- 1Department of Cell Differentiation, Institute of Molecular Embryology and Genetics, Kumamoto University, Kumamoto, Japan
- 2Microscopic and Developmental Anatomy, Tokyo Women’s Medical University, Tokyo, Japan
Most blood cells derive from hematopoietic stem cells (HSCs), originating from endothelial cells. The induction of HSCs from endothelial cells occurs during mid-gestation, and research has revealed multiple steps in this induction process. Hemogenic endothelial cells emerge within the endothelium, transition to hematopoietic cells (pre-HSCs), and subsequently mature into functional HSCs. Reports indicate transcription factors and external signals are involved in these processes. In this review, we discuss the timing and role of these transcription factors and summarize the external signals that have demonstrated efficacy in an in vitro culture. A precise understanding of the signals at each step is expected to advance the development of methods for inducing HSCs from pluripotent stem cells.
Introduction
Various types of blood cells, such as lymphoid and myeloid cells, are produced from hematopoietic stem cells (HSCs) in the bone marrow, which is not the site of HSC development. During mouse embryogenesis, HSC development begins with endothelial cell differentiation. Some endothelial cells undergo an endothelial-to-hematopoietic transition (EHT), giving rise to HSC-precursors (pre-HSCs) (Taoudi et al., 2008; Zhou et al., 2016). The pre-HSCs then migrate to the fetal liver and mature into HSCs, which ultimately move to the bone marrow and maintain lifelong hematopoiesis (Dzierzak and Speck, 2008; Orkin and Zon, 2008).
HSCs arise from endothelial cells in the aorta-gonad-mesonephros (AGM) region (Muller et al., 1994; Cumano et al., 1996; Medvinsky and Dzierzak, 1996; de Bruijn et al., 2002; Zovein et al., 2008). Although the HSC developmental process appears simple, there are branching points at each differentiation stage that determine the cell fate. Most endothelial cells engage in the formation of blood vessels, but a small proportion of these cells differentiate into hemogenic endothelial cells. These hemogenic endothelial cells undergo an EHT and differentiate into pre-HSCs as well as hematopoietic progenitor cell precursors (pre-HPCs), which enter into processes that lead to the formation of intra-aortic hematopoietic clusters (IAHCs) in the dorsal aorta of the AGM region between the embryonic day (E) 9.5 and E11.5 (Zovein et al., 2008; Chen et al., 2009; Boisset et al., 2010; Yokomizo and Dzierzak, 2010; Boisset et al., 2015). Here, we focus on the transcription factors and their target genes, and discuss hemogenic endothelium development, EHT, and IAHC formation. We also summarize the signaling molecules that are expressed during HSC development and the timing of their expression, focusing primarily on findings obtained from in vitro culture studies.
Developmental pathway of HSCs
Development of hemogenic endothelium
The first HSCs were observed in the AGM region during midgestation (Muller et al., 1994; Medvinsky and Dzierzak, 1996), and were shown to originate from IAHCs derived from endothelial cells in the innermost layer of the AGM region (Jaffredo et al., 1998; Zovein et al., 2008; Boisset et al., 2010). Whereas most endothelial cells eventually contribute to blood vessel formation, some differentiate into hemogenic endothelium, which undergoes an EHT to generate IAHCs. Amongst several transcription factors, RUNX1 has been shown to be a key factor during this sequence. RUNX1 is important for hematopoietic development and is lethal in knockout mice around E12.5 due to extensive hemorrhages (Okuda et al., 1996). North et al. showed the generation of HSCs from Runx1-expressing cells (North et al., 2002), with RUNX1 contributing to HSC production via an enhancer regulated by GATA/ETS/SCL (Nottingham et al., 2007). The activation of this enhancer was initiated in the endothelial cell layer, suggesting that hemogenic endothelium development likely occurs in the endothelial cells (Swiers et al., 2013). Indeed, when Runx1 is deleted in cells expressing the endothelial cell markers Cdh5 or Tie2, HSCs are not generated (Li et al., 2006; Chen et al., 2009), indicating that endothelial cells are the origin of HSCs and that RUNX1 contributes to their developmental process.
RUNX1 functions as a transcription factor by forming a dimer with CBFB; thus, Cbfb-deficient mice exhibit the same phenotype of definitive hematopoietic deficiency as Runx1-deficient mice. Chen et al. attempted to rescue this hematopoietic defect in Cbfb-deficient mice by introducing transgenes but found that whereas the introduction of Tie2-Cbfb induced erythro-myeloid progenitors (EMPs), only the introduction of Ly6a-Cbfb induced HSCs (Chen et al., 2011). These findings strongly suggest that hemogenic endothelial cells producing EMPs and HSCs are distinct.
In the yolk sac, EOMES transcription factor is expressed earlier than RUNX1, and regulates hematopoietic cell production through RUNX1 (Harland et al., 2021). Additionally, Meis1 is reported to be expressed before RUNX1 in the AGM region, and is important in regulating pre-hemogenic endothelium development (Coulombe et al., 2023). Meis1-knockout mice show reduced hematopoietic cells, including HSC production, and are embryonic lethal by E14.5 due to defective hematopoiesis and angiogenesis (Hisa et al., 2004; Azcoitia et al., 2005). Thus, studies suggest that fate determination from endothelial cells to hemogenic endothelial cells is initiated prior to the expression of RUNX1, and further elucidation of the mechanism underlying hemogenic endothelium development is necessary to expound upon these findings.
The search for hemogenic endothelium markers working alongside RUNX1 is ongoing (Fadlullah et al., 2022). Several groups are focusing on cell-surface markers to isolate select cells for experimentation. CD44 is expressed in endothelial cells, including hemogenic endothelial cells, and is also maintained in IAHCs formed by EHT from hemogenic endothelium (Oatley et al., 2020). Recently, it was reported that CD32 is characteristically expressed in hemogenic endothelial cells in human embryonic and human iPS-derived endothelial cells (Scarfo et al., 2024). Many CD32+ endothelial cells differentiate into hematopoietic cells, which enriches the hemogenic endothelium. However, it remains unclear whether CD32+ hemogenic endothelial cells can differentiate into HSCs, because an in vitro system that demonstrates the differentiation of human hemogenic endothelial cells into HSCs has not been established. Other works indicate the existence of different types of hemogenic endothelial cells (Chen et al., 2011; Dignum et al., 2021; Kobayashi et al., 2023), and thus we expect to see future work describing HSC-specific hemogenic endothelial cells.
Molecular mechanism of EHT
EHT is based on two events: the loss of endothelial cell characteristics and the acquisition of hematopoietic cell characteristics. As IAHCs were not formed from hemogenic endothelium in Runx1-deficient mouse embryos (North et al., 1999; Yokomizo et al., 2001), many studies have since sought to investigate the role of RUNX1 in EHT. In an in vitro differentiation system using ES cells, one group showed that the loss of Gfi1 and Gfi1b—the target genes of RUNX1—results in the persistence of endothelial cell morphology instead of the spherical shape characteristic of hematopoietic cells (Lancrin et al., 2012). The same group has also shown that the loss of Gfi1 and Gfi1b in the AGM region prevents EHT and the formation of IAHCs, and thus a failure to produce HSCs (Thambyrajah et al., 2016). In their report, the authors showed that Gfi1 is specifically expressed in hemogenic endothelium from E10.5 and that its expression gradually decreases. However, contrastingly, the expression of Gfi1b increases, and this is consistent with the expression pattern of hematopoietic cell markers, such as KIT and CD41. The authors also show that a complex comprising LSD1 generates epigenetic changes that contribute to the loss of endothelial cell characteristics by GFI1 and GFI1B. Yet, despite these findings, inducing Gfi1 and Gfi1b expression in Runx1 KO cells to produce hematopoietic-like spherical cells leads to low colony-forming capacity. Collectively, these results suggest that molecules other than GFI1 and GFI1B might be involved in the acquisition of hematopoietic cell characteristics (Lancrin et al., 2012).
In parallel with the loss of endothelial cell characteristics by GFI1 and GFI1B, several hematopoietic-related genes become activated. ETS transcription factor Spi1 is one of the target genes activated by RUNX1 (Okada et al., 1998; Huang et al., 2008; Hoogenkamp et al., 2009). In Spi1-deficient mice, there is a reduction in the proportion of pre-HSCs (CD31+KIT+CD45+ cells) in the AGM region as well as HSCs in the fetal liver (Kim et al., 2004; Zhang et al., 2024). The requirement of SPI1 for the acquisition of hematopoietic competence is conserved across species. Single-cell RNA-sequencing (scRNA-seq) data analyses of the human fetal AGM region or of endothelial cells and hematopoietic cells derived from the human iPS cells show that the decrease in endothelial cell marker genes, Cdh5 and Sox17, is accompanied by an increase in Runx1, Spi1, and Gata2 (Qu et al., 2024). Spi1 regulates the heterogeneity of hematopoietic cell differentiation pathways, and the Spi1 target genes, Lyl1 and Klf1, determine the direction of hematopoietic cell differentiation (Qu et al., 2024).
