- 1Basic Medical Experimental Teaching Center, Zhejiang University, Hangzhou, China
- 2Key Laboratory of Gastroenterology of Zhejiang Province, Zhejiang Provincial People’s Hospital, Affiliated People’s Hospital, Hangzhou Medical College, Hangzhou, China
- 3Cancer Center, General Surgery, Department of Gastrointestinal and Pancreatic Surgery, Zhejiang Provincial People’s Hospital, Affiliated People’s Hospital, Hangzhou Medical College, Hangzhou, China
- 4Center for Plastic and Reconstructive Surgery, Department of Hand and Reconstruction Surgery, Zhejiang Provincial People’s Hospital, Affiliated People’s Hospital, Hangzhou Medical College, Hangzhou, China
Digestive system malignancies, the most common types of cancer and a major cause of death in the worldwide, are generally characterized by high morbidity, insidious symptoms and poor prognosis. NLRP3 inflammasome, the most studied inflammasome member, is considered to be crucial in tumorigenesis. In this paper, we reviewed its pro-tumorigenic and anti-tumorigenic properties in different types of digestive system malignancy depending on the types of cells, tissues and organs involved, which would provide promising avenue for exploring new anti-cancer therapies.
Introduction
Digestive system malignancies, including gastric cancer (GC), hepatocellular carcinoma (HCC), colorectal cancer (CRC), pancreatic cancer (PC), etc., are one of the main factors that endanger human health. According to statistics from the American Cancer Society, in 2022, the number of new cases of digestive system malignancies in the United States is expected to be 343,040 accounting for 18% of all incident cases, and the number of deaths will be 609,360 accounting for over one-half (28%) (Siegel et al., 2022). It is generally characterized by high morbidity, insidious symptoms and poor prognosis (Koc et al., 2020; Nagtegaal et al., 2020; Assarzadegan and Montgomery, 2021; Washington et al., 2021; Kushima, 2022). Therefore, early diagnosis, effective treatment and prognosis are the current focus of clinical prevention and treatment of digestive system tumors, which all need in-depth mechanism research as theoretical support.
Inflammasomes are a complex group of multiprotein complexes in the cytoplasm which could activate inflammation-associated caspases and induce the processing, maturation and secretion of the key pro-inflammatory cytokines, interleukin-1β (IL-1β) and IL-18, which thereby would initiate inflammatory responses, promote innate immune responses, and regulate acquired immunity (Watanabe et al., 2008; Schroder, 2010; Lamkanfi and Dixit, 2014; Guo et al., 2015; Broz and Dixit, 2016; Deets and Vance, 2021). Therefore, the inflammasome is also an important link and bridge between innate immunity and acquired immunity, and is considered to be the signal transduction center of the immune system.
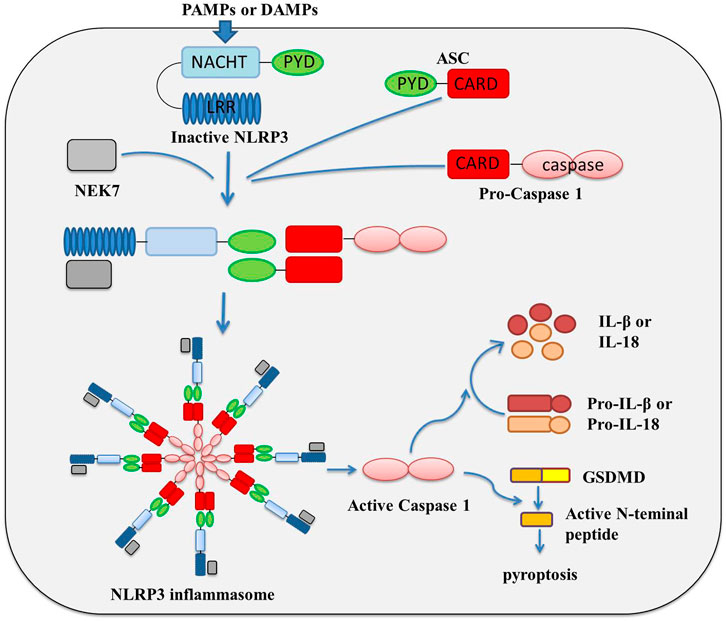
The inflammasome is composed of three key components: platform proteins, adaptor proteins and effector proteins. The classification of inflammasomes basically depends on the difference in platform proteins. At present, a variety of inflammatory complexes have been isolated and identified, including the Nod-like receptor (NLR) family pyrin domain containing protein 1 (NLRP1), NLRP3, NLR family CARD domain containing 4 (NLRC4), absent in melanoma 2 (AIM2) and RNA sensor RIG-I, etc., among which, NLRP3 is the most detailed inflammasome studied at present (Schroder, 2010; Lamkanfi and Dixit, 2014; Guo et al., 2015; Broz and Dixit, 2016; Deets and Vance, 2021). NLRP3 inflammasome is composed of NLRP3 protein, adaptor apoptosis-associated speck-like protein (ASC) and the protein kinase NIMA related kinase 7 (NEK7), and effector pro-caspase-1 (Bae and Park, 2011; He et al., 2016). NLRP3 protein contains an N-terminal pyrin domain (PYD), a central NAIP, CIITA, HET-E, and TP1 (NACHT) domain, and a C-terminal leucine-rich repeats (LRRs) (Proell et al., 2008; Martinon et al., 2009). The activation and assembly of NLRP3 and other inflammasome are largely similar. Under normal condition, NLRP3 protein is thought to be auto-repressed by interaction of NACHT domain with LRRs; once it is activated upon pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs), NLRP3 oligomerizes and triggers inflammasome assembly by recruiting ASC, pro-caspase-1 and NEK7 via homotypic domain interactions, involving NACHT, PYD, and CARD domain (Lamkanfi and Dixit, 2014; Martinon et al., 2009; Latz et al., 2013; Rahman et al., 2020) the assembly of the inflammatory complex realizes the aggregation of caspase 1, which greatly shortens the molecular distance, promotes the intermolecular self-cleavage of caspase 1, and then completes the activation of caspase 1 (Bae and Park, 2011; Shao et al., 2015; Swanson et al., 2019; Zhen and Zhang, 2019); activated caspase-1 causes the activation of inflammation-related transcription factors, and activation and releasing of inflammatory factors IL-1β and IL-18; in the meanwhile, by interaction with caspase-1, gasdermin-D (GSDMD) was cleaved and multimerized to form a peptide segment containing GSDMD, which could punch holes in the cell membrane, cause cell perforation, and induce pyroptosis (Figure 1) (Shi et al., 2015; Liu et al., 2017; Schneider et al., 2017; Wang et al., 2019; Chauhan et al., 2020; Downs et al., 2020; Karmakar et al., 2020; Shi et al., 2021a; Ding et al., 2021; Gao et al., 2021).
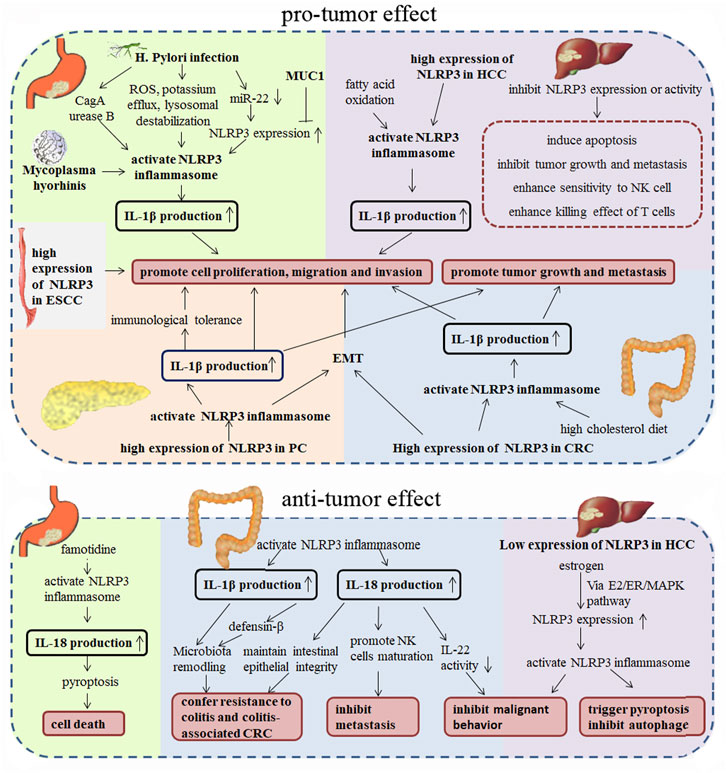
Many studies have shown that chronic inflammatory response was associated with a variety of tumors, and inflammation and persistent infection play important roles in different stages of tumor occurrence, development, malignant transformation, invasion, and metastasis (Oguma et al., 2010; Salem et al., 2018). The NLRP3 inflammasome, as an important intrinsic component of the human immune system, has attracted increasing attention in its functions in tumors. More and more studies have shown that NLRP3 inflammasome was closely associated with the progression of various tumors (such as gastric cancer (Castano-Rodriguez et al., 2014a; Castano-Rodriguez et al., 2014b; Zhang et al., 2022a), colorectal cancer (Dupaul-Chicoine et al., 2015; Perera et al., 2018; Shi et al., 2021b; Li et al., 2021; Marandi et al., 2021), liver cancer (Wei et al., 2014; Chen et al., 2020; Lee et al., 2021), etc.). NLRP3 is not only involved in the regulation of tumor itself, it also participates in the composition of the tumor microenvironment, and has dual effects of promoting and inhibiting tumorigenesis in different tissues or cell types. In this article, we review the research status of NLRP3 in digestive system malignancies (Figure 2), which might provide theoretical basis for the potential future clinical applications.
