- 1School of Geoscience, University of South Florida, Tampa, FL, United States
- 2Department of Earth and Environmental Sciences, Rensselaer Polytechnic Institute, Troy, NY, United States
Phosphorus plays a multifaceted role for all known life and hence understanding its sources on the early Earth provides constraints on how life developed to incorporate this element into its biochemistry. Currently, the major phosphorus mineral group on the surface of the Earth are the apatites, which are poorly soluble calcium phosphates and hence may not have been a good source of phosphorus on the early Earth. An alternative source of phosphorus may be the mineral olivine. Given that olivine makes up a large part of the upper mantle of Earth and presumably other rocky planets and moons, it stands to reason that olivine may be a potential phosphorus reservoir for prebiotic chemical environments. Here we examine the phosphorus content of 10 olivine samples from different terrestrial localities to determine their P content and P speciation. We find that extracts of the samples contain varying amounts of phosphate, and some contain pyrophosphate. Olivine may have served as a source of phosphate on the early Earth and possibly elsewhere in the solar system, and its dissolution could have supplied this nutrient to a nascent biosphere.
1 Introduction
Phosphorus is an element central to many aspects of modern biochemistry. The phosphate moiety is important to several classes of biomolecules such as phospholipids that make up cell membranes, metabolic molecules such as adenosine triphosphate (ATP), and as part of the backbone of DNA (with deoxyribose) allowing for its polymeric structure. In contrast to the other biogenically critical elements [carbon, hydrogen, oxygen, nitrogen, and sulfur (C, H, O, N, and S or CHONS)], phosphorus does not possess a significant volatile phase and does not participate in redox reactions over a large range of conditions commonly present on the Earth’s surface. In life, organisms extract phosphorus from mineral sources or remove it from water or recycle it from existing biomolecules. However, this presents a major hurdle for understanding how biologically important phosphorus molecules developed prior to the formation of life on rocky planets. The apatite mineral group [including hydroxyapatite (Ca5(PO4)3OH) and fluorapatite (Ca5(PO4)3F)] is considered to have been the source of phosphate on the surface of the early Earth and likely elsewhere, but the apatite mineral group is by and large unreactive and insoluble in water, which may have prevented phosphorus from being bioavailable on a large scale (Gulick, 1955). Given the importance of phosphorus to the development of terrestrial life, phosphorus’s abundance and its availability may serve as important indicator of the habitability of a planet or moon, such as Mars (Adcock et al., 2013) and Enceladus (Hao et al., 2022; Postberg et al., 2023).
If apatite was the primary source of phosphate on the early Earth this leads to what is known as the “phosphate problem” in the origin of life community (Fernández-García et al., 2017). Apatite’s poor solubility and low reactivity make it a rather difficult material from which to make the key organics of life (Pasek, 2008). Typical concentrations of phosphate in the environment are on the order of 10–6 M and below (Tyrrell, 1999) whereas many phosphorylation experiments use levels much higher than that, on the order of 0.1–1 M (Xu et al., 2019; Cheng et al., 2002; Reimann and Zubay, 1999). However, some environments may provide elevated phosphate. Recently, Last Chance Lake and Goodenough Lake in Canada were demonstrated to contain up to 37 mM of phosphate, the highest natural levels recorded thus far (Haas et al., 2024), though the source of this phosphate still ultimately was mineral dissolution during weathering. Other hypotheses have been put forth to describe how the environment may have afforded an increased phosphate reactivity including alternative solvents that are not pure water to promote dehydration, using condensing agents to promote water loss, heating phosphates with organic compounds, and the possibility that reduced phosphorus sources that oxidize to produce reactive phosphates (Lago, 2021; Schoffstall, 1976; Pasek et al., 2007; Lohrmann and Orgel, 1968).
In addition to the apatite group, the mineral olivine ((Mg,Fe)2SiO4) also bears phosphorus, and may have been a major source of phosphate on the early Earth by virtue of its ubiquity (Walton et al., 2021a). Olivine is a mineral that is a primary component of the Earth’s upper mantle and is the first to crystallize out of magma. Olivine is also common to many meteorites (Bridges and Warren, 2006). Olivine has two compositional end members, forsterite (Mg2SiO4) and fayalite (Fe2SiO4), and natural occurrences are generally a solid solution of these two, usually with more forsterite (Fo) than fayalite (Fa) in the ultramafic rock peridotite (Elias and Alderton, 2020). Peridotite is a major component of the Earth’s upper mantle believed to be formed by partial melting of the mantle with the rising molten portion of mantle (Elias and Alderton, 2020). Olivine is initially more forsteritic when it crystallizes out of magma, potentially allowing for phosphorus incorporation due to lattice mismatching (see below) (Welsch et al., 2013). Recently, olivine has been shown to incorporate phosphorus at a higher rate than previously thought due to a number of factors (Agrell et al., 1962; Bekker et al., 2021; Boesenberg and Hewins, 2010; Li et al., 2017; Lynn et al., 2017; McKibbin et al., 2019; Shea et al., 2019; Shea et al., 2015; Walton, 2022; Welsch et al., 2014; Walton et al., 2021b).
