
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 07 March 2022
Sec. Virus and Host
Volume 12 - 2022 | https://doi.org/10.3389/fcimb.2022.860559
This article is part of the Research Topic Zoonoses and Transboundary Infections: A One-Health Perspective View all 6 articles
Human adenovirus (HAdV) has a worldwide distribution and remains a major pathogen that leads to infections of the respiratory tract. No specific treatments or vaccines are yet available for HAdV infection. Sargassum fusiforme, an edible seaweed, has attracted a lot of attention for its various bioactivities. S. fusiforme has been reported to exhibit antiviral activity. However, research studies about its anti-HAdV activity are few. In this research, we found that S. fusiforme had low cytotoxicity and possessed anti-human adenovirus type 7 (HAdV7) activity in vitro, and the most effective ingredient was alginate. The time of addition assay demonstrated inhibitory effects that were observed in all life stages of the virus. In addition, we observed that the antiviral activity of alginate against HAdV7 infection might be closely related to the endoplasmic reticulum stress (ERS) pathway. Taken together, these results suggest that S. fusiforme extracts have potential application in the prevention and treatment of HAdV infection.
Human adenovirus (HAdV) belongs to the Adenoviridae mammalian adenovirus family (genus Mastadenovirus). It is a linear double-stranded DNA virus without an envelope (Rowe et al., 1953). The core of HAdV is composed of four structural proteins (PV, PVII, TP, and PX) and DNA together, with PVII being the core protein that wraps DNA. Presently, more than 85 HAdV types have been identified, which were divided into seven species (A–G) (Lu et al., 2020). Of these, about 47 types can cause acute infectious zoonoses (Ismail et al., 2019). Related literature illustrated that there were differences in the types of HAdV circulating in different countries or regions and in the same region at different times (Lynch et al., 2018; Fu et al., 2019). Acute respiratory disease induced by HADV-7 accounted for more than 60% of the global incidence rate in Asia and posed a threat to public health (Xu et al., 2018). So far, there are no effective drugs against HAdV. Commercial adenovirus vaccine is presently available only in the United States (Majee et al., 2020).
Sargassum fusiforme is a large seaweed belonging to the Sargassaceae family, Fucales order, and Phaeophyceae class (Liu et al., 2020b). It grows chiefly in coastal areas of Asian countries, such as Japan, South Korea, and North Korea (Liu et al., 2020a). There are abundant proteins, polysaccharides, and microelements in S. fusiforme, which has a wide range of biological activity in terms of antioxidant (Li Y. T. et al., 2018), antitumor (Fan et al., 2017), immunity (Sugiura et al., 2016), anti-aging (Bogie et al., 2019), bone growth and blood glucose (Zhou et al., 2021), anticoagulant, growth, and development. Moreover, Paskaleva et al. showed that S. fusiforme extract inhibited more than 90% of HIV type 1 (HIV-1) infection and replication in T cells, human macrophages, and microglia and more than 70% of pseudotyped HIV-1 [vesicular stomatitis virus (VSV)/NL4-3] infection in human astrocytes (Paskaleva et al., 2006). A separate study suggested that there exist at least two different bioactive molecules in S. fusiforme extract: one inhibiting HIV-1 fusion by interacting with the CD4 receptor and the other directly inhibiting the HIV-1 reverse transcriptase (Paskaleva et al., 2008).
In this study, we showed that S. fusiforme extracts had potential antiviral HADV-7 ability and that the most effective ingredient was alginate. At the same time, we compared three different types of anti-adenoviruses, and the stage of interaction between alginate and adenovirus was the most effective.
A549 cells were generously offered by Dr. Yiquan Li, Changchun University of Chinese Medicine. A549 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and maintained in a humidified 5% CO2 incubator at 37°C. Cells infected with HAdV7 were maintained in DMEM supplemented with 2% FBS. HAdV7 was preserved in our laboratory. S. fusiforme was kindly provided by Professor Mingjiang Wu, College of Life and Environmental Sciences, Wenzhou University. S. fusiforme was picked from a Dongtou algae farm in Wenzhou city, Zhejiang Province. The seaweeds were dried and then powdered. S. fusiforme polysaccharides (SFPSs) were extracted by boiling and soaking in diluted HCl. After boiling, 4 M CaCl2 was added to the filtrate for precipitation and the supernatant was precipitated with ethanol. The concentration was then freeze dried to obtain S. fusiforme fucoidan (Zhang Y. et al., 2020). After soaking in diluted HCl, NaOH was added to the filtrate to maintain the pH at 7.0–8.0, ethanol was added for concentration, and then the sediment was freeze dried to obtain the alginate.
The Cell Counting Kit-8 (CCK8) assay kit was purchased from Solarbio (Beijing, China). In brief, A549 cells were cultured in 96-well plates for 12 h and subsequently treated with 10 μl of S. fusiforme extracts (powder, fucoidan, and alginate) at different concentrations. Each extract was diluted in six parallel duplicate wells and cultured in humidified 5% CO2 at 37°C for 72 h. The cell viability of A549 cells was detected using the corresponding kit in strict accordance with the kit’s instructions.
