and BBB, further triggering neuroinflammatory and pathological changes in the brain (Figure 5) (Sansores-España et al., 2021).
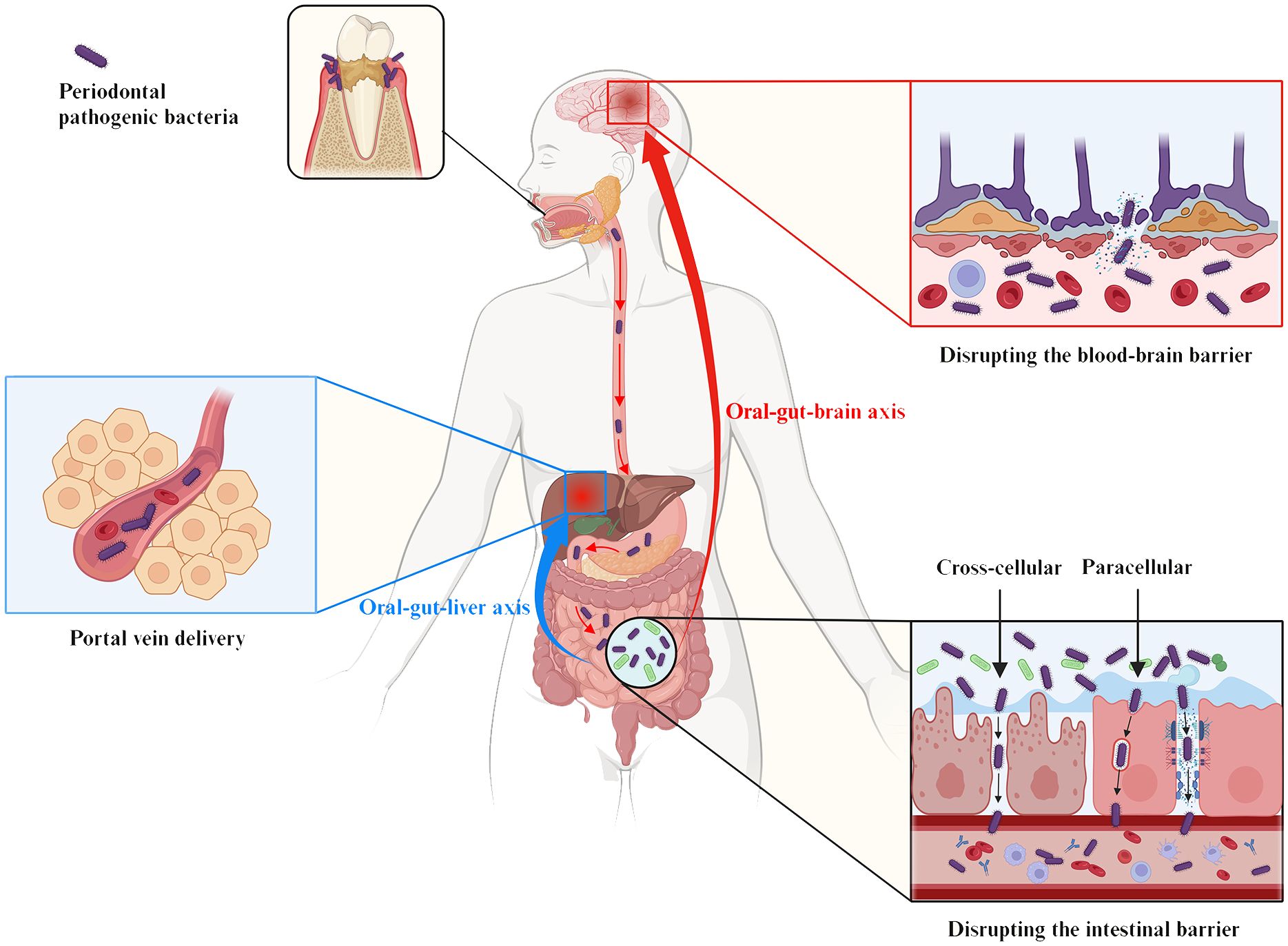
Figure 5. The oral-gut-liver axis and the oral-gut-brain axis serve as the pathophysiologic basis for the influence of periodontal pathogenic bacteria on liver and brain diseases via the intestinal pathway. The gut establishes an anatomical dependence on the liver via portal circulation for the direct delivery of pathogens or metabolites to the liver, a pathophysiologic pathway termed the “oral-gut-hepatic axis” (blue symbols). In addition, the “oral-gut-brain axis” (red symbols) refers to periodontal pathogenic bacteria that trigger neuroinflammatory and pathological changes in the brain based on the disruption of the intestinal barrier and the BBB. As shown, periodontal pathogenic bacteria are involved in the pathogenesis of various systemic diseases by either directly/indirectly reducing intestinal epithelial intercellular junctions (paracellular) or by invading IEC (transcellular), which ultimately affects and breaches the intestinal barrier.
4.3.1 Alzheimer’s disease
AD is the most common neurodegenerative disease, with progressive behavioral and cognitive impairment as the main clinical feature. Endothelial cells protecting the BBB have been shown to be infected by periodontal bacteria, and virulence factors such as P. gingivalis-LPS and gingipains can even be detected directly in the brains of AD patients (Dominy et al., 2019). Animal studies provide evidence to support this view. Mice with experimental periodontitis showed significant dysbiosis of the gut microbiota, disruption of the intestinal barrier and BBB, and increased levels of LPS in the serum and brain. Meanwhile, neuropathological alterations, including neuronal loss, synaptic injury, and glial activation, as well as progressive cognitive deficits were also observed (Xue et al., 2020).
The pathological mechanisms by which periodontal bacteria promote AD may include the following three aspects. First, periodontal bacteria trigger an excessive accumulation of Aβ in the brain. P. gingivalis may increase Aβ production by releasing gingipains-rich OMVs that drive NLRP3 inflammasome activation, ASC speck aggregation and pyroptotic cell death (Fleetwood et al., 2017; Dominy et al., 2019). Upregulation of advanced glycation end products (RAGE) expression in brain endothelial cells also mediates Aβ influx following P. gingivalis infection (Zeng et al., 2021). In addition, Intestinal-derived LPS, including P. gingivalis-LPS, has been shown to be abundant in the AD brain and has been found to be associated with Aβ plaque formation through activation of the TLR4-mediated NF-κB and MAPK pathways (Zhao et al., 2017).
Second, periodontal bacteria mediate the formation of NFTs in neuronal cells and promote AD (Arnsten et al., 2021). Formation of NFTs results from hyperphosphorylation of the tau protein (Narengaowa et al., 2021). Gingipains can cause proteolysis of tau (tau phosphorylation and cleavage) and subsequent NFTs formation by activating caspase-3. P. gingivalis and its virulence factors, such as lysine-gingipain (Kgp) and arginine-gingipain B (RgpB) are independently and positively correlated with tau load in AD brains (Dominy et al., 2019).
Finally, neuronal necrosis due to neuroinflammation is also a key mechanism for AD. Periodontal bacteria and their virulence factors may activate and accumulate large numbers of microglia through the gut, inducing synaptic toxicity and neuronal death, ultimately exacerbating neurodegeneration (Narengaowa et al., 2021). For example, intraneuronal gingipains may drive neuronal NLRP1 activation, resulting in pyroptosis of neurons and activation of caspase-1, leading to release of the neuroinflammatory interleukins IL-1β and IL-18 (Dominy et al., 2019).
Therapeutically, administration of small-molecule inhibitors of gingipain attenuates neurodegeneration and significantly decreases the host Aβ response to P. gingivalis brain infection, thereby slowing or preventing further accumulation of pathology in AD patients (Dominy et al., 2019). Another study has found a favorable effect of periodontal treatment on AD-related brain atrophy. However, more research is still needed to explore the relationship between periodontal treatment and AD (Schwahn et al., 2022).
4.3.2 Parkinson’s disease
Intestinal inflammation and leakage promoted by oral administration of periodontal bacteria (e.g. P. gingivalis) may lead to significant loss of dopaminergic neurons and microglial activation in the SNpc, thereby triggering PD (Feng et al., 2020). In animal studies, FMT treatment ameliorated gastrointestinal dysfunction and motor deficits in PD mice by restoring intestinal homeostasis, attenuating damage to the intestinal barrier and BBB and suppressing neuroinflammation and dopaminergic neuronal damage in the substantia nigra (SN) (Zhao Z. et al., 2021). Among the human studies, a recent double-blind, placebo-controlled, randomized trial also suggested that a single FMT induced mild, but long-lasting beneficial effects on motor symptoms in patients with early-stage PD (Bruggeman et al., 2024).
α-synuclein is thought to be a central molecular player involved in the pathogenesis of PD through the oral-gut-brain axis. An endoscopic biopsy study showed a significant correlation between the level of α-synuclein accumulation in neurites of the enteric nervous system and the degree of inflammation of intestinal wall (Stolzenberg et al., 2017). Periodontal bacteria may cross the IEC and induce misfolding and aggregation of α-synuclein in specific enteric neurons. The aggregated α-synuclein will then migrate to the brain via the vagus nerve (Holmqvist et al., 2014; Garrido-Gil et al., 2018). P. gingivalis-mediated activation of leucine-rich repeat kinase 2 (LRRK2) has been shown to consistently induces α-synuclein expression in the gut of R1441G mice, triggering neuroinflammation and subsequent degeneration of dopaminergic neurons (Kozina et al., 2018; Feng et al., 2020).
In addition, IL-17A may also be involved in the effect of periodontal bacteria on PD via the gut (Huang et al., 2014). For example, oral administration of P. gingivalis leads to IL-17A immunoreactivity in the peripheral system and upregulates IL-17RA protein levels in dopaminergic neurons (Feng et al., 2020). Peripheral IL-17A crosses the BBB and mediates dopaminergic neuron degeneration via IL-17RA in microglia (Liu Z. et al., 2019).
4.3.3 Autism spectrum disorder
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by abnormal language and interaction skills and repetitive stereotyped behavior (Qiao et al., 2022). Studies have observed disruption of the intestinal barrier and BBB in post-mortem brain tissue and gut in ASD subjects, as well as significant neuroinflammation in the brain (Fiorentino et al., 2016). ASD patients have been further found to have unique oral and gut microbiota distribution patterns, including elevated F/B ratio and increased abundance of opportunistic pathogens, and this bacterial community differences are positively correlated with ASD severity (Tomova et al., 2015; Kong et al., 2019; Johnson et al., 2020). A study that transferred the oral microbiota of ASD donors to an antibiotic-mediated microbiota-depleted mouse model found the induction of ASD-like behaviors (e.g. impaired social behavior) (Kong et al., 2019; Qiao et al., 2022). Significant differences in oral and gut microbiota structure and altered neurosignaling activities, including upregulation of serotonin-related gene expression and TGF-β signaling pathway, were observed in ASD microbiota recipient mice compared to typical development microbiota recipient mice. Furthermore, increased serotonin-related gene expression in ASD microbiota recipient mice was associated with both autistic behaviors and changes in abundance of specific oral microbiota, such as Porphyromonas spp. These evidences highlight the important influence of the oral microbiota in the gut-brain connection.
In addition to these, a variety of other brain disorders, including neuropsychiatric disorders (e.g. depression), neurodegenerative diseases (e.g. multiple sclerosis) and cerebrovascular diseases (e.g. ischemic stroke), may also be associated with the mediating effects of periodontal bacteria via the oral-gut-brain axis pathway (Zhang Z. et al., 2015; Cunha et al., 2019; Maitre et al., 2020; Brown et al., 2021). For example, in a rat model study, oral gavages with P. gingivalis and F. nucleatum simultaneously induced periodontitis, neuroinflammation and depression-like behavior (Martínez et al., 2021). This study further identified F. nucleatum in the frontal cortex of experimental rats and also demonstrated that gastrointestinal translocation of periodontal bacteria can lead to the entry of peripheral LPS into the rat brain and elicit TLR-4-dependent neuroinflammation by finding evidence for the existence of an APOA1-mediated transport mechanism (Martínez et al., 2021). However, clinical studies are still needed for further confirmation.
4.4 Liver diseases
The gut microbiota may transport bacteria or metabolites directly to the liver via portal circulation (Hou et al., 2022). The impaired gastric acid and bile secretion prevalent in chronic liver disease (e.g. cirrhosis) may also make the gut more susceptible to translocation and colonization by oral bacteria (e.g. P. gingivalis and T. denticola) (Kakiyama et al., 2014; Qin et al., 2014; Wang et al., 2022). The anatomical dependence of the liver on the gut through metabolic exchange and pathogen translocation is therefore the basis for the pathophysiology of periodontal bacteria affecting liver disease via the gut pathway, the “oral-gut-liver axis” referred to in many current studies (Figure 5).
Epidemiological evidence suggests that having severe periodontitis increases the prevalence of non-alcoholic fatty liver disease (NAFLD), which recently renamed Metabolic dysfunction-associated fatty liver disease (MAFLD), mortality associated with cirrhosis, and progression of NAFLD towards fibrotic liver injuries (Akinkugbe et al., 2017; Ladegaard Grønkjær et al., 2018). In addition, patients with hepatocellular carcinoma in combination with periodontitis tend to have worse cancer stage, liver function and prognosis (Tamaki et al., 2011). Periodontitis may affect liver disease by modulating the oral and gut microbiota.
Dysregulated oral and gut microbiota may act as important mediators between periodontal bacteria and liver disease. Oral administration of periodontal bacteria (e.g. P. gingivalis and A. actinomycetemcomitans) may increase the risk of liver disease by altering the F/B ratio of the gut microbiota and increasing intestinal leakage (regulating TJs) and serum endotoxin levels (Arimatsu et al., 2014; Nakajima et al., 2015; Komazaki et al., 2017; Åberg and Helenius-Hietala, 2022). Dysregulated gut microbiota can also affect chronic liver disease by regulating bile acid metabolism and the production of short-chain fatty acids (SCFAs), ethanol and choline. Significant alterations in the composition of the gut microbiomes are present in patients with liver disease, including NAFLD, non-alcoholic steatohepatitis (NASH), cirrhosis and alcoholic liver disease (ALD). In contrast, FMT treatment can prevent NASH, ALD, hepatic encephalopathy (HE), and acute liver failure (ALF) in animal models by restoring intestinal homeostasis (Ferrere et al., 2017; Wang et al., 2017; Zhou et al., 2017; Liu et al., 2021a). Furthermore, genetic tracking revealed that the majority of species causing alterations in the gut microbiota of patients with liver disease are of buccal origin (Qin et al., 2014). Supporting this view, it has been shown that systemic periodontal therapy in cirrhotic patients modulates salivary and fecal microbiota dysbiosis, improves endotoxemia, as well as systemic and local inflammation (reduces inflammatory mediators), and improves quality of life and cognition in patients with HE (Bajaj et al., 2018). Periodontal treatment also improves biochemical markers (e.g. ALT and AST) in patients with NAFLD and cirrhosis (Yoneda et al., 2012; Komazaki et al., 2017; Nakahara et al., 2018). However, whether periodontal therapy improves organic liver changes remains to be investigated.
Periodontal bacteria and their virulence factors may reach the liver via the damaged gut and act directly on hepatocytes, Kupffer cells and hepatic stellate cells (HSCs) through activation of the PRR, hence triggering downstream pro-inflammatory cascades and ultimately affecting chronic liver disease (Schroeder and Bäckhed, 2016).
Periodontal treatment has been proposed as a potential approach to improve liver disease. It has also been suggested by some that the simultaneous treatment of periodontitis and intestinal disease may have a synergistic effect on patients with liver disease (Kuraji et al., 2023). That is, treatment of intestinal inflammation and intestinal ecological dysbiosis may prevent ectopic intestinal colonization caused by oral pathology, even in the presence of periodontitis. However, more studies are still needed to confirm these findings.