Phosphoran olivine is a variety of olivine that bears a P2O5 content greater than 1 weight %. Most olivine is not phosphoran, and has a largely variable phosphorus content ranging from a lower end of 0.07–0.15 weight % (Brunet and Chazot, 2001) to an upper range of 0.3–0.6 weight % (Welsch et al., 2013). Under normal olivine crystal growth rate, phosphorus is an incompatible element in the olivine lattice, meaning due to the ionic radius and valence of phosphorus it will preferentially partition into the melt (Lynn et al., 2020). Phosphorus will slowly diffuse into the olivine crystal and only in these very small amounts (Baziotis et al., 2017). During rapid formation (growth rate of 10–6 m/s) of an olivine crystal, branch misorientations and lattice mismatching may occur potentially allowing for a higher percentage of phosphorus incorporation into the crystal structure (Welsch et al., 2014). Due to a similarity in atomic size, phosphorus (P5+) likely replaces silicon (Si4+) in the structure of olivine during this rapid growth (Welsch et al., 2013). Further, a less-stiff crystal lattice during rapid growth may be the explanation for phosphorus incorporation (Shea et al., 2019). The composition of the melt and the crystallization process of olivine seem to be the main controls on the incorporation of phosphorus into the olivine lattice (Ersoy et al., 2019).
The generally accepted substitution mechanism for most olivine is phosphorus (P5+) replaces silicon (Si4+) in the tetrahedral site (Equation 1) (Milman-Barris et al., 2008). However, Boesenberg and Hewins (2010) demonstrated a vacancy (denoted “
To this end, we investigated 10 olivine samples from around the world to check for the presence of alternative P redox states (e.g., Equations 3, 4), and to better constrain the amount of P released from olivine during extraction with a chelating agent. These data may then better constrain the role of extremely abundant olivine as a source of P on the early Earth and on other rocky planets.
2 Methods
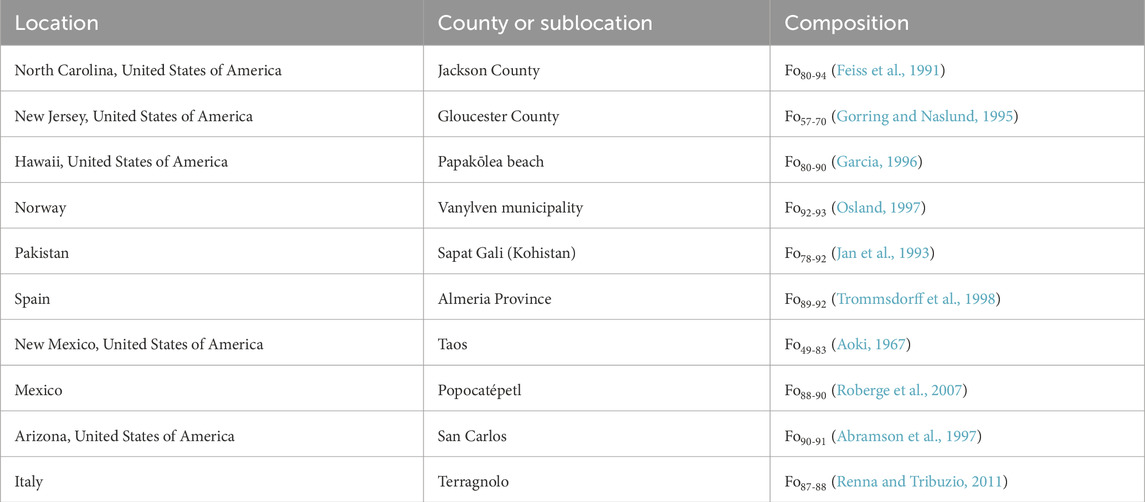
The ten olivine sample localities are provided in Table 1, along with the median composition (in terms of Fo%, or percentage Mg vs. Mg + Fe). Samples were examined by XRD to verify they were only olivine (Feng et al., 2024). Between 0.5 and 0.6 g of olivine crystals were crushed to a fine powder using a mortar and pestle, placed in glass vials with 1.5 mL of sodium ethylenediaminetetraacetic acid (Na4EDTA) (Sigma Aldrich) which was brought up to a pH of 13 using sodium hydroxide (Alfa Aesar) and placed onto a magnetic stir plate for 1 week. After extraction, the liquid was filtered using 0.22 μm filter tips (Biomed Scientific) fixed to a 1 mL syringe and placed on watch glasses to dry for 2 days. Once dried, 500 μL of D2O (Sigma Aldrich) was added to each watch glass, mixed, and using a pipet the solutions were placed into Eppendorf tubes for centrifugation to separate the solution from any remaining solid. The supernatants were placed in Wilmad Nuclear Magnetic Resonance (NMR) tubes (Sigma Aldrich) and were analyzed via Bruker Neo 600 MHz NMR running for 6,000 scans, for H-decoupled 31P NMR (242.8609732 MHz) at the University of South Florida (USF) Interdisciplinary NMR Facility. All spectra were analyzed using Mnova version 14.3.3 package.
100 mg of phosphonoacetic acid (C2H5O5P) (98%, Sigma Aldrich) was added into 1 mL of Milli-Q 18 mOhm deionized water purified in house using a Barnstead (Dubuque, IA) Nanopure Diamond Analytical combined reverse osmosis-deionization system. This resulted in a phosphonoacetic acid solution of 0.7 M. 20 μL of the phosphonoacetic acid solution was added to each NMR tube and analyzed at the same USF NMR facility.
The analysis for total P was performed on a Perkin-Elmer Nexion 2000 Quadrupole Inductively Coupled Plasma–Mass Spectrometer (ICP-MS). The same EDTA process was employed again to prepare the samples. 400 μL was diluted to 10 mL using the same deionized water. In between each sample analysis a blank of deionized water with a solution of 15% ethanol (C₂H₆O) was ran through all parts of the system followed by a 5% solution of trace metal grade nitric acid (HNO3). The solutions were used to clean the lines and the internal components of the ICP to prevent build-up of organic residue. A calibration curve was made using a solution of P (High Purity Standards, 1,000 ppm with serial dilutions to 1 ppm). A blank of EDTA was also measured to ensure no cross contamination was occurring from that source. The measurement of the EDTA measured at less than 10 ppb. A nine-point calibration curve was used to create a linear equation which was then manually applied to each sample after analysis was complete. The relative standard deviation (RSD) of the samples averaged out to be less than 3% throughout the entire run.
