- Department of Hematology, Erasmus MC Cancer Institute, Rotterdam, Netherlands
The correct maintenance and differentiation of hematopoietic stem cells (HSC) in bone marrow is vital for the maintenance and operation of the human blood system. GATA2 plays a critical role in the maintenance of HSCs and the specification of HSCs into the different hematopoietic lineages, highlighted by the various defects observed in patients with heterozygous mutations in GATA2, resulting in cytopenias, bone marrow failure and increased chance of myeloid malignancy, termed GATA2 deficiency syndrome. Despite this, the mechanisms underlying GATA2 deficiency syndrome remain to be elucidated. The detailed description of how GATA2 regulates HSC maintenance and blood lineage determination is crucial to unravel the pathogenesis of GATA2 deficiency syndrome. In this review, we summarize current advances in elucidating the role of GATA2 in hematopoietic cell fate determination and discuss the challenges of modeling GATA2 deficiency syndrome.
1 Introduction
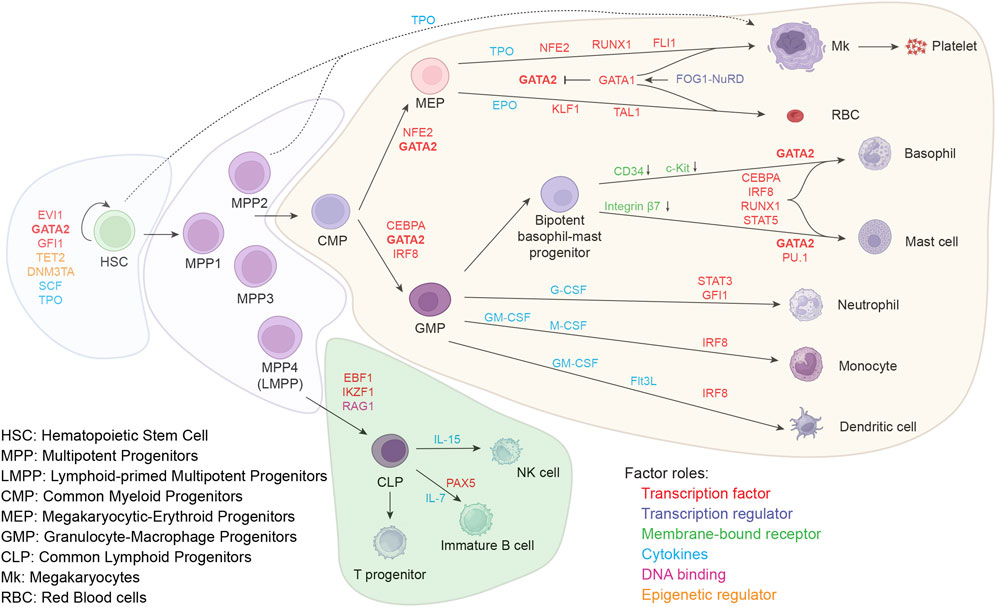
The adult hematopoietic system is derived from hematopoietic stem cells (HSCs) situated within the bone marrow (BM). According to Waddington’s epigenetic theory, various blood cell types originate from unstable stem/progenitor cells and eventually fall into a stable cell fate development track (Waddington, 1957; Ladewig et al., 2013) producing myeloid and lymphoid cells for immunity, erythrocytes for oxygen and carbon dioxide transport and platelets for coagulation. The process of hematopoietic lineage formation resembles a branching tree structure (Figure 1). Within the human bone marrow, the apex point of this classical branching structure is self-renewing HSCs which are typically characterized by the phenotype CD49f+CD90+CD45RA–CD34+CD38–LIN– (Notta et al., 2011).
Numerous genetic mutations result in hematopoietic disorders with unbalanced lineage output, such as RUNX1 mutations, leading to familial platelet disorder (Preudhomme et al., 2009), IRF8 mutations resulting in mononuclear phagocytes-related human primary immunodeficiencies (Hambleton et al., 2011), and mutations in ELANE or HAX1 resulting in severe congenital neutropenia (Ye et al., 2011). A prime example is GATA2 deficiency syndrome (Hahn et al., 2011; Hsu et al., 2011; Ostergaard et al., 2011; Spinner et al., 2014; Calvo and Hickstein, 2023). GATA2 deficiency syndrome, caused by germline mutations in the hematopoietic transcription factor GATA2, stands out because multiple lineages can be affected and patients often present with monocytopenia, B cell deficiency, NK (natural killer) cell deficiency and Dendritic Cell deficiency (Dickinson et al., 2011; Novakova et al., 2016). Neutropenia also occurs in GATA2 deficiency patients (Pasquet et al., 2013) and inversions of the CD4/CD8 T cell ratio have been reported (Mutsaers et al., 2013; Ganapathi et al., 2015), indicating that GATA2 plays a crucial role as a key component within the BM hematopoietic hierarchy, orchestrating the differentiation and maintenance of diverse hematopoietic cell lineages. Furthermore, GATA2 deficiency syndrome patients have a high predisposition to develop (pediatric) myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML) with a median age of onset of 17 years (Wlodarski et al., 2016; Homan et al., 2021), however, before the onset of malignancy, the disease is also life-threatening due to anemia, bleeding disorders, or immunodeficiency with nontuberculous mycobacterial infections (NTM), fungal infections, and human papillomavirus (HPV) infections (Spinner et al., 2014; Ganapathi et al., 2015; Calvo and Hickstein, 2023). Therefore, it is vital to understand the role of GATA2 in the molecular determinants of hematopoietic cell fate.
A schematic representation of the classical tree-like hematopoiesis model shows formation of the various lineages in human bone marrow. The HSC population forms the apex of this hierarchical model, and differentiates into distinct lineages. Important modulators of the lineage choices are depicted, such as transcription factors, transcription regulators, membrane-bound receptors, cytokines, and epigenetic regulators.
2 The role of GATA2 in HSC self-renewal and differentiation
To preserve the hematopoietic system, HSCs are required to self-renew. To preserve the self-renewal capacity of HSCs in the BM microenvironment, a variety of extracellular and intracellular factors must provide support. Extrinsically, different cellular factors, such as stem cell factor (SCF) and thrombopoietin (TPO), organize a coordinated extracellular microenvironment to preserve the self-renewal and maintenance of HSCs (Stoffel et al., 1999; Ema et al., 2000; Fox et al., 2002; Yoshihara et al., 2007; Mendelson and Frenette, 2014; Kokkaliaris et al., 2016). Intrinsically, the self-renewal of HSCs is influenced by multiple transcription factors, including GATA2, GFI1, and EVI1, and epigenetic regulatory molecules, such as TET2 and DNM3TA (Zhu and Emerson, 2002; Hock et al., 2004; Huck et al., 2014; Jeong et al., 2018; Xavier-Ferrucio and Krause, 2018; Aljoufi et al., 2022). GATA2 has various roles in supporting the maintenance of adult HSC characteristics. Complete knockout of Gata2 in mice results in apoptosis of HSCs (Tsai et al., 1994; de Pater et al., 2013; Gao et al., 2013). In proliferating HSCs, Gata2 expression is activated by EVI1 and it was shown that haploinsufficiency of Gata2 impairs cell cycle in mice (Ling et al., 2004; Yuasa et al., 2005). Interestingly, a Gata2 reporter mouse model showed that all HSCs have intermediate levels of Gata2 and that Gata2 is variable in multipotent hematopoietic progenitor cells, suggesting that different levels of Gata2 influence lineage determination (Kaimakis et al., 2016). Interestingly, Gata2 protein levels were observed to be constantly fluctuating in embryonic definitive HSPC formation during the endothelial-to-hematopoietic transition (EHT), indicating that Gata2 expression is a dynamic process in HSPC generation, likely required for normal lineage differentiation. Gata2 heterozygous animals displayed reduced Gata2 protein fluctuations and this may be the underlying cause of the lineage differentiation defects (Eich et al., 2018). Together, this shows that the gene dosage of Gata2 in embryonic and adult HSPCs is crucial for normal lineage differentiation.
As HSCs differentiate into various hematopoietic lineages, they receive extrinsic and intrinsic signals that prompt specialization towards specific blood cell lineages, resulting in the gradual reduction of self-renewal and multi-potency. Extrinsically, cytokines, including Flt3L, SCF, granulocyte colony-stimulating factor (G-CSF), interleukin-1 (IL-1), interleukin-3 (IL-3), interleukin-6 (IL-6), and interleukin-11 (IL-11), coordinate the development of multipotent progenitors (MPPs) from HSCs. SCF expression can be detected in several niche cells, including osteoblasts, endothelial cells and LepR+ perivascular stromal cells, suggesting the importance of the microenvironment for HSC maintenance and differentiation (Ding et al., 2012; Zhou et al., 2014; Zhou et al., 2017).
