
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol., 09 January 2025
Sec. Morphogenesis and Patterning
Volume 12 - 2024 | https://doi.org/10.3389/fcell.2024.1510862
This article is part of the Research TopicExtracellular Vesicles Signaling in Embryogenesis and MorphogenesisView all articles
 Mingyu Liu1,2
Mingyu Liu1,2 Teng Teng1,3,4*
Teng Teng1,3,4*Axon guidance is a key event in neural circuit development that drives the correct targeting of axons to their targets through long distances and unique patterns. Exosomes, extracellular vesicles that are smaller than 100 nm, are secreted by most cell types in the brain. Regulation of cell-cell communication, neuroregeneration, and synapse formation by exosomes have been extensively studied. However, the interaction between exosomes and axon guidance molecules is poorly understood. This review summarizes the relationship between exosomes and canonical and non-canonical guidance cues and hypothesizes a possible model for exosomes mediating axon guidance between cells. The roles of exosomes in axon outgrowth, regeneration, and neurodevelopmental disorders are also reviewed, to discuss exosome-guidance interactions as potential clinical therapeutic targets.
The complexity and correct connectivity of neural circuits ensures that the brain operates correctly. During neural circuit development, differentiating neurons extend their axons to encounter their appropriate targets to form a functional synapse, which is the basis of neural function in both health and disease (Eisenbach et al., 2004; Kandel, 2005). The faulty assembly of synapses or misdirection of axonal pathfinding leads to defects in neural circuits that may result in neural disorders, including schizophrenia and autism spectrum disorder (ASD) (Gliman et al., 2012; Bakos et al., 2015). In the last decade, genome-wide association studies (GWAS) on developmental and degenerative neural disorders have identified candidate risk genes in the developing neural circuit, highlighting the importance of axonal pathfinding in nervous system development (Bossers et al., 2009; Gliman et al., 2012; Antonell et al., 2013; Pinto et al., 2014; Ronemus et al., 2014; Kashyap et al., 2019). Thus, appropriate axon guidance establishes a heathy and well-functioning neural circuit.
The axon guidance process includes axon genesis, outgrowth, pathfinding, and regeneration. During brain development, billions of axons are guided toward their proper targets by axon guidance molecules that are subdivided into attractive and repulsive molecules. Long-range axon guidance cues are required by axons to migrate throughout the brain, whereas short-range effects are mediated by cell-cell contact-dependent ligand-receptor binding models. Long-range guidance cues are recognized at various intermediate choice points that express guidance molecules. These choice points exert either attractant or repellent effects on axons to regulate axon projection (Squarzoni et al., 2015). Short-range cues function at the growth cones in a region enriched with filopodia (Landis, 1983). Protrusion or collapse of the filopodia controls the forward movement or stopping of the axon, corresponding to attractive or repulsive guidance cues, respectively (Robles, 2005; Suter, 2011; Omotade et al., 2017). The combined long- and short-range effects lead to proper neural circuit formation.
Ramón y Cajal first described axon innervation in the brain and growth cones as the structures that guide billons of axons to their targets (Landis, 1983). What are the factors that regulate all these axons to the correct targets? This question arose a hundred years ago and has attracted the interest of numerous neuroscientists, moving from an understanding on a morphological to molecular basis. Since the 1960s, as the emphasis on guidance cues grew, numerous studies have focused on defining and identifying axon guidance molecules (Katz and Lasek, 1979). In later decades, several axon guidance molecules were discovered and well-studied, including netrin (Harris et al., 1996), ephrin (Zhu et al., 2006), semaphorin (Kolodkin et al., 1993), slit (Wu et al., 1999), and non-conventional cues, such as bone morphogenetic protein (BMP), sonic hedgehog (Shh), and wingless/int-1 (Wnt) (Yam and Charron, 2013). Recently, researchers have identified new guidance molecules, such as draxin, which was determined to provide a repulsive signal for axons in the developing brain and does not share homology with other axon guidance molecules (Shinmyo et al., 2015). Moreover, crosstalk occurs between guidance cues and significant cooperation occurs amongst molecules, such as between netrin and Shh, and netrin and ephrin (Ricolo et al., 2015; Sloan et al., 2015). Updated studies indicate that axon guidance cues also regulate axon projection by mediating changes in gene expression via RNA-binding proteins, which has been well reviewed by Kim and Kim (2020). In addition to being a fundamental feature of neural circuit development, axon guidance contributes to the nerve regeneration process. Nerve injury occurs frequently and often combines with other injury or diseases, and its recovery requires proper axon projection to the original target. Otherwise, the incorrect navigation of an axon leads to misdirection which may result in disrupted recovery (Kerschensteiner et al., 2005; de Ruiter et al., 2008; Hamilton et al., 2011). These studies have established a overview of axon guidance; however, our understanding remains incomplete. Challenges in understanding how a long-range cue precisely guides a single axon to its proper target both spatially and temporally still exist. Moreover, questions remain about how deliberate management is established among numerous intermediate choice points and in signaling crosstalk. To achieve the goals of axon guidance, a missing factor must be present in large quantities with ubiquitous distribution in the brain and contain complex signaling information, which require extracellular transport rather than being ‘fixed’ to tissues or cells and contain abundant essential molecules. On the other hand, when considering clinical treatment, the ability to engineer, isolate, and ensure re-uptake is essential. Exosomes, which are small extracellular vesicles (EVs), are a good candidate that fulfill all these criteria.
Exosomes are a subtype of small EVs with nanoscale sizes ranging between 30 and 100 nm. After their fusion with the plasma membrane, the multivesicular bodies (MVBs) deliver exosomes into the extracellular surroundings, and may undergo reuptake by receptors located on the cell membrane of the recipient (Denzer et al., 2000; Yuan et al., 2018). Exosomes can be released by different cell types, including neurons, stem cells, tumor cells, and immune cells, as well as body fluids, including cerebrospinal fluid (CSF), blood, and urine (Parolini et al., 2009; Mathivanan et al., 2010). With their unique lipid bilayer structure, exosomes are able to carry and protect cargos of biosignaling molecules, including proteins, lipids, and nuclei acids, to deliver information between cells to establish various biological signals (Xia et al., 2019; Gao et al., 2020). Exosomal activity initiates from their fusion with the plasma membrane of recipient cells or by undergoing endocytosis (Xia et al., 2022). This functions as a cell-cell communication feature that provides a contact-independent method to establish signaling between cells (Théry, 2011; Bang and Thum, 2012). Furthermore, as exosomes are detected in body fluids, potential clinical applications may use exosomes as biomarkers for pathological conditions, such as to identify mRNAs in exosomes that are isolated from the serum of patients with glioma (Skog et al., 2008).
During brain development, most types of cells secrete exosomes, including neural stem cells, neurons, astrocytes, and all other types of glia (Kang et al., 2008; Yuyama et al., 2012). Exosomes are involved in regulating cell-cell communication, cell morphology, and plasticity. An increasing number of studies are focusing on the role of exosomes in neural functions, and a study indicates a significant impact of exosomes in neurotransmission (Xia et al., 2019). This study discusses how neural and glial exosomes regulate synapse activity to control neurotransmitter release and highlights the potential function of exosome neurite growth. Brain cancers, including glioblastoma, involve interactions between different cell types in the brain, such as neuron-astrocyte or neuron-microglia interactions. Therefore, exosomes as long-range cell-cell interaction modulators have been linked with brain cancer progression (Kucharzewska et al., 2013). Conversely, exosomes may serve as biomarkers for cancer therapeutics. Moreover, the role of exosomes in Alzheimer’s and Parkinson’s diseases has been well studied, demonstrating the essential contribution of exosomes in neurodegenerative disorders. Overall, as research on exosomes continues to gain popularity, their functions are being further explored, with an increasing focus on clinical therapies (Khan et al., 2022; Zakeri et al., 2024). I believe there is a promising synergy between clinical research and basic science. However, our understanding of the role of exosomes in neural circuit development, particularly in axon guidance, remains limited. Here, we will review current progress that has been made in neuronal exosome research, with a focus on axon guidance and regeneration, summarize the interactions between exosomes and guidance cues, and highlight related neural disorders to discuss the contribution of exosomes in brain development.
Exosomes have been described to participate in neural circuit development in two ways: as vehicles that transfer molecules (receptors or ligands) or carry cargos of miRNAs to mediate the expression of relevant molecules or activate pathways. The miRNAs carried by various sources of exosomes that participate in axon guidance are summarized in Table 1. In this section, we review the most recent reports of exosomes and guidance molecules in axon guidance (Figure 1).
