- 1Inner Mongolia Key Laboratory of Disease-Related Biomarkers, The Second Affiliated Hospital, Baotou Medical College, Baotou, China
- 2School of Basic Medicine, Baotou Medical College, Baotou, China
- 3The College of Medical Technology, Shanghai University of Medicine & Health Sciences, Shanghai, China
Brucella consists of gram-negative bacteria that have the ability to invade and replicate in professional and non-professional phagocytes, and its prolonged persistence in the host leads to brucellosis, a serious zoonosis. Toll-like receptors (TLRs) are the best-known sensors of microorganisms implicated in the regulation of innate and adaptive immunity. In particular, TLRs are transmembrane proteins with a typical structure of an extracellular leucine-rich repeat (LRR) region and an intracellular Toll/interleukin-1 receptor (TIR) domain. In this review, we discuss Brucella infection and the aspects of host immune responses induced by pathogens. Furthermore, we summarize the roles of TLRs in Brucella infection, with substantial emphasis on the molecular insights into its mechanisms of action.
1 Introduction
Brucellosis is a classical bacterial zoonosis found worldwide (de Figueiredo et al., 2015). Based on clinical presentation, brucellosis can be classified as acute, subacute, or chronic. Although the mortality rate of brucellosis is low, it can cause chronic complications or even disabilities, which severely endanger human health (Willems et al., 2022).
Brucellosis is caused by Brucella infection. Brucella, a facultative intracellular pathogen belonging to the phylum Proteobacteria, was discovered by David Bruce in 1887 (Głowacka et al., 2018). Originally, Brucella was thought to contain three species: Brucella abortus, Brucella melitensis, and Brucella suis. Presently, more species have been identified in both domesticated and wildlife species, such as Brucella ceti, Brucella canis, Brucella ovis, Brucella neotomae, and Brucella microti. It has been reported that some Brucella species can be further classified into biovars. For example, B. abortus has nine biovars, and B. suis contains five biovars (Boschiroli et al., 2001).
Brucella contains various virulence factors. The type IV secretion system (T4SS) is highly conserved among Brucella species and is a major virulence factor. Additionally, lipopolysaccharide (LPS) is identified as a key pathogenicity determinant of Brucella. Furthermore, previous reports have claimed that Brucella virulence factor A (BvfA), base excision repair (BER), and BvrR/BvrS system are also essential for virulence (Boschiroli et al., 2001). It has been proven that virulence factors play key roles in chronic persistence of Brucella.
2 Brucella infection
Brucella can infect the host by inhalation or ingestion, through genital mucosa, and even via injured skin. In general, Brucella rapidly translocates in the mucosal epithelium to the body, where it is efficiently endocytosed by main target cells including mucosal macrophages and dendritic cells (DCs) and subsequently invades and survives within specialized phagocytes (such as macrophages) instead of non-specialized phagocytes (such as epithelial cells), circumventing and modulating the host’s immune responses (Dominguez-Flores et al., 2023). Recently, researchers investigated the molecular mechanisms of chronic Brucella infections using non-specialized phagocyte HeLa cells. The results showed that Brucella was able to inhibit phagosome–lysosome fusion and replicate in a different compartment (Kim et al., 2004).
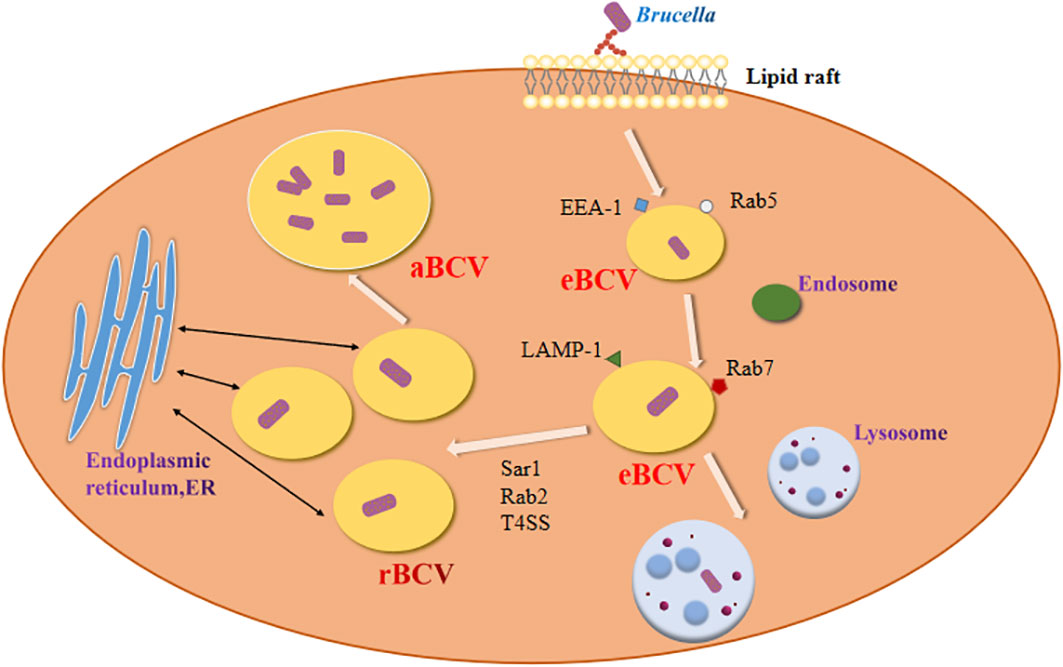
Four steps must be taken for Brucella to infect a host: adhesion, cellular internalization, intracellular growth, and transmission (Parihar et al., 2019). A number of adhesins have been identified in Brucella, such as the bacterial Ig-like (Blg-like) domain-containing proteins, the monomeric autotransporters, Bp26, the trimeric autotransporters, the sialic acid-binding proteins, and T4SS-VirB5 (Bialer et al., 2020). These bacterial cell surface proteins enable Brucella to bind to host cell surfaces. Once Brucella binds to the cell surface, it can be tested by adhering to a variety of cellular receptors. Fc gamma receptor IIa (Hashemi et al., 2007) and complement receptor 3 (CR3) (Gamazo et al., 2006), the conditioning receptors, recognize O-chain fragments of Brucella LPS. Non-conditioning receptors include the class A scavenger receptor (SR-A) and Toll-like receptors (TLRs). Compared to conditioning receptors, these receptors have a wide spectrum of biological roles because of their broad ligand-binding capacity. SR-A recognizes lipid A LPS (Kim et al., 2004). TLR2, TLR4, and TLR6 detect Brucella LPS and lipoproteins, whereas TLR3, TLR7, and TLR9 can recognize nucleic acid motifs (Oliveira et al., 2008). After detecting Brucella through the specific receptors, the host cell activates a signaling pathway that leads to the polymerization of actin filaments (Kusumawati et al., 2000). Brucella recognizes lipid rafts contained in the host cell membrane, contributing to the intracellular transport of Brucella.
Brucella enters host macrophages and forms replicative phagosomes called Brucella-containing vacuole (BCV) (Figure 1). Early BCV was defined as endosome-like BCV (eBCV), which obtains several marker molecules of the host. As eBCV matures, eBCV loses early endosomal markers and acquires late endosomal and lysosome-recognized marker molecules, which facilitates the fusion of eBCV with lysosomes (Celli, 2019). A proportion of the eBCV escapes lysosomal degradation and reaches the endoplasmic reticulum (ER) (de Bolle et al., 2012), where it then fuses with the ER to generate a replication-permissive BCV (rBCV) (Miller et al., 2017). Brucella then proliferates in rBCV. During the late period of bacterial infection, rBCV contains large amounts of Brucella and converts to autophagic BCV (aBCV) (Starr et al., 2012). Then, aBCV releases pathogens by both cleavage and non-cleavage mechanisms, and the Brucella intracellular life cycle ends (Smith et al., 2016).
