
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Allergy , 20 March 2025
Sec. Asthma
Volume 6 - 2025 | https://doi.org/10.3389/falgy.2025.1501196
 Luisa Brussino1
Luisa Brussino1 Maria Aliani2
Maria Aliani2 Elena Altieri3
Elena Altieri3 Pietro Bracciale4
Pietro Bracciale4 Maria Filomena Caiaffa5
Maria Filomena Caiaffa5 Paolo Cameli6
Paolo Cameli6 Giorgio Walter Canonica7,8
Giorgio Walter Canonica7,8 Cristiano Caruso9,10
Cristiano Caruso9,10 Stefano Centanni11
Stefano Centanni11 Fausto De Michele12
Fausto De Michele12 Stefano Del Giacco13
Stefano Del Giacco13 Fabiano Di Marco14
Fabiano Di Marco14 Laura Malerba15
Laura Malerba15 Francesco Menzella16
Francesco Menzella16 Girolamo Pelaia17
Girolamo Pelaia17 Paola Rogliani18,19
Paola Rogliani18,19 Micaela Romagnoli20
Micaela Romagnoli20 Pietro Schino21
Pietro Schino21 Jan Walter Schroeder22
Jan Walter Schroeder22 Gianenrico Senna23
Gianenrico Senna23 Alessandra Vultaggio24
Alessandra Vultaggio24 Maria D’Amato25*
Maria D’Amato25*
Introduction: Severe eosinophilic asthma (SEA) often co-occurs with chronic rhinosinusitis with nasal polyps (CRSwNP), worsening asthma symptoms. Earlier studies have shown that benralizumab improves asthma outcomes with greater efficacy if patients present CRSwNP.
Methods: This post hoc analysis of the ANANKE study (NCT04272463) reports data on the long-term effectiveness of benralizumab between SEA patients with and without CRSwNP (N = 86 and N = 75, respectively) treated for up to 96 weeks.
Results: Before benralizumab initiation, CRSwNP patients displayed longer SEA duration, greater oral corticosteroid (OCS) use and blood eosinophil count. After 96 weeks of treatment, the annual exacerbation rate (AER) decreased in both groups, with CRSwNP patients achieving considerable reductions than No-CRSwNP patients (severe AER dropped by 100% and 95.6%, respectively). While lung function improvement was comparable at week 96, CRSwNP patients showed a faster response to benralizumab, with a rise of forced expiratory volume in 1 s (FEV1) at 16 weeks that was maintained throughout the study. Median OCS daily dose decreased to 0.0 mg in both groups at 96 weeks, but benralizumab OCS-sparing effect was faster in CRSwNP patients (median OCS dose was 0.0 mg and 2.5 mg in CRSwNP and No-CRSwNP patients respectively, at 48 weeks). Although asthma control test (ACT) median scores were comparable, greater proportions of CRSwNP patients displayed well-controlled asthma (ACT ≥ 20) than No-CRSwNP patients at all time points.
Discussion: These findings show benralizumab long-term effectiveness in SEA patients with and without CRSwNP, highlighting its superior and faster-acting benefits on asthma outcomes in presence of CRSwNP.
Severe asthma (SA) is a heterogeneous chronic condition of the airways that can be accompanied by multiple comorbidities, among which chronic rhinosinusitis with nasal polyps (CRSwNP) is particularly frequent. The clinical manifestations of CRSwNP include nasal congestion, loss of smell, rhinorrhea, facial pain, headache, and sleep difficulties, which further impair asthma patients' quality of life (QoL) (1–3). The coexistence of SA and CRSwNP in the same patient implies common pathophysiological mechanisms spreading across both lower and upper airways; accordingly, asthma patients are more likely to develop CRSwNP and vice versa (4, 5). The theory of coexisting asthma and CRSwNP being a single disease, referred to as “united airways disease” (UAD), is widely embraced within the scientific community and warrants the need for a multidisciplinary approach to effectively manage comorbid patients (6, 7).
Severe eosinophilic asthma (SEA) is one of the most challenging SA pheno-endotype to treat. It is characterized by a pronounced type 2 (T2) inflammatory response driven by the activation of T helper (Th)2 cells and type 2 innate lymphoid cells (ILC2). These cells secrete elevated levels of T2 cytokines (IL4, IL5, IL13), which create a positive feedback loop by continuously recruiting eosinophils and perpetuating the inflammatory cascade in the airways (8, 9). As a result, SEA is characterized by severe symptoms, such as threatening exacerbations and progressive airflow decline (10–12). In the presence of CRSwNP, reported in 40%–60% of SEA patients, asthma symptoms are usually exacerbated and become more difficult to control, leading to a substantial worsening in the quality of life (QoL) (13–15).
Benralizumab is the only monoclonal antibody (mAb) currently approved for SEA treatment that targets the IL5 receptor alpha (IL5Rα) and induces eosinophils apoptosis via a unique mechanism of afucosylation-enhanced Ab-dependent cell-mediated cytotoxicity (ADCC) (16, 17). Apart from its anti-eosinophilic activity, benralizumab has been found to mediate additional immune-modulatory processes, such as modifications in the number of CD3+ T cell subsets and activation of NK cells, that contribute to ameliorate asthma symptoms (18). Importantly, benralizumab has been associated with superior effects in reducing asthma exacerbations, enhancing respiratory function and improving QoL in SEA and CRSwNP comorbid patients, as demonstrated by results from the randomized clinical trial (RCT) ANDHI (19) and post hoc analyses from the pivotal studies SIROCCO and CALIMA (20). Real-life studies have confirmed the enhanced effectiveness of benralizumab in SEA patients with CRSwNP compared to those without CRSwNP (21–23). Recently, Pelaia et al. reported a higher prevalence of CRSwNP in SEA patients achieving sustained clinical remission after two years of benralizumab treatment. Notably, CRSwNP emerged as a significant predictor of remission probability. Importantly, benralizumab also demonstrated significant improvements in CRSwNP outcomes. These results further support the role of CRSwNP in mediating the favourable, long-term response of SEA patients to benralizumab (24).
Consistently with the growing body of evidence, the international guidelines Global Strategy for Asthma Management (GINA) recognize CRSwNP as a predictor of benralizumab enhanced efficacy in controlling asthma (25).
ANANKE (NCT04272463) is a retrospective, multicenter Italian study in which the characteristics and clinical outcomes of SEA patients treated with benralizumab have been described (26). The differences between SEA patients with and without CRSwNP were also evaluated, revealing that comorbid patients were characterized by a higher blood eosinophil count (BEC) and a greater OCS use compared with patients without CRSwNP. After a median treatment period of 9.8 months, benralizumab treatment improved all clinical outcomes in both groups, with a more pronounced effect in reducing any and severe AER and decreasing OCS use observed in CRSwNP patients (27). Long-term data on the effectiveness of benralizumab (up to 96 weeks) have also been published, highlighting the durable response to the biologic (28). In this novel post hoc analysis, we report data on the effectiveness of benralizumab in SEA patients with and without CRSwNP, treated for up to 96 weeks.