4.5 Obesity
Obesity is a multifactorial chronic inflammatory disease characterized by the overgrowth of adipose tissue. The degree of obesity (e.g. body mass index) has been found to be positively correlated with the degree of periodontal inflammation (e.g. periodontal inflamed surface area index and the level of periodontal bacteria in the oral cavity) (Chaffee and Weston, 2010; Nascimento et al., 2015; Aoyama et al., 2021).
Obesity patients have been shown to have alterations in both oral and gut microbiota (Wu et al., 2018; Benahmed et al., 2021). Microorganisms play an essential role in digestion, absorption, metabolism, energy use and immune regulation in the body (Turnbaugh et al., 2008). Just as germ-free (GF) mice were protected against high fat diet (HFD) induced obesity, whereas receiving FMT from obese mice increases fat deposition and metabolic disturbances in GF mice (Rabot et al., 2010). Oral administration of periodontal bacteria (e.g. P. gingivalis and A. actinomycetemcomitans) significantly alters the F/B ratio in the gut microbiota, which in turn may induce insulin resistance, hepatic steatosis and macrophage infiltration in adipose tissue, and promote further increases in body weight and adipose tissue in diet-induced obese mice (Arimatsu et al., 2014; Nakajima et al., 2015; Komazaki et al., 2017; Rojas et al., 2021).
The triggering of endotoxemia via the leaky gut is one of the risk factors for periodontal bacteria affecting obesity. In a mouse model, P. gingivalis significantly elevated serum endotoxin levels after only 1 hour of single oral administration, which in turn activated pro-inflammatory genes in the adipose tissue, blood vessels and liver of mice, ultimately increasing the risk of insulin resistance, atherosclerosis (AS) and NAFLD, respectively (Arimatsu et al., 2014). Recent studies suggest that P. gingivalis-induced endotoxemia may affect obesity by altering endocrine functions in brown adipose tissue (BAT) in mice, including glucose homeostasis, insulin sensitivity, and thermogenesis (Stanford et al., 2012; Hatasa et al., 2021). In addition, P. gingivalis administration may increase inflammation-related mRNA expression in BAT, such as TNF-α and IL6, and downregulate the expression of genes related to lipolysis and metabolism in BAT, such as Lipe and Pnpla2 (Hatasa et al., 2021).
The chronic low inflammatory state caused by periodontal bacteria via the oral-gut axis promotes increased expression of pro-inflammatory adipokines (e.g. resistin) and decreased expression of anti-inflammatory adipokines (e.g. adiponectin) (Zimmermann et al., 2013). In contrast, periodontal treatment significantly reduced resistin levels and increased adiponectin levels in obesity patients (Bharti et al., 2013; Akram et al., 2017). However, there is no further evidence that periodontal therapy improves obesity by affecting adipokine secretion via the intestinal pathway.
4.6 Diabetes mellitus
DM is a group of metabolic diseases characterized by hyperglycemia caused by abnormal insulin secretion and/or action (Cho et al., 2018). A systematic review confirmed that severe periodontitis increased the prevalence of type 2 Diabetes mellitus (T2DM) by 53% (Wu et al., 2020). Periodontal patients with established DM tend to have poorer glycemic control and higher prevalence of DM-related complications and all-cause mortality (Sharma et al., 2016; Graziani et al., 2018; Ziukaite et al., 2018; Genco et al., 2020; Nguyen et al., 2020; Wu et al., 2020; Song et al., 2021). Periodontal treatment also facilitates effective glycemic management in DM patients (Chang et al., 2020).
The increased susceptibility of DM patients to certain infections due to altered innate immune response is thought to facilitate the systemic (including intestinal) dissemination of periodontal bacteria in the case of periodontitis (Toniolo et al., 2019; Barutta et al., 2022). The presence of P. gingivalis has been detected in fecal samples from DM mice following oral administration of P. gingivalis (Kashiwagi et al., 2021). DM Patients with periodontitis have significant alterations in both oral and gut microbiota, systemic pro-inflammatory cytokines and metabolic parameters (Li et al., 2020; Barutta et al., 2022; Silva et al., 2022). Studies have demonstrated that oral administration of periodontal bacteria (P. gingivalis and P. intermedia) induces altered gut microbiota and leaky gut prior to the development of systemic inflammation (Yamazaki et al., 2021).
The disruption of intestinal homeostasis mediated by oral administration of periodontal bacteria (e.g. P. gingivalis and A. actinomycetemcomitans) was confirmed by animal studies to induce systemic inflammation, metabolic changes and hepatic fat deposition in non-diabetic mice, while exacerbating fasting and postprandial hyperglycemia in DM mice (Arimatsu et al., 2014; Blasco-Baque et al., 2017; Komazaki et al., 2017; Kato et al., 2018; Sasaki et al., 2018; Kashiwagi et al., 2021). That is, in the pathogenic mechanism of periodontitis for DM, intestinal transmission of periodontal bacteria may induce/exacerbate insulin resistance and glucose intolerance by mediating entero-hepatic metabolic derangements. In addition, in a ligature-induced periodontitis mouse model, fasting blood glucose (FBG), serum glycated hemoglobin (HbA1c) and glucose intolerance levels were higher in the periodontitis group than in the control group (Li et al., 2021). In contrast, FBG, HbA1c, glucose tolerance levels and systemic inflammatory load were reversed in mice after elimination of periodontitis or depletion of the gut microbiota with antibiotics (Li et al., 2021). This further suggests that the gut microbiota may mediate the influence of periodontal bacteria on DM.
Firstly, intestinal transmission of periodontal bacteria leads to reduced diversity and altered F/B ratio in the intestinal microbiota. Individuals with low diversity of gut microbiota tend to exhibit more pronounced insulin resistance, hyperinsulinemia and increased susceptibility to DM (Cotillard et al., 2013; Le Chatelier et al., 2013). There are also mechanistic drivers for the correlation between taxa representation (Bacteroidetes and Firmicutes) of the gut microbiota and glycemic control (Larsen et al., 2010; Napolitano et al., 2014). For example, metformin is known to be a first-line treatment in patients with T2DM. Influencing bile acid metabolism and entero-endocrine hormone secretion by altering the gut microbiota is one of the important pharmacological mechanisms of metformin in the treatment of T2DM (Napolitano et al., 2014). Further studies found that serum concentrations of cholic acid and conjugates in T2DM patients were positively correlated with the microbiota abundance of Firmicutes and negatively correlated with Bacteroidetes (Napolitano et al., 2014). In addition, the F/B ratio of the gut microbiota was significantly correlated with circulating concentrations of peptide tyrosine-tyrosine (PYY) in serum (Napolitano et al., 2014). PYY, an intestinal hormone, has been shown to restore normal glucose regulation of insulin and glucagon secretion in DM rats (Ramracheya et al., 2016).
Secondly, alterations in gut metabolites mediated by periodontal bacteria may also contribute to the development of DM. In animal models, the presence of periodontitis or oral administration of P. gingivalis can disrupt the gut microbiota and serum metabolite profiles, and increase fasting hyperglycemia and entero-hepatic metabolic derangements associated with altered gut metabolism in DM mice (Kato et al., 2018; Kashiwagi et al., 2021). SCFAs are known to play an important role in mediating glucose metabolism and insulin sensitivity through multiple signaling pathways (Psichas et al., 2015). It was found that increased serum HbA1c levels in mice with periodontitis were associated with a decrease in SCFAs (e.g. butyrate) producing bacteria in the gut (Li et al., 2021). Also, butyrate supplementation reduced fasting glucose and insulin levels in mice and improved insulin sensitivity, the effects of which were mediated through inhibition of HDACs (McNabney and Henagan, 2017). Thus, reduced production of intestinal SCFAs may mediate the effect of periodontitis on diabetes. Furthermore, branched-chain amino acids (BCAA) are one of the links between periodontal bacteria and insulin resistance via the gut microbiota (Khor et al., 2021). Elevated levels of BCAA are characteristic of insulin resistance and have been suggested as a predictor of DM development (Pedersen et al., 2016; Nawaz and Siddiqui, 2020). Periodontal pathogenic bacteria (e.g. P. gingivalis) have been reported to alters the gut microbiota composition and serum metabolite profiles in mice, increasing BCAA levels and inducing insulin resistance (Tian et al., 2020). Mechanistically, BCAA activates the mTOR-S6K1 pathway, which induces insulin resistance by phosphorylating IRS-1 (Yoon, 2016).
Finally, gut dysregulation following swallowing of periodontal bacteria implies elevated levels of circulating LPS and induced/amplified systemic hypo-inflammation, which may be one of the potential mechanisms explaining the association between periodontitis and DM. Consistent with this, continuous infusion of P. gingivalis-LPS induced endotoxemia, glucose intolerance and insulin resistance in HFD-fed mice (Arimatsu et al., 2014; Blasco-Baque et al., 2017). Lowering circulating LPS concentration could be a potent strategy for the control of metabolic diseases. Gut microbiota dysbiosis may induce the expression of pro-inflammatory cytokines through the TLR4/MyD88/NF-κB signaling pathway, thereby promoting DM progression (Hou et al., 2022).
Gut dysbiosis and subsequent increased circulating levels of pro-inflammatory cytokines induced by swallowing periodontal bacteria may impair glycemic control in DM patients (Arimatsu et al., 2014; Hajishengallis and Chavakis, 2021). For example, chronic low-grade inflammation caused by periodontal bacteria can aggravate pancreatic β-cell dysfunction in DM mice through IL-12 regulation on Klotho, thereby worsening glucose control as well as glucose-stimulated insulin secretion (Liu et al., 2016). Studies have demonstrated that improved metabolic control (lower HbA1c and plasma glucose concentrations) after periodontal treatment is accompanied by a reduction in systemic inflammatory markers (D’Aiuto et al., 2018).
Rebuilding the gut microbiota may be an important way to inhibit the pathogenic effects of periodontitis on DM. Intervention studies using FMT in human and animal models have demonstrated the role of the gut microbiota in improving diabetes (Vrieze et al., 2012; Aron-Wisnewsky et al., 2019; Wang et al., 2020). Lean donor FMT in patients with metabolic syndrome showed altered gut microbiota and improved insulin sensitivity (Vrieze et al., 2012; Kootte et al., 2017). Gut microbiota from DM-resistant mice can also transfer DM protection to otherwise highly susceptible to DM hosts. A study reported that gut microbiota transfer from DM-protected MyD88-deficient non-obese diabetic (MyD88-/-NOD) mice could lead to delayed onset of DM and reduced insulitis in NOD mice (Peng et al., 2014). The study further found that the microbiota and mucosal immune system (elevated levels of IgA, TGF-β, etc.) were significantly altered in the gut of NOD mice after receiving the gut microbiota from DM-resistant mice (Peng et al., 2014). This suggests that inhibiting or reversing the alteration of the gut microbiota by periodontal bacteria might improve DM.
4.7 Atherosclerosis
AS is the pathological basis for the development of cardiovascular disease. Epidemiological studies have shown that periodontitis can significantly increase the risk and prevalence of atherosclerotic cardiovascular disease (ASCVD), including cardiovascular death, myocardial infarction, heart failure, atrial fibrillation and stroke (Linden et al., 2012; Dietrich et al., 2013; Chen et al., 2016; Liljestrand et al., 2017; Nordendahl et al., 2018; Park et al., 2019; Zhou et al., 2021). Periodontitis can also significantly increase the intima-media thickness (IMT) and atherosclerotic plaque area in the common carotid artery (Montenegro et al., 2019; Meurman and Söder, 2022). Many hypotheses have been proposed regarding the cause of AS, and it is now generally accepted that infection is an initiating factor and an important mechanism in promoting atherosclerotic changes in blood vessels (Slocum et al., 2016).
There are significant pro-inflammatory alterations in the gut microbiota of ASCVD patients, including reduced microbial diversity, altered F/B ratios and increased relative abundance of oral bacteria (Koren et al., 2011; Gregory et al., 2015; Emoto et al., 2016; Emoto et al., 2017; Jie et al., 2017; Yoshida et al., 2018). Relatively low abundance of SCFAs-producing bacteria and high abundance of LPS-producing bacteria have also been reported in the gut of ASCVD patients infected with periodontal bacteria (Arimatsu et al., 2014; Kramer et al., 2017). These intestinal alterations have not only been shown to predict and accelerate ASCVD, but have also been associated with dyslipidemia, elevated marker levels and a state of low systemic inflammation in ASCVD (Le Chatelier et al., 2013; Nie et al., 2019; Yeh et al., 2020). Conversely, FMT can reduce or transmit susceptibility to AS (Vrieze et al., 2012; Gregory et al., 2015). Quercetin has also been shown to modulate immune and metabolic function by restoring intestinal homeostasis, reducing the production of pro-inflammatory cytokines and atherosclerotic lipid metabolites, thereby significantly reducing areas of atherosclerotic lesions and sizes of plaques (Nie et al., 2019).
Pro-inflammatory alterations in the gut microbiota induced by periodontal bacteria may contribute to AS by modulating the production of its bioactive metabolites (Yeh et al., 2020). Plasma levels of trimethylamine N-oxide (TMAO) are an important predictor of adverse cardiovascular events, and a disordered gut microbiota can increase the conversion of TMAO (Tang et al., 2013; Wang et al., 2015). TMAO can inhibit reverse cholesterol transport to exacerbate foam cell accumulation, as well as induce platelet hyperactivity to increase thrombus formation, ultimately affecting atherosclerotic plaque formation and progression (Zhu et al., 2016; Yeh et al., 2020; Qian et al., 2022). In animal studies, transplantation of TMAO-rich gut microbiota feces into GF mice promotes platelet function and arterial thrombus formation (Huynh, 2020). In addition, TMAO also promotes the release of the inflammatory cytokines IL-18 and IL-1β by activating the NF-κB pathway (Liu M. et al., 2019).
The impact of infection on AS is now thought to be related to the total pathogen burden, i.e. the bacterial load determines the inflammatory status and stability of the atherosclerotic plaque (Emoto et al., 2016). Studies investigating the oral, gut and plaque microbiota in AS patients have confirmed that the atherosclerotic plaque microbiota is at least partly oral and gut in origin (Koren et al., 2011). The number and abundance of periodontal bacteria species detected in AS patients tends to be more significant and positively correlated with the size of atherosclerotic plaques (Ford et al., 2006; Otomo-Corgel et al., 2012; Rivera et al., 2013; Calandrini et al., 2014). Endotoxemia associated with the oral and gut dysbiosis may induce the recruitment of macrophages and their infiltration of adipose tissue, culminating in the formation of foam cells that accumulate in the artery walls (Diet, 2001; Michelsen et al., 2004; Mullick et al., 2005).
The pathogenic effect of periodontal bacteria on AS has been demonstrated in multiple types of animal model studies (Brodala et al., 2005; Velsko et al., 2014; Lin et al., 2015; Widziolek et al., 2016). A possible theoretical mechanism by which periodontal bacteria could reach the atherosclerotic plaque via the gut is currently mentioned, namely the phagocytosis by macrophages at epithelial linings of the gut (Schenkein and Loos, 2013; Emoto et al., 2016). Macrophage abundance and function can be regulated and driven by the gut microbiota and convey immune responses at distal organs (Yeh et al., 2020). periodontal bacteria are phagocytosed by macrophages in the dysregulated gut and carried into circulation. When they reach the activated endothelium of atherosclerotic plaques, they leave the bloodstream to enter the plaque and transform into cholesterol-laden foam cells (Wang et al., 2007; Emoto et al., 2016). P. gingivalis has been detected in circulating phagocytes from patients with severe cardiovascular disease suffering from periodontitis, supporting the possibility that phagocytes may play a role in harboring and transporting periodontal bacteria to atherosclerotic plaques (Carrion et al., 2012). However, the current study only demonstrates the theoretical validity of this periodontal bacteria transport mechanism and does not indicate the extent of its contribution in AS pathogenesis.
