
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell Dev. Biol., 23 December 2024
Sec. Cancer Cell Biology
Volume 12 - 2024 | https://doi.org/10.3389/fcell.2024.1506492
Ferroptosis, distinct from apoptosis, is primarily characterized by the accumulation of iron-dependent lipid peroxides (LPO) and reactive oxygen species (ROS). This process plays a pivotal role in the pathophysiology of various diseases and has recently emerged as a promising therapeutic strategy in oncology, garnering significant attention. Non-coding RNAs (ncRNAs), including microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs), serve as crucial regulators in numerous biological processes, particularly in cancer initiation and progression. Increasing research efforts are focused on targeting ferroptosis through modulation of these ncRNAs. This review provides an overview of the mechanisms underlying ferroptosis and explores the roles of ncRNAs in breast cancer (BC) and its regulation. Furthermore, we examine the interactions between ferroptosis and ncRNAs in BC, aiming to identify potential therapeutic targets for BC treatment.
Breast cancer (BC) represents a significant global health threat to women (Makhoul et al., 2018). Recent cancer statistics indicate that BC has surpassed lung cancer as the most prevalent malignancy among women (Nik-Zainal et al., 2016). Although BC can occur post-adolescence, its incidence increases with age (Duffy et al., 2012). Despite significant advancements in cancer diagnosis, treatment, and prevention, the global mortality rate from BC remains the fifth highest among all cancers, primarily due to the growing resistance to chemotherapy and radiotherapy (Sung et al., 2021). Approximately 90% of BC-related deaths are attributed to distant metastases from recurrent or primary tumors. Consequently, early diagnosis, prompt treatment, and the development of more effective therapeutic strategies are crucial for improving patient outcomes (Chen et al., 2018).
Ferroptosis, a novel form of regulated cell death, was first identified in 2012 (Dixon, 2017).
It is characterized by the accumulation of iron-dependent lipid peroxides (LPO) and reactive oxygen species (ROS) (Huang R. et al., 2023). However, this unique, iron-dependent mode of cell death—distinct from apoptosis, autophagy, and necrosis—had been observed prior to its formal designation. Morphologically, ferroptotic cells exhibit shrunken mitochondria, increased membrane density, and reduced cristae, while nuclear morphology remains relatively unaffected (Mou et al., 2019; Dixon et al., 2012; Li J. et al., 2020). Ferroptosis plays a pivotal role in various physiological and pathological processes, including neurodegenerative diseases, cancer, and cardiovascular disorders (Li J. et al., 2020). Interestingly, tumor cells resistant to conventional therapies still exhibit high sensitivity to ferroptosis (Hassannia et al., 2019). Therefore, understanding the mechanisms and regulation of ferroptosis is crucial for elucidating the pathogenesis of these diseases and developing novel therapeutic strategies. However, the interactions between ferroptosis and BC remain underexplored, and the impact of ferroptosis on BC prognosis is not yet fully understood.
Non-coding RNAs (ncRNAs) are RNA molecules that do not encode proteins but possess regulatory functions. These include microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs). ncRNAs play crucial roles in various biological processes, particularly in regulating cancer initiation and progression. Recent evidence suggests that ncRNAs can modulate ferroptosis by regulating ferroptosis-related genes and metabolic pathways. Through these mechanisms, ncRNAs may either promote or inhibit cancer development (Luo Y. et al., 2021).
This review discusses the mechanisms underlying ferroptosis and examines its relationship with BC. Additionally, we review the structure and function of ncRNAs and their roles in both BC and ferroptosis. Furthermore, we summarize ferroptosis-related ncRNAs in BC that are crucial for anticancer therapy, offering new insights into the development of novel therapeutic strategies for BC. Finally, we explore recent advancements and future prospects of targeting ferroptosis-related ncRNAs in BC treatment.
Ferroptosis, an iron-dependent form of regulated cell death distinct from apoptosis, is closely associated with disruptions in redox homeostasis. This process is triggered by an imbalance in redox regulation (Dixon and Olzmann, 2024). Key features of ferroptosis include the excessive accumulation of ROS and LPO, often driven by iron overload or lipoxygenase activity. The loss or reduced expression of critical antioxidant enzymes, such as glutathione peroxidase 4 (GPX4), further exacerbates lipid peroxidation, ultimately leading to cell death (Jiang et al., 2021) (Figure 1).
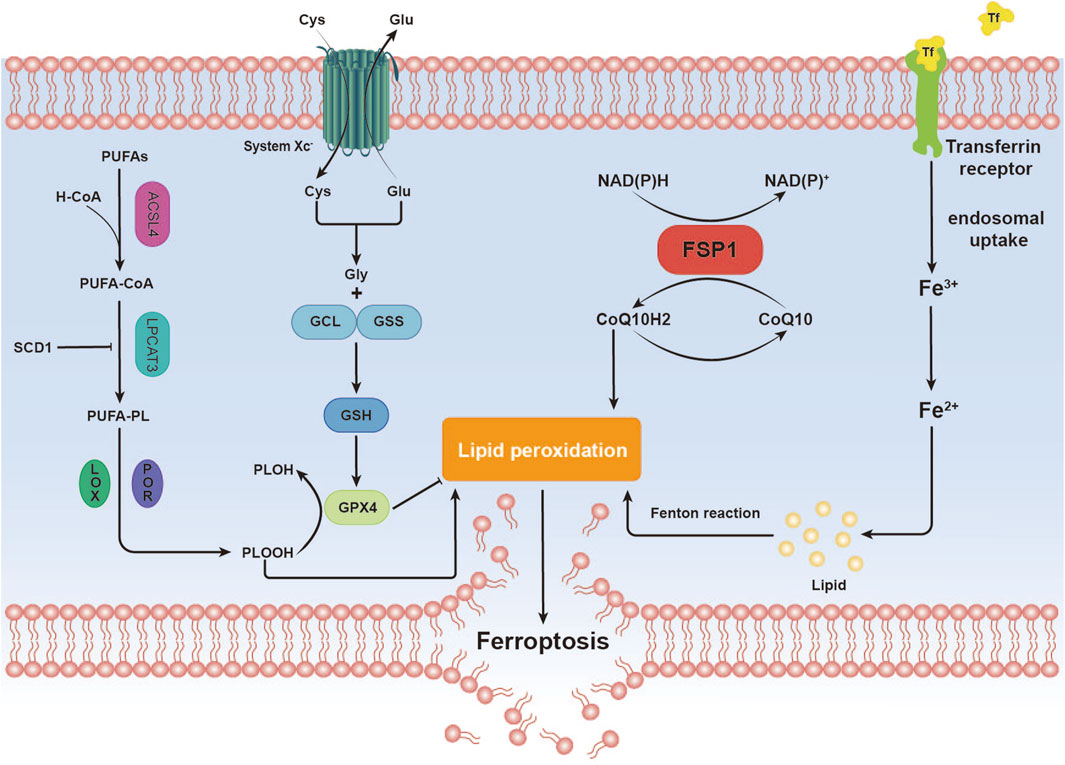
Figure 1. Overview of Ferroptosis Mechanisms. This image illustrates the mechanistic pathway of ferroptosis, a distinctive form of cell death characterized by iron-dependent lipid peroxidation. Iron (Fe) Metabolism. Polyunsaturated Fatty Acids (PUFAs) Metabolism. Antioxidant Defense. Lipid Peroxidation. These steps elucidate the mechanistic basis by which ferroptosis, through iron-dependent lipid peroxidation, culminates in the destruction of cellular membranes, leading to cell death.
Iron is an essential element in biological systems, playing key roles in oxygen transport, electron transfer, and enzymatic reactions (Morales and Xue, 2021). Within cells, iron primarily exists as ferrous (Fe2⁺) and ferric (Fe³⁺) ions (Fairweather-Tait, 2001). Under normal conditions, cells maintain iron homeostasis through tightly regulated mechanisms that govern iron uptake, storage, utilization, and export (Liu et al., 2022). However, in pathological conditions, such as genetic mutations, environmental toxin exposure, or disease states, this balance can be disrupted, leading to iron accumulation, oxidative stress, and the initiation of ferroptosis (Nakamura et al., 2019). Excessive accumulation of ferrous ions triggers the Fenton reaction, generating highly reactive hydroxyl radicals (·OH) and other ROS (Toyokuni, 2002). These ROS then target and damage various intracellular biomolecules, including lipids, proteins, and nucleic acids (Zhang K. et al., 2020).
Acyl-CoA synthetase long-chain family member 4 (ACSL4) is a key enzyme in lipid metabolism (Doll et al., 2017; Zou et al., 2019), critical for initiating lipid peroxidation and determining cellular susceptibility to ferroptosis (Cui et al., 2021). ACSL4 selectively activates specific polyunsaturated fatty acids (PUFAs), such as arachidonic acid (AA) and adrenic acid, by converting them into acyl-CoA derivatives (Xiang et al., 2024). This modification alters membrane lipid composition, increasing their susceptibility to peroxidation and promoting ferroptosis (Doll et al., 2017; Yuan et al., 2016). ACSL4-mediated incorporation of PUFAs into membrane phospholipids further enhances their vulnerability to oxidative damage (Chen X. et al., 2021).
Lipoxygenases (LOXs), a family of non-heme iron-containing dioxygenases, directly oxidize PUFAs within cellular membranes (Kuhn et al., 2018), particularly AA and adrenic acid (Gaschler and Stockwell, 2017). Due to their multiple double bonds, PUFAs are optimal substrates for LOX-mediated oxidation, leading to the generation of various LPO (Porter et al., 1979; Xie LH. et al., 2022).
Glutathione (GSH), a tripeptide composed of glutamate, cysteine, and glycine, is a critical intracellular antioxidant (Niu et al., 2021). Under normal conditions, GSH collaborates with GPX4 to inhibit ferroptosis. GPX4, a selenium-dependent enzyme, is the primary catalyst responsible for reducing phospholipid hydroperoxides (PLOOHs) to their corresponding alcohols in mammalian cells, thereby mitigating lipid peroxidation and protecting cell membranes from oxidative damage (Ursini et al., 1982; Ursini et al., 1985). Disruption of the GSH system can occur through several mechanisms:
First of all, GSH synthesis is a key determinant of GSH system function. This process is contingent on the cellular availability of cysteine, which is primarily imported into cells via the cystine/glutamate antiporter system (system Xc-) (Dixon et al., 2012; Liu et al., 2022). Dysfunction of the Xc-system reduces cysteine uptake, impairing GSH synthesis. In the absence of GSH, GPX4 loses its ability to detoxify LPO, leading to increased lipid peroxidation (Liu et al., 2022).
Secondly, increased GSH consumption disrupts the balance of GSH availability. Under pathological conditions, reduced GSH is required to neutralize enhanced cellular activities, such as iron accumulation, elevated lipoxygenase expression, increased enzyme activity, and excessive LPO (Ursini and Maiorino, 2020). During the reduction of LPO to their corresponding alcohols, GPX4 utilizes its selenocysteine residues to transfer two electrons—typically provided by GSH—to the LPO. In some cases, these electrons may also be sourced from other low-molecular-weight thiols or protein thiols (Maiorino et al., 2018). This reaction occurs as GSH binds to the active site of GPX4 via its thiol group, facilitating the reduction of the peroxyl bond in the lipid peroxide (Chen X. et al., 2021). Additionally, exogenous compounds or metabolic byproducts may bind to GSH, accelerating its depletion. For example, RSL3 has been shown to directly inhibit GPX4, destabilizing the GSH system and impairing antioxidant defense (Jiang et al., 2021).
Thirdly, reduced GSH regeneration: After its antioxidant activity, GSH is oxidized to glutathione disulfide (GSSG). Normally, intracellular glutathione reductase uses nicotinamide adenine dinucleotide phosphate (NADPH) as a reducing agent to convert GSSG back into GSH, thereby maintaining cellular GSH levels (Flohé, 2013). However, when glutathione reductase activity is suppressed or NADPH availability is limited, GSSG cannot be efficiently recycled to GSH, resulting in a decline in the pool of active GSH (Averill-Bates, 2023).
While the GPX4 pathway serves as a major antioxidant defense in ferroptosis, studies have identified ferroptosis suppressor protein 1 (FSP1) as another critical regulator of ferroptosis inhibition (Bersuker et al., 2019; Doll et al., 2019). Initially considered a pro-apoptotic gene (Wu et al., 2002), FSP1 was later found to confer resistance to ferroptosis in cells lacking GPX4. CoQ10, a lipid-soluble quinone compound, is abundant in mitochondria and cellular membranes, where it plays a key role in both antioxidant defense and energy metabolism (Raizner, 2019). CoQ10 exists in two redox states: reduced and oxidized forms. Ubiquinol scavenges lipid peroxyl radicals, effectively neutralizing lipid peroxidation and thus preventing ferroptosis. FSP1 facilitates the regeneration of reduced CoQ10, ensuring a continuous supply of this antioxidant. When the CoQ10 system is dysregulated, ubiquinol levels decrease, ubiquinone levels rise, and the risk of ferroptosis escalates (Doll et al., 2019). Dysregulation of CoQ10 can occur through several mechanisms:
CoQ10 is synthesized in various tissues and primarily stored in mitochondria. Its biosynthesis involves multiple enzymatic steps, and disruptions at any stage, due to genetic defects or pathological conditions, can lead to insufficient CoQ10 production (Raizner, 2019).
During oxidative stress, high levels of ROS and lipid hydroperoxides (LOOH) are produced (Gordan et al., 2018; Berdoukas et al., 2015; Hershko, 2010; Kruszewski, 2003), which rapidly oxidize ubiquinol to ubiquinone, depleting the active antioxidant form of CoQ10. This reaction can be represented as:CoQ10redred + LOOH → CoQ10oxox + LOH (Doll et al., 2019). The reduction in ubiquinol diminishes the cell’s capacity to neutralize LPO, exacerbating oxidative damage.
CoQ10 reductases in the body use NADH or NADPH as electron donors to reduce ubiquinone back to ubiquinol (Cadenas et al., 2022). Studies have shown that certain mitochondrial enzymes, including complexes I and II of the respiratory chain, transfer electrons to ubiquinone, regenerating it to its reduced form. Similar mechanisms may also occur in other electron transport systems, such as the endoplasmic reticulum (Martínez-Reyes et al., 2020).
When these antioxidant systems are imbalanced, susceptibility to ferroptosis increases significantly. Furthermore, these systems often interact (Fang et al., 2019). For example, GPX4, a key regulator of ferroptosis, relies on GSH to detoxify LPO. When the CoQ10 system is impaired, GPX4’s dependence on GSH intensifies, further depleting cellular GSH reserves. Excessive oxidative stress, coupled with dysfunction of antioxidant systems, leads to mitochondrial ROS accumulation (Dixon et al., 2012), exacerbating oxidative stress and creating a vicious cycle that heightens the risk of ferroptosis (Bedard and Krause, 2007).
BC can be treated through various methods, including surgery, chemotherapy, and radiotherapy. However, reducing the incidence and mortality of BC remains a significant challenge (Barzaman et al., 2020). Studies have demonstrated that BC exhibits a heterogeneous phenotype in terms of ferroptosis-related metabolites and metabolic pathways, characterized by oxidized phosphatidylethanolamine and altered GSH metabolism (Yang F. et al., 2023). Importantly, BC has been identified as a lipid- and iron-rich tumor (Martinez-Outschoorn et al., 2017; Xiao et al., 2022), making ferroptosis induction a promising therapeutic strategy. This mechanism primarily disrupts cancer cell metabolism, inducing cell death through redox imbalance and increased intracellular ROS levels (Tang et al., 2021) (Mou et al., 2019). Furthermore, research has shown that targeting the ferroptosis pathway in BC may enhance therapeutic sensitivity (Yang F. et al., 2023). Conversely, inhibiting this pathway can increase resistance to other chemotherapeutic agents (Xu et al., 2023).
