- 1Department of Cardiology, Shanxi Provincial People’s Hospital, Taiyuan, China
- 2Department of Cardiology, The First People’s Hospital of Jinzhong, Jinzhong, China
- 3Department of Cardiology, Yantai Yeda Hospital, Yantai, China
- 4Department of Cardiology, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Taiyuan, China
- 5Department of Cardiology, The First Hospital of Shanxi Medical University, Taiyuan, China
- 6Department of Cardiology, Shanxi Cardiovascular Hospital, Taiyuan, China
Background: Coronary heart disease (CHD) is one of the leading causes of mortality in the world. Although the traditional risk factors for CHD have been identified, it seems that there are still many CHD cases without these factors. Previous studies have hypothesized that Helicobacter pylori (H. pylori) infection was associated with the risk of CHD.
Objective: The association between H. pylori infection and the risk of CHD was studied using a systematic evaluation and meta-analysis method.
Methods: In order to find relevant studies, four electronic databases were systematically searched until August 2021. According to the inclusion and exclusion criteria, studies were screened and data were extracted. Under the random-effects or the fixed-effects model, the odds ratio (OR) and 95% confidence interval (95% CI) were combined. All analyses were conducted using Review Manager software (RevMan 5.4).
Results: Among the included studies, 2 studies were analyzed for H. pylori stool antigen test, 2 studies were analyzed for H. pylori histological staining test, 13 studies were analyzed for the anti-CagA test, and 38 studies were analyzed for the anti-H. pylori IgG test. The pooled results revealed that positive anti-H. pylori IgG was significantly associated with an increased risk of CHD (OR, 1.58; 95% CI: 1.34–1.87). Similarly, positive anti-CagA, positive H. pylori stool antigen, and positive H. pylori histological staining were significantly associated with the development of CHD with (OR: 1.33, 95% CI: 1.16–1.53), (OR: 3.50, 95% CI: 1.60–7.66), and (OR: 1.78, 95% CI: 1.12–2.83), respectively.
Conclusion: This meta-analysis showed that H. pylori infection increased the risk of CHD. However, more studies are needed to further investigate whether early eradication of H. pylori may reduce the morbidity of CHD.
Introduction
Helicobacter pylori (H. pylori), a gram-negative bacterium, is one of the common infections in human. More than the half of population in the world suffers from the infection (1). H. pylori infection causes a wide range of gastrointestinal diseases including chronic gastritis, gastric cancer, and duodenal ulcer (2, 3). Moreover, researchers have also recently found that H. pylori infection was closely related to atherosclerotic cardiovascular diseases, including coronary heart disease (CHD), peripheral arterial disease, and stroke (4–7).
CHD is the most common type of organ disease caused by atherosclerosis, the leading cause of mortality in many countries (8). The etiology and pathogenesis of CHD have not been fully understood until now. The classical risk factors, including diabetes, hypertension, obesity, smoking, dyslipidemia, socioeconomic status, and family history, cannot fully explain all causes (9). Chronic inflammation caused by chronic infection, such as H. pylori infection, plays an important role in the pathogenesis of CHD. Some studies have shown that H. pylori infection increased the risk of CHD (10, 11). At the same time, other studies have shown that H. pylori infection was not closely related to CHD (12, 13). Previous meta-analyses have also provided evidence for or against the relationship between H. pylori infection and the risk of CHD (14, 15).
Previous studies have been controversial, even from earlier published meta-analyses with no clear final conclusions. Therefore, we conducted a large-scale systematic review and meta-analysis to establish specific evidence about the relationship between H. pylori infection and the risk of CHD.
Materials and Methods
This meta-analysis strictly followed the recommendations of the systematic review and meta-analysis (PRISMA) list (16) of the preferred reporting items.
Literature Search
We searched four electronic databases including Web of Science, Embase, PubMed, and Cochrane Library until August 2021. We used the following search string: “ischemic heart disease (IHD)” OR “coronary heart disease (CHD)” OR “coronary artery disease (CAD)” OR “coronary atherosclerosis” OR “angina” OR “unstable angina (UA)” OR “acute myocardial infarction (AMI)” OR “Acute coronary syndrome (ACS)” OR “myocardial infarction (MI)” OR “atheroma” AND “Helicobacter” OR “Helicobacter pylori” OR “Campylobacter pylori” OR “H. pylori.” The references in the included studies were checked, and suitable studies were identified.
Literature Selection and Data Extraction
Eligible studies that reported the relationship between H. pylori infection and the risk of CHD were included in this meta-analysis. In addition to studies with unreliable data, we excluded abstract-only articles, book chapters, conference papers, theses, reviews, letters, editorials, and posters. There were no restrictions for included studies on publication year, language, place, or demographics of patients. Any discrepancy in the screening step was agreed by two reviewers. If necessary, a third reviewer was consulted. Then, the full-text screening was carried out to identify the related studies for data extraction. The extracted data included the following: the first author, publication year, category of CHD, country, settings, study design, sample size, agent, and adjustment status.
Quality and Assessment
All studies were evaluated using the modified Newcastle-Ottawa Scale (NOS). This scoring system assessed studies according to the comparability of groups, patient selection, and assessment outcomes. When an article scored >7 points in this scoring system, it was considered a high-quality article.
Statistical Analysis
The most adjusted hazard ratio (HR) or odds ratio (OR) with 95% confidence interval (CI) was extracted and combined into an equivalent measure which is expressed in the form of combined OR. The original data was extracted and used to calculate the original OR when a study did not contain the most adjusted HR or OR with 95% CI. Heterogeneity among studies was assessed by using the I-statistic (I2) test (17). When there was substantial heterogeneity (I2 > 50%), the random-effects model was used. Otherwise, the fixed-effects model was selected (18). In order to test the robustness of the results, the sensitivity analysis was evaluated by excluding one study at a time (19). In addition, several subgroup meta-analyses were performed based on study design, setting, adjustment status, quality assessment score, category of CHD, and country. All analyses were conducted using Review Manager (RevMan 5.4), and a p-value of less than 0.05 was regarded as statistically significant.
Results
Search Results
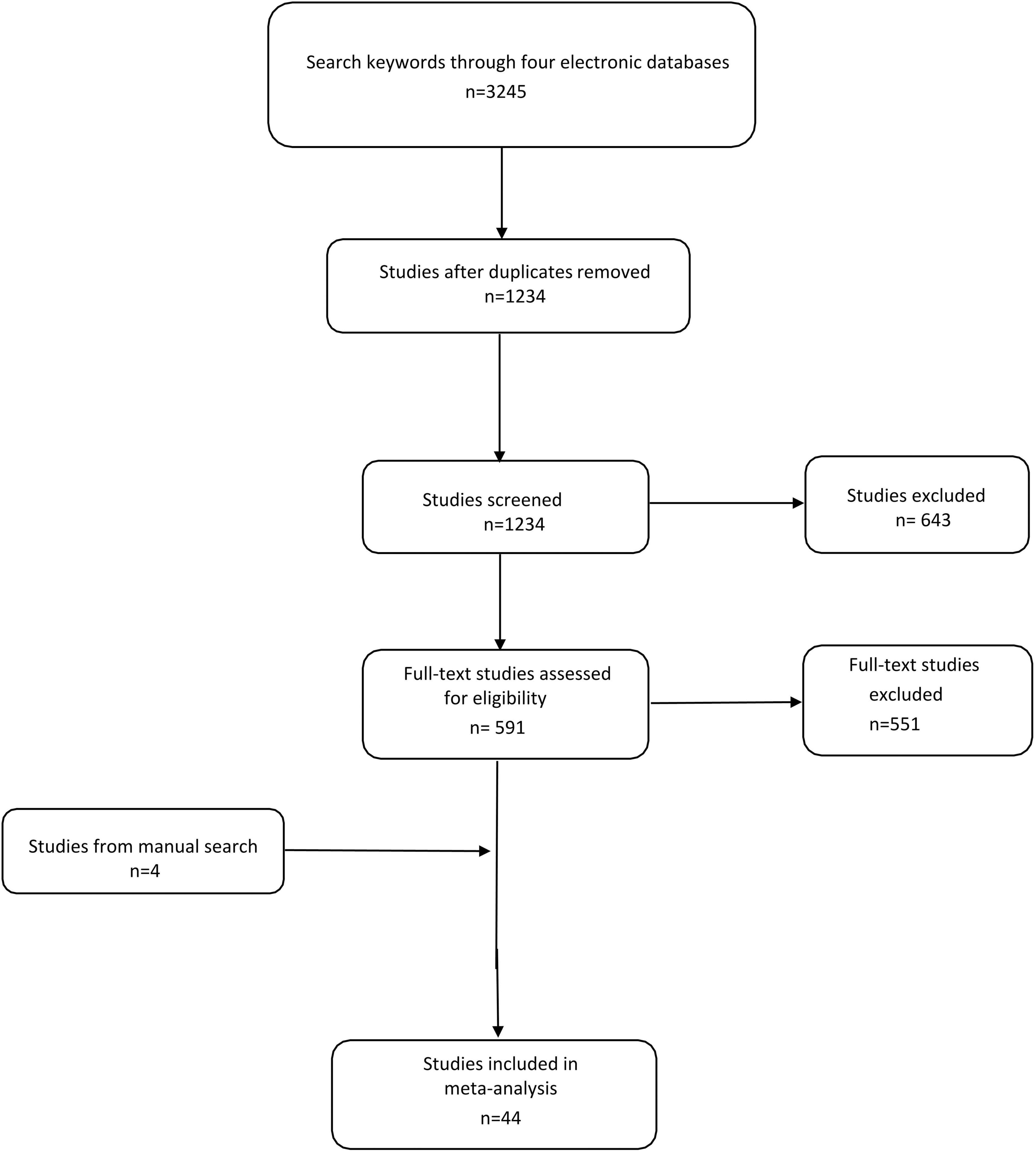
Using predefined keywords, 3,245 studies were identified from four electronic databases. After excluding duplicate studies, 1,234 studies were included and screened, of which 643 were deleted by title and abstract. We screened the remaining 591 studies and excluded 551 studies according to the exclusion criteria. In addition, the manual search yielded 4 other studies. We then extracted useful data from 44 qualified studies (20–63) (Figure 1).