After infection of A549 cells, viral DNA (hexon) was extracted using the Biospin Virus DNA/RNA Extraction Kit (BioFlux, Hangzhou, China). PCR was completed with 2×Pfu PCR Mix (TIANGEN, Beijing, China) using the following primers:
Forward primer: 5′-GCCGCAGTGGTCTTACATGCACATC-3′
Reverse primer: 5′-CAGCACGCCGCGGATAAAGT-3′
Electrophoresis was performed using PCR products of 287-bp-long segments with 2% (v/v) agarose gel.
The cell supernatant was processed using a Biospin virus DNA/RNA Extraction Kit (BioFlux, Hangzhou, China), followed by measurement of the DNA (hexon) levels in QuantStudio 3 using Top Green qPCR Supermix (Trans, Beijing, China). The PCR conditions comprised 42°C for 20 min; 95°C for 10 min; and 40 cycles of 95°C for 10 s, 58°C for 15 s, and 72°C for 20 s, with a final extension at 72°C for 5 min. The primers used to detect HAdV7 (hexon) and GAPDH (internal control) were as follows:
HAdV7 forward primer: 5′-TGAAATAGCCATAGGCAACAATC-3′
HAdV7 reverse primer: 5′-CGGGCAGAGTAATGTTAGTTGG-3′
GAPDH forward primer: 5′-AAGGTCATCCCTGAGCTGAA-3′
GAPDH reverse primer: 5′-TGACAAAGTGGTCGTTGAGG-3′
Data were analyzed using the 2−ΔΔCt method, and all PCR reactions were performed in triplicate.
Plastic 96-well plates were seeded with A549 cells and incubated at 37°C. After adherence, monolayer A549 cells were infected with a 10-fold serially diluted virus solution, each group with eight parallels. The number of cytopathic pores at each dilution was observed and recorded, until there were no new lesions. Finally, the median tissue culture infective dose (TCID50) of virus tissue cells was calculated according to the Reed–Muench method.
To examine the anti-HAdV7 effect of alginate in different stages of replication, the addition time of alginate (50 μg/ml) and HAdV7 was split into four groups: pretreatment (2 h before HAdV7 infection), co-treatment (during HAdV7 infection), posttreatment (2 h after HAdV7 infection), and full treatment (2 h before HAdV7 infection to the end of the experiment) (Figure 4A). The cell proliferation rate and antiviral activity were examined after 72 h.
Cells cultured in 10-cm dishes were harvested in RIPA lysis buffer added with a mixture of phosphatase and protease inhibitor (Cwbiotech, Beijing, China). The cell lysates were separated by 10% SDS-PAGE, and the proteins were transferred into a nitrocellulose membrane. After blocking with 5% skimmed milk for 2 h, the membrane was incubated with an anti-Bip antibody (cat. #3177s), anti-PERK antibody (cat. #3192), anti-p-PERK antibody (cat. #3179s), anti-eIf2α antibody (cat. #5324), anti-p-eIF2α antibody (cat. #3398S), and anti-IRE1α antibody (cat. #3294) (all from Cell Signaling Technology, Danvers, MA, USA), an anti-ATF4 antibody (sc-390063), anti-hexon antibody (lot #F2817), and an anti-ATF-6α antibody (sc-166659) (all from Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight under 4°C. Then, it was incubated with secondary antibodies for 1 h at room temperature. The signals were detected with an enhanced chemiluminescence (ECL) system (ECL, Solarbio, Beijing, China) reaction.
Statistical analysis was performed by applying t-test on the data between two groups. Statistical significance was considered when *p < 0.05, **p < 0.01, and ***p < 0.001.
Prior to examining the antiviral effects against HAdV7, we performed the CCK-8 assay to determine the proper concentration(s) of S. fusiforme extracts with minimal cytotoxicity on A549. The detected concentration range was 0–25,000 µg/ml. As shown in Figure 1A, the effect of S. fusiforme powder under 400 µg/ml treatment on the cell viability was not evident, while the effect of a higher concentration was significant. The 50% cytotoxic concentration (CC50) of the powder was 3,066 µg/ml (Figure 1B). Moreover, a marked effect on the cell viability was observed with fucoidan and alginate concentrations above 100 and 50 µg/ml, respectively (Figures 1C, D). Therefore, concentrations of S. fusiforme powder below 400 µg/ml, fucoidan below 100 µg/ml, and alginate below 50 µg/ml were selected for subsequent studies to prevent drug toxicity.

