
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Cardiovasc. Med. , 26 January 2023
Sec. General Cardiovascular Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.1046436
This article is part of the Research Topic Case Reports in General Cardiovascular Medicine: 2022 View all 35 articles
 Muralidharan Thoddi Ramamurthy1*
Muralidharan Thoddi Ramamurthy1* Vinod Kumar Balakrishnan1
Vinod Kumar Balakrishnan1 Sini Sunny2
Sini Sunny2 Abinayaa Rajkumar3
Abinayaa Rajkumar3 Sandhya Sundaram1
Sandhya Sundaram1 Preetam Krishnamurthy1
Preetam Krishnamurthy1 Nagendra Boopathy Senguttuvan1
Nagendra Boopathy Senguttuvan1 Arunan Murali4
Arunan Murali4 J. D. Roy Santhosham4
J. D. Roy Santhosham4 Kalaiselvi Periandavan3
Kalaiselvi Periandavan3 Namakkal S. Rajasekaran2,5*
Namakkal S. Rajasekaran2,5*The reported incidence of liposarcomas in ~2,000 cases annually results in about 30% of myxoid liposarcomas. Cardiac myoxid liposarcomas are very rare; their presentation could be cardiac tamponade, due to direct compression of the tumor and/or pericardial effusion. In this report, we describe a patient who presented with pericardial effusion secondary to myoxid liposarcomas from the right atrium, an extremely rare presentation of liposarcomas in the heart. We also present non-invasive imaging through echocardiography, CECT thorax and FDG PET scans, followed by a CT-guided mass biopsy. Histopathology of the right atrial mass demonstrated myxoid liposarcoma positive for the S100 tumor marker.
A 53-year-old woman, with a history of having been hypertensive for 8 years but displaying no prior record of cardiac illness, came to the hospital with complaints of shortness of breath (class IV) for 1 week. This was aggravated 2 days prior to her visit, associated with orthopnea, a dry cough, and a low-grade intermittent fever. On examination, Pulsus paradoxus and Kussmaul's signs were present.
