
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Bioeng. Biotechnol., 31 March 2025
Sec. Tissue Engineering and Regenerative Medicine
Volume 13 - 2025 | https://doi.org/10.3389/fbioe.2025.1557203
This article is part of the Research TopicApplication of Tissue Engineering in Bone, Joints, Ligaments Injuries and Cartilage RegenerationView all 4 articles
The management of bone defects, particularly in aging populations, remains a major clinical challenge. The immune microenvironment plays an important role in the repair of bone defects and a favorable immune environment can effectively promote the repair of bone defects. However, aging is closely associated with chronic low-grade systemic inflammation, which adversely affects bone healing. Persistent low-grade systemic inflammation critically regulates bone repair through all stages. This review explores the potential of 3D-printed bioceramic scaffolds in bone defect repair, focusing on their capacity to modulate the immune microenvironment and counteract the effects of bone aging. The scaffolds not only provide structural support for bone regeneration but also serve as effective carriers for anti-osteoporosis drugs, offering a novel therapeutic strategy for treating osteoporotic bone defects. By regulating inflammation and improving the immune response, 3D-printed bioceramic scaffolds may significantly enhance bone repair, particularly in the context of age-related bone degeneration. This approach underscores the potential of advanced biomaterials in addressing the dual challenges of bone aging and immune dysregulation, offering promising avenues for the development of effective treatments for bone defects in the elderly. We hope the concepts discussed in this review could offer novel therapeutic strategies for bone defect repair, and suggest promising avenues for the future development and optimization of bioceramic scaffolds.
Bone defects are common tissue injuries that can result from infections, trauma, or tumors (Alonso-Fernández et al., 2023). While bone tissue has some self-healing capacity, critical-sized bone defects, especially in aging people or when the damage surpasses the bone’s natural regenerative ability, are often challenging to heal spontaneously (Tam et al., 2021; Subbiah et al., 2023). The implantation of bone substitute materials is one of the primary methods for treating critical-size bone defects. This includes autografts, allografts, xenografts, and the implantation of tissue engineering scaffolds (Zou et al., 2021). Autografting is regarded as the gold standard for treating critical-size bone defects; however, its limitations include high donor-site morbidity and limited availability (Subbiah et al., 2023). Allografts and xenografts offer alternative options for bone defect repair, but they are limited by immune response complications (Wang Y. et al., 2024). With advancements in 3D printing technology, bone tissue engineering scaffolds enhance surgical precision and safety, while also enabling personalized bone repair. In recent years, 3D-printed scaffolds made from metals, ceramics, and polymers have shown promising preclinical results, with some already being used in clinical settings (Zhao et al., 2020; Zhang J. et al., 2022; Hu et al., 2023). Metal scaffolds are known for their excellent mechanical properties and corrosion resistance, but they lack osseointegration and bioactivity (Lin et al., 2023). In contrast, ceramic scaffolds are more widely used for bone defect repair due to their composition being similar to human bone, providing excellent biocompatibility and good biodegradability (Blázquez-Carmona et al., 2023). Scaffolds made from materials like hydroxyapatite (HA) and tricalcium phosphate (TCP) offer strong osteogenic potential and high biocompatibility. Furthermore, bioactive glass (BG) scaffolds demonstrate high bioactivity and rapid degradation. The properties of these scaffolds can also be improved through various processing techniques, such as phase separation, freeze-drying, solvent casting, gas foaming, electrospinning, and material blending (Gao et al., 2020; Li et al., 2020; Liu et al., 2021; Biscaia et al., 2022; Tommasino et al., 2023; Liu H. et al., 2024).
Bone repair is a complex process that is orchestrated by dynamic interactions between immune cells and bone tissue (Peng et al., 2023; Dai et al., 2024; Liu D. et al., 2024). The immune system plays an essential role in both the physiological and pathological processes of bone tissue, and the modulation of the immune microenvironment is increasingly becoming a favorable target for bone, cartilage and soft tissue regeneration (Xiong et al., 2022; Mi et al., 2024). Bone and the immune system not only share a common microenvironment but also exchange various cytokines and signaling molecules, highlighting the critical role of immune cells in the bone defect repair process (Tsukasaki and Takayanagi, 2019). In postmenopausal women or injury patients, the altered immune status can directly or indirectly result in bone destruction. Polymorphonuclear neutrophils (PMNs) rapidly infiltrate the defect site and release cytokines (e.g., IL-1, TNF-α) to recruit macrophages (Fischer and Haffner-Luntzer, 2022). The differential effects of macrophages on osteoblasts depend on their polarization curves and secreted paracrine factors. It has been shown that various immune cells interact with osteoblasts and osteoclasts either through direct cell-to-cell contact or, more likely, through paracrine mechanisms, in which TNFα increases osteoclast apoptosis and indirectly stimulates osteoclastogenesis through NF-κB ligand receptor activator of kinase (RANKL) produced by B cells (Fischer and Haffner-Luntzer, 2022; Kushioka et al., 2023). Notably, bone aging itself is accompanied by changes in the immune microenvironment and it leads to deteriorating microstructure and function, increasing the risk of osteoporosis and bone defects (Lei et al., 2024; Cui et al., 2024). Aging disrupts bone-immune crosstalk, exacerbating repair challenges and impairing transition to the M2 phenotype. Chronic low-grade inflammation perpetuates M1 polarization and oxidative stress (Mi et al., 2024). Senescent mesenchymal stem cells (MSCs) exhibit diminished proliferative capacity and secrete senescence-associated secretory phenotype (SASP) factors, further inhibiting regeneration (Tong et al., 2024).
3D-printed bioceramic scaffolds can promote bone regeneration by regulating immune microenvironment, cellular senescence and serving as carriers for anti-osteoporosis drugs (Graney et al., 2016). By synergistically inducing bone regeneration and inhibiting bone resorption, these scaffolds offer a promising approach for treating bone defects in osteoporotic patients. This review explores recent advances in the use of 3D-printed bioceramic scaffolds to promote the repair of bone defects by influencing various factors, including their impact on the osteogenic immune microenvironment and the repair of osteoporotic bone defects in the context of bone aging. Diverging from conventional fabrication-focused analyses, we establish a bone immunology-aging nexus, highlighting 3D printing’s unique spatiotemporal precision in coordinating immune-osteogenic repair for aged bone defects. It provides an in-depth analysis of the mechanisms involved and offers directions for future research and improvement of 3D-printed bioceramic scaffolds for bone regeneration following bone defect repair.
Bone healing progresses through four stages: inflammation, fibrocartilaginous callus formation, bony callus development, and remodeling (Ren et al., 2022). Immune cells primarily influence bone defect repair by affecting the processes of acute and chronic inflammation. Acute inflammation occurs in response to these external stimuli, serving as a crucial signal for the recruitment of immune cells to the injury site. This inflammatory response activates tissue-resident macrophages as well as other local immune cells, initiating an inflammatory cascade that is critical for setting the stage for subsequent tissue repair.
As primary responders to injury sites, polymorphonuclear neutrophils (PMNs) release chemoattractants and cytokines, such as interleukin (IL)-1, IL-6, tumor necrosis factor-α (TNF-α), and macrophage colony-stimulating factor (M-CSF) prior to apoptosis (Granofszky et al., 2018; Gardizani et al., 2019; Zhang et al., 2021). These molecules recruit and activate macrophages, driving chronic inflammatory responses. Macrophages, in particular, play a pivotal role in bone repair and regeneration. They are broadly classified into two functional subtypes: pro-inflammatory M1 macrophages and anti-inflammatory M2 macrophages.
M1 macrophages play a major role in the early stages of bone healing. These cells secrete pro-inflammatory factors that promote the breakdown and resorption of damaged bone tissue. While this pro-inflammatory response is essential for initiating repair, prolonged inflammation can impede healing (Holzer-Geissler et al., 2022; Kuan et al., 2025). M2 macrophages, which appear during the later stages of bone repair, are characterized by their anti-inflammatory properties because they secrete IL-10 and transforming growth factor (TGF) (Lv et al., 2017; Lu et al., 2024). With the increase in anti-inflammatory cytokines, mesenchymal stem cells (MSCs) begin to differentiate into osteoblasts, aiding in the regeneration of bone tissue, while osteoclasts work to degrade the damaged bone tissue. Therefore, one of the key factors in enhancing bone regeneration is promoting the transition from M1 to M2 macrophages. This shift not only reduces inflammation but also fosters an environment conducive to bone formation and vascularization, both of which are critical for long-term healing. Figure 1A summarizes the acute inflammatory phase of the bone defect healing process.
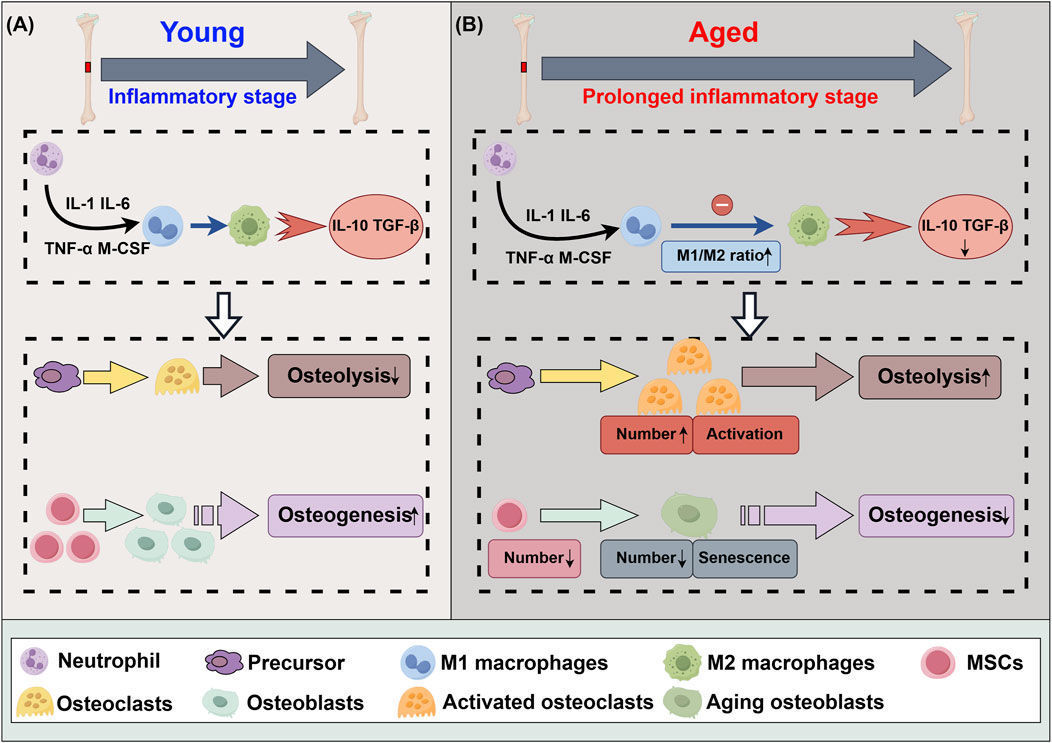
Figure 1. Differences in acute inflammatory phase of bone defect healing between youth and aging. (A) The acute inflammatory phase of the bone healing process. (B) The acute inflammatory phase of the bone healing process subsequent to senescence. IL-1: interleukin 1, IL-6: interleukin 6, IL-10: interleukin 10, CCL13: chemokine ligand 13, TNF-α: tumor necrosis factor-α, M-CSF: macrophage colony-stimulating factor, TGF-β: transforming growth factor-β, MSCs: mesenchymal stem cells.
