
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 19 December 2023
Sec. Coronary Artery Disease
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1284396
 Doo Hwan Lee1,2,3,†,‡
Doo Hwan Lee1,2,3,†,‡ Seok Oh1,2,†,‡
Seok Oh1,2,†,‡ Min Chul Kim1,2,4
Min Chul Kim1,2,4 Doo Sun Sim1,2,4
Doo Sun Sim1,2,4 Young Joon Hong1,2,4
Young Joon Hong1,2,4 Ju Han Kim1,2,4
Ju Han Kim1,2,4 Youngkeun Ahn1,2,4
Youngkeun Ahn1,2,4 Jae Bok Han3
Jae Bok Han3 In Soo Kim3*†
In Soo Kim3*† Myung Ho Jeong1,2,4*†
Myung Ho Jeong1,2,4*†
Objectives: There is no consensus regarding the optimal choice between single long stent (SLS) and overlapped double short stents (DSS) in patients with acute myocardial infarction (AMI). Therefore, we aimed to compare treatment outcomes among patients with AMI treated with these two different stenting methods.
Methods: In total, 537 patients with AMI from a single tertiary center were categorized into two groups: (1) those who received an SLS (stent length ≥38 mm) (n = 254; 47.3%) and (2) those who received overlapped DSS (individual stent lengths <38 mm) (n = 283; 52.7%). The primary outcome was the incidence of major adverse cardiac and cerebrovascular events (MACCEs) within 1 year.
Results: The mean age of participants was 65.4 years, and 75.0% were male. Patients receiving an SLS had a higher rate of serum creatinine level ≥1.5 mg/dl (16.3% vs. 8.9%, p = 0.009) but a lower rate of hypertension (46.8% vs. 55.8%, p = 0.038), lesser total stent length (38.26 ± 1.31 vs. 45.20 ± 9.25 mm, p < 0.001), total procedure time (41.40 ± 15.74 vs. 53.31 ± 21.75 min, p < 0.001) and total contrast volume (134.13 ± 30.72 vs. 160.57 ± 39.77 ml, p < 0.001) than in those receiving DSS. One-year MACCEs were comparable between the two groups before [hazard ratio (HR), 1.33; 95% confidence interval (CI), 0.80–2.24] and after adjusting for covariates (HR, 1.21; 95% CI, 0.67–2.19).
Conclusions: Stenting with an SLS demonstrated similar outcomes compared to those achieved when using stenting with overlapped DSS in patients with AMI. Therefore, if the deliverability is acceptable, stenting with an SLS appears to be a safe and effective strategy for AMI treatment.
In the modern age of percutaneous coronary intervention (PCI), many interventional cardiologists routinely encounter challenging cases of coronary artery disease (CAD) with a wide variety of complex lesions, thereby making it difficult to decide whether to implant multiple stents or a single long stent (SLS). Certain types of CAD involve extended or bifurcated coronary lesions that cannot be managed with implantation of a single stent; therefore, they tend to be treated with multiple stents (1). However, despite the widespread utilization of multiple stents, their implantation is associated with a greater risk of stent thrombosis or restenosis post-PCI (2, 3). Although newer-generation multiple drug-eluting stents (DESs) appear to provide favorable safety outcomes comparable to those of a single DES (4), these results have remained controversial (5, 6).
Among CAD, acute myocardial infarction (AMI) is an emergent medical illness that requires urgent intervention and thus necessitates well-timed and effective revascularization of the infarct-related artery (IRA) (7). In clinical settings of AMI, the primary operator must make a prompt decision regarding the stent implantation strategy during PCI, and the choice between the two stenting methods (multiple stents vs. a single stent) is challenging. However, there is a distinct lack of clinical evidence regarding the outcomes in patients with AMI treated with SLS vs. overlapped double short stents (DSS). To bridge this gap, the present study sought to examine the differences in clinical characteristics and treatment outcomes in patients with AMI receiving SLS vs. overlapped DSS.
This study is based on a non-randomized retrospective analysis in Chonnam National University Hospital (CNUH), a single tertiary cardiovascular hospital located in Gwangju, Republic of Korea. All clinical data were collected from patients with AMI undergoing PCI with 1-year clinical follow-up at CNUH. From November 2011 to June 2020, a total of 6,180 patients with AMI were initially screened. Patients who had not undergone PCI were excluded, yielding 5,134 patients. The following patients were then excluded: (1) not receiving any stent, (2) with ≥3 stents, (3) with multiple stents for different coronary vessels, (4) receiving only a single short stent, (5) receiving an SLS with any other short or long stent, (6) receiving DSS without overlapping, and (7) who were deceased during the index hospitalization. This process yielded 537 consecutive patients with AMI who were enrolled and categorized into two groups depending on the stenting method: patients in group A underwent PCI with only SLS (stent length ≥38 mm) (n = 254), and those in group B underwent PCI with overlapped DSS (individual stent lengths <38 mm) (n = 283). Representative examples are illustrated in Figure 1. All stenting procedures were performed to the IRA lesions. To compare the treatment outcomes following AMI, we excluded a total of 16 patients who were lost to follow-up from the survival analysis. Finally, the treatment outcomes in 521 consecutive survivors were analyzed. The study scheme is illustrated in Figure 2.
