
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol., 05 March 2025
Sec. Cell Death and Survival
Volume 13 - 2025 | https://doi.org/10.3389/fcell.2025.1525294
This article is part of the Research TopicFerroptosis: Intersections, Implications, and Innovations in Programmed Cell DeathView all 12 articles
Ferroptosis is a novel form of cell death that uniquely requires iron and is characterized by iron accumulation, the generation of free radicals leading to oxidative stress, and the formation of lipid peroxides, which distinguish it from other forms of cell death. The regulation of ferroptosis is extremely complex and is closely associated with a spectrum of diseases. Sirtuin 1 (SIRT1), a NAD + -dependent histone deacetylase, has emerged as a pivotal epigenetic regulator with the potential to regulate ferroptosis through a wide array of genes intricately associated with lipid metabolism, iron homeostasis, glutathione biosynthesis, and redox homeostasis. This review provides a comprehensive overview of the specific mechanisms by which SIRT1 regulates ferroptosis and explores its potential therapeutic value in the context of multiple disease pathologies, highlighting the significance of SIRT1-mediated ferroptosis in treatment strategies.
Ferroptosis, first identified in 2012, is a unique form of regulated cell death that is distinct from traditional apoptosis, necroptosis, and senescence (Dixon et al., 2012). Cells undergoing ferroptosis typically display shrunken mitochondria, increased mitochondrial membrane density, loss of mitochondrial cristae, reduced mitochondrial membrane potential, and rupture of the outer mitochondrial membrane (Li et al., 2024a; Shan et al., 2024; Lin et al., 2024a). The occurrence of ferroptosis is intricately linked to several critical biochemical processes, including the disruptions in iron homeostasis, limited synthesis of glutathione (GSH), and the accumulation of lipid peroxides (Tian et al., 2024; Cai et al., 2024; Li et al., 2024b; Yang et al., 2024). Excess iron is typically stored in ferritin to prevent it from catalyzing the formation of hydroxyl radicals, which can react with polyunsaturated fatty acids in the cell and plasma membranes. This reaction leads to the generation of a significant amount of lipid reactive oxygen species (ROS), contributing to the cellular demise that is characteristic of ferroptosis. The system XC is pivotal in sustaining cellular GSH levels by mediating the cellular uptake of cystine. GSH acts as a cofactor for antioxidant enzymes such as GPX4, which play a role in the elimination of ROS. These pathways form an interconnected network that safeguards cells against ferroptosis by maintaining a delicate balance between antioxidant defense and the production of reactive species. Ferroptosis s closely related to the occurrence and development of a variety of diseases, such as nervous system diseases, heart diseases, liver diseases, gastrointestinal diseases, lung diseases, kidney diseases, pancreatic diseases tumors, kidney injury, tumor, etc. By targeting the key components of ferroptosis, disease progression can be slowed, offering promising treatment strategies for many diseases.
Sirtuins (SIRTs), a subset of NAD + -dependent histone deacetylases, are evolutionarily conserved and consist of seven isoforms. Among these, SIRT1-3 have been implicated in the regulation of ferroptosis, with SIRT1 being the most extensively studied. SIRT1 is widely expressed across tissues and organs such as the brain, heart, liver, kidneys, and skeletal muscle, with particularly high expression in tissues vulnerable to oxidative stress and those with high metabolic activity. SIRT1 exhibits diverse subcellular localizations, being found in the nucleus, cytoplasm, or both, depending on the cell type (Sgadari et al., 2023). Structurally, it is composed of 747 amino acid (aa) residues, with both C- and N-terminal domains contributing to its structure and function. The C-terminal domain, comprising 25 aa residues, is crucial for SIRT1’s catalytic activity. It forms a hairpin that interacts with the β-sheet of the NAD + -binding domain, while the N-terminal domain enhances the enzyme’s activity (Davenport et al., 2014). As a central modulator of ferroptosis, SIRT1 modulates key ferroptosis-related proteins through deacetylation, thereby enhancing cellular resilience against ferroptotic cell death. Its regulatory role has significant implications for the treatment and management of diseases associated with oxidative stress and ferroptosis. Recent studies have highlighted SIRT1’s potential in inhibiting ferroptosis by influencing pathways related to glutathione synthesis, antioxidant mechanisms, and the metabolism of lipids and iron. Despite the growing evidence of SIRT1’s involvement in ferroptosis, a comprehensive understanding of the regulatory interplay between SIRT1 and ferroptosis signaling pathways is yet to be fully elucidated (Dang et al., 2022; Wang et al., 2021; Qiongyue et al., 2022). In this review, we delve into the current evidence of the functions of SIRT1 in regulating ferroptosis and therapeutic potential in various diseases. We aim to consolidate current understanding and explore the therapeutic implications of targeting SIRT1-mediated ferroptosis, offering insights into its promise as an innovative pathway for developing treatment strategies.
Reactive oxygen species (ROS) are central to the process of ferroptosis, as they facilitate lipid peroxidation and damage cell membranes. Therefore, regulating ROS levels may be an important strategy for controlling ferroptosis and related diseases. The System XC and GPX4 are vital in maintaining intracellular GSH levels, which are essential for ROS removal. Notably, SIRT1 functions as a sensor of redox changes and plays a critical protective role against ferroptosis by regulating redox homeostasis via Nuclear factor erythroid 2-related factor 2 (Nrf2) and p53 (Liang et al., 2023).
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a key transcription factor that maintains redox balance and protects cells from oxidative damage. Accumulating evidence points that Nrf2 upregulation can suppress the initiation of ferroptosis (Bellezza et al., 2012; Sun et al., 2016; Wang et al., 2023a; Lv et al., 2021). SIRT1 modulates various components within the antioxidant system, such as Heme Oxygenase-1(HO-1), glutathione (GSH), catalase (CAT), superoxide dismutase 1(SOD1) and SOD2 through deacetylation of Nrf2 (Zhang et al., 2018; Abukhalil et al., 2025; Xia et al., 2023). Recent studies have illustrated the role of the SIRT1-Nrf2-HO-1 pathway in the regulation of ferroptosis. Wang et al. have shown that SIRT1-Nrf2-HO-1 activation attenuated lipid peroxide accumulation and inhibited ferroptosis (Wang et al., 2023a). Another study demonstrated that SIRT1 activation positively regulates the Nrf2/HO-1 pathway, reducing mitochondrial damage and ferroptosis. Furthermore, HO-1 may play a role in modulating GPX4 levels (Xie et al., 2022). Dang et al. pointed that SIRT1 activation may mediate the upregulation of GPX4 levels by Nrf2-HO-1 axis in the alleviation of neuronal injury. Significant strides have been made in recent years to elucidate the protective effects of SIRT1-Nrf2 activation against ferroptosis (Xia et al., 2023; Xie et al., 2022), and further exploration of the underlying regulatory mechanisms is warranted.
p53 is a multifunctional protein that plays a crucial role in regulating intracellular levels of reactive oxygen species (ROS) and modulating ferroptosis through targeting downstream molecules (Latunde-Dada, 2017). The complex interplay between SIRT1 and p53 is crucial in managing ferroptosis activation, primarily through inhibition of p53’s pro-ferroptotic activity and promotion of p21 and GSH synthesis. First, overexpression of SIRT1 has been shown to repress p53 transcriptional activity, increasing SLC7A11 levels and inhibiting ferroptosis (Ma et al., 2020). Zhao et al. reported that SIRT1 participates in the development of gastric cancer by targeting p53 to regulate ferroptosis (Zhao et al., 2023a). In gastric cancer cells, silencing SIRT1 leads to upregulation of p53 and downregulation of SLC7A11, indicating that SIRT1 suppression promotes ferroptosis. Second, p21, a downstream target of p53, can form a complex with p53 and influence its transcriptional activity. SIRT1 enhances p21 expression by modulating p53 activity, which may contribute to cellular redox balance and ferroptosis resistance by mitigating oxidative stress. Through this pathway, SIRT1 indirectly supports antioxidant defenses, including GSH synthesis, further reducing ferroptosis susceptibility (Gu et al., 2022; Wang et al., 2025). While these findings highlight SIRT1’s protective role in ferroptosis regulation via the p53/p21 axis, further research is needed to elucidate its context-specific effects across different disease models.
Iron homeostasis is crucial for maintaining normal cellular and physiological metabolism. When iron supply is abundant, iron storage protein ferritin synthesis increases to store the excess. Iron (Fe2+) can be oxidized to Fe3+ by the ferroxidase hephaestin (Heph) and bind to transferrin (TF) on cell membranes, forming the TF-Fe3+ complex, which is facilitated by the presence of apo-Tf. Apo-Tf acts as an iron acceptor molecule, enhancing iron (Fe2+) efflux from cells via ferroportin (FPN1), and thus enhancing iron export and absorption (Weichhart, 2024). Most intracellular iron is either found in heme-containing and mitochondrial proteins or stored by ferritin as Fe3+, preventing iron overload that could lead to oxidative stress. Ferritin plays a vital role in preserving iron balance by storing and releasing iron. However, excessive iron levels can increase the labile iron pool (LIP), elevate intracellular reactive oxygen species (ROS), and lead to the accumulation of lipid peroxides, ultimately promoting ferroptosis. SIRT1 has been shown to influence ferroptosis by regulating iron metabolism. Activation of the SIRT1/Nrf2 pathway, such as by alpha lipoic acid, can modulate iron metabolism and mitigate ferroptosis by up-regulating ferritin and ferritin heavy chain 1 (FTH1), and down-regulating the iron import protein divalent metal transporter 1 (DMT1) (Zheng et al., 2023). Lv et al. demonstrated that suppression of the SIRT1/Nrf2/HO-1/GPX4 pathway and FTH1 protein can exacerbate ferroptosis, underscoring the significance of the SIRT1-Nrf2 signaling pathway in iron metabolism and ferroptosis (Lv et al., 2024). Additionally, SIRT1 activation is linked to altered hepcidin production and increased ferritinophagy, which can suppress ferroptosis and prevent the detrimental effects of elevated cytosolic iron (Su et al., 2021; Tziastoudi et al., 2023). However, Zhou et al. noted an exception to the inhibitory effect of SIRT1 on ferroptosis, where intestinal SIRT1 deficiency improves iron metabolism in ethanol-induced hepatic injury in mice by ameliorating iron dysfunction and alleviating ferroptosis in hepatocytes. This suggests that the role of SIRT1 can be context-dependent (Zhou et al., 2020). In summary, SIRT1 plays a multifaceted role in regulating iron metabolism and influencing ferroptosis. Modulating SIRT1 can protect against ferroptosis by influencing key iron-related proteins and pathways. However, the specific outcomes of SIRT1’s actions can vary depending on the cellular context and the type of stress involved. Further research is needed to fully understand the complex interactions between SIRT1, iron metabolism, and ferroptosis, which could potentially lead to the development of novel therapeutic strategies for iron-related diseases.
