- 1Department of Biology, Western University, London, ON, Canada
- 2Department of Molecular and Cellular Biology, University of Guelph, Guelph, ON, Canada
The holobiont theory of evolution explains how individuals are deeply symbiotic with their gut microbes, such that microbes are adapted to influence host metabolism, immunity and behaviour, as signalled from the gut to the brain. For eusocial taxa like the Western honey bee (Apis mellifera), this brain-gut axis may scale up from the individual to affect entire colonies. Here, we examine how microbial supplementation of honey bee feeds could manipulate the brain-gut axis to affect hygienic and other social behaviours relevant to beekeeping, such as foraging, recruitment (dance language) and defence. To illustrate this concept, we focus on various lactic acid-producing bacteria that can synthesize neurotransmitters such as octopamine, dopamine, serotonin and γ-aminobutyric acid, which can influence an individual bee’s behavioural cycles and responsiveness to environmental cues. If the behaviour of a worker bee can be deliberately manipulated, and this effect multiplied across many workers, microbial neurotherapeutics could conceivably render colonies more behaviourally responsive to symptoms of disease, or more motivated to forage or possibly less aggressive towards beekeepers. Drawing from the scientific literature, we infer how microbial supplements, such as neurostimulatory or neurosuppressive probiotics, could be applied or even engineered to co-opt the brain-gut axis to bolster colony health or improve performance. The mechanistic link between the gut microbiota and the collective social behaviour of single colonies remains an understudied aspect of honey bee social biology with relevance to apiculture.
Introduction
The evolutionary association between multicellular hosts and their unicellular gut microbes represents a symbiosis that supports the host’s immune, metabolic and digestive systems (Guerrero et al., 2013; Rosenberg et al., 2010). Dysbiotic shifts in the gut microbiota, typically characterized by a relative decrease in symbionts and an overgrowth of pathobionts, can detrimentally impact the host’s well-being (Carding et al., 2015). One mechanism that mediates the relationship between host and microbe is the brain-gut axis, which links the metabolic function of microbes within the gastrointestinal tract (i.e., the gut) to the central nervous system (i.e., the brain) and thus to the performance and behaviour of the organism (Schneider et al., 2024).
Studies on the brain-gut axis have primarily focused on vertebrates, but it is now established that this mechanism can influence the health and behaviour of invertebrates, including insects (Dus et al., 2015; Liberti and Engel, 2020; Cabirol et al., 2023). For social insects, where behavioural responses are coordinated among large numbers of individuals, any effects of the brain-gut axis should be amplified to influence the collective behaviour of entire colonies. This prospect of ‘social amplification’ presents an opportunity to directly manipulate the brain-gut axis of some critical subset of individuals within a colony, with the change-of-behaviour effect then ramifying throughout a larger group.
In the highly social honey bee Apis mellifera, there is massive potential for the social amplification of brain-gut axis effects (Figure 1). In a leading study, Liberti et al. (2022) demonstrated that workers with experimentally homogenized gut microbiomes interacted more frequently in a controlled setting, and that specific metabolites associated with those microbes could statistically predict the number of interactions. This association between gut microbe composition and the nature of head-to-head interactions suggests that the brain-gut axis of honey bees is functional and potentially mutable as an apicultural tool. However, few studies have examined how supplementation of colonies with bacteria known to have neurodevelopmental effects might influence aspects of beekeeping.
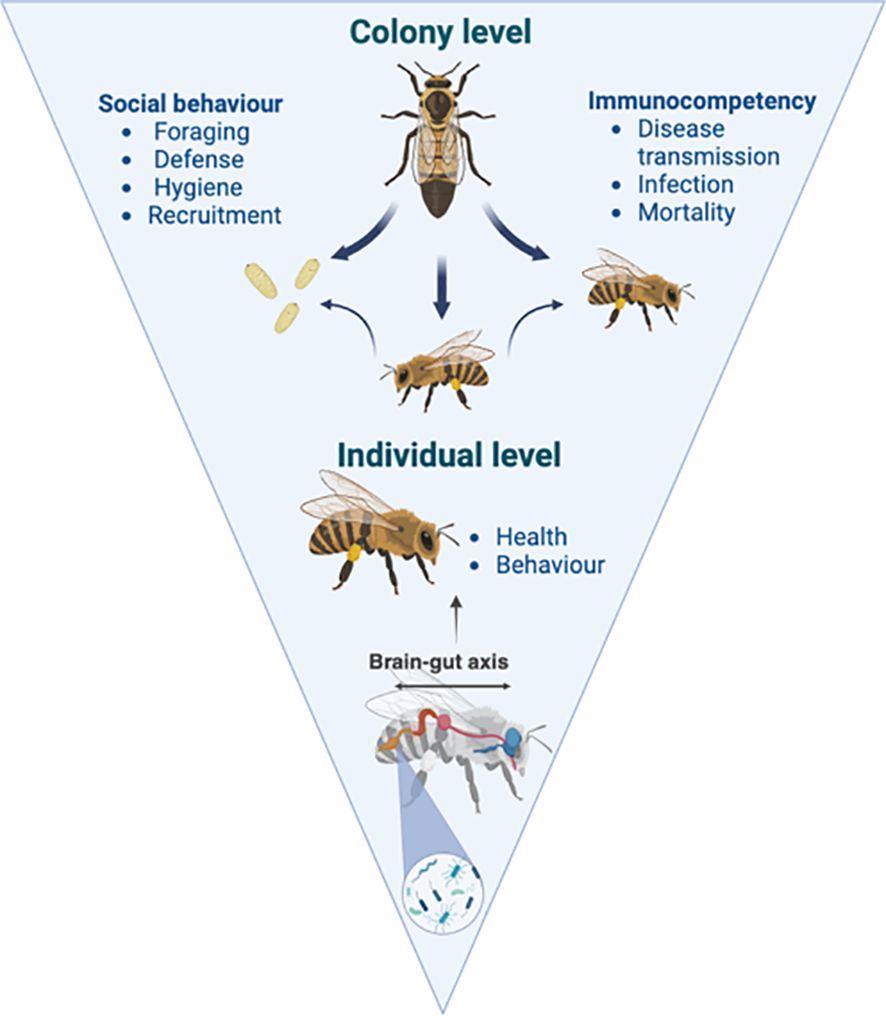
Figure 1. Social amplification from the individual to the colony level. The brain-gut axis, a bidirectional path of communication between the gut and brain, may be able to affect social behaviours and immunocompetency beyond the individual to the colony level in social-living organisms.
