- 1Division of Physiology and Pathophysiology, Otto Loewi Research Center for Vascular Biology, Immunology and Inflammation, Medical University of Graz, Graz, Austria
- 2Vetsuisse Faculty, University of Zurich, Zurich, Switzerland
Obesity is one of the major global health concerns of the 21st century, associated with many comorbidities such as type 2 diabetes mellitus (T2DM), metabolic dysfunction-associated steatotic liver disease, and early and aggressive atherosclerotic cardiovascular disease, which is the leading cause of death worldwide. Bile acids (BAs) and incretins are gut hormones involved in digestion and absorption of fatty acids, and insulin secretion, respectively. In recent years BAs and incretins are increasingly recognized as key signaling molecules, which target multiple tissues and organs, beyond the gastro-intestinal system. Moreover, incretin-based therapy has revolutionized the treatment of T2DM and obesity. This mini review highlights the current knowledge about dysregulations in BA homeostasis in obesity with a special focus on atherosclerosis as well as athero-modulating roles of incretins and currently available incretin-based therapies.
1 Introduction
Obesity is a globally increasing epidemic (1, 2) associated with comorbid conditions such as type 2 diabetes mellitus (T2DM), metabolic dysfunction-associated steatotic liver disease, and atherosclerotic cardiovascular disease (CVD) (3–6). Atherosclerosis is a systemic chronic inflammatory disease characterized by endothelial dysfunction, accumulation of lipids, immune-inflammatory cells, and fibrous neointimal tissue in the arterial wall, leading to the formation of plaques (7–9). Endothelial dysfunction, which is characterized by lower bioavailability of the vasorelaxing nitric oxide (NO) and increased production of the vasoconstricting endothelin-1 (ET-1), is, together with a dysregulated metabolism of low density lipoprotein (LDL) and high density lipoprotein (HDL), a key player in atherosclerosis onset and progression (8, 10–13). LDL cholesterol has a well-established causal role in the development of atherosclerosis (14, 15). Elevated circulating levels of LDL cholesterol after deposition in the arterial intima, undergo oxidation, becoming pro-inflammatory and attracting monocytes-macrophages (16). Macrophages engulf oxidized LDLs becoming foam cells, a hallmark of early atherosclerotic lesions (17). Over time, the accumulation of foam cells, along with other cellular debris, leads to the formation of fatty streaks and progression to advanced and rupture-prone plaques (18–20). In contrast to LDL, HDL cholesterol is often termed the “good” cholesterol. This is, however, an oversimplification of the complex physiological actions of this class of lipoprotein. The best known function of HDL is to mediate reverse cholesterol transport (RCT), by which excessive cholesterol is removed from arterial walls and peripheral tissues and transported back to the liver for excretion or reuse to synthetize hormones (21, 22). While low levels of HDL-cholesterol increase the risk for CVD, elevating HDL levels by pharmacological inhibition of cholesteryl ester transfer protein (CETP), an enzyme catalyzing the transfer of cholesterol from HDL to LDL, and triglycerides from LDL to HDL, did not result in improved cardiovascular outcome (23–25). This disappointing result highlighted that the function of the diverse molecular components of HDL rather than solely its cholesterol content is crucial in reducing cardiovascular risk (26, 27). Once dysfunctional, HDL loses its protective RCT capacity and fails to prevent LDL oxidation (oxLDL), becoming pro-inflammatory and pro-atherosclerotic (28).
BAs are amphipathic molecules synthesized from cholesterol in the liver. BAs play a crucial role in the intestinal digestion and absorption of dietary fats (29). Beyond their digestive functions, BAs are important signaling molecules. Among several receptors activated by BAs, the most studied are Farnesoid X Receptor (FXR) and G protein-coupled bile acid receptor 1 (GPBAR1), also known as TGR5, which are present in most cell types and pathophysiological processes associated with atherosclerosis development (30–32). In obesity, increased BA production in the liver and slightly elevated BA levels in the systemic circulation are reported (33, 34) as well as reduced circulating concentrations (35). Furthermore, obesity-induced changes in the gut microbiome composition are associated with altered conversion rates of primary to secondary BAs, which may alter BA-mediated FXR and TGR5 signaling (36). Physiologically, TGR5 receptor activation in the intestine by BAs promotes the release of incretins, which exert vaso-protective actions (37, 38).
Incretins, glucose-dependent insulinotropic polypeptide (GIP) and glucagon like peptide 1 (GLP-1), are gut hormones that induce insulin release from the pancreas in a glucose-dependent manner (39, 40). GIP and GLP-1 act on multiple target cells via G-protein coupled receptors GIPR and GLP1R, respectively. These receptors are expressed in numerous organs including bone, heart and blood vessels (41). The incretin signaling is impaired in obesity and T2DM (42).
Current research on the pathophysiology of atherosclerosis associated with obesity is exploring the role of BAs and incretins (43, 44). This mini review summarizes current evidence on the role of BAs, incretins, and incretin-based therapies in modulating atherosclerosis.
2 BA, incretins and atherosclerosis
2.1 Bile acids
BAs are classified into two main types: primary BA, such as cholic acid and chenodeoxycholic acid and secondary BAs, (i.e., deoxycholic acid and lithocholic acid) (45), the latter originating by bacterial modification in the intestine (46) via deconjugation and dehydroxylation processes (47). In the post-prandial phase 90%–95% of BAs are reabsorbed in the ileum and transported back to the liver. After their almost complete reabsorption, BAs are stored in the gallbladder and await to be secreted into the duodenum upon food intake. Around 5% of total BAs escape liver reabsorption and are found in the systemic circulation reaching serum concentrations of around 1–3 µM in healthy lean individuals (48). A graphical summary of BA metabolism is provided in Figure 1.
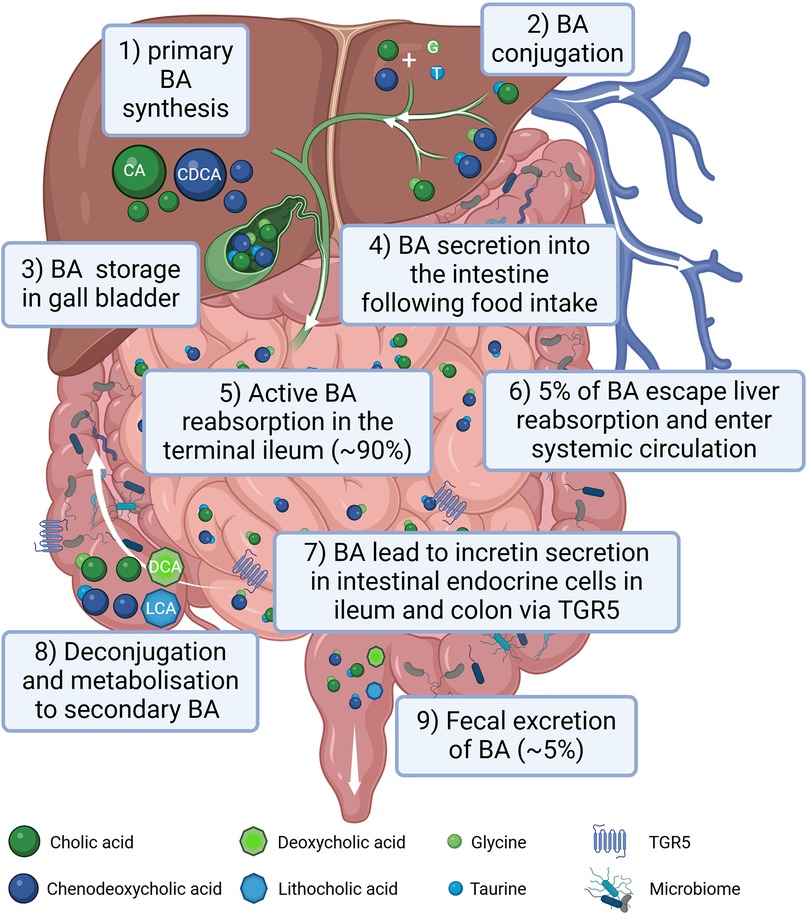
Figure 1. Graphical summary of bile acid (BA) metabolism in humans. BAs are synthesized in the liver (1), conjugated with amino acids, mainly glycine (G) and taurine (T) (2) and stored in the gall bladder (3). Following food intake, BAs are released into the small intestine to aid lipid absorption (4). Around 90% of the BA are reabsorbed in the terminal part of the ileum (5) reaching the liver via the portal circulation. Only around 5% of BAs escape liver reabsorption and are found in the systemic circulation (6). In the terminal ileum as well as in the colon BAs stimulate incretin secretion of intestinal endocrine cells via activating the TGR5 receptor (7). In the intestine BAs can be processed by the gut microbiota (8). Following an initial deconjugation the primary cholic acid (CA) is converted into deoxycholic acid (DCA) and chenodeoxycholic acid (CDCA) is converted into lithocholic acid (LCA). Only a small percentage of the BA is excreted with the feces (9). Created in BioRender. Gindlhuber, J. (2024) https://biorender.com/w38u902.