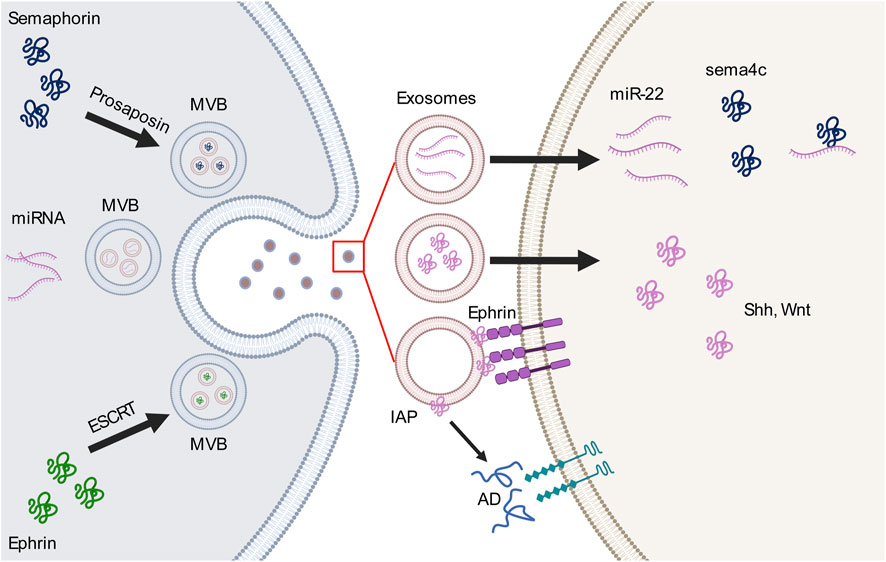
Figure 1. Interactions between exosomes and guidance molecules in the axon guidance pathway. Guidance molecules perticipate in the formation of multivesicular body (MVB) and exosome loading. For example, semaphorin 4A (sema4c) binds to prosaposin to mediate the MVB sorting pathway, whereas EphB2 interacts with the endosomal sorting complex required for transport (ESCRT) which is necessary for MVB formation. MVBs release different types of cargo-loaded exosomes, including microRNAs (miRNAs) which bind to or activate guidance molecules or ligands within the exosome or on the outer surface of the exosomes. These cargos then bind to receptors on the cell membrane or participate in other extracellular pathways.AD, adenosine; IAP, intestinal alkaline phosphatase; Shh, sonic hedgehog; Wnt, wingless-related integration site.
Researches of exosomal Eph-ephrin signaling was initiated a decade ago by Choi et al. (2007), who reported the proteomic profile of a cancer cell line that identified the presence of EphA2-8, EphB1-4, ephrin-B1, and ephrin-B2 on exosomal membranes. Next, Sun et al. (2016) added ephrin-A2 to the story. In the pathogenesis of various cancers, tumor cells release exosomes carrying biological components into the environment to mediate Eph receptor or Ephrin expression, establishing their physiological functions, including cell boundary formation, migration, and axon guidance. For instance, exosomes derived from hypoxic breast cancer cells transport miRNA-210 to neighbor cells, which regulate ephrin-A3 expression to promote angiogenesis (Jung et al., 2017). This exosome-ephrin interaction mechanism has also been confirmed by a study of ephrin-B2 function in endothelial cells (Tae et al., 2017). The most well-studied interaction is exosomal EphA2 derived from tumor serum that contributes to vesicular pathfinding, angiogenesis, and cell migration via Eph-ephrin forward signaling and is a potential diagnostic biomarker for several cancers (Fan et al., 2018; Gao et al., 2021; Han et al., 2022; Wei et al., 2020). In the nervous system, as the largest tyrosine kinase receptor family, Eph-ephrin signaling is implicated in many cellular events during neural circuit development, such as axon guidance and neuron migration via contact-dependent bidirectional signaling. Gong et al. illustrated that exosomes mediate contact-independent Eph-ephrin signaling via a long-range intracellular communication mechanism (Gong et al., 2016). The endosomal sorting complex required for transport (ESCRT) plays an essential role in MVB formation and interacts with EphB2. These EphB2+ exosomes induce ephrin-B1–EphB2 reverse signaling and provide repellent signaling to ephrin-B1+ growth cones. These findings underscore the contribution of exosomes in Eph-ephrin signaling.
A descriptive proteomic analysis of glioma-associated stem cell-derived exosomes identified semaphorin 7A on exosomal membranes. Exosomal semaphorin 7A interacts with integrin-β to increase glioma stem cell (GSC) motility, demonstrating an important role of exosomal semaphorin in neural stem cell migration. This may be a new target for disrupting the interaction between GSCs and neighbor cells (Manini et al., 2019). In addition to their contribution to neuronal migration, exosomal semaphorins regulate vascular and neural pathfinding as guidance cues. Endothelial-derived exosomes carry miRNA-22 as cargo, and the aberrant expression of miRNA-22 leads to disruptions in vascular and motor neuron pathfinding (Sheng et al., 2022). Interestingly, miRNA-22 binds to the 3′-untranslated region of the semaphorin 4C (sema4c) gene, highlighting its role in regulating vascular and neural pathfinding through an exosomal pathway (Sheng et al., 2022). Semaphorins also mediate cargo loading of endodermal exosomes. Under oxidative stress, semaphorin 4A transfers prosaposin from the Golgi apparatus to the cell periphery to be loaded and released by exosomes, and Rab11 mediates this intracellular process in retinal pigment epithelial cells. These findings underlines the role of semaphorins in mediating endosomal sorting towards the exosomal pathway (Toyofuku et al., 2012).
Netrin-1 was the first axon guidance molecule discovered in vertebrates, making it a significant factor in both central and peripheral nervous system research due to its role in axon guidance, cell migration, and morphogenesis (Serafini et al., 1994). In the peripheral nervous system (PNS), Netrin-1 plays a key role in the upregulation of the nerve stump following peripheral nerve injury (PNI). A recent study demonstrated that exosomes derived from Netrin-1-high endothelial cells (NTN1 EC-EXO) are involved in the formation of the vascular niche. Multi-omics analysis confirmed a low expression of let-7a-5p in NTN1 EC-EXO, highlighting its crucial role in establishing a microenvironment for nerve repair after PNI by activating key signaling pathways such as focal adhesion and axon guidance (Huang et al., 2024). On another hand, Exosomal netrin-1 increases the neuronal differentiation rate of bone marrow mesenchymal stem cells (BMSCs) via Hand2/Phox2b signaling. Moreover, BMSC transplantation potentially repairs the structure of damaged tissue and restores function, which may be used as a supplementary treatment in spinal cord injury or congenital spinal disorders, including spinal bifida aperta (SBA) (Ma et al., 2022). Additionally, more recent research showed that using engineered exosomes enriched with Netrin-1 in SCI rats promoted nerve recovery (Lu et al., 2023). Both results highlight the therapeutic effect of exosomal netrin in neural disorders. Roundabout homolog 1 (ROBO1) acts as the direct target of human engineered exosomal miR-29a-3p, and the interaction between miR-29a-3p and ROBO1 plays an important role in glioma migration and vasculogenic mimicry formation (Zhang et al., 2021). Both Netrin and Slit signaling are classic axon guidance modulators, similar to the ephrin family; however, there is limited research on exosomal Netrin or Slit. As mentioned, Huang’s study highlighted the key role of exosomal Netrin-1 in axon guidance within the vascular niche, underscoring a new direction for research on exosomal axon guidance signaling in the microenvironment of the PNS.
Alkaline phosphatase (AP), or tissue non-specific alkaline phosphatase (TNAP) is well known as its role in bone development and functions. Transgenic mice lacking TNAP activity display the characteristic skeletal and dental phenotype of infantile hypophosphatasia (Narisawa et al., 1997). In central nerve system, TNAP initially highly expressed in migrating primordial germ cells in neural tube (Foster et al., 2012) and had been demonstrated that played an important role in cell proliferation (Altman and Bayer, 1990), embryonic and adult neurogenesis (Langer et al., 2007), neuronal differentiation (da Silva and Dotti, 2002) and synaptogenesis (Fonta et al., 2005). The role of alkaline phosphatase (AP) in axon guidance has been poorly studied, however, Elefant et al. (2016) demonstrated the attractive role of exosomal AP in the developing avian optic chiasm. During early development of the chick embryo, intestinal alkaline phosphatase (IAP) is localized on the outer surface of exosomes that are highly concentrated in the midline of the developing diencephalon. In a following study by the same group, the attractive role of IAP was confirmed using an in vitro culture experiment showing that IAP acts on ambient extracellular adenosine triphosphate (ATP) to form an adenosine gradient from the breakdown of extracellular ATP.
Shh is a member of the hedgehog (Hh) family and acts as a key morphogen involved in many long-distance cellular events during development, including various cell behaviors and neural plasticity. In various systems, Hh is released and distinctly localized in tissues to activate target genes (Briscoe and Thérond, 2013). Therefore, the transport and release of Hh requires strict spatial and temporal control. In mammals, the Shh morphogen also directs tissue and axonal pattering according to a concentration gradient. This requires the transportation of Shh from producer to recipient cells, and exosomes are the Shh carriers in this extracellular process (Gradilla et al., 2014). Moreover, BMSC-derived exosomal Shh plays a key role in spinal cord injury (SCI) in rats (Jia et al., 2021). Compared with that in the control group, Shh knockdown by short hairpin RNA Shh-adeno-associated virus results in a significantly decreased neuroprotective effect, including a reduced amount of Nissl bodies, lower motor function (according to blood–brain barrier analysis), increased neural apoptosis, and decreased neuronal ends regeneration. This suggests a neuroprotective role of exosomal Shh. However, after regeneration of neuronal ends, axons may contact an inappropriate target, such as misdirection of motor neuron projections after SCI recovery in the case of no exercise or a inefficitent electrical stimulation (Gordon and English, 2016). The next steps in neural injury recovery require further investigation to understand neuronal pathfinding back to their original targets.
