
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 12 November 2021
Sec. Coronary Artery Disease
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.754826
This article is part of the Research TopicInsights in Coronary Artery Disease: 2021View all 15 articles
A correction has been applied to this article in:
Corrigendum: Myocardial Viability, Functional Status, and Collaterals of Patients With Chronically Occluded Coronary Arteries
 Xueyao Yang1†
Xueyao Yang1† Jinfan Tian1†
Jinfan Tian1† Lijun Zhang2
Lijun Zhang2 Wei Dong3
Wei Dong3 Hongzhi Mi3
Hongzhi Mi3 Jianan Li1
Jianan Li1 Jiahui Li1
Jiahui Li1 Ye Han4
Ye Han4 Huijuan Zuo5
Huijuan Zuo5 Jing An6
Jing An6 Yi He4*
Yi He4* Xiantao Song1*
Xiantao Song1*Objective: Viability and functional assessments are recommended for indication and intervention for chronic coronary total occlusion (CTO). We aimed to evaluate myocardial viability and left ventricular (LV) functional status by using cardiovascular magnetic resonance (CMR) and to investigate the relationship between them and collaterals in patients with CTO.
Materials and Methods: We enrolled 194 patients with one CTO artery as detected by coronary angiography. Patients were scheduled for CMR within 1 week after coronary angiography.
Results: A total of 128 CTO territories (66%) showed scar based on late gadolinium enhancement (LGE) imaging. There were 1,112 segments in CTO territory, while only 198 segments (18%) subtended by the CTO artery showed transmural scar (i.e., >50% extent on LGE). Patients with viable myocardium had higher LV ejection fraction (LVEF) (56.7 ± 13.5% vs. 48.3 ± 15.4%, p < 0.001) than those with transmural scar. Angiographically, well-developed collaterals were found in 164 patients (85%). There was no significant correlation between collaterals and the presence of myocardial scar (p = 0.680) or between collaterals and LVEF (p = 0.191). Nevertheless, more segments with transmural scar were observed in patients with poorly-developed collaterals than in those with well-developed collaterals (25 vs. 17%, p = 0.010).
Conclusion: Myocardial infarction detected by CMR is widespread among patients with CTO, yet only a bit of transmural myocardial scar was observed within CTO territory. Limited number of segments with transmural scar is associated with preserved LV function. Well-developed collaterals are not related to the prevalence of myocardial scar or systolic functioning, but could be related to reduce number of non-viable segments subtended by the CTO artery.
Coronary chronic total occlusions (CTOs) are detected in ~15–25% of patients who undergo coronary angiography (1–4). Beneficial effects of CTO revascularization include angina relief, decreased ischemia, and improved functional status (5–7). However, while better outcomes have been shown in non-randomized studies (5–7), evidence from randomized trials suggests that CTO percutaneous coronary intervention (PCI) treatment is not superior to conservative treatment with regard to functional status and long-term outcomes (8–10). Thus, an appropriate indication of CTO intervention is crucial when considering potential benefits, challenges, and risks. Therefore, baseline characteristics of patients with CTO need to be recorded in detail. Current guidelines recommend evaluation of symptoms and ischemia burden, but myocardial viability is also recommended as it is a newly recognized potential predictor of functional recovery following successful CTO PCI (11). In this study, we assessed the myocardial viability and functional status in CTO territories by using cardiovascular magnetic resonance (CMR) imaging and investigated the relationship between them and collaterals.
A total of 254 patients who underwent coronary angiography due to suspected angina or ischemic evidence between December 2014 and March 2020 were verified to have CTO in only one major epicardial coronary artery and were considered for inclusion in this study. The study protocol was approved by the Ethics Board of Beijing Anzhen Hospital, Capital Medical University. Patients with acute myocardial infarction within 3 months, patients with coagulation disorders, and patients who refused or were unable to undergo CMR (n = 46) were excluded from the study. Patients were scheduled for CMR within 1 week after coronary angiography. A total of 14 patients with poor CMR images or failed procedures were also excluded, leaving a final total of 194 eligible patients that were enrolled in the study. Figure 1 shows a schematic diagram describing the study cohort. All the patients were treated with optimal medical therapy (OMT) (medical therapy formulated by clinicians in full consideration of risk factor modification and permanent lifestyle changes) (12), regardless if the revascularization procedure succeeded or not. Written informed consent was obtained from all the patients and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
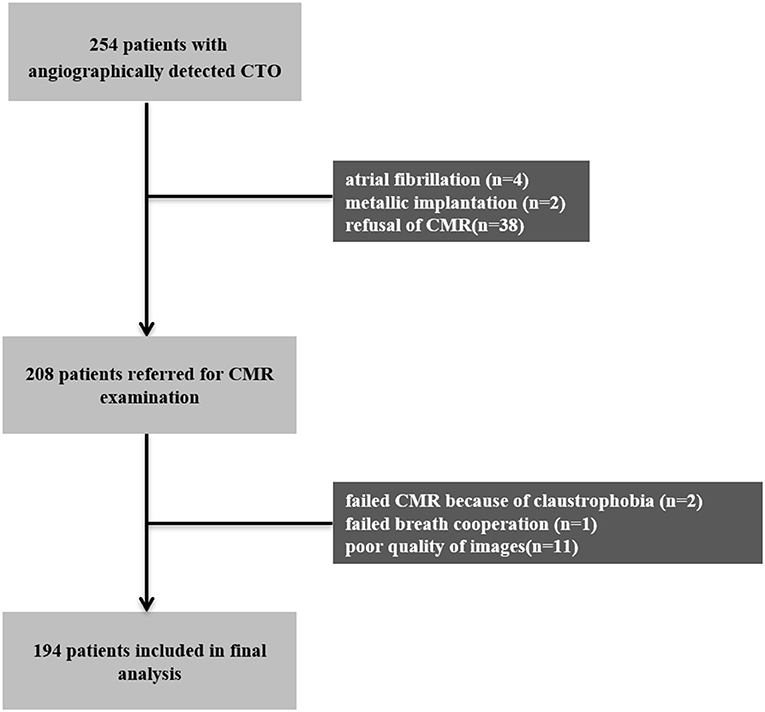
Figure 1. Patient and imaging flowchart. CMR, cardiovascular magnetic resonance; CTO, chronic total occlusion.