One stereotypic behaviour that seems potentially amenable to microbial therapeutic manipulation is hygienic behaviour − the systemic tendency to detect and dispose of diseased larvae and pupae from the colony, particularly as they are likely to cause infection (Spivak and Gilliam, 2015). Honey bees, like other social insects, live in densely populated colonies of closely related individuals, rendering them vulnerable to the spread of contagion. As such, they have evolved forms of social immunity to combat this risk (Cremer et al., 2007). The hygienic response to infection, expressed by nurse-age workers (1-2 weeks old), is likely triggered by an odour-sensitive threshold (Masterman et al., 2000) that is mediated, in part, by genetically variable loci (Oxley et al., 2010). Selecting for hygienic strains is possible (Erez et al., 2022) but in practice bee breeding can be a slow or ineffective process, requiring considerable financial considerations and expertise in bee husbandry. Further, the expression of hygiene varies beyond genetics as a function of season, food availability and other environmental factors.
As an effort to complement the bee’s natural tendency to keep their colonies disease free, many beekeepers (outside of Europe) use antibiotics, which can be immediately effective against certain pathogens, but these medicated treatments are tightly regulated due to concerns about residual accumulation in honey, as well as other off-target side effects (Lima et al., 2020), including disruption of the natural bee gut microbiota which, paradoxically, can leave colonies more vulnerable to subsequent infection (Daisley et al., 2020; Raymann et al., 2017; Zhang et al., 2022a). Alternative disease management interventions in beekeeping include essential oils (Hýbl et al., 2021), RNA interference technologies (Garbian et al., 2012) or variations of transgenerational immune priming (Dickel et al., 2022). These techniques are, however, not yet well tested or established. One remaining approach that complements or even circumvents some of these remedies involves administering living bacteria to colonies in support of native bee gut microbes (Motta et al., 2022).
In this mini-review, we explore the potential to co-opt the brain-gut axis of managed honey bees to modify hygiene and potentially other environmentally cued social responses that are relevant to beekeeping. We provide perspective on the deliberate enrichment of bee guts with bacterial strains to lower the individual response threshold to disease cues, which is an approach that, with development and testing, could enhance the colony-wide hygienic response. Although this approach has not been conclusively tested, manipulating the brain-gut axis could offer a new strategy for managing perennial bacterial diseases such as American or European foulbrood, and potentially any type of pest or pathogen that is naturally removed by hygiene.
Gut microbiota and the potential for effects on neurotransmission
The microbiota of the Western honey bee is dominated by several species of Lactobacillus and Bombilactobacillus, as well as Gilliamella apicola, Snodgrassella alvi and Bifidobacterium asteroides, all of which are consistently found in the hindgut of adult workers (Raymann and Moran, 2018; Motta et al., 2022). Other commonly detected bacteria found in association with honey bees include Frischella perrara, Bartonella apis, Bombella apis, Apilactobacillus kunkeei, and several species of Fructobacillus (Bonilla-Rosso and Engel, 2018). Within colonies, the microbiota is quite homogenous and primarily transmitted through social interactions (Powell et al., 2014), but with some variation in gut microbe composition between kin (Vernier et al., 2020), castes (Kapheim et al., 2015; Motta et al., 2022) and geographical areas (Jones et al., 2018).
The microbiota is thought to affect many systems within the host. Alberoni et al. (2016) summarize some of these effects, which include nutrient uptake, the production of fatty acids, amino acids and other metabolites, and protection of the host from pathogens and parasites, either by stimulating immune function or by directly inhibiting pathogen growth. Recent research has exploited this co-evolved relationship between microbe and host − the holobiont − to demonstrate that strategic manipulation of the worker gut microbiome can help bees recover from dysbiosis (Daisley et al., 2020) and even bolster bee immunity to protect against further gut-borne disease (Daisley et al., 2019; Raymann and Moran, 2018). Despite the prospect of microbial therapeutics, the idea of using gut microbe manipulations as a beekeeping tool has received relatively little research attention (Chmiel et al., 2021) and this despite the availability of some reportedly bee-friendly ‘probiotic’ products (Damico et al., 2023).