3 Brucella infection elicits immune responses in the host
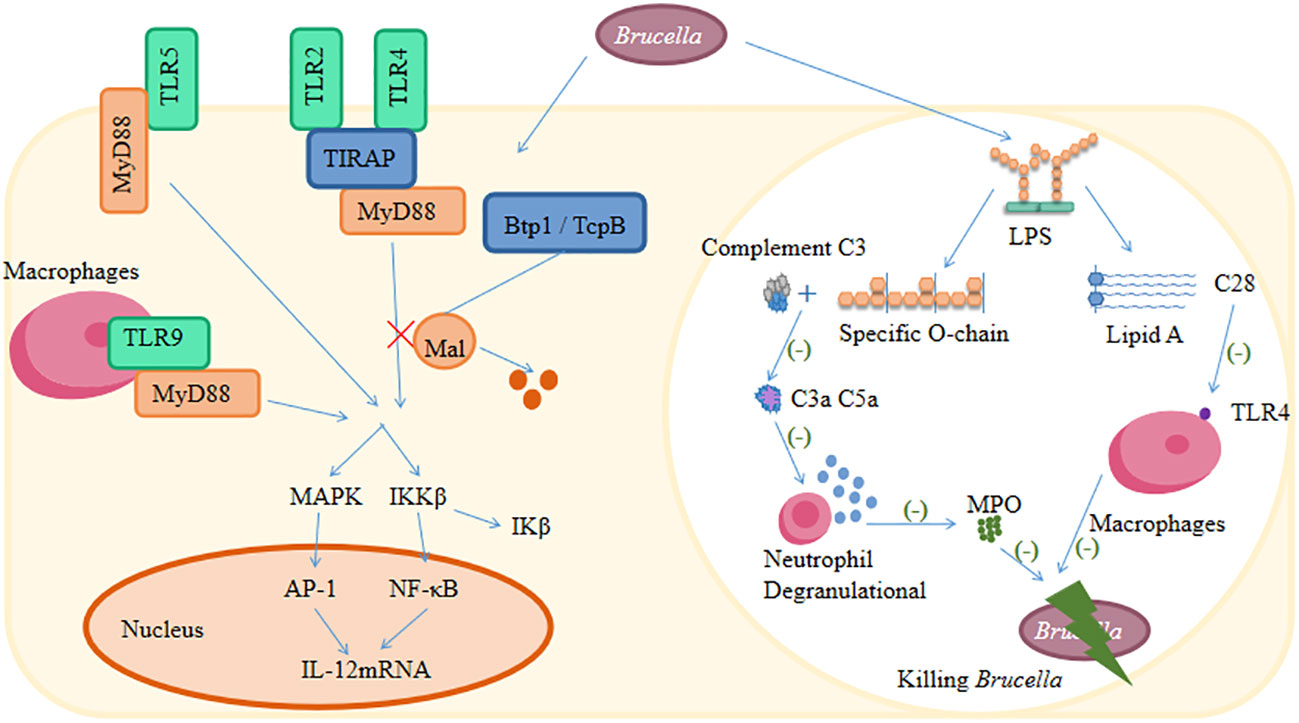
Brucella infection activates innate immunity and subsequently leads to the activation of adaptive immunity (Skendros and Boura, 2013). The innate immune response system constitutes the body’s first line of defense mechanisms that protect the host from pathogen invasion. Innate immune sensing of Brucella via pattern-recognition receptors (PRRs) that recognize specific molecular motifs called pathogen-associated molecular patterns (PAMPs) (Skendros et al., 2011; Sadeghi et al., 2023). After PRR recognition of the specific PAMP, some PRRs trigger intracellular signaling in the antigen-presenting cells (macrophages and DCs) and elicit an inflammatory response that effectively destroys the invading pathogen (Golding et al., 2001). The innate immune response against Brucella involves PRRs, such as TLRs and Nod-like receptors (NLRs) (Cerqueira et al., 2018). Among the functionally unique class of PRRs, TLRs are optimally characterized and have been intensively studied for their ability to specifically recognize distinct PAMPs from different pathogens (Sadeghi et al., 2023). Recognition of PAMPs by TLRs initiates multiple intracellular signaling cascades mediated by adapter molecules, such as myeloid differentiation factor 88 (MyD88) and Toll/interleukin-1 receptor (TIR) domain-containing adapter-inducing interferon-beta (IFN-beta) (TRIF), leading to the activation of inflammatory factors and the upregulation of co-stimulatory molecules (Sadeghi et al., 2023). For example, TLRs (except TLR3) bind to MyD88 and initiate cellular signaling, resulting in the activation of the IκB kinase complex and p38 mitogen-activated protein kinase (MAPK) (Gomes et al., 2012; Dimitrakopoulos et al., 2013). These pathways lead to the activation of the transcription factors NF-κB (Hop et al., 2017; Peng et al., 2021) and AP-1 (Jiménez de Bagüés et al., 2005), leading to the secretion of proinflammatory cytokines (Baldi and Giambartolomei, 2013). Common PAMPs include numerous microbial products, such as bacterial LPS, peptidoglycan, lipoproteins, flagellin, and nucleic acids (Kumar et al., 2011).
The cell-mediated immune response is the body’s predominant way of fighting Brucella (Ali et al., 2023). This includes the activation of antigen-presenting cells such as macrophages and DCs. Macrophages are the frontline cells for defense against Brucella (Jacob et al., 2016), and they play a critical role in innate immunity by phagocytosis and degradation of invading microorganisms (Oliveira et al., 1998). DCs are recognized as one of the most important antigen-presenting cells for eliciting effective cellular immunity. Immature DCs capture and process antigens before migrating to secondary lymphoid organs and presenting specific major histocompatibility complex (MHC) antigens. Upon maturation, DCs express high levels of MHC and costimulatory molecules and improve antigen presentation. Cytokines produced by mature DCs enhance adaptive immunity (Billard et al., 2007; Fabrik et al., 2013; Avila-Calderón et al., 2020).
TLRs play a key role in linking pathogen recognition with the activation of innate immune response and are also essential for initiating the adaptive immune response (Surendran et al., 2012). TLR stimulation can activate the innate immune response through activation of NK cells, DCs, or macrophages and secretion of IFN-α, IFN-γ, and TNF-α (Baldwin and Winter, 1994; Huang et al., 2005; de Almeida et al., 2013). TLR stimulation can also activate adaptive immune responses by promoting cross-presentation, Th1 polarization, and induction of cytotoxic T cells (Arias et al., 2017; Bin Park et al., 2023). There is evidence that the immune responses against most pathogens require multiple TLRs rather than a single TLR (Murugan et al., 2023).
It is well known that Brucella has developed a wide range of strategies to evade both innate and adaptive immune responses (Martirosyan and Gorvel, 2013; Skendros and Boura, 2013; Jiao et al., 2021). The main escape mechanisms of Brucella against the host immune system are inhibition of the complement pathway and TLR signaling pathways, interference with antigen presentation, selective subversion of the autophagy pathway, inhibition of DC stimulation, inhibition of autophagic lysosomal fusion, and macrophage apoptosis (Figure 2) (Radhakrishnan and Splitter, 2010; Rana et al., 2013; Barrionuevo and Giambartolomei, 2019; Stranahan and Arenas-Gamboa, 2021). There are strategies for modifying the LPS to evade effective recognition by TLR4 strategies (Barquero-Calvo et al., 2015; Matamoros-Recio et al., 2023). Additionally, there are also strategies to encode various outer membrane proteins to facilitate their invasion and immunomodulation (Barrionuevo et al., 2008; Velásquez et al., 2017; Pasquevich et al., 2019). Moreover, microRNAs (miRNAs) have recently been found to play a crucial role in immune evasion mechanisms in brucellosis (Ahmed et al., 2016).
4 Brucella vaccines for humans
A limited number of antibiotics are effective against Brucella because of the intracellular lifestyle of these organisms. Therefore, there is a desire to develop a safe and efficacious vaccine for human brucellosis. In order to develop Brucella vaccines for humans, an understanding of the mechanisms of the adaptive immune response in brucellosis is required. In fact, CD4+, CD8+, and γδ T cells are stimulated to produce IFN-γ to reduce the intracellular survival of Brucella. Additionally, CD8+ and γδ T cytotoxic cells were capable of killing infected macrophages. Furthermore, Th1-type immune response occurs to promote phagocytosis of Brucella (Perkins et al., 2010). Several decades ago, various Brucella vaccines for humans have been studied, such as B. abortus S19 (Vershilova, 1961), B. melitensis Rev.1 (Spink et al., 1962), B. abortus strain 19BA, and B. melitensis 104M (Perkins et al., 2010). However, these vaccines were highly reactogenic or caused brucellosis in humans and were considered unsuitable for human vaccination. Up to now, several human brucellosis vaccine candidates have been reported, including live attenuated vaccines, subunit vaccines, recombinant protein-based vaccines, vectored vaccines, and DNA vaccines (Perkins et al., 2010). An ideal vaccine for use in humans would be considered for its safety, immunogenicity, and protective efficacy (Hashemzadeh et al., 2023). It is well known that the inclusion of adjuvants within vaccines can enhance vaccine-induced protection and thus offer an alternative approach to vaccine development. Interestingly, several TLRLs are now being selectively investigated for the development of novel vaccine adjuvants within vaccines (Duthie et al., 2011).
5 Role of TLRs in Brucella infection
TLRs are essential for activating the innate immune response and initiating adaptive immunity (Fitzgerald and Kagan, 2020; Duan et al., 2022). TLRs are characterized by sharing an extracellular leucine-rich repeat (LRR) region and an intracellular TIR structural domain (Kawasaki and Kawai, 2014; Kornilov et al., 2023). The LRR structural domain is responsible for binding to PAMP, and the TIR structural domain is responsible for binding to TIR structural domains containing adapter molecules, including MyD88, TIR domain-containing adapter (TIRAP), TRIF, TRIF-related adapter molecule (TRAM), and sterile alpha and HEAT-Armadillo motifs (SARM), to initiate signaling (Brikos and O'Neill, 2008; Cui et al., 2014). MyD88 and TRIF can bind to the TLR, while TIRAP and TRAM act as bridging adapters, binding to MyD88 and TRIF, respectively (Takeda and Akira, 2004; West et al., 2006; Nilsen et al., 2023). SARM can only act as a negative regulator by interacting with TRIF (Carty and Bowie, 2019; Wang et al., 2021). The TLR is localized to the plasma membrane or endocytosis membranes. PAMP recognition of TLRs can initiate signaling and activate transcription factors, which in turn trigger the production of cytokines, chemokines, and antimicrobial peptides, ultimately controlling or clearing the infection (Chen et al., 2005; Sanjeewa et al., 2020).