Coronary angiography was performed by using the standard method. CTO was defined as 100% stenosis with the thrombolysis in myocardial infarction (TIMI) grade 0 flow in a major epicardial coronary artery (≥2.5 mm) for at least 3 months (13). Significant non-CTO coronary disease was diagnosed if there was ≥70% lumen stenosis in a major epicardial coronary artery (≥2.5 mm), except for the left main (LM) artery or ≥50% lumen stenosis in the LM artery. The presence and state of collaterals supplying the totally occluded vessel from the contralateral artery were graded by using the Rentrop classification system (14): Grade 0 referred to no collateral circulation, grade 1 referred to collateral circulation that only supplied the occluded vessel branch, grade 2 referred to collateral circulation that partially supplied the occluded vessel trunk, and grade 3 referred to collateral circulation that completely filled the occluded vessel trunk. Patients were classified as having poorly-developed collaterals (Rentrop scores of 0 and 1) or well-developed collaterals (Rentrop scores of 2 and 3). Collateral circulation scores were independently assessed by two experienced interventional cardiologists.
Cardiovascular magnetic resonance was performed with the Siemens 3.0-T Whole-body Scanner (MAGNETOM Verio, Tim System; Siemens Healthcare, Erlangen, Germany, UK). The CMR protocol included cine images and late gadolinium enhancement (LGE) imaging. As described in our previous study (15), 8-mm sections with no intersection gaps were obtained in the short-axis plane (from the base to the apex) and the long-axis plane of the left ventricle (LV) to perform cine cardiac MR and LGE imaging. Postprocessing analyses were performed by using the Siemens–Argus software.
Two experienced radiologists performed a visual analysis of all the images based on the American Heart Association 17-segment model of LV. Discrepancies were resolved by a discussion involving the presence of another senior investigator. Segments 1, 2, 7, 8, 13, 14, and 17 were considered left anterior descending (LAD) artery territory. Segments 5, 6, 11, 12, and 16 were considered left circumflex (LCX) artery territory. Segments 3, 4, 9, 10, and 15 were considered right coronary artery (RCA) territory (16). Taking coronary dominance into account, the inferoseptal segments, inferior segments, and inferolateral segments could be reassigned (17). The wall motion and extent of LGE were graded. The wall motion of each myocardial segment was scored on a scale of 1–4: 1 = normal, 2 = hypokinesia, 3 = akinesia, and 4 = dyskinesia. The extent of segmental wall enhancement was graded on a five-point scale: 1 = no hyperenhancement, 2 = hyperenhancement of 1–25% of tissue, 3 = hyperenhancement of 26–50% of tissue, 4 = hyperenhancement of 51–75% of tissue, and 5 = hyperenhancement of 76–100% of tissue (18, 19). LGE > 50% was considered to be a transmural scar, LGE between 1 and 50% was considered to be an endocardial scar with viable myocardium, and no LGE was considered to be absent of myocardial scar. Viable myocardium was defined as one with either no LGE or 1–50% LGE. Patients with left ventricular ejection fraction (LVEF) <50% were defined as having left ventricular dysfunction.
Statistical analysis was performed by using the Statistical Package for the Social Sciences (SPSS) 26.0 software (IBM Corp., Armonk, NY, USA). Normally distributed data were expressed as mean ± SD. Non-normally distributed data were expressed as median (interquartile range). Continuous variables were compared by using the t-test or the Mann–Whitney U test and categorical variables were compared by using the χ2 test or the Spearman's rank correlation test. Interobserver and intraobserver agreement were tested by using the Cohen's kappa. The correlation between wall motion score and extent of LGE was evaluated by using the Spearman's rank correlation test. A two-tailed p < 0.05 was considered to be statistically significant.