Figure 1 Cytotoxicity of Sargassum fusiforme extracts in A549 cells. (A) Powder. (C) Fucoidan. (D) Alginate. A549 cells were treated with S. fusiforme extracts at the indicated concentrations at 37°C. The cell viability was detected using the CCK-8 assay. (B) The 50% cytotoxic concentration (CC50) of powder. **p < 0.01, ***p < 0.001, and ****p < 0.0001.
To determine the anti-HAdV7 activity of S. fusiforme extracts, A549 cells were infected with HAdV7 under the indicated concentrations of S. fusiforme extracts for 72 h. Subsequently, the cytopathic effect (CPE) of the infected cells was observed. Our results revealed that the mock group cells showed morphological integrity, while the cells in the HAdV7-infected group were shrunken, rounded, and detached and the diacaustic rate was enhanced (Figure 2). The CPE in HAdV7-infected cells was remarkably reduced after treatment with S. fusiforme extracts, especially in the alginate group.
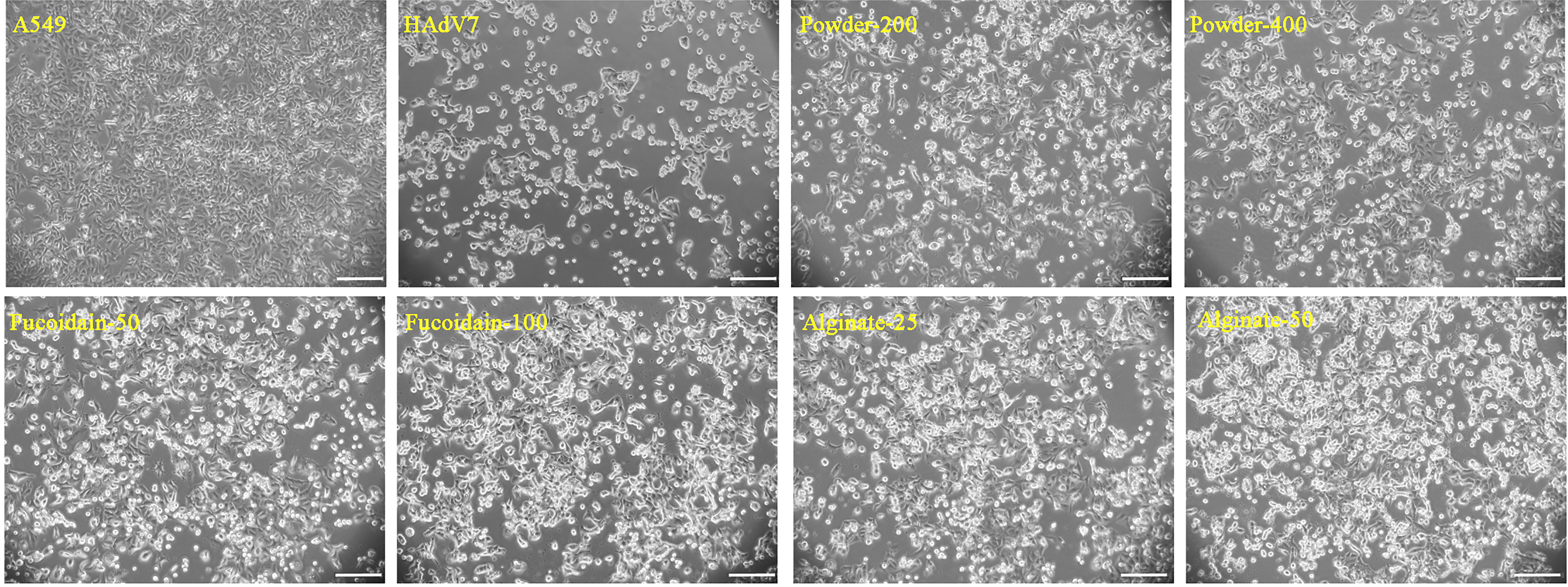
Figure 2 Sargassum fusiforme extracts reduced the human adenovirus type 7 (HAdV7)-induced cytopathic effect (CPE) in A549 cells. Scale bars, 200 µm.
The intracellular viral DNA, amplified products, and virus progeny in the supernatants were respectively detected using agarose gel electrophoresis, relative quantitative PCR (qPCR), and TCID50 assays (Figure 3). The results implied that the S. fusiforme extracts decreased viral production and the progeny virus yield, with the alginate group showing the best effect. Therefore, we focused on alginate and further explored its effect on HAdV7.