As an extension of the holobiont, the microbiota of individuals could scale-up to affect the collective behaviour of whole social groups (Sarkar et al., 2020; Jones et al., 2018; Cabirol et al., 2023). As one example, consider that worker bees have evolved an olfactory-cued sensory threshold that triggers a hygienic response; once the scent of disease becomes sufficiently intense, it can elicit a hygienic response from a proportion of the worker bees, whereby the most sensitive bees react first (Beshers and Fewell, 2001; Oldroyd and Thompson, 2006). The stronger the scent, the larger the proportion of workers that will be triggered and thus respond. What if the threshold itself could be lowered, such that a greater proportion of bees are early responders?
At a mechanistic level, the olfactory stimuli are detected by a worker’s antennae, and neurotransmitters such as octopamine, γ-aminobutyric acid (GABA), serotonin or dopamine relay that signal to the mushroom bodies and lateral horns of the bee brain for processing (Paoli and Galizia, 2021). Certain gut-borne bacteria can produce neurotransmitters or stimulate the host’s innate production of these neurotransmitters via the production of their precursors (Chen et al., 2021; Cabirol et al., 2023; Kešnerová et al., 2017; Zhang et al., 2022a; Table 1). Lactic acid-producing bacteria (LAB), including species within the genera Lactobacillus and Bifidobacterium, coevolved with bees over millions of years and are abundant in bee guts (Vásquez et al., 2012). LAB can synthesize GABA, at least in mammalian hosts, via the glutamic acid decarboxylase system (Cui et al., 2020). LAB can also modulate levels of serotonin production by regulating its precursor tryptophan (Zhang et al., 2022a). Moreover, LAB are associated with the production of dopamine in vitro (Özoğul et al., 2012) via the conversion of its precursor (levodopa) from the amino acid l-tyrosine (Sarkar et al., 2020). In the gut of the roundworm Caenorhabditis elegans, bacteria may produce octopamine indirectly by producing its amino acid precursor, tyramine (O’Donnell et al., 2020).
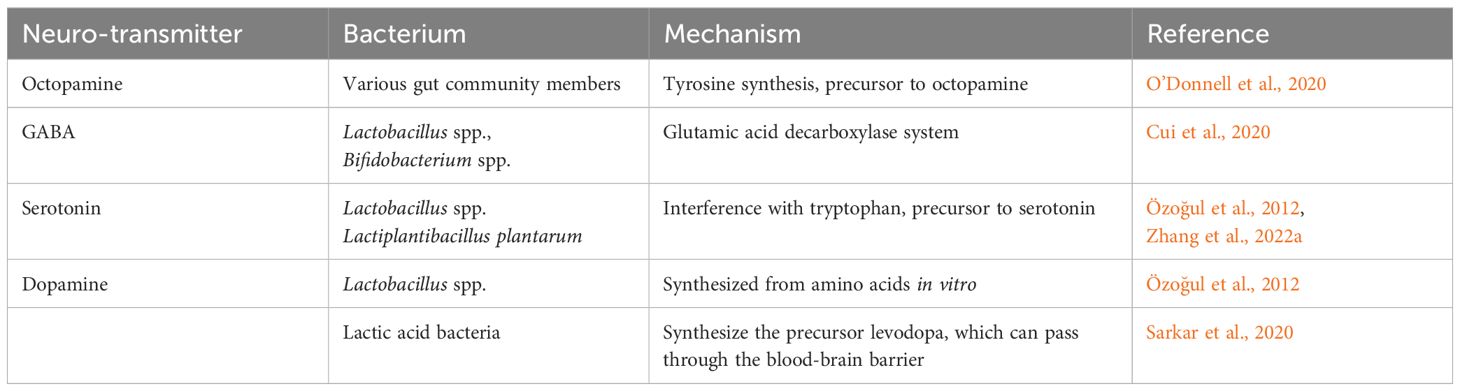
Table 1. Bacterial interactions via the production, degradation or modulation of honey bee neurotransmitters.
While a host may obtain some of these neurotransmitters or their precursors from the environment or from its own diet, it is the bacterial communities themselves that co-vary strongly with some of the most important neuroactive metabolites (Cabirol et al., 2023; Kešnerová et al., 2017). This functional linkage between the bacteria in the gut and the production of neurotransmitters suggest that LAB may be harnessed within an apicultural context to increase the neurotransmission of disease-associated olfactory cues in worker bees. If a critical number of workers could be rendered more sensitive, a probiotic supplement that specifically lowered the response threshold to disease or that affected other threshold-gated behaviours could be designed for the beekeeping community.
Octopamine and GABA
The biogenic amine octopamine may have a practical link to the hygienic response of workers. The concentration of octopamine in the worker brain tends to increase with age, which affects age-based duties performed by workers within colonies (Schulz et al., 2002). Spivak et al. (2003) observed differences in the expression of octopamine in the brains of nurse bees from hygienic and non-hygienic lines, suggesting that this neurotransmitter is functionally associated with sensitivity to cues from diseased brood. Simply knowing which gut microbes are associated with the highest concentrations of octopamine or with the most hygienic response, or both, warrants research.