Of the 13 TLRs identified in mammals (Vijay, 2018), TLR1 to TLR9 are conserved between humans and mice (Kumar and Barrett, 2022). TLR1, 2, 4, 5, and 6 are expressed in cell membranes. TLR3, 7, 8, and 9 are present in the endosomes of cells (Luchner et al., 2021; Fore et al., 2022). TLR2 recognizes a variety of microbial molecules such as peptidoglycan and lipoproteins (Venkataranganayaka Abhilasha and Kedihithlu Marathe, 2021), TLR4 recognizes LPS and several viral envelope proteins (Wu et al., 2022), TLR5 recognizes the flagellum (Clasen et al., 2023), and TLR3, 7, 8, and 9 recognize microbial and viral nucleic acid motifs (Eisenächer et al., 2007). Of these, TLR3 recognizes double-stranded RNA (Sakaniwa et al., 2023), TLR7/8 recognizes single-stranded RNA (Yu et al., 2012), and TLR9 recognizes CpG-DNA (Yu et al., 2017; Takano et al., 2023).
5.1 TLR2
The interaction of Brucella strains with TLR2 on host cells affects the induction of innate immune responses during infection (Giambartolomei et al., 2004; Macedo et al., 2008; Zwerdling et al., 2008; Pei et al., 2012; Surendran et al., 2012; Lee, K. M. et al., 2013; Weinhold et al., 2013; Ferrero et al., 2014; Arias et al., 2017; Dominguez-Flores et al., 2023). TLR2, located on the cell surface, is required for the production of tumor necrosis factor (TNF) (Huang et al., 2003; Delpino et al., 2012). Previous studies also showed that TLR2 is required for TNF production and regulates TLR9 signaling for the effective induction of IL-12 upon stimulation by heat-killed B. abortus (Zhang et al., 2012). In addition, recombinant Brucella cell-surface protein 31 (rBCSP31), an agonist of TLR2, induces cytokine production, upregulates macrophage function, and induces a Th1 immune response (Li et al., 2014). B. abortus Mdh enhanced Th2-related responses triggered by the MyD88-dependent TLR2 signaling pathway and could induce an inflammatory response in microfold cells (Shim et al., 2020). B. abortus-activated microglia induce neuronal death via Brucella lipoprotein-mediated TLR2 activation (Rodríguez et al., 2017).
Brucella uses various stealthy strategies to avoid activation of the innate immune system (Barquero-Calvo et al., 2007). Researchers identified a new Brucella protein Btp1, which down-modulates maturation of infected DCs by interfering with the TLR2 signaling pathway (Salcedo et al., 2008). Similarly, BtpB inhibits TLR2 and disrupts NLRP3 signaling pathways to inhibit host immune responses in early Brucella infections (Li, J. et al., 2022). Moreover, Brucella encodes a TIR domain-containing protein (TcpB) that mimics the properties of the TLR adaptor protein TIRAP to subvert TLR signaling (Radhakrishnan et al., 2009; Radhakrishnan and Splitter, 2010; Alaidarous et al., 2013; Jakka et al., 2017). Outer membrane vesicles (OMVs) from B. abortus also inhibit the cytokine response of monocytes to TLR2 agonists that favor the persistence of Brucella within host cells (Pollak et al., 2012). B. abortus utilizes its lipoproteins to inhibit IFN-γ-induced expression of the type I receptor for the Fc portion of IgG (FcγRI and CD64) and FcγRI-restricted phagocytosis via TLR2 and to subvert host immunonological responses (Barrionuevo et al., 2011).
5.2 TLR3
It has been shown that TLR3 signaling triggered by B. abortus RNA contributes to cytokine responses and type I IFN expression in mouse DCs, highlighting the important role of TLR3 in proinflammatory cytokine production induced by B. abortus infection (Campos et al., 2017). A previous study found that B. abortus also down-modulates TLR3 gene and dampens the type I IFN response, leading to inefficient immune response and bacterial persistence within the host (Gorvel et al., 2014).
5.3 TLR4
The interaction of Brucella with TLR4 on host cells affects the induction of the immune response, and TLR4 plays a role in resistance to Brucella infection (Campos et al., 2004; Dueñas et al., 2004; Copin et al., 2007; Martirosyan et al., 2012; Ma et al., 2015; Zhu et al., 2022). B. melitensis OMP25 interacted with ferritin heavy polypeptide 1 (FTH1) in human placenta trophoblastic cells (HPT−8) and led to the increase of the levels of TLR4 and inflammatory factors, suggesting that OMP25 serves an important role in intracellular parasitism of Brucella (Zhang et al., 2022). TLR4-linked Janus kinase 2 signaling plays a pivotal role in B. abortus phagocytosis by macrophages (Lee, J. J. et al., 2013). The cellular oncogene c-Fos also participates in host defense mechanisms against Brucella infection via TLR4 signaling (Hop et al., 2018). TLR4 agonists effectively stimulate innate immunity and enhance bacterial clearance in the mouse model of brucellosis (Hedges et al., 2023).
A recent study shows that TcpB from Brucella interferes with the MAL-TLR4 interaction. This in turn leads to suppressing host immune responses (Sengupta et al., 2010; Radhakrishnan et al., 2011; Alaidarous et al., 2014; Saqib and Baig, 2019). Brucella TcpB-derived decoy peptides (TB-8 and TB-9) also inhibited TLR4 signaling and avoided host immune recognition (Ke et al., 2016). Cytoplasmic linker protein 170 (CLIP170) was found to negatively regulate TLR4-mediated proinflammatory responses by targeting TIRAP (Jakka et al., 2018). Matamoros-Recio et al. elucidated the impact of the core oligosaccharides from α2-Proteobacteria atypical lipopolysaccharides for immune system evasion in opportunistic bacteria, including B. melitensis (Matamoros-Recio et al., 2023). B. abortus O-polysaccharide (OPS) also dictates the interactions between Brucella and TLR4 and enhances Brucella persistence (Pei et al., 2008).
The modified LPS with a defective core purified from Brucella carrying a mutated wadC gene potentiated cytokine secretion, representing a potential for vaccine development (Conde-Álvarez et al., 2012; Conde-Álvarez et al., 2013; Zhao et al., 2018). Based on the TLR4, some groups design the multi-epitope vaccine candidates against Brucella (Li, M. et al., 2022; Tarrahimofrad et al., 2022; Jalal et al., 2023; Malik et al., 2023; Yin et al., 2023). B. abortus Omp16 lipoprotein would be able to induce a protective immune response via a TLR4-dependent manner and is a promising self-adjuvanting vaccine against brucellosis (Pasquevich et al., 2010). The enzyme lumazine synthase from Brucella (BLS) can insert foreign peptides and proteins at the 10 N-termini. These chimeras induced proinflammatory cytokine secretion via TLR4, providing an excellent candidate for vaccine development (Berguer et al., 2006; Berguer et al., 2012).
5.4 TLR5
The lack of TLR5 activity of Brucella flagellin is part of the stealthy strategy of Brucella toward the innate immune system (Terwagne et al., 2013). Hiriart et al. generated a chimeric protein by fusing flagellin from Salmonella in the 10 N-termini of Brucella lumazine synthase (BLS). This fusion protein elicits the TLR5-mediated humoral response against BLS and could be exploited as a vaccine carrier/adjuvant (Hiriart et al., 2017).
5.5 TLR6
TLR6 is required to trigger innate immune responses against B. abortus in vivo through DC maturation and proinflammatory cytokine production (de Almeida LA et al., 2013). Retamal-Díaz et al. found that TLR2/6 agonist S-[2,3-bispalmitoyiloxy-(2R)-propyl]-R-cysteinyl-amido-monomethoxy polyethylene glycol (BPPcysMPEG) induced improved immunogenicity and protective efficacy of a DNA vaccine encoding B. abortus Cu,Zn superoxide dismutase (SOD) (Retamal-Díaz et al., 2014). Woodman et al. analyzed the structural characterization of TLR1 and TLR6 from both harbor and elephant seals, identifying variants that will help to understand species-specific immune responses (Woodman et al., 2016).
5.6 TLR7/8
TLR7 plays an important role in IL-12 production induced by B. abortus infection (Campos et al., 2017). Li et al. found that overexpression of melatonin synthetic enzyme acetylserotonin O-methyltransferase (ASMT) enhances the resistance of transgenic sheep to brucellosis by influencing, at least in part, the TLR7 signaling pathway (Li et al., 2021). Im et al. reported that the two B. abortus antigens, OMP19 and malate dehydrogenase (Mdh), might be involved in the TLR8 signaling pathway in human leukemic monocyte cells (Im et al., 2018). Previous reports showed that the infection of human monocytes/macrophages with B. abortus inhibits the IFN-γ-induced MHC-I surface expression by a TLR8-dependent mechanism. Thus, bacteria are able to persist and establish a chronic infection inside its host (Milillo et al., 2017).
5.7 TLR9
Signaling pathways triggered by Brucella DNA involve TLR9 (Vieira et al., 2013; Campos et al., 2014; Costa Franco et al., 2018). TLR9 recognized Brucella CpG motifs, leading to TLR9-MAPK/NF-κB signaling pathway activation and IL-12 and TNF-α production (Gomes et al., 2016). Brucella DNA can be sensed by TLR9 on the endosomal membrane of macrophages and can suppress Brucella intracellular replication by enhancing NO production (Liu et al., 2015). Copin et al. demonstrated that the induction of IFN-gamma and inducible NO synthase (iNOS) protein induced by Brucella infection required TLR4 and TLR9 stimulation (Copin et al., 2007; De Trez et al., 2009). Rahimnahal et al. developed a multi-epitope vaccine against bovine brucellosis, which is capable of being in interaction with bovine TLR4 and TLR9 (Rahimnahal et al., 2023). Our group found that repetitive extragenic palindromic DNA sequences from Brucella stimulate TLR9 signaling (Yu et al., 2017; Peng et al., 2021).