Baseline clinical and angiographic characteristics are presented in Table 1 and are ordered according to myocardial scar formation in CTO territories. Statistical analyses demonstrated that patients with myocardial scar were likely to be males and smokers and were more likely to have hypertension. The prevalence of myocardial scar in CTO territory detected by CMR (66%) was strikingly higher than traditional clinical evidence that would suggest, as only 11% of included study patients showed pathological Q waves on ECG. Angiographically, 79 patients (41%) showed at least one concomitant main artery with severe stenosis.
Of the 194 patients included in this study, a total of 128 patients (66%) had myocardial scar in CTO territory detected by CMR, while 66 patients (34%) did not have myocardial scar in CTO territory detected by CMR.
Late gadolinium enhancement imaging demonstrated myocardial scar in 417 segments (37%) out of 1,112 total segments. Over 50% LGE was observed in 198 (18%) CTO-related segments, while no scar was observed in 695 (63%) CTO-related segments (see Table 2). A total of 780 segments (70%) in CTO territory showed normal wall motion (score = 1). The Spearman's rank correlation test showed a significantly positive correlation between wall motion scores and LGE scores (r = 0.488, p < 0.001).
Left ventricular dysfunction was observed in 67 enrolled patients (35%) with an average LVEF of 36.4 ± 9.4%. Over 50% LGE was observed in 27% (103/381) of segments in CTO territory of patients with reduced LVEF (<50%), but only in 13% (95/731) of segments in CTO territory of those patients with normal systolic function (≥50%, p < 0.001). Patients with viable myocardia (LGE 0–50%) had higher LVEF (56.7 ± 13.5% vs. 48.3 ± 15.4%, p < 0.001, Figure 2A) and lower LV end-diastolic volume (LVEDV) (97.3 ± 33.8 ml vs. 121.7 ± 54.4 ml, p = 0.003, Figure 2B) and LV end-systolic volume (LVESV) (43.3 ± 24.4 ml vs. 68.6 ± 48.1 ml, p < 0.001, Figure 2C) than those with transmural scar.
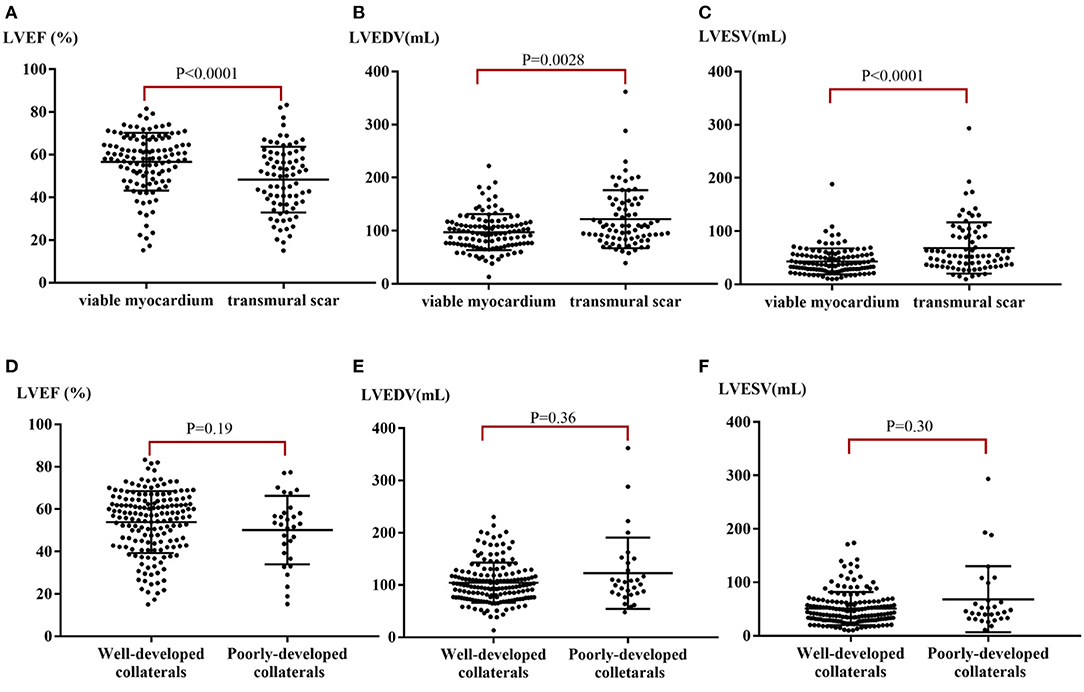
Figure 2. LV function stratified by myocardial viability and collaterals. (A) Median LVEF stratified by the extent of myocardial scar. (B) Median LVEDV stratified by the extent of myocardial scar. (C) Median LVESV stratified by the extent of myocardial scar. (D) Median LVEF stratified by well- or poorly-developed collaterals. (E) Median LVEDV stratified by well- or poorly-developed collaterals. (F) Median LVESV stratified by well- or poorly-developed collaterals. LVEF, left ventricular ejection fraction; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume.
