- Department of Gastroenterology, Changhai Hospital, Naval Medical University, Shanghai, China
Increasing attention is being paid to the unique roles gut microbes play in both physiological and pathological processes. Crohn’s disease (CD) is a chronic, relapsing, inflammatory disease of the gastrointestinal tract with unknown etiology. Currently, gastrointestinal infection has been proposed as one initiating factor of CD. Yersinia enterocolitica, a zoonotic pathogen that exists widely in nature, is one of the most common bacteria causing acute infectious gastroenteritis, which displays clinical manifestations similar to CD. However, the specific role of Y. enterocolitica in CD is controversial. In this Review, we discuss the current knowledge on how Y. enterocolitica and derived microbial compounds may link to the pathogenesis of CD. We highlight examples of Y. enterocolitica-targeted interventions in the diagnosis and treatment of CD, and provide perspectives for future basic and translational investigations on this topic.
Introduction
Crohn’s disease (CD), one of the main forms of inflammatory bowel disease, is characterized by patchy transmural inflammation that can involve any part of the digestive tract, from mouth to anus (Baumgart and Sandborn, 2012). Treatments for CD include the use of medication, alterations in diet and nutrition, and sometimes surgical procedures to repair or remove affected portions of digestive tract (Gajendran et al., 2018; Shi and Ng, 2018), however, no cure has been developed. In recent years, the global incidence of CD has been continuously increasing (Torres et al., 2017). Although a large number of studies have been conducted, the etiology of CD is still not clear. Many studies have revealed that there is a close relationship between the gut microbiota and CD. Dysbacteriosis is a typical symptom of CD patients, which includes reduced diversity, altered structural composition, and dysfunction of the gut microbiota (Gevers et al., 2014; De Cruz et al., 2015; Teh et al., 2021). The presence of gut microbes has been shown to be necessary for the occurrence of CD-related pathological changes in genetically susceptible mice (Kobayashi et al., 2014). Clinical observations from patients have demonstrated that CD lesions occur more frequently within the terminal ileum, which is an area with increased bacterial contact (Baumgart and Sandborn, 2012). Treatments, such as antibiotics and fecal microbiota transplantation, have been shown to alleviate CD, which also indicates the correlation between the intestinal microbiota and the inflammatory response (Selby et al., 2007). Genome-wide association studies have found more than 100 susceptibility loci in the genomes of CD patients (Sazonovs et al, 2022), and these susceptibility sites are mostly involved in microbial recognition and defense-related pathways (Hampe et al., 2001; Hampe et al., 2007). A nationwide case-control study showed that acute gastrointestinal infections increase the risk for CD, and speculated that the pathogens causing acute infectious gastroenteritis might play a role in the occurrence and development of CD (Axelrad et al., 2019).
Yersinia enterocolitica is the fourth most common bacterium causing acute infectious gastroenteritis in the European Union (EU) (Spackova et al., 2022). As a zoonotic pathogen which is widely distributed in nature, it is often transmitted to humans through consumption of contaminated food or water (Bottone, 1999). Compared with other bacteria, Y. enterocolitica is more frequently observed in CD lesions (e.g., CD specimens of mesenteric lymph nodes and Peyer’s plaques) (Lamps et al., 2003; Leu et al., 2013; Le Baut et al., 2018). Besides, there are many overlaps between Y. enterocolitica infection and CD in clinical symptoms and pathological manifestations, which often cause misdiagnosis and delays in treatment (Naddei et al., 2017; Cian et al., 2020). However, it is still unclear whether the presence of Y. enterocolitica in CD is a concomitant or accidental phenomenon or a contributing factor in the pathogenesis of CD. Therefore, we comprehensively investigate the relationship between Y. enterocolitica and CD from multiple aspects in this review, including pathological manifestations, diagnosis, treatment, epidemiology, and pathogenesis.
Overview of Y. enterocolitica
Y. enterocolitica is a member of the phylum Proteobacteria, family Enterobacteriaceae, and genus Yersinia, which was first discovered and named in the mid-20th century (McIver and Pike, 1934; Schleifstein and Coleman, 1939; Hässig et al., 1949; Frederiksen, 1964). Generally, there are 18 classical strains (currently, more strains have been found using whole genome sequencing and gene alignment algorithms), most of which are regarded as environmental, non-virulent human pathogens (Sulakvelidze, 2000). Of the 18 strains, 3 are pathogenic to humans, namely, Yersinia pestis (the causative agent of plague, including the medieval ‘Black Death’), Yersinia pseudotuberculosis, and Y. enterocolitica (Achtman et al., 1999). The latter two can cause gastrointestinal disorders, of which, Y. enterocolitica is more relevant to gastrointestinal infections in humans. Y. enterocolitica can be further divided into a variety of biochemical and serological heterogeneous strains. To date, six biotypes (1A, 1B, 2, 3, 4, and 5) and more than 70 serotypes have been identified. Among them, biotype 1B strains (also named ‘New World’ or American strains due to their initial isolation in the USA) are considered highly virulent and lethal in mice, while biotype 1A strains are considered non-virulent due to the absence of the virulent plasmid pYV. Biotypes 2, 3, 4, and 5 (‘Old World’ or European strains) are low-virulence. The most common strains isolated from symptomatic humans belong to serobiotypes O: 3/4, O: (5 and 27)/(biotypes 2 and 3), O: 8/1B, and O: 9/2 (Lucero-Estrada et al., 2020).
A series of studies on Y. enterocolitica from 1930 to the present have focused on the identification and comparison of strains, the molecular mechanism of effector proteins in virulence and pathogenesis, clinical symptoms, and epidemiology (Figures 1A–C). Using scientometric analysis, we found that published studies regarding Y. enterocolitica were mainly from Europe and North America before 1980 (Figure 2A). After then, a number of studies were published from Asia, South America, and Africa (Figure 2B). In 1981, Kaneko et al. isolated Y. enterocolitica in Hokkaido, Japan, while retrospectively discovering that there had been several outbreaks of Y. enterocolitica community infection have occurred in Japan (Kaneko and Hashimoto, 1981). Sun et al. reported in the Chinese Journal of Zoonoses that 107 residents of Lanzhou City were infected after consuming contaminated beef in 1987, which was the earliest publication of an outbreak of Y. enterocolitica in China (Dianbin Sun and Wei, 1987). At present, the intimate relationship between Y. enterocolitica and CD has been widely recognized (Hugot et al., 2021). The epidemiology, clinical symptoms, pathological manifestations, and molecular mechanisms of this relationship are discussed in detail below.
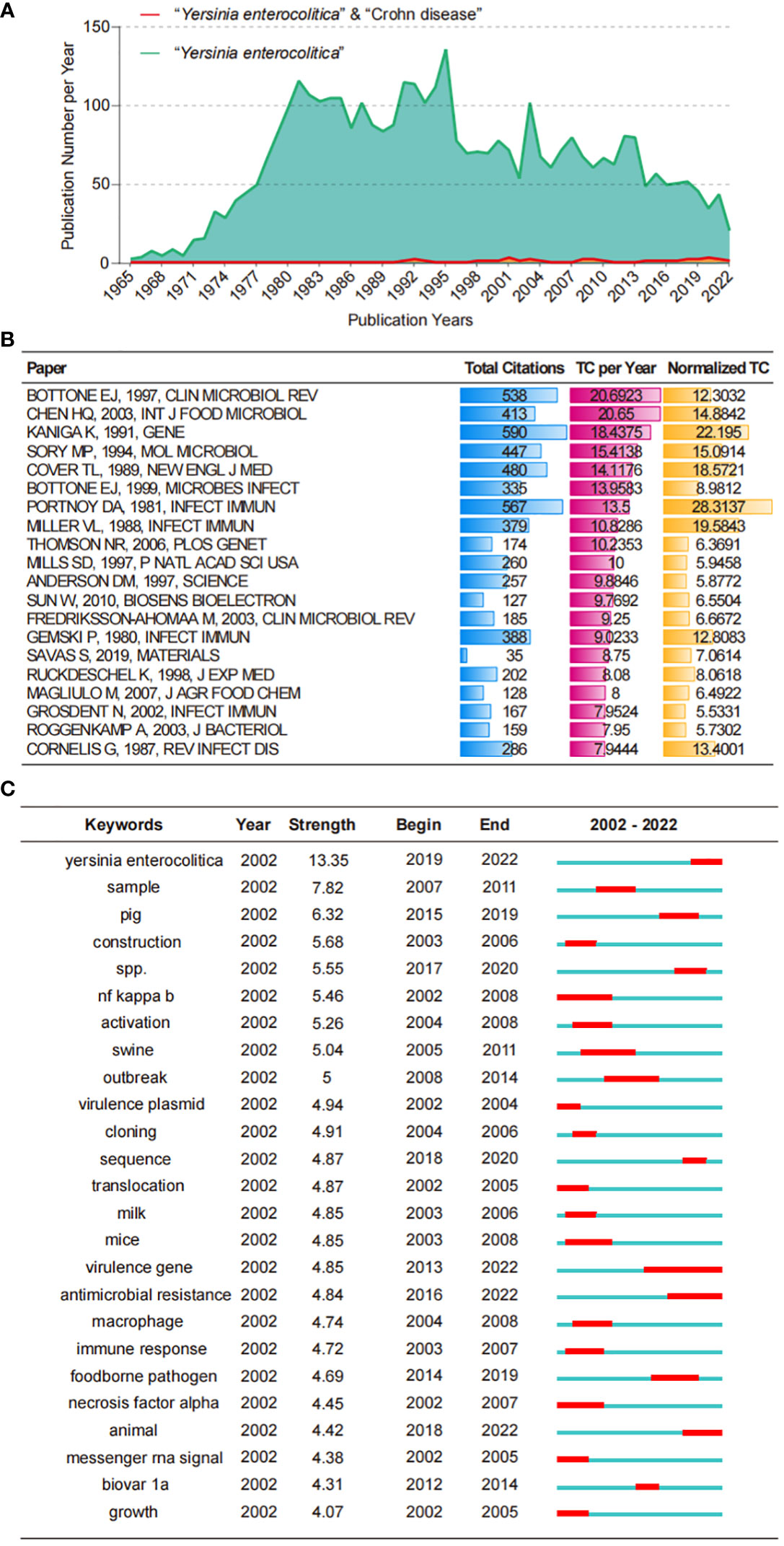
Figure 1 Bibliometric analysis of WoS core database output. (A) The growth productive trends of the topic “Y. enterocolitica” and “Y. enterocolitica” & “Crohn disease” research from 1900 to 2022. (B) Top 20 most cited papers in the topic “Y. enterocolitica” research from 1900 to 2022. (C) Top 25 Keywords with the Strongest Citation Bursts in the topic “Y. enterocolitica” research from 2002 to 2022. WoS, Web of Science.