Characteristics of the Included Studies
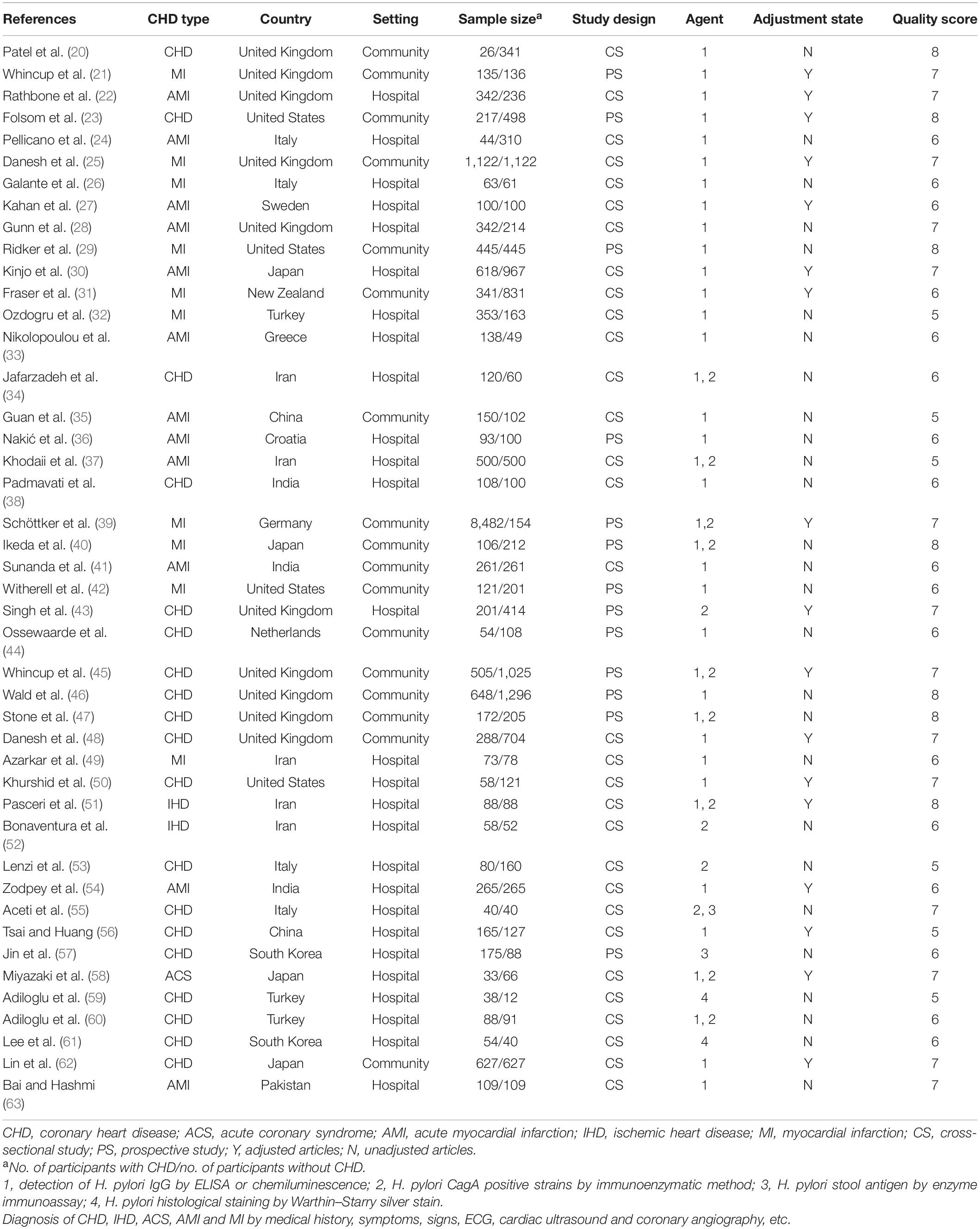
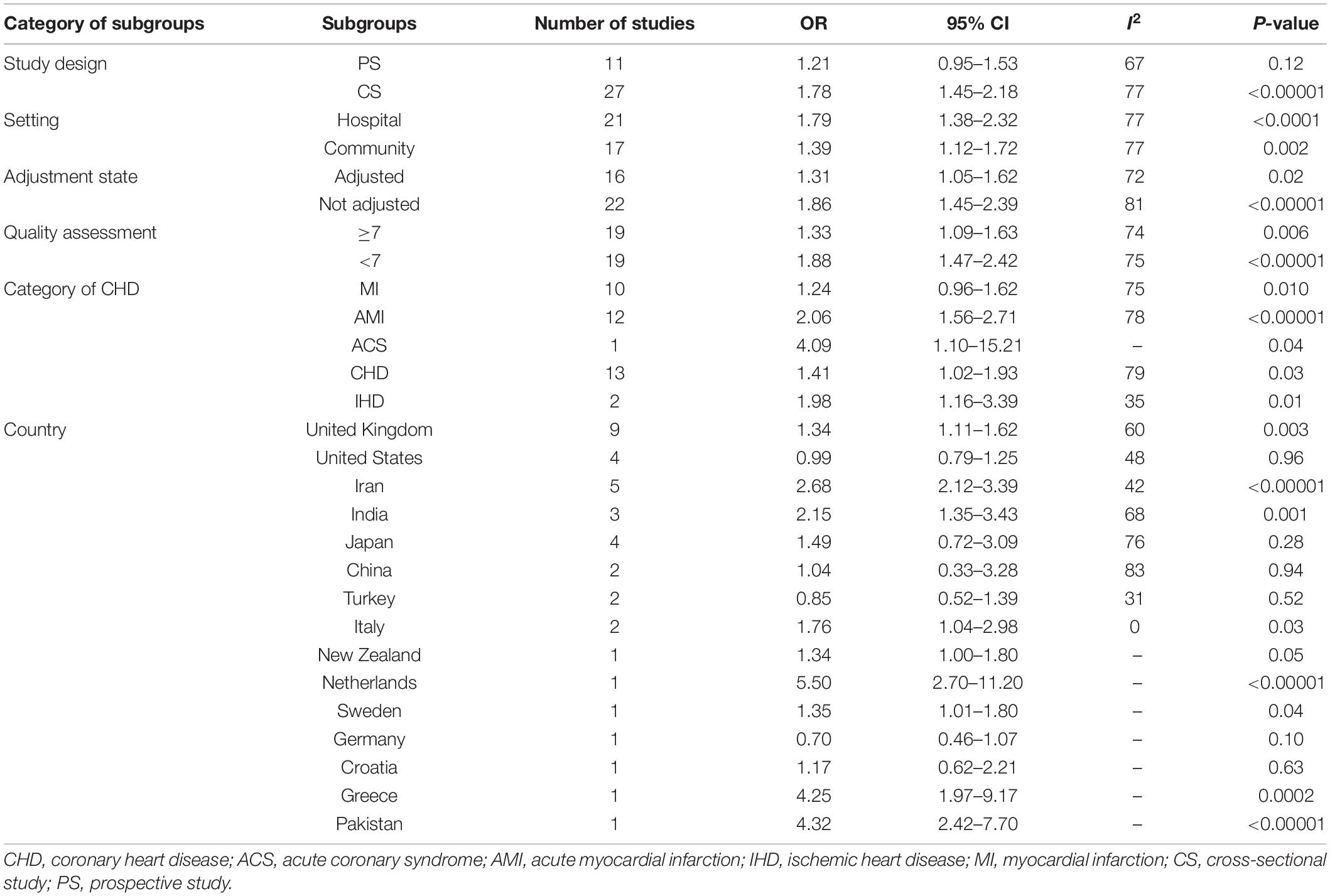
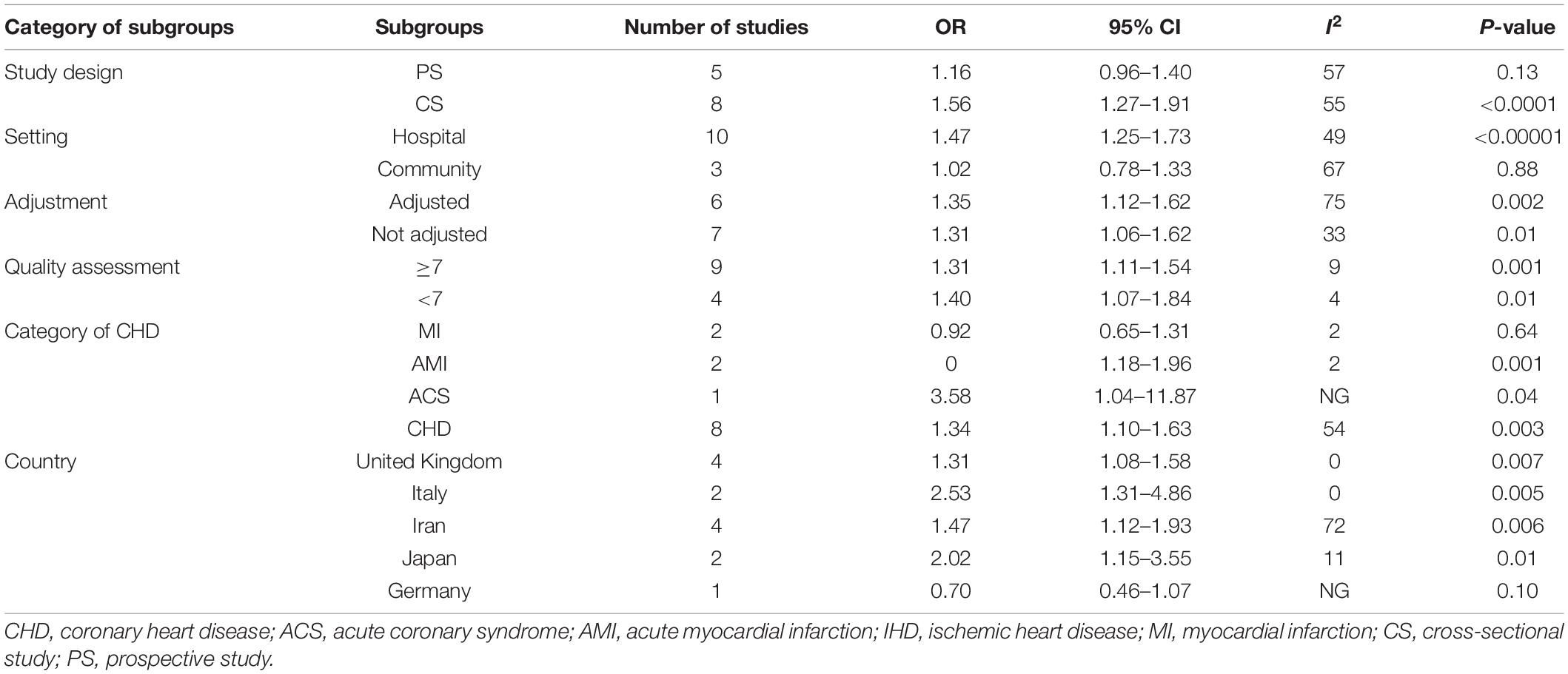
Among the included studies, 13 studies were prospective studies with a total sample size of 16,236 participants. Thirty-one studies were cross-sectional studies with 14,689 participants. Included studies were published between 1995 and 2017. The number of included studies per type of CHD were as follows: 19 studies on CHD, 12 studies on AMI, 10 studies on MI, 2 studies on IHD, and 1 study on ACS. Ten studies were conducted in the United Kingdom, 5 in Iran, 4 in the United States, 4 in Italy, 4 in Japan, 3 in India, 3 in Turkey, 2 in China, 2 in South Korea, 1 in New Zealand, 1 in the Netherlands, 1 in Sweden, 1 in Germany, 1 in Greece, 1 in Pakistan, and 1 in Croatia. We found 27 studies that