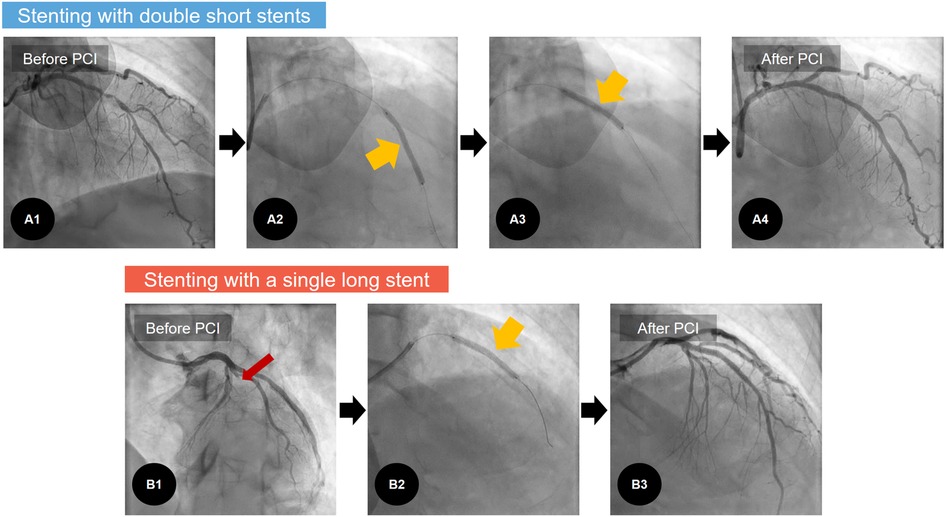
Figure 1. Representative examples of coronary stenting: stenting with double short stents. (A) Versus with a single long stent. (B) A1: in one case of AMI, diffuse coronary stenosis is noted in LAD, A2–3: With the overlapping stent technique, two short DESs (individual stent lengths <38 mm) are implanted at middle and distal parts of the LAD (yellowish arrows). A4: Post-PCI angiogram revealed good angiographic results. B1: In another case of AMI, there is 100% occlusion in the LAD (red arrow). B2: To treat this, a single long DES (stent length ≥38 mm) is implanted. B3: Thereafter, post-PCI angiogram reveals good angiographic results. AMI, acute myocardial infarction; DES, drug-eluting stent; LAD, left anterior descending coronary artery; PCI, percutaneous coronary intervention.
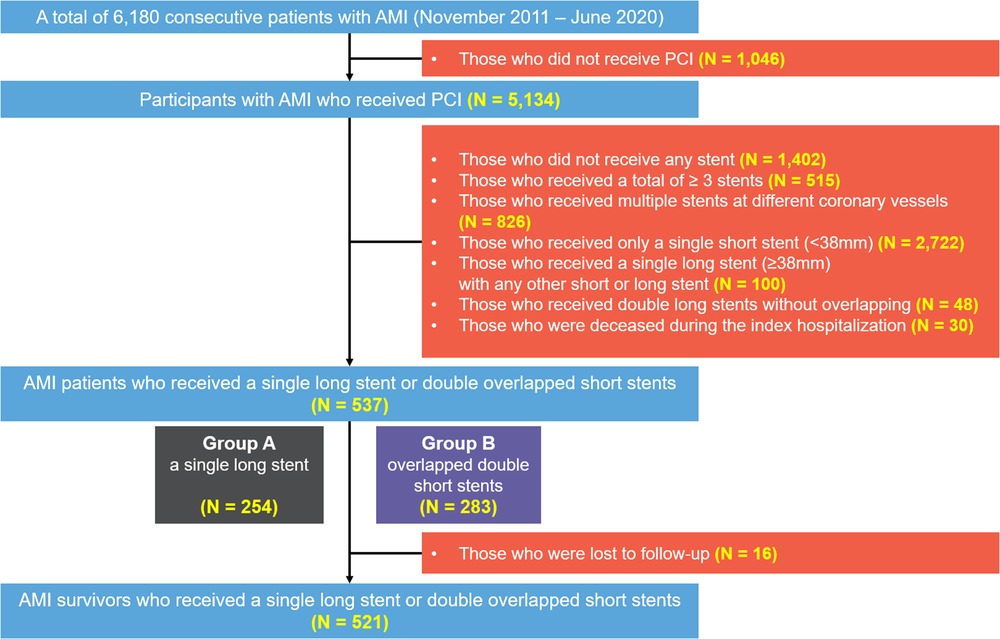
Figure 2. Flowchart of the present study. AMI, acute myocardial infarction; PCI, percutaneous coronary intervention.
As stated in several contemporary guidelines (7), AMI refers to an increase or decrease in cardiac biomarkers and associated clinical indicators, including one or more of the following conditions: (1) ischemia-driven clinical symptoms and/or signs; (2) newly identified changes on an electrocardiogram indicative of myocardial ischemia, including ST-segment deviation, T-wave inversion, or new detection of pathological Q-waves; (3) definite evidence suggesting loss of viable myocardium or regional wall motion abnormalities visualized with any cardiovascular imaging tool; and (4) presence of intracoronary thrombus during coronary angiography. Among AMI, ST-segment elevation myocardial infarction (STEMI) is a medical condition with overlapping components of the AMI definition with new-onset ST-segment elevation in at least two continuous leads (7).
Imaging guidance during the index PCI relates to adjunctive usage of any intracoronary imaging tool, such as intravascular ultrasound (IVUS) or optical coherence tomography (OCT), to evaluate intracoronary lesion characteristics. An IRA is an AMI-responsible coronary vessel that is obstructed or narrowed by atherothrombosis. Left main coronary artery (LMCA) disease refers to an LMCA lesion with a diameter stenosis ≥50%. Multivessel CAD was defined by ≥70% diameter stenosis in ≥2 coronary arteries or ≥70% stenosis in one coronary artery with LMCA disease. Coronary lesion characteristics within an IRA were stratified based on angiographic findings in consonance with the American College of Cardiology/American Heart Association (ACC/AHA) lesion complexity system (8). The antegrade intracoronary flow was stratified according to the thrombolysis in myocardial infarction (TIMI) flow grading system (9). To evaluate left ventricular systolic function, the left ventricular ejection fraction (LVEF) was assessed using a two-dimensional transthoracic echocardiogram. Peak troponin-I level was defined as the highest level of troponin-I measured within 72 h after hospital admission.