BAs-dependent activation of FXR in human liver cell lines, upregulates LDL receptor expression and activity and inhibits its degradation leading to a reduction of LDL cholesterol levels (49–51). However, FXR also reduced the main HDL apolipoprotein ApoA-I transcription, decreasing HDL levels in murine animal models (52). FXR and LDL receptor double knockout male mice were protected from atherosclerosis contrary to female double knockouts (53). FXR and apolipoprotein (Apo) E double knockouts showed severe plaque formation compared to wild-type, and single FXR-/-, and ApoE-/- mice (54). In vivo studies in rats have shown that FXR activation is beneficial in different vascular cell types [e.g., endothelial cells (ECs) and vascular smooth muscle cells] to revert their pro-constrictory and pro-inflammatory phenotype (Figure 2A), as well as neo-intima formation, all changes, which are promoting atherosclerosis development (55–58). FXR activation enhances NO production and reduces ET-1 expression contributing to vasodilation in isolated rat pulmonary ECs (59, 60). On the other hand, hepatic TGR5 stimulation prevents hepatic BA and ectopic lipid accumulation in different murine models (61–63). Activation of TGR5 in macrophages is beneficial because it attenuates foam cell formation and inhibits the activation of inflammation, as evidenced by genetically modifying TGR5 in murine peritoneal macrophages (64). An increase in specific BA subspecies has been associated with atherosclerosis in human and animal studies. For instance, in T2DM patients carotid intima media thickness (cITM), a surrogate marker of subclinical atherosclerosis, was associated with higher deoxycholic acid and taurodeoxycholic acid levels, and lower levels of taurocholic acid than patients with normal cITM (65, 66). Conversely, the glycine conjugates of cholic acid and deoxycholic as well as lithocholic acid were found to be protective when atherosclerosis patients were compared to a control cohort (67). Of note, a disrupted BA signaling impairs glucose and lipid metabolism, exacerbating conditions like insulin resistance and fatty liver disease, which are major pro-atherosclerotic metabolic derangements (68, 69).
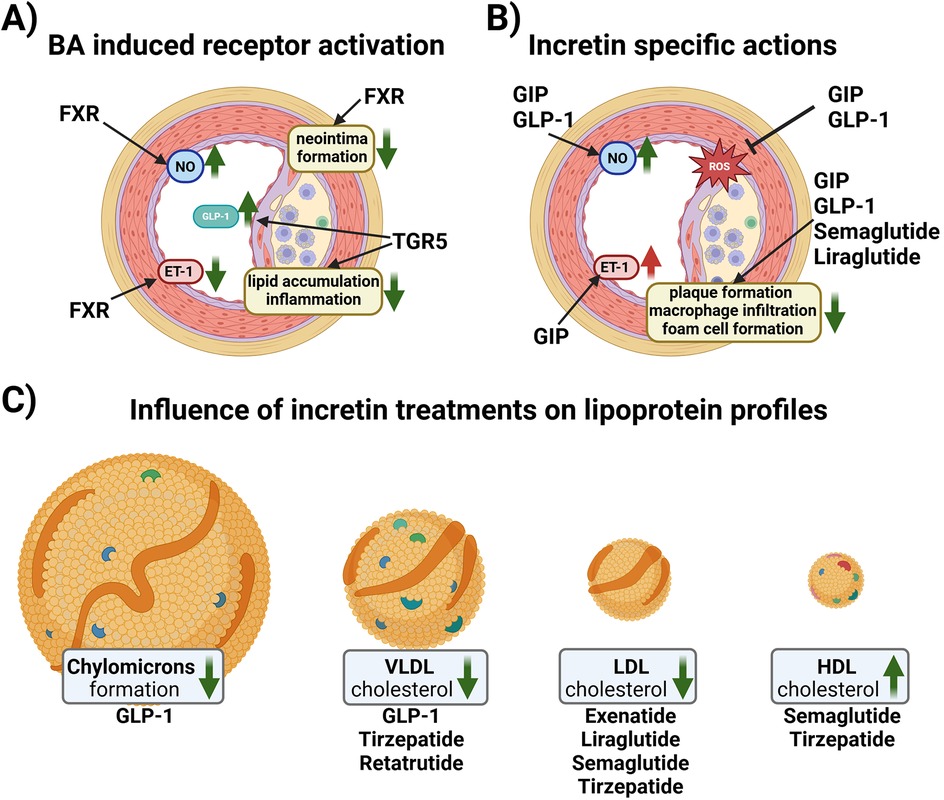
Figure 2. (A) Schematic overview of reported effects of BA receptor activation in vascular cells. In endothelial cells (ECs), Farnesoid X Receptor (FXR) activation increases nitric oxide (NO) production and reduces endothelin-1 (ET-1) expression contributing to vasorelaxation. Vascular smooth muscle cells reduce their proliferative activity upon FXR activation resulting in reduced neo intima formation. G protein-coupled bile acid receptor 1 (TGR-5) activation in endocrine cells increases the amount of glucagon-like peptide 1 (GLP-1) in the systemic circulation. Macrophages react to TGR5 activation with reduced lipid uptake and a reduction in inflammatory signaling. (B) Schematic overview of the effects of incretins and incretin-based therapy on ECs and plaque formation. GLP-1 induces NO production, while glucose-dependent insulinotropic polypeptide (GIP) induces the production of both NO and ET-1. Both GIP and GLP1 reduce the formation of reactive oxygen species (ROS). Native GIP and GLP-1, as well as GLP1Ras, semaglutide and liraglutide, reduce plaque formation, macrophage infiltration and foam cell formation. (C) Native GLP-1 decreases chylomicron formation and VLDL cholesterol levels, while GLP1RA and GLP1R/GIPR co-agonist therapy ameliorates the lipoprotein profile of patients by lowering VLDL and LDL cholesterol, and increasing HDL cholesterol. Created in BioRender. Kirsch, A. (2024) https://BioRender.com/u26n602.
2.2 Incretins
GIP is secreted by the duodenal and jejunal K cells upon ingestion of carbohydrates and lipids, while GLP-1 is secreted by the ileal L cells (70, 71). Along with the induction of insulin secretion, GIP and GLP-1 reduce gastric emptying, and GLP-1 lowers glucagon secretion (71, 72). Physiologically, these hormones have a half-life of just a few minutes upon secretion, as GIP and GLP-1 are rapidly cleaved and inactivated by diaminopeptidyl peptidase-4 (DPP4) (73).
2.3 GIP
In vitro studies in ECs have shown that GIP have both anti- and pro-atherogenic effects. In human umbilical vein ECs (HUVEC) and canine portal vein EC, GIP induced NO production (74, 75) and reduced advanced glycation end products-induced oxidative stress and inflammation (76) but was also reported to increase ET-1 (74, 77, 78), (Figure 2B).
Monocyte-macrophages transformation into foam cells contributes to the pathogenesis of atherosclerosis (79–81). GIPR is expressed in human monocytes, mouse peritoneal macrophages and human monocyte-derived macrophages, with the GIPR expression in human monocytes being higher than in the differentiated macrophages, at least in vitro (82). Moreover, GIP exerts anti-inflammatory effects by suppressing lipopolysaccharide-induced tumor necrosis factor-α (TNFα) or inducible NO synthase (iNOS) in human monocyte THP-1 cells (83), as well as suppressing the chemokine ligand 2 (CCL2)-induced migration also in mouse monocytes (84).
Animal studies using ApoE-/- deficient mice show anti-atherogenic effects of GIP. The infusion of active GIP (25 nmol/kg/day) for 4 weeks blunted the aortic plaque formation and macrophage accumulation within the plaque (82). Moreover, decreased foam cell formation and downregulation of the scavenger receptor CD36 and cholesteryl ester-forming acyl-coenzyme A: cholesterol acyltransferase-1 in macrophages was reported (82). Anti-atherogenic effects were also observed in streptozotocin-induced diabetic ApoE-/- mice, where GIP infusion led to a reduction of aortic plaque formation, intra-plaque macrophage accumulation and macrophage foam cell formation (85). Moreover, overexpression of GIP has been reported to stabilize the atherosclerotic plaque in non-diabetic ApoE-/- mice by blocking monocyte/macrophage activation (84). The anti-atherogenic effect of GIPR- agonism has been described also in LDLr -/- mice fed with a high fat, high cholesterol diet. Treatment of these mice with a long-acting acylated GIP analog reduced dyslipidemia and atherosclerotic plaque formation (86). Loss of GIPR induced aortic atherosclerosis and inflammation in ApoE−/−:Gipr−/− high fat diet-fed mice despite a reduced weight gain and preserved glucose homeostasis compared to ApoE−/−:Gipr+/+ mice (87), further confirming the anti- inflammatory role of GIP in atherosclerosis (Figure 2B).