MPPs are heterogeneous with distinct transcriptomic characteristics. Combined single-cell barcoding and transcriptional analysis reported that MPPs in mice could be further defined as MPP1, MPP2, MPP3, and MPP4, which showed different features and lineage bias through cell fate decisions (Rodriguez-Fraticelli et al., 2018). The first lineage priming separates myeloid and lymphoid differentiation from erythroid lineage differentiation (Notta et al., 2016; Belluschi et al., 2018). MPPs are gradually directed to the myeloid and lymphoid lineages (Velten et al., 2017). Upregulation of Rag1, Ikzf1, and Ebf1 in the MPP population will lead to lymphoid bias, while the upregulation of Cebpa and Irf8 will lead to myeloid bias (Wolfler et al., 2010; Pietras et al., 2015; Lenaerts et al., 2022). Although differentiation does not occur in a clear step-wise manner, several progenitors like Lymphoid-Primed Multipotent Progenitors (LMPPs), Common Myeloid Progenitors (CMPs) and Common Lymphoid Progenitors (CLPs) can be recognized and will be discussed as such.
2.1 Erythroid differentiation
Megakaryocytes (Mk) and erythrocytes are the first lineage to bifurcate from MPPs driven by the lineage-priming module of GATA2-NFE2 (Sanjuan-Pla et al., 2013; Belluschi et al., 2018) and are generated from megakaryocyte-erythroid progenitors (MEPs). EPO induces the specialization of MEPs to erythroid cells (Li et al., 2014). As development progresses, the size of erythroid cells gradually decreases, the nucleus gradually condenses, and terminally enucleates to form mature red blood cells (Sankaran et al., 2012; Wang S. et al., 2022b; Soboleva and Miharada, 2022). GATA1 plays a vital role in erythropoiesis as it is related to essential erythrocyte functions, including heme synthesis, globin synthesis/switch, and enucleation. As reported, GATA1 interacts with all known erythrocyte development-related genes (Ferreira et al., 2005; Ludwig et al., 2022).
Downregulation of GATA2 is an essential signal for Mk and erythroid lineage commitment. Downregulation of GATA2 results in a chromatin occupancy switch from GATA2 bound loci to GATA1 together with FOG1 bound loci. This change in chromatin occupation, termed “GATA factor switching,” is indispensable for differentiation towards Mk/erythrocytes and blocks mast cell differentiation (Tsai and Orkin, 1997; Grass et al., 2003; Anguita et al., 2004; Dore et al., 2012).
GATA1 is involved in the precise downregulation of GATA2 expression. GATA2 expression is promoted by the direct binding of GATA2 itself to the upstream WGATAR motif (Grass et al., 2003). During erythroid development, upregulation of GATA1 leads to the recruitment of FOG1 and NuRD, forming the GATA1-FOG1-NuRD complex that acts to repress GATA2 transcription through WGATAR motif occupation. As a result, the GATA2 level is gradually reduced alongside the increase in GATA1 expression during erythropoiesis (Ferreira et al., 2007; Gao et al., 2010; Gregory et al., 2010; Mancini et al., 2012).
For Mk maturation and platelet release, TPO induces the specialization of MEPs to Mks (Ng et al., 2012). During Mk development, the cell size continues to increase, while DNA replicates, but does not undergo mitosis. Eventually, this forms a large and lobulated mature Mk, which then releases platelets into the circulation. For Mk maturation, GATA1 and FOG1 (ZFPM1) can mediate the expression of the Mk marker CD41 (Mancini et al., 2012; Gekas and Graf, 2013). NFE2, FLI1, and RUNX1 are also critical for the terminal maturation of Mks (Zang et al., 2016). Transplantations in mice has further clarified the lineage specification of the erythroid and megakaryocyte lineage, indicating that TPO induces direct Mk development from HSCs, bypassing other hierarchical progenitors (Sanjuan-Pla et al., 2013). Although the downregulation of GATA2 is required for normal Mk differentiation, atypical Mk were observed in BM from germline GATA2 mutated patients (Ganapathi et al., 2015). This could point to a defect in correct downregulation of GATA2 in GATA2 deficiency patients. In a zebrafish model for Gata2 deficiency, such a mechanism was observed, where heterozygous loss of Gata2b (orthologue of GATA2) resulted in dysplastic erythroid lineage cells caused by excess of open chromatin at the Gata2b locus (Gioacchino et al., 2021b).
2.2 Myeloid differentiation
Common myeloid progenitors (CMPs) have the capacity to form CFU-GEMM (colony-forming unit-granulocyte erythroid macrophage megakaryocyte) in colony-forming assays under the influence of granulocyte-macrophage colony-stimulating factor (GM-CSF) and G-CSF (Pietras et al., 2015; Regan-Komito et al., 2020).
Throughout the progression from MPPs to CMPs, cytokines such as Flt3L, SCF, and IL-3 continue to sustain the proliferative capacity of progenitor cells. Under the mediation of SCF and interleukin-4 (IL-4), CMPs undergo differentiation into mast cells (Okayama and Kawakami, 2006). The development of basophils is facilitated by the sustained activity of GM-CSF and IL-3. Under the influence of GM-SCF and IL-3, CMPs develop into a mature Basophil. Single cell research showed that the differentiation of basophils and mast cells is closely linked and they share a bipotent basophil-mast cell progenitor (Hamey et al., 2021; Wanet et al., 2021; Miyake et al., 2023). In these progenitors, loss of CD34 and downregulation of c-Kit indicate differentiation in the direction of basophils, while loss of integrin β7 in c-Kit+ cells indicate differentiation in the direction of mast cells. The cooperation between GATA2 and PU.1 stimulates the lineage commitment of mast cells (Walsh et al., 2002). Additionally, several transcription factors, including CEBPA, IRF8, GATA2, RUNX1, and STAT5, play a critical role in the maturation of mast cells and basophils (Li et al., 2015; Sasaki et al., 2015). The highest expression of GATA2 in the hematopoietic system is detected in basophils and specifically the GATA2-STAT5 axis is critical for both mast cell and basophil differentiation (Zon et al., 1991; Li et al., 2015).
Monocytes and granulocytes are derived from the same progenitors, GMPs, downstream of CMPs (Rodrigues et al., 2008; Guilliams et al., 2018). The cytokines, SCF, IL-3, GM-CSF, and M-CSF, are all equally important for the differentiation of GMPs (Metcalf, 2008; Ushach and Zlotnik, 2016). M-CSF and GM-CSF induces monocyte/macrophage specialization from GMPs, while G-CSF induces neutrophil lineage specialization from GMPs via STAT3 signaling (Semerad et al., 2002; Irandoust et al., 2007; Ushach and Zlotnik, 2016; Kawano et al., 2017). The generation of dendritic cells from bone marrow progenitors is highly reliant on Flt3/Flt3L and is distinct from the further differentiation of monocytes into dendritic cells (Guilliams et al., 2014; Murphy and Murphy, 2022). Downstream of cytokines, key lineage-restricted transcription factors are critical for the hematopoietic cell fate determination, such as Irf8 (monocytes/dendritic cells) and Gfi1 (neutrophils) (Wei et al., 2008; Olsson et al., 2016; Sichien et al., 2016; Murakami et al., 2021). Additionally, heterozygous Gata2 mutated mice displayed GMP defects. It was shown that GATA2 plays a critical regulatory role in GMP function through the GATA2-HES1 signaling axis (Rodrigues et al., 2008).
The critical role that GATA2 plays in GMP formation, myeloid differentiation and maturation easily explains the regulatory mechanisms behind the dendritic cell deficiency, monocytopenia, and neutropenia frequently observed in patients that have been diagnosed with GATA2 deficiency syndromes (Hsu et al., 2011; Spinner et al., 2014; Calvo and Hickstein, 2023). Therefore, it is surprising that these phenotypes are not easily modeled in mice. This could be due to the absence of secondary injuries like infections or the fact that mice are bred in a congenic background. Interestingly, these cytopenic phenotypes have been modeled using zebrafish, where homozygous deletion of Gata2b leads to neutropenia (Gioacchino et al., 2021a; Avagyan et al., 2021), and loss of the intronic enhancer of Gata2a results in monocytopenia and neutropenia (Dobrzycki et al., 2020; Mahony et al., 2023), providing direct insights into the molecular effects of GATA2 mutation in blood lineage differentiation in the hematopoietic system. This, however, does not explain why only specific lineages are affected in zebrafish, e.g., the monocyte lineage in Gata2a enhancer mutant zebrafish, while this lineage is unaffected in Gata2b mutant zebrafish. This suggests that GATA2 is not only required for the GMP cell state, but also plays a role in the lineage differentiation choice these cells make.
2.3 Lymphoid differentiation
The adaptive immune system is indispensable for protection from invasion of pathogens, by recognition of non-self. The lymphoid lineage is derived from the common lymphoid progenitor (CLP) and this cell gives rise to natural killer (NK) cells, the B cell lineage and T cell lineage. The upregulation of CD122, a receptor for interleukin-15 (IL-15) in NK cell progenitors underscores the pivotal role IL-15 plays in orchestrating essential processes such as proliferation, metabolism, and survival throughout NK cell differentiation (Huntington et al., 2007; Carotta et al., 2011; Anton et al., 2015). Recently, the importance of the GATA2-TGF-b1 axis in regulating NK cell development was reported. In this axis, GATA2 controls the production of TGF-b1 in NK cells, showing the influence of GATA2 on NK formation and explaining the phenotype seem in patients (Wang D. et al., 2022a).