The amino acid neurotransmitter GABA is taxonomically widespread and plays a fundamental role in signal processing. For honey bees, GABAergic neurons are present in all principal olfactory centres, such as the mushroom bodies and lateral horns (Sandoz, 2011), as well as other areas of the brain (Bicker, 1999). It is associated with learning and memory of the worker caste (El Hassani et al., 2005), as well as locomotion and motor control (Mustard et al., 2020). Injection with GABA receptor antagonists can reduce bee mobility and impair their ability to right themselves after falling (Mustard et al., 2020). Injection with GABA receptor antagonists can also hinder olfactory neurons and diminish a bee’s ability to discriminate between different odours (Stopfer et al., 1997). As hygiene is a motor behaviour that is olfactory-mediated, GABA may pose an interesting candidate for modulation of hygienic behaviour of nurse bees. If colonies can be supplemented with strains that produce GABA, for example, Lactiplantibacillus plantarum (Cui et al., 2020) or certain Bifidobacterium or Bombilactobacillus (Cabirol et al., 2023; Kešnerová et al., 2017; Zhang et al., 2022b), then this effect on hygiene may be deliberately amplified.
Serotonin and dopamine
Serotonin is a biogenic amine that affects the senses of honey bees, but here the effect appears to reduce sensitivity to olfactory cues. Zhang et al. (2022b) found that enriching the native gut microbiota with Gilliamella apicola and Lactobacillus spp. reduced serotonin levels in the brains compared to gnotobiotic (i.e., gut-sterilized) bees. Like serotonin, dopamine can dampen responsiveness to stimuli and it affects locomotion and motor behaviour in honey bees (Mustard et al., 2010). Zhang et al. (2022b) demonstrated that dopamine levels can be decreased by gut microbes. The findings suggest an optimal dopamine concentration that can affect behaviour, and that the desired effect may be less, not more, of the neurotransmitter. Combinatorial enrichment of bee guts with a mix of probiotic strains that simultaneously increase octopamine and GABA while decreasing dopamine and serotonin may therefore be desirable. These complex manipulations of the bee gut microbiome could come from competition with the production of other neurotransmitters by the probiotics used to supplement the colony or by interference with the production of the precursors to these neurotransmitters (O’Donnell et al., 2020).
Testing probiotic effects on hygienic behaviour
Hygiene is a complex behaviour. Nurses share and delegate hygiene-associated tasks, specializing in areas such as uncapping of the brood cell or removing diseased offspring (Barrs et al., 2021). Our understanding of hygiene has expanded from its original descriptions around the detection and removal of chalkbrood (Ascosphaera apis) and foulbrood (Paenobacillus larvae) to include behavioural responses against other microbial sources of infection (Valizadeh et al., 2020) or against infestation by ectoparasites (e.g., Varroa mite-sensitive hygiene; Mondet et al., 2015).
We predict that administering probiotics aiding in the synthesis of olfactory-associated neurotransmitters, such as lactic acid-producing species, will modulate any genetic effects on hygiene and associated sensitivity to disease cues. This modulation may lower the hygienic threshold response of nurse bees to, in effect, render bees more hygienic. Given that LAB can help synthesize key neurotransmitters or their precursors (Table 1), we propose supplementing hives with two LAB species: Bifidobacterium asteroides, a bacterial species native to the bee gut, and Lactiplantibacillus plantarum, which is a not a species naturally found in honey bee guts. We suggest exploring the abilities of these and other candidate species and strains to affect the concentration of octopamine, GABA, serotonin and dopamine and, possibly, hygienic behaviour. Ideally, future studies would demonstrate that the specific bacteria administered can colonize, even transiently, the guts of treated bees, correlate with concentration of specific neurotransmitters or their precursors in bee brains, and ultimately affects the hygienic response. Together, these three test criteria − gut, brain, behaviour − would help to link treatment to a change in behaviour via the brain-gut axis.
The most common field assay for measuring hygiene is the freeze-kill brood (FKB) assay, which involves experimentally killing a small portion of brood with liquid nitrogen, then counting the proportion of the moribund brood removed over a set period (usually 24-48 hours). Other popular variants include the pin-killed brood assay (Leclercq et al., 2018). To investigate changes to the microbiota following treatment, researchers can employ 16S ribosomal RNA gene sequencing of the V3-V4 region to evaluate microbial community structure (as in Daisley et al., 2023) or use other forms of metagenomic sequencing to capture microbial diversity (Ellegaard and Engel, 2019). Various options are available to test the impact of bacterial supplements on the brain, such as high-performance liquid chromatography (or liquid chromatography-mass spectrometry) to determine neurotransmitter concentrations, or histochemical staining to view the distribution of neurotransmitters in the brain.
The brain-gut axis as a mechanism to modulate social behaviours
In addition to hygiene, the concepts proposed here could be extended to other honey bee behaviours, namely foraging, recruitment and defence. Recent work has demonstrated that variations in the gut microbiota of bees can influence their individual foraging behaviour (Vernier et al., 2024). As in hygiene, foraging is intricately linked to olfaction (de Brito Sanchez, 2011; Paoli and Galizia, 2021); octopamine and GABA both contribute to the foraging process (Chatterjee et al., 2021; Giray et al., 2007). Octopamine influences response thresholds to sucrose (Page and Erber, 2002), potentially increasing foraging efforts, as well as influencing food preference during foraging (Giray et al., 2007). GABA receptors are more abundant in the brains of bees scouting for new food sources, and GABAergic neuron activity increases when foragers are orienting themselves to food sources or to the colony (Kiya and Kubo, 2010). Overall, changes in GABA and its precursor, glutamate, signalling in the brain appear to modulate scouting behaviour in foragers (Chatterjee et al., 2021), although there is more to discover from this connection.
