
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med., 09 December 2022
Sec. Cardiovascular Pharmacology and Drug Discovery
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.1073148
Background: In this systematic review, we aimed to investigate the efficacy and safety of adding low-molecular-weight heparin (LMWH) or unfractionated heparin to low-dose aspirin (LDA) started ≤16 weeks'gestation in the prevention of preeclampsia (PE) in high-risk women.
Methods: PubMed, Cochrane Library, Embase, and ClinicalTrials.gov databases were searched from their inception to April 2022 for randomized controlled trials (RCTs) that to determine whether the combined treatment of LMWH and LDA is better than single anticoagulant drugs in preventing PE and improving live birth rate of fetus in high-risk women with pregnancy ≤16 weeks. We also searched Embase, OVID MEDLINE and OVID MEDLINE in-process using the OVID platform.
Results: 14 RCTs involving 1,966 women were found. The LMWH (or unfractionated heparin) and LDA groups included 1,165 wemen, and the LDA group included 960 women. The meta-analysis showed that the addition of LMWH to LDA reduced the risk of PE (RR: 0.59, 95% CI: 0.44-0.79, P < 0.05), small-for-gestational age (SGA, RR: 0.71, 95% CI: 0.52-0.97, P = 0.03), fetal and neonatal death (RR: 0.45, 95% CI: 0.23-0.88, P = 0.02) and gestational hypertension (RR: 0.47, 95% CI: 0.25-0.90, P = 0.02). It is worth emphasizing that LMWH (or unfractionated heparin) combined with LDA did not increase the risk of bleeding.
Conclusions: LMWH combined with LDA can effectively improve the pregnancy outcome of women with high risk factors for PE and its complications. Although this study showed that combined medication also did not increase the risk of bleeding, but such results lack the support of large sample size studies. The clinical safety analysis of LMWH combined with LDA in patients with PE should be more carried out.
Preeclampsia (PE) is characterized by hypertension and proteinuria, and it is one of the important factors leading to maternal and perinatal death. The incidence rate of all pregnant women is about 2–8% (1, 2). The main manifestations of PE are maternal and multiple organ and system damage, as well as adverse pregnancy outcomes such as fetal growth restriction (FGR) and placental abruption. At the same time, PE can have adverse effects on re-pregnancy. Women with a history of PE are 25–65% likely to have PE again, 3% may have placental abruption, and 10% may have FGR again (3). The causes are multifactorial, including dysinvasion of uterine spiral artery trophoblast, injury of vascular endothelial cells and transitional activation of maternal immune system (4–7). The dysfunction or disorder of hemostasis, coagulation, anticoagulation and fibrinolysis system caused by many factors can eventually lead to the abnormal increase of coagulation function and the decrease of fibrinolysis function, so that the blood is in a hypercoagulable state.
At present, there are evidence-based evidences that the application of aspirin in high-risk groups of PE can effectively prevent the onset of PE and reduce adverse pregnancy outcomes (8, 9). Studies have also confirmed that low molecular weight heparin (LMWH) reduces the incidence of PE, perinatal death and FGR in high-risk pregnant women, and improves the pregnancy outcome of patients (10, 11). In addition, because LMWH cannot pass through the placenta, there is almost no direct risk to the fetus, and massive hemorrhage or placental abruption are rarely observed in pregnant women treated with LMWH (12).
In recent years, some studies have reported the effect and safety of aspirin combined with LMWH in the prevention of PE and its complications. In this study, meta-analysis method was used to conduct a comprehensive evaluation of these related studies to make up for the small sample size of a single study. The primary purpose is to determine whether the combined treatment of LMWH and aspirin is better than single anticoagulant drugs in preventing PE and its complications in high-risk women with pregnancy ≤16 weeks.
We performed a meta-analysis and wrote the article by conforming to the requirements illustrated in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (13). We compared the treatment with LMWH or heparin (with low-dose aspirin) with LMWH alone or low-dose aspirin (LDA) alone in women at high risk of PE.
PubMed, Cochrane Library, Embase, and ClinicalTrials.gov databases were searched from their inception to April 2022. We also searched Embase, OVID MEDLINE and OVID MEDLINE in-process using the OVID platform. The search terms that we used in the Pubmed database were as follows: #1 “pre-eclampsia” [title/abstract] OR “preeclampsia” [title/abstract] OR “PE” [title/abstract] OR “eclampsia” [title/abstract]; #2 “heparin” [title/abstract] OR “Low-Molecular-Weightheparin” [title/abstract] OR “LMWH” [title/abstract]; #3 “aspirin” [title/abstract]; #4 #1 AND #2 AND #3. The databases were searched for published studies in English, including systematic reviews and meta-analysis, that were related to the treatment with LMWH or heparin (with LDA) in women at high risk of PE. After the search is complete, the documents were exported in full text format.
