- 1Division of Chinese Internal Medicine, Center for Traditional Chinese Medicine, Chang Gung Memorial Hospital, Taoyuan, Taiwan
- 2Department of Traditional Chinese Medicine, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan
- 3Department of Pediatrics, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan
- 4College of Medicine, Chang Gung University, Taoyuan, Taiwan
Background: In 2016, Lin et al. developed a prediction score of non-responsiveness to intravenous immunoglobulin (IVIG) in patients with Kawasaki disease (KD) (Lin et al., 2016). Various studies have attempted to validate the Formosa score, but inconsistent results have given us new opportunities and challenges. The aim of this meta-analysis is to explore the role of the Formosa score as a risk score in detecting IVIG-resistant KD patients and then compare the pooled sensitivity and specificity of four Asian risk scores, Egami, Formosa, Kobayashi, and Sano risk scores.
Methods: A comprehensive search of Cochrane, Embase, and PubMed was conducted through 20 December 2021, using key terms relevant to the research question “What are the sensitivities and specificities of the four Asian predicting scores, Egami, Formosa, Kobayashi, and Sano, in Kawasaki disease patients with IVIG resistance?” The reference lists of the included studies were manually reviewed to identify pertinent references. A random-effects bivariate model was used to estimate the summary of sensitivity and specificity of the tools.
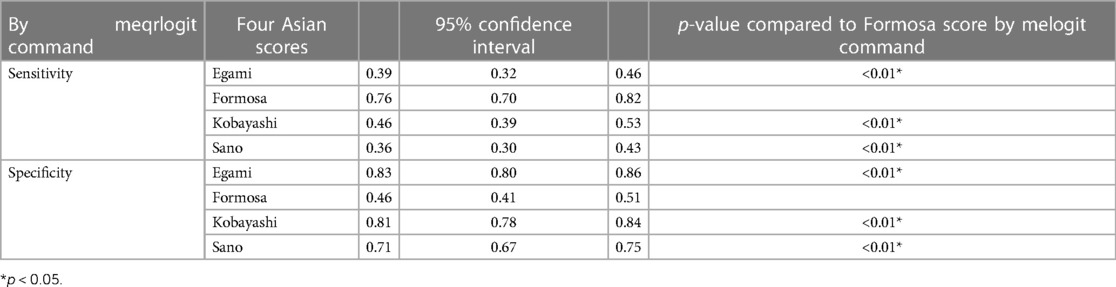
Results: We found 41 relevant studies of the four Asian risk scores that were eligible to analyze for pooled accuracy. Eleven studies involving 5,169 KD patients reported the diagnostic performance of the Formosa score for the risk of IVIG resistance. The overall performance of the Formosa score was as follows: pooled sensitivity, 0.60 [95% confidence interval (CI), 0.48–0.70]; pooled specificity, 0.59 (95% CI, 0.50–0.68); and area under the hierarchical summary receiver operating characteristic curve, 0.62. The Formosa score exhibited the highest sensitivity 0.76 (95% CI, 0.70–0.82) for detecting IVIG-resistant KD patients among the 21,389 children included in the 41 studies. In terms of specificity estimates, Formosa had the lowest specificity of 0.46 (95% CI, 0.41–0.51).
Conclusion: Patients at high risk for IVIG resistance may receive adjunctive treatment to reduce coronary lesions and thus also cardiovascular morbidity. Among all of the included studies, we found Formosa score to have the best sensitivity (0.76) but unsatisfactory specificity (0.46) for predicting IVIG resistance in Kawasaki disease. In the future, network meta-analysis should also incorporate the accuracy of the new scores after they have undergone a certain degree of validation around the world.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, PROSPERO CRD42022341410.
1. Introduction
The prevalence of Kawasaki disease (KD) is highest in Asia (1). National surveys in Japan, South Korea, and Taiwan have all confirmed this finding. KD is not just acute vascular inflammation, as long-term follow-up has found that it affects immunity and the development of allergic diseases (2–5). Intravenous immunoglobulin (IVIG) resistance is a term that was developed after the invention of IVIG treatment for KD (6). Approximately 10%–20% of patients still experience persistent or recurrent fever after completing the initial IVIG administration and are thus classified as unresponsive to IVIG treatment. This group of patients is at an increased risk of developing coronary artery lesions (CAL) (7, 8). Many scoring systems have been used to predict the risk of IVIG resistance (9). In particular, the scoring systems in Asia have been repeatedly verified for a long time in the hopes of providing initial treatment guidelines for high-risk patients in Asia (10, 11).
Many scoring systems have been developed to predict IVIG resistance in KD, such as Egami, Formosa, Kobayashi, and Sano scores (12). However, their efficacy needs to be validated given the regional and racial population (12). Fabi et al. enrolled both Caucasian and Asian children and determined that Formosa score had the highest predictive efficacy for CAL risk (13). The Kobayashi score had a sensitivity of 64.0% and specificity of 62.5% in a total of 257 patients. However, when applied to seven Asian patients, the Kobayashi score had a sensitivity of 100% and a specificity of 75% (13).
IVIG resistance scoring systems can help clinicians identify high-risk KD patients who may benefit from so-called “rescue therapies,” such as IVIG plus prednisolone or IVIG plus cyclosporine (10, 11). Adopting additional treatment before the initial use of IVIG could potentially reduce the incidence of CAL in IVIG-resistant KD patients (14).
According to the Kobayashi score, cutoff points and score points for each variable are as follows: sodium ≦133 mmol/L, 2 points; days of illness at initial treatment ≤4, 2 points; aspartate aminotransferase (AST) ≥100 IU/L, 2 points; % neutrophils ≥80%, 2 points; C-reactive protein (CRP) ≥10 mg/dL, 1 point; age ≤12 months, 1 point; and platelet count ≤30.0×104/mm3, 1 point. Patients with a total of 4 or more points are identified as being at high risk for IVIG resistance. This score has a sensitivity of 86% and specificity of 68% in predicting IVIG resistance (7). According to the Egami score, based on the odds ratios of significant predictors, 1 point is assigned for infants younger than 6 months, before 4 days of illness, platelet count of ≤30 × 1010/L, and CRP of ≥8 mg/dl, respectively. Two points are assigned for alanine transaminase (ALT) 80 IU/L. Using a cutoff point of 3 or more points with this prediction score, it could identify the IVIG-resistant group with a sensitivity of 78% and specificity of 76% (15). According to the Sano score, the criteria for at least two of the three predictors (CRP ≥7 mg/dl, total bilirubin ≥0.9 mg/dl, or AST ≥200 IU/L) are considered to be clinically useful for detecting non-responsiveness to IVIG in patients with acute KD before treatment, with a sensitivity of 77% and specificity of 86% (16). According to the Formosa score, cutoff points and score points for each variable are as follows: albumin <3.5 g/dl, 1 point; neutrophil percentage ≥60%, 2 points; and positive lymphadenopathy, 1 point. Patients with scores of ≥3 points are identified as being at high risk for IVIG resistance. Their sensitivity and specificity have been shown to be 90.9% and 81.3%, respectively (17).
In this study, we compared the predictive efficacy of the Egami, Formosa, Kobayashi, and Sano scoring systems using a bivariate meta-analysis.
