
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Allergy, 03 April 2025
Sec. Skin Allergy
Volume 6 - 2025 | https://doi.org/10.3389/falgy.2025.1569292
This article is part of the Research TopicBiomarkers in Allergic EczemaView all 7 articles
Allergic Eczema (AE) is a chronic, relapsing skin condition that significantly affects the quality of life of the AE patients and their caretakers. Decades of scientific and clinical research has helped understand the highly complex underpinnings of AE presentation wherein a multitude of variables, including the conspicuous variables such as environmental allergens, immunological triggers, genetic predisposition of individuals, to more nuanced socio-economic status, play an important part. Given the complexity of the disease, it is imperative to develop biomarkers enabling early and reliable clinical identifications and help in the active management of the disease, thereby minimizing the impact and burden of the disease on the patients. In this mini review, we provide a brief overview of AE, affected demographics, variables that trigger its onset, and summarize the discovery of various clinical biomarkers such as total and specific serum IgE levels, Th2 cytokine levels, filaggrin (FLG) mutations, periostin levels in skin, etc. that have been developed over the years to further improve the state of clinical monitoring of AE presentation and progression. Lastly, we also provide an overview of the clinical interventions and therapies, such as topical agents, phototherapy, and biologics, that are available to the patients to manage AE-related complications. While we have vastly improved the standard of care and diagnosis for the AE patients, there are still many unmet needs such as developing non-invasive, effective, and reliable clinical predictors and biomarkers which can usher better personalized treatments and provide a better quality of life to affected demographics.
Eczema is a cluster of skin conditions often characterized by inflammation, dry skin, rashes, scaly patches, and itchiness (1). Eczema affects people of all skin tones and types. In Individuals with a lighter skin tone, manifestation typically occurs in the form of erythema and skin inflammation. Eczema patients who are people of color experience brown, purple, ashen, or a gray skin tone. Though eczema affects people of all races and ethnicities, some groups are more likely to be affected (2, 3).
Around 31.6 million people, or 10% of the total population of the United States of America, suffer from some type or form of eczema. Within this population, the Asian and Pacific Islanders and Native Americans are the most affected demographics and tend to have the highest likelihood to develop eczema at 13% incidence rate, closely followed by the white population at 11%, and the black population with 10% (4).
Allergic eczema (AE) refers to a specific form of eczema which is often associated with pruritis and other IgE associated disorders such as food allergies, asthma, etc. The highest prevalence of eczema is during early childhood, with a typical onset prior to 5 years old which resolves by adulthood; however, it can re-appear later in life or continue throughout adulthood (5). Besides biological age, the gender of the person also has a bearing on the eczema incidence rates. Women have a higher incidence of eczema compared to men (8.9% and 5.7, respectively) (6). The gender discrepancy in eczema manifestation that leads to a higher incidence of allergic eczema (AE) in women is often attributed to menopause related changes. During menopause, the estrogen levels decline leading to changes in the skin which make it more prone to eczema manifestation (7).
AE pathophysiology is highly complex but is thought to primarily develop from a combination of genetic (8), immunological (9), and environmental (10) variables. Food hypersensitivity may also cause or exacerbate atopic dermatitis in 10% to 30% of patients; the majority being caused by sensitivities to egg, milk, peanuts, soy, and wheat (11, 12).
AE is one of the most common chronic, inflammatory diseases afflicting 11.3%–12.7% of children and 6.9%–7.6% adults in the USA (13)—with pruritus or skin barrier defects observed as the most common symptom in the patients (14). In AE, it is common to observe an immunological response imbalance, which usually results in an epidermal barrier defect, IgE mediated hypersensitivity, and other related conditions (15, 16).
Over the years, numerous scientific studies have explored the interplay of genetics and immune responses in driving AE development and flare-ups. Some of these studies looked at (i) atopic march—the early life presentation of the atopic disease and its progression with time (17), (ii) studies linking increased levels of total serum IgE and IgEs specific for environmental and food allergens (18), (iii) studies linking the incidence of AE with the loss of function mutations in the gene coding filaggrin (FLG) (19)—a filament-aggregating protein in skin that is responsible for binding the keratin fibers and maintaining the integrity of the skin and its barrier function. A common highlight shared by these studies was the role of skin as a peripheral lymphoid organ (20), and the importance of skin integrity in determining the severity of AE onset and progression (21, 22).
These immunologic and genetic studies have led to better patient stratification via the identification of other related traits or sub-phenotypes of AE (23, 24) allowing for better standard of care and clinical identifications. While the complete understanding of the pathophysiology of AE still eludes us, the high degree of coincidence of skin barrier dysfunction and immune dysregulation observed in clinical studies suggest their potential role in the etiology of disease progression in AE patients.
Reviews by Umehara et al. (25) and Sroka-Tomaszewska et al. (9) provide a comprehensive background on the complexities of AE, its possible causes and immunological underpinnings behind some of the commonly observed symptoms.
AE significantly affects the quality of life of both the patient and their caretakers making disease management difficult without proper planning and support. An overview of the impact and multi-faceted effects of AE disease, on patients and their caretakers alike, is provided in Figure 1.