4.8 Skin diseases
Both the skin and the gut are important barrier structures for the immune and neuroendocrine functions of the body (O’Neill et al., 2016). It is now widely accepted that there is a close bidirectional relationship between the gut and the skin, and a “gut-skin axis” has been proposed to further explore the link between gut and skin homeostasis (Salem et al., 2018).
A number of studies have referred to preliminary evidence that the oral and gut microbiota may play a role in the pathogenesis of skin diseases as regulators of the “gut-skin axis”. Sjögren’s syndrome, psoriasis, systemic lupus erythematosus (SLE) and leukoaraiosis may all be linked to dysbiosis of the oral and gut microbiota (Szymula et al., 2014; Consolandi et al., 2015; Coit et al., 2016; Ungprasert et al., 2017; Ma and Morel, 2022). Some oral microbiota species are enriched in the gut of SLE patients. In both SLE and psoriasis, the severity of skin lesions, including the SLE Disease Activity Index and the Psoriasis Area and Severity Index, were positively correlated with the severity of periodontitis and altered gut microbiota profiles (Masallat and Moemen, 2016; Azzouz et al., 2019; Qiao et al., 2019; Hussain et al., 2022).
In addition, elevated levels of markers of intestinal barrier function impairment are often observed in a variety of skin diseases. elevated levels of fecal calprotectin have been found in patients with SLE and atopic dermatitis (Seo et al., 2018; Azzouz et al., 2019; Smirnova et al., 2020). Elevated levels of claudin-3 and intestinal fatty acid-binding protein have also been found in the blood of patients with psoriasis (Sikora et al., 2019a; Sikora et al., 2019b). A compromised gut barrier allows periodontal or other bacteria to move from the gut lumen into the internal environment where they can accumulate in the skin and disrupt skin homeostasis. Studies have detected periodontal bacteria and gut-derived bacterial DNA in the blood of patients with psoriasis, while finding that those with bacterial DNA-positive psoriasis patients showed higher serum inflammatory cytokine levels, longer duration of disease and younger onset of disease (Ramírez-Boscá et al., 2015; Dalmády et al., 2020). In Sjögren’s syndrome, Sjögren’s syndrome Antigen A (SSA)/Ro60-reactive T cells can be activated by peptides originating from oral and gut bacteria, and thus may be involved in disease progression (Szymula et al., 2014).
Periodontal bacteria-induced gut dysbiosis may be involved in the pathogenesis of autoimmune (e.g. psoriasis) and allergic (e.g. atopic dermatitis) skin diseases through immune, metabolic and neuroendocrine pathways. First, disruption of Th17/Treg balance can be involved in the pathogenesis of several chronic inflammatory skin diseases (Salem et al., 2018). In particular, psoriasis is mainly considered to be a Th17 disease (Grine et al., 2015). Activation of Th17 cells and increased expression of IL17 are major factors mediating the pathogenesis of psoriasis, which could be induced by periodontal bacteria and their products (Blauvelt and Chiricozzi, 2018; Bunte and Beikler, 2019; Zhao X. et al., 2021). After antibiotic treatment, reduced skin inflammation caused by reduced Th17 activation in the gut was observed in a mouse model of imiquimod-induced psoriasis (Zákostelská et al., 2016). It was further shown that IL-17 disrupts the integrity of the skin barrier through downregulation of filaggrin and adhesion molecule expression from keratinocytes, and further induces keratinocyte hyperproliferation (Gutowska-Owsiak et al., 2012). Thus, systemic Th17 hyperactivation or overproduction of IL-17 may be the link between periodontal bacteria-induced disturbances in the gut microbiota and the development of psoriasis. In addition to this, skin diseases such as Sjögren’s syndrome, SLE, leukoplakia and scleroderma have also been linked to the pathogenic mechanisms by which periodontitis regulates Th17 cell differentiation and its key cytokines (IL-17 and IL-23) (Bunte and Beikler, 2019; Tong et al., 2019).
Secondly, periodontal bacteria may mediate skin inflammation by affecting the normal metabolic activity of the intestinal commensals. For example, elevated serum levels of free phenol and p-cresol may affect epidermal differentiation and epidermal barrier function by decreasing keratin 10 expression (Miyazaki et al., 2014). Both oral and gut microbiota dysbiosis in SLE patients may be related to amino acid metabolism. In SLE progression, amino acid (e.g. tryptophan) metabolism can modulate immune tolerance in lupus. We can therefore speculate that alterations in the oral and gut microbiota of SLE patients with periodontitis may initiate autoimmunity by manipulating amino acid metabolism. However, this needs to be verified by further studies.
Finally, some studies support the idea that alterations in the gut microbiota can be involved in the development of skin diseases by affecting the levels of circulating neuroendocrine molecules such as tryptamine, trimethylamine and serotonin, inducing skin barrier dysfunction and immune system dysregulation (Jin et al., 2014; O’Neill et al., 2016).
5 Discussion
This review suggests that periodontal bacteria gain access to systemic dissemination through the disturbed gut, thereby modulating the host’s inflammatory state and immune function, and ultimately the development of multiple systemic diseases (Table 1). The downside is that this article may not have listed all the systemic diseases that may be associated with the pathogenic mechanisms of the oral-gut axis in the setting of periodontitis, such as lung disease and other cancers of the digestive tract such as pancreatic cancer. Some diseases, such as adverse pregnancy outcomes, have been shown to be strongly associated with both periodontitis and the gut microbiota, but no studies have yet reported whether their development may be influenced by the intestinal transit of periodontal bacteria. We hope that in the future more and more evidence will emerge to help us identify more systemic diseases that may have oral-gut transit of periodontal bacteria involved in the pathogenic mechanism. In addition, this paper uses a wealth of evidence to interpret the causal role of periodontal bacteria in a variety of systemic diseases through the oral-gut axis, the oral-gut-liver axis and the oral-gut-brain axis in the pathogenesis of disease. This suggests a potential therapeutic and preventive route by blocking the transmission route. However, periodontal bacteria may also play a pathogenic role in distal organs through other parenteral routes. Further studies are needed to elucidate whether indirect regulation of periodontal bacteria through the oral-gut route is predominant.
Dysbiosis of the oral microbiota is closely associated with the development and progression of many systemic diseases. In recent years there has been an increasing interest in therapeutic interventions to regulate and re-establish the homeostasis of the human oral microbiota and thereby restore systemic health. Inspired by FMT, oral microbiota transplantation (OMT) has started to be considered by researchers as a promising treatment for improving oral and extra-oral diseases. The OMT procedure recommended in the current study consists of (i) collection of supra/subgingival plaque from a healthy donor followed by in vitro culture to obtain a sample with the healthiest oral microbiota, (ii) pre-treatment of the recipient’s oral cavity, e.g., physical plaque removal and the application of antimicrobial agents, and lastly (iii) transplantation of the donor oral microbiota is accomplished by rinsing/applying (hydrogel may be considered as a delivery vehicle) (Beikler et al., 2021; Nath et al., 2021; Xiao et al., 2021; Weyrich et al., 2024). The oral microbiota appears to be more stable than other body niches (e.g. the gut), but at the same time oral biofilms display significant phenotypic plasticity (microbial community composition fluctuates in response to changes in the oral environment). Introducing health-associated oral microbiota into the oral cavity of a diseased patient is a key factor in the potential therapeutic role of OMT by shaping and altering the composition and function of the human microbial community, which in turn affects systemic health and disease states.
The potential of OMT in the treatment of oral diseases (e.g. dental caries and periodontitis) has now been documented (Pozhitkov et al., 2015; Nascimento, 2017; Nath et al., 2021). Beikler et al. validated the safety and efficacy of OMT in the treatment of periodontitis in dogs (Beikler et al., 2021). The findings suggest that a single OMT from a healthy periodontal donor as an adjunct to mechanical and chemical full-mouth debridement has a greater modulating effect on the oral microbiota composition in dogs with naturally occurring periodontitis than full-mouth mechanical and antimicrobial debridement alone. In addition, using an experimental periodontitis mouse model, Xiao et al. showed that OMT from healthy to irradiated mice reduced OM-related epithelial damage and oral and systemic inflammation by attenuating irradiation-induced alterations in oral and gut microbiota (Xiao et al., 2021). The results of this study support the mechanism of the oral-gut axis and confirm that OMT may be employed as a novel therapeutic avenue for radiation-induced oral mucositis of head and neck cancer patients after radiotherapy in preclinical settings (Xiao et al., 2021). However, more in vivo studies are still needed to confirm the safety and efficacy of OMT in the treatment of human disease. In the meantime, OMT is only an adjunct to the treatment of systemic disease and patients will need to take other therapeutic measures to manage and treat the disease.
This article illustrates the far-reaching implications of maintaining periodontal health in reducing the risk of many intestinal and extraintestinal diseases. Some studies have already been conducted on periodontal treatment or modification of the oral microbiota as an entry point for the treatment and prevention of systemic diseases, such as targeted inhibitors against virulence factors of periodontal bacteria to reduce the ability of bacteria to translocate to distal tissues. However, more research is still needed in the future to investigate the role of periodontitis in the regulation of systemic disease pathogenesis through the oral-gut axis and to clarify the importance of oral microbiota defense in building systemic microbiota immunity. This may provide insight into the underlying pathogenesis of many systemic diseases and the search for new preventive and therapeutic strategies.
Author contributions
MX: Writing – original draft. QR: Writing – original draft. SZ: Writing – review & editing. JL: Writing – review & editing. WQ: Writing – review & editing. CX: Writing – review & editing. XW: Writing – review & editing. NA: Supervision, Writing – review & editing. JN: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Medical Scientific Research Foundation of Guangdong Province (No. B2022262, No. A2022522, and No. A2023450), Chinese Medicine Research Projects of Guangdong Province (No.20222144), Hygiene and Health Appropriate Technology Promotion Project of Guangdong Province (No. 202106241649075233), Research Incubation Program of Stomatological Hospital of Southern Medical University (No. PY2021023, No. PY2021024, No. PY2021027, and No. PY2021015), and Precision Treatment of Periodontitis Combined with Atherosclerosis, “Pearl River Scholars-Tianshan Talents” Cooperative Expert Workshop Innovation Team (No. KAYY202110).
Acknowledgments
The authors would like to thank all medical staff of the Stomatological Hospital of the Southern Medical University for their great help. Figures were created with BioRender software (https://biorender.com/).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abed, J., Emgård, J. E., Zamir, G., Faroja, M., Almogy, G., Grenov, A., et al. (2016). Fap2 mediates Fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumor-expressed Gal-GalNAc. Cell Host Microbe 20, 215–225. doi: 10.1016/j.chom.2016.07.006
Åberg, F., Helenius-Hietala, J. (2022). Oral health and liver disease: bidirectional associations—a narrative review. Dentistry J. 10, 16. doi: 10.3390/dj10020016
Abusleme, L., Hoare, A., Hong, B. Y., Diaz, P. I. (2021). Microbial signatures of health, gingivitis, and periodontitis. Periodontol. 2000 86, 57–78. doi: 10.1111/prd.12362
Akinkugbe, A. A., Slade, G. D., Barritt, A. S., Cole, S. R., Offenbacher, S., Petersmann, A., et al. (2017). Periodontitis and Non-alcoholic Fatty Liver Disease, a population-based cohort investigation in the Study of Health in Pomerania. J. Clin. Periodontol. 44, 1077–1087. doi: 10.1111/jcpe.2017.44.issue-11
Akram, Z., Baharuddin, N. A., Vaithilingam, R. D., Rahim, Z. H., Chinna, K., Krishna, V. G., et al. (2017). Effect of nonsurgical periodontal treatment on clinical periodontal variables and salivary resistin levels in obese Asians. J. Oral. Sci. 59, 93–102. doi: 10.2334/josnusd.16-0127
Albillos, A., De Gottardi, A., Rescigno, M. (2020). The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 72, 558–577. doi: 10.1016/j.jhep.2019.10.003