As a distinct but related behaviour to foraging, honey bees recruit others to food sources using intricate dances, conveying information on the distance, direction and value of the food (Wenner et al., 1967). Octopamine and dopamine can help determine how long a bee follows the dance instructions and the frequency with which a bee will ultimately be recruited (Linn et al., 2020). Waggle dance activity can be recorded by using an observation hive (Biesmeijer and Seeley, 2005) or video recording software. Octopamine and GABA are also thought to influence defensive behaviour within colonies (Hunt, 2007), suggesting that Bifidobacterium spp. and Lactobacillus spp. may affect defensive behaviour via the brain-gut axis. Characteristics of the microbiota may also influence social recognition used in defence (Vernier et al., 2020).
All core bacterial species found in the honey bee gut can be cultivated and manipulated in the laboratory (Zheng et al., 2018), making the honey bee a functional system for studying microbial effects on health and behaviour. Bees can be raised with germ-free guts in the lab (Powell et al., 2014), which allows for experimental colonization with strains of interest. In this mini review, we have highlighted potential benefits of certain lactic acid bacteria. This group is relatively well known but similar properties may also be activated by other environmental microorganisms that are in the bee sphere or by specific strains of commensal microorganisms in addition to LAB (Motta et al., 2022; Cabirol et al., 2023).
In addition to laboratory experiments, field experiments can also be conducted, as proposed here, by applying probiotics directly to colonies via probiotic-infused pollen patties (Corby-Harris et al., 2016) or probiotic sprays (Daisley et al., 2023). Further avenues of investigation could involve freeze-drying beneficial bacteria, increasing colony-forming unit counts and bacterial survivorship in different media for application to colonies.
Conclusion
The potential of honey bee probiotics is promising, with numerous studies exploring the relationship between gut bacteria and brain neurotransmitters (e.g., Cabirol et al., 2023). Despite this potential, there is not yet firm evidence that hygienic behaviour can be modified by commensal or even by exogenous bacteria. Studies are therefore required to test this idea for hygienic and other social behaviours including foraging, defence and recruitment. These research initiatives offer promising avenues to improve the health, survival and productivity of managed honey bees, while advancing our understanding of the brain-gut axis at both the individual and colony levels.
Author contributions
SK: Conceptualization, Investigation, Writing – original draft. BD: Writing – review & editing. MK: Writing – review & editing. JL: Writing – review & editing. GT: Conceptualization, Funding acquisition, Supervision, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant to GT, an Ontario Graduate Scholarship to SK, a Banting Postdoctoral Fellowship Award to BD (BPF-489532-402947) and by the Western University Sustainable Impact Fund.
Acknowledgments
We thank Marisol Vargas (Universidad de Concepción) for comments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alberoni D., Gaggìa F., Baffoni L., Di Gioia D. (2016). Beneficial microorganisms for honey bees: Problems and progresses. Appl. Microbiol. Biotechnol. 100, 9469–9482. doi: 10.1007/s00253-016-7870-4
Barrs K. R., Ani M. O., Eversman K. K., Rowell J. T., Wagoner K. M., Rueppell O. (2021). Time-accuracy trade-off and task partitioning of hygienic behavior among honey bee (Apis mellifera) workers. Behav. Ecol. Sociobiol 75, 12. doi: 10.1007/s00265-020-02940-y
Beshers S. N., Fewell J. H. (2001). Models of division of labor in social insects. Annu. Rev. Entomol 46, 413–440. doi: 10.1146/annurev.ento.46.1.413
Bicker G. (1999). Histochemistry of classical neurotransmitters in antennal lobes and mushroom bodies of the honeybee. MRT 45, 174–183. doi: 10.1002/(SICI)1097-0029(19990501)45:3<174::AID-JEMT5>3.0.CO;2-U
Biesmeijer J. C., Seeley T. D. (2005). The use of waggle dance information by honey bees throughout their foraging careers. Behav. Ecol. Sociobiol 59, 133–142. doi: 10.1007/s00265-005-0019-6
Bonilla-Rosso G., Engel P. (2018). Functional roles and metabolic niches in the honey bee gut microbiota. Curr. Opin. Microbiol. 43, 69–76. doi: 10.1016/j.mib.2017.12.009