2. Methods
We conducted this study in accordance with the guidelines of the preferred reporting items for systematic reviews and meta-analysis of diagnostic test accuracy studies (PRISMA-DTA) (18). We formulated the following patient, index test, comparison, outcome (PICO) question: “What are the sensitivities and specificities of four Asian predicting scores, Egami, Formosa, Kobayashi, and Sano, in Kawasaki disease patients with intravenous immunoglobulin resistance?” The definition of IVIG resistance varied according to different studies (Table 1) (19). We registered the study protocol at the International Prospective Register of Systematic Reviews (PROSPERO CRD42022341410).
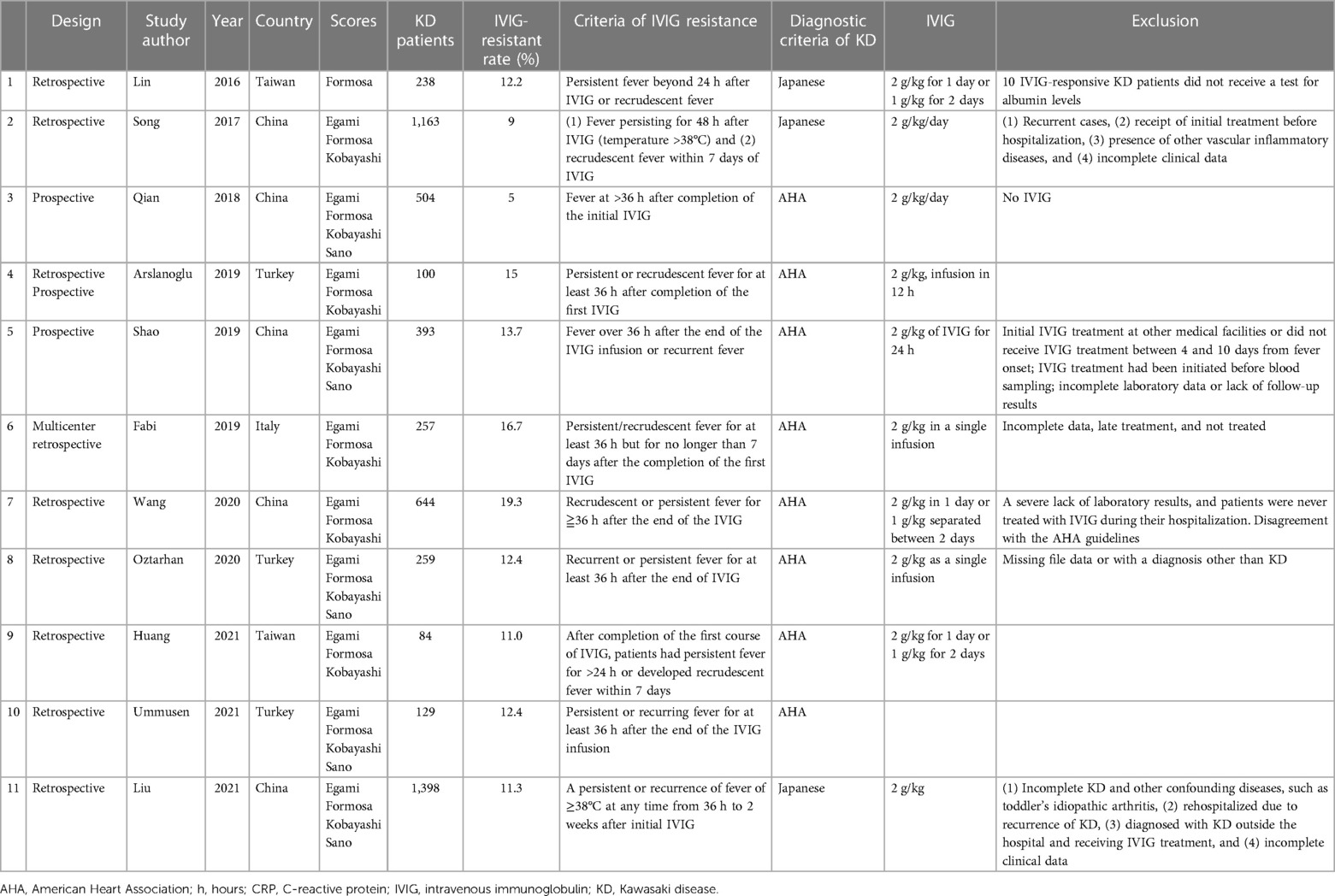
Table 1A. Clinical characteristics of the included studies: included studies with the Formosa score.
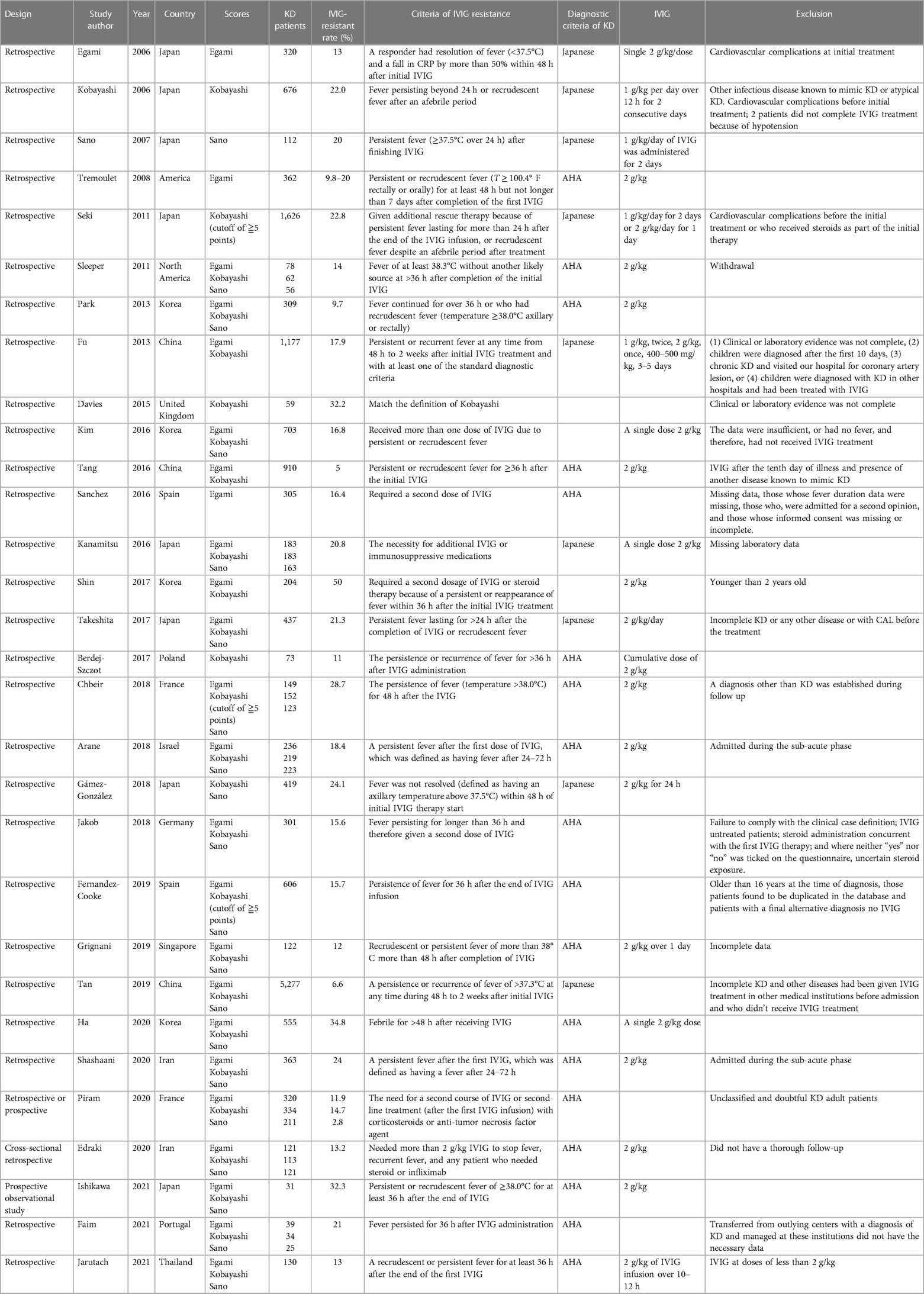
Table 1B. Clinical characteristics of the included studies: included studies without the Formosa score.