6 Conclusions
In conclusion, host TLRs play a key role in the induction of the innate immune and adaptive immune responses against Brucella infection. The development of related drugs and vaccines is of great significance for the treatment of brucellosis. However, the process of Brucella infection is complex, and the fight against Brucella infections still faces many challenges. First, Brucella has evolutionarily developed diverse strategies that allow evasion of the innate and adaptive immune systems to establish persistent infections. Second, there is no effective vaccine for human brucellosis. Despite significant progress in our understanding of the molecular mechanisms of TLRs in Brucella infection, further focused research is needed to clarify their roles in modulating various immune events against Brucella pathogens.
Author contributions
ZW: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing. HY: Funding acquisition, Supervision, Writing – original draft, Writing – review & editing. XG: Writing – original draft. DW: Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was support in part by grants from the National Natural Science Foundation of China (No. 82260092, 82170296 and 82060084) and Major Science and Technology Projects of Inner Mongolia Autonomous Region (No. 2021ZD0006).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmed, W., Zheng, K., Liu, Z. F. (2016). Establishment of chronic infection: Brucella's stealth strategy. Front. Cell Infect. Microbiol. 6, 30. doi: 10.3389/fcimb.2016.00030
Alaidarous, M., Ve, T., Casey, L. W., Valkov, E., Ericsson, D. J., Ullah, M. O., et al. (2014). Mechanism of bacterial interference with TLR4 signaling by Brucella Toll/interleukin-1 receptor domain-containing protein TcpB. J. Biol. Chem. 289 (2), 654–668. doi: 10.1074/jbc.M113.523274
Alaidarous, M., Ve, T., Ullah, M. O., Valkov, E., Mansell, A., Schembri, M. A., et al. (2013). Cloning, expression, purification, crystallization and preliminary X-ray crystallographic analysis of the TIR domain from the Brucella melitensis TIR-domain-containing protein TcpB. Acta Crystallogr. Sect F Struct. Biol. Cryst Commun. 69 (Pt 10), 1167–1170. doi: 10.1107/S1744309113024408
Ali, A., Waris, A., Khan, M. A., Asim, M., Khan, A. U., Khan, S., et al. (2023). Recent advancement, immune responses, and mechanism of action of various vaccines against intracellular bacterial infections. Life Sci. 314, 121332. doi: 10.1016/j.lfs.2022.121332
Arias, M. A., Santiago, L., Costas-Ramon, S., Jaime-Sánchez, P., Freudenberg, M., Jiménez De Bagüés, M. P., et al. (2017). Toll-Like Receptors 2 and 4 cooperate in the control of the emerging pathogen Brucella microti. Front. Cell Infect. Microbiol. 6. doi: 10.3389/fcimb.2016.00205
Avila-Calderón, E. D., Flores-Romo, L., Sharon, W., Donis-Maturano, L., Becerril-García, M. A., Arreola, M. G. A., et al. (2020). Dendritic cells and Brucella spp. interaction: the sentinel host and the stealthy pathogen. Folia Microbiol. (Praha). 65 (1), 1–16. doi: 10.1007/s12223-019-00691-6
Baldi, P. C., Giambartolomei, G. H. (2013). Immunopathology of brucella infection. Recent Pat. Antiinfect. Drug Discovery 8 (1), 18–26. doi: 10.2174/1574891x11308010005
Barquero-Calvo, E., Chaves-Olarte, E., Weiss, D. S., Guzmán-Verri, C., Chacón-Díaz, C., Rucavado, A., et al. (2007). Brucella abortus uses a stealthy strategy to avoid activation of the innate immune system during the onset of infection. PloS One 2 (7), e631. doi: 10.1371/journal.pone.0000631
Barquero-Calvo, E., Mora-Cartín, R., Arce-Gorvel, V., de Diego, J. L., Chacón-Díaz, C., Chaves-Olarte, E., et al. (2015). Brucella abortus induces the premature death of human neutrophils through the action of its lipopolysaccharide. PloS Pathog. 11 (5), e1004853. doi: 10.1371/journal.ppat.1004853
Barrionuevo, P., Cassataro, J., Delpino, M. V., Zwerdling, A., Pasquevich, K. A., García Samartino, C., et al. (2008). Brucella abortus inhibits major histocompatibility complex class II expression and antigen processing through interleukin-6 secretion via Toll-like receptor 2. Infect. Immun. 76 (1), 250–262. doi: 10.1128/IAI.00949-07
Barrionuevo, P., Delpino, M. V., Velásquez, L. N., García Samartino, C., Coria, L. M., Ibañez, A. E., et al. (2011). Brucella abortus inhibits IFN-γ-induced FcγRI expression and FcγRI-restricted phagocytosis via toll-like receptor 2 on human monocytes/macrophages. Microbes Infect. 13 (3), 239–250. doi: 10.1016/j.micinf.2010.10.020
Barrionuevo, P., Giambartolomei, G. H. (2019). Inhibition of antigen presentation by Brucella: many more than many ways. Microbes Infect. 21 (3-4), 136–142. doi: 10.1016/j.micinf.2018.12.004
Berguer, P. M., Alzogaray, V. A., Rossi, A. H., Mundiñano, J., Piazzon, I., Goldbaum, F. A. (2012). A polymeric protein induces specific cytotoxicity in a TLR4 dependent manner in the absence of adjuvants. PloS One 7 (9), e45705. doi: 10.1371/journal.pone.0045705
Berguer, P. M., Mundiñano, J., Piazzon, I., Goldbaum, F. A. (2006). A polymeric bacterial protein activates dendritic cells via TLR4. J. Immunol. 176 (4), 2366–2372. doi: 10.4049/jimmunol.176.4.2366
Bialer, M. G., Sycz, G., Muñoz González, F., Ferrero, M. C., Baldi, P. C., Zorreguieta, A. (2020). Adhesins of Brucella: Their roles in the interaction with the host. Pathogens. 9 (11), 942. doi: 10.3390/pathogens9110942
Billard, E., Dornand, J., Gross, A. (2007). Interaction of Brucella suis and Brucella abortus rough strains with human dendritic cells. Infect. Immun. 75 (12), 5916–5923. doi: 10.1128/IAI.00931-07
Bin Park, W., Kim, S., Kyung, S. M., Lee, E. S., Lee, Y. J., Yoo, H. S. (2023). Gene expression of Toll-like receptors, cytokines and a nuclear factor and cytokine secretion in DH82 canine macrophage cells infected with Brucella canis. Vet. Immunol. Immunopathol., 260. doi: 10.1016/j.vetimm.2023.110607
Boschiroli, M. L., Foulongne, V., O'Callaghan, D. (2001). Brucellosis: a worldwide zoonosis. Curr. Opin. Microbiol. 4 (1), 58–64. doi: 10.1016/s1369-5274(00)00165-x
Brikos, C., O'Neill, L. A. (2008). Signalling of toll-like receptors. Handb. Exp. Pharmacol. 183), 21–50. doi: 10.1007/978-3-540-72167-3_2
Campos, P. C., Gomes, M. T., Guimarães, G., Costa Franco, M. M., Marim, F. M., Oliveira, S. C. (2014). Brucella abortus DNA is a major bacterial agonist to activate the host innate immune system. Microbes Infect. 16 (12), 979–984. doi: 10.1016/j.micinf.2014.08.010
Campos, P. C., Gomes, M. T., Guimarães, E. S., Guimarães, G., Oliveira, S. C. (2017). TLR7 and TLR3 sense Brucella abortus RNA to induce proinflammatory cytokine production but they are dispensable for host control of infection. Front. Immunol. 8. doi: 10.3389/fimmu.2017.00028
Campos, M. A., Rosinham, G. M., Almeida, I. C., Salgueiro, X. S., Jarvis, B. W., Splitter, G. A., et al. (2004). Role of Toll-like receptor 4 in induction of cell-mediated immunity and resistance to Brucella abortus infection in mice. Infect. Immun. 72 (1), 176–186. doi: 10.1128/IAI.72.1.176-186.2004
Carty, M., Bowie, A. G. (2019). SARM: From immune regulator to cell executioner. Biochem. Pharmacol. 161, 52–62. doi: 10.1016/j.bcp.2019.01.005
Celli, J. (2019). The intracellular life cycle of Brucella spp. Microbiol. Spectr. 7 (2), 10. doi: 10.1128/microbiolspec.BAI-0006-2019
Cerqueira, D. M., Gomes, M. T. R., Silva, A. L. N., Rungue, M., Assis, N. R. G., Guimarães, E. S., et al. (2018). Guanylate-binding protein 5 licenses caspase-11 for Gasdermin-D mediated host resistance to Brucella abortus infection. PloS Pathog. 14 (12), e1007519. doi: 10.1371/journal.ppat.1007519