Poorly-developed angiographic collaterals were observed in 30 patients (15%), while well-developed collaterals were found in 164 patients (85%). Interobserver and intraobserver agreement for the Rentrop grading of collaterals in 50 (26%) randomly selected patients was high (intraobserver agreement = 90%; the Cohen's kappa = 0.62, 95% CI: 0.32–0.92).
Of the patients with poorly-developed collaterals, a total of 21 (70%) patients had a myocardial scar in CTO territory, while 65% of patients with well-developed collaterals (107/164) showed scar in CTO territory. There was no significant correlation between the presence of myocardial scar and collateral status (p = 0.680). Nevertheless, 25% of segments subtended to CTO arteries showed over 50% LGE in patients with poorly-developed collaterals, while only 17% of segments subtended to CTO arteries showed over 50% LGE in those patients with well-developed collaterals (p = 0.010; Figure 3). Additionally, more viable CTO-related segments were found in the presence of well-developed collaterals than in the presence of poorly-developed collaterals, despite the non-statistically significant difference [591 out of 934 (63%) vs. 104 out of 178 (58%) segments, p = 0.237].
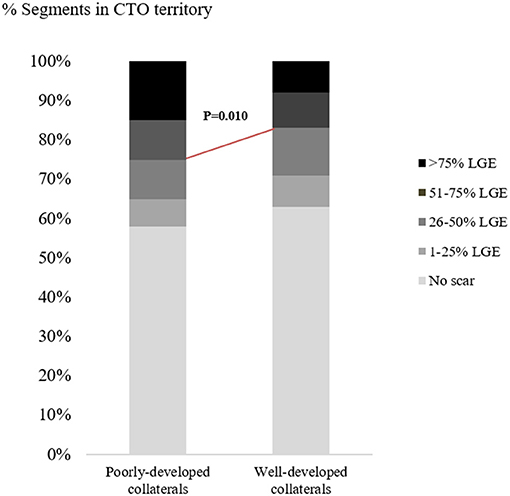
Figure 3. Distribution of myocardial segments subtended by CTO arteries according to scar formation. CTO, chronic total occlusion; LGE, late gadolinium enhancement.
There were no significant differences between mean LVEF and ventricular volume in patients with poorly-developed collaterals when compared with those with well-developed collaterals (p-values: LVEF = 0.191, LVEDV = 0.360, LVESV = 0.300, Figures 2D–F). Typical case scenarios displaying patients with well- or poorly-developed collaterals and patients with different extents of myocardial scar are shown in Figure 4.
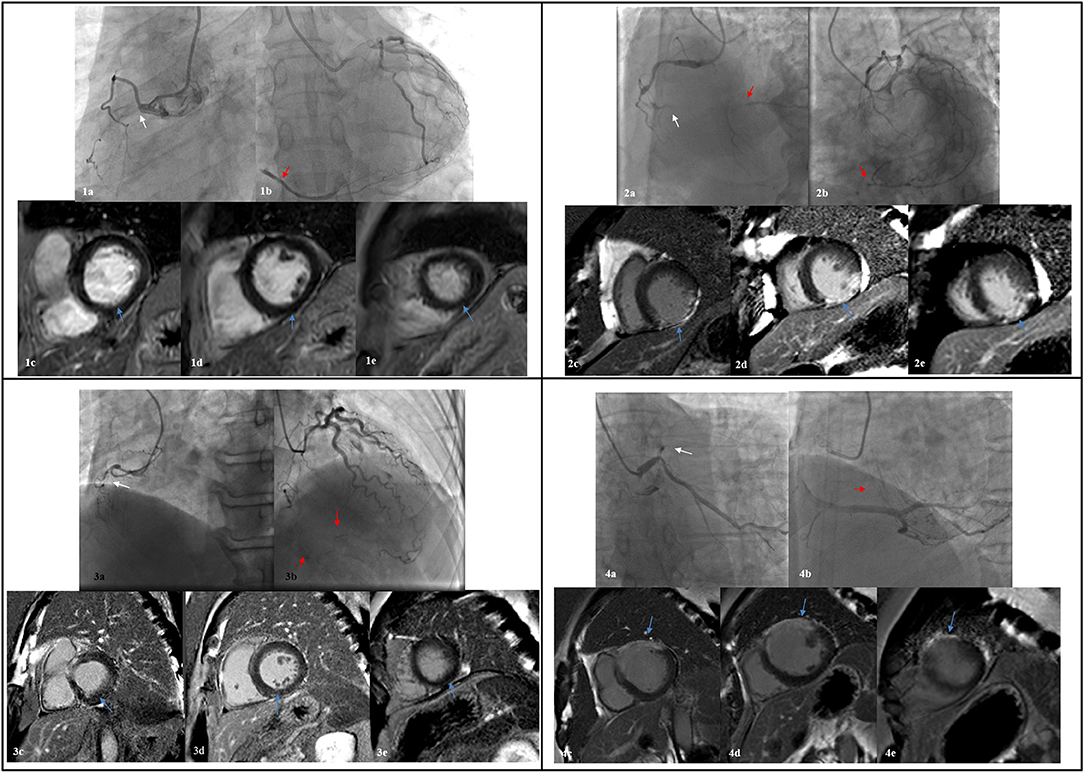
Figure 4. Four typical case scenarios displaying patients with well- or poorly-developed collaterals and different extent of myocardial scar. 1: a, CTO in proximal RCA (white arrow); b, a well-developed epicardial collateral from LCX (red arrow); c-e, no myocardial scar of the inferior wall was observed from basal, intermediate, and to apex level. 2: a, CTO in distal RCA (white arrow); b, well-developed collaterals from LCX and septum branches (red arrow); c-e, transmural scar of the inferior wall was observed from basal, intermediate, and to apex level. 3: a, CTO in proximal RCA (white arrow); b, poorly-developed collaterals from septum branches (red arrow); c-e, no myocardial scar of the inferior wall was observed from basal, intermediate, and to apex level. 4: a, CTO in proximal LAD (white arrow); b, poorly-developed collaterals from septum branches (red arrow); c-e, transmural myocardial scar of the anterior wall was observed from basal, intermediate, and to apex level. CTO, chronic total occlusion; RCA, right coronary artery; LCX, left circumflex; LAD, left anterior descending.
In this study, we assessed the myocardial viability and functional status in CTO territory by using CMR and investigated the correlation between the prevalence of myocardial viability, LV systolic function, and collateral status. The prevalence of myocardial scar in CTO territory was >50%, while only 18% of segments had transmural scar. Patients with transmural scar had worse systolic functioning. Although collateral status was not related to the presence of myocardial scar or to LV function, well-developed collaterals reduced the number of segments with transmural scar in CTO territory.