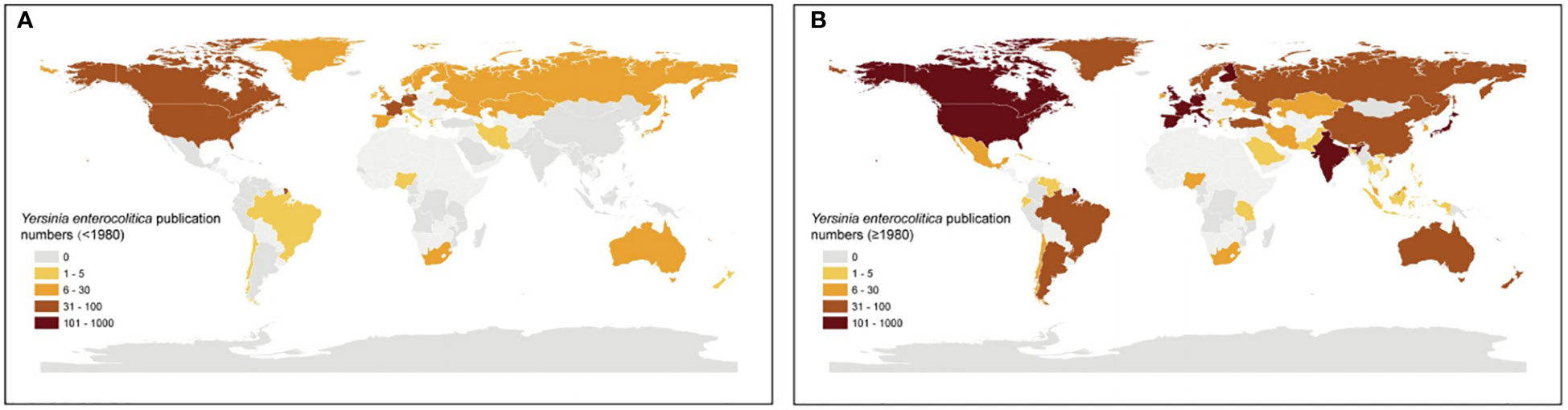
Figure 2 Worldwide Y. enterocolitica publication numbers by Bibliometric analysis. Worldwide Y. enterocolitica publication numbers for countries reporting data (A) before 1980 and (B) after 1980. Publication numbers were ranked into quintiles representing low (bright yellow) to intermediate (yellow) and high (brown).
Are Y. enterocolitica and CD companions?
The epidemiology of Y. enterocolitica and CD seem to have a strong correlation. Compared to control groups, CD patients had a significantly higher prevalence of Y. enterocolitica. As many as 63% of patients with CD were found to have Y. enterocolitica based on the investigation by Kallinowski et al. (1998). As per Lamps et al., the detection rate of pathogenic Y. enterocolitica DNA in the bowel and mesenteric lymph nodes from patients with CD reached 31% (17/54), while all control tissues (40 cases of normal intestinal specimens, 30 cases of acute appendicitis, and 50 cases of active colitis) were negative (Lamps et al., 2003). Ahmad et al. showed that Y. enterocolitica was frequently present in patients with CD (7/69, 10.14%), and was significantly associated with the disease (p = 0.02) (Khan et al., 2021). Both Y. enterocolitica infection and the onset of CD in humans have obvious familial aggregation. One infection mode of Y. enterocolitica is a family-centered, small-scale outbreak (Black and Slome, 1988). Moreover, 12% to 18% of CD patients are reported to have at least one household member who suffers from the disease (Weterman and Pena, 1984). In a study on familial CD in Belgium, Herbert et al. found that CD patients and their relatives consumed more unpasteurized milk and cheese, undercooked beef, and pork than the control population (Van Kruiningen et al., 2005). It is speculated that these food with high chance of Y. enterocolitica contaminations may be a potential trigger of CD.
Global trends in the prevalence of CD and Y. enterocolitica infection are also similar. Historically, the outbreaks and epidemics of Y. enterocolitica infection and CD cases mostly have been reported in the northern hemisphere such as North America and Northern Europe (Bottone, 1977; Molodecky et al., 2012). Currently, there has been a weakened north-south gradient in Y. enterocolitica transmission and CD in recent decades, which may be attributed to the advancement of global modernization. According to data from the European Centre for Disease Prevention and Control (ECDC) and Foodborne Diseases Active Surveillance Network (FoodNet) in the United States, the incidence of Y. enterocolitica in Europe and the United States has decreased over the past decade (Figure 3). Meanwhile, an increasing incidence of Y. enterocolitica has been found in some low-risk areas in the past. Meta-analysis of the global annual incidence of Y. enterocolitica in gastroenteritis cases between January 1, 2000 and December 31, 2019 showed that Africa and the Eastern Mediterranean rank first and second respectively in the global prevalence of Y. enterocolitica (Riahi et al., 2021). Correspondingly, newly industrialized regions from Asia, Africa, and South America are experiencing an increase in CD cases (Giegerich et al., 2018), and New Zealand and Australia are among the top four countries with the highest annual incidence of CD at present (Torres et al., 2017). Notably, bacterial infections such as Yersinia are more likely to be diagnosed and treated than those of a chronic disease such as CD, which may also contribute to global incidence changes. Taken together, these evidences suggest that Y. enterocolitica has a strong epidemiological relationship with CD. However, the mechanism underlying this association is unknown. Furthermore, it is unclear if interactions between Y. enterocolitica and CD-susceptible individuals initiate the onset of disease. Thus, further investigations regarding Y. enterocolitica infection and CD patients are needed.
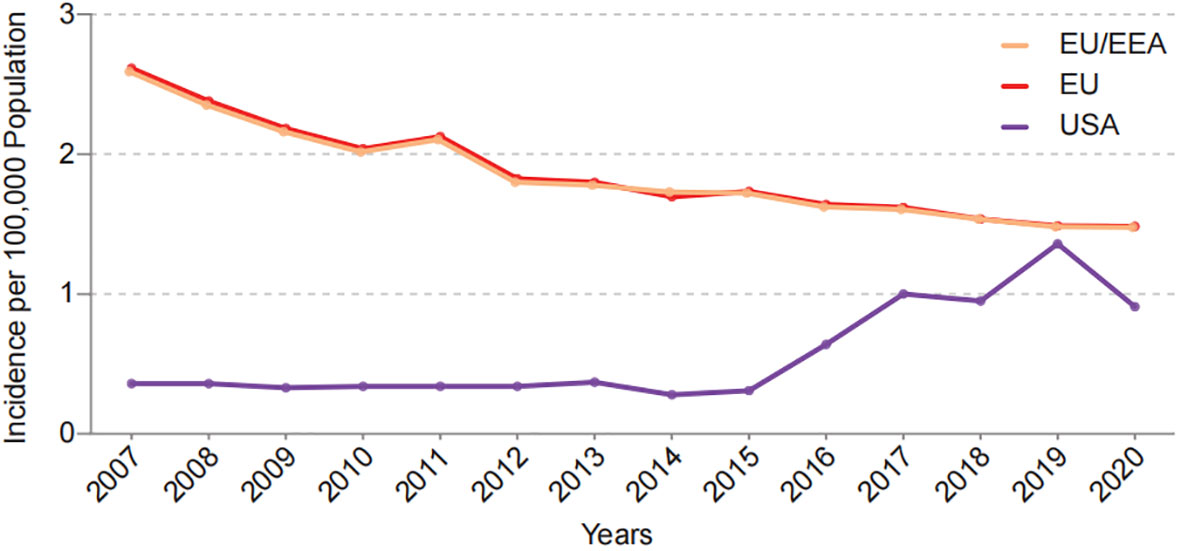
Figure 3 Incidence of Y.enterocolitica infections in EU/EEA, EU and USA per year from 2007 to 2020. Date come from European Centre for Disease Prevention and Control (ECDC) and Foodborne Diseases Active Surveillance Network (FoodNet).
Whether Y. enterocolitica and CD share pathogenesis?
