- Stanley Division of Developmental Neurovirology, Department of Pediatrics, School of Medicine, Johns Hopkins University, Baltimore, MD, United States
Toxoplasma gondii (T. gondii) is one of the most successful intracellular protozoa in that it can infect the majority of mammalian cell types during the acute phase of infection. Furthermore, it is able to establish a chronic infection for the host’s entire lifespan by developing an encysted parasite form, primarily in the muscles and brain of the host, to avoid the host immune system. The infection affects one third of the world population and poses an increased risk for people with a suppressed immune system. Despite the dormant characteristics of chronic T. gondii infection, there is much evidence suggesting that this infection leads to specific behavior changes in both humans and rodents. Although numerous hypotheses have been put forth, the exact mechanisms underlying these behavior changes have yet to be understood. In recent years, several studies revealed a strong connection between the gut microbiome and the different organ systems that are affected in T. gondii infection. While it is widely studied and accepted that acute T. gondii infection can lead to a dramatic disruption of the host’s normal, well-balanced microbial ecosystem (microbial dysbiosis), changes in the gut microbiome during the chronic stage of infection has not been well characterized. This review is intended to briefly inspect the different hypotheses that attempt to explain the behavior changes during T. gondii infection. Furthermore, this review proposes to consider the potential link between gut microbial dysbiosis, and behavior changes in T. gondii infection as a novel way to describe the underlying mechanism.
1 Introduction
Toxoplasmosis, caused by T. gondii, presents a significant global health concern, impacting approximately one-third of the world’s population (Pappas et al., 2009). While often asymptomatic, with an estimated 80 to 90% of acquired infections going unrecognized (Mittal and Ichhpujani, 2011), individuals with weakened immune systems, as well as the offspring of pregnant women, face an increased risk of severe toxoplasmosis (Wong and Remington, 1994; Wang et al., 2017). As a result, manifestations of toxoplasmosis range from mild symptoms to more severe outcomes, including congenital infection, miscarriage, chorioretinitis, and encephalitis (Dubey and Beattie, 1988; Halonen and Weiss, 2013), as well as associations with mental health conditions, such as anxiety (Markovitz et al., 2015), fear (Boillat, 2020), and schizophrenia (Torrey and Yolken, 2003; Yolken et al., 2009; Fuglewicz et al., 2017). Transmission of T. gondii occurs through congenital means, foodborne routes, zoonotic transmission, and, rarely, through organ transplant or blood transfusion from an infected donor (Hill and Dubey, 2002). The parasite’s ability to persist for extended periods, potentially throughout a lifetime, contributes to its widespread presence.
T. gondii, taxonomically classified as a coccidian, is a spore-forming, single-celled obligate intracellular organism. It undergoes both asexual and sexual reproduction, with asexual reproduction occurring in various host cells through cell division (Dubey and Beattie, 1988). Sexual reproduction is confined to the intestinal epithelium of Felidae family members, leading to the shedding of unsporulated oocysts in their feces (Dubey and Beattie, 1988). Genotyping data showed very limited sexual recombination between predominant T. gondii clonal lineages, namely Types I, II and III (Howe and Sibley, 1995), as well as non-clonal lineages, also called atypical strains (Shwab et al., 2014). Different T. gondii strains lead to different manifestations of the pathophysiology of the infection (Xiao and Yolken, 2015).
The tachyzoites, key players in acute infection, undergo rapid replication every 6 to 8 hours through endodyogeny. In the rodent host, T. gondii infection leads to severe inflammation of the small intestine, resulting in necrosis of mucosal villi and tissue destruction (Liesenfeld et al., 1996; Denkers, 2009). The parasite load correlates with the severity of this inflammation in the intestine (Dias et al., 2014). Chronic toxoplasmosis unfolds as a continuum (Cerutti et al., 2020) from acute toxoplasmosis into a persistent and latent state within immune competent individuals due to the host immune response (Dubey, 1998). During this transition from acute to chronic infectious stages, a slower replicating form (bradyzoites) of the T. gondii organism dominates. The bradyzoites are distinct in behavior and unlike tachyzoites, do not rupture host cells; instead, they form cysts, featuring a robust protective mechanism (Dubey et al., 1998). Cysts develop intracellularly and can persist for extended periods, potentially for the life of the host (Ferguson and Hutchison, 1987; Dubey et al., 1998). Brain, eyes, and muscles emerge as the primary sites for chronic, latent infection, although cysts have also been identified in various visceral organs, such as the kidney, liver, heart and lungs (Dubey et al., 1998), but parasite frequency is incredibly low. While maintaining ostensible dormancy in the host, there is a higher risk of reactivation in immunocompromised individuals (Daher et al., 2021). Despite their slow replication, bradyzoites exert a profound impact on neuronal structure and connectivity (Daher et al., 2021), as well as provoke brain-related immune responses (Xiao et al., 2016a; Li et al., 2019).
Furthermore, T. gondii can directly affect host gene expression (Carruthers and Suzuki, 2007; Müller and Howard, 2016; Abo-Al-Ela, 2019) by influencing various host signal transduction pathways (Coppens and Joiner, 2001), neurotransmission (Stibbs, 1985; Adamec et al., 1998), host immune response (Hunter and Sibley, 2012; Yarovinsky, 2014; Sasai et al., 2018; Sasai and Yamamoto, 2019), and host behavior (da Silva and Langoni, 2009; Webster and McConkey, 2010; Pittman and Knoll, 2015; Vyas, 2015; Abdulai-Saiku et al., 2021; Tong et al., 2021). This intricate interplay defines the multifaceted nature of toxoplasmosis, revealing a dynamic, complex and enduring relationship between the parasite and its host. The most intriguing interplay between the parasite and host is the parasite manipulation of the host behavior. Despite numerous studies linking toxoplasmosis with behavior changes, no satisfactory stand-alone mechanism(s) responsible for these changes has been identified. In recent years the intestinal microflora has been receiving significant attention. Following a brief discussion of previous mechanistic hypotheses, we review the evidence that gut microbial dysbiosis, immune activation, neurotransmission, endocrine signaling, and behavior changes in T. gondii infection are linked and represent key features of a conceivable new underlying mechanism (Figure 1).

Figure 1. Working hypothesis - could the microbiome offer a precise piece of the puzzle to connect the different aspects of T. gondii infection that contribute to altered behavior? T. gondii infection (regardless of the infection route) affects the immune system, the nervous system, the endocrine system along with the gut microbiome. T. gondii infection leads to microbial dysbiosis in the intestine, depicted as red epithelial cells and microbes in the figure, as well as systemic immune activation, altered neurotransmission and endocrine signaling. Throughout the infection these organ systems interact with each other through the gut brain axis (GBA) which is bidirectional communication pathways. Created with BioRender.com.
2 Previous hypotheses to explain chronic T. gondii-induced behavior changes
T. gondii has emerged as a significant focus of investigation due to its long-recognized capacity to manipulate the behavior of its hosts. Extensive research spanning decades has illuminated the intricate relationship between toxoplasmosis and behavioral alterations in a number of rodent (Berdoy et al., 2000; Gonzalez et al., 2007; Webster, 2007) and human studies (Flegr and Hrdy, 1994; Flegr et al., 1996). The behavioral manipulation hypothesis posits that T. gondii possess the ability to modify host behavior to their advantage (da Silva and Langoni, 2009; Webster and McConkey, 2010; Pittman and Knoll, 2015; Vyas, 2015; Abdulai-Saiku et al., 2021; Tong et al., 2021). The parasite’s manipulation of its host promotes the evolutionarily more advantageous sexual reproduction by enhancing the predation rates (Webster, 2007; Webster and McConkey, 2010). The most well-known behavior change induced by chronic T. gondii infection is the aversion to cat odors but no other predator odors (Berdoy et al., 2000). Despite rodents’ typical avoidance of areas with signs of cat presence, those infected with T. gondii exhibit a lack of fear, and in many instances, even show attraction to such areas (Berdoy et al., 2000). In mouse models, Torres et al. found that T. gondii infection induces anxiety-like behavior, alters spatial memory, and affects recognition of social novelty (Torres et al., 2018). Xiao et al. reported that infected mice exhibited reduced general locomotor activity, impaired object recognition memory, and lack of response to amphetamine trigger (Xiao et al., 2016b). All of the above cited works highlighted a different behavioral domain being affected in chronic T. gondii infection, suggesting that T. gondii-induced behavior changes may be variable and depends on both the host and parasite genetical background (Carruthers and Suzuki, 2007; Kannan et al., 2010; Xiao et al., 2012; Behnke et al., 2016).
The spectrum of behavioral changes associated with T. gondii infection extends beyond rodents to humans. Wong et al. reported that acute toxoplasmosis in immunocompetent adults may lead to moderately severe neurological symptoms (Wong et al., 2013). Congenital toxoplasmosis has been associated with diminished brain function and intellectual disability in humans (da Silva and Langoni, 2009). Chronic infection in men correlates with emotional instability and a disregard for social norms, while both infected men and women exhibit elevated anxiety levels (Flegr and Hrdy, 1994; Flegr et al., 1996; Flegr et al., 2000; Flegr et al., 2002; Flegr, 2007). Hinze-Selch et al. demonstrated in a study involving over 1000 subjects that toxoplasmosis can influence behavior and personality traits, particularly in psychiatric conditions (Hinze-Selch et al., 2010). The longstanding connection between toxoplasmosis and schizophrenia has been extensively documented (Torrey and Yolken, 2003; Mortensen et al., 2007; Henriquez et al., 2009; Yolken et al., 2009).
Despite numerous studies linking toxoplasmosis with behavior changes, no satisfactory explanation has been provided that can completely explain the mechanism(s) responsible for these changes. Below, we discuss the preexisting hypotheses in detail.
2.1 Immune activation hypothesis
The immune activation hypothesis emerged from the fact that T. gondii can induce an arsenal of immune responses upon infection (Yarovinsky, 2014; Sasai and Yamamoto, 2019). Briefly (Hunter and Sibley, 2012), at an early stage of the infection, the first responder host cells, in particular, dendritic cells (DCs), monocytes and macrophages, release proinflammatory cytokines. Toll-like receptors (TLRs) on these first responder cell types, have been described to have a central role in T. gondii antigen sensing. TLRs signal through myeloid differentiation primary-response gene 88 (MyD88), which is a central mediator of IL-12 secretion and the protective Th1 response to T. gondii in dendritic cells (Scanga et al., 2002). During the adaptive and innate immune response phases, IL-12 recruit interferon-γ (IFN-γ)-producing natural killer (NK) cells through the innate response, and CD4+ and CD8+ T cells through the adaptive response. The production of IFN-γ is responsible for activating cells to control parasite infection. For example, IFN-γ induces the production of nitric oxide (NO) and reactive oxygen species (ROS), both of which contribute to the control of intracellular parasite load in monocytes and macrophages. It also leads to a depletion of tryptophan and arginine, two amino acids, that are essential for T. gondii growth (Yarovinsky, 2014).
In acute infection, T. gondii induce monocytes and dendritic cell migration (Lambert et al., 2006) as well as promote the interruption of the blood-brain barrier, which allows the hijacked monocytes and dendritic cells to enter into the brain, carrying the parasite in a manner characterized as that of as a “Trojan horse” (Bierly et al., 2008; Lachenmaier et al., 2011). During acute infection parasites are observed infecting neurons, astrocytes, microglia, and infiltrating immune cells. Within the intricate landscape of the brain’s immune response, these resident cells play pivotal roles. Their contributions, marked by the production of chemokines, cytokines, and the expression of immune-regulatory cell surface molecules, collectively influence the dynamics of chronic toxoplasmosis (Daher et al., 2021). Astrocytes and microglia as well as peripheral monocytes can clear parasites through cell-autonomous immune pathways (Hunter and Sibley, 2012; Yarovinsky, 2014). Hence, as chronic infection progresses, cysts are primarily present within neurons. While immune cells in the brain contribute to T. gondii restriction, the role of cell-autonomous immunity in neurons is restricted by default to promote survival of neurons, which have an extremely limited regenerative ability. The immune response to T. gondii is sustained throughout chronic infection, resulting in elevated T. gondii–specific IgG and IFN-γ in the sera, both of which are essential to constrain the parasite growth and promote tachyzoite to bradyzoite conversion (Sturge and Yarovinsky, 2014; Zhao and Ewald, 2020).
Notably, the route of infection (intraperitoneal (IP) vs. oral) influences the sequential immune activation in rodents (French et al., 2022). The interaction of T. gondii antigens such as profilin with Toll-like receptor 11 (TLR11) on dendritic cells is important for host production of IL-12, especially in IP infection. For example, in mice that are infected IP with T. gondii, the parasite protein profilin directly binds and activates TLR11, contributing to IL-12 production and parasite restriction (Yarovinsky et al., 2005). However, profilin is an intracellular cytoskeletal protein that is crucial for movement and invasion of T. gondii. The fact that TLR11 receptors samples from endosomal compartments, implies that this pathway may be mostly activated by phagocytosed, dead, or dysfunctional parasites. In contrast, after oral infection in a TLR11-deficient mouse model, there were minimal defects in their Th1 response compared with mice deficient in MyD88 or other TLRs (Minns et al., 2006; Debierre-Grockiego et al., 2007; Denkers, 2009; Foureau et al., 2010; Zhao and Ewald, 2020). Of note, both IP and oral infection produce comparable behavior outcomes.
As described above, T. gondii is able to activate the immune system and induce a substantial immune response. Activating a robust immune response is critical to both host and parasite survival. Interestingly, an activated immune system during chronic toxoplasmosis may present as a low-grade, constant inflammatory comorbidity that can manifest in neurological symptoms and behavior changes in both rodents (Boillat, 2020) and humans (Yirmiya, 1997; Schmidt et al., 2010).