Wnt is a large family of ligands that play an important role in development, particularly in neuronal development. As a guidance cue, non-canonical Wnt signaling directs several types of axons, including neurons that express dopamine and 5-HT in the hindbrain and corticospinal tract (CST) and post-crossing commissural axons, via gradient expression of different Wnts (Lyuksyutova et al., 2003; Liu et al., 2005; Keeble et al., 2006; Fenstermaker et al., 2010). Non-canonical Wnt signaling (β-catenin-independent) includes Wnt/planar cell polarity (PCP) and Wnt/calcium (Ca2+) pathways. The Wnt/PCP pathway is one of the most popular Wnt non-canonical catenin-independent pathways involved in the axon guidance mechanism. In PCP signaling, frizzled (Fz) activates a cascade involving the small GTPases, Rac1 and RhoA, and c-Jun N-terminal kinase to regulate cell polarity and tissue morphogenesis (Yam and Charron, 2013). Dopaminergic and serotonergic axons project from the hindbrain to their midbrain and forebrain targets through a Wnt/PCP pathway (Fenstermaker et al., 2010; Shafer et al., 2011). Gradient-expressed Wnt5a and Wnt7b regulate dopaminergic axon projection as repulsive and attractive cues, respectively; however, axons are not affected in mutants of their receptor, Fz3−/−. In the Ca2+ pathway, Wnt triggers the activation of G proteins to activate the Fz-mediated phospholipase, and subsequently trigger Ca2+ release and activate Ca2+-dependent effectors (Niehrs, 2012). In the spinal cord, Wnt1 and Wnt5a are expressed in a gradient along the anterior-posterior axis to regulate CST axons that extend through the spinal cord. Data on Ryk−/− mutants indicate that receptor-like tyrosine kinase (Ryk) functions as a Wnts receptor in the CST, and pharmacological in vitro experiments reveal that Ca2+ mediates the repulsive effect between Wnts and Ryk T (Liu et al., 2005; Ian et al., 2012).
Recently, an interaction between exosomes and Wnt/PCP signaling in cancer metastasis has been described. The activation of exosomes that are released by fibroblasts stimulate the protrusive activity and motility of breast cancer cells via Wnt/PCP signaling (Luga et al., 2012). Furthermore, exosomes mediate non-directional cell migration primarily in an actin-dependent, centrosome-independent manner (Luo et al., 2019). The Wnt/Ca2+ pathway induces exosome secretion in melanoma cells (Ekstrom et al., 2014). Moreover, in melanoma cells, Wnt5a stimulates exosomes to release their cargo, including immunomodulatory and pro-angiogenic proteins such as vascular endothelial growth factor and matrix metalloproteinase-2. This induction is blocked by the Ca2+ chelator BAPTA, inhibited by a dominant negative version of the small Rho-GTPase Cdc42, and is accompanied by cytoskeletal reorganization. Co-culture experiments demonstrate that blocking Wnt5a expression leads to endothelial cell morphological defects, whereas expressing Wnt5a in endothelial cells induces exosomes release from melanoma cells. These data suggest a role for the interaction between the Wnt/Ca2+ pathway and exosomes in immunosuppressive and angiogenic functions.
In the nervous system, active Wnt proteins are released by exosomes at the neuromuscular junction in Drosophila (Gross et al., 2012). Hippocampal interneuron-derived exosomes release endogenous proline-rich 7 (PRR7) which is the antagonist of Wnt signaling (Lee et al., 2018). Exosomal PRR7 is a transmembrane protein that can be taken up by neighboring neurons to eliminate excitatory synapses. PRR7 can block the exosomal secretion of Wnts and activation of glycogen synthase kinase-3 β by promoting proteasomal degradation of postsynaptic density proteins. Conversely, exosomes mediate Wnt signaling to contribute to axonal regeneration in central nervous system (CNS) injury (Tassew et al., 2017). Fibroblast-derived (FD) exosomes rescue neurite outgrowth defects when they are applied to cultured cortical neurons with inhibitory myelin substrate. This rescue ability is driven by the interaction between exosomes and Wnt10b, as FD exosomes promote the recruitment of Wnt10b towards lipid rafts which induces a Wnt10b autocrine signaling pathway that activates the mammalian target of rapamycin in neurons. Hence, applying FD exosomes to the eye promotes optic nerve regeneration after injury (Tassew et al., 2017). A recent bioinformatic analysis demonstrates that exosomes released by epiblast-derived stem cells carrying miRNA cargo that mediate Wnt signaling are significantly enriched during dopaminergic neuron differentiation (Jin et al., 2020). These studies highlight a key role for the exosome-Wnt interaction in both the developing and adult nervous system, including neurogenesis, axon outgrowth, and neuron differentiation, providing a new therapeutic target for CNS disorders. However, our understanding of exosomal Wnt in axon guidance remains limited.
Similar to long-range regulation by ephrins, non-canonical Wnt pathways may also act as long-range cues in guiding long-distance axonal pathfinding. In dopaminergic projections from the brainstem to midbrain targets, axons are extended along a gradient pattern of Wnt expression distributed along the existing tissue. This process may promote non-canonical Wnt signaling via neighboring neuron- or astrocyte-derived exosomes. Conversely, exosomal non-canonical cue interactions may indirectly mediate axonal pathfinding via crosstalk between guidance cues. For instance, Shh regulates the Wnt expression level to generate a Wnt expression gradient that regulates post-crossing commissural axons by inducing the expression of the Wnt antagonist Sfrp1 (Domanitskaya et al., 2010). As described previously, Wnt signaling mediates exosome release, which may activate exosomal guidance cues to regulate axonal pathfinding, suggesting a new model of exosome-dependent guidance cue crosstalk.
Nerve injuries, including traumatic brain, spinal cord, and peripheral injury, involve damage to nerve tissue that leads to communication defects within the brain or between the brain and other organs. Regenerating injured axons from the CNS injury and rebuilding functional circuits that connect to their original targets are difficult, and thus injury often results in permanent disability. However, peripheral nerves perform dramatic regeneration after injury leading to recovery of the function of sensory and motor innervations. Diverse surgical procedures have attempted to repair nerve injuries; however, several disadvantages remain due to donor tissue absence, functional nerve damage, and the risk of neuroma generation. Thus, a new therapeutic target for promoting or improving axonal outgrowth and regeneration is required.
Recently, exosomes have been widely studied and used as a popular therapeutic target. Exosomes can be used as an alternative non-cell-based therapy for nerve regeneration to reduce the risks of dysfunction and transformation of transplanted stem cells (Thirabanjasak et al., 2010). To achieve their contribution in nerve regeneration, exosomes act as vehicles for remyelinating and regenerative factors or miRNAs that mediate nerve regenerating events. Retinoic acid (RA), a well-known axon guidance molecule, plays an essential role in axon/neurite outgrowth and guidance during development and adulthood (Dmetrichuk et al., 2006; Farrar et al., 2009). The RA signaling pathway regulates remyelination and axon/neurite outgrowth after spinal cord injury, and, interestingly, exosomes mediate the neural-glial crosstalk that contributes to this process (Goncalves et al., 2018). Neuronal RA receptor beta (RARβ) activation is required for the RA signaling pathway in neural injury recovery and leads to the upregulation of endogenous RA synthesis and release of RA in exosomes as either cargo or anchored to the membrane to serve as a positive cue for axon growth, suggesting the importance of exosomes in nerve regeneration as guidance molecule carriers. As exosomes may carry miRNAs as cargo, numerous studies are focused on the molecular mechanism of exosomes in nerve regeneration on the gene level. Traumatic brain injury (TBI), the primary cause of acquired permanent neuronal disability worldwide, induces axonal transection and synapse dysfunction which results in neurological function deficits (Tsitsopoulos et al., 2017). Therefore, major therapeutic strategies for TBI focus on axonal regeneration and synapse recovery.
Several studies demonstrate a key role of microglia in axon outgrowth and synaptic plasticity via crosstalk with neighboring neurons (Arnoux and Audinat, 2015), which is regulated by microglia-derived exosomes. After TBI, miR-5121 carried by microglial exosomes is significantly decreased, which may suppress neurite outgrowth and synapse recovery (Zhao et al., 2021). Overexpression of miR-5121 in a TBI model partly rescues the axonal and synaptic defects both in vitro and in vivo. Moreover, motor coordination in mice treated with exosomes overexpressing miR-5121 is significantly improved after fluid percussion injury compared with that in untreated animals. The results of gene ontology and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis revealed that repulsive guidance molecule A is the downstream direct target of miR-5121 through which miR-5121 promotes axonal outgrowth and synapse recovery. In the spinal cord, interestingly, exosomes often promote neurite outgrowth by reducing inflammation at the lesion site, creating a favorable environment for neurite outgrowth (Wang et al., 2021). Exosomes carrying miR-199a-3p/145-5p contribute to SCI (Wang et al., 2021). miR-199a-3p/145-5p is highly expressed in exosomes that are derived from by-products of human umbilical cord mesenchymal stem cells and modulates the nerve growth factor/tropomyosin receptor kinase A (TrkA) pathway to regulate neuronal differentiation. Moreover, miR-199a-3p/145-5p increases TrkA expression at the lesion site to alleviate damage to the lesion site and facilitate locomotor function in vivo. Huang et al. (2018) highlighted the role of exosomal miR-124-3p in inhibiting neuronal inflammation and promoting neurite outgrowth. Furthermore, microglial exosomal miR-124-3p is significantly upregulated following TBI. miR-124-3p promotes anti-inflammatory M2 polarization in microglia to inhibit inflammation in scratch-injured neurons. Lastly, treatment with exosomal miR-124-3p rescues the decline in neurite number and length in a scratch injury model, accompanied by additional decreases in RhoA, amyloid precursor protein (APP), and Tau. These findings indicate a key role for exosomes in regulating axonal outgrowth and regeneration via their cargo of guidance cues or miRNAs in a direct or indirect manner (Figure 2).
