- 1Graduate school, Shandong Sport University, Jinan, China
- 2School of Human Movement Science, Beijing Sport University, Beijing, China
- 3School of Sports and Health, Shandong Sport University, Jinan, China
- 4Division of Physical Education, The Chinese University of Hong Kong, Shenzhen, China
- 5School of Arts, Shanghai University of Sport, Shanghai, China
Introduction: Chronic Ankle Instability (CAI) is a chronic syndrome resulting from repeated ankle sprains that lead to persistent dysfunction.the purpose of this study is to determine whether visual disruption could influence static and dynamic postural control in people with and without chronic ankle instability (CAI), with the objective of gaining a comprehensive understanding of the interactions between visual inputs and postural control.
Methods: Thirty people with CAI (21 males and 9 females, age = 22.0 ± 1.8 years, height = 174.4 ± 10.2 cm, body mass = 72.5 ± 15.4 kg; Cumberland Ankle Instability Tool (CAIT) score = 19.7 ± 1.8) and twenty-nine without CAI (24 males and 5 females, age = 22.9 ± 1.6 years, height = 172.8 ± 8.0 cm, body mass = 69.0 ± 11.3 kg; CAIT score = 29.0 ± 0.7) were recruited. Their static and dynamic postural control was measured in two conditions with or without visual disruption, simulated using stroboscopic glasses. Static postural control was measured during single-limb standing and represented by root mean square (RMS) of the plantar center of pressure (CoP), dynamic postural control was measured during a Y-balance test and represented by the relative reach distance. Two-way mixed ANOVA (between group: CAI vs non-CAI, within group: normal vision vs visual disruption) was used to analyze data.
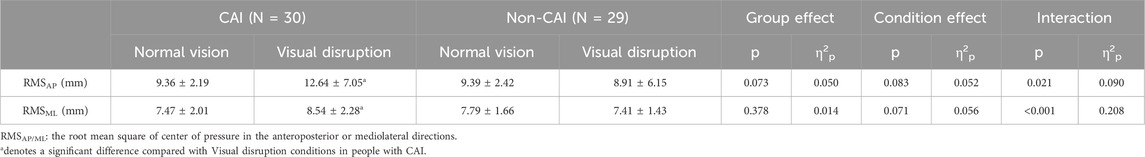
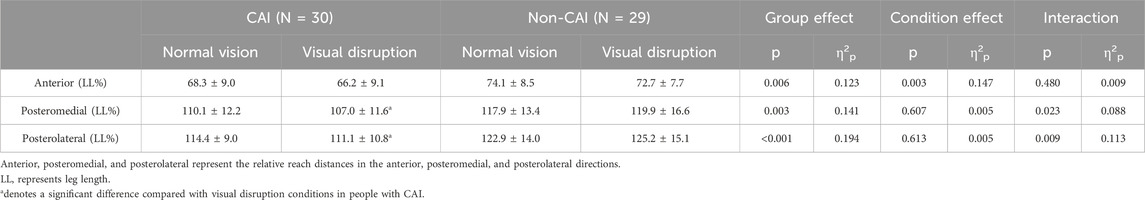
Results and discussion: Significant interactions were detected in the CoP-RMS in the anteroposterior (AP) (p = 0.021, η2p = 0.090) and mediolateral (ML) (p < 0.001, η2p = 0.208) directions, and the relative reach distances in the posteromedial (PM) p = 0.023, η2p = 0.088) and posterolateral (PL) (p = 0.009, η2p = 0.113) directions, from normal vision to visual disruption. The CoP-RMS in the AP and ML directions significantly increased and the relative reach distances in the PM and PL directions significantly decreased in people with CAI while remaining unchanged in those without CAI. People with CAI are susceptible to visual disruption on postural control, highlighting the importance of visual input in maintaining stable posture in this population.
