- 1Department of Respiratory and Critical Care Medicine, Zhengzhou University People's Hospital, Zhengzhou, China
- 2Henan Provincial People’s Hospital, Zhengzhou, China
- 3Xinxiang City Central Hospital, Xinxiang, Henan, China
- 4Department of Respiratory and Critical Care Medicine, Henan Provincial People’s Hospital, Zhengzhou, China
- 5Zhengzhou University People’s Hospital, Zhengzhou, Henan, China
- 6Henan University People’s Hospital, Zhengzhou, Henan, China
Objective: To explore the clinical utility of metagenomic next-generation sequencing (mNGS) in diagnosing invasive pulmonary aspergillosis (IPA) among patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD) in the intensive care unit (ICU).
Methods: A retrospective analysis was conducted on patients with AECOPD admitted to the ICU of Xinxiang Central Hospital in Henan Province, China, between March 2020 and September 2023, suspected of having IPA. Bronchoalveolar lavage fluid (BALF) samples were collected for fungal culture, the galactomannan (GM) test, and mNGS. Based on host factors, clinical features, and microbiological test results, patients were categorized into 62 cases of IPA and 64 cases of non-IPA. Statistical analysis was performed to compare the diagnostic efficacy of fungal culture, the serum and BALF GM test, and mNGS detection for IPA in patients with AECOPD.
Results: 1. The sensitivity and specificity of mNGS in diagnosing IPA were 70.9% and 71.8% respectively, with the sensitivity of mNGS surpassing that of fungal culture (29.0%, P<0.01), serum GM test (35.4%, P<0.01), and BALF GM test (41.9%, P<0.05), albeit with slightly lower specificity compared to fungal culture (90.6%, P >0.05), serum GM test (87.5%, P >0.05), and BALF GM test (85.9%, P >0.05).Combining fungal culture with the GM test and mNGS resulted in a sensitivity of 80.6% and a specificity of 92.2%, underscoring a superior diagnostic rate compared to any single detection method. 2.mNGS accurately distinguished strains of the Aspergillus genus. 3.The area under the ROC curves of mNGS was 0.73, indicating good diagnostic performance. 4.The detection duration for mNGS is shorter than that of traditional fungal culture and GM testing.
Conclusion: mNGS presents a pragmatic and highly sensitive approach, serving as a valuable complementary tool to conventional microbiological tests (CMT). Our research demonstrated that, compared to fungal culture and GM testing, mNGS exhibits superior diagnostic capability for IPA among patients with AECOPD. Integration of mNGS with established conventional methods holds promise for improving the diagnosis rate of IPA.
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic, non-specific inflammatory airway disease characterized by persistent and progressively worsening airflow obstruction and alveolar destruction. The 2023 Global Initiative for Chronic Obstructive Lung Disease (GOLD) version defines acute exacerbation of COPD(AECOPD) as an acute event characterized by deterioration in dyspnea and/or cough with sputum production among patients with COPD, occurring within 14 days, possibly accompanied by tachypnea and/or tachycardia (Zantah et al., 2020). In the ICU, certain critically ill patients are often subjected to prolonged courses of broad-spectrum antibiotics, glucocorticoids, invasive mechanical ventilation, and central venous catheterization (Huang et al., 2017). These factors collectively contribute to a mounting incidence of IPA among patients experiencing AECOPD, significantly compromising their prognosis and survival duration. Literature reports indicate that over 10% of COPD patients develop IPA, with mortality rates ranging from 50% to 100% when COPD is compounded by IPA. Therefore, there is an urgent need for early diagnosis and treatment of IPA.
Currently, common diagnostic methods for aspergillosis include fungal culture, the (1,3)-β-D glucan (G test), and the galactomannan (GM) test. However, these methods exhibit certain limitations, such as time-consuming procedures and low diagnostic rates. Additionally, the clinical manifestations of IPA lack specificity, leading to a decreased rate of diagnosis and delayed antifungal treatment, ultimately resulting in elevated mortality rates.Therefore, early diagnosis of IPA is essential.
The rapid advancements in genome sequencing technologies and bioinformatics approaches offer robust solutions to tackle these clinical diagnostic challenges. mNGS emerges as a promising microbial identification technique. It operates independently of culture and hypotheses, serving as a broad-spectrum sequencing method capable of circumventing the limitations of existing diagnostic tests. It facilitates direct pathogen detection without reliance on primers or probes. While mNGS has found widespread application in diagnosing microbial pathogens, its clinical utility in pulmonary aspergillosis remains relatively unexplored (Denning, 2015; Bao et al., 2022). Previous research findings predominantly stem from case reports and small case series, providing constrained clinical insights and evidence.
In this study, we investigated patients with AECOPD complicated by IPA. We compared the diagnostic efficacy of mNGS with that of fungal culture, as well as that of serum and BALF GM tests.