were conducted in hospital-based settings and 17 studies conducted in a community-based setting. H. pylori detection method included anti-H. pylori IgG, anti-CagA, H. pylori stool antigen, and H. pylori histological staining. According to the adjustment status, there were 17 adjusted studies and 27 unadjusted studies. Moreover, the included studies were divided into 21 studies with ≥7 points and 23 studies with <7 points according to the quality score (Table 1).
Main Results
We revealed the relationship between the risk of CHD and H. pylori infection by using different H. pylori detection methods.
Anti-Helicobacter pylori IgG Test and Coronary Heart Disease
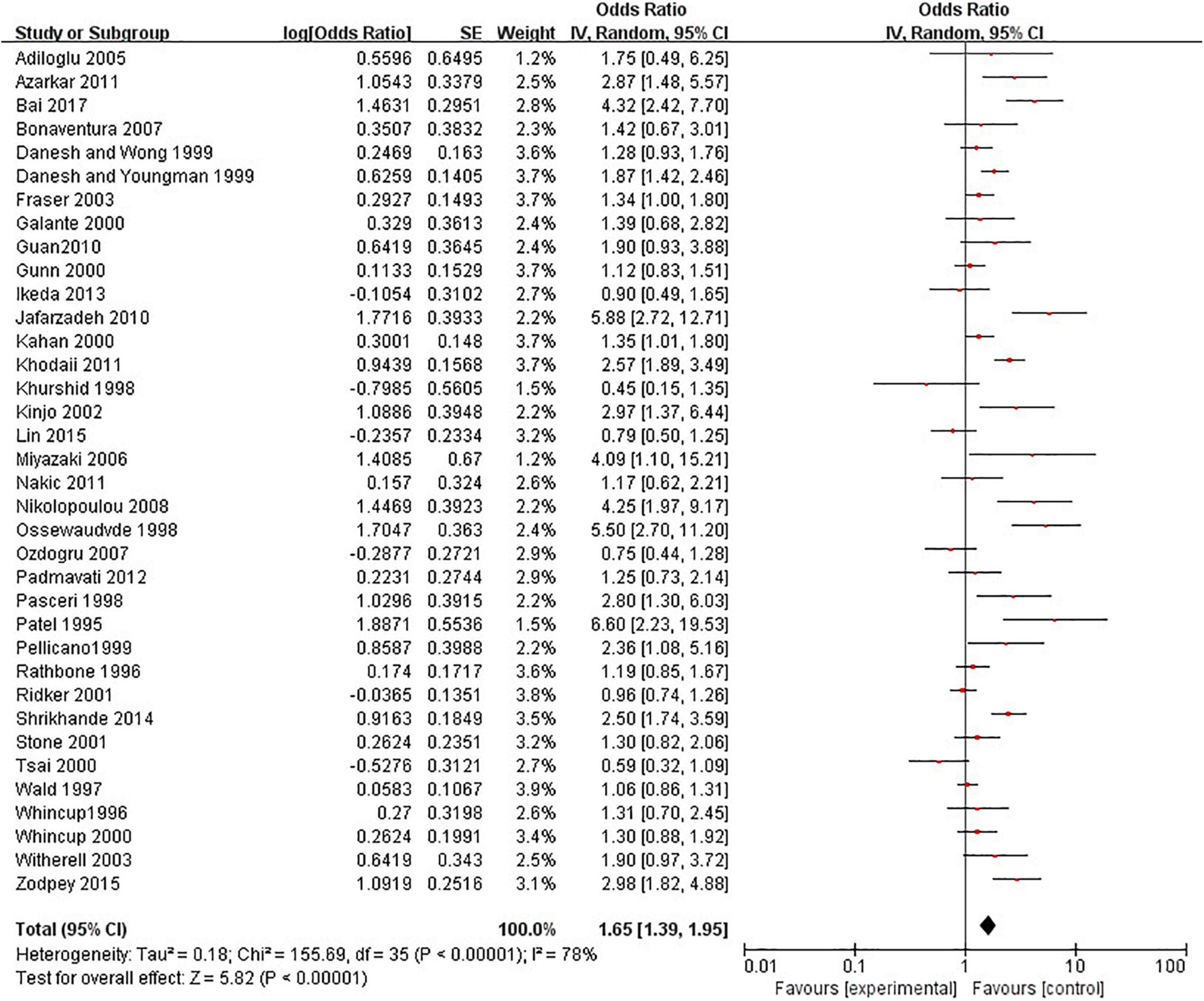
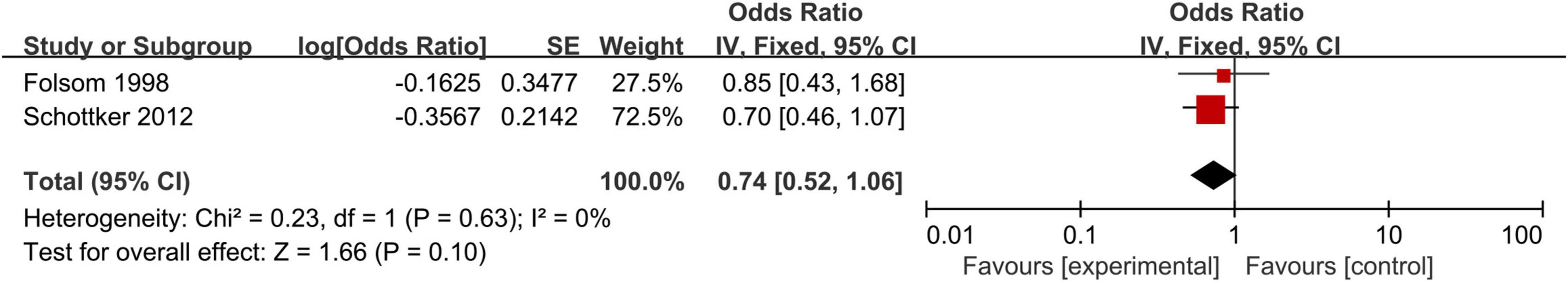