Anderson, A. C., Rothballer, M., Altenburger, M. J., Woelber, J. P., Karygianni, L., Vach, K., et al. (2020). Long-term fluctuation of oral biofilm microbiota following different dietary phases. Appl. Environ. Microbiol. 86, e01421–e01420. doi: 10.1128/AEM.01421-20
Aoyama, N., Fujii, T., Kida, S., Nozawa, I., Taniguchi, K., Fujiwara, M., et al. (2021). Association of periodontal status, number of teeth, and obesity: A cross-sectional study in Japan. J. Clin. Med. 10, 208. doi: 10.3390/jcm10020208
Arimatsu, K., Yamada, H., Miyazawa, H., Minagawa, T., Nakajima, M., Ryder, M. I., et al. (2014). Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci. Rep. 4, 4828. doi: 10.1038/srep04828
Arnsten, A. F., Datta, D., Del Tredici, K., Braak, H. (2021). Hypothesis: Tau pathology is an initiating factor in sporadic Alzheimer’s disease. Alzheimer’s Dementia. 17, 115–124. doi: 10.1002/alz.12192
Aron-Wisnewsky, J., Clément, K., Nieuwdorp, M. (2019). Fecal microbiota transplantation: a future therapeutic option for obesity/diabetes? Curr. Diabetes Rep. 19, 1–9. doi: 10.1007/s11892-019-1180-z
Azzouz, D., Omarbekova, A., Heguy, A., Schwudke, D., Gisch, N., Rovin, B. H., et al. (2019). Lupus nephritis is linked to disease-activity associated expansions and immunity to a gut commensal. Ann. Rheum. Dis. 78, 947–956. doi: 10.1136/annrheumdis-2018-214856
Baima, G., Ferrocino, I., Del Lupo, V., Colonna, E., Thumbigere-Math, V., Caviglia, G., et al. (2024). Effect of periodontitis and periodontal therapy on Oral and gut microbiota. J. Dent. Res. 103, 359–368. doi: 10.1177/00220345231222800
Bajaj, J. S., Betrapally, N. S., Hylemon, P. B., Heuman, D. M., Daita, K., White, M. B., et al. (2015). Salivary microbiota reflects changes in gut microbiota in cirrhosis with hepatic encephalopathy. Hepatology 62, 1260–1271. doi: 10.1002/hep.27819
Bajaj, J. S., Matin, P., White, M. B., Fagan, A., Deeb, J. G., Acharya, C., et al. (2018). Periodontal therapy favorably modulates the oral-gut-hepatic axis in cirrhosis. Am. J. Physiology-Gastrointestinal Liver Physiol. 315, 824-837. doi: 10.1152/ajpgi.00230.2018
Bao, J., Li, L., Zhang, Y., Wang, M., Chen, F., Ge, S., et al. (2022). Periodontitis may induce gut microbiota dysbiosis via salivary microbiota. Int. J. Oral. sci. 14, 32. doi: 10.1038/s41368-022-00183-3
Barutta, F., Bellini, S., Durazzo, M., Gruden, G. (2022). Novel insight into the mechanisms of the bidirectional relationship between diabetes and periodontitis. Biomedicines 10, 178. doi: 10.3390/biomedicines10010178
Beikler, T., Bunte, K., Chan, Y., Weiher, B., Selbach, S., Peters, U., et al. (2021). Oral microbiota transplant in dogs with naturally occurring periodontitis. J. Dent. Res. 100, 764–770. doi: 10.1177/0022034521995423
Bellando-Randone, S., Russo, E., Venerito, V., Matucci-Cerinic, M., Iannone, F., Tangaro, S., et al. (2021). Exploring the oral microbiome in rheumatic diseases, state of art and future prospective in personalized medicine with an AI approach. J. Personalized Med. 11, 625. doi: 10.3390/jpm11070625
Benahmed, A. G., Gasmi, A., Doşa, A., Chirumbolo, S., Mujawdiya, P. K., Aaseth, J., et al. (2021). Association between the gut and oral microbiome with obesity. Anaerobe 70, 102248. doi: 10.1016/j.anaerobe.2020.102248
Bharti, P., Katagiri, S., Nitta, H., Nagasawa, T., Kobayashi, H., Takeuchi, Y., et al. (2013). Periodontal treatment with topical antibiotics improves glycemic control in association with elevated serum adiponectin in patients with type 2 diabetes mellitus. Obes. Res. Clin. Pract. 7, e129–ee38. doi: 10.1016/j.orcp.2011.11.005
Birchenough, G. M., Johansson, M. E., Gustafsson, J. K., Bergström, J. H., Hansson, G. (2015). New developments in goblet cell mucus secretion and function. Mucosal Immunol. 8, 712–719. doi: 10.1038/mi.2015.32
Blasco-Baque, V., Garidou, L., Pomié, C., Escoula, Q., Loubieres, P., Le Gall-David, S., et al. (2017). Periodontitis induced by Porphyromonas gingivalis drives periodontal microbiota dysbiosis and insulin resistance via an impaired adaptive immune response. Gut 66, 872–885. doi: 10.1136/gutjnl-2015-309897
Blauvelt, A., Chiricozzi, A. (2018). The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin. Rev. Allergy Immunol. 55, 379–390. doi: 10.1007/s12016-018-8702-3
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. doi: 10.3322/caac.21492
Brennan, C. A., Garrett, W. S. (2019). Fusobacterium nucleatum—symbiont, opportunist and oncobacterium. Nat. Rev. Microbiol. 17, 156–166. doi: 10.1038/s41579-018-0129-6
Brewer, R. C., Lanz, T. V., Hale, C. R., Sepich-Poore, G. D., Martino, C., Swafford, A. D., et al. (2023). Oral mucosal breaks trigger anti-citrullinated bacterial and human protein antibody responses in rheumatoid arthritis. Sci. Transl. Med. 15, eabq8476. doi: 10.1126/scitranslmed.abq8476
Brodala, N., Merricks, E. P., Bellinger, D. A., Damrongsri, D., Offenbacher, S., Beck, J., et al. (2005). Porphyromonas gingivalis bacteremia induces coronary and aortic atherosclerosis in normocholesterolemic and hypercholesterolemic pigs. Arterioscler. Thromb. Vasc. Biol. 25, 1446–1451. doi: 10.1161/01.ATV.0000167525.69400.9c
Brown, J., Quattrochi, B., Everett, C., Hong, B.-Y., Cervantes, J. (2021). Gut commensals, dysbiosis, and immune response imbalance in the pathogenesis of multiple sclerosis. Multiple Sclerosis J. 27, 807–811. doi: 10.1177/1352458520928301
Bruggeman, A., Vandendriessche, C., Hamerlinck, H., De Looze, D., Tate, D. J., Vuylsteke, M., et al. (2024). Safety and efficacy of faecal microbiota transplantation in patients with mild to moderate Parkinson’s disease (GUT-PARFECT): a double-blind, placebo-controlled, randomised, phase 2 trial. EClinicalMedicine 71, 102563. doi: 10.1016/j.eclinm.2024.102563
Bullman, S., Pedamallu, C. S., Sicinska, E., Clancy, T. E., Zhang, X., Cai, D., et al. (2017). Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 358, 1443–1448. doi: 10.1126/science.aal5240
Bunte, K., Beikler, T. (2019). Th17 cells and the IL-23/IL-17 axis in the pathogenesis of periodontitis and immune-mediated inflammatory diseases. Int. J. Mol. Sci. 20, 3394. doi: 10.3390/ijms20143394
Calandrini, C., Ribeiro, A., Gonnelli, A., Ota-Tsuzuki, C., Rangel, L., Saba-Chujfi, E., et al. (2014). Microbial composition of atherosclerotic plaques. Oral. Dis. 20, e128–ee34. doi: 10.1111/odi.12205
Cao, P., Chen, Y., Guo, X., Chen, Y., Su, W., Zhan, N., et al. (2020). Fusobacterium nucleatum activates endoplasmic reticulum stress to promote Crohn’s disease development via the upregulation of CARD3 expression. Front. Pharmacol. 11, 106. doi: 10.3389/fphar.2020.00106
Carrion, J., Scisci, E., Miles, B., Sabino, G. J., Zeituni, A. E., Gu, Y., et al. (2012). Microbial carriage state of peripheral blood dendritic cells (DCs) in chronic periodontitis influences DC differentiation, atherogenic potential. J. Immunol. 189, 3178–3187. doi: 10.4049/jimmunol.1201053
Carrouel, F., Viennot, S., Santamaria, J., Veber, P., Bourgeois, D. (2016). Quantitative molecular detection of 19 major pathogens in the interdental biofilm of periodontally healthy young adults. Front. Microbiol. 7, 840. doi: 10.3389/fmicb.2016.00840
Chaffee, B. W., Weston, S. J. (2010). Association between chronic periodontal disease and obesity: a systematic review and meta-analysis. J. Periodontol. 81, 1708–1724. doi: 10.1902/jop.2010.100321
Chang, Y., Lee, J. S., Lee, K.-J., Woo, H. G., Song, T.-J. (2020). Improved oral hygiene is associated with decreased risk of new-onset diabetes: a nationwide population-based cohort study. Diabetologia 63, 924–933. doi: 10.1007/s00125-020-05112-9
Chen, Y., Chen, Y., Cao, P., Su, W., Zhan, N., Dong, W. (2020a). Fusobacterium nucleatum facilitates ulcerative colitis through activating IL-17F signaling to NF-κB via the upregulation of CARD3 expression. J. pathol. 250, 170–182. doi: 10.1002/path.v250.2
Chen, Y., Chen, Y., Zhang, J., Cao, P., Su, W., Deng, Y., et al. (2020b). Fusobacterium nucleatum promotes metastasis in colorectal cancer by activating autophagy signaling via the upregulation of CARD3 expression. Theranostics 10, 323. doi: 10.7150/thno.38870
Chen, D.-Y., Lin, C.-H., Chen, Y.-M., Chen, H.-H. (2016). Risk of atrial fibrillation or flutter associated with periodontitis: a nationwide, population-based, cohort study. PloS One 11, e0165601. doi: 10.1371/journal.pone.0165601
Chen, W., Liu, F., Ling, Z., Tong, X., Xiang, C. (2012). Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PloS One 7, e39743. doi: 10.1371/journal.pone.0039743
Chen, J., Pitmon, E., Wang, K. (2017). Microbiome, inflammation and colorectal cancer. Semin. Immunol. 32, 43-53. doi: 10.1016/j.smim.2017.09.006
Chen, S., Su, T., Zhang, Y., Lee, A., He, J., Ge, Q., et al. (2020). Fusobacterium nucleatum promotes colorectal cancer metastasis by modulating KRT7-AS/KRT7. Gut Microbes 11, 511–525. doi: 10.1080/19490976.2019.1695494
Chen, Q., Wu, J., Dong, X., Yin, H., Shi, X., Su, S., et al. (2021). Gut flora-targeted photobiomodulation therapy improves senile dementia in an Aß-induced Alzheimer’s disease animal model. J. Photochem. Photobiol. B: Biol. 216, 112152.
Chidambaram, S. B., Rathipriya, A. G., Mahalakshmi, A. M., Sharma, S., Hediyal, T. A., Ray, B., et al. (2022). The influence of gut dysbiosis in the pathogenesis and management of ischemic stroke. Cells 11, 1239. doi: 10.3390/cells11071239
Cho, N. H., Shaw, J. E., Karuranga, S., Huang, Y., da Rocha Fernandes, J. D., Ohlrogge, A., et al. (2018). IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 138, 271–281. doi: 10.1016/j.diabres.2018.02.023
Ciesielska, A., Matyjek, M., Kwiatkowska, K. (2021). TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol. Life Sci. 78, 1233–1261. doi: 10.1007/s00018-020-03656-y
Coit, P., Mumcu, G., Ture-Ozdemir, F., Unal, A. U., Alpar, U., Bostanci, N., et al. (2016). Sequencing of 16S rRNA reveals a distinct salivary microbiome signature in Behcet’s disease. Clin. Immunol. 169, 28–35. doi: 10.1016/j.clim.2016.06.002
Consolandi, C., Turroni, S., Emmi, G., Severgnini, M., Fiori, J., Peano, C., et al. (2015). Behçet’s syndrome patients exhibit specific microbiome signature. Autoimmun. Rev. 14, 269–276. doi: 10.1016/j.autrev.2014.11.009
Coquant, G., Aguanno, D., Pham, S., Grellier, N., Thenet, S., Carrière, V., et al. (2021). Gossip in the gut: Quorum sensing, a new player in the host-microbiota interactions. World J. Gastroenterol. 27, 7247. doi: 10.3748/wjg.v27.i42.7247
Cotillard, A., Kennedy, S. P., Kong, L. C., Prifti, E., Pons, N., Le Chatelier, E., et al. (2013). Dietary intervention impact on gut microbial gene richness. Nature 500, 585–588. doi: 10.1038/nature12480
Crawford, C. K., Lopez Cervantes, V., Quilici, M. L., Armién, A. G., Questa, M., Matloob, M. S., et al. (2022). Inflammatory cytokines directly disrupt the bovine intestinal epithelial barrier. Sci. Rep. 12, 14578. doi: 10.1038/s41598-022-18771-y
Cryan, J. F., O’Riordan, K. J., Cowan, C. S., Sandhu, K. V., Bastiaanssen, T. F., Boehme, M., et al. (2019). The microbiota-gut-brain axis. Physiol. Rev. 99, 1877-2013. doi: 10.1152/physrev.00018.2018
Cunha, F. A., Cota, L. O., Cortelli, S. C., Miranda, T. B., Neves, F. S., Cortelli, J. R., et al. (2019). Periodontal condition and levels of bacteria associated with periodontitis in individuals with bipolar affective disorders: A case-control study. J. Periodontal Res. 54, 63–72. doi: 10.1111/jre.2019.54.issue-1
D’Aiuto, F., Gkranias, N., Bhowruth, D., Khan, T., Orlandi, M., Suvan, J., et al. (2018). Systemic effects of periodontitis treatment in patients with type 2 diabetes: a 12 month, single-centre, investigator-masked, randomised trial. Lancet Diabetes endocrinol. 6, 954–965. doi: 10.1016/S2213-8587(18)30038-X
Dalmády, S., Kemény, L., Antal, M., Gyulai, R. (2020). Periodontitis: a newly identified comorbidity in psoriasis and psoriatic arthritis. Expert Rev. Clin. Immunol. 16, 101–108. doi: 10.1080/1744666X.2019.1700113
Daneman, R., Rescigno, M. (2009). The gut immune barrier and the blood-brain barrier: are they so different? Immunity 31, 722–735. doi: 10.1016/j.immuni.2009.09.012
Dang, A. T., Marsland, B. J. (2019). Microbes, metabolites, and the gut–lung axis. Mucosal Immunol. 12, 843–850. doi: 10.1038/s41385-019-0160-6
Darveau, R., Hajishengallis, G., Curtis, M. (2012). Porphyromonas gingivalis as a potential community activist for disease. J. Dent. Res. 91, 816–820. doi: 10.1177/0022034512453589
de Aquino, S. G., Abdollahi-Roodsaz, S., Koenders, M. I., van de Loo, F. A., Pruijn, G. J., Marijnissen, R. J., et al. (2014). Periodontal pathogens directly promote autoimmune experimental arthritis by inducing a TLR2-and IL-1–driven Th17 response. J. Immunol. 192, 4103–4111. doi: 10.4049/jimmunol.1301970
Devaux, C. A., Mezouar, S., Mege, J.-L. (2019). The E-cadherin cleavage associated to pathogenic bacteria infections can favor bacterial invasion and transmigration, dysregulation of the immune response and cancer induction in humans. Front. Microbiol. 10, 2598. doi: 10.3389/fmicb.2019.02598
Diet, H. (2001). Immunopathogenesis of atherosclerosis. Circulation 1524, 4539. doi: 10.1161/hc3401.093153
Dietrich, T., Sharma, P., Walter, C., Weston, P., Beck, J. (2013). The epidemiological evidence behind the association between periodontitis and incident atherosclerotic cardiovascular disease. J. Periodontol. 84, S70–S84. doi: 10.1902/jop.2013.134008
Di Stefano, M., Santonocito, S., Polizzi, A., Mauceri, R., Troiano, G., Lo Giudice, A., et al. (2023). A reciprocal link between oral, gut microbiota during periodontitis: the potential role of probiotics in reducing dysbiosis-induced inflammation. Int. J. Mol. Sci. 24, 1084. doi: 10.3390/ijms24021084
