- 1Department of Cardiology, The First Affiliated Hospital of Ningbo University, Ningbo, China
- 2Department of Cardiology, The Second Affiliated Hospital of Zhejiang University, Hangzhou, China
Calcific aortic valve disease (CAVD) is a common cardiovascular condition in the elderly population. The aortic valve, influenced by factors such as endothelial dysfunction, inflammation, oxidative stress, lipid metabolism disorders, calcium deposition, and extracellular matrix remodeling, undergoes fibrosis and calcification, ultimately leading to stenosis. In recent years, long non-coding RNAs (lncRNAs) have emerged as significant regulators of gene expression, playing crucial roles in the occurrence and progression of various diseases. Research has shown that lncRNAs participate in the pathological process underlying CAVD by regulating osteogenic differentiation and inflammatory response of valve interstitial cells. Specifically, lncRNAs, such as H19, MALAT1, and TUG1, are closely associated with CAVD. Some lncRNAs can act as miRNA sponges, form complex regulatory networks, and modulate the expression of calcification-related genes. In brief, this review discusses the mechanisms and potential therapeutic targets of lncRNAs in CAVD.
1 Introduction
Calcific aortic valve disease (CAVD) is a prevalent heart valve condition, particularly among the elderly, which is associated with high morbidity and mortality rates (1). This disease is associated with significant economic and social burdens. The primary risk factors for CAVD are bicuspid aortic valve (BAV) and advanced age (2). CAVD is characterized by fibrous calcification of the aortic valve leaflets. In the early stages, the disease presents with valve thickening and mild calcification that do not impair the valve function. However, calcification exacerbates over time, resulting in aortic stenosis (AS) (3). Severe AS is often accompanied by shortness of breath, angina, and syncope. Treatment options for severe AS include surgical aortic valve replacement (SAVR) and transcatheter aortic valve replacement (TAVR) (4).
Long noncoding RNAs (lncRNAs) are noncoding RNAs greater than 200 nucleotides in length. As significant and widely used regulatory elements, they play important roles in various cellular processes and development of numerous diseases (5–7). Initially, lncRNAs were thought to be “transcriptional noise,” but they were later recognized to have precise regulatory functions (8). As competitive endogenous RNA (ceRNA), lncRNAs exert gene regulatory functions by sponging miRNAs, directly binding to proteins to alter their function, or serving as scaffolds to recruit transcriptional inhibitors or activators (9, 10). An increasing number of studies have shown that lncRNAs play critical roles in the occurrence and development of cardiovascular diseases, including CAVD, acute myocardial infarction, and heart failure (5, 11). Specific biomarkers may predict calcification progression or disease prognosis, thereby aiding in diagnosis and treating CAVD. This article reviews the research progress regarding lncRNAs in CAVD.
2 Pathogenesis of CAVD
Although CAVD pathogenesis is not fully understood, it is believed to result from cellular dysfunction and dysregulation (12). This highly regulated process occurs at the molecular and cellular levels and involves various factors, such as endothelial dysfunction and injury, inflammation, oxidative stress, lipid metabolism disorders, calcium deposition, and extracellular matrix remodeling (12–15). These factors ultimately lead to fibrosis and ossification of valve interstitial cells (VICs), the main cellular components of the aortic valve leaflets (16). Inactive VICs are usually activated and undergo phenotypic transformation into osteoblast-like VICs, which calcify the aortic valves (17). Valvular endothelial cells (VECs) cover the valve surface to form an endothelial monolayer and may undergo endothelial-to-mesenchymal transformation (End-MT), expressing mesenchymal characteristics and plays a pathological role in CAVD development (18, 19). During the occurrence and development stages of CAVD, the abnormal accumulation of different lipid types and increased expression of inflammatory factors such as TNF-α and IL-6 promote osteogenic processes and calcification of VICs (2, 20). Increasing evidence suggests that various signaling pathways such as the NF-κB, TGF-β, Wnt/β-catenin, Notch, and BMP pathways, are involved in aortic valve fibrosis and calcification (21–25). These signaling pathways interact with each other to regulate the occurrence and development of CAVD.
In other cardiovascular diseases, certain lncRNAs have been implicated in the regulation of inflammation, oxidative stress responses and lipid metabolism (26). For example, H19 represses oxidative stress to improve diabetic cardiomyopathy (27), HOTAIR regulates oxidative stress and cardiac myocyte apoptosis during ischemia-reperfusion injury (28), and MALAT1 is involved in the regulation of lipid accumulation and chronic inflammation in atherosclerosis (29). This suggests that lncRNAs may interact with other associated factors to collaboratively modulate the progression of CAVD.
3 Roles and molecular mechanisms of lncRNAs in CAVD
3.1 HOTAIR
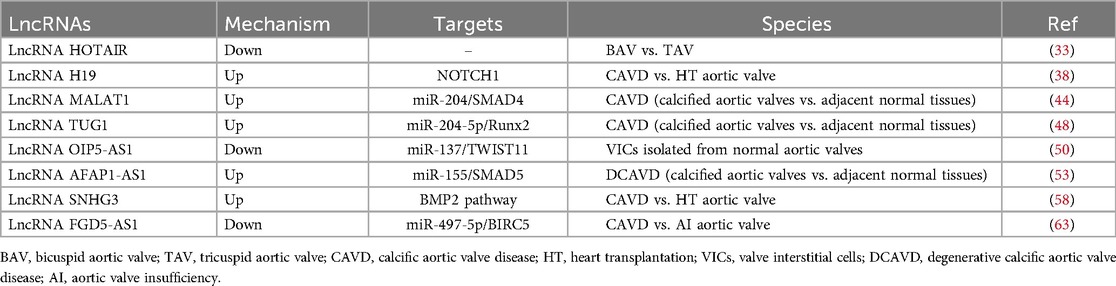
HOX transcript antisense intergenic RNA (HOTAIR) is a 2.2 kb lncRNA believed to regulate HOX expression (30). It can bind to polycomb repressive complex 2 (PRC2) and inhibit the transcription of specific genes by promoting epigenetic modifications of H3K27me3 (30, 31). HOTAIR has received widespread attention as a biomarker for tumor diagnosis (32). Recent studies have shown that HOTAIR plays an important regulatory role in CAVD. Compared to the normal tricuspid aortic valve (TAV), BAV leaflets are subjected to greater mechanical stress, resulting in a significant decrease in HOTAIR expression (33). This decreased expression increases the expression of calcification genes such as ALPL and BMP2, promoting calcification of the aortic valve (33, 34). The WNT/β-catenin signaling pathway plays a crucial role in this process, and mechanical stress can activate this signaling pathway, inhibiting the expression of HOTAIR (33). However, further studies are needed to determine whether HOTAIR directly regulates CAVD.