The metabolic balance of bone tissue is increasingly disrupted, leading to a decline in bone density and an increased risk of fragility, a phenomenon known as bone aging (Kruger and Wolber, 2016). As individuals age, factors such as telomere shortening, oxidative stress, DNA damage, and epigenetic changes contribute to cellular senescence, reducing the regenerative and repair capabilities of cells (Wang et al., 2022; Kwiatkowska et al., 2023; Harley et al., 2024; Zhu et al., 2024). Senescence is associated with a phenomenon known as “inflammation,” which describes the occurrence of persistent, low-grade systemic inflammation (Li X. et al., 2023). Inflammation resulting from the failure of M1 to M2 polarization increases osteoclast activity, decreases osteoblast formation, and exacerbates bone resorption, ultimately impairing bone healing (Mi et al., 2022; Giraldo-Osorno et al., 2024). Additionally, the number and proliferative capacity of MSCs decline, further aggravating the imbalance between bone formation and resorption, thereby prolonging bone defect healing (Peng et al., 2022). Bone aging serves as a foundation for osteoporosis and other age-related bone diseases. Figure 1B summarizes the acute inflammatory phase of the bone defect healing process subsequent to senescence.
In the treatment of bone defects, bioceramic scaffolds serve as the structural foundation for tissue regeneration. However, since these scaffolds are recognized by the body as foreign objects, they can trigger an immune rejection response. The nature and intensity of the immune reaction elicited by the scaffold significantly impact the overall success of the treatment. An uncontrolled or excessive immune response could result in chronic inflammation, impaired healing, or even scaffold rejection, while a well-modulated immune response can support tissue regeneration and integration (Wu et al., 2022; Sun et al., 2023). Thus, the scaffold’s ability to regulate the immune microenvironment is paramount to achieving favorable therapeutic outcomes. Changing scaffold structure and bioactive factor modification are crucial approaches for reducing foreign body reactions, modulating the immune microenvironment, and promoting bone regeneration (Figure 2). Table 1 summarizes recent research progress on the use of 3D-printed bioceramic scaffolds to promote bone defect repair by modulating the immune microenvironment.
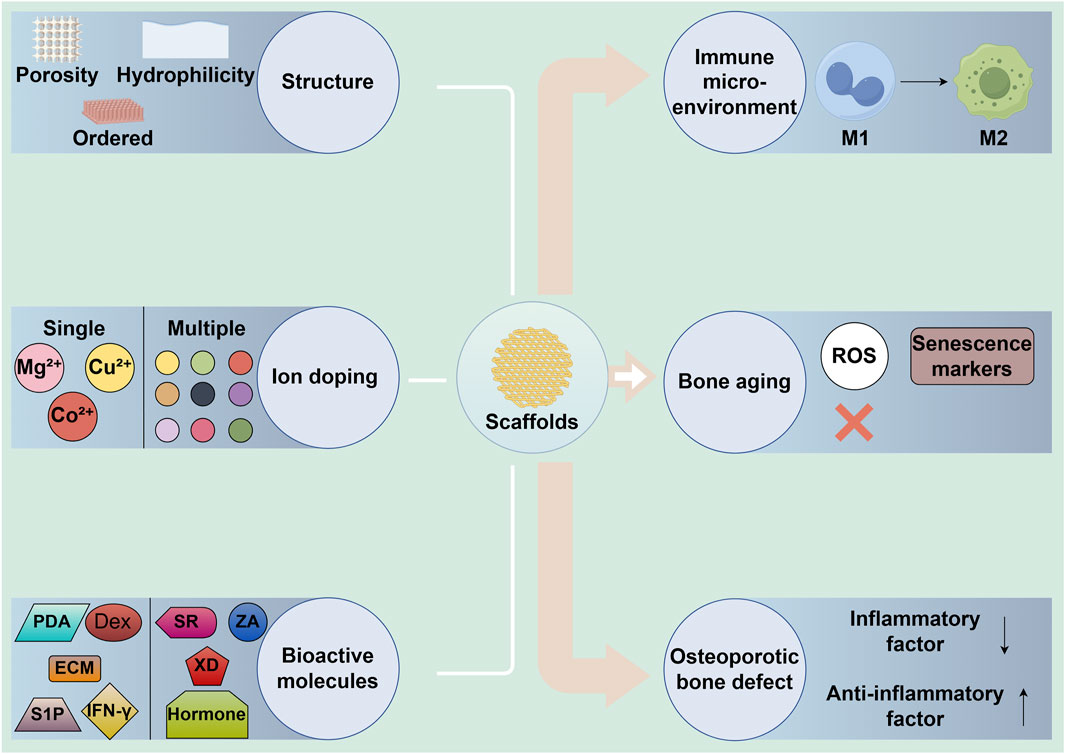
Figure 2. The role of 3D-printed bioceramic scaffolds in regulating immune microenvironment and bone regeneration through structural and bioactive factor modifications. M1: M1 macrophages, M2: M2 macrophages, MSCs: mesenchymal stem cells, ECM: extracellular matrix, IFN-γ: interferon-γ, S1P: sphingosine-1-phosphate, Dex: dexamethasone, PDA: polydopamine.
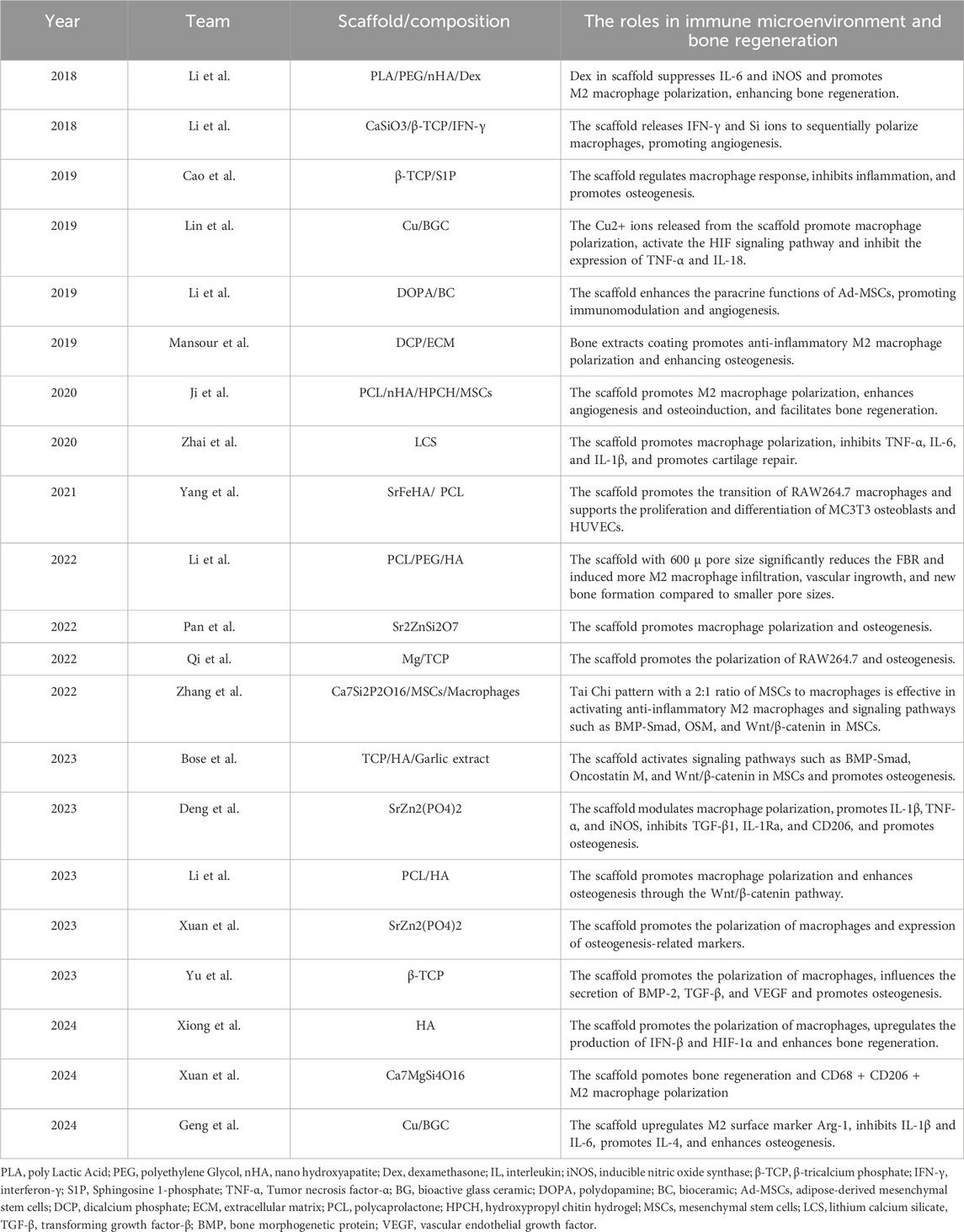
Table 1. The application of 3D-printed bioceramic scaffolds in regulating the immune microenvironment for the repair of bone defects.
Scaffold structural features directly regulate immune cell interactions. Advancements in 3D printing technology have opened up new possibilities for precisely engineering the structure of bioceramic scaffolds to modulate immune responses.
Ordered scaffolds not only enhance immune cell adhesion and migration but also optimize the immune response by modulating macrophage behavior. Xuan et al. (Xuan et al., 2023) compared ordered Bredigite (BRT-O) scaffolds with random BRT (BRT-R) scaffolds and β-TCP scaffolds and found that BRT-O scaffold could enhance the proliferation, migration, and osteogenic differentiation of bone marrow stromal cells (BMSCs) by promoting M2 macrophage polarization and creating an anti-inflammatory healing environment. This was evidenced by the upregulation of osteogenic markers such as BMP2 and RUNX2, resulting in significantly improved bone regeneration in a rat critical-sized bone defect model. Building on these findings, the authors further investigated the application of the BRT scaffold’s ordered structure in bone graft models (Xuan et al., 2024). The increased expression of anti-inflammatory markers such as CD206 and IL-10 further confirmed that the BRT-O scaffold promotes BMSCs’ osteogenic differentiation and significantly enhances bone regeneration in a rabbit calvarial graft model by inducing M2 macrophage polarization. These two studies highlights the potential of BRT scaffolds in clinical practice and further underscores the critical role of ordered microstructures in immunomodulation. Additionally, Yu et al. (2023) designed a novel gear-inspired 3D-printed bioceramic scaffold with well-ordered surface microstructure. Research indicates that this scaffold can promote M2 macrophage polarization, reduce inflammation, and stimulate the osteogenic differentiation of BMSCs.