Meanwhile, we also investigated results from quantitative coronary angiography (QCA) in all study participants. These were derived from artificial intelligence-based automated QCA by a novel software (MPXA-2000, Medipixel) using a deep learning algorithm to segment and analyze angiogram images. Data on QCA included lesion length, mean/proximal/distal reference vessel diameter, minimum lumen diameter, and percent diameter stenosis.
As mentioned earlier, a long stent was defined based on a stent length ≥38 mm, whereas a short stent was defined based on a stent length <38 mm. In other words, DSS were defined as individual stents <38 mm in length (i.e., two short stents). Total stent length was defined as the sum of lengths of all the stents implanted into the lesion site.
The baseline characteristics, angiographic and procedural profiles, and post-discharge treatment outcomes were evaluated through a retrospective review and analysis of the database from CNUH.
Baseline characteristics data included age, sex, utilization of emergency medical services, Killip functional class, body mass index, past medical history, smoking history, family history of premature ischemic heart disease, serum creatinine (Cr) level, peak troponin-I level, medications at hospital discharge, use of thrombolysis, LVEF, and final diagnosis. Information collected about discharge medication included aspirin, P2Y12 inhibitors, beta blockers, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, and statins. Among the angiographic profiles, IRA, presence of LMCA disease and multivessel CAD, ACC/AHA lesion classification, TIMI flow grade, and all QCA results were included. Procedural information included stent diameter, stent length, vascular access, the use of glycoprotein IIb/IIIa inhibitors (GPIs), thrombus aspiration, and intracoronary imaging guidance.
Clinical follow-up was conducted for approximately 12 months. For treatment estimates, the primary endpoint was defined as a major adverse cardiac and cerebrovascular event (MACCE), which was a composite of the following outcomes: all-cause death, non-fatal myocardial infarction (NFMI), any revascularization, cerebrovascular accident (CVA), rehospitalization, and stent thrombosis. The secondary endpoints included each component of MACCE, including all-cause death, cardiac/non-cardiac death, NFMI, any revascularization [repeated revascularization (PCI or coronary artery bypass graft) of any portion of the entire coronary vasculature], culprit-lesion-related revascularization (repeated revascularization of culprit lesion), CVA, rehospitalization (first-time hospitalization with the chief complaint of angina pectoris or heart failure), and stent thrombosis [a definite or probable stent thrombosis, as stated in the definitions of the Academic Research Consortium (10)]. The independent clinical event monitoring committee, consisting of independent interventional cardiologists, adjudicated all clinical events in this study.
Participants were classified into group A (patients who underwent PCI with SLS only) or group B (those who underwent PCI with overlapped DSS). The two groups were compared for baseline characteristics and treatment outcomes. For each parameter, continuous variables are described as the mean with standard deviation and were analyzed using the student's t-test or analysis of variance. Discrete variables are described as frequencies with percentages and were analyzed using Pearson's chi-square test and Fisher's two-by-two exact test. A p-value < 0.05 was considered statistically significant.
To reduce the effects of selection bias due to different backgrounds between groups, inverse probability of treatment weighting (IPTW) was applied to adjust for different results in these variables and examine whether the stenting method affected the incidence of each treatment outcome independently. In IPTW, a matching ratio of 1:1 was applied, and the propensity score was constructed with a total of 42 covariates, including age, sex, utilization of emergency medical services, Killip functional class, body mass index, past medical history, smoking history, family history of premature ischemic heart disease, serum Cr level, peak troponin-I level, medications at hospital discharge, use of thrombolysis, LVEF, final diagnosis, IRA, presence of LMCA disease and multivessel CAD, ACC/AHA lesion classification, TIMI flow grade, all QCA results, stent diameter, vascular access, the use of GPIs, thrombus aspiration, and intracoronary imaging guidance.
Multivariable logistic regression analysis was conducted to assess variables that were correlated with stenting with overlapped DSS. Univariable logistic regression analysis was initially performed using baseline covariates, except for stent diameter, stent length, and medications at hospital discharge. Thereafter, the variables with a p-value < 0.2 were rendered for entry in the backward stepwise conditional logistic regression analysis.
All data were analyzed using STATA version 15.0 (StataCorp, College Station, TX, United States) and SPSS version 25.0 (IBM Corp., Armonk, NY, United States).
This study was conducted in accordance with the ethical standards of the World Medical Association's Declaration of Helsinki. The present study was approved by the Institutional Review Board of CNUH (IRB No. CNUH-2022-136). The need for informed consent was waived because of the retrospective study design.
A total of 537 patients with AMI who underwent stent implantation were included in the analysis. Of these, 254 (47.3%) patients were treated with an SLS and 283 (52.7%) with overlapped DSS. The distribution of stent types among the participants is detailed in Figure 3. In group A, the use of one everolimus-eluting stent was the most predominant type (n = 176), followed by one zotarolimus-eluting stent (n = 50), one sirolimus-eluting stent (n = 26), and one novolimus-eluting stent (n = 2). In group B, the use of two everolimus-eluting stents was the most predominant type (n = 119), followed by two zotarolimus-eluting stents (n = 67), two sirolimus-eluting stents (n = 42), and so forth.