We first performed a systematic literature search in all fields in international electronic databases, including Cochrane, Embase, and PubMed (20). We applied the combinations of keywords used, respectively, with the “Kobayashi score,” “Egami score,” “Sano score,” “Formosa score,” “sensitivity,” and “specificity” to identify relevant articles (21). Our search only included papers published in the English language. The reference lists of the included studies were manually reviewed to identify cited articles of these four Asian scores (7, 15–17). Original articles would be included in this meta-analysis if they met the following criteria: (1) examination of patients with KD; (2) assessment of the sensitivity and specificity of the Egami, Formosa, Kobayashi, or Sano scores; and (3) received treatment with a total IVIG of 2 g/kg including one single dose or 1 g/kg per day for 2 consecutive days. When the study reported Kobayashi score with a cutoff value of ≧4 and ≧5, we recorded the value with the cutoff of ≧4 for analysis. We ruled out case reports and studies that predicted IVIG resistance with a predictive score after diagnosing KD and then prescribing different treatments. Studies that did not report sensitivity or specificity values and sample sizes were excluded. Two investigators (Wan-Ni Chiang and Dr. Ling-Sai Chang) independently extracted data from each included study by using a predesigned data extraction form, including the authors, publication year, the country where the study was conducted, study design, age, percentage of male participants, number of participants, and cutoff value for the analysis of sensitivity and specificity. The same two investigators (Wan-Ni Chiang and Dr. Ling-Sai Chang) independently performed a systematic literature search and evaluated all relevant studies for eligibility criteria. Any disagreement was resolved through discussion.
After the full systematic literature search was performed, we used bivariate statistical analysis to obtain the logit-transformed sensitivity and specificity of the Formosa score. To estimate the summary of sensitivity and specificity, we adopted a random-effects bivariate model. All analyses were performed using Stata version 17.0 (StataCorp LP, College Station, TX, United States) with meqrlogit for network calculation based on the ANOVA model proposed by Nyaga et al.; metandi for making the graph of the hierarchical summary receiver operating characteristic curve (HSROC); midas for calculating sensitivity and specificity of the Formosa score, heterogeneity measures, I2 estimation, the area under the curve (AUC), and subgroup calculation; and melogit for comparing the Formosa score and the other three Asian scores’ user-written commands (20, 22). Furthermore, we accessed the publication bias for evaluating the accuracy of the Formosa score using Deeks’ funnel plot asymmetry test (23).
We adopted the revised Quality Assessment of Diagnostic Accuracy Studies to evaluate the methodological quality of selected studies according to four domains comprising 14 items rated as “yes,” “no,” or “unclear” (24).
3. Results
3.1. Study selection
We identified a total of 345 articles through database searching (PubMed = 150, Embase = 82, Cochrane Library = 113) and 12 additional records through manual retrieval of articles, citing the original articles that invented the four scores (8, 16, 25–34). Of the 177 records initially identified through title and abstract screening after removing duplicates, 131 were removed for failing to fulfill the inclusion criteria (Figure 1). Further full-text assessment of the potential 46 articles led to the exclusion of five studies, which were excluded for the following reasons: two not in English, one using risk scoring systems in patients unresponsive to the second IVIG, one without case number, and one design with different treatments for low- and high-risk patients (35–39). Ultimately, a total of 41 studies were included in the network meta-analysis.
3.2. Study characteristics
A total of 41 articles met the inclusion criteria in Table 1, which provides broad details of the studies. Eleven studies were included in both the bivariate meta-analysis for the Formosa score (Table 1A) and network meta-analysis for the four Asian scores. All included studies were written in English. The median number of patients was 305 (interquartile range, IQR: 125.5–580.5), while the median prevalence of IVIG resistance was 15.7% (IQR: 12.1%–21.2%). Thirty-four studies with 18,170 KD patients evaluated the Egami score; 11 studies with 5,169 KD patients evaluated the Formosa score; 36 studies with 20,006 KD patients evaluated the Kobayashi score; and 25 studies with 12,970 patients evaluated the Sano score.
Of the 41 studies, three conducted prospective studies, two conducted retrospective or prospective studies, and the remaining 36 were retrospective studies. All studies provided detailed information on the reference standard for diagnosing IVIG resistance. The definition of reference was persistent or recrudescent fever at least 24, 36, or 48 h after completion of the first IVIG or the necessity for additional IVIG or immunosuppressive medications. These 41 studies were conducted between 2006 and 2021. Four of the studies excluded incomplete KD patients (7, 31, 40, 41). Furthermore, four studies excluded cardiovascular complications before or at initial treatment (7, 15, 41, 42). While three studies adopted thresholds of ≧5, other studies evaluating Kobayashi score used Kobayashi-specified thresholds (≧4) to classify the results (28, 29, 42). This study consists of four different Asian scores, namely, 3 studied Egami score, 1Formosa, 4 Kobayashi, 1 Sano, 3 Egami + Kobayashi, 1 Kobayashi + Sano, 5 Egami + Formosa + Kobayashi, 18 Egami + Obayashii + Sano, and 5 Egami + Formosa + Kobayashi + Sano scores.
3.3. Results of meta-analysis for the sensitivity and specificity of the Formosa score
In the analysis, we identified 11 studies involving 5,169 KD patients that reported the diagnostic performance of the Formosa score for IVIG-resistant risk (12, 13, 17, 30, 34, 40, 43–47). Figure 2 shows the overall performance of Formosa score: pooled sensitivity, 0.60 [95% confidence interval (CI), 0.48–0.70]; pooled specificity, 0.59 (95% CI, 0.50–0.68); and area under the summary receiver operating characteristic curve (SROC), 0.62, as illustrated in Figure 3.