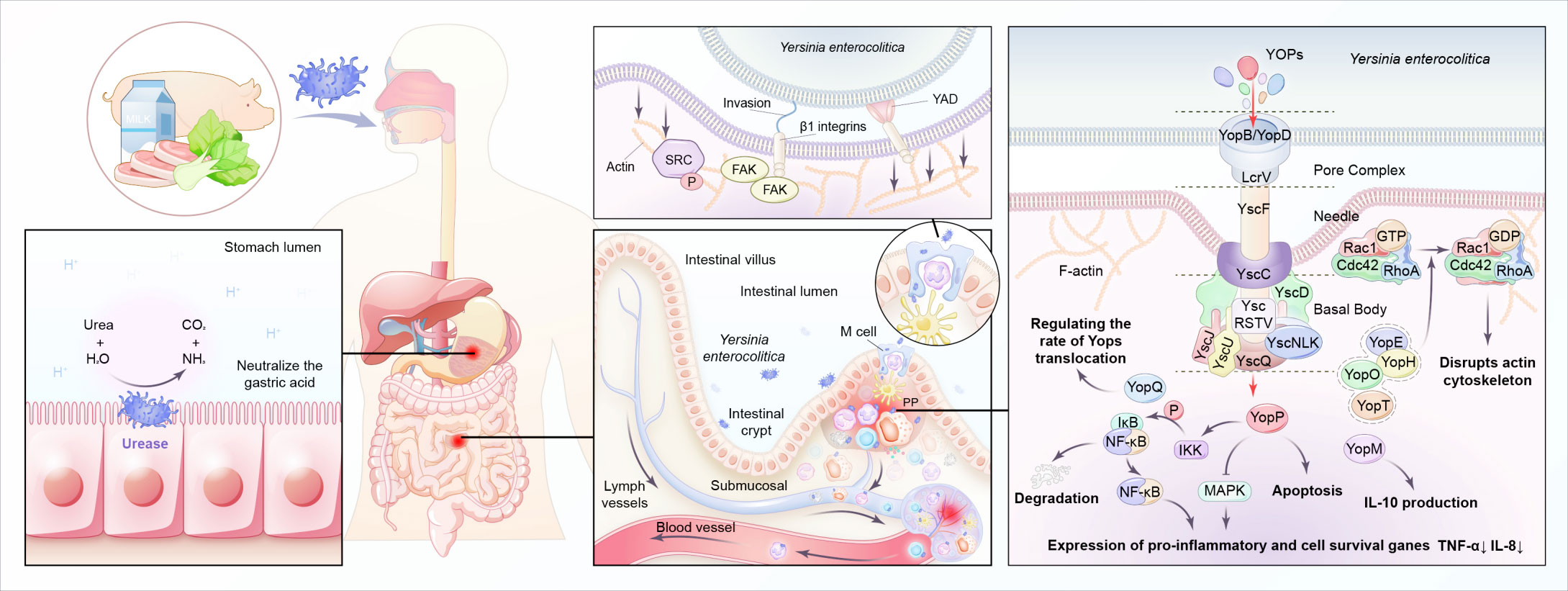
Infection of Y. enterocolitica disturbs immune balance in the host. The pathogenesis depends on multiple chromosome-encoded and plasmid-encoded virulence determinants (Table 1) (Figure 4). Once entering the digestive tract, Y. enterocolitica first passes through the gastric acid barrier with the help of urease (Righetto et al., 2020). After reaching the distal small intestine, it preferentially adheres to and invades M cells in the follicular-associated epithelium (FAE). The interactions between bacterial adhesins and β1-integrins on the surface of M cells induce Y. enterocolitica internalization by stimulating the remodeling of actin filaments and forming a polymerized actin vacuole (Schulte et al., 2000). Then the vacuole transports from the apical to the basal side of the M cell, where Y. enterocolitica is expelled into the lymphoid follicles of the Peyer’s patches (Autenrieth and Firsching, 1996). With the help of Yad and invasion proteins, Y. enterocolitica adheres to the host immune cells (including macrophages, neutrophils, dendritic cells, and monocytes) surfaces (El Tahir and Skurnik, 2001; Heise and Dersch, 2006). At the same time, the T3SS injection system forms a channel between Y. enterocolitica and the target cell and injects cytotoxic effector Yersinia outer-membrane proteins (Yops) into the cytoplasm of the host cell, enabling the bacteria to evade the host immune system (Cornelis, 2006; Cornelis, 2010). For example, YOPs, such as YopE, YopH, YopT, and YopO, inhibit the phagocytosis of macrophages and leukocytes by disrupting the actin cytoskeleton. YopP inhibits multiple signaling pathways, including TNF-α and IL-8, and exploits lipopolysaccharide signaling to trigger apoptosis in infected macrophages and dendritic cells (Denecker et al., 2002). In addition, the immunomodulatory protein Gal-1 can promote the replication of Y. enterocolitica in PP, and this protein has been shown to play a role in limiting bacterial clearance (Davicino et al., 2017). After the incubation period of 4–7 days, the engulfed bacteria migrate to the mesenteric lymph nodes, where they multiply and cause abscess formation (Hein et al., 2000). Systemic infection occurs with the migration of Y. enterocolitica along with the lymphatic fluid or blood and may cause extraintestinal complications, such as sepsis (Lai et al., 2014), deep organ abscess (Viteri et al., 1981; Demes et al., 2021), and arthritis (Appel et al., 2002). Moreover, YadA-mediated complement evasion may result in persistent pathogen presence (China et al., 1993).
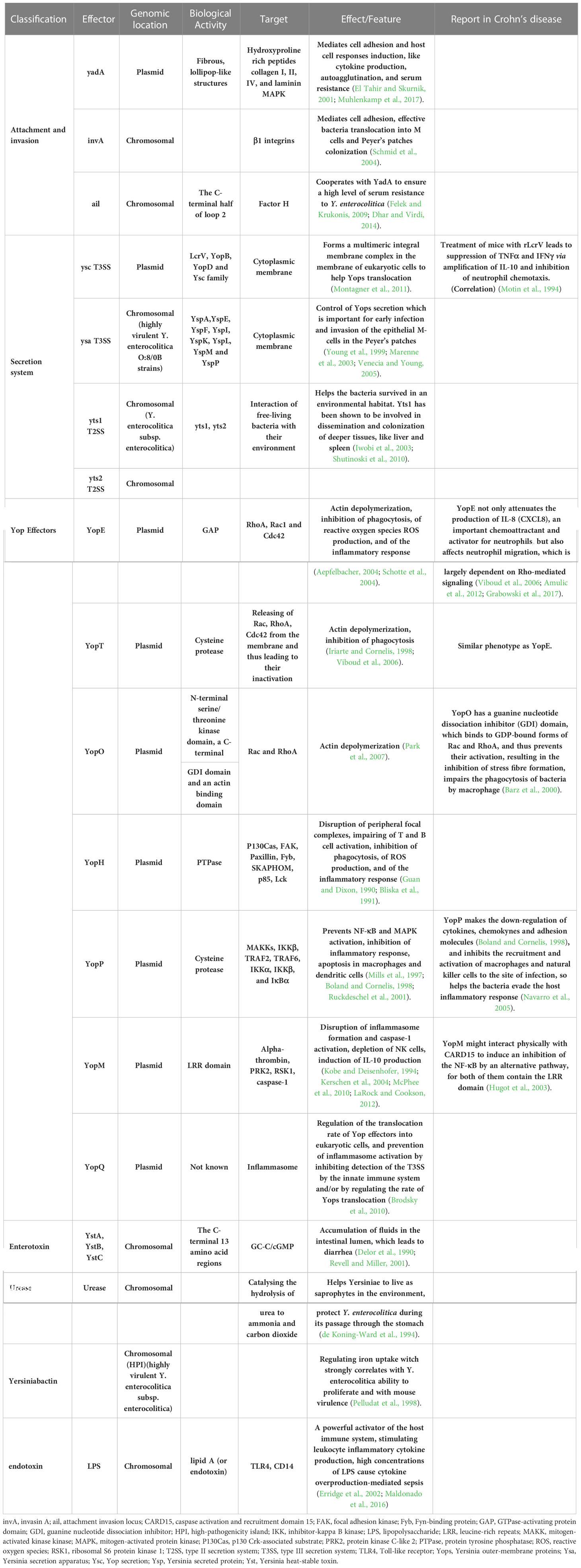
Table 1 Comparative analysis of virulence factors in Y. enterocolitica and their potential effect in Crohn’s disease.
Both Y. enterocolitica infection and CD exhibit immune defects during pathogenesis, with some immune responses shared. A break of the balance between immunosurveillance and immune escape of the intestinal microbiota is crucial in the pathogenesis of CD. For example, both mutations in MUC2, which encodes for intestinal mucus, and the emulsifiers commonly found in Western diets are risk factors for CD, as they both lead to the destruction of the mucus layer and create opportunities for the translocation of intestinal pathogens, such as Y. enterocolitica (Boltin et al., 2013; Chassaing et al., 2015). The susceptibility genes of CD are mostly related to microbial recognition and defense. CARD15, originally named NOD2, is the first identified susceptibility gene of CD. Its encoding protein NOD-like receptors (NLRs) expressed in the cytoplasm of many innate immune cells, respectively, is a main group of pattern recognition receptors (PRRs) responsible for recognizing intestinal microbiota (Al Nabhani et al., 2017). Individuals carrying CARD15 mutations were reported to present with abnormal immune responses to Y. enterocolitica infection and are subsequently diagnosed with CD (Safa et al., 2008). Toll-like receptors (TLR) expressed in the membrane of many innate immune cells, is another main group of PRRs. TLR1-/- mice with acute Y. enterocolitica infection exhibit CD-like symptoms post-infection, including poor weight gain, dysbiosis, long-term chronic inflammation, and an increased anti-commensal immunity as compared to their wild-type (WT) littermate control mice (Kamdar et al., 2016). The autophagy process, which is also affected by Y. enterocolitica infection, has a vital role in downstream antibacterial mechanisms mediated by the TLR and NLR signaling pathways. Murthy et al. found that Y. enterocolitica could activate caspase 3 after infection, leading to the accelerated degradation of the CD-susceptible autophagy gene ATG16L1 (T316A), which in turn, led to reduced autophagy, impaired pathogen clearance of ileal Y. enterocolitica, and increased secretion of TNF-α and IL-1β (Murthy et al., 2014). The detailed molecular mechanisms involved are the effects of Y. enterocolitica and derived microbial compounds on immune cells. For example, YopE has a Rho GTPase-activating protein domain (GAP), which targets RhoA, Rac1 and Cdc42. By hitting Rho-GTPases, YopE not only attenuates the production of IL-8 (CXCL8), an important chemoattractant and activator for neutrophils, but also affects neutrophil migration, which is largely dependent on Rho-mediated signaling (Viboud et al., 2006; Amulic et al., 2012; Grabowski et al., 2017). YopO has a guanine nucleotide dissociation inhibitor (GDI) domain, which binds to GDP-bound forms of Rac and RhoA, and thus prevents their activation, resulting in the inhibition of stress fiber formation, impairs the phagocytosis of bacteria by macrophage (Barz et al., 2000). YopP inhibits multiple signaling pathways, including the NF-κB and MAPKs pathways, and exploits lipopolysaccharide -related signaling pathway to trigger apoptosis in infected macrophages and DC (Ruckdeschel et al., 2001). YopP makes the down-regulation of cytokines, chemokynes and adhesion molecules (Boland and Cornelis, 1998), and inhibits the recruitment and activation of macrophages and natural killer cells to the site of infection, so helps the bacteria evade the host inflammatory response (Navarro et al., 2005).
Y. enterocolitica infection can evoke a long-term immune response and gut microbiota alteration, then lead to developing of CD in genetically susceptible individuals. As Netea et al. reported, the defects in the recognition pathways of TLR5 and NOD2 led to a defective inflammatory response to Y. enterocolitica and long-term abdominal inflammation (Netea et al., 2010). Denise et al. suggested that constant disruption in the communication between the immune system and tissue systems following clearance of an acute infection denotes a turning point in which both tissue and immune homeostasis were altered via long-term reprogramming (Fonseca et al., 2015). Therefore, we suggested that long-term reprogramming of immune cells after Y. enterocolitica infection, as well as profound and persistent remodeling of MAT and MLN, leads to the development of chronic inflammatory conditions through a phenomenon known as immunological scarring. Of course, this speculation needs to be confirmed by further studies.