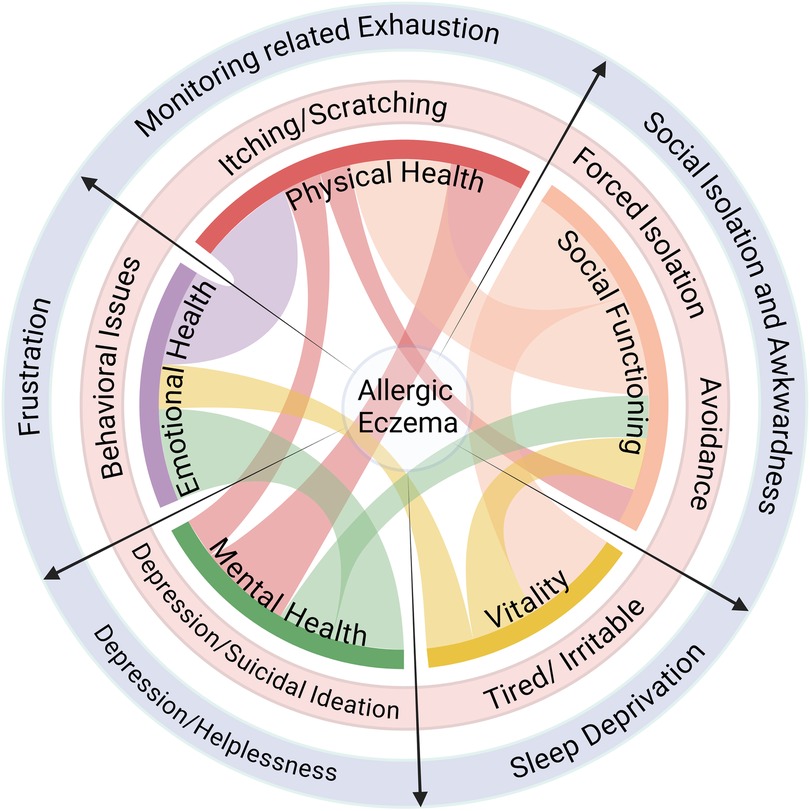
Figure 1. Impact of AE on quality of life. AE affects multiple facets of life and compromises the holistic well-being of the patient (light red circle) as well as their caretakers (light purple circle). Created with Biorender.com.
Insomnia is frequently associated with AE (26, 27), which often leads to increased itching urges at night further disrupting sleep. Melatonin production is reduced by the lack of sleep which inhibits the body's ability to decrease temperature, resulting in the increased skin temperature during sleep, causing itching episodes (28). Irregular sleep habits can cause systemic imbalances, exhaustion, and impact the overall quality of life of the patients and their caretakers (27).
AE clinical characteristics change depending on age and disease duration. The characteristics in childhood centers on eczematous changes that are accompanied by serous papules which often cause a strong urge to itch, and upon scratching, may lead to excoriation and development of new papules (29).
When AE progresses from childhood to adulthood, the clinical presentation changes and the Skin lesions transition to a more varied phenotype compared to those in childhood, a condition referred to as Besnier's prurigo or disseminated neurodermatitis (30). Mental nervousness, continuous itch prior to abnormal skin, distribution of skin lesions, dryness of the skin, visible papillae of hypertrophy of skin pigment, and an apparent circumscribed plaque that appears in the same site as the original itch are all characteristics of a chronic condition of disseminated neurodermatitis (1, 29–33).
The predominant criteria used for AE diagnosis in clinical setting remains the Hanifin-Rajka criteria (23)—first introduced in 1980, and included almost 30 signs, symptoms, and laboratory abnormalities (34), of which 3 out of 4 major criteria and 3 out of 23 minor criteria need to be met for a conclusive diagnosis. Given challenges around the difficult interpretation of the Hanifin-Rajka criteria outside a clinical setting, the United Kingdom (UK) Working Party Criteria was introduced in 1994 (35, 36). This simplified and condensed the Hanifin-Rajka criteria for a broader application to a range of ethnic groups, with pruritus being elevated to be the sole mandatory criteria in addition to coincidence of 3 or more of other 5 major criteria. A further distillation of diagnosis criteria was observed in the millennium criteria, introduced in 1998 by Bos et al. (37), and was the first to include the presence of allergen-specific IgE as a diagnostic biomarker.
Later, in 2011 Schram et al. elaborated on the millennium criteria and proposed meeting ≥5 of the criteria outlined in the original millennium criteria (35). A head-to-head assessment of the modified millennium criteria with the established UK working party criteria and Hanifin-Rajka criteria, over a cohort of 201 patients and observed that the modified minimum criteria showed a sensitivity of 81.8% with a specificity of 98.8%, compared to 100% and 48.8% for the Hanifin-Rajka criteria and 97.7% and 72.9% for the UK working party criteria (35, 38). In 2014, the American Academy of Dermatology laid out the guidelines for AE diagnosis and assessments which emphasized the role of hallmark features of AE opposed to features that are non-specific while excluding the conditions that mimic AE, such as scabies, seborrheic dermatitis, psoriasis, etc. (23).
To determine the severity of the disease in the clinic, the following two tests are commonly used and have been validated: Scoring Atopic Dermatitis (SCORAD) index, and the Eczema Area severity index (EASI) (39, 40). These tests are typically used to measure the extent of erythema, edema, papulation, excoriation, and the degree of lichenification. Additionally, other factors, such as pruritus and loss of sleep, are incorporated into the diagnosis and severity assessment (22, 26, 27).
Many of the current clinical practices for diagnosing cases of AE rely on previous clinical learnings and established biomarker tracking in order properly identify and stratify the patient and recommend therapeutic interventions accordingly.
Identification and characterization of appropriate biomarkers allows for a precise understanding of multi-factorial disease such as AE and its complex underpinnings. This understanding is crucial for enabling tailored therapeutic approaches for individual patient profiles. The utility of such biomarkers ranges from the assessment of disease severity to predicting flares and guiding treatment decisions, which in turn aids in improvement of patient outcomes overall.