GATA2 is another transcription factor that regulates EHT. Deletion of the transcriptional regulatory region of Gata2 can inhibit EHT and the formation of IAHCs (Gao et al., 2013). Interestingly, the authors show that repression of Gata2 transcription activity can suppress the expression of other transcription factors, such as Runx1 and Tal1. Previous reports showed that deletion of Tal1 causes embryonic lethality due to hematopoietic failure and TAL1 contributes to erythroid differentiation (Shivdasani et al., 1995; Mikkola et al., 2003). Thus, it is known that there are multiple transcription factors involved in EHT, and they cause EHT in a coordinated manner. This intricate network of transcription factors has been shown to continuously play a crucial role throughout the developmental process of HSCs (Wilson et al., 2010). On the other hand, studies using zebrafish and mice have also reported that GATA2 causes HSC development independently of RUNX1 (Bresciani et al., 2021). Gata2-knockout mice have been reported to die of hematopoietic failure at around E10.5 (Tsai et al., 1994), and Gata2 heterozygous knockout mice also show abnormalities in HSC generation and function (Ling et al., 2004). More interestingly, Gata2 loss specifically in Cdh5-expressing endothelial cells inhibits HSC generation, and Gata2 loss in Vav-expressing hematopoietic cells after EHT results in an inability to maintain HSCs. These reports suggest that GATA2 plays a dual role in the regulation of HSCs from their generation to their maintenance (de Pater et al., 2013). On the other hand, the deletion of Runx1 specifically in Vav-expressing hematopoietic cells does not reduce HSCs (Chen et al., 2009), suggesting that RUNX1 is important for differentiation progression up to EHT before E11.5.
Regarding EHT process, many scRNA-seq analyses have been conducted using pre- and post-EHT cells to explore its mechanisms and specific markers (Zhou et al., 2016; Baron et al., 2018; Hou et al., 2020; Vink et al., 2020; Zhu et al., 2020; Calvanese et al., 2022; Fadlullah et al., 2022; Hadland et al., 2022; Yokomizo et al., 2022; Menegatti et al., 2023). These analyses have confirmed the dynamic expression patterns of transcription factors such as Runx1 and Gfi1, which have previously been implicated in EHT. Furthermore, similarities between mice and humans have been observed in the expression of HSC-related transcription factors (Hlf and Mecom) (Zhou et al., 2016; Calvanese et al., 2022; Yokomizo et al., 2022). However, no mention has been made regarding the potential involvement of novel transcription factors in EHT. On the other hand, these studies have been highly effective in identifying new surface markers. Hadland et al. identified VE-cadherin+CD61+EPCR+ cells as a precursor to functional HSCs (Hadland et al., 2022), Vink et al. reported that the earliest functional HSCs are marked by CD27 (Vink et al., 2020), and Menegatti et al. identified CD82 as a novel surface marker specific to EHT (Menegatti et al., 2023).
Formation of IAHCs and their heterogeneity
EHT causes the formation of specific cell clusters in arteries called IAHCs (Boisset et al., 2010; Yokomizo and Dzierzak, 2010). The cells constituting these IAHCs express the transcription factor Hlf, the expression of which is maintained after the cells differentiate into HSCs (Yokomizo et al., 2019). Hlf expression is regulated by the transcription factor Evi1, as evidenced by the reduction in Hlf-positive cells in Evi1-deficient mice. Thus, Evi1 activation and the subsequent expression of Hlf in cells within IAHCs may be a pathway for HSC differentiation. Interestingly, Evi1 expression is heterogeneous within IAHCs and cells with high Evi1 expression can differentiate into HSCs (Yokomizo et al., 2022). Therefore, it is possible that cells with high Evi1 expression within IAHCs are pre-HSCs. Future studies are expected to reveal the differentiation pathway of pre-HSCs to HSCs using a combination of transcription factors and cell-surface antigens as markers.
HSC development has been defined using cell-surface antigens (Figure 1). Hemogenic endothelial cells transform into hematopoietic cells by EHT, and demonstrate a significant change in cell-surface antigen expression. Hemogenic endothelium before EHT expresses the endothelial cell marker VE-cadherin and the arterial endothelial cell marker DLL4, while the blood cell markers KIT, CD41, and CD45 are not yet expressed (Hadland et al., 2015; Hadland et al., 2017; Morino-Koga et al., 2024). When EHT occurs and IAHCs are formed, DLL4 expression is downregulated (Porcheri et al., 2020) and this downregulation is inversely correlated with the increased expression of KIT and CD41. On the contrary, VE-cadherin expression is maintained after EHT (Rybtsov et al., 2011; Rybtsov et al., 2014; Porcheri et al., 2020; Morino-Koga et al., 2024). Cells that eventually become CD45+ and migrate to the liver will acquire the ability to become HSCs (Rybtsov et al., 2011; Rybtsov et al., 2014; Morino-Koga et al., 2024). After liver migration, VE-cadherin expression gradually decreases and HSCs expressing so-called HSC markers are detected (Morrison et al., 1995; Kim et al., 2005; Taoudi et al., 2005; Papathanasiou et al., 2009).
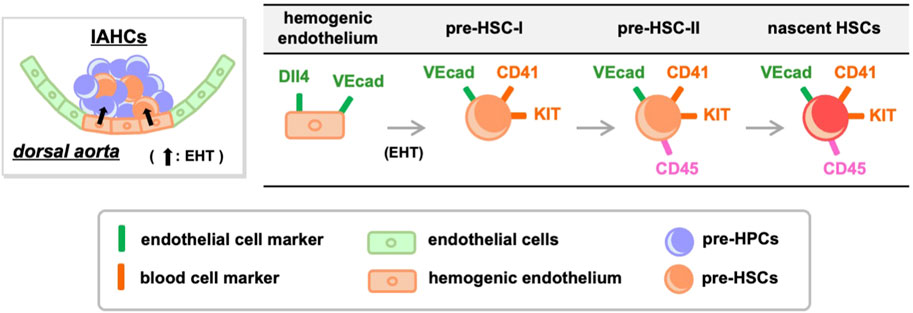
Figure 1. Transition of cell-surface antigens during HSC development. Hemogenic endothelium expressing endothelial cell markers undergoes EHT and differentiates into pre-HSC-I and pre-HPCs. During this process, some endothelial cell markers are retained while the expression of blood cell markers increases. Pre-HSC-I then differentiates into pre-HSC-II, which express CD45, and ultimately mature into HSCs. VEcad, VE-cadherin.
Of note, IAHCs are not a cell mass containing only pre-HSCs but also contain heterogeneous cell populations, most of which are pre-HPCs that do not differentiate into HSCs (Baron et al., 2018; Yokomizo et al., 2022). Consequently, it is difficult to distinguish pre-HSCs from pre-HPCs using the current definition of pre-HSCs through cell-surface antigens. The search for specific marker molecules for pre-HSCs is an upcoming challenge.
HSC development and signaling molecules
Thus far, HSC development via the intricate interactions of nuclear transcription factors has been summarized. The next question is, how do extracellular signals control HSC development? Unlike transcription factors, it is challenging to clarify the roles of signals because knockout mice do not exhibit clear phenotypes. A common approach is to add signaling molecules to culture systems to observe their effects. Specifically, tissues or cell populations that are precursors to HSCs are isolated from mouse embryos and transferred to culture systems where different signaling molecules are introduced to assess whether transplantable HSCs can be induced. This method helps to infer the signaling pathways functioning in vivo.
SCF
After studies showed evidence for HSCs within the AGM region, many groups sought to differentiate AGM-derived cells into HSCs in vitro. Medvinsky’s group developed an ex-vivo culture system to demonstrate the existence of pre-HSCs. They co-cultured pre-HSC candidate cells (VE-cadherin+CD45+ cells) with AGM stromal elements (including endothelial cells) in the presence of stem cell factor (SCF), Fms-related tyrosine kinase 3 ligand (Flt3l), and interleukin-3 (IL-3), and showed induction of transplantable HSCs (Taoudi et al., 2008). These AGM stromal elements could be replaced by simply using the OP9 stromal cell line (Rybtsov et al., 2011), with HSCs induced from E9.5 pre-HSC-I (VE-cadherin+CD45−CD41lo cells) (Rybtsov et al., 2014). This report also examined the necessity for signaling molecules, and showed that E9.5 pre-HSC-I differentiated into HSCs after supplementing SCF with OP9 stromal cells and serum, and this occurred even in the absence of other signaling molecules. Others have shown production of HSCs from E9.5 hemogenic endothelium under serum-free culture conditions following the addition of four signaling factors—SCF, Flt3l, IL-3, and thrombopoietin (TPO)—as well as AGM-derived endothelial feeder cells overexpressing Akt (Hadland et al., 2015). More recently, we showed that E11.5 pre-HSC-I differentiated into HSCs in the presence of SCF and TPO, even under serum-free and feeder-free conditions (Morino-Koga et al., 2024). These results indicate that SCF consistently contributes to HSC development from E9.5. KIT, an SCF receptor, is persistently expressed from E9.5 pre-HSC-I to E12.5 HSCs (Hadland et al., 2015; Morino-Koga et al., 2024), suggesting that SCF is continuously required for HSC development.
Scf deficiency is known to cause perinatal lethality due to severe anemia (Ding et al., 2012), and loss of Scf activity can impair HSC generation and function (Azzoni et al., 2018). Therefore, SCF might be important for HSC development and hematopoietic function. However, does SCF reside in the AGM region? Through scRNA-seq of periaortic tissues and livers from E10.5 and E11.5 mouse embryos, we recently revealed that Scf is expressed in various tissues, including endothelial cells, stromal cells, genital ridge progenitor cells, nephric duct, and hepatoblasts (Morino-Koga et al., 2024). These results are consistent with the earlier histological findings showing Scf expression to be strongly observed in the ventral wall of the dorsal aorta (Souilhol et al., 2016a). In that report, Scf expression was also detected in surrounding cells, such as stromal cells and genital ridge cells, supporting that Scf is present in the microenvironment where IAHC formation occurs. Among these, Scf produced by endothelial cells is important for HSC development (Azzoni et al., 2018).