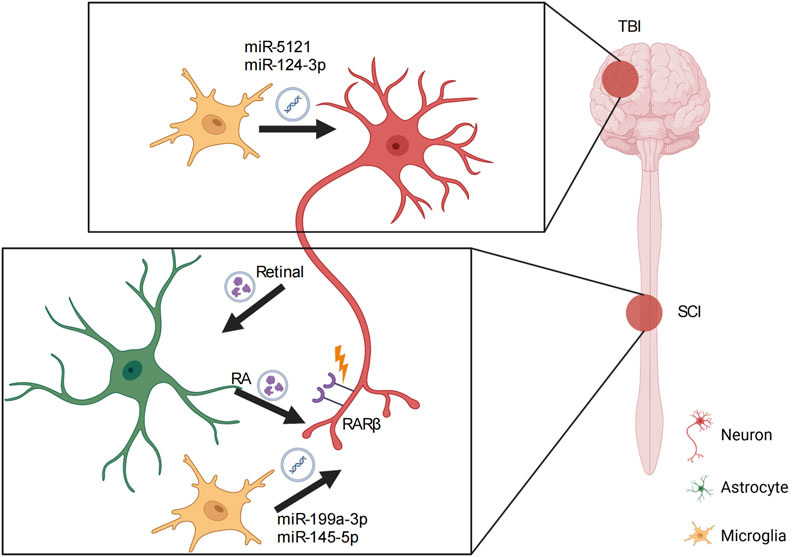
Figure 2. Role of exosomes in nervous system injury. In traumatic brain injury (TBI), microglial exosomes transfer miRNAs to the target neuron to induce neurite outgrowth and synapse recovery. In spinal cord injury (SCI), exosomes act as vehicles that carry both miRNAs and other molecules to support the interaction between neurons and astrocytes or neurons and microglia, contributing to axon recovery and subsequent axon pathfinding. RA, retinoic acid; RARβ, retinoic acid receptor beta.
Exosomes are thought to be expressed by all cell types in the nervous system to modulate crosstalk between them. During nervous system development, exosomes play key roles in neurogenesis, synaptogenesis, and circuit assembly to regulate neural circuit development (Sharma et al., 2019). During neural circuit development, defects in firing assembly and synapse formation result in numerous neurodevelopmental disorders (Gilman et al., 2012; Antonell et al., 2013; Iossifov et al., 2014; Parenti et al., 2020). This section summarizes the role of exosomes in some of these disorders and discusses the utility of exosomes as a cell-free therapy tool.
ASD is a well-known neurodevelopmental disorder that impacts emotion control, language learning, cognitive behavior, and social information perception abilities in children (Fodstad et al., 2009; Elsabbagh et al., 2011; Pierce et al., 2016). Increasing research has revealed that ASD is a largely heritable, multi-stage, and prenatal disorder (Courchesne et al., 2020). ASD is associated with abnormalities in multiple brain regions and other organs. Moreover, ASD is related to a combination of abnormal nerve development events, including neurogenesis, cell migration, axon outgrowth, spine development, and synaptogenesis in prenatal and early postnatal life (Courchesne et al., 2011; Kaushik and Zarbalis, 2016; Packer, 2016; Marchetto et al., 2017). Studies on inducible pluripotent stem cells (iPSC) derived from individuals with ASD have demonstrated disruptions in neural activity, cell proliferation, and synaptogenesis in children with ASD. An iPSC study has revealed the misregulation of genes involved in neuronal differentiation, axon guidance, cell migration, and regional patterning, however, there are barely no genes have a definite relation with ASD and this disease is eventuated by hundreds of genses in neurodevelopment and synaptic proteins (Iakoucheva et al., 2019; DeRosa et al., 2018). Consistently, a GWAS analysis validated ASD risk genes and determined that most of these genes were expressed in ASD-implicated brain regions, including the neocortex, cerebellum, amygdala, hippocampus, and striatum (Krishnan et al., 2016).
Currently, exosomes have been implicated as potential biomarkers to diagnose ASD in early childhood, as no specific biomarker of ASD exists and reliable biomarkers found in exosome cargos, such as abundant miRNAs, may be easily obtained from different types of body fluid (Nouri et al., 2024; Chen et al., 2023; Liu et al., 2024). Moreover, exosomes play key roles in regulating immune system imbalances in patients with ASD, and thus may be used as a drug delivery system to reverse immunological defects in patients with ASD (Chen et al., 2016; Li et al., 2019; Xian et al., 2019). However, the role of exosomes in regulating neural circuit defects in ASD is unknown. Several studies show that diverse resources of exosomes play key roles in axon outgrowth, cell migration, neurogenesis, and synaptogenesis during development. Furthermore, Zhdanova et al. (2021) intranasally administered exosomes derived from multipotent mesenchymal stromal cells in patients with Alzheimer’s Disease (AD) and detected the labeled exosomes in the neocortex and hippocampus. These findings demonstrate the potential therapeutic utility of exosomes in treating ASD at an early postnatal stage.
Intellectual disability is commonly defined as below-average intellectual functioning before 18 years of age, IQ score below 70, and defects in communication, self-care, and social skills. Several factors are linked to the development of intellectual disability, including genetic disorders, trauma, and prenatal events, including maternal infection and alcohol exposure. However, in almost half of these cases, the pathological mechanisms leading to intellectual disability are unknown (Daily et al., 2000; Schroeder, 2000). DS is the most prevalent intellectual disability that is also associated with phenotypical defects, including congenital heart disease and other developmental abnormalities. DS is a non-lethal genetic developmental disorder with a high incidence rate and a global morbidity of 1/800. It is described as a cognitive defect with development of age-dependent neuropathology, such as Alzheimer’s disease (AD). Before dementia occurs, biomarkers, including amyloid-β (Aβ) peptides, p-Tau protein, and interleukin-6, can be detected in the CSF of young patients with DS (Counts et al., 2017). However, collecting CSF poses risks of invasiveness and post-lumbar puncture headaches, particularly in young patients (Hamlett et al., 2017).
In the last 5 years, research on exosomal secretion in DS using post mortem brain tissue has demonstrated an approximate 40% increase in exosomes detected in DS samples compared with that in control tissue (Gauthier et al., 2017). Hamlett et al. (2018) showed a similar increase in neuron-derived exosomes in blood samples from patients with DS and determined that the Aβ1-42, p-T181-Tau, and p-S396-Tau cargos in DS neuron-derived exosomes were significantly increased compared with that in the control. These findings suggest that exosomes may be used as potential biomarkers to diagnose the early dementia phenotype of DS while avoiding the risks associated with CSF collection. Furthermore, these data reveal a new source of APP-related protein transport by neuron-derived exosomes in the brain and potentially non-brain regions in the body. Lastly, peripheral metabolism of Aβ is associated with the risk of AD, and Aβ synthesis in the liver may result in a neurodegenerative phenotype (Lam et al., 2021). Together, a new model of bidirectional brain-body transport of Aβ loaded in exosomes may be hypothesized.
Numerous studies have highlighted the relevant roles of exosomes in nervous system development and neurodegenerative diseases. As a potential biomarker, exosomes are easily collected in body fluids such as blood and saliva, providing an advantage over other collection methods. Furthermore, exosomal miRNAs are more resistant to degradation and easier to isolate than free miRNAs in body fluids. As exosomes are small, present low immunogenicity, lack the ability to transform cells, and are highly therapeutic, they can be used as disease diagnosis markers, drug delivery vehicles, and therapeutic agents for various diseases involving CNS pathology and injury.
Various miRNAs related to axon guidance and neuroplasticity are loaded in exosomes derived from many cell types. In addition to these miRNAs, exosomes carry or bind to canonical guidance cue ligands or receptors resulting in the regulation of long-range axonal pathfinding. Moreover, exosome cargo miRNAs mediate non-canonical guidance cues, such as Wnt and Shh, to activate neurite/axon outgrowth and regeneration. Most axon guidance cues, non-conventional cues, and related miRNAs are carried by, expressed, or interact with exosomes. However, studies on the role of exosomes in neural circuit development is focused on cell-cell communication, nerve regeneration, and synapse formation. Axon guidance plays a key role in neural circuit development to regulate the correct targeting of 80 billion neurons in the brain. Clinically, axon guidance is also implicated in numerous neurodevelopmental and neurodegenerative disorders, including ASD, DS, AD, and Parkinson’s disease. Axon guidance is a key step in ensuring that the regenerated axons project to their original targets during nerve injury recovery. In the latest studies, researchers seem to prefer using specifically derived exosomes as a “pathway candidate pool” to better understand the contents of functional exosomes, which have been shown to have positive therapeutic effects on certain disorders (Tang et al., 2024; Wu et al., 2024; Coy-Dibley et al., 2024; Liu et al., 2024). This trend illustrates a new approach to characterizing exosomes from “inside”, focusing not on generalities, but on their specific functions.
