- 1Department of Cardiology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China
- 2Montefiore Medical Center, New York, NY, United States
- 3Key Laboratory of Targeted Intervention of Cardiovascular Disease, Collaborative Innovation Center for Cardiovascular Disease Translational Medicine, Nanjing Medical University, Nanjing, China
Background: Observational studies have shown that central obesity is associated with adverse cardiac structure and function. However, causal association between central obesity and left ventricular (LV) structure and function in preserved ejection fraction (EF) population is still uncertain.
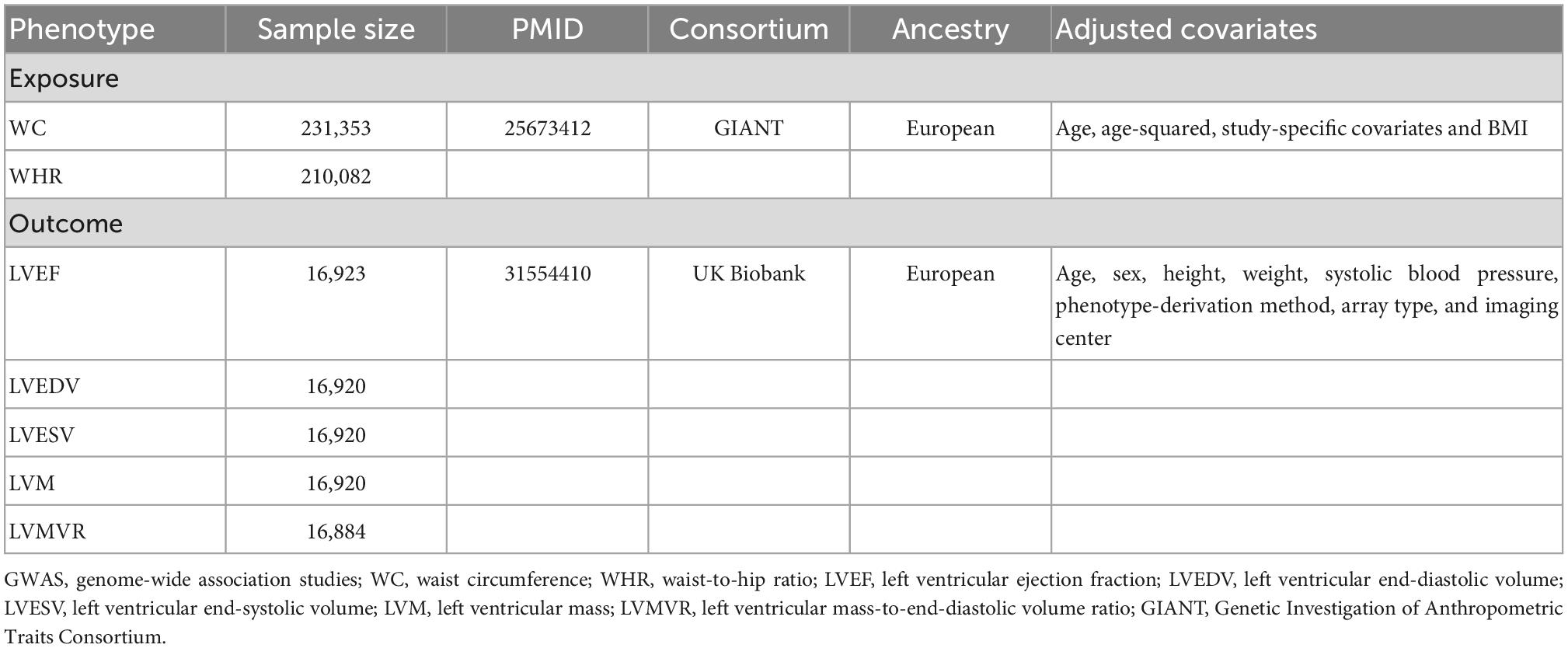
Methods: Genome-wide association studies summary data of waist circumference adjusted for body mass index (WCadjBMI) and waist-to-hip ratio adjusted for body mass index (WHRadjBMI) were selected as instrumental variables from the Genetic Investigation of Anthropometric Traits (GIANT) Consortium (n = 224,459). Outcome datasets for LV parameters including LV end-diastolic volume (LVEDV), LV end-systolic volume (LVESV), LV ejection fraction (LVEF), LV mass (LVM), and LV mass-to-end-diastolic volume ratio (LVMVR) were obtained from the participants without prevalent myocardial infarction or heart failure (LVEF ≥ 50%) in UK Biobank Cardiovascular Magnetic Resonance sub-study (n = 16,923). Two-sample Mendelian randomization (MR) was performed with the inverse-variance weighted (IVW) method as the primary estimate and with the weighted median and MR-Egger as the supplemental estimates. Sensitivity analysis was used to assess the heterogeneity and pleiotropic bias in the MR results.
Results: In the IVW analysis, every 1-standard deviation (SD) higher WHRadjBMI was significantly associated with higher LVMVR (β = 0.4583; 95% confidence interval [CI]: 0.2921 to 0.6244; P = 6.418 × 10–8) and lower LVEDV (β = –0.2395; 95% CI: –0.3984 to –0.0807; P = 0.0031) after Bonferroni adjustment. No heterogeneity and horizontal pleiotropy were detected in the analysis. No association of WCadjBMI was found with LVEF, LVEDV, LVESV, LVM, or LVMVR.
Conclusion: Our findings provide evidence of significant causal association between WHRadjBMI and adverse changes in LV structure and function in preserved EF population.