IL-7 acts as the primary cytokine of B cell lineage differentiation in fetal and adult stages in mice, although IL-7 independent B cell differentiation is described in human, highly reliant on FLT3 ligand (Carvalho et al., 2001; Jensen et al., 2008; von Muenchow et al., 2016). Besides cytokines, intracellular factors will also facilitate B lymphopoiesis. The simultaneous expression of Lhx2, Hox9 and Runx1 could drive B lineage fate commitment using pluripotent stem cells (PSCs) as cell source (Zhang et al., 2022). Bone marrow is the primary development location of immature B cells. Subsequently immature B cells can give rise to secondary B cell development in secondary lymphoid organs, like the spleen and tissue lymph nodes, where immature B cells continuously develop into naïve mature B cells (Mueller and Germain, 2009). In secondary lymphoid organs, naïve mature B cells differentiate into plasma cells (PCs), germinal center (GC) B cells, and GC-independent memory B cells (MBCs) by antigen receptor signaling in combination with T follicular helper cells (Ochiai et al., 2013; Krautler et al., 2017; Ise et al., 2018). PAX5 is a pivotal transcription factor for B lineage decision, but shows downregulation during PC generation (Chan et al., 2017; Calderon et al., 2021). Furthermore, high level of Irf4 is required for PC differentiation in mice (Ochiai et al., 2013). The differentiation of GC B cell can also be initiated by the dynamic expression of Irf4, while GC B cell generated from naïve B would develop into various B cell subpopulations like memory B and long-lived plasma cells undergoing complicated primary and secondary immune response (Ochiai et al., 2013; Akkaya et al., 2020).
Progenitor-T cells are double negative (DN) for CD4 and CD8 and can be divided into several well-defined cell stages orderly following the expression of CD44 and CD25: DN1 with CD44+ CD25-, DN2 with CD44+ CD25+, DN3 with CD44-CD25+, DN4 with CD44- CD25- (Olariu et al., 2021). These progenitor-T cell subpopulations are transcriptionally and functionally distinct. Proliferation mainly occurs in DN1 and DN2, while T cell receptor gene arrangement starts from DN3 (Olariu et al., 2021). A single-cell study in mice indicated that the “early T cell precursor”-DN2 population is characterized by the expression of Mpo and Bcl11b and gives rise in the middle stage of DN2, while in the DN3 population the expression of Flt3, Kit, and Spi1 is absent (Zhou et al., 2019). Subsequently, Naïve T population that double CD4 and CD8 positive cells are generated from the DN4 subpopulation. By expressing T cell receptor, alpha/beta T cells acquire maturation (the formation of CD4+ T or CD8+ T) in the thymus (Miller, 1961), then act as various types of effector T cells in the peripheral blood system (Fang et al., 2018).
The role of GATA2 in lymphoid lineage differentiation is poorly described. However, GATA2 deficiency patients do present with B/NK lymphopenia and inversions of the CD4/CD8 T cell ratio have been observed (Mutsaers et al., 2013; Ganapathi et al., 2015; Calvo and Hickstein, 2023). So far, there is some evidence that GATA2 plays a role in T cell development in a mouse study showing that loss of the intronic enhancer leads to defects in MPP3 resulting in defective T cell development (You et al., 2022). Interestingly, GATA2 plays a key role in lymphatic vessel and valve formation through binding with the key lymphatic transcriptional regulator Prox1, Foxc2, and Neatc1 in mice (Kazenwadel et al., 2012; Yamazaki et al., 2014; Kazenwadel et al., 2015). But if and how this influences the CD4/CD8 ratio is unclear. B cell lineage differentiation defects were observed in a zebrafish model for GATA2 deficiency syndrome after deletion of Gata2b (Avagyan et al., 2021; Gioacchino et al., 2021b). Gata2b deficiency resulted in increased lymphoid differentiation, but incomplete B cell differentiation due to a loss of B cell lineage transcription factor accessibility. What the underlying molecular mechanism is, is still under investigation.
3 The challenges of modelling defects in cell fate determination of GATA2 deficiency syndrome
Human primary cells remain a treasured resource and are widely used to understand the molecular processes underlying hematopoietic cell fate. For instance single cell RNA sequencing from primary patient samples showed clear lineage differentiation defects that reflect the defects observed in patients due to increases in Mk/erythroid priming genes such as GATA1 and decreases in myeloid priming genes and lymphoid priming genes such as SPI1 originating in the HSPC population (Wu et al., 2020). Furthermore, recent methylation data from GATA2 deficiency patient samples clearly distinguished symptomatic and asymptomatic patients from healthy donors and highlighted the changes in methylation that underlie leukemia development in these patients (Marin-Bejar et al., 2023).
Unfortunately, due to genetic heterogeneity and the inability to genetically alter these cells, a true comparative study remains impossible with primary cells. Therefore, there will always be a need for model systems to study human disease. Current zebrafish and mouse models of GATA2 deficiency syndrome were only able to partially phenocopy the lineage differentiation defects observed in patients (Dobrzycki et al., 2020; Abdelfattah et al., 2021; Gioacchino et al., 2021a; Avagyan et al., 2021; Gioacchino et al., 2021b; You et al., 2022; Mahony et al., 2023). This could be due to significant disparities between animal models and humans concerning adult size, aging and niche components. The differences may be caused by variations in the quantities of hematopoietic progenitor cells (HPCs) and osteoblasts between these species (Jung et al., 2005; Asada et al., 2015). Differences in population sizes may have impact on the concentration of signaling molecules in human and mouse bone marrow. Furthermore, there are significant differences in cytokine release by the various niche components and differences in cytokine requirements of HSPCs between mouse and human (Scheerlinck, 2014).
To solve these barriers, ex vivo human cell models can be used like induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs). In iPSCs and ESCs the embryonic hematopoiesis can be simulated, but up to a limited extent. The three waves of embryonic hematopoiesis, from 1) early primitive hematopoiesis, to 2) definitive progenitor hematopoiesis, to 3) definitive HSC formation can only be partially simulated (Sturgeon et al., 2014; Ng et al., 2016; Dzierzak and Bigas, 2018), and the formation of definitive HSCs has not been documented to date. A clear advantage of these models is the fact that these cell models can easily be genetically altered. Atkins et al. also utilized both human ESCs and iPSCs to understand human primitive embryonic hematopoiesis and further detailed the developmental mechanisms of erythroid-myeloid progenitor and lymphoid lineages (Atkins et al., 2022). iPSCs have been used to investigate lineage development (Carcamo-Orive et al., 2017), of B cell (Zhang et al., 2022), T cell (Wang Z. et al., 2022c), NK cell (Zhu et al., 2020; Woan et al., 2021), erythroid lineage (Xin et al., 2021) and myeloid lineage (Mulero-Navarro et al., 2015), providing insights into the molecular mechanisms of various lineages decisions, cellular maturation and cell function. Besides modelling normal hematopoiesis, pluripotent stem cells have been used to study malignant hematopoiesis. Patient-specific iPSCs have been used to understand the mechanisms of leukemic transformation, as well as screening patient-specific drugs (Turhan et al., 2019; Olofsen et al., 2020; Bigas et al., 2022; Olofsen et al., 2023). It must be noted that iPSC of patients with GATA2 mutations showed only marginal differences. Specifically, GATA2 patient-specific iPSCs exhibited nuanced differentiation phenotypes dependent upon the tissue which the iPSCs were derived from. Hematopoietic maturation was reduced from iPSC where GATA2 was mutated using CRISPR/Cas9. This heterogeneity in differentiation outcomes hampers the investigation of the role of GATA2 in lineage differentiation using this model system (Jung et al., 2018).
In addition to employing human cell models, the utilization of humanized animal models represent a valuable method to investigate the functional role of GATA2 in hematopoietic lineage determination. The most common humanized animal model to study hematopoiesis is the NSG immunodeficient mouse model, which allows us to study the mechanism of hematopoietic lineage determination with human cells in vivo (Adigbli et al., 2020). By xenotransplantation, it is possible to trace the differentiation of HSPCs carrying GATA2 mutations. However, as previously elucidated, it is crucial to consider the impact of microenvironmental components on hematopoiesis. Although the differentiation of human HSPCs can be activated in mouse bone marrow by the expression of human cytokines, the biological difference between mouse and human should be considered and may not represent the best model to study lineage differentiation defects.
