
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Infect. Microbiol. , 20 January 2025
Sec. Clinical Infectious Diseases
Volume 14 - 2024 | https://doi.org/10.3389/fcimb.2024.1428491
This article is part of the Research Topic Clostridioides difficile Infections and Beyond View all 6 articles
Co-infection with human immunodeficiency virus (HIV) significantly increases the incidence of human papillomavirus (HPV) infection and HPV-related cancers among men who have sex with men (MSM). Conversely, HPV infection can also influence HIV acquisition rates. HIV-induced immune suppression may affect chromosomal stability, gene expression, protein function and other molecular components in MSM with HPV-related cancers. Additionally, HIV infection also alters cellular mechanisms by compromising immune responses and epithelial integrity. In this review, we reviewed the influence of HIV on specific HPV-related cancers in MSM, including oropharyngeal squamous cell carcinoma, penile cancer, and anal cancer. We integrated epidemiological data from the past five years and discussed diagnosis and treatment strategies. Overall, our review offers crucial insights into the underlying molecular and cellular mechanisms of these co-infection MSM patients. Our review aims to assist future research in developing effective treatment strategies for MSM with HIV/HPV co-infection.
Human papillomavirus (HPV) is a widespread DNA virus with considerable public health consequences, accounting for nearly all cases of cervical cancer and a significant portion of other anogenital and oropharyngeal cancers (Ranjit et al., 2020). HPV has a prevalence of over 20% in men and accounts for 2% of male cancer cases (Ventimiglia et al., 2016). It is transmitted through sexual contact, skin-to-skin contact, and by infecting infants during delivery. Among them, sexual transmission is the most common way of transmission, primarily through vaginal and anal mucosa (Burchell et al., 2006), highlighting the significant cancer risk associated with men who have sex with men (MSM).
HPV can be classified into low-risk HPV (LR-HPV) and high-risk HPV (HR-HPV) according to its oncogenic potential (Handisurya et al., 2009). LR-HPV, including subtypes 6, 11, 42, and 43, is mainly associated with the formation of genital warts and may also cause warts in the oral cavity and throat area. HR-HPV subtypes, encompassing HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59, can lead to intraepithelial lesions and a range of HPV-related cancers, including cervical, oropharyngeal, penile, and anal cancers. Among these, HPV 16 and HPV 18 are the most carcinogenic (de Sanjose et al., 2018).
The HPV genome consists of three major regions. The long control region (LCR) is a non-coding region that regulates viral replication and transcription. The other two regions encode eight open reading frames (ORFs), including six early ORFs and two late ORFs. Early ORFs produce E1, E2, E4, E5, E6, and E7, which are involved in viral replication and tumorigenesis. The late ORFs are L1 and L2, which encode viral capsid proteins (Molina et al., 2024). HPV can invade the damaged epithelium, replicates extensively within it. Along with the amplification of HPV and cell division, resulting in a large number of cells becoming infected with HPV (Graham, 2017).
Persistent HR-HPV infection may lead to the development of cancer (Brickman and Palefsky, 2015). Due to the special physiological structure of the foreskin, men are more susceptible to HPV infection. In a study of 379 adult males, HPV infection rates varied by anatomical site and circumcision status. Uncircumcised men have higher rates of HPV in the glans or corona, as well as increased risk of oncogenic HPV and multiple HPV type infections (Hernandez et al., 2008). The excessive foreskin or phimosis, the inner foreskin and glans penis create a warm, humid, and anaerobic environment. This condition facilitates the proliferation of various anaerobic bacteria and viruses, including HPV (Mehta et al., 2021).
Human immunodeficiency virus (HIV) mainly invades CD4+T lymphocytes and destroys the host immune system, causing acquired immune deficiency syndrome (AIDS) (Deeks et al., 2015). HIV-1 and HIV-2 share similarities in genetic arrangement, transmission, replication, and clinical outcomes, both leading to AIDS. Although HIV-2 is less contagious and less likely to progress to AIDS, with its prevalence primarily confined to West Africa (Nyamweya et al., 2013), both HIV-1 and HIV-2 significantly influence HPV infection. Specifically, the incidence of HR-HPV is nearly doubled in HIV-positive individuals, with a pooled RR of 2.20 and a 95% confidence interval (CI) of 1.90 to 2.54, while the clearance rate of HPV is approximately halved, with a pooled RR of 0.53 (95% CI: 0.42 to 0.67) (Looker et al., 2018). HIV-positive MSM face a higher risk of HPV infections. An analysis enrolled 1,559 participants, including 300 HIV-positive MSM, 600 HIV-negative MSM, and 659 MSW (men who have sex with women), with HPV prevalence rates of 62.0%, 53.7%, and 8.3%, respectively (p < 0.001) (Bai et al., 2024).The incidence of HPV-related lesions and malignant cancers in individuals infected with HIV is significantly higher than in individuals not infected with HIV. A meta-analysis of 34 studies among Chinese MSM found high rates of multiple HPV infections in anogenital warts: 75.9% in HIV-positive and 41.7% in HIV-negative individuals (Zhou et al., 2021). A study identified 502 HPV-related cancers in HIV-positive Hispanics across 864,067 person-years. Excluding oropharyngeal cancer, the risk of HPV-related cancers was higher in HIV-infected patients than in the general population, with SIRs ranging from 3.59 for cervical cancer to 18.7 for anal cancer in men (Ortiz et al., 2018). HPV infection occurring before HIV can also impact HIV acquisition rates. A meta‐analytic systematic review of 14 publications showed HIV incidence was almost doubled (pooled RR = 1.91, 95% CI 1.38 to 2.65) in the presence of prevalent HPV infection (Looker et al., 2018). Understanding the bidirectional relationship between HIV and HPV is essential for effective prevention and treatment strategies for both infections.
In this review, we summarized the molecular mechanism of HIV affecting HPV carcinogenesis from chromosome, gene expression and protein levels (Figures 1, 2). We further elaborate on the mechanisms of immune cell heterogeneity in the immunological microenvironment where HIV infection is prone to be complicated by HPV infection (Figures 3, 4). Additionally, we collected epidemiological data from the past 3-5 years including anal cancer, penile cancer, and head and neck cancer. This data was scrutinized to identify the connections between HIV-positive MSM, HPV infection, and the associated cancers. We also categorized the diagnosis and treatment, and HPV vaccines for HPV-related cancers, offering insights for future prevention and intervention strategies.
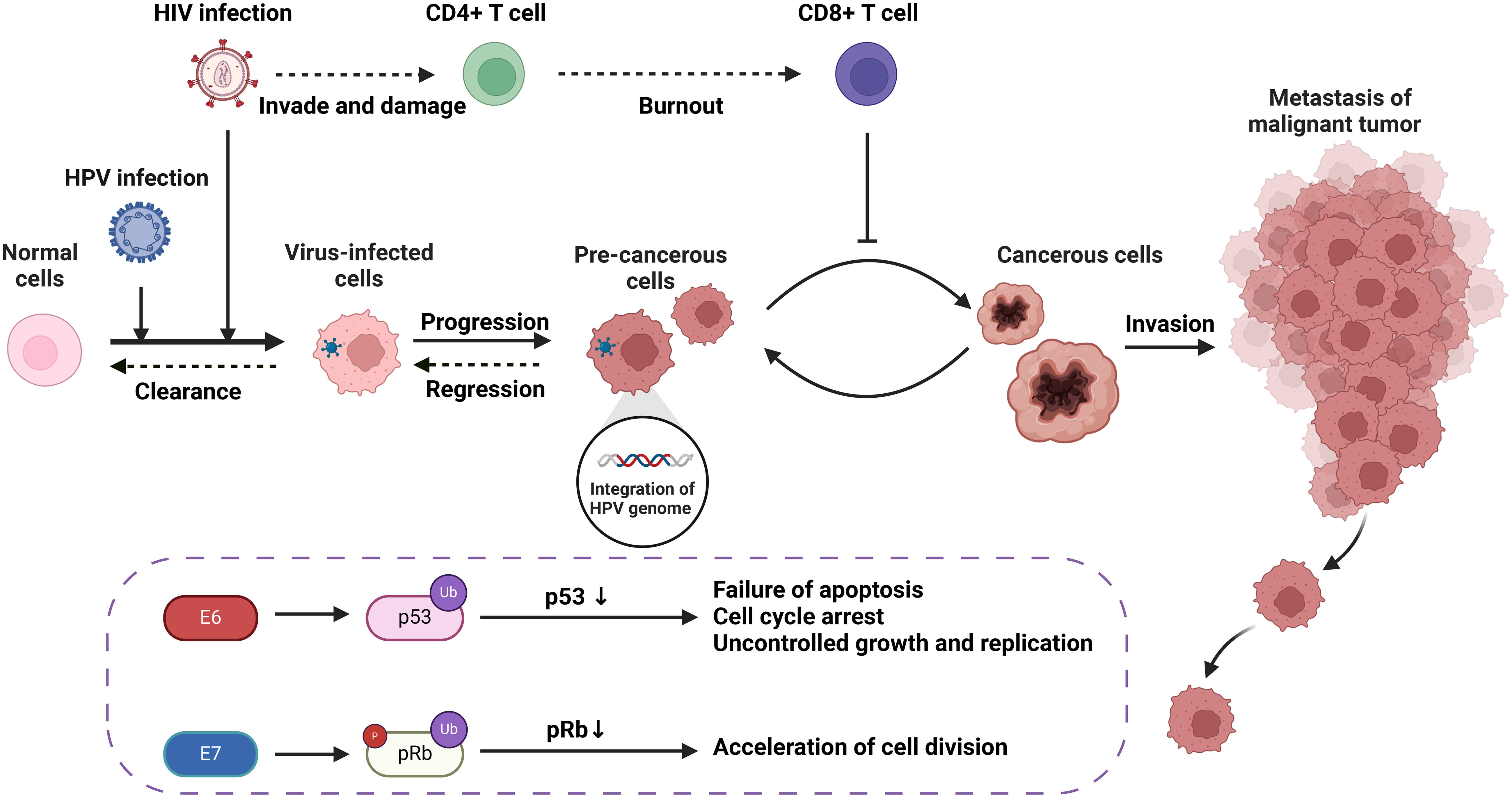
Figure 1. The carcinogenic progression of HPV-associated tumors resulting from HIV/HPV co-infection, including molecular and cellular mechanisms.