2.2 Neurotransmitter hypotheses
In this section, we provide an overview of the rationale supporting that altered neurotransmitter abundances and activities could explain T. gondii-induced host behavioral changes. In this context, we focus on dopamine (Prandovszky et al., 2011), glutamate (David et al., 2016; Kannan et al., 2016; Lang et al., 2018; Li et al., 2018), and GABA (Brooks et al., 2015; Kannan et al., 2016). Furthermore, there are reports that neurotransmitter release upon T. gondii infection happens in sex-dependent fashion in mice, thus adding another variable to consider (Gatkowska et al., 2013). We expand on the sex differences in the following section.
2.2.1 Dopamine
The discovery of two T. gondii enzymes (TgAAH1 & TgAAH2) that closely resemble mammalian tyrosine hydroxylases, enzymes that catalyze the rate limiting step in dopamine synthesis, shook the scientific community (Gaskell et al., 2009). Elevated dopamine signaling has been linked to T. gondii-induced behavior changes, where haloperidol (dopamine antagonist) rescued the predator aversion behavioral phenotype in rodents (Webster et al., 2006). Higher dopamine concentrations were detected in vitro in T. gondii infected neuronal cells, as well as in vivo in T. gondii infected mice brains (Prandovszky et al., 2011). Moreover, the host enzyme, dopamine decarboxylase (DDC), that converts L-DOPA to dopamine was also colocalized with the parasite cyst in this model (Martin et al., 2015). However, reports on the impact of T. gondii infection on dopamine metabolism have been subject to disagreement. The field was hoping to get a definitive answer by utilizing a knockout T. gondii strain. However, knocking out one of the tyrosine hydroxylase genes (TgAAH2), which is highly active in the bradyzoite stage, did not eliminate the T. gondii-induced behavioral effect (Wang et al., 2015; McFarland et al., 2018). Notably, since no double knockdown mutant has yet been created, having at least one of these two enzymes expressed seems to be crucial for the parasite’s survival (McConkey et al., 2015; Wang et al., 2015) and cannot completely rule out the role of the TgAAH genes in T. gondii-induced dopamine escalation. The inability of other groups to replicate these findings with different mouse and parasite strains suggests that the genetic background of the host and parasite strain needs to be considered (Carruthers and Suzuki, 2007; Kannan et al., 2010; Xiao et al., 2012; Behnke et al., 2016). Remarkably, McConkey’s group found that in parallel with dopamine increase, there is a decrease in noradrenaline due to a T. gondii-induced downregulation in dopamine ß-hydroxylase (DBH), which is the key enzyme catalyzing the dopamine to noradrenalin conversion (Alsaady et al., 2019). Specifically, T. gondii infected cells release extracellular vesicles containing a DBH antisense lncRNA, which is complementary to the DBH gene’s promoter region and crosses the transcription start site (Tedford et al., 2023), preventing DBH transcription and subsequently contributing to dopamine increase. Interestingly, DBH regulation takes place in a sex specific fashion caused by an estrogen receptor binding response element at the 5’ flanking region of the DBH gene (Alsaady et al., 2019).
2.2.2 Glutamate
Glutamate is the main excitatory neurotransmitter in the brain, and its receptor (N-methyl-D-aspartate receptor (NMDAR)) plays a crucial role in synaptic plasticity and cognition, including learning and memory progression. This receptor system also plays a pivotal role in glutamate excitotoxicity, when excessive glutamate causes neuronal dysfunction and degeneration (Lau and Tymianski, 2010). Several groups reported an excess of glutamate and compromised NMDAR signaling in T. gondii infection (David et al., 2016; Kannan et al., 2016; Lang et al., 2018; Li et al., 2018), suggesting an increased risk for excitotoxicity and contribution to cognitive impairment. Interestingly, NMDA receptors have been implicated in anxiety-like behavior (Adamec et al., 1998).
2.2.3 GABA
On the other hand, gamma-aminobutyric acid (GABA) is the main inhibitory neurotransmitter in the human brain and derives from glutamate (Schousboe et al., 2014). Through GABAergic signaling, T. gondii can modify the motility of host cells, in particular dendritic cells and microglia cells, thus potentially increasing the systemic propagation of the parasite (Fuks et al., 2012; Kanatani et al., 2017; Bhandage et al., 2019). In addition, T. gondii infection appears to alter the inhibitory function of GABAergic signaling in mice through changes in the distribution of an enzyme (GAD67) that catalyzes GABA synthesis in the brain (Brooks et al., 2015; Kannan et al., 2016). GABA hypofunction could decrease GABAergic activity, and consequently, reduce neuronal inhibitory control and contribute to excitotoxicity.
Neurotransmitter alteration exerts a more localized effect on T. gondii-infected host brains. Regions in the brain crucial for fear and anxiety responses, such as the medial amygdala, basolateral amygdala, and ventral hippocampus, according to some groups, exhibit higher cyst density, highlighting their role in behavioral alterations (da Silva and Langoni, 2009; McConkey et al., 2013), while others have not found a correlation between cyst location and these particular areas (Berenreiterová et al., 2011; McConkey et al., 2013). Furthermore, behavioral changes persist even after parasite cysts are cleared from the brain, hinting that tropism alone may not wholly account for these alterations (Ingram et al., 2013), and the effect is more systemic than local (Abdulai-Saiku et al., 2021).
2.3 Endocrine hypothesis
In addition to rapid acting neurotransmitters, long-acting hormones also affect the brain (Tong et al., 2021). Tong et al. proposed that T. gondii’s presence in rat ejaculate, coupled with increased testosterone synthesis, contributes to behavioral changes (Tong et al., 2021). Arousal naturally deters innate fear, and a shift towards attraction potentially favors T. gondii’s transmission. Additionally, the involvement of dopamine and arginine vasopressin in inducing impulsivity and recklessness is suggested (Tong et al., 2021). Even though sex differences in behavior changes are well documented in T. gondii infection (Flegr et al., 2008; Xiao et al., 2012), testosterone increase cannot easily explain changes in female behavior. Female mice show reduced survival rates and lower cytokine levels in comparison to male mice during acute T. gondii infection (Roberts et al., 1995). With respect to female hormones, treatment with estradiol and estrogen increase the number of tissue cysts in brain of both male and female mice (Pung and Luster, 1986). While greater resistance to T. gondii infection was found in the gonadectomized mice than sham-operated controls of both sexes (Kittas and Henry, 1980). Overall, sex hormones may have a complex vital role in the manifestation of T. gondii infection including T. gondii-induced behavior changes.
While the above listed hypotheses address certain aspects of these behavioral changes, the exact mechanism is still yet to be elucidated. Interestingly, these previously examined hypotheses involving T. gondii-associated immune activation, neurotransmitter functions and endocrine dysregulation, are consistent with an increasingly scrutinized role of the microbiome and its resident microbiota in shaping these very same processes. Could this burgeoning field of microbiome research offer a potential novel link to consider between T. gondii infection and altered behavior?
3 T. gondii-induced microbial dysbiosis in acute and chronic infection
The metagenome, otherwise known as the microbiome, includes the entire genetic content of all microorganisms, including bacteria, fungi, and viruses, that live inside or on the surface of the host (Marchesi and Ravel, 2015). The potential role of the microbiome in health and disease has been receiving increasing attention in recent years (Pflughoeft and Versalovic, 2012; Jovel et al., 2018; Gomaa, 2020). Due to the extensive impact of the microbiome on host physiology, it is considered to be the newest organ system in the body (Baquero and Nombela, 2012). The microbiome exhibits species/strain-specific and sex-specific differences, both of which are frequently encountered in studies of T. gondii infection. Since the primary infection route of T. gondii is likely to be ingestion, among the different microbiome sites, the gut microbiome is the most involved. The mammalian intestine contains 5 major anatomical areas starting from the stomach and extending distally: the duodenum, jejunum, ileum, cecum, and colon. The first three areas make up the small intestine, while the last two make up the large intestine. These individual sections of the intestine differ in pH (Evans et al., 1988) and oxygen saturation (Zheng et al., 2015), as well as cell surface receptors (Hickey et al., 2023), thus providing a series of unique niches, for the resident microorganisms.
The gut microbiome interacts with the nervous system through the gut-brain axis (GBA). The GBA can be envisioned as a bidirectional multilane highway, where the lanes represent the different routes of communication, via nerves, hormones, and microbial metabolites (e.g., short-chain fatty acids) (Carabotti et al., 2015; Clapp et al., 2017; Dinan and Cryan, 2017; Silva et al., 2020a). Evidence of microbiota-GBA interactions comes from the association of gastrointestinal (GI) dysbiosis with central nervous system (CNS) disorders, including autism and anxiety-depressive behaviors (Carabotti et al., 2015; Clapp et al., 2017). Due to the robust interactions between the gut microbiome and the immune system, infection-induced immune activation can also lead to intestinal dysbiosis which can manifest as functional GI disorders (French et al., 2019; Tomal et al., 2023). While it is widely studied and accepted that acute T. gondii infection leads to substantial microbial dysbiosis in the gut, changes in the gut microbiome during the chronic stage of infection is poorly characterized and often debated by the scientific community (Taggart et al., 2022).
3.1 T. gondii-induced bacterial dysbiosis in the gut during the acute phase of infection
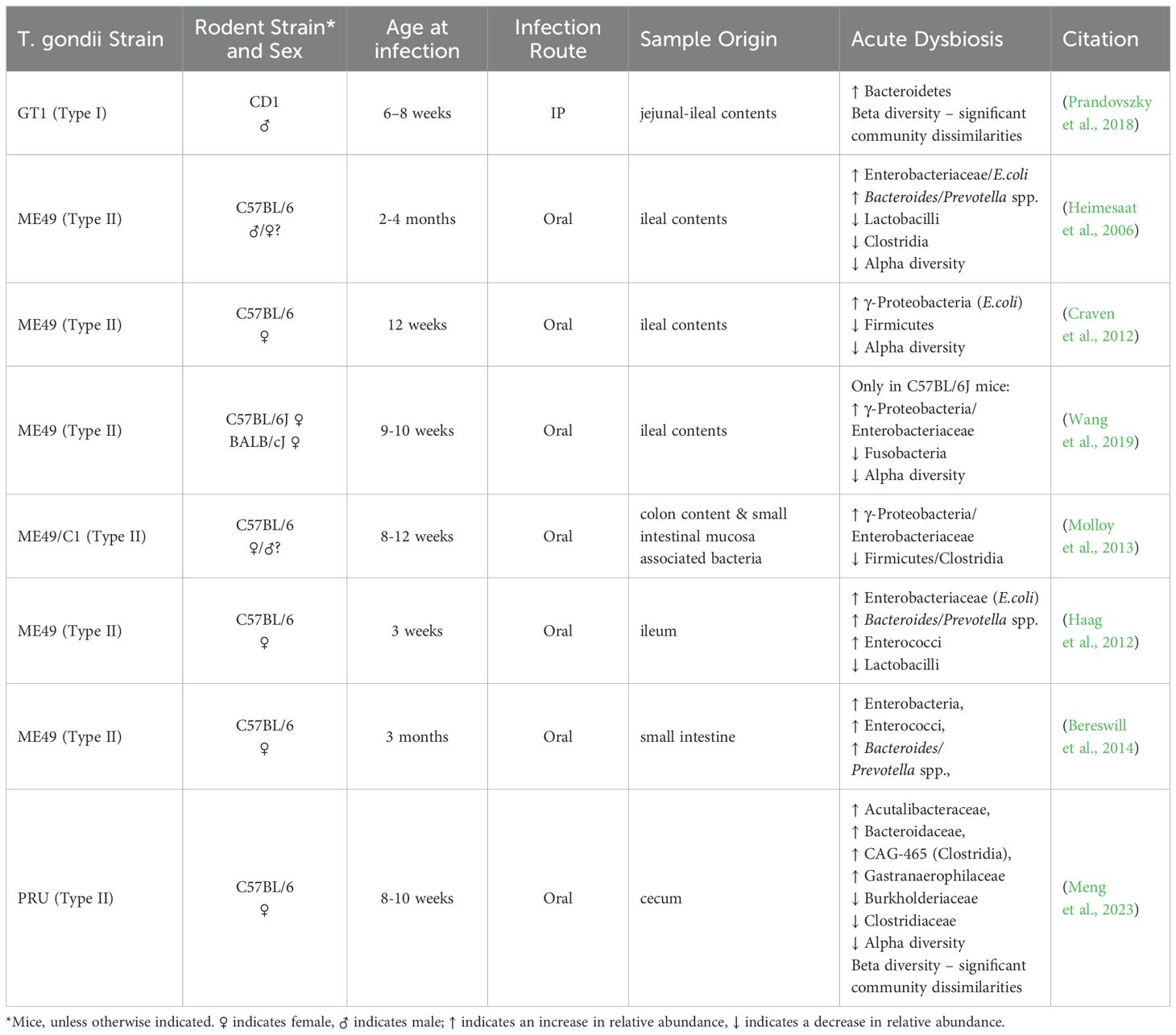
T. gondii invasion of the epithelium in the small intestine leads to intense inflammation, dysbiosis, and tissue necrosis followed by the spread of gut bacteria to peripheral organs and subsequent sepsis (Wang et al., 2019). Paneth cells, in the small intestine constitutively produce antimicrobial peptides and growth factors that help maintain the status quo between the host and the microbiota. Araujo et al. and Raetz et al. demonstrated that IFN-γ acts directly on murine Paneth cells via the m-TOR pathway, resulting in subsequent cell death (Raetz et al., 2013; Araujo et al., 2021). Since natural T. gondii infection mainly affects the small intestine and causes severe ileitis in susceptible mice, acute T. gondii infection has become a widely utilized model to study the type 1 (Th1) immune response in functional GI diseases like irritable bowel syndrome (IBS) and Crohn’s disease (Frank et al., 2007; Rolhion and Darfeuille-Michaud, 2007; Cohen and Denkers, 2015). Thus, the effect of acute T. gondii infection on the intestinal bacteriome has been well described (Heimesaat et al., 2006; Benson et al., 2009; McKnite et al., 2012; Raetz et al., 2013; Burger et al., 2018).