Introduction
Heart failure (HF) is a global health epidemic and burden, leading to increased morbidity and mortality (1). The total number of HF patients is still rising, especially with an alarming trend in young population, possibly related to the prevalence of obesity (2). The link between obesity and the risk of HF was first confirmed in Framingham Heart Study and is stronger than those for other types of cardiovascular disease (3, 4). Obesity predicts HF with preserved ejection fraction (HFpEF) but not HF with reduced ejection fraction (HFrEF) among those who develop HF (5, 6), and more than 80% of HFpEF patients in the US are overweight or obese (7).
Obesity is commonly defined by body mass index (BMI) to describe the total adipose accumulation. However, regional fat distribution may play a pivotal role in the development of HF (6). Central obesity, usually measured by waist circumference (WC) or waist-to-hip ratio (WHR), has a more prevalence in patients with HFpEF and a more association with increased risk of HF hospitalization or death than general obesity (8–10). Adverse cardiac remodeling, in the form of structural and functional abnormalities of the heart (mainly left ventricular, LV) in response to various stimuli, is associated with the development of HF (11). Observational studies have shown that central obesity is associated with cardiac remodeling independent of BMI (8, 12). Eschalier et al. have observed that cardiac concentric remodeling was associated with central obesity in asymptomatic and normotensive healthy subjects with central obesity (13). However, due to potential residual confounding and reverse causality in observational studies, whether a causal relationship exists between central obesity and cardiac remodeling and dysfunction in preserved ejection fraction (EF) population remains unclear.
Mendelian randomization (MR) is an epidemiological technique capable of elucidating causal estimate of exposures to outcomes, using genetic variants as instrumental variables (IVs) (14). As genetic variants are randomly allocated at conception, genetically predicted exposure in MR is minimally affected by confounders or reverse causation. We conducted a MR study to investigate the potential effects of genetic liability to central obesity measured by WC and WHR on LV structure and function in preserved EF population.
Materials and methods
Study design
As shown in Figure 1, a two-sample MR model was used to evaluate the causal effect of central obesity on left ventricular structure and function. The study was based on summary-level data on WC adjusted for BMI (WCadjBMI), WHR adjusted for BMI (WHRadjBMI), and parameters of left ventricular structure and function from the published genome-wide association studies (GWASs). The MR design fulfilled three assumptions: (1) genetic instruments are closely related to exposures; (2) genetic instruments are independent of confounders; (3) genetic instruments only affect outcomes via the exposures of interest (15).
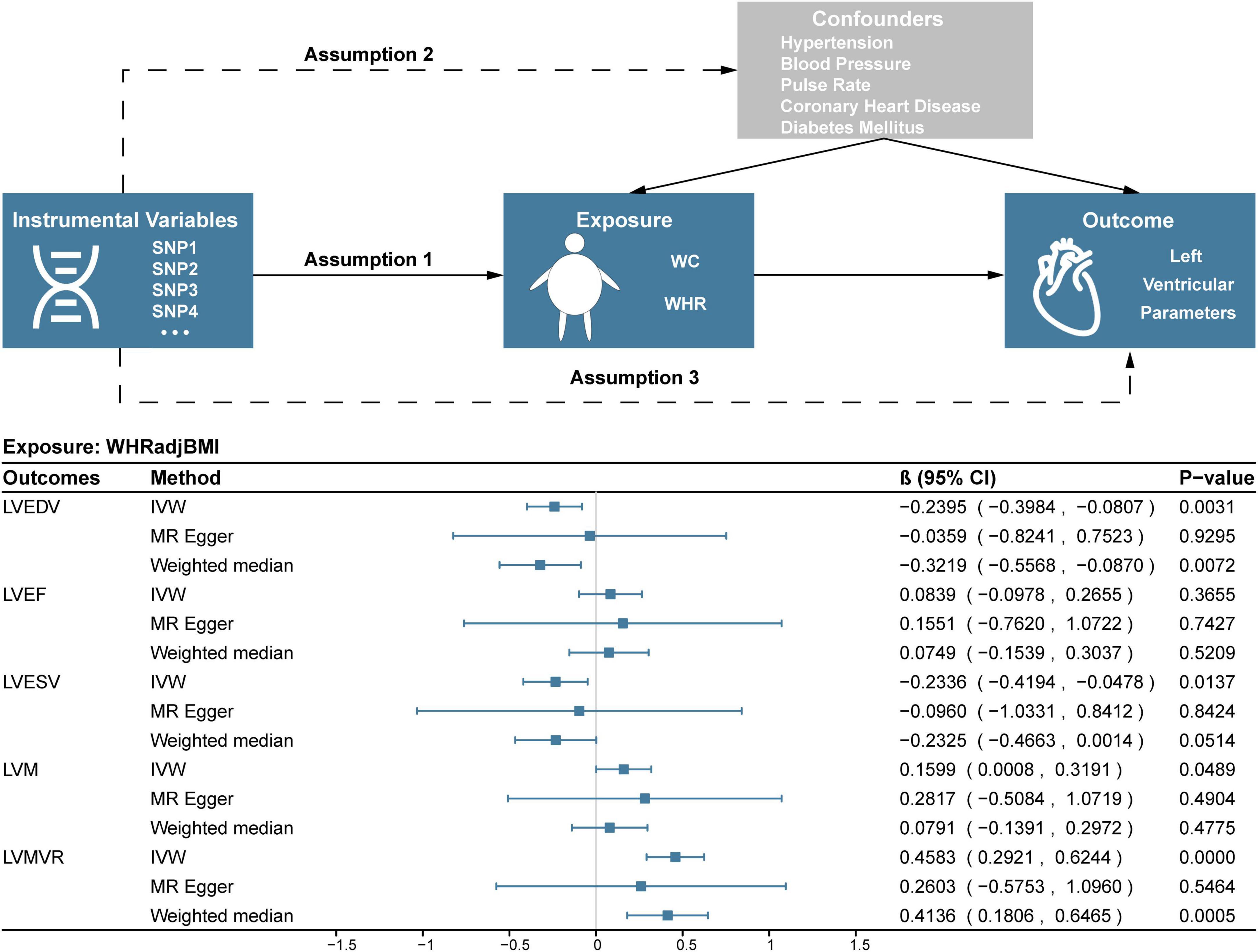
Figure 1. Study design and results of MR analysis of the association between WHRadjBMI and left ventricular parameters. SNP, single nucleotide polymorphism; WC, waist circumference; WHR, waist-to-hip ratio; WHRadjBMI, waist-to-hip ratio adjusted for body mass index; LVEF, left ventricular ejection fraction; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVM, left ventricular mass; LVMVR, left ventricular mass-to-end-diastolic volume ratio.