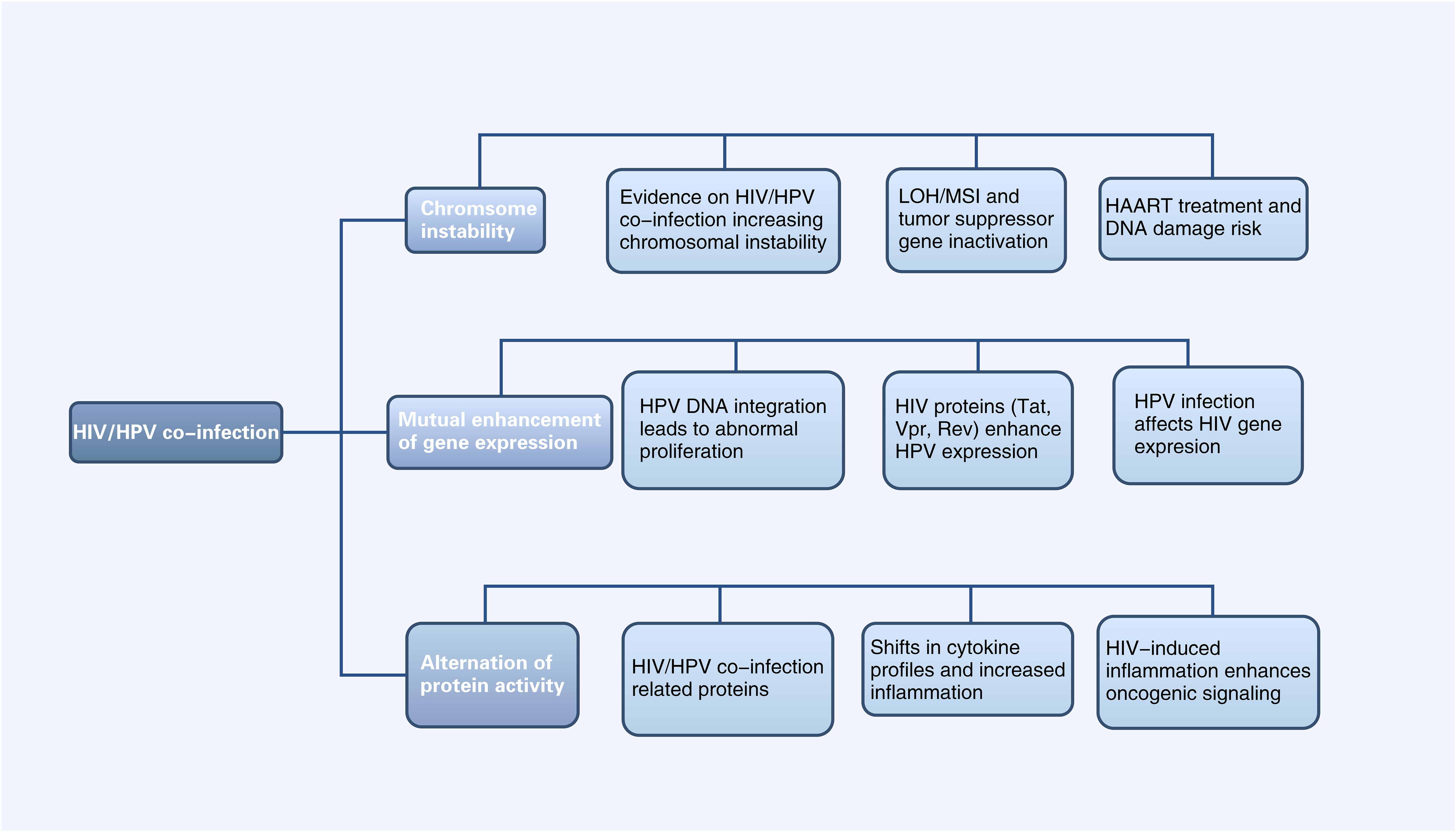
Figure 2. Diagram of Alterations at the molecular level of HPV-related cancers by HIV Infection (HIV infection can affect HPV-related tumor progression at the chromosomal, gene expression, and protein levels, and vice versa. LOH, Chromosomal heterozygous deletion; MSI, Microsatellite instability; HAART, Highly active antiretroviral therapy).
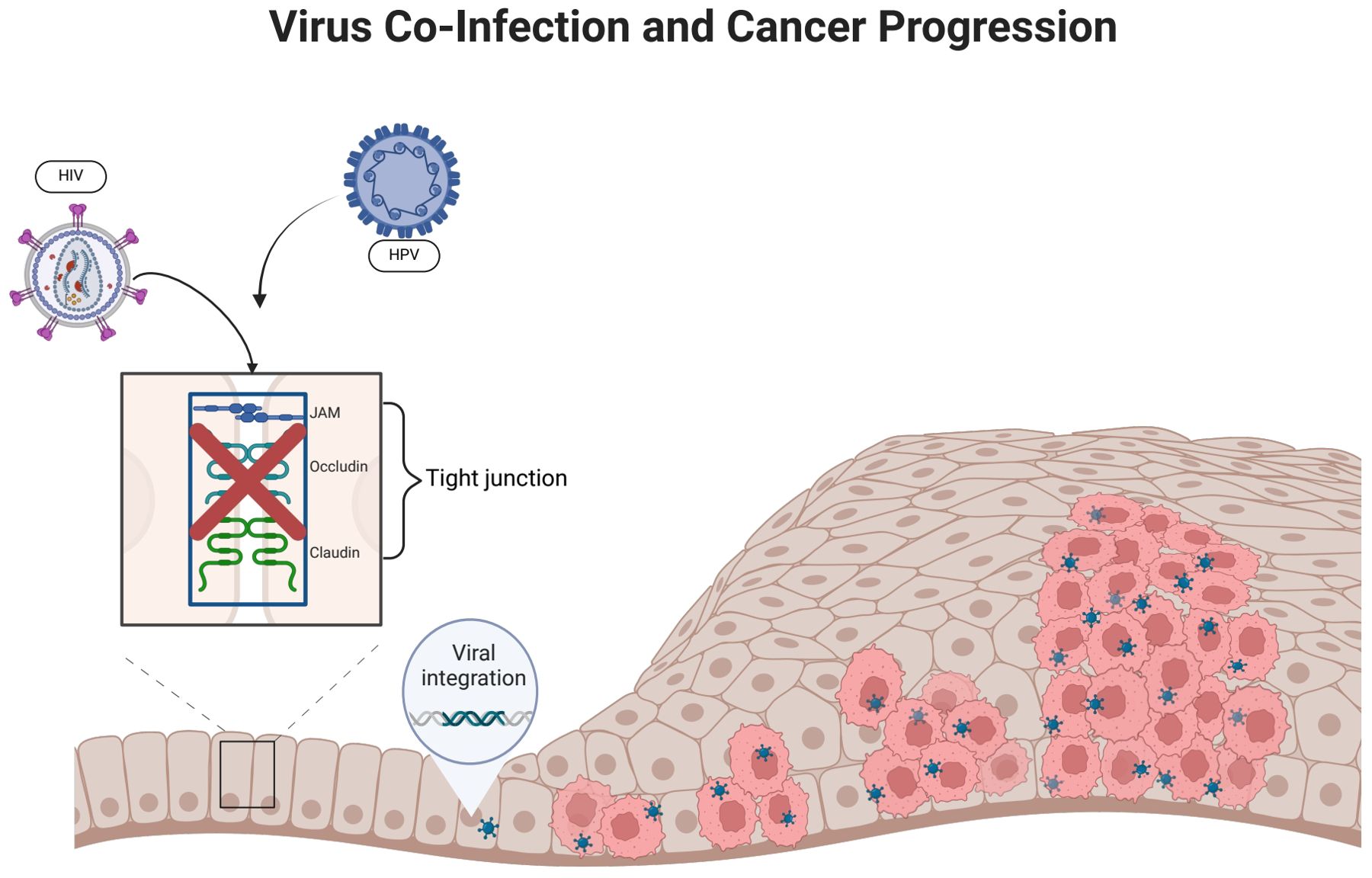
Figure 3. HIV infection promotes HPV infection. (HIV infection can disrupt epithelial tight junctions, thereby facilitating initial HPV infection. HPV enters epithelial basal cells with the help of surface proteins that bind to cellular receptors. The incorporation of HPV DNA into the host cell genome promotes abnormal cellular proliferation and differentiation).
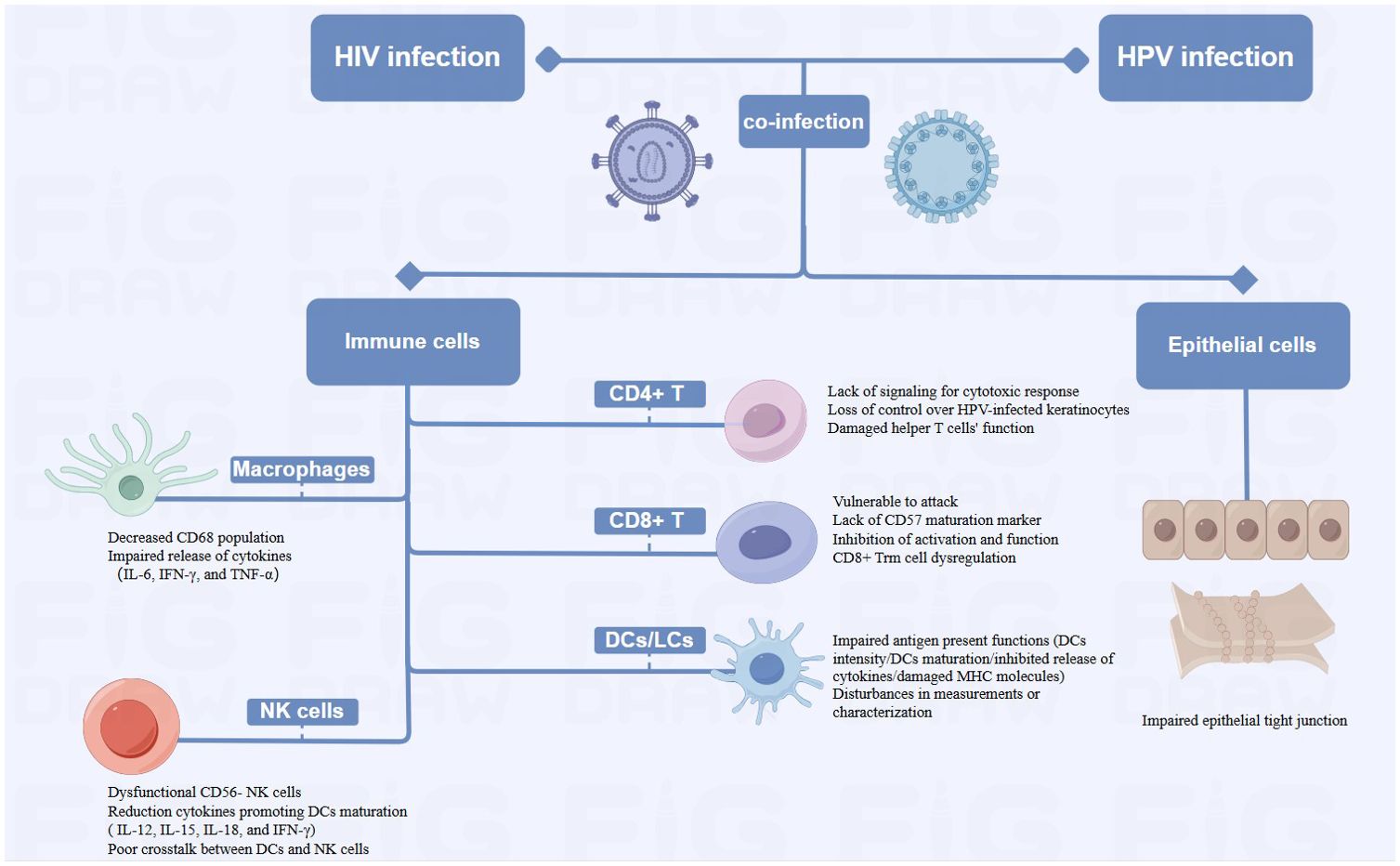
Figure 4. Diagram of Alterations at the cellular level of HPV-related cancers by HIV Infection (HIV-related immunosuppression, involving immune cells such as T cells, DCs/LCs, NK cells and Macrophages, can delay HPV clearance and increase cancer risk by reactivating or sustaining HPV infection. HIV damages epithelial tight junctions, facilitating HPV penetration and promoting HPV-related cancers).