Flt3l
Flt3l was identified as a ligand for FLT3 (Lyman et al., 1993) and, since Flt3-deficient mice exhibit hematopoietic abnormalities (Mackarehtschian et al., 1995), it is thought to be involved in lineage commitment. Additionally, FLT3 is often used as a marker for hematopoietic progenitors immediately after differentiation from HSCs (Adolfsson et al., 2005; Forsberg et al., 2006). Tracing experiments using Flt3-Cre BAC transgenic mice have shown that nearly all hematopoietic lineages, except HSCs, are marked (Boyer et al., 2011; Buza-Vidas et al., 2011). Interestingly, a subset of HSCs in the embryonic stage is marked by Flt3-Cre, but these FLT3+ cell-derived HSCs disappear after birth (Beaudin et al., 2016). These results suggest that Flt3l/FLT3 signaling is not essential for the development and maintenance of the types of HSCs that are preserved into adulthood. On the other hand, in HSC induction culture systems, Flt3l is added along with SCF and IL-3 and is used for the induction of HSCs from pre-HSCs after E9.5 (Rybtsov et al., 2011; Rybtsov et al., 2014). In an aggregation culture system developed later using OP9 stromal cells, the authors suggested that Flt3l was not needed (Rybtsov et al., 2014) and suggested that Flt3l may not be essential for the in vitro development (maturation) of HSCs. Indeed, it is possible that the same effect afforded by Flt3l is compensated by other pathways.
IL-3
Early findings by Robin et al. highlighted IL-3 as an inducer of transplantable HSCs in an in vitro culture system using E11.5 AGM region isolated from Runx1+/− mice (Robin et al., 2006). Since then, IL-3 is touted to play a role in HSC development through RUNX1. In the same report, the authors also showed that lL-3 was expressed in the cells of the AGM region, with expression of the IL-3 receptor noted on some hematopoietic cells in that region. Later work showed IL-3 as an effective inducer of HSC differentiation of E11.5 pre-HSCs in a co-aggregation culture with OP9 stromal cells (Rybtsov et al., 2014). Collectively, these findings suggest a contribution by IL-3 in the differentiation of pre-HSCs into HSCs after E11.5.
Inflammatory signaling
Inflammatory signaling has been reported to be involved in the development and maintenance of HSCs during embryogenesis (Espin-Palazon et al., 2014; Sawamiphak et al., 2014; He et al., 2015; Mariani et al., 2019; Frame et al., 2020; Zhang et al., 2024). Both Interferon-γ (Ifn-γ)- and Ifn-γ receptor-deficient mouse embryos show a reduced proportion of functional HSCs (Li et al., 2014). However, bone marrow-derived HSCs in Ifn-γ-deficient mice are normal (Baldridge et al., 2010), suggesting that the HSCs produced are functional. It is surmised that the addition of IFN-γ may be effective in inducing functional HSCs in vitro. One group showed that the addition of interferon-α (IFN-α) to AGM-derived cells using an in vitro culture system could induce HSCs with high engraftment capacity in the bone marrow (Kim et al., 2016). In that report, Ifn-α was minimally expressed in the E11.5 AGM region but upregulated in the E13.5 fetal liver, suggesting IFN-α involvement in the maturation of HSCs in the fetal liver.
Notch signaling
Recently, we reported that E11.5 pre-HSC-I could induce HSCs by adding only SCF and TPO even in the absence of serum and feeder cells (Morino-Koga et al., 2024). However, we also showed that E10.5 pre-HSC-I and E10.5 hemogenic endothelium could not differentiate into HSCs with SCF and TPO, requiring co-culture with the endothelial cell line. These results are consistent with previous results obtained using Akt-overexpressing embryonic endothelial cell lines (Hadland et al., 2015; Hadland et al., 2017), suggesting that co-culture with endothelial cells might be necessary to induce HSCs from E10.5 hemogenic endothelium in an in vitro culture system. Furthermore, HSCs have been successfully produced from E9.5-E10.5 CD45−VE-cadherin+CD41-Dll4+ cells, which are defined as arterial endothelial cells, by co-culturing with endothelial feeder cells (Hadland et al., 2017; Morino-Koga et al., 2024). Thus, it might support the process of generating HSCs from arterial endothelial cells via EHT.
What kind of environment do endothelial cells provide for hemogenic endothelium? One of the most promising candidates is Notch signaling (Clements et al., 2011; Kanz et al., 2016; Rho et al., 2019; Thambyrajah and Bigas, 2022). Notch signaling is also important for angiogenesis, and thus it is not easy to distinguish whether hematopoietic cells are directly affected or if their absence is due to a defect in angiogenesis. Studies using ES cells or endothelial cells derived from mice lacking Notch1 have reported that Notch1 is required for endothelial cells to undergo EHT and differentiate into HSCs (Kumano et al., 2003; Hadland et al., 2004). These results suggest that hemogenic endothelium and/or pre-HSCs might express Notch1, since cells lacking Notch1 cannot differentiate into HSCs. HSCs can be induced from E10.5 CD45−VE-cadherin+ cells with activated Notch signaling by aggregate culture with OP9 stromal cells and suppressed by either adding the Notch inhibitor DAPT to the AGM tissue culture at E10.5, or by treating a reaggregated culture of E10.5 AGM-derived cells with a Notch1 inhibitor antibody (Souilhol et al., 2016b). Collectively, these findings suggest that Notch1-expressing cells differentiate into HSCs. Let us return to the main question: Does the endothelial cell line express a ligand acting on the Notch1 receptor? Whole-embryo immunohistochemical staining of mice at E9.5 and E10.5 shows that endothelial cells in the P-Sp/AGM region express the Notch ligands (DLL4, JAG1, and JAG2) and receptors (Notch1 and Notch4) (Robert-Moreno et al., 2005). Notch1 is expressed in the ventral side of the dorsal aorta where IAHCs form, whereas Notch4 is uniformly expressed throughout the vessel. Therefore, these Notch1-expressing cells are expected to undergo EHT and differentiate into HSCs. On the other hand, all three Notch ligands are expressed throughout the endothelial cells. Compared with primary endothelial cells, the authors highlighted an upregulation in the expression of DLL1, DLL4, JAG1, and JAG2 in Akt-overexpressing endothelial cells (Hadland et al., 2015). In addition, endothelial cell lines that contributed to HSC development showed expression of Dll1, Dll4, and Jag1, but not Jag2; albeit the expression intensity varied (Morino-Koga et al., 2024). Thus, the endothelial cell line may not only serve as a scaffold but may also contribute to the HSC differentiation via the Notch ligands, DLL4 and JAG1.
For Notch signaling, we analyzed the scRNA-seq dataset using endothelial cells and hematopoietic cells from E10.5-E11.5 mouse embryos (Figure 2) (Yokomizo et al., 2022; Morino-Koga et al., 2024). Consistent with previous histological findings (Robert-Moreno et al., 2005), expression of Dll4, Jag1, and Jag2 was observed in arterial endothelial cells, but not Dll1 expression. Furthermore, Notch4, which is reported to be uniformly expressed in vascular endothelial cells, was highly expressed in endothelial cells. Interestingly, Notch1 was expressed not only in endothelial cells but also in regions reported to be hemogenic endothelium and in pre-HSCs, consistent with previous reports that Notch1 is expressed in HSC precursors (Kumano et al., 2003; Hadland et al., 2004; Souilhol et al., 2016b). Thus, in the developmental environment of HSCs—particularly in the AGM region of E10.5-E11.5 where pre-HSC-I is produced—the innermost layer, the arterial endothelium, expresses the Notch ligand. Although Notch signaling remains important, Hadland et al. recently reported on the contributions of factors other than Notch signaling in the role of the stromal environment within the in vitro HSC induction system (Hadland et al., 2022). This report demonstrated that Notch signaling alone could not induce HSCs and that additional signal activation from fibronectin and CXCL12 was required, supporting that multiple signaling molecules are coordinately involved in HSC development.