ANANKE (NCT04272463) is an Italian multicenter, observational, retrospective study whose methods have been extensively described (26). As explained by Vultaggio et al. (28), the observation period was extended up to 96 weeks, and patients signed the additional and amended study informed consent and privacy forms. During the observation period, data were collected at 4, 16, 24, 48, and 96 weeks after benralizumab initiation (i.e., the index date). ANANKE was performed following the principles of the Declaration of Helsinki, as well as the laws and guidelines regulating Italian medical practice. The ethics committees/institutional review boards of the participating centers approved the study.
Inclusion and exclusion criteria have been previously detailed (26). Patients were adults (≥18 years old) affected by SEA and requiring treatment with high doses of inhaled corticosteroids (ICS) and a long-acting beta2-agonist (LABA), with or without additional asthma controllers. Benralizumab treatment was initiated at least 3 months before patients' enrollment. Patients were excluded if they participated in other studies with a specific patient management strategy that differed from the site's normal clinical practice.
Eligible patients satisfied all inclusion, exclusion, and amendment criteria. Evaluable patients comprehended all eligible patients with BEC data available at the index date.
Data were recorded in the electronic case report form (eCRF) after being collected from hospital medical charts.
The primary endpoint aimed to characterize the socio-demographic and clinical characteristics of the two groups of patients at the index date. As previously specified (26), data at the index date were gathered during the 12 months before benralizumab initiation. Among patients' exacerbations, severe exacerbations were distinguished from other exacerbations based on the need for at least one of the following: (a) treatment with systemic corticosteroids for 3 days or more, or an increase in the dosage of maintenance OCS; (b) an emergency department or urgent care visit during which systemic corticosteroids were administered; (c) hospitalization.
Secondarily, the variation in the following clinical and laboratory outcomes was assessed during benralizumab treatment: BEC, AER (any and severe), proportion of patients without any exacerbations, whether mild or severe, predicted forced expiratory volume in the first second (FEV1), measured pre-bronchodilator (BD), OCS use and daily dosage (expressed as prednisone-equivalent), proportion of patients reducing or permanently interrupting OCS, ACT score, the proportion of patients reaching ACT score ≥20 (well-controlled asthma).
Measurements were taken at the index date and after 48 and 96 weeks of treatment; data at earlier time points (4, 16, 24, weeks) were included when available.
In line with previous ANANKE studies, statistical analyses were descriptive only and were performed among patients with CRSwNP and without CRSwNP. Data are expressed as either mean ± standard deviation (SD), median [interquartile range (IQR)], or absolute numbers and frequencies. Demographic and clinical characteristics at the index date were assessed in evaluable patients; secondary endpoints were assessed in evaluable patients for secondary analyses at 48 and 96 weeks.
Patient disposition in the ANANKE study has been previously detailed (28). Briefly, 218 SEA patients were enrolled in the ANANKE study between December 2019 and July 2020, and 167 patients were eligible for analysis at 96 weeks. For this post hoc analysis, SEA patients were divided into two groups, CRSwNP and No-CRSwNP. Among these, evaluable patients with consistent data were N = 161 (CRSwNP, N = 86; No-CRSwNP, N = 75) at the index date, N = 153 (CRSwNP, N = 79; No-CRSwNP, N = 74) at 48 weeks, and N = 113 (CRSwNP, N = 59; No-CRSwNP, N = 54) at 96 weeks.
Table 1 shows socio-demographic and clinical characteristics of CRSwNP and No-CRSwNP patients at the index date. Female patients were more prevalent in the No-CRSwNP group than in the CRSwNP group (74.7% and 50%, respectively). The two groups were comparable in terms of mean age (56 and 58 years old in CRSwNP and No-CRSwNP, respectively) and mean age at asthma onset (38 and 41 years in CRSwNP and No-CRSwNP, respectively). CRSwNP patients experienced a longer median duration of asthma compared with No-CRSwNP patients (15.8 and 11.9 years in CRSwNP and No-CRSwNP, respectively); consistently, they were also characterized by a longer median duration of SEA (2.1 and 1.6 years in CRSwNP and No-CRSwNP, respectively). Analysis of relevant comorbidities besides CRSwNP revealed similar prevalences between CRSwNP (69.8%) and No-CRSwNP (72%) groups, with eosinophilic granulomatosis with polyangiitis (EGPA) abeing more frequent among CRSwNP patients than No-CRSwNP patients (9.3% and 0.0%, respectively). A higher percentage of patients with BMI above 30 was found in the No-CRSwNP group (22.7%) compared to the CRSwNP group (9.3%). Median BEC was slightly higher in the CRSwNP group [600 (460–800) cells/mm3] when compared to the No-CRSwNP patients [525 (355–960) cells/mm3] whereas median IgE levels were similar (CRSwNP, 198.5 IU/ml; No-CRSwNP, 227.5 IU/ml).
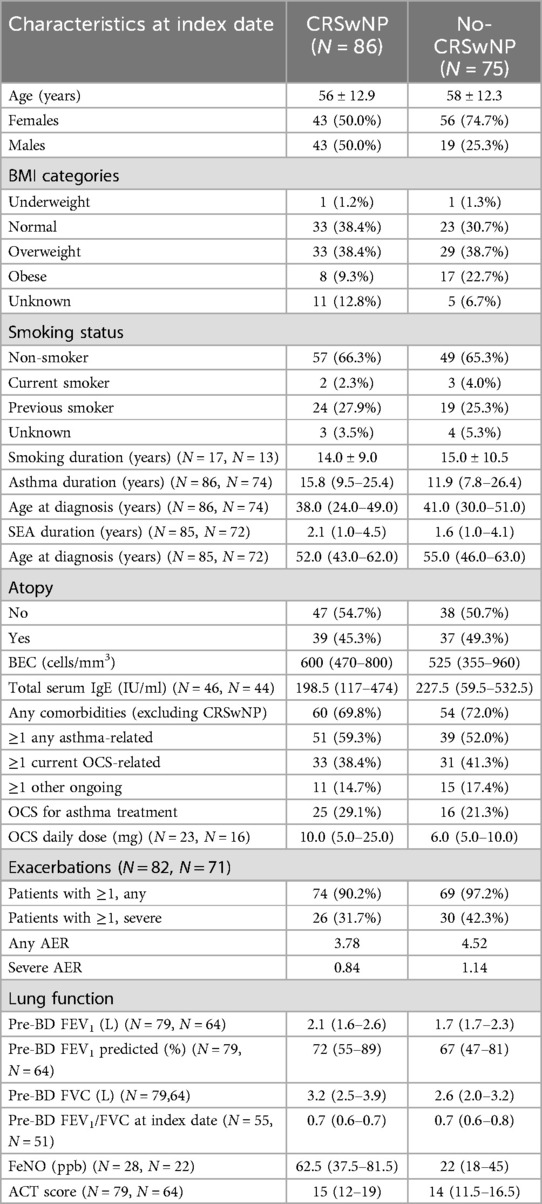
Table 1. Socio-demographic and clinical characteristics of CRSwNP and No-CRSwNP patients at the index date. Data were collected at the index date (either at benralizumab initiation or during the previous 12 months) and are expressed as N (%), mean ± SD, or median (IQR). Unless otherwise stated, evaluated patients were N = 86 and N = 75 in the CRSwNP and No-CRSwNP groups, respectively.
