- 1Department of Cardiology, Japanese Red Cross Musashino Hospital, Tokyo, Japan
- 2Department of Cardiovascular Medicine, Tokyo Medical and Dental University, Tokyo, Japan
In patients undergoing percutaneous coronary intervention (PCI), severely calcified lesions remain a great challenge even in the drug-eluting stent (DES) era. Intravascular lithotripsy (IVL) is effective for modification of severely calcified lesions prior to DES implantation. However, the efficacy of PCI with drug-coated balloon (DCB) following IVL has not been fully elucidated. Here, we present a case of severely calcified de novo coronary artery lesion successfully underwent PCI with DCB following IVL under optical coherence tomography (OCT) guidance as well as mid-term follow-up OCT. DCB following IVL might be a potential revascularization strategy for patients with heavily calcified de novo coronary artery lesions.
Introduction
Severely calcified lesions remain a great challenge for percutaneous coronary intervention (PCI) even in the drug-eluting stent (DES) era, because coronary calcification interferes with the delivery and expansion of the stent (1). Debulking devices such as rotational atherectomy (RA) or orbital atherectomy (OA) have been used to facilitate the lesion preparation of heavily calcified plaques, leading to larger stent area. Previous studies demonstrated that the efficacy and the safety of drug-coated balloon (DCB) treatment after RA for calcified coronary lesions might be comparable to those of DES following RA (2, 3). Furthermore, clinical outcome of OA combined with DCB for coronary calcification has been reported to be comparable to those of OA followed by DES (4).
Recently, the effectiveness and the safety of intravascular lithotripsy (IVL) prior to DES implantation have been reported and IVL devices have been introduced to clinical practice to obtain adequate modification of severe calcification (5–7). Moreover, IVL indications are quickly increasing over the last years not only for the treatment of coronary stenosis but even for peripheral intervention, for the treatment of severe aortic stenosis or severely calcified carotid artery stenosis (8–10). However, it remains unclear whether DCB following IVL treatment can be used as an alternative strategy for the revascularization of heavily calcified coronary artery lesions. Herein, we presented a patient with a severely calcified de novo coronary lesion that successfully underwent PCI with DCB following IVL under optical coherence tomography (OCT) guidance as well as mid-term follow-up OCT.
Case presentation
A 63-year-old male with past medical history of diabetes mellitus and dyslipidemia, was admitted to our hospital for non-ST-elevation myocardial infarction with subacute occlusion of the distal right coronary artery (RCA) and severely calcified coronary stenosis in the mid-left circumflex artery (LCX) (Figure 1A). A few days after primary PCI of RCA, we performed staged PCI of LCX. The left coronary artery was cannulated by a 6F JL3.5 guide catheter via the left distal radial artery approach. A 0.014-inch guidewire was advanced into the distal LCX. Pre-PCI OCT showed a severely calcified coronary stenosis in the mid-LCX with 279° of calcium angle, 0.81 mm of calcium thickness, 5.1 mm of calcified segment length, and 1.87 mm2 of minimum lumen area (Figure 1B). Because of the OCT-based calcium score of 4 with 2.8 mm of proximal reference lumen diameter and 2.3 mm of distal reference lumen diameter (11), we decided to use a 2.5 mm × 12 mm IVL balloon (Shockwave C2, Shockwave Medical, Inc. Santa Clara, CA, USA) to modify the heavily calcified plaque. The IVL balloon was deployed to the calcified lesion and inflated with 4 atm, then 10 pulses were delivered at a rate of 1 pulse per second, after which the balloon was inflated further to 6 atm. We stopped IVL at 60 pulses because severely calcified coronary lesion was fully enlarged by 60 pulses of IVL on coronary angiogram (CAG). Post-IVL CAG and OCT demonstrated the improvement of the stenotic lesion with the multiple fracture in almost circumferential thick calcified plaques (Figures 1C,D). According to the proximal reference lumen diameter as 2.8 mm on OCT imaging, a 2.75 mm × 13 mm scoring balloon dilatation was then performed for further lumen enlargement (Figures 1E,F). After scoring balloon dilatation, acceptable angiographic and OCT results, which include thrombolysis in myocardial infarction grade 3 flow, residual stenosis ≤30%, and the absence of major coronary dissection without hematoma were observed. We considered the optimal lesion preparation was obtained as reported in the expert consensus statement (12). Thus, a 2.5 mm × 30 mm paclitaxel-coated balloon (SeQuent Please NEO; B. Braun, Melsungen, Germany) was used as a final device, because of the distal reference lumen diameter as 2.3 mm on OCT imaging. Post-PCI CAG and OCT findings demonstrated acceptable lumen expansion with thrombolysis in myocardial infarction grade 3 coronary flow (Figures 1G,H). At 8 months later follow-up CAG, there was no restenosis in the LCX (Figure 1I). In addition, follow-up OCT showed that calcified plaque with fracture was replaced by homogeneous neointima with late lumen enlargement (Figure 1J).