1 Introduction
Acute ankle sprains are among the most prevalent musculoskeletal injuries (Waterman et al., 2010). Up to 70% of people who experience an acute lateral ankle sprain may develop chronic ankle instability (CAI) within a year following the initial injury (Herzog et al., 2019). Annually, the United States spends approximately 152 million USD on the treatment of ankle sprains (Feger et al., 2017).
The acute ankle sprains experienced by people with CAI may impact their static and dynamic postural control (Sierra-Guzmán et al., 2018). Diminished postural control is associated with falls (Moncada and Mire, 2017) and injuries (Hrysomallis, 2007; Paterno et al., 2010), particularly among those with CAI (Prawiradilaga et al., 2020). Static balance control, typically quantified by the root mean square (RMS) of plantar center of pressure (CoP) during single-leg standing (Song et al., 2021), may be compromised by an acute ankle sprain, due to the decreased tactile sensation that follows the injury (Liu et al., 2024). Similarly, dynamic postural control, often assessed by the relative reach distance during the Y-balance test (YBT) (Sierra-Guzmán et al., 2018), may also be adversely affected by an acute ankle sprain. This may be attributed to the reduction in lower extremity muscle strength after the injury (Hou et al., 2020).
The static and dynamic postural control of people with CAI may be more significantly influenced by visual disruption than those without CAI. The visual system plays a pivotal role in enhancing postural control, as it is adept at object motion perception and recognition, and is highly sensitive to dynamic scenes (Guerraz and Bronstein, 2008). A study has demonstrated reduced ankle proprioception among people with CAI (Xue et al., 2021), potentially necessitating a greater reliance on visual cues to maintain postural control (Song et al., 2016). People inevitably encounter environments or conditions that cause visual disruption, such as reduced visual stimulation due to dim lighting (Honeine and Schieppati, 2014) or decreased visual perception as a result of aging (Nguyen et al., 2021), people with CAI may particularly vulnerable due to their existing proprioception deficits, which may be used to compensate for other decreased sensations (Liu et al., 2023), like vision.
The specific impact of visual disruption on their postural control has not been fully understood. Further exploration in this area is crucial for gaining a comprehensive understanding of the complex interaction between visual input and postural control in this specific population. This study aims to determine whether visual disruption would affect static and dynamic postural control in people with and without CAI. We hypothesized that compared to those without CAI, visual disruption might result in #1. greater increases in the CoP-RMS; and #2. greater decreases in the relative reach distance during the Y balance test in people with CAI.
2 Methods
2.1 Participants
To the best of our knowledge, no prior studies have examined the impact of visual disruption on the CoP-RMS during single-limb standing, and the relative reach distance during the Y Balance Test in people with and without CAI. Data from our pilot study have been used for sample size estimating, where 3 people with CAI and another 3 without CAI participated. The effect size (η2p) for the group-by-condition interaction pertaining to the CoP-RMS and relative reach distance of YBT were 0.064 and 0.037. An a priori power analysis utilizing G*Power (3.1, Universität Düsseldorf, Düsseldorf, Germany) revealed that a sample size of 54 participants (with 27 participants in each group) would be necessary to achieve an alpha level of 0.05 and a statistical power of 0.80.
Thirty people with CAI (9 females and 21 males, aged 22.0 ± 1.8 years, with a height of 174.4 ± 10.2 cm and a body mass of 72.5 ± 15.4 kg; Cumberland Ankle Instability Tool (CAIT) score = 19.7 ± 1.8) and twenty-nine people without CAI (5 females and 24 males, aged 22.9 ± 1.6 years, with a height of 172.8 ± 8.0 cm and a body mass of 69.0 ± 11.3 kg; CAIT score = 29.0 ± 0.7) participated in this study. The inclusion criteria for people with CAI adhered to the guidelines by the International Ankle Consortium (Gribble et al., 2013), which included: (a) experiencing at least one lateral ankle sprain 12 months prior to enrollment, leading to cessation of physical activity for at least 1 day; (b) experiencing at least two lateral ankle sprains; (c) experiencing at least two episodes of ankle “giving way” within the 6 months preceding enrollment; and (d) a CAIT score of less than 24. The inclusion criteria for people without CAI were: (a) matching the CAI participants in terms of gender, age (±3 years), height (±5 cm), and weight (±5 kg); (b) having no history of lateral ankle sprains; and (c) a CAIT score of 28 or higher. The exclusion criteria for both groups were (Song et al., 2017): (a) having a lower extremity fracture or surgery; (b) sustaining an acute injury to the lower extremity within the last 3 months; (c) having neurological disorders, diabetes mellitus, or vestibular disorders; and (d) having bilateral CAI. This study received approval from the ethics committee of Shandong Sport University (No. 2022044), and all participants provided written informed consent.