The ECG showed sinus tachycardia with non-specific ST-T changes. Moreover, the echocardiogram showed massive pericardial effusion with features suggestive of cardiac tamponade and homogenous mass adjacent to the right atrium (Figures 1A, B). Emergency pericardiocentesis was done, and hemorrhagic pericardial fluid was sent for analysis. The patient became asymptomatic; a chest roentgenogram revealed bilateral minimal pleural effusion. Anti-tuberculous treatment was initiated, given the high suspicion of tuberculosis and mildly raised adenosine deaminase levels in the pericardial fluid sample.
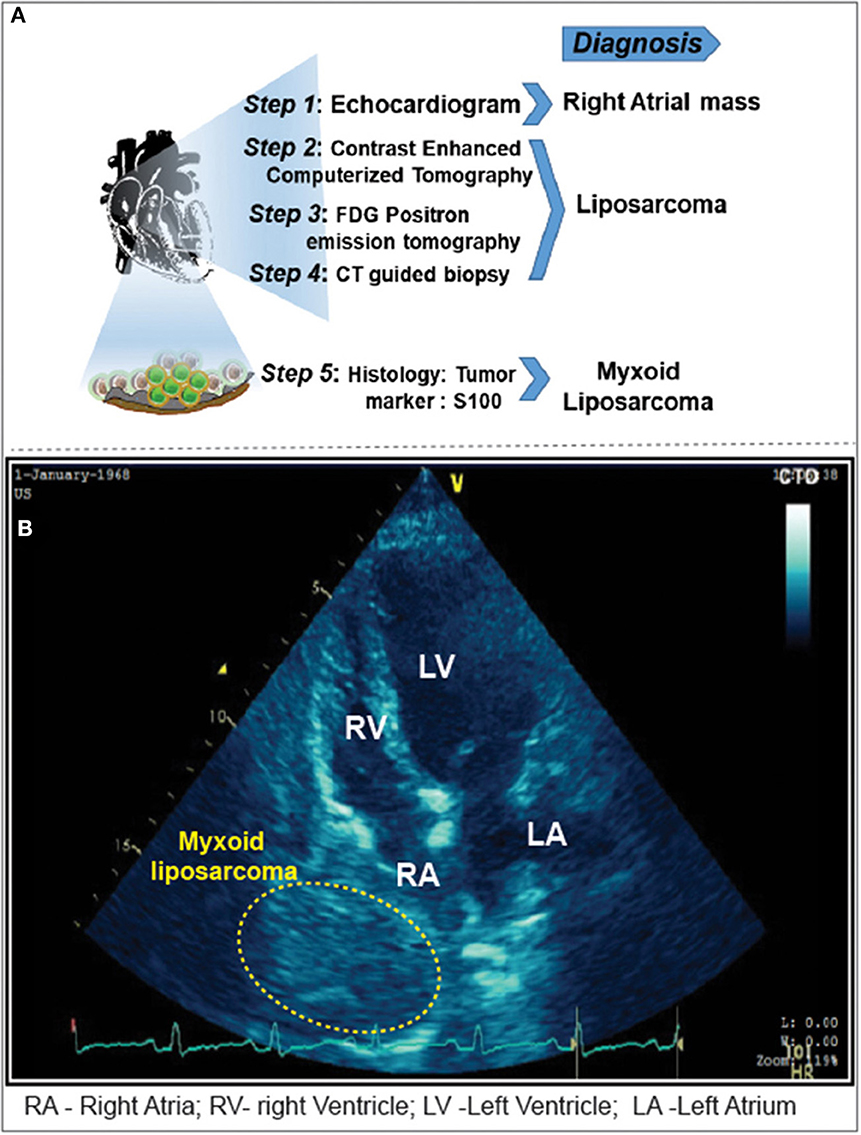
Figure 1. (A) Imaging modalities and approaches carried out for the diagnosis of cardiac tamponade. (B) Pericardiocentesis followed by 2D-Echocardiogram revealed a 5 x 4 cm sized mass adjacent to the right atrium. Yellow highlighted area locates the myxoid liposarcoma in the right atrium.
CECT thorax (Figures 2A, B) revealed minimally enhancing mass lesion in the right atrial wall with a small intraluminal component projecting into the right atrial chamber, with multiple enlarged retroperitoneal lymph nodes. As it was initially suspected to be lymphoma, a PET CT scan (Figures 2C–F) was performed to rule out metastasis, which showed mildly FDG-avid hypodensity along the lateral wall of the right atrium with mass effect over it.
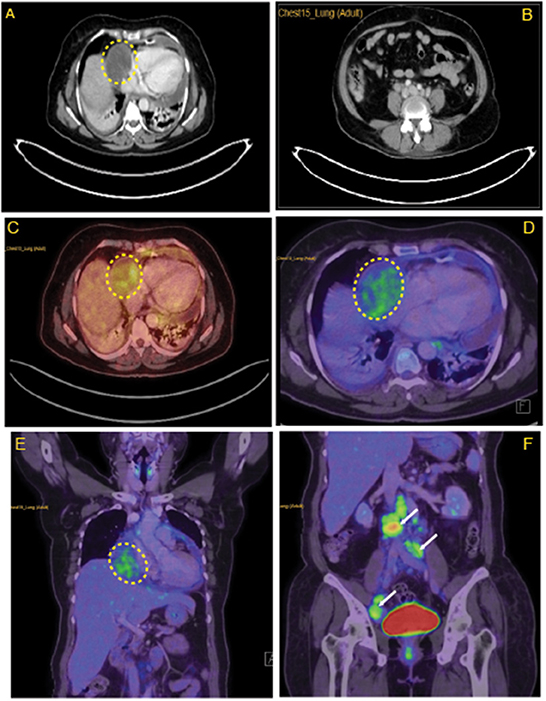
Figure 2. Lymph node characteristics in the right atrium. (A) CECT thorax revealing minimally enhancing mass lesion in the right atrial wall. (B) Multiple enlarged retroperitoneal lymph nodes. (C) Horizontal sections of FDG PET scan revealing mildly FDG avid hypodensity (5.8 × 5 cm, SUV max −3.40) along the lateral wall of the right atrium with mass effect over it. (D) Coronal section of FDG PET scan revealing mildly FDG avid mass over the lateral wall of the right atrium. (E) Enlarged FDG avid lymph nodes at perivascular, aortocaval, retrocaval, and bilateral common iliac and external iliac levels (F). Yellow highlighted area locates the myxoid liposarcoma in the right atrium. White arrows in (F) indicates metastasis in abdomen areas.