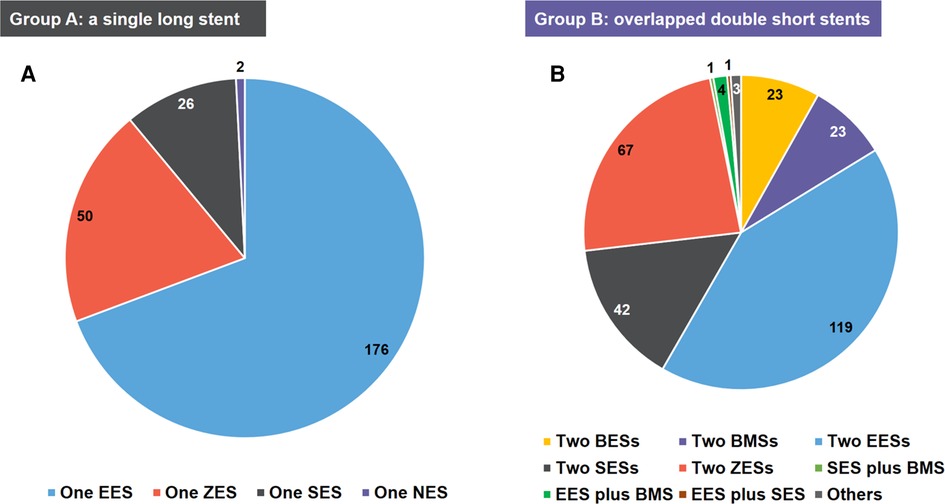
Figure 3. The distribution of stent types of the study population. (A) group A: a single long stent; (B) group B: overlapped double short stents. BES, biolimus A9-eluting stent; BMS, bare-metal stent; EES, everolimus-eluting stent; NES, novolimus-eluting stent; SES, sirolimus-eluting stent; ZES, zotarolimus-eluting stent.
Regarding baseline demographics and clinical characteristics (Table 1), most variables were comparable between the two groups, except for hypertension prevalence and serum Cr level. The prevalence of hypertension was higher in group B than in group A, whereas the rate of Cr ≥ 1.5 mg/dl was higher in group A than in group B. Angiographic and procedural profiles are summarized in Table 2. Compared with group A, group B had a greater total stent length. Group A had shorter total procedure time and lower total contrast volume than group B.
After IPTW, the different trends in all covariates of baseline clinical and procedural characteristics were adequately balanced between groups (Tables 1, 2).
The median follow-up interval was 364 days. Treatment outcomes during the 1-year follow-up, including MACCE and its individual components (all-cause death, cardiac death, noncardiac death, NFMI, any revascularization, culprit-lesion-related revascularization, CVA, rehospitalization, and stent thrombosis) were recorded (Table 3; Figure 4). Comparable outcomes were observed between groups. Regarding the IPTW-adjusted data, no significant difference between groups was evident for any treatment outcome.
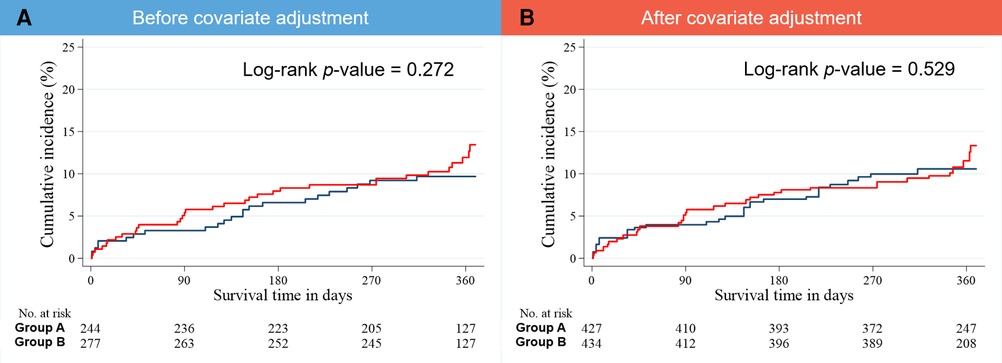
Figure 4. Kaplan–Meier estimates of the cumulative incidence of 1-year MACCE (A) before and (B) after covariate adjustment. MACCE, major adverse cardiac and cerebrovascular event.
When assessing correlates of stenting with overlapped DSS during PCI using multivariable logistic regression analysis, it was shown that hypertension [adjusted odds ratio (OR), 2.16; 95% confidence interval (CI), 1.39–3.36], serum Cr level ≥1.5 mg/dl (adjusted OR, 0.37; 95% CI, 0.19–0.74), and the use of GPIs (adjusted OR, 2.09; 95% CI, 1.09–3.99) were strongly associated with this stenting method (Table 4).
The present study utilized clinical information from patients with AMI treated with either an SLS or overlapped DSS from a single-center database and evaluated 1-year treatment outcomes. The main findings of the present study are that SLS provides 1-year treatment outcomes comparable with those in overlapped DSS in patients with AMI.
Since the first report of balloon angioplasty in 1977, the PCI procedure has markedly evolved, changing reperfusion practices for patients with CAD. Because coronary artery stenting becomes widely used, the chance of encountering and treating complex coronary lesions in real-world PCI practices has increased. The diffuse CAD is more frequently encountered (1, 11); therefore, many interventional cardiologists are occasionally forced to choose interventional strategies, including the single-stent technique with an SLS or the overlapping stent technique (OST) with DSS, to cover diffuse long-length coronary lesions (11).