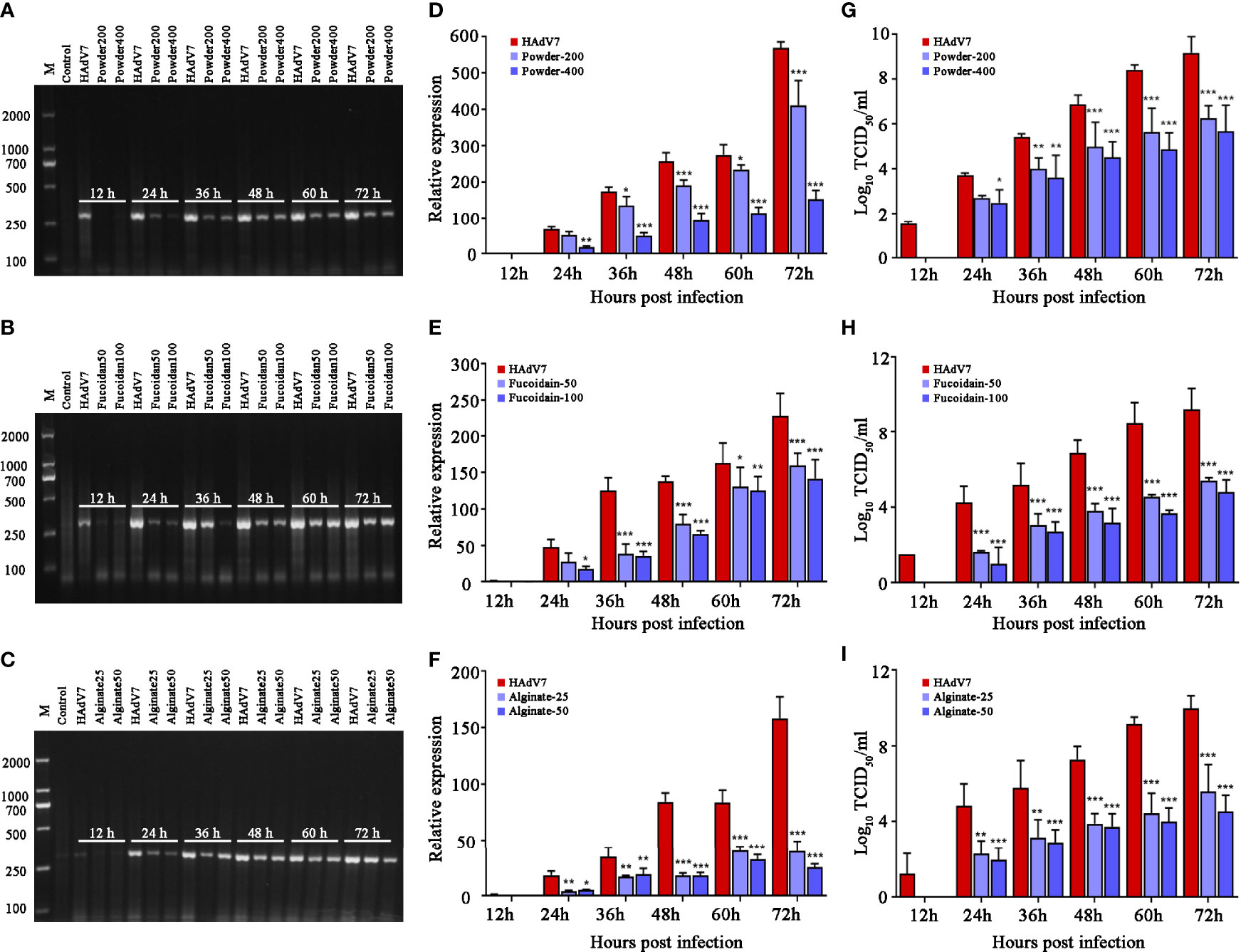
Figure 3 Antiviral activity of Sargassum fusiforme extracts on human adenovirus type 7 (HAdV7) infection in A549 cells. (A–C) Agarose gel electrophoresis of DNA amplification (hexon) in A549 cells treated with different concentrations of S. fusiforme extracts. (D–F) Relative quantitative PCR (qPCR) analysis of the virus DNA level. (G–I) Analysis of the median tissue culture infective dose (TCID50) of viral titers. *p < 0.05, **p < 0.01, and ***p < 0.001.
To pinpoint at which stage of the life cycle of the virus do the S. fusiforme extracts work, a time of addition assay was performed in accordance with the scheme (Figure 4A). The HAdV7 group was set as a negative control and the full-treatment group as a positive control. CPE was observed under a microscope after 72 h treatment. The results showed that HAdV7-caused CPE were clearly reduced under the full-treatment and co-treatment conditions (Figure 4B). Subsequently, the cell viability, as well as the levels of HAdV7 DNA in cells, was determined. The results showed that alginate had the strongest inhibitory effect in the co-treatment stage, indicating that the compound may exert its inhibitory effect mainly during the early stage of HAdV7 infection. In addition, partial effects were observed under the posttreatment stage, suggesting that the compound may also have impact during the late stage of infection with HAdV7 (Figures 4C, D).

Figure 4 Inhibitory effect of Sargassum fusiforme extracts on the life cycle of the virus in A549 cells. (A) Experimental scheme of the time of addition assay. (B) Human adenovirus type 7 (HAdV7)-induced cytopathic effect (CPE) on A549 cells with the addition of S. fusiforme extracts at different time points. Scale bar, 200 µm. (C) Cell viability was detected using the CCK-8 assay. (D) The virus DNA level was detected using relative quantitative PCR (qPCR). **p < 0.01 and ****p < 0.0001.