3.2 H19
H19 is another lncRNA involved in CAVD. Its mature product has a total length of 2.3 kb, does not encode proteins but can act on multiple gene regulatory levels to exert its biological functions (35, 36). H19 is the first imprinted gene to be discovered and shows parent-of-origin epigenetic signatures (37).
Hadji et al. found that the expression of H19 is significantly increased in calcified aortic valves and can be upregulated in the early stages of the disease (38). Its expression level is positively correlated with the progression rate of aortic valve stenosis. In CAVD tissue, the CpG methylation level in the H19 promoter region is significantly reduced, which is the main reason for the significant increase in H19 expression. Osteogenic genes, such as RUNX2, bone morphogenetic protein-2 (BMP2), and osteocalcin (BGLAP) are involved in calcification (39–41). In vitro experiments have shown that H19 overexpression can promote the BMP2 and RUNX2 expression, as well as the osteogenic phenotype, in VICs (38). Notch1 signaling is an important regulatory pathway for the transdifferentiation of VICs into osteoblast-like cells (42, 43). H19 can inhibit the binding of transcription factor p53 to the promoter region of Notch1, upregulating the expression of the calcification-promoting genes BMP2 and RUNX2 by inhibiting the Notch1 signaling pathway and promoting the occurrence of CAVD (38).
3.3 MALAT1
The metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) was initially identified in early non-small cell lung cancer. Recently, MALAT1 has been shown to be involved in the pathological regulation of cardiovascular diseases at various levels (29).
Xiao et al. compared the MALAT1 expression levels in calcified and adjacent normal tissues in 20 pairs of calcified aortic valves (44). They found, for the first time, that MALAT1 expression is significantly increased in the calcified leaflet tissue and shows a gradually increasing trend after osteogenic induction in VICs. The expression levels of MALAT1 and osteogenesis-specific genes such as ALP and osteocalcin are highly positively correlated. Further research has found that MALAT1 overexpression can promote the osteogenic differentiation of VICs, whereas silencing MALAT1 can inhibit ALP activity induced by the osteogenic medium, calcified nodule formation, and osteocalcin expression. They also found that MALAT1 sponges miR-204, thereby inhibiting its expression and activity. Smad4 is a direct target of miR-204. Overexpression of MALAT1 upregulates Smad4 by inhibiting miR-204 expression, thereby promoting the osteogenic differentiation of VICs.
3.4 TUG1
Taurine-upregulated gene 1 (TUG1) is located on chromosome 22q12 and is approximately 7.1 kb in length (45). As a competitive endogenous RNA (ceRNA), it regulates gene expression by sponging miRNAs and is involved in the development of various cancers (46). Increasing evidence indicates that TUG1 is involved in the development and prognosis of cardiovascular diseases (47).
Yu et al. have reported the mechanism of action of TUG1 in CAVD (48). They compared the expression levels of TUG1 in 40 pairs of calcified aortic valve and adjacent normal valve tissues and found that its expression is significantly increased in calcified valve tissues. In vitro cell experiments have shown that TUG1 is significantly upregulated during the calcification of VICs and is positively correlated with the expression levels of osteogenic markers, including ALP, osteocalcin, and osteopontin. Silencing TUG1 can inhibit the differentiation of VICs into osteoblast-like cells and calcified nodule formation, whereas its overexpression leads to the opposite result. The study also found that TUG1 interacts with miR-204-5p and promote RUNX2 expression by regulating miR-204-5p. Additionally, the regulatory effect of TUG1 on aortic valve calcification was confirmed in an ApoE−/− mouse model. They also found that targeting of TUG1 significantly reduces high-cholesterol diet-induced aortic valve calcification in ApoE−/− mice, which can provide evidence for a therapeutic strategy in CAVD treatment.
3.5 OIP5-AS1
OIP5 antisense transcript 1 (OIP5-AS1) is highly expressed in the nervous system and plays a crucial role in tumor transformation (49). OIP5-AS1 also plays a regulatory role in heart diseases. Hadji et al. performed RNA sequencing on 9 tricuspid valve CAVDs and 10 control non-calcified aortic valves and found that, compared with the control non-calcified aortic valves, OIP5-AS1 is significantly downregulated in calcified aortic valves (38). Zheng et al. conducted further research on its regulatory mechanisms (50). In vitro experiments have shown that during the osteogenic differentiation of VICs, the expression of OIP5-AS1 is significantly reduced, whereas the mRNA levels of osteogenic differentiation markers, including ALP, osteocalcin, and osteopontin, are increased. In addition, the overexpression of OIP5-AS1 in VICs significantly decreased the levels of these osteogenic differentiation markers. Mechanistically, further experiments showed that OIP5-AS1 could alleviate the osteogenic differentiation of VICs by upregulating TWIST1, the target gene of miR-137.
3.6 AFAP1-AS1
Actin filament-associated protein 1 antisense RNA 1 (AFAP1-AS1) is a 6810-nt lncRNA transcribed from the antisense DNA strand of AFAP1 (51). It is upregulated in various tumors and is associated with tumorigenesis (52).
In terms of heart disease, Hadji et al. found that AFAP1-AS1 level in calcified aortic valves is significantly higher than those in non-calcified aortic valves (38). Further research by He et al. showed that the expression level of AFAP1-AS1 significantly increased after calcification of the aortic valves and osteogenic induction of human VICs (53). Additionally, overexpression of AFAP1-AS1 promoted the osteogenic differentiation of VICs, whereas knockdown of AFAP1-AS1 inhibited osteogenic differentiation. Mechanistically, AFAP1-AS1 can sponge miR-155, thereby increasing the expression of SMAD5 to promote the osteogenic differentiation of VICs.