Dominy, S. S., Lynch, C., Ermini, F., Benedyk, M., Marczyk, A., Konradi, A., et al. (2019). Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 5, eaau3333. doi: 10.1126/sciadv.aau3333
Drago, L., Zuccotti, G. V., Romanò, C. L., Goswami, K., Villafañe, J. H., Mattina, R., et al. (2019). Oral–gut microbiota and arthritis: is there an evidence-based axis? J. Clin. Med. 8, 1753. doi: 10.3390/jcm8101753
du Teil Espina, M., Gabarrini, G., Harmsen, H. J., Westra, J., van Winkelhoff, A. J., van Dijl, J. M. (2019). Talk to your gut: the oral-gut microbiome axis and its immunomodulatory role in the etiology of rheumatoid arthritis. FEMS Microbiol. Rev. 43, 1–18. doi: 10.1093/femsre/fuy035
Emoto, T., Yamashita, T., Kobayashi, T., Sasaki, N., Hirota, Y., Hayashi, T., et al. (2017). Characterization of gut microbiota profiles in coronary artery disease patients using data mining analysis of terminal restriction fragment length polymorphism: gut microbiota could be a diagnostic marker of coronary artery disease. Heart Vessels 32, 39–46. doi: 10.1007/s00380-016-0841-y
Emoto, T., Yamashita, T., Sasaki, N., Hirota, Y., Hayashi, T., So, A., et al. (2016). Analysis of gut microbiota in coronary artery disease patients: a possible link between gut microbiota and coronary artery disease. J. Atheroscl. thrombosis 23, 908–921. doi: 10.5551/jat.32672
Fan, X., Alekseyenko, A. V., Wu, J., Peters, B. A., Jacobs, E. J., Gapstur, S. M., et al. (2018). Human oral microbiome and prospective risk for pancreatic cancer: a population-based nested case-control study. Gut 67, 120–127. doi: 10.1136/gutjnl-2016-312580
Feng, Y.-K., Wu, Q.-L., Peng, Y.-W., Liang, F.-Y., You, H.-J., Feng, Y.-W., et al. (2020). Oral P. gingivalis impairs gut permeability and mediates immune responses associated with neurodegeneration in LRRK2 R1441G mice. J. Neuroinflammation 17, 1–12. doi: 10.1186/s12974-020-02027-5
Ferrere, G., Wrzosek, L., Cailleux, F., Turpin, W., Puchois, V., Spatz, M., et al. (2017). Fecal microbiota manipulation prevents dysbiosis and alcohol-induced liver injury in mice. J. Hepatol. 66, 806–815. doi: 10.1016/j.jhep.2016.11.008
Fiorentino, M., Sapone, A., Senger, S., Camhi, S. S., Kadzielski, S. M., Buie, T. M., et al. (2016). Blood–brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol. autism 7, 1–17. doi: 10.1186/s13229-016-0110-z
Flanagan, L., Schmid, J., Ebert, M., Soucek, P., Kunicka, T., Liska, V., et al. (2014). Fusobacterium nucleatum associates with stages of colorectal neoplasia development, colorectal cancer and disease outcome. Eur. J. Clin. Microbiol. Infect. Dis. 33, 1381–1390. doi: 10.1007/s10096-014-2081-3
Fleetwood, A. J., Lee, M. K., Singleton, W., Achuthan, A., Lee, M.-C., O’Brien-Simpson, N. M., et al. (2017). Metabolic remodeling, inflammasome activation, and pyroptosis in macrophages stimulated by Porphyromonas gingivalis and its outer membrane vesicles. Front. Cell. Infect. Microbiol. 7, 351. doi: 10.3389/fcimb.2017.00351
Ford, P., Gemmell, E., Chan, A., Carter, C., Walker, P., Bird, P., et al. (2006). Inflammation, heat shock proteins and periodontal pathogens in atherosclerosis: an immunohistologic study. Oral. Microbiol. Immunol. 21, 206–211. doi: 10.1111/j.1399-302X.2006.00276.x
Freire, M., Nelson, K. E., Edlund, A. (2021). The oral host–microbial interactome: an ecological chronometer of health? Trends Microbiol. 29, 551–561. doi: 10.1016/j.tim.2020.11.004
Garrido-Gil, P., Rodriguez-Perez, A. I., Dominguez-Meijide, A., Guerra, M. J., Labandeira-Garcia, J. L. (2018). Bidirectional neural interaction between central dopaminergic and gut lesions in Parkinson’s disease models. Mol. Neurobiol. 55, 7297–7316. doi: 10.1007/s12035-018-0937-8
Genco, R. J., Graziani, F., Hasturk, H. (2020). Effects of periodontal disease on glycemic control, complications, and incidence of diabetes mellitus. Periodontol. 2000 83, 59–65. doi: 10.1111/prd.12271
Graves, D. T., Corrêa, J. D., Silva, T. A. (2019). The oral microbiota is modified by systemic diseases. J. Dent. Res. 98, 148–156. doi: 10.1177/0022034518805739
Graziani, F., Gennai, S., Solini, A., Petrini, M. (2018). A systematic review and meta-analysis of epidemiologic observational evidence on the effect of periodontitis on diabetes An update of the EFP-AAP review. J. Clin. Periodontol. 45, 167–187. doi: 10.1111/jcpe.2018.45.issue-2
Gregory, J. C., Buffa, J. A., Org, E., Wang, Z., Levison, B. S., Zhu, W., et al. (2015). Transmission of atherosclerosis susceptibility with gut microbial transplantation. J. Biol. Chem. 290, 5647–5660. doi: 10.1074/jbc.M114.618249
Grine, L., Dejager, L., Libert, C., Vandenbroucke, R. E. (2015). An inflammatory triangle in psoriasis: TNF, type I IFNs and IL-17. Cytokine Growth Factor Rev. 26, 25–33. doi: 10.1016/j.cytogfr.2014.10.009
Guggenheimer, J., Eghtesad, B., Close, J. M., Shay, C., Fung, J. J. (2007). Dental health status of liver transplant candidates. Liver Transpl. 13, 280–286. doi: 10.1002/(ISSN)1527-6473
Guo, S., Chen, J., Chen, F., Zeng, Q., Liu, W.-L., Zhang, G. (2021). Exosomes derived from Fusobacterium nucleatum-infected colorectal cancer cells facilitate tumour metastasis by selectively carrying miR-1246/92b-3p/27a-3p and CXCL16. Gut 70, 1507–1519. doi: 10.1136/gutjnl-2020-321187
Guo, P., Tian, Z., Kong, X., Yang, L., Shan, X., Dong, B., et al. (2020). FadA promotes DNA damage and progression of Fusobacterium nucleatum-induced colorectal cancer through up-regulation of chk2. J. Exp. Clin. Cancer Res. 39, 1–13. doi: 10.1186/s13046-020-01677-w
Gur, C., Ibrahim, Y., Isaacson, B., Yamin, R., Abed, J., Gamliel, M., et al. (2015). Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 42, 344–355. doi: 10.1016/j.immuni.2015.01.010
Gutowska-Owsiak, D., Schaupp, A. L., Salimi, M., Selvakumar, T. A., McPherson, T., Taylor, S., et al. (2012). IL-17 downregulates filaggrin and affects keratinocyte expression of genes associated with cellular adhesion. Exp. Dermatol. 21, 104–110. doi: 10.1111/j.1600-0625.2011.01412.x
Hajishengallis, G. (2014). Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 35, 3–11. doi: 10.1016/j.it.2013.09.001
Hajishengallis, G., Chavakis, T. (2021). Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 21, 426–440. doi: 10.1038/s41577-020-00488-6
Hajishengallis, G., Li, X., Divaris, K., Chavakis, T. (2022). Maladaptive trained immunity and clonal hematopoiesis as potential mechanistic links between periodontitis and inflammatory comorbidities. Periodontol. 2000 89, 215–230. doi: 10.1111/prd.12421
Hamamoto, Y., Ouhara, K., Munenaga, S., Shoji, M., Ozawa, T., Hisatsune, J., et al. (2020). Effect of Porphyromonas gingivalis infection on gut dysbiosis and resultant arthritis exacerbation in mouse model. Arthritis Res. Ther. 22, 1–15. doi: 10.1186/s13075-020-02348-z
Hatasa, M., Ohsugi, Y., Katagiri, S., Yoshida, S., Niimi, H., Morita, K., et al. (2021). Endotoxemia by Porphyromonas gingivalis alters endocrine functions in brown adipose tissue. Front. Cell. Infection Microbiol. 10, 580577. doi: 10.3389/fcimb.2020.580577
Higashi, D. L., Qin, H., Borland, C., Kreth, J., Merritt, J. (2024). An inflammatory paradox: strategies inflammophilic oral pathobionts employ to exploit innate immunity via neutrophil manipulation. Front. Oral. Health 5, 1413842. doi: 10.3389/froh.2024.1413842
Hollander, D., Kaunitz, J. D. (2020). The “leaky gut”: tight junctions but loose associations? Dig Dis. Sci. 65, 1277–1287. doi: 10.1007/s10620-019-05777-2
Holmqvist, S., Chutna, O., Bousset, L., Aldrin-Kirk, P., Li, W., Björklund, T., et al. (2014). Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 128, 805–820. doi: 10.1007/s00401-014-1343-6
Hong, M., Li, Z., Liu, H., Zheng, S., Zhang, F., Zhu, J., et al. (2023). Fusobacterium nucleatum aggravates rheumatoid arthritis through FadA-containing outer membrane vesicles. Cell Host Microbe 31, 798–810. e7. doi: 10.1016/j.chom.2023.03.018
Hou, K., Wu, Z.-X., Chen, X.-Y., Wang, J.-Q., Zhang, D., Xiao, C., et al. (2022). Microbiota in health and diseases. Signal Transduct. Target. Ther. 7, 1–28. doi: 10.1038/s41392-022-00974-4
Huang, Y., Liu, Z., Wang, X.-Q., Qiu, Y.-H., Peng, Y.-P. (2014). A dysfunction of CD4+ T lymphocytes in peripheral immune system of Parkinson’s disease model mice. Zhongguo ying yong sheng li xue za zhi= Zhongguo yingyong shenglixue zazhi= Chin. J. Appl. Physiol. 30, 567–576. doi: 10.13459/j.cnki.cjap.2014.06.013
Hussain, S. B., Leira, Y., Zehra, S. A., Botelho, J., MaChado, V., Ciurtin, C., et al. (2022). Periodontitis and Systemic Lupus Erythematosus: A systematic review and meta-analysis. J. Periodontal Res. 57, 1–10. doi: 10.1111/jre.12936
Hussain, M., Stover, C. M., Dupont, A. P. (2015). gingivalis in periodontal disease and atherosclerosis–scenes of action for antimicrobial peptides and complement. Front. Immunol. 6, 45. doi: 10.3389/fimmu.2015.00045
Huynh, K. (2020). Novel gut microbiota-derived metabolite promotes platelet thrombosis via adrenergic receptor signalling. Nat. Rev. Cardiol. 17, 265. doi: 10.1038/s41569-020-0367-y
Ji, S., Hyun, J., Park, E., Lee, B. L., Kim, K. K., Choi, Y. (2007). Susceptibility of various oral bacteria to antimicrobial peptides and to phagocytosis by neutrophils. J. Periodontal Res. 42, 410–419. doi: 10.1111/j.1600-0765.2006.00962.x
Jia, L., Jiang, Y., Wu, L., Fu, J., Du, J., Luo, Z., et al. (2024). Porphyromonas gingivalis aggravates colitis via a gut microbiota-linoleic acid metabolism-Th17/Treg cell balance axis. Nat. Commun. 15, 1617. doi: 10.1038/s41467-024-45473-y
Jie, Z., Xia, H., Zhong, S.-L., Feng, Q., Li, S., Liang, S., et al. (2017). The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 8, 845. doi: 10.1038/s41467-017-00900-1
Jin, U.-H., Lee, S.-O., Sridharan, G., Lee, K., Davidson, L. A., Jayaraman, A., et al. (2014). Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol. Pharmacol. 85, 777–788. doi: 10.1124/mol.113.091165
Jin, M., Qian, Z., Yin, J., Xu, W., Zhou, X. (2019). The role of intestinal microbiota in cardiovascular disease. J. Cell Mol. Med. 23, 2343–2350. doi: 10.1111/jcmm.2019.23.issue-4
Johnson, D., Letchumanan, V., Thurairajasingam, S., Lee, L.-H. (2020). A revolutionizing approach to autism spectrum disorder using the microbiome. Nutrients 12, 1983. doi: 10.3390/nu12071983
Kageyama, S., Sakata, S., Ma, J., Asakawa, M., Takeshita, T., Furuta, M., et al. (2023). High-resolution detection of translocation of oral bacteria to the gut. J. Dent. Res. 102, 752–758. doi: 10.1177/00220345231160747
Kakiyama, G., Hylemon, P. B., Zhou, H., Pandak, W. M., Heuman, D. M., Kang, D. J., et al. (2014). Colonic inflammation and secondary bile acids in alcoholic cirrhosis. Am. J. Physiology-Gastrointestinal Liver Physiol. 306, G929–GG37. doi: 10.1152/ajpgi.00315.2013
Kashiwagi, Y., Aburaya, S., Sugiyama, N., Narukawa, Y., Sakamoto, Y., Takahashi, M., et al. (2021). Porphyromonas gingivalis induces entero-hepatic metabolic derangements with alteration of gut microbiota in a type 2 diabetes mouse model. Sci. Rep. 11, 18398. doi: 10.1038/s41598-021-97868-2
Kato, T., Yamazaki, K., Nakajima, M., Date, Y., Kikuchi, J., Hase, K., et al. (2018). Oral administration of Porphyromonas gingivalis alters the gut microbiome and serum metabolome. Msphere 3, e00460-18. doi: 10.1128/msphere.00460-18
Khor, B., Snow, M., Herrman, E., Ray, N., Mansukhani, K., Patel, K. A., et al. (2021). Interconnections between the oral and gut microbiomes: reversal of microbial dysbiosis and the balance between systemic health and disease. Microorganisms 9, 496. doi: 10.3390/microorganisms9030496
Kinashi, Y., Hase, K. (2021). Partners in leaky gut syndrome: intestinal dysbiosis and autoimmunity. Front. Immunol. 12, 673708. doi: 10.3389/fimmu.2021.673708
Kitamoto, S., Kamada, N. (2022a). Periodontal connection with intestinal inflammation: Microbiological and immunological mechanisms. Periodontol. 2000 89, 142–153. doi: 10.1111/prd.12424
Kitamoto, S., Kamada, N. (2022b). Untangling the oral–gut axis in the pathogenesis of intestinal inflammation. Int. Immunol. 34, 485–490. doi: 10.1093/intimm/dxac027
Kitamoto, S., Nagao-Kitamoto, H., Jiao, Y., Gillilland, M. G., Hayashi, A., Imai, J., et al. (2020). The intermucosal connection between the mouth and gut in commensal pathobiont-driven colitis. Cell 182, 447–62. e14. doi: 10.1016/j.cell.2020.05.048
Komazaki, R., Katagiri, S., Takahashi, H., Maekawa, S., Shiba, T., Takeuchi, Y., et al. (2017). Periodontal pathogenic bacteria, Aggregatibacter actinomycetemcomitans affect non-alcoholic fatty liver disease by altering gut microbiota and glucose metabolism. Sci. Rep. 7, 1–14. doi: 10.1038/s41598-017-14260-9
Kong, X., Liu, J., Cetinbas, M., Sadreyev, R., Koh, M., Huang, H., et al. (2019). New and preliminary evidence on altered oral and gut microbiota in individuals with autism spectrum disorder (ASD): implications for ASD diagnosis and subtyping based on microbial biomarkers. Nutrients 11, 2128. doi: 10.3390/nu11092128
Kong, C., Yan, X., Zhu, Y., Zhu, H., Luo, Y., Liu, P., et al. (2021). Fusobacterium nucleatum promotes the development of colorectal cancer by activating a cytochrome P450/epoxyoctadecenoic acid axis via TLR4/Keap1/NRF2 signaling. Cancer Res. 81, 4485–4498. doi: 10.1158/0008-5472.CAN-21-0453