Cabirol A., Moriano-Gutierrez S., Engel P. (2023). Neuroactive metabolites modulated by the gut microbiota in honey bees. Mol. Microbiol. 122 (3), 284–293. doi: 10.1111/mmi.15167
Carding S., Verbeke K., Vipond D. T., Corfe B. M., Owen L. J. (2015). Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 26, 26191. doi: 10.3402/mehd.v26.26191
Chatterjee A., Bais D., Brockmann A., Ramesh D. (2021). Search behavior of individual foragers involves neurotransmitter systems characteristic for social scouting. Front. Insect Sci. 1. doi: 10.3389/finsc.2021.664978
Chen Y., Xu J., Chen Y. (2021). Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders. Nutrients 13 (6), 2099. doi: 10.3390/nu13062099
Chmiel J. A., Pitek A. P., Burton J. P., Thompson G. J., Reid G. (2021). Meta-analysis on the effect of bacterial interventions on honey bee productivity and the treatment of infection. Apidologie 52, 960–972. doi: 10.1007/s13592-021-00879-1
Corby-Harris V., Snyder L., Meador C. A. D., Naldo R., Mott B., Anderson K. E. (2016). Parasaccharibacter apium, gen. nov., sp. nov., Improves honey bee (Hymenoptera: Apidae) resistance to Nosema. J. Econ Entomol 109, 537–543. doi: 10.1093/jee/tow012
Cremer S., Armitage S. A. O., Schmid-Hempel P. (2007). Social immunity. Curr. Biol. 17, R693–R702. doi: 10.1016/j.cub.2007.06.008
Cui Y., Miao K., Niyaphorn S., Qu X. (2020). Production of gamma-aminobutyric acid from lactic acid bacteria: A systematic review. Int. J. Mol. Sci. 21, 995. doi: 10.3390/ijms21030995
Daisley B. A., Pitek A. P., Chmiel J. A., Al K. F., Chernyshova A. M., Faragalla K. M., et al. (2019). Novel probiotic approach to counter Paenibacillus larvae infection in honey bees. ISMEJ 14, 476–491. doi: 10.1038/s41396-019-0541-6
Daisley B. A., Pitek A. P., Chmiel J. A., Gibbons S., Chernyshova A. M., Al K. F., et al. (2020). Lactobacillus spp. attenuate antibiotic-induced immune and microbiota dysregulation in honey bees. Commun. Biol. 3 (1), 534. doi: 10.1038/s42003-020-01259-8
Daisley B. A., Pitek A. P., Torres C., Lowery R., Adair B. A., Al K. F., et al. (2023). Delivery mechanism can enhance probiotic activity against honey bee pathogens. ISMEJ 17 (9), 1382–1395. doi: 10.1038/s41396-023-01422-z
Damico M. E., Beasley B., Greenstein D., Raymann K. (2023). Testing the effectiveness of a commercially sold probiotic on restoring the gut microbiota of honey bees: A field study. Probiotics Antimicrobial Proteins. doi: 10.1007/s12602-023-10203-1
de Brito Sanchez M. G. (2011). Taste perception in honey bees. Chem. Senses 36, 675–692. doi: 10.1093/chemse/bjr040
Dickel F., Bos N. M. P., Hughes H., Martín-Hernández R., Higes M., Kleiser A., et al. (2022). The oral vaccination with Paenibacillus larvae bacterin can decrease susceptibility to American Foulbrood infection in honey bees—A safety and efficacy study. Front. Vet. Sci. 9. doi: 10.3389/fvets.2022.946237
Dus M., Lai J. S. Y., Gunapala K. M., Min S., Tayler T. D., Hergarden A. C., et al. (2015). Nutrient sensor in the brain directs the action of the brain-gut axis in drosophila. Neuron 87, 139–151. doi: 10.1016/j.neuron.2015.05.032
El Hassani A. K., Dacher M., Gauthier M., Armengaud C. (2005). Effects of sublethal doses of fipronil on the behavior of the honeybee (Apis mellifera). Pharmacol. Biochem. Behav. 82, 30–39. doi: 10.1016/j.pbb.2005.07.008
Ellegaard K. M., Engel P. (2019). Genomic diversity landscape of the honey bee gut microbiota. Nat. Commun. 10, 1–13. doi: 10.1038/s41467-019-08303-0
Erez T., Bonda E., Kahanov P., Rueppell O., Wagoner K., Chejanovsky N., et al. (2022). Multiple benefits of breeding honey bees for hygienic behavior. J. Invertebr Pathol. 193, 107788. doi: 10.1016/j.jip.2022.107788
Garbian Y., Maori E., Kalev H., Shafir S., Sela I. (2012). Bidirectional transfer of RNAi between honey bee and Varroa destructor: Varroa gene silencing reduces Varroa population. PloS Pathog. 8, e1003035. doi: 10.1371/journal.ppat.1003035
Giray T., Galindo A., Oskay D. (2007). Octopamine influences honey bee foraging preference. J. Insect Physiol. 53, 691–698. doi: 10.1016/j.jinsphys.2007.03.016
Guerrero R., Margulis L., Berlanga M. (2013). Symbiogenesis: The holobiont as a unit of evolution. Int. Microbiol. 16, 133–143. doi: 10.2436/20.1501.01.188
Hunt G. J. (2007). Flight and fight: A comparative view of the neurophysiology and genetics of honey bee defensive behavior. J. Insect Physiol. 53, 399–410. doi: 10.1016/j.jinsphys.2007.01.010