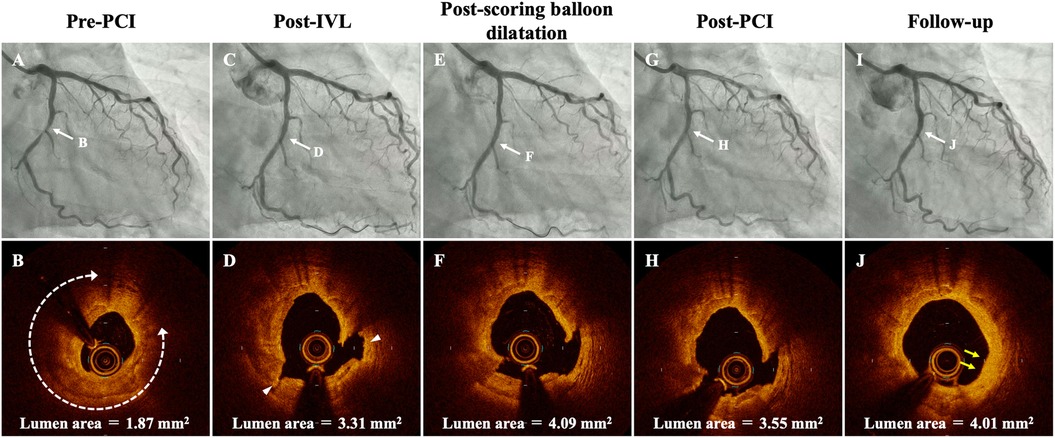
Figure 1. Serial coronary angiogram (CAG) and corresponding optical coherence tomography (OCT) images of the patient. (A,B) Pre-PCI CAG and OCT shows a severely calcified coronary stenosis in the mid-left circumflex artery (LCX) with 279° of calcium angle (white-dotted double headed arrows), 0.81 mm of calcium thickness, 5.1 mm of calcified segment length, and 1.87 mm2 of minimum lumen area. (C,D) Post-IVL CAG and OCT demonstrates good expansion at the lesion with two calcium fractures (arrowhead). (E,F) Post-scoring balloon dilatation CAG and OCT showed that lumen area was significantly enlarged. (G,H) CAG and OCT after PCI shows no significant residual stenosis and acceptable lumen area. (I,J) Follow-up CAG and OCT at 8 months after the procedure. Restenosis is not observed at the mid-LCX, and OCT demonstrates homogeneous neointima with late lumen enlargement in the modified calcified lesions (yellow arrows). IVL, intravascular lithotripsy; PCI, percutaneous coronary intervention.
Discussion
We reported a case of heavily calcified stenosis treated with IVL followed by DCB. To the best of our knowledge, this is the first case report demonstrating the efficacy of stent-less strategy with DCB following IVL by mid-term OCT assessment.
Severely calcified lesions remain a significant challenge in patients undergoing PCI. Although the optimal strategy for severely calcified lesions was controversial, recent studies reported that clinical outcome after PCI using debulking devices prior to DCB treatment for severe calcification was not inferior compared with DES implantation (2–4). Additionally, late lumen loss observed after DES implantation was significantly larger than that observed after DCB treatment in patients with severe calcification (2, 4). While RA or OA can reduce the calcium burden resulted in the luminal area gain, these effect for calcific plaque modification is limited by guidewire bias (13), and the peri-procedural complications remain a great challenge (14).