Moreover, compared to normal aortic valves, calcified ones are often accompanied by a large amount of macrophage infiltration, and the number of macrophages is positively correlated with the degree of calcification and diseases severity (54–56). In addition, studies by He et al. have shown that AFAP1-AS1 can aggravate the osteogenic differentiation of VICs by promoting the polarization of M1 macrophages, playing an important role in CAVD (56).
3.7 SNHG3
Small nucleolar RNA host gene 3 (SNHG3) is located on chromosome 1p35 and is mainly associated with the occurrence and development of various malignant tumors (57). Chen et al. found that SNHG3 is highly expressed in the calcified valves of patients with CAVD and in VICs undergoing osteogenic differentiation, and that SNHG3 expression is positively correlated with CAVD progression (58). Silencing of SNHG3 can inhibit ALP activity induced by the osteogenic differentiation medium, calcium ion concentration, calcified nodule formation, and protein levels of osteogenic differentiation markers. Conversely, SNHG3 overexpression promotes the osteogenic differentiation of VICs. In vivo experiments show that SNHG3 silencing ameliorates aortic valve calcification independent of metabolic regulation in ApoE−/− mice. Further research demonstrated that SNHG3 inhibits trimethylation of the BMP2 promoter by interacting with EZH2 (the core component of PRC2) and activates the BMP2 signaling pathway, thereby promoting the osteogenic differentiation of VICs.
3.8 FGD5-AS1
FGD5 antisense RNA 1 (FGD5-AS1) is a newly discovered lncRNA that plays an important role in cardiovascular diseases, including congenital heart diseases, myocardial ischemia/reperfusion injury, acute myocardial infarction, and dilated cardiomyopathy (59–62). Recently, Wei et al. found that the expression levels of FGD5-AS1 and BIRC5 are decreased in patients with CAVD, whereas the expression level of miR-497-5p increased (63). In vitro cell experiments demonstrated that osteogenic differentiation of VICs leads to increased ALP activity and calcium nodules, with gradually increasing levels of osteogenic differentiation markers (RUNX2 and OPN). Subsequent results showed that overexpression of FGD5-AS1 results in decreased ALP activity and calcium nodules, as well as expression of RUNX2 and OPN, thereby alleviating CAVD. Further mechanistic studies revealed that FGD5-AS1 regulates the osteogenic differentiation of VICs through the miR-497-5p/BIRC5 pathway. In vivo experiments have shown that enhancement of FGD5-AS1 effectively alleviates aortic valve leaflet thickness and calcium deposition in ApoE−/− mice (Table 1).
4 Treatment of CAVD
Currently, apart from TAVR and SAVR, no effective drug treatment is available to prevent or slow CAVD progression (4, 64, 65). Identifying sensitive and specific biomarkers like lncRNAs for the early detection of CAVD is crucial. Early detection could enable the timely application of therapeutic interventions, potentially slowing or halting the progression of CAVD and preventing irreversible damage to the aortic valve (24). Studies have demonstrated that the repression of TUG1 and SNHG3, as well as enhancement of FGD5-AS1, can ameliorate aortic valve calcification in mice, offering potential therapeutic avenues for lncRNA-based treatment of CAVD (48, 58, 63).
Although no treatments directly targeting lncRNA have yet reached clinical application, their therapeutic potential is incontestable (66). The approaches to lncRNA targeting such as antisense oligonucleotides (ASOs), small interfering RNAs (siRNAs), and CRISPR-Cas9 may become novel treatment strategies for CAVD (67). These techniques are frequently employed in cancer research and treatment. For example, ASOs targeting MALAT1 have been shown to effectively reduce tumor growth and metastasis in lung cancer (68). Silencing HOTAIR through RNA interference (RNAi) inhibits the invasion and proliferation of human colon cancer cells (69). The CRISPR-Cas9 system facilitates targeted gene editing by generating double-strand breaks (DSBs) in the DNA (70). Several approaches have been developed to manipulate lncRNA expression using the CRISPR-Cas9 system, including CRISPR interference (CRISPRi), CRISPR activation (CRISPRa), and CRISPR-Cas9 mediated knockout (70). Targeting MALAT1 by CRISPR/Cas9 technique inhibits the cell proliferation and migration in prostate cancer (71). Targeting lncRNAs with the CRISPR-Cas9 system may be a promising strategy for developing novel therapeutic interventions.
The delivery system is crucial for the therapeutic potential of lncRNAs. It must exhibit satisfactory specificity, stability, cell permeability, and low immunogenicity (72). Nanoformulation-mediated delivery and exosome-mediated delivery of targeted lncRNAs are promising avenues (73). A study reveals the therapeutic potential of lipid nanoparticles (LNPs) for targeted OIP5-AS1 delivery in mitigating MI/R injury (74). In another study, it was demonstrated that exosomes containing oxaliplatin and lncRNA PGM5-AS1 can reverse oxaliplatin resistance in colorectal cancer therapy (75). And exosome-mediated delivery of CRISPR-Cas9 has been identified as a biocompatible delivery system for gene editing in oncology (76).
However, the development of lncRNA-targeted therapeutics for CAVD will encounter challenges such as off-target effects, delivery specificity and stability. Modifications of Cas9 or sgRNA, along with sgRNA truncation, are effective strategies to reduce off-target effects (77–79). The chemical modification of ASOs may overcome the challenges associated with poor stability (72). And before proceeding to clinical trials, safety and toxicity studies must be conducted. Therefore, further technological progress is required.
5 Discussion
CAVD is a common heart valve disease characterized by multifactorial and multistep pathological processes including endothelial dysfunction, inflammation, lipid metabolism disorders, calcium deposition, and other complex biological mechanisms (12). Currently, the research significance of lncRNAs has increased, and growing attention has been focused on relationship between CAVD and lncRNAs. As biomarkers, lncRNAs have certain advantages. In a study published in Science in 2017, 499 lncRNA molecules with important functions for cell growth were identified, of which 89% functioned in specific cell types, indicating that lncRNA has strong specificity (80). And lncRNAs can be detected in the early stages of the disease, offering a potential basis for early diagnosis (38). In addition, the stability of lncRNA in the bloodstream makes it an ideal candidate for non-invasive biomarkers (81).