Y. enterocolitica in the diagnosis of CD
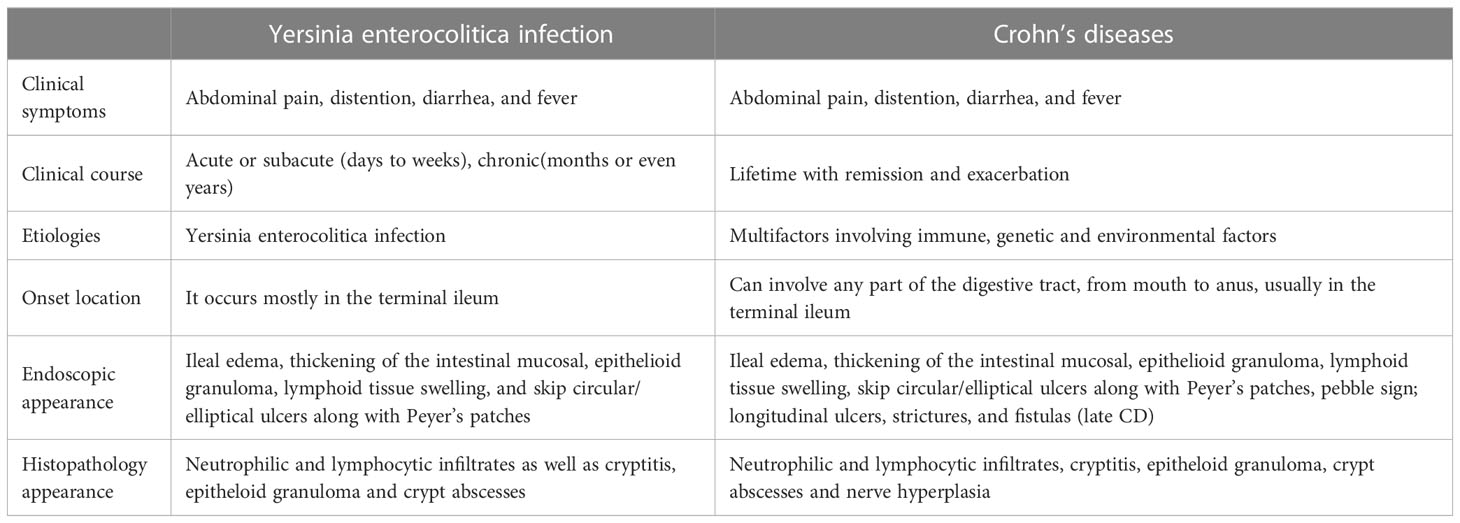
It was noted decades ago that there were many similar pathological manifestations and clinical symptoms between CD and Y. enterocolitica infection (Table 2). For instance, the location of lesions in both diseases is most frequently presented in the terminal ileum - areas with increased bacterial contact (Payne et al., 1987). Specifically, the Peyer’s patches and isolated lymphoid follicles in the small intestine, where Y. enterocolitica primarily colonizes (Revell and Miller, 2001), are also sites for typical aphthoid lesions during the early stage of CD (Lockhart-Mummery and Morson, 1960; Fujimura et al., 1996; Krauss et al., 2012). Different from the ulcers caused by local intestinal ischemia, Behcet’s disease and intestinal tuberculosis tend to occur on the mesenteric side of the intestinal lumen (Sunada et al., 2009). The main clinical symptoms in humans infected with Y. enterocolitica include abdominal pain, diarrhea, and vomiting. Endoscopic observation shows ileal edema, thickening of the intestinal mucosal, epithelioid granuloma, lymphoid tissue swelling, and an increased number of transmural blood vessels. Microscopic observation shows fossa, crypt abscess, and plasma cell and lymphocyte infiltration. All of which are consistent with the clinical and histological manifestations of CD (Gleason and Patterson, 1982; Bronner, 2004; Franczak et al., 2018). Creeping fat, an extra-intestinal manifestation of CD, is manifested by the migration and wrapping of mesenteric adipose tissue (MAT) to around sites of intestinal inflammation. Creeping fat is visually striking alongside the patchy lesions (Sheehan et al., 1992; Peyrin-Biroulet et al., 2007). Connie et al. showed that translocation of the gut microbiota is the main driving factor leading to the formation of creeping fat (Ha et al., 2020). Interestingly, the remodeling of MAT was also found in Y. enterocolitica-infected mice (Antonopoulos et al., 2008; Han et al., 2017).
In current clinical practice, the diagnosis of CD always needs to determine whether Y. enterocolitica infection exists. While positive cultures obtained from the mesenteric lymph nodes, pharyngeal exudates, peritoneal fluid, or blood can successfully diagnose Y. enterocolitica infection (Centers for Disease, 2011), this laborious and time-consuming method has been gradually replaced by molecular detection, including serological testing, such ELISA (Laporte et al., 2015) and agglutination (Paerregaard et al., 1991; Sonnevend et al., 2005) detection of the presence of specific antibodies (such as IgG, IgG, IgA, and IgM) against YOPs (such as LorV (V antigen), YopD, and Yop M) produced by B cells with the help of intestinal dendritic cells. Triantafillidis et al. recommended that all patients with terminal-ileitis as evidenced by endoscopic and histological images should be tested for YOP-specific antibodies to determine whether the terminal-ileal CD is associated with Y. enterocolitica infection (Triantafillidis et al., 2020). However, serological testing also has many concomitant difficulties, including cross-reactions with other intestinal microbes (Golkocheva-Markova et al., 2008). Currently, more sensitive, highly accurate and precise methods, such as PCR and multiplex PCR, have been used to clinically and preclinically detect the transposon and virulence genes of pathogens within mucosal tissues (Zakharchuk, 1965; Ye et al., 2014; Engberg et al., 2021). High-throughput sequencing technologies, such as metagenomic sequencing (Kim et al., 2021), will help to increase our understanding of the diversity and function of the gut microbiota and promote the development of new molecular diagnostic tools, thereby achieving accurate diagnosis and treatment of diseases.
Y. enterocolitica in the treatment of CD
Y. enterocolitica infection should be considered in the treatment of CD. Although Y. enterocolitica infection is self-limiting in most cases, there is a risk of systemic infection if patients receive immunosuppressive therapy due to presumptive CD. Timely antibiotic treatment is necessary for some patients with Y. enterocolitica infection complications. A study by George et al (Arnold et al., 2002). showed that ciprofloxacin, a first-line anti-Y. enterocolitica antibiotic may be an effective agent when added to the treatment of moderately active, resistant CD. We speculated that this result might be related to the clearance effect of ciprofloxacin on Y. enterocolitica. In addition, the presence of the high-pathogenicity island (HPI) in highly virulent strains (e.g., type 1B/O:8), which is responsible for the siderophore yersiniabactin-mediated iron uptake, facilitates the absorption and utilization of iron by Y. enterocolitica and promotes its growth under iron-limiting conditions (Bancerz-Kisiel et al., 2018). Thus, although iron-deficiency anemia is a common extraintestinal complication of CD (Stein et al., 2010; Azghari et al., 2016), Y. enterocolitica infection should be excluded prior to administering iron supplementation to anemic CD patients, as increased iron levels may increase the risk for sepsis (Cross et al., 2015). In addition, Y. enterocolitica should be considered in the treatment of CD, for it may alter drug activity through metabolism, thereby enhancing or inhibiting the clinical effect of the treatment.
Eliminating Y. enterocolitica may improve the symptoms of CD patients. For example, exclusive enteral nutrition (EEN) is used as an effective first-line treatment for inducing remission in pediatric Crohn’s disease (Ruemmele et al., 2014; Levine et al., 2019). It consists only of liquid nutrition that eliminates suspected food causative agents such as allergenic proteins, refined sugar, and pathogenic microorganisms including the Y. enterocolitica. In addition, there is evidence that EEN modulates the activity and structure of intestinal microbiota and consequently attenuates inflammation (Lionetti et al., 2005). The treatment of CD with probiotics has also gained substantial research interest (Guslandi et al., 2000; Sokol et al., 2008), as they exhibit high safety. Probiotics also have inhibitory effects on the presence of Y. enterocolitica infection through several mechanisms: 1) production of inhibitory substances, 2) blockade of intestinal surface adhesion sites, 3) nutrient competition, and 4) stimulation of mucosal and systemic immunity. For example, Bujalance et al. found that 20 strains of lactic acid bacteria had inhibitory effects on Y. enterocolitica and this inhibition was mainly attributed to the decrease in pH caused by glucose fermentation by lactic acid bacteria (Bujalance et al., 2014). Lactobacillus fermentum attenuates the proinflammatory effect of Y. enterocolitica on human epithelial cells (Frick et al., 2007). Both in vivo and in vitro experiments have shown that the probiotic Escherichia coli strain Nissle 1917 inhibits the invasion of Y. enterocolitica by secreting antibacterial compounds (Altenhoefer et al., 2004). Within the patient‐based studies, administration of Saccharomyces boulardii has been reported helpful in maintaining remission and bowel sealing (Garcia Vilela et al., 2008). However, we do note that, in some studies, probiotics did not perform better than placebo in inducing the remission of CD (Limketkai et al., 2020). More researches are needed to determine what strains and at what dose probiotics become more useful as part of a clinical intervention.
As described above, some strategies targeting Y. enterocolitica may have unexpected potential diagnostic and therapeutic applications in the treatment of CD. Development of agents with specific toxicity for Y. enterocolitica or its virulent strains is promising and merits more investment. In addition, based on the immunosuppressive effect of some Yops, these proteins may become innovative biological agents for the treatment of CD by inhibiting the release of chemokines and inflammatory factors and affecting the migration of neutrophils (Grabowski et al., 2017), thereby alleviating CD inflammation. The establishment of engineered bacteria or vaccines of Y. enterocolitica for reliable targeted drug delivery and intestinal immune regulation may provide unexpected potential treatments for abnormal submucosal immune responses in CD.
Discussion
In the past decades, there has been a substantial increase in the understanding of microbiota-host interactions. Specifically, there has been considerable advancements regarding the molecular mechanisms of Y. enterocolitica in host immune disorders. However, the role of Y. enterocolitica in CD pathogenesis remains unclear for several reasons.
First, some studies have declared that they failed to find differences in the Y. enterocolitica infection rates between CD patients and controls (Hugot et al., 2021). One reason for this different conclusion may be the detection method. Some clinical analyses (16S rRNA and metagenomic sequencing) of the gut microbiota in CD patients have primarily focused on stool samples (Khan et al., 2021). Using only stool to assess the gut microbiota can potentially dilute the signal of low-abundance bacteria, such as Y. enterocolitica, which are typically diluted by the high abundance of “transient bacteria”. Therefore, more appropriate clinical samples, such as mucosal lesions, Peyer’s patches, mesenteric lymph nodes, and creeping fat should be detected. Another factor that hinders understanding the close association between Y. enterocolitica and CD is the complex and diverse clinical manifestations of Y. enterocolitica infection. The clinical pathogenicity of different serotypes of Y. enterocolitica is different, and similarities of Y. enterocolitica infection with CD may only manifest with highly virulent strains. Thus, focusing on the role of pathogenic Y. enterocolitica in CD is essential to furthering this area of research. Additionally, other pathogens are also involved in the pathology of CD, such as adherent-invasive Escherichia coli, as reviewed by Rolhion et al (Rolhion and Darfeuille-Michaud, 2007). and Palmel et al (Palmela et al., 2018).