Materials and methods
Study design and participants
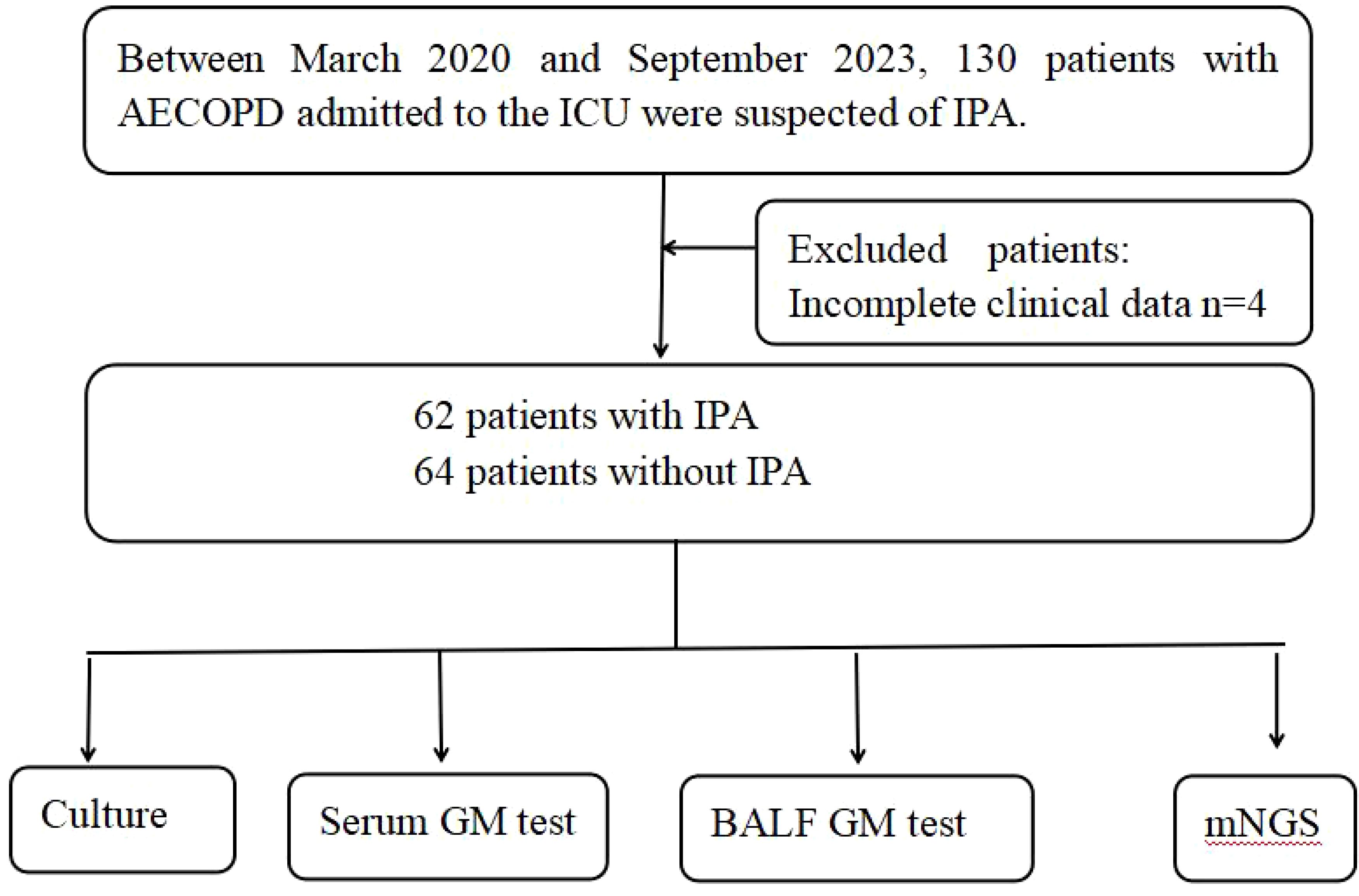
This is a retrospective study involved patients with AECOPD admitted to the ICU of the Xinxiang Central Hospital between March 2020 and September 2023. Eligible patients were those suspected of having IPA, meeting specific criteria: (1) presence of host factors: such as prolonged corticosteroid use exceeding 3 weeks, AECOPD, extended ICU stay, prolonged mechanical ventilation, indwelling catheters, total parenteral nutrition, and prolonged broad-spectrum antibiotic usage; (2)Primary clinical characteristics, including: a. Fever persisting for >96 hours with no improvement despite aggressive antibiotic therapy. b. Symptoms and signs of pulmonary infection. c. Chest CT showing lesions such as “halo sign” and “crescent sign”. (3) completion of bronchoalveolar lavage; (4) availability of culture, GM, and mNGS results (Russo et al., 2011; Otu et al., 2023). The final diagnosis was established based on the patients’ host factors, clinical characteristics, and microbiological test results, the final diagnosis was established. These patients were suspected of IPA, with a total of 126 patients included (62 with IPA and 64 without IPA) (Figure 1).
This study was approved by the Ethics Committee of Xinxiang city Central Hospital, Henan Province, China. Individual consent for this retrospective analysis was waived.
Data collection
Demographic and clinical data were extracted from electronic medical records (EMRs), including age, gender, diagnosis of underlying diseases, smoking history, dosage of steroids used, duration of antibiotic therapy, laboratory test results, characteristics during ICU treatment (such as length of ICU stay, duration of central venous catheterization, SOFA score, duration of mechanical ventilation, rapid shallow breathing index (RSBI), etc.).
Criteria of diagnosis of IPA and identification of pathogens
The diagnostic criteria for IPA were as follows: (1) Confirmed diagnosis: histopathology confirmed as fungi (independent of host factors and clinical features); or the presence of at least one host factor coupled with one major clinical feature or two minor clinical features, along with fungal identification in blood and secretions cultures. (2) Clinical diagnosis: simultaneous manifestation of at least one host factor, one major clinical feature, or two minor clinical features indicative of invasive pulmonary fungal disease, and microbiological evidence. (3) Possible diagnosis: simultaneous occurrence of at least one host risk factor, one major clinical feature, or two minor clinical features indicative of invasive pulmonary fungal disease (Ader et al., 2005; Tiew et al., 2022).
mNGS pathogen detection: BALF samples were obtained from all ICU-admitted patients within 48 hours of admission.While patients were under sedation, BALF samples were collected from the bronchial segment displaying severe lesions based on imaging findings. Each BALF sample had a minimum volume of 10 mL.Samples from the experimental group were dispatched to the Guangzhou Weiyuan Genomics Company for mNGS analysis.The specific steps were as follows: The BALF was processed using the Bioprep-24 biological sample homogenizer (/Tiangen Biotech Co., Ltd.China). Subsequently, 300μL of processed BALF was subjected to total DNA extraction using a DNA extraction kit. The extracted RNA was reverse transcribed into cDNA using reverse transcriptase (Thermo Fisher Scientific) and dNTPs, and DNA libraries were constructed. The prepared libraries underwent purification, amplification, and further purification. The concentration of the libraries was quantified using the Qubit 4.0 Fluorometer (Thermo Fisher Scientific).The libraries were then loaded onto the Illumina Nextseq CN500 sequencer for 75 single-end sequencing cycles. After sequencing, low-quality sequences were removed using Trimmomatic software to obtain high-quality data. The remaining microbial sequences were aligned to the microbial database constructed by Weiyuan Gene Company (Guangzhou, China). Subsequently, the aligned data were categorized and sorted according to viruses, bacteria, fungi, and parasites. Species-specific sequence reads (SSRN), relative abundances, identification confidences, and other information were obtained.
Traditional fungal culture: BALF specimens were inoculated onto Sabouraud’s dextrose agar plates and incubated in a fungal culture incubator at 28°C for 4–7 days. A fungal culture count of ≥105 CFU/mL was deemed positive.