Another complication in the study of GATA2 deficiency syndrome is the vast variety between patients. Some patients remain asymptomatic, while others suffer from immune deficiencies and yet others develop myeloid malignancies at an early age. Important considerations are the many different mutation types that are found between these patients, but also the environmental factors that contribute to our health, i.e., secondary injuries like infection. Inflammation has been recognized as driver of leukemogenesis and could contribute to disease progression and the variety observed between these patients (Essers et al., 2009; Rodriguez-Meira et al., 2023). A more likely model for GATA2 deficiency may thus be the current mouse models with an addition of a secondary injury like transplantation or stimulation with LPS, know to induce inflammation (Abdelfattah et al., 2021).
4 Conclusion and discussion
GATA2 has a pivotal role in HSC self-renewal and hematopoietic lineage determination. The precise expression regulation of GATA2 has a profound impact on hematopoietic development, as high expression of GATA2 is required for HSC self-renewal and maintenance, while procedural downregulation is imperative to facilitate downstream lineage differentiation. Recent advances in the field in terms of new animal models to understand the precise role of GATA2 in lineage differentiation can significantly contribute to the development of treatments for the life-threatening cytopenias from which the majority of GATA2 deficiency syndrome patients suffer (Gioacchino, et al., 2021a; Gioacchino et al., 2021b; Mahony et al., 2023). In recent years, the employment of single cell sequencing technologies resulted in remarkable progress in the comprehension of the molecular regulatory processes governing hematopoietic cell fate determination (Ranzoni et al., 2021; Zeller et al., 2023; Zhang et al., 2023). These technological advancements have supported and continue to support the deconstruction of the functions of GATA2 in hematopoiesis, and the pathophysiological mechanisms behind GATA2 deficiency syndrome. The precise mechanism behind GATA2 deficiency-related immunodeficiency, the variation between patients and the progression to myeloid leukemia remains to be elucidated. Furthermore, considering the different genetic backgrounds and inflammatory burden, caution should be exercised when addressing research questions and conclusions between human and animal models. Thus, there is a need to continue the development of animal or human cell-based research models.
Author contributions
IP wrote the manuscript, WZ and EP conceived and wrote the manuscript. IP and WZ contributed the figure. All authors contributed to the article and approved the submitted version.
Funding
EP is supported by the Junior and Senior research grants of the European Hematology Association (EHA) and a Dutch Cancer Society (KWF) grant number 15340.
Acknowledgments
Figure is created using BioRender.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelfattah, A., Hughes-Davies, A., Clayfield, L., Menendez-Gonzalez, J. B., Almotiri, A., Alotaibi, B., et al. (2021). Gata2 haploinsufficiency promotes proliferation and functional decline of hematopoietic stem cells with myeloid bias during aging. Blood Adv., 5(20), 4285–4290. doi:10.1182/bloodadvances.2021004726
Adigbli, G., Menoret, S., Cross, A. R., Hester, J., Issa, F., and Anegon, I. (2020). Humanization of immunodeficient animals for the modeling of transplantation, graft versus host disease, and regenerative medicine. Transplantation, 104(11), 2290–2306. doi:10.1097/TP.0000000000003177
Akkaya, M., Kwak, K., and Pierce, S. K. (2020). B cell memory: building two walls of protection against pathogens. Nat. Rev. Immunol. 20 (4), 229–238. doi:10.1038/s41577-019-0244-2
Aljoufi, A., Zhang, C., Ropa, J., Chang, W., Palam, L. R., Cooper, S., et al. (2022). Physioxia-induced downregulation of Tet2 in hematopoietic stem cells contributes to enhanced self-renewal. Blood 140 (11), 1263–1277. doi:10.1182/blood.2022015499
Anguita, E., Hughes, J., Heyworth, C., Blobel, G. A., Wood, W. G., and Higgs, D. R. (2004). Globin gene activation during haemopoiesis is driven by protein complexes nucleated by GATA-1 and GATA-2. EMBO J. 23 (14), 2841–2852. doi:10.1038/sj.emboj.7600274
Anton, O. M., Vielkind, S., Peterson, M. E., Tagaya, Y., and Long, E. O. (2015). NK cell proliferation induced by IL-15 transpresentation is negatively regulated by inhibitory receptors. J. Immunol. 195 (10), 4810–4821. doi:10.4049/jimmunol.1500414
Asada, N., Sato, M., and Katayama, Y. (2015). Communication of bone cells with hematopoiesis, immunity and energy metabolism. Bonekey Rep. 4, 748. doi:10.1038/bonekey.2015.117
Atkins, M. H., Scarfo, R., McGrath, K. E., Yang, D., Palis, J., Ditadi, A., et al. (2022). Modeling human yolk sac hematopoiesis with pluripotent stem cells. J. Exp. Med. 219 (3). doi:10.1084/jem.20211924
Avagyan, S., Weber, M. C., Ma, S., Prasad, M., Mannherz, W. P., Yang, S., et al. (2021). Single-cell ATAC-seq reveals GATA2-dependent priming defect in myeloid and a maturation bottleneck in lymphoid lineages. Blood Adv. 5 (13), 2673–2686. doi:10.1182/bloodadvances.2020002992
Belluschi, S., Calderbank, E. F., Ciaurro, V., Pijuan-Sala, B., Santoro, A., Mende, N., et al. (2018). Myelo-lymphoid lineage restriction occurs in the human haematopoietic stem cell compartment before lymphoid-primed multipotent progenitors. Nat. Commun. 9 (1), 4100. doi:10.1038/s41467-018-06442-4
Bigas, A., Galan Palma, L., Kartha, G. M., and Giorgetti, A. (2022). Using pluripotent stem cells to understand normal and leukemic hematopoietic development. Stem Cells Transl. Med. 11 (11), 1123–1134. doi:10.1093/stcltm/szac071
Calderon, L., Schindler, K., Malin, S. G., Schebesta, A., Sun, Q., Schwickert, T., et al. (2021). Pax5 regulates B cell immunity by promoting PI3K signaling via PTEN down-regulation. Sci. Immunol. 6 (61). doi:10.1126/sciimmunol.abg5003
Calvo, K. R., and Hickstein, D. D. (2023). The spectrum of GATA2 deficiency syndrome. Blood 141 (13), 1524–1532. doi:10.1182/blood.2022017764
Carcamo-Orive, I., Hoffman, G. E., Cundiff, P., Beckmann, N. D., D'Souza, S. L., Knowles, J. W., et al. (2017). Analysis of transcriptional variability in a large human iPSC library reveals genetic and non-genetic determinants of heterogeneity. Cell Stem Cell 20 (4), 518–532. doi:10.1016/j.stem.2016.11.005
Carotta, S., Pang, S. H., Nutt, S. L., and Belz, G. T. (2011). Identification of the earliest NK-cell precursor in the mouse BM. Blood 117 (20), 5449–5452. doi:10.1182/blood-2010-11-318956
Carvalho, T. L., Mota-Santos, T., Cumano, A., Demengeot, J., and Vieira, P. (2001). Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7(-/)- mice. J. Exp. Med. 194 (8), 1141–1150. doi:10.1084/jem.194.8.1141
Chan, L. N., Chen, Z., Braas, D., Lee, J. W., Xiao, G., Geng, H., et al. (2017). Metabolic gatekeeper function of B-lymphoid transcription factors. Nature 542 (7642), 479–483. doi:10.1038/nature21076
de Pater, E., Kaimakis, P., Vink, C. S., Yokomizo, T., Yamada-Inagawa, T., van der Linden, R., et al. (2013). Gata2 is required for HSC generation and survival. J. Exp. Med. 210 (13), 2843–2850. doi:10.1084/jem.20130751
Dickinson, R. E., Griffin, H., Bigley, V., Reynard, L. N., Hussain, R., Haniffa, M., et al. (2011). Exome sequencing identifies GATA-2 mutation as the cause of dendritic cell, monocyte, B and NK lymphoid deficiency. Blood 118 (10), 2656–2658. doi:10.1182/blood-2011-06-360313