Loss of heterozygosity (LOH) of chromosomes and microsatellite instability (MSI) are important mechanisms leading to the inactivation of tumor suppressor genes (Edelmann et al., 2004). In cervical cancer, HPV can induce LOH/MSI at the DNA HLA-I locus, which is exacerbated when co-infected with HIV-1. A study revealed a significantly higher frequency of LOH/MSI at the HLA-I locus on chromosome 6p21.21 in HIV-1/HPV co-infected tumor DNA compared to HIV-1 seronegative patients (Chambuso et al., 2019). In addition, the integration of HPV genes in the host leads to chromosomal genetic changes, including chromatin reorganization and chromosome rearrangement (Li et al., 2019; Singh et al., 2024). The E6 and E7 proteins encoded by the HPV genome have been shown to lead to host chromosomal instability (Duensing and Münger, 2002). HIV infection can increase the expression of HPV E6 and E7 proteins (Tornesello et al., 2008), thus leading to host chromosomal instability. This mechanism will be further discussed in section 3.1.2. On the other hand, the current mainstay of treatment for HIV is highly active antiretroviral therapy (HAART), which includes some drugs, such as AZT, that have properties that may lead to DNA damage (Palefsky, 2006). Therefore, HIV/HPV-co-infected individuals treated with HAART may be at increased risk of chromosomal instability and, thus, more susceptible to cancer. In conclusion, HIV/HPV co-infection leads to chromosomal instability and increase the risk of HPV-related cancers.
The integration of HPV DNA into the host cell genome is a key event leading to abnormal proliferation and malignant progression in HPV-mediated carcinogenesis (McBride and Warburton, 2017). E6 and E7, two viral oncogenes that interfere with the pathways regulated by p53 and other members of the pRB family, are the primary cancer suppressor genes that cause neoplastic conversion (Chow, 2015). E6 inactivates p53, which is a transcription factor regulating the expression of genes encoding cell cycle, DNA repair mechanisms, metabolism, and apoptosis (Hafner et al., 2019). E7 inactivates pRb, keeping infected cells in a proliferative state. As a result, the continued activity of the E6 and E7 proteins leads to abnormal cell proliferation, the accumulation of oncogene mutations, and eventually HPV-related cancers (Faraji et al., 2017).
HIV proteins Tat, Vpr and Rev participate in HPV-related pathogenesis and increase the risk of HPV-associated cancers by affecting HPV gene expression (Nyagol et al., 2006). Tat encodes a transcription activator protein that can activate the HPV LCR and increase the expression of HPV E6, E7, E1, and L1 (Nyagol et al., 2006; Kim et al., 2008; Tornesello et al., 2008). Studies have shown that HIV-1 Tat1 protein can significantly upregulate HPV 16 gene expression by enhancing the activity of HPV 16 upstream regulatory region and its associated promoter (P97) (Vernon et al., 1993). Rev protein can increase HPV L1 gene expression by overcoming post-transcriptional inhibition. HPV-16 L1 gene expression is inhibited by specific sequences on mRNA. Rev protein can bind to the Rev-responsive element (RRE) on mRNA and effectively increase the expression of HPV L1 (Zheng and Baker, 2006). Vpr and E6 are linked in cell cycle signaling pathways. Cervical cancer cells are susceptible to HIV-1 vpr-induced cell cycle arrest, while coexpression of HPV-16 E6 exacerbates this effect (Toy et al., 2000). Currently there are no other studies on possible interactions between HPV and other HIV genes and the proteins they encode. While data are limited, studies suggested that the weakened immune response caused by HIV allows HPV-related diseases such as high-grade intraepithelial neoplasia to persist, providing time for genetic changes that lead to cancer progression (Palefsky, 2006).
HPV infection can also affect HIV gene expression: some studies reported that extracellular vesicles secreted by HPV-infected cervical cancer cells can enhance HIV-1 replication through oxidative stress pathways, suggesting that HPV infection could make cervical cancer cells more susceptible to HIV and facilitating a potential vicious cycle of synergistic expression between HIV and HPV (Ranjit et al., 2020).
p53 is a pro-apoptotic tumor suppressor factor, controlling cell proliferation, senescence, DNA repair, and cell death in cancer (Mao and Jiang, 2023). In cervical cancers induced by HR-HPV, p53 is degraded by HPV protein E6 (Martinez-Zapien et al., 2016). Current research indicates that extracellular HIV-1 Tat protein can be taken up by human cervical cancer cells, followed by an increase in the expression of HPV E6 protein and a decrease in the levels of the cellular suppressor protein p53 (Barillari et al., 2016). The impact of HIV on p53 may due to the activation of cellular pathways that are different from those in HIV-negative lesions; however, no studies currently exist, and more research and further validation is needed. HIV/HPV co-infection significantly increases VEGF and p27 expression (Nicol et al., 2008). VEGF serves as an early indicator of cervical cancer development (Branca et al., 2006), while p27, a cyclin-dependent kinase (CDK) inhibitor, is crucial for cancer prognosis in various cancer types (Tjalma et al., 2005). ADAR1, an adenosine deaminase, regulates RNA editing and its dysregulation may contribute to cancer. A genetic study in HPV/HIV co-infected individuals found ADAR1 variants linked to HPV relapse (Pujantell et al., 2019).
It is known that HPV-infected patients have increased expression of IL-6 and TNF-α, and HIV infection may further enhance the carcinogenicity of HPV through the expression of pro-inflammatory cytokines such as IL-6 and TNF-α (Nicol et al., 2005a). Another study found that HIV/HPV co-infection was more predictive of a predominance of type 2 cytokines (IL-4, IL-10 and IL-6) compared to HPV-monoinfection (Behbahani et al., 2007). This shift of cytokines weakens the immune response to HPV-related cancers. Type 1 cytokines (IL-2, IFN-γ) enhance cellular and humoral immunity, leading to better clinical outcomes in HPV-related cancers. In contrast, Type 2 cytokines (IL-4, IL-10, IL-6), more prominent locally or peripherally, are often associated with humoral immune responses and suppress cell-mediated immune responses, which may promote the development of cervical squamous intraepithelial neoplasia (SIL) and change cancers (Nicol et al., 2005a). Cyclooxygenase-2 (COX-2) is an enzyme that plays a crucial role in inflammation and pain by catalyzing the conversion of arachidonic acid into prostaglandins. HPV proteins E6 and E7 can trigger the transcription of COX-2 (Subbaramaiah and Dannenberg, 2007). In cervical intraepithelial neoplasia (CIN) and cervical cancer, COX-2 is overexpressed and associated with poor prognosis (Sales et al., 2001). The combination of HIV infection may increase the carcinogenic risk of HPV-related lesions and tumors by inducing COX-2 levels. Research shows patients with HIV/HPV co-infection and squamous intraepithelial neoplasia have higher levels of COX-2 in their cervical cells than those infected only with HIV (Fitzgerald et al., 2012).
In addition, HIV infection activates signaling pathways associated with epithelial-mesenchymal transition (EMT), which increases the proliferative and metastatic capabilities of epithelial cancer cells, thus promoting the development and metastasis of cancer in the context of HIV/HPV coinfection (Tugizov, 2016). Research demonstrated that exposure to HIV-1 gp120 and tat or cell-free virions in HPV-immortalized epithelial cells results in the disruption of epithelial junctions, which initiates the EMT. This transition is a significant contributor to tumorigenesis. The transforming growth factor-beta (TGF-β) signaling pathway serves as the principal canonical network governing EMT in cancer contexts, which is predominantly regulated by the transcription factor in response to mitogen-activated protein kinase (MAPK) signaling. Inhibition of the MAPK and TGF-β pathways has been found to prevent EMT triggered by HIV-1 in oral epithelial cells, potentially leading to new treatment methods for cancers linked to HIV/HPV coinfection (Tugizov, 2016).
HIV-infected patients may reactivate or sustain an HPV infection due to immune system suppression, which delays HPV clearance and raises the probability of cancer (Frisch et al., 2000; Mooij et al., 2016). HIV-related immunosuppression primarily involves a variety of immune cells, CD4+ T cells, CD8+ T cells, dendritic cells (DCs), natural killer (NK) cells and macrophages.
HIV infection causes a decrease in CD4+ T cells, which subsequently reduces the amount of immune cells in the body and exacerbates immunosuppression (Petry et al., 1994). Low CD4+ cell counts impair the immune system’s ability to clear HR-HPV infections, due to the lack of effective signaling for a robust cytotoxic response and increase the incidence of HPV-related cancers (Chaturvedi et al., 2009; Hewavisenti et al., 2023). Consequently, HIV-infected individuals experience reduced host clearance of HPV and relatively undisturbed epithelial growth, contributing to the progression of HPV-related cancers (Frisch et al., 2000). Several studies have also shown that failure to develop an effective cell-mediated immune (CMI) response leaves HPV-positive individuals vulnerable to persistent infection and increases the probability of progression to invasive carcinoma. HPV-infected keratinocytes downregulate their innate immune signaling pathways, leading to a failure in the release of proinflammatory cytokine, particularly type I interferons. Moreover, there are insufficient or absent signals for the activation and migration of langerhans cells (LCs), as well as for the recruitment of macrophages and stromal dendritic cells (DCs) (Stanley, 2012). The loss of CMI response control over HPV-infected keratinocytes began when CD4+ levels were significantly higher than 200/μL (Frisch et al., 2000). Additionally, the helper T cell function may be compromised by the functional deficit with reduced expression of IL-2, IFN-γ, and IL-4 in HIV-infected patients (Nicol et al., 2005b; Dhasmana et al., 2008). Th2 phenotype may predominate in the immune response. This shift may result in an inadequate cellular immune response necessary for clearing HPV infections, making co-infected individuals more susceptible to persistent HPV (Mahnke et al., 2012). In addition to supporting tissue homeostasis and immune defense, resident memory T (Trm) cells exert antimicrobial and anticancer properties (Gebhardt et al., 2018). Studies have revealed that CXCR3+ CD4+ T cells were critical in preventing persistent HPV infection and cancer progression (Bodily and Laimins, 2011; Hickman et al., 2015), while CXCR3+ CD4+ Trm cells were irreversibly depleted in patients with advanced HIV disease (Saluzzo et al., 2021). The irreversibly depleted cells may impede HPV control, which in turn increased cancer risk. Although HIV may accelerate the growth of HPV-related cancers by reducing CD4+ T cells, this mechanism is still up for debate. Several studies have shown that high CD4+ T cell counts did not significantly reduce HPV incidence. Reactivation of a latent infection and recent sexual encounter were two possible causes of incident infections (Critchlow et al., 1998). Poor immune management of precancerous lesions may also encourage the development of cancer (Palefsky and Holly, 2003), while HIV-mediated immunosuppression may not always result in late progression to invasive cancer. The progression of anogenital carcinoma in situ may be depend on the accumulation of extra genetic damage which occurs more quickly in rapidly proliferating epithelial cells with defective cell cycle regulation (Critchlow et al., 1995). These findings emphasized the complex immunologic relationship between HIV infection and HPV-related cancer development.