In recent years, studies have gradually revealed the roles of lncRNAs in regulating gene expression in CAVD. Some studies have confirmed that lncRNAs regulate the osteogenic differentiation of VICs or participate in valve calcification by sponging miRNAs. However, the regulatory mechanisms of lncRNAs are complex, and there may be multiple regulatory modes. For example, some researchers have speculated that HOTAIR may improve CAVD by sponging miR-29b (82), whereas MALAT1 may exacerbate CAVD by sponging miR-195 (83). Additionally, MALAT1 participates in osteogenic differentiation through MAPK and Wnt/β-catenin signaling pathways in osteoporosis (84, 85) and H19 induces an osteogenic phenotype in isolated human mesenchymal stem cells via the Wnt pathway (86), indicating that they may also be involved in aortic valve calcification. However, the proposed mechanism requires further experimental validation.
In addition to the previously mentioned lncRNAs that have regulatory effects on CAVD, other lncRNAs, such as myocardial infarction-associated transcript (MIAT) and antisense non-coding RNA in the INK4 locus (ANRIL), may regulate osteogenic differentiation and inflammatory responses in various cells, suggesting that they could have similar mechanisms in the aortic valve. MIAT is closely related to cardiovascular disease and is involved in the NF-κB pathway, promoting the expression of pro-inflammatory cytokines like IL-6 and TNF-α, and influencing the osteogenic differentiation of human adipose-derived and bone marrow mesenchymal stem cells (87–92). ANRIL, associated with atherosclerosis, regulates osteogenic differentiation in periodontal ligament and stem cells, and may also be involved in the Wnt/β-catenin and NF-κB signaling pathways (93–97). Therefore, both MIAT and ANRIL may play a role in the valve calcification seen in CAVD; however, further research is needed to clarify their specific contributions. We can identify novel lncRNAs through high-throughput RNA sequencing and profiling as well as bioinformatic analysis.
In summary, lncRNAs play crucial roles in the occurrence and development of CAVD. The mechanisms of action of lncRNAs in CAVD are complex and not fully understood, complicating their translation to therapeutics. Further investigation of their mechanisms of action will help elucidate the pathological processes underlying CAVD and provide new insights to enable early diagnosis and targeted therapy.
Author contributions
YS: Writing – original draft. JL: Writing – original draft. ZZ: Writing – review & editing. XC: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Ningbo Science and Technology Plan Project (Grant No. 2022Z149).
Acknowledgments
The authors would like to thank the teachers for their help and guidance in writing this review, as well as the financial support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yi B, Zeng W, Lv L, Hua P. Changing epidemiology of calcific aortic valve disease: 30-year trends of incidence, prevalence, and deaths across 204 countries and territories. Aging. (2021) 13(9):12710–32. doi: 10.18632/aging.202942
2. Lindman BR, Clavel MA, Mathieu P, Iung B, Lancellotti P, Otto CM, et al. Calcific aortic stenosis. Nat Rev Dis Primers. (2016) 2:16006. doi: 10.1038/nrdp.2016.6
3. Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, et al. Calcific aortic valve disease: not simply a degenerative process: a review and agenda for research from the national heart and lung and blood institute aortic stenosis working group. Executive summary: calcific aortic valve disease-2011 update. Circulation. (2011) 124(16):1783–91. doi: 10.1161/CIRCULATIONAHA.110.006767
4. Toff WD, Hildick-Smith D, Kovac J, Mullen MJ, Wendler O, Mansouri A, et al. Effect of transcatheter aortic valve implantation vs surgical aortic valve replacement on all-cause mortality in patients with aortic stenosis. JAMA. (2022) 327(19):1875–87. doi: 10.1001/jama.2022.5776
5. Uchida S, Dimmeler S. Long noncoding rnas in cardiovascular diseases. Circ Res. (2015) 116(4):737–50. doi: 10.1161/CIRCRESAHA.116.302521
6. McCabe EM, Rasmussen TP. Lncrna involvement in cancer stem cell function and epithelial-mesenchymal transitions. Semin Cancer Biol. (2021) 75:38–48. doi: 10.1016/j.semcancer.2020.12.012
7. Tan YT, Lin JF, Li T, Li JJ, Xu RH, Ju HQ. Lncrna-mediated posttranslational modifications and reprogramming of energy metabolism in cancer. Cancer Commun (Lond). (2021) 41(2):109–20. doi: 10.1002/cac2.12108
8. Schmitt AM, Garcia JT, Hung T, Flynn RA, Shen Y, Qu K, et al. An inducible long noncoding RNA amplifies DNA damage signaling. Nat Genet. (2016) 48(11):1370–6. doi: 10.1038/ng.3673
9. Gupta SK, Kumari S, Singh S, Barthwal MK, Singh SK, Thum T. Non-coding RNAs: regulators of valvular calcification. J Mol Cell Cardiol. (2020) 142:14–23. doi: 10.1016/j.yjmcc.2020.03.015
10. Salviano-Silva A, Lobo-Alves SC, Almeida RC, Malheiros D, Petzl-Erler ML. Besides pathology: long non-coding RNA in cell and tissue homeostasis. Noncoding RNA. (2018) 4(1):3. doi: 10.3390/ncrna4010003