Iron can exacerbate the accumulation of lipid peroxides by catalyzing Fenton reaction, which in turn disrupts the intracellular redox balance, leading to an attack on biomolecules and ultimately culminating in ferroptosis. SIRT1, a key metabolic factor in energy regulation, can stimulate various endocrine signals related to lipid metabolism. and an increasing number of studies have shown that SIRT1 is involved in endocrine and metabolic diseases (Lu et al., 2023). The long-chain fatty acyl CoA synthetase (ACSLs) family of enzymes, which significantly contributes to lipid metabolism, has been recognized as a crucial regulator in the process of ferroptosis (Dixon et al., 2015). Emerging research suggests that SIRT1 activation may reduce ACSL4 expression levels, potentially alleviating the effects of ferroptosis (Majeed et al., 2021; Wang et al., 2020; Yu et al., 2023a). The work by Chen et al. provides compelling evidence that overexpression of SIRT1 can inhibit lipid peroxidation and decrease malondialdehyde (MDA) levels, a marker of lipid peroxidation. Furthermore, SIRT1 overexpression can reverse the typical upregulation of ACSL4 and acetylated p53, and the downregulation of SLC7A11 and GPX4 observed in ferroptosis, thus inhibiting ferroptotic cell death (Chen et al., 2022a). These findings underscore SIRT1’s potential in modulating lipid metabolism and its protective role against ferroptosis. However, the precise mechanisms by which SIRT1 interacts with ACSL4 to regulate ferroptosis remain to be fully understood, indicating a need for further research in this area. This research could pave the way for developing novel therapeutic strategies that target the SIRT1-ACSL4 axis to combat diseases associated with ferroptosis (Figure 1).
Inflammation and ferroptosis are closely interconnected, with inflammation often promoting ferroptosis through the release of pro-inflammatory cytokines and the induction of oxidative stress. Nuclear Factor kappa B (NF-κB), a key transcription factor, is involved in the regulation of both inflammation and oxidative stress. Upon activation, NF-κB translocates to the nucleus, where it induces the expression of pro-inflammatory and pro-ferroptotic genes, promote iron accumulation and reactive ROS production, leading to lipid peroxidation and ferroptosis (Chen et al., 2024a). By deacetylating the p65 subunit of NF-κB at lysine 310, SIRT1 inhibits its nuclear translocation and transcriptional activity, thereby reducing inflammation, decreasing ROS production and lipid peroxidation, ultimately inhibiting ferroptosis and alleviating cell injury (Min et al., 2025). Conversely, the downregulation of SIRT1 has been shown to activate the NLRP3 inflammasome, leading to the release of pro-inflammatory cytokines like IL-1β and the subsequent disruption of iron homeostasis. This disruption is characterized by increased lipid peroxidation, and the depletion of key antioxidants such as GPX4 and GSH, further weakening the cellular defense against oxidative stress and rendering cells more susceptible to ferroptosis (Hacioglu, 2024). Additionally, under pathological conditions, SIRT1 activation by adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK), a key energy sensor that regulates cellular metabolism and stress responses, further attenuates inflammatory signaling. Activation of the AMPK/SIRT1 signaling pathway alleviates the degradation of GSH, thus inhibiting ferroptosis. Selective inhibition of SIRT1 weakens the protective effect of the AMPK/SIRT1 signaling pathway against endoplasmic reticulum stress and ferroptosis (Xu et al., 2023). Restoring the activity of AMPK and SIRT1 can effectively inhibit ferroptosis, providing a potential therapeutic strategy for treating related diseases. Interestingly, SIRT1 inhibition subsequently affects the phosphorylation of AMPK, leading to downstream activation of acetyl-CoA carboxylase, promotes the synthesis of polyunsaturated fatty acids, which serve as substrates for lipid peroxidation and ferroptosis induction (Zhang et al., 2023a). Taken together, these findings underscore the critical role of SIRT1 in modulating inflammation-driven ferroptosis through NF-κB, NLRP3 and AMPK signaling. Enhancing SIRT1 activity could serve as a potential therapeutic strategy for mitigating ferroptosis-associated diseases by reducing inflammation, preserving antioxidant defenses, and regulating lipid metabolism. Further studies are warranted to fully elucidate the therapeutic potential of targeting the SIRT-mediated inflammation in ferroptosis-related pathologies.
Studies have delineated the neuroprotective role of SIRT1 in various pathological conditions, including early brain injury following subarachnoid hemorrhage. SIRT1’s role in ferroptosis within brain injury is primarily through the regulation of cellular oxidative stress and iron metabolic balance. By deacetylating a variety of key proteins, SIRT1 enhances the antioxidant capacity of cells, reduces the production of ROS, and inhibits lipid peroxidation, thus alleviating the damage caused by ferroptosis to nerve cells (Hao et al., 2022; Conde et al., 2023; Xie et al., 2022; Liang et al., 2023; Guo et al., 2022). SIRT1 activation also helps to maintain homeostasis of intracellular iron ions, prevent Fenton reaction induced by excess iron ions, and reduce oxidative DNA damage and protein degeneration. Furthermore, SIRT1 inhibits ferroptosis and protects nerve cells from oxidative stress by promoting the expression of antioxidant enzymes like glutathione peroxidase 4 (GPX4) and activating pathways such as Nrf2/HO-1 and ferroptosis suppressor protein 1 (FSP1), playing a vital role in neuroprotection and repair post-brain injury (Liu et al., 2023; Yuan et al., 2022; Chen et al., 2024b; Zhang et al., 2024a). Additionally, SIRT1-mediated ferroptosis is significant in neurodegenerative diseases and cognitive disorders, with activation of the SIRT1/Nrf2 pathway shown to inhibit oxidative stress and ferroptosis, improving cognitive function (Chen et al., 2023a). Treatments such as propofol, ketogenic diets, mesenchymal stem cell-derived exosomes, and components like ferulate acid from traditional Chinese medicine have demonstrated potential in reducing hippocampal neuron ferroptosis and improving cognitive function by enhancing the SIRT1/Nrf2/GPX4 pathway (Wen et al., 2024; Yang et al., 2022; Liu et al., 2022; Wang et al., 2023b; Yan et al., 2024). miR-30a-5p also regulates ferroptosis by targeting SIRT1, affecting cognitive dysfunction in conditions like chronic cerebral hypoperfusion (Wang et al., 2024a). In neurodegenerative diseases such as Friedreich’s ataxia and Parkinson’s disease, SIRT1 activation helps maintain cellular iron balance, reducing oxidative stress and lipid peroxidation via Nrf2, GPX4 and FTH1, thereby protecting neurons from damage caused by ferroptosis (Lv et al., 2024; Zheng et al., 2023; Sanz-Alcázar et al., 2024). The therapeutic potential of targeting the SIRT1/Nrf2/HO-1/GPX4 pathway is further supported by research indicating that edaravone, a widely used anesthetic, mitigates depression and anxiety by inhibiting ferroptosis through this pathway (Dang et al., 2022; Shen et al., 1826). In gliomas, SIRT1-mediated ferroptosis plays a key role by finely regulating intracellular iron metabolism, redox balance, and autophagy processes. Activation of SIRT1 can enhance the antioxidant capacity of cells by deacetylating key proteins such as Nrf2 and p53, reduce the production of ROS, and inhibit lipid peroxidation, protecting nerve cells from the damage caused by ferroptosis by regulating proteins related to iron metabolism and antioxidant defense mechanisms such as Nrf2, GPX4, and FTH1. Moreover, SIRT1 activates transcription factor activating transcription factor 3 (ATF3) through its interaction with active regulator and regulation of NAD + levels, thereby inhibiting the expression of SLC7A11 and GPX4, promoting the accumulation of iron ions and lipid peroxides in cells, and aggravating ferroptosis (Chen et al., 2024c; Sun et al., 2022). Collectively, targeting SIRT1 in neuro diseases offers promising therapeutic avenues. SIRT1 activators such as resveratrol, SRT1720, and SRT2104 have been shown to alleviate neurodegenerative disease symptoms by reducing oxidative stress, enhancing autophagy flux, and promoting neuronal survival (Su et al., 2021; Zhu et al., 2022; Bai et al., 2023; Rao et al., 2024). In addition, propofol, ketogenic diet, mesenchymal stem cell-derived exosomes, and the traditional Chinese medicine were able to reduce ferroptosis in hippocampal neurons and improve cognitive function (Wen et al., 2024; Yang et al., 2022; Liu et al., 2022; Wang et al., 2023b; Yan et al., 2024). These findings underscore the multifaceted role of SIRT1 in the regulation of ferroptosis and highlight its therapeutic potential value in the treatment of neuro diseases, providing a scientific basis for the development of new therapies targeting SIRT1-mediated ferroptosis (Figure 2).
SIRT1 is a pivotal regulator of ferroptosis in liver disease, playing a crucial role in maintaining hepatic health. In human acute liver failure tissue, SIRT1 levels are diminished, however, its activation can mitigate cell damage by modulating the ferroptosis and pyroptosis processes in hepatocytes through the regulation of the p53/GPX4/GSDMD and Nrf2/p53 signaling pathway, thereby exerting a protective effect on the liver (Zhou et al., 2024a). In a mouse model of sepsis-induced liver failure, the upregulation of SIRT1 indicates a potential role in safeguarding hepatocytes from ferroptosis, lessening liver damage, and enhancing the clinical prognosis for liver failure (Chen et al., 2022b). Furthermore, SIRT1 activators such as salidroside and ulinastatin, as well as therapeutic agents like rosa rugosa and dihydroquercetin, have been shown to significantly ameliorate liver pathological changes associated with ferroptosis by reducing oxidative stress and inflammatory responses, thus safeguarding liver cells (Wang et al., 2021; Xu et al., 2023; Lei et al., 2023; Zeng et al., 2024a). In the pathogenesis of non-alcoholic steatohepatitis (NASH), SIRT1 expression levels inversely correlate with disease progression, hinting at a significant regulatory function in ferroptosis. Activation of SIRT1 has been shown to boost the expression of antioxidant genes by deacetylating and activating Nrf2, subsequently mitigates cell damage caused by oxidative stress and dysregulated iron metabolism. SIRT1 may also curb ferroptosis in NASH by modulating other molecular pathways associated with ferroptosis, such as inhibiting lipid peroxidation and fostering iron metabolic equilibrium, offering new potential targets for NASH treatment (He et al., 2023; Yang et al., 2023). Interestingly, contrary to the hepatoprotective effects of SIRT1 activation, the loss of intestinal SIRT1 in mice shields them from ethanol-induced inflammation and liver damage by reducing liver ferroptosis. Targeting intestinal SIRT1 or alleviating ferroptosis signals in the liver may offer promising avenues for the treatment of human alcoholic liver disease (Zhou et al., 2020). Collectively, SIRT1 activation has emerged as a key therapeutic strategy. SIRT1 activators such as salidroside, ulinastatin, rosa rugosa and dihydroquercetin, have been shown to significantly improve liver pathological changes associated with ferroptosis by reducing oxidative stress and inflammatory responses (Wang et al., 2021; Xu et al., 2023; Lei et al., 2023; Zeng et al., 2024a). Interestingly, targeting intestinal SIRT1 has also been proposed as a novel approach for treating alcoholic liver disease by reducing liver ferroptosis. The findings above indicate that SIRT1-targeted therapies could serve as innovative approaches for treating various liver diseases by modulating ferroptosis pathways.
