- 1Guangdong Provincial Key Laboratory of Allergy & Clinical Immunology, Department of Allergy, The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
- 2Department of Nuclear Medicine, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
Allergic rhinitis (AR) is a global disease with high prevalence. It reduces the patient's quality of life seriously. The health care and management of AR was also a heavy social burden. Specific immunotherapy (SIT) is the only curative treatment for AR that may alter the natural course of this disease. However, acceptance and compliance of SIT in AR patients are still not high and many patients are not effectively controlled. Disease prevention based on known risk factors is much more cost-effective compared to post-diagnosis treatment. There have been some reports on the risk factors of AR up to now, but the information is fragmented. This review systemically clarified the risk factors of AR including hereditary factors and family history, maternal situation & mode of delivery and feeding, personal characteristics, nutrition and food intake, personal behavior and habits, acquired environmental and chemical exposure, diseases and health status. The preventive strategies were also proposed briefly. This review was hopeful to improve people's awareness of the risk factors of AR and put forward AR prevention.
1 Introduction
Allergic rhinitis (AR) is a global disease characterized by symptoms of sneezing, rhinorrhea, nasal obstruction, and pruritus (1, 2). It affects about 5%–50% population in the world (3, 4). AR is associated with the onset of rhinosinusitis and asthma. They are all united airways disease and shared some similar mechanism according to the concept that upper and lower airways form a single organ (5). AR seriously reduces the quality of life of AR patients (6, 7). In addition, the health care and management of AR cause a large economic burden to the society (8).
AR is known to be a disease mediated by Th2 type immune response. Genetic factors and environmental factors are both involved in the occurrence and development of AR, but its specific pathogenesis still needs further research (9). Pharmacology and specific immunotherapy can effectively alleviate the symptoms of AR and slow down the process of AR, but there are still many AR patients who are not under good control with low and bad compliance for the treatment in the real world (9, 10). Compared to treatment after diagnosis, prevention of AR based on the potential risk factors should be much more cost-effective and valuable. However, practice on AR prevention is still blank now. So far as we know, there have been some reports on the risk factors of AR. The information is fragmented, and readers couldn't clearly and systematically understand. This review clarified the risk factors of AR through the human's whole life (Figure 1). The important risk factors of AR with larger consensus are discussed, while some ambiguous viewpoints are not involved. The preventive strategies based on these risk factors were also proposed briefly. We hope this narrative review be helpful to improve people's awareness of the risk factors of AR and put prevention strategies to move forward.
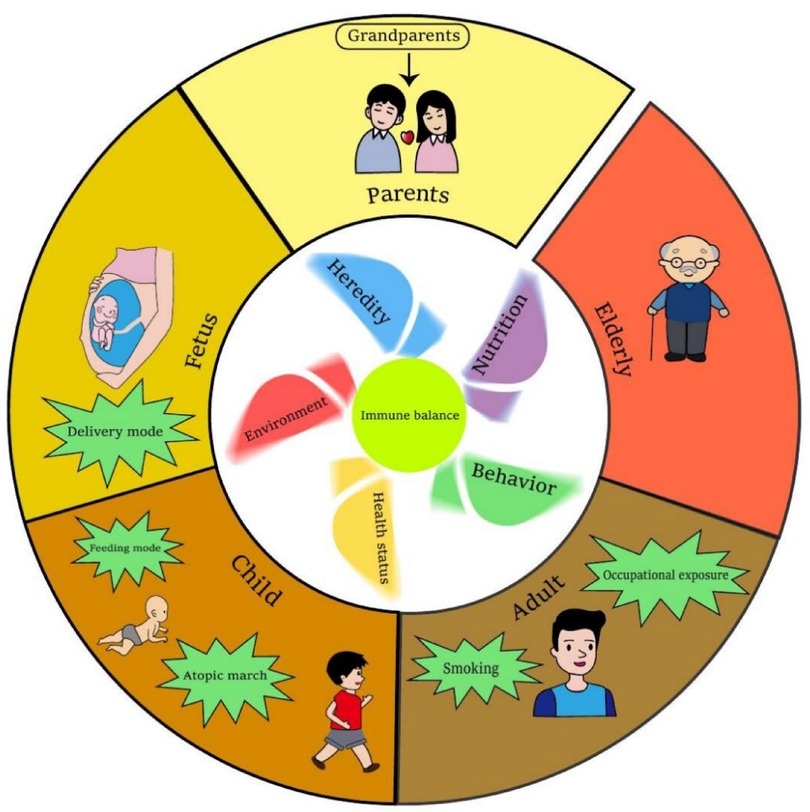
Figure 1. Risk of AR in humans’ whole life. The windmill in the inner ring has five blades which stand for heredity, environment, behavior, nutrition and health status of an individual. All of these aspects will influence the immune balance of human. Abnormal of these five blades contribute more or less to the immune imbalance, which induce AR development. The outer ring stands the different stages of a human's whole life. Main risk factors of AR at certain stage are highlighted. This figure was created with Adobe Illustrator.