GM Test: The GM test employs a chemiluminescence method, wherein a GM concentration of ≤0.25 ug/L is deemed negative, while a concentration of ≥0.45 ug/L is considered positive. For concentrations ranging from 0.25 to 0.45 ug/L, results are considered indeterminate and should be evaluated comprehensively based on clinical findings.
Evaluation criteria and observational indices
(1)Analyze the distribution of pathogens detected by mNGS and traditional Aspergillus culture<n.o></no> (2) The diagnostic efficacy of mNGS, traditional fungal detection, and combination testing was compared. The sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV) and J-index of mNGS, traditional fungal detection, the serum GM test, the BALF GM test,and combination testing were calculated and compared. (3) Finally, the time required for Aspergillus detection using mNGS, traditional fungal culture, and the GM test was compared.
Statistical analyses
SPSS version 23.0 software was utilized for analysis. Normally distributed continuous data were expressed as mean ± standard deviation (x̅ ± s), and compared between groups employing independent sample t-tests.
Results
Clinical characteristics
During the study period, a total of 130 patients with AECOPD suspected of harboring IPA infection were enrolled. Four patients were excluded owing to incomplete clinical data, resulting in 126 patients being included in the final analysis, with 62 classified as patients with IPA and 64 as non-IPA patients. A comparison and summary of demographic and clinical characteristics between the two groups were conducted, encompassing gender, age, Body Mass Index(BMI), hypoalbuminemia, neutrophil (NEUT#)/lymphocyte (LYMPH#) ratio, revealing no significant differences between the IPA and non-IPA groups. The levels of the inflammatory factor IL-6 were significantly higher in the IPA group compared to the non-IPA group(P < 0.05). However, patients with AECOPD with a history of smoking, corticosteroid use, and antibiotic use exhibited a higher propensity for IPA development compared to non-IPA patients (P < 0.01). Throughout their ICU hospitalization, clinical indicators of both patient groups, such as length of ICU stay, duration of indwelling central venous catheter, duration of mechanical ventilation, rapid shallow breathing index, and incidence of septic shock, exhibited statistically significant differences (P < 0.05).The primary characteristics of all eligible patients are detailed in Table 1.
Classification of Aspergillus detected through mNGS and traditional fungal culture
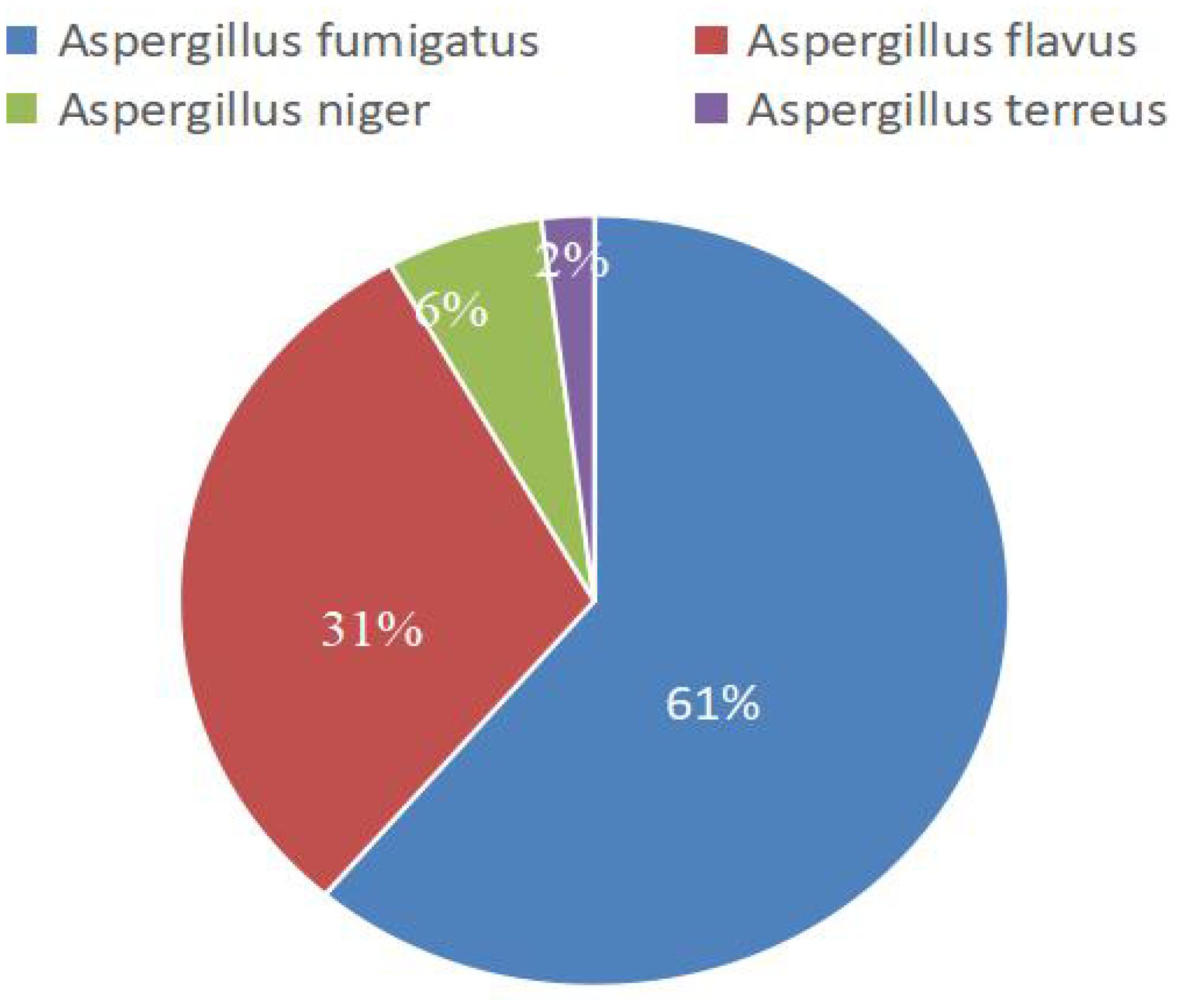
mNGS detected 49 strains encompassing various Aspergillus species, whereas traditional culture identified 22 fungal strains. Table 2 illustrates the distribution of these various Aspergillus species, and Figure 2 shows the composition ratios of these different Aspergillus species..
