
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Cardiovasc. Med. , 20 December 2023
Sec. Cardiac Rhythmology
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1243574
 Aishwarya Pastapur1*
Aishwarya Pastapur1* Nicole A. Pescatore2
Nicole A. Pescatore2 Nirav Shah2
Nirav Shah2 Sachin Kheterpal2
Sachin Kheterpal2 Brahmajee K. Nallamothu3,4,5
Brahmajee K. Nallamothu3,4,5 Jessica R. Golbus3,4
Jessica R. Golbus3,4
Background: The rising adoption of wearable technology increases the potential to identify arrhythmias. However, specificity of these notifications is poorly defined and may cause anxiety and unnecessary resource utilization. Herein, we report results of a follow-up screening protocol for incident atrial fibrillation/flutter (AF) within a large observational digital health study.
Methods: The MIPACT Study enrolled 6,765 adult patients who were provided an Apple Watch and blood pressure (BP) monitors. From March to July 2019, participants were asked to contact the study team for any irregular heart rate (HR) notification. They were assessed using structured questionnaires and asked to provide 6 Apple Watch EKGs. Those with arrhythmias or non-diagnostic EKGs were sent 7-day monitors. The EHR was reviewed after 3 years to determine if participants developed arrhythmias.
Results: 86 participants received notifications and met inclusion criteria. Mean age was 50.5 (SD 16.9) years, and 46 (53.3%) were female. Of 76 participants assessed by the study team, 32 (42.1%) reported anxiety surrounding notifications. Of 59 participants who sent at least 1 EKG, 52 (88.1%) were in sinus rhythm, 3 (5.1%) AF, 2 (3.4%) indeterminate, and 2 (3.4%) sinus bradycardia. Cardiac monitor demonstrated AF in 2 of 3 participants with AF on Apple Watch EKGs. 2 contacted their PCPs and were diagnosed with AF. In total, 5 cases of AF were diagnosed with 1 additional case identified during EHR review.
Conclusion: Wearable devices produce alarms that can frequently be anxiety provoking. Research is needed to determine the implications of these alarms and appropriate follow-up.
Atrial fibrillation (AF) is a common arrhythmia associated with increased risk for stroke, heart failure, and mortality (1). The incidence and prevalence of AF are increasing along with AF-associated mortality and hospitalizations (2). Appropriate diagnosis and treatment can decrease AF-related morbidity though this is confounded by the asymptomatic nature of the disease, requiring efforts to improve detection (3).
Adoption of wearable devices (i.e., smartwatches) and home blood pressure (BP) monitors has increased and offers the potential to detect previously undiagnosed arrhythmias such as AF. Studies evaluating the sensitivity and specificity of these devices, however, have demonstrated variable performance and have been limited by their small sample sizes, highly-selective and variable populations, and differing requirements for clinical follow-up to validate test results (4, 5). This is concerning because detection of rhythms such as sinus arrhythmia, premature atrial contractions (PACs), and premature ventricular contractions (PVCs) can also lead to irregular heart rate (HR) notifications (i.e., false positives) which are not due to AF. As wearable device use increases, this has the potential to overwhelm clinician resources.
Herein, we present the results from a secondary analysis of an observational digital health study in which we evaluated the frequency of alarms and then implemented a standardized protocol to address irregular heart rate notifications from wearable devices. In addition to using a rigorous protocol to follow-up on irregular heart rate notifications from either a smartwatch or home BP monitor, we also evaluated participants' electronic health records (EHR) to determine subsequent AF diagnoses in extended 3-year follow-up. We hypothesized that a structured protocol to evaluate irregular heart rate notifications would miss few clinically relevant AF cases.