OST accounts for up to 30% of PCI due to extensive lesion length, stent edge dissections, or incomplete stent coverage (6, 12–14). In the bare-metal stent (BMS) era, OST was associated with increased target lesion revascularization rates compared with those of the single-stent technique (6). However, with the advent of first-generation DESs, clinical and angiographic restenosis were markedly declined with the potent suppression of neointimal hyperplasia (15, 16). Furthermore, OST demonstrated improved and acceptable safety and efficacy in several DES-based studies (17, 18), leading to its wider application. Despite this revolutionary advancement, potential concerns with OST remain (11). First, OST increases the risk both of side branch compromise and periprocedural myonecrosis from the stent struts double layer or plaque shifting (19, 20). Second, OST forms an inadvertent gap between the stents, increasing the risk of acute or subacute stent thrombosis and late restenosis at this site (11). Third, while overlapping more stent platforms may minimize the stented area in complex calcified lesion, it may also increase the chances of more malapposed or more damaged struts in lesions where stent advancement is made with difficulty (6, 21, 22). Moreover, stent strut fractures can occur in overlapping zones, especially in the presence of coronary arterial curvature (23). Finally, OST increases the risk of repeat revascularization or adverse ischemic events (6). According to two DES-based large-scale clinical studies: the SIRTAX (Sirolimus-Eluting Vs. Paclitaxel-Eluting Stents for Coronary Revascularization) trial and LESSON (Long-term comparison of Everolimus-eluting and Sirolimus-eluting Stents for cOronary revascularizatioN) registry (5, 6), OST demonstrated worse clinical outcomes with more prevalent adverse cardiac events; nonetheless, these findings were not evident among patients treated using only second-generation DESs.
Based on these factors and clinical evidence, the clinical outcomes of multiple overlapping DESs remain debatable, even in the DES era. As such, the use of one long stent, rather than two short stents, may be preferable, given its appropriate implementation. Therefore, we compared these two stenting methods in patients with AMI undergoing PCI. In this study, implantations of SLS and DSS were performed in participants with similar clinical severity of their conditions, except for a few variables. Group A had worse kidney function with a higher proportion of Cr ≥ 1.5 mg/dl, a lower prevalence of hypertension, and shorter stent length than group B. Despite these differences, stenting with SLS showed a similar incidence of MACCEs compared to its counterpart; these trends were maintained even after IPTW adjustment. Also, considering that our ‘real-world’ data included a total of 28 cases with any BMS, and it is widely accepted that BMS has higher rates of restenosis than DES, a statistical analysis excluding those with any BMS was additionally conducted, demonstrating consistent results (Supplementary Table S1).
Based on the literature review, the outcomes shown in some previous clinical studies are consistent with those of the current study. Mori and colleagues showed that everolimus-eluting stents had similar angiographic and 1-year follow-up outcomes between SLS and overlapped DSS (24). Moreover, Jurado-Román et al. compared clinical outcomes of long stents and overlapped stents in diffuse CAD, emphasizing some advantages of PCI with those of long stents (1). Yano et al. demonstrated that long DES implantation had both acceptable and comparable outcomes for up to 2 years after PCI (25), and Sim et al. presented similar results (26). One meta-analysis also demonstrated that the use of SLS showed lower rates of cardiac death and target lesion revascularization than that of two or more short stents (27). Despite these prior studies, there is insufficient real-world clinical evidence concerning comparative treatment outcomes of these two stenting techniques in AMI settings. Since the present study only included patients with AMI mostly treated by second-generation DESs, our results underscore that SLS shows comparable outcomes to those of DSS, even in the clinical setting of AMI—the most severe form of CAD.
Despite similarities in treatment outcomes, PCI with SLS may have more potential treatment advantages than that with DSS. First, treatment is more economical and efficient because fewer stents are required. Second, very long stents may simplify the procedure, reducing total procedure time, fluoroscopy time, radiation exposure, and the amount of contrast media. Regarding Jurado-Román et al.'s study (1), PCI with long stents had lower contrast volume, shorter procedure duration, and shorter fluoroscopy time than that with multiple shorter stents, which may align with our results demonstrating that group A had shorter total procedure time but lower contrast volume than group B. Third, SLS can prevent potential complications due to stent overlapping. Considering these aspects, SLS appears to be a good choice for PCI in this population.
Three independent factors for stenting with DSS during PCI were identified. Interestingly, both hypertension and the use of GPIs were positive factors, whereas serum Cr was a negative factor. Given that impaired kidney function is associated with poor peri-procedural outcomes during PCI (28), it is reasonable to choose the SLS method for simplicity. Conversely, it is somewhat difficult to clarify why both hypertension and the use of GPIs were independently associated with stenting with DSS. Pre-existing hypertension is a well-established risk factor for atherosclerotic cardiovascular diseases and is associated with diffuse atherosclerosis (29). According to one clinical study, hypertension seemingly has the potential to aggravate the extent and severity of CAD, although this effect was limited to patients with diabetes. Therefore, it is plausible that hypertension may induce diffuse CAD (30), having extended atherosclerotic plaques, thereby increasing the requirement for OST with DSS. Meanwhile, since GPIs are effective on lowering thrombus burden (31), they have been shown to provide clinical benefits in high-risk patients undergoing PCI (32–34), their use is recommended in cases of a high thrombus burden to minimize the risk of the no-reflow phenomenon (35, 36). Given that stent overlapping is related to delayed arterial healing and increased inflammation (6, 37), it is plausible that OST may also promote intracoronary thrombus formation (38, 39), consequently requiring downstream administration of GPI. Of course, since these explanations are speculative at present, further investigation is required to elucidate this association.