Comparison of Sensitivity, Specificity, PPV, NPV, and J-index among fungal culture, serum GM test, BALF GM test, mNGS, and combination test
We compared the differences in sensitivity, specificity, positive predictive value, negative predictive value, and J-index among fungal culture, serum GM test, BALF GM test, mNGS, and combination test (Table 3). The sensitivity of mNGS for diagnosing IPA was 70.9%, higher than that of traditional fungal culture (29.0%), serum GM test (35.4%) and BALF GM test (41.9%) (P<0.05) (Figures 3A–C). The sensitivity of BALF GM testing (41.9%) exceeds that of serum GM testing (35.4%), with no statistically significant difference between the two groups(P>0.05) (Figure 3D). When mNGS was combined with the GM test, and fungal culture, the sensitivity increased to 80.6%, surpassing that of any single method.
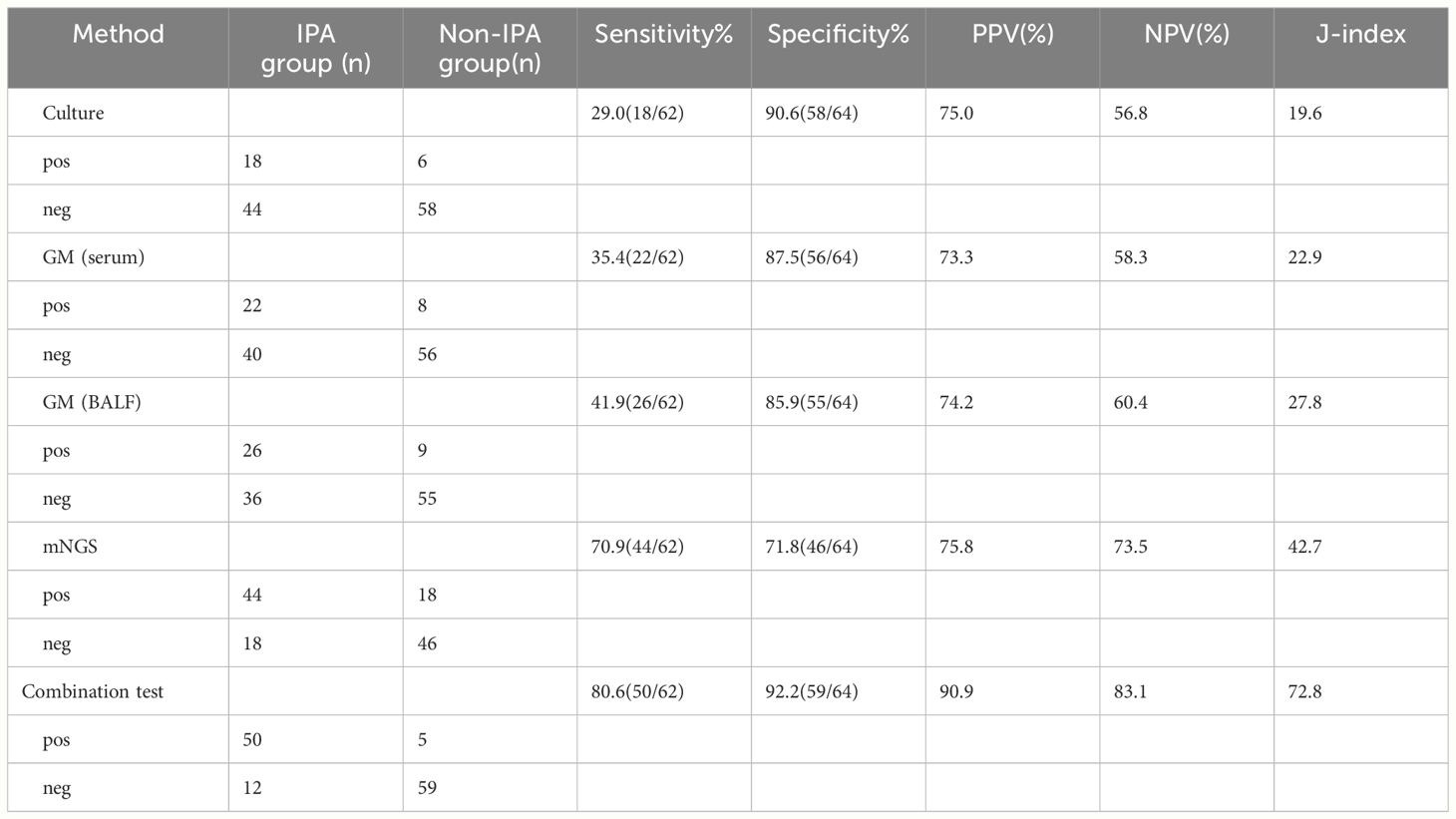
Table 3 Comparison of sensitivity, specificity, PPV, NPV, and J-index among fungal culture, serum GM test, BALF GM test, mNGS, and combination test.