We reported a higher prevalence of myocardial scar detected by CMR than traditional clinical evidence in this study. However, this finding was consistent with previous work (20). We attribute this to the high sensitivity of CMR for recognizing endocardial scar, while the occurrence of myocardial infarction symptoms or Q waves on ECG relates more to transmural necrosis. Despite the considerable prevalence of myocardial scar, less than one-fifth of the segments showed transmural scar, suggesting that severe ischemic injury is limited in CTO territory. However, since the benefits of revascularization for CTO are still controversial, many researchers pay more attention to factors that predict better outcomes following CTO PCI. Studies have shown that myocardial viability is an important outcome predictor, as confined endocardial scar or absolute viability has been associated with functional recovery (21, 22). Schumacher et al. previously reported that 76% of patients had evidence of LGE and that only 5% of CTO segments demonstrated transmural scar tissue that could be detected by CMR (23). The notion that the ischemic myocardial scar is common but most limited within the endocardium would be consistent with our results. Particularly, an evident extensive myocardial scar was observed beyond CTO territories in a few patients, despite adjustment according to the dominance of coronary artery. Indeed, the complexity of vascular variability made it difficult to assign the 17 segments of LV to specific coronary arteries. As previously reported (17), the most specific segments including anterior, anteroseptal segments correspond to LAD, but no segments can be exclusively attributed to RCA or LCX. Additionally, Yajima et al. (24) also reported myocardial scar in territories adjacent or remote to CTO territories in a chronic myocardial infarction (MI) adult porcine model and explained it by endothelium swelling and microvasculature disruption. Therefore, the variable vascular territories and extensive microvasculature injury could account for the bias of myocardial viability analysis.
Ischemia is estimated to be responsible for around two-thirds of heart failure cases (25). In patients with ischemic ventricular dysfunction, the presence of CTO is associated with higher morbidity and poor prognosis (26). In this study, we reported that 35% of patients had impaired systolic functioning and that patients with transmural scar had worse functional statuses. A few observational studies investigating the outcomes of CTO PCI among patients with LV dysfunction have shown substantial LVEF improvement and decreased cardiac mortality, especially in those with severely impaired systolic functions (27–30). Interventional procedures are more challenging in these complicated patients. A study demonstrated that PCI was a safe strategy in patients with low LVEF ( ≤ 35%), as the angiographic success rate was high and similar to that in patients with LVEF > 35% without more periprocedural complications (31). However, these outcomes are still limited in non-randomized controlled trials (RCTs). The REVASC trial (A Randomized Trial to Assess Regional Left Ventricular Function After Stent Implantation in Chronic Total Occlusion) did not show any benefits related to CTO PCI. Therefore, experts recommend both the viability and functional assessment in patients with ischemic cardiomyopathy for CTO PCI (32).