Besides our analysis demonstrated that the rates of intracoronary imaging guidance were very low (4.7% in group A, and 8.1% in group B). Despite the fact that both IVUS and OCT are well-established tools to guide and optimize PCI (40), and their use for PCI is rapidly increasing (41, 42), they are used in a small proportion of all PCI in the setting of AMI (41, 42). According to 2023 European Society of Cardiology guidelines on acute coronary syndromes, both of them should be considered for culprit lesions (Class IIa), and may also be considered for non-culprit lesions (Class IIb) (40). Hence, if they become more widely used, it may help achieve optimized stent expansion with more acceptable values of post-PCI minimum stent area, which may reduce underexpansion-related complications and demonstrate more acceptable outcomes, especially in OST with DSS (43, 44). Since these strengths are necessary in clinical situations that require relatively sophisticated stenting techniques, their utilization may contribute to minimizing OST-related complications mentioned earlier.
Although similar outcomes for both SLS and DSS in PCI in patients with AMI were observed, our results must be interpreted with caution owing to several methodological limitations. First, this study was a non-randomized retrospective analysis of a single-center database. Second, although covariate adjustment was conducted to minimize selection bias, it may have remained due to several reasons, such as inclusion and exclusion criteria, intentional exclusion of data in cases of missing information, and other potential unmeasured confounders. Third, establishing causation between the stenting method and each treatment estimate was difficult due to the non-randomized and retrospective nature of the study. Fourth, while many previous studies reported more detailed information on lesion characteristics (5, 6, 26), some detailed angiographic information including tortuosity, eccentricity, angulation, vessel diameter variability, presence of side branches, and degree of coronary artery calcification, was missing from the present study. Considering these limitations, the results may not be generalizable but must be interpreted as hypothesis-generating.
In this study, we evaluated the baseline characteristics and treatment outcomes of an SLS vs. overlapped DSS in the clinical setting of AMI. We demonstrated that stenting with an SLS produced comparable outcomes to those of stenting with DSS. Some demographic and clinical conditions seem to be independently associated with these stenting methods. Given the more potential treatment advantages with SLS, if its deliverability is acceptable, stenting with an SLS may be a safe and effective treatment strategy for patients with AMI, as opposed to that with DSS.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Institutional Review Board of Chonnam National University Hospital (IRB No. CNUH-2022-136). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
DL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. SO: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. MK: Writing – review & editing. DS: Writing – review & editing. YH: Writing – review & editing. JK: Writing – review & editing. YA: Writing – review & editing. JH: Writing – review & editing. IK: Conceptualization, Methodology, Supervision, Writing – review & editing. MJ: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was funded by the Chonnam National University Hospital Biomedical Research Institute (BCRI-22052 and BCRI-23096). This study was supported by grants from the Korean Health Technology R&D Project, Ministry of Health & Welfare (HI13C1527), and Research of Korea Centers for Disease Control and Prevention (2016-ER6304-01), Republic of Korea.
We disclose that this original article is a continuation of the work presented in DL's Doctorate dissertation. We also disclose that patients were not involved in the design or conduct of the present study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1284396/full#supplementary-material
1. Jurado-Román A, Abellán-Huerta J, Requena JA, Sánchez-Pérez I, López-Lluva MT, Maseda-Uriza R, et al. Comparison of clinical outcomes between very long stents and overlapping stents for the treatment of diffuse coronary disease in real clinical practice. Cardiovasc Revasc Med. (2019) 20(8):681–6. doi: 10.1016/j.carrev.2018.09.009
2. Bourassa MG, Lesperance J, Eastwood C, Schwartz L, Cote G, Kazim F, et al. Clinical, physiologic, anatomic and procedural factors predictive of restenosis after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. (1991) 18(2):368–76. doi: 10.1016/0735-1097(91)90588-z
3. Gwon HC, Jeon DW, Kang HJ, Jang JS, Park DW, Shin DH, et al. The practice pattern of percutaneous coronary intervention in Korea: based on year 2014 cohort of Korean percutaneous coronary intervention (K-PCI) registry. Korean Circ J. (2017) 47(3):320–7. doi: 10.4070/kcj.2017.0070
4. Farooq V, Vranckx P, Mauri L, Cutlip DE, Belardi J, Silber S, et al. Impact of overlapping newer generation drug-eluting stents on clinical and angiographic outcomes: pooled analysis of five trials from the international global resolute program. Heart. (2013) 99(9):626–33. doi: 10.1136/heartjnl-2012-303368