The removal of HPV-infected epithelial cells and the regression of lesions caused by infection may be aided by T lymphocytes, especially cytotoxic T lymphocyte activation (Chihu-Amparan et al., 2023). HPV oncoproteins E6 and E7 are presented by major histocompatibility complex I (MHC-1), rendering infected cells vulnerable to assault by CD8+ T lymphocytes (Scott et al., 2001). HIV-induced chronic inflammation depletes CD8+ T cells by up-regulating PD-1 expression, weakening the anti-cancer response (Bushara et al., 2022). Cervical biopsies from HIV-positive women show an increase in CD8+ T cells, however, most of these cells are CD45RO+ and lack CD57 markers, which reduces their capacity for cytolysis (Olaitan et al., 1996). High levels of type 2 cytokines induced by HIV infection also diminish CD8+ T cell function (Kim et al., 2008). CD8+ Trm is highest in the epidermis, but patients with advanced HIV develop irreversible CD8 Trm cell dysregulation, which may be one of the important reasons for the cancer susceptibility environment (Saluzzo et al., 2021).
Dendritic cells, particularly langerhans cells capture antigens and stimulate T cells to initiate an immune response. HIV affects their ability to stimulate T cells by inhibiting the release of cytokines like IL-12, lowering DC density, attenuating DC maturation, and deducing MHC molecule expression (Pachiadakis et al., 2005; Guimarães et al., 2011). In addition, disturbances in the measurement and/or characterization of LCs could potentially impede the immunological monitoring of HPV-related cervical lesions (Hubert et al., 2001), hence contributing to the local and systemic immune responses to HPV-induced cancers (Wright-Browne et al., 1997; Hachisuga et al., 2001).
In HIV-positive individuals, NK cell function is impaired, with a shift towards dysfunctional CD56- NK cells. The cells exhibit diminished cytotoxic capabilities and impaired cytokine production (Mavilio et al., 2005). This dysfunction correlates with the plasma HIV load (Bere et al., 2014), and weakens the immune system’s overall antiviral response, facilitating persistent HPV infections. Additionally, DCs functioning is impaired during acute HIV infection, producing fewer IL-12, 15 and -18, and lowers IFN-γ produced by NK cells, which retrospectively results in poor DC maturation. Poor crosstalk between DCs and NK cells, leads to weakened, non-specific and abnormal immunity, resulting in poor control of opportunistic infections like HPV (Hens et al., 2016).
Macrophages, like CD4+ T cells, serve as the primary target cells for HIV and facilitate in the virus’s dissemination throughout the body. HIV infection significantly alters the body’s immune response to HPV infection, as evidenced by a decrease in the local CD68 population, and by affecting the expression of IL-6, IFN-γ, and TNF-α by macrophages. These changes collectively contribute to the cancer progression (Nicol et al., 2005c).
A stratified squamous epithelium covers the mucous membranes of the oral cavity, cervix, and genital tract. It forms tight junctions, maintains morphology and physiological function, and acts as a physical barrier against infection (Sufiawati and Tugizov, 2014). HIV can damage epithelial tight junctions by interacting with mucosal epithelial cells through its envelope proteins gp120 and Tat (Tugizov, 2016). Following this interaction, HPV reaches the basal cell layer, where the life cycle of HPV infection begins. This mechanism not only enhances the penetration of HPV pseudoviral particles into the epithelium and causes an initial HPV infection, but it also facilitates the entry of HPV into the mucosal epithelium and promotes the development of HPV-related cancers. It may have been due to inflammatory factors such as tumor necrosis factor-α and TGF-β, as well as calcium mucus proteins and tightly linked proteins, which have been adjusted to the upper membrane cells after HIV infection (Hewavisenti et al., 2023). In addition, it was discovered that in approximately 60% of HIV-infected patients, epithelial junctions in the oral and anal mucosa epithelial tissues were disrupted, thereby confirming the role of HIV in amplifying the oncogenic potential of HPV (Tugizov et al., 2013).
HPV is thought to be responsible for approximately 70% of oropharyngeal squamous cell carcinoma (OPSCCs) (Brickman et al., 2019). According to the global statistics reported in 2021, the percentage of HPV-positive OPSCCs was 33%, with significant regional variations in prevalence, ranging from 0% to 85% (Carlander et al., 2021), and men were far more likely than women to develop HPV-positive OPSCCs due to sexual behavior and number of sexual partners (Lechner et al., 2022).Furthermore, HIV-positive MSM patient are 1.5 to 2 times more likely to be infected with HR-HPV types or even have OPSCCs than HIV-negative MSM. The global prevalence of oral/oropharyngeal HPV infection in men was about 5%. Additionally, the overall prevalence of oral HPV infection was found to be 17.3% (95% CI: 13.6%-21.7%) in 9619 MSM from 14 different countries in the world, with a larger prevalence range of 3.8%-81.9% (p < 0.01). Furthermore, HIV-positive MSM had a greater pooled prevalence of oral HPV than HIV-negative MSM (22.5% vs. 14.5%; p = 0.1) (Farahmand et al., 2021). HPV 16, the most common HPV genotype in OPSCCs (Lechner et al., 2022), and the prevalence of HPV 16 was higher among HIV-positive MSM (4.7%; 95%CI 2.1–7.3) vs. 3.0%; 95%CI 0.5–5.5) in HIV-negative MSM. Furthermore, the analysis of 26 publications focusing on MSM concluded that HIV-positive MSM patients were susceptible to all types of HPV (28.9% vs. 17.1%), especially HR-HPV (16.5% vs. 9.1%) (King et al., 2016; Rossotti et al., 2024).
According to the UK National Multidisciplinary Guidelines, clinical examination includes direct flexible endoscopy of the upper aerodigestive tract and imageological examination, especially PET-CT and MRI. The former is used to assess the size and respectability of the primary tumor, while the latter is employed to assess the extent of lymph node disease and bone invasion and to detect distant metastases of the lung and liver (Mehanna et al., 2016). Confirmation of the diagnosis requires routine histopathologic examination, and immunohistochemistry may be added if necessary. PCR, DNA in situ hybridization and other methods can be utilized to evaluate the HPV status (Umudum et al., 2005; Bishop et al., 2012; Holzinger et al., 2012; Guo et al., 2014; Johnson et al., 2020). The combination of immunohistochemical staining of p16 and in situ hybridization of HR-HPV showed acceptable levels of sensitivity (97%) and specificity (94%) and could be performed using formalin fixed paraffin-embedded tissue (Lechner et al., 2022). Positive cases can also be identified by immunocytochemical p16INK4a/Ki67 double staining (Linxweiler et al., 2015). Treatment options for HPV-related OPSCC include surgery, radiotherapy, and chemoradiotherapy (CRT) (Lee et al., 2018; Gillison et al., 2019; Mehanna et al., 2019). Surgery includes open surgery and minimally invasive surgery, the latter mainly including transoral laser microsurgery and transoral robotic surgery (Lechner et al., 2022). Early (T1-T2, N0) OPSCCs can be treated with primary surgery or radiation therapy alone. Locally advanced (T3-T4, N0 and T1-T4, N1-N3) OPSCCs require multimodal treatment, including pre-operative surgery followed by RT or CRT, or eventual CRT, depending on pathological findings (surgical margin, extratodular extension) (Bozec et al., 2021). Three-weekly (100 mg/m²) cisplatin-based concurrent CRT should be the standard of care for patients with locally advanced HPV-related OPSCC (Gillison et al., 2019; Mehanna et al., 2019). Additionally, immunotherapy (Vermorken et al., 2008; Fakhry et al., 2014; Ferris et al., 2016) and HPV vaccination (Berenson et al., 2022) are also therapeutic and preventive tools. In two prospective studies, the anti-EGFR monoclonal antibody cetuximab has been investigated as an alternative to cisplatin to reduce the risk of treatment-related toxicity and morbidity. The anti-PD-1 antibodies nivolumab and pembrolizumab were first approved by the FDA in 2016 for patients with metastatic platinum-resistant OPSCC. Several therapeutic vaccines targeting the E6 and/or E7 have entered clinical trials in HPV OPSCC patients (Lechner et al., 2022). The prognosis for HPV-positive OPSCC is good, but 10-25% of patients will relapse. The National Comprehensive Cancer Network recommends screening every 1 to 3 months during the first year, every 2 to 6 months in the second year, every 4 to 8 months until the fifth year, and then annually thereafter. HPV DNA has proven to be a useful biomarker for monitoring disease status after treatment (Ellis et al., 2021).
Based on the statistics, the incidence of penile cancer worldwide was expected to be 0.8/100,000 in 2020, with Asia accounting for 56.3% of total cases (Sung et al., 2021). Furthermore, HPV is responsible for more than 75% of penile intraepithelial neoplasia and over 50% of penile cancers. According to the results of a recent meta-analysis, the overall pooled prevalence of penile HPV infection in MSM was 36.2% (95% CI:29.1%-44.0%), and the most frequent HR-HPV types in the penis were HPV 16 (4.9%, 95% CI: 3.6%–6.7%) and HPV 18 (3.2%, 95% CI: 2.4%–4.0%). Furthermore, HIV status increases the risk of penile HPV infection. According to the statistics, the pooled prevalence of penile HPV was substantially higher in HIV-positive MSM than it was in HIV-negative MSM (45.4%, 95% CI: 35.2%–56.0% vs. 28.6%, 95% CI: 19.4%–39.9%, respectively; p = 0.02) (Farahmand et al., 2020). HIV infection, as an independent risk factor, had a higher risk of penile cancer (RR = 3.7-5.8, three studies; SIR = 3.8-11.1, four studies) and had a four-fold increased risk of death. Additionally, progression from intra-epithelial neoplasia to cancer occurs 6 years earlier in HIV-positive men compared to HIV-negative men (Amini et al., 2023).
Penile cancer can be diagnosed by detecting HPV DNA using PCR, hybrid capture (HC) and 5% acetic acid. The first two methods demonstrate good sensitivity and correlation, while penoscopy has good specificity but low sensitivity (Figliuolo et al., 2012). The key to confirming the diagnosis is biopsy, and prognostic factors such as tissue subtype, grading, and cancer staging affect the outcome. Lymph node evaluation has important prognostic value and is closely related to the selection of adjuvant chemotherapy (Hakenberg et al., 2018). Treatment of penile cancer includes surgery, radiotherapy, chemotherapy, and immunotherapy. Local immunotherapy, chemotherapy, and laser ablation for in situ penile cancer (Manjunath et al., 2017). For patients with locally advanced penile cancer, and for those with fixed inguinal lymph nodes confirmed by biopsy, large >4 cm, bilateral and/or positive pelvic lymph nodes, neoadjuvant chemotherapy (NAC) is recommended, followed by surgical lymph node treatment, including inguinal lymph node dissection (ILND)/pelvic lymph node dissection (PLND). The preferred NAC standard schemes are four cycles of paclitaxel, ifosfamide, and cisplatin. These have been recommended by the National Comprehensive Cancer Network (NCCN) and European Association of Urology (EAU) guidelines (Chadha et al., 2022). In the case of locally advanced cancer, current NCCN guidelines recommend adjuvant chemotherapy in the form of TIP or 5-fluorouracil (5-FU) for patients who do not receive first-line NAC and exhibit ≥2 positive lymph nodes or extratodular expansion at ILND. For penile cancer patients with distant metastatic disease, the NCCN recommends chemotherapy as first-line treatment, using either the TIP or 5-FU plus cisplatin, followed by surgery or salvage systemic therapy for responders (Joshi et al., 2022a). Immunotherapy includes immune checkpoint blockade (ICB), HPV vaccines, adoptive T-cell therapies and engineered T-cell therapies, as well as tyrosine kinase inhibitors and other targeted therapies (Joshi et al., 2022b). In penile cancer, ICB is performed with anti-PD-L1, anti-PD-1, or anti-CTLA4 drugs. However, ICB approval is limited to second-line therapy for patients with relapsed or metastatic disease. Adoptive T-cell therapies to enhance T-cell-mediated tumor destruction, such as tumor infiltrating lymphocytes (TIL) therapy, chimeric antigen receptor T cell (CAR-T) therapy and engineered T cell receptor T cell (TCR) therapy. At present, the application of these therapies in the treatment of penile cancer remains in the research and exploratory stages. Current Phase I trials demonstrate the safety and efficacy of HPV targeting E6 or E7 cancer proteins. In terms of targeted therapy, anti-EGFR, and pan-HER TKI agents may be viable options for patients who are not candidates for standard of care combination chemotherapy or for those who have undergone platinum chemotherapy (Chadha et al., 2022).