Konig, M. F., Abusleme, L., Reinholdt, J., Palmer, R. J., Teles, R. P., Sampson, K., et al. (2016). Aggregatibacter actinomycetemcomitans–induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci. Transl. Med. 8, 369ra176–369ra176. doi: 10.1126/scitranslmed.aaj1921
Kootte, R. S., Levin, E., Salojärvi, J., Smits, L. P., Hartstra, A. V., Udayappan, S. D., et al. (2017). Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 26, 611–9. e6. doi: 10.1016/j.cmet.2017.09.008
Koren, O., Spor, A., Felin, J., Fåk, F., Stombaugh, J., Tremaroli, V., et al. (2011). Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc. Natl. Acad. Sci. 108, 4592–4598. doi: 10.1073/pnas.1011383107
Kostic, A. D., Chun, E., Robertson, L., Glickman, J. N., Gallini, C. A., Michaud, M., et al. (2013). Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 14, 207–215. doi: 10.1016/j.chom.2013.07.007
Kozina, E., Sadasivan, S., Jiao, Y., Dou, Y., Ma, Z., Tan, H., et al. (2018). Mutant LRRK2 mediates peripheral and central immune responses leading to neurodegeneration in vivo. Brain 141, 1753–1769. doi: 10.1093/brain/awy077
Kramer, C. D., Simas, A. M., He, X., Ingalls, R. R., Weinberg, E. O., Genco, C. A. (2017). Distinct roles for dietary lipids and Porphyromonas gingivalis infection on atherosclerosis progression and the gut microbiota. Anaerobe 45, 19–30. doi: 10.1016/j.anaerobe.2017.04.011
Kuraji, R., Shiba, T., Dong, T. S., Numabe, Y., Kapila, Y. L. (2023). Periodontal treatment and microbiome-targeted therapy in management of periodontitis-related nonalcoholic fatty liver disease with oral and gut dysbiosis. World J. Gastroenterol. 29, 967. doi: 10.3748/wjg.v29.i6.967
Kurushima, Y., Wells, P., Bowyer, R., Zoheir, N., Doran, S., Richardson, J., et al. (2023). Host genotype links to salivary and gut microbiota by periodontal status. J. Dent. Res. 102, 146–156. doi: 10.1177/00220345221125402
Ladegaard Grønkjær, L., Holmstrup, P., Schou, S., Jepsen, P., Vilstrup, H. (2018). Severe periodontitis and higher cirrhosis mortality. United Eur. Gastroenterol. J. 6, 73–80. doi: 10.1177/2050640617715846
Lappin, D. F., Apatzidou, D., Quirke, A. M., Oliver-Bell, J., Butcher, J. P., Kinane, D. F., et al. (2013). Influence of periodontal disease, Porphyromonas gingivalis and cigarette smoking on systemic anti-citrullinated peptide antibody titres. J. Clin. Periodontol. 40, 907–915. doi: 10.1111/jcpe.2013.40.issue-10
Larsen, N., Vogensen, F. K., Van Den Berg, F. W., Nielsen, D. S., Andreasen, A. S., Pedersen, B. K., et al. (2010). Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PloS One 5, e9085. doi: 10.1371/journal.pone.0009085
Le Chatelier, E., Nielsen, T., Qin, J., Prifti, E., Hildebrand, F., Falony, G., et al. (2013). Richness of human gut microbiome correlates with metabolic markers. Nature 500, 541–546. doi: 10.1038/nature12506
Lee, Y.-C., Liu, C.-Y., Lee, C.-L., Zhang, R.-H., Huang, C.-J., Yen, T.-L. (2022). The periodontopathic pathogen, porphyromonas gingivalis, involves a gut inflammatory response and exacerbates inflammatory bowel disease. Pathogens 11, 84. doi: 10.3390/pathogens11010084
Li, L., Bao, J., Chang, Y., Wang, M., Chen, B., Yan, F. (2021). Gut microbiota may mediate the influence of periodontitis on prediabetes. J. Dent. Res. 100, 1387–1396. doi: 10.1177/00220345211009449
Li, J., Lu, H., Wu, H., Huang, S., Chen, L., Gui, Q., et al. (2020). Periodontitis in elderly patients with type 2 diabetes mellitus: impact on gut microbiota and systemic inflammation. Aging (Albany NY). 12, 25956. doi: 10.18632/aging.202174
Li, L., Michel, R., Cohen, J., DeCarlo, A., Kozarov, E. (2008). Intracellular survival and vascular cell-to-cell transmission of Porphyromonas gingivalis. BMC Microbiol. 8, 1–11. doi: 10.1186/1471-2180-8-26
Liljestrand, J. M., Paju, S., Buhlin, K., Persson, G. R., Sarna, S., Nieminen, M. S., et al. (2017). Lipopolysaccharide, a possible molecular mediator between periodontitis and coronary artery disease. J. Clin. Periodontol. 44, 784–792. doi: 10.1111/jcpe.2017.44.issue-8
Lin, G., Chen, S., Lei, L., You, X., Huang, M., Luo, L., et al. (2015). Effects of intravenous injection of Porphyromonas gingivalis on rabbit inflammatory immune response and atherosclerosis. Mediators Inflamm. 2015, 364391. doi: 10.1155/2015/364391
Lin, C.-Y., Tseng, K.-S., Liu, J.-M., Chuang, H.-C., Lien, C.-H., Chen, Y.-C., et al. (2018). Increased risk of ulcerative colitis in patients with periodontal disease: a nationwide population-based cohort study. Int. J. Environ. Res. Public Health 15, 2602. doi: 10.3390/ijerph15112602
Lin, D., Yang, L., Wen, L., Lu, H., Chen, Q., Wang, Z. (2021). Crosstalk between the oral microbiota, mucosal immunity, and the epithelial barrier regulates oral mucosal disease pathogenesis. Mucosal Immunol. 14, 1247–1258. doi: 10.1038/s41385-021-00413-7
Linden, G. J., Linden, K., Yarnell, J., Evans, A., Kee, F., Patterson, C. C. (2012). All-cause mortality and periodontitis in 60–70-year-old men: a prospective cohort study. J. Clin. Periodontol. 39, 940–946. doi: 10.1111/j.1600-051X.2012.01923.x
Liu, Y., Fan, L., Cheng, Z., Yu, L., Cong, S., Hu, Y., et al. (2021a). Fecal transplantation alleviates acute liver injury in mice through regulating Treg/Th17 cytokines balance. Sci. Rep. 11, 1611. doi: 10.1038/s41598-021-81263-y
Liu, M., Han, Q., Yang, J. (2019). Trimethylamine-N-oxide (TMAO) increased aquaporin-2 expression in spontaneously hypertensive rats. Clin. Exp. Hypertens. 41, 312–322. doi: 10.1080/10641963.2018.1481420
Liu, Y., Huang, W., Wang, J., Ma, J., Zhang, M., Lu, X., et al. (2021b). Multifaceted impacts of periodontal pathogens in disorders of the intestinal barrier. Front. Immunol. 12, 693479. doi: 10.3389/fimmu.2021.693479
Liu, Z., Qiu, A.-W., Huang, Y., Yang, Y., Chen, J.-N., Gu, T.-T., et al. (2019). IL-17A exacerbates neuroinflammation and neurodegeneration by activating microglia in rodent models of Parkinson’s disease. Brain Behav. Immun. 81, 630–645. doi: 10.1016/j.bbi.2019.07.026
Liu, X., Zeng, B., Zhang, J., Li, W., Mou, F., Wang, H., et al. (2016). Role of the gut microbiome in modulating arthritis progression in mice. Sci. Rep. 6, 30594. doi: 10.1038/srep30594
Lucas López, R., Grande Burgos, M. J., Gálvez, A., Pérez Pulido, R. (2017). The human gastrointestinal tract and oral microbiota in inflammatory bowel disease: a state of the science review. APMIS 125, 3–10. doi: 10.1111/apm.2017.125.issue-1
Lundmark, A., Hu, Y. O., Huss, M., Johannsen, G., Andersson, A. F., Yucel-Lindberg, T. (2019). Identification of salivary microbiota and its association with host inflammatory mediators in periodontitis. Front. Cell. infection Microbiol. 9, 216. doi: 10.3389/fcimb.2019.00216
Ma, L., Morel, L. (2022). Loss of gut barrier integrity in lupus. Front. Immunol. 13, 919792. doi: 10.3389/fimmu.2022.919792
Maitre, Y., Micheneau, P., Delpierre, A., Mahalli, R., Guerin, M., Amador, G., et al. (2020). Did the brain and oral microbiota talk to each other? A review of the literature. J. Clin. Med. 9, 3876. doi: 10.3390/jcm9123876
Martínez, M., Martín-Hernández, D., Virto, L., MacDowell, K. S., Montero, E., González-Bris, Á, et al. (2021). Periodontal diseases and depression: A pre-clinical in vivo study. J. Clin. Periodontol. 48, 503–527. doi: 10.1111/jcpe.13420
Martinez-Martinez, R. E., Abud-Mendoza, C., Patiño-Marin, N., Rizo-Rodríguez, J. C., Little, J. W., Loyola-Rodríguez, J. P. (2009). Detection of periodontal bacterial DNA in serum and synovial fluid in refractory rheumatoid arthritis patients. J. Clin. Periodontol. 36, 1004–1010. doi: 10.1111/j.1600-051X.2009.01496.x
Masallat, D., Moemen, D. (2016). Gut bacterial microbiota in psoriasis: A case control study. Afr. J. Microbiol. Res. 10, 1337–1343. doi: 10.5897/AJMR2016.8046
McNabney, S. M., Henagan, T. M. (2017). Short chain fatty acids in the colon and peripheral tissues: a focus on butyrate, colon cancer, obesity and insulin resistance. Nutrients 9, 1348. doi: 10.3390/nu9121348
Meilian, X., Ting, Y., Ying, Z., Xin, H., Baoyi, X., Dongying, X., et al. (2016). Periodontal inflammation affects the mechanical and immune barrier functions of mice gut. Hua xi kou Qiang yi xue za zhi= Huaxi Kouqiang Yixue Zazhi= West China J. Stomatol. 34, 414–418. doi: 10.7518/hxkq.2016.04.019
Mesa, F., Magan-Fernandez, A., Castellino, G., Chianetta, R., Nibali, L., Rizzo, M. (2019). Periodontitis and mechanisms of cardiometabolic risk: Novel insights and future perspectives. Biochim. Biophys. Acta (BBA)-Molecular Basis Disease 1865, 476–484. doi: 10.1016/j.bbadis.2018.12.001
Meurman, J. H., Söder, B. (2022). The stockholm study: over 30 years’ Observation of the effect of oral infections on systemic health. Dentistry J. 10, 68. doi: 10.3390/dj10040068
Michelsen, K. S., Wong, M. H., Shah, P. K., Zhang, W., Yano, J., Doherty, T. M., et al. (2004). Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc. Natl. Acad. Sci. 101, 10679–10684. doi: 10.1073/pnas.0403249101
Mima, K., Cao, Y., Chan, A. T., Qian, Z. R., Nowak, J. A., Masugi, Y., et al. (2016). Fusobacterium nucleatumin colorectal carcinoma tissue according to tumor location. Clin. Trans. gastroenterol. 7, e200. doi: 10.1038/ctg.2016.53
Miyazaki, K., Masuoka, N., Kano, M., Iizuka, R. (2014). Bifidobacterium fermented milk and galacto-oligosaccharides lead to improved skin health by decreasing phenols production by gut microbiota. Beneficial Microbes 5, 121–128. doi: 10.3920/BM2012.0066
Möller, B., Kollert, F., Sculean, A., Villiger, P. M. (2020). Infectious triggers in periodontitis and the gut in rheumatoid arthritis (RA): A complex story about association and causality. Front. Immunol. 11, 1108. doi: 10.3389/fimmu.2020.01108
Momen-Heravi, F., Babic, A., Tworoger, S. S., Zhang, L., Wu, K., Smith-Warner, S. A., et al. (2017). Periodontal disease, tooth loss and colorectal cancer risk: Results from the Nurses’ Health Study. Int. J. Cancer 140, 646–652. doi: 10.1002/ijc.v140.3
Montenegro, M. M., Ribeiro, I. W., Kampits, C., Saffi, M. A., Furtado, M. V., Polanczyk, C. A., et al. (2019). Randomized controlled trial of the effect of periodontal treatment on cardiovascular risk biomarkers in patients with stable coronary artery disease: Preliminary findings of 3 months. J. Clin. Periodontol. 46, 321–331. doi: 10.1111/jcpe.2019.46.issue-3
Mullick, A. E., Tobias, P. S., Curtiss, L. K. (2005). Modulation of atherosclerosis in mice by Toll-like receptor 2. J. Clin. Invest. 115, 3149–3156. doi: 10.1172/JCI25482
Nakahara, T., Hyogo, H., Ono, A., Nagaoki, Y., Kawaoka, T., Miki, D., et al. (2018). Involvement of Porphyromonas gingivalis in the progression of non-alcoholic fatty liver disease. J. Gastroenterol. 53, 269–280. doi: 10.1007/s00535-017-1368-4
Nakajima, M., Arimatsu, K., Kato, T., Matsuda, Y., Minagawa, T., Takahashi, N., et al. (2015). Oral administration of P. gingivalis induces dysbiosis of gut microbiota and impaired barrier function leading to dissemination of enterobacteria to the liver. PloS One 10, e0134234. doi: 10.1371/journal.pone.0134234
Napolitano, A., Miller, S., Nicholls, A. W., Baker, D., Van Horn, S., Thomas, E., et al. (2014). Novel gut-based pharmacology of metformin in patients with type 2 diabetes mellitus. PloS One 9, e100778. doi: 10.1371/journal.pone.0100778
Narengaowa, Kong, W., Lan, F., Awan, U. F., Qing, H., Ni, J. (2021). The oral-gut-brain AXIS: the influence of microbes in Alzheimer’s disease. Front. Cell Neurosci. 15, 633735. doi: 10.3389/fncel.2021.633735
Nascimento, M. (2017). Oral microbiota transplant: a potential new therapy for oral diseases. J. California Dental Assoc. 45, 565–568. doi: 10.1080/19424396.2017.12222506
Nascimento, G. G., Leite, F. R., Do, L. G., Peres, K. G., Correa, M. B., Demarco, F. F., et al. (2015). Is weight gain associated with the incidence of periodontitis? A systematic review and meta-analysis. J. Clin. Periodontol. 42, 495–505. doi: 10.1111/jcpe.2015.42.issue-6
Nath, S., Zilm, P., Jamieson, L., Kapellas, K., Goswami, N., Ketagoda, K., et al. (2021). Development and characterization of an oral microbiome transplant among Australians for the treatment of dental caries and periodontal disease: A study protocol. PloS One 16, e0260433. doi: 10.1371/journal.pone.0260433
Nawaz, S. S., Siddiqui, K. (2020). The emerging role of branch chain amino acids in the prediction of diabetes: a brief review. Curr. Diabetes Rev. 16, 532–537. doi: 10.2174/1573399815666190502113632
Negrut, R. L., Cote, A., Maghiar, A. M. (2023). Exploring the potential of oral microbiome biomarkers for colorectal cancer diagnosis and prognosis: A systematic review. Microorganisms 11, 1586. doi: 10.3390/microorganisms11061586
Nguyen, A. T. M., Akhter, R., Garde, S., Scott, C., Twigg, S. M., Colagiuri, S., et al. (2020). The association of periodontal disease with the complications of diabetes mellitus. A systematic review. Diabetes Res. Clin. Pract. 165, 108244. doi: 10.1016/j.diabres.2020.108244
Nie, J., Zhang, L., Zhao, G., Du, X. (2019). Quercetin reduces atherosclerotic lesions by altering the gut microbiota and reducing atherogenic lipid metabolites. J. Appl. Microbiol. 127, 1824–1834. doi: 10.1111/jam.v127.6
Nordendahl, E., Gustafsson, A., Norhammar, A., Näsman, P., Rydén, L., Kjellström, B. (2018). Severe periodontitis is associated with myocardial infarction in females. J. Dent. Res. 97, 1114–1121. doi: 10.1177/0022034518765735
O’Neill, C. A., Monteleone, G., McLaughlin, J. T., Paus, R. (2016). The gut-skin axis in health and disease: a paradigm with therapeutic implications. Bioessays 38, 1167–1176. doi: 10.1002/bies.201600008