Altered taxonomical composition in the gut microbiome during acute T. gondii infection is marked by elevated abundance of Gram positive Bacteroidetes at the expense of decreased Firmicutes. Several studies have reported on the outgrowth of Gram negative Proteobacteria, such as E. coli, during acute T. gondii infection (Heimesaat et al., 2006; Craven et al., 2012; Molloy et al., 2013). Although Proteobacteria ssp. contribute to the development of ileitis (Heimesaat et al., 2006), their presence also magnifies the parasite-induced IFN-γ driven immune response in the gut. In so doing, the bacteria prevent a faster systemic dissemination of T. gondii which protects the host in the long run (Benson et al., 2009). In germ-free mice, where commensal bacteria are not present, T. gondii infection resulted in systematic inflammation that was not constrained to the intestine (Nascimento et al., 2017).
The effect of acute T. gondii infection on the gut microbiome can have varying outcomes. The results highly depend on the following: 1) the genetic background of the host and the parasite, 2) the host age and gender, 3) the route of infection, and 4) the anatomical location of where the samples were collected from within the intestine (Table 1). For example, Lv et al. reported significantly higher bacterial diversity (species richness) in the fecal pellet of Wistar rats infected with a PSY strain of T. gondii (Lv et al., 2022), whereas utilization of the PRU strain instead of PYS showed a lower trend, but no significant differences in bacterial diversity compared to uninfected animals in concordance with others (Prandovszky et al., 2018; Shao et al., 2020; Lv et al., 2022; Meng et al., 2023). The plausible explanation of this difference might be the use of an atypical strain (PYS) in Lv’s study that produced a more robust infection, compared to the other studies where more common T. gondii strains were utilized.
3.2 T. gondii-induced bacterial dysbiosis in the gut during the chronic phase of infection
While it is widely studied and accepted that acute T. gondii infection leads to substantial microbial dysbiosis in the gut, changes in the gut microbiome during the chronic stage of infection are not well characterized. Some groups reported that as inflammation resolved in the ileum, and the infection progressed from the acute to the chronic stage, Firmicutes became the most abundant phylum, accompanied by a decrease in the abundance of Proteobacteria, the Gram negative taxa that led to substantial dysbiosis and intestinal pathology (Shao et al., 2020; French et al., 2022; Yan et al., 2022). Even if changes between IP and per oral infection in bacterial composition were similar, meaning the same bacterial phyla were affected but in different ratios, the route of infection can leave a distinctive imprint on the microbial community (French et al., 2022). As inflammation resolves in the ileum, the infection progresses from the acute to the chronic stage, giving the impression that the bacterial microflora in the intestine is restored. Yet, Hatter et al. found that changes in the commensal populations, notably an outgrowth of Clostridia spp., were sustained in T. gondii chronic infection (Hatter et al., 2018). Several studies also reported an enrichment in the taxa Verrucomicrobia (Akkermansiaceae) in animals with chronic T. gondii infection (McConkey et al., 2015; Prandovszky et al., 2018; Shao et al., 2020; Yan et al., 2022; Meng et al., 2023). This finding was universally present regardless of the sex and genetic background of the host, the route of infection, or the type of parasite strain; however, noticeable individual differences between animals were also observed. These findings suggest that changes in the composition of the gut microflora are not limited to the acute phase of T. gondii infection when the parasite induced pathology mainly localizes to the intestinal tract.
Interestingly, in numerous studies, bacterial diversity in the gut during chronic T. gondii infection was similar to acute infection, where infected animals showed lower bacterial diversity compared to uninfected controls (Shao et al., 2020; French et al., 2022; Lv et al., 2022; Meng et al., 2023). On the other hand, Prandovszky et al. showed significantly higher bacterial diversity in infected animals (Prandovszky et al., 2018). It’s of note that Prandovszky et al. used males in the experiment while all of the other groups used females.
The aforementioned studies collectively indicate that chronic T. gondii infection does indeed induce long-term microbiome changes in the gut, which are different from the acute stage of T. gondii infection. Although, in many instances, there is no clear concordance in changes affecting the bacterial microbiome, the variances likely depend on 1) the genetic background and sex of the host, 2) the host age, 3) the route of infection, and 4) the anatomical location of where the samples were collected from within the intestine (Table 2). Furthermore, understanding the microorganisms role are ultimately more important than simply running a taxonomical inventory. Gaining a truly meaningful insight to the function of the gut microbiome calls for species/strain level metagenomics sequencing of the intestinal content accompanied by metabolomics analysis of the gut and blood of the host.
Due to the pathological impact of T. gondii infection on the gut, microbiome studies are mainly profiling the ileum, and very few studies are assessing the large intestine (Table 2), where microbial metabolites, such as short chain fatty acids (Silva et al., 2020b), and dopamine as well as other neurotransmitters (Eisenhofer et al., 1997; Asano et al., 2012) are being produced. These microbial metabolites can have a critical influence on the brain (Needham et al., 2020). More research is needed to systematically examine and sample each section of the gut in a longitudinal fashion to reveal common trends and differences. Furthermore, this sampling should be implemented across multiple hosts with different genetic backgrounds and sexes as well as utilizing different parasite strains so that the effects of chronic T. gondii infection on the gut microbiome can be fully characterized. Studies utilizing different infection routes, will help in the understanding of the interplay between gut commensals and the immune activation in the behavioral outcomes.
The above-mentioned studies collectively underscore the complexity of microbiota-GBA interactions and shed light on the dynamic interplay between infectious agents, gut microbiome composition, and neurological outcomes in chronic infection. Further exploration of these relationships holds promise for advancing our understanding of the intricate mechanisms governing gut-brain communication in underlying T. gondii-induced behavioral traits (O'Mahony et al., 2009; Cryan and Dinan, 2012).
4 Connecting the dots between the different mechanistic approaches of T. gondii induced behavior and bacterial dysbiosis
The behavioral changes induced by T. gondii have been a focal point of research, with rodents and humans showing altered behavior that put the host at risk and provide survival advantages for the parasite. While the direct infection of the CNS and the parasite’s tropism to immune-privileged organs are notable considerations, the underlying mechanisms driving these changes remain unclear. Neuroinflammation, changes in neurotransmission, and endocrine signaling stand as pieces of the puzzle, awaiting precise connections, connections that the gut microbiome and GBA may be able to clarify. The GBA serves as a vital conduit for communication between the microbiome, gut, and brain, orchestrating an array of interactions through immune, neural and endocrine routes.
4.1 Bacterial dysbiosis impact on the immune activation
There is a well-established relationship between the immune system and the gut microbiota (Hooper et al., 2012). We described earlier the immune response to T. gondii in acute and chronic infection, focusing on MyD88 and TLR11, the main TLR that expressed on rodent dendritic cells, but not present in human macrophages. We pointed out, that in TLR11-deficient mice, the Th1 was not affected as significantly compared with mice deficient in MyD88 or other TLRs. What we haven’t mentioned, treating TLR11-deficient mice with antibiotics showed the same phenotype as MyD88-deficient mice (Flegr et al., 2000; Flegr et al., 2002) suggesting that commensals have a pivotal role in inducing Th1 immune response.
The immune response to toxoplasmosis involves a complex interplay of cytokines, chemokines, and lymphoid cells, with IFN-γ playing a central role in activating the host’s defense mechanisms, which involves a parasite-induced dysbiosis in the gut bacterial microflora, in particular Proteobacteria outgrowth, to combat T. gondii infection at the early stage (Benson et al., 2009). In germ-free mice, where commensal bacteria is not present, T. gondii infection resulted in systematic inflammation that was not constrained to the intestine (Nascimento et al., 2017).
Due to an intricate and continuous communication between the gut microbiota and the immune system, the outcome of this conversation does not leave the neurosystem unaffected and chronic changes in this communication can manifest in behavior traits.
4.2 Bacterial dysbiosis impact on neurotransmission
GBA profoundly influences central processes such as neurotransmission and behavior (Sherwin et al., 2016). Gut bacteria contribute to this dialogue by releasing an array of neuroactive compounds such as dopamine, glutamate, GABA (Lyte, 2011). These neurotransmitters seem to play an important role in microbial ecology (Lyte, 2013; Wall et al., 2014; Hulme et al., 2022), adding complexity when it comes to understanding the exact mechanisms by which intestinal microbes communicate with the CNS during T. gondii infection. Nevertheless, the evidence does strongly support that gut symbionts are key to CNS function, and therefore, alterations in their composition, diversity, and richness may play a role in the pathophysiology of the CNS during T. gondii infection.
4.2.1 Dopamine
Alterations in dopaminergic transmission have been related to severe CNS disorders, such as anxiety (Zweifel et al., 2011; Zarrindast and Khakpai, 2015). Alteration of the gut microbiota impact dopamine signaling in the hippocampus and the amygdala (González-Arancibia et al., 2019). The dorsal hippocampus is a center of learning, memory, and spatial navigation in the brain, while the ventral hippocampus is associated with the emotional and motivational consequences of stress, including depression and anxiety (Bagot et al., 2015). The hippocampus communicates with subcortical structures, like the amygdala and the striatum. The amygdala is also involved in emotional behavior, participates in fear modulation, fear-associated memory, and attention (Phelps, 2004; Roozendaal et al., 2009), behavior traits that are associated with T. gondii infection.
In the absence of intestinal microbes, Heijtz et al. observed higher dopamine metabolism (increased dopamine turnover) in the striatum of adult germ free mice compared to specific pathogen-free (SPF) controls (Heijtz et al., 2011). Furthermore, they found that germ-free mice had higher expression of D1 mRNA expression in the hippocampus, while lower levels of expression of this receptor were found in the striatum when compared to SPF controls (Heijtz et al., 2011). In addition, germ-free mice showed fewer anxiety-like behaviors in comparison to their respective controls (Heijtz et al., 2011), while Nishino et al. observed the reversal of these outcomes perhaps due to the use of different mice strains in each study (Nishino et al., 2013).
Among several bacteria, E. coli has been reported to produce dopamine in the gut (Cryan and Dinan, 2012). It is known that acute T. gondii infection leads to Proteobacteria outgrowth, which in oral infection is likely to be E. coli. Excess of E. coli could contribute to the production of excess of dopamine in the gut. Excess dopamine can go through autooxidation that leads to the generation of reactive oxygen or nitrogen species (Meiser et al., 2013) which in turn might contribute to the necrotic pathology of the small intestine during acute toxoplasmosis (Liesenfeld et al., 1996; Denkers, 2009; French et al., 2022). Excess of dopamine can also signal the CNS through the gut-brain axis (González-Arancibia et al., 2019). On the other hand, Hatter et al. reported Clostridia outgrowth during chronic T. gondii infection (Hatter et al., 2018). Notably, metabolites produced by pathogenic Clostridia spp. can inhibit the conversion of dopamine to norepinephrine (Shaw, 2004; Shaw, 2017) resulting in elevated dopamine levels (Hamamah et al., 2022).
Other evidence for a complementary role of gut microbes in T. gondii infection comes from studies of probiotics such as Lactobacillus rhamnosus JB-1, Bifidobacterium longum NCC3001 in mice, and Lactobacillus helveticus R0052 and B. longum R0175 in rats. These probiotic cocktails were found to reduce anxiety-like behavior in both models (Bercik et al., 2011; Bravo et al., 2011; Messaoudi et al., 2011). Moreover, administration of Lactobacillus plantarum PS128 to germ-free mice decreases anxiety-like behaviors, and these changes were accompanied by an increase in dopamine and homovanilicacid, as well as an increase in 5-HT in the striatum (Liu et al., 2016). Many groups have also detected Akkermansia enrichment in the gut of chronically infected mice (McConkey et al., 2015; Prandovszky et al., 2018; Shao et al., 2020; Yan et al., 2022; Meng et al., 2023). Akkermansia has been considered a next generation probiotic (Zhai et al., 2019), one that may have significant impact on the brain (Xu et al., 2023). If chronic T. gondii infection leads to an outgrowth of a bacteria with probiotic potential in the gut that would probably affect the brain.
4.2.2 Glutamate
In the GI tract, dietary glutamate not only serves as a major source for glutamate, but it is also the most abundant (8%–10%) among dietary amino acids (Tomé, 2018). Several Lactobacillus strains have the ability to produce glutamate, with many of these Lactobacillus strains representing environmental bacteria or strains used in food fermentation (Sanchez et al., 2018). Interestingly, chronic T. gondii infection has also been linked to Lactobacilli overgrowth in mice (Prandovszky et al., 2018). About 75%–96% of enteral glutamate is removed during portal circulation in both humans and rodents for the production of energy (Haÿs et al., 2007). The brain is not exposed to an excess of glutamate due to low concentrations of glutamate reaching systemic circulation (Tomé, 2018). While dietary glutamate does not cross the blood brain barrier under normal conditions (Brosnan et al., 2014), an altered gut microbiota can cause changes in barrier permeability, as we saw with T. gondii infection, which can compromise the barrier and lead to the transfer of luminal glutamate into the CNS (Braniste et al., 2014; Kelly et al., 2015; Mazzoli and Pessione, 2016). The concentration of glutamate in neuronal cytoplasm is about 5 mM. However, the concentration of glutamate in astrocytes is lower (around 2–3 mM), and this is due to the function of an intact blood brain barrier. Excitatory amino acid transporters (EAAT) actively remove glutamate from the synaptic cleft and transport glutamate into the cytosol. Among these EAAT are GLAST and GLT-1, which are both expressed readily by astrocytes and are also both expressed by neurons and endothelial cells in the brain, although to a lesser extent. The homeostatic control of extracellular glutamate prevents its accumulation, as excess extracellular glutamate results in excitotoxicity (Pál, 2018), including excessive postsynaptic excitation (Brassai et al., 2015; Miladinovic et al., 2015), which has been linked to chronic inflammation (Kaszaki et al., 2012; Miladinovic et al., 2015). In addition, several groups have reported that there is an excess of glutamate, attributable to an impaired GLT-1 transport, and compromised glutamate receptor signaling during T. gondii infection (David et al., 2016; Kannan et al., 2016; Lang et al., 2018; Li et al., 2018). This suggests that there is an increased risk for glutamate-mediated excitotoxicity during T. gondii infection, which can potentially contribute to cognitive impairment.