2.4 GLP-1
Native GLP-1 has been shown to be atheroprotective in vitro as it stimulates the production of vasodilatory NO in ECs (35, 75). Similar to GIPR, GLP1R is also expressed in macrophages, and treatment with native GLP-1 decreased the uptake of oxLDL and expression of CD36 in human monocyte-derived macrophages (88). Administration of active GLP-1 to ApoE -/- mice significantly suppressed atherosclerotic lesions and macrophage infiltration in the aortic wall compared to vehicle controls (82). Infusions of recombinant GLP-1 in rats dramatically decreases intestinal lymph flow and reduces triglyceride absorption and ApoB and ApoA-IV production (89). Moreover, portal vein injections of GLP-1 in hamsters and mice decreases postprandial chylomicron (CM) and VLDL secretion via vagal afferent nerves originating in the portal vein (90). These GLP-1 effects could contribute to its atheroprotection, as remnant CM and VLDL have atherogenic properties (91) (Figure 2C).
3 Incretin-based therapy and modulation of atherosclerosis
Several classes of incretin-based drugs have been developed to treat T2DM, including DPP4-inhibitors and GLP1R agonists (GLP1RAs). DPP4-inhibitors will not be discussed in detail in this mini review; for an overview on the atheroprotective role of DPP-4 inhibitors in both human and animal models see (92, 93). Incretin-based drugs, especially GLP1R agonists, beyond improving glucose levels, have shown beneficial effects on the lipid profile (Figure 2C), weight reduction, and cardiovascular protective effects (94). The most commonly reported side effects are delayed gastric emptying, bloating, diarrhea and vomiting, although drug titration mitigates the incidence of these side effects (94). GLP1RA and the dual GIPR and GLP1R agonist, tirzepatide, are currently also used for weight management of overweight/obese patients with and without CVD (95).
3.1 GLP1R agonists
GLP1RAs activate the GLP1R and are resistant to inactivation by DPP-4 (96). The first GLP1R agonist in clinical use was exenatide (exendin-4) (97), subsequently, various GLP1RAs were developed based on the human GLP-1 peptide, including liraglutide, dulaglutide and semaglutide, which have different characteristics pertaining to route and frequency of administration, and pharmacokinetics (98).
3.1.1 Preclinical studies
Mechanistic studies have addressed the effect of GLP1RAs on atherosclerosis in rodent models. GLP-1 peptide analogues CNTO3649 and exendin-4 reduced VLDL production and hepatic steatosis after 4 weeks of treatment in high fat diet-fed APOE*3-Leiden transgenic mice, a mouse model with human-like lipoprotein metabolism (i.e., high triglycerides, LDL and VLDL, low HDL) and accelerated atherosclerosis development (99). Semaglutide and liraglutide reduced atherosclerotic plaque formation in aortas of ApoE -/- and LDLr -/- mice, and semaglutide blunted gene expression of pro-inflammatory and osteogenic proteins, such as TNFα and osteopontin (100). Liraglutide alone inhibited the progression of early onset, low-burden atherosclerotic disease (101) as well as attenuated pre-established atherosclerosis in ApoE -/- mice by reducing proinflammatory immune cells and mediators (102), suppressing foam cell formation (103) and lowering the endothelial expression of the proinflammatory vascular cell adhesion molecule 1 (104).
3.1.2 Clinical trials
Several randomized cardiovascular outcome trials have been conducted, showing positive effects of GLP1RA on cardiovascular risk reduction (105–111). In addition to enhancing insulin secretion, GLP1RAs may reduce postprandial chylomicron overproduction in T2DM patients by reducing intestinal absorption of dietary lipids and enhancing hepatic fatty acid oxidation (112). Exenatide and liraglutide have been reported to be equally effective in lowering postprandial dyslipidaemia, an effect observed immediately after initial administration, as well as after a two-week treatment period (113). In a double-blind, randomized, placebo-controlled, crossover study with subjects who exhibited impaired glucose tolerance or had recent-onset T2DM, a single subcutaneous injection of exenatide strongly and consistently inhibited the postprandial increase of proatherogenic lipids and lipoproteins (114). A clinical study in patients with T2DM treatment with a long-lasting release exenatide on top of metformin, a first-line therapy for T2DM, led to improved cardiometabolic parameters, including cITM and flow-mediated dilation (115). In two prospective studies, liraglutide treatment decreased cITM, total- and LDL-cholesterol as well as triglycerides after 8 months of treatment in T2DM patients, as well as during an 18-month follow-up in subjects with T2DM and metabolic syndrome (116), thereby improving cardiometabolic risk factors. Moreover, liraglutide reduced the level of atherogenic small dense LDL-3 subfraction in association with a lower cITM (117). Semaglutide also reduced cITM (118), and improved the cholesterol profile, triglyceride levels (119, 120) and reduced oxLDL (121) in T2DM patients. Further studies are needed to assess the effect of GLP1RA on other atherogenic lipoproteins such as lipoprotein(a) or electronegative LDL.
3.2 Dual GIPR/GLP1R agonism
Tirzepatide is the first unimolecular dual GIPR/GLP1R agonist for the treatment of T2DM and overweight/obesity (122). The co-agonism of GLP-1 and GIP results in significantly greater blood glucose and weight reduction than for GLP1R agonism alone (123, 124). Moreover, tirzepatide treatment in patients with obesity and prediabetes resulted in a lower risk of progression to T2DM compared to placebo (125). The mechanism behind the greater body weight reduction in humans is still being investigated (126).
3.2.1 Preclinical studies
Animal studies suggest that GIP suppresses food intake via neural GIPR activation, although it is still not clear especially for the peripheral actions whether or not continuous GIPR agonism causes functional antagonism of the GIPR (126).
To the best of our knowledge, there are no published studies regarding the mechanism of lipid lowering effect by tirzepatide in humans. However, a recent study in APOE*3-Leiden. CETP mice, a transgenic mouse model with accelerated atherosclerosis, showed that combined GIPR/GLP1R agonism attenuated the development of severe atherosclerotic lesions (127, 128). GIPR/GLP1R agonism decreased markers of low-grade inflammation and lowered plasma triglyceride levels by increasing VLDL-derived fatty acid uptake by adipose tissue, as wells as increasing the liver uptake of VLDL remnants. In comparison, treatments with single agonists showed non-significant improvements.
3.2.2 Clinical trials
SURPASS trials in T2DM patients showed that tirzepatide was superior compared to placebo and insulin glargine in lowering triglycerides, LDL-, and VLDL- cholesterol levels (129) as well as increasing HDL-cholesterol (130, 131). When compared to semaglutide or insulin degludec, tirzepatide significantly reduced VLDL cholesterol and increased HDL cholesterol, while total cholesterol and LDL cholesterol did not differ among treatments (124, 132). Similarly, in clinical trials with the focus on obesity treatment (SURMOUNT trials), tirzepatide was superior compared to placebo in lowering triglycerides, total-, LDL-, and VLDL- cholesterol levels as well as increasing HDL cholesterol (95, 133–135).
The lipid lowering effect of tirzepatide would be expected to have benefits in reducing clinical outcomes from atherosclerotic and non-atherosclerotic CVD. The recently concluded SUMMIT trial showed that tirzepatide lowered the risk of a composite death from cardiovascular causes or worsening heart failure than placebo in patients with heart failure with preserved ejection fraction and obesity (136). Other ongoing clinical trials are exploring potential cardiovascular benefits of tirzepatide in diabetic and overweight/obese participants with established CVD or high cardiovascular risk (137–139), as well as the effect of tirzepatide on the progression of coronary atherosclerosis (140).
3.3 Future incretin-based therapies
Tirzepatide's superiority over its mono-agonist equivalents has triggered the development of additional multi-agonistic medications as the next generation of therapeutics for metabolic disease (94).