To elucidate the mechanism of S. fusiforme against HAdV7 infection, we focused on the endoplasmic reticulum stress (ERS) and unfolded protein response (UPR) pathways. Bip is a sensitive marker of ERS. Therefore, we first examined the changes of the protein expression of Bip in A549 cells. The results indicated that HAdV7 infection upregulated the expression of the Bip protein, while it was reduced by alginate treatment (Figure 5). We then detected which branch of the three UPR signaling pathways (PERK, ATF6, and IRE1) was activated. Our results showed that the levels of p-PERK, p-eIF2α, ATF4, ATF6α, and XBP1 were obviously increased in HAdV7-infected cells, whereas the levels of total PERK, eIF2α, and IRE1α were unaltered. Moreover, the increased expressions of p-PERK, p-eIF2α, ATF4, and ATF6α, which were enhanced by HAdV7 infection, could be reduced with alginate treatment. These results indicate that the suppressive effect of alginate on HAdV7 infection depended in part on the activation of the PERK and ATF6 pathways, but not the IRE1 pathway.

Figure 5 Sargassum fusiforme extracts inhibited the endoplasmic reticulum stress (ERS) pathway activated by human adenovirus type 7 (HAdV7) infection.
HAdV is widely distributed and is a major cause of respiratory infections in humans. HAdV infection is often self-limiting; however, for children with low immunity and those in special populations, the infection may cause more serious consequences (Li J. et al., 2018; Bautista-Gogel et al., 2020). There are no specific drugs or vaccines available to control HAdV infection; the focus of clinical treatments of adenovirus infections is mostly to reduce the symptoms of patients (Liu et al., 2018; Tian et al., 2018). However, inadequate efficacy and numerous adverse reactions have been observed with such treatments in many cases (Lynch and Kajon, 2021). Consequently, discovering new and effective anti-HAdV agents is particularly urgent. Herein, we demonstrated, for the first time, the antiviral effect of S. fusiforme against HAdV7 infection.
S. fusiforme is a nutrient-rich edible brown alga that has attracted much attention due to its various biological activities, being widely used in the field of functional food (Zhang R. et al., 2020). Moreover, it has also exhibited antiviral activity (Sun et al., 2021). A previous study demonstrated that treatment of HEp2 cells with S. fusiforme extracts in non-cytotoxic concentrations could led to an obvious decrease of respiratory syncytial virus (RSV) replication, RSV gene transcription, RSV protein synthesis, RSV-induced cell death, and syncytium formation (Chathuranga et al., 2021). Sun et al. also reported that, upon treatment with S. fusiforme extracts, the gene and protein expressions of the avian leukosis virus subgroup J (ALV-J) were clearly reduced (Sun et al., 2019). Thus, the anti-HAdV7 effect of the S. fusiforme extracts was discussed in the present study.
Excessive concentrations of medicinal herbs could lead to cytotoxicity and complicate clinical trials (Palasamudram Shekar et al., 2019). Therefore, we firstly isolated three S. fusiforme extracts and then determined their cytotoxic effects on A549 cells. The concentrations of the S. fusiforme extracts used in this anti-HAdV7 study were no more than 400 µg/ml (powder), 100 µg/ml (fucoidan), and 50 µg/ml (alginate) in order to prevent drug-induced cytotoxicity and maintain A549 cells showing over 90% vitality.
Thereafter, the anti-HAdV7 effect under different concentrations of S. fusiforme extracts was assessed. The results suggested that treatment with S. fusiforme extracts suppressed the viral DNA production, amplified products, and progeny yield. Moreover, the results implied that alginate showed the best effect and was therefore chosen in the subsequent study. The time of addition experiment was performed in order to justify at what stage of the HAdV7 life cycle did alginate work. The results suggested that alginate exerted its anti-HAdV7 activity in all life stages of the virus. Noticeably, the inhibition of the intracellular viral DNA level and cell viability was the strongest at the co-treatment stage.