Inclusion criteria were: (1) randomized controlled trials (RCTs) studies comparing LDA with LMWH or heparin for prevention of PE; (2) studies including women who had any known high risk factors for PE and its complications, such as adverse obstetric history of previous PE, small-for-gestational age (SGA) or FGR, placental abruption, and medical history including thrombophilia, autoimmune disease, and other chronic diseases; (3) published paper.
Animal experiments and non-RCTs were excluded from the meta-analysis. In addition, studies were excluded if they included other treatment options.
The primary outcomes were PE and live birth rate, with secondary outcomes including placental abruption, severe PE (sPE), gestational hypertension, FGR or SGA, fetal and neonatal death and HELLP (hemolysis, elevated liver enzymesand low platelet count) syndrome. We also analyzed the presence of adverse events such as intrapartum or postpartum hemorrhage.
In this context, it is recommended that women are diagnosed with PE if they present with the following severe features: systolic blood pressure of 140 mmHg or more or diastolic blood pressure of 90 mmHg or more on two occasions at least 4 h apart after 20weeks of gestation in a woman with a previously normal blood pressure plus proteinuria, defined asurinary excretion of 300 mg or more per 24 h urine collection (or this amount extrapolated from a timed collection)orc Protein/creatinine ratio of 0.3 mg/dL or more orc Dipstick reading of 2+(used only if other quantitativemethods not available) (14). The women are diagnosed with sPE when the following conditions are met: systolic blood pressure ≥160 mm Hg and/or diastolic blood pressure ≥110 mm Hg; Urine protein ic blood pressure more or the meria was strongly positive; brain nerve symptoms persist and worsen; persistent epigastric pain; serum aspartate aminotransferase significantly increased, creatinine >106 μmol/L; hypoalbuminemia, pleural or peritoneal effusion, heart failure, pulmonary edema, oligohydramnios, abnormal blood system indicators and other manifestations may occur at the same time. In addition, the main distinction between SGA and FGR is that a SGA fetus may be small but not at increased risk of adverse perinatal outcome, while a fetus with size above the 10th percentile may be FGR and at increased risk of adverse perinatal and long-term outcome (15).
Two authors (BX and YY) independently screened and retrieved the relevant literatures. Then, after reading the title and abstract of the article, they excluded the studies that did not meet the inclusion criteria. Full texts of final selected articles and data extraction were performed independently by other 2 researchers (LZ and YW), and any discrepancies were resolved by discussion.
The extracted data included names of the first authors of selected articles, basic information on studies (research design, patient groups, interventions, doses of medications, number of patients, duration of treatment), characteristics of patients (gestational age, participant condition), and reported outcomes (PE, live birth rate, sPE, gestational hypertension, placental abruption, FGR or SGA, fetal and neonatal death, HELLP syndrome and intrapartum or postpartum hemorrhage).
The risk of bias of RCTs was assessed using the Cochrane Collaboration's tool (16) based on seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. Two reviewers (YY and BX) independently finished the quality assessments. In the case of a disagreement, a third reviewer (LZ) was involved in finally reaching a consensus through negotiation.
RevMan 5.3 software provided by the Cochrane Collaboration Network was used for meta-analysis of the included studies. The software was also used to draw a forest map. For continuous variables, data have been presented as the mean difference (MD) with 95% confidence interval (CI) between the experimental and control groups. For dichotomous outcome data, the risk ratio (RR) or odds ratio (OR) with 95% CI was calculated. The chi-square test was used to assess heterogeneity between studies, and combined with I2 to quantitatively judge the extent of heterogeneity. The fixed-effects model was applied at P > 0.1 and I2 < 40%, which showed that there was little heterogeneity between studies. Otherwise, the random-effects model was used for analysis (17).
Begg's and Egger's tests of asymmetry were performed using the statistical software Stata 12.0 to assess potential publication bias. The tests were also used to identify outliers (18).