Ding, L., Saunders, T. L., Enikolopov, G., and Morrison, S. J. (2012). Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481 (7382), 457–462. doi:10.1038/nature10783
Dobrzycki, T., Mahony, C. B., Krecsmarik, M., Koyunlar, C., Rispoli, R., Peulen-Zink, J., et al. (2020). Deletion of a conserved Gata2 enhancer impairs haemogenic endothelium programming and adult Zebrafish haematopoiesis. Commun. Biol. 3 (1), 71. doi:10.1038/s42003-020-0798-3
Dore, L. C., Chlon, T. M., Brown, C. D., White, K. P., and Crispino, J. D. (2012). Chromatin occupancy analysis reveals genome-wide GATA factor switching during hematopoiesis. Blood 119 (16), 3724–3733. doi:10.1182/blood-2011-09-380634
Dzierzak, E., and Bigas, A. (2018). Blood development: hematopoietic stem cell dependence and independence. Cell Stem Cell 22 (5), 639–651. doi:10.1016/j.stem.2018.04.015
Eich, C., Arlt, J., Vink, C. S., Solaimani Kartalaei, P., Kaimakis, P., Mariani, S. A., et al. (2018). In vivo single cell analysis reveals Gata2 dynamics in cells transitioning to hematopoietic fate. J. Exp. Med. 215 (1), 233–248. doi:10.1084/jem.20170807
Ema, H., Takano, H., Sudo, K., and Nakauchi, H. (2000). In vitro self-renewal division of hematopoietic stem cells. J. Exp. Med. 192 (9), 1281–1288. doi:10.1084/jem.192.9.1281
Essers, M. A., Offner, S., Blanco-Bose, W. E., Waibler, Z., Kalinke, U., Duchosal, M. A., et al. (2009). IFNalpha activates dormant haematopoietic stem cells in vivo. Nature 458 (7240), 904–908. doi:10.1038/nature07815
Fang, P., Li, X., Dai, J., Cole, L., Camacho, J. A., Zhang, Y., et al. (2018). Immune cell subset differentiation and tissue inflammation. J. Hematol. Oncol. 11 (1), 97. doi:10.1186/s13045-018-0637-x
Ferreira, R., Ohneda, K., Yamamoto, M., and Philipsen, S. (2005). GATA1 function, a paradigm for transcription factors in hematopoiesis. Mol. Cell Biol. 25 (4), 1215–1227. doi:10.1128/MCB.25.4.1215-1227.2005
Ferreira, R., Wai, A., Shimizu, R., Gillemans, N., Rottier, R., von Lindern, M., et al. (2007). Dynamic regulation of Gata factor levels is more important than their identity. Blood 109 (12), 5481–5490. doi:10.1182/blood-2006-11-060491
Fox, N., Priestley, G., Papayannopoulou, T., and Kaushansky, K. (2002). Thrombopoietin expands hematopoietic stem cells after transplantation. J. Clin. Invest. 110 (3), 389–394. doi:10.1172/JCI15430
Ganapathi, K. A., Townsley, D. M., Hsu, A. P., Arthur, D. C., Zerbe, C. S., Cuellar-Rodriguez, J., et al. (2015). GATA2 deficiency-associated bone marrow disorder differs from idiopathic aplastic anemia. Blood 125 (1), 56–70. doi:10.1182/blood-2014-06-580340
Gao, X., Johnson, K. D., Chang, Y. I., Boyer, M. E., Dewey, C. N., Zhang, J., et al. (2013). Gata2 cis-element is required for hematopoietic stem cell generation in the mammalian embryo. J. Exp. Med. 210 (13), 2833–2842. doi:10.1084/jem.20130733
Gao, Z., Huang, Z., Olivey, H. E., Gurbuxani, S., Crispino, J. D., and Svensson, E. C. (2010). FOG-1-mediated recruitment of NuRD is required for cell lineage re-enforcement during haematopoiesis. EMBO J. 29 (2), 457–468. doi:10.1038/emboj.2009.368
Gekas, C., and Graf, T. (2013). CD41 expression marks myeloid-biased adult hematopoietic stem cells and increases with age. Blood 121 (22), 4463–4472. doi:10.1182/blood-2012-09-457929
Gioacchino, E., Koyunlar, C., Zink, J., de Looper, H., de Jong, M., Dobrzycki, T., et al. (2021a). Essential role for Gata2 in modulating lineage output from hematopoietic stem cells in zebrafish. Blood Adv. 5 (13), 2687–2700. doi:10.1182/bloodadvances.2020002993
Gioacchino, E., Zhang, W., Koyunlar, C., Zink, J., de Looper, H., Gussinklo, K. J., et al. (2021b). GATA2 haploinsufficiency causes an epigenetic feedback mechanism resulting in myeloid and erythroid dysplasi. BioRxiv. doi:10.1101/2021.10.29.466416
Grass, J. A., Boyer, M. E., Pal, S., Wu, J., Weiss, M. J., and Bresnick, E. H. (2003). GATA-1-dependent transcriptional repression of GATA-2 via disruption of positive autoregulation and domain-wide chromatin remodeling. Proc. Natl. Acad. Sci. U. S. A. 100 (15), 8811–8816. doi:10.1073/pnas.1432147100
Gregory, G. D., Miccio, A., Bersenev, A., Wang, Y., Hong, W., Zhang, Z., et al. (2010). FOG1 requires NuRD to promote hematopoiesis and maintain lineage fidelity within the megakaryocytic-erythroid compartment. Blood 115 (11), 2156–2166. doi:10.1182/blood-2009-10-251280
Guilliams, M., Ginhoux, F., Jakubzick, C., Naik, S. H., Onai, N., Schraml, B. U., et al. (2014). Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat. Rev. Immunol. 14 (8), 571–578. doi:10.1038/nri3712
Guilliams, M., Mildner, A., and Yona, S. (2018). Developmental and functional heterogeneity of monocytes. Immunity 49 (4), 595–613. doi:10.1016/j.immuni.2018.10.005
Hahn, C. N., Chong, C. E., Carmichael, C. L., Wilkins, E. J., Brautigan, P. J., and Li, X. C. (2011). Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat. Genet. 43 (10), 1012–1017. doi:10.1038/ng.913
Hambleton, S., Salem, S., Bustamante, J., Bigley, V., Boisson-Dupuis, S., Azevedo, J., et al. (2011). IRF8 mutations and human dendritic-cell immunodeficiency. N. Engl. J. Med. 365 (2), 127–138. doi:10.1056/NEJMoa1100066
Hamey, F. K., Lau, W. W. Y., Kucinski, I., Wang, X., Diamanti, E., Wilson, N. K., et al. (2021). Single-cell molecular profiling provides a high-resolution map of basophil and mast cell development. Allergy 76 (6), 1731–1742. doi:10.1111/all.14633
Hock, H., Hamblen, M. J., Rooke, H. M., Schindler, J. W., Saleque, S., Fujiwara, Y., et al. (2004). Gfi-1 restricts proliferation and preserves functional integrity of haematopoietic stem cells. Nature 431 (7011), 1002–1007. doi:10.1038/nature02994
Homan, C. C., Venugopal, P., Arts, P., Shahrin, N. H., Feurstein, S., Rawlings, L., et al. (2021). GATA2 deficiency syndrome: a decade of discovery. Hum. Mutat. 42 (11), 1399–1421. doi:10.1002/humu.24271
Hsu, A. P., Sampaio, E. P., Khan, J., Calvo, K. R., Lemieux, J. E., Patel, S. Y., et al. (2011). Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood 118 (10), 2653–2655. doi:10.1182/blood-2011-05-356352
Huck, V., Schneider, M. F., Gorzelanny, C., and Schneider, S. W. (2014). The various states of von Willebrand factor and their function in physiology and pathophysiology. Thromb. Haemost. 111 (4), 598–609. doi:10.1160/TH13-09-0800
Huntington, N. D., Puthalakath, H., Gunn, P., Naik, E., Michalak, E. M., Smyth, M. J., et al. (2007). Interleukin 15-mediated survival of natural killer cells is determined by interactions among Bim, Noxa and Mcl-1. Nat. Immunol. 8 (8), 856–863. doi:10.1038/ni1487
Irandoust, M. I., Aarts, L. H., Roovers, O., Gits, J., Erkeland, S. J., and Touw, I. P. (2007). Suppressor of cytokine signaling 3 controls lysosomal routing of G-CSF receptor. EMBO J. 26 (7), 1782–1793. doi:10.1038/sj.emboj.7601640
Ise, W., Fujii, K., Shiroguchi, K., Ito, A., Kometani, K., Takeda, K., et al. (2018). T follicular helper cell-germinal center B cell interaction strength regulates entry into plasma cell or recycling germinal center cell fate. Immunity 48 (4), 702–715 e704. doi:10.1016/j.immuni.2018.03.027
Jensen, C. T., Kharazi, S., Boiers, C., Cheng, M., Lubking, A., Sitnicka, E., et al. (2008). FLT3 ligand and not TSLP is the key regulator of IL-7-independent B-1 and B-2 B lymphopoiesis. Blood 112 (6), 2297–2304. doi:10.1182/blood-2008-04-150508
Jeong, M., Park, H. J., Celik, H., Ostrander, E. L., Reyes, J. M., Guzman, A., et al. (2018). Loss of Dnmt3a immortalizes hematopoietic stem cells in vivo. Cell Rep. 23 (1), 1–10. doi:10.1016/j.celrep.2018.03.025