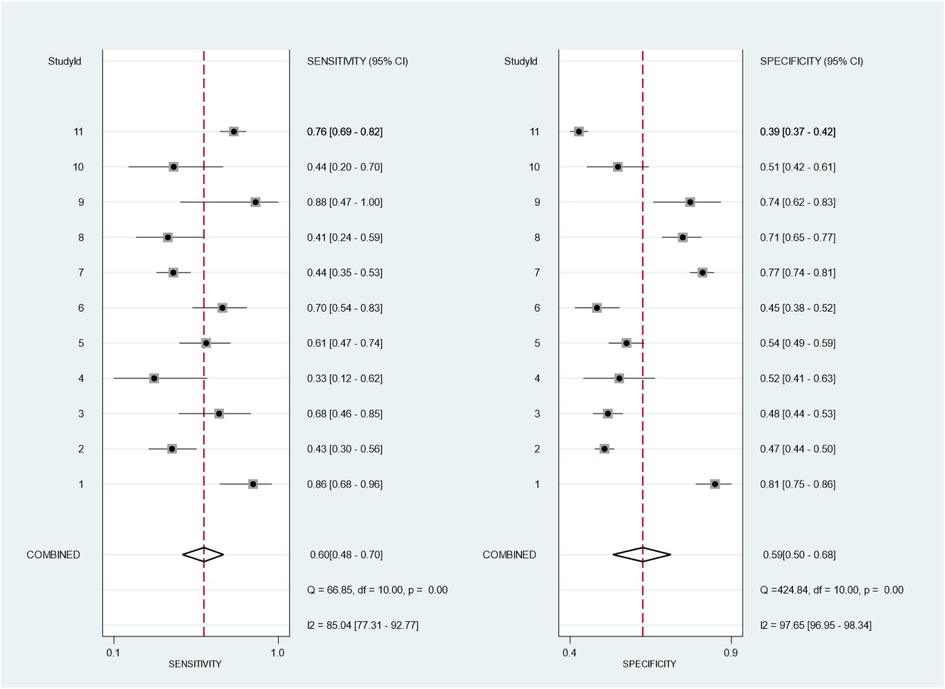
Figure 2. Bivariate meta-analysis of the Formosa score for pooled sensitivity and specificity of 11 included studies. The study ID is identified in Table 1A.
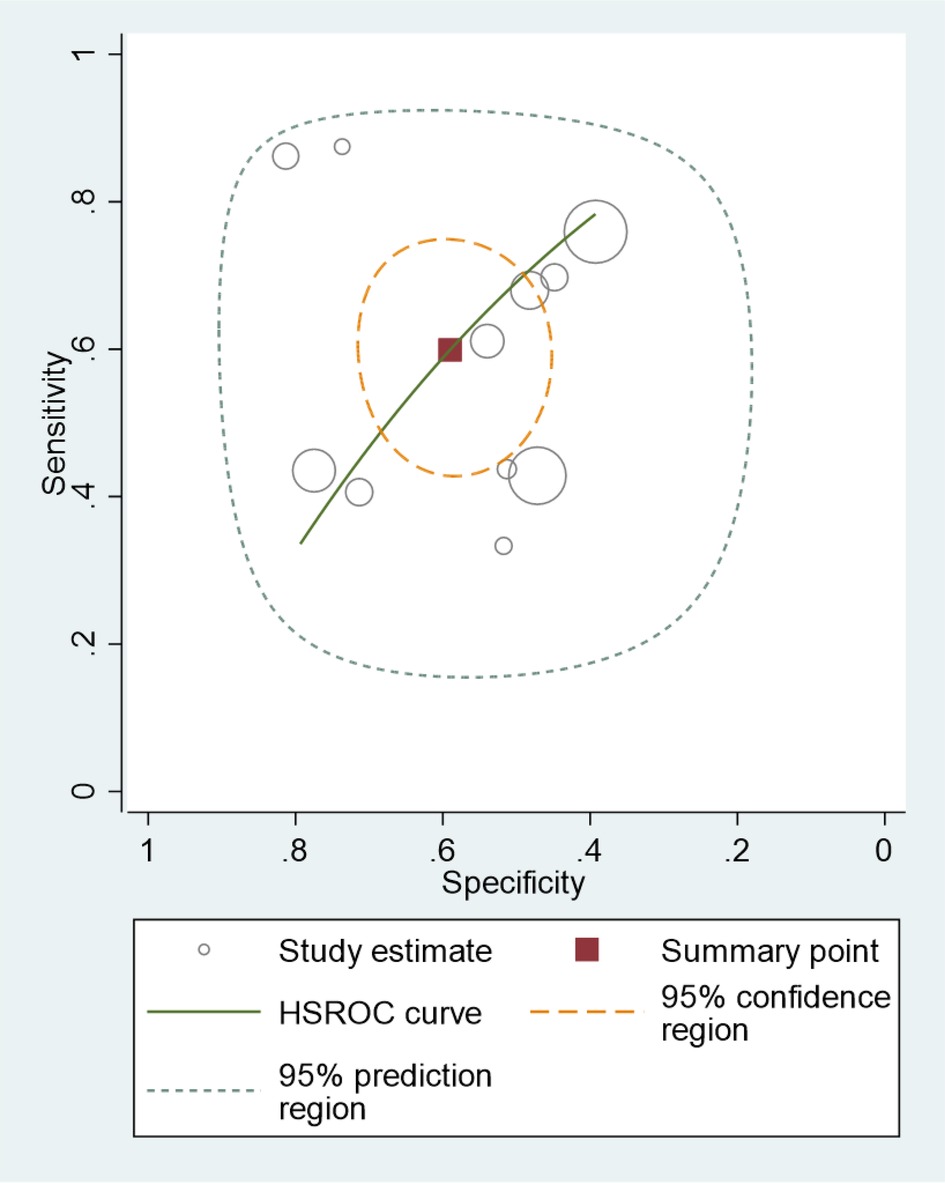
Figure 3. Hierarchical summary receiver operating curve (HSROC) of the sensitivity vs. the specificity of the performance of the Formosa score for predicting intravenous immunoglobulin resistance in Kawasaki disease patients. Each included study is represented by a circle; squares represent the summary test accuracy.
The potential sources of significantly statistical heterogeneity were IVIG-resistant rate, the definition of IVIG resistance, and the diagnostic criteria of KD. The factors that may explain the heterogeneity must be further evaluated by subgroup analysis (Table 2). The meta-regression suggested that the sensitivity and specificity of Asian studies (n = 7) were not significantly greater than that of the non-Asian studies (n = 4) (sensitivity in Asian, 0.65, with 95% CI, 0.53–0.78, and sensitivity in non-Asian, 0.48, with 95% CI, 0.30–0.67, p = 0.16; specificity in Asian, 0.61, with 95% CI, 0.50–0.72, and specificity in non-Asian, 0.55, with 95% CI, 0.40–0.71, p = 0.35). A trend of lower sensitivity in Turkey was also found (sensitivity in Turkey, 0.39, with 95% CI, 0.53–0.78, and sensitivity in non-Turkey, 0.66, with 95% CI, 0.30–0.67, p = 0.11). No significant difference was observed between subgroups according to ethics, diagnostic criteria, total scores, or IVIG-resistant rate, as shown in Table 2 (p > 0.05).