2.2 Protocols
The participants wore form-fitting T-shirts and shorts, with their tested limbs designated as the affected limbs for people with CAI and the corresponding limbs on the same side for those without CAI. Each participant underwent two tests: the single-limb standing test and the YBT, under both normal vision and visual disruption conditions. We randomly tested the single-limb standing test and Y-balance test with normal vision and visual disruption by using a computer-generated randomized sequence. Visual disruption was simulated using stroboscopic glasses (Sibokeji, Zhuhai, China) at 3 Hz, alternating between 0.100 s of clarity and 0.233 s of opacity (Han et al., 2022).
2.2.1 Single-limb standing test
The static balance control was quantified using the single-limb standing test. In this test, participants stood with their tested foot placed on a force platform (AMTI, BP600900, United States), maintaining their body as still as possible with their hands resting on their hips. The untested hip was flexed at 30° and the knee at 45°, and this position was held for 30 s under normal vision and visual disruption conditions. Throughout the test, participants fixated their gaze on a black dot positioned 1.8 m above a wall located 2.5 m away (Sun et al., 2018). The CoP data were recorded for approximately 30 s at a 1,000 Hz. One trial was conducted separately under normal vision and visual disruption conditions, separately.
2.2.2 Y-balance test
The Y-Balance Test Kit was utilized to evaluate the participants’ postural control capabilities (Plisky et al., 2009). This comprehensive kit consists of a central plate and three extendable tubes positioned in the anterior (ANT), posteromedial (PM), and posterolateral (PL) directions, each tube terminating in a unique plastic plate. During the assessment, participants stood barefoot on the central plastic plate, with their tested leg as the base of support, and pushed each of the three movable plates as far as possible with their non-tested leg. Each direction (ANT, PM, PL) was tested separately, with three trials conducted in each under normal vision and visual disruption conditions (Shaffer et al., 2013), the mean value of the three trials for each direction was then calculated for data analysis.
2.3 Data reduction
During the single-limb standing test, the CoP data were subjected to low-pass filtering at a cutoff frequency of 10 Hz (Zéronian et al., 2021) for a duration of 10 s, commencing approximately 3–5 s after the onset of the standing task (Zéronian et al., 2021). Equation 1 was used to calculate these filtered data to calculate the CoP-RMS.
where
During the Y-balance test, participants’ leg lengths were measured from the anterior-superior iliac spine to the distal end of the medial malleolus. The relative reach distance was normalized by leg length using Equation 2 (Johnston et al., 2018):
2.4 Statistics
Data were analyzed using SPSS (26.0, IBM, New York, United States) and G*Power (3.1, Universität Düsseldorf, Düsseldorf, German). Shapiro-Wilk tests were used to verify the normality of the data. Two-way mixed-design ANOVAs, distinguishing between-group factors (CAI vs. non-CAI) and within-group factors (normal vision vs. visual disruption), were utilized to ascertain the main effects of group and condition, as well as the group-by-condition interactions. If significant interactions were identified, simple effects analyses were employed to perform pairwise comparisons. When the data did not follow a normal distribution, the non-parametric Mann-Whitney U test was used to compare the differences between the two groups under normal vision and visual disruption conditions. Partial eta squared (η2p) was used to indicate the effect size of the two-way ANOVA’s interactions and main effects with the thresholds: 0.01–0.06 for small, 0.06–0.14 for moderate, and >0.14 for large effect size (Richardson, 2011). Cohen’s d was used to indicate the effect size of post hoc pairwise comparison with the thresholds: <0.20 for trivial, 0.21–0.50 for small, 0.51–0.80 for medium, and >0.81 for large effect size (Cohen, 1988). Data are presented as mean ± standard deviation and the significance level was set at 0.05.