CT-guided biopsy from the right atrial mass was subjected to histopathological examination. The findings revealed the presence of myxoid liposarcoma (Figure 3A). The histological findings were further confirmed by tumor markers Pan CK and S100, denoting that the sample stained positive for S100 (Figures 3A, B).
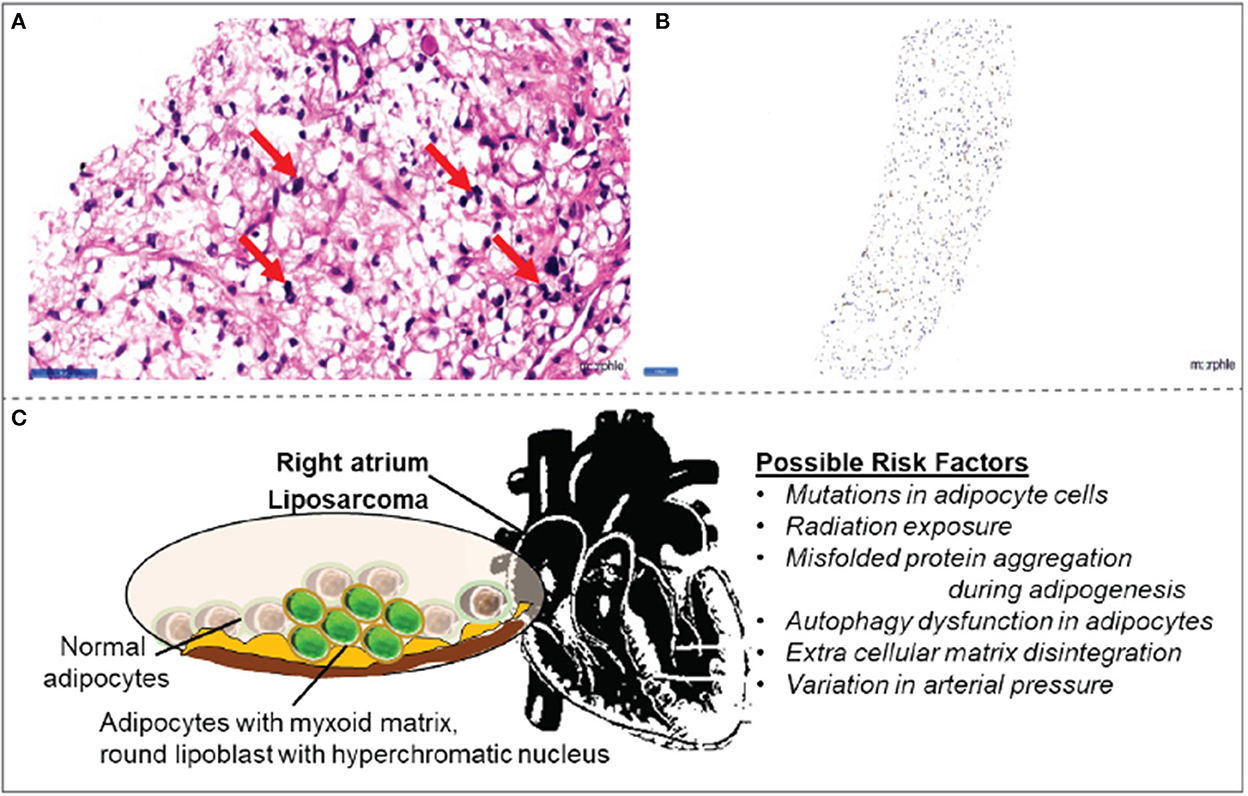
Figure 3. Histopathology observations in right atrial mass (A) biopsy collected from right atrial mass demonstrated signet-ring-shaped lipoblasts in mucoid matrix suggestive of myxoid liposarcoma. (B) The tumor cells are positive for the S100 tumor marker. (C) Schematic illustration of possible risk factors associated with myxoid liposarcomas in the right atrium.