Jung, M., Cordes, S., Zou, J., Yu, S. J., Guitart, X., Hong, S. G., et al. (2018). GATA2 deficiency and human hematopoietic development modeled using induced pluripotent stem cells. Blood Adv. 2 (23), 3553–3565. doi:10.1182/bloodadvances.2018017137
Jung, Y., Wang, J., Havens, A., Sun, Y., Wang, J., Jin, T., et al. (2005). Cell-to-cell contact is critical for the survival of hematopoietic progenitor cells on osteoblasts. Cytokine 32 (3-4), 155–162. doi:10.1016/j.cyto.2005.09.001
Kaimakis, P., de Pater, E., Eich, C., Solaimani Kartalaei, P., Kauts, M. L., Vink, C. S., et al. (2016). Functional and molecular characterization of mouse Gata2-independent hematopoietic progenitors. Blood 127 (11), 1426–1437. doi:10.1182/blood-2015-10-673749
Kawano, Y., Fukui, C., Shinohara, M., Wakahashi, K., Ishii, S., Suzuki, T., et al. (2017). G-CSF-induced sympathetic tone provokes fever and primes antimobilizing functions of neutrophils via PGE2. Blood 129 (5), 587–597. doi:10.1182/blood-2016-07-725754
Kazenwadel, J., Betterman, K. L., Chong, C. E., Stokes, P. H., Lee, Y. K., Secker, G. A., et al. (2015). GATA2 is required for lymphatic vessel valve development and maintenance. J. Clin. Invest. 125 (8), 2979–2994. doi:10.1172/JCI78888
Kazenwadel, J., Secker, G. A., Liu, Y. J., Rosenfeld, J. A., Wildin, R. S., Cuellar-Rodriguez, J., et al. (2012). Loss-of-function germline GATA2 mutations in patients with MDS/AML or MonoMAC syndrome and primary lymphedema reveal a key role for GATA2 in the lymphatic vasculature. Blood 119 (5), 1283–1291. doi:10.1182/blood-2011-08-374363
Kokkaliaris, K. D., Drew, E., Endele, M., Loeffler, D., Hoppe, P. S., Hilsenbeck, O., et al. (2016). Identification of factors promoting ex vivo maintenance of mouse hematopoietic stem cells by long-term single-cell quantification. Blood 128 (9), 1181–1192. doi:10.1182/blood-2016-03-705590
Krautler, N. J., Suan, D., Butt, D., Bourne, K., Hermes, J. R., Chan, T. D., et al. (2017). Differentiation of germinal center B cells into plasma cells is initiated by high-affinity antigen and completed by Tfh cells. J. Exp. Med. 214 (5), 1259–1267. doi:10.1084/jem.20161533
Ladewig, J., Koch, P., and Brustle, O. (2013). Leveling Waddington: the emergence of direct programming and the loss of cell fate hierarchies. Nat. Rev. Mol. Cell Biol. 14 (4), 225–236. doi:10.1038/nrm3543
Lenaerts, A., Kucinski, I., Deboutte, W., Derecka, M., Cauchy, P., Manke, T., et al. (2022). EBF1 primes B-lymphoid enhancers and limits the myeloid bias in murine multipotent progenitors. J. Exp. Med. 219 (11). doi:10.1084/jem.20212437
Li, J., Hale, J., Bhagia, P., Xue, F., Chen, L., Jaffray, J., et al. (2014). Isolation and transcriptome analyses of human erythroid progenitors: BFU-E and CFU-E. Blood 124 (24), 3636–3645. doi:10.1182/blood-2014-07-588806
Li, Y., Qi, X., Liu, B., and Huang, H. (2015). The STAT5-GATA2 pathway is critical in basophil and mast cell differentiation and maintenance. J. Immunol. 194 (9), 4328–4338. doi:10.4049/jimmunol.1500018
Ling, K. W., Ottersbach, K., van Hamburg, J. P., Oziemlak, A., Tsai, F. Y., Orkin, S. H., et al. (2004). GATA-2 plays two functionally distinct roles during the ontogeny of hematopoietic stem cells. J. Exp. Med. 200 (7), 871–882. doi:10.1084/jem.20031556
Ludwig, L. S., Lareau, C. A., Bao, E. L., Liu, N., Utsugisawa, T., Tseng, A. M., et al. (2022). Congenital anemia reveals distinct targeting mechanisms for master transcription factor GATA1. Blood 139 (16), 2534–2546. doi:10.1182/blood.2021013753
Mahony, C. B., Copper, L., Vrljicak, P., Noyvert, B., Constantinidou, C., Browne, S., et al. (2023). Lineage skewing and genome instability underlie marrow failure in a zebrafish model of GATA2 deficiency. Cell Rep. 42 (6), 112571. doi:10.1016/j.celrep.2023.112571
Mancini, E., Sanjuan-Pla, A., Luciani, L., Moore, S., Grover, A., Zay, A., et al. (2012). FOG-1 and GATA-1 act sequentially to specify definitive megakaryocytic and erythroid progenitors. EMBO J. 31 (2), 351–365. doi:10.1038/emboj.2011.390
Marin-Bejar, O., Romero-Moya, D., Rodriguez-Ubreva, J., Distefano, M., Lessi, F., and Aretini, P. (2023). Epigenome profiling reveals aberrant DNA methylation signature in GATA2 deficiency. Haematologica. doi:10.3324/haematol.2022.282305
Mendelson, A., and Frenette, P. S. (2014). Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat. Med. 20 (8), 833–846. doi:10.1038/nm.3647
Metcalf, D. (2008). Hematopoietic cytokines. Blood 111 (2), 485–491. doi:10.1182/blood-2007-03-079681
Miller, J. F. (1961). Immunological function of the thymus. Lancet 2 (7205), 748–749. doi:10.1016/s0140-6736(61)90693-6
Miyake, K., Ito, J., Nakabayashi, J., Shichino, S., Ishiwata, K., and Karasuyama, H. (2023). Single cell transcriptomics clarifies the basophil differentiation trajectory and identifies pre-basophils upstream of mature basophils. Nat. Commun. 14 (1), 2694. doi:10.1038/s41467-023-38356-1
Mueller, S. N., and Germain, R. N. (2009). Stromal cell contributions to the homeostasis and functionality of the immune system. Nat. Rev. Immunol. 9 (9), 618–629. doi:10.1038/nri2588
Mulero-Navarro, S., Sevilla, A., Roman, A. C., Lee, D. F., and D'Souza, S. L. (2015). Myeloid dysregulation in a human induced pluripotent stem cell model of PTPN11-associated juvenile myelomonocytic leukemia. Cell Rep. 13 (3), 504–515. doi:10.1016/j.celrep.2015.09.019
Murakami, K., Sasaki, H., Nishiyama, A., Kurotaki, D., Kawase, W., Ban, T., et al. (2021). A RUNX-CBFbeta-driven enhancer directs the Irf8 dose-dependent lineage choice between DCs and monocytes. Nat. Immunol. 22 (3), 301–311. doi:10.1038/s41590-021-00871-y
Murphy, T. L., and Murphy, K. M. (2022). Dendritic cells in cancer immunology. Cell Mol. Immunol. 19 (1), 3–13. doi:10.1038/s41423-021-00741-5
Mutsaers, P. G., van de Loosdrecht, A. A., Tawana, K., Bodor, C., Fitzgibbon, J., and Menko, F. H. (2013). Highly variable clinical manifestations in a large family with a novel GATA2 mutation. Leukemia 27 (11), 2247–2248. doi:10.1038/leu.2013.105
Ng, A. P., Kauppi, M., Metcalf, D., Di Rago, L., Hyland, C. D., and Alexander, W. S. (2012). Characterization of thrombopoietin (TPO)-responsive progenitor cells in adult mouse bone marrow with in vivo megakaryocyte and erythroid potential. Proc. Natl. Acad. Sci. U. S. A. 109 (7), 2364–2369. doi:10.1073/pnas.1121385109
Ng, E. S., Azzola, L., Bruveris, F. F., Calvanese, V., Phipson, B., Vlahos, K., et al. (2016). Differentiation of human embryonic stem cells to HOXA(+) hemogenic vasculature that resembles the aorta-gonad-mesonephros. Nat. Biotechnol. 34 (11), 1168–1179. doi:10.1038/nbt.3702
Notta, F., Doulatov, S., Laurenti, E., Poeppl, A., Jurisica, I., and Dick, J. E. (2011). Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science 333 (6039), 218–221. doi:10.1126/science.1201219
Notta, F., Zandi, S., Takayama, N., Dobson, S., Gan, O. I., Wilson, G., et al. (2016). Distinct routes of lineage development reshape the human blood hierarchy across ontogeny. Science 351 (6269), aab2116. doi:10.1126/science.aab2116
Novakova, M., Zaliova, M., Sukova, M., Wlodarski, M., Janda, A., Fronkova, E., et al. (2016). Loss of B cells and their precursors is the most constant feature of GATA-2 deficiency in childhood myelodysplastic syndrome. Haematologica 101 (6), 707–716. doi:10.3324/haematol.2015.137711
Ochiai, K., Maienschein-Cline, M., Simonetti, G., Chen, J., Rosenthal, R., Brink, R., et al. (2013). Transcriptional regulation of germinal center B and plasma cell fates by dynamical control of IRF4. Immunity 38 (5), 918–929. doi:10.1016/j.immuni.2013.04.009