Lung function appeared to be slightly higher in CRSwNP patients, with a median pre-BD FEV1 volume of 2.1 L (vs. 1.7 L in No-CRSwNP patients) and median pre-BD FEV1 predicted of 72.0% (vs. 67.0% in No-CRSwNP patients). FeNO levels were higher in CRSwNP patients than in No-CRSwNP patients (62.5 ppb vs. 22.0 ppb).
No dissimilarity was observed in terms of asthma control between the two groups, measured via the ACT score [CRSwNP: 15.0 (12.0–19.0); No-CRSwNP, 14.0 (11.5–16.5)]. Similarly, there were only modest disparities in exacerbation frequency between CRSwNP and No-CRSwNP groups (any AER, 3.78 and 4.52 respectively; severe AER, 0.84 and 1.14, respectively). However, more patients in the No CRSwNP group experienced at least one or more exacerbations of any severity in the previous year (N = 74, 90.2%) compared to patients in the CRSwNP group (N = 69, 97.2%).
A higher percentage of CRSwNP patients used OCS to control their asthma compared with No-CRSwNP patients (29.1% and 21.3%, respectively); CRSwNP patients also used a higher median OCS dosage than No-CRSwNP patients (10.0 mg and 6.0 mg, respectively).
During this period, benralizumab demonstrated a substantial reduction in exacerbations in both populations, with a more pronounced decrease observed in the CRSwNP population, as early as the 48th week (Figure 1). Among CRSwNP patients, any AER drastically decreased from 3.78 to 0.11 at 48 weeks, resulting in a 97.1% reduction. Any AER remained low over the entire observation period, reaching the value of 0.10 at 96 weeks (97.3% reduction). In No-CRSwNP patients, the AER, higher at baseline compared to the CRSwNP group, decreased significantly at 48 weeks, but less markedly, with an overall reduction of 89.8% (from 4.52 to 0.46). By 96 weeks, any AER further decreased to 0.27, reflecting a 94.0% reduction.

Figure 1. AER reduction during benralizumab treatment in CRSwNP and No-CRSwNP patients. Any and severe AER are shown at index date and after 48 and 96 weeks of treatment with benralizumab.
CRSwNP patients also showed a prominent decline in severe AER, which decreased from 0.84 to 0.01 at 48 weeks (98.8% reduction), and dropped to 0.0 at 96 weeks (100% reduction). In No-CRSwNP patients, severe AER decreased from 1.14 to 0.12 at 48 weeks (89.5% reduction) and reached 0.05 at 96 weeks (95.6% reduction) (Figure 1).
The proportion of patients free from any and severe exacerbations, as well as patients who still experienced exacerbations during benralizumab treatment, were also assessed. As shown in Figure 2A, at 48 weeks, 89.9% of CRSwNP patients and 67.2% of No-CRSwNP patients experienced no exacerbations, with 98.7% and 89.1% of them being free from severe exacerbations, respectively. By week 96, 88.1% of CRSwNP patients were free from any exacerbation, and 100% were free from severe exacerbations. In contrast, 66.7% of No-CRSwNP patients were free from any exacerbations, and 90.7% were free from severe exacerbations (Figure 2B).
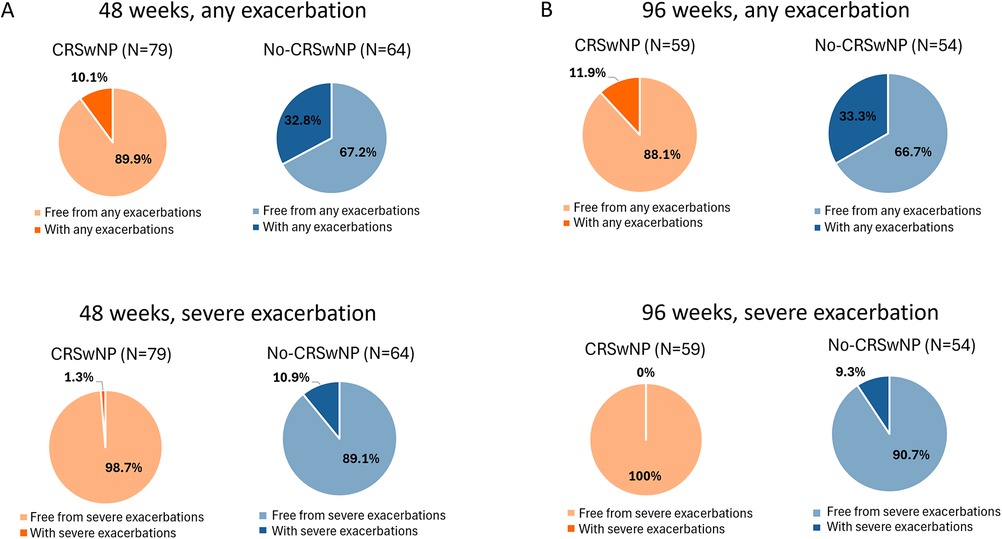
Figure 2. Percentages of CRSwNP and No-CRSwNP patients with and without any or severe exacerbations during benralizumab treatment. Data are shown after 48 (A) and 96 weeks (B) of treatment with benralizumab.
A nearly complete depletion of BEC was observed in both CRSwNP and No-CRSwNP groups at 96 weeks post-treatment initiation with benralizumab (data not shown).
Figure 3 illustrates the changes in pre-BD FEV1 predicted over the course of benralizumab treatment. Up to 48 weeks, the CRSwNP population showed improvements compared to No-CRSwNP group [89.0% (63.0–106.0) vs. 77.0% (59.0–97.0), respectively]. Remarkably, CRSwNP patients achieved a median pre-BD FEV1 predicted value of 88.0% (77.0–95.0) as early as 16 weeks, which then plateaued and remained largely stable until week 96, with values equal or greater than 87.0% throughout the treatment period.
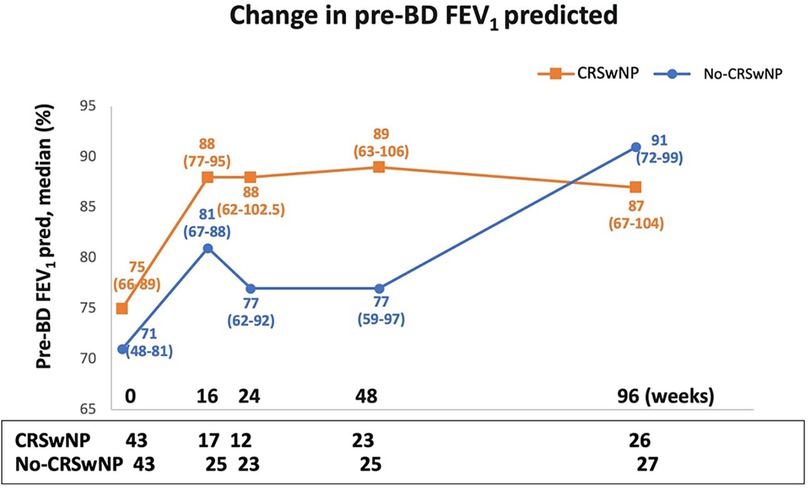
Figure 3. Change in pre-BD FEV1 pred. (%) during benralizumab treatment in CRSwNP and No-CRSwNP patients. Median (range) of pre-BD FEV1 pred. (%) is reported at the index date and after 16, 24, 48 and 96 weeks of treatment with benralizumab.