Monitoring critical biomarkers can also help in tracking the therapeutic responses of the treatment and allows for a more involved and dynamic approach in clinics, where timely modifications in treatment plan can ensure rapid alleviation of the disease condition.
The last few decades of intense research around AE and its underpinnings have allowed clinicians to come up with several biomarkers that are used in clinics to track the disease state. Some of these commonly used biomarkers have been summarized in Table 1.
In the past, significant progress was made in identifying and understanding various biomarkers associated with allergic eczema. Key studies highlighted the roles of immunological factors, genetic predispositions, cutaneous protein levels, and cytokine profiles in the disease's pathogenesis and management (21, 24, 32, 41). However, further validation and exploration of these biomarkers are needed to enhance their clinical applicability and improve patient outcomes.
Despite the identification of several potential biomarkers, significant limitations persist including issues with specificity, sensitivity, and inconsistency of findings across diverse populations. Many biomarkers were not widely validated for routine clinical use, emphasizing the need for further research into more reliable and clinically applicable biomarker candidates.
Research in the past decade has significantly advanced the understanding of biomarkers in AE. Key findings include the identification of genetic (e.g., FLG mutations), immune (e.g., Th2 cytokines), and skin barrier-related biomarkers (e.g., filaggrin, periostin) that are pivotal for diagnosing, monitoring, and personalizing treatment for AE. Additionally, the role of the microbiome, serum biomarkers, and exosomes is gaining attention as potential tools for precision medicine in AE. This ongoing research is improving both the management of the disease and the development of targeted therapies, ultimately aiming for more effective and individualized treatment strategies.
Traditional therapy for AE could be split into two types of treatments: topical or systemic. Topical agents include moisturizers, corticosteroids, antimicrobials, wet wrap therapy, and calcineurin inhibitors (1, 23, 33, 42, 43). Systemic treatments are used for more severe forms of AE. These treatments consist of systemic corticosteroids, cyclosporine, azathioprine, phototherapy, and more (21, 42, 44–46).
Use of topical emollients (lotions, creams, hydrating gels, sprays, and ointments) and anti-inflammatory agents (antihistamines, phosphodiesterase inhibitors, calcineurin inhibitors) can achieve short term control of acute symptoms and clinical improvement in mild allergic eczema and dermatitis skin diseases. Topical agents are more effective in controlling mild disease conditions as opposed to moderate-to-severe stage of AE (42). Topical corticosteroids (TCS) suppress the release of proinflammatory cytokines and inhibit antigen processing (47, 48). TCS is efficient as a maintenance therapy to help reduce the number of relapses. TCS is often clinically used along with moisturizers as the topical agent in wet wrap therapy (WWT) which is an efficient method to reduce and manage severe dermal flares.
Both traditional and prescription moisturizers have shown to decrease the symptoms of AE. They soften the skin and reduce the evaporation of water which prevents skin from drying out. These therapeutic interventions are the primary treatment for mild forms of AE but are generally included in the regimen for moderate-to-severe forms, due to the reduction of inflammation and alleviation of physical discomfort.
Systemic corticosteroids are generally reserved as a short-term bridging therapy for acute and severe flare-ups, as they can have both short- and long-term adverse effects. A systematic corticosteroid treatment consists of an oral corticosteroid with or without an additional immune suppressant such as cyclosporine, methotrexate, azathioprine, and mycophenolate mofetil (44). Three distinct clinical trials performed over varied ages and gender concluded that short-courses of systemic corticosteroid interventions could provide relief to patients with severe flare-ups or while awaiting response to other therapeutic interventions (49–51).
Phototherapy as a therapeutic intervention was first introduced in 1925 in the Mayo Clinic by William Goeckerman for treatment of psoriasis (52, 53) and falls under the umbrella of systemic therapeutic options available for patients. Phototherapy treatment decreases cutaneous inflammation and is beneficial for moderate to severe AE, with limited systemic side effects (54, 55) compared to the alternatives and is suitable for patients of all age demographics.
Phototherapy, while very effective for some patients, has its limitations such as inconvenience and potential adverse effects. An inconvenience includes the access to in-office treatments, a 3-times per week regimen for patients, which can be difficult to maintain. Rare potential adverse effects include increased risk of skin cancer and flare-ups from the excessive heat (45, 56, 57). Some studies have shown that narrowband UVB can be considered an efficient treatment with patients suffering from chronic AE exhibiting at least a 50% reduction in SCORAD index scores with the phototherapy. The disease activity reversal was associated with the elimination of the inflammatory leukocytes (58).
Learnings from the clinical studies and research have driven the introduction of novel drug delivery systems and therapeutics that address the AE-related symptoms, which were previously without a cure or as effective. While these therapies do provide a promising outlook for the patients of AE, the data on their efficacy and applicability for various demographics is limited for some. A detailed review by Waligóra-Dziwak et al. provides an excellent primer on various novel biologics currently in Phase III and Phase IV clinical trials (59).
Currently, topical application of creams or ointments are the predominant vehicle for delivery of drugs for AE. Novel delivery approaches such as electrospun patches (60, 61), sprays (62), liposomes (63), nanoparticles (64), and lasers have recently been developed to enhance transdermal delivery with a focus on increasing treatment adherence while minimizing the side effects.
New biologics and small-molecule inhibitors are being developed against key molecules for addressing the full spectrum of AE disease manifestations. Novel topical drugs that are currently approved or in late-stage clinical trials include, but are not limited to, Janus family protein kinases (JAK) inhibitor (ruxolitinib) (65), phosphodiesterase-4 inhibitors (crisaborole) (66, 67), and roflumilast (68).