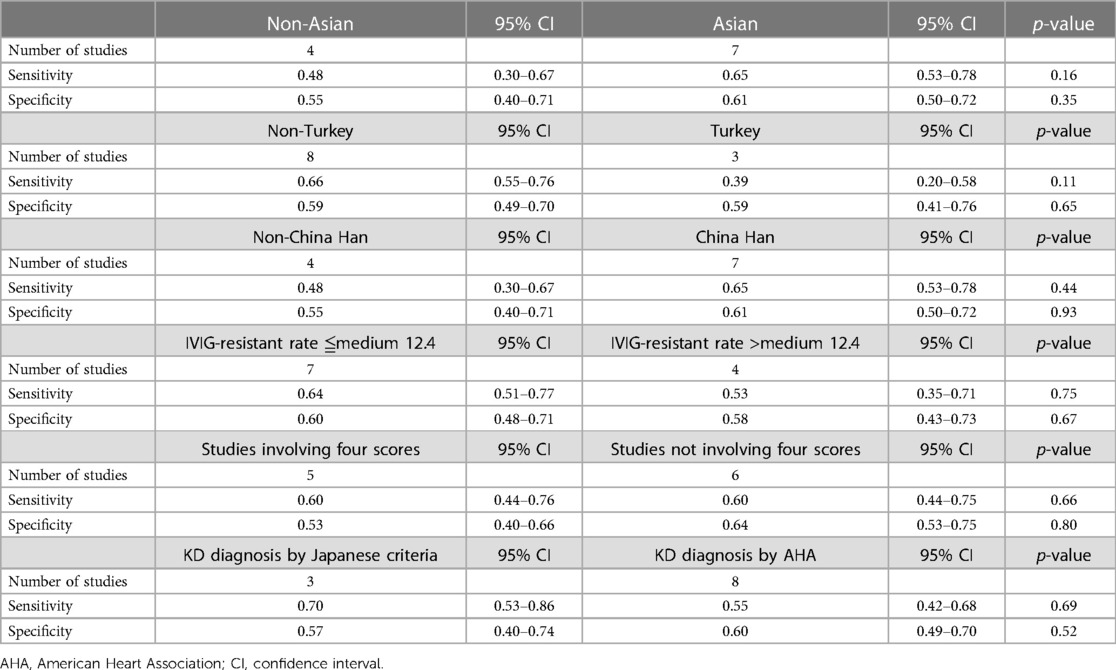
Table 2. Meta-regression results for the diagnostic performance of the Formosa score for predicting IVIG resistance.
3.4. Results of network meta-analysis of the four Asian scores
The network graph of the relationship between the four Asian scores and reference standard is shown in Figure 4. Of the children included in the network meta-analysis, 21,389 with a confirmed diagnosis of KD by the American Heart Association (AHA) or Japanese criteria were included in the comparison of sensitivity and specificity among the four Asian scores (15, 19). The current study enrolled 41 studies, and the results of the four scoring systems in predicting IVIG resistance are shown in Table 3. Based on the 41 studies, we suggest that the Formosa score has the highest sensitivity in predicting IVIG resistance among the four scores. The Formosa score has the lowest specificity. In contrast, Egami and Kobayashi score had high specificities.
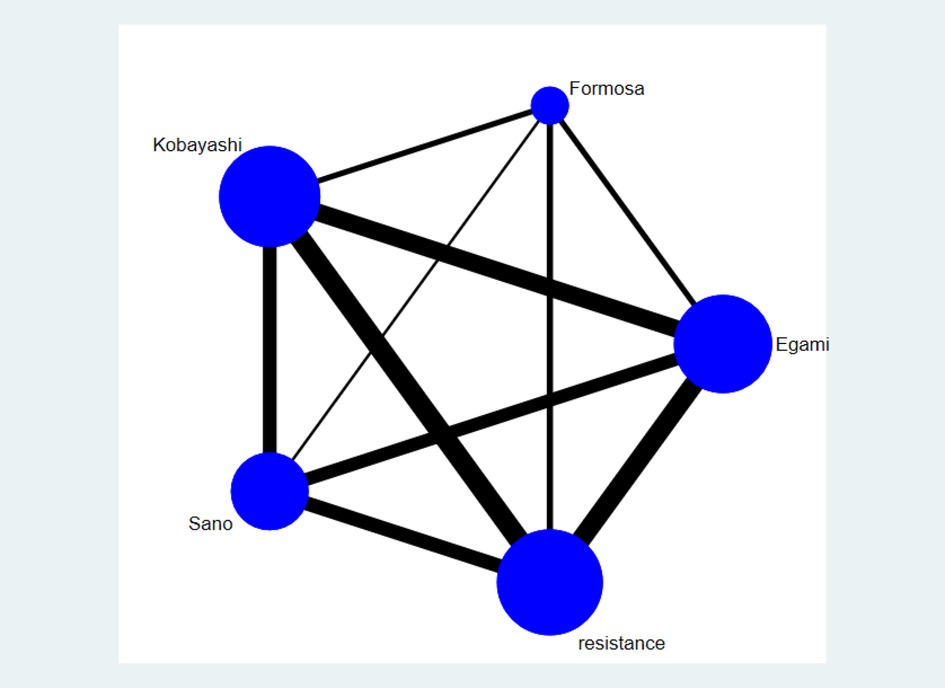
Figure 4. The network graph of the four scores only represents the comparisons of included studies. The size of the node represents the number of studies; the thickness of the line showing direct comparisons represents the number of studies. Resistance by the definition of each study is used as a reference standard.
3.5. Publication bias
Deeks’ tests revealed no significant publication bias among the included evaluation pooled results of the overall performance of Formosa score (p = 0.73), as shown in Figure 5. I2 results revealed significant between-study heterogeneity in the pooled sensitivities (I2 = 85.04%) and specificities of the Formosa score (I2 = 97.65%).
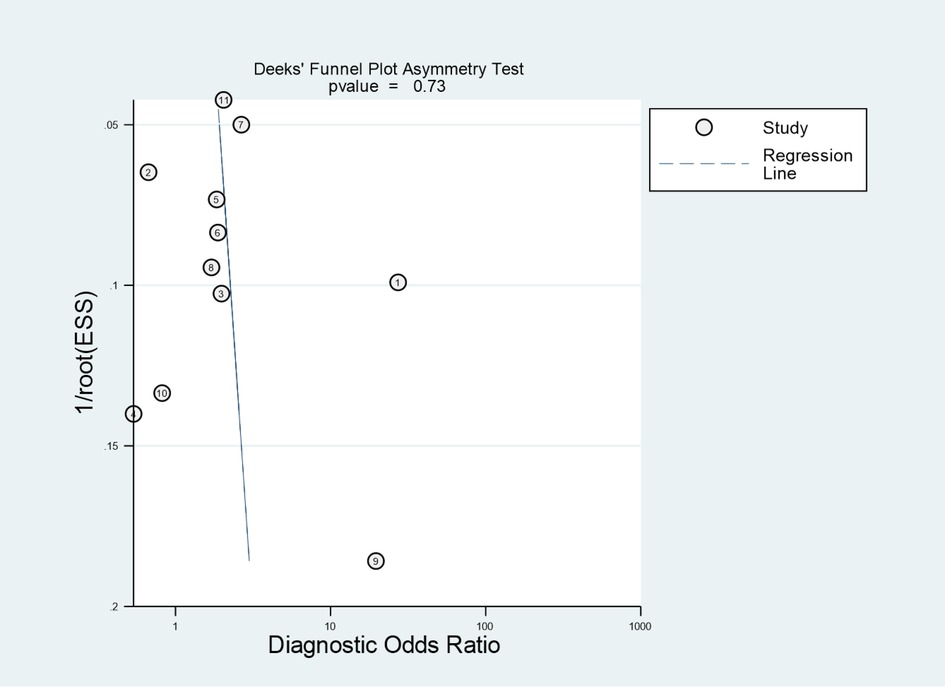
Figure 5. Deeks’ funnel plot identified potential publication bias of the eligible studies. ESS, effective sample size.
3.6. Quality of included studies
Nine studies (22.0%) had different numbers for each score, or the number of patients enrolled was not the same as the number of patients used to calculate the scores, so they introduced bias and resulted in an unclear flow and timing (Table 1 and Figure 6) (7, 17, 28, 48–53). The included studies listed the reference standard, and the KD patients received the same reference standard. No high concerns regarding applicability of index tests, reference standard, or patient selection were observed. Only a few studies adopted prospective designs (30, 33, 44). Therefore, enrolling a consecutive or random sample of patients was hard for retrospective studies. One study used a case–control design and produced an abnormally high IVIG-resistant rate (54). Regarding the Formosa score, researchers identified IVIG-resistant patients in the clinical data including physical examination (lymphadenopathy) and laboratory, while other scores did not adopt the use of the physical examination, which may influence the diagnostic accuracy of the index test (17).