Anal cancer accounts for approximately 2% of all intestinal mucosal malignant tumors. HPV infection and immunosuppression are the main risk factors for anal cancer.HPV-16 accounts for 85% of total squamous cell carcinoma of the anus (SCCA) (Muresu et al., 2020). The incidence of SCCA has increased annually over the past few decades by 2% to 6%, according to studies from high- and middle-income nations (Deshmukh et al., 2023). Anal cancer is rare in the general population, but its incidence has increased significantly among people living with HIV. A ten-year retrospective study conducted in Rome showed that HIV-positive men were about 1.4 times more likely to be HPV positive than HIV-negative men (Fracella et al). And Anal cancer is 19 times more common in HIV-positive patients than in the general population (Eng et al., 2022). HIV infection reduces the body’s clearance of HPV. HPV-16 clearance is about 1.6 times higher in HIV-negative MSM compared to HIV-positive MSM (Wei et al., 2023).
Diagnosing anal cancer involves history-taking, clinical recognition, biopsy, and MRI/CT. Recommended tests include HIV, p16, and HPV testing, along with PET-CT scans (Glynne-Jones et al., 2010). Screening methods for HIV patients include fingerprinting, anal Pap smear, HPV genotyping, and high-resolution anoscopy (Santorelli et al., 2018). Standard therapy involves chemoradiotherapy based on mitomycin C/cisplatin and 5-fluorouracil (Glynne-Jones et al., 2010). However, current treatments for anal cancer still have limitations. standard anal cancer treatments, especially in HIV-positive patients with low CD4 counts, require adjustments due to their toxicity and efficacy (Kauh et al., 2005). Many new treatment methods, such as targeted therapy, vaccination, immunotherapy, and photodynamic therapy, are undergoing clinical trials for the treatment of anal cancer (AC), and promising results have been achieved in certain indications.
HPV vaccines, including bivalent (HPV2), quadrivalent (qHPV), and nine-valent (9vHPV) offer crucial protection against HPV infection. While HPV2 targets HPV 16 and 18, qHPV extends coverage to include HPV 6, 11, 16, and 18. The latest vaccine, 9vHPV, provides protection against five additional HR-HPV types: 31, 33, 45, 52, and 58.
The Advisory Committee on Immunization Practices (ACIP) has provided corresponding HPV vaccination recommendations for different age groups and genders. MSM and individuals with compromised immune systems, including those infected with HIV, should receive the vaccine up to the age of 26 (Markowitz et al., 2014). The Merck Manual emphasizes immunocompromised individuals, including those with HIV infection, can receive the three-dose series regardless of their age at the time of the initial vaccination (Savoy, 2024).
Clinical trials have demonstrated that the quadrivalent HPV vaccine shows good efficacy in males aged 16 to 26. In the early vaccination group, the incidence of various HPV-related diseases was significantly reduced, with an incidence rate of 0.0 per 10,000 person-years for HPV-6 or 11-related external genital warts, which is lower than the control group’s rate of 137.3. The incidence of external genital lesions related to HPV-6, 11, 16, or 18 types were also significantly reduced, with an incidence rate of 0.0 per 10,000 person-years. In high-risk MSM, the incidence of anal intraepithelial neoplasia or anal cancer caused by HPV-6, 11, 16, or 18 types decreased from 906.2 to 20.5 cases per 10,000 person-years (Goldstone et al., 2022).
Vaccination-induced antibody levels are comparable in HIV-infected patients to the general population, with HIV patients who have preserved CD4+ T cell counts showing higher HPV antibody titers (Mugo et al., 2018). Furthermore, the safety of HPV vaccination is better in HIV-positive MSM, with studies finding that the highest dose of therapeutic HPV type 16 vaccine administered was demonstrated to be safe and immunogenic (Gosens et al., 2023). Adding the qHPV vaccine to a regimen for the treatment of high-grade anal intraepithelial neoplasia (HGAIN), particularly for HIV-positive MSM aged ≥27 years, significantly reduces life-cycle costs and increases quality-adjusted life years (Deshmukh et al., 2015). In addition, dual vaccination targeted at both HPV and HIV proved to be a highly cost-effective strategy and being more cost-effective compared to any other pre-exposure (PrEP) strategy (Moodley et al., 2016). These findings provide helpful scientific support for the feasibility, efficacy, and application of HPV vaccines in the context of HIV-positive patients.
The impact of HIV on HPV-related cancers in males has been comprehensively examined in this study. This study elucidates molecular interactions, cellular regulatory mechanisms, and epidemiological correlations between HIV and HPV, underscoring their synergistic roles in cancer progression. It offers insights for precision therapies and comprehensive control strategies, including HPV vaccine efficacy assessments for future prevention and treatment.
However, several questions remain to be resolved in the future. Firstly, research on the interactions between HIV-2 and HPV and the impact of this co-infection on cancer development is minimal. Additionally, the long-term effects of HAART therapy on chromosomal stability in individuals co-infected with HIV/HPV need further exploration. The molecular pathways by which other HIV proteins are involved in HPV infection and the development of related cancers have not been fully elucidated, and the potential roles of other HIV proteins in the formation of HPV-related cancers also require investigation. Finally, while HPV vaccines have shown promising efficacy and safety in HIV-infected individuals, more long-term studies are needed to confirm these findings and optimize vaccination strategies, especially the potential benefits of dual vaccination targeting both HPV and HIV.
ZZ: Writing – original draft, Writing – review & editing. YX: Writing – original draft, Writing – review & editing. TG: Writing – review & editing. WL: Writing – review & editing. SZ: Writing – review & editing. LW: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The Project was funded by Shenzhen Third People’s Hospital (No. G2022136). We also thank the Funds for Shenzhen Science and technology innovation program (KCXFZ20211020163544002) and the Natural Science Foundation of Guangdong (2023A1515220104).
We would like to thank the Shenzhen Third People’s Hospital and the School of Medicine at Southern University of Science and Technology for technical and financial support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
HPV, Human papillomavirus; HIV, Human immunodeficiency virus; MSM, Men who have sex with men; OPSCC, Oropharyngeal squamous cell carcinoma; LR-HPV, Low-risk HPV; HR-HPV, High-risk HPV; AIDS, Acquired immune deficiency syndrome; LOH, Chromosomal heterozygous deletion; MSI, Microsatellite instability; HAART, Highly active antiretroviral therapy; CMI, Cell-mediated immune; Trm, Resident memory T; MHC-1, Major Histocompatibility Complex I; DCs, Dendritic cells; LC, Langerhans cells; CRT, Chemoradiotherapy; HC, Hybrid capture; NAC, neoadjuvant chemotherapy; ILND, Inguinal lymph node dissection; PLND, Pelvic lymph node dissection; NCCN, National Comprehensive Cancer Network; EAU, European Association of Urology; 5-FU, 5-fluorouracil; ICB, Immune checkpoint blockade; TIL, Tumor infiltrating lymphocytes; CAR-T, Chimeric antigen receptor T cell; TCR, T cell receptor; SCCA, Squamous cell carcinoma of the anus; HGAIN, High-grade anal intraepithelial neoplasia; PrEP, Pre-exposure.