One promising medication is retatrutide, a triple GIP/GLP-1/glucagon receptor agonist. The treatment of obese adults with retatrutide resulted in a mean weight reduction of 24.2% after 48 weeks, and was associated with improvements in cardiometabolic measures (exploratory endpoints) including systolic and diastolic blood pressure, levels of glycated hemoglobin, fasting glucose, insulin, and lipids (141). Triglycerides, total cholesterol, LDL- and VLDL-cholesterol were lower in retatrutide groups, but no improvements in HDL cholesterol levels were observed compared to placebo. In a study in T2DM patients with a BMI 25-50 kg/m2 retatrutide treatment significantly decreased body weight from baseline compared to placebo and dulaglutide and lowered the fasting lipid profile in a dose-dependent manner at 36 weeks (142). Higher concentrations of retatrutide (8 mg and 12 mg) significantly decreased total cholesterol, triglycerides and non-HDL cholesterol compared to placebo or dulaglutide. The non-HDL cholesterol effect was driven by reductions in VLDL cholesterol concentrations, while changes in LDL- and HDL cholesterol were generally not significantly different vs. placebo or dulaglutide.
4 Outlook and conclusion
BAs act as vital metabolic regulators, rather than mere digestive aids. BAs are used in traditional Chinese medicine as anti-oxidant to treat multiple digestive and metabolic disorders and in western medicine semi-synthetic BAs like obeticholic acid are treatments for cholestatic liver diseases (143–146). BAs are commercially available as dietary aids and their assumption may lead to shift in the circulating BA pool, however, since absolute serum levels are tightly regulated long-lasting BA modulation and their effect need to be further investigated (147). By activating FXR and TGR5 as well as influencing GLP-1 secretion, BAs contribute to both energy balance and cardiovascular health and future research is examining their role in obesity-associated cardiometabolic derangements. Incretins and incretin-based therapies have a multifaceted, beneficial influence on the cardiovascular function by improving EC function, reducing inflammation, pro-atherogenic lipid and progression of atherosclerotic plaques. GIP actions have recently sparked interest based on the cardiometabolic benefits of the dual GIPR/GLP1R co-agonist tirzepatide and intense ongoing research is examining how GIP co-agonism further improves the effects of single GLP1RAs in humans. Despite the clinical efficacy of incretin-based therapies, suboptimal access, high cost, limited insurance coverage and therapeutic inertia are significant barriers to their widespread adoption. Real world data regarding the long-term effect of these drugs need to be collected to fully evaluate their multi-organ mechanism(s) of action and safety.
Author contributions
AK: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. JG: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. DZ: Writing – original draft, Writing – review & editing. EO: Supervision, Writing – original draft, Writing – review & editing, Funding acquisition.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The financial support by the Swiss National Science Foundation PRIMA: PR00P3_179861/1 and the Swiss Life Foundation, the Heubergstiftung, The Philhuman Stiftung, Novartis Foundation and the Swiss Heart Foundation, Switzerland to EO; City of Graz to AK and “Young Pilots” of the Medical University of Graz to JG are gratefully acknowledged.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Boutari C, Mantzoros CSA. 2022 update on the epidemiology of obesity and a call to action: as its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metab Clin Exp. (2022) 133:155217. doi: 10.1016/j.metabol.2022.155217
2. Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. (2011) 378(9793):815–25. doi: 10.1016/S0140-6736(11)60814-3
3. Koskinas KC, Van Craenenbroeck EM, Antoniades C, Blüher M, Gorter TM, Hanssen H, et al. Obesity and cardiovascular disease: an ESC clinical consensus statement. Eur Heart J. (2024) 45(38):4063–98. doi: 10.1093/eurheartj/ehae508
4. Volpe M, Gallo G. Obesity and cardiovascular disease: an executive document on pathophysiological and clinical links promoted by the Italian society of cardiovascular prevention (SIPREC). Front Cardiovasc Med. (2023) 10:1136340. doi: 10.3389/fcvm.2023.1136340
5. Lopez-Jimenez F, Almahmeed W, Bays H, Cuevas A, Di Angelantonio E, Le Roux CW, et al. Obesity and cardiovascular disease: mechanistic insights and management strategies. A joint position paper by the world heart federation and world obesity federation. Eur J Prev Cardiol. (2022) 29(17):2218–37. doi: 10.1093/eurjpc/zwac187
6. Akil L, Ahmad HA. Relationships between obesity and cardiovascular diseases in four southern states and Colorado. J Health Care Poor Underserved. (2011) 22(4 Suppl):61–72. doi: 10.1353/hpu.2011.0166
7. Kádár A. Development of atherosclerosis and plaque biology. Cardiovasc Surg. (2001) 9(2):109–21. doi: 10.1177/096721090100900201
8. Jebari-Benslaiman S, Galicia-García U, Larrea-Sebal A, Olaetxea JR, Alloza I, Vandenbroeck K, et al. Pathophysiology of atherosclerosis. Int J Mol Sci. (2022) 23(6):3346. doi: 10.3390/ijms23063346
9. Rafieian-Kopaei M, Setorki M, Doudi M, Baradaran A, Nasri H. Atherosclerosis: process, indicators, risk factors and new hopes. Int J Prev Med. (2014) 5(8):927–46.25489440
10. Lu Y, Cui X, Zhang L, Wang X, Xu Y, Qin Z, et al. The functional role of lipoproteins in atherosclerosis: novel directions for diagnosis and targeting therapy. Aging Dis. (2022) 13(2):491–520. doi: 10.14336/AD.2021.0929
11. Wang HH, Garruti G, Liu M, Portincasa P, Wang DQH. Cholesterol and lipoprotein metabolism and atherosclerosis: recent advances in reverse cholesterol transport. Ann Hepatol. (2017) 16:S27–42. doi: 10.5604/01.3001.0010.5495
12. Stroope C, Nettersheim FS, Coon B, Finney AC, Schwartz MA, Ley K, et al. Dysregulated cellular metabolism in atherosclerosis: mediators and therapeutic opportunities. Nat Metab. (2024) 6(4):617–38. doi: 10.1038/s42255-024-01015-w
13. Sutton G, Pugh D, Dhaun N. Developments in the role of endothelin-1 in atherosclerosis: a potential therapeutic target? Am J Hypertens. (2019) 32(9):813–5. doi: 10.1093/ajh/hpz091
14. Mhaimeed O, Burney ZA, Schott SL, Kohli P, Marvel FA, Martin SS. The importance of LDL-C lowering in atherosclerotic cardiovascular disease prevention: lower for longer is better. Am J Prev Cardiol. (2024) 18:100649. doi: 10.1016/j.ajpc.2024.100649
15. Borén J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European atherosclerosis society consensus panel. Eur Heart J. (2020) 41(24):2313–30. doi: 10.1093/eurheartj/ehz962
16. Poznyak AV, Nikiforov NG, Starodubova AV, Popkova TV, Orekhov AN. Macrophages and foam cells: brief overview of their role, linkage, and targeting potential in atherosclerosis. Biomedicines. (2021) 9(9):1221. doi: 10.3390/biomedicines9091221
17. Owsiany KM, Alencar GF, Owens GK. Revealing the origins of foam cells in atherosclerotic lesions. Arterioscler Thromb Vasc Biol. (2019) 39(5):836–8. doi: 10.1161/ATVBAHA.119.312557
18. Li M, Wang ZW, Fang LJ, Cheng SQ, Wang X, Liu NF. Programmed cell death in atherosclerosis and vascular calcification. Cell Death Dis. (2022) 13(5):467. doi: 10.1038/s41419-022-04923-5
19. He C, Kim HI, Park J, Guo J, Huang W. The role of immune cells in different stages of atherosclerosis. Int J Med Sci. (2024) 21(6):1129–43. doi: 10.7150/ijms.94570