11. Chen L, Wei K, Li J, Li Y, Cao H, Zheng Z. Integrated analysis of lncrna-mediated cerna network in calcific aortic valve disease. Cells. (2022) 11(14). doi: 10.3390/cells11142204
12. Vogl BJ, Niemi NR, Griffiths LG, Alkhouli MA, Hatoum H. Impact of calcific aortic valve disease on valve mechanics. Biomech Model Mechanobiol. (2022) 21(1):55–77. doi: 10.1007/s10237-021-01527-4
13. Mathieu P, Boulanger MC, Bouchareb R. Molecular biology of calcific aortic valve disease: towards new pharmacological therapies. Expert Rev Cardiovasc Ther. (2014) 12(7):851–62. doi: 10.1586/14779072.2014.923756
14. Towler DA. Molecular and cellular aspects of calcific aortic valve disease. Circ Res. (2013) 113(2):198–208. doi: 10.1161/CIRCRESAHA.113.300155
15. Chen H Y, Dina C, Small AM, Shaffer CM, Levinson RT, Helgadóttir A, et al. Dyslipidemia, inflammation, calcification, and adiposity in aortic stenosis: a genome-wide study. Eur Heart J. (2023) 44(21):1927–39. doi: 10.1093/eurheartj/ehad142
16. Goody PR, Hosen MR, Christmann D, Niepmann ST, Zietzer A, Adam M, et al. Aortic valve stenosis: from basic mechanisms to novel therapeutic targets. Arterioscler Thromb Vasc Biol. (2020) 40(4):885–900. doi: 10.1161/ATVBAHA.119.313067
17. Yutzey KE, Demer LL, Body SC, Huggins GS, Towler DA, Giachelli CM, et al. Calcific aortic valve disease. Arterioscler Thromb Vasc Biol. (2014) 34(11):2387–93. doi: 10.1161/ATVBAHA.114.302523
18. Deng G, Zhang L, Wang C, Wang S, Xu J, Dong J, et al. Ages-rage axis causes endothelial-to-mesenchymal transition in early calcific aortic valve disease via tgf-Β1 and Bmpr2 signaling. Exp Gerontol. (2020) 141:111088. doi: 10.1016/j.exger.2020.111088
19. Hjortnaes J, Shapero K, Goettsch C, Hutcheson JD, Keegan J, Kluin J, et al. Valvular interstitial cells suppress calcification of valvular endothelial cells. Atherosclerosis. (2015) 242(1):251–60. doi: 10.1016/j.atherosclerosis.2015.07.008
20. Mathieu P, Bouchareb R, Boulanger MC. Innate and adaptive immunity in calcific aortic valve disease. J Immunol Res. (2015) 2015:1. doi: 10.1155/2015/851945
21. Éva Sikura K, Combi Z, Potor L, Szerafin T, Hendrik Z, Méhes G, et al. Hydrogen sulfide inhibits aortic valve calcification in heart via regulating Runx2 by nf-Κb, a link between inflammation and mineralization. J Adv Res. (2021) 27:165–76. doi: 10.1016/j.jare.2020.07.005
22. Jenke A, Kistner J, Saradar S, Chekhoeva A, Yazdanyar M, Bergmann AK, et al. Transforming growth factor-Β1 promotes fibrosis but attenuates calcification of valvular tissue applied as a three-dimensional calcific aortic valve disease model. Am J Physiol Heart Circ Physiol. (2020) 319(5):H1123–h41. doi: 10.1152/ajpheart.00651.2019
23. Podolec J, Baran J, Siedlinski M, Urbanczyk M, Krupinski M, Bartus K, et al. Serum rantes, transforming growth factor-Β1 and interleukin-6 levels correlate with cardiac muscle fibrosis in patients with aortic valve stenosis. J Physiol Pharmacol. (2018) 69(4). doi: 10.26402/jpp.2018.4.12
24. Moncla LM, Briend M, Bossé Y, Mathieu P. Calcific aortic valve disease: mechanisms, prevention and treatment. Nat Rev Cardiol. (2023) 20(8):546–59. doi: 10.1038/s41569-023-00845-7
25. Dutta P, Lincoln J. Calcific aortic valve disease: a developmental biology perspective. Curr Cardiol Rep. (2018) 20(4):21. doi: 10.1007/s11886-018-0968-9
26. Petkovic A, Erceg S, Munjas J, Ninic A, Vladimirov S, Davidovic A, et al. Lncrnas as regulators of atherosclerotic plaque stability. Cells. (2023) 12(14):1832. doi: 10.3390/cells12141832
27. Wang S, Duan J, Liao J, Wang Y, Xiao X, Li L, et al. Lncrna H19 inhibits er stress induced apoptosis and improves diabetic cardiomyopathy by regulating Pi3k/akt/mtor axis. Aging. (2022) 14(16):6809–28. doi: 10.18632/aging.204256
28. Meng K, Jiao J, Zhu RR, Wang BY, Mao XB, Zhong YC, et al. The long noncoding rna hotair regulates oxidative stress and cardiac myocyte apoptosis during ischemia-reperfusion injury. Oxid Med Cell Longev. (2020) 2020:1. doi: 10.1155/2020/1645249
29. Yan Y, Song D, Song X, Song C. The role of lncrna Malat1 in cardiovascular disease. IUBMB Life. (2020) 72(3):334–42. doi: 10.1002/iub.2210
30. Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, et al. Functional demarcation of active and silent chromatin domains in human hox loci by noncoding rnas. Cell. (2007) 129(7):1311–23. doi: 10.1016/j.cell.2007.05.022
31. Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, et al. Long non-coding rna hotair reprograms chromatin state to promote cancer metastasis. Nature. (2010) 464(7291):1071–6. doi: 10.1038/nature08975
32. Fu CJ, Cong J, Sun SS, Ren MM, Xie SY, Wang PY. Diagnostic roles of hox transcript antisense intergenic RNA in cancer. Asia Pac J Clin Oncol. (2021) 17(2):e3–9. doi: 10.1111/ajco.13300
33. Carrion K, Dyo J, Patel V, Sasik R, Mohamed SA, Hardiman G, et al. The long non-coding hotair is modulated by cyclic stretch and wnt/Β-catenin in human aortic valve cells and is a novel repressor of calcification genes. PLoS One. (2014) 9(5):e96577. doi: 10.1371/journal.pone.0096577