Permeability to glutamate has been shown to increase in pathological conditions, such as in irritable bowel syndrome (IBS) and in inflammatory bowel disease (IBD). This increase in glutamate permeability has been shown to result in altered neuronal responses that are not only local in the enteric nervous system, but also remotely in the CNS by utilizing the microbiota-gut-brain axis. Elevated psychiatric co-morbidity in the development of IBS and IBD may be explained by impaired glutamate metabolism across the microbiota-gut brain axis (Holtmann et al., 2016; Mikocka-Walus et al., 2016). Notably both of these functional GI disorders have been modeled by T. gondii infection.
4.2.3 GABA
GABA is the main inhibitory neurotransmitter in the CNS, and its imbalance has been associated with a number of disorders, including anxiety (Nuss, 2015).
Some gut commensal bacteria produce GABA including Bacteroides, Bifidobacterium, Lactobacillus species (Strandwitz et al., 2019; Barrett et al., 2012). Most bacteria, including Lactobacillus and Bifidobacterium genera producing GABA use the glutamate decarboxylase (GAD) pathways, while others such as E. coli can utilize both putrescine glutamate decarboxylase pathways (Diez-Gutiérrez et al., 2020). T. gondii infection appears to alter the inhibitory function of GABAergic signaling in mice through changes in the distribution of an enzyme (GAD67) that catalyzes GABA synthesis in the brain (Brooks et al., 2015; Kannan et al., 2016). GABA hypofunction could decrease GABAergic activity, and consequently, reduce neuronal inhibitory control and contribute to excitotoxicity. Yet, GABA-producing Bifidobacterium adolescentis strain reduced serum glutamate levels in mice (Royo et al., 2023).
Some experimental evidence indicates that the gut microbiome affects the level of GABA and subsequently influences mental health. For instance, Bravo et al. reported that L. rhamnosus elevated the abundance of GABAB1b mRNA while decreasing the level of GABAAα2 mRNA in the cortex of mice, leading to the inhibition of anxiety (Bravo et al., 2011; Terunuma, 2018).
The tenth cranial nerve (n. vagus), with its afferent fibers diligently detects metabolites, including neurotransmitters, produced by the microbiome in the gut, and conveys this critical information towards the CNS (Bonaz et al., 2018). Exploring the role of the vagus nerve during T. gondii infection holds potential opportunity for understanding how T. gondii utilize this neural materialization of the gut brain axis to influence behavior. Experimental vagotomy could offer crucial insights into the mechanisms linking toxoplasmosis to behavior changes. This exploration may not only illuminate the pathophysiology of toxoplasmosis but also underscore the role of the microbiome in mediating these complex interactions.
4.3 Bacterial dysbiosis impact on the hormone system
The neuroendocrine system and microbiome interact to influence social behaviors (Sylvia and Demas, 2018). Males and females exhibit distinct patterns in energy and nutritional requirements across the lifespan. Differences in sex hormones can contribute to differences in microbial diversity and gut microbial composition (Neuman et al., 2015). Collden et al. found higher levels of glucuronidated testosterone and dihydrotestosterone but lower levels of free dihydrotestosterone in the large intestine of germ-free mice compared with conventional mice that have a normal gut microflora, which suggests that the gut microbiome plays a crucial role in testosterone metabolism in mice (Colldén et al., 2019). Shin et al. reported that sex steroid hormone levels were correlated with diversity and gut microbial composition in humans, suggesting a robust communication between the two organ systems (Shin et al., 2019). Because it is a bidirectional communication, not only do sex hormones influence the intestinal microbiome, but the gut microbiota itself also influences hormone levels. Consistent with this activity researchers have observed a decline in estrogen levels during antibiotics treatment (Adlercreutz et al., 1984). Recently it has been shown that sex differences in the microbiome present an increased risk for developing autoimmune disorders in female mice, whereby fecal transfer of male intestinal microbiota to recipient females delayed onset and lessened severity of disease (Markle et al., 2013; Yurkovetskiy et al., 2013).
These findings suggest that epigenetic factors, such as infection, may induce sex-specific alterations in the composition of the gut microbiome, that contribute to sex differences in disease risk, since these epigenetic factors are known to lead to sex-specific alterations in immune function, metabolism, stress responsiveness and behaviors (O'Mahony et al., 2009; Cryan and Dinan, 2012).
As we explore the mechanisms by which acute and chronic T. gondii infection might lead to dysfunctional neural and glial circuitries, a role for the gut microbiome in the context of the gut-brain axis becomes apparent. The collective, balanced gut microbiome provides a variety of benefits and functions integral to human health and physiology (El Aidy et al., 2014; Dinan and Cryan, 2017). When awry, however, a pathological cycle mediated by ongoing, low-level inflammation fuels imbalances and produces a chronic state of translocated microbial communities that can impact the CNS (Martel et al., 2022; Jensen et al., 2023). Up to this point, we focused on the bacterial kingdom of the indigenous microbiota, but we cannot forget about the presence of others.
5 T. gondii-induced mycobial dysbiosis (Candida)
The microbiome is composed of many microbial taxa (bacteria, fungi, viruses, Archaea, protozoa), yet most studies still largely focus on measures of bacteria. Surprisingly, the fungi, which are taxonomic powerhouses and potent instigators of dysbiosis, are often overlooked (Severance, 2023). During times of good health, the body’s fungal residents, known as the mycobiome, exist in harmony with other microbial taxa. Many fungal species, however, are opportunistic, and as pathogens, will take over when the bacterial microbiome becomes dysbiotic (Kim and Sudbery, 2011; Forbes et al., 2018; CDC, 2019; MacAlpine et al., 2022; Underhill and Braun, 2022).
A role for opportunistic fungal species, such as Candida albicans, as drivers of T. gondii-controlled altered behavior and brain biochemistry has not been extensively studied. It seems logical that not only would an acute infection with T. gondii introduce a pro-inflammatory intestinal environment primed for bacterial dysbiosis and dominance by fungal pathogens, but that this dysbiotic state and related functional deficits would be maintained chronically until the dysbiosis was reversed (Saraav et al., 2021). In studies of fungal translocation in human neuropsychiatric disorders, levels of antibodies to the fungal species, Saccharomyces cerevisiae and C. albicans, were consistently elevated versus those from comparison groups (Severance et al., 2012; Severance et al., 2014; Severance et al., 2016; Hughes and Ashwood, 2018). In fact, S. cerevisiae antibodies have been routinely referred to as indices of GI inflammation and have been used to aid in the diagnosis of Crohn’s disease and to better understand the mycobiome of ulcerative colitis and other inflammatory diseases of the bowel (Torres et al., 2020; Gao and Zhang, 2021; Cimická et al., 2022).
The routes to the brain for fungal and parasitic taxa, independently or synergistically, have not been well defined. Numerous fungal species have been found in post-mortem brain tissue from individuals with amyotrophic lateral sclerosis, Alzheimer’s Disease, and Parkinson’s Disease (Alonso et al., 2015b; Alonso et al., 2015a; Pisa et al., 2015; Phuna and Madhavan, 2022). Microbial translocation generated locally in the GI tract has been hypothesized to lead to systemic, low-grade inflammation and a loss of integrity, not only of the blood-gut barrier, but also of the blood-brain barrier, thereby exposing the brain to access by microbes (Severance and Yolken, 2020a). Another means by which gut microbes, including fungi, might travel to the brain more directly is via microbial translocation and neuroinflammation along the vagus nerve (Thapa et al., 2023).
The concept of polymicrobial invasions of the brain, and a susceptibility of certain individuals to multiple neuropathogens, thus becomes a very relevant hypothesis (Carter, 2017). T. gondii and C. albicans are known to co-occur in individuals who are immune-suppressed, such as those with HIV (Bongomin et al., 2023). Immunosuppression or immune-modulation could also be gene-based, as a common hypothesis regarding the etiologies of psychiatric disorders, such as schizophrenia, is that these disorders are products of gene-by-environmental interactions (Severance and Yolken, 2020b). A genetic predisposition involving complement immune genes, combined with environmental exposures such as T. gondii and C. albicans infections, are hypothesized to elevate one’s risk of developing schizophrenia (Severance et al., 2021; Severance et al., 2023). It is also possible that previous infections may damage specific tissues and render them especially susceptible to future infections, as demonstrated in a mouse model of T. gondii chronic infection (Saraav et al., 2021). Furthermore, viral, fungal, and parasitic pathogens including T. gondii, can infect the same cell, together inactivate the immune system, and establish themselves as latent or chronic infections. Subsequent infections can lead to reactivation of the latent pathogens and T-cell exhaustion for the newly invading pathogens (Roe, 2022).
In conclusion, gut microbiome studies are currently flourishing, although we are still very much in the beginning stages of fully appreciating the complexities of this developing field. Studies to date have underestimated the potential role of fungi, which can quickly displace bacteria, create pro-inflammatory GI environments, and generate deleterious consequences on brain function and behavior. Acute and chronic infections of T. gondii very likely accelerate the deterioration of microbial balances, implicating a partnership with fungi that is mutually beneficial. Empirical studies to test for co-occurrences and functional co-associations of these exposures could help determine the extent that these infections are related.
6 Discussion
Toxoplasmosis poses a significant global health challenge affecting a substantial portion of the world’s population. The spectrum of its impact, from asymptomatic cases to severe neurological outcomes, underscores the complexity of this parasitic infection. In the intricacies of T. gondii infection and its consequential impact on behavior and neurological conditions, the gut microbiome emerges as a potentially vital piece, contributing to the complexity of this parasitic relationship (Figure 1).
The competency of T. gondii to infect any warm-blooded animals, including humans, and its ability to alter the host behavior for its own evolutionary advantages gained significant attention in the scientific community. Over the last 25 years, T. gondii-induced behavior changes captivated many scientists studying host-parasite relationships. Numerous studies investigated this sophisticated bond and revealed the involvement of different organ systems, including the nervous, immune, and endocrine systems. However, the exact mechanism is still waiting to be unveiled. Neuroinflammation, hormonal alterations, and modulation of neurotransmission stand as pieces of the puzzle, awaiting precise connections, connections that the gut microbiome and GBA may be able to clarify. Further research is needed utilizing germ free, antibiotic treated as well as probiotic treated host to assess the relationship of these individual domains to the microbiome during T. gondii infection. Mediation analysis has yet to be performed in order to disentangle the role of the microbiome in T. gondii induced behavior in the presence of the above discussed contributing domains. As mentioned, the genetic background and sex of the host as well as the genetic back ground of the parasite needs to be considered as potential cofounders along with infection route and the part of the intestine which was sampled.
As we delve into the gut-microbiome connection, the complexity of microbiota-gut-brain axis interactions adds a new layer to our understanding. Changes in microbiome composition during T. gondii infection, both acute and chronic, highlight the dynamic nature of these relationships. The gut-brain axis, facilitating communication between the central and enteric nervous systems, presents a previously underexplored dimension in the T. gondii saga.
The gut microbiome stands as a frontier for further exploration in the intricate web of T. gondii infection, behavioral alterations, and neurological consequences. Continued research in this field has the potential to unveil missing links, providing insights into potential therapeutic interventions and preventive strategies. The holistic comprehension of the interplay between parasites, host responses, and the microbiome opens new avenues for understanding the complex mechanisms governing the impact of T. gondii on both behavior and neurological health.