Studies have shown that SIRT1-mediated ferroptosis plays a key role in lung injury. In acute lung injury (ALI) caused by heat attack, activation of SIRT1 has been shown to ameliorate ferroptosis in alveolar epithelial cells under heat stress. This activation alleviates the damage to the alveolar capillary barrier and maintains the barrier function of pulmonary microvascular endothelial cells, suggesting that the SIRT1/p53 axis plays a crucial role in regulating ferroptosis in ALI (Chen et al., 2022a). In lipopolysaccharide (LPS) -induced ALI, activation of SIRT1 by Meteorin-like/Meteorin-β and fibroblast growth factor (FGF) reduces ferroptosis and protects lung tissue by inhibiting p53 acetylation and Nrf2 (Chen et al., 2023b; Lin et al., 2024b). In ALI induced by sepsis, SIRT1 plays a role in inhibiting ferroptosis by activating the NADPH oxidase 4 signaling pathway, which reduces the production of ROS and the level of lipid peroxidation, and maintains the balance of iron metabolism in cells. The overexpression of growth differentiation factor 11 (GDF11) further inhibits ferroptosis by promoting the activity of SIRT1, providing a new molecular target and therapeutic strategy for treating sepsis related ALI (Wu et al., 2024). Moreover, quercetin has shown the potential to inhibit ferroptosis and alleviate ALI by activating SIRT1/Nrf2/GPX4 signaling pathway, providing a new strategy for the treatment of ALI (Deng et al., 2023). The role of SIRT1 in ALI is mainly realized by regulating oxidative stress and iron metabolism balance. By activating SIRT1, iron death can be effectively inhibited, inflammatory response and lung tissue injury can be alleviated. In addition, activation of SIRT1 by small molecule compounds, natural products, Meteorin-like/Meteorin-β, or upregulation of GDF11 may be potential strategies for the treatment of ALI. These studies provide an important scientific basis for the development of novel therapies for ALI.
Bioinformatics analysis has identified SIRT1 as a key gene associated with myocardial infarction, with its role in ferroptosis being particularly significant (Jiang et al., 2022). During myocardial ischemia-reperfusion, SIRT1 activation can inhibit ferroptosis and reduce cardiac cell death, thereby protecting cardiac function. SIRT1 also interacts with Nicotinamide phosphoribosyltransferase (NAMPT) and PTEN-induced putative kinase 1 (PINK1)/Parkinson disease protein 2 (Parkin) signaling pathways to maintain mitochondrial homeostasis and promote mitochondrial autophagy, which is critical for preventing ferroptosis and myocardial damage (Ma et al., 2020; Liao et al., 2023; Ju et al., 2023). In patients with sepsis-induced cardiomyopathy (SIC), lower serum levels of GPX4 and SIRT1, along with higher levels of Creatine Kinase-Muscle/Brain (CK-MB), cardiac troponin I (cTnI), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6), have been observed. Experiments showed that quercetin can reduce intracellular Fe2+ and prostaglandin-endoperoxide synthase 2 (PTGS2) levels, decrease the apoptosis rate, and upregulate GPX4 and ferritin levels by activating the SIRT1/p53/SLC7A11 signaling pathway. This action inhibits ferroptosis in H9C2 cells in vitro and alleviates SIC in vivo in a dose-dependent manner, suggesting a potential treatment strategy for SIC (Lin et al., 2023). In doxorubicin (DOX)-induced cardiomyopathy models, SIRT1 downregulation exacerbates ferroptosis in cardiomyocytes, while its activation reduces oxidative stress and inhibits ferroptosis by increasing the expression of antioxidant enzymes such as GPX4. Furthermore, SIRT1 enhances cellular antioxidant response through activation of Nrf2/Kelch-like ECH-associated protein 1(Keap1) signaling pathway, thus protecting cardiomyocytes from DOX-induced damage (Wang et al., 2023a; Abdel-Rahman et al., 2022; Yarmohammadi et al., 2024). SIRT1 also inhibits ferroptosis through p53-SLC7A11/GPX4 pathway, protects cardiomyocytes, and improves cardiac function in Type 1 Diabetes Mellitus (Tang et al., 2024). Icariin has been shown to protect against ethanol-induced atrial remodeling by activating the SIRT1 signaling pathway, reducing atrial ferroptosis, and inhibiting atrial fibrosis and oxidative stress. However, the protective effect of icariin is countered by the ferroptosis activator erastin and the SIRT1 inhibitor EX527 (Yu et al., 2023a). In heart failure models, SIRT1-mediated ferroptosis plays a crucial role in the pathogenesis, with AKG improving cardiac dysfunction through mitochondrial autophagy and ferroptosis inhibition mediated by the NAD + -SIRT1 signaling pathway (Yu et al., 2024). SIRT1 activation has been shown to inhibit ferroptosis in cardiomyocytes by reducing the acetylation of p53 protein, maintaining the stability of SLC7A11 protein, and increasing intracellular GSH and GPX4 levels, thereby reducing oxidative stress and lipid peroxidation (Tang et al., 2024). Natural compounds such as resveratrol and pterostilbene have shown inhibition of ferroptosis in cardiomyocytes via the SIRT1/p53 and SIRT1/Glycogen Synthase Kinase-3β (GSK-3β)/GPX4 signaling pathways, improving cardiac function and reducing cardiac remodeling in heart failure models (Zhang et al., 2023b; Zhang et al., 2024b). Natural compounds such as quercetin, icariin, resveratrol and pterostilbene reduce oxidative stress, inhibit ferroptosis of cardiomyocytes, and improve heart function by activating the SIRT1 signaling pathway (Lin et al., 2023; Yu et al., 2023a; Zhang et al., 2023b; Zhang et al., 2024b). Therefore, the regulation of SIRT1 and its associated signaling pathways not only provides insight into the molecular mechanisms of various heart diseases, but also provides potential targets for the development of new therapeutic strategies (Figure 3).
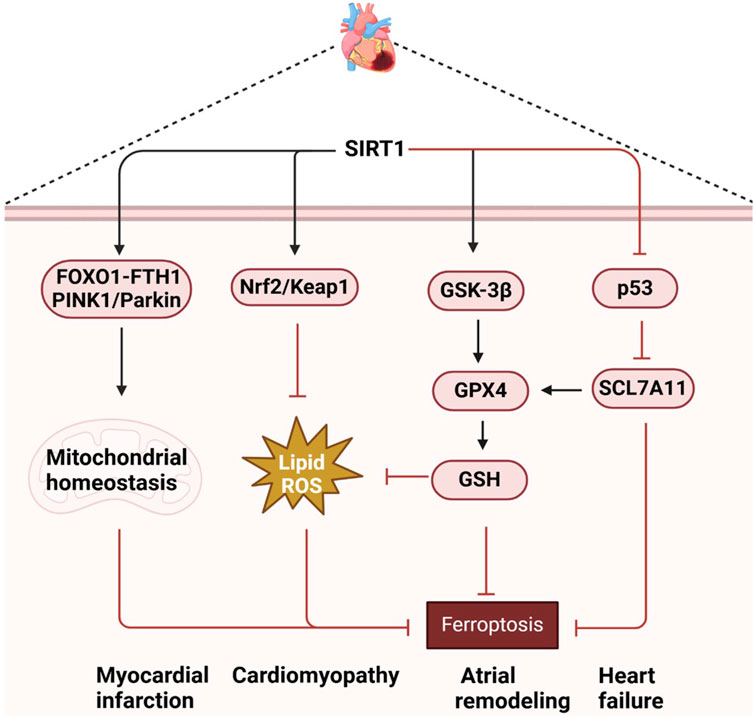
Figure 3. Mechanistic insights and therapeutic implications of SIRT1-mediated ferroptosis in cardiac diseases.
Recent studies have highlighted the key role of SIRT1-mediated ferroptosis in multiple kidney diseases. In contrast induced nephropathy (CIN), SIRT1 activated by calorie restriction was able to reduce kidney damage via the modulation GPX4 (Fang et al., 2021). In sepsis associated acute kidney injury (SA-AKI), the exercise hormone irisin mitigates ferroptosis and kidney damage through the SIRT1/Nrf2 signaling pathway (Qiongyue et al., 2022). In Polymyxin B (PMB) -induced acute kidney injury, baicalein activated SIRT1 by reducing p53 acetylation level, thereby inhibiting ferroptosis (Yu et al., 2023b). In cisplatin-induced renal toxicity, gastrodin inhibits ferroptosis through SIRT1/FOXO3A/GPX4 signaling pathway and protects the kidney from damage (Qiu et al., 2024). The water extract of earthworms alleviates oxidative stress-induced renal cell death by enhancing SIRT1/Nrf2 signaling pathways and improving mitochondrial function (Shu et al., 2024). Baicalein, gastrodin and extract of earthworms alleviates renal cell ferroptosis induced by oxidative stress by enhancing SIRT1/Nrf2 signaling pathway and improving mitochondrial function (Yu et al., 2023b; Qiu et al., 2024; Shu et al., 2024). These researches suggest that ferroptosis can be effectively inhibited by regulating SIRT1 and its associated signaling pathways, providing a new therapeutic strategy for the treatment of kidney diseases caused by different causes.