Hýbl M., Bohatá A., Rádsetoulalová I., Kopecký M., Hoštičková I., Vaníčková A., et al. (2021). Evaluating the efficacy of 30 different essential oils against Varroa destructor and honey bee workers (Apis mellifera). Insects 12 (11), 1045. doi: 10.3390/insects12111045
Jones J. C., Fruciano C., Marchant J., Hildebrand F., Forslund S., Bork P., et al. (2018). The gut microbiome is associated with behavioural task in honey bees. Insectes Sociaux 65, 419–429. doi: 10.1007/s00040-018-0624-9
Kapheim K. M., Rao V. D., Yeoman C. J., Wilson B. A., White B. A., Goldenfeld N., et al. (2015). Caste-specific differences in hindgut microbial communities of honey bees (Apis mellifera). PloS One 10, e0123911. doi: 10.1371/journal.pone.0123911
Kešnerová L., Mars R. A., Ellegaard K. M., Troilo M., Sauer U., Engel P. (2017). Disentangling metabolic functions of bacteria in the honey bee gut. PloS Biol. 15, e2003467. doi: 10.1371/journal.pbio.2003467
Kiya T., Kubo T. (2010). Analysis of GABAergic and non-GABAergic neuron activity in the optic lobes of the forager and re-orienting worker honeybee (Apis mellifera L.). PloS One 5, e8833. doi: 10.1371/journal.pone.0008833
Leclercq G., Francis F., Gengler N., Blacquière T. (2018). Bioassays to quantify hygienic behavior in honey bee (Apis Mellifera L.) colonies: A review. J. Apic Res. 57, 1–11. doi: 10.1080/00218839.2018.1494916
Liberti J., Engel P. (2020). The gut microbiota—Brain axis of insects. Curr. Opin. Insect Sci. 39, 6–13. doi: 10.1016/j.cois.2020.01.004
Liberti J., Kay T., Quinn A., Kesner L., Frank E. T., Cabirol A., et al. (2022). The gut microbiota affects the social network of honeybees. Nat. Ecol. Evol. 6 (10), 1471–1479. doi: 10.1038/s41559-022-01840-w
Lima C. M. G., Nora F. M. D., Seraglio S. K. T., Silva J. M., da Marzoque H. J., Santana R. F., et al. (2020). Antibiotic residues in honey: A public health issue. Res Soc. Dev. 9 (11), e1739119604. doi: 10.33448/rsd-v9i11.9604
Linn M., Glaser S. M., Peng T., Grüter C. (2020). Octopamine and dopamine mediate waggle dance following and information use in honeybees. Proc. R Soc. B: Biol. Sci. 287, 20201950. doi: 10.1098/rspb.2020.1950
Masterman R., Smith B. H., Spivak M. (2000). Brood odor discrimination abilities in hygienic honey bees (Apis mellifera L.) using proboscis extension reflex conditioning. J. Insect Behav. 13, 87–101. doi: 10.1023/A:1007767626594
Mondet F., Alaux C., Severac D., Rohmer M., Mercer A. R., Le Conte Y. (2015). Antennae hold a key to Varroa-sensitive hygiene behaviour in honey bees. Sci. Rep. 5, 10454. doi: 10.1038/srep10454
Motta E. V. S., Powell J. E., Leonard S. P., Moran N. A. (2022). Prospects for probiotics in social bees. Philos. Trans. R Soc B Biol. Sci. 377, 20210156. doi: 10.1098/rstb.2021.0156
Mustard J. A., Jones L., Wright G. A. (2020). GABA signaling affects motor function in the honey bee. J. Insect Physiol. 120, 103989. doi: 10.1016/j.jinsphys.2019.103989
Mustard J. A., Pham P. M., Smith B. H. (2010). Modulation of motor behavior by dopamine and the D1-like dopamine receptor AmDOP2 in the honey bee. J. Insect Phys. 56, 422–430. doi: 10.1016/j.jinsphys.2009.11.018
O’Donnell M. P., Fox B. W., Chao P. H., Schroeder F. C., Sengupta P. (2020). A neurotransmitter produced by gut bacteria modulates host sensory behaviour. Nature 583, 415–420. doi: 10.1038/s41586-020-2395-5
Oldroyd B. P., Thompson G. J. (2006). Behavioural genetics of the honey bee Apis mellifera. Adv. Insect Phys. 33, 1–49. doi: 10.1016/S0065-2806(06)33001-9
Oxley P. R., Spivak M., Oldroyd B. P. (2010). Six quantitative trait loci influence task thresholds for hygienic behaviour in honeybees (Apis mellifera). Mol. Ecol. 19, 1452–1461. doi: 10.1111/j.1365-294X.2010.04569.x
Özoğul F., Kuley E., Özoğul Y., Özoğul İ. (2012). The function of lactic acid bacteria on biogenic amines production by food-borne pathogens in arginine decarboxylase broth. Food Sci. Technol. Res. 18, 795–804. doi: 10.3136/fstr.18.795
Page R. E., Erber J. (2002). Levels of behavioral organization and the evolution of division of labor. Naturwissenschaften 89, 91–106. doi: 10.1007/s00114-002-0299-x
Paoli M., Galizia G. C. (2021). Olfactory coding in honeybees. Cell Tissue Res. 383, 35–58. doi: 10.1007/s00441-020-03385-5
Powell J. E., Martinson V. G., Urban-Mead K., Moran N. A. (2014). Routes of acquisition of the gut microbiota of the honey bee Apis mellifera. Appl. Environ. Microbiol. 80, 7378–7387. doi: 10.1128/AEM.01861-14