Genetic instrument selection
Single nucleotide polymorphisms (SNPs) as instrumental variables associated with WCadjBMI and WHRadjBMI at the genome-wide significance level (P < 5 × 10–8) were obtained in 224,459 European individuals from the Genetic Investigation of Anthropometric Traits (GIANT) Consortium (Table 1) (16). After estimating linkage disequilibrium (LD r2 < 0.001, LD distance > 10,000 kb) among the SNPs based on the 1000 Genomes European reference panel (17), we extracted 65 SNPs and 38 SNPs that genetically predicted WCadjBMI and WHRadjBMI, respectively. All SNPs were not associated with the outcome. In order to avoid specific confounders (e.g., hypertension, coronary heart disease, blood pressure, pulse rate, and diabetes mellitus), we excluded 10 of 65 SNPs (rs1344674, rs7684221, rs2071449, rs12656497, rs806794, rs6905288, rs606452, rs3786897, rs459193, rs849140) and 7 of 38 SNPs (rs2071449, rs7705502, rs7759742, rs998584, rs2820443, rs1128249, rs459193) with a threshold of P < 5 × 10––8 based on the Phenoscanner database1 (18). Additionally, one SNP (rs16957304) associated with WCadjBMI missing in the outcome datasets was excluded for its limited influence on the results with a small proportion. In the end, fifty-two WCadjBMI and thirty WHRadjBMI related independent SNPs (Supplementary Tables 1, 2) were considered as instruments for main MR analyses after removing palindromic SNPs (WCadjBMI: rs7162542, rs984222; WHRadjBMI: rs2276824) (19). F statistics for the SNPs were calculated to evaluate the strength of the instrument variables (20).
Data source for outcomes
The outcomes of the study were selected based on the GWAS conducted by Aung et al. comprising 16,923 European UK Biobank participants without prevalent myocardial infarction or heart failure (LVEF in every participant ≥ 50%) to identify the genetic loci for 6 relevant cardiac magnetic resonance (CMR)-derived LV imaging phenotypes, including LV end-diastolic volume (LVEDV), LV end-systolic volume (LVESV), LV stroke volume (LVSV), LV ejection fraction (LVEF), LV mass (LVM), and LV mass-to-end-diastolic volume ratio (LVMVR) (21). The GWAS analysis was adjusted for age, sex, height, weight, systolic blood pressure, phenotype-derivation method, array type, and imaging center. We used the summary-level data of 5 parameters (except LVSV) as the outcomes in our study.
Mendelian randomization analyses
The random effects inverse-variance weighed (IVW) was used as the main MR method in our study while MR-Egger, weighted median and MR-PRESSO were also performed for more robust estimates. IVW analysis estimates the effect of each SNP on the outcome by calculating the Wald ratio and performs a meta-analysis for the combined causal effect with the inverse variance of SNPs as weights (22). MR-Egger provides the estimate with adjustment for horizontal pleiotropy based on the assumption that the effect of the genetic instruments is uncorrected with any pleiotropic effect (23). Weighted median provides consistent estimates based on the assumption but requires more than 50% of weight from valid genetic instruments (24). MR-PRESSO method can detect and correct outlier SNPs and provide estimates after removing outliers (25). For further sensitivity analyses, we conduct the Cochran’s Q test to assess the heterogeneity, the MR-Egger intercept test to analyze the horizontal pleiotropy and leave-one-out analysis to detect high influence points (23, 26, 27). We calculated MR power using a wed-based tool2 according to Burgess’s method (28).
Statistical analysis
All analyses were performed in R software (version 4.2.1) using the packages Two SampleMR (version 0.5.6) and MR-PRESSO (version 1.0). The association with P-value < 0.005 (0.05/10) was considered a significant association, and a P-value < 0.05 and ≥ 0.005 was regarded as nominally significant after Bonferroni adjustment.
Results
A total of 52 and 30 SNPs genetically associated with WCadjBMI and WHRadjBMI were enrolled to analyze before removing outliers, respectively (Supplementary Tables 1, 2). All the F statistics (Supplementary Tables 1, 2) for instruments were over 10, indicating a good strength of each instrument.