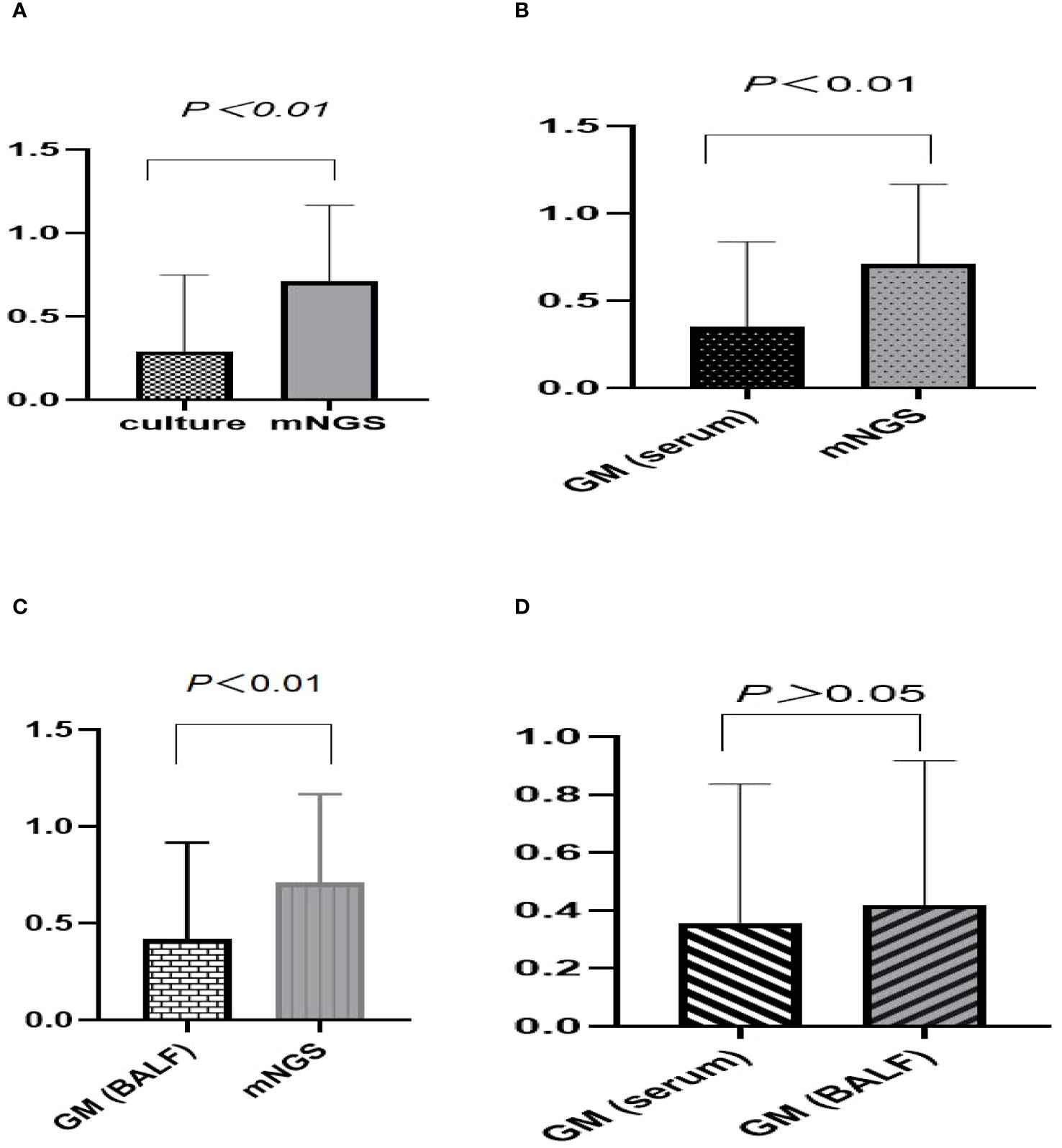
Figure 3 Comparative analysis of sensitivity across various diagnostic methods for suspected IPA. (A–C) The sensitivity of mNGS was 70.9%, higher than that of traditional fungal culture (29.0%, P<0.01), serum GM test (35.4%, P<0.01) and BALF GM test (41.9%, P<0.01). (D) The sensitivity of BALF GM testing (41.9%) exceeds that of serum GM testing (35.4%), with no statistically significant difference between the two groups (P>0.05).
Comparison of the diagnostic efficacy of mNGS, fungal culture, the GM test, and combination testing
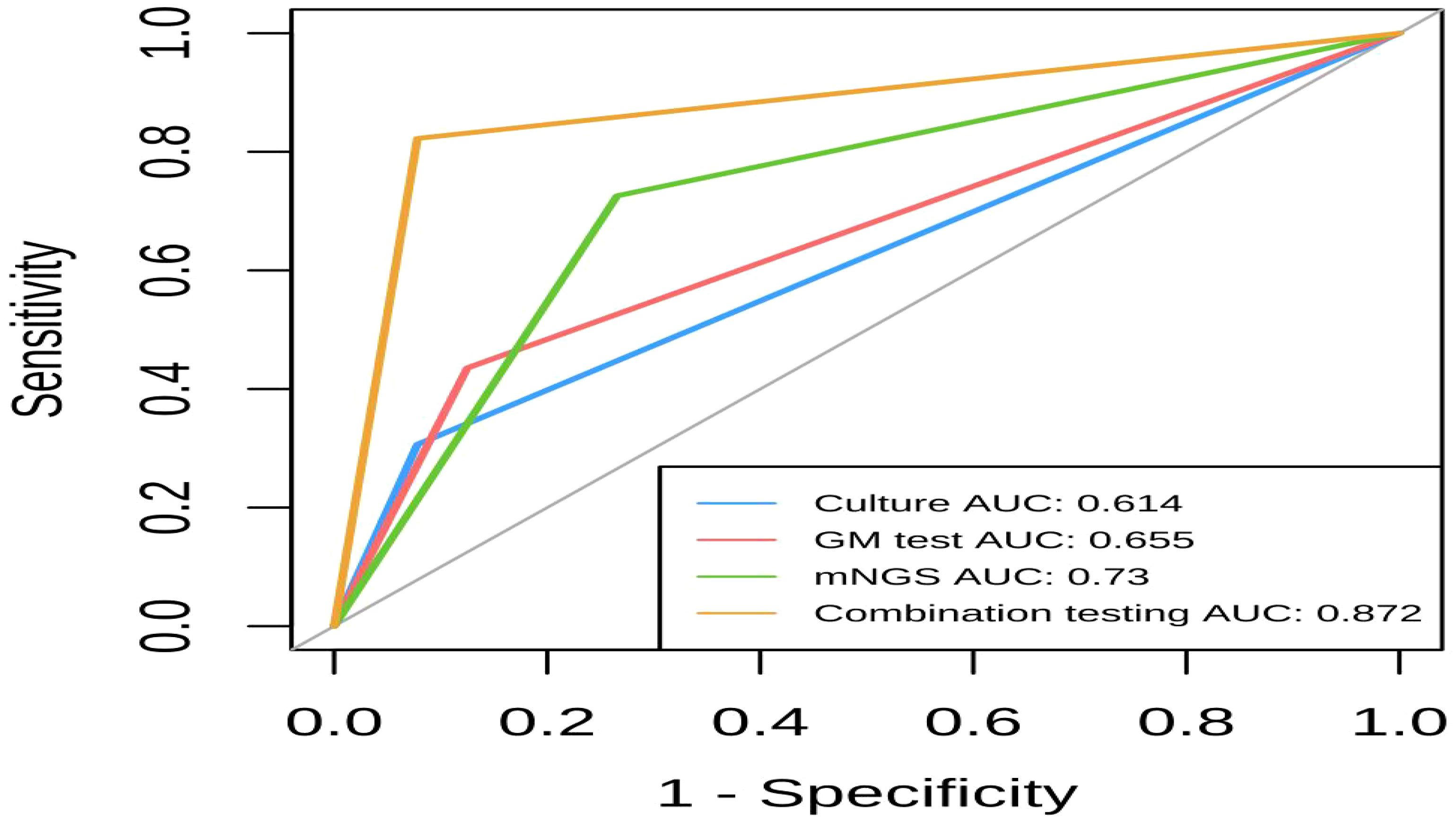
The area under the ROC curves (AUC) for combined detection, mNGS, GM, and conventional culture were 0.872, 0.73, 0.655, and 0.614, respectively. This suggests that mNGS has a higher diagnostic value for IPA compared to GM and culture alone, and combination testing has the highest diagnostic value for IPA. Figure 4 illustrates the comparative diagnostic efficacy of mNGS, fungal culture, GM test (BALF), and combination testing.