34. Mathieu P, Bossé Y, Huggins GS, Della Corte A, Pibarot P, Michelena HI, et al. The pathology and pathobiology of bicuspid aortic valve: state of the art and novel research perspectives. J Pathol Clin Res. (2015) 1(4):195–206. doi: 10.1002/cjp2.21
35. Busscher D, Boon RA, Juni RP. The multifaceted actions of the lncrna H19 in cardiovascular biology and diseases. Clin Sci (Lond). (2022) 136(15):1157–78. doi: 10.1042/CS20210994
36. Hashemi M, Moosavi MS, Abed HM, Dehghani M, Aalipour M, Heydari EA, et al. Long non-coding rna (lncrna) H19 in human cancer: from proliferation and metastasis to therapy. Pharmacol Res. (2022) 184:106418. doi: 10.1016/j.phrs.2022.106418
37. Monnier P, Martinet C, Pontis J, Stancheva I, Ait-Si-Ali S, Dandolo L. H19 lncrna controls gene expression of the imprinted gene network by recruiting Mbd1. Proc Natl Acad Sci U S A. (2013) 110(51):20693–8. doi: 10.1073/pnas.1310201110
38. Hadji F, Boulanger MC, Guay SP, Gaudreault N, Amellah S, Mkannez G, et al. Altered DNA methylation of long noncoding RNA H19 in calcific aortic valve disease promotes mineralization by silencing Notch1. Circulation. (2016) 134(23):1848–62. doi: 10.1161/CIRCULATIONAHA.116.023116
39. Guauque-Olarte S, Messika-Zeitoun D, Droit A, Lamontagne M, Tremblay-Marchand J, Lavoie-Charland E, et al. Calcium signaling pathway genes Runx2 and Cacna1c are associated with calcific aortic valve disease. Circ Cardiovasc Genet. (2015) 8(6):812–22. doi: 10.1161/CIRCGENETICS.115.001145
40. Yang X, Fullerton DA, Su X, Ao L, Cleveland JC Jr, Meng X. Pro-osteogenic phenotype of human aortic valve interstitial cells is associated with higher levels of toll-like receptors 2 and 4 and enhanced expression of bone morphogenetic protein 2. J Am Coll Cardiol. (2009) 53(6):491–500. doi: 10.1016/j.jacc.2008.09.052
41. Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, et al. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. (2003) 107(17):2181–4. doi: 10.1161/01.CIR.0000070591.21548.69
42. Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, et al. Mutations in Notch1 cause aortic valve disease. Nature. (2005) 437(7056):270–4. doi: 10.1038/nature03940
43. Toshima T, Watanabe T, Narumi T, Otaki Y, Shishido T, Aono T, et al. Therapeutic inhibition of microrna-34a ameliorates aortic valve calcification via modulation of Notch1-Runx2 signalling. Cardiovasc Res. (2019) 116(5):983–94. doi: 10.1093/cvr/cvz210
44. Xiao X, Zhou T, Guo S, Guo C, Zhang Q, Dong N, et al. Lncrna Malat1 sponges mir-204 to promote osteoblast differentiation of human aortic valve interstitial cells through up-regulating Smad4. Int J Cardiol. (2017) 243:404–12. doi: 10.1016/j.ijcard.2017.05.037
45. Ghaforui-Fard S, Vafaee R, Taheri M. Taurine-upregulated gene 1: a functional long noncoding rna in tumorigenesis. J Cell Physiol. (2019) 234(10):17100–12. doi: 10.1002/jcp.28464
46. Da M, Zhuang J, Zhou Y, Qi Q, Han S. Role of long noncoding RNA taurine-upregulated gene 1 in cancers. Mol Med. (2021) 27(1):51. doi: 10.1186/s10020-021-00312-4
47. Haybar H, Sadati NS, Purrahman D, Mahmoudian-Sani MR, Saki N. Lncrna Tug1 as potential novel biomarker for prognosis of cardiovascular diseases. Epigenomics. (2023) 15(23):1273–90. doi: 10.2217/epi-2023-0242
48. Yu C, Li L, Xie F, Guo S, Liu F, Dong N, et al. Lncrna Tug1 sponges mir-204-5p to promote osteoblast differentiation through upregulating Runx2 in aortic valve calcification. Cardiovasc Res. (2018) 114(1):168–79. doi: 10.1093/cvr/cvx180
49. Ghafouri-Fard S, Dashti S, Farsi M, Hussen BM, Taheri M. A review on the role of oncogenic lncrna Oip5-As1 in human malignancies. Biomed Pharmacother. (2021) 137:111366. doi: 10.1016/j.biopha.2021.111366
50. Zheng D, Wang B, Zhu X, Hu J, Sun J, Xuan J, et al. Lncrna Oip5-As1 inhibits osteoblast differentiation of valve interstitial cells via mir-137/Twist11 axis. Biochem Biophys Res Commun. (2019) 511(4):826–32. doi: 10.1016/j.bbrc.2019.02.109
51. Zhang F, Li J, Xiao H, Zou Y, Liu Y, Huang W. Afap1-As1: a novel oncogenic long non-coding RNA in human cancers. Cell Prolif. (2018) 51(1):e12397. doi: 10.1111/cpr.12397
52. Xiong F, Zhu K, Deng S, Huang H, Yang L, Gong Z, et al. Afap1-As1: a rising star among oncogenic long non-coding RNAs. Sci China Life Sci. (2021) 64(10):1602–11. doi: 10.1007/s11427-020-1874-6
53. He W, Li F, Zhang S, Zhu Z, Lin M, Ge S, et al. Lncrna Afap1-As1 promotes osteoblast differentiation of human aortic valve interstitial cells through regulating mir-155/Smad5 axis. Mol Cell Probes. (2020) 50:101509. doi: 10.1016/j.mcp.2020.101509
54. Raddatz MA, Madhur MS, Merryman WD. Adaptive immune cells in calcific aortic valve disease. Am J Physiol Heart Circ Physiol. (2019) 317(1):H141–h55. doi: 10.1152/ajpheart.00100.2019
55. Raddatz MA, Huffstater T, Bersi MR, Reinfeld BI, Madden MZ, Booton SE, et al. Macrophages promote aortic valve cell calcification and Alter Stat3 splicing. Arterioscler Thromb Vasc Biol. (2020) 40(6):e153–e65. doi: 10.1161/ATVBAHA.120.314360
56. He W, Che H, Jin C, Li Y, Li F, Zhou R. Lncrna Afap1-As1 promotes M1 polarization of macrophages and osteogenic differentiation of valve interstitial cells. J Physiol Biochem. (2021) 77(3):461–8. doi: 10.1007/s13105-021-00821-0