A meat-analysis of 38 studies, of which 2 studies used HR and 36 studies used OR, indicated a statistically significant relationship between the risk of CHD and positive anti-H. pylori IgG (OR, 1.58; 95% CI: 1.34–1.87; Figure 2). In addition, the meta-analysis of studies reporting OR also showed a statistically significant relationship (OR, 1.65; 95% CI: 1.39–1.95; Figure 3). However, the meta-analysis of studies reporting HR showed a statistically non-significant relationship (HR 0.74; 95% CI: 0.52–1.06; Figure 4). Subgroup analyses based on study design, setting, adjustment, quality assessment score, the category of CHD, and country are presented in Table 2. A leave-one-out sensitivity analysis showed robust results, and none of the studies had a significant impact on the pooled results.
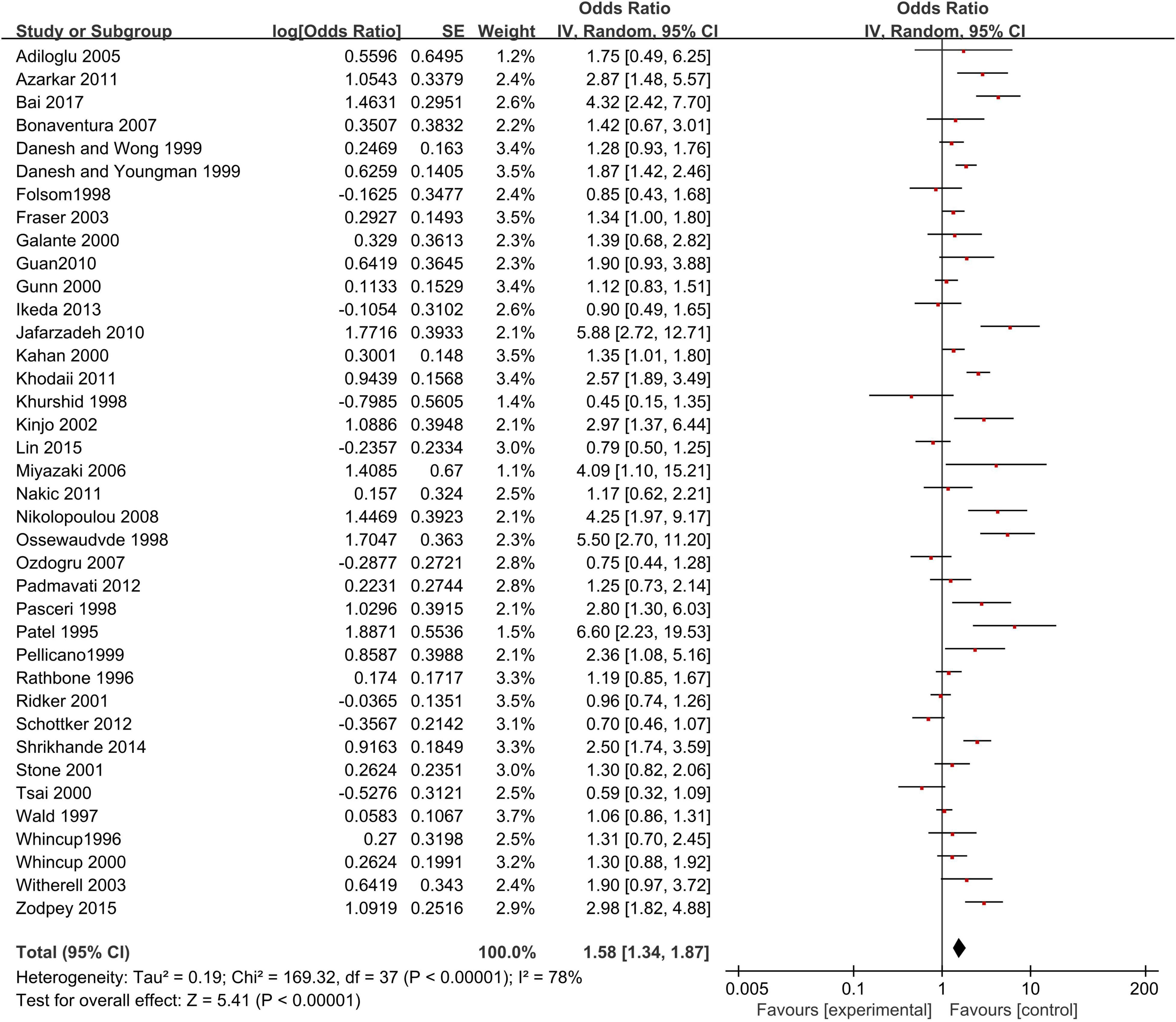
Figure 2. Relationship anti-H. pylori IgG test and coronary heart disease [CHD; studies reporting OR + studies reporting hazard ratio (HR)].