Thousand six hundred and seventy eight articles were obtained from the search through the above mentioned databases. Of these, 1,146 articles were removed due to record duplication and 447 studies were excluded based on their titles and abstracts. Finally, a total of 14 studies that included 1,966 women were considered in the systematic review and meta-analysis based on the inclusion and exclusion criteria (Figure 1).
The baseline characteristics of the included studies were presented in the Table 1. Eight articles used enoxaparin (19, 20, 22, 24, 25, 27, 29, 30), three articles used dalteparin (21, 23, 26), one article used nadroparin (28), one did not specify the type of LMWH (32) and one used unfractionated heparin (31). The gestational age of included pregnant women varied among different studies, but all women were randomly divided between the first positive pregnancy test and 16 weeks' gestation. Because patients' conditions are heterogeneous across the different studies with different baseline characteristics, which may lead to heterogeneity in outcome measures, we performed subgroup analysis.
The Cochrane Collaboration's Risk of Bias tool (16) was used to evaluate the risk of bias of included studies. The results showed that the quality of most studies is acceptable. Most RCTs had an unclear risk of bias for allocation concealment, blinding of the outcome, and incomplete outcome data, considering that detailed information was not provided.
The highest risk of bias occurred in the blinding of participants and personnel. This is because neither study personnel nor participants in five studies (20–22, 26, 29) were blinded to treatment assignment, as placebo injections were not considered to be ethically acceptable during pregnancy in. Quality assessment of the included studies is shown in Figure 2.
Among all the included studies, six studies (20–22, 25, 26, 30) recruited women with a history of PE, two of which included only women with thrombophilia; eight studies (19, 23, 24, 27–29, 31, 32) recruited women with a history of miscarriages, one of them in women with thrombophilia and one of them including women with factor V Leiden mutation (FVL, a risk factor for deep vein thrombosis and pulmonary embolism). Due to the wide heterogeneity between the inclusion criteria of different studies, we performed subgroup analysis of the results according to the entry criteria.
We observed that the data from ten studies (19–22, 25–30) included (n = 1,452), the pooled results obtained using a fixed-effects model showed that the addition of LMWH to LDA reduced the risk of PE (RR: 0.59, 95% CI: 0.44–0.79, P < 0.05). In the subgroup analysis, the addition of LMWH to LDA reduced the risk of PE in women with a history of PE (RR: 0.62, 95% CI: 0.45–0.87, P < 0.05) and miscarriages (RR: 0.50, 95% CI: 0.27–0.90, P < 0.05), respectively (Figure 3A).
Five studies (19, 27, 28, 31, 32) (n=785) reported the live birth rate, but these studies only included women with a history of miscarriages. The pooled results obtained using a fixed-effects model showed thatthe addition of LMWH to LDA did not improve the live birth rate (RR: 1.06, 95% CI: 0.96–1.16, P > 0.05) (Figure 3B).
We observed that the data from nine studies (19, 20, 22, 25–29, 32) included (n = 1,464), the pooled results obtained using a fixed-effects model showed that the addition of LMWH to LDA did not reduced the risk of placental abruption (RR: 0.58, 95% CI: 0.33–1.02, P = 0.06). In the subgroup analysis, the addition of LMWH to LDA did not reduced the risk of placental abruptionin women with a history of PE (RR: 0.96, 95% CI: 0.45–2.05, P = 0.91). Conversely, in women with a history of miscarriages, the addition of LMWH to LDA can reduce the risk of placental abruption (RR: 0.30, 95% CI: 0.12-0.77, P = 0.01) (Figure 4A).