There was no significant difference in the prevalence of myocardial scar or systolic functioning in patients with well- or poorly-developed collaterals and there were fewer non-viable segments in the CTO territory supplied by well-developed collaterals. The presence of well-developed collaterals may not directly predict myocardial viability, but could protect the myocardium from severe ischemic injury to some extent. In fact, whether collaterals could have protective effects on myocardial viability and contractility have long been a matter of debate. In a recent study, patients with well-developed collaterals showed less myocardial scar as assessed by quantitative CMR analysis and more retained systolic function in CTO territory (23). Previously, a few studies reported that collaterals had lower sensitivity for predicting myocardial viability (33, 34). The blood supply from collaterals to the totally occluded territory could be limited, especially when myocardial oxygen consumption increases. Additionally, myocardial viability and contractility could be affected by many concomitant factors including multiple-vessel stenosis and microvascular dysfunction. In the subgroup analysis of the EXPLORE (Evaluating Xience and Left Ventricular Function in Percutaneous Coronary Intervention on Occlusions After ST-Elevation Myocardial Infarction) trial, the presence of well-developed collaterals was shown to correlate with better outcomes. However, well-developed collaterals did not translate into better clinical outcomes. A recent meta-analysis showed that the presence of well-developed collaterals is not well-related to lower rates of acute MI (AMI) or mortality, but does increase the likelihood of successful CTO PCI (35, 36). These results indicated that well-developed collaterals should not be the only factor that affects prognosis.
There are several limitations inherent in this study. First, it was a single-center and observational study with a small sample size that enrolled patients with a range of LVEF—meaning the population with impaired LVEF accounted for a minority of patients. This does, however, reflect a real-world trend. Second, the LGE images were analyzed by using a semi-quantitative visual method rather than a quantitative method. However, the utility of quantitative CMR analysis by using commercial software is still limited and thresholds for signal intensity are not unified. Third, stress perfusion imaging was not performed on our patients, meaning that we lacked analysis of ischemic burden in CTO territory. Finally, collateral circulation was only assessed by using visualized angiography and functional intravascular evaluations were not employed.
This study demonstrates that myocardial injuries in CTO territory are common, but those non-viable myocardia only account for a minority. Further, transmural myocardial scar appears to be associated with worse functional outcomes. Finally, well-developed collaterals are not related to the prevalence of myocardial scar or systolic functioning, but can reduce the number of segments with non-viable scar that are subtended by CTO arteries.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Beijing Anzhen Hospital. The patients/participants provided their written informed consent to participate in this study.
XY, JT, LZ, WD, HM, JianL, JiahL, YHa, HZ, JA, YHe, and XS: conceptualization. JT and JianL: data curation. XY and JT: formal analysis. JT and XS: funding acquisition. XY, JT, and JianL: investigation. LZ, WD, HM, HZ, YHe, and XS: methodology. XY, JT, JianL, and XS: project administration. XS: resources. LZ, WD, HM, and YHe: software. YHe and XS: supervision, validation, and visualization. XY and JT: writing—original draft. LZ, YHe, and XS: writing—review and editing. All authors contributed to the article and approved the submitted version.
This work was supported by Capital Health Development Research Project (No. 2018-2-2063), National Natural Science Foundation of China (Nos. 81971569, 81670324, and 81671650), Beijing Lab for Cardiovascular Precision Medicine (PXM2018_014226_000013), Beijing Municipal Science and Technology Project (Z161100000516139), 2018 Beijing Excellent Talent Fund (NO. 2018000021469G241).