The research of NLRP3 in gastric cancer
Gastric cancer is one of the most common malignant tumors, and chronic infection with H. pylori is believed to be the most important risk factor for the development of gastric cancer (about 75% of gastric cancers were associated with it in previous research) (Correa, 2013; Wang et al., 2014; Plummer et al., 2016; Van Cutsem et al., 2016; Molina-Castro et al., 2017; Engstrand and Graham, 2020; Smyth et al., 2020; Thrift and El-Serag, 2020). Several researches have demonstrated that H. pylori infection could induce ROS production, potassium efflux and lysosomal destabilization, which would finally lead to activation of NLRP3 inflammasome (Kim et al., 2013; Semper et al., 2014; Koch et al., 2015; Li et al., 2015; Perez-Figueroa et al., 2016; Man, 2018). Further studies have shown that the cag pathogenicity island (cagPAI) and urease B subunit (the H. pylori virulence factors) could promote the IL-1β secretion by potentiating activation of the NLRP3 inflammasome in immune cells, and excessive production of IL-1β were considered to be extensively linked to gastric carcinogenesis (Kim et al., 2013; Semper et al., 2014; Koch et al., 2015). In recent research, Zhang et al. found that cytotoxin-associated gene A (CagA) encoded on the cagPAI of H. pylori could activate the NLRP3 inflammasome, promote the secretion of IL-1β and IL-18 secretion, and finally promote cell proliferation, migration and invasion in gastric cancer (Zhang et al., 2022a). Li et al. found that H. pylori infection could inhibit the expression of miR-22 in gastric epithelial cells, which would upregulated the expression of NLRP3 and enhance H. pylori-induced gastric carcinogenesis (Li et al., 2018). And Ng et al. found that MUC1 is an important negative regulator of NLRP3 inflammasome which could protect epithelial barrier from H. pylori infection by negative regulating NLRP3 expression via a nuclear factor (NF)-κB-dependent pathway (Ng et al., 2016). Moreover, Mycoplasma hyorhinis, which was detected in 56% of gastric cancer, was also found to be able to active NLRP3 inflammasome and induce IL-1β secretion, thus promote gastric cancer cell migration and invasion (Xu et al., 2013). Besides, Huang et al. reported that in gastric cancer cells, famotidine, a gastric antisecretory drug, could promote the activation of NLRP3 inflammasomes by upregulating expression of NLRP3, ASC, and Caspase-1, then enhance IL-18, not IL-1β, mature and secretion, thus trigger cell pyroptosis and aggravate inflammation, which was considered to be critical in development of gastric cancer (Huang et al., 2021). However, according to the study of West et al., in gp130 F/F mouse model, NLRP3 expression levels did not affect the development of gastric tumors, and the cellular processes associated with tumorigenesis in the gastric mucosa, such as proliferation, apoptosis, and inflammation did not changed in the NLRP3 knockout gp130 mouse, which suggested that NLRP3 might not play a major role in promoting inflammasome-driven gastric tumorigenesis (West et al., 2022).
The research of NLRP3 in hepatocellular carcinoma
NLRP3 is one of the most widely studied NLR family members in liver disease, which was widely distributed in parenchymal and non-parenchymal hepatocytes. NLRP3 was reported to play essential roles in the pathogenesis of alcohol-associated liver disease, non-alcoholic fatty liver disease/non-alcoholic steatohepatitis, cirrhosis and fibrosis, which ultimately leads to hepatocellular carcinoma (HCC) (Wei et al., 2014; Wree et al., 2014; Mridha et al., 2017; Luan et al., 2018; Pourcet et al., 2018; Chen et al., 2020; Tao et al., 2020; Xie et al., 2020; Surlin et al., 2021). Many studies have shown that the NLRP3 inflammasome has a promoting effect on HCC. NLRP3 has been found highly expressed in HCC tissues, and inhibition of NLRP3 inflammasome activation could induce apoptosis and regulate the levels of inflammatory factors, which would suppress the growth of HCC cells (Chen et al., 2022) (Li et al., 2020a). Knockout of NLRP3 in HCC cells also inhibited tumor growth and metastasis in vivo, as well as increased the sensitivity to NK cell cytotoxicity (Lee et al., 2021). While enhanced NLRP3 inflammasome activation and IL-1β secretion by fatty acid oxidation in M2 macrophages could enhance the proliferation, migration, and invasion of HCC cells (Zhang et al., 2018). Besides, Ding et al. found that inhibition of NLRP3 in Hep3B cells promoted the killing effect of T cells to cancer cells by repressing the expression of immune checkpoints (Ding et al., 2022). And in Sonohara’s research, the adjacent tissue of HCC was reported to be in a hyper-inflammatory state due to the overexpression of NLRP3, NLRC4, and AIM2 genes, which was showed to be closely related to poor overall survival, suggesting that the high expression of NLRP3 may become an independent prognostic factor for overall survival after surgery in HCC (Sonohara et al., 2017). However, there are a few studies indicated the protective role of NLRP3 inflammasome in HCC progression. Wei et al. (2014) found that the expression of NLRP3 inflammasome components was down-regulated in HCC tissues and inversely correlated with pathological grades and clinical stages; the up-regulation of NLRP3 inflammasome expression mediated by estrogen through the E2/ER/MAPK pathway, could significantly inhibit the malignant behavior of HCC cells (Wei et al., 2015; Wei et al., 2019). Therefore, the controversial role of NLRP3 in HCC needs more research and data to explore and clarify.
The research of NLRP3 in colorectal cancer
Colorectal cancer (CRC) is the most common malignant tumor of digestive system, and its mortality rate ranks second in the world (Siegel et al., 2022). Meanwhile, inflammatory bowel disease (IBD), including ulcerative colitis and Crohn’s disease, has been identified as a high-risk factor for CRC (Dekker et al., 2019). Therefore, inflammasome, as an important link of inflammatory response, plays an important role in the occurrence and development of CRC (Dupaul-Chicoine et al., 2015; Deng et al., 2019; Shao et al., 2020; Shi et al., 2021b; Li et al., 2021; Marandi et al., 2021).
Research on the NLRP3 inflammasome in colorectal tumors is mainly focused on its function in colitis-associated CRC, and it is controversial. It was reported that NLRP3 may confer protection against colitis and colitis-associated tumorigenesis, by mediating the secretion of IL-18, which could promote intestinal epithelial cell differentiation, maintain intestinal epithelial integrity, and reduce intestinal epithelial cell proliferation during colitis remission; NLRP3 deficient mice were found to be more susceptible to dextran sodium sulfate (DSS) and 2,4,6-trinitrobenzenesulfonic acid, and showed more sever colonic inflammation, and higher carcinogenesis (Allen et al., 2010; Zaki et al., 2010; Zaki et al., 2011). Hirota et al. also found that knockout of NLRP3 resulted in altered expression of colonic defensin-β, decreased antimicrobial secretions, and unique gut microbiota types change, which finally reduced intestinal resistance to enteric pathogens (Hirota et al., 2011). And Yao’s research indicated that hyperactive NLRP3 enhances IL-1β but not IL-18 secretion, leading to gut microbiota remodeling and regulatory T cells (Tregs) induction, thus confers disease resistance (Yao et al., 2017). Recently, Dupaul-Chicoine et al. reported that the NLRP3 inflammasome could reduce the occurrence of enteritis-related CRC and inhibit metastatic growth of CRC in liver (Dupaul-Chicoine et al., 2015). This study also found that the activation of IL-18 mediated by NLRP3 could enhance tumoricidal activity of NK cells, which directed to the metastasized colonic tumor cells in the mouse liver (Dupaul-Chicoine et al., 2015). Moreover, IL-18 also could fine-tune the biological activity of IL-22 via inhibit the production of the IL-22 binding protein, And IL-22 was reported to promote tumor development at later stages (Huber et al., 2012).