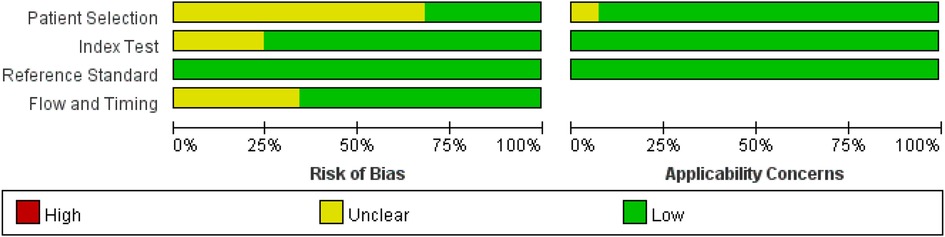
Figure 6. Bar graph for overall risk of bias and clinical applicability evaluated by the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool.
4. Discussion
To the best of our knowledge, this study is the first diagnostic meta-analysis to focus on comparing different scores for predicting IVIG resistance in KD patients. The identification of patients at high risk for IVIG resistance at the time of presentation is of significant benefit and may allow clinicians to identify those who may benefit from more intensive monitoring of their condition and who may require treatment modulation during the acute phase, with the potential addition of other anti-inflammatory agents to the conventional IVIG treatment to protect them from ongoing CAL. Many scoring systems have been used to predict the risk of IVIG resistance. However, the prediction efficacies of these scoring systems vary considerably. The results of five head-to-head studies suggested significant variations without consistent conclusion (30, 34, 40, 44, 46). Creating new scores for IVIG-resistant prediction is becoming an increasingly popular field of study. Since obtaining head-to-head evidence is difficult, diagnostic network meta-analyses are useful for incorporating direct and indirect comparisons with these scores (20).
In this diagnostic meta-analysis study, we evaluated the prediction efficacies of IVIG resistance through four existing scores, such as Egami, Formosa, Kobayashi, and Sano, based on their reported sensitivity and specificity in relation to clinical parameters of the risk of IVIG-resistant KD. This meta-analysis of 41 articles including 21,389 patients with KD showed that the Formosa score demonstrated the highest sensitivity in predicting IVIG resistance. The pooled sensitivity and specificity for the most commonly reported predicting tools (Egami, Formosa, Kawasaki, and Sano scores) ranged from 0.36 to 0.76 and from 0.46 to 0.83, respectively.
This bivariate network analysis faced some limitations. The meta-analysis estimated high heterogeneity for the Formosa score. The results of the Formosa score vary widely among different studies, with particularly high sensitivity in a Taiwanese study (AUC 0.84) and very low sensitivity in a Turkish study (Tables 1, 2) (12, 17, 34, 45, 46). Such a discrepancy implies that each region needs its own score, especially where the prevalence is high. The Formosa score has the potential to modify clinical practice and improve health outcomes if identifying a specific population improves the AUC. As the research on the Formosa score was conducted in China and Turkey, we could not apply our findings to other regions. The false-positive rate of the Formosa score was relatively high, so unnecessary medical intervention due to low specificity might occur. The Formosa score helped reduce unnecessary medications for patients responsive to IVIG when the score was negative.
Patients at high risk of IVIG resistance may receive adjunctive treatment to reduce coronary lesions and thus also cardiovascular morbidity. In the process of developing drugs, a good prediction score is necessary, and the Formosa score provides a good option of sensitivity to enroll more participants in trials since only 10%–20% of KD patients have IVIG resistance. More research is needed to analyze which group has a higher sensitivity and specificity of the Formosa score. Since the verification of many ethnic groups found that the Asian scoring systems were not applicable, many more accurate scoring systems have been developed (31). However, these prediction models showed unsatisfactory results when applied to Chinese, French, Iranian, Portuguese, Thai, and other populations (49, 51, 53, 55). Future network meta-analyses must also incorporate the accuracy of the new scores after new scores have undergone a certain degree of validation around the world.
5. Conclusion
Patients at high risk of IVIG resistance may receive adjunctive treatment to reduce coronary lesions and thus also cardiovascular morbidity. Among all of the included studies, we found that the Formosa score had the best sensitivity (0.76) but unsatisfactory specificity (0.46) for predicting IVIG resistance in Kawasaki disease.
Data availability statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding author.
Author contributions
W-NC and L-SC independently extracted data from each included study. W-NC and L-SC independently performed a systematic literature search. P-YH and L-SC adopted the revised Quality Assessment of Diagnostic Accuracy Studies to evaluate the methodological quality. H-CK and Y-HH supervised the study. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Chang Gung Memorial Hospital (CMRPG8M1451) and the Ministry of Science and Technology, Taiwan (MOST 110–2635–B–182A–004). However, these institutions had no role in the study design, data collection analysis, publication decision, or manuscript preparation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lin MT, Wu MH. The global epidemiology of Kawasaki disease: review and future perspectives. Glob Cardiol Sci Pract. (2017) 2017(3):e201720. doi: 10.21542/gcsp.2017.2029564341
2. Lei WT, Hsu CW, Chen PC, Tseng PT, Kuo HC, Guo MM, et al. Increased risk of asthma and allergic rhinitis in patients with a past history of Kawasaki disease: a systematic review and meta-analyses. Front Pediatr. (2021) 9:746856. doi: 10.3389/fped.2021.74685634988034
3. Huang PY, Huang YH, Guo MM, Chang LS, Kuo HC. Kawasaki disease and allergic diseases. Front Pediatr. (2020) 8:614386. doi: 10.3389/fped.2020.61438633490002
4. Chang LS, Guo MM, Yan JH, Huang YH, Lo MH, Kuo HC. Low FCMR mRNA expression in leukocytes of patients with Kawasaki disease six months after disease onset. Pediatr Allergy Immunol. (2020) 31(5):554–9. doi: 10.1111/pai.1323532073687
5. Chang LS, Chen YJ, Huang PY, Chen KD, Lo MH, Huang YH, et al. Significantly lower immunoglobulin M levels 6 months after disease onset in patients with Kawasaki disease with coronary artery lesions. J Am Heart Assoc. (2021) 10(12):e020505. doi: 10.1161/JAHA.120.02050534096327
6. Newburger JW, Takahashi M, Burns JC, Beiser AS, Chung KJ, Duffy CE, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. (1986) 315(6):341–7. doi: 10.1056/NEJM1986080731506012426590