IVL is a novel therapy for the treatment of severely calcified plaque by delivering circumferential pulsatile acoustic pressure waves to modify calcification (5). Instead of not ablating and reducing calcified plaque, lesion modification using IVL is not dependent on guidewire bias and no extensive training for IVL therapy is required. Previous clinical studies revealed the efficacy and safety of IVL prior to DES implantation in severely calcified lesions (5–7). Additionally, IVL is safe and effective to achieve lumen gain and stent dimensions in underexpanded stents due to heavily calcified lesions (15). Although the use of IVL prior to DCB for the treatment of severely calcified lesions remains off-label, acute luminal gain with calcium fracture by IVL theoretically may positively affect clinical outcome after stent-less PCI using IVL. The International DCB Consensus Group showed that the DCB-only strategy was feasible in case with acceptable angiographic result after optimal lesion preparation (16).
In the present case, fractured calcification was replaced by homogeneous neointima with late lumen enlargement, which was in line with the previous case report, showing that calcified plaque with fracture was replaced by homogeneous neointima 6 months after PCI using DCB following OA (17). It is possible that calcium fracture created by IVL may lead to increase drug penetration and then facilitate morphological changes in severely calcified plaque, resulting in acceptable luminal gain in the late phase. Therefore, DCB following IVL strategy might be clinically applicable if IVL could create the calcium fracture and thus achieve adequate calcium modification and acceptable angiographic result.
Conclusions
Stent-less PCI by using a combined IVL and DCB therapy might be a potential revascularization strategy for patients with heavily calcified de novo coronary artery lesions of small vessels. Further studies are needed to definitively address the efficacy of this strategy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Japanese Red Cross Musashino Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
TM: Writing – original draft. TL: Supervision, Writing – review & editing. TA: Conceptualization, Supervision, Writing – review & editing. TN: Writing – review & editing. TY: Writing – review & editing. TS: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Madhavan MV, Tarigopula M, Mintz GS, Maehara A, Stone GW, Généreux P. Coronary artery calcification: pathogenesis and prognostic implications. J Am Coll Cardiol. (2014) 63(17):1703–14. doi: 10.1016/j.jacc.2014.01.017
2. Ueno K, Morita N, Kojima Y, Takahashi H, Kawasaki M, Ito R, et al. Safety and long-term efficacy of drug-coated balloon angioplasty following rotational atherectomy for severely calcified coronary lesions compared with new generation drug-eluting stents. J Interv Cardiol. (2019) 2019:9094178. doi: 10.1155/2019/9094178
3. Dong H, Shan Y, Gong S, Li R, Li Y, Lu X, et al. Clinical research of drug-coated balloon after rotational atherectomy for severe coronary artery calcification. BMC Cardiovasc Disord. (2023) 23(1):40. doi: 10.1186/s12872-023-03071-8
4. Mitsui K, Lee T, Miyazaki R, Hara N, Nagamine S, Nakamura T, et al. Drug-coated balloon strategy following orbital atherectomy for calcified coronary artery compared with drug-eluting stent: one-year outcomes and optical coherence tomography assessment. Catheter Cardiovasc Interv. (2023) 102(1):11–7. doi: 10.1002/ccd.30689
5. Ali ZA, Nef H, Escaned J, Werner N, Banning AP, Hill JM, et al. Safety and effectiveness of coronary intravascular lithotripsy for treatment of severely calcified coronary stenoses: the disrupt CAD II study. Circ Cardiovasc Interv. (2019) 12(10):e008434. doi: 10.1161/CIRCINTERVENTIONS.119.008434
6. Hill JM, Kereiakes DJ, Shlofmitz RA, Klein AJ, Riley RF, Price MJ, et al. Intravascular lithotripsy for treatment of severely calcified coronary artery disease. J Am Coll Cardiol. (2020) 76(22):2635–46. doi: 10.1016/j.jacc.2020.09.603