3 Results
As shown in Table 1, a group-by-condition interaction was detected in CoP-RMS in the anteroposterior (AP) (p = 0.021, η2p = 0.090) and mediolateral (ML) (p < 0.001, η2p = 0.208) directions. From normal vision to visual disruption conditions, the CoP-RMS in the AP (normal vision = 9.36 ± 2.19 mm, visual disruption = 12.64 ± 7.05 mm, p = 0.005, d = 0.63) and ML (normal vision = 7.47 ± 2.01 mm, visual disruption = 8.54 ± 2.28 mm, p < 0.001, d = 0.50) directions significantly increased in people with CAI while remained unchanged in those without CAI.
As shown in Table 2, significant group-by-condition interactions were detected in the relative reach distances in PM (p = 0.023, η2p = 0.088) and PL (p = 0.009, η2p = 0.113) directions. From normal vision to visual disruption conditions, the relative reach distance in the PM (normal vision = 107.0 ± 11.6%, visual disruption = 110.1 ± 12.2%, p = 0.046, d = 0.26) and PL directions (normal vision = 111.1 ± 10.8%, visual disruption = 114.4 ± 9.0%, p = 0.026, d = 0.33) significantly decreased in people with CAI while remained unchanged in those without CAI. In addition, significant group main effects were detected. Compared with people without CAI, those with CAI have a significantly shorter relative reach distance of YBT in the PM (p = 0.003, η2p = 0.141) and PL (p = <0.001, η2p = 0.194) directions.
4 Discussion
This study aimed to investigate the impact of visual disruption on static and dynamic postural control in people with and without CAI. Our results supported hypotheses #1 and #2. Specifically, visual disruption significantly increased CoP-RMS, and significantly decreased the relative reach distance in the YBT among people with CAI, whereas these effects were not observed in people without CAI.
Our findings reveal that visual disruption leads to a decrease in static postural control, as indicated by CoP-RMS, in people with CAI, whereas this effect is not observed in those without CAI. This reduction in static postural control is often interpreted as a sign of decreased automation of static posture control (Roerdink et al., 2011; Rizzato et al., 2023). Our finding is consistent with previous studies. Song et al. investigated the effect of closed eyes on the CoP displacement during single-limb standing test in people with CAI, and their results showed that with the reduction of visual information, the static postural control during single-limb standing test in people with CAI decreased (Song et al., 2017). Furthermore, Lee et al. showed a significant decrease in static postural control in healthy people when both somatosensory and visual disruption were present, whereas there was no significant change when only visual disruption was present (Lee et al., 2022).
We propose that the impact of visual disruption on people with CAI stems from their limited ability to allocate additional attentional resources towards maintaining stability. Attentional resources are crucial for maintaining physical stability and integrating sensory information (McDowd, 2007; Katsuki and Constantinidis, 2014). When sensory information is inadequate or inaccurate, people require more attentional resources to process this information compared with people without sensory deficits (Shumway-Cook and Woollacott, 2000; Redfern et al., 2001a; Teasdale and Simoneau, 2001). Given that people with CAI require more attentional resources to process their compromised sensory information (Song et al., 2017; Terada et al., 2019), visual disruption further increases the demand for attentional resources to maintain stability, ultimately leading to a deficit in the resources allocated to physical stability and a subsequent decrease in static postural control. Several pieces of evidence support our hypothesis. Firstly, reduced relevant sensory information causes decreased static postural control in people with CAI (Song et al., 2017). Secondly, when visual information is reduced, people with impaired or absent sensory functions tend to be more concerned about maintaining physical stability (Brown et al., 1999; Lindenberger et al., 2000; Rankin et al., 2000; Marchese et al., 2003; Redfern et al., 2004). These findings suggest that the visual disruption in our study may have disproportionately affected people with CAI, leading to a reduction in static postural control and potentially increasing their risk of injury.