3 Results
The phosphorus speciation of 10 olivine samples was examined using 31P NMR (Figure 1). Phosphate of varying quantities was detected in each of the 10 samples. Pyrophosphate was clearly detected in the samples from Hawaii and Pakistan.
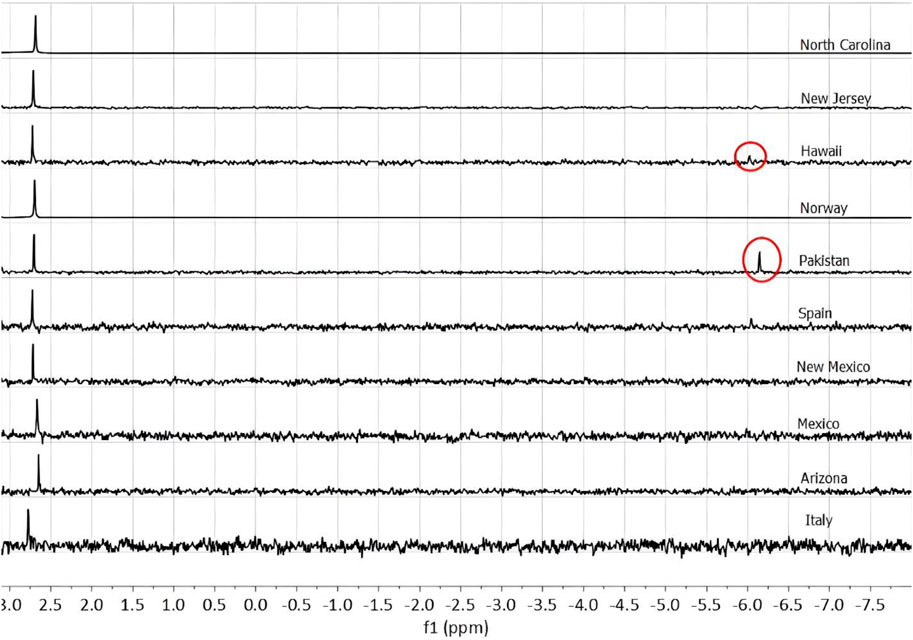
Figure 1. NMR spectra showing phosphate (PO43-) peaks at roughly 2.7 ppm. The Hawaiian and Pakistani olivine samples show pyrophosphate (P2O47-) peaks in circles. The samples from Spain and new Jersey may also have a peak corresponding to pyrophosphate.
Three methods were employed to determine the total phosphate concentration extracted for each sample (Table 2; Figure 2). In the first method, a known concentration and quantity of phosphonoacetic acid was added to each olivine extract. The samples were then run, and spectra analyzed by taking the integrations of phosphonoacetic acid and phosphate peak. The mass of P extracted was then determined using the integrated ratio and amount of initial olivine sample. In the second method, the phosphate NMR “signal” was compared at its highest intensity to three times the standard deviation of the “noise” ranges. The signal value was divided by the average noise and squared to obtain a concentration as per Pasek et al. (2007). These methods were contrasted to the concentration determined by ICP-MS.
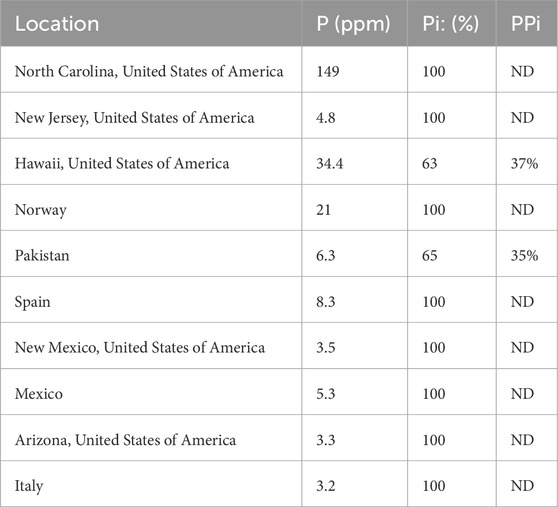
Table 2. Phosphorus content extracted and relative quantities of phosphate (Pi) and pyrophosphate (PPi).
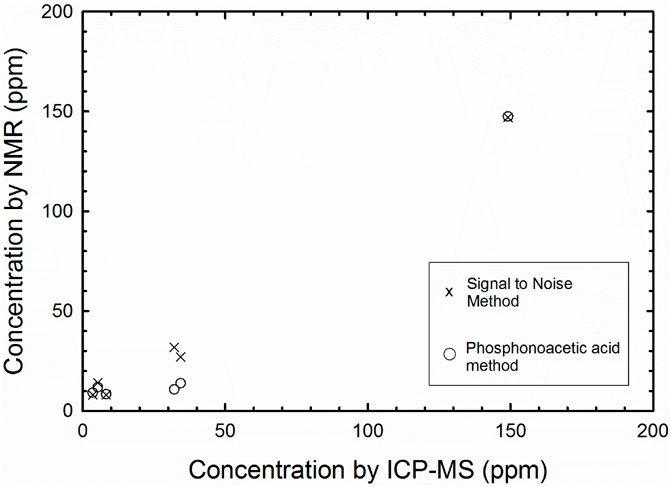
Figure 2. Scatter plot showing trend of NMR signal to noise ratio (“x”) compared to samples spiked with phosphonoacetic acid (circles), both compared to results from ICPMS.