7. Kobayashi T, Inoue Y, Takeuchi K, Okada Y, Tamura K, Tomomasa T, et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. (2006) 113(22):2606–12. doi: 10.1161/CIRCULATIONAHA.105.59286516735679
8. Tremoulet AH, Best BM, Song S, Wang S, Corinaldesi E, Eichenfield JR, et al. Resistance to intravenous immunoglobulin in children with Kawasaki disease. J Pediatr. (2008) 153(1):117–21. doi: 10.1016/j.jpeds.2007.12.02118571548
9. Rigante D, Andreozzi L, Fastiggi M, Bracci B, Natale MF, Esposito S. Critical overview of the risk scoring systems to predict non-responsiveness to intravenous immunoglobulin in Kawasaki syndrome. Int J Mol Sci. (2016) 17(3):278. doi: 10.3390/ijms1703027826927060
10. Lei WT, Chang LS, Zeng BY, Tu YK, Uehara R, Matsuoka YJ, et al. Pharmacologic interventions for Kawasaki disease in children: a network meta-analysis of 56 randomized controlled trials. EBioMedicine. (2022) 78:103946. doi: 10.1016/j.ebiom.2022.10394635306339
11. Chang LS, Kuo HC. The role of corticosteroids in the treatment of Kawasaki disease. Expert Rev Anti-Infect Ther. (2020) 18(2):155–64. doi: 10.1080/14787210.2020.171375231914832
12. Huang CN, Wu FF, Chang YM, Huang HC, Lin MT, Wang JK, et al. Comparison of risk scores for predicting intravenous immunoglobulin resistance in Taiwanese patients with Kawasaki disease. J Formos Med Assoc. (2021) 120(10):1884–9. doi: 10.1016/j.jfma.2020.12.01033358267
13. Fabi M, Andreozzi L, Corinaldesi E, Bodnar T, Lami F, Cicero C, et al. Inability of Asian risk scoring systems to predict intravenous immunoglobulin resistance and coronary lesions in Kawasaki disease in an Italian cohort. Eur J Pediatr. (2019) 178(3):315–22. doi: 10.1007/s00431-018-3297-530499051
14. Chang LS, Lin YJ, Yan JH, Guo MM, Lo MH, Kuo HC. Neutrophil-to-lymphocyte ratio and scoring system for predicting coronary artery lesions of Kawasaki disease. BMC Pediatr. (2020) 20(1):398. doi: 10.1186/s12887-020-02285-532838756
15. Egami K, Muta H, Ishii M, Suda K, Sugahara Y, Iemura M, et al. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr. (2006) 149(2):237–40. doi: 10.1016/j.jpeds.2006.03.05016887442
16. Sano T, Kurotobi S, Matsuzaki K, Yamamoto T, Maki I, Miki K, et al. Prediction of non-responsiveness to standard high-dose gamma-globulin therapy in patients with acute Kawasaki disease before starting initial treatment. Eur J Pediatr. (2007) 166(2):131–7. doi: 10.1007/s00431-006-0223-z16896641
17. Lin MT, Chang CH, Sun LC, Liu HM, Chang HW, Chen CA, et al. Risk factors and derived formosa score for intravenous immunoglobulin unresponsiveness in Taiwanese children with Kawasaki disease. J Formos Med Assoc. (2016) 115(5):350–5. doi: 10.1016/j.jfma.2015.03.01225910931
18. Salameh JP, Bossuyt PM, McGrath TA, Thombs BD, Hyde CJ, Macaskill P, et al. Preferred reporting items for systematic review and meta-analysis of diagnostic test accuracy studies (PRISMA-DTA): explanation, elaboration, and checklist. BMJ. (2020) 370:m2632. doi: 10.1136/bmj.m263232816740
19. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. (2017) 135(17):e927–99. doi: 10.1161/CIR.000000000000048428356445
20. Chang LS, Huang PY, Kuo HC, Tu YK, Tseng PT, Liang CS, et al. Diagnostic accuracy of the American College of Rheumatology-1997, the systemic lupus international collaborating clinics-2012, and the European league against rheumatism-2019 criteria for juvenile systemic lupus erythematosus: a systematic review and network meta-analysis. Autoimmun Rev. (2022):103144. doi: 10.1016/j.autrev.2022.10314435842200
21. Chan H, Chi H, You H, Wang M, Zhang G, Yang H, et al. Indirect-comparison meta-analysis of treatment options for patients with refractory Kawasaki disease. BMC Pediatr. (2019) 19(1):158. doi: 10.1186/s12887-019-1504-931101091
22. Nyaga VN, Aerts M, Arbyn M. ANOVA model for network meta-analysis of diagnostic test accuracy data. Stat Methods Med Res. (2018) 27(6):1766–84. doi: 10.1177/096228021666918227655805
23. Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. (2005) 58(9):882–93. doi: 10.1016/j.jclinepi.2005.01.01616085191
24. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-0000922007046
25. Park HM, Lee DW, Hyun MC, Lee SB. Predictors of nonresponse to intravenous immunoglobulin therapy in Kawasaki disease. Korean J Pediatr. (2013) 56(2):75–9. doi: 10.3345/kjp.2013.56.2.7523482814
26. Kim BY, Kim D, Kim YH, Ryoo E, Sun YH, Jeon IS, et al. Non-responders to intravenous immunoglobulin and coronary artery dilatation in Kawasaki disease: predictive parameters in Korean children. Korean Circ J. (2016) 46(4):542–9. doi: 10.4070/kcj.2016.46.4.54227482264
27. Berdej-Szczot E, Małecka-Tendera E, Gawlik T, Firek-Pędras M, Szydłowski L, Gawlik A. Risk factors of immunoglobulin resistance and coronary complications in children with Kawasaki disease. Kardiol Pol. (2017) 75(3):261–6. doi: 10.5603/KP.a2016.017927995598
28. Chbeir D, Gaschignard J, Bonnefoy R, Beyler C, Melki I, Faye A, et al. Kawasaki disease: abnormal initial echocardiogram is associated with resistance to IV Ig and development of coronary artery lesions. Pediatr Rheumatol Online J. (2018) 16(1):48. doi: 10.1186/s12969-018-0264-730021610
29. Fernandez-Cooke E, Barrios Tascón A, Sánchez-Manubens J, Antón J, Grasa Lozano CD, Aracil Santos J, et al. Epidemiological and clinical features of Kawasaki disease in Spain over 5 years and risk factors for aneurysm development. (2011–2016): KAWA-RACE study group. PloS One. (2019) 14(5):e0215665. doi: 10.1371/journal.pone.021566531107862
30. Shao S, Luo C, Zhou K, Hua Y, Wu M, Liu L, et al. The role of age-specific N-terminal pro-brain natriuretic peptide cutoff values in predicting intravenous immunoglobulin resistance in Kawasaki disease: a prospective cohort study. Pediatr Rheumatol Online J. (2019) 17(1):65. doi: 10.1186/s12969-019-0368-831533770
31. Tan XH, Zhang XW, Wang XY, He XQ, Fan C, Lyu TW, et al. A new model for predicting intravenous immunoglobin-resistant Kawasaki disease in Chongqing: a retrospective study on 5277 patients. Sci Rep. (2019) 9(1):1722. doi: 10.1038/s41598-019-39330-y30742060
32. Ha KS, Lee J, Lee KC. Prediction of intravenous immunoglobulin resistance in patients with Kawasaki disease according to the duration of illness prior to treatment. Eur J Pediatr. (2020) 179(2):257–64. doi: 10.1007/s00431-019-03474-w31713683
33. Ishikawa T, Wada Y, Namba H, Kawai T. Hepcidin in Kawasaki disease: upregulation by acute inflammation in patients having resistance to intravenous immunoglobulin therapy. Clin Rheumatol. (2021) 40(12):5019–24. doi: 10.1007/s10067-021-05822-434148165