JA was employed by the company Siemens Shenzhen Magnetic Resonance Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Råmunddal T, Hoebers LP, Henriques JP, Dworeck C, Angerås O, Odenstedt J, et al. Chronic total occlusions in Sweden–a report from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). PLoS ONE. (2014) 9:e103850. doi: 10.1371/journal.pone.0103850
2. Fefer P, Knudtson ML, Cheema AN, Galbraith PD, Osherov AB, Yalonetsky S, et al. Current perspectives on coronary chronic total occlusions: the Canadian Multicenter Chronic Total Occlusions Registry. J Am Coll Cardiol. (2012) 59:991–7. doi: 10.1016/j.jacc.2011.12.007
3. Tomasello SD, Boukhris M, Giubilato S, Marzà F, Garbo R, Contegiacomo G, et al. Management strategies in patients affected by chronic total occlusions: results from the Italian Registry of Chronic Total Occlusions. Eur Heart J. (2015) 36:3189–98. doi: 10.1093/eurheartj/ehv450
4. Azzalini L, Jolicoeur EM, Pighi M, Millán X, Picard F, Tadros VX, et al. Epidemiology, management strategies, and outcomes of patients with chronic total coronary occlusion. Am J Cardiol. (2016) 118:1128–35. doi: 10.1016/j.amjcard.2016.07.023
5. Grantham JA, Jones PG, Cannon L, Spertus JA. Quantifying the early health status benefits of successful chronic total occlusion recanalization: results from the FlowCardia's Approach to Chronic Total Occlusion Recanalization (FACTOR) trial. Circ Cardiovasc Qual Outcomes. (2010) 3:284–90. doi: 10.1161/CIRCOUTCOMES.108.825760
6. Stuijfzand WJ, Biesbroek PS, Raijmakers PG, Driessen RS, Schumacher SP, van Diemen P, et al. Effects of successful percutaneous coronary intervention of chronic total occlusions on myocardial perfusion and left ventricular function. Eurointervention. (2017) 13:345–54. doi: 10.4244/EIJ-D-16-01014
7. Roth C, Goliasch G, Aschauer S, Gangl C, Ayoub M, Distelmaier K, et al. Impact of treatment strategies on long-term outcome of CTO patients. Eur J Intern Med. (2020) 77:97–104. doi: 10.1016/j.ejim.2020.03.008
8. Lee SW, Lee PH, Ahn JM, Park DW, Yun SC, Han S, et al. Randomized trial evaluating percutaneous coronary intervention for the treatment of chronic total occlusion. Circulation. (2019) 139:1674–83. doi: 10.1161/CIRCULATIONAHA.118.031313
9. Werner GS, Martin-Yuste V, Hildick-Smith D, Boudou N, Sianos G, Gelev V, et al. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur Heart J. (2018) 39:2484–93. doi: 10.1093/eurheartj/ehy220
10. Henriques JP, Hoebers LP, Råmunddal T, Laanmets P, Eriksen E, Bax M, et al. Percutaneous intervention for concurrent chronic total occlusions in patients with STEMI: the EXPLORE trial. J Am Coll Cardiol. (2016) 68:1622–32. doi: 10.1016/j.jacc.2016.07.744
11. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. (2019) 40:87–165. doi: 10.1093/eurheartj/ehy855
12. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. (2016). 37:2315–81. doi: 10.1093/eurheartj/ehw106
13. Di Mario C, Werner GS, Sianos G, Galassi AR, Büttner J, Dudek D, et al. European perspective in the recanalisation of Chronic Total Occlusions (CTO): consensus document from the EuroCTO Club. Eurointervention. (2007) 3:30–43.
14. Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol. (1985) 5:587–92. doi: 10.1016/S0735-1097(85)80380-6
15. Li JN, He Y, Dong W, Zhang LJ, Mi HZ, Zhang DF, et al. Comparison of cardiac MRI with PET for assessment of myocardial viability in patients with coronary chronic total occlusion. Clin Radiol. (2019) 74:410.e411-19. doi: 10.1016/j.crad.2019.01.021
16. Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Int J Cardiovasc Imaging. (2002) 18:539–542. doi: 10.1161/hc0402.102975
17. Pereztol-Valdés O, Candell-Riera J, Santana-Boado C, Angel J, Aguadé-Bruix S, Castell-Conesa J, et al. Correspondence between left ventricular 17 myocardial segments and coronary arteries. Eur Heart J. (2005) 26:2637–43. doi: 10.1093/eurheartj/ehi496
18. Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. (2000) 343:1445–53. doi: 10.1056/NEJM200011163432003
19. Thiele H, Kappl MJ, Conradi S, Niebauer J, Hambrecht R, Schuler G. Reproducibility of chronic and acute infarct size measurement by delayed enhancement-magnetic resonance imaging. J Am Coll Cardiol. (2006) 47:1641–5. doi: 10.1016/j.jacc.2005.11.065
20. Choi JH, Chang SA, Choi JO, Song YB, Hahn JY, Choi SH, et al. Frequency of myocardial infarction and its relationship to angiographic collateral flow in territories supplied by chronically occluded coronary arteries. Circulation. (2013) 127:703–9. doi: 10.1161/CIRCULATIONAHA.112.092353
21. Nandalur KR, Dwamena BA, Choudhri AF, Nandalur MR, Carlos RC. Diagnostic performance of stress cardiac magnetic resonance imaging in the detection of coronary artery disease: a meta-analysis. J Am Coll Cardiol. (2007) 50:1343–53. doi: 10.1016/j.jacc.2007.06.030