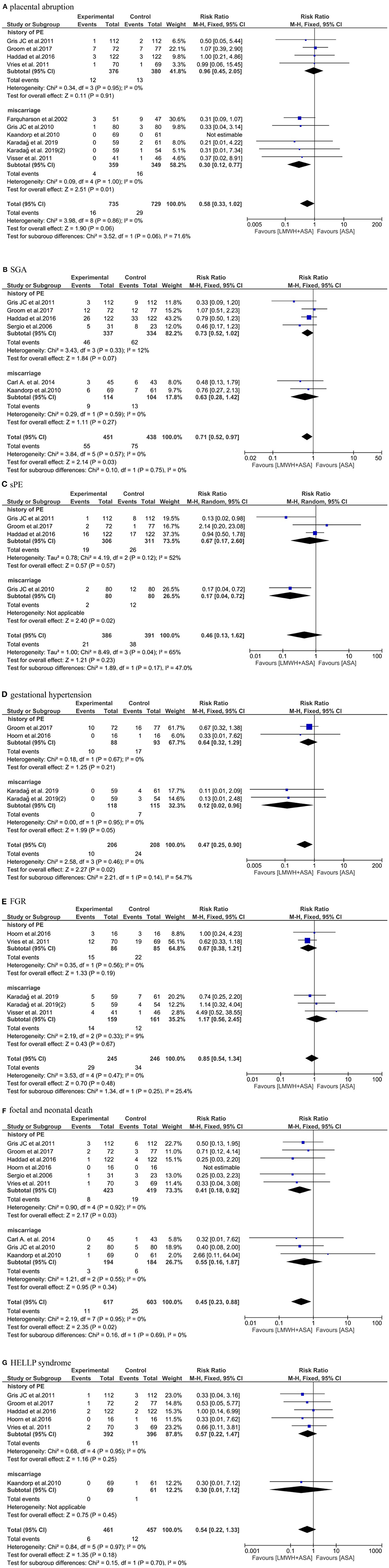
Figure 4. Meta-analysis of (A) placental abruption. (B) SGA. (C) sPE. (D) gestational hypertension. (E) FGR. (F) fetal and neonatal death. (G) HELLP syndrome.
Six studies (20, 22, 23, 25, 28, 30) (n = 889) reported the SGA, the pooled results obtained using a fixed-effects model showed that the addition of LMWH to LDA reduced the the risk of SGA (RR: 0.71, 95% CI: 0.52–0.97, P = 0.03). But in the subgroup analysis, the addition of LMWH to LDA did not reduced the risk of SGA in women with a history of PE (RR: 0.73, 95% CI: 0.52–1.02, P = 0.07) and miscarriages (RR: 0.63, 95% CI: 0.28–1.42, P = 0.27), respectively (Figure 4B).
Four studies (20, 22, 25, 29) (n = 777) reported the sPE, the pooled results obtained using a random-effects model showed that the addition of LMWH to LDA did not reduced the the risk of sPE (RR: 0.46, 95% CI: 0.13–1.62, P = 0.23). In the subgroup analysis, the addition of LMWH to LDA did not reduced the risk of sPE in women with a history of PE (RR: 0.67, 95% CI: 0.17–2.60, P = 0.57). Conversely, in women with a history of miscarriages, the addition of LMWH to LDA can reduce the risk of sPE (RR: 0.17, 95% CI: 0.04–0.72, P = 0.02) (Figure 4C).
Three studies (19–21) (n = 414) reported the gestational hypertension, the pooled results obtained using a fixed-effects model showed that the addition of LMWH to LDA reduced the the risk of gestational hypertension (RR: 0.47, 95% CI: 0.25–0.90, P = 0.02). In the subgroup analysis, the addition of LMWH to LDA did not reduced the risk of gestational hypertension in women with a history of PE (RR: 0.64, 95% CI: 0.32–1.29, P = 0.21). However, in women with a history of miscarriages, the addition of LMWH to LDA can reduce the risk of gestational hypertension (RR: 0.12, 95% CI: 0.02–0.96, P = 0.05) (Figure 4D).
Four studies (19, 21, 26, 27) (n = 491) reported the FGR, the pooled results obtained using a fixed-effects model showed that the addition of LMWH to LDA did not reduced the the risk of FGR (RR: 0.85, 95% CI: 0.54–1.34, P = 0.48). In the subgroup analysis, the addition of LMWH to LDA also did not reduced the risk of FGR in women with a history of PE (RR: 0.67, 95% CI: 0.38–1.21, P = 0.19) and miscarriages (RR: 1.17, 95% CI: 0.56–2.45, P = 0.67), respectively (Figure 4E).
Nine studies (20–23, 25, 26, 28–30) (n = 1,220) reported the fetal and neonatal death, the pooled results obtained using a fixed-effects model showed that the addition of LMWH to LDA reduced the risk of fetal and neonatal death (RR: 0.45, 95% CI: 0.23–0.88, P = 0.02). In the subgroup analysis, the addition of LMWH to LDA also can reduced the risk of fetal and neonatal deathin women with a history of PE (RR: 0.41, 95% CI: 0.18–0.92, P = 0.03), but the addition of LMWH to LDA did not reduce the risk of fetal and neonatal death in women with a history of miscarriages (RR: 0.55, 95% CI: 0.16–1.87, P = 0.34) (Figure 4F).