20. Neels JG, Gollentz C, Chinetti G. Macrophage death in atherosclerosis: potential role in calcification. Front Immunol. (2023) 14:1215612. doi: 10.3389/fimmu.2023.1215612
21. Ouimet M, Barrett TJ, Fisher EA. HDL and reverse cholesterol transport. Circ Res. (2019) 124(10):1505–18. doi: 10.1161/CIRCRESAHA.119.312617
22. Marques LR, Diniz TA, Antunes BM, Rossi FE, Caperuto EC, Lira FS, et al. Reverse cholesterol transport: molecular mechanisms and the non-medical approach to enhance HDL cholesterol. Front Physiol. (2018) 9:526. doi: 10.3389/fphys.2018.00526
23. Dastmalchi LN, German CA, Taub PR. High density lipoprotein: when to rethink too much of a good thing. Am J Prev Cardiol. (2023) 15:100511. doi: 10.1016/j.ajpc.2023.100511
24. Sheridan S, Pignone M, Mulrow C. Framingham-based tools to calculate the global risk of coronary heart disease: a systematic review of tools for clinicians. J Gen Intern Med. (2003) 18(12):1039–52. doi: 10.1111/j.1525-1497.2003.30107.x
25. Miller GJ, Miller NE. Plasma-high-density-lipoprotein concentration and development of ischæmic heart-disease. Lancet. (1975) 305(7897):16–9. doi: 10.1016/S0140-6736(75)92376-4
26. Chiesa ST, Charakida M. High-density lipoprotein function and dysfunction in health and disease. Cardiovasc Drugs Ther. (2019) 33(2):207–19. doi: 10.1007/s10557-018-06846-w
27. Jomard A, Osto E. High density lipoproteins: metabolism, function, and therapeutic potential. Front Cardiovasc Med. (2020) 7:39. doi: 10.3389/fcvm.2020.00039
28. Navab M, Reddy ST, Van Lenten BJ, Anantharamaiah GM, Fogelman AM. The role of dysfunctional HDL in atherosclerosis. J Lipid Res. (2009) 50:S145–9. doi: 10.1194/jlr.R800036-JLR200
30. Duboc H, Taché Y, Hofmann AF. The bile acid TGR5 membrane receptor: from basic research to clinical application. Dig Liver Dis. (2014) 46(4):302–12. doi: 10.1016/j.dld.2013.10.021
31. Claudel T, Staels B, Kuipers F. The farnesoid X receptor: a molecular link between bile acid and lipid and glucose metabolism. Arterioscler Thromb Vasc Biol. (2005) 25(10):2020–30. doi: 10.1161/01.ATV.0000178994.21828.a7
32. Wang YD, Chen WD, Moore DD, Huang W. FXR: a metabolic regulator and cell protector. Cell Res. (2008) 18(11):1087–95. doi: 10.1038/cr.2008.289
33. Ma H, Patti ME. Bile acids, obesity, and the metabolic syndrome. Best Pract Res Clin Gastroenterol. (2014) 28(4):573–83. doi: 10.1016/j.bpg.2014.07.004
34. Haeusler RA, Camastra S, Nannipieri M, Astiarraga B, Castro-Perez J, Xie D, et al. Increased bile acid synthesis and impaired bile acid transport in human obesity. J Clin Endocrinol Metab. (2016) 101(5):1935–44. doi: 10.1210/jc.2015-2583
35. Osto E, Doytcheva P, Corteville C, Bueter M, Dörig C, Stivala S, et al. Rapid and body weight-independent improvement of endothelial and high-density lipoprotein function after roux-en-Y gastric bypass: role of glucagon-like peptide-1. Circulation. (2015) 131(10):871–81. doi: 10.1161/CIRCULATIONAHA.114.011791
36. Li R, Andreu-Sánchez S, Kuipers F, Fu J. Gut microbiome and bile acids in obesity-related diseases. Best Pract Res Clin Endocrinol Metab. (2021) 35(3):101493. doi: 10.1016/j.beem.2021.101493
37. Harach T, Pols TWH, Nomura M, Maida A, Watanabe M, Auwerx J, et al. TGR5 Potentiates GLP-1 secretion in response to anionic exchange resins. Sci Rep. (2012) 2(1):430. doi: 10.1038/srep00430
38. Ticho AL, Malhotra P, Dudeja PK, Gill RK, Alrefai WA. Bile acid receptors and gastrointestinal functions. Liver Res. (2019) 3(1):31–9. doi: 10.1016/j.livres.2019.01.001
39. Drucker DJ, Habener JF, Holst JJ. Discovery, characterization, and clinical development of the glucagon-like peptides. J Clin Invest. (2017) 127(12):4217–27. doi: 10.1172/JCI97233
40. Dupre J, Ross SA, Watson D, Brown JC. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab. (1973) 37(5):826–8. doi: 10.1210/jcem-37-5-826
41. Drucker DJ, Yusta B. Physiology and pharmacology of the enteroendocrine hormone glucagon-like peptide-2. Annu Rev Physiol. (2014) 76:561–83. doi: 10.1146/annurev-physiol-021113-170317
42. Rabbani N, Thornalley PJ. Unraveling the impaired incretin effect in obesity and type 2 diabetes: key role of hyperglycemia-induced unscheduled glycolysis and glycolytic overload. Diabetes Res Clin Pract. (2024) 217:111905. doi: 10.1016/j.diabres.2024.111905
43. Lintom M, Yancey P, Davies S. The Role of Lipids and Lipoproteins in Atherosclerosis. South Dartmouth, MA: MDText.com, Inc. (2000). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK343489/
44. Bezsonov E, Khotina V, Glanz V, Sobenin I, Orekhov A. Lipids and lipoproteins in atherosclerosis. Biomedicines. (2023) 11(5):1424. doi: 10.3390/biomedicines11051424
45. Hofmann AF, Sjövall J, Kurz G, Radominska A, Schteingart CD, Tint GS, et al. A proposed nomenclature for bile acids. J Lipid Res. (1992) 33(4):599–604. doi: 10.1016/S0022-2275(20)41624-4
46. Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol. (2014) 30(3):332–8. doi: 10.1097/MOG.0000000000000057
47. Guzior DV, Quinn RA. Review: microbial transformations of human bile acids. Microbiome. (2021) 9(1):140. doi: 10.1186/s40168-021-01101-1
48. Di Ciaula A, Garruti G, Lunardi Baccetto R, Molina-Molina E, Bonfrate L, Wang DQH, et al. Bile acid physiology. Ann Hepatol. (2017) 16:S4–14. doi: 10.5604/01.3001.0010.5493
49. Langhi C, Le May C, Kourimate S, Caron S, Staels B, Krempf M, et al. Activation of the farnesoid X receptor represses PCSK9 expression in human hepatocytes. FEBS Lett. (2008) 582(6):949–55. doi: 10.1016/j.febslet.2008.02.038
50. Nakahara M, Fujii H, Maloney PR, Shimizu M, Sato R. Bile acids enhance low density lipoprotein receptor gene expression via a MAPK cascade-mediated stabilization of mRNA. J Biol Chem. (2002) 277(40):37229–34. doi: 10.1074/jbc.M206749200
51. Taniguchi T, Chen J, Cooper AD. Regulation of cholesterol 7 alpha-hydroxylase gene expression in hep-G2 cells. Effect of serum, bile salts, and coordinate and noncoordinate regulation with other sterol-responsive genes. J Biol Chem. (1994) 269(13):10071–8. doi: 10.1016/S0021-9258(17)36991-0
52. Claudel T, Sturm E, Duez H, Torra IP, Sirvent A, Kosykh V, et al. Bile acid-activated nuclear receptor FXR suppresses apolipoprotein A-I transcription via a negative FXR response element. J Clin Invest. (2002) 109(7):961–71. doi: 10.1172/JCI0214505
53. Zhang Y, Wang X, Vales C, Lee FY, Lee H, Lusis AJ, et al. FXR Deficiency causes reduced atherosclerosis in Ldlr −/− mice. Arterioscler Thromb Vasc Biol. (2006) 26(10):2316–21. doi: 10.1161/01.ATV.0000235697.35431.05
54. Hanniman EA, Lambert G, McCarthy TC, Sinal CJ. Loss of functional farnesoid X receptor increases atherosclerotic lesions in apolipoprotein E-deficient mice. J Lipid Res. (2005) 46(12):2595–604. doi: 10.1194/jlr.M500390-JLR200
55. Nakajima M, Hutchinson HG, Fujinaga M, Hayashida W, Morishita R, Zhang L, et al. The angiotensin II type 2 (AT2) receptor antagonizes the growth effects of the AT1 receptor: gain-of-function study using gene transfer. Proc Natl Acad Sci. (1995) 92(23):10663–7. doi: 10.1073/pnas.92.23.10663
56. Bishop-Bailey D, Walsh DT, Warner TD. Expression and activation of the farnesoid X receptor in the vasculature. Proc Natl Acad Sci. (2004) 101(10):3668–73. doi: 10.1073/pnas.0400046101
57. Zhang Q, He F, Kuruba R, Gao X, Wilson A, Li J, et al. FXR-mediated regulation of angiotensin type 2 receptor expression in vascular smooth muscle cells. Cardiovasc Res. (2008) 77(3):560–9. doi: 10.1093/cvr/cvm068