In contrast, the No-CRSwNP group experienced a more gradual improvement, with lower pre-BD FEV1 predicted median values than CRSwNP patients [81.0% (67.0–88.0) and 77.0% (62.0–92.0) at 16 and 24 weeks, respectively]. However, by week 96, the No-CRSwNP group reached pre-BD FEV1 predicted values similar to the CRSwNP group [91.0% (72.0–99.0)].
Benralizumab OCS-sparing effect was evaluated in CRSwNP and No-CRSwNP groups (Figure 4). At 48 weeks, CRSwNP patients exhibited a median OCS dose of 0.0 mg/day, indicating a 100% reduction from the median dose of 10.0 mg/day used at the index date. During the same timeframe, No-CRSwNP patients reduced their median OCS dose to 2.5 mg/day (60% reduction from the starting dose of 6.25 mg/day). By week 96, both groups achieved a 100% reduction in median OCS dose.
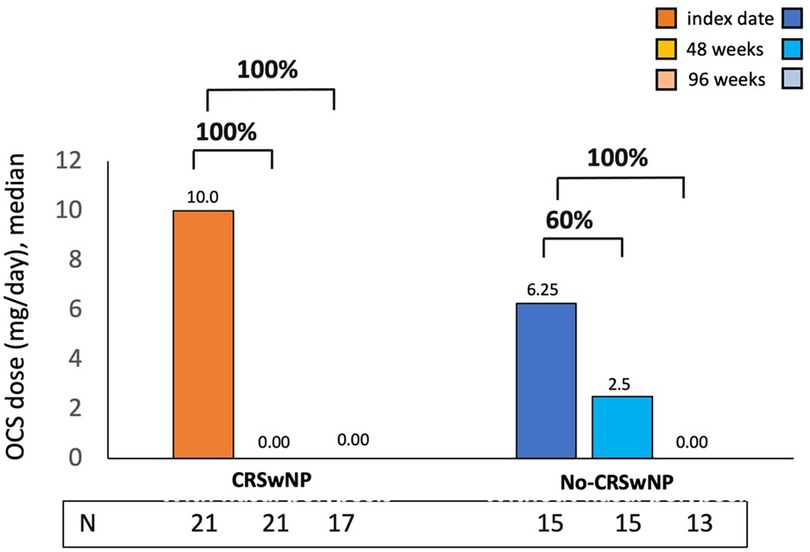
Figure 4. OCS reduction during benralizumab treatment in CRSwNP and No-CRSwNP patients. The median (IQR) OCS daily dose (mg of prednisone equivalent) is reported at the index date and after 4, 16, 24, 48 and 96 weeks of treatment with benralizumab.
The proportion of patients who reduced and/or eliminated the use of OCS during benralizumab treatment was examined and is shown in Table 2. At 48 weeks, 61.9% of CRSwNP patients and 46.6% of No-CRSwNP patients had completely discontinued OCS treatment; these percentages further increased at 96 weeks, reaching 70.5% and 53.8% in the CRSwNP and No-CRSwNP groups, respectively. Overall, 76.4% of CRSwNP patients and 61.5% of No-CRSwNP patients reduced the OCS daily dose to some extent by 96 weeks. Consistently, a smaller percentage of patients in both groups showed no reduction in OCS use, with slightly higher percentages observed in the No-CRSwNP group (N = 7, 46.7% at 48 weeks; N = 5, 38.5% at 96 weeks) compared to the CRSwNP group (N = 6, 28.6% at 48 weeks, N = 4, 23.6% at 96 weeks) (Table 2).
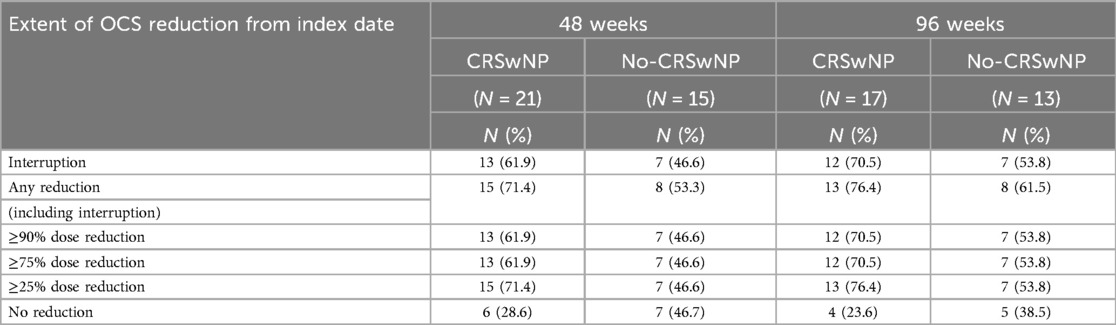
Table 2. OCS dose reduction and/or interruption in CRSwNP and No-CRSwNP patients during benralizumab treatment. Data were collected after 48 (A) and 96 weeks (B) of benralizumab treatment. OCS dosage is expressed in daily mg (prednisone equivalent). Data are expressed as N (%).
Changes in asthma control were monitored via the ACT during benralizumab treatment. As depicted in Figure 5, the ACT score improved and increased rapidly after just 4 weeks of benralizumab treatment, reaching median values equal to or greater than 20 in both groups [CRSwNP, 21.5 (17.0–24.0)] and No-CRSwNP, 20.0 [18.0–24.0]). Between 16 and 48 weeks, median ACT scores were slightly higher in CRSwNP patients [from 21.0 (18.0–23.0) at 16 weeks to 23.5 (20.0–25.0) at 48 weeks], compared to No-CRSwNP patients [from 20.5 (18.0–23.0) at 16 weeks, to 21.5 (18.0–23.0) at 48 weeks]. However, at 96 weeks, both groups reached identical median ACT scores [CRSwNP, 23.0 (20.0–24.0); No-CRSwNP, 23.0 (18.0–24.0)].
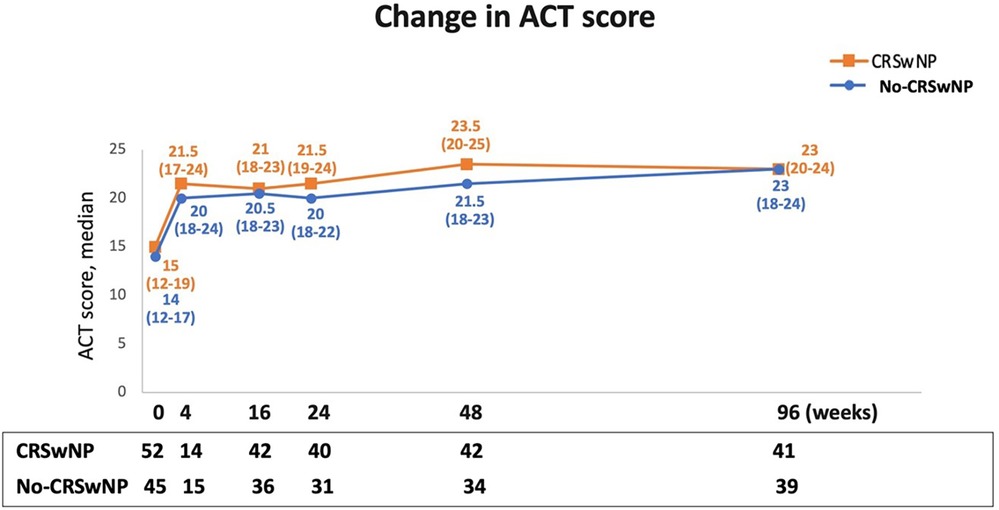
Figure 5. Improvement in asthma control during benralizumab treatment in CRSwNP and No-CRSwNP patients. Change in ACT score is expressed as median (IQR). Data are reported at index date and after 4, 16, 24, 48, and 96 weeks of treatment with benralizumab.