Apart from the ordered microstructure, altering the pore size and porosity of the scaffold can also modulate immune responses. The size of pores within the scaffold can significantly impact immune cell behavior, particularly in the modulation of macrophage activity, which is essential for tissue healing and immune regulation. Li et al. (2022) compared polycaprolactone/polyethylene glycol/hydroxyapatite (PCL/PEG/HA) scaffolds with different pore sizes and found that P600 significantly reduced foreign body reactions, induced M2 macrophage infiltration, and promoted vascularization and bone regeneration. Also, macrophage polarization may be related to the MyD88 protein. In another study, Xiong et al. (2024) fabricated three different pore-sized hydroxyapatite (HA) bioceramic scaffolds to investigate the systematic effects of pore size on the immune microenvironment. The results indicate that the 600 μm pore-sized scaffold most effectively promoted macrophage M2 polarization and improved the inflammatory response by upregulating interferon-β and HIF-1α production.
Another important strategy for regulating the immune response is to alter the hydrophilicity of the scaffold surface. Compared to hydrophilicity, the hydrophobicity of the scaffold surface reduces cell adhesion and bioactivity, significantly impacting the osteogenic capacity of bioceramic scaffolds (Meng et al., 2022). Li et al. (2023a) enhanced the hydrophilicity of PCL/HA scaffolds through alkali treatment and found that the scaffold significantly reduced the foreign body response and promoted M2 macrophage polarization. In addition, it was found that the regulation of osteogenesis by hydrophilic surface scaffolds may be related to the Wnt/β-catenin signaling pathway.
The above research results show that modifying the structure of 3D-printed bioceramic scaffolds can guide macrophage polarization toward an anti-inflammatory phenotype and enhance the activity of key cell types such as BMSCs, thereby actively facilitating tissue repair and regeneration. However, the balance between the bioactivity and mechanical properties of the scaffold will need to be further optimized in the future.
In addition to the structural characteristics of 3D-printed bioceramic scaffolds, scaffold bioactive factor modification also plays a significant role in modulating the immune response during bone regeneration.
Bioactive bioceramics interact with the cellular environment via surface contact and ion release into the tissue microenvironment. The various ions released from the scaffold, such as magnesium (Mg2+), zinc (Zn2+), and copper (Cu2+), have been shown to modulate the immune microenvironment, particularly by influencing macrophage polarization and promoting bone regeneration (Yang J. et al., 2023; Li et al., 2024; Sun et al., 2024).
Mg2+ is a key component in bone tissue, contributing to bone metabolism and maintaining homeostasis (Qiao et al., 2021). A deficiency in Mg2+ can lead to insufficient bone formation and metabolic disturbances in bone. Therefore, the incorporation of Mg2+ into bioceramic scaffolds to promote osteogenesis and regulate the immune microenvironment has garnered widespread attention. For instance, Qi et al. (2022) developed a 3D-printed magnesium-doped β-TCP scaffold for bone regeneration. Varying MgO contents (0%, 1%, 3%, 5%) were tested, with 3% Mg-TCP showing the best biological performance. In vitro experiments have demonstrated that 3 Mg-TCP enhanced the osteogenic and angiogenic differentiation of BMSCs and endothelial progenitor cells. It also promoted M2 macrophage polarization, supporting tissue regeneration. In vivo experiments have demonstrated that 3 Mg-TCP demonstrated superior bone repair capabilities in a rabbit femoral defect model. Cu2+ is another important ion for regulating immune responses and promoting tissue regeneration. Lin et al. (2019) demonstrated that copper-incorporated bioactive glass ceramics (Cu-BGC) enhance cartilage and bone regeneration by promoting chondrocyte proliferation and inducing macrophage polarization towards the anti-inflammatory M2 phenotype. The potential mechanism may involve released Cu2+ stimulating cartilage immune responses through the HIF-1α pathway and inhibiting tissue inflammation. In a subsequent study, Geng et al. (2024) found that Cu-BGC significantly inhibited the expression of pro-inflammatory cytokines (e.g., IL-1β and IL-6) by inducing M2 macrophage polarization, while upregulating anti-inflammatory cytokines (e.g., IL-4), indicating its anti-inflammatory effects. Additionally, Cu-BGC significantly reduced the expression of matrix-degrading enzymes (e.g., MMP3, MMP13), suggesting that Cu-BGC effectively protects cartilage matrix from degradation in inflammatory conditions. The above research indicates that bioceramic scaffolds with single-ion doping can promote bone regeneration by enhancing macrophage polarization, which holds significant value in the treatment of bone defects.
Based on the foundation of single ion doping, the complementary doping of multiple beneficial elements to enhance biological effects is now considered a promising new strategy. Yang et al. (2021) investigated the synergistic biological effects of Sr2+/Fe3+ by employing extrusion-based low-temperature 3D printing technology to fabricate SrFeHA/PCL scaffolds, comparing them with scaffolds doped solely with Sr10HA and Fe10HA. The results indicated that the synergistic effect of Sr2+/Fe3+ not only modulates the immune response by promoting M2 macrophage polarization but also enhances the functionality of MC3T3 osteoblasts and HUVECs. Zhai et al. (2020) investigated whether lithium calcium silicate (LCS) bioceramic scaffolds could promote the repair and regeneration of cartilage tissue by inducing the differentiation of macrophages in a specific direction. The experimental results showed that LCS scaffolds promote M2 macrophage polarization by reducing the activity of inflammatory-related genes TNFα, IL-6, and IL-1β, while enhancing the expression of the IL-10 gene, thereby promoting the growth of chondrocytes. Pan et al. (2022) developed 3D-printed Sr2ZnSi2O7 (SZS) scaffolds. Research found that these scaffolds possess good immunomodulatory functions, modulating the inflammatory response of macrophages by releasing bioactive ions. This is specifically manifested by promoting an immune environment conducive to healing and reducing the release of inflammatory factors. However, the issues of rapid degradation rate and low mechanical strength remain challenges to be addressed in the future.
The multi-ion doping method still faces certain challenges in precisely controlling the proportions and distribution of each dopant ion. In contrast, the single-phased bioceramics with a fixed composition offer greater stability. Deng et al. (2023) compared 3D-printed strontium-zinc-phosphate (SZP) bioceramic scaffolds with β-TCP scaffolds and found that SZP scaffolds possess superior osteogenic, angiogenic, and antibacterial properties, and promote M2 macrophage polarization.
The above research results reveal the significant potential of ion-doped bioceramic scaffolds in immune modulation. By releasing specific bioactive ions, these scaffolds can effectively modulate the inflammatory microenvironment of macrophages, promoting the phenotypic transformation of macrophages with anti-inflammatory properties. This transformation is crucial for facilitating the healing process of bone defects. However, the synergistic effects between ions and the underlying mechanisms of their interactions still need to be further explored, and the accuracy and stability of the proportion and distribution of doped ions require enhancement.
Bioceramic scaffolds loaded with bioactive molecules not only provide structural support for tissue regeneration but also promote tissue repair by leveraging various immunomodulatory mechanisms.
The extracellular matrix (ECM), composed of organic components like proteins, polysaccharides, and growth factors, plays a pivotal role in regulating cellular behavior and directing tissue regeneration and development (Wang J. et al., 2024). Mansour et al. (2019) demonstrated that coating dicalcium phosphate bioceramic scaffolds with bone ECM extracts, especially calcium-binding E-extract, enhances bone regeneration through immunomodulation. E-extract-coated scaffolds reduced inflammatory responses, promoted anti-inflammatory macrophage activity, and significantly improved bone formation in rat tibial defects. The study also shows that 3D-printed hydrogel scaffolds incorporating E-extract support better bone regeneration, suggesting future applications for personalized, ECM-based scaffolds in complex bone repair. However, whether this process is related to the conversion of M1 to M2 macrophages remains to be verified.
Interferon-γ (IFN-γ) and sphingosine-1-phosphate (S1P), among others, regulate host immune responses through different mechanisms to promote bone regeneration. Li et al. (2018b) presented a 3D-printed calcium silicate-β-tricalcium phosphate (CaSiO3-β-TCP) scaffold loaded with IFN-γ. The research results indicate that the scaffold sequentially activates M1 and M2 macrophage polarization: M1 for early inflammation and M2 for tissue repair. In vitro experiments have demonstrated that it enhances macrophage-driven angiogenesis and bone regeneration by increasing the secretion of VEGF and PDGF-BB. In vivo experiments have demonstrated that the scaffold improves blood vessel formation and bone healing in a mouse model. Additionally, S1P is an important immunomodulatory molecule that, when coated on β-tricalcium phosphate scaffolds, effectively inhibits inflammation and promotes osteogenesis. S1P plays a crucial role in bone formation by regulating macrophage migration and the expression of inflammation-related genes. Cao et al. (2019) evaluated 3D-printed β-TCP scaffolds coated with S1P for immunomodulation and bone regeneration. The research results indicate that the S1P-coated scaffolds reduce inflammation by downregulating pro-inflammatory cytokines and promote osteogenesis by upregulating osteogenic genes like OCN, OPN, and RUNX2. Additionally, this scaffold also promotes the differentiation of BMSCs into osteoblasts. This dual action of reducing inflammation and enhancing bone regeneration makes S1P-coated scaffolds a promising option for treating large bone defects.
Dexamethasone (Dex), as a potent glucocorticoid, not only promotes osteogenesis but also effectively controls local inflammation due to its immunosuppressive properties. Li et al. (2018a) developed a composite scaffold made from PLA, PEG, nHA, and Dex using 3D printing for bone regeneration and found that Dex release from the scaffold modulated inflammation by promoting M2 macrophage polarization and enhanced osteogenesis by increasing late alkaline phosphatase secretion and calcium deposition. In a rat calvarial defect model, the scaffold improved bone regeneration without adverse effects on vital organs.
Due to its excellent biocompatibility and degradability, polydopamine (PDA) shows great potential for applications in the field of tissue engineering. Li et al. (2019) conducted a comparative study by culturing adipose-derived mesenchymal stem cells (Ad-MSCs) on BC scaffolds and PDA-modified BC scaffolds and found that polydopamine biomimetic coating significantly enhanced the paracrine capabilities of mesenchymal stem cells. Specifically, Ad-MSCs cultured on polydopamine-modified BC scaffolds (DOPA-BC) were able to secrete a greater amount of immunomodulatory factors. This phenomenon promoted the polarization of M2-type macrophages, thereby playing a positive role in immunomodulation.
3D-printed bioceramic scaffolds loaded with immune cells have shown unique immunomodulatory potential in bone tissue engineering. Using 3D printing technology to load macrophages or MSCs into bioceramic scaffolds can create a complex network of cellular interactions. The spatial distribution and arrangement of these cells on the scaffold can significantly affect their immunomodulatory functions. For example, Zhang et al. (2022a) in order to observe the intercellular “cross-talk” between macrophages and MSCs within a three-dimensional structure, used digital light processing-based 3D printing technology to create a multi-channel honeycomb-like bioceramic scaffold. The study results showed that the “Taiji” pattern with a 2:1 ratio of MSCs to macrophages more effectively stimulated M2 polarization and promoted the osteogenic differentiation of MSCs. They also found that this effect may be associated with the activation of the BMP-Smad, Oncostatin M, and Wnt/β-catenin signaling pathways.