ML: Conceptualization, Investigation, Visualization, Writing–original draft. TT: Conceptualization, Funding acquisition, Supervision, Visualization, Writing–original draft, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Hainan Province Science and Technology Special Fund, grant number 823MS049.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Altman, J., and Bayer, S. A. (1990). Horizontal compartmentation in the germinal matrices and intermediate zone of the embryonic rat cerebral cortex. Exp. Neurol. 107, 36–47. doi:10.1016/0014-4886(90)90061-v
Antonell, A., Lladó, A., Altirriba, J., Botta-Orfila, T., Balasa, M., Fernández, M., et al. (2013). A preliminary study of the whole-genome expression profile of sporadic and monogenic early-onset Alzheimer's disease. Neurobiol. Aging 34 (7), 1772–1778. doi:10.1016/j.neurobiolaging.2012.12.026
Arnoux, I., and Audinat, E. (2015). Fractalkine signaling and microglia functions in the developing brain. Neural Plast. 2015, 689404. doi:10.1155/2015/689404
Bakos, J., Bacova, Z., Grant, S. G., Castejon, A. M., and Ostatnikova, D. (2015). Are molecules involved in neuritogenesis and axon guidance related to autism pathogenesis? Neuromolecular Med. 17 (3), 297–304. doi:10.1007/s12017-015-8357-7
Bang, C., and Thum, T. (2012). Exosomes: new players in cell-cell communication. Int. J. Biochem. Cell Biol. 44 (11), 2060–2064. doi:10.1016/j.biocel.2012.08.007
Bossers, K., Meerhoff, G., Balesar, R., van Dongen, J. W., Kruse, C. G., Swaab, D. F., et al. (2009). Analysis of gene expression in Parkinson’s disease: possible involvement of neurotrophic support and axon guidance in dopaminergic cell death. Brain Pathol. 19 (1), 91–107. doi:10.1111/j.1750-3639.2008.00171.x
Briscoe, J., and Thérond, P. P. (2013). The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 14 (7), 416–429. doi:10.1038/nrm3598
Chen, L., Xiong, X. Y., Yao, T. T., Gui, L. N., Luo, F., Du, Y., et al. (2023). Blood exosome sensing via neuronal insulin-like growth factor-1 regulates autism-related phenotypes. Pharmacol. Res. 197, 106965. doi:10.1016/j.phrs.2023.106965
Chen, W., Huang, Y., Han, J., Yu, L., Li, Y., Lu, Z., et al. (2016). Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol. Res. 64 (4), 831–840. doi:10.1007/s12026-016-8798-6
Choi, D. S., Lee, J. M., Park, G. W., Lim, H. W., Bang, J. Y., Kim, Y. K., et al. (2007). Proteomic analysis of microvesicles derived from human colorectal cancer cells. J. Proteome Res. 6 (12), 4646–4655. doi:10.1021/pr070192y
Counts, S. E., Ikonomovic, M. D., Mercado, N., Vega, I. E., and Mufson, E. J. (2017). Biomarkers for the early detection and progression of Alzheimer's disease. Neurotherapeutics 14 (1), 35–53. doi:10.1007/s13311-016-0481-z
Courchesne, E., Campbell, K., and Solso, S. (2011). Brain growth across the life span in autism: age-specific changes in anatomical pathology. Brain Res. 1380, 138–145. doi:10.1016/j.brainres.2010.09.101
Courchesne, E., Gazestani, V. H., and Lewis, N. E. (2020). Prenatal origins of ASD: the when, what, and how of ASD development. Trends Neurosci. 43 (5), 326–342. doi:10.1016/j.tins.2020.03.005
Coy-Dibley, J., Jayaraj, N. D., Ren, D., Pacifico, P., Belmadani, A., Wang, Y. Z., et al. (2024). Keratinocyte-derived exosomes in painful diabetic neuropathy. bioRxiv. doi:10.1101/2024.08.21.608803
Daily, D. K., Ardinger, H. H., and Holmes, G. E. (2000). Identification and evaluation of mental retardation [published correction appears in Am Fam Physician 2000 Sep 1;62(5):961-3]. Am. Fam. Physician 61 (4), 1059–1070.
da Silva, J. S., and Dotti, C. G. (2002). Breaking the neuronal sphere: regulation of the actin cytoskeleton in neuritogenesis. Nat. Rev. Neurosci. 3, 694–704. doi:10.1038/nrn918
Denzer, K., van Eijk, M., Kleijmeer, M. J., Jakobson, E., de Groot, C., and Geuze, H. J. (2000). Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. J. Immunol. 165 (3), 1259–1265. doi:10.4049/jimmunol.165.3.1259
DeRosa, B. A., El Hokayem, J., Artimovich, E., Garcia-Serje, C., Phillips, A. W., Van Booven, D., et al. (2018). Convergent pathways in idiopathic autism revealed by time course transcriptomic analysis of patient-derived neurons. Sci. Rep. 8 (1), 8423. doi:10.1038/s41598-018-26495-1
de Ruiter, G. C., Malessy, M. J., Alaid, A. O., Spinner, R. J., Engelstad, J. K., Sorenson, E. J., et al. (2008). Misdirection of regenerating motor axons after nerve injury and repair in the rat sciatic nerve model. Exp. Neurol. 211 (2), 339–350. doi:10.1016/j.expneurol.2007.12.023
Dmetrichuk, J. M., Carlone, R. L., and Spencer, G. E. (2006). Retinoic acid induces neurite outgrowth and growth cone turning in invertebrate neurons. Dev. Biol. 294 (1), 39–49. doi:10.1016/j.ydbio.2006.02.018
Domanitskaya, E., Wacker, A., Mauti, O., Baeriswyl, T., Esteve, P., Bovolenta, P., et al. (2010). Sonic hedgehog guides post-crossing commissural axons both directly and indirectly by regulating Wnt activity. J. Neurosci. 30 (33), 11167–11176. doi:10.1523/JNEUROSCI.1488-10.2010
Eisenbach, M., Lengeler, J. W., Varon, M., Gutnick, D., Meili, R., Firtel, R. A., et al. (2004). “Chemotropic guidance of axons in the nervous system,” in Chemotaxis (World Scientific Publishing Company).
Ekström, E. J., Bergenfelz, C., von Bülow, V., Serifler, F., Carlemalm, E., Jönsson, G., et al. (2014). WNT5A induces release of exosomes containing pro-angiogenic and immunosuppressive factors from malignant melanoma cells. Mol. Cancer 13, 88. doi:10.1186/1476-4598-13-88
Elefant, D., Hernandez-Morato, I., Kumaratilake, J., Sharma, S. C., and Newman, S. A. (2016). Alkaline phosphatase enriched exosomes as guidance cues in the developing avian optic chiasm. Invest Ophthalmol. Vis. Sci. 57 (12), 3616a.