Chen, X. M., O'Hara, S. P., Nelson, J. B., Splinter, P. L., Small, A. J., Tietz, P. S., et al. (2005). Multiple TLRs are expressed in human cholangiocytes and mediate host epithelial defense responses to Cryptosporidium parvum via activation of NF-kappaB. J. Immunol. 175 (11), 7447–7456. doi: 10.4049/jimmunol.175.11.7447
Clasen, S. J., Bell, M. E. W., Borbón, A., Lee, D. H., Henseler, Z. M., de la Cuesta-Zuluaga, J., et al. (2023). Silent recognition of flagellins from human gut commensal bacteria by Toll-like receptor 5. Sci. Immunol. 8 (79), eabq7001. doi: 10.1126/sciimmunol.abq7001
Conde-Álvarez, R., Arce-Gorvel, V., Gil-Ramírez, Y., Iriarte, M., Grilló, M. J., Gorvel, J. P., et al. (2013). Lipopolysaccharide as a target for brucellosis vaccine design. Microb. Pathog. 58, 29–34. doi: 10.1016/j.micpath.2012.11.011
Conde-Álvarez, R., Arce-Gorvel, V., Iriarte, M., Manček-Keber, M., Barquero-Calvo, E., Palacios-Chaves, L., et al. (2012). The lipopolysaccharide core of Brucella abortus acts as a shield against innate immunity recognition. PloS Pathog. 8 (5), e1002675. doi: 10.1371/journal.ppat.1002675
Copin, R., De Baetselier, P., Carlier, Y., Letesson, J. J., Muraille, E. (2007). MyD88-dependent activation of B220-CD11b+LY-6C+ dendritic cells during Brucella melitensis infection. J. Immunol. 178 (8), 5182–5191. doi: 10.4049/jimmunol.178.8.5182
Costa Franco, M. M., Marim, F., Guimarães, E. S., Assis, N. R. G., Cerqueira, D. M., Alves-Silva, J., et al. (2018). Brucella abortus triggers a cGAS-independent STING pathway to induce host protection that involves guanylate-binding proteins and inflammasome activation. J. Immunol. 200 (2), 607–622. doi: 10.4049/jimmunol.1700725
Cui, J., Chen, Y., Wang, H. Y., Wang, R. F. (2014). Mechanisms and pathways of innate immune activation and regulation in health and cancer. Hum. Vaccin Immunother. 10 (11), 3270–3285. doi: 10.4161/21645515.2014.979640
de Almeida, L. A., Macedo, G. C., Marinho, F. A., Gomes, M. T., Corsetti, P. P., Silva, A. M., et al. (2013). Toll-like receptor 6 plays an important role in host innate resistance to Brucella abortus infection in mice. Infect. Immun. 81 (5), 1654–1662. doi: 10.1128/IAI.01356-12
de Bolle, X., Letesson, J. J., Gorvel, J. P. (2012). Small GTPases and Brucella entry into the endoplasmic reticulum. Biochem. Soc. Trans. 40 (6), 1348–1352. doi: 10.1042/BST20120156
de Figueiredo, P., Ficht, T. A., Rice-Ficht, A., Rossetti, C. A., Adams, L. G. (2015). Pathogenesis and immunobiology of brucellosis: review of Brucella-host interactions. Am. J. Pathol. 185 (6), 1505–1517. doi: 10.1016/j.ajpath.2015.03.003
Delpino, M. V., Barrionuevo, P., Macedo, G. C., Oliveira, S. C., Genaro, S. D., Scian, R., et al. (2012). Macrophage-elicited osteoclastogenesis in response to Brucella abortus infection requires TLR2/MyD88-dependent TNF-α production. J. Leukoc. Biol. 91 (2), 285–298. doi: 10.1189/jlb.04111185
De Trez, C., Magez, S., Akira, S., Ryffel, B., Carlier, Y., Muraille, E. (2009). iNOS-producing inflammatory dendritic cells constitute the major infected cell type during the chronic Leishmania major infection phase of C57BL/6 resistant mice. PloS Pathog. 5 (6), e1000494. doi: 10.1371/journal.ppat.1000494
Dimitrakopoulos, O., Liopeta, K., Dimitracopoulos, G., Paliogianni, F. (2013). Replication of Brucella melitensis inside primary human monocytes depends on mitogen activated protein kinase signaling. Microbes Infect. 15 (6-7), 450–460. doi: 10.1016/j.micinf.2013.04.007
Dominguez-Flores, A., Rodríguez López, G. M., Soria-Castro, R., López-Santiago, R., Rodríguez-Cortés, O., Pérez-Tapia, S. M., et al. (2023). Brucella abortus induces mast cell activation through TLR-2 and TLR-4. Microb. Pathog. 176, 106005. doi: 10.1016/j.micpath.2023.106005
Duan, T., Du, Y., Xing, C., Wang, H. Y., Wang, R. F. (2022). Toll-like receptor signaling and its role in cell-mediated immunity. Front. Immunol. 13. doi: 10.3389/fimmu.2022.812774
Dueñas, A. I., Orduña, A., Crespo, M. S., García-Rodríguez, C. (2004). Interaction of endotoxins with Toll-like receptor 4 correlates with their endotoxic potential and may explain the proinflammatory effect of Brucella spp. LPS. Int. Immunol. 16 (10), 1467–1475. doi: 10.1093/intimm/dxh148
Duthie, M. S., Windish, H. P., Fox, C. B., Reed, S. G. (2011). Use of defined TLR ligands as adjuvants within human vaccines. Immunol. Rev. 239 (1), 178–196. doi: 10.1111/j.1600-065X.2010.00978.x
Eisenächer, K., Steinberg, C., Reindl, W., Krug, A. (2007). The role of viral nucleic acid recognition in dendritic cells for innate and adaptive antiviral immunity. Immunobiology. 212 (9-10), 701–714. doi: 10.1016/j.imbio.2007.09.007
Fabrik, I., Härtlova, A., Rehulka, P., Stulik, J. (2013). Serving the new masters - dendritic cells as hosts for stealth intracellular bacteria. Cell Microbiol. 15 (9), 1473–1483. doi: 10.1111/cmi.12160
Ferrero, M. C., Hielpos, M. S., Carvalho, N. B., Barrionuevo, P., Corsetti, P. P., Giambartolomei, G. H., et al. (2014). Key role of Toll-like receptor 2 in the inflammatory response and major histocompatibility complex class ii downregulation in Brucella abortus-infected alveolar macrophages. Infect. Immun. 82 (2), 626–639. doi: 10.1128/IAI.01237-13
Fitzgerald, K. A., Kagan, J. C. (2020). Toll-like receptors and the control of immunity. Cell. 180 (6), 1044–1066. doi: 10.1016/j.cell.2020.02.041
Fore, F., Budipranama, M., Destiawan, R. A. (2022). TLR10 and its role in immunity. Handb. Exp. Pharmacol. 276, 161–174. doi: 10.1007/164_2021_541
Gamazo, C., Lecároz, M. C., Prior, S., Vitas, A. I., Campanero, M. A., Irache, J. M., et al. (2006). Chemical and biological factors in the control of Brucella and brucellosis. Curr. Drug Deliv. 3 (4), 359–365. doi: 10.2174/156720106778559038
Giambartolomei, G. H., Zwerdling, A., Cassataro, J., Bruno, L., Fossati, C. A., Philipp, M. T. (2004). Lipoproteins, not lipopolysaccharide, are the key mediators of the proinflammatory response elicited by heat-killed Brucella abortus. J. Immunol. 173 (7), 4635–4642. doi: 10.4049/jimmunol.173.7.4635
Głowacka, P., Żakowska, D., Naylo, R. K., Niemcewicz, M., Bielawska-Drózd, A. (2018). Brucella - virulence factors, pathogenesis and treatment. Pol. J. Microbiol. 67 (2), 151–161. doi: 10.21307/pjm-2018-029
Golding, B., Scott, D. E., Scharf, O., Huang, L. Y., Zaitseva, M., Lapham, C., et al. (2001). Immunity and protection against Brucella abortus. Microbes Infect. 3 (1), 43–48. doi: 10.1016/s1286-4579(00)01350-2
Gomes, M. T., Campos, P. C., de Almeida, L. A., Oliveira, F. S., Costa, M. M., Marim, F. M., et al. (2012). The role of innate immune signals in immunity to Brucella abortus. Front. Cell Infect. Microbiol. 2. doi: 10.3389/fcimb.2012.00130
Gomes, M. T., Campos, P. C., Pereira Gde, S., Bartholomeu, D. C., Splitter, G., Oliveira, S. C. (2016). TLR9 is required for MAPK/NF-κB activation but does not cooperate with TLR2 or TLR6 to induce host resistance to Brucella abortus. J. Leukoc. Biol. 99 (5), 771–780. doi: 10.1189/jlb.4A0815-346R
Gorvel, L., Textoris, J., Banchereau, R., Ben Amara, A., Tantibhedhyangkul, W., von Bargen, K., et al. (2014). Intracellular bacteria interfere with dendritic cell functions: role of the type I interferon pathway. PloS One 9 (6), e99420. doi: 10.1371/journal.pone.0099420
Hashemi, S. H., Hajilooi, M., Mamani, M., Jamal-Omidi, S. (2007). Fc gamma receptor IIa polymorphism in patients with brucellosis. Jpn J. Infect. Dis. 60 (4), 196–197.