Studies have shown that mutations in the tumor suppressor genes BRCA1 and BRCA2 increase the risk of BC (Liu L. et al., 2020). The loss of function in these genes impairs DNA repair, thereby promoting tumorigenesis (Ben Ayed-Guerfali et al., 2021). Similarly, deletion of the tumor suppressor gene PTEN, which normally inhibits cellular proliferation, invasion, and metastasis, accelerates tumor progression (Chen et al., 2022a). Mutations in other genes, such as CHEK2, ATM, PALB2, BRIP1, and CDH1, allow cancer cells to evade immune surveillance and develop resistance to conventional therapies (Neves et al., 2023; Hansen et al., 2015; Piccolo et al., 2014). In response, researchers have focused on discovering novel anticancer compounds, leading to the identification of ferroptosis inducers (Dolma et al., 2003; Yagoda et al., 2007). Notably, cancer cells resistant to standard treatments have shown increased sensitivity to ferroptosis inducers (Viswanathan et al., 2017; Hangauer et al., 2017; Tsoi et al., 2018).
The development of ferroptosis-based therapies for BC remains in its early stages. Current research focuses on several non-targeted approaches aimed at promoting ferroptosis by enhancing cellular uptake of iron, peroxides, and other substances to eliminate BC cells. Concurrently, efforts are being made to develop targeted therapies that modulate ferroptosis-related molecules, such as enzymes (Hassannia et al., 2019; Hassannia et al., 2018). For example, silencing the GPX4 gene using RNA interference or CRISPR/Cas9, or inhibiting GPX4 activity with ferroptosis inducers like RSL3, can induce ferroptosis by reducing GPX4 levels (Yang et al., 2014; Yang et al., 2016). Targeting GSH metabolism, such as by inhibiting interferon-gamma (IFN-γ), can suppress the XC- system, reducing cystine uptake and GSH synthesis (Sato et al., 1995; Koppula et al., 2021). Similarly, inhibiting glutathione reductase or decreasing NADPH supply can hinder GSH reduction, thereby promoting ferroptosis (Niu et al., 2021). Additionally, silencing FSP1 decreases the production of ferroptosis suppressor proteins, reducing levels of reduced coenzyme Q10 and further potentiating ferroptosis (Wang H. et al., 2021). Iron metabolism plays a central role in ferroptosis, with transferrin (TF) and transferrin receptor 1 (TFR1) regulating iron uptake. Overexpression of TF and TFR1, or inhibition of the iron export pump FPN, increases intracellular iron concentration and LPO, thereby promoting ferroptosis (Bogdan et al., 2016; Gammella et al., 2017; Geng et al., 2018; Torti and Torti, 2020). Finally, certain immune cells, such as CD8+ T cells, can enhance the sensitivity of BC cells to ferroptosis (Wang W. et al., 2019). Ferroptosis can also enhance the immune function of cells like neutrophils, aiding BC treatment (Yotsumoto et al., 2017). By altering the tumor microenvironment, ferroptosis may influence tumor prognosis and suppress tumor cell proliferation (Kim et al., 2023).
miRNAs are short RNA molecules derived from longer stem-loop precursors that bind to and inhibit messenger RNA (mRNA) (Chen, 2016). miRNAs exert their function by sequence-specific binding to target RNAs, thereby repressing gene expression (Bartel, 2009; Lee et al., 2009). This process is not solely governed by direct RNA interactions but also involves effector proteins within the miRISC complex (Bartel, 2009). Beyond their role in post-transcriptional gene regulation, miRNAs can recruit their respective ribonucleoprotein complexes to modulate target translation (Vasudevan et al., 2007). miRNAs regulate numerous biological processes, including stress responses, cell adhesion, motility, inflammation (Hata and Kashima, 2016), differentiation, proliferation, senescence, apoptosis, and hematopoiesis (Jensen et al., 2013)—all of which are closely associated with tumorigenesis.
Aberrant miRNA expression is common across cancers and is regulated by epigenetic and tissue-specific mechanisms. This dysregulation is often linked to DNA methylation-mediated gene silencing and distinct cancer phenotypes (Sengupta et al., 2021). Growing evidence highlights the importance of miRNAs as crucial biomarkers in the initiation, progression, detection, and prognosis of BC. For example, in triple-negative breast cancer (TNBC), the most aggressive molecular subtype, miR-200b suppresses metastasis by targeting Rho GTPase-activating protein 18 (ARHGAP18) and promoting RhoA activation (Humphries et al., 2017), underscoring the critical role of miR-200b in BC progression. Elevated levels of miR-10b, miR-34a, and miR-155 are found in patients with metastatic BC (Roth et al., 2010), with circulating miR-10b and miR-373 significantly increased in lymph node-positive patients compared to those without nodal metastasis or healthy controls (Schwarzenbach et al., 2012). Notably, miR-10b has emerged as a potential biomarker for brain (Ahmad et al., 2014)and bone metastasis (Zhao et al., 2012), suggesting its utility for early detection and prognosis. Furthermore, in hormone receptor-positive BC cells resistant to endocrine therapy (tamoxifen), the miR-221/222 cluster is upregulated through the negative regulation of cyclin-dependent kinase inhibitor 1B (p27Kip1) (Hanna et al., 2012; Gan et al., 2014; Miller et al., 2008). p27Kip1, an enzyme inhibitor encoded by the CDKN1B gene, plays a pivotal role in regulating the human cell cycle (Kurozumi et al., 2015; Toyoshima and Hunter, 1994). Therefore, the miR-221/222 cluster has been proposed as a potential therapeutic target and predictor of tamoxifen resistance in BC treatment (Figure 2) (Table 1).
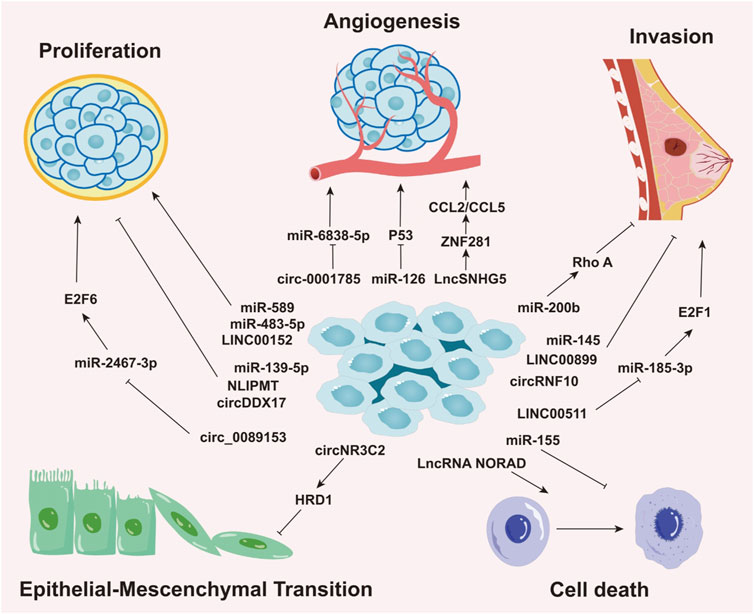
Figure 2. Roles of Non-Coding RNAs in Various Biological Processes of Breast Cancer. This image delineates the roles of different types of non-coding RNAs (ncRNAs), including miRNAs, circRNAs, and lncRNAs, in regulating distinct biological processes in breast cancer, such as cellular proliferation, angiogenesis, invasion, epithelial-mesenchymal transition (EMT), and cell death. The roles and mechanisms of these non-coding RNAs in various biological processes of breast cancer are highly intricate, involving complex regulatory networks that drive cancer progression.
lncRNAs are transcripts longer than 200 nucleotides that do not encode proteins (Mattick and Rinn, 2015). They regulate gene expression through epigenetic modifications, as well as transcriptional and post-transcriptional mechanisms. lncRNAs engage in base-pairing with other RNA molecules (e.g., mRNA, miRNA, or DNA), facilitating direct interactions at the primary structure level (Novikova et al., 2013). At the secondary structure level, lncRNAs perform their roles through base-pairing or ribonucleotide backbone interactions (Cruz and Westhof, 2009; Mercer and Mattick, 2013). In addition to protein interactions mediated by their spatial conformation, lncRNAs recognize other nucleic acids through base-pairing, guiding proteins to specific loci and thereby broadening their functional roles in cancer.
The relationship between lncRNAs and BC has attracted increasing attention due to their critical role in regulating various cancer-related processes, including cell proliferation, invasion, migration, apoptosis, epithelial-mesenchymal transition (EMT), and drug resistance across multiple malignancies (Jin et al., 2021; Wang CJ. et al., 2019; Guo et al., 2019). In BC, lncRNAs influence prognosis through several mechanisms, which include: (Makhoul et al., 2018): Peptide-Encoding lncRNAs: Recent studies have revealed that certain lncRNAs harbor hidden open reading frames capable of encoding functional peptides. For example, LINC00908 encodes a peptide, ASRPS, which exerts antitumor effects by inhibiting angiogenesis in TNBC (Wang et al., 2020). (Nik-Zainal et al., 2016) mRNA Stability Modulation: lncRNAs also regulate mRNA stability in BC cells. For instance, the PDCD4-AS1 lncRNA stabilizes the mRNA of the tumor suppressor gene PDCD4 by forming double-stranded RNA, which interacts with RNA decay factors such as HuR, thereby inhibiting BC cell proliferation and migration (Jadaliha et al., 2018). (Duffy et al., 2012) lncRNAs in Intercellular Communication: Emerging studies suggest that certain lncRNAs can be encapsulated in extracellular vesicles (EVs) and mediate intercellular communication. For instance, EVs released by cancer-associated fibroblasts (CAFs) containing the lncRNA SNHG3 are absorbed by BC cells, where SNHG3 acts as a sponge for miR-330-5p, thereby reprogramming cellular metabolic pathways (Jin et al., 2021). Overall, lncRNAs participate in multiple mechanisms that drive BC development and progression. Research suggests that lncRNAs hold significant potential as molecular biomarkers for BC, offering both research and clinical value (Yuan et al., 2021).
Through these mechanisms, lncRNAs exhibit oncogenic and tumor-promoting activities, influencing cancer development by engaging in or disrupting key cellular pathways (Zeng et al., 2022) (Figure 2) (Table 1).
circRNAs are covalently closed RNA molecules formed through the back-splicing of exons or introns (Jeck et al., 2013). circRNAs are involved in a range of biological processes. Due to their miRNA-binding sites, circRNAs can act as miRNA sponges, modulating miRNA expression (Panda, 2018). In addition to regulating transcription (Stoll et al., 2020), circRNAs may also be translated into proteins (Yang et al., 2017), influence protein function by shuttling between the nucleus and cytoplasm, and promote cellular senescence (Fang et al., 2018). Furthermore, circRNAs serve as protein scaffolds, facilitating protein-protein interactions (Du et al., 2017).
The mechanisms through which circRNAs influence BC are diverse (Wang Z. et al., 2023): (Makhoul et al., 2018) circRNAs as miRNA Sponges: circRNAs can modulate gene expression by sequestering miRNAs, either upregulating or downregulating their targets. For example, circ_0089153 sponges miR-2467-3p, alleviating its inhibitory effect on E2F6, thus promoting BC cell proliferation, migration, invasion, and EMT (Gao et al., 2022). (Nik-Zainal et al., 2016) circRNAs as RNA-Binding Protein (RBP) Complexes: circRNAs can interact with RBPs involved in pre-mRNA transcription, splicing, polyadenylation, and RNA degradation. The RBP human antigen R (HuR) stabilizes mRNA and regulates its processing (Lebedeva et al., 2011). Experimental evidence shows that circ-1073 binds to HuR in BC cells, increasing the levels of caspases 3/9 and E-cadherin, thereby inhibiting oncogenic activity (Yi et al., 2020). (Duffy et al., 2012) circRNAs as Translation Templates: A growing area of research suggests that circRNAs may also function as templates for translation (Zhang M. et al., 2018). For example, circFAM53B encodes a unique peptide in BC cells that binds to HLA-I molecules, enhancing antitumor immunity by inducing higher levels of immunoactive substances such as IFNγ and perforin (Huang et al., 2024). Additionally, circCAPG encodes a peptide known as CAPG-71AA, which promotes tumor growth in BC (Song R. et al., 2023). Numerous circRNAs have been identified as key regulators in BC pathogenesis, with potential applications in diagnosis, prognosis, and therapeutic interventions.
In summary, circRNAs play a central role in regulating tumor proliferation and the surrounding microenvironment, positioning them as crucial mediators in the initiation and progression of BC (Figure 2) (Table 1).
Research has shown that various miRNAs regulate iron metabolism by modulating intracellular iron levels, thereby influencing ferroptosis (Zuo et al., 2022). For example, miR-545 binds to TF mRNA, inhibiting its expression (Zheng et al., 2021), while miR-214 and miR-367-3p target and suppress TFRC expression, reducing iron absorption and preventing ferroptosis (Lu et al., 2020; Huang J. et al., 2023). Additionally, miR-7-5p downregulates mitochondrial TF, lowering Fe2⁺ levels and inhibiting ferroptosis (Tomita et al., 2019). In the context of ulcerative colitis (UC), miR-375-3p binds to the transmembrane iron transporter SLC11A2, downregulating its transcription and blocking iron absorption and metabolism, thus preventing ferroptosis (Luo and Chen, 2023). MiR-19a suppresses ferroptosis in colorectal cancer by modulating iron metabolism and inhibiting the ferroptosis-inducing factor iron-responsive element-binding protein 2 (IREB2) (Fan et al., 2022). Conversely, miR-761 promotes ferroptosis by reducing hepcidin levels, preventing the degradation of ferroportin 1 (FPN1) (Zheng R. et al., 2023). MiR-30b-5p suppresses Pax3 to downregulate FPN1 transcription, inducing ferroptosis in trophoblast cells, which is associated with preeclampsia (Zhang H. et al., 2020). MiR-19b-3p directly targets and reduces ferritin heavy chain 1 (FTH1) expression, resulting in increased free iron and promoting ferroptosis in lung cancer (Zhang R. et al., 2022). Furthermore, miR-129-5p targets and downregulates PROM2, inhibiting iron efflux and enhancing ferroptosis in non-small cell lung cancer (NSCLC) (Luo W. et al., 2021) (Figure 3).
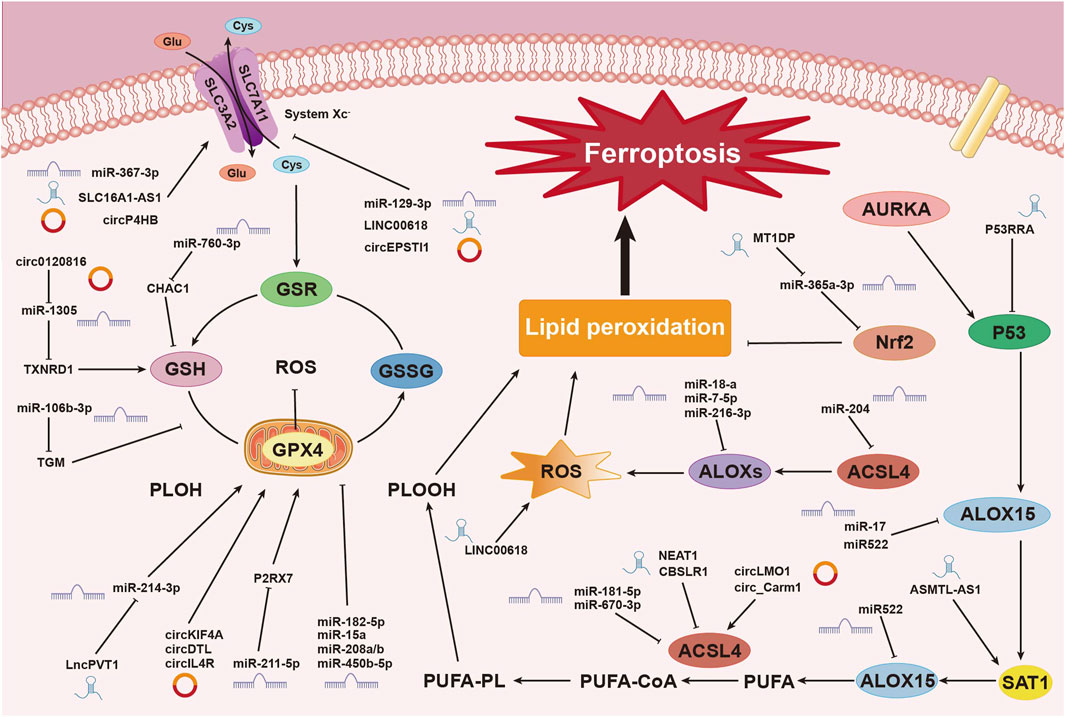
Figure 3. Mechanisms of Non-Coding RNAs in Regulating Ferroptosis. This image illustrates the mechanisms by which non-coding RNAs (ncRNAs), including miRNAs, circRNAs, and lncRNAs, regulate the process of ferroptosis, a form of cell death driven by iron-dependent lipid peroxidation. ncRNAs play pivotal roles at various stages of this process. Non-coding RNAs influence the process of ferroptosis in breast cancer cells through multiple pathways and mechanisms. These include regulating system Xc⁻, modulating glutathione metabolism and redox reactions, controlling lipid peroxidation, and influencing ROS production. These complex interactions and regulatory networks involving miRNAs, circRNAs, and lncRNAs underscore the critical role of ncRNAs in ferroptosis within the context of breast cancer.