However, in contrast to the aforementioned studies, NLRP3 inflammasome also showed the potential to promote carcinogenesis and cancer progression in CRC. Bauer et al. showed that NLRP3 gene knockout would alleviate DSS-induced colitis (Bauer et al., 2010). Besides, high cholesterol diet induced colon carcinogenesis was mediated by NLRP3 inflammasome activation (Du et al., 2016a). Moreover, NLRP3 was found highly expressed in CRC tissues and mesenchymal-like CRC cells, and promoted epithelial-mesenchymal transition (EMT) in an inflammasome-independent way; knockdown of NLRP3 in CRC cells decreased cell proliferation, migration and invasion ability (Wang et al., 2016; Shao et al., 2020; Shi et al., 2021b; Marandi et al., 2021). Furthermore, macrophages-CRC cell crosstalk activated NLRP3 inflammasomes and induced IL-1β secretion in macrophages, thus promoted the invasion and migration of CRC cell, and drive metastasis to the liver (Deng et al., 2019; Zhang et al., 2022b). The controversy maybe due to NLRP3’s broad activity in intrinsic properties and microenvironments of a tumor cell, and further studies are needed to explain the diversity of its functions.
The research of NLRP3 in pancreatic cancer
In the pancreas, NLRP3 activation was found to be necessary for the development of experimental acute pancreatitis, pancreatic fibrosis, obesity-induced insulin resistance, and to be a critical inflammatory mechanism in pancreatic cancer (Vandanmagsar et al., 2011; Wan et al., 2016; Boone et al., 2019; Li et al., 2020b; Das et al., 2020; Sendler et al., 2020; Tang et al., 2020; Gao et al., 2021). In Liu’s study, NLRP3 was reported to be upregulated in pancreatic ductal adenocarcinoma (PDA), as shown in both infiltrating immune cells and tumor cells; and the activation of NLRP3 inflammasome in macrophages would induce immunological tolerance and promote the tumor growth (Liu et al., 2020). Boone et al. found that the platelet NLRP3 inflammasome is upregulated in a murine model of pancreatic cancer which would promote platelet aggregation and tumor growth (Boone et al., 2019). Daley et al. (2017) reported that the activation of NLRP3 inflammasome in macrophages directs tolerogenic T cell differentiation, which would induce immunological tolerance and promote pancreatic tumor growth. Hu et al. (2018) showed that down-regulated NLRP3 signaling in pancreatic cancer cells would suppress progression of pancreatic cancer and decrease epithelial-mesenchymal transition-induced cell invasion. In short, NLRP3 could promote the tumor growth, progression and cell invasion in pancreatic cancer.
The research of NLRP3 in other cancers
The roles of NLRP3 in other digestive system malignancy were constantly being discovered. Yu et al. (2022) found that the level of NLRP3 inflammasome was significantly up-regulated in human esophageal squamous cell carcinoma (ESCC) tissues, which was positively correlated with tumor malignancy. And the knockdown or overexpression of NLRP3 could markedly abolish or promote cell migration and invasion and decrease or increase cell mobility, respectively, which suggested that NLRP3 inflammasome-mediated inflammation could contribute to the development and progression of ESCC (Yu et al., 2020). Matsushita et al. showed that lower expressions of NLRP3 in primary sclerosing cholangitis (PSC) patients were associated with the development of cholangiocarcinoma (Matsushita et al., 2015). Overall, the diverse roles of NLRP3 inflammasome in different digestive system tumors still need to be confirmed and explored, and its related mechanism needs to be further studied.
The NLRP3 inflammasome as a promising target in cancer therapy
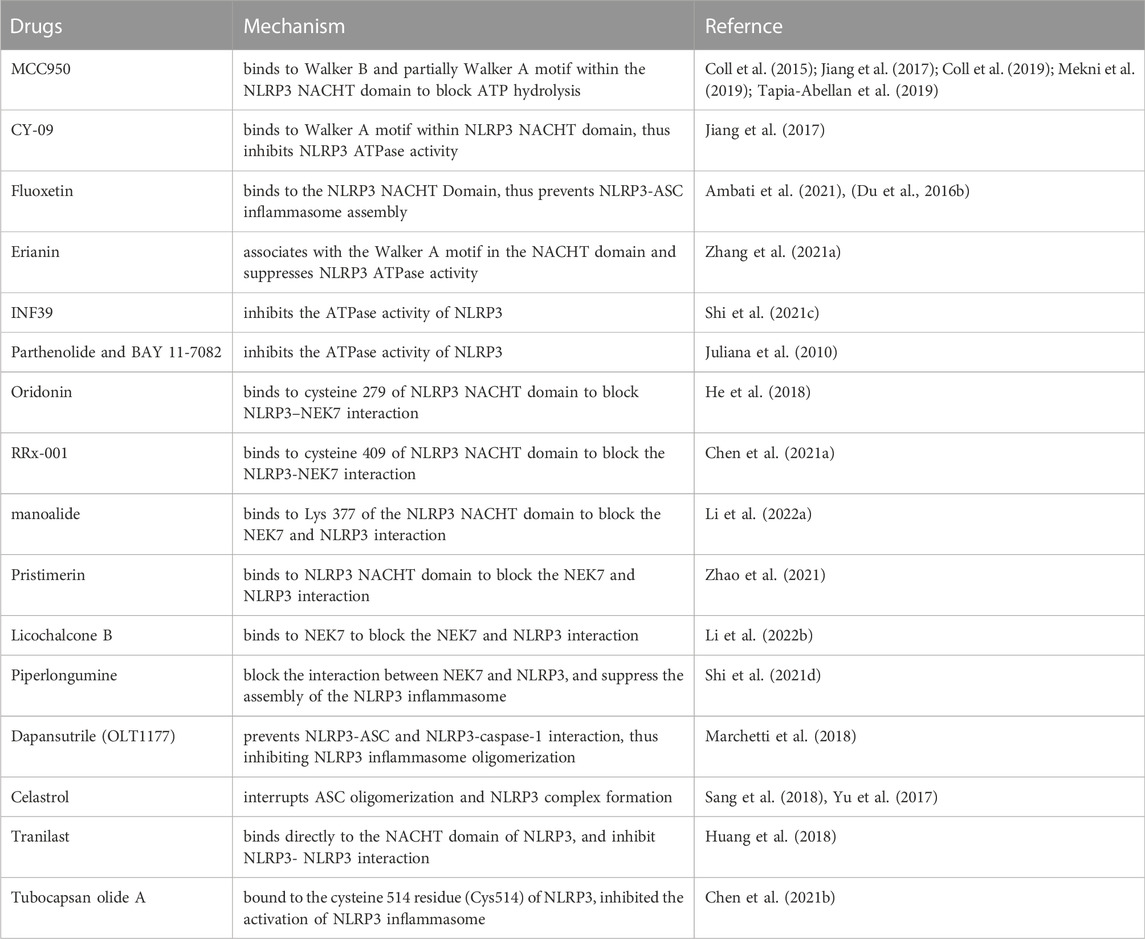
The involvement of NLRP3 inflammasome in inflammation-related diseases and cancers makes it an attractive therapeutic target. Many efforts have been made to explore effective compounds that can inhibit the NLRP3 inflammasome components, and various agents have been developed, including MCC950, CY-09, Fluoxetine, Erianin, INF39, Parthenolide and BAY 11-7082, Oridonin, RRx-001, Manoalide, Pristimerin, Piperlongumine, OLT1177, Celastrol, Tranilast, and so on (Table 1).
MCC950/CRID3/CP-456773 is the most studied selective inhibitor of NLRP3 inflammasome. MCC950 directly binds to a region crossing the Walker B and partially Walker A motifs within the NACHT domain of NLRP3, thereby blocks ATP hydrolysis and prevents NLRP3 oligomerization and activation (Coll et al., 2015; Coll et al., 2019; Mekni et al., 2019; Tapia-Abellan et al., 2019). CY-09, fluoxetine and Erianin all show the ability to directly bind to the ATP-binding motif of NLRP3 NACHT domain to inhibits NLRP3 ATPase activity and suppresses NLRP3 assembly (Du et al., 2016b; Jiang et al., 2017; Ambati et al., 2021; Zhang et al., 2021a). Besides, INF39, Parthenolide and BAY 11-7082 are also able to inhibit the ATPase activity of NLRP3, however, the detailed molecular mechanism is not yet fully understood (Juliana et al., 2010; Shi et al., 2021c).