Our results further demonstrated that visual disruption significantly impaired dynamic postural control in people with CAI, whereas it had no discernible effect on those without CAI, suggesting a heightened reliance on vision for maintaining stability among those with CAI. A previous study has shown that people with CAI are more visually dependent than those without CAI in performing the dynamic task of one-legged jumping and that somatosensory sensations other than vision are not sufficient to compensate for the detrimental effects of visual disruption in people with CAI (Han et al., 2022). In addition, a previous meta-analysis, which aggregated data on time to boundary under both eyes-open and eyes-closed conditions among healthy controls and people with CAI, corroborated our findings by emphasizing the greater visual dependence among those with CAI (Song et al., 2016).
We posit that the impact of visual disruption on postural control in people with CAI stems from proprioceptive deficits. In situations of sensory deficits, proprioception can typically compensate for the loss of other sensory cues to maintain stability (Liu et al., 2023). However, people with CAI exhibit weaker proprioception, rendering them unable to provide sufficient and effective sensory information (Kawabata et al., 2024). This, in turn, leads to an inability to adequately compensate for the reduced visual inputs, ultimately resulting in compromised postural control (Redfern et al., 2001b). Conversely, people without CAI, who possess unimpaired proprioception, may exhibit reduced reliance on visual inputs and heightened reliance on proprioception in response to visual distractions.
The first limitation of this study is that, although stroboscopic glasses have been proven effective for visual disruption, they cannot simulate the complex visual changes associated with aging, such as macular degeneration, cataracts, and glaucoma, which affect visual perception. Therefore, future research should explore changes in physiological complexity, balance control, and injury potential in older adults with clinical vision loss. The second limitation lies in the gender imbalance in our participants, with a higher proportion of males, which may restrict the generalization of our findings to female populations. Future studies should strive to recruit a more gender-balanced participants to better represent the broader population and mitigate potential gender-related biases.
5 Conclusion
People with CAI are more vulnerable to visual disruption during static and dynamic postural control than those without CAI, suggesting that visual disruptions can adversely affect postural control in people with CAI, highlighting the importance of visual input in maintaining stable posture in this population.
Data availability statement
The datasets for this study can be found in the 4TU. ResearchData doi: 10.4121/9be48906-8440-44a2-8c7a-af630e6735de.v1.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Shandong Sport University (2022044). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YM: Data curation, Investigation, Validation, Writing–original draft. YG: Data curation, Formal Analysis, Writing–review and editing. DW: Software, Visualization, Writing–review and editing. DM: Supervision, Writing–review and editing. QS: Conceptualization, Methodology, Project administration, Resources, Visualization, Writing–review and editing. RW: Conceptualization, Funding acquisition, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This project was funded by General Administration of Sport of China (23QN009), National Natural Science Foundation of China (No.12102235), and Natural Science Foundation of Shandong Province (ZR2022MH163).