Figure 6 shows the proportions of patients achieving a good control of asthma (ACT score ≥20). Compared to patients in the No-CRSwNP group, higher percentages of CRSwNP patients achieved a well-controlled asthma at all considered time points, with up to 80.5% of CRSwNP patients having an ACT score ≥20 registered at 96 weeks. The percentages of No-CRSwNP patients with well-controlled asthma were consistently lower at all time points (up 74.4% at 96 weeks) (Supplementary Table S1).
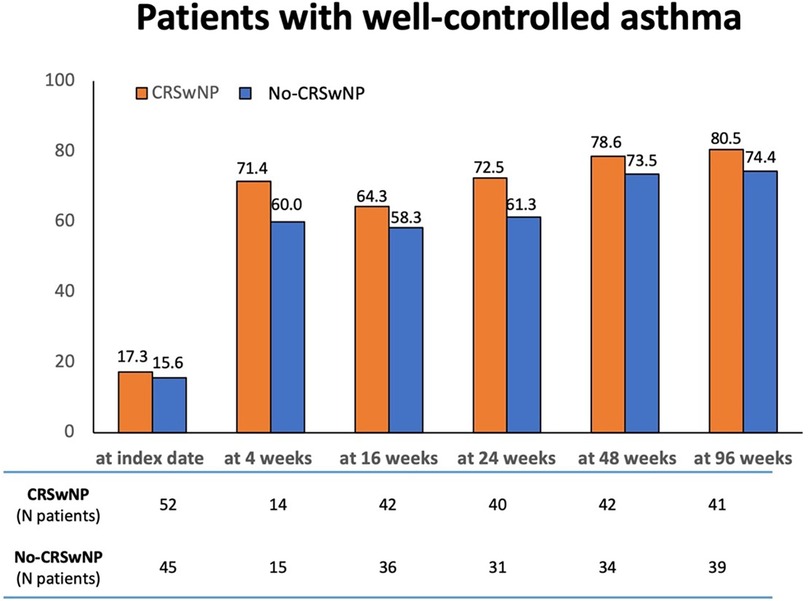
Figure 6. Percentages of patients achieving a well-controlled asthma (ACT score ≥20) in CRSwNP and No-CRSwNP groups at different timepoints. Data are reported at index date and after 4, 16, 24, 48, and 96 weeks of treatment with benralizumab.
At 96 weeks, a similar percentage of CRSwNP and No-CRSwNP patients discontinued benralizumab treatment (10.2% and 11.1%, respectively). Reasons for discontinuation included lack of clinical efficacy (6.8% vs. 3.7%, respectively), adverse events (1.7% vs. 1.9%, respectively), allergic rhinitis (0 vs. 1.9%, respectively), and patient decision (0 vs. 3.7%, respectively). Data are shown in Supplementary Table S2.
This post hoc analysis, conducted from the real-life ANANKE study, evaluated the clinical characteristics and outcomes in SEA patients with CRSwNP and those without (No-CRSwNP) treated with benralizumab for up to 96 weeks. This work builds upon the study of D'Amato et al., which assessed the efficacy of benralizumab in reducing symptoms, exacerbations, OCS use and improving lung function and asthma control in the same populations after a median exposure to benralizumab of 9.8 months (27).
Previous research has provided evidence supporting the rapid effectiveness of benralizumab in real-world settings for patients with SEA. While the ANANKE study described asthma outcomes in patients treated for up to 96 weeks (28), only recently benralizumab sustained effectiveness was confirmed over a period of 3 years (29). Recently, the real-world XALOC-1 programme demonstrated that the SEA patients treated with benralizumab had substantial improvements in clinical outcomes irrespective of previous biologic use (30). While some studies have investigated the effectiveness of benralizumab in patients with both SEA and CRSwNP, most of them reported results for treatment periods of up to 24 weeks (22, 23, 31). In the retrospective ORBE II cohort study, Padilla-Galo et al. investigated the effects of benralizumab in SEA patients with and without CRSwNP over 1-year period (32). In addition, Santomasi et al. demonstrated that benralizumab exerted beneficial effects on both SEA and CRSwNP outcomes in comorbid patients treated over a period of 20 months (33). Recently, Pelaia et al. assessed the effectiveness of benralizumab in patients treated up to 2 years. Although the authors did not compare treatment outcomes between CRSwNP and No-CRSwNP patients, their results highlight that CRSwNP patients are more likely to achieve clinical remission (24). Therefore, our study complements previous findings by providing a comprehensive 96-week comparison of benralizumab effectiveness on asthma outcomes in SEA patients with and without CRSwNP for up to 96 weeks.
A close examination of the clinical characteristics of the two patient populations in this study reveals several key findings. Firstly, the age at asthma onset was similar between the two groups. However, CRSwNP patients had a longer duration of asthma and SEA compared to those without CRSwNP, along with a higher BEC. Although the rate of comorbidities (excluding CRSwNP) was comparable between CRSwNP and No-CRSwNP patients, EGPA was more frequent in the CRSwNP group. CRSwNP patients appeared to experience fewer severe exacerbations and exhibited better lung function compared to No CRSwNP patients. A higher proportion of CRSwNP patients used OCS compared to No CRSwNP patients, potentially contributing to the observed differences in exacerbation control and lung function. These findings are in line with existing literature indicating that patients with both SEA and CRSwNP may require a more complex management compared to patients with SEA (13–15). In this study, the sustained effectiveness of benralizumab is evidenced by the decrease in AER observed in both patient cohorts at 96 weeks. When comparing the percentages of AER reduction between the two groups (both any and severe AER), benralizumab appeared to be slightly more effective in CRSwNP patients, at both 48 weeks and 96 weeks, with the greatest difference observed at 48 weeks in severe AER (98.8% in CRSwNP vs. 89.5% in No-CRSwNP). The different response to benralizumab was even more pronounced when considering the percentage of patients free from exacerbations. At 96 weeks, 100% of CRSwNP patients were free from severe exacerbations, compared to 90.7% of No-CRSwNP patients; similarly, 88.1% of CRSwNP patients were free from any exacerbations, compared to 66.7% of No-CRSwNP patients. These findings are consistent with the work by Bagnasco et al., in which exacerbation mean reduction was significantly greater in CRSwNP patients than No-CRSwNP patients (23). In the ORBE II study, patients with and without CRSwNP had a similar reduction in the mean number of severe exacerbations; however, a greater percentage of patients with CRSwNP had at least a 50% reduction in exacerbations (32). Our results are also in line with post hoc analyses of the SIROCCO and CALIMA clinical trials, in which the presence of CRSwNP was first identified as a consistent predictor of benralizumab response in terms of exacerbations and other asthma outcomes (20).