Anti-CagA Test and Coronary Heart Disease
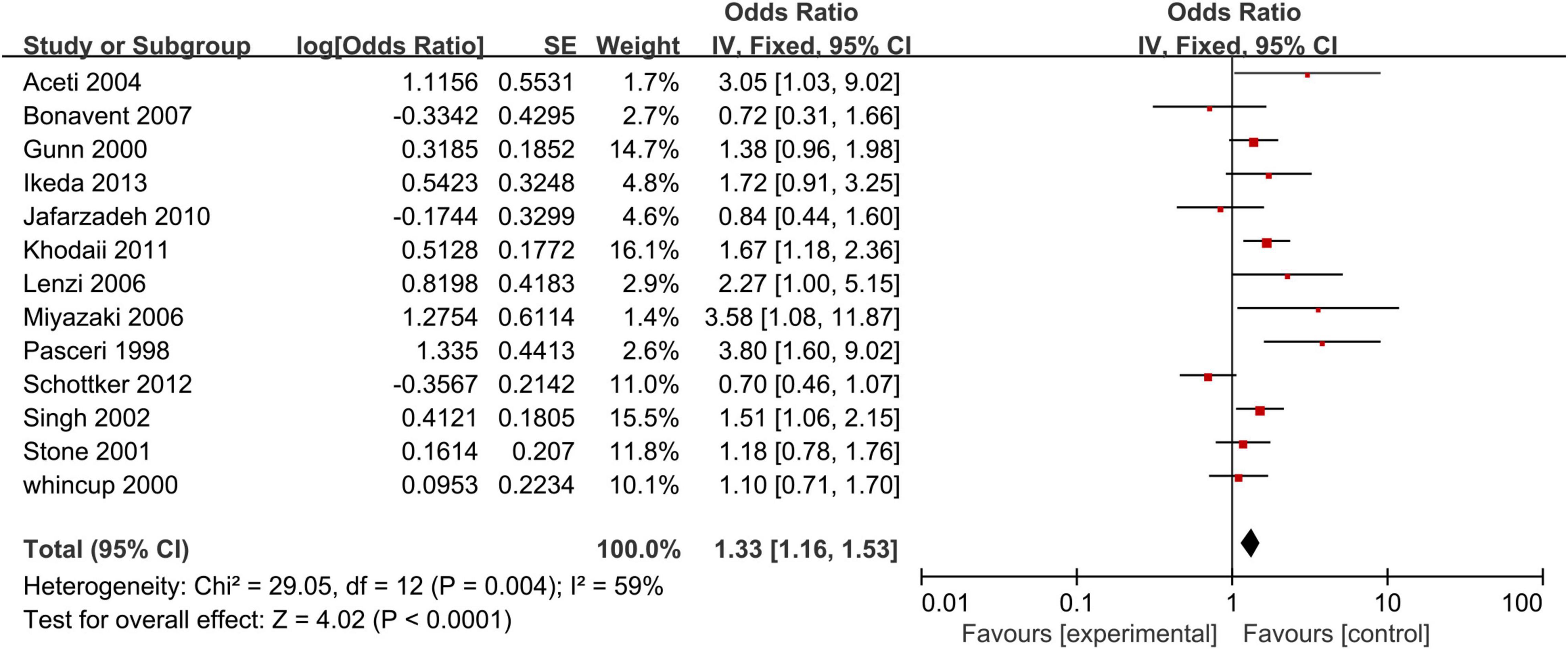
Our analysis of 13 studies showed a significant correlation between the risk of CHD and positive anti-CagA (OR, 1.33; 95% CI: 1.16–1.53; Figure 5). One study was reported using an adjusted result. Subgroup analyses based on study design, setting, adjustment, quality assessment score, the category of CHD, and country are presented in Table 3. A leave-one-out sensitivity analysis also indicated that the results are robust, and none of the studies had a significant influence on the overall results.
Helicobacter pylori Stool Antigen Test and Coronary Heart Disease
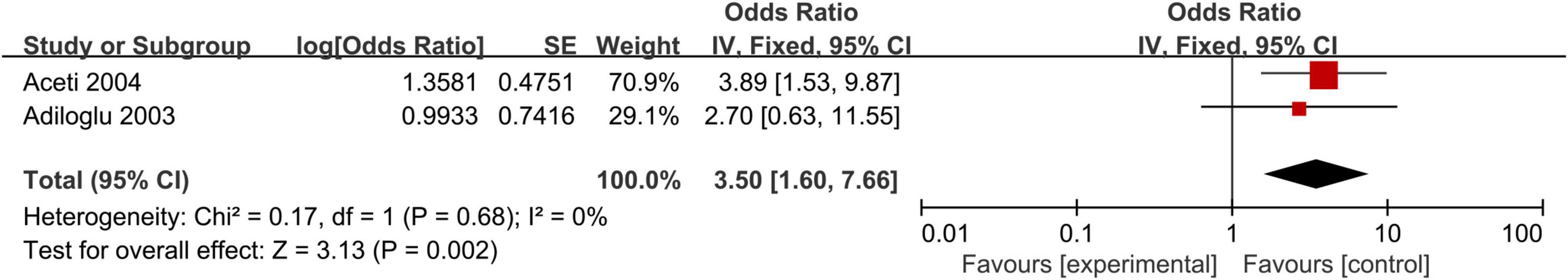
We observed a statistically significant association between positive H. pylori stool antigen and the development of CHD (OR, 3.50; 95% CI: 1.60–7.66; Figure 6). Because of the lack of data, subgroup meta-analyses and sensitivity analysis could not be conducted for H. pylori stool antigen.
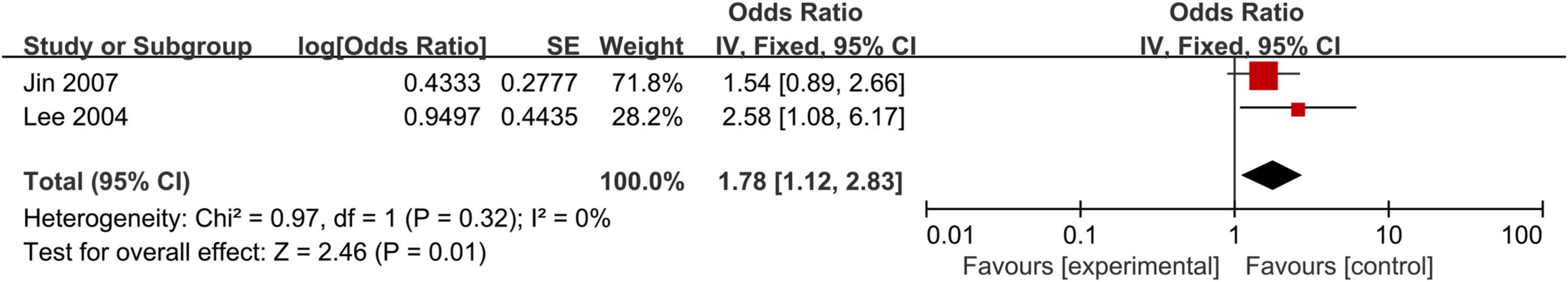
Helicobacter pylori Histological Staining Test and Coronary Heart Disease
We also observed that positive H. pylori histological staining was significantly associated with the risk of CHD (OR, 1.78; 95% CI: 1.12–2.83; Figure 7). Due to limited data, subgroup meta-analyses and sensitivity analysis of H. pylori histological staining also could not be performed.
Discussion
Over the past 30 years, there has been controversy in the literature about the impact of H. pylori infection on the risk of CHD. Therefore, we conducted this updated meta-analysis based on all available studies in order to establish a more comprehensive and stronger analysis. The final results of our meta-analysis showed that the positive anti-H. pylori IgG was closely related to the risk of CHD. In addition, this relation was also significant in analysis of positive anti-CagA, positive H. pylori stool antigen, and positive H. pylori histological staining.
Earlier studies showed that there may be a weak association between H. pylori infection and the risk of CHD (64, 65). Currently, our results are similar to those of recent meta-analyses (4, 15, 66–68) in which positive anti-H. pylori IgG was positively related to the risk of CHD. However, our study avoided the main limitations of these meta-analyses. For example, the meta-analyses performed by Wang et al. (4), Liu et al. (66), and Rahmani et al. (67) only involved myocardial infarction and did not mention other types of CHD. A meta-analysis (67) conducted in 2017 showed that H. pylori infection was associated with an increased risk of CHD, but the meta-analysis was based on Iranians and the number of studies was limited. In addition, a meta-analysis by Sun et al. (15), published in 2016, was based only on prospective studies, excluding cross-sectional studies with stronger evidence. Therefore, the sample size of the included studies was small, which makes the results more prone to confounding factors and selection bias. Similarly, our results are consistent with a previous meta-analysis that indicated a significant association between positive anti-CagA and the risk of CHD. However, our meta-analysis avoided many defects of previous studies (68, 69). For instance, a meta-analysis conducted by Zhang et al. (68) was based only on cross-sectional studies, excluding more meaningful prospective studies. A meta-analysis (69), published in 2006, had a limited number of studies included and had no subgroup analysis for finding the source of heterogeneity. In addition, we also found that positive H. pylori stool antigen and positive H. pylori histological staining were significantly associated with the risk of CHD. Subgroup analysis and sensitivity analysis were not performed due to the small number of studies.
The mechanism of H. pylori infection causing CHD mainly consists of the following aspects. H. pylori in atherosclerotic plaque can stimulate inflammatory cells and cause excessive production of cytokines, which leads to local endothelial and vascular dysfunction (30). H. pylori infection leads to non-specific stimulation of inflammatory mediators in vivo, such as interleukin-1 (IL-1), interleukin-6 (IL-6), C-reactive protein (CRP), and tumor necrosis factor alpha (TNF-α), which promote plaque instability (70). Lastly, it can not only enter endothelial cells through CagA containing exosomes, resulting in endothelial damage (71) but also secrete another virulence factor, vacuolating cytotoxin A (VacA), which can reduce nitric oxide (NO), resulting in endothelial function damage (72).