Amini, A. P., Brookes, T. S., Shah, H., Bhate, K., Alnajjar, H., Muneer, A., et al. (2023). The association between penile cancer and HIV infection: A literature review. Int. J. STD AIDS. 34, 214–228. doi: 10.1177/09564624221148622
Bai, J., Dong, X., Ning, T., Zhu, J., Wu, Z., Li, H., et al. (2024). Analysis of multi-site HPV infection and vaccination willingness among men who have sex with men in Tianjin, China. Front. Public Health 12. doi: 10.3389/fpubh.2024.1453024
Barillari, G., Palladino, C., Bacigalupo, I., Leone, P., Falchi, M., Ensoli, B. (2016). Entrance of the Tat protein of HIV-1 into human uterine cervical carcinoma cells causes upregulation of HPV-E6 expression and a decrease in p53 protein levels. Oncol. Lett. 12, 2389–2394. doi: 10.3892/ol.2016.4921
Behbahani, H., Walther-Jallow, L., Klareskog, E., Baum, L., French, A. L., Patterson, B. K., et al. (2007). Proinflammatory and type 1 cytokine expression in cervical mucosa during HIV-1 and human papillomavirus infection. JAIDS J. Acquired Immune Deficiency Syndr. 45 (1), 9–19. doi: 10.1097/QAI.0b013e3180415da7
Bere, A., Tayib, S., Kriek, J.-M., Masson, L., Jaumdally, S. Z., Barnabas, S. L., et al. (2014). Altered phenotype and function of NK cells infiltrating Human Papillomavirus (HPV)-associated genital warts during HIV infection. Clin. Immunol. 150, 210–219. doi: 10.1016/j.clim.2013.12.005
Berenson, A. B., Hirth, J. M., Chang, M. (2022). Prevalence of oral human papillomavirus infection: impact of sex, race/ethnicity, and vaccination status. Clin. Infect. Dis. 74, 1230–1236. doi: 10.1093/cid/ciab605
Bishop, J. A., Maleki, Z., Valsamakis, A., Ogawa, T., Chang, X., Pai, S. I., et al. (2012). Application of the hybrid capture 2 assay to squamous cell carcinomas of the head and neck: a convenient liquid-phase approach for the reliable determination of human papillomavirus status. Cancer Cytopathol. 120, 18–25. doi: 10.1002/cncy.v120.1
Bodily, J., Laimins, L. A. (2011). Persistence of human papillomavirus infection: keys to Malignant progression. Trends Microbiol. 19, 33–39. doi: 10.1016/j.tim.2010.10.002
Bozec, A., Culié, D., Poissonnet, G., Demard, F., Dassonville, O. (2021). Current therapeutic strategies in patients with oropharyngeal squamous cell carcinoma: impact of the tumor HPV status. Cancers (Basel). 13 (21), 5456. doi: 10.3390/cancers13215456
Branca, M., Giorgi, C., Santini, D., Bonito, L. D., Ciotti, M., Benedetto, A., et al. (2006). Aberrant expression of VEGF-C is related to grade of cervical intraepithelial neoplasia (CIN) and high risk HPV, but does not predict virus clearance after treatment of CIN or prognosis of cervical cancer. J. Clin. Pathol. 59, 40. doi: 10.1136/jcp.2005.026922
Brickman, C., Palefsky, J. M. (2015). Human papillomavirus in the HIV-infected host: epidemiology and pathogenesis in the antiretroviral era. Curr. HIV/AIDS Rep. 12, 6–15. doi: 10.1007/s11904-014-0254-4
Brickman, C. E., Propert, K. J., Merlin, J. S., Liu, J. C., Eady, S., McGhee-Jez, A., et al. (2019). Treatment and outcomes of oropharyngeal cancer in people with human immunodeficiency virus. AIDS Res. Hum. Retroviruses 35, 934–940. doi: 10.1089/aid.2019.0009
Burchell, A. N., Winer, R. L., de Sanjosé, S., Franco, E. L. (2006). Chapter 6: Epidemiology and transmission dynamics of genital HPV infection. Vaccine 24, S52–S61. doi: 10.1016/j.vaccine.2006.05.031
Bushara, O., Krogh, K., Weinberg, S. E., Finkelman, B. S., Sun, L., Liao, J., et al. (2022). Human immunodeficiency virus infection promotes human papillomavirus-mediated anal squamous carcinogenesis: an immunologic and pathobiologic review. Pathobiology 89, 1–12. doi: 10.1159/000518758
Carlander, A. F., Jakobsen, K. K., Bendtsen, S. K., Garset-Zamani, M., Lynggaard, C. D., Jensen, J. S., et al. (2021). A contemporary systematic review on repartition of HPV-positivity in oropharyngeal cancer worldwide. Viruses 13 (7), 1326. doi: 10.3390/v13071326
Chadha, J., Chahoud, J., Spiess, P. E. (2022). An update on treatment of penile cancer. Ther. Adv. Med. Oncol. 14, 17588359221127254. doi: 10.1177/17588359221127254
Chambuso, R., Kaambo, E., Denny, L., Gray, C. M., Williamson, A.-L., Migdalska-Sęk, M., et al. (2019). Investigation of cervical tumor biopsies for chromosomal loss of heterozygosity (LOH) and microsatellite instability (MSI) at the HLA II locus in HIV-1/HPV co-infected women. Front. Oncol. 9. doi: 10.3389/fonc.2019.00951
Chaturvedi, A. K., Madeleine, M. M., Biggar, R. J., Engels, E. A. (2009). Risk of human papillomavirus-associated cancers among persons with AIDS. J. Natl. Cancer Inst. 101, 1120–1130. doi: 10.1093/jnci/djp205
Chihu-Amparan, L., Pedroza-Saavedra, A., Gutierrez-Xicotencatl, L. (2023). The immune response generated against HPV infection in men and its implications in the diagnosis of cancer. Microorganisms 11 (6), 1609. doi: 10.3390/microorganisms11061609
Chow, L. T. (2015). Model systems to study the life cycle of human papillomaviruses and HPV-associated cancers. Virol. Sinica. 30, 92–100. doi: 10.1007/s12250-015-3600-9
Critchlow, C. W., Hawes, S. E., Kuypers, J. M., Goldbaum, G. M., Holmes, K. K., Surawicz, C. M., et al. (1998). Effect of HIV infection on the natural history of anal human papillomavirus infection. Aids 12, 1177–1184. doi: 10.1097/00002030-199810000-00010
Critchlow, C. W., Surawicz, C. M., Holmes, K. K., Kuypers, J., Daling, J. R., Hawes, S. E., et al. (1995). Prospective study of high grade anal squamous intraepithelial neoplasia in a cohort of homosexual men: influence of HIV infection, immunosuppression and human papillomavirus infection. Aids 9, 1255–1262. doi: 10.1097/00002030-199511000-00007
Deeks, S. G., Overbaugh, J., Phillips, A., Buchbinder, S. (2015). HIV infection. Nat. Rev. Dis. Primers. 1, 15035. doi: 10.1038/nrdp.2015.35
de Sanjose, S., Brotons, M., Pavon, M. A. (2018). The natural history of human papillomavirus infection. Best Pract. Res. Clin. Obstet Gynaecol. 47, 2–13. doi: 10.1016/j.bpobgyn.2017.08.015
Deshmukh, A. A., Chhatwal, J., Chiao, E. Y., Nyitray, A. G., Das, P., Cantor, S. B. (2015). Long-term outcomes of adding HPV vaccine to the anal intraepithelial neoplasia treatment regimen in HIV-positive men who have sex with men. Clin. Infect. Dis. 61, 1527–1535. doi: 10.1093/cid/civ628
Deshmukh, A. A., Damgacioglu, H., Georges, D., Sonawane, K., Ferlay, J., Bray, F., et al. (2023). Global burden of HPV-attributable squamous cell carcinoma of the anus in 2020, according to sex and HIV status: A worldwide analysis. Int. J. Cancer. 152, 417–428. doi: 10.1002/ijc.v152.3
Dhasmana, D. J., Dheda, K., Ravn, P., Wilkinson, R. J., Meintjes, G. (2008). Immune reconstitution inflammatory syndrome in HIV-infected patients receiving antiretroviral therapy: pathogenesis, clinical manifestations and management. Drugs 68, 191–208. doi: 10.2165/00003495-200868020-00004
Duensing, S., Münger, K. (2002). The human papillomavirus type 16 E6 and E7 oncoproteins independently induce numerical and structural chromosome instability. Cancer Res. 62, 7075–7082.
Edelmann, J., Lessig, R., Hering, S., Horn, L. C. (2004). Loss of heterozygosity and microsatellite instability of forensically used STR markers in human cervical carcinoma. Int. Congress Series. 1261, 499–501. doi: 10.1016/S0531-5131(03)01717-5
Ellis, M., Garas, G., Hardman, J., Kahn, M., Mehanna, H., Smith, M. E., et al. (2021). Post-treatment head and neck cancer care: national audit and analysis of current practice in the United Kingdom. Clin. Otolaryngol. 46, 284–294.
Eng, C., Ciombor, K. K., Cho, M., Dorth, J. A., Rajdev, L. N., Horowitz, D. P., et al. (2022). Anal cancer: emerging standards in a rare disease. J. Clin. Oncol. 40, 2774–2788. doi: 10.1200/JCO.21.02566
Fakhry, C., Zhang, Q., Nguyen-Tan, P. F., Rosenthal, D., El-Naggar, A., Garden, A. S., et al. (2014). Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J. Clin. Oncol. 32, 3365–3373. doi: 10.1200/JCO.2014.55.1937
Farahmand, M., Moghoofei, M., Dorost, A., Abbasi, S., Monavari, S. H., Kiani, S. J., et al. (2020). Prevalence and genotype distribution of genital human papillomavirus infection in female sex workers in the world: a systematic review and meta-analysis. BMC Public Health 20, 1455. doi: 10.1186/s12889-020-09570-z
Farahmand, M., Monavari, S. H., Tavakoli, A. (2021). Prevalence and genotype distribution of human papillomavirus infection in different anatomical sites among men who have sex with men: A systematic review and meta-analysis. Rev. Med. Virol. 31, e2219. doi: 10.1002/rmv.v31.6
Faraji, F., Zaidi, M., Fakhry, C., Gaykalova, D. A. (2017). Molecular mechanisms of human papillomavirus-related carcinogenesis in head and neck cancer. Microbes Infect. 19, 464–475. doi: 10.1016/j.micinf.2017.06.001
Ferris, R. L., Blumenschein, G., Jr., Fayette, J., Guigay, J., Colevas, A. D., Licitra, L., et al. (2016). Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl. J. Med. 375, 1856–1867. doi: 10.1056/NEJMoa1602252
Figliuolo, G., Maia, J., Jalkh, A. P., Miranda, A. E., Ferreira, L. C. L. (2012). Clinical and laboratorial study of HPV infection in men infected with HIV. Int. Braz. J. Urol. 38, 411–418. doi: 10.1590/S1677-55382012000300015
Fitzgerald, D. W., Bezak, K., Ocheretina, O., Riviere, C., Wright, T. C., Milne, G. L., et al. (2012). The effect of HIV and HPV coinfection on cervical COX-2 expression and systemic prostaglandin E2 levels. Cancer Prev. Res. 5, 34–40. doi: 10.1158/1940-6207.CAPR-11-0496
Fracella, M., Oliveto, G., Roberto, P., Cinti, L., Gentile, M., Coratti, E., et al. (2024). The epidemiology of anal human papillomavirus (HPV) in HIV-positive and HIV-negative women and men: A ten-year retrospective observational study in Rome (Italy). Pathogens 13 (2), 163. doi: 10.3390/pathogens13020163
Frisch, M., Biggar, R. J., Goedert, J. J. (2000). Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J. Natl. Cancer Inst. 92, 1500–1510. doi: 10.1093/jnci/92.18.1500