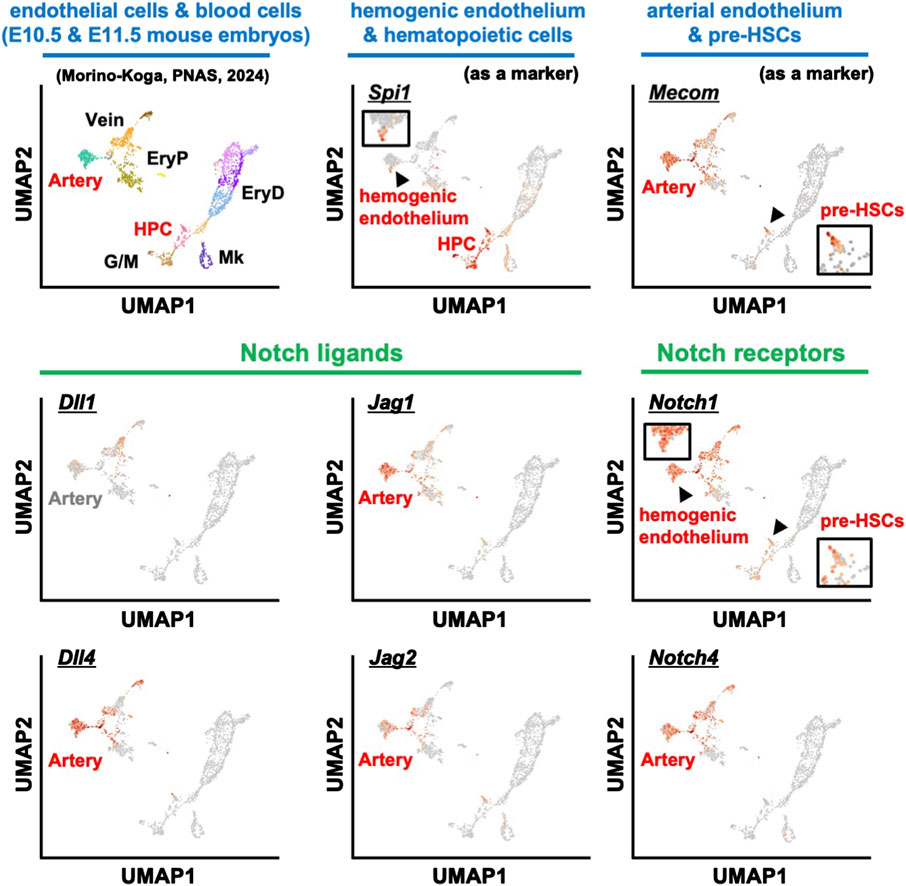
Figure 2. scRNA-seq analysis of blood and endothelial cells from E10.5 and E11.5 mouse embryos. The expression patterns of various marker genes: hemogenic endothelium and HPC (Spi1), endothelial cells and pre-HSCs (Mecom), Notch ligands (Dll1, Dll4, Jag1, Jag2), and Notch receptors (Notch1 and Notch4). HPC, hematopoietic progenitor cells; EryP, primitive erythrocytes; EryD, definitive erythrocytes; G/M, granulocytes and monocytes; Mk, megakaryocytes.
BMP4
BMP4 was originally identified as being important for mesoderm development (Winnier et al., 1995; Lengerke et al., 2008) and in the promotion of EHT. BMP4 can generate hematopoietic cells from endothelial cells using an in vitro differentiation assay (Tsuruda et al., 2021). Furthermore, cells with activated BMP signaling in the AGM region are present in IAHCs and there is evidence that transplantation of BMP-activated cells into irradiated mice results in the engraftment of HSCs (Crisan et al., 2015).
BMP4 is abundant on the ventral side of the dorsal aorta from E10.5-E11.5 (Durand et al., 2007), while the expression of BMP inhibitory molecules is increased, and indeed, BMP signaling inhibitors have been shown to promote HSC development in an in vitro culture system (Souilhol et al., 2016a; McGarvey et al., 2017). Recently, we found that HSCs were induced by adding BMP4, SCF, and TPO to E9.5 hemogenic endothelium using an in vitro culture system (Tsuruda et al., 2024). In this culture system, it was important to remove BMP4 in the latter part of the culture. On the other hand, BMP4 was not necessary when generating HSCs from E10.5 hemogenic endothelium or pre-HSC-I (Morino-Koga et al., 2024). Therefore, it is likely that BMP4 is required for HSC development before E10.5 and gradually becomes unnecessary.
Retinoic acid (RA)
In mouse embryos deficient in the RA synthase enzyme Raldh2, RA synthesis is impaired, resulting in defective hemogenic endothelium development in the yolk sac and decreased hematopoietic cellularity (Goldie et al., 2008). Raldh2-deficient mouse embryos are embryonic lethal at around E10.5 due to various morphogenetic abnormalities (Niederreither et al., 1999). The deletion of Raldh2 specifically in Cdh5-expressing endothelial cells inhibits HSC production (Chanda et al., 2013). In that report, Cdh5-expressing cells at E10.5-E11.5 were shown to express the RA receptor, and treatment of Raldh2-deficient mice with an RA receptor agonist restored HSC production. Therefore, it is expected that supplementation with RA can promote HSC production in vitro.
Sonic hedgehog (Shh)
The notochord is present in the dorsal periphery of the dorsal aorta. Shh supplied by the notochord is important for the differentiation of E10.5 AGM-derived cells into HSCs but not E11.5 AGM-derived cells (Souilhol et al., 2016a). Indeed, since the addition of Shh to the tissue culture systems using the E10 AGM region differentiates them into HSCs (Peeters et al., 2009), it is still unclear whether Shh is required before E9.5. Importantly, strong Shh signaling was observed in stromal cells located around the dorsal aorta (Peeters et al., 2009; Souilhol et al., 2016a). It is possible that Shh may not act directly on HSC progenitor cells but may indirectly influence HSC development by acting on surrounding cells.
Catecholamine
The nervous system surrounding the AGM region is known to influence HSC development (Kwan et al., 2016; Lv et al., 2017). Cells of the sympathetic nervous system developing near the aorta secrete catecholamines via the transcription factor Gata3, which promotes HSC development in the AGM region (Fitch et al., 2012). The cell cycle regulator p57Kip2 (Cdkn1c) is also a regulator of the sympathetic nervous system; its suppression promotes sympathetic nervous system development that, in turn, promotes HSC development via catecholamines (Mascarenhas et al., 2009; Kapeni et al., 2022). Although experiments adding catecholamines have been studied in tissue culture of the E11.5 AGM region and contribute to HSC production (Fitch et al., 2012), it is unclear when catecholamines begin to affect HSC development. Further insight could be gained by additional experiments in an in vitro differentiation system of hemogenic endothelium or pre-HSCs.
TPO
TPO signaling has been reported to be important for HSC maintenance and quiescence in the bone marrow and is also required for HSC engraftment capacity (Qian et al., 2007; Yoshihara et al., 2007). Yet, the source of Tpo is the liver, not the bone marrow (Decker et al., 2018); this suggests that TPO expression in the liver is important in maintaining HSCs. However, since HSC development in the fetal liver in Tpo-deficient mice is normal (Qian et al., 2007; Lee et al., 2022), evidence suggests that TPO is not required for HSC development in the fetal liver.
On the other hand, many reports have shown that TPO is important in differentiating hemogenic endothelium and pre-HSCs into HSCs in vitro culture (Kieusseian et al., 2012; Hadland et al., 2015; Mascarenhas et al., 2016). Recently, we reported that E11.5 pre-HSC-I can be induced into HSCs in an in vitro culture system without serum or feeder cells using just two signaling molecules: SCF and TPO (Morino-Koga et al., 2024). Furthermore, the cell-surface expression of MPL, the receptor for TPO, was gradually observed on E10.5 pre-HSC-I. Considering that HSC engraftment capacity was not very high, these two signaling molecules are necessary but insufficient for HSC differentiation. Interestingly, no Tpo-expressing cells were present in the AGM environment or surrounding tissues, and hepatoblasts in the fetal liver were the only Tpo-producing cells in the HSC developmental environment (Morino-Koga et al., 2024). HSCs are first produced at the AGM region but are detectable in high quantities at E12.5 in the fetal liver, suggesting that pre-HSCs can mature by migrating to the fetal liver and activating TPO signaling. As mentioned above, HSCs were produced even when TPO signaling was lost, suggesting the existence of compensatory mechanisms that activate the TPO downstream molecule, JAK2.
Conclusion
This review focused on signaling molecules in vitro differentiation systems in HSC development (Figure 3). Meanwhile, efforts are currently underway to identify transcription factors and signaling molecules that control HSC development and their engraftment in the bone marrow using human iPS cells (Doulatov et al., 2013; Piau et al., 2023; Ng et al., 2024). We believe that establishing an HSC in vitro differentiation system is important because it will lead to clinical applications of HSCs for hematopoietic disorders. However, in addition to the signaling molecules shown in this review, multiple environmental factors surrounding the AGM region are likely to support HSC development. For example, studies using mice and zebrafish show that HSC development is affected by blood flow-induced shear stress (Adamo et al., 2009; North et al., 2009). Therefore, it is crucial to investigate in detail which signaling molecules affect HSC development when recreating the microenvironment in vitro. Our serum-free culture system using only commercially available products (Morino-Koga et al., 2024) allows for the re-evaluation of key signaling molecules during HSC development under serum-containing conditions or using tissue culture systems derived from the AGM region. In the future, the identification of truly essential signaling molecules is expected to contribute to the establishment of in vitro differentiation systems for HSCs.
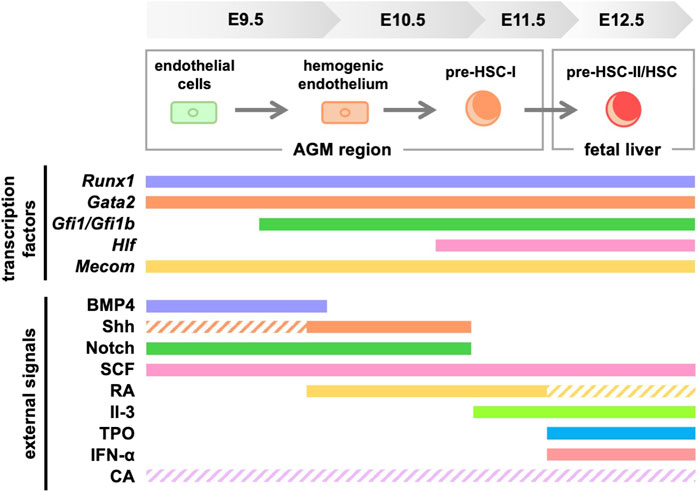
Figure 3. Signaling molecules and transcription factors required for HSC generation in mice. Solid lines indicate the signaling molecules involved in development, while dashed lines indicate those with unclear involvement. RA, retinoic acid; TPO, thrombopoietin; CA, catecholamine.