SIRT1 plays an important role in maintaining bone health by regulating ferroptosis. In disc degeneration, the SIRT1-autophagy axis may protect disc cells by inhibiting ferroptosis caused by oxidative stress (Zhou and Ruan, 2022). Moreover, in primary osteoporosis, SIRT1 is recognized as a pivotal gene linked to ferroptosis, with its expression levels potentially influencing bone metabolism and the viability of bone cells (Xia et al., 2022). Consequently, SIRT1’s role extends to Type 2 diabetic osteoporosis, where vitamin K2 promotes bone mass by activating the adenosine monophosphate-activated protein kinase (AMPK)/SIRT1 signaling pathway to suppress ferroptosis. Similarly, in postmenopausal osteoporosis, the Chinese herbal ingredient icariin is believed to have therapeutic benefits by targeting multiple ferroptosis-related pathways, including the modulation of SIRT1 (Huang et al., 2024; Wang et al., 2024b; Jin et al., 2023; Jing et al., 2019; Schluesener and Schluesener, 2014). In osteoarthritis, by deacetylating key proteins, SIRT1 activates the antioxidant response element (ARE), thereby upregulating the expression of antioxidant genes such as Nrf2, HO-1, and GPX4. This activation enhances the cellular defense against oxidative stress and suppresses the iron-mediated ROS production, which are pivotal in the pathogenesis of osteoarthritis. Consequently, the SIRT1/Nrf2 signaling axis emerges as a critical pathway in maintaining chondrocyte integrity and attenuating the degenerative processes in the joint, offering a potential therapeutic strategy for managing osteoarthritis (Zhan et al., 2023; Zhang et al., 2024c; Sun et al., 2023; Ruan et al., 2023). Together, SIRT1 activation has shown potential in maintaining bone health by inhibiting ferroptosis. Vitamin K2 and icariin have been demonstrated to promote bone mass and protect bone cells from oxidative damage by activating the AMPK/SIRT1 and SIRT1/Nrf2 pathways, respectively (Jin et al., 2023; Jing et al., 2019; Schluesener and Schluesener, 2014). These findings highlight SIRT1’s central role in regulating ferroptosis, protecting bone cells from oxidative damage, and maintaining bone health.
SIRT1-mediated ferroptosis is increasingly recognized as a pivotal factor in diverse cancer treatment strategies. Bioinformatics analysis has revealed a significant association between SIRT1 and ferroptosis in both hepatocellular carcinoma (HCC), gastric cancer and Ewing’s sarcoma, highlighting its potential as a therapeutic target in these diseases (Sui et al., 2019; Niu et al., 2023; Jiao et al., 2023). In HCC, SIRT1 inhibition by protocadherin 20 promotes ferroptosis via reducing the expression of SLC7A11, GPX4 and GSH, while increasing MDA, ROS and intracellular iron levels, resulting in a significant decrease in cell viability, colony-forming ability, and the growth and size of tumor (Jun et al., 2023). In the context of gastric cancer, SIRT1 collaborates with APE1 to stimulate cancer cell ferroptosis by repressing p53, thereby curbing cancer cell proliferation (Zhao et al., 2023a). Furthermore, the loss of long non-coding RNA DACT3-AS1 in exosomes derived from cancer-associated fibroblasts (CAFs) promotes gastric tumor malignant transformation and oxaliplatin resistance by affecting the miR-181a-5p/SIRT1 axis, a process involved in the regulation of ferroptosis (Qu et al., 2023). In addition, in colorectal cancer, ropivacaine enhances cisplatin sensitivity by inhibiting SIRT1 expression, an effect achieved in part by promoting ferroptosis (Zeng et al., 2024b). In melanoma studies, ubiquitin specific peptidase 22 controls melanoma metastasis and sensitivity to ferroptosis through the SIRT1/phosphatase and tensin homolog deleted on chromosome 10 (PTEN)/phosphoinositol-3 kinase (PI3K) signaling pathway, suggesting that activation of the SIRT1 pathway may enhance melanoma cells’ sensitivity to ferroptosis (Sun et al., 2024). In the study of lung adenocarcinoma, high doses of β-nicotinamide mononucleotide promote ferroptosis and inhibit lung cancer cell growth through the excess nicotinamide-mediated SIRT1/AMPK/acetyl-coA carboxylase (ACC) signaling pathway (Zhang et al., 2023a). In head and neck cancer, the activation of SIRT1 facilitates the epithelial-mesenchymal transition (EMT), thereby enhancing cancer cells’ susceptibility to ferroptosis. Conversely, the inhibition of SIRT1 diminishes ferroptosis. Additionally, SIRT1 plays a role in governing ferroptosis by modulating the expression of GPX4, SLC7A11, and SLC3A2 (Lee et al., 2020). In paclitaxel-tolerant persister head and neck cancer (HNC) cell lines, SIRT1 activation promotes ferroptosis by increasing mitochondrial fatty acid oxidation via facilitating the dispersion and localization of lipid droplets on mitochondria (You et al., 2021). In addition, in studies of chronic lymphocytic leukemia (CLL), activation or inhibition of SIRT1 influenced the sensitivity of CLL cells to ferroptosis, suggesting a potential role for SIRT1 in regulating ferroptosis in CLL cells (Pan et al., 2022). These results underscore the potential of SIRT1 modulators (activators or inhibitors) and the ferroptosis pathway it regulates, as a novel therapeutic strategy for cancer treatment. Future research will further explore the specific mechanisms of action of SIRT1 modulators in different types of cancer, providing a scientific basis for the development of more effective cancer therapies.
SIRT1-mediated ferroptosis plays an important role in the pathological process of mastitis. The activation of SIRT1 can inhibit the activation of inflammasome and the release of inflammatory cytokines, and reduce the inflammatory response. At the same time, SIRT1 deacetylates multiple transcription factors, such as Nrf2, promotes its entry into the nucleus and activates the expression of antioxidant stress genes, such as HO-1 and GPX4, the activation of which helps mitigate cell damage caused by ferroptosis. In mastitis, SIRT1 activation helps to reduce intracellular iron content and inhibit lipid peroxidation, thereby reducing ferroptosis and protecting breast tissue from damage (Zhou et al., 2024b; Zhao et al., 2023b; Zhao et al., 2023c). Natural compounds and small-molecule activators that enhance SIRT1 activity could be developed as novel therapeutics for mitigating inflammatory responses and protecting tissue from ferroptosis-induced damage.
In diabetes mellitus, SIRT1-mediated ferroptosis plays an important role in islet β-cell dysfunction. Research has shown that hyperglycemia inhibits the expression of SIRT1 in islet β-cells, leading to decreased levels of the antioxidant enzyme GPX4 and increased expression of the ferroptosis-related protein TFR1. This weakens the cells’ antioxidant capacity, making them more susceptible to oxidative stress and ultimately resulting in ferroptosis. Stabilizing SIRT1 activity alleviates ferroptosis in islet β-cells, improves insulin secretion, and mitigates hyperglycemia symptoms (Zhang et al., 2022). SIRT1 is also involved in the development of diabetic complications through its regulation of ferroptosis. Under high-glucose conditions, SIRT1 activity is reduced, leading to increased ferroptosis. Activating SIRT1 can inhibit ferroptosis and reduce pathological damage in diabetic complications. For example, in diabetic retinopathy (DR), SIRT1 suppresses inflammation and retinal vascular damage by regulating HMGB1 deacetylation and inhibiting ferroptosis (Peng et al., 2025). Inhibition of SIRT1 reduces Nrf2 activity, decreases the expression of antioxidant-related molecules, and exacerbates ferroptosis. Astragaloside-IV can enhance SIRT1 and Nrf2 activity, boost cellular antioxidant capacity, reduce hyperglycemia-induced ferroptosis, and protect retinal pigment epithelial (RPE) cells from damage, offering a potential therapeutic strategy for DR (Tang et al., 2022). Flavanones can increase SIRT1 activity, inhibit ferroptosis through the FOXO3a and Nrf2 signaling pathways, and alleviate renal tubular epithelial cell injury induced by high glucose (Zhou et al., 2025). Additionally, activating the SIRT1/Nrf2/p62 pathway can promote the healing of diabetic foot ulcers, possibly mediated by autophagy-dependent ferroptosis (Han et al., 2024). In diabetic peripheral neuropathy (DPN), inactivation of SIRT1 promotes the production of mitochondrial ROS, leading to dysfunction and ferroptosis in Schwann cells. Activation of the AMPK/SIRT1/PGC-1α signaling pathway by honokiol alleviates hyperglycemia-induced oxidative stress and ferroptosis, thereby improving cell function (Liang et al., 2023; Hu et al., 2023). These findings suggest that SIRT1 and its regulation of ferroptosis are crucial in diabetic complications and may represent a novel therapeutic target for these conditions.
Together, the diverse roles of SIRT1 across multiple disease types underscore its significance as a promising therapeutic target. A variety of therapeutic agents have been explored for modulating SIRT1 in ferroptosis-related diseases. Table 1 summarizes key SIRT1-targeted interventions, their mechanisms, and potential clinical applications across various pathological conditions. This consolidated information provides a reference for ongoing research and potential translational applications in ferroptosis-associated diseases (Table 1).
The role of SIRT1 in the regulation of ferroptosis has emerged as a promising area of research with significant therapeutic potential in a multiple of diseases. While significant progress has been made in understanding the role of SIRT1 in ferroptosis, there are still many challenges to overcome. The integration of new technologies and the pursuit of innovative research directions will be instrumental in advancing the field and unlocking the therapeutic potential of targeting SIRT1 and ferroptosis. Future research should focus on elucidating the precise molecular mechanisms by which SIRT1 regulates ferroptosis. This includes investigating the full spectrum of SIRT1 targets, the epigenetic changes it induces, and how these contribute to the susceptibility of target cells to ferroptosis. Research could focus on identifying SIRT1-dependent gene regulatory networks that modulate ferroptosis. Investigating the interplay between SIRT1 and other cellular pathways, such as autophagy and the unfolded protein response, will also be crucial for a comprehensive understanding of ferroptosis regulation. What’s more, the development of small molecules that specifically target SIRT1 or its regulatory pathway is a promising avenue. This includes the design of more potent and selective SIRT1 modulators and the evaluation of their efficacy in preclinical models of diseases characterized by ferroptosis, such as kidney diseases, neurodegenerative disorders, and cancer. Moreover, the development of predictive biomarkers for ferroptosis sensitivity is crucial. Future studies should aim to identify and validate biomarkers that can predict a patient’s response to ferroptosis-inducing therapies, allowing for personalized treatment strategies. Translational research efforts should be directed towards the design of clinical trials assessing the safety and efficacy of SIRT1-targeted ferroptosis induction in patients. Notably, long-term studies are needed to assess the safety and efficacy of SIRT1 modulation in inducing ferroptosis.