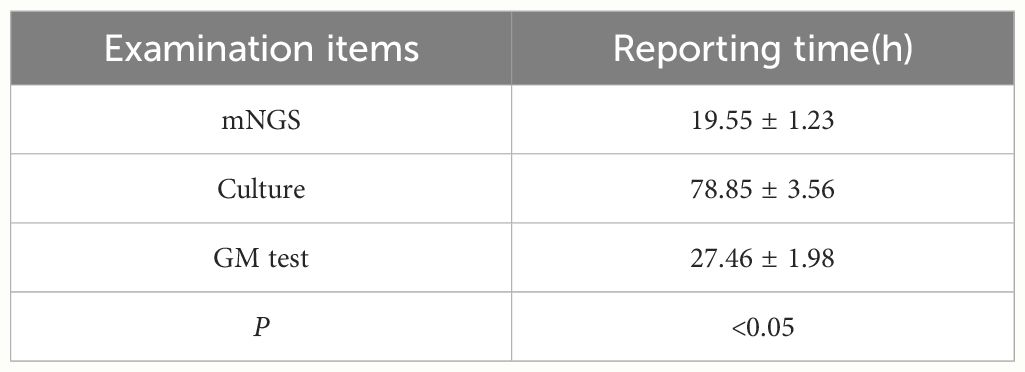
Comparison of detection time
The detection time of mNGS proved shorter compared to traditional fungal culture and GM test (P < 0.05). Table 4 compares the detection times of mNGS, fungal culture, and GM testing.
The difference was significant between culture and mNGS (P < 0.05).
The difference was significant between BALF GM test and culture (P < 0.05).
Discussion
Aspergillus is widespread in nature and can be found in moldy grains, feed, water, soil, clothing, and furniture. For healthy individuals with normal immune function, ciliary movement in the airways, and phagocytosis by immune cells effectively eliminate inhaled Aspergillus fungi (Yang et al., 2022). Despite the daily inhalation of Aspergillus spores, Aspergillus infections are rare. On the contrary, for individuals receiving immunosuppressive therapy, neutropenic following bone marrow transplantation, or undergoing chemotherapy, these individuals have weakened immune function, making them susceptible to IPA.
Recently, patients with AECOPD in the ICU have been identified as a potential high-risk population for IPA. On the one hand, declining lung function, prolonged exposure to chronic hypoxia, reduced quantity of airway epithelial cilia, and impaired ciliary motility diminish the airway’s defense and clearance mechanisms against Aspergillus, facilitating Aspergillus colonization (Niu and Zhao, 2022). On the other hand, the use of corticosteroids and antibiotics, mechanical ventilation, central venous catheterization, inadequate nutritional intake, and other measures heighten the risk of developing IPA (Chen et al., 2022).
Currently, in all patients with AECOPD, particularly those admitted to the ICU, suspicion of IPA should be raised when presenting with fever refractory to antibiotic treatment, anomalous chest CT scans, and evidence of fungal elements in respiratory samples, AECOPD complicated by IPA is associated with a mortality rate exceeding 50%, emphasizing the crucial importance of early and accurate diagnosis. However, owing to the lack of specific clinical manifestations and precise early diagnostic tools, discerning IPA from bacterial infections, other fungal infections, and malignancies can prove challenging at times (Guinea et al., 2010).
Presently, traditional methods for detecting Aspergillus encompass fungal culture, the G test, the GM test, and antibody detection techniques. Fungal culture serves as the fundamental diagnostic method for IPA, enabling species identification and in vitro drug sensitivity testing of fungal pathogens. Conventional microbial culture relies on the growth rate of live microorganisms, with the culture period for common bacterial pathogens typically requiring at least 8 hours and for fungi at least 5 days (Molinos-Castro et al., 2020). In contrast, mNGS provides test results within 24 hours of sample collection, significantly shortening the detection time. According to various studies, the positive rate of fungal culture in respiratory samples from patients with IPA varies from 11.8% to 50% (Hammond et al., 2020). However, Aspergillus colonization in the airways may lead to false-negative or false-positive results. In this study, the sensitivity of respiratory sample culture for diagnosing IPA was only 29%, significantly lower than that of mNGS. GM, present on the cell walls of various fungi, is released into the blood and other body fluids during hyphal growth and is commonly utilized for early screening and continuous monitoring of Aspergillus infections. GM serves as a reliable serum marker for clinical adjunctive diagnosis of IPA. Continuous monitoring of serum GM levels in high-risk patients, such as COPD, tumors, and organ transplant recipients, facilitates the early identification and recognition of infections (Everaerts et al., 2018; Diao et al., 2022). In immunocompetent patients, neutrophils impede Aspergillus entry into the bloodstream and can engulf GM antigens, thereby diminishing the sensitivity of serum GM testing (Huang et al., 2020). A study revealed that in patients lacking neutropenia, the sensitivity of BALF GM detection reaches 87%, whereas serum GM detection yields a sensitivity of only 42% (Jenks et al., 2020).Our experiment suggests that the sensitivity of BALF GM testing (41.9%) for diagnosing IPA surpasses that of serum GM testing (35.4%), with no statistically significant difference between the two groups, possibly due to prior antifungal therapy received by the patients.
Additionally, the definitive diagnosis of IPA necessitates a lung biopsy demonstrating invasive growth and yielding positive culture (Xie et al., 2019). However, conducting such biopsies in ICU patients with AECOPD often poses significant risks due to hemodynamic and respiratory instability, along with potential coagulation disorders (Miao et al., 2018; Chen et al., 2021). Therefore, clinicians frequently encounter challenges in obtaining adequate biopsy samples from the infection site, thereby amplifying the risk of false-negative results (Wu et al., 2020).