The expression of P-selectin increases after H. pylori infection, and the adhesion between von Willebrand factor (vWF) released by platelets and P-selectin eventually leads to platelet aggregation (73). In addition to this, H. pylori infection can affect the risk factors for CHD, such as hypertension, dyslipidemia, hyperhomocysteinemia, diabetes, and impaired glucose tolerance. A recent meta-analysis (74) showed that H. pylori infection was significantly associated with arterial hypertension. Aslan et al. (75) found that the levels of total cholesterol, triglyceride, and low-density lipoprotein cholesterol in patients infected with H. pylori increased significantly, while high-density lipoprotein cholesterol decreased significantly. The interaction between H. pylori infection and diabetes leads to the occurrence of CHD (76). After persistent infection with H. pylori, low levels of serum vitamin B12 and folic acid will lead to hyperhomocysteinemia (77).
From our perspective, our meta-analysis is by far the most comprehensive and largest study supported by its statistical ability, leading to a more reliable overall evaluation. Our meta-analysis proved the positive relationship between positive anti-H. pylori IgG and development of CHD by subgroup analyses based on setting, category of CHD, adjustment status, and quality assessment score, but this relationship did not appear in prospective studies and some countries. Similarly, we also observed a positive association between the positive anti-CagA and the risk of CHD based on subgroup analyses of adjustment status and quality assessment score, but this association did not exist in prospective studies, community, MI, and Germany. Our results are similar to a previous meta-analysis (15) based on prospective studies, which indicated that H. pylori infection increased CHD risk, but this relationship weakens over time. The development of CHD is a multi-effect, long-term process. Over time, other CHD risk factors may attenuate the risk of CHD from the infection. Reasons for other subgroups without H. pylori infection increasing the risk of CHD might be associated with fewer studies. In addition, except for the fixed model for studies on anti-H. pylori IgG studies reporting HR, H. pylori stool antigen, and H. pylori histological staining, there is substantial heterogeneity among other studies, which may be attributed to the different participants and different study designs. Hence, the random model is adopted.
Strengths and Limitations
This is the first attempt to use a meta-analysis to evaluate the relationship between the risk of CHD and H. pylori infection through different H. pylori detection methods. Most previous meta-analyses used the anti-H. pylori IgG test to detect bacterial infection. In fact, this approach fails to detect current infections and may overestimate the association between bacteria and the risk of CHD. However, H. pylori stool antigen and H. pylori histological staining tests can detect current infection, which accurately assess the relationship between bacteria and the risk of CHD. Since positive anti-CagA has a strong inflammatory response, we also analyzed its association with the risk of CHD. More importantly, the included studies did not adequately consider traditional risk factors for CHD and other microbial infections. In the future, we strongly recommend conducting more well-designed intervention trials and investigating the relationship between other sources of infection and the risk of CHD.
Conclusion
This meta-analysis revealed an evidence-based relationship between H. pylori infection and the risk of CHD, which may contribute to the arguments established in the literature to provide strong evidence. Therefore, we suggest that H. pylori infection should be regarded as a new risk factor for CHD in future guidelines related to CHD. Future clinical application needs to be further studied to determine whether early eradication of H. pylori can reduce the incidence of CHD.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
X-JD and LT designed and analyzed the meta-analysis, and contributed to the revision of the manuscript. X-JD, LT, B-BW, F-HL, S-PL, and F-FP collected the data. All authors have read and approved the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kotilea K, Bontems P, Touat E. Epidemiology, diagnosis and risk factors of Helicobacter pylori infection. Adv Exp Med Biol. (2019) 1149:17–33. doi: 10.1007/5584_2019_357
2. Watari J, Chen N, Amenta PS, Fukui H, Oshima T, Tomita T, et al. Helicobacter pylori associated chronic gastritis, clinical syndromes, precancerous lesions, and pathogenesis of gastric cancer development. World J Gastroenterol. (2014) 20:5461–73. doi: 10.3748/wjg.v20.i18.5461
3. Holleczek B, Schöttker B, Brenner H. Helicobacter pylori infection, chronic atrophic gastritis and risk of stomach and esophagus cancer: results from the prospective population-based ESTHER cohort study. Int J Cancer. (2020) 146:2773–83. doi: 10.1002/ijc.32610
4. Wang B, Yu M, Zhang R, Chen S, Xi Y, Duan G. A meta-analysis of the association between Helicobacter pylori infection and risk of atherosclerotic cardiovascular disease. Helicobacter. (2020) 25:e12761. doi: 10.1111/hel.12761
5. Doheim MF, Altaweel AA, Elgendy MG, Elshanbary AA, Dibas M, Hegil Abo Ali AA, et al. Association between Helicobacter pylori infection and stroke: a meta-analysis of 273,135 patients. J Neurol. (2021) 268:3238–48. doi: 10.1007/s00415-020-09933-x
6. Christodoulou DK, Milionis HJ, Pappa P, Katsanos KH, Sigounas D, Florentin M, et al. Association of Helicobacter pylori infection with cardiovascular disease is it just a myth? Eur J Intern Med. (2011) 22:191–4. doi: 10.1016/j.ejim.2010.11.010
7. Bloemenkamp D, Mali W, Tanis BC, Rosendaal FR, Bosch MA, Kemmeren JM, et al. Chlamydia pneumoniae, Helicobacter pylori and Cytomegalovirus infections and the risk of peripheral arterial disease in young women. Atherosclerosis. (2002) 163:149–56. doi: 10.1016/s0021-9150(01)00761-4
8. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2020 update: a report from the American heart association. Circulation. (2020) 141:e139–596. doi: 10.1161/CIR.0000000000000757
9. Helfand M, Buckley DI, Freeman M, Fu R, Rogers K, Fleming C, et al. Emerging risk factors for coronary heart disease: a summary of systematic reviews conducted for the U.S. preventive services task force. Ann Intern Med. (2009) 151:496–507. doi: 10.7326/0003-4819-151-7-200910060-00010
10. Chmiela M, Gajewski A, Rudnicka K. Helicobacter pylori vs coronary heart disease-searching for connections. World J Cardiol. (2015) 7:187–203. doi: 10.4330/wjc.v7.i4.187
11. Jiang J, Chen Y, Shi J, Song C, Zhang J, Wang K. Population attributable burden of Helicobacter pylori-related gastric cancer, coronary heart disease, and ischemic stroke in China. Eur J Clin Microbiol Infect Dis. (2017) 36:199–212. doi: 10.1007/s10096-016-2810-x
12. Al-Nozha MM, Khalil MZ, Al-Mofleh IA, Al-Ghamdi AS. Lack of association of coronary artery disease with H. pylori infection. Saudi Med J. (2003) 24:1370–3.
13. Rothenbacher D, Brenner H, Hoffmeister A, Mertens T, Persson K, Koenig W. Relationship between infectious burden, systemic inflammatory response, and risk of stable coronary artery disease: role of confounding and reference group. Atherosclerosis. (2003) 170:339–45. doi: 10.1016/s0021-9150(03)00300-9
14. Rahmani Y, Mohammadi S, Karim H, Rezazadeh M, Babanejad M, Shahmohammadi A, et al. Association of Helicobacter pylori and coronary heart disease in Iran: a meta-analysis. Med J Islam Repub Iran. (2018) 32:73. doi: 10.14196/mjiri.32.73
15. Sun J, Rangan P, Bhat SS, Liu L. A meta-analysis of the association between Helicobacter pylori infection and risk of coronary heart disease from published prospective studies. Helicobacter. (2016) 21:11–23. doi: 10.1111/hel.12234
16. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. (2009) 62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006
17. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
18. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
19. Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, NJ: Wiley-Blackwell (2008). doi: 10.1002/9780470712184.ch1
20. Patel P, Mendall MA, Carrington D, Strachan DP, Leatham E, Molineaux N, et al. Association of Helicobacter pylori and Chlamydia pneumoniae infections with coronary heart disease and cardiovascular risk factors. BMJ. (1995) 311:711–4. doi: 10.1136/bmj.311.7007.711
21. Whincup PH, Mendall MA, Perry IJ, Strachan DP, Walker M. Prospective relations between Helicobacter pylori infection, coronary heart disease, and stroke in middle aged men. Heart. (1996) 75:568–72. doi: 10.1136/hrt.75.6.568
22. Rathbone B, Martin D, Stephens J, Thompson JR, Samani NJ. Helicobacter pylori seropositivity in subjects with acute myocardial infarction. Heart. (1996) 76:308–11. doi: 10.1136/hrt.76.4.308
23. Folsom AR, Nieto FJ, Sorlie P, Chambless LE, Graham DY. Helicobacter pylori seropositivity and coronary heart disease incidence. atherosclerosis risk in communities (ARIC) study investigators. Circulation. (1998) 98:845–50. doi: 10.1161/01.cir.98.9.845
24. Pellicano R, Mazzarello MG, Morelloni S, Allegri M, Arena V, Ferrari M, et al. Acute myocardial infarction and Helicobacter pylori seropositivity. Int J Clin Lab Res. (1999) 29:141–4.