Six studies (20–22, 25, 26, 28) (n = 918) reported the HELLP syndrome, the pooled results obtained using a fixed-effects model showed that the addition of LMWH to LDA did not obviously reduced the risk of HELLP syndrome (RR: 0.54, 95% CI: 0.22–1.33, P = 0.18). In the subgroup analysis, the addition of LMWH to LDA also did not reduced the risk of HELLP syndrome in women with a history of PE (RR: 0.57, 95% CI: 0.22–1.47, P = 0.25) and miscarriages (RR: 0.30, 95% CI: 0.01–7.12, P = 0.45), respectively. Although the results were not statistically different, it can be seen from the Figure 4G that LDA combined with LMWH is more beneficial to prevent the occurrence of HELLP syndrome and is a more favorable treatment option (Figure 4G).
Three studies (25, 27, 29) (n = 471) reported the intrapartum or postpartum hemorrhage, the pooled results obtained using a fixed-effects model showed that the addition of LMWH to LDA did not increased the risk of intrapartum or postpartum hemorrhage (RR: 0.66, 95% CI: 0.34–1.26, P = 0.20) (Figure 5).
The results of Begg's and Egger's tests showed no evidence of publication bias for all the outcomes except PE, as all the results showed P > 0.05 (Table 2). However, there was evidence of publication bias for PE, as the P-value from Egger's tests was 0.007, the P value from Begg's tests was 0.02.
The results of this systematic review and meta-analysas showed that among women with a history of PE, LMWH combined with LDA can effectively reduce the incidence of PE and fetal and neonatal death. However, in the prevention of placental abruption, sPE, gestational hypertension, SGA and FGR, compared with LDA or LMWH alone, the combination of the drugs appeared to have no benefit. Nevertheless, the combination of drugs reduced the incidence of gestational hypertension, sPE, SGA and FGR in women who had previously developed PE. In women with a history of miscarriage, LMWH combined with LDA can significantly reduce the incidence of PE, placental abruption, sPE and gestational hypertension. However, there was no significant difference between the combined use of the two drugs and the use of a single drug in improving the live birth rate of fetus and reducing the incidence of SGA, FRG and fetal and neonatal death. Despite the results, the combination of drugs increased the number of newborns and decreased the number of SGA and fetal and neonatal death in women with previous miscarriages. In addition, no major side effects such as intrapartum or postpartum hemorrhage were observed in women treated with LMWH combined with LDA, and the combined medication reduced the incidence of HELLP syndrome.
During our search in the above mentioned databases, we found a meta-analysis (8) reported on LMWH combined with aspirin in preventing PE and SGA. The meta-analysis involved 8 RCTs, which were divided into two subgroups according to women's history of PE or miscarriage, which is roughly the same as our study grouping criteria. The results of this study showed that the combination of drugs could not effectively reduce the incidence of PE and SGA in women with a history of miscarriage. However, after we increased the sample size, we came to a conclusion inconsistent with Roberge et al. (8). We found that in high-risk women, combination therapy not only reduced PE, but also reduced fetal and neonatal death.
Aspirin inhibits the production of prostaglandin and thromboxane A2 by irreversible acetylation of serine residues in the active center of cyclooxygenase 1 (COX-1) and cyclooxygenase 2 (COX-2), and finally inhibits platelet aggregation (33), thereby reducing thrombosis. Aspirin can also inhibit the production of thromboxane and prostacyclin in trophoblast, thus effectively preventing the contraction of placental blood vessels and avoiding insufficient placental blood flow. Additionally, aspirin plays a therapeutic role in preeclampsia through anti platelet aggregation, prevention of oxidative stress, improvement of cell apoptosis, etc (34, 35).
LMWH strengthens its inactivation of coagulation factors IIa, IXa and Xa by combining with antithrombin, thus reducing the generation of corresponding coagulation factors. It not only has the effects of anticoagulant, preventing placental thrombosis and placental infarction (36), but also has the effects of anti-inflammatory, anti-tumor and promoting angiogenesis (10, 37). LMWH can promote the conversion of plasminogen to plasmin, reduce blood viscosity, change blood rheology, inhibit vasoconstriction, improve organ blood perfusion, protect vascular endothelium, strengthen placental exchange function, increase fetal urine volume and amniotic fluid volume, promote fetal growth and development, and improve perinatal prognosis (10). With further research, it was found that LMWH not only acts as an anticoagulant, but also has an obvious effect on the development and invasion of trophoblast (38). These results make LMWH as a potential drug for the prevention of PE and SGA.