LINC00618 has been shown to enhance ferroptosis by increasing levels of ROS and iron, both of which are hallmark indicators of ferroptosis, while also downregulating SLC7A11 expression. Deletion of LINC00618 significantly reduces ROS and iron levels in cancer cells, highlighting its role in enhancing sensitivity to ferroptosis through classical pathways (Wang Z. et al., 2021). In contrast, lncPVT1 suppresses ferroptosis by downregulating miR-214-3p, which leads to increased GPX4 expression and subsequently promotes cancer progression (He et al., 2021).
In summary, lncRNAs exert multilayered regulatory control over ferroptosis in cancer cells. As demonstrated, the mechanisms through which lncRNAs influence ferroptosis are complex and interconnected. Although categorizing these mechanisms may be challenging, their roles are of significant importance. This complexity emphasizes the need for further exploration, making this area a key focus in current cancer research (Figure 3).
circLMO1 and circ_Carm1 promote ferroptosis by upregulating ACSL4 expression (Mao and Liu, 2022). In contrast, circKIF4A (Chen W. et al., 2021), circDTL (Shanshan et al., 2021), cmmu_circRNA_000030 (Jin et al., 2022), and circIL4R (Xu et al., 2020) inhibit ferroptosis by upregulating GPX4 expression. Similarly, circ_0067934 (Wang HH. et al., 2021), and circP4HB (Pan et al., 2022) upregulate SLC7A11 expression, affecting the system Xc− and inhibiting ferroptosis. CircGFRA1 (Bazhabayi et al., 2021) upregulates AIFM2, which encodes FSP1, further suppressing ferroptosis. Recent research has identified numerous circRNAs that regulate ferroptosis through classical pathways, with ongoing studies continuing to enhance our understanding of these mechanisms (Figure 3).
Ferroptosis has become a central focus in BC research. Studies utilizing 3′UTR luciferase assays have demonstrated that miR-5096 targets SLC7A11, thereby promoting ferroptosis in BC cells by downregulating SLC7A11 expression. This mechanism increases ROS production, iron accumulation, and lipid peroxidation, while inhibiting BC cell proliferation, colony formation, migration, and invasion. Notably, miR-5096 induces ferroptosis more effectively in TNBC cells compared to other BC subtypes. Furthermore, miR-5096 reduces the metastatic potential of MDA-MB-231 cells in a zebrafish larvae xenotransplantation model. These findings suggest that miR-5096 may serve as a promising therapeutic target in BC, particularly in TNBC (Yadav et al., 2021).
MiR-128-3p, which is downregulated in BC patients, plays a critical role in various biological processes. By directly targeting SP1 mRNA, miR-128-3p inhibits TGF-β1-induced migration, invasion, and cell cycle progression (Nalla et al., 2022). CD98 heavy chain (CD98hc), a type II transmembrane glycoprotein, interacts with several light chain amino acid transporters, including xCT, LAT1, and y + LAT2, and is involved in the intersection of oxidative stress and amino acid metabolism (de la Ballina et al., 2016). Upregulation of CD98hc promotes cell proliferation, EMT, adhesion, and polarity (Feral et al., 2005), while its downregulation can trigger ferroptosis. Inhibition of SP1 affects CD98hc expression (Yan et al., 2007). Consequently, studies have shown that empagliflozin (EMPA), functioning as a miR-128-3p mimetic, suppresses SP1 expression, reduces CD98hc levels, sensitizes cells to ferroptosis, and may serve as a potential adjunct in BC chemotherapy (Nalla and Khairnar, 2024).
MiR-335-5p exhibits tumor-suppressive properties in various cancers, including BC (Gao et al., 2021; Qian et al., 2021; Zhang S. et al., 2023). Adenosine deaminase acting on RNA 1 (ADAR1), an RNA editing enzyme that converts adenosine to inosine within double-stranded RNA, plays a crucial role in the initiation and progression of several cancers and is overexpressed in BC (Li Y. et al., 2022). Studies have shown that ADAR1 downregulates miR-335-5p in an editing-independent manner, promoting Sp1 expression, upregulating GPX4 levels, and inhibiting ferroptosis in BC. These findings offer new insights into the role of ADAR1 in BC progression (Yin et al., 2024).
Lidocaine, a commonly used local anesthetic, exhibits antitumor activity under experimental conditions, including the inhibition of BC cell viability suppressed by erastin, highlighting its potential role in BC progression. Lidocaine treatment induces the accumulation of Fe2⁺, iron, and ROS in BC cells, thereby inhibiting cell proliferation, while promoting apoptosis and reducing cell invasion and migration (Gao et al., 2018; Khan et al., 2019). Studies have shown that lidocaine enhances ferroptosis by upregulating miR-382-5p, which downregulates SLC7A11 and suppresses malignant BC progression. Inhibition of miR-382-5p blocks lidocaine-induced ferroptosis in BC cells. However, the clinical potential of lidocaine in BC treatment requires further investigation (Sun et al., 2021). Metformin, a widely prescribed oral hypoglycemic agent, has demonstrated inhibitory effects on the proliferation and metastasis of various cancers, including BC (Wang et al., 2017; Mallik and Chowdhury, 2018). Metformin suppresses cell proliferation, upregulates Fe2⁺ and ROS levels, inhibits GPX4 expression, and induces ferroptosis in BC cells (Yang et al., 2021). Furthermore, metformin promotes ferroptosis by upregulating miR-324-3p, which directly targets GPX4 for downregulation (Hou et al., 2021). These findings suggest that metformin, in combination with miR-324-3p, holds promise as a novel therapeutic strategy for cancer treatment.
The investigation of ferroptosis-related miRNAs not only provides new opportunities for BC treatment but also enhances our understanding of the mechanisms through which miRNAs regulate ferroptosis. Future research is anticipated to further elucidate their clinical potential in therapeutic applications.
In the context of BC therapies, ferroptosis has emerged as a critical mechanism in tumor treatment. lncRNAs have garnered significant attention due to their multifaceted roles in tumorigenesis and the regulation of ferroptosis. Several lncRNAs have been implicated in the modulation of ferroptosis within BC cells, including AC092916.1, L133467.1, USP30-AS1, AC108474.1, LINC01235, AL365356.1, AC072039.2, AC012213.3, LIPE-AS1, MAPT-AS1, and TDRKHAS1. Notably, lncRNA USP30-AS1 exhibits a significant co-expression pattern with nine ferroptosis-related genes, including SOCS1, CAPG, IFNG, PML, TNFAIP3, NCF2, SLC2A6, GCH1, and CYBB. This co-expression suggests that upregulation of USP30-AS1 may be associated with improved survival in BC patients. Additionally, lncRNA LIPE-AS1 interacts with key ferroptosis-related genes, such as GPX4, PHKG2, EGLN2, MAPK14, and HRAS, offering new insights into potential therapeutic strategies for improving patient prognosis. Similarly, AC108474.1 has been shown to interact with five ferroptosis-related genes, including HIC1, ISCU, PLIN4, CAV1, and TAZ, suggesting its potential role as a protective factor in BC (Xiang et al., 2024). Research has also identified specific lncRNAs that are strongly associated with BC prognosis. LINC01871, LINC02384, LIPE-AS1, and HSD11B1-AS1 have been classified as low-risk ferroptosis-related lncRNAs (FRLncRNAs), while LINC00393, AC121247.2, AC010655.2, LINC01419, PTPRD-AS1, AC099329.2, OTUD6B-AS1, and LINC02266 are considered high-risk FRLncRNAs.
Recent studies have identified LncFASA as a significant regulator in BC. LncFASA interacts with a specific domain of peroxiredoxin PRDX1, promoting its liquid-liquid phase separation, a process that impairs the enzyme’s peroxidase activity and affects the SLC7A11-GPX4 signaling axis, which is crucial for maintaining cellular oxidative stress homeostasis (Lovatt et al., 2020). This disruption leads to the accumulation of lipid ROS and triggers ferroptosis (Neumann et al., 2003). Notably, elevated LncFASA expression is strongly correlated with the formation of PRDX1 droplets and a favorable prognosis in BC patients (Fan et al., 2024).
Conversely, certain lncRNAs suppress ferroptosis and promote BC progression. For example, a novel protein encoded by the lncRNA HCP5, called HCP5-132aa, promotes the growth of TNBC by regulating GPX4 and reducing ROS levels, thereby inhibiting ferroptosis. Kaplan-Meier survival analysis reveals that high HCP5-132aa expression is associated with an increased risk of BC-related mortality, positioning it as a risk factor for TNBC progression (Tong et al., 2023). Additionally, specific lncRNAs, such as RUNX1-IT1, are selectively upregulated during cancer progression. RUNX1-IT1 is significantly elevated in BC tissues and correlates with larger tumor size and more advanced clinical stages. Mechanistically, RUNX1-IT1 binds to the m6A reader protein IGF2BP1, stabilizing GPX4 mRNA and inhibiting ferroptosis (Wang S. et al., 2023). Similarly, the lncRNA DSCAM-AS1 binds to m6A-modified SLC7A11 mRNA, enhancing its stability and inhibiting ferroptosis (Yan et al., 2024).
LINC00460 expression is significantly elevated in BC tissues compared to normal tissues, promoting cancer cell proliferation and inhibiting ferroptosis by sponging miR-320a and upregulating myelin and lymphocyte protein 2 (MAL2) (Zhang C. et al., 2023). MAL2 has been implicated in cancer progression through various mechanisms (Fang et al., 2021; Jeong et al., 2021; López-Coral et al., 2020). Research suggests that MAL2 overexpression can reverse the effects of LINC00460 knockdown on both proliferation and ferroptosis in BC cells (Zhang C. et al., 2023).
Given the specificity of lncRNAs in BC cells, several prognostic models have been developed (Liu Q. et al., 2020). One such model includes nine metabolism-related lncRNAs—SIRLNT, SIAH2-AS1, MIR205HG, USP30-AS1, MIR200CHG, TFAP2A-AS1, AP005131.2, AL031316.1, and C6orf99—which shows potential for improving predictive accuracy and enabling personalized treatment for BC patients. Prospective validation of these lncRNA signatures could further enhance their clinical utility (Ge et al., 2024). Beyond prognostic models, various therapeutic strategies targeting lncRNAs have been explored. For example, metformin has been shown to downregulate lncRNA H19, inducing ferroptosis in BC cells by increasing lipid ROS levels (Alimova et al., 2009; Chen et al., 2022b). Additionally, lncRNAs contribute to overcoming drug resistance, with targeting LINC00152 demonstrated to increase BC sensitivity to tamoxifen by stabilizing PDE4D mRNA. This stabilization raises cytosolic Ca2⁺ levels, a key regulator of ferroptosis, thereby enhancing tamoxifen efficacy through the induction of ROS and ferroptosis. (Gomez et al., 2002; Pedrera et al., 2021; Saatci et al., 2023). In conclusion, lncRNAs play critical roles in BC development and ferroptosis, presenting promising opportunities for future research. Their involvement in tumor therapy, staging, and prognosis suggests they could serve as valuable therapeutic targets.
Although research on ferroptosis-related circRNAs in BC cells remains limited, we have compiled the existing findings to provide a basis for future exploration.
CircGFRA1 has been shown to promote the malignant progression of HER-2 positive BC by acting as a sponge for miR-1228 and enhancing the expression of AIFM2, an essential NADH oxidase. CircGFRA1 is upregulated in HER-2 positive BC cells and tissues, positioning it as a potential biomarker for BC diagnosis. Silencing circGFRA1 inhibits the proliferation of HER-2 positive BC cells and attenuates invasion and metastasis, highlighting its therapeutic potential (Bazhabayi et al., 2021). The mechanisms underlying this process are as follows: (Makhoul et al., 2018) circGFRA1 sponges miR-1228, leading to the upregulation of AIFM2, which inhibits ferroptosis mediated by ubiquinone and thus promotes BC progression (Bazhabayi et al., 2021; Chen and Xie, 2020); (Nik-Zainal et al., 2016) in experimental models, silencing circGFRA1 in cancer cells reduces the GSH/GSSG ratio, depletes GPX4, accumulates highly toxic lipid ROS, and induces ferroptosis (Seibt et al., 2019). Therefore, circGFRA1 modulates ferroptosis through multiple pathways in BC cells.
Circ_0000643 regulates ferroptosis in BC cells through the FOXQ1/circ_0000643/miR-153/SLC7A11 axis. FOXQ1, a member of the Forkhead box protein family, is an oncogenic transcription factor highly expressed in various tumors and associated with poor prognosis (Dong et al., 2022). SLC7A11, a downstream target of miR-153, is regulated by circ_0000643, which acts as a sponge for miR-153 in BC cells. By upregulating SLC7A11, circ_0000643 promotes BC progression and inhibits ferroptosis (Lin et al., 2020). Furthermore, FOXQ1 enhances the expression of circ_0000643 in BC by binding to the promoter region of ZFAND6, establishing circ_0000643 as a critical component in this regulatory axis (Huang X. et al., 2023).
Emerging studies indicate that circRNAs modulate ferroptosis and influence drug resistance in BC cells. For example, circ-BGN directly interacts with the deubiquitinase OTUB1 and the ferroportin-related protein SLC7A11, enhancing OTUB1-mediated deubiquitination of SLC7A11, which inhibits ferroptosis. Notably, the small-molecule ferroptosis inducer Erastin significantly reduces tumor volume in trastuzumab-resistant BC cells, with increased efficacy when circ-BGN is co-silenced. This suggests that Erastin may restore the antitumor effects of trastuzumab by inducing ferroptosis (Wang S. et al., 2022). Furthermore, SRSF1, circSEPT9, and GCH1 are upregulated in triple-negative BC (TNBC) cells. Downregulation of SRSF1 reduces the IC50 of cisplatin (DDP) in both parental and resistant TNBC cells, inhibiting cell viability and proliferation, decreasing GSH/SLC7A11 levels, and increasing Fe3+/ROS/MDA/ACSL4 levels, thereby promoting ferroptosis. SRSF1 binds to circSEPT9, which in turn upregulates GCH1 by preventing its ubiquitination, thus enhancing GCH1 protein levels. Overexpression of circSEPT9 and GCH1 suppresses ferroptosis, ultimately reducing the chemosensitivity of TNBC cells to DDP (Song et al., 2024).
In conclusion, while research in this field remains in its early stages, existing studies provide valuable insights into the diagnosis and treatment of BC, highlighting its significant potential. Further investigations are needed to explore additional therapeutic strategies, validate current approaches, and translate promising diagnostic and therapeutic targets into clinical practice (Table 2).