5. O’Sullivan CJ, Stefanini GG, Raber L, Heg D, Taniwaki M, Kalesan B, et al. Impact of stent overlap on long-term clinical outcomes in patients treated with newer-generation drug-eluting stents. EuroIntervention. (2014) 9(9):1076–84. doi: 10.4244/EIJV9I9A182
6. Raber L, Juni P, Loffel L, Wandel S, Cook S, Wenaweser P, et al. Impact of stent overlap on angiographic and long-term clinical outcome in patients undergoing drug-eluting stent implantation. J Am Coll Cardiol. (2010) 55(12):1178–88. doi: 10.1016/j.jacc.2009.11.052
7. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2016) 37(3):267–315. doi: 10.1093/eurheartj/ehv320
8. Theuerle J, Yudi MB, Farouque O, Andrianopoulos N, Scott P, Ajani AE, et al. Utility of the ACC/AHA lesion classification as a predictor of procedural, 30-day and 12-month outcomes in the contemporary percutaneous coronary intervention era. Catheter Cardiovasc Interv. (2018) 92(3):E227–234. doi: 10.1002/ccd.27411
9. Appleby MA, Michaels AD, Chen M, Michael CG. Importance of the TIMI frame count: implications for future trials. Curr Control Trials Cardiovasc Med. (2000) 1(1):31–4. doi: 10.1186/cvm-1-1-031
10. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. (2007) 115(17):2344–51. doi: 10.1161/CIRCULATIONAHA.106.685313
11. Menown IB. Very long stent technology: clinical and practical value. Future Cardiol. (2013) 9(5):641–4. doi: 10.2217/fca.13.50
12. Holmes DR Jr, Leon MB, Moses JW, Popma JJ, Cutlip D, Fitzgerald PJ, et al. Analysis of 1-year clinical outcomes in the SIRIUS trial: a randomized trial of a sirolimus-eluting stent versus a standard stent in patients at high risk for coronary restenosis. Circulation. (2004) 109(5):634–40. doi: 10.1161/01.cir.0000112572.57794.22
13. Schampaert E, Moses JW, Schofer J, Schlüter M, Gershlick AH, Cohen EA, et al. Sirolimus-eluting stents at two years: a pooled analysis of SIRIUS, E-SIRIUS, and C-SIRIUS with emphasis on late revascularizations and stent thromboses. Am J Cardiol. (2006) 98(1):36–41. doi: 10.1016/j.amjcard.2006.01.049
14. Stone GW, Ellis SG, Cox DA, Hermiller J, O’Shaughnessy C, Mann JT, et al. One-year clinical results with the slow-release, polymer-based, paclitaxel-eluting TAXUS stent: the TAXUS-IV trial. Circulation. (2004) 109(16):1942–7. doi: 10.1161/01.CIR.0000127110.49192.72
15. Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O’Shaughnessy C, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. (2003) 349(14):1315–23. doi: 10.1056/NEJMoa035071
16. Schofer J, Schluter M, Gershlick AH, Wijns W, Garcia E, Schampaert E, et al. Sirolimus-eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: double-blind, randomised controlled trial (E-SIRIUS). Lancet. (2003) 362(9390):1093–9. doi: 10.1016/S0140-6736(03)14462-5
17. Lee CW, Park KH, Kim YH, Hong MK, Kim JJ, Park SW, et al. Clinical and angiographic outcomes after placement of multiple overlapping drug-eluting stents in diffuse coronary lesions. Am J Cardiol. (2006) 98(7):918–22. doi: 10.1016/j.amjcard.2006.05.011
18. Tsagalou E, Chieffo A, Iakovou I, Ge L, Sangiorgi GM, Corvaja N, et al. Multiple overlapping drug-eluting stents to treat diffuse disease of the left anterior descending coronary artery. J Am Coll Cardiol. (2005) 45(10):1570–3. doi: 10.1016/j.jacc.2005.01.049
19. Dawkins KD, Grube E, Guagliumi G, Banning AP, Zmudka K, Colombo A, et al. Clinical efficacy of polymer-based paclitaxel-eluting stents in the treatment of complex, long coronary artery lesions from a multicenter, randomized trial: support for the use of drug-eluting stents in contemporary clinical practice. Circulation. (2005) 112(21):3306–13. doi: 10.1161/circulationaha.105.552190
20. Stone GW, Ellis SG, Cannon L, Mann JT, Greenberg JD, Spriggs D, et al. Comparison of a polymer-based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease: a randomized controlled trial. JAMA. (2005) 294(10):1215–23. doi: 10.1001/jama.294.10.1215
21. Chen X, Gao X, Kan J, Shrestha R, Han L, Lu S, et al. Overlapping drug-eluting stent is associated with increased definite stent thrombosis and revascularization: results from 15,561 patients in the authentic study. Cardiovasc Drugs Ther. (2021) 35(2):331–41. doi: 10.1007/s10557-020-07094-7
22. Achim A, Alampi C, Krivoshei L, Leibundgut G. In vitro effect of intravascular lithotripsy on the polymer of a drug-eluting stent. EuroIntervention. (2022) 18(4):e333–4. doi: 10.4244/EIJ-D-22-00300
23. Kapnisis KK, Halwani DO, Brott BC, Anderson PG, Lemons JE, Anayiotos AS. Stent overlapping and geometric curvature influence the structural integrity and surface characteristics of coronary nitinol stents. J Mech Behav Biomed Mater. (2013) 20:227–36. doi: 10.1016/j.jmbbm.2012.11.006
24. Mori N, Okamoto N, Tanaka A, Yano M, Makino N, Egami Y, et al. Comparison of angiographic and 1-year outcomes between a long single stent and overlapping double stents in patients with newer-generation drug-eluting stents for long narrowings. Am J Cardiol. (2016) 117(11):1724–8. doi: 10.1016/j.amjcard.2016.03.004
25. Yano H, Horinaka S, Ishimitsu T. Impact of everolimus-eluting stent length on long-term clinical outcomes of percutaneous coronary intervention. J Cardiol. (2018) 71(5):444–51. doi: 10.1016/j.jjcc.2017.10.011
26. Sim HW, Thong EH, Loh PH, Lee CH, Chan MY, Low AF, et al. Treating very long coronary artery lesions in the contemporary drug-eluting-stent era: single long 48mm stent versus two overlapping stents showed comparable clinical outcomes. Cardiovasc Revasc Med. (2020) 21(9):1115–8. doi: 10.1016/j.carrev.2020.02.005