Olsen, I., Yamazaki, K. (2019). Can oral bacteria affect the microbiome of the gut? J. Oral. Microbiol. 11, 1586422. doi: 10.1080/20002297.2019.1586422
Otomo-Corgel, J., Pucher, J. J., Rethman, M. P., Reynolds, M. A. (2012). State of the science: chronic periodontitis and systemic health. J. Evidence Based Dental Practice 12, 20–28. doi: 10.1016/S1532-3382(12)70006-4
Parhi, L., Alon-Maimon, T., Sol, A., Nejman, D., Shhadeh, A., Fainsod-Levi, T., et al. (2020). Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat. Commun. 11, 3259. doi: 10.1038/s41467-020-16967-2
Park, S.-Y., Hwang, B.-O., Lim, M., Ok, S.-H., Lee, S.-K., Chun, K.-S., et al. (2021). Oral–gut microbiome axis in gastrointestinal disease and cancer. Cancers (Basel) 13, 2124. doi: 10.3390/cancers13092124
Park, S.-Y., Kim, S.-H., Kang, S.-H., Yoon, C.-H., Lee, H.-J., Yun, P.-Y., et al. (2019). Improved oral hygiene care attenuates the cardiovascular risk of oral health disease: a population-based study from Korea. Eur. Heart J. 40, 1138–1145. doi: 10.1093/eurheartj/ehy836
Pedersen, H. K., Gudmundsdottir, V., Nielsen, H. B., Hyotylainen, T., Nielsen, T., Jensen, B. A., et al. (2016). Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 535, 376–381. doi: 10.1038/nature18646
Pei, J., Li, F., Xie, Y., Liu, J., Yu, T., Feng, X. (2020). Microbial and metabolomic analysis of gingival crevicular fluid in general chronic periodontitis patients: lessons for a predictive, preventive, and personalized medical approach. EPMA J. 11, 197–215. doi: 10.1007/s13167-020-00202-5
Pelaseyed, T., Bergström, J. H., Gustafsson, J. K., Ermund, A., Birchenough, G. M., Schütte, A., et al. (2014). The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 260, 8–20. doi: 10.1111/imr.2014.260.issue-1
Peng, J., Narasimhan, S., Marchesi, J. R., Benson, A., Wong, F. S., Wen, L. (2014). Long term effect of gut microbiota transfer on diabetes development. J. Autoimmun. 53, 85–94. doi: 10.1016/j.jaut.2014.03.005
Phillips, R. (2015). Microbiome reflects status of RA and response to therapy. Nat. Rev. Rheumatol. 11, 502. doi: 10.1038/nrrheum.2015.109
Pianta, A., Arvikar, S., Strle, K., Drouin, E. E., Wang, Q., Costello, C. E., et al. (2017). Evidence of the immune relevance of Prevotella copri, a gut microbe, in patients with rheumatoid arthritis. Arthritis Rheumatol. 69, 964–975. doi: 10.1002/art.40003
Pignatelli, P., Nuccio, F., Piattelli, A., Curia, M. C. (2023). The role of Fusobacterium nucleatum in oral and colorectal carcinogenesis. Microorganisms 11, 2358. doi: 10.3390/microorganisms11092358
Pozhitkov, A. E., Leroux, B. G., Randolph, T. W., Beikler, T., Flemmig, T. F., Noble, P. A. (2015). Towards microbiome transplant as a therapy for periodontitis: an exploratory study of periodontitis microbial signature contrasted by oral health, caries and edentulism. BMC Oral. Health 15, 1–11. doi: 10.1186/s12903-015-0109-4
Psichas, A., Sleeth, M., Murphy, K., Brooks, L., Bewick, G., Hanyaloglu, A., et al. (2015). The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. 39, 424–429. doi: 10.1038/ijo.2014.153
Qian, B., Zhang, K., Li, Y., Sun, K. (2022). Update on gut microbiota in cardiovascular diseases. Front. Cell. Infect. Microbiol. 12, 1059349. doi: 10.3389/fcimb.2022.1059349
Qiao, Y., Gong, W., Li, B., Xu, R., Wang, M., Shen, L., et al. (2022). Oral microbiota changes contribute to autism spectrum disorder in mice. J. Dent. Res. 101, 821–831. doi: 10.1177/00220345211070470
Qiao, P., Shi, Q., Zhang, R., Lingling, E., Wang, P., Wang, J., et al. (2019). Psoriasis patients suffer from worse periodontal status—a meta-analysis. Front. Med. 6, 212. doi: 10.3389/fmed.2019.00212
Qin, N., Yang, F., Li, A., Prifti, E., Chen, Y., Shao, L., et al. (2014). Alterations of the human gut microbiome in liver cirrhosis. Nature 513, 59–64. doi: 10.1038/nature13568
Rabot, S., Membrez, M., Bruneau, A., Gérard, P., Harach, T., Moser, M., et al. (2010). Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 24, 4948–4959. doi: 10.1096/fj.10-164921
Raghava, K. V., Shivananda, H., Mundinamane, D., Boloor, V., Thomas, B. (2013). Evaluation of periodontal status in alcoholic liver cirrhosis patients: a comparative study. J. Contemp. Dental Practice 14, 179. doi: 10.5005/jp-journals-10024-1296
Ramírez-Boscá, A., Navarro-López, V., Martínez-Andrés, A., Such, J., Francés, R., de la Parte, J. H., et al. (2015). Identification of bacterial DNA in the peripheral blood of patients with active psoriasis. JAMA Dermatol. 151, 670–671. doi: 10.1001/jamadermatol.2014.5585
Ramracheya, R. D., McCulloch, L. J., Clark, A., Wiggins, D., Johannessen, H., Olsen, M. K., et al. (2016). PYY-dependent restoration of impaired insulin and glucagon secretion in type 2 diabetes following Roux-En-Y gastric bypass surgery. Cell Rep. 15, 944–950. doi: 10.1016/j.celrep.2016.03.091
Rivera, M. F., Lee, J.-Y., Aneja, M., Goswami, V., Liu, L., Velsko, I. M., et al. (2013). Polymicrobial infection with major periodontal pathogens induced periodontal disease and aortic atherosclerosis in hyperlipidemic ApoEnull mice. PloS One 8, e57178. doi: 10.1371/journal.pone.0057178
Rojas, C., García, M. P., Polanco, A. F., González-Osuna, L., Sierra-Cristancho, A., Melgar-Rodríguez, S., et al. (2021). Humanized mouse models for the study of periodontitis: an opportunity to elucidate unresolved aspects of its immunopathogenesis and analyze new immunotherapeutic strategies. Front. Immunol. 12, 663328. doi: 10.3389/fimmu.2021.663328
Rosier, B., Marsh, P., Mira, A. (2018). Resilience of the oral microbiota in health: mechanisms that prevent dysbiosis. J. Dent. Res. 97, 371–380. doi: 10.1177/0022034517742139
Rubinstein, M. R., Wang, X., Liu, W., Hao, Y., Cai, G., Han, Y. W. (2013). Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 14, 195–206. doi: 10.1016/j.chom.2013.07.012
Salem, I., Ramser, A., Isham, N., Ghannoum, M. (2018). The gut microbiome as a major regulator of the gut-skin axis. Front. Microbiol. 9, 1459. doi: 10.3389/fmicb.2018.01459
Sansores-España, L. D., Melgar-Rodríguez, S., Olivares-Sagredo, K., Cafferata, E. A., Martínez-Aguilar, V. M., Vernal, R., et al. (2021). Oral-gut-brain axis in experimental models of periodontitis: associating gut dysbiosis with neurodegenerative diseases. Front. aging 2, 781582. doi: 10.3389/fragi.2021.781582
Santonocito, S., Giudice, A., Polizzi, A., Troiano, G., Merlo, E. M., Sclafani, R., et al. (2022). A cross-talk between diet and the oral microbiome: balance of nutrition on inflammation and immune system’s response during periodontitis. Nutrients 14, 2426. doi: 10.3390/nu14122426
Sasaki, N., Katagiri, S., Komazaki, R., Watanabe, K., Maekawa, S., Shiba, T., et al. (2018). Endotoxemia by Porphyromonas gingivalis injection aggravates non-alcoholic fatty liver disease, disrupts glucose/lipid metabolism, and alters gut microbiota in mice. Front. Microbiol. 9, 2470. doi: 10.3389/fmicb.2018.02470
Sato, K., Takahashi, N., Kato, T., Matsuda, Y., Yokoji, M., Yamada, M., et al. (2017). Aggravation of collagen-induced arthritis by orally administered Porphyromonas gingivalis through modulation of the gut microbiota and gut immune system. Sci. Rep. 7, 6955. doi: 10.1038/s41598-017-07196-7
Schenkein, H. A., Loos, B. G. (2013). Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J. Periodontol. 84, S51–S69. doi: 10.1111/jcpe.12060
Schirmer, M., Denson, L., Vlamakis, H., Franzosa, E. A., Thomas, S., Gotman, N. M., et al. (2018). Compositional and temporal changes in the gut microbiome of pediatric ulcerative colitis patients are linked to disease course. Cell Host Microbe 24, 600–10. e4. doi: 10.1016/j.chom.2018.09.009
Schmidt, T. S., Hayward, M. R., Coelho, L. P., Li, S. S., Costea, P. I., Voigt, A. Y., et al. (2019). Extensive transmission of microbes along the gastrointestinal tract. elife 8, e42693. doi: 10.7554/eLife.42693
Schroeder, B. O., Bäckhed, F. (2016). Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 22, 1079–1089. doi: 10.1038/nm.4185
Schwahn, C., Frenzel, S., Holtfreter, B., van der Auwera, S., Pink, C., Bülow, R., et al. (2022). Effect of periodontal treatment on preclinical Alzheimer’s disease—Results of a trial emulation approach. Alzheimer’s Dementia. 18, 127–141. doi: 10.1002/alz.12378
Seo, S., Ahn, S. H., Ri, S., Yoon, Y., Byeon, J. H., Kim, S. H., et al. (2018). Elevated fecal calprotectin levels are associated with severity of atopic dermatitis in children. Asian Pac J. Allergy Immunol. 36, 82–87. doi: 10.12932/AP-100317-0044
Shang, F.-M., Liu, H.-L. (2018). Fusobacterium nucleatum and colorectal cancer: A review. World J. Gastrointest Oncol. 10, 71. doi: 10.4251/wjgo.v10.i3.71
Sharma, P., Dietrich, T., Ferro, C. J., Cockwell, P., Chapple, I. L. (2016). Association between periodontitis and mortality in stages 3–5 chronic kidney disease: NHANES III and linked mortality study. J. Clin. Periodontol. 43, 104–113. doi: 10.1111/jcpe.2016.43.issue-2
Shi, Y., He, J., Tong, Z., Qian, Y., Wang, Q., Jia, D., et al. (2023). Ligature-induced periodontitis drives colorectal cancer: an experimental model in mice. J. Dent. Res. 102, 689–698. doi: 10.1177/00220345231158269
Sikora, M., Chrabąszcz, M., Waśkiel-Burnat, A., Rakowska, A., Olszewska, M., Rudnicka, L. (2019a). Claudin-3–a new intestinal integrity marker in patients with psoriasis: association with disease severity. J. Eur. Acad. Dermatol. Venereol. 33, 1907–1912. doi: 10.1111/jdv.v33.10
Sikora, M., Stec, A., Chrabaszcz, M., Waskiel-Burnat, A., Zaremba, M., Olszewska, M., et al. (2019b). Intestinal fatty acid binding protein, a biomarker of intestinal barrier, is associated with severity of psoriasis. J. Clin. Med. 8, 1021. doi: 10.3390/jcm8071021
Silva, D., Casarin, M., Monajemzadeh, S., Bezerra, B., Lux, R., Pirih, F. Q. (2022). The microbiome in periodontitis and diabetes. Front. Oral. Health 3, 859209. doi: 10.3389/froh.2022.859209
Silva, I. L., Cascales, E. (2021). Molecular strategies underlying Porphyromonas gingivalis virulence. J. Mol. Biol. 433, 166836. doi: 10.1016/j.jmb.2021.166836
Slocum, C., Kramer, C., Genco, C. (2016). Immune dysregulation mediated by the oral microbiome: potential link to chronic inflammation and atherosclerosis. J. Intern. Med. 280, 114–128. doi: 10.1111/joim.2016.280.issue-1
Smirnova, J., Montgomery, S., Lindberg, M., Svensson, Å, Von Kobyletzki, L. (2020). Associations of self-reported atopic dermatitis with comorbid conditions in adults: a population-based cross-sectional study. BMC Dermatol. 20, 1–10. doi: 10.1186/s12895-020-00117-8
Sohn, J., Li, L., Zhang, L., Settem, R. P., Honma, K., Sharma, A., et al. (2022). Porphyromonas gingivalis indirectly elicits intestinal inflammation by altering the gut microbiota and disrupting epithelial barrier function through IL9-producing CD4+ T cells. Mol. Oral. Microbiol. 37, 42–52. doi: 10.1111/omi.12359
Song, T.-J., Jeon, J., Kim, J. (2021). Cardiovascular risks of periodontitis and oral hygiene indicators in patients with diabetes mellitus. Diabetes Metab. 47, 101252. doi: 10.1016/j.diabet.2021.101252
Stanford, K. I., Middelbeek, R. J., Townsend, K. L., An, D., Nygaard, E. B., Hitchcox, K. M., et al. (2012). Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Invest. 123, 215-223. doi: 10.1172/JCI62308
Stanisic, D., Jeremic, N., Majumder, S., Pushpakumar, S., George, A., Singh, M., et al. (2021). High fat diet dysbiotic mechanism of decreased gingival blood flow. Front. Physiol. 12, 625780. doi: 10.3389/fphys.2021.625780
Stolzenberg, E., Berry, D., Yang, D., Lee, E. Y., Kroemer, A., Kaufman, S., et al. (2017). A role for neuronal alpha-synuclein in gastrointestinal immunity. J. Innate Immun. 9, 456–463. doi: 10.1159/000477990
Su, W., Chen, Y., Cao, P., Chen, Y., Guo, Y., Wang, S., et al. (2020). Fusobacterium nucleatum promotes the development of ulcerative colitis by inducing the autophagic cell death of intestinal epithelial. Front. Cell. Infect. Microbiol. 10, 594806. doi: 10.3389/fcimb.2020.594806
Suárez, L. J., Arboleda, S., Angelov, N., Arce, R. M. (2021). Oral versus gastrointestinal mucosal immune niches in homeostasis and allostasis. Front. Immunol. 12, 705206. doi: 10.3389/fimmu.2021.705206
Sultan, S., El-Mowafy, M., Elgaml, A., Ahmed, T. A., Hassan, H., Mottawea, W. (2021). Metabolic influences of gut microbiota dysbiosis on inflammatory bowel disease. Front. Physiol. 12, 715506. doi: 10.3389/fphys.2021.715506
Sureda, A., Daglia, M., Castilla, S. A., Sanadgol, N., Nabavi, S. F., Khan, H., et al. (2020). Oral microbiota and Alzheimer’s disease: Do all roads lead to Rome? Pharmacol. Res. 151, 104582. doi: 10.1016/j.phrs.2019.104582
Szymula, A., Rosenthal, J., Szczerba, B. M., Bagavant, H., Fu, S. M., Deshmukh, U. S. (2014). T cell epitope mimicry between Sjögren’s syndrome Antigen A (SSA)/Ro60 and oral, gut, skin and vaginal bacteria. Clin. Immunol. 152, 1–9. doi: 10.1016/j.clim.2014.02.004
Tajik, N., Frech, M., Schulz, O., Schälter, F., Lucas, S., Azizov, V., et al. (2020). Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat. Commun. 11, 1995. doi: 10.1038/s41467-020-15831-7
Takahashi, N., Sulijaya, B., Yamada-Hara, M., Tsuzuno, T., Tabeta, K., Yamazaki, K. (2019). Gingival epithelial barrier: regulation by beneficial and harmful microbes. Tissue Barriers 7, e1651158. doi: 10.1080/21688370.2019.1651158