34. Kaya Akca U, Arslanoglu Aydin E, Aykan HH, Serin O, Sag E, Demir S, et al. Comparison of IVIG resistance predictive models in Kawasaki disease. Pediatr Res. (2022) 91(3):621–6. doi: 10.1038/s41390-021-01459-w33753891
35. Amano Y, Akazawa Y, Yasuda J, Yoshino K, Kojima K, Kobayashi N, et al. A low-frequency IL4R locus variant in Japanese patients with intravenous immunoglobulin therapy-unresponsive Kawasaki disease. Pediatr Rheumatol Online J. (2019) 17(1):34. doi: 10.1186/s12969-019-0337-231269967
36. Tajima M, Shiozawa Y, Kagawa J. Early appearance of principal symptoms of Kawasaki disease is a risk factor for intravenous immunoglobulin resistance. Pediatr Cardiol. (2015) 36(6):1159–65. doi: 10.1007/s00246-015-1136-225753685
37. Yoshikane Y, Okuma Y, Miyamoto T, Hashimoto J, Fukazawa R, Kato T, et al. Serum tenascin-C predicts resistance to steroid combination therapy in high-risk Kawasaki disease: a multicenter prospective cohort study. Pediatr Rheumatol Online J. (2021) 19(1):82. doi: 10.1186/s12969-021-00562-w34090475
38. Kobayashi T, Inoue Y, Morikawa A. Risk stratification and prediction of resistance to intravenous immunoglobulin in Kawasaki disease. Nihon Rinsho. (2008) 66(2):332–7.18260333
39. Vágó I, Guóth G, Simon G, Szabó H. The predictive value of the Kobayashi and Kawanet score systems regarding immunoglobulin resistance and cardiac complications in patients with Kawasaki disease: a pilot study]. Orv Hetil. (2021) 162(47):1885–90. doi: 10.1556/650.2021.32270
40. Liu HH, Chen WX, Niu MM, Jiang Q, Qiu Z, Fan GZ, et al. A new scoring system for coronary artery abnormalities in Kawasaki disease. Pediatr Res. (2021):1–9. doi: 10.1038/s41390-021-01752-8
41. Takeshita S, Kanai T, Kawamura Y, Yoshida Y, Nonoyama S. A comparison of the predictive validity of the combination of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio and other risk scoring systems for intravenous immunoglobulin (IVIG)-resistance in Kawasaki disease. PLoS One. (2017) 12(5):e0176957. doi: 10.1371/journal.pone.017695728542183
42. Seki M, Kobayashi T, Kobayashi T, Morikawa A, Otani T, Takeuchi K, et al. External validation of a risk score to predict intravenous immunoglobulin resistance in patients with Kawasaki disease. Pediatr Infect Dis J. (2011) 30(2):145–7. doi: 10.1097/INF.0b013e3181f386db20802375
43. Song R, Yao W, Li X. Efficacy of four scoring systems in predicting intravenous immunoglobulin resistance in children with Kawasaki disease in a children’s hospital in Beijing, north China. J Pediatr. (2017) 184:120–4. doi: 10.1016/j.jpeds.2016.12.01828043682
44. Qian W, Tang Y, Yan W, Sun L, Lv H. A comparison of efficacy of six prediction models for intravenous immunoglobulin resistance in Kawasaki disease. Ital J Pediatr. (2018) 44(1):33. doi: 10.1186/s13052-018-0475-z29523168
45. Arslanoglu Aydin E, Ertugrul I, Bilginer Y, Batu ED, Sonmez HE, Demir S, et al. The factors affecting the disease course in Kawasaki disease. Rheumatol Int. (2019) 39(8):1343–9. doi: 10.1007/s00296-019-04336-231139951
46. Öztarhan K, Varlı YZ, Aktay Ayaz N. Usefulness of Kawasaki disease risk scoring systems to the Turkish population. Anatol J Cardiol. (2020) 24(2):97–106. doi: 10.14744/AnatolJCardiol.2020.37560
47. Wang T, Liu G, Lin H. A machine learning approach to predict intravenous immunoglobulin resistance in Kawasaki disease patients: a study based on a southeast China population. PLoS One. (2020) 15(8):e0237321. doi: 10.1371/journal.pone.0237321 32853226
48. Arane K, Mendelsohn K, Mimouni M, Mimouni F, Koren Y, Brik Simon D, et al. Japanese Scoring systems to predict resistance to intravenous immunoglobulin in Kawasaki disease were unreliable for Caucasian Israeli children. Acta Paediatrica. (2018) 107(12):2179–84. doi: 10.1111/apa.1441829797463
49. Edraki MR, Mohammadi H, Mehdizadegan N, Ghorashi M, Amoozgar H, Borzouee M, et al. Japanese Kawasaki disease scoring systems: are they applicable to the Iranian population? Arch Iran Med. (2020) 23(1):31–6.31910632
50. Kanamitsu K, Kakimoto H, Shimada A, Nakata Y, Ochi H, Watanabe H, et al. Verification of risk scores to predict i.v. Immunoglobulin resistance in incomplete Kawasaki disease. Pediatrics international: official journal of the Japan Pediatric Society. (2016) 58(2):146–51. doi: 10.1111/ped.1275526190225
51. Piram M, Darce Bello M, Tellier S, Di Filippo S, Boralevi F, Madhi F, et al. Defining the risk of first intravenous immunoglobulin unresponsiveness in non-Asian patients with Kawasaki disease. Sci Rep. (2020) 10(1):3125. doi: 10.1038/s41598-020-59972-732080307
52. Sleeper LA, Minich LL, McCrindle BM, Li JS, Mason W, Colan SD, et al. Evaluation of Kawasaki disease risk-scoring systems for intravenous immunoglobulin resistance. J Pediatr. (2011) 158(5):831–5.e3. doi: 10.1016/j.jpeds.2010.10.03121168857
53. Faim D, Henriques C, Brett A, Francisco A, Rodrigues F, Pires A. Kawasaki disease: predictors of resistance to intravenous immunoglobulin and cardiac complications. Arq Bras Cardiol. (2021) 116(3):485–91. doi: 10.36660/abc.2019075833470332
54. Shin J, Lee H, Eun L. Verification of current risk scores for Kawasaki disease in Korean children. J Korean Med Sci. (2017) 32(12):1991–6. doi: 10.3346/jkms.2017.32.12.199129115081
Keywords: diagnosis, Egami score, Formosa score, Kawasaki disease, Kobayashi score, meta-analysis, Sano score
Citation: Chiang W-N, Huang P-Y, Kuo H-C, Huang Y-H and Chang L-S (2023) Evaluation of Formosa score and diagnostic sensitivity and specificity of four Asian risk scores for predicting intravenous immunoglobulin resistance in Kawasaki disease: a bivariate meta-analysis. Front. Cardiovasc. Med. 10:1164530. doi: 10.3389/fcvm.2023.1164530
Received: 13 February 2023; Accepted: 25 May 2023;
Published: 12 June 2023.
Edited by:
Xiangbin Pan, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaReviewed by:
Peng Hu, First Affiliated Hospital of Anhui Medical University, ChinaKai-Sheng Hsieh, China Medical University, Taiwan
Avinash Sharma, Rajendra Prasad Government Medical College, India
© 2023 Chiang, Huang, Kuo, Huang and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling-Sai Chang am95Y2Vqb2huc3lva29AZ21haWwuY29t
 Wan-Ni Chiang1
Wan-Ni Chiang1 Po-Yu Huang
Po-Yu Huang Ying-Hsien Huang
Ying-Hsien Huang Ling-Sai Chang
Ling-Sai Chang