The Michigan Predictive Activity & Clinical Trajectories in Health (MIPACT) study was a prospective observational, digital health study (6). The study enrolled Michigan Medicine patients who were 18 years of age or older, fluent in English, owned an iPhone 6 or newer model, and had regular access to the internet throughout the study period. All participants were provided with an Apple Watch Series 3 or 4, an Omron Evolv Wireless BP Monitor, and the study smartphone application delivered through MyDataHelps. The study was divided into two phases. In both phases, participants were asked to wear their watches for at least 12 h/day for 15 days. Additionally, during the first phase of the study, which lasted up to 45 days, participants were asked to obtain two sets of BP readings daily, with each set consisting of two measurements. During phase two, which lasted until the end of the 3-year study, participants were asked to check at least one set of BP readings each month.
Between 12 March 2019 and 1 July 2019, all participants were provided with an Apple Watch Series 4 and asked during enrollment visits to contact the study team if they received an irregular HR notification through their Apple Watch or BP monitor. These participants were subsequently assessed using structured questionnaires to confirm their comorbidities, evaluate for associated symptoms, and assess anxiety surrounding the notifications with a modified GAD-7 questionnaire. Participants were excluded if they were younger than 22 due to FDA restrictions on use of the Apple Watch ECG feature in these individuals, had a history of AF or stroke, or were on anticoagulation. Participants with concerning symptoms as determined by a study clinician were referred to an urgent care center or to the emergency department as appropriate. Eligible participants were asked to provide 6 ECGs from their Apple Watch, ideally 2 ECGs daily for three days. These were reviewed by a study clinician. Participants with arrhythmias or non-diagnostic ECGs were sent 7-day cardiac monitors. The EHR was later reviewed to determine if participants developed arrhythmias in the greater than 3 years since the study period. Data are summarized as means and standard deviations (SDs) for continuous symmetric variables and as counts and percentages for categorical variables.