Bioceramic scaffolds loaded with bioactive molecules represent a cutting-edge approach in bone regeneration and immunomodulation, effectively reducing inflammatory responses and regulating macrophage polarization. Additionally, the rational adjustment of the spatial arrangement and distribution of immune cells can enhance immunomodulatory capabilities. However, research on the loading of multiple bioactive molecules, as well as their spatial distribution and arrangement, is insufficient, and further studies are needed in the future.
With the occurrence of bone aging, the incidence of age-related diseases such as osteoporosis has been rising year by year, posing a significant challenge to public health. Osteoporosis is a systemic degenerative bone disease associated with aging, characterized by reduced bone mass and deterioration of bone microstructure, thereby leading to increased bone fragility and risk of fracture, known as osteoporotic fractures (Patsch et al., 2011). 3D-printed bioceramic scaffolds can be used to treat bone defects in an aging environment by modulating bone aging and delivering drug therapies for osteoporotic bone defects (Figure 3). Table 2 summarizes recent research advancements in the modulation of bone aging and the treatment of osteoporotic bone defects using 3D-printed bioceramic scaffolds.
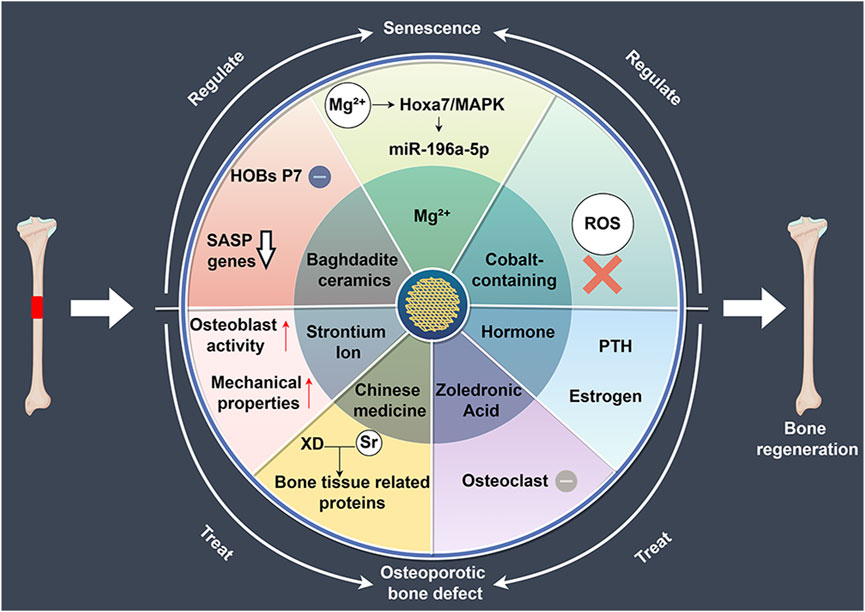
Figure 3. The role of 3D-printed bioceramic scaffolds in regulating bone regeneration in aging and osteoporosis environments. HOBs: human primary osteoblast-like cells, SASP: senescence-associated secretory phenotype, XD: Xu Duan, ROS: reactive oxygen species, PTH: parathyroid hormone.
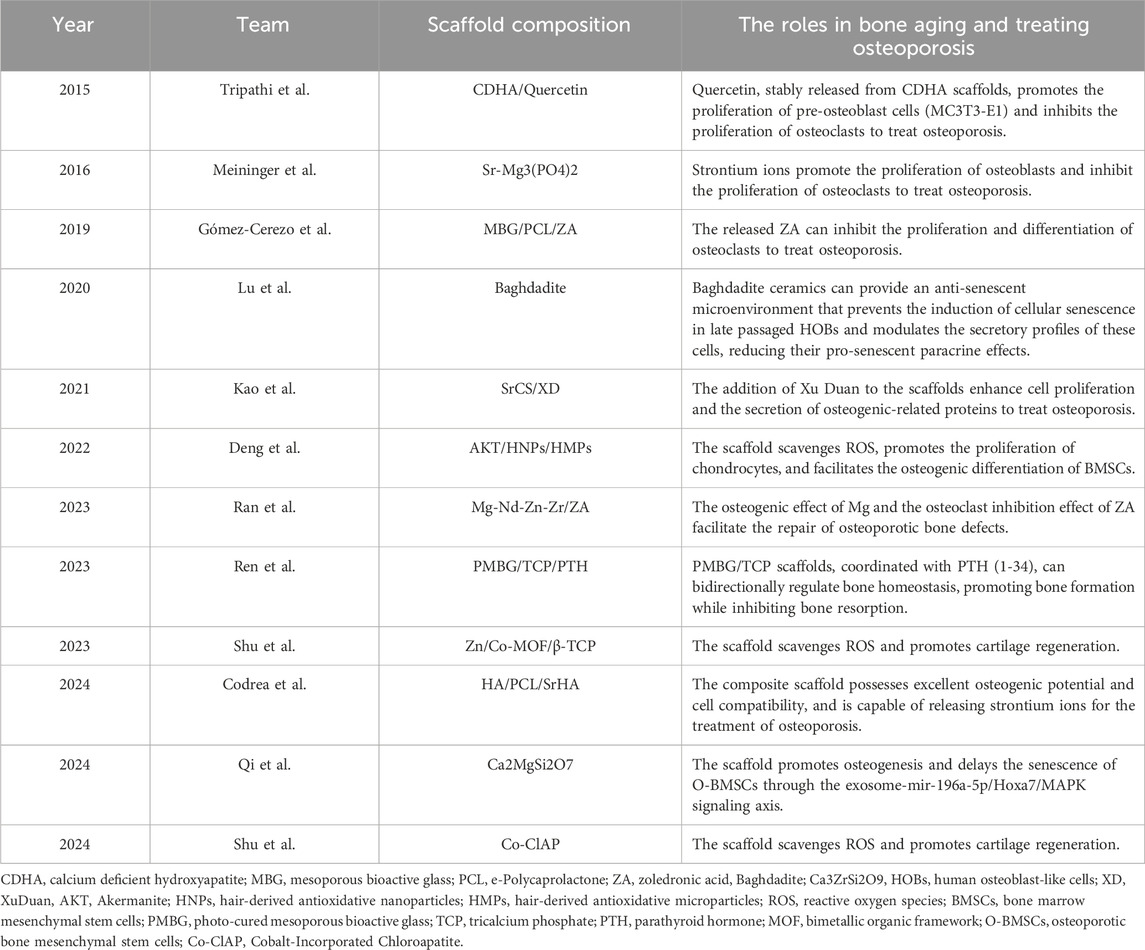
Table 2. The application of 3D-printed bioceramic scaffolds in treating aged and osteoporotic bone defects.
3D-printed bioceramic scaffolds with tailored structures and compositions effectively support bone regeneration in aging microenvironments. Lu et al. (2020) compared Baghdadite scaffolds with HA/TCP scaffolds and found that Baghdadite scaffolds significantly reduced the expression of senescence markers (P16, P21) in P7 human primary osteoblast-like cells (HOBs), as well as senescence-associated secretory phenotype markers, such as IL-1α, TNF-α, and IL-6. Additionally, Baghdadite scaffolds decreased the paracrine effects of P7 HOB secretions in inducing senescence in young cells. Notably, Baghdadite scaffolds also improved mitochondrial function. These findings suggest that Baghdadite ceramics are a promising biomaterial capable of creating an anti-senescence and pro-regenerative microenvironment.
In addition, Mg2+ can not only regulate the immune microenvironment but also play a significant role in modulating the aging microenvironment. Qi et al. (2024) in order to investigate the effects of magnesium ions on osteoporosis and osteogenesis, fabricated Akermanite bioceramics (Akt) scaffolds enriched with Mg ions. The research found that magnesium-rich bioceramics can effectively improve bone regeneration impairments caused by aging by regulating the osteogenic differentiation and angiogenesis of BMSCs. Mg2+ enhances bone regeneration by targeting the Hoxa7/MAPK signaling axis, which modulates the secretion of miR-196a-5p in exosomes, thereby influencing the expression of osteogenesis-related genes in the aging microenvironment. This provides a potential strategy for bone regeneration in an aging microenvironment.
Oxidative stress plays a critical role in aging and bone tissue degeneration, particularly in the process of bone tissue regeneration. Reactive oxygen species (ROS)-induced damage to cellular components is considered one of the key mechanisms promoting aging (He et al., 2024). Similar to other tissues, the accumulation of oxidative stress-induced cellular and tissue damage in bone increases with age, leading to a decline in bone cell function and senescence (Xu et al., 2020). Mitochondria are the primary source of ROS. With advancing age, mitochondrial ATP production capacity declines, and the efficiency of antioxidant defense systems also diminishes, resulting in elevated intracellular ROS levels (Cheng and Ristow, 2013). This oxidative stress not only damages macromolecules such as cell membranes, DNA, and proteins but also triggers a range of age-related pathological changes, including chronic inflammation, neurodegenerative diseases, cardiovascular diseases, and diabetes (Kello et al., 2020; Fang et al., 2022; Hanchang et al., 2022; Yang Y. M. et al., 2023; Prata et al., 2024). Using 3D-printed bioceramic scaffolds to alleviate oxidative stress may offer new strategies for slowing aging and preventing related diseases. Shu et al. (2023) developed Zn/Co-MOF-functionalized β-TCP scaffolds to enhance osteochondral regeneration in osteoarthritis. The research results indicate that the scaffold has a broad ability to clear ROS and promotes osteogenic and chondrogenic differentiation. In subsequent research, Shu et al. (2024) developed Co-ClAP/PLGA composite scaffolds using 3D printing technology. The research indicates that this scaffold possesses antioxidant characteristics, capable of neutralizing excessive ROS in inflammatory environments, thereby maintaining cell proliferation, adhesion, and differentiation, and concurrently promoting the regeneration of cartilage and subchondral bone. The above results demonstrate that these two cobalt-containing bioceramic scaffolds not only exhibit strong antioxidant properties, significantly clearing ROS, but also promote osteogenesis. Future research should focus on enhancing the antioxidant properties of the scaffold while improving osteogenic capacity.
3D-printed bioceramic scaffolds play a crucial role in combating bone aging. They significantly promote bone tissue regeneration under aging conditions by reducing the expression of aging markers and modulating the activity of osteogenesis-related genes in a aging environment. Additionally, these scaffolds exhibit excellent antioxidant properties, effectively neutralizing excessive ROS in inflammatory environments, thereby combating the aging process triggered by oxidative stress.
The primary factors contributing to osteoporosis include intrinsic factors related to natural aging, which heighten bone resorption and reduce bone formation, as well as external factors, such as long-term glucocorticoid use, which further disrupt bone microarchitecture and lead to osteoporosis. Over the past few decades, various pharmacological treatments have been developed to treat osteoporotic bone defects, broadly categorized into anti-resorptive agents (which prevent bone breakdown) and anabolic agents (which stimulate bone formation) (Fixen and Fixen, 2022; Shane et al., 2022; Hartz et al., 2024). In recent years, advances in 3D printing technology have revolutionized the field of bone regeneration by enabling the development of bioceramic scaffolds as drug carriers.