57. Xu B, Mei J, Ji W, Bian Z, Jiao J, Sun J, et al. Lncrna Snhg3, a potential oncogene in human cancers. Cancer Cell Int. (2020) 20(1):536. doi: 10.1186/s12935-020-01608-x
58. Chen L, Liu H, Sun C, Pei J, Li J, Li Y, et al. A novel lncrna Snhg3 promotes osteoblast differentiation through Bmp2 upregulation in aortic valve calcification. JACC Basic Transl Sci. (2022) 7(9):899–914. doi: 10.1016/j.jacbts.2022.06.009
59. Zhang X, Gao Y, Zhang X, Zhang X, Xiang Y, Fu Q, et al. Fgd5-As1 is a hub lncrna cerna in hearts with tetralogy of fallot which regulates congenital heart disease genes transcriptionally and epigenetically. Front Cell Dev Biol. (2021) 9:630634. doi: 10.3389/fcell.2021.630634
60. Hao L, Wang J, Bi SJ, Cheng C. Upregulation of long noncoding RNA Fgd5-As1 ameliorates myocardial ischemia/reperfusion injury via microrna-106a-5p and microrna-106b-5p. J Cardiovasc Pharmacol. (2021) 78(1):e45–54. doi: 10.1097/FJC.0000000000001036
61. Shen LS, Hu XF, Chen T, Shen GL, Cheng D. Integrated network analysis to explore the key mrnas and lncrnas in acute myocardial infarction. Math Biosci Eng. (2019) 16(6):6426–37. doi: 10.3934/mbe.2019321
62. Chen YX, Ding J, Zhou WE, Zhang X, Sun XT, Wang XY, et al. Identification and functional prediction of long non-coding rnas in dilated cardiomyopathy by bioinformatics analysis. Front Genet. (2021) 12:648111. doi: 10.3389/fgene.2021.648111
63. Wei J, Zhu X, Sun A, Yan X, Meng X, Ge S. Long non-coding RNA Fgd5 antisense RNA 1 targets baculovirus inhibitor 5 via microrna-497-5p to alleviate calcific aortic valve disease. Clin Hemorheol Microcirc. (2024) 86(3):285–302. doi: 10.3233/CH-221692
64. Evangelista A, Galian-Gay L, Guala A, Teixido-Tura G, Calvo-Iglesias F, Sevilla T, et al. Atorvastatin effect on aortic dilatation and valvular calcification progression in bicuspid aortic valve (bicator): a randomized clinical trial. Circulation. (2024) 149(25):1938–48. doi: 10.1161/circulationaha.123.067537
65. Kraler S, Blaser MC, Aikawa E, Camici GG, Lüscher TF. Calcific aortic valve disease: from molecular and cellular mechanisms to medical therapy. Eur Heart J. (2022) 43(7):683–97. doi: 10.1093/eurheartj/ehab757
66. Khorkova O, Stahl J, Joji A, Volmar CH, Zeier Z, Wahlestedt C. Long non-coding RNA-targeting therapeutics: discovery and development update. Expert Opin Drug Discov. (2023) 18(9):1011–29. doi: 10.1080/17460441.2023.2236552
67. Winkle M, El-Daly SM, Fabbri M, Calin GA. Noncoding RNA therapeutics—challenges and potential solutions. Nat Rev Drug Discov. (2021) 20(8):629–51. doi: 10.1038/s41573-021-00219-z
68. Gutschner T, Hämmerle M, Eissmann M, Hsu J, Kim Y, Hung G, et al. The noncoding RNA Malat1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. (2013) 73(3):1180–9. doi: 10.1158/0008-5472.CAN-12-2850
69. Wu XL, Lu RY, Wang LK, Wang YY, Dai YJ, Wang CY, et al. Long noncoding rna hotair silencing inhibits invasion and proliferation of human colon cancer lovo cells via regulating Igf2bp2. J Cell Biochem. (2019) 120(2):1221–31. doi: 10.1002/jcb.27079
70. Mahato RK, Bhattacharya S, Khullar N, Sidhu IS, Reddy PH, Bhatti GK, et al. Targeting long non-coding RNAs in cancer therapy using crispr-Cas9 technology: a novel paradigm for precision oncology. J Biotechnol. (2024) 379:98–119. doi: 10.1016/j.jbiotec.2023.12.003
71. Ahmadi-Balootaki S, Doosti A, Jafarinia M, Goodarzi HR. Targeting the Malat1 gene with the crispr/Cas9 technique in prostate cancer. Genes Environ. (2022) 44(1):22. doi: 10.1186/s41021-022-00252-3
72. Chen Y, Li Z, Chen X, Zhang S. Long non-coding RNAs: from disease code to drug role. Acta Pharm Sin B. (2021) 11(2):340–54. doi: 10.1016/j.apsb.2020.10.001
73. Ammad M, Javed Z, Sadia H, Ahmed R, Akbar A, Nadeem T, et al. Advancements in long non-coding RNA-based therapies for cancer: targeting, delivery, and clinical implications. Med Oncol. (2024) 41(11):292. doi: 10.1007/s12032-024-02534-y
74. Niu X, Zhang J, Zhang J, Bai L, Hu S, Zhang Z, et al. Lipid nanoparticle-mediated Oip5-As1 delivery preserves mitochondrial function in myocardial ischemia/reperfusion injury by inhibiting the P53 pathway. ACS Appl Mater Interfaces. (2024) 16(45):61565–82. doi: 10.1021/acsami.4c10032
75. Hui B, Lu C, Wang J, Xu Y, Yang Y, Ji H, et al. Engineered exosomes for co-delivery of Pgm5-As1 and oxaliplatin to reverse drug resistance in colon cancer. J Cell Physiol. (2022) 237(1):911–33. doi: 10.1002/jcp.30566
76. Balaraman AK, Babu MA, Moglad E, Mandaliya V, Rekha MM, Gupta S, et al. Exosome-mediated delivery of crispr-Cas9: a revolutionary approach to cancer gene editing. Pathol Res Pract. (2024) 266:155785. doi: 10.1016/j.prp.2024.155785
77. Slaymaker IM, Gao L, Zetsche B, Scott DA, Yan WX, Zhang F. Rationally engineered Cas9 nucleases with improved specificity. Science. (2016) 351(6268):84–8. doi: 10.1126/science.aad5227
78. Kocak DD, Josephs EA, Bhandarkar V, Adkar SS, Kwon JB, Gersbach CA. Increasing the specificity of crispr systems with engineered RNA secondary structures. Nat Biotechnol. (2019) 37(6):657–66. doi: 10.1038/s41587-019-0095-1