Author contributions
EP: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. ES: Writing – original draft, Writing – review & editing. JX: Writing – original draft, Writing – review & editing. VS: Writing – original draft, Writing – review & editing. HL: Writing – original draft, Writing – review & editing. RY: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding for the research, authorship, and/or publication was provided by the Stanley Medical Research Institute.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdulai-Saiku, S., Tong, W. H., Vyas, A. (2021). Behavioral manipulation by Toxoplasma gondii: does brain residence matter? Trends Parasitol. 37 (5), 381–390. doi: 10.1016/j.pt.2020.12.006
Abo-Al-Ela, H. G. (2019). Toxoplasmosis and psychiatric and neurological disorders: A step toward understanding parasite pathogenesis. ACS Chem. Neurosci. 11 (16), 2393–2406. doi: 10.1021/acschemneuro.9b00245
Adamec, R. E., Burton, P., Shallow, T., Budgell, J. (1998). NMDA receptors mediate lasting increases in anxiety-like behavior produced by the stress of predator exposure—implications for anxiety associated with posttraumatic stress disorder. Physiol. Behav. 65, 723–737. doi: 10.1016/S0031-9384(98)00226-1
Adlercreutz, H., Pulkkinen, M., Hämäläinen, E., Korpela, J. (1984). Studies on the role of intestinal bacteria in metabolism of synthetic and natural steroid hormones. J. Steroid Biochem. 20, 217–229. doi: 10.1016/0022-4731(84)90208-5
Alonso, R., Pisa, D., Marina, A. I., Morato, E., Rabano, A., Rodal, I., et al. (2015a). Evidence for fungal infection in cerebrospinal fluid and brain tissue from patients with amyotrophic lateral sclerosis. Int. J. Biol. Sci. 11, 546–558. doi: 10.7150/ijbs.11084
Alonso, R., Pisa, D., Rabano, A., Rodal, I., Carrasco, L. (2015b). Cerebrospinal fluid from Alzheimer's disease patients contains fungal proteins and DNA. J. Alzheimer's disease: JAD 47, 873–876. doi: 10.3233/JAD-150382
Alsaady, I., Tedford, E., Alsaad, M., Bristow, G., Kohli, S., Murray, M., et al. (2019). Downregulation of the central noradrenergic system by Toxoplasma gondii infection. Infection Immun. 87 (2), e00789–18. doi: 10.1128/iai.00789-18
Araujo, A., Safronova, A., Burger, E., López-Yglesias, A., Giri, S., Camanzo, E. T., et al. (2021). IFN-γ mediates Paneth cell death via suppression of mTOR. Elife 10, e60478. doi: 10.7554/eLife.60478.sa2
Asano, Y., Hiramoto, T., Nishino, R., Aiba, Y., Kimura, T., Yoshihara, K., et al. (2012). Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiology-Gastrointestinal Liver Physiol. 303 (11), G1288–G1295. doi: 10.1152/ajpgi.00341.2012
Bagot, R. C., Parise, E. M., Pena, C. J., Zhang, H.-X., Maze, I., Chaudhury, D., et al. (2015). Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat. Commun. 6, 7062. doi: 10.1038/ncomms8062
Barrett, E., Ross, R. P., O’Toole, P. W., Fitzgerald, G. F., Stanton, C.. (2012). γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 113, 411–417. doi: 10.1111/j.1365-2672.2012.05344.x
Baquero, F., Nombela, C. (2012). The microbiome as a human organ. Clin. Microbiol. Infect. 18 Suppl, 4, 2–4, 4. doi: 10.1111/j.1469-0691.2012.03916.x
Behnke, M. S., Dubey, J., Sibley, L. D. (2016). Genetic mapping of pathogenesis determinants in Toxoplasma gondii. Annu. Rev. Microbiol. 70, 63–81. doi: 10.1146/annurev-micro-091014-104353
Benson, A., Pifer, R., Behrendt, C. L., Hooper, L. V., Yarovinsky, F. (2009). Gut commensal bacteria direct a protective immune response against Toxoplasma gondii. Cell Host Microbe 6, 187–196. doi: 10.1016/j.chom.2009.06.005
Bercik, P., Denou, E., Collins, J., Jackson, W., Lu, J., Jury, J., et al. (2011). The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 141, 599–609, 609.e1-3. doi: 10.1053/j.gastro.2011.04.052
Berdoy, M., Webster, J. P., Macdonald, D. W. (2000). Fatal attraction in rats infected with Toxoplasma gondii. Proc. Biol. Sci. 267, 1591–1594. doi: 10.1098/rspb.2000.1182
Berenreiterová, M., Flegr, J., Kuběna, A. A., Němec, P. (2011). The distribution of Toxoplasma gondii cysts in the brain of a mouse with latent toxoplasmosis: implications for the behavioral manipulation hypothesis. PloS One 6, e28925. doi: 10.1371/journal.pone.0028925
Bereswill, S., Kühl, A. A., Alutis, M., Fischer, A., Möhle, L., Struck, D., et al. (2014). The impact of Toll-like-receptor-9 on intestinal microbiota composition and extra-intestinal sequelae in experimental Toxoplasma gondii induced ileitis. Gut Pathog. 6, 19. doi: 10.1186/1757-4749-6-19
Bhandage, A. K., Kanatani, S., Barragan, A. (2019). Toxoplasma-induced hypermigration of primary cortical microglia implicates GABAergic signaling. Front. Cell. infection Microbiol. 9, 73. doi: 10.3389/fcimb.2019.00073
Bierly, A. L., Shufesky, W. J., Sukhumavasi, W., Morelli, A. E., Denkers, E. Y. (2008). Dendritic cells expressing plasmacytoid marker PDCA-1 are Trojan horses during Toxoplasma gondii infection. J. Immunol. 181, 8485–8491. doi: 10.4049/jimmunol.181.12.8485
Boillat, M. (2020). Neuroinflammation-associated aspecific manipulation of mouse predator fear by toxoplasma gondii. Cell Rep. 30 (2), 320–334.e6. doi: 10.1016/j.celrep.2019.12.019
Bonaz, B., Bazin, T., Pellissier, S. (2018). Frontiers | The vagus nerve at the interface of the microbiota-gut-brain axis. Front. Neurosci. 12. doi: 10.3389/fnins.2018.00049
Bongomin, F., Kibone, W., Atulinda, L., Morgan, B., Ocansey, B., Storer, I. S. R., et al. (2023). Frequency of fungal pathogens in autopsy studies of people who died with HIV in Africa: a scoping review. Clin. Microbiol. Infect. 30 (5), 592–600. doi: 10.1016/j.cmi.2023.12.016
Braniste, V., Al-Asmakh, M., Kowal, C., Anuar, F., Abbaspour, A., Tóth, M., et al. (2014). The gut microbiota influences blood-brain barrier permeability in mice. Sci. Trans. Med. 6, 263ra158–263ra158. doi: 10.1126/scitranslmed.3009759
Brassai, A., Suvanjeiev, R.-G., Bán, E.-G., Lakatos, M. (2015). Role of synaptic and nonsynaptic glutamate receptors in ischaemia induced neurotoxicity. Brain Res. Bull. 112, 1–6. doi: 10.1016/j.brainresbull.2014.12.007
Bravo, J. A., Forsythe, P., Chew, M. V., Escaravage, E., Savignac, H. M., Dinan, T. G., et al. (2011). Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. 108, 16050–16055. doi: 10.1073/pnas.1102999108
Brooks, J. M., Carrillo, G. L., Su, J., Lindsay, D. S., Fox, M. A., Blader, I. J. (2015). Toxoplasma gondii infections alter GABAergic synapses and signaling in the central nervous system. mBio 6, e01428–e01415. doi: 10.1128/mBio.01428-15
Brosnan, J. T., Drewnowski, A., Friedman, M. I. (2014). Is there a relationship between dietary MSG obesity in animals or humans? Amino Acids 46, 2075–2087. doi: 10.1007/s00726-014-1771-6
Burger, E., Araujo, A., López-Yglesias, A., Rajala, M. W., Geng, L., Levine, B., et al. (2018). Loss of Paneth cell autophagy causes acute susceptibility to Toxoplasma gondii-mediated inflammation. Cell Host Microbe 23, 177–190.e4. doi: 10.1016/j.chom.2018.01.001
Carabotti, M., Scirocco, A., Maselli, M. A., Severi, C. (2015). The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann. gastroenterology: Q. Publ. Hellenic Soc. Gastroenterol. 28, 203.
Carruthers, V. B., Suzuki, Y. (2007). Effects of Toxoplasma gondii infection on the brain. Schizophr. Bull. 33 (3), 745–751. doi: 10.1093/schbul/sbm008
Carter, C. J. (2017). Genetic, transcriptome, proteomic, and epidemiological evidence for blood-brain barrier disruption and polymicrobial brain invasion as determinant factors in Alzheimer's disease. J. Alzheimers Dis. Rep. 1, 125–157. doi: 10.3233/adr-170017
CDC (2019). Antibiotic resistant threats in the United States, 2019 (Atlanta, GA: U.S. Department of Health and Human Services).
Cerutti, A., Blanchard, N., Besteiro, S. (2020). The bradyzoite: A key developmental stage for the persistence and pathogenesis of toxoplasmosis. Pathogens 9 (3), 234. doi: 10.3390/pathogens9030234
Cimická, J., Riegert, J., Kavková, M., Černá, K. (2022). Intestinal mycobiome associated with diagnosis of inflammatory bowel disease based on tissue biopsies. Med. Mycol 60 (1), myab076. doi: 10.1093/mmy/myab076
Clapp, M., Aurora, N., Herrera, L., Bhatia, M., Wilen, E., Wakefield, S. (2017). Gut microbiota’s effect on mental health: the gut-brain axis. Clinics Pract. 7, 987. doi: 10.4081/cp.2017.987
Cohen, S., Denkers, E. (2015). The gut mucosal immune response to Toxoplasma gondii. Parasite Immunol. 37, 108–117. doi: 10.1111/pim.12164
Colldén, H., Landin, A., Wallenius, V., Elebring, E., Fändriks, L., Nilsson, M. E., et al. (2019). The gut microbiota is a major regulator of androgen metabolism in intestinal contents. Am. J. Physiology-Endocrinology Metab. 317, E1182–E1192. doi: 10.1152/ajpendo.00338.2019
Coppens, I., Joiner, K. A. (2001). Parasite–host cell interactions in toxoplasmosis: new avenues for intervention? Expert Rev. Mol. Med. 3, 1–20. doi: 10.1017/S1462399401002277
Craven, M., Egan, C. E., Dowd, S. E., McDonough, S. P., Dogan, B., Denkers, E. Y., et al. (2012). Inflammation drives dysbiosis and bacterial invasion in murine models of ileal Crohn's disease. PloS One 7, e41594. doi: 10.1371/journal.pone.0041594
Cryan, J. F., Dinan, T. G. (2012). Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 13, 701–712. doi: 10.1038/nrn3346
Daher, D., Shaghlil, A., Sobh, E., Hamie, M., Hassan, M., Moumneh, M. B., et al. (2021). Comprehensive overview of Toxoplasma gondii-induced and associated diseases. Pathogens 10, 1351. doi: 10.3390/pathogens10111351
da Silva, R. C., Langoni, H. (2009). Toxoplasma gondii: host–parasite interaction and behavior manipulation. Parasitol. Res. 105 (4), 893–898. doi: 10.1007/s00436-009-1526-6
David, C. N., Frias, E. S., Szu, J. I., Vieira, P. A., Hubbard, J. A., Lovelace, J., et al. (2016). GLT-1-dependent disruption of CNS glutamate homeostasis and neuronal function by the protozoan parasite Toxoplasma gondii. PloS Pathog. 12 (6), e1005643. doi: 10.1371/journal.ppat.1005643
Debierre-Grockiego, F., Campos, M. A., Azzouz, N., Schmidt, J. r., Bieker, U., Resende, M. G., et al. (2007). Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. J. Immunol. 179, 1129–1137. doi: 10.4049/jimmunol.179.2.1129
Denkers, E. Y. (2009). A gut feeling for microbes: getting it going between a parasite and its host. Cell Host Microbe 6, 104–106. doi: 10.1016/j.chom.2009.07.009
Dias, R. R., Carvalho, E. C., Leite, C. C., Tedesco, R. C., Calabrese Kda, S., Silva, A. C., et al. (2014). Toxoplasma gondii oral infection induces intestinal inflammation and retinochoroiditis in mice genetically selected for immune oral tolerance resistance. PloS One 9, e113374. doi: 10.1371/journal.pone.0113374
Diez-Gutiérrez, L., San Vicente, L., Barrón, L. J. R., del Carmen Villarán, M., Chávarri, M. (2020). Gamma-aminobutyric acid and probiotics: Multiple health benefits and their future in the global functional food and nutraceuticals market. J. Funct. Foods 64, 103669. doi: 10.1016/j.jff.2019.103669
Dinan, T. G., Cryan, J. F. (2017). The microbiome-gut-brain axis in health and disease. Gastroenterol. Clin. North Am. 46, 77–89. doi: 10.1016/j.gtc.2016.09.007
Dubey, J. P. (1998). Advances in the life cycle of Toxoplasma gondii. Int. J. Parasitol. 28 (7), 1019–1024. doi: 10.1016/S0020-7519(98)00023-X
Dubey, J. P., Lindsay, D. S., Speer, C. A. (1998). Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin. Microbiol. Rev. 11 (2), 267–99. doi: 10.1128/cmr.11.2.267
Eisenhofer, G., Aneman, A., Friberg, P., Hooper, D., Fåndriks, L., Lonroth, H., et al. (1997). Substantial production of dopamine in the human gastrointestinal tract. J. Clin. Endocrinol. Metab. 82 (11), 3864–3871. doi: 10.1210/jcem.82.11.4339
El Aidy, S., Dinan, T. G., Cryan, J. F. (2014). Immune modulation of the brain-gut-microbe axis. Front. Microbiol. 5. doi: 10.3389/fmicb.2014.00146
Evans, D., Pye, G., Bramley, R., Clark, A., Dyson, T., Hardcastle, J. (1988). Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut 29, 1035–1041. doi: 10.1136/gut.29.8.1035
Ferguson, D. J. P., Hutchison, W. M. (1987). An ultrastructural study of the early development and tissue cyst formation of Toxoplasma gondii in the brains of mice. Parasitol. Res. 73 (6), 483–491. doi: 10.1007/BF00535321
Flegr, J. (2007). Effects of toxoplasma on human behavior. Schizophr. Bull. 33 (3), 757–760. doi: 10.1093/schbul/sbl074
Flegr, J., Havlícek, J., Kodym, P., Malý, M., Smahel, Z. (2002). Increased risk of traffic accidents in subjects with latent toxoplasmosis: a retrospective case-control study. BMC Infect. Dis. 2, 11. doi: 10.1186/1471-2334-2-11
Flegr, J., Hrdy, I. (1994). Influence of chronic toxoplasmosis on some human personality factors. Folia Parasitologica 41, 122–126.