Association of WCadjBMI with LV parameters
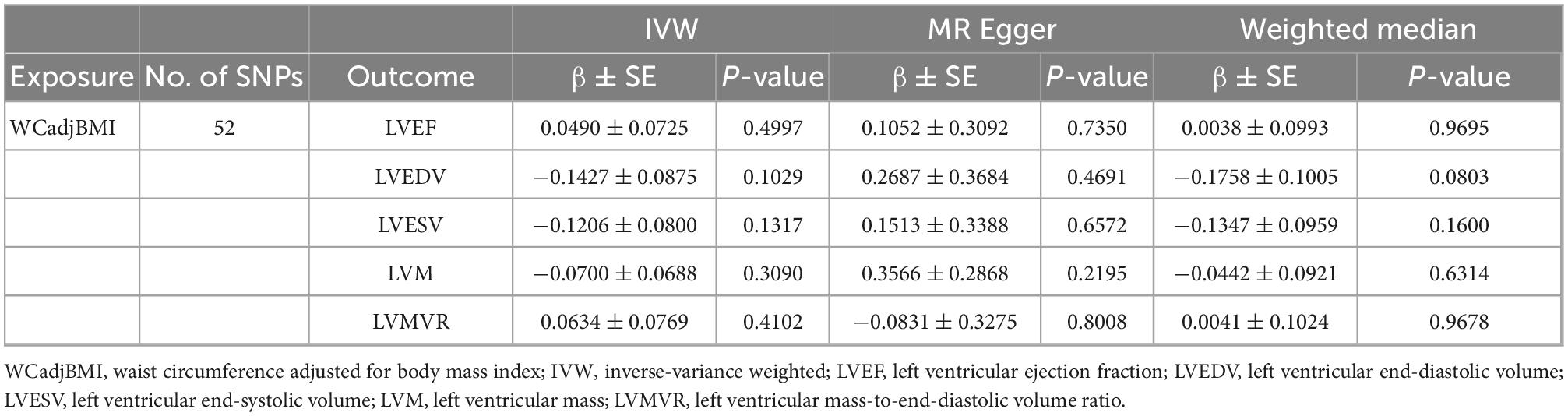
As shown in Table 2, no causal associations were found between WCadjBMI and LV parameters. Results of heterogeneity and pleiotropy tests were shown in Table 3, and there was no pleiotropy in the analysis. Scatter, leave-one-out and funnel plots were reported in the Supplementary Figures 1–6. One outlier (rs7970350) was identified with MR-PRESSO when exploring the association between WCadjBMI and LVEDV. After removing the outlier, every 1-SD increase in genetic liability to WCadjBMI was nominally significantly associated with lower LVEDV in the IVW analysis (β = –0.1718, 95% confidence interval [CI] –0.3311 to –0.0125; P = 0.0345) without horizontal pleiotropy (Supplementary Tables 3, 4).
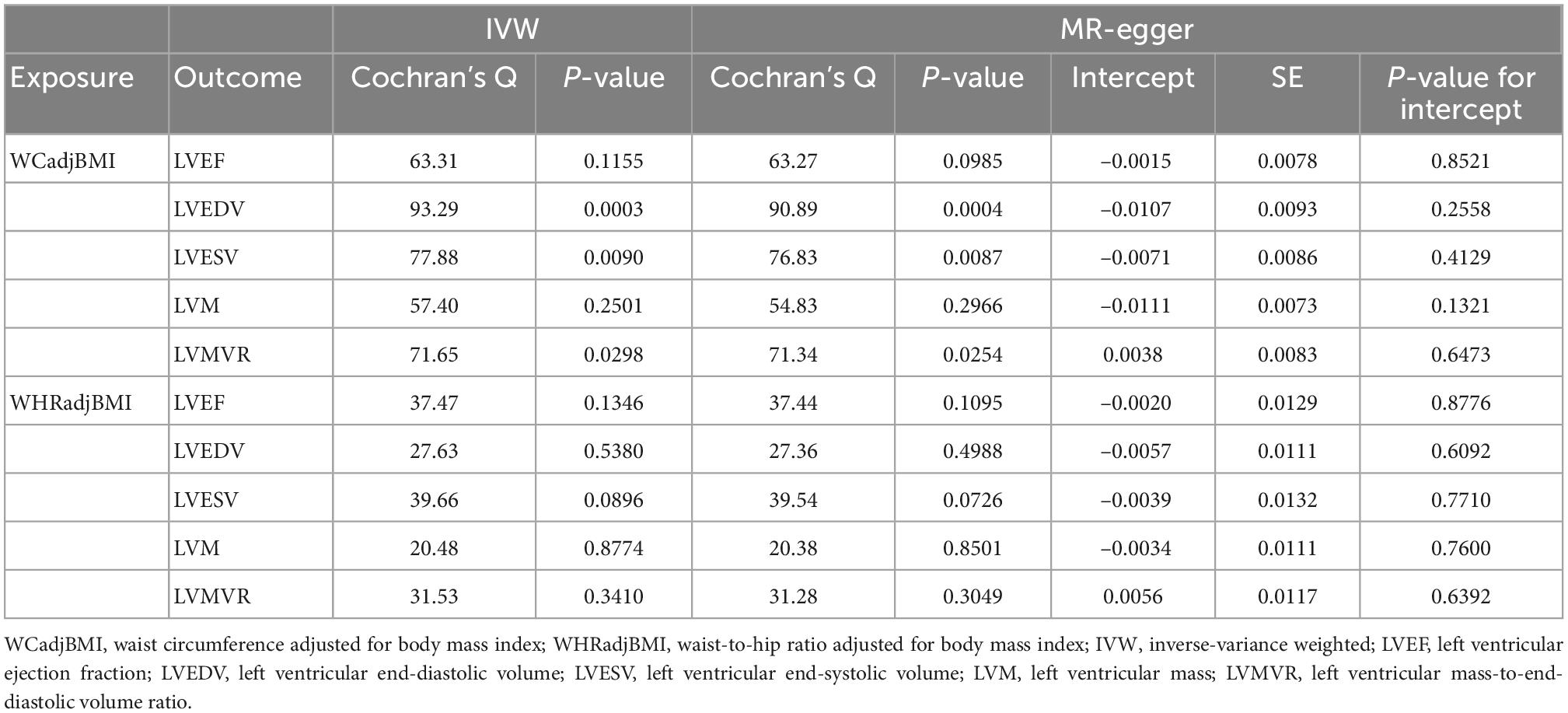
Table 3. Heterogeneity and horizontal pleiotropy test of the associations between WCadjBMI and WHRadjBMI and left ventricular parameters.