Second, the function and mechanism of Y. enterocolitica in the pathogenesis of CD are still uncertain. It has been reported that some Y. enterocolitica-positive patients were subsequently diagnosed with CD (Lamps et al., 2001; Zippi et al., 2006). However, evidence of an association between Y. enterocolitica and CD does not directly indicate a causal relationship between the two, nor does it rule out the possibility that Y. enterocolitica is only a confounding factor in the etiology of CD. In addition, CD patients are often immunodeficient, and as such, receive immunosuppressive treatment, which leads to an increased risk of infection with pathogenic bacteria, such as Y. enterocolitica. However, whether secondary infection of Y. enterocolitica affects the incidence rate, severity, and recurrence rate of CD is also worthy of further study.
Specifically, a multicenter, prospective study with large sample size, as well as additional studies using relevant genetically susceptible and gnotobiotic mice, coculture of gut-microbiota, immune cells and intestinal organoid on a chip, advanced sequencing technologies such as single-cell transcriptome and spatial transcriptome are needed to elucidate the development and progression of Y. enterocolitica in CD. Understanding the molecular mechanisms of the pathogenesis of these two diseases provides prospects for better diagnosis and treatment. More rapid and sensitive tools for the detection of Y. enterocolitica and strict food monitoring and management are conducive to reducing the chance of pathogenic Y. enterocolitica infection. They will help control gastrointestinal infections and even possibly CD. Innovative biological agents based on Y. enterocolitica, such as YOPs, genetically engineered bacteria, and vaccines, may also have unexpected potential therapeutic applications in the treatment of CD. In addition, considering the infection of Y. enterocolitica in CD patients and taking more proper management is very important. In summary, studying the relationship between Y. enterocolitica and CD may provide a basis for clarifying the etiology and pathogenesis of CD, formulating a reasonable and practical treatment plan, and determining the prognosis of the disease.
Author contributions
YB and Z-SL conceived the original idea. XF, LK, and Y-FQ collected and wrote the manuscript with contributions from all other authors. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No.81800479, No.82170568) and Shanghai Pujiang Program (No.22PJD015).
Acknowledgments
We thank Accdon (www.accdon.com) for linguistic assistance and pre-submission expert review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Achtman, M., Zurth, K., Morelli, G., Torrea, G., Guiyoule, A., Carniel, E. (1999). Yersinia pestis, the cause of plague, is a recently emerged clone of yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. U.S.A. 96, 14043–14048. doi: 10.1073/pnas.96.24.14043
Aepfelbacher, M. (2004). Modulation of rho GTPases by type III secretion system translocated effectors of yersinia. Rev. Physiol. Biochem. Pharmacol. 152, 65–77. doi: 10.1007/s10254-004-0035-3
Al Nabhani, Z., Dietrich, G., Hugot, J. P., Barreau, F. (2017). Nod2: The intestinal gate keeper. PloS Pathog. 13, e1006177. doi: 10.1371/journal.ppat.1006177
Altenhoefer, A., Oswald, S., Sonnenborn, U., Enders, C., Schulze, J., Hacker, J., et al. (2004). The probiotic escherichia coli strain nissle 1917 interferes with invasion of human intestinal epithelial cells by different enteroinvasive bacterial pathogens. FEMS Immunol. Med. Microbiol. 40, 223–229. doi: 10.1016/S0928-8244(03)00368-7
Amulic, B., Cazalet, C., Hayes, G. L., Metzler, K. D., Zychlinsky, A. (2012). Neutrophil function: from mechanisms to disease. Annu. Rev. Immunol. 30, 459–489. doi: 10.1146/annurev-immunol-020711-074942
Antonopoulos, P., Constantinidis, F., Charalampopoulos, G., Dalamarinis, K., Karanicas, I., Kokkini, G. (2008). An emergency diagnostic dilemma: A case of yersinia enterocolitica colitis mimicking acute appendicitis in a beta-thalassemia major patient: the role of CT and literature review. Emerg. Radiol. 15, 123–126. doi: 10.1007/s10140-007-0643-8
Appel, H., Rudwaleit, M., Wu, P., Grolms, M., Sieper, J., Mertz, A. (2002). Synovial T cell proliferation to the yersinia enterocolitica 19 kDa antigen differentiates yersinia triggered reactive arthritis (ReA) from ReA triggered by other enterobacteria. Ann. Rheum Dis. 61, 566–567. doi: 10.1136/ard.61.6.566
Arnold, G. L., Beaves, M. R., Pryjdun, V. O., Mook, W. J. (2002). Preliminary study of ciprofloxacin in active crohn's disease. Inflammation Bowel Dis. 8, 10–15. doi: 10.1097/00054725-200201000-00002
Autenrieth, I. B., Firsching, R. (1996). Penetration of m cells and destruction of peyer's patches by yersinia enterocolitica: an ultrastructural and histological study. J. Med. Microbiol. 44, 285–294. doi: 10.1099/00222615-44-4-285
Axelrad, J. E., Olen, O., Askling, J., Lebwohl, B., Khalili, H., Sachs, M. C., et al. (2019). Gastrointestinal infection increases odds of inflammatory bowel disease in a nationwide case-control study. Clin. Gastroenterol. Hepatol. 17, 1311–1322 e7. doi: 10.1016/j.cgh.2018.09.034
Azghari, I., Bargach, A., Billah, N. M., Essaoudi, M. A., Jahid, A., Kabbaj, N. (2016). Ileocecal resection for massive rectal bleeding due to yersinia enterocolitica: A case report and review of the literature. J. Med. Case Rep. 10, 6. doi: 10.1186/s13256-015-0786-2
Bancerz-Kisiel, A., Pieczywek, M., Łada, P., Szweda, W. (2018). The most important virulence markers of yersinia enterocolitica and their role during infection. Genes (Basel) 9 (5), 235. doi: 10.3390/genes9050235
Barz, C., Abahji, T. N., Trulzsch, K., Heesemann, J. (2000). The yersinia Ser/Thr protein kinase YpkA/YopO directly interacts with the small GTPases RhoA and rac-1. FEBS Lett. 482, 139–143. doi: 10.1016/S0014-5793(00)02045-7
Baumgart, D. C., Sandborn, W. J. (2012). Crohn's disease. Lancet 380, 1590–1605. doi: 10.1016/S0140-6736(12)60026-9
Black, R. E., Slome, S. (1988). Yersinia enterocolitica. Infect. Dis. Clin. North Am. 2, 625–641. doi: 10.1016/S0891-5520(20)30215-4
Bliska, J. B., Guan, K. L., Dixon, J. E., Falkow, S. (1991). Tyrosine phosphate hydrolysis of host proteins by an essential yersinia virulence determinant. Proc. Natl. Acad. Sci. U.S.A. 88, 1187–1191. doi: 10.1073/pnas.88.4.1187
Boland, A., Cornelis, G. R. (1998). Role of YopP in suppression of tumor necrosis factor alpha release by macrophages during yersinia infection. Infection Immun. 66, 1878–1884. doi: 10.1128/IAI.66.5.1878-1884.1998
Boltin, D., Perets, T. T., Vilkin, A., Niv, Y. (2013). Mucin function in inflammatory bowel disease: an update. J. Clin. Gastroenterol. 47, 106–111. doi: 10.1097/MCG.0b013e3182688e73
Bottone, E. J. (1977). Yersinia enterocolitica: a panoramic view of a charismatic microorganism. CRC Crit. Rev. Microbiol. 5, 211–241. doi: 10.3109/10408417709102312
Bottone, E. J. (1999). Yersinia enterocolitica: overview and epidemiologic correlates. Microbes Infect. 1, 323–333. doi: 10.1016/S1286-4579(99)80028-8
Brodsky, I. E., Palm, N. W., Sadanand, S., Ryndak, M. B., Sutterwala, F. S., Flavell, R. A., et al. (2010). A yersinia effector protein promotes virulence by preventing inflammasome recognition of the type III secretion system. Cell Host Microbe 7, 376–387. doi: 10.1016/j.chom.2010.04.009
Bronner, M. P. (2004). Granulomatous appendicitis and the appendix in idiopathic inflammatory bowel disease. Semin. Diagn. Pathol. 21, 98–107. doi: 10.1053/j.semdp.2004.12.001
Bujalance, C., Jimenez-Valera, M., Moreno, E., Ruiz-Lopez, M. D., Lasserrot, A., Ruiz-Bravo, A. (2014). Lack of correlation between in vitro antibiosis and in vivo protection against enteropathogenic bacteria by probiotic lactobacilli. Res. Microbiol. 165, 14–20. doi: 10.1016/j.resmic.2013.10.006
Centers for Disease, C. (2011). And prevention, notes from the field: Yersinia enterocolitica infections associated with pasteurized milk — southwestern Pennsylvania, march-august, 2011. MMWR Morb Mortal Wkly Rep. 60, 1428.
Chassaing, B., Koren, O., Goodrich, J. K., Poole, A. C., Srinivasan, S., Ley, R. E., et al. (2015). Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 519, 92–96. doi: 10.1038/nature14232
China, B., Sory, M. P., N'Guyen, B. T., De Bruyere, M., Cornelis, G. R. (1993). Role of the YadA protein in prevention of opsonization of yersinia enterocolitica by C3b molecules. Infect. Immun. 61, 3129–3136. doi: 10.1128/iai.61.8.3129-3136.1993
Cian, S., Tabacco, O., Costaguta, A. (2020). Terminal ileitis due to yersinia enterocolitica: differential diagnosis with crohn s disease in a 12-year-old male. Arch. Argent Pediatr. 118, e191–e193. doi: 10.5546/aap.2020.e191
Cornelis, G. R. (2006). The type III secretion injectisome. Nat. Rev. Microbiol. 4, 811–825. doi: 10.1038/nrmicro1526
Cornelis, G. R. (2010). The type III secretion injectisome, a complex nanomachine for intracellular 'toxin' delivery. Biol. Chem. 391, 745–751. doi: 10.1515/bc.2010.079
Cross, J. H., Bradbury, R. S., Fulford, A. J., Jallow, A. T., Wegmuller, R., Prentice, A. M., et al. (2015). Oral iron acutely elevates bacterial growth in human serum. Sci. Rep. 5, 16670. doi: 10.1038/srep16670
Davicino, R. C., Mendez-Huergo, S. P., Elicabe, R. J., Stupirski, J. C., Autenrieth, I., Di Genaro, M. S., et al. (2017). Galectin-1-Driven tolerogenic programs aggravate yersinia enterocolitica infection by repressing antibacterial immunity. J. Immunol. 199, 1382–1392. doi: 10.4049/jimmunol.1700579
De Cruz, P., Kang, S., Wagner, J., Buckley, M., Sim, W. H., Prideaux, L., et al. (2015). Association between specific mucosa-associated microbiota in crohn's disease at the time of resection and subsequent disease recurrence: a pilot study. J. Gastroenterol. Hepatol. 30, 268–278. doi: 10.1111/jgh.12694
de Koning-Ward, T. F., Ward, A. C., Robins-Browne, R. M. (1994). Characterisation of the urease-encoding gene complex of yersinia enterocolitica. Gene 145, 25–32. doi: 10.1016/0378-1119(94)90318-2
Delor, I., Kaeckenbeeck, A., Wauters, G., Cornelis, G. R. (1990). Nucleotide sequence of yst, the yersinia enterocolitica gene encoding the heat-stable enterotoxin, and prevalence of the gene among pathogenic and nonpathogenic yersiniae. Infect. Immun. 58, 2983–2988. doi: 10.1128/iai.58.9.2983-2988.1990
Demes, J., Kay, C., Morales-Cardona, A., Subramanian, S. (2021). Severe acute liver injury associated with yersinia enterocolitica. BMJ Case Rep. 14 (7), e242369. doi: 10.1136/bcr-2021-242369
Denecker, G., Totemeyer, S., Mota, L. J., Troisfontaines, P., Lambermont, I., Youta, C., et al. (2002). Effect of low- and high-virulence yersinia enterocolitica strains on the inflammatory response of human umbilical vein endothelial cells. Infect. Immun. 70, 3510–3520. doi: 10.1128/IAI.70.7.3510-3520.2002
Dhar, M. S., Virdi, J. S. (2014). Strategies used by yersinia enterocolitica to evade killing by the host: thinking beyond yops. Microbes Infect. 16, 87–95. doi: 10.1016/j.micinf.2013.11.002
Dianbin Sun, W. P. R. J., Wei, W. (1987). Ying zhang, xiaoqing Wang, penglin Li, minghe jia, donglou xiao, ruihe cai, xiaochun Wang. Study Outbreak Yersinia Enterocoiitica Contammated Raw Beef. Chin. J. Zoonoses 05, 2–4.