Flegr, J., Kodym, P., Tolarová, V. (2000). Correlation of duration of latent Toxoplasma gondii infection with personality changes in women. Biol. Psychol. 53, 57–68. doi: 10.1016/S0301-0511(00)00034-X
Flegr, J., Lindová, J., Kodym, P. (2008). Sex-dependent toxoplasmosis-associated differences in testosterone concentration in humans. Parasitology 135, 427–431. doi: 10.1017/S0031182007004064
Flegr, J., Zitková, Š., Kodym, P., Frynta, D. (1996). Induction of changes in human behaviour by the parasitic protozoan Toxoplasma gondii. Parasitology 113, 49–54. doi: 10.1017/S0031182000066269
Forbes, J. D., Bernstein, C. N., Tremlett, H., Van Domselaar, G., Knox, N. C. (2018). A fungal world: could the gut mycobiome be involved in neurological disease? Front. Microbiol. 9. doi: 10.3389/fmicb.2018.03249
Foureau, D. M., Mielcarz, D. W., Menard, L. C., Schulthess, J., Werts, C., Vasseur, V., et al. (2010). TLR9-dependent induction of intestinal α-defensins by Toxoplasma gondii. J. Immunol. 184, 7022–7029. doi: 10.4049/jimmunol.0901642
Frank, D. N., St. Amand, A. L., Feldman, R. A., Boedeker, E. C., N. Harpaz and, N. R. (2007). Pace: Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. 104, 13780–13785. doi: 10.1073/pnas.0706625104
French, T., Düsedau, H. P., Steffen, J., Biswas, A., Ahmed, N., Hartmann, S., et al. (2019). Neuronal impairment following chronic Toxoplasma gondii infection is aggravated by intestinal nematode challenge in an IFN-γ-dependent manner. J. Neuroinflamm. 16, 159. doi: 10.1186/s12974-019-1539-8
French, T., Steffen, J., Glas, A., Osbelt, L., Strowig, T., Schott, B. H., et al. (2022). Persisting microbiota and neuronal imbalance following T. gondii infection reliant on the infection route. Front. Immunol. 13. doi: 10.3389/fimmu.2022.920658
Fuglewicz, A. J., Piotrowski, P., Stodolak, A. (2017). Relationship between toxoplasmosis and schizophrenia: a review. Adv. Clin. Exp. Med. 26, 1031–1036. doi: 10.17219/acem/61435
Fuks, J. M., Arrighi, R. B., Weidner, J. M., Kumar Mendu, S., Jin, Z., Wallin, R. P., et al. (2012). GABAergic signaling is linked to a hypermigratory phenotype in dendritic cells infected by Toxoplasma gondii. PloS Pathog. 8, e1003051. doi: 10.1371/journal.ppat.1003051
Gao, X., Zhang, Y. (2021). Serological markers facilitate the diagnosis of Crohn's disease. Postgrad Med. 133, 286–290. doi: 10.1080/00325481.2021.1873649
Gaskell, E. A., Smith, J. E., Pinney, J. W., Westhead, D. R., McConkey, G. A. (2009). A unique dual activity amino acid hydroxylase in Toxoplasma gondii. PloS One 4 (3), e4801. doi: 10.1371/journal.pone.0004801
Gatkowska, J., Wieczorek, M., Dziadek, B., Dzitko, K., Dlugonska, H. (2013). Sex-dependent neurotransmitter level changes in brains of Toxoplasma gondii infected mice. Exp. Parasitol. 133 (1), 1–7. doi: 10.1016/j.exppara.2012.10.005
Gomaa, E. Z. (2020). Human gut microbiota/microbiome in health and diseases: a review. Antonie Van Leeuwenhoek 113, 2019–2040. doi: 10.1007/s10482-020-01474-7
Gonzalez, L. E., Rojnik, B., Urrea, F., Urdaneta, H., Petrosino, P., Colasante, C., et al. (2007). Toxoplasma gondii infection lower anxiety as measured in the plus-maze and social interaction tests in rats: A behavioral analysis. Behav. Brain Res. 177 (1), 70–79. doi: 10.1016/j.bbr.2006.11.012
González-Arancibia, C., Urrutia-Piñones, J., Illanes-González, J., Martinez-Pinto, J., Sotomayor-Zárate, R., Julio-Pieper, M., et al. (2019). Do your gut microbes affect your brain dopamine? Psychopharmacology 236, 1611–1622. doi: 10.1007/s00213-019-05265-5
Haag, L. M., Fischer, A., Otto, B., Plickert, R., Kuhl, A. A., Gobel, U. B., et al. (2012). Intestinal microbiota shifts towards elevated commensal Escherichia coli loads abrogate colonization resistance against Campylobacter jejuni in mice. PloS One 7, e35988. doi: 10.1371/journal.pone.0035988
Halonen, S. K., Weiss, L. M. (2013). Toxoplasmosis. Neuroparasitology Trop. Neurol. 114, 125–145. doi: 10.1016/B978-0-444-53490-3.00008-X
Hamamah, S., Aghazarian, A., Nazaryan, A., Hajnal, A., Covasa, M. (2022). Role of microbiota-gut-brain axis in regulating dopaminergic signaling. Biomedicines 10, 436. doi: 10.3390/biomedicines10020436
Hatter, J. A., Kouche, Y. M., Melchor, S. J., Ng, K., Bouley, D. M., Boothroyd, J. C., et al. (2018). Toxoplasma gondii infection triggers chronic cachexia and sustained commensal dysbiosis in mice. PloS One 13 (10), e0204895. doi: 10.1371/journal.pone.0204895
Haÿs, S. P., Ordonez, J. M., Burrin, D. G., Sunehag, A. L. (2007). Dietary glutamate is almost entirely removed in its first pass through the splanchnic bed in premature infants. Pediatr. Res. 62, 353–356. doi: 10.1203/PDR.0b013e318123f719
Heijtz, R. D., Wang, S., Anuar, F., Qian, Y., Björkholm, B., Samuelsson, A., et al. (2011). Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. 108, 3047–3052. doi: 10.1073/pnas.1010529108
Heimesaat, M. M., Bereswill, S., Fischer, A., Fuchs, D., Struck, D., Niebergall, J., et al. (2006). Gram-Negative Bacteria Aggravate Murine Small Intestinal Th1-Type Immunopathology following Oral Infection with Toxoplasma gondii. J. Immunol. 177, 8785–8795. doi: 10.4049/jimmunol.177.12.8785
Henriquez, S., Brett, R., Alexander, J., Pratt, J., Roberts, C. (2009). Neuropsychiatric disease and Toxoplasma gondii infection. Neuroimmunomodulation 16, 122–133. doi: 10.1159/000180267
Hickey, J. W., Becker, W. R., Nevins, S. A., Horning, A., Perez, A. E., Zhu, C., et al. (2023). Organization of the human intestine at single-cell resolution. Nature 619, 572–584. doi: 10.1038/s41586-023-05915-x
Hill, D., Dubey, J. P. (2002). Toxoplasma gondii: transmission, diagnosis and prevention. Clin. Microbiol. Infection 8 (10), 634–640. doi: 10.1046/j.1469-0691.2002.00485.x
Hinze-Selch, D., Däubener, W., Erdag, S., Wilms, S. (2010). The diagnosis of a personality disorder increases the likelihood for seropositivity to Toxoplasma gondii in psychiatric patients. Folia parasitologica 57, 129. doi: 10.14411/fp.2010.016
Holtmann, G. J., Ford, A. C., Talley, N. J. (2016). Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol. Hepatol. 1, 133–146. doi: 10.1016/S2468-1253(16)30023-1
Hooper, L. V., Littman, D. R., Macpherson, A. J. (2012). Interactions between the microbiota and the immune system. science 336, 1268–1273. doi: 10.1126/science.1223490
Howe, D. K., Sibley, L. D. (1995). Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J. Infect. Dis. 172 (6), 1561–1566. doi: 10.1093/infdis/172.6.1561
Hughes, H. K., Ashwood, P. (2018). Anti-candida albicans IgG antibodies in children with autism spectrum disorders. Front. Psychiatry 9. doi: 10.3389/fpsyt.2018.00627
Hulme, H., Meikle, L. M., Strittmatter, N., Swales, J., Hamm, G., Brown, S. L., et al. (2022). Mapping the influence of the gut microbiota on small molecules across the microbiome gut brain axis. J. Am. Soc. Mass Spectrometry 33, 649–659. doi: 10.1021/jasms.1c00298
Hunter, C. A., Sibley, L. D. (2012). Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat. Rev. Microbiol. 10 (11), 766–778. doi: 10.1038/nrmicro2858
Ingram, W. M., Goodrich, L. M., Robey, E. A., Eisen, M. B. (2013). Mice infected with low-virulence strains of Toxoplasma gondii lose their innate aversion to cat urine, even after extensive parasite clearance. PloS One 8, e75246. doi: 10.1371/journal.pone.0075246
Jensen, S. B., Sheikh, M. A., Akkouh, I. A., Szabo, A., O'Connell, K. S., Lekva, T., et al. (2023). Elevated systemic levels of markers reflecting intestinal barrier dysfunction and inflammasome activation are correlated in severe mental illness. Schizophr. Bull. 49, 635–645. doi: 10.1093/schbul/sbac191
Jovel, J., Dieleman, L. A., Kao, D., Mason, A. L., Wine, E. (2018). The human gut microbiome in health and disease. Metagenomics, 197–213. doi: 10.1016/B978-0-08-102268-9.00010-0
Kanatani, S., Fuks, J. M., Olafsson, E. B., Westermark, L., Chambers, B., Varas-Godoy, M., et al. (2017). Voltage-dependent calcium channel signaling mediates GABAA receptor-induced migratory activation of dendritic cells infected by Toxoplasma gondii. PloS Pathog. 13, e1006739. doi: 10.1371/journal.ppat.1006739
Kannan, G., Crawford, J. A., Yang, C., Gressitt, K. L., Ihenatu, C., Krasnova, I. N., et al. (2016). Anti-NMDA receptor autoantibodies and associated neurobehavioral pathology in mice are dependent on age of first exposure to Toxoplasma gondii. Neurobiol. Dis. 91, 307–314. doi: 10.1016/j.nbd.2016.03.005
Kannan, G., Moldovan, K., Xiao, J.-C., Yolken, R. H., Jones-Brando, L., Pletnikov, M. V. (2010). Toxoplasma gondii strain-dependent effects on mouse behaviour. Folia parasitologica 57, 151. doi: 10.14411/fp.2010.019
Kaszaki, J., Érces, D., Varga, G., Szabó, A., Vécsei, L., Boros, M. (2012). Kynurenines and intestinal neurotransmission: the role of N-methyl-D-aspartate receptors. J. Neural Transm. 119, 211–223. doi: 10.1007/s00702-011-0658-x
Kelly, J. R., Kennedy, P. J., Cryan, J. F., Dinan, T. G., Clarke, G., Hyland, N. P. (2015). Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 9, 166028. doi: 10.3389/fncel.2015.00392
Kim, J., Sudbery, P. (2011). Candida albicans, a major human fungal pathogen. J. Microbiol. 49, 171–177. doi: 10.1007/s12275-011-1064-7
Kittas, C., Henry, L. (1980). Effect of sex hormones on the response of mice to infection with Toxoplasma gondii. Br. J. Exp. Pathol. 61, 590.