Oridonin, RRx-001, manoalide, and pristimerin, are showed to supress the assembly and the activation of the NLRP3 inflammasome by covalently binding to the NACHT domain to block the NLRP-NEK7 interaction (He et al., 2018; Chen et al., 2021a; Zhao et al., 2021; Li et al., 2022a). While Licochalcone B is found to directly bind to NEK7 and inhibit the interaction between NLRP3 and NEK7, thus inhibit the activation of NLRP3 inflammasome (Li et al., 2022b). Piperlongumine also can suppress assembly of the NLRP3 inflammasome by interrupting NLRP3 and NEK7 interaction (Shi et al., 2021d).
OLT1177/Dapansutrile is able to prevent NLRP3-ASC and NLRP3-caspase-1 interaction, thus inhibited NLRP3 inflammasome oligomerization (Marchetti et al., 2018). Celastrol may inhibit NLRP3 inflammasome activation via interrupting of ASC oligomerization and subsequently NLRP3 inflammasome assembly (Yu et al., 2017; Sang et al., 2018). Tranilast, an old anti-allergic clinical drug, can directly bind to the NACHT domain of NLRP3 to block NLRP3-NLRP3 interaction and the subsequent ASC oligomerization (Huang et al., 2018). Tubocapsan olide A can bind to the Cys51) of NLRP3 to inhibit the activation of NLRP3 inflammasome (Chen et al., 2021b).
These NLRP3 inflammasome inhibitors exhibit efficient anti-inflammatory effect in inflammation-related diseases. Moreover, some studies also have shown that some of them even possess potent anti-tumor effect, such as MCC950, Erianin, Oridonin, RRx-001, Pristimerin, which were found able to suppress cell proliferation and migration, induce apoptosis, and delay tumor growth (Scicinski et al., 2015; Huang et al., 2017; Li et al., 2020c; Yaw et al., 2020; Shi et al., 2021b; Zhang et al., 2021b; Li et al., 2022a; Ding et al., 2022; Hong et al., 2022). MCC950, MCC950, Erianin, Oridonin and RRx-001 also showed ability to reshape the immune microenvironment by increasing the number of effector T cells and decreasing immunosuppressive cell accumulation (Brzezniak et al., 2016; Huang et al., 2017; Guo et al., 2020; Yang et al., 2021). These results indicate that NLRP3 inflammasome inhibitors have great promise as a novel cancer therapeutic agent.
Conclusion and perspective
In this review, we provided a brief overview of the biological importance of NLRP3 inflammasome in different forms of digestive system malignancy. In gastric, pancreas and esophageal cancer, NLRP3 inflammasome mainly act as a tumor promoter to regulate the tumor growth, invasion and metastasis, and so on. In cholangiocarcinoma and liver cancer, NLRP3 inflammasome could inhibit the tumor progression, and in colorectal tumor, it might have the dual effect of promoting and inhibiting. It was believed that the pro-tumorigenic and anti-tumorigenic properties of NLRP3 inflammasome are largely determined on effector cytokines (IL-1β and IL-18) and the types of target cells, tissues and organs involved. Although IL-1β and IL-18 belong to IL-1 family, they paly different roles in tumor biology and tumor immunity. Many studies have demonstrated that IL-1β can enhance tumor progression and resistance to cancer therapy; while IL-18 exerts an anti-tumor activity, as it can stimulate anti-tumor immunity by activating NK, Th1, CD4+ T cells, and CD8+ T cells (Sharma and Kanneganti, 2021; Sun et al., 2022). Besides, we also summarized the recent advances in specific inhibitors of NLRP3 inflammasome and their potential in cancer therapy.
In conclusion, NLRP3 inflammasome played a significant part in occurrence and development of various digestive system tumors, which requires more in-depth and comprehensive research in the future. And the biological relationship between NLRP3 inflammasomes and cancer would might open up new directions for anti-cancer therapies.
Author contributions
H-JW and LW managed the article design, C-CS and LL drafted the manuscript, Z-CJ collected the references, H-QT reviewed the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Zhejiang Provincial Natural Science Foundation of China (Grant Number LY19H160033, LY20H160042, and LQ20H160060) and the Zhejiang Province Bureau of Health (2019KY031, 2021KY531, 2018ZA011, and 2021ZQ010).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Allen, I. C., TeKippe, E. M., Woodford, R. M., Uronis, J. M., Holl, E. K., Rogers, A. B., et al. (2010). The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J. Exp. Med. 207 (5), 1045–1056. doi:10.1084/jem.20100050
Ambati, M., Apicella, I., Wang, S. B., Narendran, S., Leung, H., Pereira, F., et al. (2021). Identification of fluoxetine as a direct NLRP3 inhibitor to treat atrophic macular degeneration. Proc. Natl. Acad. Sci. U. S. A. 118 (41), e2102975118. doi:10.1073/pnas.2102975118
Assarzadegan, N., and Montgomery, E. (2021). What is new in the 2019 world Health organization (WHO) classification of tumors of the digestive system: Review of selected updates on neuroendocrine neoplasms, appendiceal tumors, and molecular testing. Archives pathology laboratory Med. 145 (6), 664–677. doi:10.5858/arpa.2019-0665-RA
Bae, J. Y., and Park, H. H. (2011). Crystal structure of NALP3 protein pyrin domain (PYD) and its implications in inflammasome assembly. J. Biol. Chem. 286 (45), 39528–39536. doi:10.1074/jbc.M111.278812
Bauer, C., Duewell, P., Mayer, C., Lehr, H. A., Fitzgerald, K. A., Dauer, M., et al. (2010). Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 59 (9), 1192–1199. doi:10.1136/gut.2009.197822
Boone, B. A., Murthy, P., Miller-Ocuin, J. L., Liang, X., Russell, K. L., Loughran, P., et al. (2019). The platelet NLRP3 inflammasome is upregulated in a murine model of pancreatic cancer and promotes platelet aggregation and tumor growth. Ann. Hematol. 98 (7), 1603–1610. doi:10.1007/s00277-019-03692-0
Broz, P., and Dixit, V. M. (2016). Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 16 (7), 407–420. doi:10.1038/nri.2016.58
Brzezniak, C., Schmitz, B. A., Peterson, P. G., Degesys, A., Oronsky, B. T., Scicinski, J. J., et al. (2016). RRx-001-Induced tumor necrosis and immune cell infiltration in an egfr mutation-positive nsclc with resistance to egfr tyrosine kinase inhibitors: A case report. Case Rep. Oncol. 9 (1), 45–50. doi:10.1159/000443605
Castano-Rodriguez, N., Kaakoush, N. O., Goh, K. L., Fock, K. M., and Mitchell, H. M. (2014). The NOD-like receptor signalling pathway in Helicobacter pylori infection and related gastric cancer: A case-control study and gene expression analyses. PloS one 9 (6), e98899. doi:10.1371/journal.pone.0098899
Castano-Rodriguez, N., Kaakoush, N. O., and Mitchell, H. M. (2014). Pattern-recognition receptors and gastric cancer. Front. Immunol. 5, 336. doi:10.3389/fimmu.2014.00336
Chauhan, D., Vande Walle, L., and Lamkanfi, M. (2020). Therapeutic modulation of inflammasome pathways. Immunol. Rev. 297 (1), 123–138. doi:10.1111/imr.12908
Chen, C., Liu, X., Gong, L., Zhu, T., Zhou, W., Kong, L., et al. (2021). Identification of Tubocapsanolide A as a novel NLRP3 inhibitor for potential treatment of colitis. Biochem. Pharmacol. 190, 114645. doi:10.1016/j.bcp.2021.114645