The endoplasmic reticulum (ER) is an essential organelle in eukaryotes that is mainly responsible for protein assembly, modification, folding, calcium storage, and lipid synthesis (Metcalf et al., 2020). Studies have shown that when viruses interact with cells, large amounts of viral proteins accumulate in the ER lumen, which perturbs the homeostasis and function of the ER, triggering the UPR (Prasad and Greber, 2021; Shaban et al., 2021; Shi et al., 2021). Regulation of the ERS on UPR signals is mainly dependent on three pathways (ATF6, PERK, and IRE1). A previous study demonstrated that the porcine reproductive and respiratory syndrome virus (PRRSV) targets the UPR master regulator Bip for degradation and activates both the XBP1s and ATF4 signaling branches at the early stage of infection (Gao et al., 2019). Subsequent studies showed that adenovirus infection also enhanced the UPR (Prasad et al., 2020). Therefore, we wondered whether S. fusiforme extracts could inhibit HAdV7 via the UPR pathway. The Western blot analysis data revealed that alginate inhibited the Bip level in HAdV7-infected A549 cells. Furthermore, HAdV7-infected cells activated the PERK and ATF6 pathways, but not the IRE1 pathway. This effect also occurred in hepatitis C virus (HCV)- or encephalomyocarditis virus (EMCV)-infected cells (Hou et al., 2014; Wang et al., 2014), indicating that the PERK and ATF6 pathways play a major role in the infection process of multiple viruses. However treatment with alginate blocked the activation of the PERK and ATF6 pathways. Possibly, it can be speculated that S. fusiforme may effectively inhibit HAdV7 infection by altering the activation of the UPR pathway.
In conclusion, our study demonstrated that S. fusiforme had low cytotoxicity and possessed anti-HAdV7 activity in vitro, and the antiviral effects were likely associated with the ERS pathway. Our current study was limited to the application of S. fusiforme in HAdV7 infection in vitro. Therefore, it is also necessary to establish a virus-sensitive animal model for in vivo experiments and to clarify its potential mechanism so as to provide a theoretical basis to guide the clinical application of antiviral drugs.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Conceptualization: NL, PX, and GF. Data curation: GF and NL. Funding acquisition: NL and PX. Investigation: GF, CP, DZ, MW, and PX. Validation: GF and PX. Writing—original draft: GF. Writing—review and editing: NL and PX. All authors have read and agreed to the published version of the manuscript.
This study was supported by the Natural Science Foundation of Zhejiang Province (LQ21H160001), the National Natural Science Foundation of China (32002312), and Science and Technology Project of Wenzhou, Zhejiang, China (Y20210080 and Y2020103).
The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bautista-Gogel, J., Madsen, C. M., Lu, X., Sakthivel, S. K., Froh, I., Kamau, E., et al. (2020). Outbreak of Respiratory Illness Associated With Human Adenovirus Type 7 Among Persons Attending Officer Candidates School, Quantico, Virgini. J. Infect. Dis. 221 (5), 697–700. doi: 10.1093/infdis/jiz060
Bogie, J., Hoeks, C., Schepers, M., Tiane, A., Cuypers, A., Leijten, F., et al. (2019). Dietary Sargassum Fusiforme Improves Memory and Reduces Amyloid Plaque Load in an Alzheimer's Disease Mouse Model. Sci. Rep. 9 (1), 4908. doi: 10.1038/s41598-019-41399-4
Chathuranga, K., Weerawardhana, A., Dodantenna, N., Ranathunga, L., Cho, W. K., Ma, J. Y., et al. (2021). Inhibitory Effect of Sargassum Fusiforme and Its Components on Replication of Respiratory Syncytial Virus In Vitro and In Vivo. Viruses 13 (4), 548. doi: 10.3390/v13040548
Fan, S., Zhang, J., Nie, W., Zhou, W., Jin, L., Chen, X., et al. (2017). Antitumor Effects of Polysaccharide From Sargassum Fusiforme Against Human Hepatocellular Carcinoma HepG2 Cells. Food Chem. Toxicol. 102, 53–62. doi: 10.1016/j.fct.2017.01.020
Fu, Y., Tang, Z., Ye, Z., Mo, S., Tian, X., Ni, K., et al. (2019). Human Adenovirus Type 7 Infection Causes a More Severe Disease Than Type 3. BMC Infect. Dis. 19 (1), 36. doi: 10.1186/s12879-018-3651-2