Monoclonal antibodies that target specifically offer a focused and highly efficacious therapeutic option. mAb Tx against cytokine receptor IL-4Rα (e.g dupilumab, CM310, and CBP201) have also been developed to address unmet AE-related conditions. Th2 cytokines, IL-4 and IL-13, have a significant impact on the role in pathogenesis of allergic eczema (69–71). The IL-4Rα binds the IL-4 and IL-13 cytokines via the formation of type I and type II receptors, respectively. This binding event leads to the activation of the JAK inhibitors and its downstream counterpart—signal transducer and activator of transcription (STAT)—that acts as an activator of transcription leading to downstream signaling pathways associated with IL-14 and IL-13 (72).
JAK inhibitors can block multiple cytokine-signaling pathways and is thus the preferred target when aiming for broad immunomodulation (73). Historically, topical JAK inhibitors have exhibited fewer adverse events compared to oral JAK inhibitors (69); however, the US Food and Drug Administration (FDA) recently placed a black box warning on this class of medications due to safety concerns based on data from studies investigating tofacitinib in patients with rheumatoid arthritis (74). This news along with the inadequate guidance on communicating the merits and drawbacks of JAK inhibitors has been a source of hesitation for the dermatologists in recommending this treatment modality (75).
Eczema cases have seen a steady rise in the past decades with about 10% of the total population of the United States exhibiting some form of eczema. Incidences of AE have been closely linked to genetic and environmental factors leading to a loss of proper regulation of the housekeeping immunological functions, which results in eventual compromise in the dermal integrity and presentation of AE symptoms. Most of the AE patients experience a chronic, relapsing disease course with hallmark remissions and flare-ups. Clinical presentation of AE can be varied and nuanced, and early and accurate clinical diagnosis is imperative in ensuring an appropriate standard of care is provided to the patient. To this end, several biomarkers have historically been identified which have had a wide range of successes and clinical applications. While we have come a long way in terms of the biomarker reliability and effectiveness, there is still a huge scope of improvement in their performance and applicability across the multiple facets of AE. Recent advancements in therapeutic and palliative interventions have allowed patients to manage flare-ups and minimize the impact of AE on their quality of life. These interventions range from (i) topical emollients to moisturize and soothe the affected skin areas, (ii) phototherapy to decrease cutaneous inflammation with very limited side effects, and (iii) biologic modalities, such as JAK inhibitors and antibody-based therapeutics, which directly address the cytokine signaling pathways thereby putting the brakes on the progressive inflammatory immune responses.
Developing a better understanding of the underpinnings of AE pathogenesis will allow for the development of novel treatment options with breakthrough potential, personalized treatment plans, and recovery strategies, all of which will help address the unmet needs of a huge patient demographic and allow them and their caretakers to live a more enjoyable and holistic life.
JL: Writing – original draft. BB: Writing – review & editing. PB: Conceptualization, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
We want to thank Dr. Amitava Das for his input on the manuscript and in the ideation phase. The authors also thank numerous colleagues in Biotherapeutics Discovery and Development DMPK groups at Boehringer Ingelheim Pharmaceuticals Inc. for excellent discussions and support.
JL, BB and PB are employees of Boehringer Ingelheim Pharmaceuticals Inc.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AE, allergic eczema; FLG, filaggrin; IgE, immunoglobulin E; TCS, topical corticosteroids; WWT, wet wrap therapy; JAK, Janus Kinase; STAT, signal transducer and activator of transcription; TARC, thymus and activation regulated chemokine; MEL, monochromatic excimer light; BBUVB, broadband ultraviolet B; NBUVB, narrowband ultraviolet B; ECP, eosinophil cationic protein; TEWL, trans-epidermal water loss; Th2, T-helper 2; mAb-TX, monoclonal antibody based therapeutics.
1. Eyerich K, Ring J. Atopic Dermatitis - Eczema, Clinics, Pathophysiology and Therapy. 2nd ed. Cham: Springer (2023). p. 35–80. doi: 10.1007/978-3-031-12499-0
2. Davis CM, Flohr C, Gupta MR, Koplin JJ. Managing atopic dermatitis in patients with skin of color. J Allergy Clin Immunol Pract. (2023) 11(5):1376–83. doi: 10.1016/j.jaip.2023.03.041
3. Mahajan VK, Handa S. Atlas of dermatology. In: Smoller BR, Mishra CS, editors. Dermatopathology and Venereology, Cutaneous Anatomy, Biology and Inherited Disorders and General Dermatologic Concepts. Cham: Springer Nature Switzerland AG (2021). p. 45–107.
4. Hanifin JM, Reed ML. A population-based survey of eczema prevalence in the United States. DERM. (2007) 18(2):82–91. doi: 10.2310/6620.2007.06034
5. Chan AR, Sandhu VK, Drucker AM, Fleming P, Lynde CW. Adult-onset atopic dermatitis: presentations and progress. J Cutan Med Surg. (2020) 24(3):267–72. doi: 10.1177/1203475420911896