Chen, W., Hu, M., Wei, T., Liu, Y., Tan, T., Zhang, C., et al. (2022). IL-1 receptor-associated kinase 1 participates in the modulation of the NLRP3 inflammasome by tumor-associated macrophages in hepatocellular carcinoma. J. Gastrointest. Oncol. 13 (3), 1317–1329. doi:10.21037/jgo-22-471
Chen, Y., He, H., Lin, B., Chen, Y., Deng, X., Jiang, W., et al. (2021). RRx-001 ameliorates inflammatory diseases by acting as a potent covalent NLRP3 inhibitor. Cell. Mol. Immunol. 18 (6), 1425–1436. doi:10.1038/s41423-021-00683-y
Chen, Z., He, M., Chen, J., Li, C., and Zhang, Q. (2020). Long non-coding RNA SNHG7 inhibits NLRP3-dependent pyroptosis by targeting the miR-34a/SIRT1 axis in liver cancer. Oncol. Lett. 20 (1), 893–901. doi:10.3892/ol.2020.11635
Coll, R. C., Hill, J. R., Day, C. J., Zamoshnikova, A., Boucher, D., Massey, N. L., et al. (2019). MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat. Chem. Biol. 15 (6), 556–559. doi:10.1038/s41589-019-0277-7
Coll, R. C., Robertson, A. A., Chae, J. J., Higgins, S. C., Munoz-Planillo, R., Inserra, M. C., et al. (2015). A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 21 (3), 248–255. doi:10.1038/nm.3806
Correa, P. (2013). Gastric cancer: Overview. Gastroenterology Clin. N. Am. 42 (2), 211–217. doi:10.1016/j.gtc.2013.01.002
Daley, D., Mani, V. R., Mohan, N., Akkad, N., Pandian, G., Savadkar, S., et al. (2017). NLRP3 signaling drives macrophage-induced adaptive immune suppression in pancreatic carcinoma. J. Exp. Med. 214 (6), 1711–1724. doi:10.1084/jem.20161707
Das, S., Shapiro, B., Vucic, E. A., Vogt, S., and Bar-Sagi, D. (2020). Tumor cell-derived IL1β promotes desmoplasia and immune suppression in pancreatic cancer. Cancer Res. 80 (5), 1088–1101. doi:10.1158/0008-5472.CAN-19-2080
Deets, K. A., and Vance, R. E. (2021). Inflammasomes and adaptive immune responses. Nat. Immunol. 22 (4), 412–422. doi:10.1038/s41590-021-00869-6
Dekker, E., Tanis, P. J., Vleugels, J. L. A., Kasi, P. M., and Wallace, M. B. (2019). Colorectal cancer. Lancet 394 (10207), 1467–1480. doi:10.1016/S0140-6736(19)32319-0
Deng, Q., Geng, Y., Zhao, L., Li, R., Zhang, Z., Li, K., et al. (2019). NLRP3 inflammasomes in macrophages drive colorectal cancer metastasis to the liver. Cancer Lett. 442, 21–30. doi:10.1016/j.canlet.2018.10.030
Ding, X., Kambara, H., Guo, R., Kanneganti, A., Acosta-Zaldivar, M., Li, J., et al. (2021). Inflammasome-mediated GSDMD activation facilitates escape of Candida albicans from macrophages. Nat. Commun. 12 (1), 6699. doi:10.1038/s41467-021-27034-9
Ding, Y., Yan, Y., Dong, Y., Xu, J., Su, W., Shi, W., et al. (2022). NLRP3 promotes immune escape by regulating immune checkpoints: A pan-cancer analysis. Int. Immunopharmacol. 104, 108512. doi:10.1016/j.intimp.2021.108512
Downs, K. P., Nguyen, H., Dorfleutner, A., and Stehlik, C. (2020). An overview of the non-canonical inflammasome. Mol. aspects Med. 76, 100924. doi:10.1016/j.mam.2020.100924
Du, Q., Wang, Q., Fan, H., Wang, J., Liu, X., Wang, H., et al. (2016). Dietary cholesterol promotes AOM-induced colorectal cancer through activating the NLRP3 inflammasome. Biochem. Pharmacol. 105, 42–54. doi:10.1016/j.bcp.2016.02.017
Du, R. H., Tan, J., Sun, X. Y., Lu, M., Ding, J. H., and Hu, G. (2016). Fluoxetine inhibits NLRP3 inflammasome activation: Implication in depression. Int. J. Neuropsychopharmacol. 19 (9), pyw037. doi:10.1093/ijnp/pyw037
Dupaul-Chicoine, J., Arabzadeh, A., Dagenais, M., Douglas, T., Champagne, C., Morizot, A., et al. (2015). The Nlrp3 inflammasome suppresses colorectal cancer metastatic growth in the liver by promoting natural killer cell tumoricidal activity. Immunity 43 (4), 751–763. doi:10.1016/j.immuni.2015.08.013
Engstrand, L., and Graham, D. Y. (2020). Microbiome and gastric cancer. Dig. Dis. Sci. 65 (3), 865–873. doi:10.1007/s10620-020-06101-z
Gao, L., Dong, X., Gong, W., Huang, W., Xue, J., Zhu, Q., et al. (2021). Acinar cell NLRP3 inflammasome and gasdermin D (GSDMD) activation mediates pyroptosis and systemic inflammation in acute pancreatitis. Br. J. Pharmacol. 178 (17), 3533–3552. doi:10.1111/bph.15499
Guo, H., Callaway, J. B., and Ting, J. P. (2015). Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 21 (7), 677–687. doi:10.1038/nm.3893
Guo, J., Chen, T., Ma, Z., Qiao, C., Yuan, F., Guo, X., et al. (2020). Oridonin inhibits 4T1 tumor growth by suppressing Treg differentiation via TGF-beta receptor. Int. Immunopharmacol. 88, 106831. doi:10.1016/j.intimp.2020.106831
He, H., Jiang, H., Chen, Y., Ye, J., Wang, A., Wang, C., et al. (2018). Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat. Commun. 9 (1), 2550. doi:10.1038/s41467-018-04947-6
He, Y., Zeng, M. Y., Yang, D., Motro, B., and Nunez, G. (2016). NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 530 (7590), 354–357. doi:10.1038/nature16959
Hirota, S. A., Ng, J., Lueng, A., Khajah, M., Parhar, K., Li, Y., et al. (2011). NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm. bowel Dis. 17 (6), 1359–1372. doi:10.1002/ibd.21478
Hong, J., Xie, Z., Yang, F., Jiang, L., Jian, T., Wang, S., et al. (2022). Erianin suppresses proliferation and migration of cancer cells in a pyruvate carboxylase-dependent manner. Fitoterapia 157, 105136. doi:10.1016/j.fitote.2022.105136
Hu, H., Wang, Y., Ding, X., He, Y., Lu, Z., Wu, P., et al. (2018). Long non-coding RNA XLOC_000647 suppresses progression of pancreatic cancer and decreases epithelial-mesenchymal transition-induced cell invasion by down-regulating NLRP3. Mol. cancer 17 (1), 18. doi:10.1186/s12943-018-0761-9
Huang, C. F., Chen, L., Li, Y. C., Wu, L., Yu, G. T., Zhang, W. F., et al. (2017). NLRP3 inflammasome activation promotes inflammation-induced carcinogenesis in head and neck squamous cell carcinoma. J. Exp. Clin. cancer Res. CR 36 (1), 116. doi:10.1186/s13046-017-0589-y
Huang, J., Fan, P., Liu, M., Weng, C., Fan, G., Zhang, T., et al. (2021). Famotidine promotes inflammation by triggering cell pyroptosis in gastric cancer cells. BMC Pharmacol. Toxicol. 22 (1), 62. doi:10.1186/s40360-021-00533-7
Huang, Y., Jiang, H., Chen, Y., Wang, X., Yang, Y., Tao, J., et al. (2018). Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. EMBO Mol. Med. 10 (4), e8689. doi:10.15252/emmm.201708689
Huber, S., Gagliani, N., Zenewicz, L. A., Huber, F. J., Bosurgi, L., Hu, B., et al. (2012). IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 491 (7423), 259–263. doi:10.1038/nature11535
Jiang, H., He, H., Chen, Y., Huang, W., Cheng, J., Ye, J., et al. (2017). Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J. Exp. Med. 214 (11), 3219–3238. doi:10.1084/jem.20171419
Juliana, C., Fernandes-Alnemri, T., Wu, J., Datta, P., Solorzano, L., Yu, J. W., et al. (2010). Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J. Biol. Chem. 285 (13), 9792–9802. doi:10.1074/jbc.M109.082305