Hashemzadeh, P., Nezhad, S. A., Khoshkhabar, H. (2023). Immunoinformatics analysis of Brucella melitensis to approach a suitable vaccine against brucellosis. J. Genet. Eng. Biotechnol. 21 (1), 152. doi: 10.1186/s43141-023-00614-6
Hedges, J. F., Snyder, D. T., Robison, A., Thompson, M. A., Aspelin, K., Plewa, J., et al. (2023). A TLR4 agonist liposome formulation effectively stimulates innate immunity and enhances protection from bacterial infection. Innate Immun. 29 (3-4), 45–57. doi: 10.1177/17534259231168725
Hiriart, Y., Rossi, A. H., Biedma, M. E., Errea, A. J., Moreno, G., Cayet, D., et al. (2017). Characterization of structural and immunological properties of a fusion protein between flagellin from Salmonella and lumazine synthase from Brucella. Protein Sci. 26 (5), 1049–1059. doi: 10.1002/pro.3151
Hop, H. T., Arayan, L. T., Huy, T. X. N., Reyes, A. W. B., Vu, S. H., Min, W., et al. (2018). The key role of c-Fos for immune regulation and bacterial dissemination in Brucella infected macrophage. Front. Cell Infect. Microbiol. 8. doi: 10.3389/fcimb.2018.00287
Hop, H. T., Reyes, A. W. B., Huy, T. X. N., Arayan, L. T., Min, W., Lee, H. J., et al. (2017). Activation of NF-kB-mediated TNF-induced antimicrobial immunity is required for the efficient Brucella abortus clearance in RAW 264.7 cells. Front. Cell Infect. Microbiol. 7. doi: 10.3389/fcimb.2017.00437
Huang, L. Y., Aliberti, J., Leifer, C. A., Segal, D. M., Sher, A., Golenbock, D. T., et al. (2003). Heat-killed Brucella abortus induces TNF and IL-12p40 by distinct MyD88-dependent pathways: TNF, unlike IL-12p40 secretion, is Toll-like receptor 2 dependent. J. Immunol. 171 (3), 1441–1446. doi: 10.4049/jimmunol.171.3.1441
Huang, L. Y., Ishii, K. J., Akira, S., Aliberti, J., Golding, B. (2005). Th1-like cytokine induction by heat-killed Brucella abortus is dependent on triggering of TLR9. J. Immunol. 175 (6), 3964–3970. doi: 10.4049/jimmunol.175.6.3964
Im, Y. B., Shim, S., Soh, S. H., Kim, S., Yoo, H. S. (2018). Cytokines production and toll-like receptors expression in human leukemic monocyte cells, THP-1, stimulated with Brucella abortus cellular antigens. Microb. Pathog. 122, 7–12. doi: 10.1016/j.micpath.2018.06.007
Jacob, J., Makou, P., Finke, A., Mielke, M. (2016). Inflammatory response of TLR4 deficient spleen macrophages (CRL 2471) to Brucella abortus S19 and an isogenic ΔmglA deletion mutant. Int. J. Med. Microbiol. 306 (3), 141–151. doi: 10.1016/j.ijmm.2016.02.006
Jakka, P., Bhargavi, B., Namani, S., Murugan, S., Splitter, G., Radhakrishnan, G. (2018). Cytoplasmic linker protein CLIP170 negatively regulates TLR4 signaling by targeting the TLR adaptor protein TIRAP. J. Immunol. 200 (2), 704–714. doi: 10.4049/jimmunol.1601559
Jakka, P., Namani, S., Murugan, S., Rai, N., Radhakrishnan, G. (2017). The Brucella effector protein TcpB induces degradation of inflammatory caspases and thereby subverts non-canonical inflammasome activation in macrophages. J. Biol. Chem. 292 (50), 20613–20627. doi: 10.1074/jbc.M117.815878
Jalal, K., Khan, K., Uddin, R. (2023). Immunoinformatic-guided designing of multi-epitope vaccine construct against Brucella Suis 1300. Immunol. Res. 71 (2), 247–266. doi: 10.1007/s12026-022-09346-0
Jiao, H., Zhou, Z., Li, B., Xiao, Y., Li, M., Zeng, H., et al. (2021). The mechanism of facultative intracellular parasitism of brucella. Int. J. Mol. Sci. 22 (7), 3673. doi: 10.3390/ijms22073673
Jiménez de Bagüés, M. P., Gross, A., Terraza, A., Dornand, J. (2005). Regulation of the mitogen-activated protein kinases by Brucella spp. expressing a smooth and rough phenotype: relationship to pathogen invasiveness. Infect. Immun. 73 (5), 3178–3183. doi: 10.1128/IAI.73.5.3178-3183.2005
Kawasaki, T., Kawai, T. (2014). Toll-like receptor signaling pathways. Front. Immunol. 5. doi: 10.3389/fimmu.2014.00461
Ke, Y., Li, W., Wang, Y., Yang, M., Guo, J., Zhan, S., et al. (2016). Inhibition of TLR4 signaling by Brucella TIR-containing protein TcpB-derived decoy peptides. Int. J. Med. Microbiol. 306 (6), 391–400. doi: 10.1016/j.ijmm.2016.05.003
Kim, S., Watarai, M., Suzuki, H., Makino, S., Kodama, T., Shirahata, T. (2004). Lipid raft microdomains mediate class A scavenger receptor-dependent infection of Brucella abortus. Microb. Pathog. 37 (1), 11–19. doi: 10.1016/j.micpath.2004.04.002
Kornilov, F. D., Shabalkina, A. V., Lin, C., Volynsky, P. E., Kot, E. F., Kayushin, A. L., et al. (2023). The architecture of transmembrane and cytoplasmic juxtamembrane regions of Toll-like receptors. Nat. Commun. 14 (1), 1503. doi: 10.1038/s41467-023-37042-6
Kumar, V., Barrett, J. E. (2022). Toll-like receptors (TLRs) in health and disease: An overview. Handb. Exp. Pharmacol. 276, 1–21. doi: 10.1007/164_2021_568
Kumar, H., Kawai, T., Akira, S. (2011). Pathogen recognition by the innate immune system. Int. Rev. Immunol. 30 (1), 16–34. doi: 10.3109/08830185.2010.529976
Kusumawati, A., Cazevieille, C., Porte, F., Bettache, S., Liautard, J. P., Sri Widada, J. (2000). Early events and implication of F-actin and annexin I associated structures in the phagocytic uptake of Brucella suis by the J-774A.1 murine cell line and human monocytes. Microb. Pathog. 28 (6), 343–352. doi: 10.1006/mpat.2000.0354
Lee, K. M., Chiu, K. B., Sansing, H. A., Didier, P. J., Ficht, T. A., Arenas-Gamboa, A. M., et al. (2013). Aerosol-induced brucellosis increases TLR-2 expression and increased complexity in the microanatomy of astroglia in rhesus macaques. Front. Cell Infect. Microbiol. 3. doi: 10.3389/fcimb.2013.00086
Lee, J. J., Kim, D. H., Kim, D. G., Lee, H. J., Min, W., Rhee, M. H., et al. (2013). Toll-like receptor 4-linked Janus kinase 2 signaling contributes to internalization of Brucella abortus by macrophages. Infect. Immun. 81 (7), 2448–2458. doi: 10.1128/IAI.00403-13
Li, J. Y., Liu, Y., Gao, X. X., Gao, X., Cai, H. (2014). TLR2 and TLR4 signaling pathways are required for recombinant Brucella abortus BCSP31-induced cytokine production, functional upregulation of mouse macrophages, and the Th1 immune response in vivo and in vitro. Cell Mol. Immunol. 11 (5), 477–494. doi: 10.1038/cmi.2014.28
Li, G., Lv, D., Yao, Y., Wu, H., Wang, J., Deng, S., et al. (2021). Overexpression of ASMT likely enhances the resistance of transgenic sheep to brucellosis by influencing immune-related signaling pathways and gut microbiota. FASEB J. 35 (9), e21783. doi: 10.1096/fj.202100651R
Li, J., Zhang, G., Zhi, F., Zhai, Y., Zhou, D., Chen, H., et al. (2022). BtpB inhibits innate inflammatory responses in goat alveolar macrophages through the TLR/NF-κB pathway and NLRP3 inflammasome during Brucella infection. Microb. Pathog. 166, 105536. doi: 10.1016/j.micpath.2022.105536
Li, M., Zhu, Y., Niu, C., Xie, X., Haimiti, G., Guo, W., et al. (2022). Design of a multi-epitope vaccine candidate against Brucella melitensis. Sci. Rep. 12 (1), 10146. doi: 10.1038/s41598-022-14427-z
Liu, N., Wang, L., Sun, C., Yang, L., Tang, B., Sun, W., et al. (2015). Macrophage activation induced by Brucella DNA suppresses bacterial intracellular replication via enhancing NO production. Microb. Pathog. 89, 177–183. doi: 10.1016/j.micpath.2015.10.011
Luchner, M., Reinke, S., Milicic, A. (2021). TLR agonists as vaccine adjuvants targeting cancer and infectious diseases. Pharmaceutics. 13 (2), 142. doi: 10.3390/pharmaceutics13020142
Ma, Q. L., Liu, A. C., Ma, X. J., Wang, Y. B., Hou, Y. T., Wang, Z. H. (2015). Brucella outer membrane protein Omp25 induces microglial cells in vitro to secrete inflammatory cytokines and inhibit apoptosis. Int. J. Clin. Exp. Med. 8 (10), 17530–17535.