4 Discussion
The mineral apatite is usually considered to be the de facto source of prebiotic phosphorus by prebiotic geochemists as it comprises 80% of the modern phosphorus reservoir, and apatite is soluble in mantle melts and common in the continental crustal (Walton et al., 2021a). The potential of olivine to be a major source of phosphorus on the early Earth has received comparatively little consideration (Walton et al., 2021a).
Pasek et al. (2022), in a study of serpentinites and phosphorus, hypothesized that in the presence of iron (II), the reduction of phosphate to phosphite could occur in the olivine itself, and that serpentinization merely releases this phosphorus as phosphite. In this study, we find no evidence of phosphite in 10 terrestrial olivine samples from different localities, suggesting that P in olivine is present only as P5+. Therefore, the serpentinization process likely leads to phosphate reduction to phosphite.
Pyrophosphate was in olivine samples from Hawaii and Pakistan, and possibly in the samples from Spain and New Jersey. Phosphate has been shown at high temperatures (∼1,000 K) in the presence of silicate glass to be polymerized into pyrophosphate at a large percentage (Mysen, 1996). At such temperatures, the phosphate begins to exchange oxygens with the silicate species, resulting in a more polymerized phosphorus and less polymerized silicate (Mysen, 2011). The relative quantity of phosphate to pyrophosphate (as measured through peak integration) in the Hawaiian olivine is 63% phosphate and 37% pyrophosphate and 65% phosphate and 35% pyrophosphate in the Pakistani olivine, suggesting some polymerization of phosphate to pyrophosphate during crystallization, or perhaps some other process is effecting this change. Given the absence of pyrophosphate in most samples, the formation of pyrophosphate may be a mineralogic or petrologic process, as opposed to being an artifact of our sample preparation procedure. Notably pyrophosphate may have been relevant to the development of life on the earth, based on the presence of pyrophosphatase enzymes (Baltscheffsky et al., 1966), where pyrophosphate serves as an energy molecule akin to ATP. However, some of these enzymes are not widespread across all domains of life (Segami et al., 2018) hence natural pyrophosphate may have had a mixed effect on early biosystems: it may have been useful as a biologic energy molecule but may have also not been easy for early organisms to use as a P source.
The presence of phosphorus in olivine provides a broader context for other planets. For example, Chassingnite meteorites from Mars (as part of the SNC Martian meteorites) are dunites [composed of ∼90% olivine (Mason, 1981)]. The Chassigny meteorite has 0.018 wt% P within it (Banin et al., 1992; Maciá et al., 1997). It is plausible that dunites on Mars could have served as P sources, given their possible wide-spread occurrence. Olivine located on the Martian surface have been postulated as indicative of primitive mantle magmas (McSween et al., 2006) and various olivine cumulate outcrops have been identified, in some cases composing up to 65% of the rock material present (Liu et al., 2022; Mustard et al., 2005; Hamilton and Christensen, 2005).
For these reasons, olivine could have been a source of phosphorus on Mars to a nascent, early biosphere. Although extractions of Mars dunites like the extractions performed above are too costly given the scarcity of this material, we would also anticipate Mars olivine to be a source of pyrophosphate, though at a lower scale than of phosphate.
Europa and Enceladus are both of particular interest when exploring the potential of olivine as a source of phosphorus on other worlds where life may have developed. Due to the subsurface oceans likely present on both moons (Carr et al., 1998; Pappalardo et al., 1999; Roberts and Nimmo, 2008; Thomas et al., 2016), water-rock interactions between olivine and the ocean may have liberated phosphate into water. Olivine present in the mantle boundary interacting with the ocean could lead to the release of phosphorus from the olivine as phosphate, as well as through the process of serpentinization (Pasek et al., 2022). The crust and mantles of both Europa and Enceladus have been theorized to be composed of olivine similar to that of Earth’s mantle (Schubert et al., 2009; Hsu et al., 2015). Hao et al. (2022) argued for the efficient release of phosphate by binding of divalent cations with carbonate from CO2 and by enhancing dissolution with lower pH (Hao et al., 2022). Evidence for higher phosphate at Saturn’s moon Enceladus is further supported by analyses by the Cassini Cosmic Dust Analyzer, which suggested that sodium phosphates were a component of some of the icy particles of Saturn’s e-ring (Postberg et al., 2023), the source of which appears to be Enceladus. This suggests the subsurface water at Enceladus may bear more phosphate than previously envisioned (Carr et al., 1998), with olivine as a potential source.
In comparison to the measured P contents of olivine (Welsch et al., 2013; Agrell et al., 1962; Bekker et al., 2021; Boesenberg and Hewins, 2010; Li et al., 2017; Lynn et al., 2017; Brunet and Chazot, 2001; Baziotis et al., 2017; Ersoy et al., 2019; Buseck and Clark, 1984), the average phosphorus content we extracted was between 3 and 150 ppm. The EDTA extraction method has been shown to work with other samples to establish phosphorus speciation including soils (Bowman and Moir, 1993; Turner et al., 2003), fulgurites (Pasek and Block, 2009), and serpentinites (Pasek et al., 2022). The amount of P extracted by this method is typically 50–90+% (Turner et al., 2003). We chose to use a soil science P extraction procedure given these prior successes, and the fact that the EDTA solution does not appear to alter phosphorus speciation. In contrast, a more aggressive extraction procedure (using a strong acid such as HCl or HF) would likely have caused the hydrolysis of P species including pyrophosphate and possibly caused oxidation of any reduced P compounds. That said, the relatively low extraction using this method contrasts with these prior measurements and suggests that the phases extracted with EDTA are likely easier to extract when linked to chelatable ions (such as Ca and Fe). The phosphate in these samples is likely bound to Mg and substitutes for Si. For this reason, we posit that the likelihood of olivine serving as a P-supplying material on the early Earth and other planets is instead limited to the dissolution of olivine.