Acknowledgments
We thank Miss Xueke Huang, a graduate student at Shandong Sport University, for their assistance in the experiment of this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Brown, L. A., Shumway-Cook, A., and Woollacott, M. H. (1999). Attentional demands and postural recovery: the effects of aging. J. Gerontol. A Biol. Sci. Med. Sci. 54 (4), M165–M171. doi:10.1093/gerona/54.4.m165
Feger, M. A., Glaviano, N. R., Donovan, L., Hart, J. M., Saliba, S. A., Park, J. S., et al. (2017). Current trends in the management of lateral ankle sprain in the United States. Clin. J. Sport Med. 27 (2), 145–152. doi:10.1097/jsm.0000000000000321
Gribble, P. A., Delahunt, E., Bleakley, C., Caulfield, B., Docherty, C. L., Fourchet, F., et al. (2013). Selection criteria for patients with chronic ankle instability in controlled research: a position statement of the International Ankle Consortium. J. Orthop. Sports Phys. Ther. 43 (8), 585–591. doi:10.2519/jospt.2013.0303
Guerraz, M., and Bronstein, A. M. (2008). Ocular versus extraocular control of posture and equilibrium. Neurophysiol. Clin. 38 (6), 391–398. doi:10.1016/j.neucli.2008.09.007
Han, S., Lee, H., Son, S. J., and Hopkins, J. T. (2022). The effects of visual feedback disruption on postural control with chronic ankle instability. J. Sci. Med. Sport 25 (1), 53–57. doi:10.1016/j.jsams.2021.07.014
Herzog, M. M., Kerr, Z. Y., Marshall, S. W., and Wikstrom, E. A. (2019). Epidemiology of ankle sprains and chronic ankle instability. J. Athl. Train. 54 (6), 603–610. doi:10.4085/1062-6050-447-17
Honeine, J. L., and Schieppati, M. (2014). Time-interval for integration of stabilizing haptic and visual information in subjects balancing under static and dynamic conditions. Front. Syst. Neurosci. 8, 190. doi:10.3389/fnsys.2014.00190
Hou, Z. C., Miao, X., Ao, Y. F., Hu, Y. L., Jiao, C., Guo, Q. W., et al. (2020). Characteristics and predictors of muscle strength deficit in mechanical ankle instability. BMC Musculoskelet. Disord. 21 (1), 730. doi:10.1186/s12891-020-03754-9
Hrysomallis, C. (2007). Relationship between balance ability, training and sports injury risk. Sports Med. 37 (6), 547–556. doi:10.2165/00007256-200737060-00007
Johnston, W., Dolan, K., Reid, N., Coughlan, G. F., and Caulfield, B. (2018). Investigating the effects of maximal anaerobic fatigue on dynamic postural control using the Y-Balance Test. J. Sci. Med. Sport 21 (1), 103–108. doi:10.1016/j.jsams.2017.06.007
Katsuki, F., and Constantinidis, C. (2014). Bottom-up and top-down attention: different processes and overlapping neural systems. Neuroscientist 20 (5), 509–521. doi:10.1177/1073858413514136
Kawabata, S., Ozone, K., Minegishi, Y., Oka, Y., Terada, H., Takasu, C., et al. (2024). Chronic ankle joint instability induces ankle sensorimotor dysfunction: a controlled laboratory study. Am. J. Sports Med. 52 (3), 739–749. doi:10.1177/03635465231217490
Lee, H., Han, S., and Hopkins, J. T. (2022). Altered visual reliance induced by stroboscopic glasses during postural control. Int. J. Environ. Res. Public Health 19 (4), 2076. doi:10.3390/ijerph19042076
Lindenberger, U., Marsiske, M., and Baltes, P. B. (2000). Memorizing while walking: increase in dual-task costs from young adulthood to old age. Psychol. Aging 15 (3), 417–436. doi:10.1037//0882-7974.15.3.417
Liu, Y., Song, Q., Liu, Z., Dong, S., Hiller, C., Fong, D. T. P., et al. (2024). Correlations of postural stability to proprioception, tactile sensation, and strength among people with chronic ankle instability. Mot. Control 28 (4), 464–479. doi:10.1123/mc.2023-0084
Liu, Z., Wang, Q., Sun, W., and Song, Q. (2023). Balancing sensory inputs: somatosensory reweighting from proprioception to tactile sensation in maintaining postural stability among older adults with sensory deficits. Front. Public Health 11, 1165010. doi:10.3389/fpubh.2023.1165010
Marchese, R., Bove, M., and Abbruzzese, G. (2003). Effect of cognitive and motor tasks on postural stability in Parkinson's disease: a posturographic study. Mov. Disord. 18 (6), 652–658. doi:10.1002/mds.10418
McDowd, J. M. (2007). An overview of attention: behavior and brain. J. Neurol. Phys. Ther. 31 (3), 98–103. doi:10.1097/npt.0b013e31814d7874
Moncada, L. V. V., and Mire, L. G. (2017). Preventing Falls in Older Persons. Am. Fam. Physician. 96 (4), 240–247.