Association of WHRadjBMI with LV parameters
In the IVW analysis, one-SD genetically determined increase in WHRadjBMI was significantly associated with lower LVEDV (β = –0.2395, 95% CI –0.3984 to –0.0807; P = 0.0031) and higher LVMVR (β = 0.4583, 95% CI 0.2921 to 0.6244; P < 0.0001). Additionally, every 1-SD increase in genetic liability to WHRadjBMI was nominally significantly associated with lower LVESV (β = –0.2336, 95% CI –0.4194 to –0.0478; P = 0.0137) and higher LVM (β = 0.1599, 95% CI 0.0008 to 0.3191; P = 0.0489). Weighted median and MR-Egger analyses also showed similar associations (Figure 1). No evidence supported that genetic liability to WHRadjBMI was associated with LVEF (Figure 1). Several sensitivity analyses showed no heterogeneity or pleiotropy in Table 3 and no outlier was found using MR-PRESSSO. Scatter, leave-one-out and funnel plots were reported in the Supplementary Figures 1–6.
Discussion
This two-sample MR study described the major finding that every 1-SD genetically determined increased WHR is causally associated with lower LVEDV and higher LVMVR in preserved EF population. The findings were robust based on different kinds of MR methods and sensitivity analyses.
Multiple population-based studies have observed that both general obesity and central obesity are major risk factors for the development of HF (3, 29–32). Several studies demonstrated general obesity is causally associated with HF (33–35). Our findings are consistent with the prior studies, while we focused on the causal effect of central obesity measured by anthropometrics on LV morphology and function in preserved EF population. With the increasing evidence in HFpEF pathogenesis, obesity has been considered as a primary and direct cause of HFpEF, instead of comorbid bystander, mediated via other metabolic syndromes (36). A recent study describing LV structural characteristics of HFpEF across different LVEF reported that individuals with higher LVEF (> 60%) presented more concentric remodeling and diastolic/systolic stiffness, while those with lower LVEF (50–60%) were more characterized by eccentric myocardial remodeling and a higher amount of myocardial fibrosis similar to HFrEF (37). This suggested that there is a dynamic variation of phenotypes with adverse changes in cardiac structure and function. Although we did not investigate the causal association of WC and WHR with the risk of both HFpEF and HFrEF due to a lack of certain GWAS summaries, LV remodeling with preserved LVEF is a critical preclinical characteristic.
Obesity cardiomyopathy increasingly attracts more attention as epidemiological, clinical, and experimental evidence support the existence of this unique disease entity, which develops independent of coronary heart disease, hypertension, and other cardiovascular diseases (38). Alterations of LV structure and function have been noted in obesity with the use of echocardiography and magnetic resonance imaging during observational studies (39–41). The Dallas Heart Study observed the impact of longitudinal changes in adiposity on concentric left ventricular remodeling (42). We further confirmed the causal association of WHR with lower LVEDV and higher LVMVR in this two-sample MR study. This also shed light on the causal relationship of the distribution of adipose tissue on LV remodeling.
Diastolic dysfunction has been reported in obese individuals without meeting diagnosis of HF (38). Yagmur et al. found transmitral deceleration time, isovolumetric relaxation time, and peak late diastolic tissue doppler velocity values, which reflect LV diastolic function, were significantly higher in obese individuals compared with normal weight subjects without significant difference in LVEF between groups (43). Similarly in children, a cross-sectional study found higher ratio of transmitral early diastolic filling velocity to septal peak early diastolic myocardial velocity (E/e’) without left ventricular hypertrophy in obese patients (44). LVMVR is a good parameter to assess the diastolic performance (45) and we further confirmed the causal effect of central obesity on diastolic dysfunction reflected by higher LVMVR with increasing WHR in our study. However, we did not find any difference in LVEF as WHR and WC increased. On one hand, the outcome data were all from those with preserved LVEF, which means the systolic function might not be impaired. On the other hand, this might suggest that central obesity mainly affects cardiac diastolic function in the early disease process. The CARDIA study, a multi-center prospective study that enrolled 5,115 white and black men and women aged between 18 and 30, found longstanding obesity for more than 20 years is associated with overtly impaired LV systolic function as well (46).
Animal studies also observed improvement in cardiac function after weight and fat mass reduction, although Partington et al. emphasized an improvement in left ventricular hypertrophy rather than diastolic dysfunction possibly due to a short follow-up duration (47, 48). Benefits of general obesity control on left ventricular diastolic function was reported in humans (49, 50). Sundström et al. demonstrated that bariatric surgery leads to a lower incidence of HF with a hazard ratio of 0.54 compared with intensive lifestyle treatment (51). Nevertheless, no further subgroup analyses were performed based HF phenotype, such as HFpEF and HFrEF. For central obesity, the Look AHEAD study found that decline in WC was significantly associated with lower risk of HFpEF in adults with type 2 diabetes (52). The Utah obesity study also suggested reverse cardiac remodeling and improved cardiac function along with significant reductions in WC after gastric bypass surgery (53). Recently, the role of WHR, another easily available measurement of central obesity, in cardiovascular diseases draws increasing attention (54). Our data suggested WHR was causally associated with more LV parameters than WC. WHR, affected by both gluteofemoral subcutaneous adipose tissue and abdominal visceral adipose tissue, is considered more accurate to evaluate central obesity than WC for those with large body size. Individuals with large body size without central obesity might be misdiagnosed due to the high WC (55). Yet, considering that both of WHR and WC are easily available, it is better to assess central obesity comprehensively via measuring both WHR and WC.