6. Ng A, Boersma P. Diagnosed Allergic Conditions in Adults: United States, 2021. United States: CDC.Gov (2023).
7. Kanda N, Hoashi T, Saeki H. The roles of sex hormones in the course of atopic dermatitis. Int J Mol Sci. (2019) 20(19):4660. doi: 10.3390/ijms20194660
8. O’Regan GM, Irvine AD. The role of filaggrin in the atopic diathesis. Clin Exp Allergy. (2010) 40(7):965–72. doi: 10.1111/j.1365-2222.2010.03522.x
9. Sroka-Tomaszewska J, Trzeciak M. Molecular mechanisms of atopic dermatitis pathogenesis. Int J Mol Sci. (2021) 22(8):4130. doi: 10.3390/ijms22084130
10. Luschkova D, Zeiser K, Ludwig A, Traidl-Hoffmann C. Atopic eczema is an environmental disease. Allergol Sel. (2021) 5(01):244–50. doi: 10.5414/ALX02258E
11. Oliveira ADT, Sodré CS, de Ferreira DC, de Abad ED, Saintive S, Ribeiro M, et al. Oral aspects identified in atopic dermatitis patients: a literature review. Open Dent J. (2018) 12(1):424–34. doi: 10.2174/1874210601812010424
12. Berg AK, Nørgaard K, Thyssen JP, Zachariae C, Hommel E, Rytter K, et al. Skin problems associated with insulin pumps and sensors in adults with type 1 diabetes: a cross-sectional study. Diabetes Technol Ther. (2018) 20(7):475–82. doi: 10.1089/dia.2018.0088
13. Premkumar M, Kalarani IB, Mohammed V, Veerabathiran R. An extensive review of vitiligo-associated conditions. Int J Dermatol Venereol. (2024) 7(1):44–51. doi: 10.1097/JD9.0000000000000346
14. Rosso JQD. Clinical cases in atopic dermatitis. In: Brownstone N, Liao W, Bhutani T, editors. Clinical Cases in Dermatology. 1st ed. Cham: Springer (2024). p. 183–96. doi: 10.1007/978-3-031-52147-8
15. Makowska K, Nowaczyk J, Blicharz L, Waśkiel-Burnat A, Czuwara J, Olszewska M, et al. Immunopathogenesis of atopic dermatitis: focus on interleukins as disease drivers and therapeutic targets for novel treatments. Int J Mol Sci. (2023) 24(1):781. doi: 10.3390/ijms24010781
16. Boothe WD, Tarbox JA, Tarbox MB. Management of atopic dermatitis, methods and challenges. Adv Exp Med Biol. (2017) 1027:21–37. doi: 10.1007/978-3-319-64804-0_3
17. Heimall J, Spergel JM. Filaggrin mutations and atopy: consequences for future therapeutics. Expert Rev Clin Immunol. (2012) 8(2):189–97. doi: 10.1586/eci.11.100
18. Tokura Y. Extrinsic and intrinsic types of atopic dermatitis. J Dermatol Sci. (2010) 58(1):1–7. doi: 10.1016/j.jdermsci.2010.02.008
19. Palmer CNA, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. (2006) 38(4):441–6. doi: 10.1038/ng1767
20. Egawa G, Kabashima K. Skin as a peripheral lymphoid organ: revisiting the concept of skin-associated lymphoid tissues. J Investig Dermatol. (2011) 131(11):2178–85. doi: 10.1038/jid.2011.198
21. Renert-Yuval Y, Thyssen JP, Bissonnette R, Bieber T, Kabashima K, Hijnen D, et al. Biomarkers in atopic dermatitis—a review on behalf of the International Eczema Council. J Allergy Clin Immunol. (2021) 147(4):1174–90.e1. doi: 10.1016/j.jaci.2021.01.013
22. Mocanu M, Vâță D, Alexa AI, Trandafir L, Patrașcu AI, Hâncu MF, et al. Atopic dermatitis—beyond the skin. Diagnostics. (2021) 11(9):1553. doi: 10.3390/diagnostics11091553
23. Eichenfield LF, Tom WL, Chamlin SL, Feldman SR, Hanifin JM, Simpson EL, et al. Guidelines of care for the management of atopic dermatitis section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. (2014) 70(2):338–51. doi: 10.1016/j.jaad.2013.10.010
24. Otsuka A, Nomura T, Rerknimitr P, Seidel JA, Honda T, Kabashima K. The interplay between genetic and environmental factors in the pathogenesis of atopic dermatitis. Immunol Rev. (2017) 278(1):246–62. doi: 10.1111/imr.12545
25. Umehara Y, Kiatsurayanon C, Trujillo-Paez JV, Chieosilapatham P, Peng G, Yue H, et al. Intractable itch in atopic dermatitis: causes and treatments. Biomedicines. (2021) 9(3):229. doi: 10.3390/biomedicines9030229
26. Lee J, Suh H, Jung H, Park M, Ahn J. Association between chronic pruritus, depression, and insomnia: a cross-sectional study. JAAD Int. (2021) 3:54–60. doi: 10.1016/j.jdin.2021.02.004
27. Khosravi A, Glińska J, Barańska-Rybak W. Sleep efficiency and neurocognitive decline in atopic dermatitis: a systematic review. Acta Derm-Venereol. (2024) 104:40459. doi: 10.2340/actadv.v104.40459
28. Erdem Y, Altunay İK, Özkur E, Şekerlisoy G, Karabay EA, Özdemir FT, et al. The association between melatonin levels and sleep quality in patients with pruritus: a potential biomarker on a candidate future treatment. Indian J Dermatol. (2021) 66(6):609–15. doi: 10.4103/ijd.ijd_31_21