Studies have shown that LDA combined with LMWH may offset the adverse reactions caused by some drugs and increase the effect of the two drugs (39). For pregnant women with underlying diseases, LDA should not be given only. For the impact of factors such as hypercoagulability and thrombophilia on placenta and fetus, anticoagulant drugs such as LMWH may also be needed (40). Our results also show that LMWH combined with LDA can significantly improve the pregnancy outcome of high-risk women without increasing the risk of bleeding.
The strengths of our study include the inclusion of more RCT studies and more patients comparing the effects of combination and single drug than other meta-analysis. Although there may be heterogeneity in our results, it also proves to some extent that our results are applicable to women with high risk factors for PE and its complications. At the same time, we also evaluated the bleeding risk of combined drugs, which is also a key safety index to be considered in the process of clinical use.
This study has some limitations. First, the quality grades of the included studies were inconsistent. Among them, five studies (20–22, 26, 29) did not adopt blind design, and the random allocation method of two studies (30, 31) was unclear. Second, because many women chose to withdraw from the trial study, there was uncertainty about the treatment compliance of the subjects. Third, four of all included RCTs in recurrent miscarriage did not report whether LMWH combined with LDA could reduce the incidence of PE. In addition, only three of the 14 included studies reported on intrapartum or postpartum hemorrhage, leading to publication bias in the results. Fourth, the study did not evaluate the impact of long-term maternal and infant outcomes. More large sample, multicenter and long-term follow-up studies are needed to confirm the long-term impact of combined drugs on maternal and infant outcomes. Finally, the Begg's and Egger's test results of PE showed potential publication bias, indicating that the statistical analysis results may be unstable, and it is recommended to carry out studies with higher quality and more rigorous experimental design.
Therefore, the efficacy of aspirin combined with LMWH is better than that of single drug, and can improve maternal and infant outcomes to a certain extent. It is worthy of clinical promotion, but it needs to be further verified by strictly designed, large sample and multi center randomized controlled trials.
The results of this meta-analysis showed that LMWH combined with LDA can effectively improve the pregnancy outcome of women with high risk factors and reduce the incidence of PE, SGA, gestational hypertension, fetal and neonatal death. At the same time, there are three studies that report women's bleeding. The results show that the combination of drugs does not increase the risk of bleeding, but such results lack the support of large sample size studies. The clinical safety analysis of LMWH combined with LDA in patients with PE should be more carried out.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
LZ and YaW planned and designed the study. LZ, BX, YY, and YuW screened studies and performed the systematic review and extracted data from included studies. YY, BX, and LZ analyzed the data and performed the quality assessment of studies. LZ drafted the manuscript. All authors contributed to the discussion section of the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the Chinese Peking Union Medical College, Basic Scientific Research Project of Central University (No. 2019XK320078, BJ-2019-092) and Sailing Project of Beijing Hospital Clinical Research in 2022 (BJ-2022-155).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. (2012) 36:56–9. doi: 10.1053/j.semperi.2011.09.011
2. Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “great obstetrical syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. (2011) 204:193–201. doi: 10.1016/j.ajog.2010.08.009
3. Leaf RK, Connors JM. The role of anticoagulants in the prevention of pregnancy complications. Clin Appl Thromb Hemost. (2017) 23:116–23. doi: 10.1177/1076029615615972
4. Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. (2006) 27:939–58. doi: 10.1016/j.placenta.2005.12.006
5. Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. (2005) 308:1592–4. doi: 10.1126/science.1111726
6. Steegers EA, Von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. (2016) 387:999–1011. doi: 10.1016/S0140-6736(15)00070-7
7. Redman CW, Sargent IL. Pre-eclampsia, the placenta and the maternal systemic inflammatory response–a review. Placenta. (2003) 24(Suppl A):S21–7. doi: 10.1053/plac.2002.0930
8. Roberge S, Demers S, Nicolaides KH, Bureau M, Côté S, Bujold E. Prevention of pre-eclampsia by low-molecular-weight heparin in addition to aspirin: a meta-analysis. Ultrasound Obstet Gynecol. (2016) 47:548–53. doi: 10.1002/uog.15789
9. Cui Y, Zhu B, Zheng F. Low-dose aspirin at ≤ 16 weeks of gestation for preventing preeclampsia and its maternal and neonatal adverse outcomes: a systematic review and meta-analysis. Exp Ther Med. (2018) 15:4361–9. doi: 10.3892/etm.2018.5972
10. McLaughlin K, Baczyk D, Potts A, Hladunewich M, Parker JD, Kingdom JC. Low molecular weight heparin improves endothelial function in pregnant women at high risk of preeclampsia. Hypertension. (2017) 69:180–8. doi: 10.1161/HYPERTENSIONAHA.116.08298
11. Rodger MA, Carrier M, Le Gal G, Martinelli I, Perna A, Rey E, et al. Meta-analysis of low-molecular-weight heparin to prevent recurrent placenta-mediated pregnancy complications. Blood. (2014) 123:822–8. doi: 10.1182/blood-2013-01-478958
12. Greer IA. Anticoagulants in pregnancy. J Thromb Thrombolysis. (2006) 21:57–65. doi: 10.1007/s11239-006-5578-5
13. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
14. Gestational hypertension and preeclampsia: ACOG practice bulletin summary Number 222. Obstet Gynecol. (2020) 135:1492–5. doi: 10.1097/AOG.0000000000003892
15. Lees CC, Stampalija T, Baschat A, da Silva Costa F, Ferrazzi E, Figueras F, et al. ISUOG Practice Guidelines: diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet Gynecol. (2020) 56:298–312. doi: 10.1002/uog.22134
16. Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
17. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ,. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons (2021). Available online at: https://training.cochrane.org/handbook/archive/v6.2
18. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
19. Karadag C, Akar B, Gönenç G, Aslancan R, Yilmaz N, Çalişkan E. Aspirin, low molecular weight heparin, or both in preventing pregnancy complications in women with recurrent pregnancy loss and factor V Leiden mutation. J Matern Fetal Neonatal Med. (2020) 33:1934–9. doi: 10.1080/14767058.2019.1671348
20. Groom KM, McCowan LM, Stone PR, Chamley LC, McLintock C. Enoxaparin for the prevention of preeclampsia and intrauterine growth restriction in women with a prior history - an open-label randomized trial (the EPPI trial): study protocol. BMC Pregnancy Childbirth. (2016) 16:367. doi: 10.1186/s12884-016-1162-y
21. van van Hoorn ME, Hague WM, van Pampus MG, Bezemer D, de Vries JI. Low-molecular-weight heparin and aspirin in the prevention of recurrent early-onset pre-eclampsia in women with antiphospholipid antibodies: the FRUIT-RCT. Eur J Obstet Gynecol Reprod Biol. (2016) 197:168–73. doi: 10.1016/j.ejogrb.2015.12.011
22. Haddad B, Winer N, Chitrit Y, Houfflin-Debarge V, Chauleur C, Bages K, et al. Enoxaparin and aspirin compared with aspirin alone to prevent placenta-mediated pregnancy complications: a randomized controlled trial. Obstet Gynecol. (2016) 128:1053–63. doi: 10.1097/AOG.0000000000001673
23. Laskin CA, Spitzer KA, Clark CA, Crowther MR, Ginsberg JS, Hawker GA, et al. Low molecular weight heparin and aspirin for recurrent pregnancy loss: results from the randomized, controlled HepLDA trial. J Rheumatol. (2009) 36:279–87. doi: 10.3899/jrheum.080763
24. Elmahashi MO, Elbareg AM, Essadi FM, Ashur BM, Adam I. Low dose aspirin and low-molecular-weight heparin in the treatment of pregnant Libyan women with recurrent miscarriage. BMC Res Notes. (2014) 7:23. doi: 10.1186/1756-0500-7-23