BC remains the leading cause of cancer-related mortality among women worldwide. Identifying effective treatment strategies and improving patient recovery rates are of critical importance. Recent studies have highlighted the pivotal role of ferroptosis in tumor development, with increased ferroptosis in cells shown to inhibit tumor progression (Bedoui et al., 2020; Shen et al., 2018). Furthermore, ncRNAs, including miRNAs, lncRNAs, and circRNAs, have been implicated in ferroptosis-related biological processes, influencing cancer growth (Chen Li, 2020).
Based on the treatment mechanisms, targeting ncRNAs (ncRNAs) plays a crucial regulatory role in cancer progression and may emerge as a novel therapeutic strategy for combating BC in the future. There are generally two main approaches for targeting ncRNAs: the first involves inhibiting the expression of overexpressed ncRNAs that act on oncogenes, thereby suppressing tumor progression. The second approach aims to activate or upregulate ncRNAs that express tumor suppressor genes to inhibit cancer development.
Despite the potential of ncRNA-based ferroptosis therapy, several challenges remain. The regulatory network of ferroptosis-related ncRNAs in cancer treatment and diagnosis is not yet fully understood. Current therapies targeting tumorigenesis via ncRNA-mediated ferroptosis have limited efficacy, and individual variability in ncRNA expression, along with differing responses to treatment, significantly affects predictability. Further research is required to balance the promotion of ferroptosis for tumor inhibition with the prevention of chemotherapy resistance through ncRNA regulation. While Erastin is a potent ferroptosis inducer, its low solubility and rapid metabolic degradation remain significant drawbacks (Larraufie et al., 2015). In terms of diagnosis, the lack of standardized reference genes for circulating lncRNAs impedes the development of reliable diagnostic methods for BC. Moreover, circulating lncRNAs often exhibit low expression levels, making detection difficult and reducing diagnostic accuracy (Schlosser et al., 2016). Additionally, the anti-breast cancer mechanisms of traditional herbal medicine remain poorly understood, and only a limited number of lncRNA functions have been elucidated. Identifying potential therapeutic targets for lncRNAs is crucial for developing more effective treatments. Identifying potential therapeutic targets for lncRNAs is crucial for developing more effective treatments (Li et al., 2023). The impact of immune cell subsets and signaling pathways on immune checkpoint inhibitor (ICI) treatment responses requires further investigation. Additionally, the relationship between genetic characteristics, immune subtypes, and specific mutations, and how these factors influence treatment outcomes or resistance, needs to be explored. In addition, further development of ICIs and targeted therapy, chemotherapy, radiotherapy, or other immunotherapies (such as new ICIs and chimeric antigen receptor T cells) is needed to more accurately select appropriate combination therapies (Xie Q. et al., 2022). Overall, the clinical application of ncRNA regulation in BC ferroptosis faces significant challenges, including the lack of effective therapeutic agents and unresolved chemotherapy resistance. The unclear targets of ncRNA regulation in ferroptosis will be a major hurdle for clinical implementation.
To address this issue, further investigation is needed into the encapsulation of ferroptosis-inducing agents within protective delivery systems, such as nanoparticles (Larraufie et al., 2015). Additionally, to explore the clinical potential of targeting ferroptosis-related ncRNAs for BC treatment, it is essential to focus on the molecular mechanisms linking these ncRNAs with iron and ROS metabolism. Further research is also required to understand the relationship between ferroptosis and other regulated cell death pathways, such as TP53-mediated apoptosis, as well as their upstream mechanisms. The role of iron-independent redox processes in ferroptosis must also be explored. Moreover, the lack of specific markers for identifying ferroptosis in living cells and intact tissues hinders the precision of ferroptosis-based treatments and may lead to unwanted side effects. Therefore, identifying specific markers to detect ferroptosis in vivo is crucial. For instance, a comprehensive analysis of PLK using databases like Oncomine, GEPIA, cBioPortal, and Kaplan-Meier plots revealed that PLK1 and PLK4 are potential targets for precision treatment in BC, while PLK2, PLK3, and PLK5 may serve as new prognostic biomarkers (Jiawei et al., 2022). Furthermore, studying the ncRNA network in cancer is essential, as dysregulation of this network can inhibit ferroptosis and promote tumor cell survival and progression. Enhancing the detection of circulating lncRNAs and improving diagnostic accuracy remains a key research direction (Schlosser et al., 2016). In addition, some drugs have been shown to regulate ncRNA expression, thereby influencing ferroptosis and exhibiting anti-cancer effects. Hence, identifying and targeting specific ncRNAs while minimizing side effects is an important area for future research. For example, Circ_0069094 enhances the sensitivity of paclitaxel (PTX) in BC by targeting and silencing the miR-136-5p/YWHAZ axis, thus regulating the malignant phenotype and paclitaxel resistance of BC cells (Kong et al., 2023). Traditional herbal medicines, along with their active ingredients and molecular mechanisms, can be identified using public databases. This approach provides a scientific foundation for the prevention and treatment of BC using traditional Chinese medicine. For example, Ecliptae Herba, known for its anti-tumor properties, has been shown through network pharmacology and cytology experiments that TGF-β1 may be a key therapeutic target, with the TGF-β1/Smad signaling pathway playing a critical role (Li et al., 2023). Moreover, nomograms or predictive models for pathologic complete response (pCR) or tumor size reduction could help identify patients likely to benefit from neoadjuvant chemotherapy (NAC) for TNBC, enabling personalized treatment strategies (Yan et al., 2020). Overall, ncRNAs hold significant potential as risk genes, diagnostic markers, prognostic indicators, nanoparticle cargos, and therapeutic targets for BC. Understanding the functions of ncRNAs in future studies may enhance our understanding of breast cancer’s pathogenesis and lead to more efficient, rapid, and precise diagnostic and therapeutic approaches.
WL: Writing–original draft, Writing–review and editing. CJ: Writing–original draft, Writing–review and editing. YM: Software, Validation, Writing–original draft, Writing–review and editing. WW: Formal Analysis, Investigation, Writing–original draft, Writing–review and editing. JP: Investigation, Project administration, Resources, Writing–original draft, Writing–review and editing. WM: Formal Analysis, Funding acquisition, Methodology, Project administration, Writing–original draft, Writing–review and editing. SX: Conceptualization, Formal Analysis, Project administration, Writing–original draft, Writing–review and editing. DW: Data curation, Funding acquisition, Writing–original draft, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Lanzhou University Innovation and Entrepreneurship training program (20240060129).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahmad, A., Sethi, S., Chen, W., Ali-Fehmi, R., Mittal, S., and Sarkar, F. H. (2014). Up-regulation of microRNA-10b is associated with the development of breast cancer brain metastasis. Am. J. Transl. Res. 6 (4), 384–390.
Alimova, I. N., Liu, B., Fan, Z., Edgerton, S. M., Dillon, T., Lind, S. E., et al. (2009). Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle 8 (6), 909–915. doi:10.4161/cc.8.6.7933
Averill-Bates, D. A. (2023). The antioxidant glutathione. Vitam. Horm. 121, 109–141. doi:10.1016/bs.vh.2022.09.002
Banerjee, K., and Resat, H. (2016). Constitutive activation of STAT3 in breast cancer cells: a review. Int. J. Cancer 138 (11), 2570–2578. doi:10.1002/ijc.29923
Bartel, D. P. (2009). MicroRNAs: target recognition and regulatory functions. Cell 136 (2), 215–233. doi:10.1016/j.cell.2009.01.002
Barzaman, K., Karami, J., Zarei, Z., Hosseinzadeh, A., Kazemi, M. H., Moradi-Kalbolandi, S., et al. (2020). Breast cancer: biology, biomarkers, and treatments. Int. Immunopharmacol. 84, 106535. doi:10.1016/j.intimp.2020.106535
Bazhabayi, M., Qiu, X., Li, X., Yang, A., Wen, W., Zhang, X., et al. (2021). CircGFRA1 facilitates the malignant progression of HER-2-positive breast cancer via acting as a sponge of miR-1228 and enhancing AIFM2 expression. J. Cell. Mol. Med. 25 (21), 10248–10256. doi:10.1111/jcmm.16963
Bedard, K., and Krause, K. H. (2007). The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 87 (1), 245–313. doi:10.1152/physrev.00044.2005
Bedoui, S., Herold, M. J., and Strasser, A. (2020). Emerging connectivity of programmed cell death pathways and its physiological implications. Nat. Rev. Mol. Cell Biol. 21 (11), 678–695. doi:10.1038/s41580-020-0270-8
Beltrán-Anaya, F. O., Romero-Córdoba, S., Rebollar-Vega, R., Arrieta, O., Bautista-Piña, V., Dominguez-Reyes, C., et al. (2019). Expression of long non-coding RNA ENSG00000226738 (LncKLHDC7B) is enriched in the immunomodulatory triple-negative breast cancer subtype and its alteration promotes cell migration, invasion, and resistance to cell death. Mol. Oncol. 13 (4), 909–927. doi:10.1002/1878-0261.12446
Ben Ayed-Guerfali, D., Ben Kridis-Rejab, W., Ammous-Boukhris, N., Ayadi, W., Charfi, S., Khanfir, A., et al. (2021). Novel and recurrent BRCA1/BRCA2 germline mutations in patients with breast/ovarian cancer: a series from the south of Tunisia. J. Transl. Med. 19 (1), 108. doi:10.1186/s12967-021-02772-y
Berdoukas, V., Coates, T. D., and Cabantchik, Z. I. (2015). Iron and oxidative stress in cardiomyopathy in thalassemia. Free Radic. Biol. Med. 88 (Pt A), 3–9. doi:10.1016/j.freeradbiomed.2015.07.019
Bersuker, K., Hendricks, J. M., Li, Z., Magtanong, L., Ford, B., Tang, P. H., et al. (2019). The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575 (7784), 688–692. doi:10.1038/s41586-019-1705-2
Bogdan, A. R., Miyazawa, M., Hashimoto, K., and Tsuji, Y. (2016). Regulators of iron homeostasis: new players in metabolism, cell death, and disease. Trends Biochem. Sci. 41 (3), 274–286. doi:10.1016/j.tibs.2015.11.012
Cadenas, E., Boveris, A., Ian Ragan, C., and A, O. M. S. (2022). Reprint of: production of superoxide radicals and hydrogen peroxide by NADH- ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Arch. Biochem. Biophys. 726, 109231. doi:10.1016/j.abb.2022.109231
Chen, D., Zeng, S., Qiu, H., Yang, M., Lin, X., Lv, X., et al. (2024). Circ-FOXO3 inhibits triple-negative breast cancer growth and metastasis via regulating WHSC1-H3K36me2-Zeb2 axis. Cell. Signal. 117, 111079. doi:10.1016/j.cellsig.2024.111079
Chen, J., Jiang, Q., Jiang, X. Q., Li, D. Q., Jiang, X. C., Wu, X. B., et al. (2020). miR-146a promoted breast cancer proliferation and invasion by regulating NM23-H1. J. Biochem. 167 (1), 41–48. doi:10.1093/jb/mvz079
Chen, J., Qin, C., Zhou, Y., Chen, Y., Mao, M., and Yang, J. (2022b). Metformin may induce ferroptosis by inhibiting autophagy via lncRNA H19 in breast cancer. FEBS Open Bio 12 (1), 146–153. doi:10.1002/2211-5463.13314
Chen, J., Sun, J., Wang, Q., Du, Y., Cheng, J., Yi, J., et al. (2022a). Systemic deficiency of PTEN accelerates breast cancer growth and metastasis. Front. Oncol. 12, 825484. doi:10.3389/fonc.2022.825484
Chen, L., and Xie, J. (2020). Ferroptosis-suppressor-protein 1: a potential neuroprotective target for combating ferroptosis. Mov. Disord. official J. Mov. Disord. Soc. 35 (3), 400. doi:10.1002/mds.27990
Chen, L. L. (2016). The biogenesis and emerging roles of circular RNAs. Nat. Rev. Mol. Cell Biol. 17 (4), 205–211. doi:10.1038/nrm.2015.32
Chen, T., Wang, X., Li, C., Zhang, H., Liu, Y., Han, D., et al. (2021c). CircHIF1A regulated by FUS accelerates triple-negative breast cancer progression by modulating NFIB expression and translocation. Oncogene 40 (15), 2756–2771. doi:10.1038/s41388-021-01739-z
Chen, W., Fu, J., Chen, Y., Li, Y., Ning, L., Huang, D., et al. (2021). Circular RNA circKIF4A facilitates the malignant progression and suppresses ferroptosis by sponging miR-1231 and upregulating GPX4 in papillary thyroid cancer. Aging 13 (12), 16500–16512. doi:10.18632/aging.203172
Chen, W., Hoffmann, A. D., Liu, H., and Liu, X. (2018). Organotropism: new insights into molecular mechanisms of breast cancer metastasis. NPJ Precis. Oncol. 2 (1), 4. doi:10.1038/s41698-018-0047-0
Chen X., X., Li, J., Kang, R., Klionsky, D. J., and Tang, D. (2021). Ferroptosis: machinery and regulation. Autophagy 17 (9), 2054–2081. doi:10.1080/15548627.2020.1810918
Chen, Z., Sang, M. X., Geng, C. Z., and Jia, H. Q. (2022c). MicroRNA-409 regulates the proliferation and invasion of breast cancer cell lines by targeting special AT-rich sequence-binding protein 1 (SATB1). Bioengineered 13 (5), 13045–13054. doi:10.1080/21655979.2022.2073320
Chen, Li, Tang (2020). Research progress on the molecular mechanism of non-coding RNA regulating ferroptosis in tumor cells. Life Sciences 32 (10), 9.