4.1 Olivine as a model phosphorus-supplying material
We investigate two scenarios to constrain the release of P from olivine dissolution in the environment. The first scenario (Equation 5) considers the low release from a global, perfectly spherical shell/crust of olivine dissolving in a global ocean. The second (Equation 6) considers a localized region where small olivine grains dissolve to release P, modeled after the green sand Papokolea Beach, Hawaii. Note that each of these cases explores the initial, linear dissolution of olivine (which is usually given in length over time). As dissolution proceeds, the rate of dissolution slows due to increasing concentrations of ions, among other factors (Hausrath and Brantley, 2010). As such, they represent maxima and will likely decrease to a steady-state system where P is released at the same rate it is removed (for instance, by crystallization of phosphates or through adsorption).
In the minimal scenario, we consider the release of P from olivine within the whole Earth’s crust, assuming the crust is a sphere.
In this calculation
In the olivine sand beach environment, the P released can be much higher locally. We determine this through:
where
We compare these olivine dissolution values to the potential phosphorus released via apatite on a global scale using Equation 7 where
It is apparent from these models that olivine dissolution may exceed phosphate release from the dissolution of apatite, especially in localized environments. It is unclear though what occurs following this release. The phosphorus could immediately crystallize into apatite, via reaction with Ca2+. It is possible that the phosphorus may react with iron and reduce to phosphite (Herschy et al., 2018). It may also adsorb onto silica gel and create microenvironments for production of prebiotically relevant molecules (Westall et al., 2018). Nonetheless, the release of phosphate from olivine dissolution may have provided an additional source of bioavailable phosphate, through the slow dissolution of abundant olivine.
5 Conclusion
Phosphorus as phosphate was present in all 10 terrestrial olivine samples from different localities. Phosphorus was mainly present as phosphate, but some amounts of pyrophosphate were in at least two samples. The phosphate in the samples ranged from 3.2–149 ppm released during these extractions. Pyrophosphate was the only other P species observed released from olivine. The potential for olivine to be a viable source of phosphorus on the early Earth seems promising. The range of phosphorus in olivine on a global scale is equal to and in some cases greater than that of apatite. This holds true on a local environment scale as well such as the one modeled for Papokolea beach, Hawaii. Although olivine beaches are uncommon today, they may have been more prevalent on an early Earth where island arc accretion would have helped form the early crustal rocks [e.g. (Korsch et al., 2011)].
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
JA: Data curation, Investigation, Methodology, Resources, Validation, Visualization, Writing–original draft, Writing–review and editing. JH: Methodology, Writing–review and editing. MP: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by NASA Emerging Worlds program (Grant 80NSSC18K0598).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fspas.2024.1441187/full#supplementary-material
References
Abramson, E., Brown, J., Slutsky, L., and Zaug, J. (1997). The elastic constants of San Carlos olivine to 17 GPa. J. Geophys. Res. Solid Earth 102 (B6), 12253–12263. doi:10.1029/97jb00682
Adcock, C., Hausrath, E., and Forster, P. (2013). Readily available phosphate from minerals in early aqueous environments on Mars. Nat. Geosci. 6 (10), 824–827. doi:10.1038/ngeo1923
Agrell, S., Charnley, N., and Chinner, G. (1962). Phosphoran olivine from pine canyon, 2. Utah Miner: Piute Co, 265–269.
Aoki, K.-i. (1967). Petrography and petrochemistry of latest Pliocene olivine-tholeiites of Taos area, northern New Mexico, USA. Contributions Mineralogy Petrology 14 (3), 190–203. doi:10.1007/bf00376639
Baltscheffsky, H., von Stedingk, L.-V., Heldt, H.-W., and Klingenberg, M. (1966). Inorganic pyrophosphate: formation in bacterial photophosphorylation. Science 153 (3740), 1120–1122. doi:10.1126/science.153.3740.1120
Banin, A., Clark, B., and Wänke, H. (1992). “Surface chemistry and mineralogy,”in Mars, Los. Angel, January 1, 1992, (United States: University of Arizona Press), 594–625. doi:10.2307/j.ctt207g59v.22
Baziotis, I., Asimow, P., Ntaflos, T., Boyce, J., McCubbin, F., Koroneos, A., et al. (2017). Phosphorus zoning as a recorder of crystal growth kinetics: application to second-generation olivine in mantle xenoliths from the Cima Volcanic Field. Contributions Mineralogy Petrology 172, 58–32. doi:10.1007/s00410-017-1376-7
Bekker, T., Litasov, K., Shatskiy, A., Sagatov, N., Podborodnikov, I., and Krinitsin, P. (2021). Experimental and ab initio investigation of the formation of phosphoran olivine. ACS Earth Space Chem. 5 (6), 1373–1383. doi:10.1021/acsearthspacechem.1c00011
Boesenberg, J. S., and Hewins, R. H. (2010). An experimental investigation into the metastable formation of phosphoran olivine and pyroxene. Geochimica Cosmochimica Acta 74 (6), 1923–1941. doi:10.1016/j.gca.2009.12.008
Bowman, R., and Moir, J. (1993). Basic EDTA as an extractant for soil organic phosphorus. Soil Sci. Soc. Am. J. 57 (6), 1516–1518. doi:10.2136/sssaj1993.03615995005700060020x
Bridges, J., and Warren, P. (2006). The SNC meteorites: basaltic igneous processes on Mars. J. Geol. Soc. 163 (2), 229–251. doi:10.1144/0016-764904-501
Brunet, F., and Chazot, G. (2001). Partitioning of phosphorus between olivine, clinopyroxene and silicate glass in a spinel lherzolite xenolith from Yemen. Chem. Geol. 176 (1-4), 51–72. doi:10.1016/s0009-2541(00)00351-x
Buseck, P. R., and Clark, J. (1984). Zaisho—a pallasite containing pyroxene and phosphoran olivine. Mineral. Mag. 48 (347), 229–235. doi:10.1180/minmag.1984.048.347.06
Carr, M. H., Belton, M. J., Chapman, C. R., Davies, M. E., Geissler, P., Greenberg, R., et al. (1998). Evidence for a subsurface ocean on Europa. Nature 391 (6665), 363–365. doi:10.1038/34857
Cheng, C., Fan, C., Wan, R., Tong, C., Miao, Z., Chen, J., et al. (2002). Phosphorylation of adenosine with trimetaphosphate under simulated prebiotic conditions. Orig. Life Evol. Biosphere 32, 219–224. doi:10.1023/a:1016513114799
Ersoy, Ö., Nikogosian, I. K., van Bergen, M. J., and Mason, P. R. (2019). Phosphorous incorporation in olivine crystallized from potassium-rich magmas. Geochimica Cosmochimica Acta 253, 63–83. doi:10.1016/j.gca.2019.03.012
Feiss, P. G., Maybin, I. I. I. A. H., Riggs, S. R., and Grosz, A. E. (1991). Mineral Resources of the carolinas. Knoxville TN: The Geology of the Carolinas: Carolina Geological Society Fiftieth Anniversary, 319.