Okayama, Y., and Kawakami, T. (2006). Development, migration, and survival of mast cells. Immunol. Res. 34 (2), 97–115. doi:10.1385/IR:34:2:97
Olariu, V., Yui, M. A., Krupinski, P., Zhou, W., Deichmann, J., Andersson, E., et al. (2021). Multi-scale dynamical modeling of T cell development from an early thymic progenitor state to lineage commitment. Cell Rep. 34 (2), 108622. doi:10.1016/j.celrep.2020.108622
Olofsen, P. A., Bosch, D. A., de Looper, H. W. J., van Strien, P. M. H., Hoogenboezem, R. M., Roovers, O., et al. (2023). Truncated CSF3 receptors induce pro-inflammatory responses in severe congenital neutropenia. Br. J. Haematol. 200 (1), 79–86. doi:10.1111/bjh.18477
Olofsen, P. A., Fatrai, S., van Strien, P. M. H., Obenauer, J. C., de Looper, H. W. J., Hoogenboezem, R. M., et al. (2020). Malignant transformation involving CXXC4 mutations identified in a leukemic progression model of severe congenital neutropenia. Cell Rep. Med. 1 (5), 100074. doi:10.1016/j.xcrm.2020.100074
Olsson, A., Venkatasubramanian, M., Chaudhri, V. K., Aronow, B. J., Salomonis, N., Singh, H., et al. (2016). Single-cell analysis of mixed-lineage states leading to a binary cell fate choice. Nature 537 (7622), 698–702. doi:10.1038/nature19348
Ostergaard, P., Simpson, M. A., Connell, F. C., Steward, C. G., Brice, G., Woollard, W. J., et al. (2011). Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nat. Genet. 43 (10), 929–931. doi:10.1038/ng.923
Pasquet, M., Bellanne-Chantelot, C., Tavitian, S., Prade, N., Beaupain, B., Larochelle, O., et al. (2013). High frequency of GATA2 mutations in patients with mild chronic neutropenia evolving to MonoMac syndrome, myelodysplasia, and acute myeloid leukemia. Blood 121 (5), 822–829. doi:10.1182/blood-2012-08-447367
Pietras, E. M., Reynaud, D., Kang, Y. A., Carlin, D., Calero-Nieto, F. J., Leavitt, A. D., et al. (2015). Functionally distinct subsets of lineage-biased multipotent progenitors control blood production in normal and regenerative conditions. Cell Stem Cell 17 (1), 35–46. doi:10.1016/j.stem.2015.05.003
Preudhomme, C., Renneville, A., Bourdon, V., Philippe, N., Roche-Lestienne, C., Boissel, N., et al. (2009). High frequency of RUNX1 biallelic alteration in acute myeloid leukemia secondary to familial platelet disorder. Blood 113 (22), 5583–5587. doi:10.1182/blood-2008-07-168260
Ranzoni, A. M., Tangherloni, A., Berest, I., Riva, S. G., Myers, B., Strzelecka, P. M., et al. (2021). Integrative single-cell RNA-seq and ATAC-seq analysis of human developmental hematopoiesis. Cell Stem Cell 28 (3), 472–487 e477. doi:10.1016/j.stem.2020.11.015
Regan-Komito, D., Swann, J. W., Demetriou, P., Cohen, E. S., Horwood, N. J., Sansom, S. N., et al. (2020). GM-CSF drives dysregulated hematopoietic stem cell activity and pathogenic extramedullary myelopoiesis in experimental spondyloarthritis. Nat. Commun. 11 (1), 155. doi:10.1038/s41467-019-13853-4
Rodrigues, N. P., Boyd, A. S., Fugazza, C., May, G. E., Guo, Y., Tipping, A. J., et al. (2008). GATA-2 regulates granulocyte-macrophage progenitor cell function. Blood 112 (13), 4862–4873. doi:10.1182/blood-2008-01-136564
Rodriguez-Fraticelli, A. E., Wolock, S. L., Weinreb, C. S., Panero, R., Patel, S. H., Jankovic, M., et al. (2018). Clonal analysis of lineage fate in native haematopoiesis. Nature 553 (7687), 212–216. doi:10.1038/nature25168
Rodriguez-Meira, A., Norfo, R., Wen, S., Chedeville, A. L., Rahman, H., O'Sullivan, J., et al. (2023). Single-cell multi-omics identifies chronic inflammation as a driver of TP53-mutant leukemic evolution. Nat. Genet. 55 (9), 1531–1541. doi:10.1038/s41588-023-01480-1
Sanjuan-Pla, A., Macaulay, I. C., Jensen, C. T., Woll, P. S., Luis, T. C., Mead, A., et al. (2013). Platelet-biased stem cells reside at the apex of the haematopoietic stem-cell hierarchy. Nature 502 (7470), 232–236. doi:10.1038/nature12495
Sankaran, V. G., Ludwig, L. S., Sicinska, E., Xu, J., Bauer, D. E., Eng, J. C., et al. (2012). Cyclin D3 coordinates the cell cycle during differentiation to regulate erythrocyte size and number. Genes Dev. 26 (18), 2075–2087. doi:10.1101/gad.197020.112
Sasaki, H., Kurotaki, D., Osato, N., Sato, H., Sasaki, I., Koizumi, S., et al. (2015). Transcription factor IRF8 plays a critical role in the development of murine basophils and mast cells. Blood 125 (2), 358–369. doi:10.1182/blood-2014-02-557983
Scheerlinck, J.-P. Y. (2014). “Cytokine species-specificity and humanized mice,” in Humanized mice for HIV research. Editors L. Y. Poluektova, J. V. Garcia, Y. Koyanagi, M. G. Manz, and A. M. Tager (New York: Springer), 93–108. doi:10.1007/978-1-4939-1655-9_9
Semerad, C. L., Liu, F., Gregory, A. D., Stumpf, K., and Link, D. C. (2002). G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity 17 (4), 413–423. doi:10.1016/s1074-7613(02)00424-7
Sichien, D., Scott, C. L., Martens, L., Vanderkerken, M., Van Gassen, S., Plantinga, M., et al. (2016). IRF8 transcription factor controls survival and function of terminally differentiated conventional and plasmacytoid dendritic cells, respectively. Immunity 45 (3), 626–640. doi:10.1016/j.immuni.2016.08.013
Soboleva, S., and Miharada, K. (2022). Induction of enucleation in primary and immortalized erythroid cells. Int. J. Hematol. 116 (2), 192–198. doi:10.1007/s12185-022-03386-w
Spinner, M. A., Sanchez, L. A., Hsu, A. P., Shaw, P. A., Zerbe, C. S., Calvo, K. R., et al. (2014). GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood 123 (6), 809–821. doi:10.1182/blood-2013-07-515528
Stoffel, R., Ziegler, S., Ghilardi, N., Ledermann, B., de Sauvage, F. J., and Skoda, R. C. (1999). Permissive role of thrombopoietin and granulocyte colony-stimulating factor receptors in hematopoietic cell fate decisions in vivo. Proc. Natl. Acad. Sci. U. S. A. 96 (2), 698–702. doi:10.1073/pnas.96.2.698
Sturgeon, C. M., Ditadi, A., Awong, G., Kennedy, M., and Keller, G. (2014). Wnt signaling controls the specification of definitive and primitive hematopoiesis from human pluripotent stem cells. Nat. Biotechnol. 32 (6), 554–561. doi:10.1038/nbt.2915
Tsai, F. Y., Keller, G., Kuo, F. C., Weiss, M., Chen, J., Rosenblatt, M., et al. (1994). An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature 371 (6494), 221–226. doi:10.1038/371221a0
Tsai, F. Y., and Orkin, S. H. (1997). Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood 89 (10), 3636–3643. https://www.ncbi.nlm.nih.gov/pubmed/9160668.
Turhan, A., Foudi, A., Hwang, J. W., Desterke, C., Griscelli, F., and Bennaceur-Griscelli, A. (2019). Modeling malignancies using induced pluripotent stem cells: from chronic myeloid leukemia to hereditary cancers. Exp. Hematol. 71, 61–67. doi:10.1016/j.exphem.2019.01.003
Ushach, I., and Zlotnik, A. (2016). Biological role of granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) on cells of the myeloid lineage. J. Leukoc. Biol. 100 (3), 481–489. doi:10.1189/jlb.3RU0316-144R
Velten, L., Haas, S. F., Raffel, S., Blaszkiewicz, S., Islam, S., Hennig, B. P., et al. (2017). Human haematopoietic stem cell lineage commitment is a continuous process. Nat. Cell Biol. 19 (4), 271–281. doi:10.1038/ncb3493
von Muenchow, L., Alberti-Servera, L., Klein, F., Capoferri, G., Finke, D., Ceredig, R., et al. (2016). Permissive roles of cytokines interleukin-7 and Flt3 ligand in mouse B-cell lineage commitment. Proc. Natl. Acad. Sci. U. S. A. 113 (50), E8122–E8130. doi:10.1073/pnas.1613316113
Waddington, C. H. (1957). The strategy of the genes: a discussion of some aspects of theoretical Biology. Allen Unwin. https://books.google.nl/books?id=PdU9AAAAIAAJ.