Choi, S., An, H. J., Yeo, H. J., Sung, M. J., Oh, J., Lee, K., et al. (2024). MicroRNA-606 inhibits the growth and metastasis of triple-negative breast cancer by targeting Stanniocalcin 1. Oncol. Rep. 51 (1), 2. doi:10.3892/or.2023.8661
Cruz, J. A., and Westhof, E. (2009). The dynamic landscapes of RNA architecture. Cell 136 (4), 604–609. doi:10.1016/j.cell.2009.02.003
Cui, Y., Zhang, Y., Zhao, X., Shao, L., Liu, G., Sun, C., et al. (2021). ACSL4 exacerbates ischemic stroke by promoting ferroptosis-induced brain injury and neuroinflammation. Brain Behav. Immun. 93, 312–321. doi:10.1016/j.bbi.2021.01.003
Davenport, M. L., Davis, M. R., Davenport, B. N., Crossman, D. K., Hall, A., Pike, J., et al. (2022). Suppression of SIN3A by miR-183 promotes breast cancer metastasis. Mol. cancer Res. MCR 20 (6), 883–894. doi:10.1158/1541-7786.MCR-21-0508
de la Ballina, L. R., Cano-Crespo, S., González-Muñoz, E., Bial, S., Estrach, S., Cailleteau, L., et al. (2016). Amino acid transport associated to cluster of differentiation 98 heavy chain (CD98hc) is at the cross-road of oxidative stress and amino acid availability. J. Biol. Chem. 291 (18), 9700–9711. doi:10.1074/jbc.M115.704254
Dixon, S. J. (2017). Ferroptosis: bug or feature? Immunol. Rev. 277 (1), 150–157. doi:10.1111/imr.12533
Dixon, S. J., Lemberg, K. M., Lamprecht, M. R., Skouta, R., Zaitsev, E. M., Gleason, C. E., et al. (2012). Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149 (5), 1060–1072. doi:10.1016/j.cell.2012.03.042
Dixon, S. J., and Olzmann, J. A. (2024). The cell biology of ferroptosis. Nat. Rev. Mol. Cell Biol. 25 (6), 424–442. doi:10.1038/s41580-024-00703-5
Doll, S., Freitas, F. P., Shah, R., Aldrovandi, M., da Silva, M. C., Ingold, I., et al. (2019). FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575 (7784), 693–698. doi:10.1038/s41586-019-1707-0
Doll, S., Proneth, B., Tyurina, Y. Y., Panzilius, E., Kobayashi, S., Ingold, I., et al. (2017). ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 13 (1), 91–98. doi:10.1038/nchembio.2239
Dolma, S., Lessnick, S. L., Hahn, W. C., and Stockwell, B. R. (2003). Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 3 (3), 285–296. doi:10.1016/s1535-6108(03)00050-3
Dong, Q., Yan, L., Xu, Q., Hu, X., Yang, Y., Zhu, R., et al. (2022). Pan-cancer analysis of forkhead box Q1 as a potential prognostic and immunological biomarker. Front. Genet. 13, 944970. doi:10.3389/fgene.2022.944970
Dong, S., Ma, M., Li, M., Guo, Y., Zuo, X., Gu, X., et al. (2021). LncRNA MEG3 regulates breast cancer proliferation and apoptosis through miR-141-3p/RBMS3 axis. Genomics 113 (4), 1689–1704. doi:10.1016/j.ygeno.2021.04.015
Dou, D., Ren, X., Han, M., Xu, X., Ge, X., Gu, Y., et al. (2020). CircUBE2D2 (hsa_circ_0005728) promotes cell proliferation, metastasis and chemoresistance in triple-negative breast cancer by regulating miR-512-3p/CDCA3 axis. Cancer cell Int. 20, 454. doi:10.1186/s12935-020-01547-7
Du, W. W., Fang, L., Yang, W., Wu, N., Awan, F. M., Yang, Z., et al. (2017). Induction of tumor apoptosis through a circular RNA enhancing Foxo3 activity. Cell Death Differ. 24 (2), 357–370. doi:10.1038/cdd.2016.133
Duffy, M. J., McGowan, P. M., and Crown, J. (2012). Targeted therapy for triple-negative breast cancer: where are we? Int. J. Cancer 131 (11), 2471–2477. doi:10.1002/ijc.27632
Fairweather-Tait, S. J. (2001). Iron. J. Nutr. 131 (4 Suppl. l), 1383s–6S. doi:10.1093/jn/131.4.1383S
Fan, D., Ma, Y., Qi, Y., Yang, X., and Zhao, H. (2023). TMEM189 as a target gene of MiR-499a-5p regulates breast cancer progression through the ferroptosis pathway. J. Clin. Biochem. Nutr. 73 (2), 154–160. doi:10.3164/jcbn.22-130
Fan, H., Ai, R., Mu, S., Niu, X., Guo, Z., and Liu, L. (2022). MiR-19a suppresses ferroptosis of colorectal cancer cells by targeting IREB2. Bioengineered 13 (5), 12021–12029. doi:10.1080/21655979.2022.2054194
Fan, X., Liu, F., Wang, X., Wang, Y., Chen, Y., Shi, C., et al. (2024). LncFASA promotes cancer ferroptosis via modulating PRDX1 phase separation. Sci. China Life Sci. 67 (3), 488–503. doi:10.1007/s11427-023-2425-2
Fan, Y., Wang, J., Jin, W., Sun, Y., Xu, Y., Wang, Y., et al. (2021). CircNR3C2 promotes HRD1-mediated tumor-suppressive effect via sponging miR-513a-3p in triple-negative breast cancer. Mol. cancer 20 (1), 25. doi:10.1186/s12943-021-01321-x
Fang, L., Du, W. W., Lyu, J., Dong, J., Zhang, C., Yang, W., et al. (2018). Enhanced breast cancer progression by mutant p53 is inhibited by the circular RNA circ-Ccnb1. Cell Death and Differ. 25 (12), 2195–2208. doi:10.1038/s41418-018-0115-6
Fang, W., Chi, Z., Li, W., Zhang, X., and Zhang, Q. (2019). Comparative study on the toxic mechanisms of medical nanosilver and silver ions on the antioxidant system of erythrocytes: from the aspects of antioxidant enzyme activities and molecular interaction mechanisms. J. Nanobiotechnology 17 (1), 66. doi:10.1186/s12951-019-0502-2
Fang, Y., Wang, L., Wan, C., Sun, Y., Van der Jeught, K., Zhou, Z., et al. (2021). MAL2 drives immune evasion in breast cancer by suppressing tumor antigen presentation. J. Clin. Invest 131 (1), e140837. doi:10.1172/JCI140837
Feral, C. C., Nishiya, N., Fenczik, C. A., Stuhlmann, H., Slepak, M., and Ginsberg, M. H. (2005). CD98hc (SLC3A2) mediates integrin signaling. Proc. Natl. Acad. Sci. U. S. A. 102 (2), 355–360. doi:10.1073/pnas.0404852102
Flohé, L. (2013). The fairytale of the GSSG/GSH redox potential. Biochim. Biophys. Acta 1830 (5), 3139–3142. doi:10.1016/j.bbagen.2012.10.020
Gammella, E., Buratti, P., Cairo, G., and Recalcati, S. (2017). The transferrin receptor: the cellular iron gate. Metallomics 9 (10), 1367–1375. doi:10.1039/c7mt00143f
Gan, R., Yang, Y., Yang, X., Zhao, L., Lu, J., and Meng, Q. H. (2014). Downregulation of miR-221/222 enhances sensitivity of breast cancer cells to tamoxifen through upregulation of TIMP3. Cancer Gene Ther. 21 (7), 290–296. doi:10.1038/cgt.2014.29
Gao, S. L., Fan, Y., Liu, X. D., Liu, W., and Zhao, M. (2022). circ_0089153 exacerbates breast cancer cells proliferation and metastasis via sponging miR-2467-3p/E2F6. Environ. Toxicol. 37 (6), 1458–1471. doi:10.1002/tox.23498
Gao, X., Yang, H., Wu, M., Shi, K., Zhou, C., Peng, J., et al. (2018). Targeting delivery of lidocaine and cisplatin by nanogel enhances chemotherapy and alleviates metastasis. ACS Appl. Mater Interfaces 10 (30), 25228–25240. doi:10.1021/acsami.8b09376
Gao, Y., Wang, Y., Wang, X., Zhao, C., Wang, F., Du, J., et al. (2021). miR-335-5p suppresses gastric cancer progression by targeting MAPK10. Cancer Cell Int. 21 (1), 71. doi:10.1186/s12935-020-01684-z
Gaschler, M. M., and Stockwell, B. R. (2017). Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 482 (3), 419–425. doi:10.1016/j.bbrc.2016.10.086
Ge, X., Lei, S., Wang, P., Wang, W., and Wang, W. (2024). The metabolism-related lncRNA signature predicts the prognosis of breast cancer patients. Sci. Rep. 14 (1), 3500. doi:10.1038/s41598-024-53716-7
Geng, N., Shi, B. J., Li, S. L., Zhong, Z. Y., Li, Y. C., Xua, W. L., et al. (2018). Knockdown of ferroportin accelerates erastin-induced ferroptosis in neuroblastoma cells. Eur. Rev. Med. Pharmacol. Sci. 22 (12), 3826–3836. doi:10.26355/eurrev_201806_15267
Gomez, E., Pritchard, C., and Herbert, T. P. (2002). cAMP-dependent protein kinase and Ca2+ influx through L-type voltage-gated calcium channels mediate Raf-independent activation of extracellular regulated kinase in response to glucagon-like peptide-1 in pancreatic beta-cells. J. Biol. Chem. 277 (50), 48146–48151. doi:10.1074/jbc.M209165200
Gordan, R., Wongjaikam, S., Gwathmey, J. K., Chattipakorn, N., Chattipakorn, S. C., and Xie, L. H. (2018). Involvement of cytosolic and mitochondrial iron in iron overload cardiomyopathy: an update. Heart Fail Rev. 23 (5), 801–816. doi:10.1007/s10741-018-9700-5
Guo, F. X., Wu, Q., Li, P., Zheng, L., Ye, S., Dai, X. Y., et al. (2019). The role of the LncRNA-FA2H-2-MLKL pathway in atherosclerosis by regulation of autophagy flux and inflammation through mTOR-dependent signaling. Cell Death Differ. 26 (9), 1670–1687. doi:10.1038/s41418-018-0235-z
Hangauer, M. J., Viswanathan, V. S., Ryan, M. J., Bole, D., Eaton, J. K., Matov, A., et al. (2017). Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 551 (7679), 247–250. doi:10.1038/nature24297
Hanna, J. A., Wimberly, H., Kumar, S., Slack, F., Agarwal, S., and Rimm, D. L. (2012). Quantitative analysis of microRNAs in tissue microarrays by in situ hybridization. Biotechniques. 52 (4), 235–245. doi:10.2144/000113837
Hansen, C. G., Moroishi, T., and Guan, K. L. (2015). YAP and TAZ: a nexus for Hippo signaling and beyond. Trends Cell Biol. 25 (9), 499–513. doi:10.1016/j.tcb.2015.05.002
Hao, C., Sheng, Z., Wang, W., Feng, R., Zheng, Y., Xiao, Q., et al. (2023). Tumor-derived exosomal miR-148b-3p mediates M2 macrophage polarization via TSC2/mTORC1 to promote breast cancer migration and invasion. Thorac. cancer 14 (16), 1477–1491. doi:10.1111/1759-7714.14891
Hassannia, B., Vandenabeele, P., and Vanden Berghe, T. (2019). Targeting ferroptosis to iron out cancer. Cancer Cell 35 (6), 830–849. doi:10.1016/j.ccell.2019.04.002
Hassannia, B., Wiernicki, B., Ingold, I., Qu, F., Van Herck, S., Tyurina, Y. Y., et al. (2018). Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma. J. Clin. Invest 128 (8), 3341–3355. doi:10.1172/JCI99032
Hata, A., and Kashima, R. (2016). Dysregulation of microRNA biogenesis machinery in cancer. Crit. Rev. Biochem. Mol. Biol. 51 (3), 121–134. doi:10.3109/10409238.2015.1117054
He, G. N., Bao, N. R., Wang, S., Xi, M., Zhang, T. H., and Chen, F. S. (2021). Ketamine induces ferroptosis of liver cancer cells by targeting lncRNA PVT1/miR-214-3p/GPX4. Drug Des. Devel Ther. 15, 3965–3978. doi:10.2147/DDDT.S332847
Hershko, C. (2010). Pathogenesis and management of iron toxicity in thalassemia. Ann. N. Y. Acad. Sci. 1202, 1–9. doi:10.1111/j.1749-6632.2010.05544.x
Hou, Y., Cai, S., Yu, S., and Lin, H. (2021). Metformin induces ferroptosis by targeting miR-324-3p/GPX4 axis in breast cancer. Acta Biochim. Biophys. Sin. (Shanghai) 53 (3), 333–341. doi:10.1093/abbs/gmaa180
Huang, D., Zhu, X., Ye, S., Zhang, J., Liao, J., Zhang, N., et al. (2024). Tumour circular RNAs elicit anti-tumour immunity by encoding cryptic peptides. Nature 625 (7995), 593–602. doi:10.1038/s41586-023-06834-7
Huang, J., Deng, C., Guo, T., Chen, X., Chen, P., Du, S., et al. (2023). Cinobufotalin induces ferroptosis to suppress lung cancer cell growth by lncRNA linc00597/hsa-miR-367-3p/TFRC pathway via resibufogenin. Anticancer Agents Med. Chem. 23 (6), 717–725. doi:10.2174/1871520622666221010092922
Huang, R., Wu, J., Ma, Y., and Kang, K. (2023). Molecular mechanisms of ferroptosis and its role in viral pathogenesis. Viruses 15 (12), 2373. doi:10.3390/v15122373
Huang, X., Wu, J., Wang, Y., Xian, Z., Li, J., Qiu, N., et al. (2023). FOXQ1 inhibits breast cancer ferroptosis and progression via the circ_0000643/miR-153/SLC7A11 axis. Exp. Cell Res. 431 (1), 113737. doi:10.1016/j.yexcr.2023.113737
Humphries, B., Wang, Z., Li, Y., Jhan, J. R., Jiang, Y., and Yang, C. (2017). ARHGAP18 downregulation by miR-200b suppresses metastasis of triple-negative breast cancer by enhancing activation of RhoA. Cancer Res. 77 (15), 4051–4064. doi:10.1158/0008-5472.CAN-16-3141
Jadaliha, M., Gholamalamdari, O., Tang, W., Zhang, Y., Petracovici, A., Hao, Q., et al. (2018). A natural antisense lncRNA controls breast cancer progression by promoting tumor suppressor gene mRNA stability. PLoS Genet. 14 (11), e1007802. doi:10.1371/journal.pgen.1007802
Jeck, W. R., Sorrentino, J. A., Wang, K., Slevin, M. K., Burd, C. E., Liu, J., et al. (2013). Circular RNAs are abundant, conserved, and associated with ALU repeats. Rna 19 (2), 141–157. doi:10.1261/rna.035667.112
Jensen, K., Brusletto, B. S., Aass, H. C., Olstad, O. K., Kierulf, P., and Gautvik, K. M. (2013). Transcriptional profiling of mRNAs and microRNAs in human bone marrow precursor B cells identifies subset- and age-specific variations. PLoS One 8 (7), e70721. doi:10.1371/journal.pone.0070721
Jeong, J., Shin, J. H., Li, W., Hong, J. Y., Lim, J., Hwang, J. Y., et al. (2021). MAL2 mediates the formation of stable HER2 signaling complexes within lipid raft-rich membrane protrusions in breast cancer cells. Cell Rep. 37 (13), 110160. doi:10.1016/j.celrep.2021.110160
Jia, H., Liang, K., Liu, G., Zhang, Z., Shi, Y., Liang, H., et al. (2022). lncRNA DANCR promotes proliferation and metastasis of breast cancer cells through sponging miR-4319 and upregulating VAPB. Cancer biotherapy and Radiopharm. 37 (8), 650–661. doi:10.1089/cbr.2020.3675
Jia, H., Wu, D., Zhang, Z., and Li, S. (2021). Regulatory effect of the MAFG-AS1/miR-150-5p/MYB axis on the proliferation and migration of breast cancer cells. Int. J. Oncol. 58 (1), 33–44. doi:10.3892/ijo.2020.5150
Jiang, X., Stockwell, B. R., and Conrad, M. (2021). Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 22 (4), 266–282. doi:10.1038/s41580-020-00324-8