Strontium ions, the active component of strontium ranelate, enhance bone formation while suppressing resorption. Codrea et al. (2024) utilized 3D printing technology to fabricate scaffolds made of PCL and strontium-substituted hydroxyapatite (SrHA) with the aim of enhancing the recovery of osteoporotic bone defects. The results indicated that scaffolds incorporating SrHA exhibited improved mechanical properties and osteogenic potential, suggesting their potential application in the treatment of osteoporotic bone defects. Meininger et al. (2016) fabricated 3D-printed strontium-substituted magnesium phosphate (SrMPC) scaffolds designed for bone regeneration. These biodegradable scaffolds show good mechanical strength (36.7 MPa compressive) and a porous architecture ideal for bone ingrowth. In vitro studies confirmed controlled degradation and a sustained release of Sr2+, which promotes osteoblast activity. In both studies, it has been demonstrated that strontium-doped bioceramic scaffolds possess superior mechanical properties, offering significant advantages in the repair of osteoporotic bone defects. However, the stability of strontium release rates still needs to be further improved.
Zoledronic acid (ZA), a third-generation bisphosphonate with high affinity for bone tissue, is a first-line treatment for osteoporotic bone defects. It inhibits osteoclast differentiation and induces osteoclast apoptosis, thereby suppressing bone resorption. Ran et al. (2023) developed a 3D-printed biodegradable Mg2+ scaffold with a ceramic coating loaded with ZA for the treatment of osteoporotic bone defects. Studies have shown that the ZA coating not only reduces the corrosion rate of the scaffold but also achieves precise and slow drug release. Furthermore, due to the combined action of Mg2+ and ZA, the scaffold promotes bone formation while inhibiting osteoclast activity, effectively facilitating the repair of osteoporotic bone defects. In another study, Gómez-Cerezo et al. (2019) developed mesoporous bioactive glass (MBG)/PCL scaffolds for bone regeneration in osteoporotic sheep. The scaffold demonstrated good biocompatibility in vivo, promoting both bone formation and angiogenesis. Nonetheless, when loaded with 1% ZA, the scaffold induced a strong inflammatory response and impaired bone healing. The above results indicate that bioceramic scaffolds loaded with ZA show great potential in promoting the repair of osteoporotic bone defects, but further research and optimization are needed in terms of drug dosage to achieve better therapeutic effects.
Traditional Chinese medicine has long been valued for its holistic approach to health, and its integration with modern medical technologies presents an innovative avenue for treating osteoporotic bone defects. Kao et al. (2021) investigated the efficacy of 3D-printed scaffolds made from poly-ε-caprolactone, strontium-doped calcium silicate, and Xu Duan (a traditional Chinese medicine) for bone regeneration in osteoporosis. The results indicate that XD and Sr ions have a synergistic effect. In vitro and in vivo, the scaffold, especially those with a higher concentration of strontium (XD10), can increase the secretion of bone tissue related proteins and promote the repair of osteoporotic bone defects. These scaffolds promote bone healing by enhancing osteoblast activity and bone mineralization. This approach opens up new possibilities for combining traditional medicine with advanced materials, offering a promising strategy for treating osteoporotic bone defects. However, research on the combination of traditional Chinese medicine with 3D-printed bioceramic scaffolds is insufficient, and future studies should place greater emphasis on the use of traditional Chinese medicine in bone tissue engineering and the treatment of osteoporotic bone defects.
Anabolic bone therapies aim to enhance bone mass by stimulating bone formation. One widely used strategy is the intermittent activation of parathyroid hormone (PTH), which encourages osteogenesis by promoting osteoblast activity. Ren et al. (2023) studied a 3D-printed scaffold composed of photocurable mesoporous bioactive glass (PMBG) and TCP loaded with PTH (1-34). The research findings indicate that the scaffold, in conjunction with PTH (1-34), regulates bone homeostasis bidirectionally by activating the Wnt/β-catenin pathway and inhibiting fibroblast activation protein. This study underscores the potential of combining advanced materials like PMBG and TCP with anabolic agents such as PTH to create scaffolds that not only support bone regeneration but also modulate the underlying cellular mechanisms to optimize healing. Further research is necessary to fully elucidate the underlying molecular pathways and optimize the therapeutic potential of PTH in clinical applications for the treatment of osteoporotic bone defects.
Postmenopausal estrogen deficiency accelerates bone resorption, driving significant bone loss. Hormone replacement therapy has been a key method for preventing and treating osteoporotic bone defects in women (Chen et al., 2020). Tripathi et al. (2015) developed 3D-printed calcium-deficient hydroxyapatite scaffolds loaded with quercetin for the treatment of osteoporotic bone defects. The research findings indicate that the quercetin-loaded scaffolds significantly enhanced pre-osteoblast cell (MC3T3-E1) activity and suppressed osteoclast proliferation, outperforming traditional treatments like alendronate. The potential advantage of this type of scaffold may be its capacity to offer more effective solutions for the treatment of osteoporotic bone defects, particularly in the treatment of postmenopausal osteoporotic bone defects.
As the population ages, bone defects from various causes continue to be a clinical challenge. Due to their excellent biocompatibility and potential for personalized design, 3D-printed bioceramic scaffolds show great promise in addressing these defects. In this review, we aim to explore the critical role of 3D-printed bioceramic scaffolds in the repair of bone defects, highlighting their ability to regulate the immune microenvironment, combat bone aging, and their application in the regeneration of osteoporotic bone defects. By optimizing scaffold design and modifying the scaffold, it is possible to effectively regulate the polarization state of macrophages, reduce inflammatory responses, and promote the process of bone regeneration (Mansour et al., 2019; Xuan et al., 2023). Also, 3D-printed bioceramic scaffolds exhibit good antioxidant properties, capable of neutralizing excessive ROS in the inflammatory environment, and combating aging phenomena caused by oxidative stress (Shu et al., 2024). More importantly, 3D-printed bioceramic scaffolds not only reduce the expression of senescence markers but also promote bone regeneration in a senescent environment (Lu et al., 2020). By delivering osteoporosis drugs, bioceramic scaffolds have also shown a promising application prospect in the treatment of osteoporotic bone defects (Gómez-Cerezo et al., 2019; Kao et al., 2021).
In comparison with conventional artificial molding or phase separation techniques, 3D printing technology facilitates the precise fabrication of complex structures for personalised defect repair and immunomodulation, as well as the integration of multifunctional active ingredients during the fabrication process to further promote bone defect repair by modulating the immune microenvironment and bone ageing. Although 3D-printed bioceramic scaffolds have made significant breakthroughs in the treatment of bone defects, there are still several key issues that need in-depth exploration. Firstly, the inadequate alignment of materials’ biocompatibility, degradability, and mechanical properties with bone defects hinders clinical translation. Secondly, current research lacks a detailed and in-depth analysis of the mechanisms by which 3D-printed bioceramic scaffolds induce macrophage polarization. Research on the role of 3D-printed bioceramic scaffolds in regulating cellular senescence remains limited. It is recommended that subsequent studies investigate the manner in which the surface topology of 3D-printed bioceramic scaffolds and the microenvironment loaded with actives coordinates macrophage polarisation with cellular senescence. Such studies should also explore the synergistic intervention of conventional RANKL, NF-κB, TGF-β/Smad, p53, and other pathways, as well as explore new possible mechanisms.
The process of bone repair is a complex physiological process that involves not only the repair of the bone itself but also has a close relationship with surrounding tissues such as muscles and blood vessels (Zhai et al., 2021; Toita et al., 2024). The importance of blood vessels in bone regeneration lies in their creation of a microenvironment conducive to bone regeneration, which provides sufficient nutrients, growth factors, and oxygen for bone tissue repair. Vascular endothelial growth factor (VEGF) is a key regulatory factor in angiogenesis, promoting the migration and proliferation of endothelial cells through the regulation of osteogenic growth factor release and paracrine signaling, thereby indirectly promoting the osteogenic process (Burger et al., 2022). Studies have found that VEGF-decorated crystalline SiHA scaffolds can effectively treat osteoporotic bone defects (Casarrubios et al., 2020). Enhanced vascularization observed in VEGF-decorated scaffolds not only aids in bone regeneration but also in the overall integration of the scaffold with surrounding tissues. Therefore, an ideal 3D-printed bioceramic scaffold should induce vascularization to optimize the bone repair process. Additionally, studies have shown that skeletal muscle also plays a crucial role in the process of bone repair. The expression of bone morphogenetic protein 2 (BMP-2) in autologous muscle tissue significantly enhances its ability in bone regeneration, which has a significant therapeutic effect on the treatment of bone defects (Kong et al., 2020).
Therefore, future scaffold designs should take a comprehensive approach, considering the regulation of the immune microenvironment, the mitigation of bone aging, the promotion of vascularization, and the modulation of surrounding muscle tissues. Regulating M1/M2 polarization will be one of the key factors in promoting bone defect repair. M1 macrophages are usually associated with inflammatory responses, and inhibiting M1 polarization or inducing M2 macrophage polarization helps reduce chronic inflammation and promote tissue repair. M2 macrophages not only play a role in anti-inflammatory processes but also promote bone reconstruction and vascularization by secreting growth factors and cytokines. By optimizing the surface properties and microstructure of the scaffold, designing materials that promote M2 macrophage polarization will help improve immune responses and accelerate bone repair processes. Additionally, bone aging is a critical factor influencing the bone defect repair process. The design of scaffold materials should have characteristics that combate or even reverse bone aging, such as enhancing bone density and rejuvenating senescent bone cells. Vascularization is another important factor for successful scaffold implantation, as a robust vascular network is essential for supplying the necessary nutrients and oxygen to the repair site, thereby facilitating bone tissue regeneration. In addition, the control of surrounding muscle tissues is also an indispensable dimension in the bone defect repair process, as muscle function recovery has a direct impact on the bone repair. Therefore, the design of future bioceramic scaffolds should integrate these multifaceted biological needs, promoting the research and clinical application of multifunctional scaffolds.
3D-printed bioceramic scaffolds demonstrate significant potential in regulating the immune microenvironment and mitigating bone aging. By optimising scaffold structures and incorporating bioactive factors, these scaffolds can modulate the immune microenvironment, effectively target specific immune pathways, and promote tissue healing and osteogenic differentiation. In addition, the modified bioceramic scaffolds reduce cellular senescence and oxidative stress, enhance bone regeneration and serve as carriers for anti-osteoporotic drugs, further contributing to bone regeneration. In summary, bioceramic scaffolds present innovative solutions for the treatment of bone defects and promise to provide multiple strategies for maintaining bone health.