Macedo, G. C., Magnani, D. M., Carvalho, N. B., Bruna-Romero, O., Gazzinelli, R. T., Oliveira, S. C. (2008). Central role of MyD88-dependent dendritic cell maturation and proinflammatory cytokine production to control Brucella abortus infection. J. Immunol. 2180 (2), 1080–1087. doi: 10.4049/jimmunol.180.2.1080
Malik, M., Khan, S., Ullah, A., Hassan, M., Haq, M. U., Ahmad, S., et al. (2023). Proteome-wide screening of potential vaccine targets against Brucella melitensis. Vaccines (Basel). 11 (2), 263. doi: 10.3390/vaccines11020263
Martirosyan, A., Gorvel, J. P. (2013). Brucella evasion of adaptive immunity. Future Microbiol. 8 (2), 147–154. doi: 10.2217/fmb.12.140
Martirosyan, A., Pérez-Gutierrez, C., Banchereau, R., Dutartre, H., Lecine, P., Dullaers, M., et al. (2012). Brucella β 1,2 cyclic glucan is an activator of human and mouse dendritic cells. PloS Pathog. 8 (11), e1002983. doi: 10.1371/journal.ppat.1002983
Matamoros-Recio, A., Merino, J., Gallego-Jiménez, A., Conde-Alvarez, R., Fresno, M., Martín-Santamaría, S. (2023). Immune evasion through Toll-like receptor 4: The role of the core oligosaccharides from α2-Proteobacteria atypical lipopolysaccharides. Carbohydr Polym. 318, 121094. doi: 10.1016/j.carbpol.2023.121094
Milillo, M. A., Velásquez, L. N., Trotta, A., Delpino, M. V., Marinho, F. V., Balboa, L., et al. (2017). B. abortus RNA is the component involved in the down-modulation of MHC-I expression on human monocytes via TLR8 and the EGFR pathway. PloS Pathog. 13 (8), e1006527. doi: 10.1371/journal.ppat.1006527
Miller, C. N., Smith, E. P., Cundiff, J. A., Knodler, L. A., Bailey Blackburn, J., Lupashin, V., et al. (2017). A Brucella type IV effector targets the COG tethering complex to remodel host secretory traffic and promote intracellular replication. Cell Host Microbe 22 (3), 317–329.e7. doi: 10.1016/j.chom.2017.07.017
Murugan, S., Nandi, B. R., Mazumdar, V., Joshi, K., Nandini, P., Namani, S., et al. (2023). Outer membrane protein 25 of Brucella suppresses TLR-mediated expression of proinflammatory cytokines through degradation of TLRs and adaptor proteins. J. Biol. Chem. 299 (11), 105309. doi: 10.1016/j.jbc.2023.105309
Nilsen, K. E., Zhang, B., Skjesol, A., Ryan, L., Vagle, H., Bøe, M. H., et al. (2023). Peptide derived from SLAMF1 prevents TLR4-mediated inflammation in vitro and in vivo. Life Sci. Alliance. 6 (12), e202302164. doi: 10.26508/lsa.202302164
Oliveira, S. C., de Oliveira, F. S., Macedo, G. C., de Almeida, L. A., Carvalho, N. B. (2008). The role of innate immune receptors in the control of Brucella abortus infection: toll-like receptors and beyond. Microbes Infect. 10 (9), 1005–1009. doi: 10.1016/j.micinf.2008.07.005
Oliveira, S. C., Harms, J. S., Rech, E. L., Rodarte, R. S., Bocca, A. L., Goes, A. M., et al. (1998). The role of T cell subsets and cytokines in the regulation of intracellular bacterial infection. Braz. J. Med. Biol. Res. 31 (1), 77–84. doi: 10.1590/s0100-879x1998000100010
Parihar, S. P., Guler, R., Brombacher, F. (2019). Statins: a viable candidate for host-directed therapy against infectious diseases. Nat. Rev. Immunol. 19 (2), 104–117. doi: 10.1038/s41577-018-0094-3
Pasquevich, K. A., Carabajal, M. V., Guaimas, F. F., Bruno, L., Roset, M. S., Coria, L. M., et al. (20191436). Omp19 enables Brucella abortus to evade the antimicrobial activity from host's proteolytic defense system. Front. Immunol. 10. doi: 10.3389/fimmu.2019.01436
Pasquevich, K. A., García Samartino, C., Coria, L. M., Estein, S. M., Zwerdling, A., Ibañez, A. E., et al. (2010). The protein moiety of Brucella abortus outer membrane protein 16 is a new bacterial pathogen-associated molecular pattern that activates dendritic cells in vivo, induces a Th1 immune response, and is a promising self-adjuvanting vaccine against systemic and oral acquired brucellosis. J. Immunol. 184 (9), 5200–5212. doi: 10.4049/jimmunol.0902209
Pei, J., Ding, X., Fan, Y., Rice-Ficht, A., Ficht, T. A. (2012). Toll-like receptors are critical for clearance of Brucella and play different roles in development of adaptive immunity following aerosol challenge in mice. Front. Cell Infect. Microbiol. 2. doi: 10.3389/fcimb.2012.00115
Pei, J., Turse, J. E., Ficht, T. A. (2008). Evidence of Brucella abortus OPS dictating uptake and restricting NF-kappaB activation in murine macrophages. Microbes Infect. 10 (6), 582–590. doi: 10.1016/j.micinf.2008.01.005
Peng, Y., Bai, W., Wang, Z., Yu, H. (2021). TLR9/NF-kB pathway regulates Brucella CpG DNA-mediated cytokine response in human peripheral blood mononuclear cells. Iran J. Immunol. 18 (4), 268–278. doi: 10.22034/IJI.2021.84578.1665
Perkins, S. D., Smither, S. J., Atkins, H. S. (2010). Towards a Brucella vaccine for humans. FEMS Microbiol. Rev. 34 (3), 379–394. doi: 10.1111/j.1574-6976.2010.00211.x
Pollak, C. N., Delpino, M. V., Fossati, C. A., Baldi, P. C. (2012). Outer membrane vesicles from Brucella abortus promote bacterial internalization by human monocytes and modulate their innate immune response. PloS One 7 (11), e50214. doi: 10.1371/journal.pone.0050214
Radhakrishnan, G. K., Harms, J. S., Splitter, G. A. (2011). Modulation of microtubule dynamics by a TIR domain protein from the intracellular pathogen Brucella melitensis. Biochem. J. 439 (1), 79–83. doi: 10.1042/BJ20110577
Radhakrishnan, G. K., Splitter, G. A. (2010). Biochemical and functional analysis of TIR domain containing protein from Brucella melitensis. Biochem. Biophys. Res. Commun. 397 (1), 59–63. doi: 10.1016/j.bbrc.2010.05.056
Radhakrishnan, G. K., Yu, Q., Harms, J. S., Splitter, G. A. (2009). Brucella TIR domain-containing protein mimics properties of the Toll-like receptor adaptor protein TIRAP. J. Biol. Chem. 284 (15), 9892–9898. doi: 10.1074/jbc.M805458200
Rahimnahal, S., Yousefizadeh, S., Mohammadi, Y. (2023). Novel multi-epitope vaccine against bovine brucellosis: approach from immunoinformatics to expression. J. Biomol Struct. Dyn. 16, 1–25. doi: 10.1080/07391102.2023.2188962
Rana, R. R., Zhang, M., Spear, A. M., Atkins, H. S., Byrne, B. (2013). Bacterial TIR-containing proteins and host innate immune system evasion. Med. Microbiol. Immunol. 202 (1), 1–10. doi: 10.1007/s00430-012-0253-2
Retamal-Díaz, A., Riquelme-Neira, R., Sáez, D., Rivera, A., Fernández, P., Cabrera, A., et al. (2014). Use of S-[2,3-bispalmitoyiloxy-(2R)-propyl]- R-cysteinyl-amido-monomethoxy polyethylene glycol as an adjuvant improved protective immunity associated with a DNA vaccine encoding Cu, Zn superoxide dismutase of Brucella abortus in mice. Clin. Vaccine Immunol. 21 (11), 1474–1480. doi: 10.1128/CVI.00554-14
Rodríguez, A. M., Delpino, M. V., Miraglia, M. C., Costa Franco, M. M., Barrionuevo, P., Dennis, V. A., et al. (2017). Brucella abortus-activated microglia induce neuronal death through primary phagocytosis. Glia. 65 (7), 1137–1151. doi: 10.1002/glia.23149
Sadeghi, Z., Fasihi-Ramandi, M., Davoudi, Z., Bouzari, S. (2023). Multi-epitope vaccine candidates associated with mannosylated chitosan and LPS conjugated chitosan nanoparticles against Brucella infection. J. Pharm. Sci. 112 (4), 991–999. doi: 10.1016/j.xphs.2022.12.025
Sakaniwa, K., Fujimura, A., Shibata, T., Shigematsu, H., Ekimoto, T., Yamamoto, M., et al. (2023). TLR3 forms a laterally aligned multimeric complex along double-stranded RNA for efficient signal transduction. Nat. Commun. 14 (1), 164. doi: 10.1038/s41467-023-35844-2
Salcedo, S. P., Marchesini, M. I., Lelouard, H., Fugier, E., Jolly, G., Balor, S., et al. (2008). Brucella control of dendritic cell maturation is dependent on the TIR-containing protein Btp1. PloS Pathog. 4 (2), e21. doi: 10.1371/journal.ppat.0040021
Sanjeewa, K. K. A., Nagahawatta, D. P., Yang, H. W., Oh, J. Y., Jayawardena, T. U., Jeon, Y. J., et al. (2020). Octominin inhibits LPS-induced chemokine and pro-inflammatory cytokine secretion from RAW 264.7 macrophages via blocking TLRs/NF-κB signal transduction. Biomolecules. 10 (4), 511. doi: 10.3390/biom10040511
Saqib, U., Baig, M. S. (2019). Scaffolding role of TcpB in disrupting TLR4-Mal interactions: Three to tango. J. Cell Biochem. 120 (3), 3455–3458. doi: 10.1002/jcb.27619
Sengupta, D., Koblansky, A., Gaines, J., Brown, T., West, A. P., Zhang, D., et al. (2010). Subversion of innate immune responses by Brucella through the targeted degradation of the TLR signaling adapter, MAL. J. Immunol. 184 (2), 956–964. doi: 10.4049/jimmunol.0902008
Shim, S., Park, H. E., Soh, S. H., Im, Y. B., Yoo, H. S. (2020). Induction of Th2 response through TLR2-mediated MyD88-dependent pathway in human microfold cells stimulated with chitosan nanoparticles loaded with Brucella abortus Mdh. Microb. Pathog. 142, 104040. doi: 10.1016/j.micpath.2020.104040
Skendros, P., Boura, P. (2013). Immunity to brucellosis. Rev. Sci. Tech. 32 (1), 137–147. doi: 10.20506/rst.32.1.2190
Skendros, P., Pappas, G., Boura, P. (2011). Cell-mediated immunity in human brucellosis. Microbes Infect. 13 (2), 134–142. doi: 10.1016/j.micinf.2010.10.015
Smith, E. P., Miller, C. N., Child, R., Cundiff, J. A., Celli, J. (2016). Postreplication roles of the Brucella VirB type IV secretion system uncovered via conditional expression of the VirB11 ATPase. mBio. 7 (6), e01730-16. doi: 10.1128/mBio.01730-16
Spink, W. W., Hall, J. W., Finstad, J., Mallet, E. (1962). Immunization with viable Brucella organisms. Results of a safety test in humans. Bull. World Health Organ. 26 (3), 409–419.