Anti-tuberculous treatment was terminated. The case was discussed on the tumor board and a decision to obtain a biopsy from the mesenteric lymph node adjacent to the left iliac artery with laparoscopic assistance was made. However, the patient and her family were not in agreement; hence, she was discharged against medical advice.
Primary cardiac tumors are rare (1–3), found in about 0.001–0.3% of autopsies performed (4–6). Exact incidence of these tumors are unknown or not reported, but 1 in 500 cardiac surgical cases found to have primary cardiac tumors, out of which 75% are benign and about 25% are malignant (7, 8). Of these malignant tumors, around 75% are sarcomas (4, 9). Cardiac sarcoma is classified into right heart sarcomas, left heart sarcomas, and pulmonary artery sarcomas (10). Right heart sarcomas are generally bulkier, exophytic, infiltrate easily, remain asymptomatic, and cause heart failure belatedly and metastasize earlier (11). Therefore, the clinical diagnosis is challenging. The heart is an unusual site for liposarcoma and very few cases of metastatic primary cardiac sarcomas have been reported to date. WHO classification was proposed in 2004 for cardiac tumors (12). This may arise in either of the atria, but also from ventricles and the pericardium. Pericardial involvement may manifest as pericardial effusion or irregular nodules over pericardium (13, 14).
Non-invasive imaging plays a critical role in the diagnosis and surgical interventions of cardiac tamponade (15). Although echocardiography is a simple non-invasive method for cardiac mass evaluation, the area of tissue that could be covered using standard transducers may not be sufficient to assess the extent of the tumor mass (16). In the present case, our multimodal approach involving FDG PET scan followed by CT-guided biopsy facilitated the accurate assessment of size, location, depth of infiltration, and lesion margins of cardiac mass. Histopathology (H&E) of liposarcomas showed a traditional myxoid pattern of the matrix, round lipoblast cells with a hyperchromatic nucleus in the periphery. These cells had scanty cytoplasm, displaced by clear, large lipid droplets appearing as signet-ring shapes. Immunohistochemistry confirmed the S100, a known marker of malignant cells (17, 18). Thus, we recommend the use of an FDG-PET scan followed by histological analysis for the evaluation of size, morphology, tissue characteristics, and clinical correlation of cardiac masses.
Even though there are more innovations in diagnosis (19), the imaging characteristics of myxoid liposarcomas remain inconclusive. Usually, the malignancy tends to arise in atria as a large infiltrating mass; in time, it infiltrates into the ventricles and pericardium. Usually, the pericardium is the most vulnerable site for effusion and tumor nodules (14). While the exact cause of liposarcoma is unclear, variation in arterial pressure, genetic mutations in adipocytes, misfolded protein aggregation during adipogenesis, autophagy dysfunction, and extracellular matrix disintegration are likely to be the molecular mechanisms of the pathology (Figure 3C). In the present case, cardiac tamponade was characterized by pericardial effusion occurring in the right atrium. Of note, the myxoid liposarcoma is positive for S100 tumor markers even in the atrium itself, without any signs of infiltration to ventricles, a very rare occurrence.
Diagnosing myxoid liposarcomas remains a challenge (20, 21). Imaging modalities like echocardiography, cardiac MRI, and CT scans are extremely useful tools for identifying these tumors. FDG-PET scan is the newly evolved tool to diagnose both the tumor and metastasis (22, 23). In this case, we progressed schematically using an echocardiogram for diagnosing cardiac tamponade along with right atrial mass, then imaging with CECT thorax and FDG-PET scan, followed by CT-guided biopsy of the mass. There are limited data and guidelines for the diagnosis and treatment of this rare condition. Total or partial surgical resection may rarely benefit for patients with cardiac tumors. Here, since the sarcoma invaded the whole right atrium of the patient at the time of first hospitalization, a surgical resection or reconstruction was unlikely. However, because the patient was discharged against medical advice, there was no follow-up due to loss of communication.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by CSP-MED/20/FEB/59/53 DATED 07.03.2020. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
MTR and NSR contributed to the intellectual content and revised the manuscript. VK drafted the manuscript. VK and NB were responsible for acquisition and interpretation of the data. SSunn and AR reconstructed the images, prepared the legends, and updated the literature. SSund and PK reviewed the histology data and interpreted the results. JS was responsible for acquisition of CT biopsy. AM performed and interpreted PET scanning. All authors made substantial contribution to the preparation of the manuscript, as well as read and approved the final version of the manuscript.