The pulmonary function (measured as pre-BD FEV1% predicted) improved already at 16 weeks in both populations. At 48 weeks, CRSwNP patients improved pre-BD FEV1 predicted values compared with No-CRSwNP patients; only at 96 weeks, the two populations reached similar values. These findings suggest that benralizumab may exert rapid effects in patients with CRSwNP, with improvement in pulmonary function observed as early as 16 weeks and maintained throughout the observation period. Conversely, the response to benralizumab in patients without CRSwNP appeared to be more gradual. A plateau was not reached, but a peak was observed at 96 weeks. These findings are consistent with other real-life observations, which showed that benralizumab has a more rapid (22) and pronounced effect on respiratory parameters in CRSwNP than No-CRSwNP patients (22, 32). In contrast, Bagnasco et al. reported a greater improvement in both volume and predicted volume of pre-BD FEV1 in patients without CRSwNP than patients with CRSwNP after 24 weeks of treatment (23). Differences in the length of observation period and clinical characteristics at baseline may account for these discrepant results (23).
A profound OCS-sparing effect of benralizumab was observed in all patients of our study. Although both groups were characterized by a median OCS reduction of 100% at 96 weeks, the action appears to be more pronounced in CRSwNP patients, as this group used a higher median OCS dose at the index date (10 mg) compared with No-CRSwNP patients (6.5 mg) (22, 23, 32).
Both CRSwNP and No-CRSwNP populations achieved asthma control already at 4 weeks after starting benralizumab. This positive outcome persisted throughout the study, with both groups maintaining well-controlled asthma for up to 96 weeks, as evidenced by median ACT scores ≥20. While median ACT scores were almost identical between the two populations, CRSwNP group featured the highest percentage of patients with ACT ≥ 20 at all time points considered up to 96 weeks, compared to No-CRSwNP group. This observation suggests that the presence of CRSwNP may contribute to a more favorable response to benralizumab in terms of asthma control. This finding gains even greater relevance in light of the well-established negative interplay between CRSwNP and asthma; regardless of the presence of nasal polyps, benralizumab could effectively control asthma symptoms, leading to a meaningful improvement of ACT score. Importantly, our results are in line with other real-life studies. Among these, Padilla-Galo et al. showed that a greater proportion of CRSwNP patients (76.9%) achieved a clinically meaningful improvement in asthma control (defined as an increase in ACT score of ≥3 points), compared to patients without CRSwNP (70.0%) (32).
This study describes the long-term improvement of asthma outcomes in CRSwNP and No-CRSwNP patients treated with benralizumab. At 96 weeks, CRSwNP and No-CRSwNP responded similarly to benralizumab; however, more pronounced effects and rapid onset of action have been noticed for all outcomes in CRSwNP compared to No-CRSwNP patients. Differences in clinical characteristics at baseline (i.e., more frequent exacerbations and worse respiratory function in No CRSwNP vs. CRSwNP patients) may have contributed to the enhanced response to benralizumab observed in the CRSwNP population. Nevertheless, previous studies have reported similar results. Since the No CRSwNP group consisted predominantly of female patients, whereas the CRSwNP group had a balanced male-to-female ratio, we cannot exclude the potential influence of this gender imbalance between the two groups on the observed differences in benralizumab effectiveness. Indeed, female sex hormones may play a role in asthma pathogenesis and have been associated with poor outcomes (34). To date, few studies have investigated the impact of gender on biologics treatment response, and existing evidence suggests that gender is not a determining factor in treatment effectiveness (35).
In addition to the literature discussed above, the 24-week data obtained by Nolasco et al. potentially suggest that patients with CRSwNP may experience a quicker response to benralizumab compared to those without (22). Recently, benralizumab capacity to attenuate eosinophilic inflammation has been associated with its ability to restore NK cell function independently of its OCS-sparing effect. In addition, the recovery of NK cell activity exhibited a positive correlation with improvements in FEV1. Restoring NK cell function not only helps improve respiratory function, but also strengthens the body's defenses against infections and relapses, which can trigger severe asthma attacks. This dual benefit highlights how benralizumab can effectively manage severe eosinophilic asthma (18). This unique mode of action of benralizumab may provide a more comprehensive approach to restoring immune balance and achieving asthma control in SEA patients with CRSwNP even in the most difficult to manage patients, such as those with CRSwNP. This potential for enhanced effectiveness is supported by the observation that CRSwNP patients exhibit more severe asthma characteristics at the index date, as evidenced by their higher OCS use, compared to the No-CRSwNP group. The excessive eosinophilic accumulation and T2 inflammation characterizing comorbid patients could further explain the superior and faster effects observed with benralizumab treatment (36). Therefore, the presence of CRSwNP may amplify the positive effects of benralizumab's broad mechanism of action.
This study has a few limitations, the main ones represented by its retrospective observational design, the lack of a control arm and its restriction to data available in routine clinical practice. Nevertheless, this study provides the first long-term, real-world insights into the utilization and effectiveness of benralizumab in a large cohort of SEA patients with and without CRSwNP in Italy. Secondly, the retrospective design and the extended follow-up period have contributed to the loss of patients observed during the follow-up. As highlighted in previous publications (26–28), the absence of statistical analysis represents a considerable drawback in all the ANANKE studies, although it does not diminish the relevance of the observations. Indeed, ANANKE is an observational retrospective study whose primary endpoint is to describe the clinical profile of patients eligible for treatment with benralizumab in a real-world setting; therefore, the analyses of the variables were descriptive only, and no formal hypotheses were pre-specified and tested.
This study explores for the first time the long-term impact of benralizumab on patients with SEA and CRSwNP, compared to those without CRSwNP. The results not only reinforce the established effectiveness of benralizumab in SEA with CRSwNP (25), but also suggest a potentially rapid onset of action in this specific patient population. Further research is warranted to confirm this observation and to understand the mechanisms underlying benralizumab superior efficacy and rapid action in the presence of CRSwNP.
Overall, these results emphasize the importance of considering the presence of comorbidities when making therapeutic decisions, further highlighting the importance of tailored treatment approaches based on individual patient characteristics, ultimately optimizing clinical outcomes in the management of severe asthma.
The data analyzed in this study is subject to the following licenses/restrictions: The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request. Requests to access these datasets should be directed tobWFyaWVsbGFkYW1AaG90bWFpbC5pdA==.
Ethical approval was provided by the ethics committees/institutional review boards at each participating site. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
LB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. EA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. PB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. PC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. GC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. CC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. FM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SRG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. FM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. FM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. GP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. PR: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MR: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. PS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. GS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AV: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MD: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. Financial support for the preparation of the article was provided by AstraZeneca SpA Italy, which had a role in the study design and in the collection and analysis of data.
We are grateful to the patients and physicians who participated in the ANANKE study. Writing and editorial assistance were provided by Raffaella Gatta, PhD, and Alessandra Rossi, PhD, on behalf of EDRA SpA.