Elsabbagh, M., Holmboe, K., Gliga, T., Mercure, E., Hudry, K., Charman, T., et al. (2011). Social and attention factors during infancy and the later emergence of autism characteristics. Prog. Brain Res. 189, 195–207. doi:10.1016/B978-0-444-53884-0.00025-7
Fan, J., Wei, Q., Koay, E. J., Liu, Y., Ning, B., Bernard, P. W., et al. (2018). Chemoresistance transmission via exosome-mediated EphA2 transfer in pancreatic cancer. Theranostics 8 (21), 5986–5994. doi:10.7150/thno.26650
Farrar, N. R., Dmetrichuk, J. M., Carlone, R. L., and Spencer, G. E. (2009). A novel, nongenomic mechanism underlies retinoic acid-induced growth cone turning. J. Neurosci. 29 (45), 14136–14142. doi:10.1523/JNEUROSCI.2921-09.2009
Fenstermaker, A. G., Prasad, A. A., Bechara, A., Adolfs, Y., Tissir, F., Goffinet, A., et al. (2010). Wnt/planar cell polarity signaling controls the anterior-posterior organization of monoaminergic axons in the brainstem. J. Neurosci. 30 (47), 16053–16064. doi:10.1523/JNEUROSCI.4508-10.2010
Fodstad, J. C., Matson, J. L., Hess, J., and Neal, D. (2009). Social and communication behaviours in infants and toddlers with autism and pervasive developmental disorder-not otherwise specified. Dev. Neurorehabil 12 (3), 152–157. doi:10.1080/17518420902936748
Fonta, C., Negyessy, L., Renaud, L., and Barone, P. (2005). Postnatal development of alkaline phosphatase activity correlates with the maturation of neurotransmission in the cerebral cortex. J. Comp. Neurol. 486, 179–196. doi:10.1002/cne.20524
Foster, B. L., Nagatomo, K. J., Nociti, Jr F. H., Fong, H., Dunn, D., Tran, A. B., et al. (2012). Central role of pyrophosphate in acellular cementum formation. PLoS One 7, e38393. doi:10.1371/journal.pone.0038393
Gao, G., Li, C., Zhu, J., Wang, Y., Huang, Y., Zhao, S., et al. (2020). Glutaminase 1 regulates neuroinflammation after cerebral ischemia through enhancing microglial activation and pro-inflammatory exosome release. Front. Immunol. 11, 161. doi:10.3389/fimmu.2020.00161
Gao, Z., Han, X., Zhu, Y., Zhang, H., Tian, R., Wang, Z., et al. (2021). Drug-resistant cancer cell-derived exosomal EphA2 promotes breast cancer metastasis via the EphA2-Ephrin A1 reverse signaling. Cell Death Dis. 12 (5), 414. doi:10.1038/s41419-021-03692-x
Gauthier, S. A., Pérez-González, R., Sharma, A., Huang, F. K., Alldred, M. J., Pawlik, M., et al. (2017). Enhanced exosome secretion in Down syndrome brain - a protective mechanism to alleviate neuronal endosomal abnormalities. Acta Neuropathol. Commun. 5 (1), 65. doi:10.1186/s40478-017-0466-0
Gilman, S. R., Chang, J., Xu, B., Bawa, T. S., Gogos, J. A., Karayiorgou, M., et al. (2012). Diverse types of genetic variation converge on functional gene networks involved in schizophrenia. Nat. Neurosci. 15 (12), 1723–1728. doi:10.1038/nn.3261
Goncalves, M. B., Wu, Y., Trigo, D., Clarke, E., Malmqvist, T., Grist, J., et al. (2018). Retinoic acid synthesis by NG2 expressing cells promotes a permissive environment for axonal outgrowth. Neurobiol. Dis. 111, 70–79. doi:10.1016/j.nbd.2017.12.016
Gong, J., Körner, R., Gaitanos, L., and Klein, R. (2016). Exosomes mediate cell contact-independent ephrin-Eph signaling during axon guidance. J. Cell Biol. 214 (1), 35–44. doi:10.1083/jcb.201601085
Gordon, T., and English, A. W. (2016). Strategies to promote peripheral nerve regeneration: electrical stimulation and/or exercise. Eur. J. Neurosci. 43 (3), 336–350. Epub 2015 Aug 14. doi:10.1111/ejn.13005
Goswami, S., Banerjee, A., Kumari, B., Bandopadhyay, B., Bhattacharya, N., Basu, N., et al. (2017). Differential expression and significance of circulating microRNAs in cerebrospinal fluid of acute encephalitis patients infected with Japanese Encephalitis Virus. Mol. Neurobiol. 54 (2), 1541–1551. doi:10.1007/s12035-016-9764-y
Gradilla, A. C., González, E., Seijo, I., Andrés, G., Bischoff, M., González-Mendez, L., et al. (2014). Exosomes as Hedgehog carriers in cytoneme-mediated transport and secretion. Nat. Commun. 5, 5649. doi:10.1038/ncomms6649
Gross, J. C., Chaudhary, V., Bartscherer, K., and Boutros, M. (2012). Active Wnt proteins are secreted on exosomes. Nat. Cell Biol. 14 (10), 1036–1045. doi:10.1038/ncb2574
Hamilton, S. K., Hinkle, M. L., Nicolini, J., Rambo, L. N., Rexwinkle, A. M., Rose, S. J., et al. (2011). Misdirection of regenerating axons and functional recovery following sciatic nerve injury in rats. J. Comp. Neurol. 519 (1), 21–33. doi:10.1002/cne.22446
Hamlett, E. D., Goetzl, E. J., Ledreux, A., Vasilevko, V., Boger, H. A., LaRosa, A., et al. (2017). Neuronal exosomes reveal Alzheimer's disease biomarkers in Down syndrome. Alzheimers Dement. 13 (5), 541–549. doi:10.1016/j.jalz.2016.08.012
Hamlett, E. D., Ledreux, A., Potter, H., Chial, H. J., Patterson, D., Espinosa, J. M., et al. (2018). Exosomal biomarkers in Down syndrome and Alzheimer's disease. Free Radic. Biol. Med. 114, 110–121. doi:10.1016/j.freeradbiomed.2017.08.028
Han, B., Zhang, H., Tian, R., Liu, H., Wang, Z., Wang, Z., et al. (2022). Exosomal EPHA2 derived from highly metastatic breast cancer cells promotes angiogenesis by activating the AMPK signaling pathway through Ephrin A1-EPHA2 forward signaling. Theranostics 12 (9), 4127–4146. doi:10.7150/thno.72404
Harris, R., Sabatelli, L. M., and Seeger, M. A. (1996). Guidance cues at the Drosophila CNS midline: identification and characterization of two Drosophila Netrin/UNC-6 homologs. Neuron 17 (2), 217–228. doi:10.1016/s0896-6273(00)80154-3
Huang, J., Li, J., Li, S., Yang, X., Huo, N., Chen, Q., et al. (2024). Netrin-1-engineered endothelial cell exosomes induce the formation of pre-regenerative niche to accelerate peripheral nerve repair. Sci. Adv. 10 (26), eadm8454. Epub 2024 Jun 28. doi:10.1126/sciadv.adm8454
Huang, S., Ge, X., Yu, J., Han, Z., Yin, Z., Li, Y., et al. (2018). Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J. 32 (1), 512–528. doi:10.1096/fj.201700673R
Iakoucheva, L. M., Muotri, A. R., and Sebat, J. (2019). Getting to the cores of autism. Cell 178 (6), 1287–1298. doi:10.1016/j.cell.2019.07.037
Ian, H. B., Li, L., and Katherine, K. (2012). Wnt/calcium signaling mediates axon growth and guidance in the developing corpus callosum. Dev. Neurobiol. 5, pt1. doi:10.1002/dneu.20846
Iossifov, I., O’Roak, B. J., Sanders, S. J., Ronemus, M., Krumm, N., Levy, D., et al. (2014). The contribution of de novo coding mutations to autism spectrum disorder. Nature 515, 216–221. doi:10.1038/nature13908
Jia, Y., Yang, J., Lu, T., Pu, X., Chen, Q., Ji, L., et al. (2021). Repair of spinal cord injury in rats via exosomes from bone mesenchymal stem cells requires sonic hedgehog. Regen. Ther. 18, 309–315. doi:10.1016/j.reth.2021.08.007
Jin, T., Gu, J., Xia, H., Chen, H., Xu, X., Li, Z., et al. (2020). Differential expression of microRNA profiles and Wnt Signals in stem cell-derived exosomes during dopaminergic neuron differentiation [Epub ahead of print, 2020 Oct 16]. DNA Cell Biol. doi:10.1089/dna.2020.5931
Jung, K. O., Youn, H., Lee, C. H., Kang, K. W., and Chung, J. K. (2017). Visualization of exosome-mediated miR-210 transfer from hypoxic tumor cells. Oncotarget 8 (6), 9899–9910. doi:10.18632/oncotarget.14247
Kandel, E. R. (2005). The molecular biology of memory storage: a dialogue between genes and synapses. Science 294, 1030–1038. doi:10.1126/science.1067020
Kang, D., Oh, S., Ahn, S. M., Lee, B. H., and Moon, M. H. (2008). Proteomic analysis of exosomes from human neural stem cells by flow field-flow fractionation and nanoflow liquid chromatography-tandem mass spectrometry. J. Proteome Res. 7 (8), 3475–3480. doi:10.1021/pr800225z
Kashyap, G., Bapat, D., Das, D., Gowaikar, R., Amritkar, R. E., Rangarajan, G., et al. (2019). Synapse loss and progress of Alzheimer's disease -A network model. Sci. Rep. 9 (1), 6555. doi:10.1038/s41598-019-43076-y
Katz, M., and Lasek, R. A basal substrate pathway demonstrated by transplanted mauthner axons. 1979.