Jiang, Y., Lin, L., Zhong, S., Cai, Y., Zhang, F., Wang, X., et al. (2019). Overexpression of novel lncRNA NLIPMT inhibits metastasis by reducing phosphorylated glycogen synthase kinase 3β in breast cancer. J. Cell. physiology 234 (7), 10698–10708. doi:10.1002/jcp.27738
Jiawei, W., Xiajun, B., Tian, S., Xuzheng, G., and Zhenwang, Z. (2022). Comprehensive analysis of PLKs expression and prognosis in breast cancer. Cancer Genet. 268-269, 83–92. doi:10.1016/j.cancergen.2022.09.007
Jin, H., Du, W., Huang, W., Yan, J., Tang, Q., Chen, Y., et al. (2021). lncRNA and breast cancer: progress from identifying mechanisms to challenges and opportunities of clinical treatment. Mol. Ther. Nucleic Acids 25, 613–637. doi:10.1016/j.omtn.2021.08.005
Jin, J., Wang, Y., Zheng, D., Liang, M., and He, Q. (2022). A novel identified circular RNA, mmu_mmu_circRNA_0000309, involves in germacrone-mediated improvement of diabetic nephropathy through regulating ferroptosis by targeting miR-188-3p/GPX4 signaling Axis. Antioxidants and redox Signal. 36 (10-12), 740–759. doi:10.1089/ars.2021.0063
Jin, Y., Yang, L., Li, X., and Liu, F. (2020). Circular RNA KIF4A promotes cell migration, invasion and inhibits apoptosis through miR-152/ZEB1 axis in breast cancer. Diagn. Pathol. 15 (1), 55. doi:10.1186/s13000-020-00963-7
Khan, J. S., Hodgson, N., Choi, S., Reid, S., Paul, J. E., Hong, N. J. L., et al. (2019). Perioperative pregabalin and intraoperative lidocaine infusion to reduce persistent neuropathic pain after breast cancer surgery: a multicenter, factorial, randomized, controlled pilot trial. J. Pain 20 (8), 980–993. doi:10.1016/j.jpain.2019.02.010
Kim, R., Taylor, D., Vonderheide, R. H., and Gabrilovich, D. I. (2023). Ferroptosis of immune cells in the tumor microenvironment. Trends Pharmacol. Sci. 44 (8), 542–552. doi:10.1016/j.tips.2023.06.005
Kong, Z., Han, Q., Zhu, B., Wan, L., and Feng, E. (2023). Circ_0069094 regulates malignant phenotype and paclitaxel resistance in breast cancer cells via targeting the miR-136-5p/YWHAZ axis. Thorac. Cancer 14 (19), 1831–1842. doi:10.1111/1759-7714.14928
Koppula, P., Zhuang, L., and Gan, B. (2021). Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 12 (8), 599–620. doi:10.1007/s13238-020-00789-5
Kruszewski, M. (2003). Labile iron pool: the main determinant of cellular response to oxidative stress. Mutat. Res. 531 (1-2), 81–92. doi:10.1016/j.mrfmmm.2003.08.004
Kuhn, H., Humeniuk, L., Kozlov, N., Roigas, S., Adel, S., and Heydeck, D. (2018). The evolutionary hypothesis of reaction specificity of mammalian ALOX15 orthologs. Prog. Lipid Res. 72, 55–74. doi:10.1016/j.plipres.2018.09.002
Kurozumi, S., Inoue, K., Takei, H., Matsumoto, H., Kurosumi, M., Horiguchi, J., et al. (2015). ER, PgR, Ki67, p27(Kip1), and histological grade as predictors of pathological complete response in patients with HER2-positive breast cancer receiving neoadjuvant chemotherapy using taxanes followed by fluorouracil, epirubicin, and cyclophosphamide concomitant with trastuzumab. BMC Cancer 15, 622. doi:10.1186/s12885-015-1641-y
Larraufie, M.-H., Yang, W. S., Jiang, E., Thomas, A. G., Slusher, B. S., and Stockwell, B. R. (2015). Incorporation of metabolically stable ketones into a small molecule probe to increase potency and water solubility. Bioorg. and Med. Chem. Lett. 25 (21), 4787–4792. doi:10.1016/j.bmcl.2015.07.018
Lebedeva, S., Jens, M., Theil, K., Schwanhäusser, B., Selbach, M., Landthaler, M., et al. (2011). Transcriptome-wide analysis of regulatory interactions of the RNA-binding protein HuR. Mol. cell 43 (3), 340–352. doi:10.1016/j.molcel.2011.06.008
Lee, I., Ajay, S. S., Yook, J. I., Kim, H. S., Hong, S. H., Kim, N. H., et al. (2009). New class of microRNA targets containing simultaneous 5'-UTR and 3'-UTR interaction sites. Genome Res. 19 (7), 1175–1183. doi:10.1101/gr.089367.108
Li, H., Li, H. H., Chen, Q., Wang, Y. Y., Fan, C. C., Duan, Y. Y., et al. (2022b). miR-142-5p inhibits cell invasion and migration by targeting DNMT1 in breast cancer. Oncol. Res. 28 (9), 885–897. doi:10.3727/096504021X16274672547967
Li, H., Shi, W., Shen, T., Hui, S., Hou, M., Wei, Z., et al. (2023). Network pharmacology-based strategy for predicting therapy targets of Ecliptae Herba on breast cancer. Med. Baltim. 102 (41), e35384. doi:10.1097/MD.0000000000035384
Li, J., Cao, F., Yin, H. L., Huang, Z. J., Lin, Z. T., Mao, N., et al. (2020). Ferroptosis: past, present and future. Cell Death Dis. 11 (2), 88. doi:10.1038/s41419-020-2298-2
Li, Q., Wang, X., Zhou, L., Jiang, M., Zhong, G., Xu, S., et al. (2021). A positive feedback loop of long noncoding RNA LINC00152 and KLF5 facilitates breast cancer growth. Front. Oncol. 11, 619915. doi:10.3389/fonc.2021.619915
Li, S., Zhou, J., Wang, Z., Wang, P., Gao, X., and Wang, Y. (2018). Long noncoding RNA GAS5 suppresses triple negative breast cancer progression through inhibition of proliferation and invasion by competitively binding miR-196a-5p. Biomed. and Pharmacother. = Biomedecine and Pharmacother. 104, 451–457. doi:10.1016/j.biopha.2018.05.056
Li Y., Y., Ma, H. Y., Hu, X. W., Qu, Y. Y., Wen, X., Zhang, Y., et al. (2020). LncRNA H19 promotes triple-negative breast cancer cells invasion and metastasis through the p53/TNFAIP8 pathway. Cancer cell Int. 20, 200. doi:10.1186/s12935-020-01261-4
Li, Y., Wang, N. X., Yin, C., Jiang, S. S., Li, J. C., and Yang, S. Y. (2022a). RNA editing enzyme ADAR1 regulates METTL3 in an editing dependent manner to promote breast cancer progression via METTL3/ARHGAP5/YTHDF1 Axis. Int. J. Mol. Sci. 23 (17), 9656. doi:10.3390/ijms23179656
Liang, Z., Liu, L., Gao, R., Che, C., and Yang, G. (2022). Downregulation of exosomal miR-7-5p promotes breast cancer migration and invasion by targeting RYK and participating in the atypical WNT signalling pathway. Cell. and Mol. Biol. Lett. 27 (1), 88. doi:10.1186/s11658-022-00393-x
Lin, W., Wang, C., Liu, G., Bi, C., Wang, X., Zhou, Q., et al. (2020). SLC7A11/xCT in cancer: biological functions and therapeutic implications. Am. J. cancer Res. 10 (10), 3106–3126.
Liu, J., Kang, R., and Tang, D. (2022). Signaling pathways and defense mechanisms of ferroptosis. Febs J. 289 (22), 7038–7050. doi:10.1111/febs.16059
Liu, L., Matsunaga, Y., Tsurutani, J., Akashi-Tanaka, S., Masuda, H., Ide, Y., et al. (2020a). BRCAness as a prognostic indicator in patients with early breast cancer. Sci. Rep. 10 (1), 21173. doi:10.1038/s41598-020-78016-8
Liu, Q., Wang, Z., Kong, X., Wang, X., Qi, Y., Gao, R., et al. (2020b). A novel prognostic signature of mRNA-lncRNA in breast cancer. DNA Cell Biol. 39 (4), 671–682. doi:10.1089/dna.2019.5223
López-Coral, A., Del Vecchio, G. J., Chahine, J. J., Kallakury, B. V., and Tuma, P. L. (2020). MAL2-Induced actin-based protrusion formation is anti-oncogenic in hepatocellular carcinoma. Cancers (Basel) 12 (2), 422. doi:10.3390/cancers12020422
Lovatt, M., Adnan, K., Kocaba, V., Dirisamer, M., Peh, G. S. L., and Mehta, J. S. (2020). Peroxiredoxin-1 regulates lipid peroxidation in corneal endothelial cells. Redox Biol. 30, 101417. doi:10.1016/j.redox.2019.101417
Lu, J., Xu, F., and Lu, H. (2020). LncRNA PVT1 regulates ferroptosis through miR-214-mediated TFR1 and p53. Life Sci. 260, 118305. doi:10.1016/j.lfs.2020.118305
Luo, H., and Chen, G. (2023). Neutrophil extracellular traps promote the proliferation, invasion and migration of prostate cancer cells by upregulating IL-8 expression in DU145 human prostate cancer cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 39 (3), 261–267.
Luo, W., Wang, J., Xu, W., Ma, C., Wan, F., Huang, Y., et al. (2021). LncRNA RP11-89 facilitates tumorigenesis and ferroptosis resistance through PROM2-activated iron export by sponging miR-129-5p in bladder cancer. Cell Death Dis. 12 (11), 1043. doi:10.1038/s41419-021-04296-1
Luo, Y., Huang, Q., He, B., Liu, Y., Huang, S., and Xiao, J. (2021). Regulation of ferroptosis by non-coding RNAs in the development and treatment of cancer (Review). Oncol. Rep. 45 (1), 29–48. doi:10.3892/or.2020.7836
Ma, Y., Zhu, Y., Shang, L., Qiu, Y., Shen, N., Wang, J., et al. (2023). LncRNA XIST regulates breast cancer stem cells by activating proinflammatory IL-6/STAT3 signaling. Oncogene 42 (18), 1419–1437. doi:10.1038/s41388-023-02652-3
Maiorino, M., Conrad, M., and Ursini, F. (2018). GPx4, lipid peroxidation, and cell death: discoveries, rediscoveries, and open issues. Antioxid. Redox Signal 29 (1), 61–74. doi:10.1089/ars.2017.7115
Makhoul, I., Atiq, M., Alwbari, A., and Kieber-Emmons, T. (2018). Breast cancer immunotherapy: an update. Breast Cancer (Auckl) 12, 1178223418774802. doi:10.1177/1178223418774802
Mallik, R., and Chowdhury, T. A. (2018). Metformin in cancer. Diabetes Res. Clin. Pract. 143, 409–419. doi:10.1016/j.diabres.2018.05.023
Mao, C., Wang, X., Liu, Y., Wang, M., Yan, B., Jiang, Y., et al. (2018). A G3BP1-interacting lncRNA promotes ferroptosis and apoptosis in cancer via nuclear sequestration of p53. Cancer Res. 78 (13), 3484–3496. doi:10.1158/0008-5472.CAN-17-3454
Mao, R., and Liu, H. (2022). Depletion of mmu_circ_0001751 (circular RNA Carm1) protects against acute cerebral infarction injuries by binding with microRNA-3098-3p to regulate acyl-CoA synthetase long-chain family member 4. Bioengineered 13 (2), 4063–4075. doi:10.1080/21655979.2022.2032971
Martinez-Outschoorn, U. E., Peiris-Pagés, M., Pestell, R. G., Sotgia, F., and Lisanti, M. P. (2017). Cancer metabolism: a therapeutic perspective. Nat. Rev. Clin. Oncol. 14 (1), 11–31. doi:10.1038/nrclinonc.2016.60
Martínez-Reyes, I., Cardona, L. R., Kong, H., Vasan, K., McElroy, G. S., Werner, M., et al. (2020). Mitochondrial ubiquinol oxidation is necessary for tumour growth. Nature 585 (7824), 288–292. doi:10.1038/s41586-020-2475-6
Mathias, C., Muzzi, J. C. D., Antunes, B. B., Gradia, D. F., Castro, M. A. A., and Carvalho de Oliveira, J. (2021). Unraveling immune-related lncRNAs in breast cancer molecular subtypes. Front. Oncol. 11, 692170. doi:10.3389/fonc.2021.692170
Mattick, J. S., and Rinn, J. L. (2015). Discovery and annotation of long noncoding RNAs. Nat. Struct. Mol. Biol. 22 (1), 5–7. doi:10.1038/nsmb.2942
Mercer, T. R., and Mattick, J. S. (2013). Structure and function of long noncoding RNAs in epigenetic regulation. Nat. Struct. Mol. Biol. 20 (3), 300–307. doi:10.1038/nsmb.2480
Miller, T. E., Ghoshal, K., Ramaswamy, B., Roy, S., Datta, J., Shapiro, C. L., et al. (2008). MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27Kip1. J. Biol. Chem. 283 (44), 29897–29903. doi:10.1074/jbc.M804612200
Morales, M., and Xue, X. (2021). Targeting iron metabolism in cancer therapy. Theranostics 11 (17), 8412–8429. doi:10.7150/thno.59092
Mou, Y., Wang, J., Wu, J., He, D., Zhang, C., Duan, C., et al. (2019). Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J. Hematol. Oncol. 12 (1), 34. doi:10.1186/s13045-019-0720-y
Nakamura, T., Naguro, I., and Ichijo, H. (2019). Iron homeostasis and iron-regulated ROS in cell death, senescence and human diseases. Biochim. Biophys. Acta Gen. Subj. 1863 (9), 1398–1409. doi:10.1016/j.bbagen.2019.06.010
Nalla, L. V., Gondaliya, P., Kalia, K., and Khairnar, A. (2022). Targeting specificity protein 1 with miR-128-3p overcomes TGF-β1 mediated epithelial-mesenchymal transition in breast cancer: an in vitro study. Mol. Biol. Rep. 49 (7), 6987–6996. doi:10.1007/s11033-022-07466-2
Nalla, L. V., and Khairnar, A. (2024). Empagliflozin drives ferroptosis in anoikis-resistant cells by activating miR-128-3p dependent pathway and inhibiting CD98hc in breast cancer. Free Radic. Biol. Med. 220, 288–300. doi:10.1016/j.freeradbiomed.2024.05.018
Neumann, C. A., Krause, D. S., Carman, C. V., Das, S., Dubey, D. P., Abraham, J. L., et al. (2003). Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature 424 (6948), 561–565. doi:10.1038/nature01819
Neves, R. A. L., Dummer Meira, D., Poppe, M. L., Correia Casotti, M., do Prado Ventorim, D., Ferreira Figueiredo, A. J., et al. (2023). Biomarkers in breast cancer: an old story with a new end. Genes (Basel) 14 (7), 1364. doi:10.3390/genes14071364
Nik-Zainal, S., Davies, H., Staaf, J., Ramakrishna, M., Glodzik, D., Zou, X., et al. (2016). Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature 534 (7605), 47–54. doi:10.1038/nature17676
Niu, B., Liao, K., Zhou, Y., Wen, T., Quan, G., Pan, X., et al. (2021). Application of glutathione depletion in cancer therapy: enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials 277, 121110. doi:10.1016/j.biomaterials.2021.121110
Novikova, I. V., Hennelly, S. P., Tung, C. S., and Sanbonmatsu, K. Y. (2013). Rise of the RNA machines: exploring the structure of long non-coding RNAs. J. Mol. Biol. 425 (19), 3731–3746. doi:10.1016/j.jmb.2013.02.030
Ouyang, Q., Cui, Y., Yang, S., Wei, W., Zhang, M., Zeng, J., et al. (2020). lncRNA MT1JP suppresses biological activities of breast cancer cells in vitro and in vivo by regulating the miRNA-214/RUNX3 Axis. OncoTargets Ther. 13, 5033–5046. doi:10.2147/OTT.S241503
Pan, C. F., Wei, K., Ma, Z. J., He, Y. Z., Huang, J. J., Guo, Z. Z., et al. (2022). CircP4HB regulates ferroptosis via SLC7A11-mediated glutathione synthesis in lung adenocarcinoma. Transl. lung cancer Res. 11 (3), 366–380. doi:10.21037/tlcr-22-138