SDG received fee for Advisory Board and as Speaker from AstraZeneca, Chiesi, GSK, Novartis, Sanofi. PC received grants and fees as speaker from AstraZeneca-MedImmune, Guidotti-Malesci and GlaxoSmithKline in the last 3 years; GWC received research grants and lecture or advisory board fees from A. Menarini, Allergy—Therapeutics, AstraZeneca-MedImmune, Boehringer Ingelheim, Chiesi, Faes, Genentech, Guidotti-Malesci, GlaxoSmithKline, HAL Allergy, Novartis, Sanofi-Aventis, Sanofi-Genzyme/Regeneron, Stallergenes-Greer, Thermo Fisher, Valeas, Vifor Pharma in the last 3 years; SC received grants and/or personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Glaxo Smith Kline, Guidotti, Menarini, Novartis, Valeas; FDMa received lectures fees at national and international meetings and consultancy fees from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, Dompé, Guidotti/Malesci, GlaxoSmithKline, Menarini, Novartis, and Zambon; SDG received grants and/or personal fees from AstraZeneca, Chiesi, Glaxo Smith Kline, Menarini, Novartis, Sanofi; GP received lecture fees and consultancy fees from AlfaSigma, AstraZeneca, Chiesi, GlaxoSmithKline, Guidotti-Malesci, Menarini, Mundipharma, Novartis, Sanofi, Zambon; PR has been lecturer, speaker, and advisor in scientific meetings and courses under the sponsorship of Almirall, AstraZeneca, Biofutura, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Menarini Group, Mundipharma, and Novartis, her department has received funding from Almirall, Boehringer Ingelheim, Chiesi, Novartis, and Zambon; MR declares grants and personal fees from Boehringer Ingelheim, Roche, AstraZeneca, Novartis, Chiesi, GSK, Menarini, Guidotti, AlfaSigma, Zambon; PS has nothing to declare; AV received fees as speaker/lecturer by AstraZeneca, Chiesi Farmaceutici, GSK, Novartis, Sanofi; MB and SB are AstraZeneca employees; FM received research funding as Principal investigator by AstraZeneca, Chiesi Farmaceutici, Novartis, Sanofi; fees as speaker/lecturer by AstraZeneca, Chiesi Farmaceutici, GlaxoSmithKline, Novartis, Sanofi.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/falgy.2025.1501196/full#supplementary-material
1. Canonica GW, Malvezzi L, Blasi F, Paggiaro P, Mantero M, Senna G, et al. Chronic rhinosinusitis with nasal polyps impact in severe asthma patients: evidences from the severe asthma network Italy (SANI) registry. Respir Med. (2020) 166:105947. doi: 10.1016/j.rmed.2020.105947
2. Laidlaw TM, Mullol J, Woessner KM, Amin N, Mannent LP. Chronic rhinosinusitis with nasal polyps and asthma. J Allergy Clin Immunol. (2021) 9:1133–41. doi: 10.1016/j.jaip.2020.09.063
3. Bachert C, Bhattacharyya N, Desrosiers M, Khan AH. Burden of disease in chronic rhinosinusitis with nasal polyps. JAA. (2021) 14:127–34. doi: 10.2147/JAA.S290424
4. Kanda A, Kobayashi Y, Asako M, Tomoda K, Kawauchi H, Iwai H. Regulation of interaction between the upper and lower airways in united airway disease. Med Sci (Basel). (2019) 7:27. doi: 10.3390/medsci7020027
5. Ryu G, Min C, Park B, Choi HG, Mo J-H. Bidirectional association between asthma and chronic rhinosinusitis: two longitudinal follow-up studies using a national sample cohort. Sci Rep. (2020) 10:9589. doi: 10.1038/s41598-020-66479-8
6. Seccia V, D’Amato M, Scioscia G, Bagnasco D, Di Marco F, Fadda G, et al. Management of patients with severe asthma and chronic rhinosinusitis with nasal polyps: a multidisciplinary shared approach. JPM. (2022) 12:1096. doi: 10.3390/jpm12071096
7. Mullol J, Maldonado M, Castillo JA, Miguel-Blanco C, Dávila I, Domínguez-Ortega J, et al. Management of united airway disease focused on patients with asthma and chronic rhinosinusitis with nasal polyps: a systematic review. J Allergy Clin Immunol. (2022) 10:2438–2447.e9. doi: 10.1016/j.jaip.2022.04.039
8. Hussain M, Liu G. Eosinophilic asthma: pathophysiology and therapeutic horizons. Cells. (2024) 13:384. doi: 10.3390/cells13050384
9. Peters MC, Wenzel SE. Intersection of biology and therapeutics: type 2 targeted therapeutics for adult asthma. Lancet. (2020) 395:371–83. doi: 10.1016/S0140-6736(19)33005-3
10. Bakakos A, Loukides S, Bakakos P. Severe eosinophilic asthma. J Clin Med. (2019) 8:1375. doi: 10.3390/jcm8091375
11. de Groot JC, Ten Brinke A, Bel EHD. Management of the patient with eosinophilic asthma: a new era begins. ERJ Open Res. (2015) 1:00024–2015. doi: 10.1183/23120541.00024-2015
12. Heaney LG, Perez de Llano L, Al-Ahmad M, Backer V, Busby J, Canonica GW, et al. Eosinophilic and noneosinophilic asthma: an expert consensus framework to characterize phenotypes in a global real-life severe asthma cohort. Chest. (2021) 160:814–30. doi: 10.1016/j.chest.2021.04.013
13. Denlinger LC, Phillips BR, Ramratnam S, Ross K, Bhakta NR, Cardet JC, et al. Inflammatory and comorbid features of patients with severe asthma and frequent exacerbations. Am J Respir Crit Care Med. (2017) 195:302–13. doi: 10.1164/rccm.201602-0419OC
14. John Staniorski C, Price CPE, Weibman AR, Welch KC, Conley DB, Shintani-Smith S, et al. Asthma onset pattern and patient outcomes in a chronic rhinosinusitis population: asthma onset and patient characteristics in CRS. Int Forum Allergy Rhinol. (2018) 8:495–503. doi: 10.1002/alr.22064
15. Heffler E, Blasi F, Latorre M, Menzella F, Paggiaro P, Pelaia G, et al. The severe asthma network in Italy: findings and perspectives. J Allergy Clin Immunol. (2019) 7:1462–8. doi: 10.1016/j.jaip.2018.10.016
16. Dagher R, Kumar V, Copenhaver AM, Gallagher S, Ghaedi M, Boyd J, et al. Novel mechanisms of action contributing to benralizumab’s potent anti-eosinophilic activity. Eur Respir J. (2022) 59:2004306. doi: 10.1183/13993003.04306-2020
17. Caminati M, Bagnasco D, Vaia R, Senna G. New horizons for the treatment of severe, eosinophilic asthma: benralizumab, a novel precision biologic. BTT. (2019) 13:89–95. doi: 10.2147/BTT.S157183
18. Bergantini L, d’Alessandro M, Pianigiani T, Cekorja B, Bargagli E, Cameli P. Benralizumab affects NK cell maturation and proliferation in severe asthmatic patients. Clin Immunol. (2023) 253:109680. doi: 10.1016/j.clim.2023.109680