El Tahir, Y., Skurnik, M. (2001). YadA, the multifaceted yersinia adhesin. Int. J. Med. Microbiol. IJMM 291, 209–218. doi: 10.1078/1438-4221-00119
Engberg, J., Vejrum, L. K., Madsen, T. V., Nielsen, X. C. (2021). Verification of analytical bacterial spectrum of QIAstat-Dx(R) GI V2 and Novodiag(R) bacterial GE+ V2-0 diagnostic panels. J. Antimicrob. Chemother. 76, iii50–iii57. doi: 10.1093/jac/dkab242
Erridge, C., Bennett-Guerrero, E., Poxton, I. R. (2002). Structure and function of lipopolysaccharides. Microbes Infect. 4, 837–851. doi: 10.1016/S1286-4579(02)01604-0
Felek, S., Krukonis, E. S. (2009). The yersinia pestis ail protein mediates binding and yop delivery to host cells required for plague virulence. Infect. Immun. 77, 825–836. doi: 10.1128/IAI.00913-08
Fonseca, D. M., Hand, T. W., Han, S. J., Gerner, M. Y., Glatman Zaretsky, A., Byrd, A. L., et al. (2015). Microbiota-dependent sequelae of acute infection compromise tissue-specific immunity. Cell 163, 354–366. doi: 10.1016/j.cell.2015.08.030
Franczak, P., Witzling, M., Siczewski, W. (2018). Yersiniosis or lesniowski-crohn's disease. Pol. Przegl Chir 90, 52–54. doi: 10.5604/01.3001.0011.5966
Frederiksen, W. (1964). A study of some yersinia pseudotuberculosis-like bacteria (Bacterium enterocoliticum and pasteurella X). Scand. Congr. Pathol. Microbiol. 14, 103–104.
Frick, J. S., Schenk, K., Quitadamo, M., Kahl, F., Koberle, M., Bohn, E., et al. (2007). Lactobacillus fermentum attenuates the proinflammatory effect of yersinia enterocolitica on human epithelial cells. Inflammation Bowel Dis. 13, 83–90. doi: 10.1002/ibd.20009
Fujimura, Y., Kamoi, R., Iida, M. (1996). Pathogenesis of aphthoid ulcers in crohn's disease: correlative findings by magnifying colonoscopy, electron microscopy, and immunohistochemistry. Gut 38, 724–732. doi: 10.1136/gut.38.5.724
Gajendran, M., Loganathan, P., Catinella, A. P., Hashash, J. G. (2018). A comprehensive review and update on crohn's disease. Dis. Mon 64, 20–57. doi: 10.1016/j.disamonth.2017.07.001
Garcia Vilela, E., De Lourdes De Abreu Ferrari, M., Oswaldo Da Gama Torres, H., Guerra Pinto, A., Carolina Carneiro Aguirre, A., Paiva Martins, F., et al. (2008). Influence of saccharomyces boulardii on the intestinal permeability of patients with crohn's disease in remission. Scandinavian J. Gastroenterol. 43, 842–848. doi: 10.1080/00365520801943354
Gevers, D., Kugathasan, S., Denson, L. A., Vazquez-Baeza, Y., Van Treuren, W., Ren, B., et al. (2014). The treatment-naive microbiome in new-onset crohn's disease. Cell Host Microbe 15, 382–392. doi: 10.1016/j.chom.2014.02.005
Giegerich, E., Ayodele, O., John, A., Hughes, M., van der Pluijm, W., Gezmu, T. (2018). Estimating the global diagnosed prevalence of crohn's disease 2017-2027. Value Health 21, S222. doi: 10.1016/j.jval.2018.04.1500
Gleason, T. H., Patterson, S. D. (1982). The pathology of yersinia enterocolitica ileocolitis. Am. J. Surg. Pathol. 6, 347–355. doi: 10.1097/00000478-198206000-00007
Golkocheva-Markova, E., Christova, I., Stoilov, R., Najdenski, H. (2008). Cross-reaction between Yersinia outer membrane proteins and anti-Borreli antibodies in sera of patients with Lyme disease. Clin. Microbiol. Infect. 14 (9), 873–875. doi: 10.1111/j.1469-0691.2008.02051.x
Grabowski, B., Schmidt, M. A., Ruter, C. (2017). Immunomodulatory yersinia outer proteins (Yops)-useful tools for bacteria and humans alike. Virulence 8, 1124–1147. doi: 10.1080/21505594.2017.1303588
Guan, K. L., Dixon, J. E. (1990). Protein tyrosine phosphatase activity of an essential virulence determinant in yersinia. Science 249, 553–556. doi: 10.1126/science.2166336
Guslandi, M., Mezzi, G., Sorghi, M., Testoni, P. A. (2000). Saccharomyces boulardii in maintenance treatment of crohn’s disease. Digestive Dis. Sci. 45, 1462–1464. doi: 10.1023/A:1005588911207
Ha, C. W. Y., Martin, A., Sepich-Poore, G. D., Shi, B., Wang, Y., Gouin, K., et al. (2020). Translocation of viable gut microbiota to mesenteric adipose drives formation of creeping fat in humans. Cell 183, 666–683 e17. doi: 10.1016/j.cell.2020.09.009
Hampe, J., Cuthbert, A., Croucher, P. J., Mirza, M. M., Mascheretti, S., Fisher, S., et al. (2001). Association between insertion mutation in NOD2 gene and crohn's disease in German and British populations. Lancet 357, 1925–1928. doi: 10.1016/S0140-6736(00)05063-7
Hampe, J., Franke, A., Rosenstiel, P., Till, A., Teuber, M., Huse, K., et al. (2007). A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for crohn disease in ATG16L1. Nat. Genet. 39, 207–211. doi: 10.1038/ng1954
Han, S. J., Glatman Zaretsky, A., Andrade-Oliveira, V., Collins, N., Dzutsev, A., Shaik, J., et al. (2017). White adipose tissue is a reservoir for memory T cells and promotes protective memory responses to infection. Immunity 47, 1154–1168 e6. doi: 10.1016/j.immuni.2017.11.009
Hässig, A., Karrer, J., Pusterla, F. (1949). Über pseudotuberkulose beim menschen. Schweiz. Med. Wschr 79, 971–973.
Hein, J., Kempf, V. A., Diebold, J., Bucheler, N., Preger, S., Horak, I., et al. (2000). Interferon consensus sequence binding protein confers resistance against yersinia enterocolitica. Infect. Immun. 68, 1408–1417. doi: 10.1128/IAI.68.3.1408-1417.2000
Heise, T., Dersch, P. (2006). Identification of a domain in yersinia virulence factor YadA that is crucial for extracellular matrix-specific cell adhesion and uptake. Proc. Natl. Acad. Sci. United States America 103, 3375–3380. doi: 10.1073/pnas.0507749103
Hugot, J. P., Alberti, C., Berrebi, D., Bingen, E., Cezard, J. P. (2003). Crohn's disease: the cold chain hypothesis. Lancet 362, 2012–2015. doi: 10.1016/S0140-6736(03)15024-6
Hugot, J. P., Dumay, A., Barreau, F., Meinzer, U. (2021). Crohn's disease: Is the cold chain hypothesis still hot? J. Crohns Colitis 15, 678–686. doi: 10.1093/ecco-jcc/jjaa192
Iriarte, M., Cornelis, G. R. (1998). YopT, a new yersinia yop effector protein, affects the cytoskeleton of host cells. Mol. Microbiol. 29, 915–929. doi: 10.1046/j.1365-2958.1998.00992.x
Iwobi, A., Heesemann, J., Garcia, E., Igwe, E., Noelting, C., Rakin, A. (2003). Novel virulence-associated type II secretion system unique to high-pathogenicity yersinia enterocolitica. Infect. Immun. 71, 1872–1879. doi: 10.1128/IAI.71.4.1872-1879.2003
Kallinowski, F., Wassmer, A., Hofmann, M. A., Harmsen, D., Heesemann, J., Karch, H., et al. (1998). Prevalence of enteropathogenic bacteria in surgically treated chronic inflammatory bowel disease. Hepatogastroenterology 45, 1552–1558.