In such circumstances, the importance of developing and implementing faster and more accurate detection methods is highlighted, mNGS technology demonstrates unique advantages (Zhu et al., 2023). mNGS, a high-throughput DNA sequencing technique, enables rapid and comprehensive pathogen detection without requiring primers or probes. It identifies the genomes of all microorganisms, including bacteria, viruses, and fungi, irrespective of prior knowledge of microbial species in the sample (Zhan et al., 2021). This renders mNGS advantageous in diagnosing complex infections. It is undeniable that NGS has received considerable attention in the field of respiratory infections and fundamentally transformed our comprehension of the airway (Kim et al., 2021).
However, despite numerous studies reporting the clinical applications of mNGS, most of them have summarized retrospective analyses of various patient populations. Moreover, there are fewer reports on the utilization of mNGS in early diagnosis of IPA. Some studies have shown that in patients with neutropenia, the sensitivity of mNGS for diagnosing IPA in BALF is 92.31%, higher than traditional detection methods or GM testing. The AUC of BALF mNGS is 0.925, indicating good performance. In the diagnosis of invasive fungal infections (IFIs), the ROC curve indicates that the performance of mNGS (particularly lg(RPKM)) surpasses that of CMTs (Deng et al., 2023; Jia et al., 2023).In this study, we collected data from patients with AECOPD admitted to the ICU. Most of these patients exhibited characteristics such as long-term use of antibiotics and steroids, mechanical ventilation, central venous catheterization, and risk of malnutrition, all of which increase the risk of invasive aspergillosis infection. Through analysis, the diagnostic sensitivity of BALF mNGS was found to be 70.9%, higher than that of the BALF GM test (41.9%), serum GM test (35.4%) and traditional fungal culture (29.0%). BALF mNGS exhibited higher sensitivity and a shorter detection time, showing promise for improving treatment effectiveness in patients with AECOPD, however, its specificity falls below that of conventional culture methods.
This study also has some limitations. Firstly, it is a single-center study with a small sample size, potentially limiting its generalizability to a broader population. Consequently, the findings primarily reflect the detection performance of mNGS within a subset of patients from a single hospital (Wang et al., 2022; Shi et al., 2023). Future research should aim for multicenter mNGS testing to address the insufficient sample size. Secondly, the interpretation of mNGS results lacks a universally recognized standard, necessitating physicians to integrate patients’ medical histories, clinical presentations, and other laboratory test results to arrive at a final clinical diagnosis. This process often entails subjective judgment. Moreover, the high cost associated with mNGS limits its widespread implementation and imposes a significant financial burden on patients to some extent.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The Ethics Committee of Xinxiang City Central Hospital, Henan Province, China. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Since retrospective studies primarily involve the utilization of existing data or records without actively intervening with patients or collecting new data, the requirement for written informed consent is typically waived. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article because Since retrospective studies primarily involve the utilization of existing data or records without actively intervening with patients or collecting new data, the requirement for written informed consent is typically waived.
Author contributions
SN: Writing – original draft, Writing – review & editing. DL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. YY: Writing – original draft. LZ: Writing – original draft, Conceptualization, Data curation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by China International Medical Exchange Foundation (Grant No. Z-2018-35-2101).
Acknowledgments
We thank the patients for cooperating with our investigation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ader, F., Nseir, S., Le Berre, R., Leroy, S., Tillie-Leblond, I., Marquette, C. H., et al. (2005). Invasive pulmonary aspergillosis in chronic obstructive pulmonary disease. An emerging fungal pathogen. Clin. Microbiol. Infect. 11, 427–429. doi: 10.1111/j.1469–0691.2005.01143.x
Bao, S., Song, H., Chen, Y., Zhong, C., Tang, H. (2022). Metagenomic next generation sequencing for the diagnosis of: A retrospective study. Front. Cell.Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.925982
Chen, J., Zhao, Y., Shang, Y., Lin, Z., Xu, G., Bai, B., et al. (2021). The clinical significance of simultaneous detection of pathogens from bronchoalveolar lavage fluid and blood samples by metagenomic next-generation sequencing in patients with severe pneumonia. J. Med. Microbiol. 70(1), 1–8. doi: 10.1099/jmm.0.001259
Chen, S., Kang, Y., Li, D., Li, Z. (2022). Diagnostic performance of metagenomic next-generation sequencing for the detection of pathogens in bronchoalveolar lavage fluid in patients with pulmonary infections: Systematic review and meta-analysis. Int. J.Infect. Dis. 122, 867–873. doi: 10.1016/j.ijid.2022.07.054
Deng, Z., Tang, Y., Tu, Y., Liu, M., Cheng, Q., Zhang, J., et al. (2023). BALF metagenomic next-generation sequencing analysis in hematological Malignancy patients with suspected pulmonary infection: clinical significance of negative results. Front. Med. (Lausanne). 10. doi: 10.3389/fmed.2023.1195629
Denning, D. W. (2015). The ambitious ‘95–95 by 2025’ roadmap for the diagnosis and management of fungal diseases. Thorax 70, 613–614. doi: 10.1136/thoraxjnl-2015–207305
Diao, Z., Han, D., Zhang, R., Li, J. (2022). Metagenomics next-generation sequencing tests take the stage in the diagnosis of lower respiratory tract infections. J. Adv. Res. 38, 201–212. doi: 10.1016/j.jare.2021.09.012