Author contributions
SM-K: Writing–original draft. TY: Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by JSPS Kakenhi Grant (JP21K08398, JP24K11519, JP23K27632, JP23K18305), the Chemo-Sero-Therapeutic Research Institute (to TY), Astellas Foundation for Research on Metabolic Disorders (to TY), the program of the Joint Usage/Research Center for Developmental Medicine and High Depth Omics, Institute of Molecular Embryology and Genetics (IMEG), Kumamoto University (to SM-K and TY), and the program of the Inter-University Research Network for High Depth Omics, IMEG, Kumamoto University (to SM-K).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
IAHC, intra-aortic hematopoietic cluster; HSC, hematopoietic stem cell; EMP, erythro-myeloid progenitor; EHT, endothelial-to-hematopoietic transition; pre-HSC, hematopoietic stem cell precursor; AGM, aorta-gonad-mesonephros.
References
Adamo, L., Naveiras, O., Wenzel, P. L., McKinney-Freeman, S., Mack, P. J., Gracia-Sancho, J., et al. (2009). Biomechanical forces promote embryonic haematopoiesis. Nature 459 (7250), 1131–1135. doi:10.1038/nature08073
Adolfsson, J., Mansson, R., Buza-Vidas, N., Hultquist, A., Liuba, K., Jensen, C. T., et al. (2005). Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell 121 (2), 295–306. doi:10.1016/j.cell.2005.02.013
Azcoitia, V., Aracil, M., Martinez, A. C., and Torres, M. (2005). The homeodomain protein Meis1 is essential for definitive hematopoiesis and vascular patterning in the mouse embryo. Dev. Biol. 280 (2), 307–320. doi:10.1016/j.ydbio.2005.01.004
Azzoni, E., Frontera, V., McGrath, K. E., Harman, J., Carrelha, J., Nerlov, C., et al. (2018). Kit ligand has a critical role in mouse yolk sac and aorta-gonad-mesonephros hematopoiesis. EMBO Rep. 19 (10), e45477. doi:10.15252/embr.201745477
Baldridge, M. T., King, K. Y., Boles, N. C., Weksberg, D. C., and Goodell, M. A. (2010). Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature 465 (7299), 793–797. doi:10.1038/nature09135
Baron, C. S., Kester, L., Klaus, A., Boisset, J. C., Thambyrajah, R., Yvernogeau, L., et al. (2018). Single-cell transcriptomics reveal the dynamic of haematopoietic stem cell production in the aorta. Nat. Commun. 9 (1), 2517. doi:10.1038/s41467-018-04893-3
Beaudin, A. E., Boyer, S. W., Perez-Cunningham, J., Hernandez, G. E., Derderian, S. C., Jujjavarapu, C., et al. (2016). A transient developmental hematopoietic stem cell gives rise to innate-like B and T cells. Cell Stem Cell 19 (6), 768–783. doi:10.1016/j.stem.2016.08.013
Boisset, J. C., Clapes, T., Klaus, A., Papazian, N., Onderwater, J., Mommaas-Kienhuis, M., et al. (2015). Progressive maturation toward hematopoietic stem cells in the mouse embryo aorta. Blood 125 (3), 465–469. doi:10.1182/blood-2014-07-588954
Boisset, J. C., van Cappellen, W., Andrieu-Soler, C., Galjart, N., Dzierzak, E., and Robin, C. (2010). In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature 464 (7285), 116–120. doi:10.1038/nature08764
Boyer, S. W., Schroeder, A. V., Smith-Berdan, S., and Forsberg, E. C. (2011). All hematopoietic cells develop from hematopoietic stem cells through Flk2/Flt3-positive progenitor cells. Cell Stem Cell 9 (1), 64–73. doi:10.1016/j.stem.2011.04.021
Bresciani, E., Carrington, B., Yu, K., Kim, E. M., Zhen, T., Guzman, V. S., et al. (2021). Redundant mechanisms driven independently by RUNX1 and GATA2 for hematopoietic development. Blood Adv. 5 (23), 4949–4962. doi:10.1182/bloodadvances.2020003969
Buza-Vidas, N., Woll, P., Hultquist, A., Duarte, S., Lutteropp, M., Bouriez-Jones, T., et al. (2011). FLT3 expression initiates in fully multipotent mouse hematopoietic progenitor cells. Blood 118 (6), 1544–1548. doi:10.1182/blood-2010-10-316232
Calvanese, V., Capellera-Garcia, S., Ma, F., Fares, I., Liebscher, S., Ng, E. S., et al. (2022). Mapping human haematopoietic stem cells from haemogenic endothelium to birth. Nature 604 (7906), 534–540. doi:10.1038/s41586-022-04571-x
Chanda, B., Ditadi, A., Iscove, N. N., and Keller, G. (2013). Retinoic acid signaling is essential for embryonic hematopoietic stem cell development. Cell 155 (1), 215–227. doi:10.1016/j.cell.2013.08.055
Chen, M. J., Li, Y., De Obaldia, M. E., Yang, Q., Yzaguirre, A. D., Yamada-Inagawa, T., et al. (2011). Erythroid/myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells. Cell Stem Cell 9 (6), 541–552. doi:10.1016/j.stem.2011.10.003
Chen, M. J., Yokomizo, T., Zeigler, B. M., Dzierzak, E., and Speck, N. A. (2009). Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature 457 (7231), 887–891. doi:10.1038/nature07619
Clements, W. K., Kim, A. D., Ong, K. G., Moore, J. C., Lawson, N. D., and Traver, D. (2011). A somitic Wnt16/Notch pathway specifies haematopoietic stem cells. Nature 474 (7350), 220–224. doi:10.1038/nature10107
Coulombe, P., Cole, G., Fentiman, A., Parker, J. D. K., Yung, E., Bilenky, M., et al. (2023). Meis1 establishes the pre-hemogenic endothelial state prior to Runx1 expression. Nat. Commun. 14 (1), 4537. doi:10.1038/s41467-023-40283-0
Crisan, M., Kartalaei, P. S., Vink, C. S., Yamada-Inagawa, T., Bollerot, K., van, I. W., et al. (2015). BMP signalling differentially regulates distinct haematopoietic stem cell types. Nat. Commun. 6, 8040. doi:10.1038/ncomms9040
Cumano, A., Dieterlen-Lievre, F., and Godin, I. (1996). Lymphoid potential, probed before circulation in mouse, is restricted to caudal intraembryonic splanchnopleura. Cell 86 (6), 907–916. doi:10.1016/s0092-8674(00)80166-x
de Bruijn, M. F., Ma, X., Robin, C., Ottersbach, K., Sanchez, M. J., and Dzierzak, E. (2002). Hematopoietic stem cells localize to the endothelial cell layer in the midgestation mouse aorta. Immunity 16 (5), 673–683. doi:10.1016/s1074-7613(02)00313-8
Decker, M., Leslie, J., Liu, Q., and Ding, L. (2018). Hepatic thrombopoietin is required for bone marrow hematopoietic stem cell maintenance. Science 360 (6384), 106–110. doi:10.1126/science.aap8861
de Pater, E., Kaimakis, P., Vink, C. S., Yokomizo, T., Yamada-Inagawa, T., van der Linden, R., et al. (2013). Gata2 is required for HSC generation and survival. J. Exp. Med. 210 (13), 2843–2850. doi:10.1084/jem.20130751
Dignum, T., Varnum-Finney, B., Srivatsan, S. R., Dozono, S., Waltner, O., Heck, A. M., et al. (2021). Multipotent progenitors and hematopoietic stem cells arise independently from hemogenic endothelium in the mouse embryo. Cell Rep. 36 (11), 109675. doi:10.1016/j.celrep.2021.109675
Ding, L., Saunders, T. L., Enikolopov, G., and Morrison, S. J. (2012). Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481 (7382), 457–462. doi:10.1038/nature10783
Doulatov, S., Vo, L. T., Chou, S. S., Kim, P. G., Arora, N., Li, H., et al. (2013). Induction of multipotential hematopoietic progenitors from human pluripotent stem cells via respecification of lineage-restricted precursors. Cell Stem Cell 13 (4), 459–470. doi:10.1016/j.stem.2013.09.002
Durand, C., Robin, C., Bollerot, K., Baron, M. H., Ottersbach, K., and Dzierzak, E. (2007). Embryonic stromal clones reveal developmental regulators of definitive hematopoietic stem cells. Proc. Natl. Acad. Sci. U. S. A. 104 (52), 20838–20843. doi:10.1073/pnas.0706923105
Dzierzak, E., and Speck, N. A. (2008). Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat. Immunol. 9 (2), 129–136. doi:10.1038/ni1560
Espin-Palazon, R., Stachura, D. L., Campbell, C. A., Garcia-Moreno, D., Del Cid, N., Kim, A. D., et al. (2014). Proinflammatory signaling regulates hematopoietic stem cell emergence. Cell 159 (5), 1070–1085. doi:10.1016/j.cell.2014.10.031