Gao, P., Chai, Y., Song, J., Liu, T., Chen, P., Zhou, L., et al. (2019). Reprogramming the Unfolded Protein Response for Replication by Porcine Reproductive and Respiratory Syndrome Virus. PLoS Pathog. 15 (11), e1008169. doi: 10.1371/journal.ppat.1008169
Hou, L., Ge, X., Xin, L., Zhou, L., Guo, X., Yang, H. (2014). Nonstructural Proteins 2C and 3D Are Involved in Autophagy as Induced by the Encephalomyocarditis Virus. Virol. J. 11, 156. doi: 10.1186/1743-422X-11-156
Ismail, A. M., Zhou, X., Dyer, D. W., Seto, D., Rajaiya, J., Chodosh, J. (2019). Genomic Foundations of Evolution and Ocular Pathogenesis in Human Adenovirus Species D. FEBS Lett. 593 (24), 3583–3608. doi: 10.1002/1873-3468.13693
Li, Y. T., Chen, B. J., Wu, W. D., Ge, K., Wei, X. Y., Kong, L. M., et al. (2018). Antioxidant and Antimicrobial Evaluation of Carboxymethylated and Hydroxamated Degraded Polysaccharides From Sargassum Fusiforme. Int. J. Biol. Macromol 118 (Pt B), 1550–1557. doi: 10.1016/j.ijbiomac.2018.06.196
Li, J., Lu, X., Jiang, B., Du, Y., Yang, Y., Qian, H., et al. (2018). Adenovirus-Associated Acute Conjunctivitis in Beijing, Chin-2013. BMC Infect. Dis. 18 (1), 135. doi: 10.1186/s12879-018-3014-z
Liu, J., Luthuli, S., Yang, Y., Cheng, Y., Zhang, Y., Wu, M., et al. (2020a). Therapeutic and Nutraceutical Potentials of a Brown Seaweed Sargassum Fusiforme. Food Sci. Nutr. 8 (10), 5195–5205. doi: 10.1002/fsn3.1835
Liu, J., Wu, S. Y., Chen, L., Li, Q. J., Shen, Y. Z., Jin, L., et al. (2020b). Different Extraction Methods Bring About Distinct Physicochemical Properties and Antioxidant Activities of Sargassum Fusiforme Fucoidans. Int. J. Biol. Macromol. 155, 1385–1392. doi: 10.1016/j.ijbiomac.2019.11.113
Liu, T., Zhou, Z., Tian, X., Liu, W., Xu, D., Fan, Y., et al. (2018). A Recombinant Trivalent Vaccine Candidate Against Human Adenovirus Types 3, 7, and 55. Vaccine. 36 (16), 2199–2206. doi: 10.1016/j.vaccine.2018.02.050
Lu, J., Wang, R., Huang, Y., Yu, Y., Zhou, X., Huang, P., et al. (2020). A Novel Human Monoclonal Antibody Potently Neutralizes Human Adenovirus Serotype 7 by Primarily Targeting the Adenovirus Hexon Protein. Virol. 543, 20–26. doi: 10.1016/j.virol.2019.12.005
Lynch, B. L., Dean, J., Brady, D., De Gascun, C. (2018). Adenovirus Type 4 Respiratory Infections Among Civilian Adults, Northeastern United State-2015. Emerg. Infect. Dis. 24 (7), 1392–1393. doi: 10.3201/eid2407.180137
Lynch, J. P., 3rd, Kajon, A. E. (2021). Adenovirus: Epidemiology, Global Spread of Novel Types, and Approach to Treatment. Semin. Respir. Crit. Care Med. 42 (6), 800–821. doi: 10.1055/s-0041-1733802
Majee, P., Shankar, U., Pasadi, S., Muniyappa, K., Nayak, D., Kumar, A. (2020). Genome-Wide Analysis Reveals a Regulatory Role for G-Quadruplexes During Adenovirus Multiplication. Virus Res. 283, 197960. doi: 10.1016/j.virusres.2020.197960
Metcalf, M. G., Higuchi-Sanabria, R., Garcia, G., Tsui, C. K., Dillin, A. (2020). Beyond the Cell Factory: Homeostatic Regulation of and by the UPR(Er). Sci. Adv. 6 (29), eabb9614. doi: 10.1126/sciadv.abb9614
Palasamudram Shekar, S., Rojas, E. E., D'Angelo, C. C., Gillenwater, S. R., Martinez Galvis, N. P. (2019). Legally Lethal Kratom: A Herbal Supplement With Overdose Potential. J. Psychoact. Drugs 51 (1), 28–30. doi: 10.1080/02791072.2018.1562591
Paskaleva, E. E., Lin, X., Duus, K., McSharry, J. J., Veille, J. C., Thornber, C., et al. (2008). Sargassum Fusiforme Fraction Is a Potent and Specific Inhibitor of HIV-1 Fusion and Reverse Transcriptase. Virol. J. 5, 8. doi: 10.1186/1743-422x-5-8
Paskaleva, E. E., Lin, X., Li, W., Cotter, R., Klein, M. T., Roberge, E., et al. (2006). Inhibition of Highly Productive HIV-1 Infection in T Cells, Primary Human Macrophages, Microglia, and Astrocytes by Sargassum Fusiforme. AIDS Res. Ther. 3, 15. doi: 10.1186/1742-6405-3-15
Prasad, V., Greber, U. F. (2021). The Endoplasmic Reticulum Unfolded Protein Response - Homeostasis, Cell Death and Evolution in Virus Infections. FEMS Microbiol. Rev. 45 (5), fuab016. doi: 10.1093/femsre/fuab016