Through the diligent exploration of these research avenues, the scientific community can potentially unlock the therapeutic potential of SIRT1 and ferroptosis as novel strategies in human disease. This advancement may provide renewed hope for patients who have exhausted conventional treatment options and are in dire need of innovative therapeutic interventions.
Our review underscores the multifaceted role of SIRT1 in modulating ferroptosis, highlighting its potential as a therapeutic target. The intricate interplay between SIRT1 and the molecular machinery governing ferroptosis offers a rich avenue for therapeutic intervention. SIRT1’s ability to deacetylate and thereby activate key proteins involved in ferroptosis, such as GPX4 and FOXO3A, positions it as a nodal point in the regulation of this cell death process. By maintaining cellular redox balance and influencing iron homeostasis, SIRT1 contributes to the cellular resistance against ferroptosis. The therapeutic potential of SIRT1 in modulating ferroptosis is vast and spans a range of diseases, including neurodegenerative disorders, cancer, and kidney diseases. The activation of SIRT1 has been shown to ameliorate ferroptosis-associated cell death in various models, suggesting its utility in developing protective strategies against diseases where ferroptosis plays a pathogenic role. However, the field faces challenges, including the need for a deeper understanding of the molecular underpinnings of SIRT1’s role in ferroptosis and the development of targeted therapies that can effectively harness this enzyme’s activity. In conclusion, the regulation of ferroptosis by SIRT1 represents a burgeoning frontier in cellular biology with significant therapeutic implications. As we continue to unravel the complexities of this process, we edge closer to a future where the modulation of ferroptosis through SIRT1 activation may offer novel treatment strategies for a host of diseases. Our review establish a foundation for future research and pave the way for novel therapeutic strategies that could harness the potential of ferroptosis in biological and medical contexts.
YZ: Writing–original draft, Funding acquisition. FK: Visualization, Writing–original draft. NL: Supervision, Writing–review and editing. LT: Supervision, Writing–review and editing. JZ: Conceptualization, Writing–review and editing. JM: Conceptualization, Writing–review and editing. SZ: Conceptualization, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Department of Science and Technology of Jilin Province (grant No. YDZJ202201ZYTS068 to Lina Tao), the Department of Education of Jilin Province (grant No. JJKH20221106KJ to Yueming Zhang), Jilin Provincial Health Commission (grant No. J2023JC006 to Jie Ma) and the Department of Science and Technology of Jilin Province (grant No. 20210401091YY to Sixi Zhang).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdel-Rahman, A., Soliman, E., Testai, L., Sun, Z., Yu, L., Xu, C., et al. (2022). Fisetin attenuates doxorubicin-induced cardiomyopathy in vivo and in vitro by inhibiting ferroptosis through SIRT1/nrf2 signaling pathway activation. Front. Pharmacol. 12. doi:10.3389/fphar.2021.808480
Abukhalil, M. H., Al-Alami, Z., Alfwuaires, M. A., Imran, M. R., Aladaileh, S. H., and Althunibat, O. Y. (2025). Taxifolin protects against 5-fluorouracil-induced cardiotoxicity in mice through mitigating oxidative stress, inflammation, and apoptosis: possible involvement of sirt1/Nrf2/HO-1 signaling. Cardiovasc Toxicol. doi:10.1007/s12012-025-09962-w
Bai, X., Ye, D., Shi, Y., Fan, M., Lu, P., Feng, Y., et al. (2023). Neuroprotection of SRT2104 in murine ischemia/reperfusion injury through the enhancement of Sirt1-mediated deacetylation. Invest. Ophthalmol. Vis. Sci. 64, 31. doi:10.1167/iovs.64.4.31
Bellezza, I., Tucci, A., Galli, F., Grottelli, S., Mierla, A. L., Pilolli, F., et al. (2012). Inhibition of NF-κB nuclear translocation via HO-1 activation underlies α-tocopheryl succinate toxicity. J. Nutr. Biochem. 23, 1583–1591. doi:10.1016/j.jnutbio.2011.10.012
Cai, M., Fu, T., Zhu, R., Hu, P., Kong, J., Liao, S., et al. (2024). An iron-based metal-organic framework nanoplatform for enhanced ferroptosis and oridonin delivery as a comprehensive antitumor strategy. Acta Pharm. Sin. B 14, 4073–4086. doi:10.1016/j.apsb.2024.05.015
Chen, H., Lin, X., Yi, X., Liu, X., Yu, R., Fan, W., et al. (2022a). SIRT1-mediated p53 deacetylation inhibits ferroptosis and alleviates heat stress-induced lung epithelial cells injury. Int. J. Hyperth. 39, 977–986. doi:10.1080/02656736.2022.2094476
Chen, J., Chen, P., Song, Y., Wei, J., Wu, F., Sun, J., et al. (2024a). STING upregulation mediates ferroptosis and inflammatory response in lupus nephritis by upregulating TBK1 and activating NF-κB signal pathway. J. Biosci. 49, 9. doi:10.1007/s12038-023-00381-z
Chen, J., Xiao, L., Chen, Y., Li, W., Liu, Y., Zhou, Y., et al. (2023a). YT521-B homology domain containing 1 ameliorates mitochondrial damage and ferroptosis in sleep deprivation by activating the sirtuin 1/nuclear factor erythroid-derived 2-like 2/heme oxygenase 1 pathway. Brain Res. Bull. 197, 1–12. doi:10.1016/j.brainresbull.2023.03.008
Chen, L., Xu, H., Zhang, C., He, J., and Wang, Y. (2024b). Semaglutide alleviates early brain injury following subarachnoid hemorrhage by suppressing ferroptosis and neuroinflammation via SIRT1 pathway. Am. J. Transl. Res. 16, 1102–1117. doi:10.62347/IZGJ1332
Chen, Q., Liu, L., and Ni, S. (2022b). Screening of ferroptosis-related genes in sepsis-induced liver failure and analysis of immune correlation. PeerJ 10, e13757. doi:10.7717/peerj.13757
Chen, X., Wang, Z., Li, C., Zhang, Z., Lu, S., Wang, X., et al. (2024c). SIRT1 activated by AROS sensitizes glioma cells to ferroptosis via induction of NAD+ depletion-dependent activation of ATF3. Redox Biol. 69, 103030. doi:10.1016/j.redox.2024.103030
Chen, Z., Li, J., Peng, H., Zhang, M., Wu, X., Gui, F., et al. (2023b). Meteorin-like/Meteorin-β protects LPS-induced acute lung injury by activating SIRT1-P53-SLC7A11 mediated ferroptosis pathway. Mol. Med. 29, 144. doi:10.1186/s10020-023-00714-6
Conde, M. A., Alza, N. P., Funk, M. I., Maniscalchi, A., Benzi Juncos, O. N., Berge, I., et al. (2023). α-Synuclein attenuates maneb Neurotoxicity through the modulation of redox-Sensitive transcription factors. Oxidative Med. Cell. Longev. 2023, 5803323–5803415. doi:10.1155/2023/5803323
Dang, R., Wang, M., Li, X., Wang, H., Liu, L., Wu, Q., et al. (2022). Edaravone ameliorates depressive and anxiety-like behaviors via Sirt1/Nrf2/HO-1/Gpx4 pathway. J. Neuroinflammation 19, 41. doi:10.1186/s12974-022-02400-6
Davenport, A. M., Huber, F. M., and Hoelz, A. (2014). Structural and functional analysis of human SIRT1. J Mol Biol 426 (3), 526–41. doi:10.1016/j.jmb.2013.10.009
Deng, S., Li, J., Li, L., Lin, S., Yang, Y., Liu, T., et al. (2023). Quercetin alleviates lipopolysaccharide-induced acute lung injury by inhibiting ferroptosis via the Sirt1/Nrf2/Gpx4 pathway. Int. J. Mol. Med. 52, 118. doi:10.3892/ijmm.2023.5321
Dixon, S. J., Lemberg, K. M., Lamprecht, M. R., Skouta, R., Zaitsev, E. M., Gleason, C. E., et al. (2012). Ferroptosis: an iron-dependent form of Nonapoptotic cell death. Cell 149, 1060–1072. doi:10.1016/j.cell.2012.03.042
Dixon, S. J., Winter, G. E., Musavi, L. S., Lee, E. D., Snijder, B., Rebsamen, M., et al. (2015). Human Haploid cell Genetics reveals roles for lipid metabolism genes in Nonapoptotic cell death. ACS Chem. Biol. 10, 1604–1609. doi:10.1021/acschembio.5b00245
Fang, D., Wang, Y., Zhang, Z., Yang, D., Gu, D., He, B., et al. (2021). Calorie restriction protects against contrast-induced nephropathy via SIRT1/GPX4 activation. Oxid Med Cell Longev. 9846101. doi:10.1155/2021/2999296
Gu, X., Zhang, G., Qin, Z., Yin, M., Chen, W., Zhang, Y., et al. (2022). Safinamide protects against amyloid β (Aβ)-induced oxidative stress and cellular senescence in M17 neuronal cells. Bioengineered 13, 1921–1930. doi:10.1080/21655979.2021.2022262
Guo, J., Xue, H., Zhong, H., Sun, W., Zhao, S., Meng, J., et al. (2022). Involvement of LARP7 in activation of SIRT1 to inhibit NF-κB signaling protects microglia from Acrylamide-induced neuroinflammation. Neurotox. Res. 40, 2016–2026. doi:10.1007/s12640-022-00624-1
Hacioglu, C. (2024). Long-term exposure of sucralose induces neuroinflammation and ferroptosis in human microglia cells via SIRT1/NLRP3/IL-1β/GPx4 signaling pathways. Food Sci. and Nutr. 12, 9094–9107. doi:10.1002/fsn3.4488