Kaushik, G., and Zarbalis, K. S. (2016). Prenatal neurogenesis in autism spectrum disorders. Front. Chem. 4, 12. doi:10.3389/fchem.2016.00012
Keeble, T. R., Halford, M. M., Seaman, C., Kee, N., Macheda, M., Anderson, R. B., et al. (2006). The Wnt receptor Ryk is required for Wnt5a-mediated axon guidance on the contralateral side of the corpus callosum. J. Neurosci. 26 (21), 5840–5848. doi:10.1523/JNEUROSCI.1175-06.2006
Kerschensteiner, M., Schwab, M. E., Lichtman, J. W., and Misgeld, T. (2005). In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat. Med. 11 (5), 572–577. doi:10.1038/nm1229
Khan, S. U., Khan, M. I., Khan, M. U., Khan, N. M., Bungau, S., and Hassan, S. S. U. (2022). Applications of extracellular vesicles in nervous system disorders: an overview of recent advances. Bioeng. (Basel) 10 (1), 51. doi:10.3390/bioengineering10010051
Kim, S. W., and Kim, K. T. (2020). Expression of genes involved in axon guidance: how much have we learned? Int. J. Mol. Sci. 21 (10), 3566. doi:10.3390/ijms21103566
Kolodkin, A. L., Matthes, D. J., and Goodman, C. S. (1993). The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell 75 (7), 1389–1399. doi:10.1016/0092-8674(93)90625-z
Krishnan, A., Zhang, R., Yao, V., Theesfeld, C. L., Wong, A. K., Tadych, A., et al. (2016). Genome-wide prediction and functional characterization of the genetic basis of autism spectrum disorder. Nat. Neurosci. 19 (11), 1454–1462. doi:10.1038/nn.4353
Kucharzewska, P., Christianson, H. C., Welch, J. E., Svensson, K. J., Fredlund, E., Ringnér, M., et al. (2013). Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc. Natl. Acad. Sci. U. S. A. 110 (18), 7312–7317. doi:10.1073/pnas.1220998110
Lam, V., Takechi, R., Hackett, M. J., Francis, R., Bynevelt, M., Celliers, L. M., et al. (2021). Synthesis of human amyloid restricted to liver results in an Alzheimer disease-like neurodegenerative phenotype. PLoS Biol. 19 (9), e3001358. doi:10.1371/journal.pbio.3001358
Landis, S. C. (1983). Neuronal growth cones. Annu. Rev. Physiol. 45, 567–580. doi:10.1146/annurev.ph.45.030183.003031
Langer, D., Ikehara, Y., Takebayashi, H., Hawkes, R., and Zimmermann, H. (2007). The ectonucleotidases alkaline phosphatase and nucleoside triphosphate diphosphohydrolase 2 are associated with subsets of progenitor cell populations in the mouse embryonic, postnatal and adult neurogenic zones. Neuroscience 150, 863–879. doi:10.1016/j.neuroscience.2007.07.064
Lee, S. H., Shin, S. M., Zhong, P., Kim, H. T., Kim, D. I., Kim, J. M., et al. (2018). Reciprocal control of excitatory synapse numbers by Wnt and Wnt inhibitor PRR7 secreted on exosomes. Nat. Commun. 9 (1), 3434. doi:10.1038/s41467-018-05858-2
Li, Q., Wang, H., Peng, H., Huyan, T., and Cacalano, N. A. (2019). Exosomes: versatile nano mediators of immune regulation. Cancers (Basel) 11 (10), 1557. doi:10.3390/cancers11101557
Li, Z., Yang, H., Ye, L., Quan, R., and Chen, M. (2021). Role of exosomal miRNAs in brain metastasis affected by radiotherapy. Transl. Neurosci. 12 (1), 127–137. doi:10.1515/tnsci-2020-0163
Liu, C., Sun, L., Worden, H., Ene, J., Zeng, O. Z., Bhagu, J., et al. (2024). Profiling biomanufactured extracellular vesicles of human forebrain spheroids in a Vertical-Wheel Bioreactor. J. Extracell. Biol. 3 (9), e70002. doi:10.1002/jex2.70002
Liu, S., Chen, L., Guo, M., Li, Y., Liu, Q., and Cheng, Y. (2024). Targeted delivery of engineered RVG-BDNFexosomes: a novel neurobiological approach for ameliorating depression and regulating neurogenesis. Research 7. doi:10.34133/research.0402
Liu, Y., Shi, J., Lu, C. C., Wang, Z. B., Lyuksyutova, A. I., Song, X. J., et al. (2005). Ryk-mediated Wnt repulsion regulates posterior-directed growth of corticospinal tract. Nat. Neurosci. 8 (9), 1151–1159. doi:10.1038/nn1520
Lu, X., Xu, G., Lin, Z., Zou, F., Liu, S., Zhang, Y., et al. (2023). Engineered exosomes enriched in netrin-1 modRNA promote axonal growth in spinal cord injury by attenuating inflammation and pyroptosis. Biomater. Res. 27 (1), 3. doi:10.1186/s40824-023-00339-0
Luga, V., Zhang, L., Viloria-Petit, A. M., Ogunjimi, A. A., Inanlou, M. R., Chiu, E., et al. (2012). Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 151 (7), 1542–1556. doi:10.1016/j.cell.2012.11.024
Luo, Y., Barrios-Rodiles, M., Gupta, G. D., Zhang, Y. Y., Ogunjimi, A. A., Bashkurov, M., et al. (2019). Atypical function of a centrosomal module in WNT signalling drives contextual cancer cell motility. Nat. Commun. 10 (1), 2356. doi:10.1038/s41467-019-10241-w
Lyuksyutova, A. I., Lu, C. C., Milanesio, N., King, L. A., Guo, N., Wang, Y., et al. (2003). Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science 302 (5652), 1984–1988. doi:10.1126/science.1089610
Ma, L., Wei, X., Ma, W., Liu, Y., Wang, Y., He, Y., et al. (2022). Neural stem cell-derived exosomal Netrin1 contributes to neuron differentiation of mesenchymal stem cells in therapy of spinal bifida aperta. Stem Cells Transl. Med. 11 (5), 539–551. doi:10.1093/stcltm/szac009
Manini, I., Ruaro, M. E., Sgarra, R., Bartolini, A., Caponnetto, F., Ius, T., et al. (2019). Semaphorin-7A on Exosomes: a promigratory signal in the glioma microenvironment. Cancers (Basel) 11 (6), 758. doi:10.3390/cancers11060758
Marchetto, M. C., Belinson, H., Tian, Y., Freitas, B. C., Fu, C., Vadodaria, K., et al. (2017). Altered proliferation and networks in neural cells derived from idiopathic autistic individuals. Mol. Psychiatry 22 (6), 820–835. doi:10.1038/mp.2016.95
Mathivanan, S., Ji, H., and Simpson, R. J. (2010). Exosomes: extracellular organelles important in intercellular communication. J. Proteomics 73 (10), 1907–1920. doi:10.1016/j.jprot.2010.06.006
Muroya, S., Ogasawara, H., and Hojito, M. (2015). Grazing affects exosomal circulating microRNAs in cattle. PLoS One 10 (8), e0136475. doi:10.1371/journal.pone.0136475
Narisawa, S., Frohlander, N., and Millan, J. L. (1997). Inactivation of two mouse alkaline phosphatase genes and establishment of a model of infantile hypophosphatasia. Dev. Dyn. 208, 432–446. doi:10.1002/(SICI)1097-0177(199703)208:3<432::AID-AJA13>3.0.CO;2-1
Niehrs, C. (2012). The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol. 13 (12), 767–779. doi:10.1038/nrm3470
Nouri, Z., Barfar, A., Perseh, S., Motasadizadeh, H., Maghsoudian, S., Fatahi, Y., et al. (2024). Exosomes as therapeutic and drug delivery vehicle for neurodegenerative diseases. J. Nanobiotechnology 22, 463. doi:10.1186/s12951-024-02681-4
Omotade, O. F., Pollitt, S. L., and Zheng, J. Q. (2017). Actin-based growth cone motility and guidance. Mol. Cell Neurosci. 84, 4–10. doi:10.1016/j.mcn.2017.03.001
Packer, A. (2016). Neocortical neurogenesis and the etiology of autism spectrum disorder. Neurosci. Biobehav Rev. 64, 185–195. doi:10.1016/j.neubiorev.2016.03.002
Parenti, I., Rabaneda, L. G., Schoen, H., and Novarino, G. (2020). Neurodevelopmental disorders: from genetics to functional pathways. Trends Neurosci. 43 (8), 608–621. Epub 2020 Jun 5. doi:10.1016/j.tins.2020.05.004
Parolini, I., Federici, C., Raggi, C., Lugini, L., Palleschi, S., De Milito, A., et al. (2009). Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 284 (49), 34211–34222. doi:10.1074/jbc.M109.041152
Pierce, K., Marinero, S., Hazin, R., McKenna, B., Barnes, C. C., and Malige, A. (2016). Eye tracking reveals abnormal visual preference for geometric images as an early biomarker of an autism spectrum disorder subtype associated with increased symptom severity. Biol. Psychiatry 79 (8), 657–666. doi:10.1016/j.biopsych.2015.03.032
Pinto, D., Delaby, E., Merico, D., Barbosa, M., Merikangas, A., Klei, L., et al. (2014). Convergence of genes and cellular pathways dysregulated in autism spectrum disorders. Am. J. Hum. Genet. 94 (5), 677–694. doi:10.1016/j.ajhg.2014.03.018
Ricolo, D., Butí, E., and Araújo, S. J. (2015). Drosophila melanogaster Hedgehog cooperates with Frazzled to guide axons through a non-canonical signalling pathway. Mech. Dev. 137, 11–22. doi:10.1016/j.mod.2015.04.003
Robles, E. (2005). Regulation of growth cone motility and guidance by tyrosine kinase signaling. The University of Wisconsin-Madison. [PhD thesis].