Feng, T., Omran, A., Gull, M., Schaible, M. J., Orlando, T. M., and Pasek, M. A. (2024). Schreibersite oxidation under varied oxygen buffers. Geochimica Cosmochimica Acta 380, 140–148. doi:10.1016/j.gca.2024.07.022
Fernández-García, C., Coggins, A. J., and Powner, M. W. (2017). A chemist’s perspective on the role of phosphorus at the origins of life. Life 7 (3), 31. doi:10.3390/life7030031
Fuhr, M., Geilert, S., Schmidt, M., Liebetrau, V., Vogt, C., Ledwig, B., et al. (2022). Kinetics of olivine weathering in seawater: an experimental study. Front. Clim. 4, 39. doi:10.3389/fclim.2022.831587
Garcia, M. O. (1996). Petrography and olivine and glass chemistry of lavas from the Hawaii Scientific Drilling Project. J. Geophys. Res. Solid Earth 101 (B5), 11701–11713. doi:10.1029/95jb03846
Gorring, M. L., and Naslund, H. (1995). Geochemical reversals within the lower 100 m of the Palisades sill, New Jersey. Contributions Mineralogy Petrology 119 (2-3), 263–276. doi:10.1007/bf00307286
Haas, S., Sinclair, K. P., and Catling, D. C. (2024). Biogeochemical explanations for the world’s most phosphate-rich lake, an origin-of-life analog. Commun. Earth & Environ. 5 (1), 28. doi:10.1038/s43247-023-01192-8
Hamilton, V. E., and Christensen, P. R. (2005). Evidence for extensive, olivine-rich bedrock on Mars. Geology 33 (6), 433–436. doi:10.1130/g21258.1
Hao, J., Glein, C. R., Huang, F., Yee, N., Catling, D. C., Postberg, F., et al. (2022). Abundant phosphorus expected for possible life in Enceladus’s ocean. Proc. Natl. Acad. Sci. 119 (39), e2201388119. doi:10.1073/pnas.2201388119
Hausrath, E., and Brantley, S. (2010). Basalt and olivine dissolution under cold, salty, and acidic conditions: what can we learn about recent aqueous weathering on Mars? J. Geophys. Res. Planets 115 (E12). doi:10.1029/2010je003610
Herschy, B., Chang, S. J., Blake, R., Lepland, A., Abbott-Lyon, H., Sampson, J., et al. (2018). Archean phosphorus liberation induced by iron redox geochemistry. Nat. Commun. 9 (1), 1346. doi:10.1038/s41467-018-03835-3
Hsu, H.-W., Postberg, F., Sekine, Y., Shibuya, T., Kempf, S., Horányi, M., et al. (2015). Ongoing hydrothermal activities within Enceladus. Nature 519 (7542), 207–210. doi:10.1038/nature14262
Jahnke, R. A. (1992). “14 the phosphorus cycle,” in International geophysics (Elsevier), 50, 301–315. doi:10.1016/s0074-6142(08)62697-2
Jan, M. Q., Khan, M. A., and Qazi, M. S. (1993). The Sapat mafic-ultramafic complex, Kohistan arc, north Pakistan. Geol. Soc. Lond. Spec. Publ. 74 (1), 113–121. doi:10.1144/gsl.sp.1993.074.01.09
Jusino-Maldonado, M., Rianço-Silva, R., Mondal, J. A., Pasek, M., Laneuville, M., and Cleaves, H. J. (2022). A global network model of abiotic phosphorus cycling on Earth through time. Sci. Rep. 12 (1), 9348. doi:10.1038/s41598-022-12994-9
Korsch, R., Kositcin, N., and Champion, D. (2011). Australian island arcs through time: geodynamic implications for the Archean and Proterozoic. Gondwana Res. 19 (3), 716–734. doi:10.1016/j.gr.2010.11.018
Lago, J. Revisiting Darwin’s little pond as a method to liberate phosphorus from apatite under prebiotic hadean earth conditions. (2021).
Langmuir, C. H., and Broecker, W. (2012). How to build a habitable planet: the story of earth from the big bang to humankind-revised and expanded edition. Princeton University Press.