Fadlullah, M. Z. H., Neo, W. H., Lie, A. L. M., Thambyrajah, R., Patel, R., Mevel, R., et al. (2022). Murine AGM single-cell profiling identifies a continuum of hemogenic endothelium differentiation marked by ACE. Blood 139 (3), 343–356. doi:10.1182/blood.2020007885
Fitch, S. R., Kimber, G. M., Wilson, N. K., Parker, A., Mirshekar-Syahkal, B., Gottgens, B., et al. (2012). Signaling from the sympathetic nervous system regulates hematopoietic stem cell emergence during embryogenesis. Cell Stem Cell 11 (4), 554–566. doi:10.1016/j.stem.2012.07.002
Forsberg, E. C., Serwold, T., Kogan, S., Weissman, I. L., and Passegue, E. (2006). New evidence supporting megakaryocyte-erythrocyte potential of flk2/flt3+ multipotent hematopoietic progenitors. Cell 126 (2), 415–426. doi:10.1016/j.cell.2006.06.037
Frame, J. M., Kubaczka, C., Long, T. L., Esain, V., Soto, R. A., Hachimi, M., et al. (2020). Metabolic regulation of inflammasome activity controls embryonic hematopoietic stem and progenitor cell production. Dev. Cell 55 (2), 133–149.e6. doi:10.1016/j.devcel.2020.07.015
Gao, X., Johnson, K. D., Chang, Y. I., Boyer, M. E., Dewey, C. N., Zhang, J., et al. (2013). Gata2 cis-element is required for hematopoietic stem cell generation in the mammalian embryo. J. Exp. Med. 210 (13), 2833–2842. doi:10.1084/jem.20130733
Goldie, L. C., Lucitti, J. L., Dickinson, M. E., and Hirschi, K. K. (2008). Cell signaling directing the formation and function of hemogenic endothelium during murine embryogenesis. Blood 112 (8), 3194–3204. doi:10.1182/blood-2008-02-139055
Hadland, B., Varnum-Finney, B., Dozono, S., Dignum, T., Nourigat-McKay, C., Heck, A. M., et al. (2022). Engineering a niche supporting hematopoietic stem cell development using integrated single-cell transcriptomics. Nat. Commun. 13 (1), 1584. doi:10.1038/s41467-022-28781-z
Hadland, B. K., Huppert, S. S., Kanungo, J., Xue, Y., Jiang, R., Gridley, T., et al. (2004). A requirement for Notch1 distinguishes 2 phases of definitive hematopoiesis during development. Blood 104 (10), 3097–3105. doi:10.1182/blood-2004-03-1224
Hadland, B. K., Varnum-Finney, B., Mandal, P. K., Rossi, D. J., Poulos, M. G., Butler, J. M., et al. (2017). A common origin for B-1a and B-2 lymphocytes in clonal pre- hematopoietic stem cells. Stem Cell Rep. 8 (6), 1563–1572. doi:10.1016/j.stemcr.2017.04.007
Hadland, B. K., Varnum-Finney, B., Poulos, M. G., Moon, R. T., Butler, J. M., Rafii, S., et al. (2015). Endothelium and NOTCH specify and amplify aorta-gonad-mesonephros-derived hematopoietic stem cells. J. Clin. Invest 125 (5), 2032–2045. doi:10.1172/JCI80137
Harland, L. T. G., Simon, C. S., Senft, A. D., Costello, I., Greder, L., Imaz-Rosshandler, I., et al. (2021). The T-box transcription factor Eomesodermin governs haemogenic competence of yolk sac mesodermal progenitors. Nat. Cell Biol. 23 (1), 61–74. doi:10.1038/s41556-020-00611-8
He, Q., Zhang, C., Wang, L., Zhang, P., Ma, D., Lv, J., et al. (2015). Inflammatory signaling regulates hematopoietic stem and progenitor cell emergence in vertebrates. Blood 125 (7), 1098–1106. doi:10.1182/blood-2014-09-601542
Hisa, T., Spence, S. E., Rachel, R. A., Fujita, M., Nakamura, T., Ward, J. M., et al. (2004). Hematopoietic, angiogenic and eye defects in Meis1 mutant animals. Embo J. 23 (2), 450–459. doi:10.1038/sj.emboj.7600038
Hoogenkamp, M., Lichtinger, M., Krysinska, H., Lancrin, C., Clarke, D., Williamson, A., et al. (2009). Early chromatin unfolding by RUNX1: a molecular explanation for differential requirements during specification versus maintenance of the hematopoietic gene expression program. Blood 114 (2), 299–309. doi:10.1182/blood-2008-11-191890
Hou, S., Li, Z., Zheng, X., Gao, Y., Dong, J., Ni, Y., et al. (2020). Embryonic endothelial evolution towards first hematopoietic stem cells revealed by single-cell transcriptomic and functional analyses. Cell Res. 30 (5), 376–392. doi:10.1038/s41422-020-0300-2
Huang, G., Zhang, P., Hirai, H., Elf, S., Yan, X., Chen, Z., et al. (2008). PU.1 is a major downstream target of AML1 (RUNX1) in adult mouse hematopoiesis. Nat. Genet. 40 (1), 51–60. doi:10.1038/ng.2007.7
Jaffredo, T., Gautier, R., Eichmann, A., and Dieterlen-Lievre, F. (1998). Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development 125 (22), 4575–4583. doi:10.1242/dev.125.22.4575
Kanz, D., Konantz, M., Alghisi, E., North, T. E., and Lengerke, C. (2016). Endothelial-to-hematopoietic transition: Notch-ing vessels into blood. Ann. N. Y. Acad. Sci. 1370 (1), 97–108. doi:10.1111/nyas.13030
Kapeni, C., Nitsche, L., Kilpatrick, A. M., Wilson, N. K., Xia, K., Mirshekar-Syahkal, B., et al. (2022). p57Kip2 regulates embryonic blood stem cells by controlling sympathoadrenal progenitor expansion. Blood 140 (5), 464–477. doi:10.1182/blood.2021014853
Kieusseian, A., Brunet de la Grange, P., Burlen-Defranoux, O., Godin, I., and Cumano, A. (2012). Immature hematopoietic stem cells undergo maturation in the fetal liver. Development 139 (19), 3521–3530. doi:10.1242/dev.079210
Kim, H. G., de Guzman, C. G., Swindle, C. S., Cotta, C. V., Gartland, L., Scott, E. W., et al. (2004). The ETS family transcription factor PU.1 is necessary for the maintenance of fetal liver hematopoietic stem cells. Blood 104 (13), 3894–3900. doi:10.1182/blood-2002-08-2425
Kim, I., Yilmaz, O. H., and Morrison, S. J. (2005). CD144 (VE-cadherin) is transiently expressed by fetal liver hematopoietic stem cells. Blood 106 (3), 903–905. doi:10.1182/blood-2004-12-4960
Kim, P. G., Canver, M. C., Rhee, C., Ross, S. J., Harriss, J. V., Tu, H. C., et al. (2016). Interferon-α signaling promotes embryonic HSC maturation. Blood 128 (2), 204–216. doi:10.1182/blood-2016-01-689281
Kobayashi, M., Wei, H., Yamanashi, T., Azevedo Portilho, N., Cornelius, S., Valiente, N., et al. (2023). HSC-independent definitive hematopoiesis persists into adult life. Cell Rep. 42 (3), 112239. doi:10.1016/j.celrep.2023.112239
Kumano, K., Chiba, S., Kunisato, A., Sata, M., Saito, T., Nakagami-Yamaguchi, E., et al. (2003). Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity 18 (5), 699–711. doi:10.1016/s1074-7613(03)00117-1
Kwan, W., Cortes, M., Frost, I., Esain, V., Theodore, L. N., Liu, S. Y., et al. (2016). The central nervous system regulates embryonic HSPC production via stress-responsive glucocorticoid receptor signaling. Cell Stem Cell 19 (3), 370–382. doi:10.1016/j.stem.2016.06.004
Lancrin, C., Mazan, M., Stefanska, M., Patel, R., Lichtinger, M., Costa, G., et al. (2012). GFI1 and GFI1B control the loss of endothelial identity of hemogenic endothelium during hematopoietic commitment. Blood 120 (2), 314–322. doi:10.1182/blood-2011-10-386094
Lee, Y., DiMaulo-Milk, E., Leslie, J., and Ding, L. (2022). Hematopoietic stem cells temporally transition to thrombopoietin dependence in the fetal liver. Sci. Adv. 8 (11), eabm7688. doi:10.1126/sciadv.abm7688
Lengerke, C., Schmitt, S., Bowman, T. V., Jang, I. H., Maouche-Chretien, L., McKinney-Freeman, S., et al. (2008). BMP and Wnt specify hematopoietic fate by activation of the Cdx-Hox pathway. Cell Stem Cell 2 (1), 72–82. doi:10.1016/j.stem.2007.10.022
Li, Y., Esain, V., Teng, L., Xu, J., Kwan, W., Frost, I. M., et al. (2014). Inflammatory signaling regulates embryonic hematopoietic stem and progenitor cell production. Genes Dev. 28 (23), 2597–2612. doi:10.1101/gad.253302.114
Li, Z., Chen, M. J., Stacy, T., and Speck, N. A. (2006). Runx1 function in hematopoiesis is required in cells that express Tek. Blood 107 (1), 106–110. doi:10.1182/blood-2005-05-1955