We thank Snekha N. Rajasekaran for language editing. Department of Pathology and School of Medicine at University of Alabama at Birmingham (UAB) provided travel support to NSR (PATH-MCP#3111315-PDF#2085) for this collaborative research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CECT, contrast enhanced computerized tomography; FDG–PET, fluoro deoxy glucose—positron emission tomography; PET–CT scan, positron emission tomography—computed tomography.
1. Silverman N. Primary cardiac tumors. Ann Surg. (1980) 191:127. doi: 10.1097/00000658-198002000-00001
2. Maraj S, Pressman GS, Figueredo VM. Primary cardiac tumors. Int J Cardiol. (2009) 133:152-6. doi: 10.1016/j.ijcard.2008.11.103
3. Simpson L, Kumar SK, Okuno SH, Schaff HV, Porrata LF, Buckner JC, et al. Malignant primary cardiac tumors: review of a single institution experience. Cancer. (2008) 112:2440–6. doi: 10.1002/cncr.23459
4. Reardon MJ, DeFelice CA, Sheinbaum R, Baldwin JC. Cardiac autotransplant for surgical treatment of a malignant neoplasm. Ann Thorac Surg. (1999) 67:1793–5. doi: 10.1016/S0003-4975(99)00343-4
5. Patel J, Sheppard MN. Pathological study of primary cardiac and pericardial tumours in a specialist UK Centre: surgical and autopsy series. Cardiovasc Pathol. (2010) 19:343–52. doi: 10.1016/j.carpath.2009.07.005
6. Yanagawa B, Chan EY, Cusimano RJ, Reardon MJ. Approach to surgery for cardiac tumors: primary simple, primary complex, and secondary. Cardiol Clin. (2019) 37:525–31. doi: 10.1016/j.ccl.2019.07.001
7. Blackmon SH, Reardon MJ. Surgical treatment of primary cardiac sarcomas. Texas Heart Inst J. (2009) 36:451.
8. Reardon MJ, Walkes J-C, Benjamin R. Therapy insight: malignant primary cardiac tumors. Nat Clin Pract Cardiovasc Med. (2006) 3:548–53. doi: 10.1038/ncpcardio0653
9. Orbach D, Rey A, Oberlin O, Toledo JSd, Terrier-Lacombe MJ, Unnik Av, et al. Soft tissue sarcoma or malignant mesenchymal tumors in the first year of life: experience of the international society of pediatric oncology (SIOP) malignant mesenchymal tumor committee. J Clin Oncol. (2005) 23:4363–71. doi: 10.1200/JCO.2005.12.009
10. Blackmon SH, Patel A, Reardon MJ. Management of primary cardiac sarcomas. Expert Rev Cardiovasc Ther. (2008) 6:1217–22. doi: 10.1586/14779072.6.9.1217
11. Ramlawi B, Leja MJ, Saleh WKA, Al Jabbari O, Benjamin R, Ravi V, et al. Surgical treatment of primary cardiac sarcomas: review of a single-institution experience. Ann Thorac Surg. (2016) 101:698–702. doi: 10.1016/j.athoracsur.2015.07.087
12. Amano J, Nakayama J, Yoshimura Y, Ikeda U. Clinical classification of cardiovascular tumors and tumor-like lesions, and its incidences. Gen Thorac Cardiovasc Surg. (2013) 61:435–47. doi: 10.1007/s11748-013-0214-8
13. Lococo F, Cesario A, Meacci E, Vita L, Porziella V, Margaritora S, et al. Huge primary pericardial liposarcoma. Thorac Cardiovasc Surg. (2011) 59:172–3. doi: 10.1055/s-0030-1250187