HQ: Investigation, Writing–original draft, Writing–review and editing. BZ: Investigation, Writing–review and editing. FL: Funding acquisition, Supervision, Writing–review and editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Research Funds of Centre for Leading Medicine and Advanced Technologies of IHM (2023IHMO1072), and the Natural Science Foundation of Heilongjiang Province (PL2024H141).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alonso-Fernández, I., Haugen, H. J., López-Peña, M., González-Cantalapiedra, A., and Muñoz, F. (2023). Use of 3D-printed polylactic acid/bioceramic composite scaffolds for bone tissue engineering in preclinical in vivo studies: a systematic review. Acta Biomater. 168, 1–21. doi:10.1016/j.actbio.2023.07.013
Biscaia, S., Branquinho, M. V., Alvites, R. D., Fonseca, R., Sousa, A. C., Pedrosa, S. S., Caseiro, A. R., Guedes, F., Patrício, T., Viana, T., Mateus, A., Maurício, A. C., and Alves, N. (2022). 3D printed Poly(ε-caprolactone)/Hydroxyapatite Scaffolds for Bone Tissue Engineering: A Comparative Study on a Composite Preparation by Melt Blending or Solvent Casting Techniques and the Influence of Bioceramic Content on Scaffold Properties. Int J. M.ol S.ci 2.3, 2318. doi:10.3390/ijms23042318
Blázquez-Carmona, P., Mora-Macías, J., Martínez-Vázquez, F.J., Morgaz, J., Domínguez, J., and Reina-Romo, E. (2023). Mechanics Predicts Effective Critical-Size Bone Regeneration Using 3D-Printed Bioceramic Scaffolds. Tissue Eng R.egen M.ed 2.0, 893–904. doi:10.1007/s13770-023-00577-2
Burger, M.G., Grosso, A., Briquez, P.S., Born, G.M. E., Lunger, A., Schrenk, F., et al. (2022). Robust coupling of angiogenesis and osteogenesis by VEGF-decorated matrices for bone regeneration. Acta Biomater 1.49, 111–125. doi:10.1016/j.actbio.2022.07.014
Cao, Y., Xiao, L., Cao, Y., Nanda, A., Xu, C., and Ye, Q. (2019). 3D printed β-TCP scaffold with sphingosine 1-phosphate coating promotes osteogenesis and inhibits inflammation. Biochem B.iophys R.es C.ommun 5.12, 889–895. doi:10.1016/j.bbrc.2019.03.132
Casarrubios, L., Gómez-Cerezo, N., Sánchez-Salcedo, S., Feito, M.J., Serrano, M.C., Saiz-Pardo, M., et al. (2020). Silicon substituted hydroxyapatite/VEGF scaffolds stimulate bone regeneration in osteoporotic sheep. Acta Biomater 1.01, 544–553. doi:10.1016/j.actbio.2019.10.033
Chen, X., Zhu, X., Hu, Y., Yuan, W., Qiu, X., Jiang, T., et al. (2020). EDTA-Modified 17β-Estradiol-Laden Upconversion Nanocomposite for Bone-Targeted Hormone Replacement Therapy for Osteoporosis. Theranostics 10, 3281–3292. doi:10.7150/thno.37599
Cheng, Z., and Ristow, M. (2013). Mitochondria and metabolic homeostasis. Antioxid R.edox Signal 19, 240–242. doi:10.1089/ars.2013.5255
Codrea, C.I., Lincu, D., Ene, V.L., Nicoară, A.I., Stan, M.S., Ficai, D., et al. (2024). Three-Dimensional-Printed Composite Scaffolds Containing Poly-ε-Caprolactone and Strontium-Doped Hydroxyapatite for Osteoporotic Bone Restoration. Polymer.Basel 16, 1511. doi:10.3390/polym16111511
Cui, Y., Lv, B., Li, Z., Ma, C., Gui, Z., Geng, Y., et al. (2024). Bone-Targeted Biomimetic Nanogels Re-Establish Osteoblast/Osteoclast Balance to Treat Postmenopausal Osteoporosis. Small 20, e2303494. doi:10.1002/smll.202303494
Dai, Y., Wu, J., Wang, J., Wang, H., Guo, B., Jiang, T., et al. (2024). Magnesium Ions Promote the Induction of Immunosuppressive Bone Microenvironment and Bone Repair through HIF-1α-TGF-β Axis in Dendritic Cells. Small 20, e2311344. doi:10.1002/smll.202311344
Deng, L., Huang, L., Pan, H., Zhang, Q., Que, Y., Fan, C., et al. (2023). 3D printed strontium-zinc-phosphate bioceramic scaffolds with multiple biological functions for bone tissue regeneration. J M.ater Chem B. 11, 5469–5482. doi:10.1039/d2tb02614g
Fang, T., Huang, Y.K., Wei, J., Monterrosa Mena, J.E., Lakey, P.S. J., Kleinman, M.T., et al. (2022). Superoxide Release by Macrophages through NADPH Oxidase Activation Dominating Chemistry by Isoprene Secondary Organic Aerosols and Quinones to Cause Oxidative Damage on Membranes. Environ S.ci T.echnol 5.6, 17029–17038. doi:10.1021/acs.est.2c03987
Fischer, V., and Haffner-Luntzer, M. (2022). Interaction between bone and immune cells: Implications for postmenopausal osteoporosis. Semin C.ell Dev B.iol 1.23, 14–21. doi:10.1016/j.semcdb.2021.05.014
Fixen, C.W., and Fixen, D.R. (2022). Renal safety of zoledronic acid for osteoporosis in adults 75 years and older. Osteoporos I.nt 3.3, 2417–2422. doi:10.1007/s00198-022-06499-4
Gao, D., Wang, Z., Wu, Z., Guo, M., Wang, Y., Gao, Z., et al. (2020). 3D-printing of solvent exchange deposition modeling (SEDM) for a bilayered flexible skin substitute of poly (lactide-co-glycolide) with bioorthogonally engineered EGF. Mater Sci E.ng C. Mater Biol A.ppl 1.12, 110942. doi:10.1016/j.msec.2020.110942
Gardizani, T.P., Della Coletta, A.M., Romagnoli, G.G., Puccia, R., Serezani, A.P. M., De Campos Soares Â, M.V., et al. (2019). 43 kDa Glycoprotein (gp43) from Paracoccidioides brasiliensis Induced IL-17A and PGE2 Production by Human Polymorphonuclear Neutrophils: Involvement of TLR2 and TLR4. J I.mmunol R.es 2.019, 1–9. doi:10.1155/2019/1790908
Geng, D., Lin, R., Wei, P., Tang, C., Xu, Y., and Wang, L. (2024). The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in Osteoarthritis. Med S.ci M.onit 3.0, e943738. doi:10.12659/msm.943738
Giraldo-Osorno, P.M., Wirsig, K., Asa'ad, F., Omar, O., Trobos, M., Bernhardt, A., et al. (2024). Macrophage-to-osteocyte communication: Imiact in a 3D inin vitromplant-associated infection model. Acta Biomater 1.86, 141–155. doi:10.1016/j.actbio.2024.08.005
Gómez-Cerezo, N., Casarrubios, L., Saiz-Pardo, M., Ortega, L., De Pablo, D., Díaz-Güemes, I., et al. (2019). Mesoporous bioactive glass/ɛ-polycaprolactone scaffolds promote bone regeneration in osteoporotic sheep. Acta Biomater 9.0, 393–402. doi:10.1016/j.actbio.2019.04.019
Graney, P.L., Roohani-Esfahani, S.I., Zreiqat, H., and Spiller, K.L. (2016). InIn vitroesponse of macrophages to ceramic scaffolds used for bone regeneration. J R. S.oc I.nterface 13, 20160346. doi:10.1098/rsif.2016.0346
Granofszky, N., Lang, M., Khare, V., Schmid, G., Scharl, T., Ferk, F., et al. (2018). Identification of PMN-released mutagenic factors in a co-culture model for colitis-associated cancer. Carcinogenesis 39, 146–157. doi:10.1093/carcin/bgx118
Hanchang, W., Wongmanee, N., Yoopum, S., and Rojanaverawong, W. (2022). Protective role of hesperidin against diabetes induced spleen damage: Memhanism associated with oxidative stress and inflammation. J F.ood Biochem 4.6, e14444. doi:10.1111/jfbc.14444
Harley, J., Santosa, M.M., Ng, C.Y., Grinchuk, O.V., Hor, J.H., Liang, Y., et al. (2024). Telomere shortening induces aging-associated phenotypes in hiPSC-derived neurons and astrocytes. Biogerontology 25, 341–360. doi:10.1007/s10522-023-10076-5
Hartz, M.C., Johannessen, F.B., Harsløf, T., and Langdahl, B.L. (2024). The Effectiveness and Safety of Romosozumab and Teriparatide in Postmenopausal Women with Osteoporosis. J C.lin E.ndocrinol M.etab, .dgae484. doi:10.1210/clinem/dgae484
He, Z., Sun, C., Ma, Y., Chen, X., Wang, Y., Chen, K., et al. (2024). Rejuvenating Aged Bone Repair through Multihierarchy Reactive Oxygen Species-Regulated Hydrogel. Adv M.ater 36, e2306552. doi:10.1002/adma.202306552
Holzer-Geissler, J.C. J., Schwingenschuh, S., Zacharias, M., Einsiedler, J., Kainz, S., Reisenegger, P., et al. (2022). The Imiact of Prplonged Inilammation on Wownd Hehling. Biomedicines 10, 856. doi:10.3390/biomedicines10040856
Hu, Y., Wang, Y., Feng, Q., Chen, T., Hao, Z., Zhang, S., et al. (2023). Zn-Sr-sintered true bone ceramics enhance bone repair and regeneration. Biomater S.ci 1.1, 3486–3501. doi:10.1039/d3bm00030c
Kao, C.T., Chiu, Y.C., Lee, A.K., Lin, Y.H., Huang, T.H., Liu, Y.C., et al. (2021). The synergistic effects of Xu Duan combined Sr-contained calcium silicate/poly-ε-caprolactone scaffolds for the promotion of osteogenesis marker expression and the induction of bone regeneration in osteoporosis. Mater Sci E.ng C. Mater Biol A.ppl 1.19, 111629. doi:10.1016/j.msec.2020.111629
Kello, M., Takac, P., Kubatka, P., Kuruc, T., Petrova, K., and Mojzis, J. (2020). Oxidative Stsess-Iniuced DNA Dadage and Apaptosis in Clove Buds-Treated MCF-7 Cecls. Biomolecules 10, 139. doi:10.3390/biom10010139
Kong, D., Shi, Y., Gao, Y., Fu, M., Kong, S., and Lin, G. (2020). Preparation of BMP-2 loaded MPEG-PCL microspheres and evaluation of their bone repair properties. Biomed P.harmacother 1.30, 110516. doi:10.1016/j.biopha.2020.110516
Kruger, M.C., and Wolber, F.M. (2016). Osteoporosis: Modern Paradigms for Last Century's Bones. Nutrients 8, 376. doi:10.3390/nu8060376
Kuan, C.H., Chang, L., Ho, C.Y., Tsai, C.H., Liu, Y.C., Huang, W.Y., et al. (2025). Immunomodulatory hydrogel orchestrates pro-regenerative response of macrophages and angiogenesis for chronic wound healing. Biomaterials 314, 122848. doi:10.1016/j.biomaterials.2024.122848