Limitations
Our two-sample MR analysis had several strengths: (1) The MR method could minimize confounding and reverse causality compared with conventional observational studies; (2) all summary data were based on the population from European descent, which effectively mitigated the bias of population stratification; (3) to reduce pleiotropic effects, we selected the IVs through a rigorous procedure and no significant pleiotropy was observed via MR-Egger intercept test and MR-PRESSO analysis. Our study had certain limitations: (1) we could not further explore the causal association upon gender, age, etc. via subgroup analyses because our study used the summary-level data rather than individual-level data; (2) our study was confined to individuals of European descent, which limits the generalizability of the findings to other populations; (3) central obesity in our study did not include measurement of visceral fat, which is closely associated with cardiac structure and function as well; (4) all exposure data from the individuals with preserved LVEF in UK Biobank led to healthy worker effect.
Conclusion
In summary, this MR study supports the genetic causality between WHRadjBMI and adverse changes in LV structure and function in preserved EF population. Our findings may strengthen our understanding of the critical role of central obesity in cardiac remodeling.
Data availability statement
Data on WCadjBMI and WHRadjBMI have been contributed by the GIANT investigators and have been downloaded from the IEU OpenGWAS Project (GWAS ID: WCadjBMI, ieu-a-67 and WHRadjBMI, ieu-a-79) at: https://gwas.mrcieu.ac.uk/. Data on LV parameters were contributed by the UK Biobank investigators and have been downloaded from https://www.ebi.ac.uk/gwas/home.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
JZo and YG designed the study. YG and JZe performed the data analysis and drafted the manuscript. FZ, XZ, ZQ, YW, and XH revised the manuscript. JZo supervised the study and acquired funding for the work. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Clinical Competence Improvement Project of Jiangsu Province Hospital (JSPH-MA-2020-3) and Project on New Technology of Jiangsu Province (JX233C202103).
Acknowledgments
We gratefully acknowledge the authors and participants of all GWASs from which we used summary statistics data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1103011/full#supplementary-material
Footnotes
References
1. Kunutsor S, Laukkanen J. Heart failure risk reduction: is fit and overweight or obese better than unfit and normal weight? Eur J Heart Fail. (2019) 21:445–8. doi: 10.1002/ejhf.1440
2. Groenewegen A, Rutten F, Mosterd A, Hoes A. Epidemiology of heart failure. Eur J Heart Fail. (2020) 22:1342–56. doi: 10.1002/ejhf.1858
3. Kenchaiah S, Evans J, Levy D, Wilson P, Benjamin E, Larson M, et al. Obesity and the risk of heart failure. N Engl J Med. (2002) 347:305–13. doi: 10.1056/NEJMoa020245
4. Ndumele C, Matsushita K, Lazo M, Bello N, Blumenthal R, Gerstenblith G, et al. Obesity and subtypes of incident cardiovascular disease. J Am Heart Assoc. (2016) 5:e003921. doi: 10.1161/JAHA.116.003921
5. Eaton C, Pettinger M, Rossouw J, Martin L, Foraker R, Quddus A, et al. Risk factors for incident hospitalized heart failure with preserved versus reduced ejection fraction in a multiracial cohort of postmenopausal women. Circ Heart Fail. (2016) 9:e002883. doi: 10.1161/CIRCHEARTFAILURE.115.002883
6. Rao V, Zhao D, Allison M, Guallar E, Sharma K, Criqui M, et al. Adiposity and incident heart failure and its subtypes: mesa (Multi-Ethnic Study of Atherosclerosis). JACC Heart Fail. (2018) 6:999–1007. doi: 10.1016/j.jchf.2018.07.009
7. Shah S, Borlaug B, Kitzman D, McCulloch A, Blaxall B, Agarwal R, et al. Research priorities for heart failure with preserved ejection fraction: national heart, lung, and blood institute working group summary. Circulation. (2020) 141:1001–26. doi: 10.1161/CIRCULATIONAHA.119.041886
8. Rao V, Fudim M, Mentz R, Michos ED, Felker G. Regional adiposity and heart failure with preserved ejection fraction. Eur J Heart Fail. (2020) 22:1540–50. doi: 10.1002/ejhf.1956
9. Parente E, Harjutsalo V, Forsblom C, Groop P, FinnDiane Study G. The impact of central obesity on the risk of hospitalization or death due to heart failure in type 1 diabetes: a 16-year cohort study. Cardiovasc Diabetol. (2021) 20:153. doi: 10.1186/s12933-021-01340-4
10. Sorimachi H, Omote K, Omar M, Popovic D, Verbrugge F, Reddy Y, et al. Sex and central obesity in heart failure with preserved ejection fraction. Eur J Heart Fail. (2022) 24:1359–70. doi: 10.1002/ejhf.2563
11. Yang D, Liu H, Liu F, Tang N, Guo Z, Ma S, et al. The roles of noncardiomyocytes in cardiac remodeling. Int J Biol Sci. (2020) 16:2414–29. doi: 10.7150/ijbs.47180
12. Shah R, Abbasi S, Heydari B, Rickers C, Jacobs D Jr., Wang L, et al. Insulin resistance, subclinical left ventricular remodeling, and the obesity paradox: mesa (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. (2013) 61:1698–706. doi: 10.1016/j.jacc.2013.01.053
13. Eschalier R, Rossignol P, Kearney-Schwartz A, Adamopoulos C, Karatzidou K, Fay R, et al.. Features of cardiac remodeling, associated with blood pressure and fibrosis biomarkers, are frequent in subjects with abdominal obesity. Hypertension. (2014) 63:740–6. doi: 10.1161/HYPERTENSIONAHA.113.02419
14. Lawlor D, Harbord R, Sterne J, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. (2008) 27:1133–63. doi: 10.1002/sim.3034
15. Boef A, Dekkers O, Le Cessie S. Mendelian randomization studies: a review of the approaches used and the quality of reporting. Int J Epidemiol. (2015) 44:496–511. doi: 10.1093/ije/dyv071
16. Shungin D, Winkler T, Croteau-Chonka D, Ferreira T, Locke A, Magi R, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature. (2015) 518:187–96. doi: 10.1038/nature14132
17. Clarke L, Zheng-Bradley X, Smith R, Kulesha E, Xiao C, Toneva I, et al. The 1000 genomes project: data management and community access. Nat Methods. (2012) 9:459–62. doi: 10.1038/nmeth.1974
18. Staley J, Blackshaw J, Kamat M, Ellis S, Surendran P, Sun B, et al. Phenoscanner: a database of human genotype-phenotype associations. Bioinformatics. (2016) 32:3207–9. doi: 10.1093/bioinformatics/btw373
19. Hemani G, Zheng J, Elsworth B, Wade K, Haberland V, Baird D, et al. The Mr-base platform supports systematic causal inference across the human phenome. Elife. (2018) 7:e34408. doi: 10.7554/eLife.34408
20. Burgess S, Thompson S. Bias in causal estimates from mendelian randomization studies with weak instruments. Stat Med. (2011) 30:1312–23.