19. Harrison TW, Chanez P, Menzella F, Canonica GW, Louis R, Cosio BG, et al. Onset of effect and impact on health-related quality of life, exacerbation rate, lung function, and nasal polyposis symptoms for patients with severe eosinophilic asthma treated with benralizumab (ANDHI): a randomised, controlled, phase 3b trial. Lancet Respir Med. (2021) 9:260–74. doi: 10.1016/S2213-2600(20)30414-8
20. Bleecker ER, Wechsler ME, FitzGerald JM, Menzies-Gow A, Wu Y, Hirsch I, et al. Baseline patient factors impact on the clinical efficacy of benralizumab for severe asthma. Eur Respir J. (2018) 52:1800936. doi: 10.1183/13993003.00936-2018
21. Menzella F, Ruggiero P, Galeone C, Scelfo C, Bagnasco D, Facciolongo N. Significant improvement in lung function and asthma control after benralizumab treatment for severe refractory eosinophilic asthma. Pulm Pharmacol Ther. (2020) 64:101966. doi: 10.1016/j.pupt.2020.101966
22. Nolasco S, Crimi C, Pelaia C, Benfante A, Caiaffa MF, Calabrese C, et al. Benralizumab effectiveness in severe eosinophilic asthma with and without chronic rhinosinusitis with nasal polyps: a real-world multicenter study. J Allergy Clin Immunol. (2021) 9:4371–4380.e4. doi: 10.1016/j.jaip.2021.08.004
23. Bagnasco D, Brussino L, Bonavia M, Calzolari E, Caminati M, Caruso C, et al. Efficacy of Benralizumab in severe asthma in real life and focus on nasal polyposis. Respir Med. (2020) 171:106080. doi: 10.1016/j.rmed.2020.106080
24. Pelaia C, Crimi C, Benfante A, Caiaffa MF, Campisi R, Candia C, et al. Sustained remission induced by 2 years of treatment with benralizumab in patients with severe eosinophilic asthma and nasal polyposis. Respirology. (2024) 29(10):869–79. doi: 10.1111/resp.14767
25. 2024 GINA MAIN REPORT. Available online at: https://ginasthma.org/wp-content/uploads/2024/05/GINA-2024-Strategy-Report-24_05_22_WMS.pdf. Available at: https://ginasthma.org/wp-content/uploads/2024/05/GINA-2024-Strategy-Report-24_05_22_WMS.pdf (Accessed February 19, 2025).
26. Menzella F, Bargagli E, Aliani M, Bracciale P, Brussino L, Caiaffa MF, et al. Characterization of Italian severe uncontrolled asthmatic patieNts key features when receiving benralizumab in a real-life setting: the observational rEtrospective ANANKE study. Respir Res. (2022) 23:36. doi: 10.1186/s12931-022-01952-8
27. D’Amato M, Menzella F, Altieri E, Bargagli E, Bracciale P, Brussino L, et al. Benralizumab in patients with severe eosinophilic asthma with and without chronic rhinosinusitis with nasal polyps: an ANANKE study post-hoc analysis. Front Allergy. (2022) 3:881218. doi: 10.3389/falgy.2022.881218
28. Vultaggio A, Aliani M, Altieri E, Bracciale P, Brussino L, Caiaffa MF, et al. Long-term effectiveness of benralizumab in severe eosinophilic asthma patients treated for 96-weeks: data from the ANANKE study. Respir Res. (2023) 24:135. doi: 10.1186/s12931-023-02439-w
29. Pini L, Bagnasco D, Beghè B, Braido F, Cameli P, Caminati M, et al. Unlocking the long-term effectiveness of benralizumab in severe eosinophilic asthma: a three-year real-life study. JCM. (2024) 13:3013. doi: 10.3390/jcm13103013
30. Jackson DJ, Pelaia G, Emmanuel B, Tran TN, Cohen D, Shih VH, et al. Benralizumab in severe eosinophilic asthma by previous biologic use and key clinical subgroups: real-world XALOC-1 programme. Eur Respir J. (2024) 64:2301521. doi: 10.1183/13993003.01521-2023
31. Lombardo N, Pelaia C, Ciriolo M, Della Corte M, Piazzetta G, Lobello N, et al. Real-life effects of benralizumab on allergic chronic rhinosinusitis and nasal polyposis associated with severe asthma. Int J Immunopathol Pharmacol. (2020) 34:205873842095085. doi: 10.1177/2058738420950851
32. Padilla-Galo A, Moya Carmona I, Ausín P, Carazo Fernández L, García-Moguel I, Velasco-Garrido JL, et al. Achieving clinical outcomes with benralizumab in severe eosinophilic asthma patients in a real-world setting: ORBE II study. Respir Res. (2023) 24:235. doi: 10.1186/s12931-023-02539-7
33. Santomasi C, Buonamico E, Dragonieri S, Iannuzzi L, Portacci A, Quaranta N, et al. Effects of benralizumab in a population of patients affected by severe eosinophilic asthma and chronic rhinosinusitis with nasal polyps: a real life study. Acta Biomedica Atenei Parmensis. (2023) 94:e2023028. doi: 10.23750/abm.v94i1.13474
34. Jenkins CR, Boulet L-P, Lavoie KL, Raherison-Semjen C, Singh D. Personalized treatment of asthma: the importance of sex and gender differences. J Allergy Clin Immunol. (2022) 10:963–971.e3. doi: 10.1016/j.jaip.2022.02.002
35. Benoni R, Panunzi S, Batani V, Moretti F, Fuggini S, Todesco M, et al. Clinical response to biologicals for severe asthma: any relevance for sex in different age ranges? ERJ Open Res. (2022) 8:00670–2021. doi: 10.1183/23120541.00670-2021
Keywords: benralizumab, asthma, eosinophils, CRSwNP, long-term
Citation: Brussino L, Aliani M, Altieri E, Bracciale P, Caiaffa MF, Cameli P, Canonica GW, Caruso C, Centanni S, De Michele F, Del Giacco S, Di Marco F, Malerba L, Menzella F, Pelaia G, Rogliani P, Romagnoli M, Schino P, Schroeder JW, Senna G, Vultaggio A and D’Amato M (2025) Durability of benralizumab effectiveness in severe eosinophilic asthma patients with and without chronic rhinosinusitis with nasal polyps: a post hoc analysis from the ANANKE study. Front. Allergy 6:1501196. doi: 10.3389/falgy.2025.1501196
Received: 24 September 2024; Accepted: 25 February 2025;
Published: 20 March 2025.
Edited by:
Li Ping Chung, Fiona Stanley Hospital, AustraliaReviewed by:
Jesús Miguel García-Menaya, University Hospital of Badajoz, SpainCopyright: © 2025 Brussino, Aliani, Altieri, Bracciale, Caiaffa, Cameli, Canonica, Caruso, Centanni, De Michele, Del Giacco, Di Marco, Malerba, Menzella, Pelaia, Rogliani, Romagnoli, Schino, Schroeder, Senna, Vultaggio and D’Amato. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria D’Amato, bWFyaWVsbGFkYW1AaG90bWFpbC5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.