Gebhardt, T., Palendira, U., Tscharke, D. C., Bedoui, S. (2018). Tissue-resident memory T cells in tissue homeostasis, persistent infection, and cancer surveillance. Immunol. Rev. 283, 54–76. doi: 10.1111/imr.2018.283.issue-1
Gillison, M. L., Trotti, A. M., Harris, J., Eisbruch, A., Harari, P. M., Adelstein, D. J., et al. (2019). Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet 393, 40–50. doi: 10.1016/S0140-6736(18)32779-X
Glynne-Jones, R., Northover, J. M., Cervantes, A., Group, E. G. W. (2010). Anal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 21 Suppl 5, v87–v92. doi: 10.1093/annonc/mdq171
Goldstone, S. E., Giuliano, A. R., Palefsky, J. M., Lazcano-Ponce, E., Penny, M. E., Cabello, R. E., et al. (2022). Efficacy, immunogenicity, and safety of a quadrivalent HPV vaccine in men: results of an open-label, long-term extension of a randomised, placebo-controlled, phase 3 trial. Lancet Infect. Dis. 22, 413–425. doi: 10.1016/S1473-3099(21)00327-3
Gosens, K. C. M., Burg, S., Welters, M. J. P., Boekestijn, S., Loof, N. M., Quint, W. G. V., et al. (2023). Therapeutic vaccination against human papillomavirus type 16 for the treatment of high-grade anal intraepithelial neoplasia in HIV+ Men. Clin. Cancer Res. 29 (20), 4109–4117. doi: 10.1158/1078-0432.CCR-22-3361
Graham, S. V. (2017). The human papillomavirus replication cycle, and its links to cancer progression: a comprehensive review. Clin. Sci. 131, 2201–2221. doi: 10.1042/CS20160786
Guimarães, A. G., Silva Junior, R. M., Costa, O. T., Silva, I. T., Gimenez, F. S., Araujo, J. R., et al. (2011). Morphometric analysis of dendritic cells from anal mucosa of HIV-positive patients and the relation to intraepithelial lesions and cancer seen at a tertiary health institution in Brazil. Acta Cir Bras. 26, 521–529. doi: 10.1590/S0102-86502011000600019
Guo, M., Khanna, A., Dhillon, J., Patel, S. J., Feng, J., Williams, M. D., et al. (2014). Cervista HPV assays for fine-needle aspiration specimens are a valid option for human papillomavirus testing in patients with oropharyngeal carcinoma. Cancer Cytopathol. 122, 96–103. doi: 10.1002/cncy.v122.2
Hachisuga, T., Fukuda, K., Kawarabayashi, T. (2001). Local immune response in squamous cell carcinoma of the uterine cervix. Gynecol. Obstet Invest. 52, 3–8. doi: 10.1159/000052931
Hafner, A., Bulyk, M. L., Jambhekar, A., Lahav, G. (2019). The multiple mechanisms that regulate p53 activity and cell fate. Nat. Rev. Mol. Cell Biol. 20, 199–210. doi: 10.1038/s41580-019-0110-x
Hakenberg, O. W., Dräger, D. L., Erbersdobler, A., Naumann, C. M., Jünemann, K. P., Protzel, C. (2018). The diagnosis and treatment of penile cancer. Dtsch Arztebl Int. 115, 646–652. doi: 10.3238/arztebl.2018.0646
Handisurya, A., Schellenbacher, C., Kirnbauer, R. (2009). Diseases caused by human papillomaviruses (HPV). JDDG: J. der Deutschen Dermatologischen Gesellschaft. 7, 453–466. doi: 10.1111/j.1610-0387.2009.06988.x
Hens, J., Jennes, W., Kestens, L. (2016). The role of NK cells in HIV-1 protection: autologous, allogeneic or both? AIDS Res. Ther. 13, 15. doi: 10.1186/s12981-016-0099-6
Hernandez, B. Y., Wilkens, L. R., Zhu, X., McDuffie, K., Thompson, P., Shvetsov, Y. B., et al. (2008). Circumcision and human papillomavirus infection in men: a site-specific comparison. J. Infect. Dis. 197, 787–794. doi: 10.1086/528379
Hewavisenti, R. V., Arena, J., Ahlenstiel, C. L., Sasson, S. C. (2023). Human papillomavirus in the setting of immunodeficiency: Pathogenesis and the emergence of next-generation therapies to reduce the high associated cancer risk. Front. Immunol. 14. doi: 10.3389/fimmu.2023.1112513
Hickman, H. D., Reynoso, G. V., Ngudiankama, B. F., Cush, S. S., Gibbs, J., Bennink, J. R., et al. (2015). CXCR3 chemokine receptor enables local CD8(+) T cell migration for the destruction of virus-infected cells. Immunity 42, 524–537. doi: 10.1016/j.immuni.2015.02.009
Holzinger, D., Schmitt, M., Dyckhoff, G., Benner, A., Pawlita, M., Bosch, F. X. (2012). Viral RNA patterns and high viral load reliably define oropharynx carcinomas with active HPV16 involvement. Cancer Res. 72, 4993–5003. doi: 10.1158/0008-5472.CAN-11-3934
Hubert, P., Giannini, S. L., Vanderplasschen, A., Franzen-Detrooz, E., Jacobs, N., Boniver, J., et al. (2001). Dendritic cells induce the death of human papillomavirus-transformed keratinocytes. FASEB J. 15, 2521–2523. doi: 10.1096/fj.00-0872fje
Johnson, D. E., Burtness, B., Leemans, C. R., Lui, V. W. Y., Bauman, J. E., Grandis, J. R. (2020). Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers. 6, 92. doi: 10.1038/s41572-020-00224-3
Joshi, V. B., Chadha, J., Chahoud, J. (2022a). Penile cancer: Updates in systemic therapy. Asian J. Urol. 9, 374–388. doi: 10.1016/j.ajur.2022.03.006
Joshi, V. B., Spiess, P. E., Necchi, A., Pettaway, C. A., Chahoud, J. (2022b). Immune-based therapies in penile cancer. Nat. Rev. Urol. 19, 457–474. doi: 10.1038/s41585-022-00617-x
Kauh, J., Koshy, M., Gunthel, C., Joyner, M. M., Landry, J., Thomas, C. R. (2005). Management of anal cancer in the HIV-positive population. Oncology-Williston Park Then Huntington Melville New York 19, 1634.
Kim, R. H., Yochim, J. M., Kang, M. K., Shin, K.-H., Christensen, R., Park, N.-H. (2008). HIV-1 Tat enhances replicative potential of human oral keratinocytes harboring HPV-16 genome. Int. J. Oncol. 33, 777–782. doi: 10.3892/ijo_00000064
King, E. M., Oomeer, S., Gilson, R., Copas, A., Beddows, S., Soldan, K., et al. (2016). Oral human papillomavirus infection in men who have sex with men: A systematic review and meta-analysis. PloS One 11, e0157976. doi: 10.1371/journal.pone.0157976
Lechner, M., Liu, J., Masterson, L., Fenton, T. R. (2022). HPV-associated oropharyngeal cancer: epidemiology, molecular biology and clinical management. Nat. Rev. Clin. Oncol. 19, 306–327. doi: 10.1038/s41571-022-00603-7
Lee, N. C. J., Kelly, J. R., Park, H. S., An, Y., Judson, B. L., Burtness, B. A., et al. (2018). Patterns of failure in high-metastatic node number human papillomavirus-positive oropharyngeal carcinoma. Oral. Oncol. 85, 35–39. doi: 10.1016/j.oraloncology.2018.08.001
Li, W., Tian, S., Wang, P., Zang, Y., Chen, X., Yao, Y., et al. (2019). The characteristics of HPV integration in cervical intraepithelial cells. J. Cancer. 10, 2783–2787. doi: 10.7150/jca.31450
Linxweiler, M., Bochen, F., Wemmert, S., Lerner, C., Hasenfus, A., Bohle, R. M., et al. (2015). Combination of p16(INK4a)/Ki67 immunocytology and HPV polymerase chain reaction for the noninvasive analysis of HPV involvement in head and neck cancer. Cancer Cytopathol. 123, 219–229. doi: 10.1002/cncy.v123.4
Looker, K. J., Rönn, M. M., Brock, P. M., Brisson, M., Drolet, M., Mayaud, P., et al. (2018). Evidence of synergistic relationships between HIV and Human Papillomavirus (HPV): systematic reviews and meta-analyses of longitudinal studies of HPV acquisition and clearance by HIV status, and of HIV acquisition by HPV status. J. Int. AIDS Soc. 21, e25110. doi: 10.1002/jia2.2018.21.issue-6
Mahnke, Y. D., Greenwald, J. H., DerSimonian, R., Roby, G., Antonelli, L. R. V., Sher, A., et al. (2012). Selective expansion of polyfunctional pathogen-specific CD4+ T cells in HIV-1–infected patients with immune reconstitution inflammatory syndrome. Blood 119, 3105–3112. doi: 10.1182/blood-2011-09-380840
Manjunath, A., Brenton, T., Wylie, S., Corbishley, C. M., Watkin, N. A. (2017). Topical Therapy for non-invasive penile cancer (Tis)-updated results and toxicity. Transl. Androl. Urol. 6, 803–808. doi: 10.21037/tau.2017.06.24
Mao, Y., Jiang, P. (2023). The crisscross between p53 and metabolism in cancer. Acta Biochim. Biophys. Sin. (Shanghai). 55, 914–922. doi: 10.3724/abbs.2023109
Markowitz, L. E., Dunne, E. F., Saraiya, M., Chesson, H. W., Curtis, C. R., Gee, J., et al. (2014). Human papillomavirus vaccination recommendations of the advisory committee on immunization practices (ACIP). Morbidity and mortality weekly report: recommendations and reports. MMWR-Morbidity and Mortality Weekly Report. 63 (5), 1–30.
Martinez-Zapien, D., Ruiz, F. X., Poirson, J., Mitschler, A., Ramirez, J., Forster, A., et al. (2016). Structure of the E6/E6AP/p53 complex required for HPV-mediated degradation of p53. Nature 529, 541–545. doi: 10.1038/nature16481
Mavilio, D., Lombardo, G., Benjamin, J., Kim, D., Follman, D., Marcenaro, E., et al. (2005). Characterization of CD56–/CD16+ natural killer (NK) cells: A highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc. Natl. Acad. Sci. 102, 2886–2891. doi: 10.1073/pnas.0409872102
McBride, A. A., Warburton, A. (2017). The role of integration in oncogenic progression of HPV-associated cancers. PloS Pathog. 13, 1, e100621. doi: 10.1371/journal.ppat.1006211
Mehanna, H., Evans, M., Beasley, M., Chatterjee, S., Dilkes, M., Homer, J., et al. (2016). Oropharyngeal cancer: United Kingdom national multidisciplinary guidelines. J. Laryngol. Otol. 130, S90–Ss6. doi: 10.1017/S0022215116000505
Mehanna, H., Robinson, M., Hartley, A., Kong, A., Foran, B., Fulton-Lieuw, T., et al. (2019). Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet 393, 51–60. doi: 10.1016/S0140-6736(18)32752-1
Mehta, K. S., Marfatia, Y. S., Jain, A. P., Shah, D. J., Baxi, D. S. (2021). Male circumcision and Sexually transmitted Infections - An update. Indian J. Sex Transm. Dis. AIDS. 42, 1–6. doi: 10.4103/ijstd.ijstd_20_21
Molina, M. A., Steenbergen, R. D. M., Pumpe, A., Kenyon, A. N., Melchers, W. J. G. (2024). HPV integration and cervical cancer: a failed evolutionary viral trait. Trends Mol. Med. 30 (9), 890–902. doi: 10.1016/j.molmed.2024.05.009
Moodley, N., Gray, G., Bertram, M. (2016). The price of prevention: cost effectiveness of biomedical HIV prevention strategies in South Africa. Clin. Res. HIV AIDS 3 (1), 1031.