Everaerts, S., Lagrou, K., Vermeersch, K., Dupont, L. J., Vanaudenaerde, B. M., Janssens, W. (2018). Aspergillus fumigatus detection and risk factors in patients with COPD–bronchiectasis overlap. Int. J. Mol. Sci. 19, 523. doi: 10.3390/ijms19020523
Guinea, J., Torres-Narbona, M., Gijón, P., Mun˜oz, P., Pozo, F., Peláez, T., et al. (2010). Pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: Incidence, risk factors and outcome. Clin. Microbiol. Infect. 16, 870–877. doi: 10.1111/j.1469-0691.2009.03015.x
Hammond, E. E., McDonald, C. S., Vestbo, J., Denning, D. W. (2020). Theglobal impact of Aspergillus infection on COPD. BMC Pulm. Med. 20, 241. doi: 10.1186/s12890-020-01259-8
Huang, L. N., He, H. Y., Jin, J. J., et al. (2017). Is Bulpa criteria suitable for the diagnosis of probable invasive pulmonary Aspergillosis in critically ill patients with chronic obstructive pulmonary disease? A comparative study with EORTC/MSG and ICU criteria. BMC Infect. Dis. 17(1), 209. doi: 10.1186/s12879–017-2307-y
Huang, J., Jiang, E., Yang, D., Wei, J., Zhao, M., Feng, J., et al. (2020). Metagenomic next-generation sequencing versus traditional pathogen detection in the diagnosis of peripheral pulmonary infectious lesions. Infect. Drug Resistance. 13, 567–576. doi: 10.2147/IDR.S235182
Jenks, J. D., Miceli, M. H., Prattes, J., Mercier, T., Hoenigl, M. (2020). The aspergillus lateral flow assay for the diagnosis of invasive aspergillosis: an update. Curr. Fungal Infect. Rep. 14, 378–383. doi: 10.1007/s12281–020-00409-z
Jia, H., Liu, H., Tu, M., Wang, Y., Wang, X., Li, J., et al. (2023). Diagnostic efficacy of metagenomic next generation sequencing in bronchoalveolar lavage fluid for proven invasive pulmonary aspergillosis. Front. Cell Infect. Microbiol. 13. doi: 10.3389/fcimb.2023.1223576
Kim, S., Min, J., Cho, J., Kang, H., Yang, B., Shin, Y., et al. (2021). Clinical profiles and outcomes of pulmonary tuberculosis patients with delayed treatment at a tertiary hospital in South Korea. Ann. Palliat. Med. 10, 2948–2957. doi: 10.21037/apm-20–1521
Miao, Q., Ma, Y. Y., Wang, Q. Q. (2018). Microbiological diagnostic performance of metagenomic next-generation sequencing whenapplied to clinical practice. Clin. Infect. Dis. 67, S231–S240. doi: 10.1093/cid/ciy693
Molinos-Castro, S., Pesqueira-Fontán, P. M., Rodríguez-Fernández, S., Rodríguez-Framil, M., Barbeito-Castiñeiras, G., Carmen Gayol-Fernández, M., et al. (2020). Clinical factors associated with pulmonary aspergillosis in patients with chronic obstructive pulmonary disease. Enferm. Infecc. Microbiol. Clin. (England. Ed). 38, 4–10. doi: 10.1016/j.eimc.2019.06.007
Niu, S., Zhao, L. (2022). Metagenomic next-generation sequencing clinches the diagnosis of Legionella pneumonia in a patient with acute myeloid leukemia: A case report and literature review. Front. Cell. Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.924597
Otu, A., Kosmidis, C., Mathioudakis, A. G., Ibe, C., Denning, D. W. (2023). The clinical spectrum of aspergillosis in chronic obstructive pulmonary disease. Infection 51, 813–829. doi: 10.1007/s15010–022-01960–2
Russo, A., Falcone, M., Vena, A., et al. (2011). Invasive pulmonary aspergillosis in non-neutropenic patients. Analysis of a 14-month prospective clinical experience. J. Chemother. 23(5), 290–294. doi: 10.1179/joc.2011.23.5.290
Shi, Y., Peng, J.-M., Hu, X.-Y., Yang, Q.-W., Wang, Y. (2023). Metagenomic next-generation sequencing for detecting Aspergillosis pneumonia in immunocompromised patients: a retrospective study. Front. Cell Infect. Microbiol. 13. doi: 10.3389/fcimb.2023.1209724
Tiew, P. Y., Thng, K. X., Chotirmall, S. H. (2022). Clinical aspergillus signatures in COPD and bronchiectasis. J. Fungi. (Basel). 8, 480. doi: 10.3390/jof8050480
Wang, C., You, Z., Fu, J., Chen, S., Bai, D., Zhao, H., et al. (2022). Application of metagenomic next-generation sequencing in the diagnosis of pulmonary invasive fungal disease. Front. Cell Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.949505
Wu, X. D., Li, Y. Y., Zhang, M. (2020). Etiology of severe community-acquired pneumonia in adults based on metagenomic next-generation sequencing: a prospective multicenter study. Infect. Dis. Ther. 9, 1003–1015. doi: 10.1007/s40121-020-00353-y
Xie, Y., Du, J., Jin, W., Teng, X., Cheng, R., Huang, P., et al. (2019). Next generation sequencing for diagnosis of severe pneumonia: China, 2010–2018. J. Infect. 78, 158–169. doi: 10.1016/j.jinf.2018.09.004
Yang, A., Wang, C., Chen, P., Zheng, G., Zhao, Z., Liu, J., et al. (2022). Diagnosis by metagenomic next-generation sequencing of invasive pulmonary aspergillosis in an infant with chronic granulomatous disease. Respir. Med. Case Rep. 41, 101792. doi: 10.1016/j.rmcr.2022.101792
Zantah, M., Dotan, Y., Dass, C., Zhao, H., Marchetti, N., Criner, G. J. (2020). Acute exacerbations of COPD versus IPF in patients with combined pulmonary fibrosis and emphysema. Respir. Res. 21, 164. doi: 10.1186/s12931–020-01432-x
Zhan, Y., Xu, T., He, F., Guan, W., Li, Z., Li, S., et al. (2021). Clinical evaluation of a metagenomics-based assay for pneumonia management [J]. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.751073
Keywords: invasive pulmonary aspergillosis, diagnosis, acute exacerbation of chronic obstructive pulmonary disease, metagenomic next-generation sequencing, traditional tests, GM test
Citation: Niu S, Liu D, Yang Y and Zhao L (2024) Clinical utility of metagenomic next-generation sequencing in the diagnosis of invasive pulmonary aspergillosis in acute exacerbation of chronic obstructive pulmonary disease patients in the intensive care unit. Front. Cell. Infect. Microbiol. 14:1397733. doi: 10.3389/fcimb.2024.1397733
Received: 08 March 2024; Accepted: 20 June 2024;
Published: 12 July 2024.
Edited by:
Beiwen Zheng, Zhejiang University, ChinaReviewed by:
Huaping Tang, Qingdao Municipal Hospital, ChinaYi Jiang, First Hospital of Shanxi Medical University, China
Copyright © 2024 Niu, Liu, Yang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Limin Zhao, emxtOTg5OEAxMjYuY29t
 Siqiang Niu
Siqiang Niu Dezhi Liu3
Dezhi Liu3