Prasad, V., Suomalainen, M., Jasiqi, Y., Hemmi, S., Hearing, P., Hosie, L., et al. (2020). The UPR Sensor IRE1α and the Adenovirus E3-19K Glycoprotein Sustain Persistent and Lytic Infections. Nat. Commun. 11 (1), 1997. doi: 10.1038/s41467-020-15844-2
Rowe, W. P., Huebner, R. J., Gilmore, L. K., Parrott, R. H., Ward, T. G. (1953). Isolation of a Cytopathogenic Agent From Human Adenoids Undergoing Spontaneous Degeneration in Tissue Culture. Proc. Soc. Exp. Biol. Med. 84 (3), 570–573. doi: 10.3181/00379727-84-20714
Shaban, M. S., Müller, C., Mayr-Buro, C., Weiser, H., Meier-Soelch, J., Albert, B. V., et al. (2021). Multi-Level Inhibition of Coronavirus Replication by Chemical ER Stress. Nat. Commun. 12 (1), 5536. doi: 10.1038/s41467-021-25551-1
Shi, J., Li, Z., Xu, R., Zhang, J., Zhou, Q., Gao, R., et al. (2021). The PERK/PKR-Eif2α Pathway Negatively Regulates Porcine Hemagglutinating Encephalomyelitis Virus Replication by Attenuating Global Protein Translation and Facilitating Stress Granule Formation. J. Virol. 96 (1), e0169521. doi: 10.1128/jvi.01695-21
Sugiura, Y., Kinoshita, Y., Abe, M., Murase, N., Tanaka, R., Matsushita, T., et al. (2016). Suppressive Effects of the Diethyl Ether Fraction From a Brown Alga Sargassum Fusiforme on Allergic and Inflammatory Reactions. Fish Sci. 82 (2), 369–377. doi: 10.1007/s12562-016-0969-9
Sun, Y., Chen, X., Liu, H., Liu, S., Yu, H., Wang, X., et al. (2021). Preparation of New Sargassum Fusiforme Polysaccharide Long-Chain Alkyl Group Nanomicelles and Their Antiviral Properties Against ALV-J. Molecules 26 (11), 3265. doi: 10.3390/molecules26113265
Sun, Y., Chen, X., Zhang, L., Liu, H., Liu, S., Yu, H., et al. (2019). The Antiviral Property of Sargassum Fusiforme Polysaccharide for Avian Leukosis Virus Subgroup J In Vitro and In Vivo. Int. J. Biol. Macromol 138, 70–78. doi: 10.1016/j.ijbiomac.2019.07.073
Tian, X., Jiang, Z., Fan, Y., Qiu, S., Zhang, L., Li, X., et al. (2018). A Tetravalent Vaccine Comprising Hexon-Chimeric Adenoviruses Elicits Balanced Protective Immunity Against Human Adenovirus Types 3, 7, 14 and 55. Antiviral Res. 154, 17–25. doi: 10.1016/j.antiviral.2018.04.001
Wang, J., Kang, R., Huang, H., Xi, X., Wang, B., Wang, J., et al. (2014). Hepatitis C Virus Core Protein Activates Autophagy Through EIF2AK3 and ATF6 UPR Pathway-Mediated MAP1LC3B and ATG12 Expression. Autophagy 10 (5), 766–784. doi: 10.4161/auto.27954
Xu, L., Liu, J., Liu, C., Duan, Y., Zhu, Y., Xu, B., et al. (2018). Case-Control Study of the Epidemiological and Clinical Features of Human Adenovirus 55 and Human Adenovirus 7 Infection in Children With Acute Lower Respiratory Tract Infections in Beijing, China 2008-2013. BMC Infect. Dis. 18 (1), 634. doi: 10.1186/s12879-018-3520-z
Zhang, R., Zhang, X., Tang, Y., Mao, J. (2020). Composition, Isolation, Purification and Biological Activities of Sargassum Fusiforme Polysaccharides: A Review. Carbohydr Polym. 228, 115381. doi: 10.1016/j.carbpol.2019.115381
Zhang, Y., Zuo, J., Yan, L., Cheng, Y., Li, Q., Wu, S., et al. (2020). Sargassum Fusiforme Fucoidan Alleviates High-Fat Diet-Induced Obesity and Insulin Resistance Associated With the Improvement of Hepatic Oxidative Stress and Gut Microbiota Profile. J. Agric. Food Chem. 68 (39), 10626–10638. doi: 10.1021/acs.jafc.0c02555
Keywords: human adenovirus, Sargassum fusiforme, antiviral activity, endoplasmic reticulum stress, in vitro
Citation: Feng G, Zhang D, Peng C, Wu M, Xiao P and Li N (2022) Study on the Anti-Adenovirus Mechanism of Sargassum fusiforme. Front. Cell. Infect. Microbiol. 12:860559. doi: 10.3389/fcimb.2022.860559
Received: 23 January 2022; Accepted: 08 February 2022;
Published: 07 March 2022.
Edited by:
Bruno Tilocca, University of Catanzaro, ItalyReviewed by:
Bhakti Tanna, Gujarat Biotechnology Research Centre (GBRC), IndiaCopyright © 2022 Feng, Zhang, Peng, Wu, Xiao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nan Li, MjAxOTAyODNAd3p1LmVkdS5jbg==; Pengpeng Xiao, MjAxOTAwMDJAd3p1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.