Karmakar, M., Minns, M., Greenberg, E. N., Diaz-Aponte, J., Pestonjamasp, K., Johnson, J. L., et al. (2020). N-GSDMD trafficking to neutrophil organelles facilitates IL-1β release independently of plasma membrane pores and pyroptosis. Nat. Commun. 11 (1), 2212. doi:10.1038/s41467-020-16043-9
Kim, D. J., Park, J. H., Franchi, L., Backert, S., and Nunez, G. (2013). The Cag pathogenicity island and interaction between TLR2/NOD2 and NLRP3 regulate IL-1β production in Helicobacter pylori infected dendritic cells. Eur. J. Immunol. 43 (10), 2650–2658. doi:10.1002/eji.201243281
Koc, C., Akbulut, S., Akatli, A. N., Turkmen Samdanci, E., Tuncer, A., and Yilmaz, S. (2020). Nomenclature of appendiceal mucinous lesions according to the 2019 WHO classification of tumors of the digestive system. Turkish J. gastroenterology 31 (9), 649–657. doi:10.5152/tjg.2020.20537
Koch, K. N., Hartung, M. L., Urban, S., Kyburz, A., Bahlmann, A. S., Lind, J., et al. (2015). Helicobacter urease-induced activation of the TLR2/NLRP3/IL-18 axis protects against asthma. J. Clin. investigation 125 (8), 3297–3302. doi:10.1172/JCI79337
Kushima, R. (2022). The updated WHO classification of digestive system tumours-gastric adenocarcinoma and dysplasia. Der Pathol. 43 (1), 8–15. doi:10.1007/s00292-021-01023-7
Lamkanfi, M., and Dixit, V. M. (2014). Mechanisms and functions of inflammasomes. Cell 157 (5), 1013–1022. doi:10.1016/j.cell.2014.04.007
Latz, E., Xiao, T. S., and Stutz, A. (2013). Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 13 (6), 397–411. doi:10.1038/nri3452
Lee, H. H., Kim, D., Jung, J., Kang, H., and Cho, H. (2021). NLRP3 deficiency in hepatocellular carcinoma enhances surveillance of NK-92 through a modulation of MICA/B. Int. J. Mol. Sci. 22 (17), 9285. doi:10.3390/ijms22179285
Li, C., Lin, H., He, H., Ma, M., Jiang, W., and Zhou, R. (2022). Inhibition of the NLRP3 inflammasome activation by manoalide ameliorates experimental autoimmune encephalomyelitis pathogenesis. Front. Cell Dev. Biol. 10, 822236. doi:10.3389/fcell.2022.822236
Li, J., Guo, Q., Lei, X., Zhang, L., Su, C., Liu, Y., et al. (2020). Pristimerin induces apoptosis and inhibits proliferation, migration in H1299 Lung Cancer Cells. J. Cancer 11 (21), 6348–6355. doi:10.7150/jca.44431
Li, P., Liu, Y., and He, Q. (2020). Anisodamine suppressed the growth of hepatocellular carcinoma cells, induced apoptosis and regulated the levels of inflammatory factors by inhibiting NLRP3 inflammasome activation. Drug Des. Dev. Ther. 14, 1609–1620. doi:10.2147/DDDT.S243383
Li, Q., Feng, H., Wang, H., Wang, Y., Mou, W., Xu, G., et al. (2022). Licochalcone B specifically inhibits the NLRP3 inflammasome by disrupting NEK7-NLRP3 interaction. EMBO Rep. 23 (2), e53499. doi:10.15252/embr.202153499
Li, S., Liang, X., Ma, L., Shen, L., Li, T., Zheng, L., et al. (2018). MiR-22 sustains NLRP3 expression and attenuates H. pylori-induced gastric carcinogenesis. Oncogene 37 (7), 884–896. doi:10.1038/onc.2017.381
Li, T., Fu, B., Zhang, X., Zhou, Y., Yang, M., Cao, M., et al. (2021). Overproduction of gastrointestinal 5-HT promotes colitis-associated colorectal cancer progression via enhancing NLRP3 inflammasome activation. Cancer Immunol. Res. 9 (9), 1008–1023. doi:10.1158/2326-6066.CIR-20-1043
Li, X., He, C., Li, N., Ding, L., Chen, H., Wan, J., et al. (2020). The interplay between the gut microbiota and NLRP3 activation affects the severity of acute pancreatitis in mice. Gut microbes 11 (6), 1774–1789. doi:10.1080/19490976.2020.1770042
Li, X., Liu, S., Luo, J., Liu, A., Tang, S., Liu, S., et al. (2015). Helicobacter pylori induces IL-1β and IL-18 production in human monocytic cell line through activation of NLRP3 inflammasome via ROS signaling pathway. Pathogens Dis. 73 (4), ftu024. doi:10.1093/femspd/ftu024
Liu, H., Xu, Y., Liang, K., and Liu, R. (2020). Immune cells combined with NLRP3 inflammasome inhibitor exert better antitumor effect on pancreatic ductal adenocarcinoma. Front. Oncol. 10, 1378. doi:10.3389/fonc.2020.01378
Liu, Z., Gan, L., Xu, Y., Luo, D., Ren, Q., Wu, S., et al. (2017). Melatonin alleviates inflammasome-induced pyroptosis through inhibiting NF-κB/GSDMD signal in mice adipose tissue. J. pineal Res. 63 (1), e12414. doi:10.1111/jpi.12414
Luan, J., Zhang, X., Wang, S., Li, Y., Fan, J., Chen, W., et al. (2018). NOD-like receptor protein 3 inflammasome-dependent IL-1β accelerated ConA-induced hepatitis. Front. Immunol. 9, 758. doi:10.3389/fimmu.2018.00758
Man, S. M. (2018). Inflammasomes in the gastrointestinal tract: Infection, cancer and gut microbiota homeostasis. Nat. Rev. Gastroenterology hepatology 15 (12), 721–737. doi:10.1038/s41575-018-0054-1
Marandi, Y., Hashemzade, S., Tayebinia, H., Karimi, J., Zamani, A., and Khodadadi, I. (2021). NLRP3-inflammasome activation is associated with epithelial-mesenchymal transition and progression of colorectal cancer. Iran. J. basic Med. Sci. 24 (4), 483–492. doi:10.22038/ijbms.2021.52355.11835
Marchetti, C., Swartzwelter, B., Gamboni, F., Neff, C. P., Richter, K., Azam, T., et al. (2018). OLT1177, a beta-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc. Natl. Acad. Sci. U. S. A. 115 (7), E1530–E1539. doi:10.1073/pnas.1716095115
Martinon, F., Mayor, A., and Tschopp, J. (2009). The inflammasomes: Guardians of the body. Annu. Rev. Immunol. 27, 229–265. doi:10.1146/annurev.immunol.021908.132715
Matsushita, H., Miyake, Y., Takaki, A., Yasunaka, T., Koike, K., Ikeda, F., et al. (2015). TLR4, TLR9, and NLRP3 in biliary epithelial cells of primary sclerosing cholangitis: Relationship with clinical characteristics. J. gastroenterology hepatology 30 (3), 600–608. doi:10.1111/jgh.12711
Mekni, N., De Rosa, M., Cipollina, C., Gulotta, M. R., De Simone, G., Lombino, J., et al. (2019). In silico insights towards the identification of NLRP3 druggable hot spots. Int. J. Mol. Sci. 20 (20), 4974. doi:10.3390/ijms20204974
Molina-Castro, S., Pereira-Marques, J., Figueiredo, C., Machado, J. C., and Varon, C. (2017). Gastric cancer: Basic aspects. Helicobacter 22, e12412. doi:10.1111/hel.12412
Mridha, A. R., Wree, A., Robertson, A. A. B., Yeh, M. M., Johnson, C. D., Van Rooyen, D. M., et al. (2017). NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J. hepatology 66 (5), 1037–1046. doi:10.1016/j.jhep.2017.01.022
Nagtegaal, I. D., Odze, R. D., Klimstra, D., Paradis, V., Rugge, M., Schirmacher, P., et al. (2020). The 2019 WHO classification of tumours of the digestive system. Histopathology 76 (2), 182–188. doi:10.1111/his.13975
Ng, G. Z., Menheniott, T. R., Every, A. L., Stent, A., Judd, L. M., Chionh, Y. T., et al. (2016). The MUC1 mucin protects against Helicobacter pylori pathogenesis in mice by regulation of the NLRP3 inflammasome. Gut 65 (7), 1087–1099. doi:10.1136/gutjnl-2014-307175
Oguma, K., Oshima, H., and Oshima, M. (2010). Inflammation, tumor necrosis factor and Wnt promotion in gastric cancer development. Future Oncol. 6 (4), 515–526. doi:10.2217/fon.10.13