29. Murota H, Katayama I. Exacerbating factors of itch in atopic dermatitis. Allergol Int. (2017) 66(1):8–13. doi: 10.1016/j.alit.2016.10.005
30. Lorenzini D, Lorenzini FK, Muller KR, Sanvido SD. Dermatology in public health environments. In: Bonamigo RR, editor. A Comprehensive Textbook. 2nd ed. Cham: Springer (2023). p. 1637–50. doi: 10.1007/978-3-031-13505-7
31. Zeiser K, Hammel G, Kirchberger I, Traidl-Hoffmann C. Social and psychosocial effects on atopic eczema symptom severity—a scoping review of observational studies published from 1989 to 2019. J Eur Acad Dermatol Venereol. (2021) 35(4):835–43. doi: 10.1111/jdv.16950
32. Ogulur I, Pat Y, Ardicli O, Barletta E, Cevhertas L, Fernandez-Santamaria R, et al. Advances and highlights in biomarkers of allergic diseases. Allergy. (2021) 76(12):3659–86. doi: 10.1111/all.15089
33. Leung DYM, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Investig. (2004) 113(5):651–7. doi: 10.1172/JCI21060
34. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm-Venereol. (1980) 60:44–7. doi: 10.2340/00015555924447
35. Schram ME, Leeflang MMG, Ottolander JPSD, Spuls PI, Bos JD. Validation and refinement of the millennium criteria for atopic dermatitis. J Dermatol. (2011) 38(9):850–8. doi: 10.1111/j.1346-8138.2011.01202.x
36. Williams HC, Jburney PG, Pembroke AC, Hay RJ, Party ADDCW. The U.K. working party’s diagnostic criteria for atopic dermatitis. III. Independent hospital validation. Br J Dermatol. (1994) 131(3):406–16. doi: 10.1111/j.1365-2133.1994.tb08532.x
37. Bos JD, van Leent EJM, Smitt JHS. The millennium criteria for the diagnosis of atopic dermatitis. Exp Dermatol. (1998) 7(4):132–8. doi: 10.1111/j.1600-0625.1998.tb00313.x
38. Reynolds M, Gorelick J, Bruno M. Atopic dermatitis: a review of current diagnostic criteria and a proposed update to management. J Drugs Dermatol. (2020) 19(3):244–8. doi: 10.36849/JDD.2020.4737
39. Jacobson ME, Morimoto RY, Leshem YA, Howells L, Williams HC, Grinich E, et al. The eczema area and severity Index: an update of progress and challenges in its measurement of atopic dermatitis after 20 years of use. J Eur Acad Dermatol Venereol. (2025) 39(1):70–85. doi: 10.1111/jdv.20248
40. Silverberg JI, Lei D, Yousaf M, Janmohamed SR, Vakharia PP, Chopra R, et al. What are the best endpoints for eczema area and severity index and scoring atopic dermatitis in clinical practice? A prospective observational study*. Br J Dermatol. (2021) 184(5):888–95. doi: 10.1111/bjd.19457
41. Yu L, Li L. Potential biomarkers of atopic dermatitis. Front Med. (2022) 9:1028694. doi: 10.3389/fmed.2022.1028694
42. Rodenbeck DL, Silverberg JI, Silverberg NB. Phototherapy for atopic dermatitis. Clin Dermatol. (2016) 34(5):607–13. doi: 10.1016/j.clindermatol.2016.05.011
43. Lee JH, Son SW, Cho SH. A comprehensive review of the treatment of atopic eczema. Allergy Asthma Immunol Res. (2015) 8(3):181–90. doi: 10.4168/aair.2016.8.3.181
44. Calabrese G, Licata G, Gambardella A, Rosa AD, Alfano R, Argenziano G. Topical and conventional systemic treatments in atopic dermatitis: have they gone out of fashion? Dermatol Pract Concept. (2022) 12(1):e2022155. doi: 10.5826/dpc.1201a155
45. Davis DMR, Drucker AM, Alikhan A, Bercovitch L, Cohen DE, Darr JM, et al. Guidelines of care for the management of atopic dermatitis in adults with phototherapy and systemic therapies. J Am Acad Dermatol. (2024) 90(2):e43–56. doi: 10.1016/j.jaad.2023.08.102
46. Panel AJADG, Chu DK, Schneider L, Asiniwasis RN, Boguniewicz M, Benedetto AD, et al. Atopic dermatitis (eczema) guidelines: 2023 American Academy of Allergy, Asthma and Immunology/American College of Allergy, Asthma and Immunology Joint Task Force on Practice Parameters GRADE– and Institute of Medicine–based recommendations. Ann Allergy Asthma Immunol. (2024) 132(3):274–312. doi: 10.1016/j.anai.2023.11.009
47. Zhang J, Xu X, Wang X, Zhang L, Hu M, Le Y, et al. Topical emollient prevents the development of atopic dermatitis and atopic march in mice. Exp Dermatol. (2023) 32(7):1007–15. doi: 10.1111/exd.14806
48. Axon E, Chalmers JR, Santer M, Ridd MJ, Lawton S, Langan SM, et al. Safety of topical corticosteroids in atopic eczema: an umbrella review. BMJ Open. (2021) 11(7):e046476. doi: 10.1136/bmjopen-2020-046476
49. Rosa ML, Musarra I, Ranno C, Maiello N, Negri L, del Giudice MM, et al. A randomized, double-blind, placebo-controlled, crossover trial of systemic flunisolide in the treatment of children with severe atopic dermatitis. Curr Ther Res. (1995) 56(7):720–6. doi: 10.1016/0011-393X(95)85143-7