The MIPACT study enrolled 6,765 eligible participants between 14 August 2018, and 19 December 2019. From 12 March 2019 to 1 July 2019, participants were advised to reach out to the study team for any irregular HR notifications. 2,615 patients were enrolled in this time period (2,435 participants >22 years of age; Supplementary Table S1), of whom 97 (3.7%) contacted the study team for irregular HR notifications. Of these, 5 were younger than 22, 2 had a known history of AF, 3 a previous stroke, and 1 was already on anticoagulation, leaving a final cohort of 86 participants (Figure 1). Participants were 50.5 (SD 16.9) years of age (Supplementary Table S2), and 46 (53.5%) were female. Seventy-one (71; 82.6%) participants received an alarm on the BP monitor, 1 (1.2%) on their Apple Watch, 3 (3.5%) on both devices, and 11 (12.8%) did not recall from which device they received a notification.
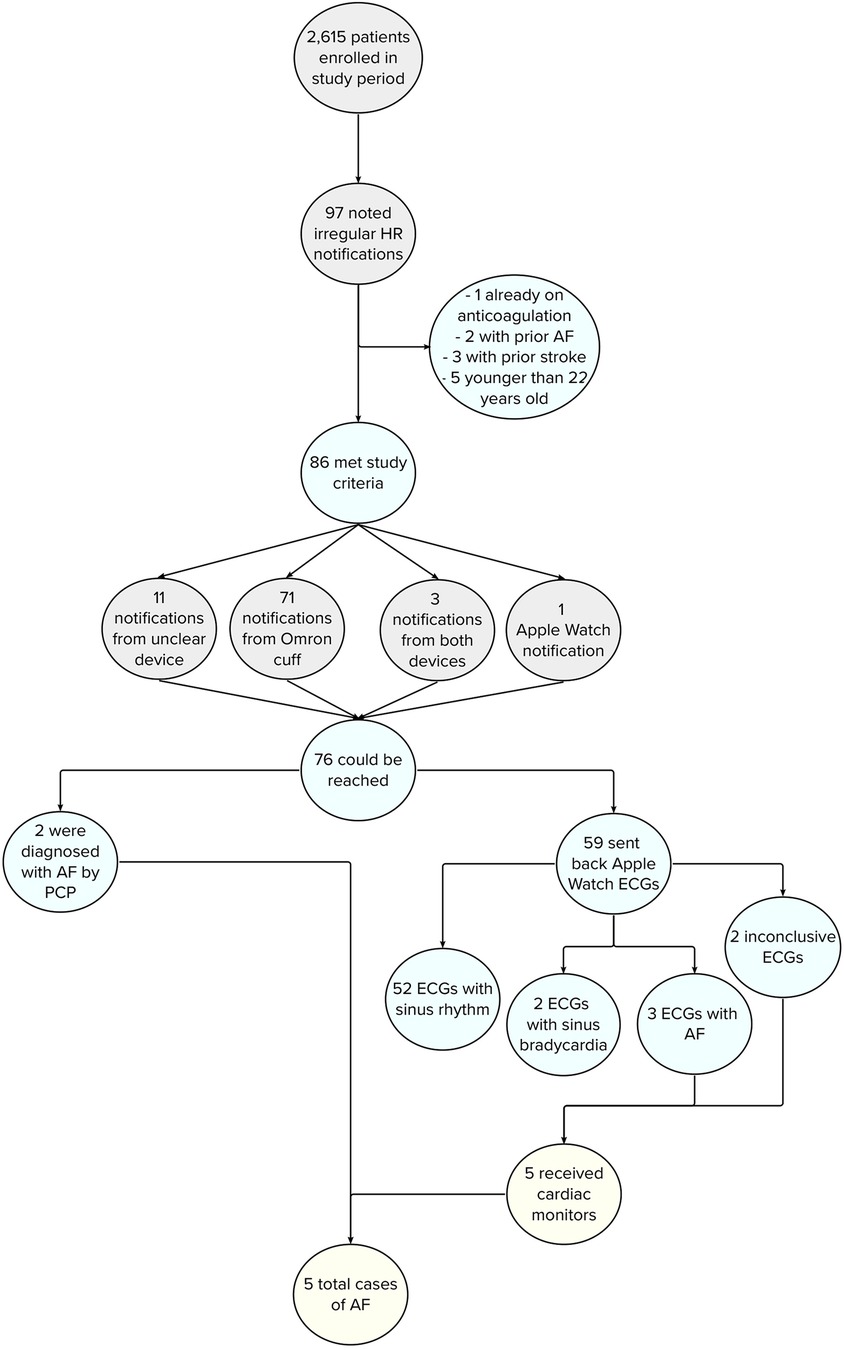
Figure 1. Flowchart depicting incidence of AF in study participants after EHR review. Of the 5 patients who were found to have AF, 4 initially received a notification from their Omron BP cuff and 1 from their Apple Watch.
Amongst the 86 participants, 76 (89.4%) could be reached and were evaluated by the study team. The mean modified GAD-7 score was 4.27 (SD 4.12), corresponding to no anxiety disorder. However, 26 (34.2%) participants noted mild anxiety surrounding irregular HR notifications and 6 (7.9%) moderate anxiety. Two participants contacted their primary care clinicians after receiving the alarm and were diagnosed with AF. Thus, 74 participants were asked to provide 6 Apple Watch ECGs. Of these participants, 59 (79.7%) sent back at least 1 ECG and 55 (74.3%) 6 ECGs. ECGs revealed sinus rhythm for 52 (88.1%) participants, sinus bradycardia for 2 (3.4%) participants, AF for 3 (5.1%) participants, and inconclusive results for 2 (3.4%) participants (Figure 1). A 7-day cardiac monitor was subsequently mailed to the 5 participants with AF or inconclusive ECGs, confirming AF in 2 of 3 participants with AF noted on their Apple Watch ECGs. In total, 5 (6.6%) cases of AF were diagnosed, 3 by Apple Watch ECGs and 2 by participants' primary care clinicians. In these instances, 4 participants received the initial alarm from the Omron BP cuff and 1 from the Apple Watch.