Raymann K., Moran N. A. (2018). The role of the gut microbiome in health and disease of adult honey bee workers. Curr. Opin. Insect Sci. 26, 97–104. doi: 10.1016/j.cois.2018.02.012
Raymann K., Shaffer Z., Moran N. A. (2017). Antibiotic exposure perturbs the gut microbiota and elevates mortality in honeybees. PloS Biol. 15, e2001861. doi: 10.1371/journal.pbio.2001861
Rosenberg E., Sharon G., Atad I., Zilber-Rosenberg I. (2010). The evolution of animals and plants via symbiosis with microorganisms. Environ. Microbiol. Rep. 2, 500–506. doi: 10.1111/j.1758-2229.2010.00177.x
Sandoz J. C. (2011). Behavioral and neurophysiological study of olfactory perception and learning in honeybees. Front. Syst. Neurosci. 5. doi: 10.3389/fnsys.2011.00098
Sarkar A., Harty S., Johnson K. V. A., Moeller A. H., Carmody R. N., Lehto S. M., et al. (2020). The role of the microbiome in the neurobiology of social behaviour. Biol. Rev. 95, 1131–1166. doi: 10.1111/brv.12603
Schneider E., O’Riordan K. J., Clarke G., Cryan J. F. (2024). Feeding gut microbes to nourish the brain: unravelling the diet–microbiota–gut–brain axis. Nat. Metab. 6, 1454–1478. doi: 10.1038/s42255-024-01108-6
Schulz D. J., Barron A. B., Robinson G. E. (2002). A role for octopamine in honey bee division of labor. Brain Behav. Evol. 60, 350–359. doi: 10.1159/000067788
Spivak M., Gilliam M. (2015). Hygienic behaviour of honey bees and its application for control of brood diseases and varroa: Part II. Studies on hygienic behaviour since the Rothenbuhler era. Bee World 79, 169–186. doi: 10.1080/0005772X.1998.11099408
Spivak M., Masterman R., Ross R., Mesce K. A. (2003). Hygienic behavior in the honey bee (Apis mellifera L.) and the modulatory role of octopamine. J. Neurobiol. 55, 341–354. doi: 10.1002/neu.10219
Stopfer M., Bhagavan S., Smith B. H., Laurent G. (1997). Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature 390, 6655. doi: 10.1038/36335
Valizadeh P., Guzman-Novoa E., Goodwin P. H. (2020). Effect of immune inducers on Nosema ceranae multiplication and their impact on honey bee (Apis mellifera L.) survivorship and behaviors. Insects 11, 9. doi: 10.3390/insects11090572
Vásquez A., Forsgren E., Fries I., Paxton R. J., Flaberg E., Szekely L., et al. (2012). Symbionts as major modulators of insect health: Lactic acid bacteria and honeybees. PloS One 7, e33188. doi: 10.1371/journal.pone.0033188
Vernier C. L., Chin I. M., Adu-Oppong B., Krupp J. J., Levine J., Dantas G., et al. (2020). The gut microbiome defines social group membership in honey bee colonies. Sci. Adv. 6, eabd3431. doi: 10.1126/sciadv.abd3431
Vernier C. L., Nguyen L. A., Gernat T., Ahmed A. C., Chen Z., Robinson G. E. (2024). Gut microbiota contribute to variations in honey bee foraging intensity. ISME J. 18 (1), wrae030. doi: 10.1093/ismejo/wrae030
Wenner A. M., Wells P. H., Rohlf F. J. (1967). An analysis of the waggle dance and recruitment in honey bees. Physiol. Zool 40, 317–344. doi: 10.1086/physzool.40.4.30158452
Zhang Z., Mu X., Cao Q., Shi Y., Hu X., Zheng H. (2022a). Honeybee gut Lactobacillus modulates host learning and memory behaviors via regulating tryptophan metabolism. Nat. Commun. 13, 1–13. doi: 10.1038/s41467-022-29760-0
Zhang Z., Mu X., Shi Y., Zheng H. (2022b). Distinct roles of honeybee gut bacteria on host metabolism and neurological processes. Microbiol. Spectr. 10, e02438–e02421. doi: 10.1128/spectrum.02438-21
Keywords: probiotics, neurotransmitters, microbiota, brain-gut axis, insect behaviour, hostmicrobe interactions, eusociality
Citation: Killam SM, Daisley BA, Kleiber ML, Lacika JF and Thompson GJ (2024) A case for microbial therapeutics to bolster colony health and performance of honey bees. Front. Bee Sci. 2:1422265. doi: 10.3389/frbee.2024.1422265
Received: 23 April 2024; Accepted: 17 September 2024;
Published: 04 October 2024.
Edited by:
Karina Antúnez, Instituto de Investigaciones Biológicas Clemente Estable (IIBCE), Montevideo, UruguayReviewed by:
Daniele Alberoni, University of Bologna, Bologna, ItalyCopyright © 2024 Killam, Daisley, Kleiber, Lacika and Thompson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Graham J. Thompson, Z3JhaGFtLnRob21wc29uQHV3by5jYQ==
 Sophie M. Killam
Sophie M. Killam Brendan A. Daisley1,2
Brendan A. Daisley1,2 Morgan L. Kleiber
Morgan L. Kleiber Graham J. Thompson
Graham J. Thompson