Han, Q., Gu, Y., and Qian, Y. (2024). Study on the mechanism of activating SIRT1/Nrf2/p62 pathway to mediate autophagy-dependent ferroptosis to promote healing of diabetic foot ulcers. Schmiedeb. Arch. Pharmacol. doi:10.1007/s00210-024-03400-4
Hao, R., Ge, J., Song, X., Li, F., Sun-Waterhouse, D., and Li, D. (2022). Cadmium induces ferroptosis and apoptosis by modulating miR -34a-5p/Sirt1axis in PC12 cells. Environ. Toxicol. 37, 41–51. doi:10.1002/tox.23376
He, L., Wang, J., Tao, B., Zhu, R., Li, C., and Ning, B. (2023). Identification of ferroptosis-related genes in the progress of NASH. Front. Endocrinol. 14, 1184280. doi:10.3389/fendo.2023.1184280
Hu, M., Jiang, W., Ye, C., Hu, T., Yu, Q., Meng, M., et al. (2023). Honokiol attenuates high glucose-induced peripheral neuropathy via inhibiting ferroptosis and activating AMPK/SIRT1/PGC -1α pathway in Schwann cells. Phytotherapy Res. 37, 5787–5802. doi:10.1002/ptr.7984
Huang, C., Li, Y., Li, B., Liu, X., Luo, D., Liu, Y., et al. (2024). Identifying potential ferroptosis key genes for diagnosis and treatment of postmenopausal osteoporosis through competitive endogenous RNA network analysis. Heliyon 10, e23672. doi:10.1016/j.heliyon.2023.e23672
Jiang, Y.-H., Wu, S.-Y., Wang, Z., Zhang, L., Zhang, J., Li, Y., et al. (2022). Bioinformatics analysis identifies ferroptosis-related genes in the regulatory mechanism of myocardial infarction. Exp. Ther. Med. 24, 748. doi:10.3892/etm.2022.11684
Jiao, X., Li, Q., and Xu, X. (2023). Prognostic implication of a ferroptosis-related gene signature associates with immunity in Ewing’s sarcoma. Electron. J. Biotechnol. 64, 42–58. doi:10.1016/j.ejbt.2023.01.004
Jin, C., Tan, K., Yao, Z., Lin, B., Zhang, D., Chen, W.-K., et al. (2023). A novel anti-osteoporosis mechanism of VK2: Interfering with ferroptosis via AMPK/SIRT1 pathway in type 2 diabetic osteoporosis. J. Agric. Food Chem. 71, 2745–2761. doi:10.1021/acs.jafc.2c05632
Jing, X., Du, T., Chen, K., Guo, J., Xiang, W., Yao, X., et al. (2019). Icariin protects against iron overload-induced bone loss via suppressing oxidative stress. J. Cell Physiol. 234, 10123–10137. doi:10.1002/jcp.27678
Ju, J., Li, X.-M., Zhao, X.-M., Li, F.-H., Wang, S.-C., Wang, K., et al. (2023). Circular RNA FEACR inhibits ferroptosis and alleviates myocardial ischemia/reperfusion injury by interacting with NAMPT. J. Biomed. Sci. 30, 45. doi:10.1186/s12929-023-00927-1
Jun, L., Chen, W., Han, L., Yanmin, L., Qinglei, Z., and Pengfei, Z. (2023). Protocadherin 20 promotes ferroptosis by suppressing the expression of Sirtuin 1 and promoting the acetylation of nuclear factor erythroid 2-related factor 2 in hepatocellular carcinoma. Int. J. Biochem. and Cell Biol. 156, 106363. doi:10.1016/j.biocel.2023.106363
Latunde-Dada, G. O. (2017). Ferroptosis: role of lipid peroxidation, iron and ferritinophagy. Biochim. Biophys. Acta Gen. Subj. 1861, 1893–1900. doi:10.1016/j.bbagen.2017.05.019
Lee, J., You, J. H., Kim, M.-S., and Roh, J.-L. (2020). Epigenetic reprogramming of epithelial-mesenchymal transition promotes ferroptosis of head and neck cancer. Redox Biol. 37, 101697. doi:10.1016/j.redox.2020.101697
Lei, Y., Lei, X., Zhu, A., Xie, S., Zhang, T., Wang, C., et al. (2023). Ethanol extract of rosa rugosa ameliorates acetaminophen-induced liver injury via upregulating Sirt1 and subsequent Potentiation of LKB1/AMPK/Nrf2 cascade in hepatocytes. Molecules 28, 7307. doi:10.3390/molecules28217307
Li, C., Cui, K., Zhu, X., Wang, S., Yang, Q., and Fang, G. (2024a). 8-weeks aerobic exercise ameliorates cognitive deficit and mitigates ferroptosis triggered by iron overload in the prefrontal cortex of APP Swe/PSEN 1dE9 mice through Xc-/GPx4 pathway. Front. Neurosci. 18, 1453582. doi:10.3389/fnins.2024.1453582
Li, R., Yuan, H., Zhang, C., Han, D., Wang, Y., and Feng, L. (2024b). Induced ferroptosis pathway by regulating cellular lipid peroxidation with Peroxynitrite generator for reversing “Cold” tumors. “Cold” Tumors, Small 20, e2404807. doi:10.1002/smll.202404807
Liang, Z., Zhang, N., Wang, X., Zhang, J., Li, K., and Lei, T. (2023). Epothilone B inactivation of Sirtuin1 promotes mitochondrial reactive oxygen species to induce dysfunction and ferroptosis of Schwann cells. Eur. J. Pharm. Sci. 181, 106350. doi:10.1016/j.ejps.2022.106350
Liao, Y., Ke, B., Long, X., Xu, J., and Wu, Y. (2023). Abnormalities in the SIRT1-SIRT3 axis promote myocardial ischemia-reperfusion injury through ferroptosis caused by silencing the PINK1/Parkin signaling pathway. BMC Cardiovasc Disord. 23, 582. doi:10.1186/s12872-023-03603-2
Lin, L., Yang, L., Wang, N., Chen, S., Du, X., Chen, R., et al. (2024b). FGF10 protects against LPS-induced epithelial barrier injury and inflammation by inhibiting SIRT1-ferroptosis pathway in acute lung injury in mice. Int. Immunopharmacol. 127, 111426. doi:10.1016/j.intimp.2023.111426
Lin, X., Zhao, X., Chen, Q., Wang, X., Wu, Y., and Zhao, H. (2023). Quercetin ameliorates ferroptosis of rat cardiomyocytes via activation of the SIRT1/p53/SLC7A11 signaling pathway to alleviate sepsis-induced cardiomyopathy. Int. J. Mol. Med. 52, 116. doi:10.3892/ijmm.2023.5319
Lin, Y.-S., Tsai, Y.-C., Li, C.-J., Wei, T.-T., Wang, J.-L., Lin, B.-W., et al. (2024a). Overexpression of NUDT16L1 sustains proper function of mitochondria and leads to ferroptosis insensitivity in colorectal cancer. Redox Biol. 77, 103358. doi:10.1016/j.redox.2024.103358
Liu, J., Huang, J., Zhang, Z., Zhang, R., Sun, Q., Zhang, Z., et al. (2022). Mesenchymal stem cell-derived exosomes ameliorate Delayed Neurocognitive Recovery in aged mice by inhibiting Hippocampus ferroptosis via activating SIRT1/Nrf2/HO-1 signaling pathway. Oxidative Med. Cell. Longev. 2022, 3593294–3593322. doi:10.1155/2022/3593294
Liu, Q., Liu, Y., Li, Y., Hong, Z., Li, S., and Liu, C. (2023). PUM2 aggravates the neuroinflammation and brain damage induced by ischemia–reperfusion through the SLC7A11-dependent inhibition of ferroptosis via suppressing the SIRT1. Mol. Cell Biochem. 478, 609–620. doi:10.1007/s11010-022-04534-w
Lu, C., Zhao, H., Liu, Y., Yang, Z., Yao, H., Liu, T., et al. (2023). Novel role of the SIRT1 in endocrine and metabolic diseases. Int. J. Biol. Sci. 19, 484–501. doi:10.7150/ijbs.78654
Lv, Q.-K., Tao, K.-X., Yao, X.-Y., Pang, M.-Z., Cao, B.-E., Liu, C.-F., et al. (2024). Melatonin MT1 receptors regulate the Sirt1/Nrf2/Ho-1/Gpx4 pathway to prevent α-synuclein-induced ferroptosis in Parkinson’s disease. J. Pineal Res. 76, e12948. doi:10.1111/jpi.12948
Lv, Z., Wang, F., Zhang, X., Zhang, X., Zhang, J., and Liu, R. (2021). Etomidate attenuates the ferroptosis in myocardial ischemia/reperfusion rat model via Nrf2/HO-1 pathway. Shock 56, 440–449. doi:10.1097/SHK.0000000000001751
Ma, S., Sun, L., Wu, W., Wu, J., Sun, Z., and Ren, J. (2020). USP22 protects against myocardial ischemia–reperfusion injury via the SIRT1-p53/SLC7A11–dependent inhibition of ferroptosis–induced cardiomyocyte death. Front. Physiol. 11, 551318. doi:10.3389/fphys.2020.551318
Majeed, Y., Halabi, N., Madani, A. Y., Engelke, R., Bhagwat, A. M., Abdesselem, H., et al. (2021). SIRT1 promotes lipid metabolism and mitochondrial biogenesis in adipocytes and coordinates adipogenesis by targeting key enzymatic pathways. Sci. Rep. 11, 8177. doi:10.1038/s41598-021-87759-x
Min, X., Shi, Y., Xu, Y., Li, Y., Dong, Y., Chen, F., et al. (2025). Mechanism of sirtuin1-mediated deacetylation of p65-mediated ferroptosis of hippocampal neurons in cerebral injury after Cardiopulmonary Resuscitation in rats. Neurochem. Res. 50, 66. doi:10.1007/s11064-024-04297-4
Niu, J., Guo, W., Lu, A., Han, G., Wang, G., Peng, B., et al. (2023). Comparison with gastric cancer-associated genes reveals the role of ferroptosis-related genes in eosinophils of asthma patients: a bioinformatic study. Medicine 102, e35002. doi:10.1097/MD.0000000000035002
Pan, B., Li, Y., Xu, Z., Miao, Y., Yin, H., Kong, Y., et al. (2022). Identifying a novel ferroptosis-related prognostic score for predicting prognosis in chronic lymphocytic leukemia. Front. Immunol. 13, 962000. doi:10.3389/fimmu.2022.962000
Peng, Y., Hu, L., Xu, H., Fang, J., and Zhong, H. (2025). Resveratrol alleviates reactive oxygen species and inflammation in diabetic retinopathy via SIRT1/HMGB1 pathway-mediated ferroptosis. Toxicol. Appl. Pharmacol. 495, 117214. doi:10.1016/j.taap.2024.117214