Ling, K. W., Ottersbach, K., van Hamburg, J. P., Oziemlak, A., Tsai, F. Y., Orkin, S. H., et al. (2004). GATA-2 plays two functionally distinct roles during the ontogeny of hematopoietic stem cells. J. Exp. Med. 200 (7), 871–882. doi:10.1084/jem.20031556
Lv, J., Wang, L., Gao, Y., Ding, Y. Q., and Liu, F. (2017). 5-hydroxytryptamine synthesized in the aorta-gonad-mesonephros regulates hematopoietic stem and progenitor cell survival. J. Exp. Med. 214 (2), 529–545. doi:10.1084/jem.20150906
Lyman, S. D., James, L., Vanden Bos, T., de Vries, P., Brasel, K., Gliniak, B., et al. (1993). Molecular cloning of a ligand for the flt3/flk-2 tyrosine kinase receptor: a proliferative factor for primitive hematopoietic cells. Cell 75 (6), 1157–1167. doi:10.1016/0092-8674(93)90325-k
Mackarehtschian, K., Hardin, J. D., Moore, K. A., Boast, S., Goff, S. P., and Lemischka, I. R. (1995). Targeted disruption of the flk2/flt3 gene leads to deficiencies in primitive hematopoietic progenitors. Immunity 3 (1), 147–161. doi:10.1016/1074-7613(95)90167-1
Mariani, S. A., Li, Z., Rice, S., Krieg, C., Fragkogianni, S., Robinson, M., et al. (2019). Pro-inflammatory aorta-associated macrophages are involved in embryonic development of hematopoietic stem cells. Immunity 50 (6), 1439–1452.e5. doi:10.1016/j.immuni.2019.05.003
Mascarenhas, M. I., Bacon, W. A., Kapeni, C., Fitch, S. R., Kimber, G., Cheng, S. W., et al. (2016). Analysis of Jak2 signaling reveals resistance of mouse embryonic hematopoietic stem cells to myeloproliferative disease mutation. Blood 127 (19), 2298–2309. doi:10.1182/blood-2015-08-664631
Mascarenhas, M. I., Parker, A., Dzierzak, E., and Ottersbach, K. (2009). Identification of novel regulators of hematopoietic stem cell development through refinement of stem cell localization and expression profiling. Blood 114 (21), 4645–4653. doi:10.1182/blood-2009-06-230037
McGarvey, A. C., Rybtsov, S., Souilhol, C., Tamagno, S., Rice, R., Hills, D., et al. (2017). A molecular roadmap of the AGM region reveals BMPER as a novel regulator of HSC maturation. J. Exp. Med. 214 (12), 3731–3751. doi:10.1084/jem.20162012
Medvinsky, A., and Dzierzak, E. (1996). Definitive hematopoiesis is autonomously initiated by the AGM region. Cell 86 (6), 897–906. doi:10.1016/s0092-8674(00)80165-8
Menegatti, S., Potts, B., Paredes, R., Garcia-Alegria, E., Baker, S. M., and Kouskoff, V. (2023). CD82 expression marks the endothelium to hematopoietic transition at the onset of blood specification in human. iScience 26 (9), 107583. doi:10.1016/j.isci.2023.107583
Mikkola, H. K., Klintman, J., Yang, H., Hock, H., Schlaeger, T. M., Fujiwara, Y., et al. (2003). Haematopoietic stem cells retain long-term repopulating activity and multipotency in the absence of stem-cell leukaemia SCL/tal-1 gene. Nature 421 (6922), 547–551. doi:10.1038/nature01345
Morino-Koga, S., Tsuruda, M., Zhao, X., Oshiro, S., Yokomizo, T., Yamane, M., et al. (2024). Transition of signal requirement in hematopoietic stem cell development from hemogenic endothelial cells. Proc. Natl. Acad. Sci. U. S. A. 121 (31), e2404193121. doi:10.1073/pnas.2404193121
Morrison, S. J., Hemmati, H. D., Wandycz, A. M., and Weissman, I. L. (1995). The purification and characterization of fetal liver hematopoietic stem cells. Proc. Natl. Acad. Sci. U. S. A. 92 (22), 10302–10306. doi:10.1073/pnas.92.22.10302
Muller, A. M., Medvinsky, A., Strouboulis, J., Grosveld, F., and Dzierzak, E. (1994). Development of hematopoietic stem cell activity in the mouse embryo. Immunity 1 (4), 291–301. doi:10.1016/1074-7613(94)90081-7
Ng, E. S., Sarila, G., Li, J. Y., Edirisinghe, H. S., Saxena, R., Sun, S., et al. (2024). Long-term engrafting multilineage hematopoietic cells differentiated from human induced pluripotent stem cells. Nat. Biotechnol. doi:10.1038/s41587-024-02360-7
Niederreither, K., Subbarayan, V., Dolle, P., and Chambon, P. (1999). Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat. Genet. 21 (4), 444–448. doi:10.1038/7788
North, T., Gu, T. L., Stacy, T., Wang, Q., Howard, L., Binder, M., et al. (1999). Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development 126 (11), 2563–2575. doi:10.1242/dev.126.11.2563
North, T. E., de Bruijn, M. F., Stacy, T., Talebian, L., Lind, E., Robin, C., et al. (2002). Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity 16 (5), 661–672. doi:10.1016/s1074-7613(02)00296-0
North, T. E., Goessling, W., Peeters, M., Li, P., Ceol, C., Lord, A. M., et al. (2009). Hematopoietic stem cell development is dependent on blood flow. Cell 137 (4), 736–748. doi:10.1016/j.cell.2009.04.023
Nottingham, W. T., Jarratt, A., Burgess, M., Speck, C. L., Cheng, J. F., Prabhakar, S., et al. (2007). Runx1-mediated hematopoietic stem-cell emergence is controlled by a Gata/Ets/SCL-regulated enhancer. Blood 110 (13), 4188–4197. doi:10.1182/blood-2007-07-100883
Oatley, M., Bolukbasi, O. V., Svensson, V., Shvartsman, M., Ganter, K., Zirngibl, K., et al. (2020). Single-cell transcriptomics identifies CD44 as a marker and regulator of endothelial to haematopoietic transition. Nat. Commun. 11 (1), 586. doi:10.1038/s41467-019-14171-5
Okada, H., Watanabe, T., Niki, M., Takano, H., Chiba, N., Yanai, N., et al. (1998). AML1(-/-) embryos do not express certain hematopoiesis-related gene transcripts including those of the PU.1 gene. Oncogene 17 (18), 2287–2293. doi:10.1038/sj.onc.1202151
Okuda, T., van Deursen, J., Hiebert, S. W., Grosveld, G., and Downing, J. R. (1996). AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell 84 (2), 321–330. doi:10.1016/s0092-8674(00)80986-1
Orkin, S. H., and Zon, L. I. (2008). Hematopoiesis: an evolving paradigm for stem cell biology. Cell 132 (4), 631–644. doi:10.1016/j.cell.2008.01.025
Papathanasiou, P., Attema, J. L., Karsunky, H., Xu, J., Smale, S. T., and Weissman, I. L. (2009). Evaluation of the long-term reconstituting subset of hematopoietic stem cells with CD150. Stem Cells 27 (10), 2498–2508. doi:10.1002/stem.170
Peeters, M., Ottersbach, K., Bollerot, K., Orelio, C., de Bruijn, M., Wijgerde, M., et al. (2009). Ventral embryonic tissues and Hedgehog proteins induce early AGM hematopoietic stem cell development. Development 136 (15), 2613–2621. doi:10.1242/dev.034728
Piau, O., Brunet-Manquat, M., L'Homme, B., Petit, L., Birebent, B., Linard, C., et al. (2023). Generation of transgene-free hematopoietic stem cells from human induced pluripotent stem cells. Cell Stem Cell 30 (12), 1610–1623 e1617. doi:10.1016/j.stem.2023.11.002
Porcheri, C., Golan, O., Calero-Nieto, F. J., Thambyrajah, R., Ruiz-Herguido, C., Wang, X., et al. (2020). Notch ligand Dll4 impairs cell recruitment to aortic clusters and limits blood stem cell generation. EMBO J. 39 (8), e104270. doi:10.15252/embj.2019104270
Qian, H., Buza-Vidas, N., Hyland, C. D., Jensen, C. T., Antonchuk, J., Mansson, R., et al. (2007). Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell 1 (6), 671–684. doi:10.1016/j.stem.2007.10.008
Qu, K., Mo, S., Huang, J., Liu, S., Zhang, S., Shen, J., et al. (2024). SPI1-KLF1/LYL1 axis regulates lineage commitment during endothelial-to-hematopoietic transition from human pluripotent stem cells. iScience 27 (8), 110409. doi:10.1016/j.isci.2024.110409
Rho, S. S., Kobayashi, I., Oguri-Nakamura, E., Ando, K., Fujiwara, M., Kamimura, N., et al. (2019). Rap1b promotes notch-signal-mediated hematopoietic stem cell development by enhancing integrin-mediated cell adhesion. Dev. Cell 49 (5), 681–696.e6. doi:10.1016/j.devcel.2019.03.023
Robert-Moreno, A., Espinosa, L., de la Pompa, J. L., and Bigas, A. (2005). RBPjkappa-dependent Notch function regulates Gata2 and is essential for the formation of intra-embryonic hematopoietic cells. Development 132 (5), 1117–1126. doi:10.1242/dev.01660