50. Heddle RJ, Soothill JF, Bulpitt CJ, Atherton DJ. Combined oral and nasal beclomethasone diproprionate in children with atopic eczema: a randomised controlled trial. Br Méd J (Clin Res Ed). (1984) 289(6446):651. doi: 10.1136/bmj.289.6446.651
51. Schmitt J, Schäkel K, Fölster-Holst R, Bauer A, Oertel R, Augustin M, et al. Prednisolone vs. Ciclosporin for severe adult eczema. An investigator-initiated double-blind placebo-controlled multicentre trial. Br J Dermatol. (2010) 162(3):661–8. doi: 10.1111/j.1365-2133.2009.09561.x
52. Goeckerman W. The treatment of psoriasis. Northwest Med. (1925) 24:229–31. Available online at: https://cir.nii.ac.jp/crid/1571417125226207872
53. Goeckerman WH. Treatment of psoriasis: continued observations on the use of crude coal tar and ultraviolet light. Arch Dermatol Syphilol. (1931) 24(3):446–50. doi: 10.1001/archderm.1931.01450010455012
54. Branisteanu DE, Dirzu DS, Toader MP, Branisteanu DC, Nicolescu AC, Brihan I, et al. Phototherapy in dermatological maladies (Review). Exp Ther Med. (2022) 23(4):259. doi: 10.3892/etm.2022.11184
55. Borgia F, Pomi FL, Vaccaro M, Alessandrello C, Papa V, Gangemi S. Oxidative stress and phototherapy in atopic dermatitis: mechanisms, role, and future perspectives. Biomolecules. (2022) 12(12):1904. doi: 10.3390/biom12121904
56. de Barros NM, Sbroglio LL, de Buffara MO, Baka JLCES, de Pessoa AS, Azulay-Abulafia L. Phototherapy. An Bras Dermatol. (2021) 96(4):397–407. doi: 10.1016/j.abd.2021.03.001
57. Mahajan RK, Kokare DM, Raut NA, Itankar PR. Photophysics and Nanophysics in Therapeutics. Mahajan NM, Saini A, Raut NA, Dhoble SJ, editors. Amsterdam: Elsevier Science (2022). p. 15–30. doi: 10.1016/C2020-0-02664-2
58. Tintle S, Shemer A, Suárez-Fariñas M, Fujita H, Gilleaudeau P, Sullivan-Whalen M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. (2011) 128(3):583–93.e4. doi: 10.1016/j.jaci.2011.05.042
59. Waligóra-Dziwak K, Dańczak-Pazdrowska A, Jenerowicz D. A comprehensive review of biologics in phase III and IV clinical trials for atopic dermatitis. J Clin Med. (2024) 13(14):4001. doi: 10.3390/jcm13144001
60. Dharmaraj D, Chavan N, Likhitha U, Nayak UY. Electrospun nanofibers for dermatological delivery. J Drug Deliv Sci Technol. (2024) 99:105981. doi: 10.1016/j.jddst.2024.105981
61. Gürtler AL, Rades T, Heinz A. Electrospun fibers for the treatment of skin diseases. J Control Release. (2023) 363:621–40. doi: 10.1016/j.jconrel.2023.10.009
62. Godse K, Dethe G, Sawant S, Sharma A, Pereira R, Ghate S, et al. Clinical evaluation of the safety and tolerability of film-forming sprays in patients with psoriasis and eczema. Cureus. (2024) 16(3):e57020. doi: 10.7759/cureus.57020
63. Shen S, Qu X, Liu Y, Wang M, Zhou H, Xia H. Evaluation of antioxidant activity and treatment of eczema by berberine hydrochloride-loaded liposomes-in-gel. Molecules. (2024) 29(7):1566. doi: 10.3390/molecules29071566
64. Lee JH, Chandrawati R, Lee NA. Nanoparticles in allergen-delivery systems for allergen-specific immunotherapy. Adv Ther. (2024) 8(3):2400223. doi: 10.1002/adtp.202400223
65. Kim BS, Howell MD, Sun K, Papp K, Nasir A, Kuligowski ME, et al. Treatment of atopic dermatitis with ruxolitinib cream (JAK1/JAK2 inhibitor) or triamcinolone cream. J Allergy Clin Immunol. (2020) 145(2):572–82. doi: 10.1016/j.jaci.2019.08.042
66. Cheape AC, Murrell DF. 2% Crisaborole topical ointment for the treatment of mild-to-moderate atopic dermatitis. Expert Rev Clin Immunol. (2017) 13(5):415–23. doi: 10.1080/1744666X.2017.1304820
67. Gold LFS, Spelman L, Spellman MC, Hughes MH, Zane LT. A phase 2, randomized, controlled, dose-ranging study evaluating crisaborole topical ointment, 0.5% and 2% in adolescents with mild to moderate atopic dermatitis. J Drugs Dermatol. (2015) 14(12):1394–9.26659931
68. Simpson EL, Eichenfield LF, Alonso-Llamazares J, Draelos ZD, Ferris LK, Forman SB, et al. Roflumilast cream, 0.15%, for atopic dermatitis in adults and children. JAMA Dermatol. (2024) 160(11):1161–70. doi: 10.1001/jamadermatol.2024.3121
69. Tsuji G, Yamamura K, Kawamura K, Kido-Nakahara M, Ito T, Nakahara T. Novel therapeutic targets for the treatment of atopic dermatitis. Biomedicines. (2023) 11(5):1303. doi: 10.3390/biomedicines11051303
70. Pappa G, Sgouros D, Theodoropoulos K, Kanelleas A, Bozi E, Gregoriou S, et al. The IL-4/-13 axis and its blocking in the treatment of atopic dermatitis. J Clin Med. (2022) 11(19):5633. doi: 10.3390/jcm11195633
71. Mitroi GG, Pleșea EL, Mitroi GF, Mitroi MR, Neagoe CD, Ianoși SL. Exploring the potential of IL-4 and IL-13 plasma levels as biomarkers in atopic dermatitis. Life. (2024) 14(3):352. doi: 10.3390/life14030352