Mooij, S. H., van Santen, D. K., Geskus, R. B., van der Sande, M. A., Coutinho, R. A., Stolte, I. G., et al. (2016). The effect of HIV infection on anal and penile human papillomavirus incidence and clearance: a cohort study among MSM. Aids 30, 121–132. doi: 10.1097/QAD.0000000000000909
Mugo, N. R., Eckert, L., Magaret, A. S., Cheng, A., Mwaniki, L., Ngure, K., et al. (2018). Quadrivalent HPV vaccine in HIV-1-infected early adolescent girls and boys in Kenya: Month 7 and 12 post vaccine immunogenicity and correlation with immune status. Vaccine 36, 7025–7032. doi: 10.1016/j.vaccine.2018.09.059
Muresu, N., Sotgiu, G., Saderi, L., Sechi, I., Cossu, A., Marras, V., et al. (2020). Distribution of HPV genotypes in patients with a diagnosis of anal cancer in an italian region. Int. J. Environ. Res. Public Health 17, 4516. doi: 10.3390/ijerph17124516
Nicol, A. F., Fernandes, A. T. G., Bonecini-Almeida, M. (2005a). Immune response in cervical dysplasia induced by human papillomavirus: the influence of human immunodeficiency virus-1 co-infection - review. Memórias do Instituto Oswaldo Cruz 100. doi: 10.1590/S0074-02762005000100001
Nicol, A. F., Fernandes, A. T., Bonecini-Almeida Mda, G. (2005b). Immune response in cervical dysplasia induced by human papillomavirus: the influence of human immunodeficiency virus-1 co-infection – review. Mem Inst Oswaldo Cruz. 100, 1–12. doi: 10.1590/S0074-02762005000100001
Nicol, A. F., Fernandes, A. T. G., Grinsztejn, B. G. J., Russomano, F. B., E Silva, J. R. L., Tristão, A., et al. (2005c). Distribution of immune cell subsets and cytokine-producing cells in the uterine cervix of human papillomavirus (HPV)-infected women: influence of HIV-1 coinfection. Diagn. Mol. Pathol. 14, 39–47. doi: 10.1097/01.pas.0000143309.81183.6c
Nicol, A. F., Pires, A. R. C., de Souza, S. R., Nuovo, G. J., Grinsztejn, B., Tristão, A., et al. (2008). Cell-cycle and suppressor proteins expression in uterine cervix in HIV/HPV co-infection: comparative study by tissue micro-array (TMA). BMC Cancer. 8 (1), 289. doi: 10.1186/1471-2407-8-289
Nyagol, J., Leucci, E., Omnis, A., De Falco, G., Tigli, C., Sanseverino, F., et al. (2006). The effects of HIV-1 Tat protein on cell cycle during cervical carcinogenesis. Cancer Biol. Ther. 5, 684–690. doi: 10.4161/cbt.5.6.2907
Nyamweya, S., Hegedus, A., Jaye, A., Rowland-Jones, S., Flanagan, K. L., Macallan, D. C. (2013). Comparing HIV-1 and HIV-2 infection: Lessons for viral immunopathogenesis. Rev. Med. Virol. 23, 221–240. doi: 10.1002/rmv.v23.4
Olaitan, A., Johnson, M. A., MacLean, A., Poulter, L. W. (1996). The distribution of immunocompetent cells in the genital tract of HIV-positive women. Aids 10, 759–764. doi: 10.1097/00002030-199606001-00010
Ortiz, A. P., Engels, E. A., Nogueras-González, G. M., Colón-López, V., Soto-Salgado, M., Vargas, A., et al. (2018). Disparities in human papillomavirus-related cancer incidence and survival among human immunodeficiency virus-infected Hispanics living in the United States. Cancer 124, 4520–4528. doi: 10.1002/cncr.31702
Pachiadakis, I., Pollara, G., Chain, B. M., Naoumov, N. V. (2005). Is hepatitis C virus infection of dendritic cells a mechanism facilitating viral persistence? Lancet Infect. Dis. 5, 296–304. doi: 10.1016/s1473-3099(05)70114-6
Palefsky, J. (2006). Biology of HPV in HIV infection. Adv. Dent. Res. 19, 99–105. doi: 10.1177/154407370601900120
Palefsky, J. M., Holly, E. A. (2003). Chapter 6: immunosuppression and co-infection with HIV. J. Natl. Cancer Inst Monogr. 31, 41–46. doi: 10.1093/oxfordjournals.jncimonographs.a003481
Petry, K. U., Scheffel, D., Bode, U., Gabrysiak, T., Köchel, H., Kupsch, E., et al. (1994). Cellular immunodeficiency enhances the progression of human papillomavirus-associated cervical lesions. Int. J. Cancer. 57, 836–840. doi: 10.1002/ijc.2910570612
Pujantell, M., Badia, R., Galván-Femenía, I., Garcia-Vidal, E., de Cid, R., Alcalde, C., et al. (2019). ADAR1 function affects HPV replication and is associated to recurrent human papillomavirus-induced dysplasia in HIV coinfected individuals. Sci. Rep. 9, 19848. doi: 10.1038/s41598-019-56422-x
Ranjit, S., Kodidela, S., Sinha, N., Chauhan, S., Kumar, S. (2020). Extracellular vesicles from human papilloma virus-infected cervical cancer cells enhance HIV-1 replication in differentiated U1 cell line. Viruses 12 (2), 239. doi: 10.3390/v12020239
Rossotti, R., Nava, A., Baiguera, C., Baldassari, L., Moioli, M. C., Fanti, D., et al. (2024). Oral HPV infection clearance and acquisition after nonavalent vaccination in men who have sex with men and transgender women: a prospective analysis. Eur. J. Clin. Microbiol. Infect. Dis. 43, 1847–1854. doi: 10.1007/s10096-024-04887-8
Sales, K. J., Katz, A. A., Davis, M., Hinz, S., Soeters, R. P., Hofmeyr, M. D., et al. (2001). Cyclooxygenase-2 expression and prostaglandin E(2) synthesis are up-regulated in carcinomas of the cervix: a possible autocrine/paracrine regulation of neoplastic cell function via EP2/EP4 receptors. J. Clin. Endocrinol. Metab. 86, 2243–2249. doi: 10.1210/jcem.86.5.7442
Saluzzo, S., Pandey, R. V., Gail, L. M., Dingelmaier-Hovorka, R., Kleissl, L., Shaw, L., et al. (2021). Delayed antiretroviral therapy in HIV-infected individuals leads to irreversible depletion of skin- and mucosa-resident memory T cells. Immunity 54, 2842–58.e5. doi: 10.1016/j.immuni.2021.10.021
Santorelli, C., Leo, C. A., Hodgkinson, J. D., Baldelli, F., Cantarella, F., Cavazzoni, E. (2018). Screening for squamous cell anal cancer in HIV positive patients: A five-year experience. J. Invest. Surg. 31, 378–384. doi: 10.1080/08941939.2017.1334845
Savoy, M. L. (2024). Human Papillomavirus (HPV) Vaccine Merck Manual Professional Edition: Merck Manual. Available online at: https://www.merckmanuals.com/professional/infectious-diseases/immunization/human-papillomavirus-hpv-vaccine (Accessed October 10, 2024).
Scott, M., Nakagawa, M., Moscicki, A. B. (2001). Cell-mediated immune response to human papillomavirus infection. Clin. Diagn. Lab. Immunol. 8, 209–220. doi: 10.1128/CDLI.8.2.209-220.2001
Singh, A. K., Walavalkar, K., Tavernari, D., Ciriello, G., Notani, D., Sabarinathan, R. (2024). Cis-regulatory effect of HPV integration is constrained by host chromatin architecture in cervical cancers. Mol Oncol. (2024) 18 (5), 1189–1208. doi: 10.1002/1878-0261.13559
Stanley, M. A. (2012). Epithelial cell responses to infection with human papillomavirus. Clin. Microbiol. Rev. 25, 215–222. doi: 10.1128/CMR.05028-11
Subbaramaiah, K., Dannenberg, A. J. (2007). Cyclooxygenase-2 transcription is regulated by human papillomavirus 16 E6 and E7 oncoproteins: evidence of a corepressor/coactivator exchange. Cancer Res. 67, 3976–3985. doi: 10.1158/0008-5472.CAN-06-4273
Sufiawati, I., Tugizov, S. M. (2014). HIV-associated disruption of tight and adherens junctions of oral epithelial cells facilitates HSV-1 infection and spread. PloS One 9, e88803. doi: 10.1371/journal.pone.0088803
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. doi: 10.3322/caac.21660
Tjalma, W. A. A., Van Waes, T. R., Van den Eeden, L. E. M., Bogers, J. J. P. M. (2005). Role of human papillomavirus in the carcinogenesis of squamous cell carcinoma and adenocarcinoma of the cervix. Best Pract. Res. Clin. Obstet. Gynaecol. 19, 469–483. doi: 10.1016/j.bpobgyn.2005.02.002
Tornesello, M. L., Buonaguro, F. M., Beth-Giraldo, E., Giraldo, G. (2008). Human immunodeficiency virus type 1 tat gene enhances human papillomavirus early gene expression. Intervirology 36, 57–64. doi: 10.1159/000150322
Toy, E. P., Rodríguez-Rodríguez, L., McCance, D., Ludlow, J., Planelles, V. (2000). Induction of cell-cycle arrest in cervical cancer cells by the human immunodeficiency virus type 1 viral protein R11Named a Searle-Donald F. Richardson Prize Paper after presentation at the ACOG District II Junior Fellows Annual Meeting, New York, October 1998, and Annual Clinical Meeting of ACOG, Philadelphia, May 1999. Obstet. Gynecol. 95, 141–146. doi: 10.1016/s0029-7844(99)00464-0
Tugizov, S. (2016). Human immunodeficiency virus-associated disruption of mucosal barriers and its role in HIV transmission and pathogenesis of HIV/AIDS disease. Tissue Barriers. 4, e1159276. doi: 10.1080/21688370.2016.1159276
Tugizov, S. M., Herrera, R., Chin-Hong, P., Veluppillai, P., Greenspan, D., Michael Berry, J., et al. (2013). HIV-associated disruption of mucosal epithelium facilitates paracellular penetration by human papillomavirus. Virology 446, 378–388. doi: 10.1016/j.virol.2013.08.018
Umudum, H., Rezanko, T., Dag, F., Dogruluk, T. (2005). Human papillomavirus genome detection by in situ hybridization in fine-needle aspirates of metastatic lesions from head and neck squamous cell carcinomas. Cancer 105, 171–177. doi: 10.1002/cncr.21027
Ventimiglia, E., Horenblas, S., Muneer, A., Salonia, A. (2016). Human papillomavirus infection and vaccination in males. Eur. Urol. Focus. 2, 355–362. doi: 10.1016/j.euf.2016.08.012
Vermorken, J. B., Mesia, R., Rivera, F., Remenar, E., Kawecki, A., Rottey, S., et al. (2008). Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl. J. Med. 359, 1116–1127. doi: 10.1056/NEJMoa0802656
Vernon, S. D., Hart, C. E., Reeves, W. C., Icenogle, J. P. (1993). The HIV-1 tat protein enhances E2-dependent human papillomavirus 16 transcription. Virus Res. 27, 133–145. doi: 10.1016/0168-1702(93)90077-Z
Wei, F., Goodman, M. T., Xia, N., Zhang, J., Giuliano, A. R., D’Souza, G., et al. (2023). Incidence and clearance of anal human papillomavirus infection in 16 164 individuals, according to human immunodeficiency virus status, sex, and male sexuality: an international pooled analysis of 34 longitudinal studies. Clin. Infect. Dis. 76, e692–e701. doi: 10.1093/cid/ciac581
Wright-Browne, V., McClain, K. L., Talpaz, M., Ordonez, N., Estrov, Z. (1997). Physiology and pathophysiology of dendritic cells. Hum. Pathol. 28, 563–579. doi: 10.1016/S0046-8177(97)90079-4
Zheng, Z. M., Baker, C. C. (2006). Papillomavirus genome structure, expression, and post-transcriptional regulation. Front. Biosci. 11, 2286–2302. doi: 10.2741/1971
Keywords: human papillomavirus, human immunodeficiency virus, MSM, co-infection, cancer
Citation: Zhang Z, Xing Y, Gong T, Li W, Zhang S and Wei L (2025) Impact of HIV on HPV-related cancers in men who have sex with men: a review. Front. Cell. Infect. Microbiol. 14:1428491. doi: 10.3389/fcimb.2024.1428491
Received: 06 May 2024; Accepted: 23 December 2024;
Published: 20 January 2025.
Edited by:
Jianhong Zhao, Second Hospital of Hebei Medical University, ChinaReviewed by:
Erica Diani, University of Verona, ItalyCopyright © 2025 Zhang, Xing, Gong, Li, Zhang and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Siwei Zhang, c3dfemhhbmcyNTI4QDE2My5jb20=; Lanlan Wei, d2VpbGFubGFuQG1haWwuc3VzdGVjaC5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.