Kamdar, K., Khakpour, S., Chen, J., Leone, V., Brulc, J., Mangatu, T., et al. (2016). Genetic and metabolic signals during acute enteric bacterial infection alter the microbiota and drive progression to chronic inflammatory disease. Cell Host Microbe 19, 21–31. doi: 10.1016/j.chom.2015.12.006
Kaneko, K., Hashimoto, N. (1981). Occurrence of yersinia enterocolitica in wild animals. Appl. Environ. Microbiol. 41, 635–638. doi: 10.1128/aem.41.3.635-638.1981
Kerschen, E. J., Cohen, D. A., Kaplan, A. M., Straley, S. C. (2004). The plague virulence protein YopM targets the innate immune response by causing a global depletion of NK cells. Infect. Immun. 72, 4589–4602. doi: 10.1128/IAI.72.8.4589-4602.2004
Khan, I. A., Nayak, B., Markandey, M., Bajaj, A., Verma, M., Kumar, S., et al. (2021). Differential prevalence of pathobionts and host gene polymorphisms in chronic inflammatory intestinal diseases: Crohn's disease and intestinal tuberculosis. PloS One 16, e0256098. doi: 10.1371/journal.pone.0256098
Kim, H., Cho, J. H., Song, M., Cho, J. H., Kim, S., Kim, E. S., et al. (2021). Evaluating the prevalence of foodborne pathogens in livestock using metagenomics approach. J. Microbiol. Biotechnol. 31, 1701–1708. doi: 10.4014/jmb.2109.09038
Kobayashi, T., Steinbach, E. C., Russo, S. M., Matsuoka, K., Nochi, T., Maharshak, N., et al. (2014). NFIL3-deficient mice develop microbiota-dependent, IL-12/23-driven spontaneous colitis. J. Immunol. 192, 1918–1927. doi: 10.4049/jimmunol.1301819
Kobe, B., Deisenhofer, J. (1994). The leucine-rich repeat: a versatile binding motif. Trends Biochem. Sci. 19, 415–421. doi: 10.1016/0968-0004(94)90090-6
Krauss, E., Agaimy, A., Neumann, H., Schulz, U., Kessler, H., Hartmann, A., et al. (2012). Characterization of lymphoid follicles with red ring signs as first manifestation of early crohn's disease by conventional histopathology and confocal laser endomicroscopy. Int. J. Clin. Exp. Pathol. 5, 411–421.
Lai, C. H., Lin, J. N., Chen, Y. H., Chang, L. L., Huang, W. Y., Ku, H. P., et al. (2014). The first imported human case of yersinia pseudotuberculosis serotype O1 septicemia presents with acute appendicitis-like syndrome in Taiwan. J. Formos Med. Assoc. 113, 656–659. doi: 10.1016/j.jfma.2011.06.020
Lamps, L. W., Madhusudhan, K. T., Greenson, J. K., Pierce, R. H., Massoll, N. A., Chiles, M. C., et al. (2001). The role of yersinia enterocolitica and yersinia pseudotuberculosis in granulomatous appendicitis: a histologic and molecular study. Am. J. Surg. Pathol. 25, 508–515. doi: 10.1097/00000478-200104000-00011
Lamps, L. W., Madhusudhan, K. T., Havens, J. M., Greenson, J. K., Bronner, M. P., Chiles, M. C., et al. (2003). Pathogenic yersinia DNA is detected in bowel and mesenteric lymph nodes from patients with crohn's disease. Am. J. Surg. Pathol. 27, 220–227. doi: 10.1097/00000478-200302000-00011
Laporte, J., Savin, C., Lamourette, P., Devilliers, K., Volland, H., Carniel, E., et al. (2015). Fast and sensitive detection of enteropathogenic yersinia by immunoassays. J. Clin. Microbiol. 53, 146–159. doi: 10.1128/JCM.02137-14
LaRock, C. N., Cookson, B. T. (2012). The yersinia virulence effector YopM binds caspase-1 to arrest inflammasome assembly and processing. Cell Host Microbe 12, 799–805. doi: 10.1016/j.chom.2012.10.020
Le Baut, G., O'Brien, C., Pavli, P., Roy, M., Seksik, P., Treton, X., et al. (2018). Prevalence of yersinia species in the ileum of crohn's disease patients and controls. Front. Cell Infect. Microbiol. 8, 336. doi: 10.3389/fcimb.2018.00336
Leu, S. B., Shulman, S. C., Steelman, C. K., Lamps, L. W., Bulut, O. P., Abramowsky, C. R., et al. (2013). Pathogenic yersinia DNA in intestinal specimens of pediatric patients with crohn's disease. Fetal Pediatr. Pathol. 32, 367–370. doi: 10.3109/15513815.2013.768744
Levine, A., Wine, E., Assa, A., Sigall Boneh, R., Shaoul, R., Kori, M., et al. (2019). Crohn's disease exclusion diet plus partial enteral nutrition induces sustained remission in a randomized controlled trial. Gastroenterology 157, 440–450 e8. doi: 10.1053/j.gastro.2019.04.021
Limketkai, B. N., Akobeng, A. K., Gordon, M., Adepoju, A. A. (2020). Probiotics for induction of remission in crohn's disease. Cochrane Database Syst. Rev. 7, CD006634. doi: 10.1002/14651858.CD006634.pub3
Lionetti, P., Callegari, M. L., Ferrari, S., Cavicchi, M. C., Pozzi, E., de Martino, M., et al. (2005). Enteral nutrition and microflora in pediatric crohn's disease. JPEN. J. parenteral enteral Nutr. 29, S173–S175. doi: 10.1177/01486071050290S4S173
Lockhart-Mummery, H. E., Morson, B. C. (1960). Crohn's disease (regional enteritis) of the large intestine and its distinction from ulcerative colitis. Gut 1, 87–105. doi: 10.1136/gut.1.2.87
Lucero-Estrada, C., Favier, G. I., Escudero, M. E. (2020). An overview of yersinia enterocolitica and related species in samples of different origin from San Luis, Argentina. Food Microbiol. 86, 103345. doi: 10.1016/j.fm.2019.103345
Maldonado, R. F., Sa-Correia, I., Valvano, M. A. (2016). Lipopolysaccharide modification in gram-negative bacteria during chronic infection. FEMS Microbiol. Rev. 40, 480–493. doi: 10.1093/femsre/fuw007
Marenne, M. N., Journet, L., Mota, L. J., Cornelis, G. R. (2003). Genetic analysis of the formation of the ysc-yop translocation pore in macrophages by yersinia enterocolitica: role of LcrV, YscF and YopN. Microb. Pathog. 35, 243–258. doi: 10.1016/S0882-4010(03)00154-2
McIver, M., Pike, R. (1934). Chronic glanders-like infection of face caused by an organism resembling flavobacterium pseudomallei whitmore. Clin. miscellany 1, 16–21.
McPhee, J. B., Mena, P., Bliska, J. B. (2010). Delineation of regions of the yersinia YopM protein required for interaction with the RSK1 and PRK2 host kinases and their requirement for interleukin-10 production and virulence. Infect. Immun. 78, 3529–3539. doi: 10.1128/IAI.00269-10
Mills, S. D., Boland, A., Sory, M. P., van der Smissen, P., Kerbourch, C., Finlay, B. B., et al. (1997). Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc. Natl. Acad. Sci. U.S.A. 94, 12638–12643. doi: 10.1073/pnas.94.23.12638
Molodecky, N. A., Soon, I. S., Rabi, D. M., Ghali, W. A., Ferris, M., Chernoff, G., et al. (2012). Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142, 46–54 e42. doi: 10.1053/j.gastro.2011.10.001
Montagner, C., Arquint, C., Cornelis, G. R. (2011). Translocators YopB and YopD from yersinia enterocolitica form a multimeric integral membrane complex in eukaryotic cell membranes. J. Bacteriol 193, 6923–6928. doi: 10.1128/JB.05555-11
Motin, V. L., Nakajima, R., Smirnov, G. B., Brubaker, R. R. (1994). Passive immunity to yersiniae mediated by anti-recombinant V antigen and protein a-V antigen fusion peptide. Infect. Immun. 62, 4192–4201. doi: 10.1128/iai.62.10.4192-4201.1994
Muhlenkamp, M. C., Hallstrom, T., Autenrieth, I. B., Bohn, E., Linke, D., Rinker, J., et al. (2017). Vitronectin binds to a specific stretch within the head region of yersinia adhesin a and thereby modulates yersinia enterocolitica host interaction. J. Innate Immun. 9, 33–51. doi: 10.1159/000449200
Murthy, A., Li, Y., Peng, I., Reichelt, M., Katakam, A. K., Noubade, R., et al. (2014). A crohn's disease variant in Atg16l1 enhances its degradation by caspase 3. Nature 506, 456–462. doi: 10.1038/nature13044
Naddei, R., Martinelli, M., Strisciuglio, C., D'Armiento, M., Vollaro, A., Staiano, A., et al. (2017). Yersinia enterocolitica ileitis mimicking pediatric crohn's disease. Inflammation Bowel Dis. 23, E15–E16. doi: 10.1097/MIB.0000000000001052
Navarro, L., Alto, N. M., Dixon, J. E. (2005). Functions of the yersinia effector proteins in inhibiting host immune responses. Curr. Opin. Microbiol. 8, 21–27. doi: 10.1016/j.mib.2004.12.014
Netea, M. G., van der Leij, F., Drenth, J. P., Joosten, L. A., te Morsche, R., Verweij, P., et al. (2010). Chronic yersiniosis due to defects in the TLR5 and NOD2 recognition pathways. Neth J. Med. 68, 310–315.
Paerregaard, A., Shand, G. H., Gaarslev, K., Espersen, F. (1991). Comparison of crossed immunoelectrophoresis, enzyme-linked immunosorbent assays, and tube agglutination for serodiagnosis of yersinia enterocolitica serotype O:3 infection. J. Clin. Microbiol. 29, 302–309. doi: 10.1128/jcm.29.2.302-309.1991
Palmela, C., Chevarin, C., Xu, Z., Torres, J., Sevrin, G., Hirten, R., et al. (2018). Adherent-invasive escherichia coli in inflammatory bowel disease. Gut 67, 574–587. doi: 10.1136/gutjnl-2017-314903
Park, H., Teja, K., O'Shea, J. J., Siegel, R. M. (2007). The yersinia effector protein YpkA induces apoptosis independently of actin depolymerization. J. Immunol. 178, 6426–6434. doi: 10.4049/jimmunol.178.10.6426
Payne, M., Girdwood, A. H., Roost, R. W., Freson, M. J., Kottler, R. E. (1987). Yersinia enterocolitica and crohn's disease. A Case Rep. S Afr Med. J. 72, 53–55.