72. Kelly-Welch AE, Hanson EM, Boothby MR, Keegan AD. Interleukin-4 and interleukin-13 signaling connections maps. Science. (2003) 300(5625):1527–8. doi: 10.1126/science.1085458
73. Speeckaert R, Belpaire A, Lambert J, Speeckaert M, van Geel N. Th pathways in immune-mediated skin disorders: a guide for strategic treatment decisions. Immune Netw. (2024) 24(5):e33. doi: 10.4110/in.2024.24.e33
74. Ytterberg SR, Bhatt DL, Mikuls TR, Koch GG, Fleischmann R, Rivas JL, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. (2022) 386(4):316–26. doi: 10.1056/NEJMoa2109927
75. Teixeira AJ, Duong JQ, Parraga SP, Feldman SR. Presenting JAK inhibitor safety information to dermatology patients. JEADV Clin Practice. (2025) 4(1):12–20. doi: 10.1002/jvc2.551
76. Brehler R. Clinic and diagnostics of house dust mite allergy. Allergo J Int. (2023) 32(1):1–4. doi: 10.1007/s40629-022-00232-7
77. Martorano L, Erwin EA. Aeroallergen exposure and spread in the modern era. J Allergy Clin Immunol: Pr. (2018) 6(6):1835–42. doi: 10.1016/j.jaip.2018.08.014
78. Shah SN, Grunwell JR, Mohammad AF, Stephenson ST, Lee GB, Vickery BP, et al. Performance of eosinophil cationic protein as a biomarker in asthmatic children. J Allergy Clin Immunol: Pr. (2021) 9(7):2761–9.e2. doi: 10.1016/j.jaip.2021.02.053
79. Hozumi I, Hasegawa T, Honda A, Ozawa K, Hayashi Y, Hashimoto K, et al. Patterns of levels of biological metals in CSF differ among neurodegenerative diseases. J Neurol Sci. (2011) 303(1–2):95–9. doi: 10.1016/j.jns.2011.01.003
80. Brown SJ, Asai Y, Cordell HJ, Campbell LE, Zhao Y, Liao H, et al. Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut allergy. J Allergy Clin Immunol. (2011) 127(3):661–7. doi: 10.1016/j.jaci.2011.01.031
81. Frølunde AS, Vestergaard C, Deleuran M. Skin barrier abnormalities in atopic dermatitis. Curr Treat Options Allergy. (2022) 9(3):107–17. doi: 10.1007/s40521-022-00310-9
82. Flohr C, England K, Radulovic S, McLean WHI, Campbell LE, Barker J, et al. Filaggrin loss-of-function mutations are associated with early-onset eczema, eczema severity and transepidermal water loss at 3 months of age. Br J Dermatol. (2010) 163(6):1333–6. doi: 10.1111/j.1365-2133.2010.10068.x
83. Tokura Y, Yunoki M, Kondo S, Otsuka M. What is “eczema”? J Dermatol. (2025) 52(2):192–203. doi: 10.1111/1346-8138.17439
84. Kou K, Okawa T, Yamaguchi Y, Ono J, Inoue Y, Kohno M, et al. Periostin levels correlate with disease severity and chronicity in patients with atopic dermatitis. Br J Dermatol. (2014) 171(2):283–91. doi: 10.1111/bjd.12943
85. Wollenberg A, Klein E. Current aspects of innate and adaptive immunity in atopic dermatitis. Clin Rev Allergy Immunol. (2007) 33(1–2):35–44. doi: 10.1007/s12016-007-0032-9
86. Kabashima K. New concept of the pathogenesis of atopic dermatitis: interplay among the barrier, allergy, and pruritus as a Trinity. J Dermatol Sci. (2013) 70(1):3–11. doi: 10.1016/j.jdermsci.2013.02.001
87. Schuler CF, Billi AC, Maverakis E, Tsoi LC, Gudjonsson JE. Novel insights into atopic dermatitis. J Allergy Clin Immunol. (2023) 151(5):1145–54. doi: 10.1016/j.jaci.2022.10.023
88. Carlier TDB, Badloe FMS, Ring J, Gutermuth J, Krohn IK. Autoreactive T cells and their role in atopic dermatitis. J Autoimmun. (2021) 120:102634. doi: 10.1016/j.jaut.2021.102634
89. Ong PY. Atopic dermatitis: is innate or adaptive immunity in control? A clinical perspective. Front Immunol. (2022) 13:943640. doi: 10.3389/fimmu.2022.943640
Keywords: allergic eczema, biomarkers, clinical research, skin disorders, allergy, IgE-mediated disease, atopic dermatatis, phototherapy
Citation: Lazor JE, Bozsoki BA and Bharadwaj P (2025) Cure for the itch: current clinical standards and therapies in allergic eczema. Front. Allergy 6:1569292. doi: 10.3389/falgy.2025.1569292
Received: 31 January 2025; Accepted: 11 March 2025;
Published: 3 April 2025.
Edited by:
Junling Wang, Henan Provincial People's Hospital, ChinaReviewed by:
Nurit P. Azouz, Cincinnati Children's Hospital Medical Center, United StatesCopyright: © 2025 Lazor, Bozsoki and Bharadwaj. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pranay Bharadwaj, cHJhbmF5LmJoYXJhZHdhakBib2VocmluZ2VyLWluZ2VsaGVpbS5jb20=
†These authors contributed equally to this work
‡ORCID:
Jennifer E. Lazor
orcid.org/0009-0005-0643-7769
Bree A. Bozsoki
orcid.org/0009-0005-7412-1061
Pranay Bharadwaj
orcid.org/0000-0003-3768-3557
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.