58. Molavi B, Chen J, Mehta JL. Cardioprotective effects of rosiglitazone are associated with selective overexpression of type 2 angiotensin receptors and inhibition of p42/44 MAPK. Am J Physiol-Heart Circ Physiol. (2006) 291(2):H687–93. doi: 10.1152/ajpheart.00926.2005
59. Li J, Wilson A, Kuruba R, Zhang Q, Gao X, He F, et al. FXR-mediated regulation of eNOS expression in vascular endothelial cells. Cardiovasc Res. (2008) 77(1):169–77. doi: 10.1093/cvr/cvm016
60. He F, Li J, Mu Y, Kuruba R, Ma Z, Wilson A, et al. Downregulation of endothelin-1 by farnesoid X receptor in vascular endothelial cells. Circ Res. (2006) 98(2):192–9. doi: 10.1161/01.RES.0000200400.55539.85
61. Maruyama T, Tanaka K, Suzuki J, Miyoshi H, Harada N, Nakamura T, et al. Targeted disruption of G protein-coupled bile acid receptor 1 (Gpbar1/M-bar) in mice. J Endocrinol. (2006) 191(1):197–205. doi: 10.1677/joe.1.06546
62. Li T, Holmstrom SR, Kir S, Umetani M, Schmidt DR, Kliewer SA, et al. The G protein-coupled bile acid receptor, TGR5, stimulates gallbladder filling. Mol Endocrinol. (2011) 25(6):1066–71. doi: 10.1210/me.2010-0460
63. Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. (2009) 10(3):167–77. doi: 10.1016/j.cmet.2009.08.001
64. Pols TWH, Nomura M, Harach T, Lo Sasso G, Oosterveer MH, Thomas C, et al. TGR5 Activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab. (2011) 14(6):747–57. doi: 10.1016/j.cmet.2011.11.006
65. Zhang F, Jia Z, Gao P, Kong H, Li X, Chen J, et al. Metabonomics study of atherosclerosis rats by ultra fast liquid chromatography coupled with ion trap-time of flight mass spectrometry. Talanta. (2009) 79(3):836–44. doi: 10.1016/j.talanta.2009.05.010
66. Su J, Zhao Q, Zhao A, Jia W, Zhu W, Lu J, et al. Serum metabolic signatures of subclinical atherosclerosis in patients with type 2 diabetes mellitus: a preliminary study. Acta Diabetol. (2021) 58(9):1217–24. doi: 10.1007/s00592-021-01717-7
67. Cheng X, Zhang R, Qi X, Wang H, Gao T, Zheng L, et al. Metabolomics and network pharmacology exploration of the effects of bile acids on carotid atherosclerosis and potential underlying mechanisms. Front Endocrinol. (2024) 15:1430720. doi: 10.3389/fendo.2024.1430720
68. Porez G, Prawitt J, Gross B, Staels B. Bile acid receptors as targets for the treatment of dyslipidemia and cardiovascular disease. J Lipid Res. (2012) 53(9):1723–37. doi: 10.1194/jlr.R024794
69. Kuipers F, Stroeve JH, Caron S, Staels B. Bile acids, farnesoid X receptor, atherosclerosis and metabolic control. Curr Opin Lipidol. (2007) 18(3):289–97. doi: 10.1097/MOL.0b013e3281338d08
70. Zhu X, Zhou A, Dey A, Norrbom C, Carroll R, Zhang C, et al. Disruption of PC1/3 expression in mice causes dwarfism and multiple neuroendocrine peptide processing defects. Proc Natl Acad Sci U S A. (2002) 99(16):10293–8. doi: 10.1073/pnas.162352599
71. Camilleri M. Gastrointestinal hormones and regulation of gastric emptying. Curr Opin Endocrinol Diabetes Obes. (2019) 26(1):3–10. doi: 10.1097/MED.0000000000000448
72. Hammoud R, Drucker DJ. Beyond the pancreas: contrasting cardiometabolic actions of GIP and GLP1. Nat Rev Endocrinol. (2023) 19(4):201–16. doi: 10.1038/s41574-022-00783-3
73. Drucker DJ. Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: preclinical biology and mechanisms of action. Diabetes Care. (2007) 30(6):1335–43. doi: 10.2337/dc07-0228
74. Ding KH, Zhong Q, Xu J, Isales CM. Glucose-dependent insulinotropic peptide: differential effects on hepatic artery vs. portal vein endothelial cells. Am J Physiol Endocrinol Metab. (2004) 286(5):E773–779. doi: 10.1152/ajpendo.00507.2003
75. Lim DM, Park KY, Hwang WM, Kim JY, Kim BJ. Difference in protective effects of GIP and GLP-1 on endothelial cells according to cyclic adenosine monophosphate response. Exp Ther Med. (2017) 13(5):2558–64. doi: 10.3892/etm.2017.4279
76. Ojima A, Matsui T, Maeda S, Takeuchi M, Yamagishi S. Glucose-dependent insulinotropic polypeptide (GIP) inhibits signaling pathways of advanced glycation end products (AGEs) in endothelial cells via its antioxidative properties. Horm Metab Res Horm Stoffwechselforschung Horm Metab. (2012) 44(7):501–5. doi: 10.1055/s-0032-1312595
77. Berglund LM, Lyssenko V, Ladenvall C, Kotova O, Edsfeldt A, Pilgaard K, et al. Glucose-dependent insulinotropic polypeptide stimulates osteopontin expression in the vasculature via endothelin-1 and CREB. Diabetes. (2016) 65(1):239–54. doi: 10.2337/db15-0122
78. Ding KH, Zhong Q, Isales CM. Glucose-dependent insulinotropic peptide stimulates thymidine incorporation in endothelial cells: role of endothelin-1. Am J Physiol Endocrinol Metab. (2003) 285(2):E390–396. doi: 10.1152/ajpendo.00509.2002
79. Chinetti-Gbaguidi G, Colin S, Staels B. Macrophage subsets in atherosclerosis. Nat Rev Cardiol. (2015) 12(1):10–7. doi: 10.1038/nrcardio.2014.173
80. Chistiakov DA, Bobryshev YV, Orekhov AN. Macrophage-mediated cholesterol handling in atherosclerosis. J Cell Mol Med. (2016) 20(1):17–28. doi: 10.1111/jcmm.12689
81. Libby P, Ridker PM, Hansson GK. Leducq transatlantic network on atherothrombosis. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. (2009) 54(23):2129–38. doi: 10.1016/j.jacc.2009.09.009
82. Nagashima M, Watanabe T, Terasaki M, Tomoyasu M, Nohtomi K, Kim-Kaneyama J, et al. Native incretins prevent the development of atherosclerotic lesions in apolipoprotein E knockout mice. Diabetologia. (2011) 54(10):2649–59. doi: 10.1007/s00125-011-2241-2
83. Suzuki Y, Nakamura N, Miyabe M, Nishikawa T, Miyajima SI, Adachi K, et al. Anti-inflammatory role of glucose-dependent insulinotropic polypeptide in periodontitis. J Diabetes Investig. (2016) 7(4):497–505. doi: 10.1111/jdi.12450
84. Kahles F, Liberman A, Halim C, Rau M, Möllmann J, Mertens RW, et al. The incretin hormone GIP is upregulated in patients with atherosclerosis and stabilizes plaques in ApoE-/- mice by blocking monocyte/macrophage activation. Mol Metab. (2018) 14:150–7. doi: 10.1016/j.molmet.2018.05.014
85. Nogi Y, Nagashima M, Terasaki M, Nohtomi K, Watanabe T, Hirano T. Glucose-dependent insulinotropic polypeptide prevents the progression of macrophage-driven atherosclerosis in diabetic apolipoprotein E-null mice. PLoS One. (2012) 7(4):e35683. doi: 10.1371/journal.pone.0035683
86. Sachs S, Götz A, Finan B, Feuchtinger A, DiMarchi RD, Döring Y, et al. GIP receptor agonism improves dyslipidemia and atherosclerosis independently of body weight loss in preclinical mouse model for cardio-metabolic disease. Cardiovasc Diabetol. (2023) 22(1):217. doi: 10.1186/s12933-023-01940-2
87. Pujadas G, Baggio LL, Kaur KD, McLean BA, Cao X, Drucker DJ. Genetic disruption of the gipr in apoe-/- mice promotes atherosclerosis. Mol Metab. (2022) 65:101586. doi: 10.1016/j.molmet.2022.101586
88. Dai Y, Dai D, Wang X, Ding Z, Li C, Mehta JL. GLP-1 agonists inhibit ox-LDL uptake in macrophages by activating protein kinase A. J Cardiovasc Pharmacol. (2014) 64(1):47–52. doi: 10.1097/FJC.0000000000000087
89. Qin X, Shen H, Liu M, Yang Q, Zheng S, Sabo M, et al. GLP-1 reduces intestinal lymph flow, triglyceride absorption, and apolipoprotein production in rats. Am J Physiol Gastrointest Liver Physiol. (2005) 288(5):G943–949. doi: 10.1152/ajpgi.00303.2004
90. Hoffman S, Alvares D, Adeli K. GLP-1 attenuates intestinal fat absorption and chylomicron production via vagal afferent nerves originating in the portal vein. Mol Metab. (2022) 65:101590. doi: 10.1016/j.molmet.2022.101590