25. Danesh J, Youngman L, Clark S, Parish S, Peto R, Collins R. Helicobacter pylori infection and early onset myocardial infarction: case-control and sibling pairs study. BMJ. (1999) 319:1157–62. doi: 10.1136/bmj.319.7218.1157
26. Galante A, Pietroiusti A, Carta S, Franceschelli L, Piccolo P, Mastino A, et al. Infection with Helicobacter pylori and leukocyte response in patients with myocardial infarction. Eur J Clin Microbiol Infect Dis. (2000) 19:298–300. doi: 10.1007/s100960050479
27. Kahan T, Lundman P, Olsson G, Wendt M. Greater than normal prevalence of seropositivity for Helicobacter pylori among patients who have suffered myocardial infarction. Coron Artery Dis. (2000) 11:523–6. doi: 10.1097/00019501-200010000-00002
28. Gunn M, Stephens JS, Thompson JR, Rathbone BJ, Samani NJ. Significant association of cagA positive Helicobacter pylori strains with risk of premature myocardial infarction. Heart. (2000) 84:267–71. doi: 10.1136/heart.84.3.267
29. Ridker PM, Danesh J, Youngman L, Collins R, Stampfer MJ, Peto R, et al. A prospective study of Helicobacter pylori seropositivity and the risk for future myocardial infarction among socioeconomically similar U.S. men. Ann Intern Med. (2001) 135:184–8. doi: 10.7326/0003-4819-135-3-200108070-00010
30. Kinjo K, Sato H, Sato H, Shiotani I, Kurotobi T, Ohnishi Y, et al. Prevalence of Helicobacter pylori infection and its link to coronary risk factors in Japanese patients with acute myocardial infarction. Circ J. (2002) 66:805–10. doi: 10.1253/circj.66.805
31. Fraser AG, Scragg RK, Cox B, Jackson RT. Helicobacter pylori, Chlamydia pneumoniae and myocardial infarction. Intern Med J. (2003) 33:267–72. doi: 10.1046/j.1445-5994.2003.00349.x
32. Ozdogru I, Kalay N, Dogan A, Inanc MT, Kaya MG, Topsakal R, et al. The relationship between Helicobacter pylori IgG titre and coronary atherosclerosis. Acta Cardiol. (2007) 62:501–5. doi: 10.2143/AC.62.5.2023414
33. Nikolopoulou A, Tousoulis D, Antoniades C, Petroheilou K, Vasiliadou C, Papageorgiou N, et al. Common community infections and the risk for coronary artery disease and acute myocardial infarction: evidence for chronic over-expression of tumor necrosis factor alpha and vascular cells adhesion molecule-1. Int J Cardiol. (2008) 130:246–50. doi: 10.1016/j.ijcard.2007.08.052
34. Jafarzadeh A, Esmaeeli-Nadimi A, Nemati M, Tahmasbi M, Ahmadi P. Serum concentrations of Helicobacter pylori IgG and the virulence factor CagA in patients with ischaemic heart disease. East Mediterr Health J. (2010) 16:1039–44. doi: 10.26719/2010.16.10.1039
35. Guan XR, Jiang LX, Ma XH, Wang LF, Quan H, Li HY. Respiratory syncytial virus infection and risk of acute myocardial infarction. Am J Med Sci. (2010) 340:356–9. doi: 10.1097/MAJ.0b013e3181eecf29
36. Nakić D, Vcev A, Jović A, Patrk J, Zekanović D, Klarin I, et al. Helicobacter pylori infection and acute myocardial infarction. Coll Antropol. (2011) 35: 781–5.
37. Khodaii Z, Vakili H, Ghaderian SMH, Najar RA, Panah AST. Association of Helicobacter pylori infection with acute myocardial infarction. Coron Artery Dis. (2011) 22:6–11. doi: 10.1097/mca.0b013e3283402360
38. Padmavati S, Gupta U, Agarwal HK. Chronic infections & coronary artery disease with special reference to Chalmydia pneumoniae. Indian J Med Res. (2012) 135:228–32.
39. Schöttker B, Adamu MA, Weck MN, Müller H, Brenner H. Helicobacter pylori infection, chronic atrophic gastritis and major cardiovascular events: a population-based cohort study. Atherosclerosis. (2012) 220:569–74. doi: 10.1016/j.atherosclerosis.2011.11.029
40. Ikeda A, Iso H, Sasazuki S, Inoue M, Tsugane S, Jphc Study Group. The combination of Helicobacter pylori- and cytotoxin-associated gene-A seropositivity in relation to the risk of myocardial infarction in middle-aged Japanese: the Japan public health center-based study. Atherosclerosis. (2013) 230:67–72. doi: 10.1016/j.atherosclerosis.2013.06.013
41. Sunanda N, Shrikhande, Zodpey SP, Negandhi H. A case-control study examining association between infectious agents and acute myocardial infarction. Indian J Public Health. (2014) 58:106–9. doi: 10.4103/0019-557X.132285
42. Witherell HL, Smith KL, Friedman GD, Ley C, Thom DH, Orentreich N, et al. C-reactive protein, Helicobacter pylori, Chlamydia pneumoniae, cytomegalovirus and risk for myocardial infarction. Ann Epidemiol. (2003) 13:170–7. 00276-4 doi: 10.1016/s1047-2797(02)
43. Singh PK, McMahon AD, Patel H, Packard CJ, Rathbone BJ, Saman NJ. Prospective analysis of the association of infection with CagA bearing strains of Helicobacter pylori and coronary heart disease. Heart. (2002) 88:43–6. doi: 10.1136/heart.88.1.43
44. Ossewaarde JM, Feskens EJ, Vries AD, Vallinga CE, Kromhout D. Chlamydia pneumoniae is a risk factor for coronary heart disease in symptom-free elderly men, but Helicobacter pylori and cytomegalovirus are not epidemiology and infection. Epidemiol Infect. (1998) 120:93–9. doi: 10.1017/s0950268897008303
45. Whincup P, Danesh J, Walker M, Lennon L, Thomson A, Appleby P, et al. Prospective study of potentially virulent strains of Helicobacter pylori and coronary heart disease in middle-aged men. Circulation. (2000) 101:1647–52. doi: 10.1161/01.cir.101.14.1647
46. Wald NJ, Law MR, Morris JK, Bagnall AM. Helicobacter pylori infection and mortality from ischaemic heart disease: negative result from a large, prospective study. BMJ. (1997) 315:1199–201. doi: 10.1136/bmj.315.7117.1199
47. Stone AF, Risley P, Markus HS, Butland BK, Strachan DP, Elwood PC, et al. Ischaemic heart disease and Cag A strains of Helicobacter pylori in the Caerphilly heart disease study. Heart. (2001) 86:506–9.
48. Danesh J, Wong Y, Ward M, Muir J. Chronic infection with Helicobacter pylori, Chlamydia pneumoniae, or Cytomegalovirus: population based study of coronary heart disease. Heart. (1999) 81:245–7. doi: 10.1136/hrt.81.3.245
49. Azarkar Z, Jafarnejad M, Sharifzadeh G. The relationship between Helicobacter pylori infection and myocardial infarction. Caspian J Intern Med. (2011) 2:222–5.