Ronemus, M., Iossifov, I., Levy, D., and Wigler, M. (2014). The role of de novo mutations in the genetics of autism spectrum disorders. Nat. Rev. Genet. 15 (2), 133–141. doi:10.1038/nrg3585
Schroeder, S. R. (2000). Mental retardation and developmental disabilities influenced by environmental neurotoxic insults. Environ. Health Perspect. 108 (3), 395–399. doi:10.1289/ehp.00108s3395
Serafini, T., Kennedy, T. E., Galko, M. J., Mirzayan, C., Jessell, T. M., and Tessier-Lavigne, M. (1994). The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans unc-6. Cell 78, 409–424. doi:10.1016/0092-8674(94)90420-0
Shafer, B., Onishi, K., Lo, C., Colakoglu, G., and Zou, Y. (2011). Vangl2 promotes Wnt/planar cell polarity-like signaling by antagonizing Dvl1-mediated feedback inhibition in growth cone guidance. Dev. Cell 20 (2), 177–191. doi:10.1016/j.devcel.2011.01.002
Sharma, P., Mesci, P., Carromeu, C., McClatchy, D. R., Schiapparelli, L., Yates, J. R., et al. (2019). Exosomes regulate neurogenesis and circuit assembly. Proc. Natl. Acad. Sci. U. S. A. 116 (32), 16086–16094. doi:10.1073/pnas.1902513116
Sheng, J., Gong, J., Shi, Y., Wang, X., and Liu, D. (2022). MicroRNA-22 coordinates vascular and motor neuronal pathfinding via sema4 during zebrafish development. Open Biol. 12 (4), 210315. doi:10.1098/rsob.210315
Shinmyo, Y., Asrafuzzaman Riyadh, M., Ahmed, G., Bin Naser, I., Hossain, M., Takebayashi, H., et al. (2015). Draxin from neocortical neurons controls the guidance of thalamocortical projections into the neocortex. Nat. Commun. 6, 10232. doi:10.1038/ncomms10232
Skog, J., Würdinger, T., van Rijn, S., Meijer, D. H., Gainche, L., Sena-Esteves, M., et al. (2008). Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 10 (12), 1470–1476. doi:10.1038/ncb1800
Sloan, T. F., Qasaimeh, M. A., Juncker, D., Yam, P. T., and Charron, F. (2015). Integration of shallow gradients of Shh and Netrin-1 guides commissural axons. PLoS Biol. 13 (3), e1002119. doi:10.1371/journal.pbio.1002119
Squarzoni, P., Thion, M. S., and Garel, S. (2015). Neuronal and microglial regulators of cortical wiring: usual and novel guideposts. Front. Neurosci. 9, 248. doi:10.3389/fnins.2015.00248
Sun, W., Zhao, C., Li, Y., Wang, L., Nie, G., Peng, J., et al. (2016). Osteoclast-derived microRNA-containing exosomes selectively inhibit osteoblast activity. Cell Discov. 2, 16015. doi:10.1038/celldisc.2016.15
Suter, D. M. (2011). Live cell imaging of neuronal growth cone motility and guidance in vitro. Methods Mol. Biol. 769, 65–86. doi:10.1007/978-1-61779-207-6_6
Tae, N., Lee, S., Kim, O., Park, J., Na, S., and Lee, J. H. (2017). Syntenin promotes VEGF-induced VEGFR2 endocytosis and angiogenesis by increasing ephrin-B2 function in endothelial cells. Oncotarget 8 (24), 38886–38901. doi:10.18632/oncotarget.16452
Tang, H., Li, J., Wang, H., Ren, J., Ding, H., Shang, J., et al. (2024). Human umbilical cord mesenchymal stem cell-derived exosomes loaded into a composite conduit promote functional recovery after peripheral nerve injury in rats. Neural Regen. Res. 19 (4), 900–907. doi:10.4103/1673-5374.380911
Tassew, N. G., Charish, J., Shabanzadeh, A. P., Luga, V., Harada, H., Farhani, N., et al. (2017). Exosomes mediate mobilization of autocrine Wnt10b to promote axonal regeneration in the injured CNS. Cell Rep. 20 (1), 99–111. doi:10.1016/j.celrep.2017.06.009
Théry, C. (2011). Exosomes: secreted vesicles and intercellular communications. F1000 Biol. Rep. 3, 15. doi:10.3410/B3-15
Thirabanjasak, D., Tantiwongse, K., and Thorner, P. S. (2010). Angiomyeloproliferative lesions following autologous stem cell therapy. J. Am. Soc. Nephrol. 21 (7), 1218–1222. doi:10.1681/ASN.2009111156
Toyofuku, T., Nojima, S., Ishikawa, T., Takamatsu, H., Tsujimura, T., Uemura, A., et al. (2012). Endosomal sorting by Semaphorin 4A in retinal pigment epithelium supports photoreceptor survival. Genes Dev. 26 (8), 816–829. doi:10.1101/gad.184481.111
Tsitsopoulos, P. P., Abu Hamdeh, S., and Marklund, N. (2017). Current opportunities for clinical monitoring of axonal pathology in traumatic brain injury. Front. Neurol. 8, 599. doi:10.3389/fneur.2017.00599
Wang, Y., Lai, X., Wu, D., Liu, B., Wang, N., and Rong, L. (2021). Umbilical mesenchymal stem cell-derived exosomes facilitate spinal cord functional recovery through the miR-199a-3p/145-5p-mediated NGF/TrkA signaling pathway in rats. Stem Cell Res. Ther. 12 (1), 117. doi:10.1186/s13287-021-02148-5
Wei, Q., Zhang, J., Li, Z., Wei, L., and Ren, L. (2020). Serum Exo-EphA2 as a potential diagnostic biomarker for pancreatic cancer. Pancreas 49 (9), 1213–1219. doi:10.1097/MPA.0000000000001660
Wu, D., Sun, H., Yang, B., Song, E., Song, Y., and Tan, W. (2024). Exosome heterogeneity affects the distal “Barrier-Crossing” trafficking of exosome encapsulated quantum dots. ACS Nano 18 (11), 7907–7922. Epub 2024 Feb 23. doi:10.1021/acsnano.3c09378
Wu, W., Wong, K., Chen, J., Jiang, Z., Dupuis, S., Wu, J. Y., et al. (1999). Directional guidance of neuronal migration in the olfactory system by the protein Slit. Nature 400 (6742), 331–336. doi:10.1038/22477
Xia, X., Wang, Y., Huang, Y., Zhang, H., Lu, H., and Zheng, J. C. (2019). Exosomal miRNAs in central nervous system diseases: biomarkers, pathological mediators, protective factors and therapeutic agents. Prog. Neurobiol. 183, 101694. doi:10.1016/j.pneurobio.2019.101694
Xia, X., Wang, Y., Qin, Y., Zhao, S., and Zheng, J. C. (2022). Exosome: a novel neurotransmission modulator or non-canonical neurotransmitter? Ageing Res. Rev. 74, 101558. doi:10.1016/j.arr.2021.101558
Xian, P., Hei, Y., Wang, R., Wang, T., Yang, J., Li, J., et al. (2019). Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics 9 (20), 5956–5975. doi:10.7150/thno.33872
Yam, P. T., and Charron, F. (2013). Signaling mechanisms of non-conventional axon guidance cues: the Shh, BMP and Wnt morphogens. Curr. Opin. Neurobiol. 23 (6), 965–973. doi:10.1016/j.conb.2013.09.002
Yan, S., Zhang, H., Xie, W., Meng, F., Zhang, K., Jiang, Y., et al. (2017). Altered microRNA profiles in plasma exosomes from mesial temporal lobe epilepsy with hippocampal sclerosis. Oncotarget 8 (3), 4136–4146. doi:10.18632/oncotarget.13744
Yuan, Y., Du, W., Liu, J., Ma, W., Zhang, L., Du, Z., et al. (2018). Stem cell-derived exosome in cardiovascular diseases: macro roles of micro particles. Front. Pharmacol. 9, 547. doi:10.3389/fphar.2018.00547
Yuyama, K., Sun, H., Mitsutake, S., and Igarashi, Y. (2012). Sphingolipid-modulated exosome secretion promotes clearance of amyloid-β by microglia. J. Biol. Chem. 287 (14), 10977–10989. doi:10.1074/jbc.M111.324616
Zakeri, Z., Heiderzadeh, M., Kocaarslan, A., Metin, E., Hosseini Karimi, S. N., Saghati, S., et al. (2024). Exosomes encapsulated in hydrogels for effective central nervous system drug delivery. Biomater. Sci. 12 (10), 2561–2578. doi:10.1039/d3bm01055d
Zhang, Z., Guo, X., Guo, X., Yu, R., Qian, M., Wang, S., et al. (2021). MicroRNA-29a-3p delivery via exosomes derived from engineered human mesenchymal stem cells exerts tumour suppressive effects by inhibiting migration and vasculogenic mimicry in glioma. Aging (Albany NY) 13 (4), 5055–5068. doi:10.18632/aging.202424
Zhao, C., Deng, Y., He, Y., Huang, X., Wang, C., and Li, W. (2021). Decreased level of exosomal miR-5121 released from microglia suppresses neurite outgrowth and synapse recovery of neurons following traumatic brain injury. Neurotherapeutics 18 (2), 1273–1294. doi:10.1007/s13311-020-00999-z
Zhdanova, D. Y., Poltavtseva, R. A., Svirshchevskaya, E. V., and Bobkova, N. V. (2021). Effect of intranasal administration of multipotent mesenchymal stromal cell exosomes on memory of mice in Alzheimer's disease model. Bull. Exp. Biol. Med. 170 (4), 575–582. doi:10.1007/s10517-021-05109-3
Keywords: axon guidance, central nervous system development, exosomes, neurodevelopmental disorders, neuronal circuit formation
Citation: Liu M and Teng T (2025) Exosomes: new targets for understanding axon guidance in the developing central nervous system. Front. Cell Dev. Biol. 12:1510862. doi: 10.3389/fcell.2024.1510862
Received: 14 October 2024; Accepted: 19 December 2024;
Published: 09 January 2025.
Edited by:
Carol Margaret Trim, Canterbury Christ Church University, United KingdomReviewed by:
Rita Teodoro, NOVA University of Lisbon, PortugalCopyright © 2025 Liu and Teng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Teng Teng, aHkwMjA2MjA1QG11aG4uZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.