91. Yanai H, Adachi H, Hakoshima M, Katsuyama H. Postprandial hyperlipidemia: its pathophysiology, diagnosis, atherogenesis, and treatments. Int J Mol Sci. (2023) 24(18):13942. doi: 10.3390/ijms241813942
92. Duan L, Rao X, Xia C, Rajagopalan S, Zhong J. The regulatory role of DPP4 in atherosclerotic disease. Cardiovasc Diabetol. (2017) 16(1):76. doi: 10.1186/s12933-017-0558-y
93. Liu H, Guo L, Xing J, Li P, Sang H, Hu X, et al. The protective role of DPP4 inhibitors in atherosclerosis. Eur J Pharmacol. (2020) 875:173037. doi: 10.1016/j.ejphar.2020.173037
94. Psaltis JP, Marathe JA, Nguyen MT, Le R, Bursill CA, Marathe CS, et al. Incretin-based therapies for the management of cardiometabolic disease in the clinic: past, present, and future. Med Res Rev. (2024) 45:29–65. doi: 10.1002/med.22070
95. Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. (2022) 387(3):205–16. doi: 10.1056/NEJMoa2206038
96. Sposito AC, Berwanger O, de Carvalho LSF, Saraiva JFK. GLP-1RAs in type 2 diabetes: mechanisms that underlie cardiovascular effects and overview of cardiovascular outcome data. Cardiovasc Diabetol. (2018) 17(1):157. doi: 10.1186/s12933-018-0800-2
97. Parkes DG, Mace KF, Trautmann ME. Discovery and development of exenatide: the first antidiabetic agent to leverage the multiple benefits of the incretin hormone, GLP-1. Expert Opin Drug Discov. (2013) 8(2):219–44. doi: 10.1517/17460441.2013.741580
98. Marx N, Husain M, Lehrke M, Verma S, Sattar N. GLP-1 receptor agonists for the reduction of atherosclerotic cardiovascular risk in patients with type 2 diabetes. Circulation. (2022) 146(24):1882–94. doi: 10.1161/CIRCULATIONAHA.122.059595
99. Parlevliet ET, Wang Y, Geerling JJ, Schröder-Van der Elst JP, Picha K, O’Neil K, et al. GLP-1 receptor activation inhibits VLDL production and reverses hepatic steatosis by decreasing hepatic lipogenesis in high-fat-fed APOE*3-Leiden mice. PLoS One. (2012) 7(11):e49152. doi: 10.1371/journal.pone.0049152
100. Rakipovski G, Rolin B, Nøhr J, Klewe I, Frederiksen KS, Augustin R, et al. The GLP-1 analogs liraglutide and semaglutide reduce atherosclerosis in ApoE-/- and LDLr-/- mice by a mechanism that includes inflammatory pathways. JACC Basic Transl Sci. (2018) 3(6):844–57. doi: 10.1016/j.jacbts.2018.09.004
101. Gaspari T, Welungoda I, Widdop RE, Simpson RW, Dear AE. The GLP-1 receptor agonist liraglutide inhibits progression of vascular disease via effects on atherogenesis, plaque stability and endothelial function in an ApoE(-/-) mouse model. Diab Vasc Dis Res. (2013) 10(4):353–60. doi: 10.1177/1479164113481817
102. Bruen R, Curley S, Kajani S, Lynch G, O’Reilly ME, Dillon ET, et al. Liraglutide attenuates preestablished atherosclerosis in apolipoprotein E-deficient mice via regulation of immune cell phenotypes and proinflammatory mediators. J Pharmacol Exp Ther. (2019) 370(3):447–58. doi: 10.1124/jpet.119.258343
103. Tashiro Y, Sato K, Watanabe T, Nohtomi K, Terasaki M, Nagashima M, et al. A glucagon-like peptide-1 analog liraglutide suppresses macrophage foam cell formation and atherosclerosis. Peptides. (2014) 54:19–26. doi: 10.1016/j.peptides.2013.12.015
104. Punjabi M, Kosareva A, Xu L, Ochoa-Espinosa A, Decembrini S, Hofmann G, et al. Liraglutide lowers endothelial vascular cell adhesion molecule-1 in murine atherosclerosis independent of glucose levels. JACC Basic Transl Sci. (2023) 8(2):189–200. doi: 10.1016/j.jacbts.2022.08.002
105. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JFE, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. (2016) 375(4):311–22. doi: 10.1056/NEJMoa1603827
106. Husain M, Bain SC, Jeppesen OK, Lingvay I, Sørrig R, Treppendahl MB, et al. Semaglutide (SUSTAIN and PIONEER) reduces cardiovascular events in type 2 diabetes across varying cardiovascular risk. Diabetes Obes Metab. (2020) 22(3):442–51. doi: 10.1111/dom.13955
107. Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. (2015) 373(23):2247–57. doi: 10.1056/NEJMoa1509225
108. Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. (2017) 377(13):1228–39. doi: 10.1056/NEJMoa1612917
109. Hernandez AF, Green JB, Janmohamed S, D’Agostino RB, Granger CB, Jones NP, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (harmony outcomes): a double-blind, randomised placebo-controlled trial. Lancet Lond Engl. (2018) 392(10157):1519–29. doi: 10.1016/S0140-6736(18)32261-X
110. Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet Lond Engl. (2019) 394(10193):121–30. doi: 10.1016/S0140-6736(19)31149-3
111. Gerstein HC, Sattar N, Rosenstock J, Ramasundarahettige C, Pratley R, Lopes RD, et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N Engl J Med. (2021) 385(10):896–907. doi: 10.1056/NEJMoa2108269
112. Farr S, Taher J, Adeli K. Glucagon-like peptide-1 as a key regulator of lipid and lipoprotein metabolism in fasting and postprandial states. Cardiovasc Hematol Disord Drug Targets. (2014) 14(2):126–36. doi: 10.2174/1871529X14666140505125300
113. Voukali M, Kastrinelli I, Stragalinou S, Tasiopoulou D, Paraskevopoulou P, Katsilambros N, et al. Study of postprandial lipaemia in type 2 diabetes mellitus: exenatide versus liraglutide. J Diabetes Res. (2014) 2014:304032. doi: 10.1155/2014/304032
114. Schwartz EA, Koska J, Mullin MP, Syoufi I, Schwenke DC, Reaven PD. Exenatide suppresses postprandial elevations in lipids and lipoproteins in individuals with impaired glucose tolerance and recent onset type 2 diabetes mellitus. Atherosclerosis. (2010) 212(1):217–22. doi: 10.1016/j.atherosclerosis.2010.05.028
115. Patti AM, Nikolic D, Magan-Fernandez A, Giglio RV, Castellino G, Chianetta R, et al. Exenatide once-weekly improves metabolic parameters, endothelial dysfunction and carotid intima-media thickness in patients with type-2 diabetes: an 8-month prospective study. Diabetes Res Clin Pract. (2019) 149:163–9. doi: 10.1016/j.diabres.2019.02.006
116. Rizzo M, Rizvi AA, Patti AM, Nikolic D, Giglio RV, Castellino G, et al. Liraglutide improves metabolic parameters and carotid intima-media thickness in diabetic patients with the metabolic syndrome: an 18-month prospective study. Cardiovasc Diabetol. (2016) 15(1):162. doi: 10.1186/s12933-016-0480-8
117. Nikolic D, Giglio RV, Rizvi AA, Patti AM, Montalto G, Maranta F, et al. Liraglutide reduces carotid intima-media thickness by reducing small dense low-density lipoproteins in a real-world setting of patients with type 2 diabetes: a novel anti-atherogenic effect. Diabetes Ther Res Treat Educ Diabetes Relat Disord. (2021) 12(1):261–74. doi: 10.1007/s13300-020-00962-3
118. Patti AM, Giglio RV, Allotta A, Bruno A, Di Bella T, Pantea Stoian A, et al. Effect of semaglutide on subclinical atherosclerosis and cardiometabolic compensation: a real-world study in patients with type 2 diabetes. Biomedicines. (2023) 11(5):1362. doi: 10.3390/biomedicines11051362
119. Yamada H, Yoshida M, Funazaki S, Morimoto J, Tonezawa S, Takahashi A, et al. Retrospective analysis of the effectiveness of oral semaglutide in type 2 diabetes mellitus and its effect on cardiometabolic parameters in Japanese clinical settings. J Cardiovasc Dev Dis. (2023) 10(4):176. doi: 10.3390/jcdd10040176
120. Aroda VR, Ahmann A, Cariou B, Chow F, Davies MJ, Jódar E, et al. Comparative efficacy, safety, and cardiovascular outcomes with once-weekly subcutaneous semaglutide in the treatment of type 2 diabetes: insights from the SUSTAIN 1-7 trials. Diabetes Metab. (2019) 45(5):409–18. doi: 10.1016/j.diabet.2018.12.001