50. Khurshid A, Fenske T, Bajwa T, Bourgeois K, Vakil N. A prospective, controlled study of Helicobacter pylori seroprevalence in coronary artery disease. Am J Gastroenterol. (1998) 93:717–20. doi: 10.1111/j.1572-0241.1998.212_a.x
51. Pasceri V, Cammarota G, Patti G, CuocoL L, Gasbarrini A, Grillo RL, et al. Association of virulent Helicobacter pylori strains with ischemic heart disease. Circulation. (1998) 97:1675–9. doi: 10.1161/01.cir.97.17.1675
52. Bonaventura GD, Piccolomini R, Pompilio A, Zappacosta R, Piccolomini M, Neri M. Serum and mucosal cytokine profiles in patients with active Helicobacter pylori and ischemic heart disease: is there a relationship? Int J Immunopathol Pharmacol. (2007) 20:163–72. doi: 10.1177/039463200702000119
53. Lenzi C, Palazzuoli A, Giordano N, Alegente G, Gonnelli C, Campagna MS, et al. H pylori infection and systemic antibodies to CagA and heat shock protein 60 in patients with coronary heart disease. World J Gastroenterol. (2006) 12:7815–20. doi: 10.3748/wjg.v12.i48.7815
54. Zodpey SP, Shrikhande SN, Negandhi HN, Ughade SN, Joshi PP. Risk factors for acute myocardial infarction in central India: a case-control study. Indian J Community Med. (2015) 40:19–26. doi: 10.4103/0970-0218.149265
55. Aceti A, Are R, Sabino G, Fenu L, Pasquazzi C, Quaranta G, et al. Helicobacter pylori active infection in patients with acute coronary heart disease. J Infect. (2004) 49:8–12. doi: 10.1016/j.jinf.2004.01.011
56. Tsai CJ, Huang TY. Relation of Helicobacter pylori infection and angiographically demonstrated coronary artery disease. Dig Dis Sci. (2000) 45:1227–32.
57. Jin JS, Her SH, Lee JM, Yoon HJ, Moon SJ, Kim PJ, et al. The association between current Helicobacter pylori infection and coronary artery disease. Korean J Intern Med. (2007) 22:152–6. doi: 10.3904/kjim.2007.22.3.152
58. Miyazaki M, Babazono A, Kadowak K, Kato M, Takata T, Une H. Is Helicobacter pylori infection a risk factor for acute coronary syndromes? J Infect. (2006) 52:86–91. doi: 10.1016/j.jinf.2005.04.009
59. Adiloglu AK, Nazli C, Cicioglu-Aridogan B, Kinay O, Can R, Ergene O. Gastroduodenal Helicobacter pylori infection diagnosed by Helicobacter pylori stool antigen is related to atherosclerosis. Acta Cardiol. (2003) 58:335–9. doi: 10.2143/AC.58.4.2005291
60. Adiloglu AK, Can R, Nazli C, Ocal A, Ergene O, Tinaz G, et al. Ectasia and severe atherosclerosis: relationships with Chlamydia pneumoniae, Helicobacter pylori, and inflammatory markers. Tex Heart Inst J. (2005) 32:21–7. doi: 10.2307/2232228
61. Lee SY, Kim DK, Son HJ, Lee JH, Kim YH, Kim JJ, et al. The impact of Helicobacter pylori infection on coronary heart disease in a Korean population. Korean J Gastroenterol. (2004) 44:193–8. doi: 10.1016/S0304-4017(00)00332-0
62. Lin Y, Obata Y, Kikuchi S, Tamakoshi A, Iso H, Jacc Study Group. Helicobacter pylori infection and risk of death from cardiovascular disease among the Japanese population: a nested case-control study within the JACC study. J Atheroscler Thromb. (2015) 22:1207–13. doi: 10.5551/jat.27987
63. Bai S, Hashmi SFA. Association between Helicobacter pylori infection and acute myocardial infarction (AMI). Pak Heart J. (2017) 50:33–8.
64. Pellicano R, Mladenova I, Broutet N, Salmi LR, Mégraud F. Is there an association between Helicobacter pylori infection and coronary heart disease? Eur J Epidemiol. (1999) 15:611–9. doi: 10.1023/a:1007640609707
65. Danesh J, Peto R. Risk factors for coronary heart disease and infection with Helicobacter pylori: meta-analysis of 18 studies. BMJ. (1998) 316:1130–2. doi: 10.1136/bmj.316.7138.1130
66. Liu J, Wang F, Shi S. Helicobacter pylori infection increase the risk of myocardial infarction: a meta-analysis of 26 studies involving more than 20,000 participants. Helicobacter. (2015) 20:176–83. doi: 10.1111/hel.12188
67. Rahmani Y, Mohammadi S, Babanejad M, Rai A, Zalei B, Shahmohammadi A. Association of Helicobacter pylori with presence of myocardial infarction in Iran: a systematic review and meta-analysis. Ethiop J Health Sci. (2017) 27:433–40. doi: 10.4314/ejhs.v27i4.15
68. Zhang S, Guo Y, Ma Y, Teng Y. Cytotoxin-associated gene-A-seropositive virulent strains of Helicobacter pylori and atherosclerotic diseases: a systematic review. Chin Med J (Engl). (2008) 121:946–51. doi: 10.1023/A:1016079505458
69. Pasceri V, Patti G, Cammarota G, Pristipino C, Richichi G, Sciascio GD. Virulent strains of Helicobacter pylori and vascular diseases: a meta-analysis. Am Heart J. (2006) 151:1215–22. doi: 10.1016/j.ahj.2005.06.041
70. Figura N, Palazzuoli A, Vaira D, Campagna M, Moretti E, Iacoponi F, et al. Cross-sectional study: CagA-positive Helicobacter pylori infection, acute coronary artery disease and systemic levels of B-type natriuretic peptide. J Clin Pathol. (2014) 67:251–7. doi: 10.1136/jclinpath-2013-201743
71. Xia XJ, Zhang LF, Chi JS, Li H, Liu XM, Hu TZ, et al. Helicobacter pylori infection impairs endothelial function through an exosome-mediated mechanism. J Am Heart Assoc. (2020) 9:e014120. doi: 10.1161/JAHA.119.014120
72. Tobin NP, Henehan GT, Murphy RP, Atherton JC, Guinan AF, Kerrigan SW, et al. Helicobacter pylori-induced inhibition of vascular endothelial cell functions: a role for VacA-dependent nitric oxide reduction. Am J Physiol Heart Circ Physiol. (2008) 295:H1403–13. doi: 10.1152/ajpheart.00240.2008
73. Yeh JJ, Tsai S, Wu DC, Wu JY, Liu TC, Chen A. P-selectin-dependent platelet aggregation and apoptosis may explain the decrease in platelet count during Helicobacter pylori infection. Blood. (2010) 115:4247–53. doi: 10.1182/blood-2009-09-241166
74. Huang MY, Zhu LJ, Jin YL, Fang ZM, Chen Y, Yao YS. Association between Helicobacter pylori infection and systemic arterial hypertension: a meta-analysis. Arq Bras Cardiol. (2021) 117:626–36. doi: 10.36660/abc.20200186
75. Aslan M, Nazligul Y, Horoz M, Bolukbas C, Bolukbas FF, Gur M, et al. Serum paraoxonase-1 activity in Helicobacter pylori infected subjects. Atherosclerosis. (2008) 196:270–4. doi: 10.1016/j.atherosclerosis.2006.10.024
76. Nodoushan S, Nabavi A. The interaction of Helicobacter pylori infection and type 2 diabetes mellitus. Adv Biomed Res. (2019) 8:15. doi: 10.4103/abr.abr_37_18
77. Chen YH, Xu CL, Xu HF, Chen WL, Wang HH, Wang ZT, et al. Persistent Helicobacter pylori infection for more than 3 years leads to elevated serum homocysteine concentration: a retrospective cohort study based on a healthy Chinese population. J Gastroenterol Hepatol. (2021) 36:3077–83. doi: 10.1111/jgh.15603
Keywords: coronary heart disease, Helicobacter pylori, anti-H. pylori IgG test, anti-CagA test, H. pylori stool antigen test, H. pylori histological staining test, systematic review, meta-analysis
Citation: Tong L, Wang B-B, Li F-H, Lv S-P, Pan F-F and Dong X-J (2022) An Updated Meta-Analysis of the Relationship Between Helicobacter pylori Infection and the Risk of Coronary Heart Disease. Front. Cardiovasc. Med. 9:794445. doi: 10.3389/fcvm.2022.794445
Received: 13 October 2021; Accepted: 14 February 2022;
Published: 29 April 2022.
Edited by:
Yuefei Jin, Zhengzhou University, ChinaReviewed by:
Rongguang Zhang, Hainan Medical University, ChinaZeng-hong Wu, Huazhong University of Science and Technology, China
Copyright © 2022 Tong, Wang, Li, Lv, Pan and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin-Jiang Dong, MTUyMjE4MjQ2NkBxcS5jb20=
 Ling Tong1
Ling Tong1 Xin-Jiang Dong
Xin-Jiang Dong