Nguyen, T., Combs, E. M., Wright, P. J., and Corbett, C. F. (2021). Reducing fall risks among visually impaired older adults. Home Healthc. Now. 39 (4), 186–193. doi:10.1097/nhh.0000000000000995
Prawiradilaga, R. S., Madsen, A. O., Jørgensen, N. R., and Helge, E. W. (2010). Acute response of biochemical bone turnover markers and the associated ground reaction forces to high-impact exercise in postmenopausal women. Biol. Sport 37 (1), 41–48. doi:10.5114/biolsport.2020.91497
Paterno, M. V., Schmitt, L. C., Ford, K. R., Rauh, M. J., Myer, G. D., Huang, B., et al. (2010). Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Am. J. Sports Med. 38 (10), 1968–1978. doi:10.1177/0363546510376053
Plisky, P. J., Gorman, P. P., Butler, R. J., Kiesel, K. B., Underwood, F. B., and Elkins, B. (2009). The reliability of an instrumented device for measuring components of the star excursion balance test. N. Am. J. Sports Phys. Ther. 4 (2), 92–99.
Rankin, J. K., Woollacott, M. H., Shumway-Cook, A., and Brown, L. A. (2000). Cognitive influence on postural stability: a neuromuscular analysis in young and older adults. J. Gerontol. A Biol. Sci. Med. Sci. 55 (3), M112–M119. doi:10.1093/gerona/55.3.m112
Redfern, M. S., Jennings, J. R., Martin, C., and Furman, J. M. (2001a). Attention influences sensory integration for postural control in older adults. Gait Posture 14 (3), 211–216. doi:10.1016/s0966-6362(01)00144-8
Redfern, M. S., Talkowski, M. E., Jennings, J. R., and Furman, J. M. (2004). Cognitive influences in postural control of patients with unilateral vestibular loss. Gait Posture 19 (2), 105–114. doi:10.1016/s0966-6362(03)00032-8
Redfern, M. S., Yardley, L., and Bronstein, A. M. (2001b). Visual influences on balance. J. Anxiety Disord. 15 (1-2), 81–94. doi:10.1016/s0887-6185(00)00043-8
Richardson, J. T. E. (2011). Eta squared and partial eta squared as measures of effect size in educational research. Educ. Res. Rev. 6 (2), 135–147. doi:10.1016/j.edurev.2010.12.001
Rizzato, A., Benazzato, M., Cognolato, M., Grigoletto, D., Paoli, A., and Marcolin, G. (2023). Different neuromuscular control mechanisms regulate static and dynamic balance: a center-of-pressure analysis in young adults. Hum. Mov. Sci. 90, 103120. doi:10.1016/j.humov.2023.103120
Roerdink, M., Hlavackova, P., and Vuillerme, N. (2011). Center-of-pressure regularity as a marker for attentional investment in postural control: a comparison between sitting and standing postures. Hum. Mov. Sci. 30 (2), 203–212. doi:10.1016/j.humov.2010.04.005
Shaffer, S. W., Teyhen, D. S., Lorenson, C. L., Warren, R. L., Koreerat, C. M., Straseske, C. A., et al. (2013). Y-balance test: a reliability study involving multiple raters. Mil. Med. 178 (11), 1264–1270. doi:10.7205/milmed-d-13-00222
Shumway-Cook, A., and Woollacott, M. (2000). Attentional demands and postural control: the effect of sensory context. J. Gerontol. A Biol. Sci. Med. Sci. 55 (1), M10–M16. doi:10.1093/gerona/55.1.m10