Pelludat, C., Rakin, A., Jacobi, C. A., Schubert, S., Heesemann, J. (1998). The yersiniabactin biosynthetic gene cluster of yersinia enterocolitica: organization and siderophore-dependent regulation. J. Bacteriol 180, 538–546. doi: 10.1128/JB.180.3.538-546.1998
Peyrin-Biroulet, L., Chamaillard, M., Gonzalez, F., Beclin, E., Decourcelle, C., Antunes, L., et al. (2007). Mesenteric fat in crohn's disease: a pathogenetic hallmark or an innocent bystander? Gut 56, 577–583. doi: 10.1136/gut.2005.082925
Revell, P. A., Miller, V. L. (2001). Yersinia virulence: more than a plasmid. FEMS Microbiol. Lett. 205, 159–164. doi: 10.1111/j.1574-6968.2001.tb10941.x
Riahi, S. M., Ahmadi, E., Zeinali, T. (2021). Global prevalence of yersinia enterocolitica in cases of gastroenteritis: A systematic review and meta-analysis. Int. J. Microbiol. 2021, 1499869. doi: 10.1155/2021/1499869
Righetto, R. D., Anton, L., Adaixo, R., Jakob, R. P., Zivanov, J., Mahi, M. A., et al. (2020). High-resolution cryo-EM structure of urease from the pathogen yersinia enterocolitica. Nat. Commun. 11, 5101. doi: 10.1038/s41467-020-18870-2
Rolhion, N., Darfeuille-Michaud, A. (2007). Adherent-invasive escherichia coli in inflammatory bowel disease. Inflammation Bowel Dis. 13, 1277–1283. doi: 10.1002/ibd.20176
Ruckdeschel, K., Mannel, O., Richter, K., Jacobi, C. A., Trulzsch, K., Rouot, B., et al. (2001). Yersinia outer protein p of yersinia enterocolitica simultaneously blocks the nuclear factor-kappa b pathway and exploits lipopolysaccharide signaling to trigger apoptosis in macrophages. J. Immunol. 166, 1823–1831. doi: 10.4049/jimmunol.166.3.1823
Ruemmele, F. M., Veres, G., Kolho, K. L., Griffiths, A., Levine, A., Escher, J. C., et al. (2014). Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric crohn's disease. J. Crohns Colitis 8, 1179–1207. doi: 10.1016/j.crohns.2014.04.005
Safa, G., Loppin, M., Tisseau, L., Lamoril, J. (2008). Cutaneous aseptic neutrophilic abscesses and yersinia enterocolitica infection in a case subsequently diagnosed as crohn's disease. Dermatology 217, 340–342. doi: 10.1159/000155646
Sazonovs, A., Stevens, C. R., Venkataraman, G. R., Yuan, K., Avila, B., Abreu, M. T., et al. (2022). Large-scale sequencing identifies multiple genes and rare variants associated with Crohn's disease susceptibility. Nat. Genet. 54 (9), 1275–1283. doi: 10.1038/s41588-022-01156-2
Schleifstein, J., Coleman, M. (1939). An unidentified microorganism resembling b. lignieri and past, pseudotuberculosis, and pathogenic for man. New York State J. Med. 39, 1749–1753.
Schmid, Y., Grassl, G. A., Buhler, O. T., Skurnik, M., Autenrieth, I. B., Bohn, E. (2004). Yersinia enterocolitica adhesin a induces production of interleukin-8 in epithelial cells. Infect. Immun. 72, 6780–6789. doi: 10.1128/IAI.72.12.6780-6789.2004
Schotte, P., Denecker, G., Van Den Broeke, A., Vandenabeele, P., Cornelis, G. R., Beyaert, R. (2004). Targeting Rac1 by the yersinia effector protein YopE inhibits caspase-1-mediated maturation and release of interleukin-1beta. J. Biol. Chem. 279, 25134–25142. doi: 10.1074/jbc.M401245200
Schulte, R., Kerneis, S., Klinke, S., Bartels, H., Preger, S., Kraehenbuhl, J. P., et al. (2000). Translocation of yersinia entrocolitica across reconstituted intestinal epithelial monolayers is triggered by yersinia invasin binding to beta1 integrins apically expressed on m-like cells. Cell Microbiol. 2, 173–185. doi: 10.1046/j.1462-5822.2000.00047.x
Selby, W., Pavli, P., Crotty, B., Florin, T., Radford-Smith, G., Gibson, P., et al. (2007). Antibiotics in crohn's disease study, two-year combination antibiotic therapy with clarithromycin, rifabutin, and clofazimine for crohn's disease. Gastroenterology 132, 2313–2319. doi: 10.1053/j.gastro.2007.03.031
Sheehan, A. L., Warren, B. F., Gear, M. W., Shepherd, N. A. (1992). Fat-wrapping in crohn's disease: pathological basis and relevance to surgical practice. Br. J. Surg. 79, 955–958. doi: 10.1002/bjs.1800790934
Shi, H. Y., Ng, S. C. (2018). The state of the art on treatment of crohn's disease. J. Gastroenterol. 53, 989–998. doi: 10.1007/s00535-018-1479-6
Shutinoski, B., Schmidt, M. A., Heusipp, G. (2010). Transcriptional regulation of the Yts1 type II secretion system of yersinia enterocolitica and identification of secretion substrates. Mol. Microbiol. 75, 676–691. doi: 10.1111/j.1365-2958.2009.06998.x
Sokol, H., Pigneur, B., Watterlot, L., Lakhdari, O., Bermudez-Humaran, L. G., Gratadoux, J. J., et al. (2008). Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of crohn disease patients. Proc. Natl. Acad. Sci. U.S.A. 105, 16731–16736. doi: 10.1073/pnas.0804812105
Sonnevend, A., Czirok, E., Pal, T. (2005). Yersinia yop-specific IgA antibodies in Hungarian blood donors. Folia Microbiol. (Praha) 50, 269–272. doi: 10.1007/BF02931576
Spackova, M., Daniel, O., Klimesova, P., Ileninova, Z. (2022). Overview of basic epidemiological characteristics and descriptive analysis of the incidence of human yersiniosis in the Czech republic in 2018-2020. Epidemiol. Mikrobiol Imunol 71, 32–39.
Stein, J., Hartmann, F., Dignass, A. U. (2010). Diagnosis and management of iron deficiency anemia in patients with IBD. Nat. Rev. Gastroenterol. Hepatol. 7, 599–610. doi: 10.1038/nrgastro.2010.151
Sulakvelidze, A. (2000). Yersiniae other than y. enterocolitica, y. pseudotuberculosis and y. pestis: the ignored species. Microbes Infect. 2, 497–513. doi: 10.1016/S1286-4579(00)00311-7
Sunada, K., Yamamoto, H., Yano, T., Sugano, K. (2009). Advances in the diagnosis and treatment of small bowel lesions with crohn's disease using double-balloon endoscopy. Therap Adv. Gastroenterol. 2, 357–366. doi: 10.1177/1756283X09343542
Teh, J. J., Berendsen, E. M., Hoedt, E. C., Kang, S., Zhang, J., Zhang, F., et al. (2021). Novel strain-level resolution of crohn's disease mucosa-associated microbiota via an ex vivo combination of microbe culture and metagenomic sequencing. ISME J. 15, 3326–3338. doi: 10.1038/s41396-021-00991-1
Torres, J., Mehandru, S., Colombel, J. F., Peyrin-Biroulet, L. (2017). Crohn's disease. Lancet 389, 1741–1755. doi: 10.1016/S0140-6736(16)31711-1
Triantafillidis, J. K., Thomaidis, T., Papalois, A. (2020). Terminal ileitis due to yersinia infection: An underdiagnosed situation. BioMed. Res. Int. 2020, 1240626. doi: 10.1155/2020/1240626
Van Kruiningen, H. J., Joossens, M., Vermeire, S., Joossens, S., Debeugny, S., Gower-Rousseau, C., et al. (2005). Environmental factors in familial crohn's disease in Belgium. Inflammation Bowel Dis. 11, 360–365. doi: 10.1097/01.MIB.0000158536.31557.90
Venecia, K., Young, G. M. (2005). Environmental regulation and virulence attributes of the ysa type III secretion system of yersinia enterocolitica biovar 1B. Infect. Immun. 73, 5961–5977. doi: 10.1128/IAI.73.9.5961-5977.2005
Viboud, G. I., Mejia, E., Bliska, J. B. (2006). Comparison of YopE and YopT activities in counteracting host signalling responses to yersinia pseudotuberculosis infection. Cell Microbiol. 8, 1504–1515. doi: 10.1111/j.1462-5822.2006.00729.x
Viteri, A. L., Howard, P. H., May, J. L., Ramesh, G. S., Roberts, J. W. (1981). Hepatic abscess due to yersinia enterocolitica without bacteremia. Gastroenterology 81, 592–593. doi: 10.1016/0016-5085(81)90615-6
Weterman, I. T., Pena, A. S. (1984). Familial incidence of crohn's disease in the Netherlands and a review of the literature. Gastroenterology 86, 449–452. doi: 10.1016/S0016-5085(84)80014-1
Ye, Y. W., Ling, N., Han, Y. J., Wu, Q. P. (2014). Detection and prevalence of pathogenic yersinia enterocolitica in refrigerated and frozen dairy products by duplex PCR and dot hybridization targeting the virF and ail genes. J. Dairy Sci. 97, 6785–6791. doi: 10.3168/jds.2014-8382
Young, G. M., Schmiel, D. H., Miller, V. L. (1999). A new pathway for the secretion of virulence factors by bacteria: the flagellar export apparatus functions as a protein-secretion system. Proc. Natl. Acad. Sci. U.S.A. 96, 6456–6461. doi: 10.1073/pnas.96.11.6456
Zakharchuk, S. S. (1965). [Fundamentals of the problem of congenital toxoplasmosis and tasks concerning their further study]. Med. Parazitol (Mosk) 34, 597–601.
Zippi, M., Colaiacomo, M. C., Marcheggiano, A., Pica, R., Paoluzi, P., Iaiani, G., et al. (2006). Mesenteric adenitis caused by yersinia pseudotubercolosis in a patient subsequently diagnosed with crohn's disease of the terminal ileum. World J. Gastroenterol. 12, 3933–3935. doi: 10.3748/wjg.v12.i24.3933
Keywords: Yersinia enterocolitica, Crohn’s disease, immune system, inflammation bowel disease, gut microbiota
Citation: Fang X, Kang L, Qiu Y-F, Li Z-S and Bai Y (2023) Yersinia enterocolitica in Crohn’s disease. Front. Cell. Infect. Microbiol. 13:1129996. doi: 10.3389/fcimb.2023.1129996
Received: 22 December 2022; Accepted: 13 February 2023;
Published: 08 March 2023.
Edited by:
Shiming Yang, Xinqiao Hospital, ChinaReviewed by:
Michael Bording-Jorgensen, University of Alberta, CanadaHong Guo, Chongqing General Hospital, China
Copyright © 2023 Fang, Kang, Qiu, Li and Bai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhao-Shen Li, bGkuemhhb3NoZW5AaG90bWFpbC5jb20=; Yu Bai, YmFpeXUxOTk4QGhvdG1haWwuY29t
†These authors have contributed equally to this work
 Xue Fang
Xue Fang Le Kang†
Le Kang† Yi-Fan Qiu
Yi-Fan Qiu Zhao-Shen Li
Zhao-Shen Li Yu Bai
Yu Bai