Robin, C., Ottersbach, K., Durand, C., Peeters, M., Vanes, L., Tybulewicz, V., et al. (2006). An unexpected role for IL-3 in the embryonic development of hematopoietic stem cells. Dev. Cell 11 (2), 171–180. doi:10.1016/j.devcel.2006.07.002
Rybtsov, S., Batsivari, A., Bilotkach, K., Paruzina, D., Senserrich, J., Nerushev, O., et al. (2014). Tracing the origin of the HSC hierarchy reveals an SCF-dependent, IL-3-independent CD43(-) embryonic precursor. Stem Cell Rep. 3 (3), 489–501. doi:10.1016/j.stemcr.2014.07.009
Rybtsov, S., Sobiesiak, M., Taoudi, S., Souilhol, C., Senserrich, J., Liakhovitskaia, A., et al. (2011). Hierarchical organization and early hematopoietic specification of the developing HSC lineage in the AGM region. J. Exp. Med. 208 (6), 1305–1315. doi:10.1084/jem.20102419
Sawamiphak, S., Kontarakis, Z., and Stainier, D. Y. (2014). Interferon gamma signaling positively regulates hematopoietic stem cell emergence. Dev. Cell 31 (5), 640–653. doi:10.1016/j.devcel.2014.11.007
Scarfo, R., Randolph, L. N., Abou Alezz, M., El Khoury, M., Gersch, A., Li, Z. Y., et al. (2024). CD32 captures committed haemogenic endothelial cells during human embryonic development. Nat. Cell Biol. 26 (5), 719–730. doi:10.1038/s41556-024-01403-0
Shivdasani, R. A., Mayer, E. L., and Orkin, S. H. (1995). Absence of blood formation in mice lacking the T-cell leukaemia oncoprotein tal-1/SCL. Nature 373 (6513), 432–434. doi:10.1038/373432a0
Souilhol, C., Gonneau, C., Lendinez, J. G., Batsivari, A., Rybtsov, S., Wilson, H., et al. (2016a). Inductive interactions mediated by interplay of asymmetric signalling underlie development of adult haematopoietic stem cells. Nat. Commun. 7, 10784. doi:10.1038/ncomms10784
Souilhol, C., Lendinez, J. G., Rybtsov, S., Murphy, F., Wilson, H., Hills, D., et al. (2016b). Developing HSCs become Notch independent by the end of maturation in the AGM region. Blood 128 (12), 1567–1577. doi:10.1182/blood-2016-03-708164
Swiers, G., Baumann, C., O'Rourke, J., Giannoulatou, E., Taylor, S., Joshi, A., et al. (2013). Early dynamic fate changes in haemogenic endothelium characterized at the single-cell level. Nat. Commun. 4, 2924. doi:10.1038/ncomms3924
Taoudi, S., Gonneau, C., Moore, K., Sheridan, J. M., Blackburn, C. C., Taylor, E., et al. (2008). Extensive hematopoietic stem cell generation in the AGM region via maturation of VE-cadherin+CD45+ pre-definitive HSCs. Cell Stem Cell 3 (1), 99–108. doi:10.1016/j.stem.2008.06.004
Taoudi, S., Morrison, A. M., Inoue, H., Gribi, R., Ure, J., and Medvinsky, A. (2005). Progressive divergence of definitive haematopoietic stem cells from the endothelial compartment does not depend on contact with the foetal liver. Development 132 (18), 4179–4191. doi:10.1242/dev.01974
Thambyrajah, R., and Bigas, A. (2022). Notch signaling in HSC emergence: when, why and how. Cells 11 (3), 358. doi:10.3390/cells11030358
Thambyrajah, R., Mazan, M., Patel, R., Moignard, V., Stefanska, M., Marinopoulou, E., et al. (2016). GFI1 proteins orchestrate the emergence of haematopoietic stem cells through recruitment of LSD1. Nat. Cell Biol. 18 (1), 21–32. doi:10.1038/ncb3276
Tsai, F. Y., Keller, G., Kuo, F. C., Weiss, M., Chen, J., Rosenblatt, M., et al. (1994). An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature 371 (6494), 221–226. doi:10.1038/371221a0
Tsuruda, M., Morino-Koga, S., and Ogawa, M. (2021). Bone morphogenetic protein 4 differently promotes distinct VE-cadherin(+) precursor stages during the definitive hematopoietic development from embryonic stem cell-derived mesodermal cells. Exp. Hematol. 103, 40–51.e7. doi:10.1016/j.exphem.2021.08.008
Tsuruda, M., Morino-Koga, S., Zhao, X., Usuki, S., Yasunaga, K. I., Yokomizo, T., et al. (2024). Bone morphogenetic protein 4 induces hematopoietic stem cell development from murine hemogenic endothelial cells in culture. Stem Cell Rep. doi:10.1016/j.stemcr.2024.10.005
Vink, C. S., Calero-Nieto, F. J., Wang, X., Maglitto, A., Mariani, S. A., Jawaid, W., et al. (2020). Iterative single-cell analyses define the transcriptome of the first functional hematopoietic stem cells. Cell Rep. 31 (6), 107627. doi:10.1016/j.celrep.2020.107627
Wilson, N. K., Foster, S. D., Wang, X., Knezevic, K., Schutte, J., Kaimakis, P., et al. (2010). Combinatorial transcriptional control in blood stem/progenitor cells: genome-wide analysis of ten major transcriptional regulators. Cell Stem Cell 7 (4), 532–544. doi:10.1016/j.stem.2010.07.016
Winnier, G., Blessing, M., Labosky, P. A., and Hogan, B. L. (1995). Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 9 (17), 2105–2116. doi:10.1101/gad.9.17.2105
Yokomizo, T., and Dzierzak, E. (2010). Three-dimensional cartography of hematopoietic clusters in the vasculature of whole mouse embryos. Development 137 (21), 3651–3661. doi:10.1242/dev.051094
Yokomizo, T., Ideue, T., Morino-Koga, S., Tham, C. Y., Sato, T., Takeda, N., et al. (2022). Independent origins of fetal liver haematopoietic stem and progenitor cells. Nature 609 (7928), 779–784. doi:10.1038/s41586-022-05203-0
Yokomizo, T., Ogawa, M., Osato, M., Kanno, T., Yoshida, H., Fujimoto, T., et al. (2001). Requirement of Runx1/AML1/PEBP2alphaB for the generation of haematopoietic cells from endothelial cells. Genes cells. 6 (1), 13–23. doi:10.1046/j.1365-2443.2001.00393.x
Yokomizo, T., Watanabe, N., Umemoto, T., Matsuo, J., Harai, R., Kihara, Y., et al. (2019). Hlf marks the developmental pathway for hematopoietic stem cells but not for erythro-myeloid progenitors. J. Exp. Med. 216 (7), 1599–1614. doi:10.1084/jem.20181399
Yoshihara, H., Arai, F., Hosokawa, K., Hagiwara, T., Takubo, K., Nakamura, Y., et al. (2007). Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell 1 (6), 685–697. doi:10.1016/j.stem.2007.10.020
Zhang, Y., Kang, Z., Liu, M., Wang, L., and Liu, F. (2024). Single-cell omics identifies inflammatory signaling as a trans-differentiation trigger in mouse embryos. Dev. Cell 59 (8), 961–978.e7. doi:10.1016/j.devcel.2024.02.010
Zhou, F., Li, X., Wang, W., Zhu, P., Zhou, J., He, W., et al. (2016). Tracing haematopoietic stem cell formation at single-cell resolution. Nature 533 (7604), 487–492. doi:10.1038/nature17997
Zhu, Q., Gao, P., Tober, J., Bennett, L., Chen, C., Uzun, Y., et al. (2020). Developmental trajectory of prehematopoietic stem cell formation from endothelium. Blood 136 (7), 845–856. doi:10.1182/blood.2020004801
Keywords: intra-aortic hematopoietic cluster (IAHC), hematopoietic stem cell (HSC), erythro-myeloid progenitor (EMP), endothelial-to-hematopoietic transition (EHT), hematopoietic stem cell precursor (pre-HSC), aorta-gonad-mesonephros (AGM)
Citation: Morino-Koga S and Yokomizo T (2024) Deciphering hematopoietic stem cell development: key signaling pathways and mechanisms. Front. Cell Dev. Biol. 12:1510198. doi: 10.3389/fcell.2024.1510198
Received: 12 October 2024; Accepted: 22 November 2024;
Published: 09 December 2024.
Edited by:
Pawan Kumar Raghav, University of California, San Francisco, United StatesReviewed by:
Benjamin Dannenmann, University Hospital Tuebingen, GermanyNeslihan Meriç, Kutahya Health Sciences University, Türkiye
Gurudutta Gangenahalli, Netaji Subhas University of Technology, India
Hideo Ema, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2024 Morino-Koga and Yokomizo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saori Morino-Koga, a29nYXNAa3VtYW1vdG8tdS5hYy5qcA==; Tomomasa Yokomizo, dG9tb3lva29taXpvQGdtYWlsLmNvbQ==
 Saori Morino-Koga
Saori Morino-Koga Tomomasa Yokomizo
Tomomasa Yokomizo