Perera, A. P., Sajnani, K., Dickinson, J., Eri, R., and Korner, H. (2018). NLRP3 inflammasome in colitis and colitis-associated colorectal cancer. Mammalian genome official J. Int. Mammalian Genome Soc. 29 (11-12), 817–830. doi:10.1007/s00335-018-9783-2
Perez-Figueroa, E., Torres, J., Sanchez-Zauco, N., Contreras-Ramos, A., Alvarez-Arellano, L., and Maldonado-Bernal, C. (2016). Activation of NLRP3 inflammasome in human neutrophils by Helicobacter pylori infection. Innate Immun. 22 (2), 103–112. doi:10.1177/1753425915619475
Plummer, M., de Martel, C., Vignat, J., Ferlay, J., Bray, F., and Franceschi, S. (2016). Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob. health 4 (9), e609–e616. doi:10.1016/S2214-109X(16)30143-7
Pourcet, B., Zecchin, M., Ferri, L., Beauchamp, J., Sitaula, S., Billon, C., et al. (2018). Nuclear receptor subfamily 1 group D member 1 regulates circadian activity of NLRP3 inflammasome to reduce the severity of fulminant hepatitis in mice. Gastroenterology 154 (5), 1449–1464. e1420. doi:10.1053/j.gastro.2017.12.019
Proell, M., Riedl, S. J., Fritz, J. H., Rojas, A. M., and Schwarzenbacher, R. (2008). The nod-like receptor (NLR) family: A tale of similarities and differences. PloS one 3 (4), e2119. doi:10.1371/journal.pone.0002119
Rahman, T., Nagar, A., Duffy, E. B., Okuda, K., Silverman, N., and Harton, J. A. (2020). NLRP3 sensing of diverse inflammatory stimuli requires distinct structural features. Front. Immunol. 11, 1828. doi:10.3389/fimmu.2020.01828
Salem, A., Mistry, H., Backen, A., Hodgson, C., Koh, P., Dean, E., et al. (2018). Cell death, inflammation, tumor burden, and proliferation blood biomarkers predict lung cancer radiotherapy response and correlate with tumor volume and proliferation imaging. Clin. lung cancer 19 (3), 239–248. doi:10.1016/j.cllc.2017.12.002
Sang, X., Chen, Y., Chen, W., Xie, J., Meng, G., Zhong, J., et al. (2018). Celastrol specifically inhibits the activation of NLRP3 inflammasome. Sci. China Life Sci. 61 (3), 355–357. doi:10.1007/s11427-017-9048-8
Schneider, K. S., Gross, C. J., Dreier, R. F., Saller, B. S., Mishra, R., Gorka, O., et al. (2017). The inflammasome drives GSDMD-independent secondary pyroptosis and IL-1 release in the absence of caspase-1 protease activity. Cell Rep. 21 (13), 3846–3859. doi:10.1016/j.celrep.2017.12.018
Scicinski, J., Fisher, G., Carter, C., Cho-Phan, C., Kunz, P., Ning, S., et al. (2015). The development of RRx-001, A novel nitric-oxide-mediated epigenetically active anticancer agent. Redox Biol. 5, 422. doi:10.1016/j.redox.2015.09.035
Semper, R. P., Mejias-Luque, R., Gross, C., Anderl, F., Muller, A., Vieth, M., et al. (2014). Helicobacter pylori-induced IL-1β secretion in innate immune cells is regulated by the NLRP3 inflammasome and requires the cag pathogenicity island. J. Immunol. 193 (7), 3566–3576. doi:10.4049/jimmunol.1400362
Sendler, M., van den Brandt, C., Glaubitz, J., Wilden, A., Golchert, J., Weiss, F. U., et al. (2020). NLRP3 inflammasome regulates development of systemic inflammatory response and compensatory anti-inflammatory response syndromes in mice with acute pancreatitis. Gastroenterology 158 (1), 253–269. doi:10.1053/j.gastro.2019.09.040
Shao, B. Z., Xu, Z. Q., Han, B. Z., Su, D. F., and Liu, C. (2015). NLRP3 inflammasome and its inhibitors: A review. Front. Pharmacol. 6, 262. doi:10.3389/fphar.2015.00262
Shao, X., Lei, Z., and Zhou, C. (2020). NLRP3 promotes colorectal cancer cell proliferation and metastasis via regulating epithelial mesenchymal transformation. Anti-cancer agents Med. Chem. 20 (7), 820–827. doi:10.2174/1871520620666200220112741
Sharma, B. R., and Kanneganti, T. D. (2021). NLRP3 inflammasome in cancer and metabolic diseases. Nat. Immunol. 22 (5), 550–559. doi:10.1038/s41590-021-00886-5
Shi, F., Wei, B., Lan, T., Xiao, Y., Quan, X., Chen, J., et al. (2021). Low NLRP3 expression predicts a better prognosis of colorectal cancer. Biosci. Rep. 41 (4), BSR20210280. doi:10.1042/BSR20210280
Shi, H., Gao, Y., Dong, Z., Yang, J., Gao, R., Li, X., et al. (2021). GSDMD-mediated cardiomyocyte pyroptosis promotes myocardial I/R injury. Circulation Res. 129 (3), 383–396. doi:10.1161/CIRCRESAHA.120.318629
Shi, J., Xia, Y., Wang, H., Yi, Z., Zhang, R., and Zhang, X. (2021). Piperlongumine is an NLRP3 inhibitor with anti-inflammatory activity. Front. Pharmacol. 12, 818326. doi:10.3389/fphar.2021.818326
Shi, J., Zhao, Y., Wang, K., Shi, X., Wang, Y., Huang, H., et al. (2015). Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526 (7575), 660–665. doi:10.1038/nature15514
Shi, Y., Lv, Q., Zheng, M., Sun, H., and Shi, F. (2021). NLRP3 inflammasome inhibitor INF39 attenuated NLRP3 assembly in macrophages. Int. Immunopharmacol. 92, 107358. doi:10.1016/j.intimp.2020.107358
Siegel, R. L., Miller, K. D., Fuchs, H. E., and Jemal, A. (2022). Cancer statistics, 2016. CA a cancer J. Clin. 72 (1), 7–30. doi:10.3322/caac.21332
Smyth, E. C., Nilsson, M., Grabsch, H. I., van Grieken, N. C., and Lordick, F. (2020). Gastric cancer. Lancet 396 (10251), 635–648. doi:10.1016/S0140-6736(20)31288-5
Sonohara, F., Inokawa, Y., Kanda, M., Nishikawa, Y., Yamada, S., Fujii, T., et al. (2017). Association of inflammasome components in background liver with poor prognosis after curatively-resected hepatocellular carcinoma. Anticancer Res. 37 (1), 293–300. doi:10.21873/anticanres.11320
Sun, R., Gao, D. S., Shoush, J., and Lu, B. (2022). The IL-1 family in tumorigenesis and antitumor immunity. Seminars cancer Biol. 86, 280–295. doi:10.1016/j.semcancer.2022.05.002
Surlin, P., Lazar, L., Sincar, C., Gheorghe, D. N., Popescu, D. M., Boldeanu, V. M., et al. (2021). NLRP3 inflammasome expression in gingival crevicular fluid of patients with periodontitis and chronic hepatitis C. Mediat. Inflamm. 2021, 6917919. doi:10.1155/2021/6917919
Swanson, K. V., Deng, M., and Ting, J. P. (2019). The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 19 (8), 477–489. doi:10.1038/s41577-019-0165-0
Tang, Y., Cao, G., Zhao, G., Wang, C., and Qin, Q. (2020). LncRNA differentiation antagonizing non-protein coding RNA promotes proliferation and invasion through regulating miR-135a/NLRP37 axis in pancreatic cancer. Investig. new drugs 38 (3), 714–721. doi:10.1007/s10637-019-00798-0
Tao, Y., Wang, N., Qiu, T., and Sun, X. (2020). The role of autophagy and NLRP3 inflammasome in liver fibrosis. BioMed Res. Int. 2020, 7269150. doi:10.1155/2020/7269150
Tapia-Abellan, A., Angosto-Bazarra, D., Martinez-Banaclocha, H., de Torre-Minguela, C., Ceron-Carrasco, J. P., Perez-Sanchez, H., et al. (2019). MCC950 closes the active conformation of NLRP3 to an inactive state. Nat. Chem. Biol. 15 (6), 560–564. doi:10.1038/s41589-019-0278-6
Thrift, A. P., and El-Serag, H. B. (2020). Burden of gastric cancer. Clin. gastroenterology hepatology 18 (3), 534–542. doi:10.1016/j.cgh.2019.07.045
Van Cutsem, E., Sagaert, X., Topal, B., Haustermans, K., and Prenen, H. (2016). Gastric cancer. Lancet 388 (10060), 2654–2664. doi:10.1016/S0140-6736(16)30354-3
Vandanmagsar, B., Youm, Y. H., Ravussin, A., Galgani, J. E., Stadler, K., Mynatt, R. L., et al. (2011). The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 17 (2), 179–188. doi:10.1038/nm.2279