22. Baks T, van Geuns RJ, Duncker DJ, Cademartiri F, Mollet NR, Krestin GP, et al. Prediction of left ventricular function after drug-eluting stent implantation for chronic total coronary occlusions. J Am Coll Cardiol. (2006) 47:721–5. doi: 10.1016/j.jacc.2005.10.042
23. Schumacher SP, Everaars H, Stuijfzand WJ, Huynh JW, van Diemen PA, Bom MJ, et al. Coronary collaterals and myocardial viability in patients with chronic total occlusions. Eurointervention. (2020) 16:e453–61. doi: 10.4244/EIJ-D-19-01006
24. Yajima S, Miyagawa S, Fukushima S, Isohashi K, Watabe T, Ikeda H, et al. Microvascular dysfunction related to progressive left ventricular remodeling due to chronic occlusion of the left anterior descending artery in an adult porcine heart. Int Heart J. (2019) 60:715–27. doi: 10.1536/ihj.18-346
25. Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. (2011) 8:30–41. doi: 10.1038/nrcardio.2010.165
26. Tajstra M, Pyka Ł, Gorol J, Pres D, Gierlotka M, Gadula-Gacek E, et al. Impact of chronic total occlusion of the coronary artery on long-term prognosis in patients with ischemic systolic heart failure: insights from the COMMIT-HF registry. JACC Cardiovasc Interv. (2016) 9:1790–7. doi: 10.1016/j.jcin.2016.06.007
27. Cardona M, Martín V, Prat-Gonzalez S, Ortiz JT, Perea RJ, de Caralt TM, et al. Benefits of chronic total coronary occlusion percutaneous intervention in patients with heart failure and reduced ejection fraction: insights from a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. (2016) 18:78. doi: 10.1186/s12968-016-0287-5
28. Samy M, El Awady WS, Al-Daydamony MM, Abd El Samei MM, Shokry K. Echocardiographic assessment of left ventricular function recovery post percutaneous coronary intervention of chronic total occlusions in patients with low and mid-range left ventricular ejection fractions. Echocardiography. (2020) 37:239–246. doi: 10.1111/echo.14582
29. Chadid P, Markovic S, Bernhardt P, Hombach V, Rottbauer W, Wöhrle J. Improvement of regional and global left ventricular function in magnetic resonance imaging after recanalization of true coronary chronic total occlusions. Cardiovasc Revasc Med. (2015) 16:228–32. doi: 10.1016/j.carrev.2015.03.003
30. Toma A, Stähli BE, Gick M, Gebhard C, Kaufmann BA, Mashayekhi K, et al. Comparison of benefit of successful percutaneous coronary intervention for chronic total occlusion in patients with versus without reduced ( ≤40%) left ventricular ejection fraction. Am J Cardiol. (2017) 120:1780–6. doi: 10.1016/j.amjcard.2017.07.088
31. Galassi AR, Boukhris M, Toma A, Elhadj Z, Laroussi L, Gaemperli O, et al. Percutaneous coronary intervention of chronic total occlusions in patients with low left ventricular ejection fraction. JACC Cardiovasc Interv. (2017) 10:2158–70. doi: 10.1016/j.jcin.2017.06.058
32. Brilakis ES, Mashayekhi K, Tsuchikane E, Abi Rafeh N, Alaswad K, Araya M, et al. Guiding principles for chronic total occlusion percutaneous coronary intervention. Circulation. (2019) 140:420–33. doi: 10.1161/CIRCULATIONAHA.119.039797
33. Dong W, Li J, Mi H, Song X, Jiao J, Li Q. Relationship between collateral circulation and myocardial viability of (18)F-FDG PET/CT subtended by chronic total occluded coronary arteries. Ann Nucl Med. (2018) 32:197–205. doi: 10.1007/s12149-018-1234-3
34. Wang L, Lu MJ, Feng L, Wang J, Fang W, He ZX, et al. Relationship of myocardial hibernation, scar, and angiographic collateral flow in ischemic cardiomyopathy with coronary chronic total occlusion. J Nucl Cardiol. (2019) 26:1720–30. doi: 10.1007/s12350-018-1241-8
35. van Dongen IM, Elias J, van Houwelingen KG, Agostoni P, Claessen B, Hoebers LP, et al. Impact of collateralisation to a concomitant chronic total occlusion in patients with ST-elevation myocardial infarction: a subanalysis of the EXPLORE randomised controlled trial. Open Heart. (2018) 5:e000810. doi: 10.1136/openhrt-2018-000810
Keywords: chronic total occlusion, myocardial viability, coronary artery disease, cardiovascular magnetic resonance, cardiac function
Citation: Yang X, Tian J, Zhang L, Dong W, Mi H, Li J, Li J, Han Y, Zuo H, An J, He Y and Song X (2021) Myocardial Viability, Functional Status, and Collaterals of Patients With Chronically Occluded Coronary Arteries. Front. Cardiovasc. Med. 8:754826. doi: 10.3389/fcvm.2021.754826
Received: 07 August 2021; Accepted: 11 October 2021;
Published: 12 November 2021.
Edited by:
Minjie Lu, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaReviewed by:
Hsin-jung Yang, Cedars Sinai Medical Center, United StatesCopyright © 2021 Yang, Tian, Zhang, Dong, Mi, Li, Li, Han, Zuo, An, He and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiantao Song, c29uZ3hpYW50YW8wOTI5QHFxLmNvbQ==; Yi He, aGV5aTEzOUBzaW5hLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.