25. Gris JC, Chauleur C, Molinari N, Mares P, Fabbro-Peray P, Quere I. Addition of enoxaparin to aspirin for the secondary prevention of placental vascular complications in women with severe pre-eclampsia. The pilot randomised controlled NOH-PE trial. Thromb Haemost. (2011) 106:1053–61. doi: 10.1160/TH11-05-0340
26. de Vries JI, Van Pampus MG, Hague WM, Bezemer PD, Joosten JH, Fruit Investigators. Low-molecular-weight heparin added to aspirin in the prevention of recurrent early-onset pre-eclampsia in women with inheritable thrombophilia: the FRUIT-RCT. J Thromb Haemost. (2012) 10:64–72. doi: 10.1111/j.1538-7836.2011.04553.x
27. Visser J, Ulander VM, Helmerhorst FM, et al. Thromboprophylaxis for recurrent miscarriage in women with or without thrombophilia. HABENOX: a randomised multicentre trial. Thromb Haemost. (2011) 105:295–301. doi: 10.1160/TH10-05-0334
28. Kaandorp SP, Goddijn M, Van Der Post JA, Hutten BA, Verhoeve HR, Hamulyák K, et al. Aspirin plus heparin or aspirin alone in women with recurrent miscarriage. N Engl J Med. (2010) 362:1586–96. doi: 10.1056/NEJMoa1000641
29. Gris JC, Chauleur C, Faillie JL, Baer G, Marès P, Fabbro-Peray P, et al. Enoxaparin for the secondary prevention of placental vascular complications in women with abruptio placentae. The pilot randomised controlled NOH-AP trial. Thromb Haemost. (2010) 104:771–9. doi: 10.1160/TH10-03-0167
30. Sergio F, Maria Clara DA, Gabriella F, Giorgia S, Sara DC, Giancarlo P, et al. Prophylaxis of recurrent preeclampsia: low-molecular-weight heparin plus low-dose aspirin versus low-dose aspirin alone. Hypertens Pregnancy. (2006) 25:115–27. doi: 10.1080/10641950600745517
31. Goel N, Tuli A, Choudhry R. The role of aspirin versus aspirin and heparin in cases of recurrent abortions with raised anticardiolipin antibodies. Med Sci Monit. (2006) 12:CR132–6.
32. Farquharson RG, Quenby S, Greaves M. Antiphospholipid syndrome in pregnancy: a randomized, controlled trial of treatment. Obstet Gynecol. (2002) 100:408–13. doi: 10.1097/00006250-200212000-00032
33. Hossain N, Paidas MJ. Adverse pregnancy outcome, the uteroplacental interface, and preventive strategies. Semin Perinatol. (2007) 31:208–12. doi: 10.1053/j.semperi.2007.05.002
34. Orendi K, Kivity V, Sammar M, Grimpel Y, Gonen R, Meiri H, et al. Placental and trophoblastic in vitro models to study preventive and therapeutic agents for preeclampsia. Placenta. (2011) 32:S49–54. doi: 10.1016/j.placenta.2010.11.023
35. Schröder H. Nitric oxide and aspirin: a new mediator for an old drug. Am J Ther. (2009) 16:17–23. doi: 10.1097/MJT.0b013e318164bd60
36. ACOG Committee Opinion No. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. (2018) 132:e44–52. doi: 10.1097/AOG.0000000000002708
37. Mastrolia SA, Mazor M, Holcberg G. The physiologic anticoagulant and anti-inflammatory role of heparins and their utility in the prevention of pregnancy complications. Thromb Haemost. (2015) 113:1236–46. doi: 10.1160/TH14-10-0848
38. Bolnick AD, Bolnick JM, Kohan-Ghadr HR, Kilburn BA, Pasalodos OJ, Singhal PK, et al. Enhancement of trophoblast differentiation and survival by low molecular weight heparin requires heparin-binding EGF-like growth factor. Hum Reprod. (2017) 32:1218–29. doi: 10.1093/humrep/dex069
39. Han CS, Mulla MJ, Brosens JJ, Chamley LW, Paidas MJ, Lockwood CJ, et al. Aspirin and heparin effect on bLDAl and antiphospholipid antibody modulation of trophoblast function. Obstet Gynecol. (2011) 118:1021–8. doi: 10.1097/AOG.0b013e31823234ad
Keywords: low-dose-aspirin, low-molecular-weight heparin, prevention, preeclampsia, meta-analysis
Citation: Zheng L, Xia B, Yuan Y, Wang Y and Wang Y (2022) Low-molecular-weight heparin in addition to low-dose aspirin for preventing preeclampsia and its complications: A systematic review and meta-analysis. Front. Cardiovasc. Med. 9:1073148. doi: 10.3389/fcvm.2022.1073148
Received: 18 October 2022; Accepted: 23 November 2022;
Published: 09 December 2022.
Edited by:
Yu Sun, Princeton University, United StatesReviewed by:
Yixiao Wang, Southeast University, ChinaCopyright © 2022 Zheng, Xia, Yuan, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Zheng, zhenglis1270755@163.com; Yan Wang, wanyan4127@bjhmoh.cn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.