7. Saito S, Yamazaki S, Takahashi A, Namiki A, Kawasaki T, Otsuji S, et al. Intravascular lithotripsy for vessel preparation in severely calcified coronary arteries prior to stent placement—primary outcomes from the Japanese disrupt CAD IV study. Circ J. (2021) 85(6):826–33. doi: 10.1253/circj.CJ-20-1174
8. Tepe G, Brodmann M, Werner M, Bachinsky W, Holden A, Zeller T, et al. Intravascular lithotripsy for peripheral artery calcification: 30-day outcomes from the randomized disrupt PAD III trial. JACC Cardiovasc Interv. (2021) 14(12):1352–61. doi: 10.1016/j.jcin.2021.04.010
9. Ponna PK, Gonuguntla A, Botta RK, Fischell T, Agrawal Y. Shockwave lithotripsy of calcific stenosis of the distal abdominal aorta to facilitate transcatheter aortic valve replacement. Cardiovasc Revasc Med. (2023) 53S:S134–8. doi: 10.1016/j.carrev.2023.04.021
10. Vadalà G, Galassi AR, Nerla R, Micari A. Shockwave intravascular lithoplasty for the treatment of calcified carotid artery stenosis: a very early single-center experience. Catheter Cardiovasc Interv. (2020) 96(6):E608–13. doi: 10.1002/ccd.28963
11. Fujino A, Mintz GS, Matsumura M, Lee T, Kim SY, Hoshino M, et al. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention. (2018) 13(18):e2182–9. doi: 10.4244/EIJ-D-17-00962
12. Muramatsu T, Kozuma K, Tanabe K, Morino Y, Ako J, Nakamura S, et al. Clinical expert consensus document on drug-coated balloon for coronary artery disease from the Japanese Association of Cardiovascular Intervention and Therapeutics. Cardiovasc Interv Ther. (2023) 38(2):166–76. doi: 10.1007/s12928-023-00921-2
13. Yamamoto MH, Maehara A, Karimi Galougahi K, Mintz GS, Parviz Y, Kim SS, et al. Mechanisms of orbital versus rotational atherectomy plaque modification in severely calcified lesions assessed by optical coherence tomography. JACC Cardiovasc Interv. (2017) 10(24):2584–6. doi: 10.1016/j.jcin.2017.09.031
14. Abdel-Wahab M, Richardt G, Joachim Büttner H, Toelg R, Geist V, Meinertz T, et al. High-speed rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: the randomized ROTAXUS (rotational atherectomy prior to Taxus stent treatment for Complex native coronary artery disease) trial. JACC Cardiovasc Interv. (2013) 6(1):10–9. doi: 10.1016/j.jcin.2012.07.017
15. Tovar Forero MN, Sardella G, Salvi N, Cortese B, di Palma G, Werner N, et al. Coronary lithotripsy for the treatment of underexpanded stents: the international & multicentre CRUNCH registry. EuroIntervention. (2022) 18(7):574–81. doi: 10.4244/EIJ-D-21-00545
16. Jeger RV, Eccleshall S, Wan Ahmad WA, Ge J, Poerner TC, Shin ES, et al. Drug-Coated balloons for coronary artery disease: third report of the international DCB consensus group. JACC Cardiovasc Interv. (2020) 13(12):1391–402. doi: 10.1016/j.jcin.2020.02.043
17. Miyazaki R, Lee T, Nagamine S, Nagata Y, Nozato T, Ashikaga T. Optical coherence tomography findings on intima healing reaction using drug-coated balloon after orbital atherectomy for a heavily calcified coronary artery lesion. Cardiovasc Interv Ther. (2022) 37(3):566–8. doi: 10.1007/s12928-021-00810-6
Keywords: severely calcified lesions, intravascular lithotripsy, drug-coated balloon, percutaneous coronary intervention, optical coherence tomography
Citation: Misawa T, Lee T, Ashikaga T, Nozato T, Yonetsu T and Sasano T (2024) Case Report: Drug-coated balloon after intravascular lithotripsy for the treatment of severely calcified de novo coronary artery lesion. Front. Cardiovasc. Med. 11:1470785. doi: 10.3389/fcvm.2024.1470785
Received: 26 July 2024; Accepted: 1 October 2024;
Published: 18 November 2024.
Edited by:
Saraschandra Vallabhajosyula, Brown University, United StatesReviewed by:
Giuseppe Vadalà, Azienda Ospedaliera Universitaria Policlinico Paolo Giaccone, ItalyWilliam Kongto Hau, The Chinese University of Hong Kong, China
Copyright: © 2024 Misawa, Lee, Ashikaga, Nozato, Yonetsu and Sasano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Toru Misawa, bWlzYXdhLnRvcnUxMTJAZ21haWwuY29t
 Toru Misawa
Toru Misawa Tetsumin Lee
Tetsumin Lee Takashi Ashikaga
Takashi Ashikaga Toshihiro Nozato1
Toshihiro Nozato1 Taishi Yonetsu
Taishi Yonetsu Tetsuo Sasano
Tetsuo Sasano