79. Fu Y, Sander JD, Reyon D, Cascio VM, Joung JK. Improving crispr-cas nuclease specificity using truncated guide RNAs. Nat Biotechnol. (2014) 32(3):279–84. doi: 10.1038/nbt.2808
80. Liu SJ, Horlbeck MA, Cho SW, Birk HS, Malatesta M, He D, et al. Crispri-based genome-scale identification of functional long noncoding RNA loci in human cells. Science. (2017) 355(6320):aah7111. doi: 10.1126/science.aah7111
81. Badowski C, He B, Garmire LX. Blood-derived lncrnas as biomarkers for cancer diagnosis: the good, the bad and the beauty. NPJ Precis Oncol. (2022) 6(1):40. doi: 10.1038/s41698-022-00283-7
82. Guo Y, Li H, Huang B, Hou L. Lncrna hotair ameliorates calcific aortic valve disease by sponging mir-29b. Int J Cardiol. (2024) 408:132094. doi: 10.1016/j.ijcard.2024.132094
83. Xie MY, Hou LJ. Lncrna Malat1 aggravates calcific aortic valve disease by sponging mir-195. Int J Cardiol. (2021) 333:161. doi: 10.1016/j.ijcard.2021.02.061
84. Zheng S, Wang YB, Yang YL, Chen BP, Wang CX, Li RH, et al. Lncrna Malat1 inhibits osteogenic differentiation of mesenchymal stem cells in osteoporosis rats through mapk signaling pathway. Eur Rev Med Pharmacol Sci. (2019) 23(11):4609–17. doi: 10.26355/eurrev_201906_18038
85. Li X. Lncrna metastasis-associated lung adenocarcinoma transcript-1 promotes osteogenic differentiation of bone marrow stem cells and inhibits osteoclastic differentiation of mø in osteoporosis via the mir-124-3p/Igf2bp1/wnt/Β-catenin axis. J Tissue Eng Regen Med. (2022) 16(3):311–29. doi: 10.1002/term.3279
86. Liang WC, Fu WM, Wang YB, Sun YX, Xu LL, Wong CW, et al. H19 activates wnt signaling and promotes osteoblast differentiation by functioning as a competing endogenous RNA. Sci Rep. (2016) 6:20121. doi: 10.1038/srep20121
87. Cao X, Ma Q, Wang B, Qian Q, Liu N, Liu T, et al. Silencing long non-coding RNA miat ameliorates myocardial dysfunction induced by myocardial infarction via miat/mir-10a-5p/Egr2 axis. Aging (Albany NY). (2021) 13(8):11188–206. doi: 10.18632/aging.202785
88. Yan ZS, Zhang NC, Li K, Sun HX, Dai XM, Liu GL. Upregulation of long non-coding RNA myocardial infarction-associated transcription is correlated with coronary artery stenosis and elevated inflammation in patients with coronary atherosclerotic heart disease. Kaohsiung J Med Sci. (2021) 37(12):1038–47. doi: 10.1002/kjm2.12444
89. Liu Y, Wang T, Zhang M, Chen P, Yu Y. Down-regulation of myocardial infarction associated transcript 1 improves myocardial ischemia-reperfusion injury in aged diabetic rats by inhibition of activation of nf-Κb signaling pathway. Chem Biol Interact. (2019) 300:111–22. doi: 10.1016/j.cbi.2019.01.001
90. Dong Q, Wang Q, Yan X, Wang X, Li Z, Zhang L. Long noncoding RNA miat inhibits the progression of diabetic nephropathy and the activation of nf-Κb pathway in high glucose-treated renal tubular epithelial cells by the mir-182-5p/Gprc5a axis. Open Med (Wars). (2021) 16(1):1336–49. doi: 10.1515/med-2021-0328
91. Wang F, Deng H, Chen J, Wang Z, Yin R. Lncrna miat can regulate the proliferation, apoptosis, and osteogenic differentiation of bone marrow-derived mesenchymal stem cells by targeting mir-150-5p. Bioengineered. (2022) 13(3):6343–52. doi: 10.1080/21655979.2021.2011632
92. Jin C, Zheng Y, Huang Y, Liu Y, Jia L, Zhou Y. Long non-coding RNA miat knockdown promotes osteogenic differentiation of human adipose-derived stem cells. Cell Biol Int. (2017) 41(1):33–41. doi: 10.1002/cbin.10697
93. Razeghian-Jahromi I, Karimi Akhormeh A, Zibaeenezhad MJ. The role of anril in atherosclerosis. Dis Markers. (2022) 2022:8859677. doi: 10.1155/2022/8859677
94. Liu X, Zhou Y. Downregulation of lncrna anril inhibits osteogenic differentiation of periodontal ligament cells via sponging mir-7 through nf-Κb pathway. Anal Cell Pathol (Amst). (2021) 2021:7890674. doi: 10.1155/2021/7890674
95. Bian M, Yu Y, Li Y, Zhou Z, Wu X, Ye X, et al. Upregulating the expression of lncrna anril promotes osteogenesis via the mir-7-5p/igf-1r axis in the inflamed periodontal ligament stem cells. Front Cell Dev Biol. (2021) 9:604400. doi: 10.3389/fcell.2021.604400
96. Guo F, Tang C, Li Y, Liu Y, Lv P, Wang W, et al. The interplay of lncrna anril and mir-181b on the inflammation-relevant coronary artery disease through mediating nf-Κb signalling pathway. J Cell Mol Med. (2018) 22(10):5062–75. doi: 10.1111/jcmm.13790
Keywords: calcific aortic valve disease, long non-coding RNA, valve interstitial cells, osteogenic differentiation, mechanisms
Citation: Shen Y, Li J, Zhao Z and Chen X (2025) Progress on long non-coding RNAs in calcific aortic valve disease. Front. Cardiovasc. Med. 12:1522544. doi: 10.3389/fcvm.2025.1522544
Received: 4 November 2024; Accepted: 2 January 2025;
Published: 17 January 2025.
Edited by:
Pompilio Faggiano, Fondazione Poliambulanza Istituto Ospedaliero, ItalyReviewed by:
Kenji Miki, Osaka University, JapanGloria Santangelo, IRCCS Ca 'Granda Foundation Maggiore Policlinico Hospital, Italy
Copyright: © 2025 Shen, Li, Zhao and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaomin Chen, Y2h4bWluQGhvdG1haWwuY29t
 Yan Shen
Yan Shen Jiahui Li1
Jiahui Li1 Xiaomin Chen
Xiaomin Chen