Lachenmaier, S. M., Deli, M. A., Meissner, M., Liesenfeld, O. (2011). Intracellular transport of Toxoplasma gondii through the blood–brain barrier. J. neuroimmunology 232, 119–130. doi: 10.1016/j.jneuroim.2010.10.029
Lambert, H., Hitziger, N., Dellacasa, I., Svensson, M., Barragan, A. (2006). Induction of dendritic cell migration upon Toxoplasma gondii infection potentiates parasite dissemination. Cell. Microbiol. 8, 1611–1623. doi: 10.1111/j.1462-5822.2006.00735.x
Lang, D., Schott, B. H., van Ham, M., Morton, L., Kulikovskaja, L., Herrera-Molina, R., et al. (2018). Chronic Toxoplasma infection is associated with distinct alterations in the synaptic protein composition. J. Neuroinflamm. 15 (1), 216. doi: 10.1186/s12974-018-1242-1
Lau, A., Tymianski, M. (2010). Glutamate receptors, neurotoxicity and neurodegeneration. Pflügers Archiv-European J. Physiol. 460, 525–542. doi: 10.1007/s00424-010-0809-1
Li, Y., Severance, E. G., Viscidi, R. P., Yolken, R. H., Xiao, J. (2019). Persistent Toxoplasma infection of the brain induced neurodegeneration associated with activation of complement and microglia. Infection Immun. 87 (8), e00139–19. doi: 10.1128/iai.00139-19
Li, Y., Viscidi, R. P., Kannan, G., McFarland, R., Pletnikov, M. V., Severance, E. G., et al. (2018). Chronic Toxoplasma gondii infection induces anti-N-methyl-d-aspartate receptor autoantibodies and associated behavioral changes and neuropathology. Infection Immun. 86 (10), e00398–18. doi: 10.1128/iai.00398-18
Liesenfeld, O., Kosek, J., Remington, J. S., Suzuki, Y. (1996). Association of CD4+ T cell-dependent, interferon-gamma-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J. Exp. Med. 184, 597–607. doi: 10.1084/jem.184.2.597
Liu, Y.-W., Liu, W.-H., Wu, C.-C., Juan, Y.-C., Wu, Y.-C., Tsai, H.-P., et al. (2016). Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naïve adult mice. Brain Res. 1631, 1–12. doi: 10.1016/j.brainres.2015.11.018
Lv, Q.-B., Ma, H., Wei, J., Qin, Y.-F., Qiu, H.-Y., Ni, H.-B., et al. (2022). Changes of gut microbiota structure in rats infected with Toxoplasma gondii. Front. Cell. Infection Microbiol. 12. doi: 10.3389/fcimb.2022.969832
Lyte, M. (2011). Probiotics function mechanistically as delivery vehicles for neuroactive compounds: microbial endocrinology in the design and use of probiotics. Bioessays 33, 574–581. doi: 10.1002/bies.201100024
Lyte, M. (2013). Microbial endocrinology in the microbiome-gut-brain axis: how bacterial production and utilization of neurochemicals influence behavior. PloS Pathog. 9, e1003726. doi: 10.1371/journal.ppat.1003726
MacAlpine, J., Robbins, N., Cowen, L. E. (2022). Bacterial-fungal interactions and their impact on microbial pathogenesis. Mol. Ecol. 32 (10), 2565–2581. doi: 10.1111/mec.16411
Marchesi, J. R., Ravel, J. (2015). The vocabulary of microbiome research: a proposal. Microbiome 3, 31. doi: 10.1186/s40168-015-0094-5
Markle, J. G., Frank, D. N., Mortin-Toth, S., Robertson, C. E., Feazel, L. M., Rolle-Kampczyk, U., et al. (2013). Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339, 1084–1088. doi: 10.1126/science.1233521
Markovitz, A. A., Simanek, A. M., Yolken, R. H., Galea, S., Koenen, K. C., Chen, S., et al. (2015). Toxoplasma gondii and anxiety disorders in a community-based sample. Brain behavior Immun. 43, 192–197. doi: 10.1016/j.bbi.2014.08.001
Martel, J., Chang, S. H., Ko, Y. F., Hwang, T. L., Young, J. D., Ojcius, D. M. (2022). Gut barrier disruption and chronic disease. Trends Endocrinol. Metab. 33, 247–265. doi: 10.1016/j.tem.2022.01.002
Martin, H. L., Alsaady, I., Howell, G., Prandovszky, E., Peers, C., Robinson, P., et al. (2015). Effect of parasitic infection on dopamine biosynthesis in dopaminergic cells. Neuroscience 306, 50–62. doi: 10.1016/j.neuroscience.2015.08.005
Mazzoli, R., Pessione, E. (2016). The neuro-endocrinological role of microbial glutamate and GABA signaling. Front. Microbiol. 7, 210784. doi: 10.3389/fmicb.2016.01934
McConkey, G. A., Martin, H. L., Bristow, G. C., Webster, J. P. (2013). Toxoplasma gondii infection and behaviour–location, location, location? J. Exp. Biol. 216, 113–119. doi: 10.1242/jeb.074153
McConkey, G. A., Peers, C., Prandovszky, E. (2015). Reproducing increased dopamine with infection to evaluate the role of parasite-encoded tyrosine hydroxylase activity. Infection Immun. 83 (8), 3334–3335. doi: 10.1128/iai.00605-15
McFarland, R., Wang, Z. T., Jouroukhin, Y., Li, Y., Mychko, O., Coppens, I., et al. (2018). AAH2 gene is not required for dopamine-dependent neurochemical and behavioral abnormalities produced by Toxoplasma infection in mouse. Behav. Brain Res. 347, 193–200. doi: 10.1016/j.bbr.2018.03.023
McKnite, A. M., Perez-Munoz, M. E., Lu, L., Williams, E. G., Brewer, S., Andreux, P. A., et al. (2012). Murine gut microbiota is defined by host genetics and modulates variation of metabolic traits. PloS One 7 (6), e39191. doi: 10.1371/journal.pone.0039191
Meiser, J., Weindl, D., Hiller, K. (2013). Complexity of dopamine metabolism. Cell Communication Signaling 11, 34. doi: 10.1186/1478-811X-11-34
Meng, J. X., Wei, X. Y., Guo, H., Chen, Y., Wang, W., Geng, H. L., et al. (2023). Metagenomic insights into the composition and function of the gut microbiota of mice infected with Toxoplasma gondii. Front. Immunol. 14. doi: 10.3389/fimmu.2023.1156397
Messaoudi, M., Lalonde, R., Violle, N., Javelot, H., Desor, D., Nejdi, A., et al. (2011). Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br. J. Nutr. 105, 755–764. doi: 10.1017/S0007114510004319
Mikocka-Walus, A., Knowles, S. R., Keefer, L., Graff, L. (2016). Controversies revisited: a systematic review of the comorbidity of depression and anxiety with inflammatory bowel diseases. Inflammatory bowel Dis. 22, 752–762. doi: 10.1097/MIB.0000000000000620
Miladinovic, T., Nashed, M. G., Singh, G. (2015). Overview of glutamatergic dysregulation in central pathologies. Biomolecules 5, 3112–3141. doi: 10.3390/biom5043112
Minns, L. A., Menard, L. C., Foureau, D. M., Darche, S., Ronet, C., Mielcarz, D. W., et al. (2006). TLR9 is required for the gut-associated lymphoid tissue response following oral infection of Toxoplasma gondii. J. Immunol. 176, 7589–7597. doi: 10.4049/jimmunol.176.12.7589
Mittal, V., Ichhpujani, R. L. (2011). Toxoplasmosis – an update. Trop. Parasitol. 1 (1), 9–14. doi: 10.4103/2229-5070.72109
Molloy, M. J., Grainger, J. R., Bouladoux, N., Hand, T. W., Koo, L. Y., Naik, S., et al. (2013). Intraluminal containment of commensal outgrowth in the gut during infection-induced dysbiosis. Cell Host Microbe 14, 318–328. doi: 10.1016/j.chom.2013.08.003
Mortensen, P. B., Nørgaard-Pedersen, B., Waltoft, B. L., Sørensen, T. L., Hougaard, D., Yolken, R. H. (2007). Early infections of Toxoplasma gondii and the later development of schizophrenia. Schizophr. Bull. 33, 741–744. doi: 10.1093/schbul/sbm009
Müller, U. B., Howard, J. C. (2016). The impact of Toxoplasma gondii on the mammalian genome. Curr. Opin. Microbiol. 32, 19–25. doi: 10.1016/j.mib.2016.04.009
Nascimento, B. B., Cartelle, C. T., d. L. Noviello, M., Pinheiro, B. V., de Almeida Vitor, R. W., d. G. Souza, D., et al. (2017). Influence of indigenous microbiota on experimental toxoplasmosis in conventional and germ-free mice. Int. J. Exp. Pathol. 98, 191–202. doi: 10.1111/iep.12236
Needham, B. D., Kaddurah-Daouk, R., Mazmanian, S. K. (2020). Gut microbial molecules in behavioural and neurodegenerative conditions. Nat. Rev. Neurosci. 21, 717–731. doi: 10.1038/s41583-020-00381-0
Neuman, H., Debelius, J. W., Knight, R., Koren, O. (2015). Microbial endocrinology: the interplay between the microbiota and the endocrine system. FEMS Microbiol. Rev. 39, 509–521. doi: 10.1093/femsre/fuu010
Nishino, R., Mikami, K., Takahashi, H., Tomonaga, S., Furuse, M., Hiramoto, T., et al. (2013). Commensal microbiota modulate murine behaviors in a strictly contamination-free environment confirmed by culture-based methods. Neurogastroenterol. Motil. 25, 521–e371. doi: 10.1111/nmo.12110
Nuss, P. (2015). Anxiety disorders and GABA neurotransmission: a disturbance of modulation. Neuropsychiatr. Dis. treatment 11, 165–175. doi: 10.2147/NDT.S58841
O'Mahony, S. M., Marchesi, J. R., Scully, P., Codling, C., Ceolho, A.-M., Quigley, E. M., et al. (2009). Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol. Psychiatry 65, 263–267. doi: 10.1016/j.biopsych.2008.06.026
Pál, B. (2018). Involvement of extrasynaptic glutamate in physiological and pathophysiological changes of neuronal excitability. Cell. Mol. Life Sci. 75, 2917–2949. doi: 10.1007/s00018-018-2837-5
Pappas, G., Roussos, N., Falagas, M. E. (2009). Toxoplasmosis snapshots: Global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int. J. Parasitol. 39 (12), 1385–1394. doi: 10.1016/j.ijpara.2009.04.003
Pflughoeft, K. J., Versalovic, J. (2012). Human microbiome in health and disease. Annu. Rev. Pathology: Mech. Dis. 7, 99–122. doi: 10.1146/annurev-pathol-011811-132421
Phelps, E. A. (2004). Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr. Opin. Neurobiol. 14, 198–202. doi: 10.1016/j.conb.2004.03.015
Phuna, Z. X., Madhavan, P. (2022). A closer look at the mycobiome in Alzheimer's disease: Fungal species, pathogenesis and transmission. Eur. J. Neurosci. 55 (5), 1291–1321. doi: 10.1111/ejn.15599
Pisa, D., Alonso, R., Rabano, A., Rodal, I., Carrasco, L. (2015). Different brain regions are infected with fungi in Alzheimer's disease. Sci. Rep. 5, 15015. doi: 10.1038/srep15015
Pittman, K. J., Knoll, L. J. (2015). Long-Term Relationships: the Complicated Interplay between the Host and the Developmental Stages of Toxoplasma gondii during Acute and Chronic Infections. Microbiol. Mol. Biol. Rev. 79 (4), 387–401. doi: 10.1128/mmbr.00027-15
Prandovszky, E., Gaskell, E., Martin, H., Dubey, J. P., Webster, J. P., McConkey, G. A. (2011). The neurotropic parasite Toxoplasma gondii increases dopamine metabolism. PloS One 6, e23866. doi: 10.1371/journal.pone.0023866
Prandovszky, E., Li, Y., Sabunciyan, S., Steinfeldt, C. B., Avalos, L. N., Gressitt, K. L., et al. (2018). Toxoplasma gondii-Induced Long-Term Changes in the Upper Intestinal Microflora during the Chronic Stage of Infection. Scientifica 2018, 2308619. doi: 10.1155/2018/2308619
Pung, O. J., Luster, M. I. (1986). Toxoplasma gondii: decreased resistance to infection in mice due to estrogen. Exp. Parasitol. 61, 48–56. doi: 10.1016/0014-4894(86)90134-7
Raetz, M., S.-h. Hwang, C. L., Kirkland, D., Benson, A., Sturge, C. R., Mirpuri, J., et al. (2013). DeFranco: Parasite-induced TH1 cells and intestinal dysbiosis cooperate in IFN-γ-dependent elimination of Paneth cells. Nat. Immunol. 14, 136–142. doi: 10.1038/ni.2508
Roberts, C. W., Cruickshank, S. M., Alexander, J. (1995). Sex-determined resistance to Toxoplasma gondii is associated with temporal differences in cytokine production. Infection Immun. 63, 2549–2555. doi: 10.1128/iai.63.7.2549-2555.1995
Roe, K. (2022). Concurrent infections of cells by two pathogens can enable a reactivation of the first pathogen and the second pathogen's accelerated T-cell exhaustion. Heliyon 8, e11371. doi: 10.1016/j.heliyon.2022.e11371
Rolhion, N., Darfeuille-Michaud, A. (2007). Adherent-invasive Escherichia coli in inflammatory bowel disease. Inflammatory bowel Dis. 13, 1277–1283. doi: 10.1002/ibd.20176
Roozendaal, B., McEwen, B. S., Chattarji, S. (2009). Stress, memory and the amygdala. Nat. Rev. Neurosci. 10, 423–433. doi: 10.1038/nrn2651
Royo, F., Tames, H., Bordanaba-Florit, G., Cabrera, D., Azparren-Angulo, M., Garcia-Vallicrosa, C., et al. (2023). Orally administered Bifidobacterium adolescentis diminishes serum glutamate concentration in mice. Microbiol. Spectr. 11, e05063–e05022. doi: 10.1128/spectrum.05063-22
Sanchez, S., Rodríguez-Sanoja, R., Ramos, A., Demain, A. L. (2018). Our microbes not only produce antibiotics, they also overproduce amino acids. J. antibiotics 71, 26–36. doi: 10.1038/ja.2017.142
Saraav, I., Cervantes-Barragan, L., Olias, P., Fu, Y., Wang, Q., Wang, L., et al. (2021). Chronic Toxoplasma gondii infection enhances susceptibility to colitis. Proc. Natl. Acad. Sci. 118 (36), e2106730118. doi: 10.1073/pnas.2106730118
Sasai, M., Pradipta, A., Yamamoto, M. (2018). Host immune responses to Toxoplasma gondii. Int. Immunol. 30 (3), 113–119. doi: 10.1093/intimm/dxy004
Sasai, M., Yamamoto, M. (2019). Innate, adaptive, and cell-autonomous immunity against Toxoplasma gondii infection. Exp. Mol. Med. 51, 1–10. doi: 10.1038/s12276-019-0353-9
Scanga, C. A., Aliberti, J., Jankovic, D., Tilloy, F., Bennouna, S., Denkers, E. Y., et al. (2002). Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J. Immunol. 168, 5997–6001. doi: 10.4049/jimmunol.168.12.5997
Schmidt, D., Reber, S. O., Botteron, C., Barth, T., Peterlik, D., Uschold, N., et al. (2010). Chronic psychosocial stress promotes systemic immune activation and the development of inflammatory Th cell responses. Brain behavior Immun. 24, 1097–1104. doi: 10.1016/j.bbi.2010.04.014
Schousboe, A., Scafidi, S., Bak, L. K., Waagepetersen, H. S., McKenna, M. C. (2014). Glutamate metabolism in the brain focusing on astrocytes. Adv. Neurobiol. 11, 13–30. doi: 10.1007/978-3-319-08894-5_2
Severance, E. G. (2023). Fungal forces in mental health: microbial meddlers or function fixers? Curr. Top. Behav. Neurosci. 61, 163–179. doi: 10.1007/7854_2022_364
Severance, E. G., Alaedini, A., Yang, S., Halling, M., Gressitt, K. L., Stallings, C. R., et al. (2012). Gastrointestinal inflammation and associated immune activation in schizophrenia. Schizophr. Res. 138, 48–53. doi: 10.1016/j.schres.2012.02.025
Severance, E. G., Gressitt, K. L., Stallings, C. R., Katsafanas, E., Schweinfurth, L. A., Savage, C. L., et al. (2016). Candida albicans exposures, sex specificity and cognitive deficits in schizophrenia and bipolar disorder. NPJ Schizophr. 2, 16018. doi: 10.1038/npjschz.2016.18
Severance, E. G., Gressitt, K. L., Yang, S., Stallings, C. R., Origoni, A. E., Vaughan, C., et al. (2014). Seroreactive marker for inflammatory bowel disease and associations with antibodies to dietary proteins in bipolar disorder. Bipolar Disord. 16, 230–240. doi: 10.1111/bdi.12159
Severance, E. G., Leister, F., Lea, A., Yang, S., Dickerson, F., Yolken, R. H. (2021). Complement C4 associations with altered microbial biomarkers exemplify gene-by-environment interactions in schizophrenia. Schizophr. Res. 234, 87–93. doi: 10.1016/j.schres.2021.02.001
Severance, E. G., Prandovszky, E., Yang, S., Leister, F., Lea, A., Wu, C. L., et al. (2023). Prospects and pitfalls of plasma complement C4 in schizophrenia: building a better biomarker. Dev. Neurosci. 45, 349–360. doi: 10.1159/000534185
Severance, E. G., Yolken, R. H. (2020a). From infection to the microbiome: an evolving role of microbes in schizophrenia. Curr. Top. Behav. Neurosci. 44, 67–84. doi: 10.1007/7854_2018_84
Severance, E. G., Yolken, R. H. (2020b). Deciphering microbiome and neuroactive immune gene interactions in schizophrenia. Neurobiol. Dis. 135, 104331. doi: 10.1016/j.nbd.2018.11.016
Shao, D. Y., Bai, X., Tong, M. W., Zhang, y., L., Y. ,. X., Zhou, Y.h., et al. (2020). Changes to the gut microbiota in mice induced by infection with Toxoplasma gondii. Acta Tropica 203, 105301. doi: 10.1016/j.actatropica.2019.105301
Shaw, W. (2004). Inhibition of dopamine conversion to norepinephrine by Clostridia metabolites appears to be a (the) major cause of autism, schizophrenia, and other neuropsychiatric disorders. Great Plains Lab. 13, 1–7.