Panda, A. C. (2018). Circular RNAs act as miRNA sponges. Adv. Exp. Med. Biol. 1087, 67–79. doi:10.1007/978-981-13-1426-1_6
Park, M. K., Zhang, L., Min, K. W., Cho, J. H., Yeh, C. C., Moon, H., et al. (2021). NEAT1 is essential for metabolic changes that promote breast cancer growth and metastasis. Cell metab. 33 (12), 2380–2397.e9. doi:10.1016/j.cmet.2021.11.011
Pedrera, L., Espiritu, R. A., Ros, U., Weber, J., Schmitt, A., Stroh, J., et al. (2021). Ferroptotic pores induce Ca(2+) fluxes and ESCRT-III activation to modulate cell death kinetics. Cell Death Differ. 28 (5), 1644–1657. doi:10.1038/s41418-020-00691-x
Peng, H. H., and Wen, Y. G. (2020). CircDDX17 acts as a competing endogenous RNA for miR-605 in breast cancer progression. Eur. Rev. Med. Pharmacol. Sci. 24 (12), 6794–6801. doi:10.26355/eurrev_202006_21668
Piccolo, S., Dupont, S., and Cordenonsi, M. (2014). The biology of YAP/TAZ: hippo signaling and beyond. Physiol. Rev. 94 (4), 1287–1312. doi:10.1152/physrev.00005.2014
Porter, N. A., Wolf, R. A., Yarbro, E. M., and Weenen, H. (1979). The autoxidation of arachidonic acid: formation of the proposed SRS-A intermediate. Biochem. Biophys. Res. Commun. 89 (4), 1058–1064. doi:10.1016/0006-291x(79)92115-6
Qi, L., Sun, B., Yang, B., and Lu, S. (2021). circHIPK3 (hsa_circ_0000284) promotes proliferation, migration and invasion of breast cancer cells via miR-326. OncoTargets Ther. 14, 3671–3685. doi:10.2147/OTT.S299190
Qian, J., Lei, X., Sun, Y., Zheng, L., Li, J., Zhang, S., et al. (2021). Long non-coding RNA SNHG8 enhances triple-negative breast cancer cell proliferation and migration by regulating the miR-335-5p/PYGO2 axis. Biol. Direct 16 (1), 13. doi:10.1186/s13062-021-00295-6
Qian, W., Yang, L., Ni, Y., Yin, F., Qin, L., and Yang, Y. (2022). LncRNA LINC01857 reduces metastasis and angiogenesis in breast cancer cells via regulating miR-2052/CENPQ axis. Open Med. Wars. Pol. 17 (1), 1357–1367. doi:10.1515/med-2022-0525
Qiu, X., Wang, Q., Song, H., Shao, D., and Xue, J. (2020). circ_103809 promotes breast cancer progression by regulating the PI3K/AKT signaling pathway. Oncol. Lett. 19 (6), 3725–3730. doi:10.3892/ol.2020.11507
Rahbari, R., Rahimi, K., Rasmi, Y., Khadem-Ansari, M. H., and Abdi, M. (2022). miR-589-5p inhibits cell proliferation by targeting histone deacetylase 3 in triple negative breast cancer. Archives Med. Res. 53 (5), 483–491. doi:10.1016/j.arcmed.2022.06.006
Raizner, A. E. (2019). Coenzyme Q(10). Methodist Debakey Cardiovasc J. 15 (3), 185–191. doi:10.14797/mdcj-15-3-185
Ren, J., Chen, W., Zhou, Y., Sun, J., and Jiang, G. (2024). The novel circRNA circ_0045881 inhibits cell proliferation and invasion by targeting mir-214-3p in triple-negative breast cancer. BMC cancer 24 (1), 278. doi:10.1186/s12885-024-12007-0
Roth, C., Rack, B., Müller, V., Janni, W., Pantel, K., and Schwarzenbach, H. (2010). Circulating microRNAs as blood-based markers for patients with primary and metastatic breast cancer. Breast Cancer Res. 12 (6), R90. doi:10.1186/bcr2766
Saatci, O., Alam, R., Huynh-Dam, K. T., Isik, A., Uner, M., Belder, N., et al. (2023). Targeting LINC00152 activates cAMP/Ca(2+)/ferroptosis axis and overcomes tamoxifen resistance in ER+ breast cancer. bioRxiv, 2023.11.05.565697. doi:10.1101/2023.11.05.565697
Sang, M., Meng, L., Liu, S., Ding, P., Chang, S., Ju, Y., et al. (2018). Circular RNA ciRS-7 maintains metastatic phenotypes as a ceRNA of miR-1299 to target MMPs. Mol. cancer Res. MCR 16 (11), 1665–1675. doi:10.1158/1541-7786.MCR-18-0284
Sang, Y., Chen, B., Song, X., Li, Y., Liang, Y., Han, D., et al. (2019). circRNA_0025202 regulates tamoxifen sensitivity and tumor progression via regulating the miR-182-5p/FOXO3a Axis in breast cancer. Mol. Ther. J. Am. Soc. Gene Ther. 27 (9), 1638–1652. doi:10.1016/j.ymthe.2019.05.011
Sato, H., Fujiwara, K., Sagara, J., and Bannai, S. (1995). Induction of cystine transport activity in mouse peritoneal macrophages by bacterial lipopolysaccharide. Biochem. J. 310, 547–551. doi:10.1042/bj3100547
Schlosser, K., Hanson, J., Villeneuve, P. J., Dimitroulakos, J., McIntyre, L., Pilote, L., et al. (2016). Assessment of circulating LncRNAs under physiologic and pathologic conditions in humans reveals potential limitations as biomarkers. Sci. Rep. 6 (1), 36596. doi:10.1038/srep36596
Schwarzenbach, H., Milde-Langosch, K., Steinbach, B., Müller, V., and Pantel, K. (2012). Diagnostic potential of PTEN-targeting miR-214 in the blood of breast cancer patients. Breast Cancer Res. Treat. 134 (3), 933–941. doi:10.1007/s10549-012-1988-6
Seibt, T. M., Proneth, B., and Conrad, M. (2019). Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic. Biol. and Med. 133, 144–152. doi:10.1016/j.freeradbiomed.2018.09.014
Sengupta, D., Deb, M., Kar, S., Pradhan, N., Parbin, S., Kirtana, R., et al. (2021). Dissecting miRNA facilitated physiology and function in human breast cancer for therapeutic intervention. Semin. Cancer Biol. 72, 46–64. doi:10.1016/j.semcancer.2020.05.017
Shanshan, W., Hongying, M., Jingjing, F., Yiming, Y., Yu, R., and Rui, Y. (2021). CircDTL functions as an oncogene and regulates both apoptosis and ferroptosis in non-small cell lung cancer cells. Front. Genet. 12, 743505. doi:10.3389/fgene.2021.743505
Shen, Z., Song, J., Yung, B. C., Zhou, Z., Wu, A., and Chen, X. (2018). Emerging strategies of cancer therapy based on ferroptosis. Adv. Mater. 30 (12), e1704007. doi:10.1002/adma.201704007
Shi, F., Chen, X., Wang, Y., Xie, Y., Zhong, J., Su, K., et al. (2022). HOTAIR/miR-203/CAV1 crosstalk influences proliferation, migration, and invasion in the breast cancer cell. Int. J. Mol. Sci. 23 (19), 11755. doi:10.3390/ijms231911755
Shi, G., Cheng, Y., Zhang, Y., Guo, R., Li, S., and Hong, X. (2021). Long non-coding RNA LINC00511/miR-150/MMP13 axis promotes breast cancer proliferation, migration and invasion. Biochimica biophysica acta Mol. basis Dis. 1867 (3), 165957. doi:10.1016/j.bbadis.2020.165957
Song, B., Xu, C., Zhang, Y., and Shan, Y. (2023b). Circ_ATAD3B inhibits cell proliferation of breast cancer via mediating the miR-570-3p/MX2 axis. Prev. Med. 173, 107568. doi:10.1016/j.ypmed.2023.107568
Song, R., Guo, P., Ren, X., Zhou, L., Li, P., Rahman, N. A., et al. (2023a). A novel polypeptide CAPG-171aa encoded by circCAPG plays a critical role in triple-negative breast cancer. Mol. cancer 22 (1), 104. doi:10.1186/s12943-023-01806-x
Song, X., Liu, Z., and Yu, Z. (2019). LncRNA NEF is downregulated in triple negative breast cancer and correlated with poor prognosis. Acta biochimica biophysica Sinica 51 (4), 386–392. doi:10.1093/abbs/gmz021
Song, X., Wang, X., Chen, X., Yu, Z., and Zhou, Y. (2024). SRSF1 inhibits ferroptosis and reduces cisplatin chemosensitivity of triple-negative breast cancer cells through the circSEPT9/GCH1 axis. J. proteomics 292, 105055. doi:10.1016/j.jprot.2023.105055
Stoll, L., Rodríguez-Trejo, A., Guay, C., Brozzi, F., Bayazit, M. B., Gattesco, S., et al. (2020). A circular RNA generated from an intron of the insulin gene controls insulin secretion. Nat. Commun. 11, 5611. doi:10.1038/s41467-020-19381-w
Sun, D., Li, Y. C., and Zhang, X. Y. (2021). Lidocaine promoted ferroptosis by targeting miR-382-5p/SLC7A11 Axis in ovarian and breast cancer. Front. Pharmacol. 12, 681223. doi:10.3389/fphar.2021.681223
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Tan, B. S., Yang, M. C., Singh, S., Chou, Y. C., Chen, H. Y., Wang, M. Y., et al. (2019). LncRNA NORAD is repressed by the YAP pathway and suppresses lung and breast cancer metastasis by sequestering S100P. Oncogene 38 (28), 5612–5626. doi:10.1038/s41388-019-0812-8
Tang, D., Chen, X., Kang, R., and Kroemer, G. (2021). Ferroptosis: molecular mechanisms and health implications. Cell Res. 31 (2), 107–125. doi:10.1038/s41422-020-00441-1
Tomita, K., Fukumoto, M., Itoh, K., Kuwahara, Y., Igarashi, K., Nagasawa, T., et al. (2019). MiR-7-5p is a key factor that controls radioresistance via intracellular Fe(2+) content in clinically relevant radioresistant cells. Biochem. Biophys. Res. Commun. 518 (4), 712–718. doi:10.1016/j.bbrc.2019.08.117
Tong, X., Yu, Z., Xing, J., Liu, H., Zhou, S., Huang, Y., et al. (2023). LncRNA HCP5-encoded protein regulates ferroptosis to promote the progression of triple-negative breast cancer. Cancers (Basel) 15 (6), 1880. doi:10.3390/cancers15061880
Torti, S. V., and Torti, F. M. (2020). Iron: the cancer connection. Mol. Asp. Med. 75, 100860. doi:10.1016/j.mam.2020.100860
Toyokuni, S. (2002). Iron and carcinogenesis: from Fenton reaction to target genes. Redox Rep. 7 (4), 189–197. doi:10.1179/135100002125000596
Toyoshima, H., and Hunter, T. (1994). p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 78 (1), 67–74. doi:10.1016/0092-8674(94)90573-8
Tsoi, J., Robert, L., Paraiso, K., Galvan, C., Sheu, K. M., Lay, J., et al. (2018). Multi-stage differentiation defines melanoma subtypes with differential vulnerability to drug-induced iron-dependent oxidative stress. Cancer Cell 33 (5), 890–904. doi:10.1016/j.ccell.2018.03.017
Ursini, F., and Maiorino, M. (2020). Lipid peroxidation and ferroptosis: the role of GSH and GPx4. Free Radic. Biol. Med. 152, 175–185. doi:10.1016/j.freeradbiomed.2020.02.027
Ursini, F., Maiorino, M., and Gregolin, C. (1985). The selenoenzyme phospholipid hydroperoxide glutathione peroxidase. Biochim. Biophys. Acta 839 (1), 62–70. doi:10.1016/0304-4165(85)90182-5
Ursini, F., Maiorino, M., Valente, M., Ferri, L., and Gregolin, C. (1982). Purification from pig liver of a protein which protects liposomes and biomembranes from peroxidative degradation and exhibits glutathione peroxidase activity on phosphatidylcholine hydroperoxides. Biochim. Biophys. Acta 710 (2), 197–211. doi:10.1016/0005-2760(82)90150-3
Vasudevan, S., Tong, Y., and Steitz, J. A. (2007). Switching from repression to activation: microRNAs can up-regulate translation. Science 318 (5858), 1931–1934. doi:10.1126/science.1149460
Viswanathan, V. S., Ryan, M. J., Dhruv, H. D., Gill, S., Eichhoff, O. M., Seashore-Ludlow, B., et al. (2017). Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 547 (7664), 453–457. doi:10.1038/nature23007
Wang, C. J., Zhu, C. C., Xu, J., Wang, M., Zhao, W. Y., Liu, Q., et al. (2019). The lncRNA UCA1 promotes proliferation, migration, immune escape and inhibits apoptosis in gastric cancer by sponging anti-tumor miRNAs. Mol. Cancer 18 (1), 115. doi:10.1186/s12943-019-1032-0
Wang, H., Cheng, Y., Mao, C., Liu, S., Xiao, D., Huang, J., et al. (2021). Emerging mechanisms and targeted therapy of ferroptosis in cancer. Mol. Ther. 29 (7), 2185–2208. doi:10.1016/j.ymthe.2021.03.022
Wang, H., Tan, Z., Hu, H., Liu, H., Wu, T., Zheng, C., et al. (2019). microRNA-21 promotes breast cancer proliferation and metastasis by targeting LZTFL1. BMC Cancer 19 (1), 738. doi:10.1186/s12885-019-5951-3
Wang, H. H., Ma, J. N., and Zhan, X. R. (2021). Circular RNA Circ_0067934 attenuates ferroptosis of thyroid cancer cells by miR-545-3p/slc7a11 signaling. Front. Endocrinol. 12, 670031. doi:10.3389/fendo.2021.670031
Wang, S., Wang, Y., Li, Q., Li, X., and Feng, X. (2022). A novel circular RNA confers trastuzumab resistance in human epidermal growth factor receptor 2-positive breast cancer through regulating ferroptosis. Environ. Toxicol. 37 (7), 1597–1607. doi:10.1002/tox.23509
Wang, S., Wang, Y., Li, Q., Zeng, K., Li, X., and Feng, X. (2023). RUNX1-IT1 favors breast cancer carcinogenesis through regulation of IGF2BP1/GPX4 axis. Discov. Oncol. 14 (1), 42. doi:10.1007/s12672-023-00652-z
Wang, W., Green, M., Choi, J. E., Gijón, M., Kennedy, P. D., Johnson, J. K., et al. (2019). CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 569 (7755), 270–274. doi:10.1038/s41586-019-1170-y
Wang, W. B., Ren, P., Ren, F. H., Huang, M., and Cheng, X. (2021). Circ_0000526 blocks the progression of breast cancer by sponging miR-492. Cancer biotherapy and Radiopharm. 36 (6), 467–476. doi:10.1089/cbr.2019.3513
Wang, Y., Shi, S., Wang, Y., Zhang, X., Liu, X., Li, J., et al. (2022). miR-223-3p targets FBXW7 to promote epithelial-mesenchymal transition and metastasis in breast cancer. Thorac. cancer 13 (3), 474–482. doi:10.1111/1759-7714.14284
Wang, Y., Wu, S., Zhu, X., Zhang, L., Deng, J., Li, F., et al. (2020). LncRNA-encoded polypeptide ASRPS inhibits triple-negative breast cancer angiogenesis. J. Exp. Med. 217 (3), jem.20190950. doi:10.1084/jem.20190950
Wang, Y. W., He, S. J., Feng, X., Cheng, J., Luo, Y. T., Tian, L., et al. (2017). Metformin: a review of its potential indications. Drug Des. Devel Ther. 11, 2421–2429. doi:10.2147/DDDT.S141675
Wang, Z., Chen, X., Liu, N., Shi, Y., Liu, Y., Ouyang, L., et al. (2021). A nuclear long non-coding RNA LINC00618 accelerates ferroptosis in a manner dependent upon apoptosis. Mol. Ther. 29 (1), 263–274. doi:10.1016/j.ymthe.2020.09.024
Wang, Z., Deng, H., Jin, Y., Luo, M., Huang, J., Wang, J., et al. (2023). Circular RNAs: biology and clinical significance of breast cancer. RNA Biol. 20 (1), 859–874. doi:10.1080/15476286.2023.2272468