Li, Y., Zhang, A.-C., Chen, J.-N., Gu, L.-X., and Wang, R.-C. (2017). Formation of phosphorus-rich olivine in Dar al Gani 978 carbonaceous chondrite through fluid-assisted metamorphism. Am. Mineralogist 102 (1), 98–107. doi:10.2138/am-2017-5881
Liu, Y., Tice, M., Schmidt, M., Treiman, A., Kizovski, T., Hurowitz, J., et al. (2022). An olivine cumulate outcrop on the floor of Jezero crater, Mars. Science 377 (6614), 1513–1519. doi:10.1126/science.abo2756
Lohrmann, R., and Orgel, L. (1968). Prebiotic synthesis: phosphorylation in aqueous solution. Science 161 (3836), 64–66. doi:10.1126/science.161.3836.64
Lynn, K. J., Garcia, M. O., and Shea, T. (2020). Phosphorus coupling obfuscates lithium Geospeedometry in olivine. Front. Earth Sci. 8, 525919. doi:10.3389/feart.2020.00135
Lynn, K. J., Shea, T., and Garcia, M. O. (2017). Nickel variability in Hawaiian olivine: evaluating the relative contributions from mantle and crustal processes. Am. Mineralogist 102 (3), 507–518. doi:10.2138/am-2017-5763
Maciá, E., Hernández, M., and Oró, J. (1997). Primary sources of phosphorus and phosphates in chemical evolution. Orig. Life Evol. Biosphere 27, 459–480. doi:10.1023/A:1006523226472
McKibbin, S. J., Pittarello, L., Makarona, C., Hamann, C., Hecht, L., Chernonozhkin, S. M., et al. (2019). Petrogenesis of main group pallasite meteorites based on relationships among texture, mineralogy, and geochemistry. Meteorit. & Planet. Sci. 54 (11), 2814–2844. doi:10.1111/maps.13392
McSween, H. Y., Wyatt, M. B., Gellert, R., Bell, I. I. I. J. F., Morris, R. V., Herkenhoff, K. E., et al. (2006). Characterization and petrologic interpretation of olivine rich basalts at Gusev Crater, Mars. J. Geophys. Res. Planets 111 (E2). doi:10.1029/2005je002477
Milman-Barris, M. S., Beckett, J. R., Baker, M. B., Hofmann, A. E., Morgan, Z., Crowley, M. R., et al. (2008). Zoning of phosphorus in igneous olivine. Contributions Mineralogy Petrology 155, 739–765. doi:10.1007/s00410-007-0268-7
Mustard, J., Poulet, F., Gendrin, A., Bibring, J.-P., Langevin, Y., Gondet, B., et al. (2005). Olivine and pyroxene diversity in the crust of Mars. Science 307 (5715), 1594–1597. doi:10.1126/science.1109098
Mysen, B. (1996). Phosphorus speciation changes across the glass transition in highly polymerized alkali silicate glasses and melts. Am. Mineralogist 81 (11-12), 1531–1534. doi:10.2138/am-1996-11-1226
Mysen, B. O. (2011). Amorphous Materials: an experimental study of phosphorous and aluminosilicate speciation in and partitioning between aqueous fluids and silicate melts determined in-situ at high temperature and pressure. Am. Mineralogist 96 (10), 1636–1649. doi:10.2138/am.2011.3728
Olsen, A. A., Hausrath, E. M., and Rimstidt, J. D. (2015). Forsterite dissolution rates in Mg sulfate rich Mars analog brines and implications of the aqueous history of Mars. J. Geophys. Res. Planets 120 (3), 388–400. doi:10.1002/2014je004664
Osland, R. (1997). Modelling of variations in Norwegian olivine deposits. Doktor Ingeniør Thesis. Trondheim, Norway: Norwegian Univ of Science and Technology.
Pappalardo, R. T., Belton, M. J., Breneman, H., Carr, M., Chapman, C. R., Collins, G., et al. (1999). Does Europa have a subsurface ocean? Evaluation of the geological evidence. J. Geophys. Res. Planets. 104 (E10), 24015–24055. doi:10.1029/1998je000628
Pasek, M., and Block, K. (2009). Lightning-induced reduction of phosphorus oxidation state. Nat. Geosci. 2 (8), 553–556. doi:10.1038/ngeo580
Pasek, M. A. (2008). Rethinking early Earth phosphorus geochemistry. Proc. Natl. Acad. Sci. 105 (3), 853–858. doi:10.1073/pnas.0708205105
Pasek, M. A., Dworkin, J. P., and Lauretta, D. S. (2007). A radical pathway for organic phosphorylation during schreibersite corrosion with implications for the origin of life. Geochimica Cosmochimica Acta 71 (7), 1721–1736. doi:10.1016/j.gca.2006.12.018
Pasek, M. A., Omran, A., Feng, T., Gull, M., Lang, C., Abbatiello, J., et al. (2022). Serpentinization as a route to liberating phosphorus on habitable worlds. Geochimica Cosmochimica Acta 336, 332–340. doi:10.1016/j.gca.2022.09.027
Paytan, A., and McLaughlin, K. (2007). The oceanic phosphorus cycle. Chem. Rev. 107 (2), 563–576. doi:10.1021/cr0503613
Pokrovsky, O. S., and Schott, J. (2000). Kinetics and mechanism of forsterite dissolution at 25°C and pH from 1 to 12. Geochimica Cosmochimica Acta 64 (19), 3313–3325. doi:10.1016/s0016-7037(00)00434-8
Postberg, F., Sekine, Y., Klenner, F., Glein, C. R., Zou, Z., Abel, B., et al. (2023). Detection of phosphates originating from Enceladus’s ocean. Nature 618 (7965), 489–493. doi:10.1038/s41586-023-05987-9
Reimann, R., and Zubay, G. (1999). Nucleoside phosphorylation: a feasible step in the prebiotic pathway to RNA. Orig. Life Evol. Biosphere 29, 229–247. doi:10.1023/a:1006580009791
Renna, M. R., and Tribuzio, R. (2011). Olivine-rich troctolites from Ligurian ophiolites (Italy): evidence for impregnation of replacive mantle conduits by MORB-type melts. J. Petrology 52 (9), 1763–1790. doi:10.1093/petrology/egr029
J. Roberge, H. Delgado-Granados, P. Wallace, and A. Kent (2007). Pre-eruptive volatile contents of mafic magma at Popocatepetl volcano, Mexico, from olivine-hosted melt inclusions (San Francisco, California: AGU Fall Meeting Abstracts).