Three years after the last participant was enrolled, the EHR was reviewed to determine subsequent AF diagnosis. Only 1 additional case of AF was diagnosed in a participant who was initially alerted by an Omron BP cuff and had inconclusive ECGs and a 7-day cardiac monitor revealing supraventricular tachycardia but no AF. Notably, 1 other participant was noted to be in AF on Apple Watch ECG but cardiac monitor revealed only sinus rhythm, and per EHR review, they were not diagnosed with AF on long-term follow-up. An additional 5 participants were found to have other arrhythmias, including ventricular tachycardia, atrial tachycardia, ectopic atrial rhythm, sinus bradycardia, and premature ventricular contractions. In total, 80 irregular heart rate notifications were felt to potentially represent false positive alarms, with 67 related to the Omron BP cuff notification, 4 from both the BP cuff and Apple Watch, and 9 from an unrecalled device. Furthermore, the positive and negative predictive values of our irregular HR alarm follow-up protocol for detecting AF was 100% (3 of 3) and 98.2% (55 of 56), respectively.
Wearable devices and remote monitoring have the potential to increase AF diagnoses. To mitigate this, clinicians need a standardized protocol to address the increasing incidence of irregular HR alarms, which we implemented and evaluated. Of the 2,615 participants enrolled in the study period, nearly 4% experienced alarms. Of 76 participants who could be contacted after reporting an irregular HR by their devices, only 5 were ultimately found to have AF, and only 1 additional participant was diagnosed in the 3 years after the study. This suggests that a standardized algorithm using home-based ECGs coupled with clinician review can help to detect and diagnose AF without overwhelming clinic resources. Additionally, over one third of participants had mild or moderate anxiety surrounding irregular HR notifications. Efficient and structured evaluation of these notifications may help with alarm-associated anxiety though we did not evaluate the impact of our intervention on participant anxiety after implementation of the protocol.
The Apple Heart Study and Fitbit Heart Study revealed that 30%–35% of patients who received irregular HR notifications on their wearable devices had AF on continuous ECG monitoring (7). BP monitors have also been studied for AF detection though results are highly variable especially in light of their evolving capabilities (8, 9). In all prior studies, participants ultimately required clinician follow-up after an irregular HR notification. Follow-up protocols are thus needed to provide guidance on how to address the increasing alarm burden and assuage patient and clinician concerns.
This study has the benefit of long-term follow-up greater than 3 years since the initial study period. However, limitations include limited study period duration and the inclusion of English-speaking patients only. The study also enrolled younger patients and required them to self-report irregular HR notifications. It is also unknown whether the alarms represent false positive alarms or are due to the paroxysmal nature of AF, which can be missed on follow-up in the absence of standardized monitoring. Our study suggests, however, that a standardized protocol using participant obtained ECGs from a smartwatch may be useful for diagnosing AF while minimizing critical resource utilization. With a high positive predictive value, our protocol was generally effective in detecting AF in ambulatory patients who experienced irregular HR notifications, with only 1 case clinically diagnosed after study completion. More notably, our protocol had a high negative predictive value, eliminating the need for further downstream testing for most participants. Additional research is still needed to determine the implications of these alarms and to develop appropriate follow-up protocols, including those that account for participant baseline risk.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the University of Michigan Health IRB (HUM00169702). The patients/participants provided their written informed consent to participate in this study.
AP performed the chart review. AP and JG co-wrote the manuscript with editing and advice provided by BN, NS, SK, and NP. All authors contributed to the article and approved the submitted version.