Wei, X., Tao, S., Mao, H., Zhu, H., Mao, L., Pei, W., et al. (2023). Exosomal lncRNA NEAT1 induces paclitaxel resistance in breast cancer cells and promotes cell migration by targeting miR-133b. Gene 860, 147230. doi:10.1016/j.gene.2023.147230
Wu, M., Xu, L. G., Li, X., Zhai, Z., and Shu, H. B. (2002). AMID, an apoptosis-inducing factor-homologous mitochondrion-associated protein, induces caspase-independent apoptosis. J. Biol. Chem. 277 (28), 25617–25623. doi:10.1074/jbc.M202285200
Xiang, S., Yan, W., Ren, X., Feng, J., and Zu, X. (2024). Role of ferroptosis and ferroptosis-related long non'coding RNA in breast cancer. Cell Mol. Biol. Lett. 29 (1), 40. doi:10.1186/s11658-024-00560-2
Xiao, Y., Ma, D., Yang, Y. S., Yang, F., Ding, J. H., Gong, Y., et al. (2022). Comprehensive metabolomics expands precision medicine for triple-negative breast cancer. Cell Res. 32 (5), 477–490. doi:10.1038/s41422-022-00614-0
Xie, D., Li, S., Wu, T., Wang, X., and Fang, L. (2022c). MiR-181c suppresses triple-negative breast cancer tumorigenesis by targeting MAP4K4. Pathology, Res. Pract. 230, 153763. doi:10.1016/j.prp.2022.153763
Xie, L. H., Fefelova, N., Pamarthi, S. H., and Gwathmey, J. K. (2022). Molecular mechanisms of ferroptosis and relevance to cardiovascular disease. Cells 11 (17), 2726. doi:10.3390/cells11172726
Xie, Q., Zhang, P., Wang, Y., Mei, W., and Zeng, C. (2022). Overcoming resistance to immune checkpoint inhibitors in hepatocellular carcinoma: challenges and opportunities. Front. Oncol. 12, 958720. doi:10.3389/fonc.2022.958720
Xu, Q., Zhou, L., Yang, G., Meng, F., Wan, Y., Wang, L., et al. (2020). CircIL4R facilitates the tumorigenesis and inhibits ferroptosis in hepatocellular carcinoma by regulating the miR-541-3p/GPX4 axis. Cell Biol. Int. 44 (11), 2344–2356. doi:10.1002/cbin.11444
Xu, Z., Jiang, S., Ma, J., Tang, D., Yan, C., and Fang, K. (2021). Comprehensive analysis of ferroptosis-related LncRNAs in breast cancer patients reveals prognostic value and relationship with tumor immune microenvironment. Front. Surg. 8, 742360. doi:10.3389/fsurg.2021.742360
Xu, Z., Wang, X., Sun, W., Xu, F., Kou, H., Hu, W., et al. (2023). RelB-activated GPX4 inhibits ferroptosis and confers tamoxifen resistance in breast cancer. Redox Biol. 68, 102952. doi:10.1016/j.redox.2023.102952
Yadav, P., Sharma, P., Sundaram, S., Venkatraman, G., Bera, A. K., and Karunagaran, D. (2021). SLC7A11/xCT is a target of miR-5096 and its restoration partially rescues miR-5096-mediated ferroptosis and anti-tumor effects in human breast cancer cells. Cancer Lett. 522, 211–224. doi:10.1016/j.canlet.2021.09.033
Yagoda, N., von Rechenberg, M., Zaganjor, E., Bauer, A. J., Yang, W. S., Fridman, D. J., et al. (2007). RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 447 (7146), 864–868. doi:10.1038/nature05859
Yan, S., Wang, W., Zhu, B., Pan, X., Wu, X., and Tao, W. (2020). Construction of nomograms for predicting pathological complete response and tumor shrinkage size in breast cancer. Cancer Manag. Res. 12, 8313–8323. doi:10.2147/CMAR.S270687
Yan, Y., Dalmasso, G., Sitaraman, S., and Merlin, D. (2007). Characterization of the human intestinal CD98 promoter and its regulation by interferon-gamma. Am. J. Physiol. Gastrointest. Liver Physiol. 292 (2), G535–G545. doi:10.1152/ajpgi.00385.2006
Yan, Z., Liang, Z., Luo, K., Yu, L., Chen, C., Yu, M., et al. (2024). METTL3-modified lncRNA DSCAM-AS1 promotes breast cancer progression through inhibiting ferroptosis. J. Bioenerg. Biomembr. 56 (4), 451–459. doi:10.1007/s10863-024-10024-z
Yang, F., Xiao, Y., Ding, J. H., Jin, X., Ma, D., Li, D. Q., et al. (2023). Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy. Cell Metab. 35 (1), 84–100.e8. doi:10.1016/j.cmet.2022.09.021
Yang, H., Wang, Z., Hu, S., Chen, L., Li, W., and Yang, Z. (2023). miRNA-874-3p inhibits the migration, invasion and proliferation of breast cancer cells by targeting VDAC1. Aging (Albany NY) 15 (3), 705–717. doi:10.18632/aging.204474
Yang, J., Zhou, Y., Xie, S., Wang, J., Li, Z., Chen, L., et al. (2021). Metformin induces Ferroptosis by inhibiting UFMylation of SLC7A11 in breast cancer. J. Exp. Clin. Cancer Res. 40 (1), 206. doi:10.1186/s13046-021-02012-7
Yang, K., Li, D., Jia, W., Song, Y., Sun, N., Wang, J., et al. (2022). MiR-379-5p inhibits the proliferation, migration, and invasion of breast cancer by targeting KIF4A. Thorac. cancer 13 (13), 1916–1924. doi:10.1111/1759-7714.14437
Yang, W. S., Kim, K. J., Gaschler, M. M., Patel, M., Shchepinov, M. S., and Stockwell, B. R. (2016). Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. U. S. A. 113 (34), E4966–E4975. doi:10.1073/pnas.1603244113
Yang, W. S., SriRamaratnam, R., Welsch, M. E., Shimada, K., Skouta, R., Viswanathan, V. S., et al. (2014). Regulation of ferroptotic cancer cell death by GPX4. Cell. 156 (1-2), 317–331. doi:10.1016/j.cell.2013.12.010
Yang, Y., Fan, X., Mao, M., Song, X., Wu, P., Zhang, Y., et al. (2017). Extensive translation of circular RNAs driven by N6-methyladenosine. Cell Res. 27 (5), 626–641. doi:10.1038/cr.2017.31
Ye, Q., Wang, X., Yuan, M., Cui, S., Chen, Y., Hu, Z., et al. (2021). miR-219-5p targets TBXT and inhibits breast cancer cell EMT and cell migration and invasion. Biosci. Rep. 41 (8). doi:10.1042/BSR20210318
Yi, Z., Li, Y., Wu, Y., Zeng, B., Li, H., Ren, G., et al. (2020). Circular RNA 0001073 attenuates malignant biological behaviours in breast cancer cell and is delivered by nanoparticles to inhibit mice tumour growth. OncoTargets Ther. 13, 6157–6169. doi:10.2147/OTT.S248822
Yin, C., Zhang, M. M., Wang, G. L., Deng, X. Y., Tu, Z., Jiang, S. S., et al. (2024). Loss of ADAR1 induces ferroptosis of breast cancer cells. Cell Signal 121, 111258. doi:10.1016/j.cellsig.2024.111258
Yotsumoto, S., Muroi, Y., Chiba, T., Ohmura, R., Yoneyama, M., Magarisawa, M., et al. (2017). Hyperoxidation of ether-linked phospholipids accelerates neutrophil extracellular trap formation. Sci. Rep. 7 (1), 16026. doi:10.1038/s41598-017-15668-z
Yuan, C., Yuan, H., Chen, L., Sheng, M., and Tang, W. (2021). A novel three-long noncoding RNA risk score system for the prognostic prediction of triple-negative breast cancer. Biomark. Med. 15 (1), 43–55. doi:10.2217/bmm-2020-0505
Yuan, H., Li, X., Zhang, X., Kang, R., and Tang, D. (2016). Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem. Biophys. Res. Commun. 478 (3), 1338–1343. doi:10.1016/j.bbrc.2016.08.124
Zeng, H., Hou, Y., Zhou, X., Lang, L., Luo, H., Sun, Y., et al. (2022). Cancer-associated fibroblasts facilitate premetastatic niche formation through lncRNA SNHG5-mediated angiogenesis and vascular permeability in breast cancer. Theranostics 12 (17), 7351–7370. doi:10.7150/thno.74753
Zeng, K., He, B., Yang, B. B., Xu, T., Chen, X., Xu, M., et al. (2018). The pro-metastasis effect of circANKS1B in breast cancer. Mol. cancer 17 (1), 160. doi:10.1186/s12943-018-0914-x
Zhai, Z., Mu, T., Zhao, L., Li, Y., Zhu, D., and Pan, Y. (2022). MiR-181a-5p facilitates proliferation, invasion, and glycolysis of breast cancer through NDRG2-mediated activation of PTEN/AKT pathway. Bioengineered 13 (1), 83–95. doi:10.1080/21655979.2021.2006974
Zhang, C., Xu, L., Li, X., Chen, Y., Shi, T., and Wang, Q. (2023b). LINC00460 facilitates cell proliferation and inhibits ferroptosis in breast cancer through the miR-320a/MAL2 Axis. Technol. Cancer Res. Treat. 22, 15330338231164359. doi:10.1177/15330338231164359
Zhang, H., He, Y., Wang, J. X., Chen, M. H., Xu, J. J., Jiang, M. H., et al. (2020). miR-30-5p-mediated ferroptosis of trophoblasts is implicated in the pathogenesis of preeclampsia. Redox Biol. 29, 101402. doi:10.1016/j.redox.2019.101402
Zhang, K., Fan, C., Cai, D., Zhang, Y., Zuo, R., Zhu, L., et al. (2020). Contribution of TGF-beta-mediated NLRP3-HMGB1 activation to tubulointerstitial fibrosis in rat with angiotensin II-induced chronic kidney disease. Front. Cell Dev. Biol. 8, 1. doi:10.3389/fcell.2020.00001
Zhang, L., Sun, D., Zhang, J., and Tian, Y. (2021). Circ-UBR1 facilitates proliferation, metastasis, and inhibits apoptosis in breast cancer by regulating the miR-1299/CCND1 axis. Life Sci. 266, 118829. doi:10.1016/j.lfs.2020.118829
Zhang, M., Zhao, K., Xu, X., Yang, Y., Yan, S., Wei, P., et al. (2018a). A peptide encoded by circular form of LINC-PINT suppresses oncogenic transcriptional elongation in glioblastoma. Nat. Commun. 9 (1), 4475. doi:10.1038/s41467-018-06862-2
Zhang, Q., Jin, X., Shi, W., Chen, X., Pang, W., Yu, X., et al. (2020c). A long non-coding RNA LINC00461-dependent mechanism underlying breast cancer invasion and migration via the miR-144-3p/KPNA2 axis. Cancer cell Int. 20, 137. doi:10.1186/s12935-020-01221-y
Zhang, R., Pan, T., Xiang, Y., Zhang, M., Xie, H., Liang, Z., et al. (2022a). Curcumenol triggered ferroptosis in lung cancer cells via lncRNA H19/miR-19b-3p/FTH1 axis. Bioact. Mater 13, 23–36. doi:10.1016/j.bioactmat.2021.11.013
Zhang, S., Liu, Y., Wang, M., Ponikwicka-Tyszko, D., Ma, W., Krentowska, A., et al. (2023a). Role and mechanism of miR-335-5p in the pathogenesis and treatment of polycystic ovary syndrome. Transl. Res. 252, 64–78. doi:10.1016/j.trsl.2022.07.007
Zhang, Y., Tan, D., Xie, Y., Wu, L., Wu, S., Tang, Y., et al. (2022b). CircEPSTI1 promotes the proliferation of HER2-positive breast cancer cells via circEPSTI1/miR-145/ERBB3 Axis. J. Oncol. 2022, 1028851. doi:10.1155/2022/1028851
Zhang, Z., Sun, L., Zhang, Y., Lu, G., Li, Y., and Wei, Z. (2018b). Long non-coding RNA FEZF1-AS1 promotes breast cancer stemness and tumorigenesis via targeting miR-30a/Nanog axis. J. Cell. physiology 233 (11), 8630–8638. doi:10.1002/jcp.26611
Zhao, C., Ling, X., Li, X., Hou, X., and Zhao, D. (2019). MicroRNA-138-5p inhibits cell migration, invasion and EMT in breast cancer by directly targeting RHBDD1. Breast cancer Tokyo, Jpn. 26 (6), 817–825. doi:10.1007/s12282-019-00989-w
Zhao, F. L., Hu, G. D., Wang, X. F., Zhang, X. H., Zhang, Y. K., and Yu, Z. S. (2012). Serum overexpression of microRNA-10b in patients with bone metastatic primary breast cancer. J. Int. Med. Res. 40 (3), 859–866. doi:10.1177/147323001204000304
Zheng, R., Lin, C., Mao, Y., and Jin, F. (2023a). miR-761-hepcidin/Gpx4 pathway contribute to unexplained liver dysfunction in polycystic ovary syndrome by regulating liver iron overload and ferroptosis. Gynecol. Endocrinol. 39 (1), 2166483. doi:10.1080/09513590.2023.2166483
Zheng, S., Hu, L., Song, Q., Shan, Y., Yin, G., Zhu, H., et al. (2021). miR-545 promotes colorectal cancer by inhibiting transferring in the non-normal ferroptosis signaling. Aging (Albany NY) 13 (24), 26137–26147. doi:10.18632/aging.203801
Zheng, S., Li, M., Miao, K., and Xu, H. (2020a). lncRNA GAS5-promoted apoptosis in triple-negative breast cancer by targeting miR-378a-5p/SUFU signaling. J. Cell. Biochem. 121 (3), 2225–2235. doi:10.1002/jcb.29445
Zheng, W., Wang, X., Yu, Y., Ji, C., and Fang, L. (2023b). CircRNF10-DHX15 interaction suppressed breast cancer progression by antagonizing DHX15-NF-κB p65 positive feedback loop. Cell. and Mol. Biol. Lett. 28 (1), 34. doi:10.1186/s11658-023-00448-7
Zheng, X., Huang, M., Xing, L., Yang, R., Wang, X., Jiang, R., et al. (2020b). The circRNA circSEPT9 mediated by E2F1 and EIF4A3 facilitates the carcinogenesis and development of triple-negative breast cancer. Mol. cancer 19 (1), 73. doi:10.1186/s12943-020-01183-9
Zhong, S., Xu, H., Wang, D., Yang, S., Li, H., Zhang, H., et al. (2024). circNFIB decreases synthesis of arachidonic acid and inhibits breast tumor growth and metastasis. Eur. J. Pharmacol. 963, 176221. doi:10.1016/j.ejphar.2023.176221
Zhou, H., Gan, M., Jin, X., Dai, M., Wang, Y., Lei, Y., et al. (2022a). miR-382 inhibits breast cancer progression and metastasis by affecting the M2 polarization of tumor-associated macrophages by targeting PGC-1α. Int. J. Oncol. 61 (4), 126. doi:10.3892/ijo.2022.5416
Zhou, R., Luo, Z., Yin, G., Yu, L., and Zhong, H. (2022b). MiR-556-5p modulates migration, invasion, and epithelial-mesenchymal transition in breast cancer cells via targeting PTHrP. J. Mol. histology 53 (2), 297–308. doi:10.1007/s10735-021-10056-4
Zou, Y., Palte, M. J., Deik, A. A., Li, H., Eaton, J. K., Wang, W., et al. (2019). A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat. Commun. 10 (1), 1617. doi:10.1038/s41467-019-09277-9
Keywords: breast cancer, ferroptosis, noncoding RNAs, miRNAs, CircRNAs, lncRNAs
Citation: Liu W, Jiang C, Ma Y, Wang W, Peng J, Ma W, Xu S and Wu D (2024) The impact of ferroptosis and ferroptosis-related non-coding RNAs on breast cancer progression. Front. Cell Dev. Biol. 12:1506492. doi: 10.3389/fcell.2024.1506492
Received: 05 October 2024; Accepted: 10 December 2024;
Published: 23 December 2024.
Edited by:
José Díaz-Chávez, Instituto Nacional de Cancerología (INCAN), MexicoReviewed by:
Josephine Ataa Hinneh, University of California San Francisco, United StatesCopyright © 2024 Liu, Jiang, Ma, Wang, Peng, Ma, Xu and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Duoming Wu, OTkzNDY1NUBxcS5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.