Roberts, J. H., and Nimmo, F. (2008). Tidal heating and the long-term stability of a subsurface ocean on Enceladus. Icarus 194 (2), 675–689. doi:10.1016/j.icarus.2007.11.010
Schoffstall, A. M. (1976). Prebiotic phosphorylation of nucleosides in formamide. Orig. life 7 (4), 399–412. doi:10.1007/bf00927935
Schubert, G., Sohl, F., and Hussmann, H. (2009). “Interior of europa,” in Europa, Editors R. T. Pappalardo, W. B. McKinnon, and K. Khurana (Tucson, AZ: University of Arizona press), 353–367.
Segami, S., Asaoka, M., Kinoshita, S., Fukuda, M., Nakanishi, Y., and Maeshima, M. (2018). Biochemical, structural and physiological characteristics of vacuolar H+-pyrophosphatase. Plant Cell Physiology 59 (7), 1300–1308. doi:10.1093/pcp/pcy054
Shea, T., Hammer, J. E., Hellebrand, E., Mourey, A. J., Costa, F., First, E. C., et al. (2019). Phosphorus and aluminum zoning in olivine: contrasting behavior of two nominally incompatible trace elements. Contributions Mineralogy Petrology 174, 85–24. doi:10.1007/s00410-019-1618-y
Shea, T., Lynn, K. J., and Garcia, M. O. (2015). Cracking the olivine zoning code: distinguishing between crystal growth and diffusion. Geology 43 (10), 935–938. doi:10.1130/g37082.1
Thomas, P., Tajeddine, R., Tiscareno, M., Burns, J., Joseph, J., Loredo, T., et al. (2016). Enceladus’s measured physical libration requires a global subsurface ocean. Icarus 264, 37–47. doi:10.1016/j.icarus.2015.08.037
Trommsdorff, V., Sánchez-Vizcaíno, V. L., Gómez-Pugnaire, M., and Müntener, O. (1998). High pressure breakdown of antigorite to spinifex-textured olivine and orthopyroxene, SE Spain. Contributions Mineralogy Petrology 132, 139–148. doi:10.1007/s004100050412
Turner, B. L., Mahieu, N., and Condron, L. M. (2003). The phosphorus composition of temperate pasture soils determined by NaOH–EDTA extraction and solution 31P NMR spectroscopy. Org. Geochem. 34 (8), 1199–1210. doi:10.1016/s0146-6380(03)00061-5
Tyrrell, T. (1999). The relative influences of nitrogen and phosphorus on oceanic primary production. Nature 400 (6744), 525–531. doi:10.1038/22941
Walton, C. R., Baziotis, I., Cernok, A., Ferrière, L., Asimow, P. D., Shorttle, O., et al. (2021b). Microtextures in the Chelyabinsk impact breccia reveal the history of Phosphorus Olivine Assemblages in chondrites. Meteorit. & Planet. Sci. 56 (4), 742–766. doi:10.1111/maps.13648
Walton, C. R., Shorttle, O., Jenner, F. E., Williams, H. M., Golden, J., Morrison, S. M., et al. (2021a). Phosphorus mineral evolution and prebiotic chemistry: from minerals to microbes. Earth-Science Rev. 221, 103806. doi:10.1016/j.earscirev.2021.103806
Welsch, B., Faure, F., Famin, V., Baronnet, A., and Bachèlery, P. (2013). Dendritic crystallization: a single process for all the textures of olivine in basalts? J. Petrology 54 (3), 539–574. doi:10.1093/petrology/egs077
Welsch, B., Hammer, J., and Hellebrand, E. (2014). Phosphorus zoning reveals dendritic architecture of olivine. Geology 42 (10), 867–870. doi:10.1130/g35691.1
Westall, F., Hickman-Lewis, K., Hinman, N., Gautret, P., Campbell, K. A., Bréhéret, J.-G., et al. (2018). A hydrothermal-sedimentary context for the origin of life. Astrobiology 18 (3), 259–293. doi:10.1089/ast.2017.1680
Keywords: phosphorus, astrobiology, habitability, olivine, dissolution
Citation: Abbatiello J, Henson JE and Pasek M (2024) Phosphorus availability from olivine for biospheres. Front. Astron. Space Sci. 11:1441187. doi: 10.3389/fspas.2024.1441187
Received: 30 May 2024; Accepted: 26 August 2024;
Published: 16 September 2024.
Edited by:
David Benoit, University of Hull, United KingdomReviewed by:
Nathan Oborny, NASA Jet Propulsion Laboratory (JPL), United StatesMaría Colín-García, National Autonomous University of Mexico, Mexico
Copyright © 2024 Abbatiello, Henson and Pasek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthew Pasek, bXBhc2VrQHVzZi5lZHU=
 Josh Abbatiello
Josh Abbatiello John E. Henson1
John E. Henson1 Matthew Pasek
Matthew Pasek