Starr, T., Child, R., Wehrly, T. D., Hansen, B., Hwang, S., López-Otin, C., et al. (2012). Selective subversion of autophagy complexes facilitates completion of the Brucella intracellular cycle. Cell Host Microbe 11 (1), 33–45. doi: 10.1016/j.chom.2011.12.002
Stranahan, L. W., Arenas-Gamboa, A. M. (2021). When the going gets rough: The significance of Brucella lipopolysaccharide phenotype in host-pathogen interactions. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.713157
Surendran, N., Hiltbold, E. M., Heid, B., Akira, S., Standiford, T. J., Sriranganathan, N., et al. (2012). Role of TLRs in Brucella mediated murine DC activation in vitro and clearance of pulmonary infection in vivo. Vaccine. 30 (8), 1502–1512. doi: 10.1016/j.vaccine.2011.12.036
Takano, S., Miyashima, Y., Fujii, S., Sakurai, K. (2023). Molecular bottlebrushes for immunostimulatory CpG ODN delivery: Relationship among cation density, complex formation ability, and cytotoxicity. Biomacromolecules. 24 (3), 1299–1309. doi: 10.1021/acs.biomac.2c01348
Takeda, K., Akira, S. (2004). Microbial recognition by Toll-like receptors. J. Dermatol. Sci. 34 (2), 73–82. doi: 10.1016/j.jdermsci.2003.10.002
Tarrahimofrad, H., Zamani, J., Hamblin, M. R., Darvish, M., Mirzaei, H. (2022). A designed peptide-based vaccine to combat Brucella melitensis, B. suis and B. abortus: Harnessing an epitope mapping and immunoinformatics approach. BioMed. Pharmacother. 155, 113557. doi: 10.1016/j.biopha.2022.113557
Terwagne, M., Ferooz, J., Rolán, H. G., Sun, Y. H., Atluri, V., Xavier, M. N., et al. (2013). Innate immune recognition of flagellin limits systemic persistence of Brucella. Cell Microbiol. 15 (6), 942–960. doi: 10.1111/cmi.12088
Velásquez, L. N., Milillo, M. A., Delpino, M. V., Trotta, A., Fernández, P., Pozner, R. G., et al. (2017). Brucella abortus down-regulates MHC class II by the IL-6-dependent inhibition of CIITA through the downmodulation of IFN regulatory factor-1 (IRF-1). J. Leukoc. Biol. 101 (3), 759–773. doi: 10.1189/jlb.4A0416-196R
Venkataranganayaka Abhilasha, K., Kedihithlu Marathe, G. (2021). Bacterial lipoproteins in sepsis. Immunobiology. 226 (5), 152128. doi: 10.1016/j.imbio.2021.152128
Vershilova, P. A. (1961). The use of live vaccine for vaccination of human beings against brucellosis in the USSR. B World Health Organ. 24, 85–89.
Vieira, A. L., Silva, T. M., Mol, J. P., Oliveira, S. C., Santos, R. L., Paixão, T. A. (2013). MyD88 and TLR9 are required for early control of Brucella ovis infection in mice. Res. Vet. Sci. 94 (3), 399–405. doi: 10.1016/j.rvsc.2012.10.028
Vijay, K. (2018). Toll-like receptors in immunity and inflammatory diseases: Past, present, and future. Int. Immunopharmacol. 59, 391–412. doi: 10.1016/j.intimp.2018.03.002
Wang, K. L., Chen, S. N., Li, L., Huo, H. J., Nie, P. (2021). Functional characterization of four TIR domain-containing adaptors, MyD88, TRIF, MAL, and SARM in mandarin fish Siniperca chuatsi. Dev. Comp. Immunol. 122, 104110. doi: 10.1016/j.dci.2021.104110
Weinhold, M., Eisenblätter, M., Jasny, E., Fehlings, M., Finke, A., Gayum, H., et al. (2013). The attenuated Brucella abortus strain 19 invades, persists in, and activates human dendritic cells, and induces the secretion of IL-12p70 but not IL-23. PloS One 8 (6), e65934. doi: 10.1371/journal.pone.0065934
West, A. P., Koblansky, A. A., Ghosh, S. (2006). Recognition and signaling by toll-like receptors. Annu. Rev. Cell Dev. Biol. 22, 409–437. doi: 10.1146/annurev.cellbio.21.122303.115827
Willems, S. A., Brouwers, J. J. W. M., Eefting, D. (2022). Aortic and iliac involvement in Brucellosis - A rare but life threatening manifestation: A review of the literature. Eur. J. Vasc. Endovasc Surg. 63 (5), 743–750. doi: 10.1016/j.ejvs.2022.02.004
Woodman, S., Gibson, A. J., García, A. R., Contreras, G. S., Rossen, J. W., Werling, D., et al. (2016). Structural characterisation of Toll-like receptor 1 (TLR1) and Toll-like receptor 6 (TLR6) in elephant and harbor seals. Vet. Immunol. Immunopathol. 169, 10–14. doi: 10.1016/j.vetimm.2015.11.006
Wu, Z., Hu, T., Merits, A., He, Y., Wang, M., Jia, R., et al. (2022). Toll-like receptor 4 and lipopolysaccharide from commensal microbes regulate Tembusu virus infection. J. Biol. Chem. 298 (12), 102699. doi: 10.1016/j.jbc.2022.102699
Yin, Z., Li, M., Niu, C., Yu, M., Xie, X., Haimiti, G., et al. (2023). Design of multi-epitope vaccine candidate against Brucella type IV secretion system (T4SS). PloS One 18 (8), e0286358. doi: 10.1371/journal.pone.0286358
Yu, H., Bai, L., Zhang, Y., Wang, Z., Yu, Y. (2017). Repetitive extragenic palindromic DNA sequences from Brucella melitensis stimulate Toll-like receptor 9 signaling in macrophages. Mol. Med. Rep. 15 (1), 271–276. doi: 10.3892/mmr.2016.5990
Yu, H., Wang, Z., Sun, G., Yu, Y. (2012). Recognition of nucleic acid ligands by toll-like receptors 7/8: importance of chemical modification. Curr. Med. Chem. 19 (9), 1365–1377. doi: 10.2174/092986712799462603
Zhang, C. Y., Bai, N., Zhang, Z. H., Liang, N., Dong, L., Xiang, R., et al. (2012). TLR2 signaling subpathways regulate TLR9 signaling for the effective induction of IL-12 upon stimulation by heat-killed Brucella abortus. Cell Mol. Immunol. 9 (4), 324–333. doi: 10.1038/cmi.2012.11
Zhang, Y., Wang, X., Li, Z., Zhang, J., Wang, Y., Wu, C., et al. (2022). Brucella melitensis outer membrane protein 25 interacts with ferritin heavy polypeptide 1 in human trophoblast cells. Mol. Med. Rep. 26 (1), 224. doi: 10.3892/mmr.2022.12740
Zhao, Y., Hanniffy, S., Arce-Gorvel, V., Conde-Alvarez, R., Oh, S., Moriyón, I., et al. (2018). Immunomodulatory properties of Brucella melitensis lipopolysaccharide determinants on mouse dendritic cells in vitro and in vivo. Virulence. 9 (1), 465–479. doi: 10.1080/21505594.2017.1386831
Zhu, Y., Shi, L., Zeng, Y., Piao, D., Xie, Y., Du, J., et al. (2022). Key immunity characteristics of diverse stages of brucellosis in rural population from Inner Mongolia, China. Infect. Dis. Poverty. 11 (1), 63. doi: 10.1186/s40249-022-00989-7
Keywords: Brucella, Toll-like receptor, innate immunity, pathogen-associated molecular pattern, vaccine
Citation: Yu H, Gu X, Wang D and Wang Z (2024) Brucella infection and Toll-like receptors. Front. Cell. Infect. Microbiol. 14:1342684. doi: 10.3389/fcimb.2024.1342684
Received: 22 November 2023; Accepted: 17 January 2024;
Published: 12 March 2024.
Edited by:
Collins Ouma, Maseno University, KenyaReviewed by:
Isaac Ndede, Moi University, KenyaJames Miser Akoko, International Livestock Research Institute (ILRI), Kenya
Bernard Ochieng Guyah, Maseno University, Kenya
Copyright © 2024 Yu, Gu, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhanli Wang, d2FuZy56aGFubGlAaG90bWFpbC5jb20=
 Hui Yu1,2
Hui Yu1,2 Zhanli Wang
Zhanli Wang