Wan, X., Xu, C., Lin, Y., Lu, C., Li, D., Sang, J., et al. (2016). Uric acid regulates hepatic steatosis and insulin resistance through the NLRP3 inflammasome-dependent mechanism. J. hepatology 64 (4), 925–932. doi:10.1016/j.jhep.2015.11.022
Wang, F., Meng, W., Wang, B., and Qiao, L. (2014). Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 345 (2), 196–202. doi:10.1016/j.canlet.2013.08.016
Wang, H., Wang, Y., Du, Q., Lu, P., Fan, H., Lu, J., et al. (2016). Inflammasome-independent NLRP3 is required for epithelial-mesenchymal transition in colon cancer cells. Exp. Cell Res. 342 (2), 184–192. doi:10.1016/j.yexcr.2016.03.009
Wang, Y., Shi, P., Chen, Q., Huang, Z., Zou, D., Zhang, J., et al. (2019). Mitochondrial ROS promote macrophage pyroptosis by inducing GSDMD oxidation. J. Mol. Cell Biol. 11 (12), 1069–1082. doi:10.1093/jmcb/mjz020
Washington, M. K., Goldberg, R. M., Chang, G. J., Limburg, P., Lam, A. K., Salto-Tellez, M., et al. (2021). Diagnosis of digestive system tumours. Int. J. cancer 148 (5), 1040–1050. doi:10.1002/ijc.33210
Watanabe, H., Gehrke, S., Contassot, E., Roques, S., Tschopp, J., Friedmann, P. S., et al. (2008). Danger signaling through the inflammasome acts as a master switch between tolerance and sensitization. J. Immunol. 180 (9), 5826–5832. doi:10.4049/jimmunol.180.9.5826
Wei, Q., Guo, P., Mu, K., Zhang, Y., Zhao, W., Huai, W., et al. (2015). Estrogen suppresses hepatocellular carcinoma cells through ERβ-mediated upregulation of the NLRP3 inflammasome. Lab. Investig. 95 (7), 804–816. doi:10.1038/labinvest.2015.63
Wei, Q., Mu, K., Li, T., Zhang, Y., Yang, Z., Jia, X., et al. (2014). Deregulation of the NLRP3 inflammasome in hepatic parenchymal cells during liver cancer progression. Laboratory investigation; a J. Tech. methods pathology 94 (1), 52–62. doi:10.1038/labinvest.2013.126
Wei, Q., Zhu, R., Zhu, J., Zhao, R., and Li, M. (2019). E2-Induced activation of the NLRP3 inflammasome triggers pyroptosis and inhibits autophagy in HCC cells. Oncol. Res. 27 (7), 827–834. doi:10.3727/096504018X15462920753012
West, A. J., Deswaerte, V., West, A. C., Gearing, L. J., Tan, P., and Jenkins, B. J. (2022). Inflammasome-associated gastric tumorigenesis is independent of the NLRP3 pattern recognition receptor. Front. Oncol. 12, 830350. doi:10.3389/fonc.2022.830350
Wree, A., Eguchi, A., McGeough, M. D., Pena, C. A., Johnson, C. D., Canbay, A., et al. (2014). NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology 59 (3), 898–910. doi:10.1002/hep.26592
Xie, W. H., Ding, J., Xie, X. X., Yang, X. H., Wu, X. F., Chen, Z. X., et al. (2020). Hepatitis B virus X protein promotes liver cell pyroptosis under oxidative stress through NLRP3 inflammasome activation. Inflamm. Res. 69 (7), 683–696. doi:10.1007/s00011-020-01351-z
Xu, Y., Li, H., Chen, W., Yao, X., Xing, Y., Wang, X., et al. (2013). Mycoplasma hyorhinis activates the NLRP3 inflammasome and promotes migration and invasion of gastric cancer cells. PloS one 8 (11), e77955. doi:10.1371/journal.pone.0077955
Yang, A., Li, M. Y., Zhang, Z. H., Wang, J. Y., Xing, Y., Ri, M., et al. (2021). Erianin regulates programmed cell death ligand 1 expression and enhances cytotoxic T lymphocyte activity. J. Ethnopharmacol. 273, 113598. doi:10.1016/j.jep.2020.113598
Yao, X., Zhang, C., Xing, Y., Xue, G., Zhang, Q., Pan, F., et al. (2017). Remodelling of the gut microbiota by hyperactive NLRP3 induces regulatory T cells to maintain homeostasis. Nat. Commun. 8 (1), 1896. doi:10.1038/s41467-017-01917-2
Yaw, A. C. K., Chan, E. W. L., Yap, J. K. Y., and Mai, C. W. (2020). The effects of NLRP3 inflammasome inhibition by MCC950 on LPS-induced pancreatic adenocarcinoma inflammation. J. cancer Res. Clin. Oncol. 146 (9), 2219–2229. doi:10.1007/s00432-020-03274-y
Yu, S., Yin, J. J., Miao, J. X., Li, S. G., Huang, C. Z., Huang, N., et al. (2020). Activation of NLRP3 inflammasome promotes the proliferation and migration of esophageal squamous cell carcinoma. Oncol. Rep. 43 (4), 1113–1124. doi:10.3892/or.2020.7493
Yu, X., Zhao, Q., Zhang, X., Zhang, H., Liu, Y., Wu, X., et al. (2017). Celastrol ameliorates inflammation through inhibition of NLRP3 inflammasome activation. Oncotarget 8 (40), 67300–67314. doi:10.18632/oncotarget.18619
Zaki, M. H., Boyd, K. L., Vogel, P., Kastan, M. B., Lamkanfi, M., and Kanneganti, T. D. (2010). The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 32 (3), 379–391. doi:10.1016/j.immuni.2010.03.003
Zaki, M. H., Lamkanfi, M., and Kanneganti, T. D. (2011). The Nlrp3 inflammasome: Contributions to intestinal homeostasis. Trends Immunol. 32 (4), 171–179. doi:10.1016/j.it.2011.02.002
Zhang, H. Q., Xie, X. F., Li, G. M., Chen, J. R., Li, M. T., Xu, X., et al. (2021). Erianin inhibits human lung cancer cell growth via PI3K/Akt/mTOR pathway in vitro and in vivo. Phytotherapy Res. PTR 35 (8), 4511–4525. doi:10.1002/ptr.7154
Zhang, L., Wang, Y., Liu, X., and Zhang, Y. (2022). NLRP3 inflammasome activation in mfs-CRC crosstalk promotes colorectal cancer metastasis. Ann. Clin. laboratory Sci. 52 (4), 571–579.
Zhang, Q., Wang, H., Mao, C., Sun, M., Dominah, G., Chen, L., et al. (2018). Fatty acid oxidation contributes to IL-1β secretion in M2 macrophages and promotes macrophage-mediated tumor cell migration. Mol. Immunol. 94, 27–35. doi:10.1016/j.molimm.2017.12.011
Zhang, X., Hu, L., Xu, S., Ye, C., and Chen, A. (2021). Erianin: A direct NLRP3 inhibitor with remarkable anti-inflammatory activity. Front. Immunol. 12, 739953. doi:10.3389/fimmu.2021.739953
Zhang, X., Li, C., Chen, D., He, X., Zhao, Y., Bao, L., et al. (2022). H. pylori CagA activates the NLRP3 inflammasome to promote gastric cancer cell migration and invasion. Inflamm. Res. 71 (1), 141–155. doi:10.1007/s00011-021-01522-6
Zhao, Q., Bi, Y., Guo, J., Liu, Y. X., Zhong, J., Pan, L. R., et al. (2021). Pristimerin protects against inflammation and metabolic disorder in mice through inhibition of NLRP3 inflammasome activation. Acta Pharmacol. Sin. 42 (6), 975–986. doi:10.1038/s41401-020-00527-x
Keywords: digestive system malignancy, pro-tumorigenic, anti-tumorigenic, NLRP3, inflammasome
Citation: Sun C-C, Li L, Tao H-Q, Jiang Z-C, Wang L and Wang H-J (2022) The role of NLRP3 inflammasome in digestive system malignancy. Front. Cell Dev. Biol. 10:1051612. doi: 10.3389/fcell.2022.1051612
Received: 23 September 2022; Accepted: 13 December 2022;
Published: 23 December 2022.
Edited by:
Zhi-Gang Zhang, Shanghai Jiao Tong University, ChinaReviewed by:
Balaji Banoth, St. Jude Children's Research Hospital, United StatesFan Xin, Affiliated Hospital of Jiangsu University, China
Copyright © 2022 Sun, Li, Tao, Jiang, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui-Ju Wang, d2hqMzM0NEAxNjMuY29t; Liang Wang, MTM4NTgwNzIxMDdAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Cen-Cen Sun1†
Cen-Cen Sun1† Li Li
Li Li Zhi-Chen Jiang
Zhi-Chen Jiang Hui-Ju Wang
Hui-Ju Wang