14. Wang J-G, Wei Z-M, Liu H, Li Y-J. Primary pleomorphic liposarcoma of pericardium. Interact CardioVasc Thorac Surg. (2010) 11:325–7. doi: 10.1510/icvts.2010.239723
15. O'Connor MJ. Imaging the itis: endocarditis, myocarditis, and pericarditis. Curr Opin Cardiol. (2019) 34:57–64. doi: 10.1097/HCO.0000000000000577
16. Sinagra G, Merlo M, Pinamonti B. Dilated Cardiomyopathy: From Genetics to Clinical Management. (2019). doi: 10.1007/978-3-030-13864-6
17. Salama I, Malone P, Mihaimeed F, Jones JL. A review of the S100 proteins in cancer. Eur J Surg Oncol. (2008) 34:357–64. doi: 10.1016/j.ejso.2007.04.009
18. Gaynor R, Irie R, Morton D, Herschman H, Jones P, Cochran A. S100 protein: a marker for human malignant melanomas? Lancet. (1981) 317:869–71. doi: 10.1016/S0140-6736(81)92142-5
19. Abaricia S, Hirbe AC. Diagnosis and treatment of myxoid liposarcomas: histology matters. Curr Treat Opt Oncol. (2018) 19:1–13. doi: 10.1007/s11864-018-0590-5
20. Narendra S, Valente A, Tull J, Zhang S. DDIT3 gene break-apart as a molecular marker for diagnosis of myxoid liposarcoma—assay validation and clinical experience. Diagn Mol Pathol. (2011) 20:218–24. doi: 10.1097/PDM.0b013e3182107eb9
21. Anderson WJ, Jo VY, editors. Pleomorphic liposarcoma: updates and current differential diagnosis. Semin Diagn Pathol. (2019) 36:122–8. doi: 10.1053/j.semdp.2019.02.007
22. Paydary K, Seraj SM, Zadeh MZ, Emamzadehfard S, Shamchi SP, Gholami S, et al. The evolving role of FDG-PET/CT in the diagnosis, staging, and treatment of breast cancer. Mol Imaging Biol. (2019) 21:1–10. doi: 10.1007/s11307-018-1181-3
Keywords: liposarcoma—diagnosis, cardiac tamponade, right atrium (RA), cardiac tumor diagnosis, FDG (18F-fluorodeoxyglucose)-PET/CT
Citation: Thoddi Ramamurthy M, Kumar Balakrishnan V, Sunny S, Rajkumar A, Sundaram S, Krishnamurthy P, Boopathy Senguttuvan N, Murali A, Santhosham JDR, Periandavan K and Rajasekaran NS (2023) Case report: Metastatic myxoid liposarcoma arising from the right atrium extends as cardiac tamponade—A rare case of atrial oncology. Front. Cardiovasc. Med. 9:1046436. doi: 10.3389/fcvm.2022.1046436
Received: 16 September 2022; Accepted: 20 December 2022;
Published: 26 January 2023.
Edited by:
Junjie Xiao, Shanghai University, ChinaReviewed by:
Thomas Theologou, Istituto Cardiocentro Ticino, Ospedale Regionale di Lugano, SwitzerlandCopyright © 2023 Thoddi Ramamurthy, Kumar Balakrishnan, Sunny, Rajkumar, Sundaram, Krishnamurthy, Boopathy Senguttuvan, Murali, Santhosham, Periandavan and Rajasekaran. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Namakkal S. Rajasekaran,  cmFqbnNyQHVhYm1jLmVkdQ==; Muralidharan Thoddi Ramamurthy,
cmFqbnNyQHVhYm1jLmVkdQ==; Muralidharan Thoddi Ramamurthy,  bXVyYWxpZGhhcmFudHJAc3JpcmFtYWNoYW5kcmEuZWR1Lmlu
bXVyYWxpZGhhcmFudHJAc3JpcmFtYWNoYW5kcmEuZWR1Lmlu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.