Shaw, W. (2017). Elevated urinary glyphosate and clostridia metabolites with altered dopamine metabolism in triplets with autistic spectrum disorder or suspected seizure disorder: A case study. Integr. Medicine: A Clinician's J. 16, 50.
Sherwin, E., Sandhu, K. V., Dinan, T. G., Cryan, J. F. (2016). May the force be with you: the light and dark sides of the microbiota–gut–brain axis in neuropsychiatry. CNS Drugs 30, 1019–1041. doi: 10.1007/s40263-016-0370-3
Shin, J.-H., Park, Y.-H., Sim, M., Kim, S.-A., Joung, H., Shin, D.-M. (2019). Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Res. Microbiol. 170, 192–201. doi: 10.1016/j.resmic.2019.03.003
Shwab, E. K., Zhu, X.-Q., Majumdar, D., Pena, H. F., Gennari, S. M., Dubey, J. P., et al. (2014). Geographical patterns of Toxoplasma gondii genetic diversity revealed by multilocus PCR-RFLP genotyping. Parasitology 141, 453–461. doi: 10.1017/S0031182013001844
Silva, Y. P., Bernardi, A., Frozza, R. L. (2020a). The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 11, 25. doi: 10.3389/fendo.2020.00025
Silva, Y. P., Bernardi, A., Frozza, R. L. (2020b). Frontiers | The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 11. doi: 10.3389/fendo.2020.00025
Stibbs, H. (1985). Changes in brain concentrations of catecholamines and indoleamines in Toxoplasma gondii infected mice. Ann. Trop. Med. Parasitol. 79, 153–157. doi: 10.1080/00034983.1985.11811902
Strandwitz, P., Kim, K. H., Terekhova, D., Liu, J. K., Sharma, A., Levering, J., et al. (2019). GABA-modulating bacteria of the human gut microbiota. Nature Microbiol. 43(3), 396–403.
Sturge, C. R., Yarovinsky, F. (2014). Complex immune cell interplay in the gamma interferon response during Toxoplasma gondii infection. Infection Immun. 82, 3090–3097. doi: 10.1128/IAI.01722-14
Sylvia, K. E., Demas, G. E. (2018). A gut feeling: microbiome-brain-immune interactions modulate social and affective behaviors. Hormones Behav. 99, 41–49. doi: 10.1016/j.yhbeh.2018.02.001
Taggart, P. L., Liddicoat, C., Tong, W. H., Breed, M. F., Weinstein, P., Wheeler, D., et al. (2022). Gut microbiota composition does not associate with toxoplasma infection in rats. Mol. Ecol. 31, 3963–3970. doi: 10.1111/mec.16552
Tedford, E., Badya, N. B., Laing, C., Asaoka, N., Kaneko, S., Filippi, B. M., et al. (2023). Infection-induced extracellular vesicles evoke neuronal transcriptional and epigenetic changes. Sci. Rep. 13, 6913. doi: 10.1038/s41598-023-34074-2
Terunuma, M. (2018). Diversity of structure and function of GABAB receptors: a complexity of GABAB-mediated signaling. Proc. Japan Academy Ser. B 94, 390–411. doi: 10.2183/pjab.94.026
Thapa, M., Kumari, A., Chin, C. Y., Choby, J. E., Jin, F., Bogati, B., et al. (2023). Translocation of gut commensal bacteria to the brain. bioRxiv. doi: 10.1101/2023.08.30.555630
Tomal, F., Sadrin, G., Gaboriaud, P., Guitton, E., Sedano, L., Lallier, N., et al. (2023). The caecal microbiota promotes the acute inflammatory response and the loss of the intestinal barrier integrity during severe Eimeria tenella infection. Front. Cell Infect. Microbiol. 13. doi: 10.3389/fcimb.2023.1250080
Tomé, D. (2018). The roles of dietary glutamate in the intestine. Ann. Nutr. Metab. 73, 15–20. doi: 10.1159/000494777
Tong, W. H., Pavey, C., O’Handley, R., Vyas, A. (2021). Behavioral biology of Toxoplasma gondii infection. Parasites Vectors 14 (1), 77. doi: 10.1186/s13071-020-04528-x
Torres, J., Petralia, F., Sato, T., Wang, P., Telesco, S. E., Choung, R. S., et al. (2020). Serum biomarkers identify patients who will develop inflammatory bowel diseases up to 5 years before diagnosis. Gastroenterology 159, 96–104. doi: 10.1053/j.gastro.2020.03.007
Torres, L., Robinson, S.-A., Kim, D.-G., Yan, A., Cleland, T. A., Bynoe, M. S. (2018). Toxoplasma gondii alters NMDAR signaling and induces signs of Alzheimer’s disease in wild-type, C57BL/6 mice. J. Neuroinflamm. 15 (1), 57. doi: 10.1186/s12974-018-1086-8
Torrey, E. F., Yolken, R. H. (2003). Toxoplasma gondii and schizophrenia. Emerging Infect. Dis. 9, 1375–1380. doi: 10.3201/eid0911.030143
Underhill, D. M., Braun, J. (2022). Fungal microbiome in inflammatory bowel disease: a critical assessment. J. Clin. Invest. 132 (5), e155786. doi: 10.1172/jci155786
Vyas, A. (2015). Mechanisms of host behavioral change in Toxoplasma gondii rodent association. PloS Pathog. 11 (7), e1004935. doi: 10.1371/journal.ppat.1004935
Wall, R., Cryan, J. F., Ross, R. P., Fitzgerald, G. F., Dinan, T. G., Stanton, C. (2014). Bacterial neuroactive compounds produced by psychobiotics. Microbial endocrinology: microbiota-gut-brain axis Health Dis. 817, 221–239. doi: 10.1007/978-1-4939-0897-4_10
Wang, S., El-Fahmawi, A., Christian, D. A., Fang, Q., Radaelli, E., Chen, L., et al. (2019). Infection-induced intestinal dysbiosis is mediated by macrophage activation and nitrate production. mBio 10 (3), e00935–19. doi: 10.1128/mBio.00935-19
Wang, Z. T., Harmon, S., O'Malley, K. L., Sibley, L. D. (2015). Reassessment of the role of aromatic amino acid hydroxylases and the effect of infection by Toxoplasma gondii on host dopamine. Infection Immun. 83, 1039–1047. doi: 10.1128/IAI.02465-14
Wang, Z.-D., Liu, H.-H., Ma, Z.-X., Ma, H.-Y., Li, Z.-Y., Yang, Z.-B., et al. (2017). Toxoplasma gondii infection in immunocompromised patients: A systematic review and meta-analysis. Front. Microbiol. 8. doi: 10.3389/fmicb.2017.00389
Webster, J. P. (2007). The effect of Toxoplasma gondii on animal behavior: playing cat and mouse. Schizophr. Bull. 33 (3), 752–756. doi: 10.1093/schbul/sbl073
Webster, J., Lamberton, P., Donnelly, C., Torrey, E. (2006). Parasites as causative agents of human affective disorders? The impact of anti-psychotic, mood-stabilizer and anti-parasite medication on Toxoplasma gondii's ability to alter host behaviour. Proc. R. Soc. B: Biol. Sci. 273, 1023–1030. doi: 10.1098/rspb.2005.3413
Webster, J. P., McConkey, G. A. (2010). Toxoplasma gondii-altered host behaviour: clues as to mechanism of action. Folia parasitologica 57, 95. doi: 10.14411/fp.2010.012
Wong, S.-Y., Remington, J. S. (1994). Toxoplasmosis in pregnancy. Clin. Infect. Dis. 18 (6), 853–861. doi: 10.1093/clinids/18.6.853
Wong, W. K., Upton, A., Thomas, M. G. (2013). Neuropsychiatric symptoms are common in immunocompetent adult patients with Toxoplasma gondii acute lymphadenitis. Scandinavian J. Infect. Dis. 45 (5), 357–361. doi: 10.3109/00365548.2012.737017
Xiao, J., Kannan, G., Jones-Brando, L., Brannock, C., Krasnova, I., Cadet, J., et al. (2012). Sex-specific changes in gene expression and behavior induced by chronic Toxoplasma infection in mice. Neuroscience 206, 39–48. doi: 10.1016/j.neuroscience.2011.12.051
Xiao, J., Li, Y., Gressitt, K. L., He, H., Kannan, G., Schultz, T. L., et al. (2016a). Cerebral complement C1q activation in chronic Toxoplasma infection. Brain behavior Immun. 58, 52–56. doi: 10.1016/j.bbi.2016.04.009
Xiao, J., Li, Y., Prandovszky, E., Kannan, G., Viscidi, R. P., Pletnikov, M. V., et al. (2016b). Behavioral abnormalities in a mouse model of chronic toxoplasmosis are associated with MAG1 antibody levels and cyst burden. PloS Negl. Trop. Dis. 10, e0004674. doi: 10.1371/journal.pntd.0004674
Xiao, J., Yolken, R. H. (2015). Strain hypothesis of Toxoplasma gondii infection on the outcome of human diseases. Acta physiologica 213, 828–845. doi: 10.1111/apha.12458
Xu, R., Zhang, Y., Chen, S., Zeng, Y., Fu, X., Chen, T., et al. (2023). The role of the probiotic Akkermansia muciniphila in brain functions: insights underpinning therapeutic potential. Crit. Rev. Microbiol. 49, 151–176. doi: 10.1080/1040841X.2022.2044286
Yan, X., Han, W., Jin, X., Sun, Y., Gao, J., Yu, X., et al. (2022). Study on the effect of koumiss on the intestinal microbiota of mice infected with Toxoplasma gondii. Sci. Rep. 12, 1271. doi: 10.1038/s41598-022-05454-x
Yarovinsky, F. (2014). Innate immunity to Toxoplasma gondii infection. Nat. Rev. Immunol. 14 (2), 109–121. doi: 10.1038/nri3598
Yarovinsky, F., Zhang, D., Andersen, J. F., Bannenberg, G. L., Serhan, C. N., Hayden, M. S., et al. (2005). TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 308, 1626–1629. doi: 10.1126/science.1109893
Yirmiya, R. (1997). Behavioral and psychological effects of immune activation: implications for'depression due to a general medical condition'. Curr. Opin. Psychiatry 10, 470–476. doi: 10.1097/00001504-199711000-00011
Yolken, R. H., Dickerson, F. B., Torrey, E. F. (2009). Toxoplasma and schizophrenia. Parasite Immunol. 31 (11), 706–715. doi: 10.1111/j.1365-3024.2009.01131.x
Yurkovetskiy, L., Burrows, M., Khan, A. A., Graham, L., Volchkov, P., Becker, L., et al. (2013). Gender bias in autoimmunity is influenced by microbiota. Immunity 39, 400–412. doi: 10.1016/j.immuni.2013.08.013
Zarrindast, M. R., Khakpai, F. (2015). The modulatory role of dopamine in anxiety-like behavior. Arch Iran Med. 18 (9).
Zhai, Q., Feng, S., Arjan, N., Chen, W. (2019). A next generation probiotic, Akkermansia muciniphila. Crit. Rev. Food Sci. Nutr. 59, 3227–3236. doi: 10.1080/10408398.2018.1517725
Zhao, X.-Y., Ewald, S. E. (2020). The molecular biology and immune control of chronic Toxoplasma gondii infection. J. Clin. Invest. 130, 3370–3380. doi: 10.1172/JCI136226
Zheng, L., Kelly, C. J., Colgan, S. P. (2015). Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A review in the theme: cellular responses to hypoxia. Am. J. Physiology-Cell Physiol. 309, C350–C360. doi: 10.1152/ajpcell.00191.2015
Keywords: toxoplasma, microbiome, behavior, neurotransmitters, immune activation
Citation: Prandovszky E, Severance EG, Splan VW, Liu H, Xiao J and Yolken RH (2024) Toxoplasma-induced behavior changes - is microbial dysbiosis the missing link? Front. Cell. Infect. Microbiol. 14:1415079. doi: 10.3389/fcimb.2024.1415079
Received: 09 April 2024; Accepted: 13 August 2024;
Published: 30 September 2024.
Edited by:
Glenn McConkey, University of Leeds, United KingdomReviewed by:
Geoff Hide, University of Salford, United KingdomIldiko Rita Dunay, University Hospital Magdeburg, Germany
Copyright © 2024 Prandovszky, Severance, Splan, Liu, Xiao and Yolken. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emese Prandovszky, ZW9kb25uZTNAamhtaS5lZHU=
 Emese Prandovszky
Emese Prandovszky Emily G. Severance
Emily G. Severance Victor W. Splan
Victor W. Splan Hua Liu
Hua Liu Jianchun Xiao
Jianchun Xiao Robert H. Yolken
Robert H. Yolken