Qiongyue, Z., Xin, Y., Meng, P., Sulin, M., Yanlin, W., Xinyi, L., et al. (2022). Post-treatment with irisin attenuates acute kidney injury in sepsis mice through anti-ferroptosis via the SIRT1/Nrf2 pathway. Front. Pharmacol. 13, 857067. doi:10.3389/fphar.2022.857067
Qiu, C.-W., Chen, B., Zhu, H.-F., Liang, Y.-L., and Mao, L.-S. (2024). Gastrodin alleviates cisplatin nephrotoxicity by inhibiting ferroptosis via the SIRT1/FOXO3A/GPX4 signaling pathway. J. Ethnopharmacol. 319, 117282. doi:10.1016/j.jep.2023.117282
Qu, X., Liu, B., Wang, L., Liu, L., Zhao, W., Liu, C., et al. (2023). Loss of cancer-associated fibroblast-derived exosomal DACT3-AS1 promotes malignant transformation and ferroptosis-mediated oxaliplatin resistance in gastric cancer. Drug Resist. Updat. 68, 100936. doi:10.1016/j.drup.2023.100936
Rao, Y. L., Ganaraja, B., Suresh, P. K., Joy, T., Ullal, S. D., Manjrekar, P. A., et al. (2024). Outcome of resveratrol and resveratrol with donepezil combination on the β-amyloid plaques and neurofibrillary tangles in Alzheimer's disease. 3 Biotech. 14, 190. doi:10.1007/s13205-024-04034-2
Ruan, Q., Wang, C., Zhang, Y., and Sun, J. (2023). Brevilin A attenuates cartilage destruction in osteoarthritis mouse model by inhibiting inflammation and ferroptosis via SIRT1/Nrf2/GPX4 signaling pathway. Int. Immunopharmacol. 124, 110924. doi:10.1016/j.intimp.2023.110924
Sanz-Alcázar, A., Portillo-Carrasquer, M., Delaspre, F., Pazos-Gil, M., Tamarit, J., Ros, J., et al. (2024). “Deciphering the ferroptosis pathways in dorsal root ganglia of Friedreich ataxia models,” in The role of LKB1/AMPK, KEAP1, and GSK3β in the impairment of the NRF2 response. doi:10.1101/2024.05.10.593481
Schluesener, J. K., and Schluesener, H. (2014). Plant polyphenols in the treatment of age-associated diseases: revealing the pleiotropic effects of icariin by network analysis. Mol. Nutr. Food Res. 58, 49–60. doi:10.1002/mnfr.201300409
Sgadari, M., Cacciola, N. A., Power, K., Martano, M., and Restucci, B. (2023). Sirtuin 1 expression in canine Mammary tumors: a Pilot study. Anim. (Basel) 13, 2609. doi:10.3390/ani13162609
Shan, H., Gao, L., Zhao, S., Dou, Z., and Pan, Y. (2024). Bone marrow mesenchymal stem cells with PTBP1 knockdown protect against cerebral ischemia-reperfusion injury by inhibiting ferroptosis via the JNK/P38 pathway in rats. Neuroscience S0306-4522 (24), 130–142. doi:10.1016/j.neuroscience.2024.09.038
Shen, J., Hao, C., Yuan, S., Chen, W., Tong, T., Chen, Y., et al. (1826). Acupuncture alleviates CUMS-induced depression-like behaviors of rats by regulating oxidative stress, neuroinflammation and ferroptosis. Brain Res. 1826, 148715. doi:10.1016/j.brainres.2023.148715
Shu, G., Wang, C., Song, A., Zheng, Z., Zheng, S., Song, Y., et al. (2024). Water extract of earthworms mitigates kidney injury triggered by oxidative stress via activating intrarenal Sirt1/Nrf2 cascade and ameliorating mitochondrial damage. J. Ethnopharmacol. 335, 118648. doi:10.1016/j.jep.2024.118648
Su, G., Yang, W., Wang, S., Geng, C., and Guan, X. (2021). SIRT1-autophagy axis inhibits excess iron-induced ferroptosis of foam cells and subsequently increases IL-1Β and IL-18. Biochem. Biophysical Res. Commun. 561, 33–39. doi:10.1016/j.bbrc.2021.05.011
Sui, S., Zhang, J., Xu, S., Wang, Q., Wang, P., and Pang, D. (2019). Ferritinophagy is required for the induction of ferroptosis by the bromodomain protein BRD4 inhibitor (+)-JQ1 in cancer cells. Cell Death Dis. 10, 331. doi:10.1038/s41419-019-1564-7
Sun, H., Meng, Y., Yao, L., Du, S., Li, Y., Zhou, Q., et al. (2024). Ubiquitin-specific protease 22 controls melanoma metastasis and vulnerability to ferroptosis through targeting SIRT1/PTEN/PI3K signaling. MedComm 5, e684. doi:10.1002/mco2.684
Sun, J., Zhang, Y., Wang, C., and Ruan, Q. (2023). Kukoamine A protects mice against osteoarthritis by inhibiting chondrocyte inflammation and ferroptosis via SIRT1/GPX4 signaling pathway. Life Sci. 332, 122117. doi:10.1016/j.lfs.2023.122117
Sun, W., Yan, J., Ma, H., Wu, J., and Zhang, Y. (2022). Autophagy-dependent ferroptosis-related signature is closely associated with the prognosis and tumor immune Escape of patients with glioma. IJGM 15, 253–270. doi:10.2147/IJGM.S343046
Sun, X., Ou, Z., Chen, R., Niu, X., Chen, D., Kang, R., et al. (2016). Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 63, 173–184. doi:10.1002/hep.28251
Tang, X., Li, X., Zhang, D., and Han, W. (2022). Astragaloside-IV alleviates high glucose-induced ferroptosis in retinal pigment epithelial cells by disrupting the expression of miR-138-5p/Sirt1/Nrf2. Bioengineered 13, 8240–8254. doi:10.1080/21655979.2022.2049471
Tang, Y., Zhang, Z., Yan, T., Chen, K., Xu, G., Xiong, S., et al. (2024). Irisin attenuates type 1 diabetic cardiomyopathy by anti-ferroptosis via SIRT1-mediated deacetylation of p53. Cardiovasc Diabetol. 23, 116. doi:10.1186/s12933-024-02183-5
Tian, Y., He, X., Yuan, Y., Zhang, S., Wang, C., Dong, J., et al. (2024). TME-responsive nanoplatform with glutathione depletion for enhanced tumor-specific Mild Photothermal/gene/ferroptosis Synergistic Therapy. Int. J. Nanomedicine 19, 9145–9160. doi:10.2147/IJN.S475698
Tziastoudi, M., Pissas, G., Golfinopoulos, S., Filippidis, G., Dousdampanis, P., Eleftheriadis, T., et al. (2023). Sodium-glucose transporter 2 (SGLT2) inhibitors and iron deficiency in heart failure and chronic kidney disease: a Literature review. Life (Basel) 13, 2338. doi:10.3390/life13122338
Wang, C., Liu, T., Tong, Y., Cui, R., Qu, K., Liu, C., et al. (2021). Ulinastatin protects against acetaminophen-induced liver injury by alleviating ferroptosis via the SIRT1/NRF2/HO-1 pathway. Am. J. Transl. Res. 13, 6031–6042.
Wang, L., Li, M., Liu, B., Zheng, R., Zhang, X., and Yu, S. (2024a). miR-30a-5p mediates ferroptosis of hippocampal neurons in chronic cerebral hypoperfusion-induced cognitive dysfunction by modulating the SIRT1/NRF2 pathway. Brain Res. Bull. 212, 110953. doi:10.1016/j.brainresbull.2024.110953
Wang, M., Huang, J., Zou, J., Xu, Z., Yang, A., Liu, Z., et al. (2025). Electroacupuncture regulates SIRT1/p53/p21 signaling pathway to prevent stress-induced premature senescence of nucleus pulposus cells in degenerative intervertebral discs. Int. Immunopharmacol. 148, 114114. doi:10.1016/j.intimp.2025.114114
Wang, W., Li, X., Ding, N., Teng, J., Zhang, S., Zhang, Q., et al. (2020). miR-34a regulates adipogenesis in porcine intramuscular adipocytes by targeting ACSL4. BMC Genet. 21, 33. doi:10.1186/s12863-020-0836-7
Wang, W., Zhong, X., Fang, Z., Li, J., Li, H., Liu, X., et al. (2023a). Cardiac sirtuin1 deficiency exacerbates ferroptosis in doxorubicin-induced cardiac injury through the Nrf2/Keap1 pathway. Chem-Biol Interact. 377, 110469. doi:10.1016/j.cbi.2023.110469
Wang, X., Shao, N., Zhang, X., Chen, H., Chang, Z., Xie, D., et al. (2023b). Ferulic acid activates SIRT1-mediated ferroptosis signaling pathway to improve cognition dysfunction in Wilson’s disease. NDT 19, 2681–2696. doi:10.2147/NDT.S443278
Wang, X., Wei, K., Wang, M., and Zhang, L. (2024b). Identification of potential key ferroptosis- and autophagy-related genes in myelomeningocele through bioinformatics analysis. Heliyon 10, e29654. doi:10.1016/j.heliyon.2024.e29654
Weichhart, T. (2024). Transferrin: the iron transporter takes control. Blood 144, 9–10. doi:10.1182/blood.2024024731
Wen, Y., Zhang, W., Wang, D., and Lu, M. (2024). Propofol ameliorates cognitive deficits following splenectomy in aged rats by inhibiting ferroptosis via the SIRT1/Nrf2/GPX4 pathway. NeuroReport 35, 846–856. doi:10.1097/WNR.0000000000002074
Wu, Z., Xi, Q., Zhao, Q., and Zhu, S. (2024). GDF11 overexpression alleviates sepsis-induced lung microvascular endothelial barrier damage by activating SIRT1/NOX4 signaling to inhibit ferroptosis. Shock 62, 245–254. doi:10.1097/SHK.0000000000002391
Xia, Y., Li, S., Wang, X., Zhao, B., Chen, S., Jiang, Q., et al. (2023). Astilbin targeted Sirt1 to inhibit acetylation of Nrf2 to alleviate grass carp hepatocyte apoptosis caused by PCB126-induced mitochondrial kinetic and metabolism dysfunctions. Fish and Shellfish Immunol. 141, 109000. doi:10.1016/j.fsi.2023.109000
Xia, Y., Zhang, H., Wang, H., Wang, Q., Zhu, P., Gu, Y., et al. (2022). Identification and validation of ferroptosis key genes in bone mesenchymal stromal cells of primary osteoporosis based on bioinformatics analysis. Front. Endocrinol. 13, 980867. doi:10.3389/fendo.2022.980867
Xie, R., Zhao, W., Lowe, S., Bentley, R., Hu, G., Mei, H., et al. (2022). Quercetin alleviates kainic acid-induced seizure by inhibiting the Nrf2-mediated ferroptosis pathway. Free Radic. Bio Med. 191, 212–226. doi:10.1016/j.freeradbiomed.2022.09.001