21. Aung N, Vargas J, Yang C, Cabrera C, Warren H, Fung K, et al. Genome-wide analysis of left ventricular image-derived phenotypes identifies fourteen loci associated with cardiac morphogenesis and heart failure development. Circulation. (2019) 140:1318–30. doi: 10.1161/CIRCULATIONAHA.119.041161
22. Lee C, Cook S, Lee J, Han B. Comparison of two meta-analysis methods: inverse-variance-weighted average and weighted sum of Z-scores. Genomics Inform. (2016) 14:173–80. doi: 10.5808/GI.2016.14.4.173
23. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
24. Bowden J, Davey Smith G, Haycock P, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
25. Verbanck M, Chen C, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8.
26. Hemani G, Bowden J, Davey Smith G. Evaluating the potential role of pleiotropy in mendelian randomization studies. Hum Mol Genet. (2018) 27:R195–208. doi: 10.1093/hmg/ddy163
27. Bowden J, Del Greco M, Minelli C, Zhao Q, Lawlor D, Sheehan N, et al. Improving the accuracy of two-sample summary-data mendelian randomization: moving beyond the nome assumption. Int J Epidemiol. (2019) 48:728–42. doi: 10.1093/ije/dyy258
28. Burgess S. Sample size and power calculations in mendelian randomization with a single instrumental variable and a binary outcome. Int J Epidemiol. (2014) 43:922–9. doi: 10.1093/ije/dyu005
29. Kenchaiah S, Sesso H, Gaziano J. Body mass index and vigorous physical activity and the risk of heart failure among men. Circulation. (2009) 119:44–52. doi: 10.1161/CIRCULATIONAHA.108.807289
30. Hu G, Jousilahti P, Antikainen R, Katzmarzyk P, Tuomilehto J. Joint effects of physical activity, body mass index, waist circumference, and waist-to-hip ratio on the risk of heart failure. Circulation. (2010) 121:237–44. doi: 10.1161/CIRCULATIONAHA.109.887893
31. Ebong I, Goff D Jr., Rodriguez C, Chen H, Bluemke D, Szklo M, et al. The relationship between measures of obesity and incident heart failure: the multi-ethnic study of atherosclerosis. Obesity (Silver Spring). (2013) 21:1915–22. doi: 10.1002/oby.20298
32. Pandey A, Cornwell W III, Willis B, Neeland I, Gao A, Leonard D, et al. Body mass index and cardiorespiratory fitness in mid-life and risk of heart failure hospitalization in older age: findings from the cooper center longitudinal study. JACC Heart Fail. (2017) 5:367–74. doi: 10.1016/j.jchf.2016.12.021
33. Larsson S, Back M, Rees J, Mason A, Burgess S. Body mass index and body composition in relation to 14 cardiovascular conditions in Uk biobank: a mendelian randomization study. Eur Heart J. (2020) 41:221–6. doi: 10.1093/eurheartj/ehz388
34. Benn M, Marott S, Tybjaerg-Hansen A, Nordestgaard B. Obesity increases heart failure incidence and mortality: observational and mendelian randomisation studies totalling over 1 million individuals. Cardiovasc Res. (2021) doi: 10.1093/cvr/cvab368 [Epub ahead of print].
35. Kim M, Kim W, Khera A, Kim J, Yon D, Lee S, et al. Association between adiposity and cardiovascular outcomes: an umbrella review and meta-analysis of observational and mendelian randomization studies. Eur Heart J. (2021) 42:3388–403. doi: 10.1093/eurheartj/ehab454
36. Borlaug B, Jensen M, Kitzman D, Lam C, Obokata M, Rider O. Obesity and heart failure with preserved ejection fraction: new insights and pathophysiologic targets. Cardiovasc Res. (2022) doi: 10.1093/cvr/cvac120 [Epub ahead of print].