121. Hachuła M, Kosowski M, Ryl S, Basiak M, Okopień B. Impact of glucagon-like peptide 1 receptor agonists on biochemical markers of the initiation of atherosclerotic process. Int J Mol Sci. (2024) 25(3):1854. doi: 10.3390/ijms25031854
122. Willard FS, Douros JD, Gabe MB, Showalter AD, Wainscott DB, Suter TM, et al. Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight. (2020) 5(17):e140532. doi: 10.1172/jci.insight.140532
123. Baggio LL, Drucker DJ. Glucagon-like peptide-1 receptor co-agonists for treating metabolic disease. Mol Metab. (2021) 46:101090. doi: 10.1016/j.molmet.2020.101090
124. Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. (2021) 385(6):503–15. doi: 10.1056/NEJMoa2107519
125. Jastreboff AM, le Roux CW, Stefanski A, Aronne LJ, Halpern B, Wharton S, et al. Tirzepatide for obesity treatment and diabetes prevention. N Engl J Med. (2024). doi: 10.1056/NEJMoa2410819
126. Nauck MA, D’Alessio DA. Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes with unmatched effectiveness regrading glycaemic control and body weight reduction. Cardiovasc Diabetol. (2022) 21(1):169. doi: 10.1186/s12933-022-01604-7
127. van Eenige R, Ying Z, Tramper N, Wiebing V, Siraj Z, de Boer JF, et al. Combined glucose-dependent insulinotropic polypeptide receptor and glucagon-like peptide-1 receptor agonism attenuates atherosclerosis severity in APOE*3-Leiden.CETP mice. Atherosclerosis. (2023) 372:19–31. doi: 10.1016/j.atherosclerosis.2023.03.016
128. Ilyas I, Little PJ, Liu Z, Xu Y, Kamato D, Berk BC, et al. Mouse models of atherosclerosis in translational research. Trends Pharmacol Sci. (2022) 43(11):920–39. doi: 10.1016/j.tips.2022.06.009
129. Dahl D, Onishi Y, Norwood P, Huh R, Bray R, Patel H, et al. Effect of subcutaneous tirzepatide vs placebo added to titrated insulin glargine on glycemic control in patients with type 2 diabetes: the SURPASS-5 randomized clinical trial. JAMA. (2022) 327(6):534–45. doi: 10.1001/jama.2022.0078
130. Rosenstock J, Wysham C, Frías JP, Kaneko S, Lee CJ, Fernández Landó L, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet Lond Engl. (2021) 398(10295):143–55. doi: 10.1016/S0140-6736(21)01324-6
131. Del Prato S, Kahn SE, Pavo I, Weerakkody GJ, Yang Z, Doupis J, et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet Lond Engl. (2021) 398(10313):1811–24. doi: 10.1016/S0140-6736(21)02188-7
132. Ludvik B, Giorgino F, Jódar E, Frias JP, Fernández Landó L, Brown K, et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet Lond Engl. (2021) 398(10300):583–98. doi: 10.1016/S0140-6736(21)01443-4
133. Garvey WT, Frias JP, Jastreboff AM, le Roux CW, Sattar N, Aizenberg D, et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet Lond Engl. (2023) 402(10402):613–26. doi: 10.1016/S0140-6736(23)01200-X
134. Wadden TA, Chao AM, Machineni S, Kushner R, Ard J, Srivastava G, et al. Tirzepatide after intensive lifestyle intervention in adults with overweight or obesity: the SURMOUNT-3 phase 3 trial. Nat Med. (2023) 29(11):2909–18. doi: 10.1038/s41591-023-02597-w
135. Aronne LJ, Sattar N, Horn DB, Bays HE, Wharton S, Lin WY, et al. Continued treatment with tirzepatide for maintenance of weight reduction in adults with obesity: the SURMOUNT-4 randomized clinical trial. JAMA. (2024) 331(1):38–48. doi: 10.1001/jama.2023.24945
136. Packer M, Zile MR, Kramer CM, Baum SJ, Litwin SE, Menon V, et al. Tirzepatide for heart failure with preserved ejection fraction and obesity. N Engl J Med. (2024). doi: 10.1056/NEJMoa2410027
137. Nicholls SJ, Bhatt DL, Buse JB, Prato SD, Kahn SE, Lincoff AM, et al. Comparison of tirzepatide and dulaglutide on major adverse cardiovascular events in participants with type 2 diabetes and atherosclerotic cardiovascular disease: sURPASS-CVOT design and baseline characteristics. Am Heart J. (2024) 267:1–11. doi: 10.1016/j.ahj.2023.09.007
138. Eli Lilly and Company. Study of Tirzepatide (LY3298176) on the Reduction on Morbidity and Mortality in Adults With Obesity (SURMOUNT-MMO). Bethesda, MD: National Library of Medicine (2022). Available online at: https://clinicaltrials.gov/study/NCT05556512#study-overview (cited July 10, 2024).
139. Lundquist Institute for Biomedical Innovation at Harbor-UCLA Medical Center. Effect of Tirzepatide on Progression of Coronary Atherosclerosis Using MDCT (T-PLAQUE). Bethesda, MD: National Library of Medicine (2024). Available online at: https://clinicaltrials.gov/study/NCT05708859 (cited July 10, 2024).
140. Hamidi H, Bagheri M, Benzing T, Krishnan S, Kianoush S, Ichikawa K, et al. Effect of tirzepatide on the progression of coronary atherosclerosis using MDCT: rationale and design of the tirzepatide treatment on coronary atherosclerosis progression: the (T-plaque) randomized-controlled trial design. Am Heart J. (2024) 278:24–32. doi: 10.1016/j.ahj.2024.08.015
141. Jastreboff AM, Kaplan LM, Frías JP, Wu Q, Du Y, Gurbuz S, et al. Triple-hormone-receptor agonist retatrutide for obesity - A phase 2 trial. N Engl J Med. (2023) 389(6):514–26. doi: 10.1056/NEJMoa2301972
142. Rosenstock J, Frias J, Jastreboff AM, Du Y, Lou J, Gurbuz S, et al. Retatrutide, a GIP, GLP-1 and glucagon receptor agonist, for people with type 2 diabetes: a randomised, double-blind, placebo and active-controlled, parallel-group, phase 2 trial conducted in the USA. Lancet Lond Engl. (2023) 402(10401):529–44. doi: 10.1016/S0140-6736(23)01053-X
143. Zhang HY, Tian JX, Lian FM, Li M, Liu WK, Zhen Z, et al. Therapeutic mechanisms of traditional Chinese medicine to improve metabolic diseases via the gut microbiota. Biomed Pharmacother. (2021) 133:110857. doi: 10.1016/j.biopha.2020.110857
144. Wang DQH. Therapeutic uses of animal biles in traditional Chinese medicine: an ethnopharmacological, biophysical chemical and medicinal review. World J Gastroenterol. (2014) 20(29):9952. doi: 10.3748/wjg.v20.i29.9952
145. Ðanić M, Stanimirov B, Pavlović N, Goločorbin-Kon S, Al-Salami H, Stankov K, et al. Pharmacological applications of bile acids and their derivatives in the treatment of metabolic syndrome. Front Pharmacol. (2018) 9:1382. doi: 10.3389/fphar.2018.01382
146. D’Amato D, De Vincentis A, Malinverno F, Viganò M, Alvaro D, Pompili M, et al. Real-world experience with obeticholic acid in patients with primary biliary cholangitis. JHEP Rep. (2021) 3(2):100248. doi: 10.1016/j.jhepr.2021.100248
Keywords: cardiovascular disease, obesity, gut hormones, lipoproteins, atherosclerosis
Citation: Kirsch A, Gindlhuber J, Zabini D and Osto E (2025) Bile acids and incretins as modulators of obesity-associated atherosclerosis. Front. Cardiovasc. Med. 11:1510148. doi: 10.3389/fcvm.2024.1510148
Received: 12 October 2024; Accepted: 17 December 2024;
Published: 6 January 2025.
Edited by:
Alexander Akhmedov, University of Zurich, SwitzerlandReviewed by:
Nadezhda Sabeva, Central University of the Caribbean, Puerto RicoChu-Huang Chen, Texas Heart Institute, United States
Copyright: © 2025 Kirsch, Gindlhuber, Zabini and Osto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elena Osto, ZWxlbmEub3N0b0BtZWR1bmlncmF6LmF0
†These authors have contributed equally to this work
‡ORCID:
Andrijana Kirsch
orcid.org/0000-0002-6623-0234
Juergen Gindlhuber
orcid.org/0000-0002-6155-5208
Elena Osto
orcid.org/0000-0001-8196-5696
 Andrijana Kirsch
Andrijana Kirsch Juergen Gindlhuber1,†,‡
Juergen Gindlhuber1,†,‡ Elena Osto
Elena Osto