Walsh, J. C., DeKoter, R. P., Lee, H. J., Smith, E. D., Lancki, D. W., Gurish, M. F., et al. (2002). Cooperative and antagonistic interplay between PU.1 and GATA-2 in the specification of myeloid cell fates. Immunity 17 (5), 665–676. doi:10.1016/s1074-7613(02)00452-1
Wanet, A., Bassal, M. A., Patel, S. B., Marchi, F., Mariani, S. A., Ahmed, N., et al. (2021). E-cadherin is regulated by GATA-2 and marks the early commitment of mouse hematopoietic progenitors to the basophil and mast cell fates. Sci. Immunol. 6 (56). doi:10.1126/sciimmunol.aba0178
Wang, D., Malarkannan, S., Myers, K. C., Bresnick, E. H., Verbsky, J., and Thakar, M. (2022a). GATA2-TGF-b Axis in human NK cell development. Blood 140, 2622–2623. doi:10.1182/blood-2022-166834
Wang, S., Zhao, H., Zhang, H., Gao, C., Guo, X., Chen, L., et al. (2022b). Analyses of erythropoiesis from embryonic stem cell-CD34(+) and cord blood-CD34(+) cells reveal mechanisms for defective expansion and enucleation of embryomic stem cell-erythroid cells. J. Cell Mol. Med. 26 (8), 2404–2416. doi:10.1111/jcmm.17263
Wang, Z., McWilliams-Koeppen, H. P., Reza, H., Ostberg, J. R., Chen, W., Wang, X., et al. (2022c). 3D-organoid culture supports differentiation of human CAR(+) iPSCs into highly functional CAR T cells. Cell Stem Cell 29 (4), 515–527. doi:10.1016/j.stem.2022.02.009
Wei, W., Wen, L., Huang, P., Zhang, Z., Chen, Y., Xiao, A., et al. (2008). Gfi1.1 regulates hematopoietic lineage differentiation during zebrafish embryogenesis. Cell Res. 18 (6), 677–685. doi:10.1038/cr.2008.60
Wlodarski, M. W., Hirabayashi, S., Pastor, V., Stary, J., Hasle, H., Masetti, R., et al. (2016). Prevalence, clinical characteristics, and prognosis of GATA2-related myelodysplastic syndromes in children and adolescents. Blood 127 (11), 1387–1397. doi:10.1182/blood-2015-09-669937
Woan, K. V., Kim, H., Bjordahl, R., Davis, Z. B., Gaidarova, S., Goulding, J., et al. (2021). Harnessing features of adaptive NK cells to generate iPSC-derived NK cells for enhanced immunotherapy. Cell Stem Cell 28 (12), 2062–2075 e2065. doi:10.1016/j.stem.2021.08.013
Wolfler, A., Danen-van Oorschot, A. A., Haanstra, J. R., Valkhof, M., Bodner, C., Vroegindeweij, E., et al. (2010). Lineage-instructive function of C/EBPalpha in multipotent hematopoietic cells and early thymic progenitors. Blood 116 (20), 4116–4125. doi:10.1182/blood-2010-03-275404
Wu, Z., Gao, S., Diamond, C., Kajigaya, S., Chen, J., Shi, R., et al. (2020). Sequencing of RNA in single cells reveals a distinct transcriptome signature of hematopoiesis in GATA2 deficiency. Blood Adv. 4 (12), 2656–2670. doi:10.1182/bloodadvances.2019001352
Xavier-Ferrucio, J., and Krause, D. S. (2018). Concise review: bipotent megakaryocytic-erythroid progenitors: concepts and controversies. Stem Cells 36 (8), 1138–1145. doi:10.1002/stem.2834
Xin, Z., Zhang, W., Gong, S., Zhu, J., Li, Y., Zhang, Z., et al. (2021). Mapping human pluripotent stem cell-derived erythroid differentiation by single-cell transcriptome analysis. Genomics Proteomics Bioinforma. 19 (3), 358–376. doi:10.1016/j.gpb.2021.03.009
Yamazaki, H., Suzuki, M., Otsuki, A., Shimizu, R., Bresnick, E. H., Engel, J. D., et al. (2014). A remote GATA2 hematopoietic enhancer drives leukemogenesis in inv(3)(q21;q26) by activating EVI1 expression. Cancer Cell 25 (4), 415–427. doi:10.1016/j.ccr.2014.02.008
Ye, Y., Carlsson, G., Wondimu, B., Fahlen, A., Karlsson-Sjoberg, J., Andersson, M., et al. (2011). Mutations in the ELANE gene are associated with development of periodontitis in patients with severe congenital neutropenia. J. Clin. Immunol. 31 (6), 936–945. doi:10.1007/s10875-011-9572-0
Yoshihara, H., Arai, F., Hosokawa, K., Hagiwara, T., Takubo, K., Nakamura, Y., et al. (2007). Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell 1 (6), 685–697. doi:10.1016/j.stem.2007.10.020
You, X., Zhou, Y., Chang, Y. I., Kong, G., Ranheim, E. A., Johnson, K. D., et al. (2022). Gata2 +9.5 enhancer regulates adult hematopoietic stem cell self-renewal and T-cell development. Blood Adv. 6 (4), 1095–1099. doi:10.1182/bloodadvances.2021004311
Yuasa, H., Oike, Y., Iwama, A., Nishikata, I., Sugiyama, D., Perkins, A., et al. (2005). Oncogenic transcription factor Evi1 regulates hematopoietic stem cell proliferation through GATA-2 expression. EMBO J. 24 (11), 1976–1987. doi:10.1038/sj.emboj.7600679
Zang, C., Luyten, A., Chen, J., Liu, X. S., and Shivdasani, R. A. (2016). NF-E2, FLI1 and RUNX1 collaborate at areas of dynamic chromatin to activate transcription in mature mouse megakaryocytes. Sci. Rep. 6, 30255. doi:10.1038/srep30255
Zeller, P., Yeung, J., Vinas Gaza, H., de Barbanson, B. A., Bhardwaj, V., Florescu, M., et al. (2023). Single-cell sortChIC identifies hierarchical chromatin dynamics during hematopoiesis. Nat. Genet. 55 (2), 333–345. doi:10.1038/s41588-022-01260-3
Zhang, Q., Wu, B., Weng, Q., Hu, F., Lin, Y., Xia, C., et al. (2022). Regeneration of immunocompetent B lymphopoiesis from pluripotent stem cells guided by transcription factors. Cell Mol. Immunol. 19 (4), 492–503. doi:10.1038/s41423-021-00805-6
Zhang, S., Pyne, S., Pietrzak, S., Halberg, S., McCalla, S. G., Siahpirani, A. F., et al. (2023). Inference of cell type-specific gene regulatory networks on cell lineages from single cell omic datasets. Nat. Commun. 14 (1), 3064. doi:10.1038/s41467-023-38637-9
Zhou, B. O., Yu, H., Yue, R., Zhao, Z., Rios, J. J., Naveiras, O., et al. (2017). Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat. Cell Biol. 19 (8), 891–903. doi:10.1038/ncb3570
Zhou, B. O., Yue, R., Murphy, M. M., Peyer, J. G., and Morrison, S. J. (2014). Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15 (2), 154–168. doi:10.1016/j.stem.2014.06.008
Zhou, W., Yui, M. A., Williams, B. A., Yun, J., Wold, B. J., Cai, L., et al. (2019). Single-cell analysis reveals regulatory gene expression dynamics leading to lineage commitment in early T cell development. Cell Syst. 9 (4), 321–337 e329. doi:10.1016/j.cels.2019.09.008
Zhu, H., Blum, R. H., Bjordahl, R., Gaidarova, S., Rogers, P., Lee, T. T., et al. (2020). Pluripotent stem cell-derived NK cells with high-affinity noncleavable CD16a mediate improved antitumor activity. Blood 135 (6), 399–410. doi:10.1182/blood.2019000621
Zhu, J., and Emerson, S. G. (2002). Hematopoietic cytokines, transcription factors and lineage commitment. Oncogene 21 (21), 3295–3313. doi:10.1038/sj.onc.1205318
Keywords: GATA2, hematopoietic stem cell (HSC), GATA2 deficiency syndrome, myelodysplastic syndrome (MDS), acute myeloic leukemia (AML), immune deficiency
Citation: Peters IJA, de Pater E and Zhang W (2023) The role of GATA2 in adult hematopoiesis and cell fate determination. Front. Cell Dev. Biol. 11:1250827. doi: 10.3389/fcell.2023.1250827
Received: 30 June 2023; Accepted: 31 October 2023;
Published: 14 November 2023.
Edited by:
Anna Silvia Pistocchi, University of Milan, ItalyReviewed by:
Giorgio Anselmi, University of Oxford, United KingdomMarcin Wlodarski, University of Freiburg Medical Center, Germany
Copyright © 2023 Peters, de Pater and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Zhang, dy56aGFuZ0BlcmFzbXVzbWMubmw=; Emma de Pater, ZS5kZXBhdGVyQGVyYXNtdXNtYy5ubA==
†These authors have contributed equally to this work
 Iris J. A. Peters
Iris J. A. Peters Emma de Pater
Emma de Pater Wei Zhang
Wei Zhang