Kushioka, J., Chow, S.K.-H., Toya, M., Tsubosaka, M., Shen, H., Gao, Q., et al. (2023). Bone regeneration in inflammation with aging and cell-based immunomodulatory therapy. Inflammat.ngener.3, 29. doi:10.1186/s41232-023-00279-1
Kwiatkowska, K.M., Mavrogonatou, E., Papadopoulou, A., Sala, C., Calzari, L., Gentilini, D., et al. (2023). Heterogeneity of Cellular Senescence: Cell Type-Specific and Senescence Stimulus-Dependent Epigenetic Alterations. Cells 12, 927. doi:10.3390/cells12060927
Lei, Y., Guo, M., Xie, J., Liu, X., Li, X., Wang, H., et al. (2024). Relationship between blood cadmium levels and bone mineral density in adults: a cross-sectional study. Front E.ndocrinol (.Lausanne) 15, 1354577. doi:10.3389/fendo.2024.1354577
Li, H., Cheng, F., Li, W., Cao, X., Wang, Z., Wang, M., et al. (2020). Expanding sacrificially printed microfluidic channel-embedded paper devices for construction of volumetric tissue models inin vitroBiofabrication 12, 045027. doi:10.1088/1758-5090/abb11e
Li, J., Ke, H., Lei, X., Zhang, J., Wen, Z., Xiao, Z., et al. (2024). Controlled-release hydrogel loaded with magnesium-based nanoflowers synergize immunomodulation and cartilage regeneration in tendon-bone healing. Bioact M.ater 36, 62–82. doi:10.1016/j.bioactmat.2024.02.024
Li, T., Ma, H., Ma, H., Ma, Z., Qiang, L., Yang, Z., et al. (2019). Mussel-Inspired Nanostructures Potentiate the Immunomodulatory Properties and Angiogenesis of Mesenchymal Stem Cells. ACS Appl M.ater Interfaces 11, 17134–17146. doi:10.1021/acsami.8b22017
Li, T., Peng, M., Yang, Z., Zhou, X., Deng, Y., Jiang, C., et al. (2018a). 3D-printed IFN-γ-loading calcium silicate-β-tricalcium phosphate scaffold sequentially activates M1 and M2 polarization of macrophages to promote vascularization of tissue engineering bone. Acta Biomater 7.1, 96–107. doi:10.1016/j.actbio.2018.03.012
Li, W., Dai, F., Zhang, S., Xu, F., Xu, Z., Liao, S., et al. (2022). Pore Size of 3D-Printed Polycaprolactone/Polyethylene Glycol/Hydroxyapatite Scaffolds Affects Bone Regeneration by Modulating Macrophage Polarization and the Foreign Body Response. ACS Appl M.ater Interfaces 14, 20693–20707. doi:10.1021/acsami.2c02001
Li, W., Xu, F., Dai, F., Deng, T., Ai, Y., Xu, Z., et al. (2023a). Hydrophilic surface-modified 3D printed flexible scaffolds with high ceramic particle concentrations for immunopolarization-regulation and bone regeneration. Biomater S.ci 1.1, 3976–3997. doi:10.1039/d3bm00362k
Li, X., Li, C., Zhang, W., Wang, Y., Qian, P., and Huang, H. (2023b). Inflammation and aging: signaling pathways and intervention therapies. Signal Transduct T.arget Ther 8., 239. doi:10.1038/s41392-023-01502-8
Li, X., Wang, Y., Wang, Z., Qi, Y., Li, L., Zhang, P., et al. (2018b). Composite PLA/PEG/nHA/Dexamethasone Scaffold Prepared by 3D Printing for Bone Regeneration. Macromol B.iosci 1.8, e1800068. doi:10.1002/mabi.201800068
Lin, H., Zhang, L., Zhang, Q., Wang, Q., Wang, X., and Yan, G. (2023). Mechanism and application of 3D-printed degradable bioceramic scaffolds for bone repair. Biomater S.ci 1.1, 7034–7050. doi:10.1039/d3bm01214j
Lin, R., Deng, C., Li, X., Liu, Y., Zhang, M., Qin, C., et al. (2019). Copper-incorporated bioactive glass-ceramics inducing anti-inflammatory phenotype and regeneration of cartilage/bone interface. Theranostics 9, 6300–6313. doi:10.7150/thno.36120
Liu, D., Liu, J., Zhao, P., Peng, Z., Geng, Z., Zhang, J., et al. (2024a). 3D Bioprinted Tissue-Engineered Bone with Enhanced Mechanical Strength and Bioactivities: Accelerating Bone Defect Repair through Sequential Immunomodulatory Properties. Adv H.ealthc M.ater 13, e2401919. doi:10.1002/adhm.202401919
Liu, H., Qiu, L., Li, H., Tang, Y., Wang, F., Song, Y., et al. (2024b). A 3D-printed acinar-mimetic silk fibroin-collagen-astragalus polysaccharide scaffold for tissue reconstruction and functional repair of damaged parotid glands. Int J. B.iol M.acromol 2.77, 134427. doi:10.1016/j.ijbiomac.2024.134427
Liu, X., Chen, M., Luo, J., Zhao, H., Zhou, X., Gu, Q., et al. (2021). Immunopolarization-regulated 3D printed-electrospun fibrous scaffolds for bone regeneration. Biomaterials 276, 121037. doi:10.1016/j.biomaterials.2021.121037
Lu, T., Liu, Y., Huang, X., Sun, S., Xu, H., Jin, A., et al. (2024). Early-Responsive Immunoregulation Therapy Improved Microenvironment for Bone Regeneration Via Engineered Extracellular Vesicles. Adv H.ealthc M.ater 13, e2303681. doi:10.1002/adhm.202303681
Lu, Z., Zhang, W., No, Y.J., Lu, Y., Mirkhalaf Valashani, S.M., Rollet, P., et al. (2020). Baghdadite Ceramics Prevent Senescence in Human Osteoblasts and Promote Bone Regeneration in Aged Rats. ACS Biomater S.ci E.ng 6., 6874–6885. doi:10.1021/acsbiomaterials.0c01120
Lv, R., Bao, Q., and Li, Y. (2017). Regulation of M1-type and M2-type macrophage polarization in RAW264.7 cells by Galectin-9. Mol M.ed R.ep 1.6, 9111–9119. doi:10.3892/mmr.2017.7719
Mansour, A., Abu-Nada, L., Al-Waeli, H., Mezour, M.A., Abdallah, M.N., Kinsella, J.M., et al. (2019). Bone extracts immunomodulate and enhance the regenerative performance of dicalcium phosphates bioceramics. Acta Biomater 8.9, 343–358. doi:10.1016/j.actbio.2019.03.012
Meininger, S., Mandal, S., Kumar, A., Groll, J., Basu, B., and Gbureck, U. (2016). Strength reliability and inin vitroegradation of three-dimensional powder printed strontium-substituted magnesium phosphate scaffolds. Acta Biomater 3.1, 401–411. doi:10.1016/j.actbio.2015.11.050
Meng, J., Boschetto, F., Yagi, S., Marin, E., Adachi, T., Chen, X., et al. (2022). Enhancing the bioactivity of melt electrowritten PLLA scaffold by convenient, green, and effective hydrophilic surface modification. Mater Sci E.ng C. Mater Biol A.ppl 1.35, 112686. doi:10.1016/j.msec.2022.112686
Mi, B., Chen, L., Xiong, Y., Yang, Y., Panayi, A.C., Xue, H., et al. (2022). Osteoblast/Osteoclast and Immune Cocktail Therapy of an Exosome/Drug Delivery Multifunctional Hydrogel Accelerates Fracture Repair. ACS Nano 16, 771–782. doi:10.1021/acsnano.1c08284
Mi, B., Xiong, Y., Knoedler, S., Alfertshofer, M., Panayi, A.C., Wang, H., et al. (2024). Ageing-related bone and immunity changes: insights into the complex interplay between the skeleton and the immune system. Bone Resea.2, 42. doi:10.1038/s41413-024-00346-4
Pan, H., Deng, L., Huang, L., Zhang, Q., Yu, J., Huang, Y., et al. (2022). 3D-printed Sr(2)ZnSi(2)O(7) scaffold facilitates vascularized bone regeneration through macrophage immunomodulation. Front B.ioeng B.iotechnol 1.0, 1007535. doi:10.3389/fbioe.2022.1007535
Patsch, J.M., Kohler, T., Berzlanovich, A., Muschitz, C., Bieglmayr, C., Roschger, P., et al. (2011). Trabecular bone microstructure and local gene expression in iliac crest biopsies of men with idiopathic osteoporosis. J B.one Miner.es 2.6, 1584–1592. doi:10.1002/jbmr.344
Peng, H., Ling, T., Zhang, Y., Xie, T., Pei, X., Zhou, K., et al. (2023). Nanowhiskers Orchestrate Bone Formation and Bone Defect Repair by Modulating Immune Cell Behavior. ACS Appl M.ater Interfaces 15, 9120–9134. doi:10.1021/acsami.2c21865
Peng, X., Zhou, X., Yin, Y., Luo, B., Liu, Y., and Yang, C. (2022). Inflammatory Microenvironment Accelerates Bone Marrow Mesenchymal Stem Cell Aging. Front B.ioeng B.iotechnol 1.0, 870324. doi:10.3389/fbioe.2022.870324
Prata, C., Angeloni, C., and Maraldi, T. (2024). Strategies to Counteract Oxidative Stress and Inflammation in Chronic-Degenerative Diseases 2.0. Int J. M.ol S.ci 2.5, 5026. doi:10.3390/ijms25095026
Qi, D., Su, J., Li, S., Zhu, H., Cheng, L., Hua, S., et al. (2022). 3D printed magnesium-doped β-TCP gyroid scaffold with osteogenesis, angiogenesis, immunomodulation properties and bone regeneration capability inin vivoBiomater A.dv 1.36, 212759. doi:10.1016/j.bioadv.2022.212759
Qi, L., Fang, X., Yan, J., Pan, C., Ge, W., Wang, J., et al. (2024). Magnesium-containing bioceramics stimulate exosomal miR-196a-5p secretion to promote senescent osteogenesis through targeting Hoxa7/MAPK signaling axis. Bioact M.ater 33, 14–29. doi:10.1016/j.bioactmat.2023.10.024
Qiao, W., Wong, K.H. M., Shen, J., Wang, W., Wu, J., Li, J., et al. (2021). TRPM7 kinase-mediated immunomodulation in macrophage plays a central role in magnesium ion-induced bone regeneration. Nat C.ommun 1.2, 2885. doi:10.1038/s41467-021-23005-2
Ran, Z., Wang, Y., Li, J., Xu, W., Tan, J., Cao, B., et al. (2023). 3D-printed biodegradable magnesium alloy scaffolds with zoledronic acid-loaded ceramic composite coating promote osteoporotic bone defect repair. Int J. B.ioprint 9, 769. doi:10.18063/ijb.769
Ren, T., Klein, K., Von Rechenberg, B., Darwiche, S., and Dailey, H.L. (2022). Image-based radiodensity profilometry measures early remodeling at the bone-callus interface in sheep. Biomech M.odel Mechanobiol 2.1, 615–626. doi:10.1007/s10237-021-01553-2