27. Şaylık F, Çınar T, Selçuk M, Çiçek V, Hayıroğlu MI, Orhan AL. Comparison of outcomes between single long stent and overlapping stents: a meta-analysis of the literature. Herz. (2023) 48(5):376–83. doi: 10.1007/s00059-022-05152-4
28. Best PJ, Lennon R, Ting HH, Bell MR, Rihal CS, Holmes DR, et al. The impact of renal insufficiency on clinical outcomes in patients undergoing percutaneous coronary interventions. J Am Coll Cardiol. (2002) 39(7):1113–9. doi: 10.1016/s0735-1097(02)01745-x
29. Meco JF, Pinto X, Escriba JM, Vela M, Jara F, Pallares C, et al. Cardiovascular risk factors associated with clinically isolated and diffuse atherosclerosis in Spanish patients with coronary artery disease. Eur J Clin Invest. (1998) 28(8):643–50. doi: 10.1046/j.1365-2362.1998.00350.x
30. Jiang Y, Li Y, Shi K, Wang J, Qian WL, Yan WF, et al. The additive effect of essential hypertension on coronary artery plaques in type 2 diabetes mellitus patients: a coronary computed tomography angiography study. Cardiovasc Diabetol. (2022) 21(1):1. doi: 10.1186/s12933-021-01438-9
31. Iancu AC, Ober C. Intracoronary glycoprotein IIb/IIIa inhibitors downstream of the coronary occlusion: the “highway” to periphery. J Am Coll Cardiol. (2013) 62(5):481. doi: 10.1016/j.jacc.2013.04.052
32. Cuisset T, Frere C, Quilici J, Morange PE, Mouret JP, Bali L, et al. Glycoprotein IIb/IIIa inhibitors improve outcome after coronary stenting in clopidogrel nonresponders: a prospective, randomized study. JACC Cardiovasc Interv. (2008) 1(6):649–53. doi: 10.1016/j.jcin.2008.08.018
33. Cura FA, Bhatt DL, Lincoff AM, Kapadia SR, L'Allier PL, Ziada KM, et al. Pronounced benefit of coronary stenting and adjunctive platelet glycoprotein IIb/IIIa inhibition in complex atherosclerotic lesions. Circulation. (2000) 102(1):28–34. doi: 10.1161/01.cir.102.1.28
34. Winchester DE, Wen X, Brearley WD, Park KE, Anderson RD, Bavry AA. Efficacy and safety of glycoprotein IIb/IIIa inhibitors during elective coronary revascularization: a meta-analysis of randomized trials performed in the era of stents and thienopyridines. J Am Coll Cardiol. (2011) 57(10):1190–9. doi: 10.1016/j.jacc.2010.10.030
35. Hahn J, Jeon J, Geum MJ, Lee HW, Shin J, Chung WY, et al. Intracoronary versus intravenous glycoprotein IIb/IIIa inhibitors during primary percutaneous coronary intervention in patients with STEMI: a systematic review and meta-analysis. Thromb J. (2023) 21(1):76. doi: 10.1186/s12959-023-00519-x
36. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European society of cardiology (ESC). Eur Heart J. (2018) 39(2):119–77. doi: 10.1093/eurheartj/ehx393
37. Lim SY, Jeong MH, Hong SJ, Lim DS, Moon JY, Hong YJ, et al. Inflammation and delayed endothelization with overlapping drug-eluting stents in a porcine model of in-stent restenosis. Circ J. (2008) 72(3):463–8. doi: 10.1253/circj.72.463
38. Honda Y, Fitzgerald PJ. Stent thrombosis: an issue revisited in a changing world. Circulation. (2003) 108(1):2–5. doi: 10.1161/01.CIR.0000075929.79964.D8
39. Schuhlen H, Kastrati A, Dirschinger J, Hausleiter J, Elezi S, Wehinger A, et al. Intracoronary stenting and risk for major adverse cardiac events during the first month. Circulation. (1998) 98(2):104–11. doi: 10.1161/01.cir.98.2.104
40. Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, et al. 2023 ESC guidelines for the management of acute coronary syndromes: developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J. (2023) 44(38):3720–826. doi: 10.1093/eurheartj/ehad191
41. Park DY, Vemmou E, An S, Nikolakopoulos I, Regan CJ, Cambi BC, et al. Trends and impact of intravascular ultrasound and optical coherence tomography on percutaneous coronary intervention for myocardial infarction. Int J Cardiol Heart Vasc. (2023) 45:101186. doi: 10.1016/j.ijcha.2023.101186
42. Kim Y, Johnson TW, Akasaka T, Jeong MH. The role of optical coherence tomography in the setting of acute myocardial infarction. J Cardiol. (2018) 72(3):186–92. doi: 10.1016/j.jjcc.2018.03.004
43. Katagiri Y, De Maria GL, Kogame N, Chichareon P, Takahashi K, Chang CC, et al. Impact of post-procedural minimal stent area on 2-year clinical outcomes in the SYNTAX II trial. Catheter Cardiovasc Interv. (2019) 93(4):E225–34. doi: 10.1002/ccd.28105
Keywords: comparative study, coronary intervention, stents, myocardial infarction, percutaneous coronary intervention
Citation: Lee DH, Oh S, Kim MC, Sim DS, Hong YJ, Kim JH, Ahn Y, Han JB, Kim IS and Jeong MH (2023) Comparative treatment outcomes of a single long stent vs. overlapped short stents in acute myocardial infarction. Front. Cardiovasc. Med. 10:1284396. doi: 10.3389/fcvm.2023.1284396
Received: 28 August 2023; Accepted: 29 November 2023;
Published: 19 December 2023.
Edited by:
Tommaso Gori, University Medical Centre, Johannes Gutenberg University Mainz, GermanyReviewed by:
Alexandru Achim, Cantonal Hospital Baselland (KSBL), Switzerland© 2023 Lee, Oh, Kim, Sim, Hong, Kim, Ahn, Han, Kim and Jeong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: In Soo Kim a2ltaXMxMjNAaGFubWFpbC5uZXQ= Myung Ho Jeong bXl1bmdob0BjaG9sbGlhbi5uZXQ=
†These authors have contributed equally to this work
‡These authors have contributed equally to this work and share co-first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.