Tamaki, N., Takaki, A., Tomofuji, T., Endo, Y., Kasuyama, K., Ekuni, D., et al. (2011). Stage of hepatocellular carcinoma is associated with periodontitis. J. Clin. Periodontol. 38, 1015–1020. doi: 10.1111/j.1600-051X.2011.01777.x
Tang, W. W., Wang, Z., Levison, B. S., Koeth, R. A., Britt, E. B., Fu, X., et al. (2013). Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl. J. Med. 368, 1575–1584. doi: 10.1056/NEJMoa1109400
Tennert, C., Reinmuth, A. C., Bremer, K., Al-Ahmad, A., Karygianni, L., Hellwig, E., et al. (2020). An oral health optimized diet reduces the load of potential cariogenic and periodontal bacterial species in the supragingival oral plaque: A randomized controlled pilot study. Microbiologyopen 9, e1056. doi: 10.1002/mbo3.1056
Thompson, J. A., Oliveira, R. A., Djukovic, A., Ubeda, C., Xavier, K. B. (2015). Manipulation of the quorum sensing signal AI-2 affects the antibiotic-treated gut microbiota. Cell Rep. 10, 1861–1871. doi: 10.1016/j.celrep.2015.02.049
Tian, J., Liu, C., Zheng, X., Jia, X., Peng, X., Yang, R., et al. (2020). Porphyromonas gingivalis induces insulin resistance by increasing BCAA levels in mice. J. Dent. Res. 99, 839–846. doi: 10.1177/0022034520911037
Tomova, A., Husarova, V., Lakatosova, S., Bakos, J., Vlkova, B., Babinska, K., et al. (2015). Gastrointestinal microbiota in children with autism in Slovakia. Physiol. Behav. 138, 179–187. doi: 10.1016/j.physbeh.2014.10.033
Tong, B., Liu, X., Xiao, J., Su, G. (2019). Immunopathogenesis of behcet’s disease. Front. Immunol. 10, 665. doi: 10.3389/fimmu.2019.00665
Toniolo, A., Cassani, G., Puggioni, A., Rossi, A., Colombo, A., Onodera, T., et al. (2019). The diabetes pandemic and associated infections: suggestions for clinical microbiology. Rev. Res. Med. Microbiol. 30, 1–17. doi: 10.1097/MRM.0000000000000155
Tsuzuno, T., Takahashi, N., Yamada-Hara, M., Yokoji-Takeuchi, M., Sulijaya, B., Aoki-Nonaka, Y., et al. (2021). Ingestion of Porphyromonas gingivalis exacerbates colitis via intestinal epithelial barrier disruption in mice. J. Periodontal Res. 56, 275–288. doi: 10.1111/jre.12816
Tu, Y., Yang, R., Xu, X., Zhou, X. (2021). The microbiota-gut-bone axis and bone health. J. Leukoc. Biol. 110, 525–537. doi: 10.1002/JLB.3MR0321-755R
Turnbaugh, P. J., Bäckhed, F., Fulton, L., Gordon, J. I. (2008). Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 3, 213–223. doi: 10.1016/j.chom.2008.02.015
Ungprasert, P., Wijarnpreecha, K., Wetter, D. (2017). Periodontitis and risk of psoriasis: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 31, 857–862. doi: 10.1111/jdv.2017.31.issue-5
van der Post, S., Subramani, D. B., Bäckström, M., Johansson, M. E., Vester-Christensen, M. B., Mandel, U., et al. (2013). Site-specific O-glycosylation on the MUC2 mucin protein inhibits cleavage by the Porphyromonas gingivalis secreted cysteine protease (RgpB). J. Biol. Chem. 288, 14636–14646. doi: 10.1074/jbc.M113.459479
Vavricka, S. R., Manser, C. N., Hediger, S., Vögelin, M., Scharl, M., Biedermann, L., et al. (2013). Periodontitis and gingivitis in inflammatory bowel disease: a case–control study. Inflammation Bowel Dis. 19, 2768–2777. doi: 10.1097/01.MIB.0000438356.84263.3b
Velsko, I. M., Chukkapalli, S. S., Rivera, M. F., Lee, J.-Y., Chen, H., Zheng, D., et al. (2014). Active invasion of oral and aortic tissues by Porphyromonas gingivalis in mice causally links periodontitis and atherosclerosis. PloS One 9, e97811. doi: 10.1371/journal.pone.0097811
Vernal, R., Díaz-Zúñiga, J., Melgar-Rodríguez, S., Pujol, M., Diaz-Guerra, E., Silva, A., et al. (2014). Activation of RANKL-induced osteoclasts and memory T lymphocytes by Porphyromonas gingivalis is serotype dependant. J. Clin. Periodontol. 41, 451–459. doi: 10.1111/jcpe.2014.41.issue-5
Vrieze, A., Van Nood, E., Holleman, F., Salojärvi, J., Kootte, R. S., Bartelsman, J. F., et al. (2012). Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143, 913–6. e7. doi: 10.1053/j.gastro.2012.06.031
Wang, L., Guan, N., Jin, Y., Lin, X., Gao, H. (2015). Subcutaneous vaccination with Porphyromonas gingivalis ameliorates periodontitis by modulating Th17/Treg imbalance in a murine model. Int. Immunopharmacol. 25, 65–73. doi: 10.1016/j.intimp.2015.01.007
Wang, T., Ishikawa, T., Sasaki, M., Chiba, T. (2022). Oral and gut microbial dysbiosis and non-alcoholic fatty liver disease: the central role of Porphyromonas gingivalis. Front. Med. 9, 822190. doi: 10.3389/fmed.2022.822190
Wang, S., Liu, Y., Li, J., Zhao, L., Yan, W., Lin, B., et al. (2021). Fusobacterium nucleatum acts as a pro-carcinogenic bacterium in colorectal cancer: from association to causality. Front. Cell Dev. Biol. 9, 710165. doi: 10.3389/fcell.2021.710165
Wang, H., Lu, Y., Yan, Y., Tian, S., Zheng, D., Leng, D., et al. (2020). Promising treatment for type 2 diabetes: fecal microbiota transplantation reverses insulin resistance and impaired islets. Front. Cell. Infect. Microbiol. 9, 455. doi: 10.3389/fcimb.2019.00455
Wang, M., Shakhatreh, M.-A. K., James, D., Liang, S., Nishiyama, S.-i., Yoshimura, F., et al. (2007). Fimbrial proteins of Porphyromonas gingivalis mediate in vivo virulence and exploit TLR2 and complement receptor 3 to persist in macrophages. J. Immunol. 179, 2349–2358. doi: 10.4049/jimmunol.179.4.2349
Wang, Z., Sun, W., Hua, R., Wang, Y., Li, Y., Zhang, H. (2024). Promising dawn in tumor microenvironment therapy: engineering oral bacteria. Int. J. Oral. Sci. 16, 24. doi: 10.1038/s41368-024-00282-3
Wang, W.-W., Zhang, Y., Huang, X.-B., You, N., Zheng, L., Li, J. (2017). Fecal microbiota transplantation prevents hepatic encephalopathy in rats with carbon tetrachloride-induced acute hepatic dysfunction. World J. Gastroenterol. 23, 6983. doi: 10.3748/wjg.v23.i38.6983
Weyrich, L. S., Nath, S., Jamieson, L. (2024). Commercializing equitable, accessible oral microbiome transplantation therapy. Community Dent. Health 41, 83–88. doi: 10.1172/JCI62308
Widziolek, M., Prajsnar, T. K., Tazzyman, S., Stafford, G. P., Potempa, J., Murdoch, C. (2016). Zebrafish as a new model to study effects of periodontal pathogens on cardiovascular diseases. Sci. Rep. 6, 36023. doi: 10.1038/srep36023
Wu, Y., Chi, X., Zhang, Q., Chen, F., Deng, X. (2018). Characterization of the salivary microbiome in people with obesity. PeerJ 6, e4458. doi: 10.7717/peerj.4458
Wu, C.-z., Yuan, Y.-h., Liu, H.-h., Li, S.-s., Zhang, B.-w., Chen, W., et al. (2020). Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral. Health 20, 1–15. doi: 10.1186/s12903-020-01180-w
Xiao, H., Fan, Y., Li, Y., Dong, J., Zhang, S., Wang, B., et al. (2021). Oral microbiota transplantation fights against head and neck radiotherapy-induced oral mucositis in mice. Comput. Struct. Biotechnol. J. 19, 5898–5910. doi: 10.1016/j.csbj.2021.10.028
Xiao, E., Mattos, M., Vieira, G. H. A., Chen, S., Corrêa, J. D., Wu, Y., et al. (2017). Diabetes enhances IL-17 expression and alters the oral microbiome to increase its pathogenicity. Cell Host Microbe 22, 120–8. e4. doi: 10.1016/j.chom.2017.06.014
Xue, Y., Xiao, H., Guo, S., Xu, B., Liao, Y., Wu, Y., et al. (2018). Indoleamine 2, 3-dioxygenase expression regulates the survival and proliferation of Fusobacterium nucleatum in THP-1-derived macrophages. Cell Death Dis. 9, 355. doi: 10.1038/s41419-018-0389-0
Xue, L., Zou, X., Yang, X.-Q., Peng, F., Yu, D.-K., Du, J.-R. (2020). Chronic periodontitis induces microbiota-gut-brain axis disorders and cognitive impairment in mice. Exp. Neurol. 326, 113176. doi: 10.1016/j.expneurol.2020.113176
Yamazaki, K., Kato, T., Tsuboi, Y., Miyauchi, E., Suda, W., Sato, K., et al. (2021). Oral pathobiont-induced changes in gut microbiota aggravate the pathology of nonalcoholic fatty liver disease in mice. Front. Immunol. 12, 766170. doi: 10.3389/fimmu.2021.766170
Yang, Y., Weng, W., Peng, J., Hong, L., Yang, L., Toiyama, Y., et al. (2017). Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating toll-like receptor 4 signaling to nuclear factor– κB, and up-regulating expression of microRNA-21. Gastroenterology 152, 851–66. e24. doi: 10.1053/j.gastro.2016.11.018
Yeh, C.-F., Chen, Y.-H., Liu, S.-F., Kao, H.-L., Wu, M.-S., Yang, K.-C., et al. (2020). Mutual interplay of host immune system and gut microbiota in the immunopathology of atherosclerosis. Int. J. Mol. Sci. 21, 8729. doi: 10.3390/ijms21228729
Yilmaz, Oz, Jungas, T., Verbeke, P., Ojcius, D. M. (2004). Activation of the phosphatidylinositol 3-kinase/Akt pathway contributes to survival of primary epithelial cells infected with the periodontal pathogen Porphyromonas gingivalis. Infect. Immun. 72, 3743–3751. doi: 10.1128/IAI.72.7.3743-3751.2004
Yoneda, M., Naka, S., Nakano, K., Wada, K., Endo, H., Mawatari, H., et al. (2012). Involvement of a periodontal pathogen, Porphyromonas gingivalis on the pathogenesis of non-alcoholic fatty liver disease. BMC Gastroenterol. 12, 1–10. doi: 10.1186/1471-230X-12-16
Yoon, M.-S. (2016). The emerging role of branched-chain amino acids in insulin resistance and metabolism. Nutrients 8, 405. doi: 10.3390/nu8070405
Yoshida, N., Emoto, T., Yamashita, T., Watanabe, H., Hayashi, T., Tabata, T., et al. (2018). Bacteroides vulgatus and Bacteroides dorei reduce gut microbial lipopolysaccharide production and inhibit atherosclerosis. Circulation 138, 2486–2498. doi: 10.1161/CIRCULATIONAHA.118.033714
Yu, T., Guo, F., Yu, Y., Sun, T., Ma, D., Han, J., et al. (2017). Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell 170, 548–63. e16. doi: 10.1016/j.cell.2017.07.008
Zákostelská, Z., Málková, J., Klimešová, K., Rossmann, P., Hornová, M., Novosádová, I., et al. (2016). Intestinal microbiota promotes psoriasis-like skin inflammation by enhancing Th17 response. PloS One 11, e0159539. doi: 10.1371/journal.pone.0159539
Zeng, F., Liu, Y., Huang, W., Qing, H., Kadowaki, T., Kashiwazaki, H., et al. (2021). Receptor for advanced glycation end products up-regulation in cerebral endothelial cells mediates cerebrovascular-related amyloid β accumulation after Porphyromonas gingivalis infection. J. Neurochem. 158, 724–736. doi: 10.1111/jnc.v158.3
Zhang, Z., Ma, N., Zheng, Y., Zhang, L. (2015). Association of serum immunoglobulin-G to Porphyromonas gingivalis with acute cerebral infarction in the Chinese population. J. Indian Soc. Periodontol. 19, 628–632. doi: 10.4103/0972-124X.164750
Zhang, S., Yang, Y., Weng, W., Guo, B., Cai, G., Ma, Y., et al. (2019). Fusobacterium nucleatum promotes chemoresistance to 5-fluorouracil by upregulation of BIRC3 expression in colorectal cancer. J. Exp. Clin. Cancer Res. 38, 1–13. doi: 10.1186/s13046-018-0985-y
Zhang, X., Zhang, D., Jia, H., Feng, Q., Wang, D., Liang, D., et al. (2015). The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 21, 895–905. doi: 10.1038/nm.3914
Zhao, Y., Jaber, V., Lukiw, W. J. (2017). Secretory products of the human GI tract microbiome and their potential impact on Alzheimer’s disease (AD): detection of lipopolysaccharide (LPS) in AD hippocampus. Front. Cell. Infect. Microbiol. 7, 318. doi: 10.3389/fcimb.2017.00318
Zhao, X., Liu, J., Zhang, C., Yu, N., Lu, Z., Zhang, S., et al. (2021). Porphyromonas gingivalis exacerbates ulcerative colitis via Porphyromonas gingivalis peptidylarginine deiminase. Int. J. Oral. Sci. 13, 31. doi: 10.1038/s41368-021-00136-2
Zhao, Z., Ning, J., Bao, X.-q., Shang, M., Ma, J., Li, G., et al. (2021). Fecal microbiota transplantation protects rotenone-induced Parkinson’s disease mice via suppressing inflammation mediated by the lipopolysaccharide-TLR4 signaling pathway through the microbiota-gut-brain axis. Microbiome 9, 1–27. doi: 10.1186/s40168-021-01107-9
Zhou, M., Dong, J., Zha, L., Liao, Y. (2021). Causal association between periodontal diseases and cardiovascular diseases. Genes 13, 13. doi: 10.3390/genes13010013
Zhou, D., Pan, Q., Shen, F., Cao, H.-x., Ding, W.-j., Chen, Y.-w., et al. (2017). Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci. Rep. 7, 1529. doi: 10.1038/s41598-017-01751-y
Zhu, W., Gregory, J. C., Org, E., Buffa, J. A., Gupta, N., Wang, Z., et al. (2016). Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 165, 111–124. doi: 10.1016/j.cell.2016.02.011
Zimmermann, G. S., Bastos, M. F., Dias Gonçalves, T. E., Chambrone, L., Duarte, P. M. (2013). Local and circulating levels of adipocytokines in obese and normal weight individuals with chronic periodontitis. J. Periodontol. 84, 624–633. doi: 10.1902/jop.2012.120254
Keywords: periodontal bacteria, oral dysbiosis, gut dysbiosis, oral-gut axis, systemic diseases, fecal microbiota transplantation
Citation: Xi M, Ruan Q, Zhong S, Li J, Qi W, Xie C, Wang X, Abuduxiku N and Ni J (2024) Periodontal bacteria influence systemic diseases through the gut microbiota. Front. Cell. Infect. Microbiol. 14:1478362. doi: 10.3389/fcimb.2024.1478362
Received: 09 August 2024; Accepted: 29 October 2024;
Published: 15 November 2024.
Edited by:
Soumyadev Sarkar, Arizona State University, United StatesReviewed by:
Nanda Kumar Navalpur Shanmugam, Harvard Medical School, United StatesYashika Kamte, University of California, Los Angeles, United States
Himani Dey, University of Texas Health Science Center at Houston, United States
Copyright © 2024 Xi, Ruan, Zhong, Li, Qi, Xie, Wang, Abuduxiku and Ni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nuerbiya Abuduxiku, bnVlcmJpeWFhYnVkdXhpa3VAMTYzLmNvbQ==; Jia Ni, TkoxOTkyNzYzMzM2MUBvdXRsb29rLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Mengying Xi
Mengying Xi Qijun Ruan
Qijun Ruan Sulan Zhong1
Sulan Zhong1 Xiaoyan Wang
Xiaoyan Wang