37. Rosch S, Kresoja K, Besler C, Fengler K, Schober A, von Roeder M, et al. Characteristics of heart failure with preserved ejection fraction across the range of left ventricular ejection fraction. Circulation. (2022) 146:506–18. doi: 10.1161/CIRCULATIONAHA.122.059280
38. Ren J, Wu N, Wang S, Sowers J, Zhang Y. Obesity cardiomyopathy: evidence, mechanisms, and therapeutic implications. Physiol Rev. (2021) 101:1745–807. doi: 10.1152/physrev.00030.2020
39. Peterson L, Waggoner A, Schechtman K, Meyer T, Gropler R, Barzilai B, et al. Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue doppler imaging. J Am Coll Cardiol. (2004) 43:1399–404. doi: 10.1016/j.jacc.2003.10.062
40. Wong C, O’Moore-Sullivan T, Leano R, Byrne N, Beller E, Marwick T. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation. (2004) 110:3081–7. doi: 10.1161/01.CIR.0000147184.13872.0F
41. Turkbey E, McClelland R, Kronmal R, Burke G, Bild D, Tracy R, et al. The impact of obesity on the left ventricle: the multi-ethnic study of atherosclerosis (Mesa). JACC Cardiovasc Imaging. (2010) 3:266–74. doi: 10.1016/j.jcmg.2009.10.012
42. Wilner B, Garg S, Ayers C, Maroules C, McColl R, Matulevicius S, et al. Dynamic relation of changes in weight and indices of fat distribution with cardiac structure and function: the dallas heart study. J Am Heart Assoc. (2017) 6:e005897. doi: 10.1161/JAHA.117.005897
43. Yagmur J, Cansel M, Kurtoglu E, Hidayet S, Acikgoz N, Ermis N, et al. Assessment of left atrial volume and function by real time three-dimensional echocardiography in obese patients. Echocardiography. (2017) 34:210–6. doi: 10.1111/echo.13417
44. El Saiedi S, Mira M, Sharaf S, Al Musaddar M, El Kaffas R, AbdelMassih A, et al. Left ventricular diastolic dysfunction without left ventricular hypertrophy in obese children and adolescents: a tissue doppler imaging and cardiac troponin I study. Cardiol Young. (2018) 28:76–84. doi: 10.1017/S1047951117001627
45. Buakhamsri A, Popovic Z, Lin J, Lim P, Greenberg N, Borowski A, et al. Impact of left ventricular volume/mass ratio on diastolic function. Eur Heart J. (2009) 30:1213–21. doi: 10.1093/eurheartj/ehp084
46. Kishi S, Armstrong A, Gidding S, Colangelo L, Venkatesh B, Jacobs D Jr., et al. Association of obesity in early adulthood and middle age with incipient left ventricular dysfunction and structural remodeling: the cardia study (Coronary Artery Risk Development in Young Adults). JACC Heart Fail. (2014) 2:500–8. doi: 10.1016/j.jchf.2014.03.001
47. Adolphe J, Silver T, Childs H, Drew M, Weber L. Short-term obesity results in detrimental metabolic and cardiovascular changes that may not be reversed with weight loss in an obese dog model. Br J Nutr. (2014) 112:647–56. doi: 10.1017/S0007114514001214
48. Partington C, Hodgkiss-Geere H, Woods G, Dukes-McEwan J, Flanagan J, Biourge V, et al. The effect of obesity and subsequent weight reduction on cardiac structure and function in dogs. BMC Vet Res. (2022) 18:351. doi: 10.1186/s12917-022-03449-4
49. Karimian S, Stein J, Bauer B, Teupe C. Improvement of impaired diastolic left ventricular function after diet-induced weight reduction in severe obesity. Diabetes Metab Syndr Obes. (2017) 10:19–25. doi: 10.2147/DMSO.S124541
50. Lee S, Daimon M, Di Tullio M, Homma S, Hasegawa T, Chiou S, et al. Beneficial effect of body weight control on left ventricular diastolic function in the general population: an analysis of longitudinal data from a health check-up clinic. Eur Heart J Cardiovasc Imaging. (2018) 19:136–42. doi: 10.1093/ehjci/jex219
51. Sundstrom J, Bruze G, Ottosson J, Marcus C, Naslund I, Neovius M. Weight loss and heart failure: a nationwide study of gastric bypass surgery versus intensive lifestyle treatment. Circulation. (2017) 135:1577–85. doi: 10.1161/CIRCULATIONAHA.116.025629
52. Patel K, Bahnson J, Gaussoin S, Johnson K, Pi-Sunyer X, White U, et al. Association of baseline and longitudinal changes in body composition measures with risk of heart failure and myocardial infarction in type 2 diabetes: findings from the look ahead trial. Circulation. (2020) 142:2420–30. doi: 10.1161/CIRCULATIONAHA.120.050941
53. Owan T, Avelar E, Morley K, Jiji R, Hall N, Krezowski J, et al. Favorable changes in cardiac geometry and function following gastric bypass surgery: 2-year follow-up in the utah obesity study. J Am Coll Cardiol. (2011) 57:732–9. doi: 10.1016/j.jacc.2010.10.017
54. Lotta L, Wittemans L, Zuber V, Stewart I, Sharp S, Luan J, et al. Association of genetic variants related to gluteofemoral vs abdominal fat distribution with type 2 diabetes, coronary disease, and cardiovascular risk factors. JAMA. (2018) 320:2553–63. doi: 10.1001/jama.2018.19329
Keywords: central obesity, left ventricular structure and function, causal association, Mendelian randomization, waist-to-hip ratio
Citation: Gao Y, Zeng J, Zou F, Zhang X, Qian Z, Wang Y, Hou X and Zou J (2023) Causal effect of central obesity on left ventricular structure and function in preserved EF population: A Mendelian randomization study. Front. Cardiovasc. Med. 9:1103011. doi: 10.3389/fcvm.2022.1103011
Received: 19 November 2022; Accepted: 08 December 2022;
Published: 09 January 2023.
Edited by:
Gen-Min Lin, Hualien Armed Forces General Hospital, TaiwanReviewed by:
Alessandro Mengozzi, University of Pisa, ItalyTimothy P. Fitzgibbons, University of Massachusetts Medical School, United States
Copyright © 2023 Gao, Zeng, Zou, Zhang, Qian, Wang, Hou and Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiangang Zou,  amd6b3VAbmptdS5lZHUuY24=
amd6b3VAbmptdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Yue Gao
Yue Gao Jiaxin Zeng1†
Jiaxin Zeng1† Xinwei Zhang
Xinwei Zhang Zhiyong Qian
Zhiyong Qian Jiangang Zou
Jiangang Zou