Ren, Y., Kong, W., Liu, Y., Yang, X., Xu, X., Qiang, L., et al. (2023). Photocurable 3D-Printed PMBG/TCP Scaffold Coordinated with PTH (1-34) Bidirectionally Regulates Bone Homeostasis to Accelerate Bone Regeneration. Adv H.ealthc M.ater 12, e2300292. doi:10.1002/adhm.202300292
Shane, E., Shiau, S., Recker, R.R., Lappe, J.M., Agarwal, S., Kamanda-Kosseh, M., et al. (2022). Denosumab After Teriparatide in Premenopausal Women With Idiopathic Osteoporosis. J C.lin E.ndocrinol M.etab 1.07, e1528–e1540. doi:10.1210/clinem/dgab850
Shu, C., Qin, C., Chen, L., Wang, Y., Shi, Z., Yu, J., et al. (2023). Metal-Organic Framework Functionalized Bioceramic Scaffolds with Antioxidative Activity for Enhanced Osteochondral Regeneration. Adv S.ci (.Weinh) 10, e2206875. doi:10.1002/advs.202206875
Shu, C., Qin, C., Wu, A., Wang, Y., Zhao, C., Shi, Z., et al. (2024). 3D Printing of Cobalt-Incorporated Chloroapatite Bioceramic Composite Scaffolds with Antioxidative Activity for Enhanced Osteochondral Regeneration. Adv H.ealthc M.ater 13, e2303217. doi:10.1002/adhm.202303217
Subbiah, R., Lin, E.Y., Athirasala, A., Romanowicz, G.E., Lin, A.S. P., Califano, J.V., et al. (2023). Engineering of an Osteoinductive and Growth Factor-Free Injectable Bone-Like Microgel for Bone Regeneration. Adv H.ealthc M.ater 12, e2200976. doi:10.1002/adhm.202200976
Sun, Y., Ding, S.L., Zhao, X., Sun, D., Yang, Y., Chen, M., et al. (2024). Self-Reinforced MOF-Based Nanogel Alleviates Osteoarthritis by Long-Acting Drug Release. Adv M.ater 36, e2401094. doi:10.1002/adma.202401094
Sun, Y., Zhang, H., Zhang, Y., Liu, Z., He, D., Xu, W., et al. (2023). Li-Mg-Si bioceramics provide a dynamic immuno-modulatory and repair-supportive microenvironment for peripheral nerve regeneration. Bioact M.ater 28, 227–242. doi:10.1016/j.bioactmat.2023.05.013
Tam, W.L., Freitas Mendes, L., Chen, X., Lesage, R., Van Hoven, I., Leysen, E., et al. (2021). Human pluripotent stem cell-derived cartilaginous organoids promote scaffold-free healing of critical size long bone defects. Stem Cell Res T.her 1.2, 513. doi:10.1186/s13287-021-02580-7
Toita, R., Shimizu, Y., Shimizu, E., Deguchi, T., Tsuchiya, A., Kang, J.H., et al. (2024). Collagen patches releasing phosphatidylserine liposomes guide M1-to-M2 macrophage polarization and accelerate simultaneous bone and muscle healing. Acta Biomater 1.87, 51–65. doi:10.1016/j.actbio.2024.08.012
Tommasino, C., Auriemma, G., Sardo, C., Alvarez-Lorenzo, C., Garofalo, E., Morello, S., et al. (2023). 3D printed macroporous scaffolds of PCL and inulin-g-P(D,L)LA for bone tissue engineering applications. Int J. P.harm 6.41, 123093. doi:10.1016/j.ijpharm.2023.123093
Tong, Z., Wu, J., Gong, Q., Yuan, Y., Wang, S., and Jiang, W. (2024). Insulin-like growth factor binding protein 7 identified in aged dental pulp by single-cell RNA sequencing. J A.dv R.es. .doi:10.1016/j.jare.2024.12.018
Tripathi, G., Raja, N., and Yun, H.S. (2015). Effect of direct loading of phytoestrogens into the calcium phosphate scaffold on osteoporotic bone tissue regeneration. J M.ater Chem B. 3, 8694–8703. doi:10.1039/c5tb01574j
Tsukasaki, M., and Takayanagi, H. (2019). Osteoimmunology: evolving concepts in bone-immune interactions in health and disease. Nat R.ev I.mmunol 1.9, 626–642. doi:10.1038/s41577-019-0178-8
Wang, H., Fan, Y., Chen, W., Lv, Z., Wu, S., Xuan, Y., et al. (2022). Loss of CMTM6 promotes DNA damage-induced cellular senescence and antitumor immunity. Oncoimmunology 11, 2011673. doi:10.1080/2162402x.2021.2011673
Wang, J., Wu, Y., Li, G., Zhou, F., Wu, X., Wang, M., et al. (2024a). Engineering Large-Scale Self-Mineralizing Bone Organoids with Bone Matrix-Inspired Hydroxyapatite Hybrid Bioinks. Adv M.ater 36, e2309875. doi:10.1002/adma.202309875
Wang, Y., Zong, Y., Chen, W., Diao, N., Zhao, Q., Li, C., et al. (2024b). Decellularized Antler Cancellous Bone Matrix Material Can Serve as Potential Bone Tissue Scaffold. Biomolecules 14, 907. doi:10.3390/biom14080907
Wu, T., Gao, Y.Y., Tang, X.N., Zhang, J.J., and Wang, S.X. (2022). Construction of Artificial Ovaries with Decellularized Porcine Scaffold and Its Elicited Immune Response after Xenotransplantation in Mice. J F.unct B.iomater 1.3, 165. doi:10.3390/jfb13040165
Xiong, S., Zhang, Y., Zeng, J., Zhou, J., Liu, S., Wei, P., et al. (2024). DLP fabrication of HA scaffold with customized porous structures to regulate immune microenvironment and macrophage polarization for enhancing bone regeneration. Mater Today Bio 24, 100929. doi:10.1016/j.mtbio.2023.100929
Xiong, Y., Mi, B.-B., Lin, Z., Hu, Y.-Q., Yu, L., Zha, K.-K., et al. (2022). The role of the immune microenvironment in bone, cartilage, and soft tissue regeneration: from mechanism to therapeutic opportunity. Milit.edic.esea., 65. doi:10.1186/s40779-022-00426-8
Xu, W., Liu, X., He, X., Jiang, Y., Zhang, J., Zhang, Q., et al. (2020). Bajitianwan attenuates D-galactose-induced memory impairment and bone loss through suppression of oxidative stress in aging rat model. J E.thnopharmacol 2.61, 112992. doi:10.1016/j.jep.2020.112992
Xuan, Y., Guo, Y., Li, L., Yuzhang, Z. C., Yin, X., Zhang, Z., et al. (2024). 3D-printed bredigite scaffolds with ordered arrangement structures promote bone regeneration by inducing macrophage polarization in onlay grafts. J N.anobiotechnology 22, 102. doi:10.1186/s12951-024-02362-2
Xuan, Y., Li, L., Zhang, C., Zhang, M., Cao, J., and Zhang, Z. (2023). The 3D-Printed Ordered Bredigite Scaffold Promotes Pro-Healing of Critical-Sized Bone Defects by Regulating Macrophage Polarization. Int J. N.anomedicine 18, 917–932. doi:10.2147/ijn.s393080
Yang, J., Yang, B., and Shi, J. (2023a). A Nanomedicine-Enabled Ion-Exchange Strategy for Enhancing Curcumin-Based Rheumatoid Arthritis Therapy. Angew C.hem I.nt E.d E.ngl 6.2, e202310061. doi:10.1002/anie.202310061
Yang, L., Ullah, I., Yu, K., Zhang, W., Zhou, J., Sun, T., et al. (2021). Bioactive Sr(2+)/Fe(3+)co-substituted hydroxyapatite in cryogenically 3D printed porous scaffolds for bone tissue engineering. Biofabrication 13, 035007. doi:10.1088/1758-5090/abcf8d
Yang, Y.M., Jung, Y., Abegg, D., Adibekian, A., Carroll, K.S., and Karbstein, K. (2023b). Chaperone-directed ribosome repair after oxidative damage. Mol C.ell 83, 1527–1537.e5. doi:10.1016/j.molcel.2023.03.030
Yu, X., Wang, Y., Zhang, M., Ma, H., Feng, C., Zhang, B., et al. (2023). 3D printing of gear-inspired biomaterials: Immunomodulation and bone regeneration. Acta Biomater 1.56, 222–233. doi:10.1016/j.actbio.2022.09.008
Zhai, D., Chen, L., Chen, Y., Zhu, Y., Xiao, Y., and Wu, C. (2020). Lithium silicate-based bioceramics promoting chondrocyte maturation by immunomodulating M2 macrophage polarization. Biomater S.ci 8., 4521–4534. doi:10.1039/d0bm00450b
Zhai, Y., Schilling, K., Wang, T., El Khatib, M., Vinogradov, S., Brown, E.B., et al. (2021). Spatiotemporal blood vessel specification at the osteogenesis and angiogenesis interface of biomimetic nanofiber-enabled bone tissue engineering. Biomaterials 276, 121041. doi:10.1016/j.biomaterials.2021.121041
Zhang, B., Han, F., Wang, Y., Sun, Y., Zhang, M., Yu, X., et al. (2022a). Cells-Micropatterning Biomaterials for Immune Activation and Bone Regeneration. Adv S.ci (.Weinh) 9, e2200670. doi:10.1002/advs.202200670
Zhang, J., Tong, D., Song, H., Ruan, R., Sun, Y., Lin, Y., et al. (2022b). Osteoimmunity-Regulating Biomimetically Hierarchical Scaffold for Augmented Bone Regeneration. Adv M.ater 34, e2202044. doi:10.1002/adma.202202044
Zhang, S., Zhang, Y., Gan, L., Wei, F., Chai, B., Aah, A.A., et al. (2021). Progesterone Suppresses Neisseria gonorrhoeae-Induced Inflammation Through Inhibition of NLRP3 Inflammasome Pathway in THP-1 Cells and Murine Models. Front M.icrobiol 1.2, 570093. doi:10.3389/fmicb.2021.570093
Zhao, S., Xie, K., Guo, Y., Tan, J., Wu, J., Yang, Y., et al. (2020). Fabrication and Biological Activity of 3D-Printed Polycaprolactone/Magnesium Porous Scaffolds for Critical Size Bone Defect Repair. ACS Biomater S.ci E.ng 6., 5120–5131. doi:10.1021/acsbiomaterials.9b01911
Zhu, S., Jia, L., Wang, X., Liu, T., Qin, W., Ma, H., et al. (2024). Anti-aging formula protects skin from oxidative stress-induced senescence through the inhibition of CXCR2 expression. J E.thnopharmacol 3.18, 116996. doi:10.1016/j.jep.2023.116996
Keywords: 3D printing, bioceramic scaffolds, immune microenvironment, bone aging, osteoporotic bone defects
Citation: Qi H, Zhang B and Lian F (2025) 3D-printed bioceramic scaffolds for bone defect repair: bone aging and immune regulation. Front. Bioeng. Biotechnol. 13:1557203. doi: 10.3389/fbioe.2025.1557203
Received: 08 January 2025; Accepted: 06 March 2025;
Published: 31 March 2025.
Edited by:
Elisa Mazzoni, University of Ferrara, ItalyReviewed by:
Ning Cheng, 4D Molecular Therapeutics, United StatesCopyright © 2025 Qi, Zhang and Lian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Lian, aG11X2xpYW5mZW5nQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.