Xu, J., Zhao, L., Zhang, X., Ying, K., Zhou, R., Cai, W., et al. (2023). Salidroside ameliorates acetaminophen-induced acute liver injury through the inhibition of endoplasmic reticulum stress-mediated ferroptosis by activating the AMPK/SIRT1 pathway. Ecotoxicol. Environ. Saf. 262, 115331. doi:10.1016/j.ecoenv.2023.115331
Yan, C., Yang, S., Shao, S., Zu, R., Lu, H., Chen, Y., et al. (2024). Exploring the anti-ferroptosis mechanism of Kai-Xin-San against Alzheimer’s disease through integrating network pharmacology, bioinformatics, and experimental validation strategy in vivo and in vitro. J. Ethnopharmacol. 326, 117915. doi:10.1016/j.jep.2024.117915
Yang, J., Zhang, M., Zhang, X., Zhou, Y., Ma, T., Liang, J., et al. (2024). Glioblastoma-derived exosomes promote lipid accumulation and induce ferroptosis in dendritic cells via the NRF2/GPX4 pathway. Front. Immunol. 15, 1439191. doi:10.3389/fimmu.2024.1439191
Yang, M., Xia, L., Song, J., Hu, H., Zang, N., Yang, J., et al. (2023). Puerarin ameliorates metabolic dysfunction-associated fatty liver disease by inhibiting ferroptosis and inflammation. Lipids Health Dis. 22, 202. doi:10.1186/s12944-023-01969-y
Yang, Y., Wang, X., Xiao, A., Han, J., Wang, Z., and Wen, M. (2022). Ketogenic diet prevents chronic sleep deprivation-induced Alzheimer’s disease by inhibiting iron dyshomeostasis and promoting repair via Sirt1/Nrf2 pathway. Front. Aging Neurosci. 14, 998292. doi:10.3389/fnagi.2022.998292
Yarmohammadi, F., Wallace Hayes, A., and Karimi, G. (2024). Molecular mechanisms involved in doxorubicin-induced cardiotoxicity: a bibliometrics analysis by VOSviewer. Naunyn. Schmiedeb. Arch. Pharmacol. 397, 1971–1984. doi:10.1007/s00210-023-02773-2
You, J. H., Lee, J., and Roh, J. (2021). PGRMC1-dependent lipophagy promotes ferroptosis in paclitaxel-tolerant persister cancer cells. J. Exp. Clin. Cancer Res. 40, 350. doi:10.1186/s13046-021-02168-2
Yu, H., Gan, D., Luo, Z., Yang, Q., An, D., Zhang, H., et al. (2024). α-Ketoglutarate improves cardiac insufficiency through NAD+-SIRT1 signaling-mediated mitophagy and ferroptosis in pressure overload-induced mice. Mol. Med. 30, 15. doi:10.1186/s10020-024-00783-1
Yu, L.-M., Dong, X., Huang, T., Zhao, J.-K., Zhou, Z.-J., Huang, Y.-T., et al. (2023a). Inhibition of ferroptosis by icariin treatment attenuates excessive ethanol consumption-induced atrial remodeling and susceptibility to atrial fibrillation, role of SIRT1. Apoptosis 28, 607–626. doi:10.1007/s10495-023-01814-8
Yu, M., Li, H., Wang, B., Wu, Z., Wu, S., Jiang, G., et al. (2023b). Baicalein ameliorates polymyxin B-induced acute renal injury by inhibiting ferroptosis via regulation of SIRT1/p53 acetylation. Chemico-Biological Interact. 382, 110607. doi:10.1016/j.cbi.2023.110607
Yuan, B., Zhao, X.-D., Shen, J.-D., Chen, S.-J., Huang, H.-Y., Zhou, X.-M., et al. (2022). Activation of SIRT1 alleviates ferroptosis in the early brain injury after subarachnoid hemorrhage. Oxidative Med. Cell. Longev. 2022, 9069825–9069919. doi:10.1155/2022/9069825
Zeng, L., Zhao, W., Han, T., Qing, F., He, Z., Zhao, Q., et al. (2024b). Ropivacaine prompts ferroptosis to enhance the cisplatin-sensitivity of human colorectal cancer through SIRT1/Nrf2 signaling pathway. Chemico-Biological Interact. 400, 111163. doi:10.1016/j.cbi.2024.111163
Zeng, Y., He, Y., Wang, L., Xu, H., Zhang, Q., Wang, Y., et al. (2024a). Dihydroquercetin improves experimental acute liver failure by targeting ferroptosis and mitochondria-mediated apoptosis through the SIRT1/p53 axis. Phytomedicine 128, 155533. doi:10.1016/j.phymed.2024.155533
Zhan, Y., Yang, Z., Zhan, F., Huang, Y., and Lin, S. (2023). SIRT1 is transcriptionally repressed by YY1 and suppresses ferroptosis in rheumatoid arthritis. Adv. Rheumatol. 63, 9. doi:10.1186/s42358-023-00289-0
Zhang, F., Zeng, Z., Zhang, J., Li, X., Yang, W., Wei, Y., et al. (2024b). Pterostilbene attenuates heart failure by inhibiting myocardial ferroptosis through SIRT1/GSK-3β/GPX4 signaling pathway. Heliyon 10, e24562. doi:10.1016/j.heliyon.2024.e24562
Zhang, J., Zhu, Q., Peng, Z., Li, X.-J., Ding, P.-F., Gao, S., et al. (2024a). Menaquinone-4 attenuates ferroptosis by upregulating DHODH through activation of SIRT1 after subarachnoid hemorrhage. Free Radic. Biol. Med. 210, 416–429. doi:10.1016/j.freeradbiomed.2023.11.031
Zhang, M., Cui, J., Chen, H., Wang, Y., Kuai, X., Sun, S., et al. (2023a). High-dosage NMN promotes ferroptosis to suppress lung adenocarcinoma growth through the NAM-mediated SIRT1–AMPK–ACC pathway. Cancers 15, 2427. doi:10.3390/cancers15092427
Zhang, S., Liu, X., Wang, J., Yuan, F., and Liu, Y. (2022). Targeting ferroptosis with miR-144-3p to attenuate pancreatic β cells dysfunction via regulating USP22/SIRT1 in type 2 diabetes. Diabetol. Metab. Syndr. 14, 89. doi:10.1186/s13098-022-00852-7
Zhang, W., Qian, S., Tang, B., Kang, P., Zhang, H., and Shi, C. (2023b). Resveratrol inhibits ferroptosis and decelerates heart failure progression via Sirt1/p53 pathway activation. J. Cell. Mol. Medi 27, 3075–3089. doi:10.1111/jcmm.17874
Zhang, X., Wu, Q., Lu, Y., Wan, J., Dai, H., Zhou, X., et al. (2018). Cerebroprotection by salvianolic acid B after experimental subarachnoid hemorrhage occurs via Nrf2-and SIRT1-dependent pathways. Free Radic. Biol. Med. 124, 504–516. doi:10.1016/j.freeradbiomed.2018.06.035
Zhang, Z., Zhang, N., Li, M., Ma, X., and Qiu, Y. (2024c). Sappanone a alleviates osteoarthritis progression by inhibiting chondrocyte ferroptosis via activating the SIRT1/Nrf2 signaling pathway. Schmiedeb. Arch. Pharmacol. 397, 8759–8770. doi:10.1007/s00210-024-03179-4
Zhao, H., Ding, Y., and Zhang, L. (2023a). SIRT1/APE1 promotes the viability of gastric cancer cells by inhibiting p53 to suppress ferroptosis. Open Med-Warsaw 18 (1), 20220620. doi:10.1515/med-2022-0620
Zhao, L., Jin, L., and Yang, B. (2023b). Saikosaponin A alleviates Staphylococcus aureus -induced mastitis in mice by inhibiting ferroptosis via SIRT1/Nrf2 pathway. J. Cell. Mol. Medi 27, 3443–3450. doi:10.1111/jcmm.17914
Zhao, L., Jin, L., and Yang, B. (2023c). Diosmetin alleviates S. aureus-induced mastitis by inhibiting SIRT1/GPX4 mediated ferroptosis. Life Sci. 331, 122060. doi:10.1016/j.lfs.2023.122060
Zheng, Q., Ma, P., Yang, P., Zhai, S., He, M., Zhang, X., et al. (2023). Alpha lipoic acid ameliorates motor deficits by inhibiting ferroptosis in Parkinson’s disease. Neurosci. Lett. 810, 137346. doi:10.1016/j.neulet.2023.137346
Zhou, D., Sun, L., Li, J., and Yang, Y. (2024b). Schisandrin B inhibits inflammation and ferroptosis in S.aureus-induced mastitis through regulating SIRT1/p53/SLC7A11 signaling pathway. Int. Immunopharmacol. 137, 112430. doi:10.1016/j.intimp.2024.112430
Zhou, Q., and Ruan, D. (2022). SIRT1-autophagy axis may inhibit oxidative stress-induced ferroptosis in human nucleus pulposus cells. Med. Hypotheses 159, 110757. doi:10.1016/j.mehy.2021.110757
Zhou, X.-N., Zhang, Q., Peng, H., Qin, Y.-J., Liu, Y.-H., Wang, L., et al. (2024a). Silent information regulator sirtuin 1 ameliorates acute liver failure via the p53/glutathione peroxidase 4/gasdermin D axis. World J. Gastroenterol. 30, 1588–1608. doi:10.3748/wjg.v30.i11.1588
Zhou, Y., Hu, T., Zeng, H., Lin, L., Xie, H., Lin, R., et al. (2025). Naringenin inhibits ferroptosis in renal tubular epithelial cells of diabetic nephropathy through SIRT1/FOXO3a signaling pathway. Drug Dev. Res. 86, e70044. doi:10.1002/ddr.70044
Zhou, Z., Ye, T. J., DeCaro, E., Buehler, B., Stahl, Z., Bonavita, G., et al. (2020). Intestinal SIRT1 deficiency protects mice from ethanol-induced liver injury by mitigating ferroptosis. Am. J. Pathol. 190, 82–92. doi:10.1016/j.ajpath.2019.09.012
Keywords: SIRT1, ferroptosis, molecular mechanisms, therapeutic strategies, various diseases
Citation: Zhang Y, Kong F, Li N, Tao L, Zhai J, Ma J and Zhang S (2025) Potential role of SIRT1 in cell ferroptosis. Front. Cell Dev. Biol. 13:1525294. doi: 10.3389/fcell.2025.1525294
Received: 09 November 2024; Accepted: 14 February 2025;
Published: 05 March 2025.
Edited by:
Patrice X. Petit, Centre National de la Recherche Scientifique (CNRS), FranceReviewed by:
Adrianna Skoneczna, Polish Academy of Sciences, PolandCopyright © 2025 Zhang, Kong, Li, Tao, Zhai, Ma and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sixi Zhang, c2l4aUBqbHUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.