Sierra-Guzmán, R., Jiménez, F., and Abián-Vicén, J. (2018). Predictors of chronic ankle instability: analysis of peroneal reaction time, dynamic balance and isokinetic strength. Clin. Biomech. (Bristol, Avon) 54, 28–33. doi:10.1016/j.clinbiomech.2018.03.001
Song, K., Burcal, C. J., Hertel, J., and Wikstrom, E. A. (2016). Increased visual use in chronic ankle instability: a meta-analysis. Med. Sci. Sports Exerc 48 (10), 2046–2056. doi:10.1249/mss.0000000000000992
Song, K., Kang, T. K., Wikstrom, E. A., Jun, H. P., and Lee, S. Y. (2017). Effects of reduced plantar cutaneous sensation on static postural control in individuals with and without chronic ankle instability. J. Sci. Med. Sport 20 (10), 910–914. doi:10.1016/j.jsams.2016.04.011
Song, Q., Zhang, X., Mao, M., Sun, W., Zhang, C., Chen, Y., et al. (2021). Relationship of proprioception, cutaneous sensitivity, and muscle strength with the balance control among older adults. J. Sport Health Sci. 10 (5), 585–593. doi:10.1016/j.jshs.2021.07.005
Sun, W., Wang, L., Zhang, C., Song, Q., Gu, H., and Mao, D. (2018). Detraining effects of regular Tai Chi exercise on postural control ability in older women: a randomized controlled trial. J. Exerc Sci. Fit. 16 (2), 55–61. doi:10.1016/j.jesf.2018.06.003
Teasdale, N., and Simoneau, M. (2001). Attentional demands for postural control: the effects of aging and sensory reintegration. Gait Posture 14 (3), 203–210. doi:10.1016/s0966-6362(01)00134-5
Terada, M., Morgan, K. D., and Gribble, P. A. (2019). Altered movement strategy of chronic ankle instability individuals with postural instability classified based on Nyquist and Bode analyses. Clin. Biomech. (Bristol, Avon) 69, 39–43. doi:10.1016/j.clinbiomech.2019.06.020
Waterman, B. R., Owens, B. D., Davey, S., Zacchilli, M. A., and Belmont, P. J. (2010). The epidemiology of ankle sprains in the United States. J. Bone Jt. Surg. Am. 92 (13), 2279–2284. doi:10.2106/jbjs.i.01537
Xue, X., Ma, T., Li, Q., Song, Y., and Hua, Y. (2021). Chronic ankle instability is associated with proprioception deficits: a systematic review and meta-analysis. J. Sport Health Sci. 10 (2), 182–191. doi:10.1016/j.jshs.2020.09.014
Keywords: ankle sprain, sensory feedback, balance control, vision, rehabilitation
Citation: Miao Y, Ge Y, Wang D, Mao D, Song Q and Wu R (2024) Effects of visual disruption on static and dynamic postural control in people with and without chronic ankle instability. Front. Bioeng. Biotechnol. 12:1499684. doi: 10.3389/fbioe.2024.1499684
Received: 21 September 2024; Accepted: 24 October 2024;
Published: 05 November 2024.
Edited by:
Jianqiao Guo, Beijing Institute of Technology, ChinaCopyright © 2024 Miao, Ge, Wang, Mao, Song and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rentana Wu, c2hlcnJ5OTI1NEAxNjMuY29t; Qipeng Song, c29uZ3FpcGVuZ0BzZHBlaS5lZHUuY24=
 Yushan Miao
Yushan Miao Yubin Ge1
Yubin Ge1 Dongmei Wang
Dongmei Wang Qipeng Song
Qipeng Song