Partial funding for data collection was provided by Apple Inc., (Cupertino, CA) to the University of Michigan. JG receives funding from the NIH (grant no. L30HL143700 and grant no. 1K23HL168220-01) and receives salary support by an American Heart Association grant (grant no. 20SFRN35370008). BN is a principal investigator or co-investigator on research grants from the NIH, VA HSR & D and the American Heart Association. He also receives compensation as Editor-in-Chief of Circulation: Cardiovascular Quality & Outcomes, a journal of the American Heart Association. Finally, he is a co-inventor on US Utility Patent Number US15/356,012 (grant no. US20170148158A1) entitled “Automated Analysis of Vasculature in Coronary Angiograms” that uses software technology with signal processing and machine learning to automate the reading of coronary angiograms, held by the University of Michigan. The patent is licensed to AngioInsight, Inc., in which Nallamothu holds ownership shares and receives consultancy fees. SK is a principal investigator or co-investigator on research grants from the US NIH, Blue Cross Blue Shield of Michigan, the American Heart Association, Apple, Merck & Co, and Becton Dickinson & Company; and is a co-inventor on US patent number 62/791,257 entitled “Automated System To Medical Procedures”, which is held by the University of Michigan. NS is a principal investigator or co-investigator on current or recent research grants from the US NIH, Blue Cross Blue Shield of Michigan, the American Heart Association, Apple, and Edwards Lifesciences. Some of the study staff may have held stock in Apple. These researchers are not likely to personally benefit from the results of this research.
This study received funding from Apple Inc. The funder had the following involvement with the study: partial funding for data collection.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1243574/full#supplementary-material
1. Benjamin EJ, Chen PS, Bild DE, Mascette AM, Albert CM, Alonso A, et al. Prevention of atrial fibrillation: report from an NHLBI workshop. Circulation. (2009) 119(4):606–18. doi: 10.1161/CIRCULATIONAHA.108.825380
2. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, et al. Fifty-year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the community. Lancet. (2015) 386(9989):154–62. doi: 10.1016/S0140-6736(14)61774-8
3. Dilaveris PE, Kennedy HL. Silent atrial fibrillation: epidemiology, diagnosis, and clinical impact. Clin Cardiol. (2017) 40(6):413–8. doi: 10.1002/clc.22667
4. Gladstone DJ, Wachter R, Schmalstieg-Bahr K, Quinn FR, Hummers E, Ivers N, et al. Screening for atrial fibrillation in the older population: a randomized clinical trial. JAMA Cardiol. (2021) 6(5):558–67. doi: 10.1001/jamacardio.2021.0038
5. Kane SA, Blake JR, McArdle FJ, Langley P, Sims AJ. Opportunistic detection of atrial fibrillation using blood pressure monitors: a systematic review. Open Heart. (2016) 3(1):e000362. doi: 10.1136/openhrt-2015-000362
6. Golbus JR, Pescatore NA, Nallamothu BK, Shah N, Kheterpal S. Wearable device signals and home blood pressure data across age, sex, race, ethnicity, and clinical phenotypes in the Michigan predictive activity & clinical trajectories in health (MIPACT) study: a prospective, community-based observational study. Lancet Digit Health. (2021) 3(11):e707–15. doi: 10.1016/S2589-7500(21)00138-2
7. Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. (2019) 381(20):1909–17. doi: 10.1056/NEJMoa1901183
8. Park SH, June KJ, Choi YK. Predictive validity of automated oscillometric blood pressure monitors for screening atrial fibrillation: a systematic review and meta-analysis. Expert Rev Med Devices. (2019) 16(6):503–14. doi: 10.1080/17434440.2019.1620102
Keywords: atrial fibrillation, wearable devices, ambulatory monitoring, irregular heart rate notifications, standardized protocol
Citation: Pastapur A, Pescatore NA, Shah N, Kheterpal S, Nallamothu BK and Golbus JR (2023) Evaluation of atrial fibrillation using wearable device signals and home blood pressure data in the Michigan Predictive Activity & Clinical Trajectories in Health (MIPACT) Study: A Subgroup Analysis (MIPACT-AFib). Front. Cardiovasc. Med. 10:1243574. doi: 10.3389/fcvm.2023.1243574
Received: 21 June 2023; Accepted: 24 November 2023;
Published: 20 December 2023.
Edited by:
Patrick Badertscher, University Hospital of Basel, SwitzerlandReviewed by:
Emanuela Teresina Locati, IRCCS San Donato Polyclinic, Italy© 2023 Pastapur, Pescatore, Shah, Kheterpal, Nallamothu and Golbus. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aishwarya Pastapur cGFzdGFwdWFAbWVkLnVtaWNoLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.