2 Hereditary factors/family history of allergies
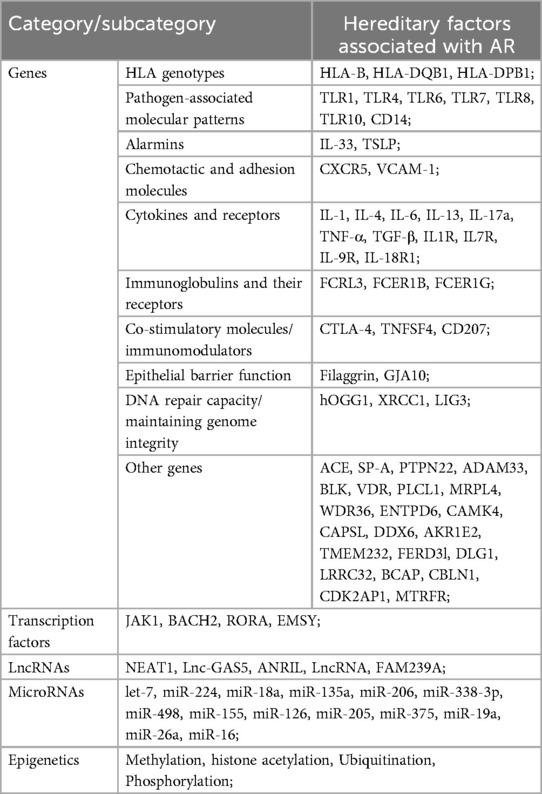
Hereditary factors play an important role in allergic diseases including AR. Parents with allergic diseases will highly increase the risk of allergic rhinitis in their offspring (11). Large-scale genome-wide association studies (GWAS) indicated that the single nucleotide polymorphisms of many human genes are associated with AR (12, 13). As shown in Table 1, these genes are divided into several categories by their feature and functions. There are pathogen-associated molecular patterns: such as TLR1, TLR4, TLR6, TLR7, TLR8, TLR10, and CD14; alarmins such as IL-33 and TSLP; chemotactic and adhesion molecules including CXCR5, and VCAM-1; cytokines and their receptors such as IL-1, IL-4, IL-6, IL-13, IL-17a, TNF-α, TGF-β, IL1R, IL7R, IL-9R, IL-18R1; immunoglobulin receptors including FCRL3, FCER1B and FCER1G; co-stimulatory molecules and immunomodulators such as CTLA-4, TNFSF4, CD207; epithelial barrier function related genes such as filaggrin and GJA10; genes related to DNA repair capacity and maintaining genome integrity such as hOGG1, XRCC1and LIG3. Other genes such as ACE, SP-A, PTPN22, ADAM33, etc. are also involved.
These genes mentioned above are involved in cell migration, chemotaxis and adhesion, maturation and activation of immune cells, antigen presentation, pathogen recognition and activation of innate immunity, pro-inflammatory effects, T helper cell function and regulation, immunoregulation, etc. Transcription factors or transcription repressors such as JAK1, BACH2, RORA, and EMSY are also reported in risk of AR, as well as some lncRNAs and microRNAs listed in Table 1. Gene-environment interaction effects and gene epigenetic modification such as methylation, histone acetylation, ubiquitination, and phosphorylation are reported strongly associated with AR (14). Furthermore, polygene rather than a single gene contributed to the occurrence and development of AR. Shared genetic mechanisms for AR and other allergic diseases such as asthma and atopic dermatitis also existed (1). And only a few genes related to disease-specific effects in AR. Although hereditary factors are critical risk factors for AR; we could not do too much for that except for reducing the negative impact of gene-environment interaction and recognizing the necessity of AR prevention.
3 Maternal situation & mode of delivery and feeding
Pregnancy is a critical time window for the early life programming period of the fetal immune system (15). Maternal situations such as food and nutrition intake, behaviors, health status, and living environment as well as the mode of delivery and feeding are all associated with the risk of AR in their offspring (as shown in Table 2).
A healthy maternal diet during pregnancy can reduce the risk of allergic rhinitis in offspring, otherwise, the opposite is also true (16). Healthy food includes a variety of seafood represented by fish, fresh fruits and vegetables, and a variety of dairy products. Beneficial components in food include vitamin D, n-3 polyunsaturated fatty acids, probiotics, etc. Unhealthy foods include a variety of improperly cooked foods or diets high in meat, carbonated drinks, and so on. Harmful components of food include heterocyclic amines, polycyclic aromatic hydrocarbons, high concentrations of sugar, etc. More detailed information was illustrated together in the section on food and nutrition intake.
Environmental tobacco smoke exposure during pregnancy may affect the occurrence and development of allergic diseases in children through epigenetic mechanisms, including histone acetylation, microRNA expression, and DNA methylation (83). The harmful components nicotine, which up-regulates a variety of inflammatory factors including IL-8 can cross the placenta and affect the development of the fetal immune system (17). Maternal short sleep duration, physical inactivity, and excessive screen exposure during pregnancy were also associated with an increased risk of AR in offspring (18). The use of oral contraceptives before pregnancy may lead to epigenetic changes, and these changes may be transmitted to the embryo, leading to the occurrence of allergic diseases such as rhinitis in childhood. Furthermore, taking contraceptives lead to abnormal secretion of progesterone and other hormones in the mother's body, which may not only affect the development of fetal immunity but also alter the composition of the vaginal microbiome (19). Other harmful substances such as phthalates and their metabolites, cadmium(Cd), and plumbum(Pb) in maternal blood also have certain effects on the pathogenesis of allergic rhinitis in offspring (20, 48).
Maternal exposure to air pollutants such as PM2.5, mold, volatiles from newly refurbished furniture, and toxic organic solvents during pregnancy may increase the risk of rhinitis in offspring (21, 84). Studies have shown that prenatal exposure to air pollutants leads to maternal Th1/Th2 imbalance through the induction of IL-25 and IL-13 (21, 85). However, prenatal exposure to a farm environment is protective, which can be explained partly by high exposure to microorganisms, particularly bacterial endotoxin in the farm environment, which is helpful to prime the development of fetal immunity (22).
Maternal anxiety and depression during pregnancy, gestational diabetes mellitus (GDM), as well as obesity, can increase the risk of allergic diseases in offspring (23–25). Depression and anxiety are often accompanied by persistent hypersecretion of cortisol (23). Mothers with GDM exhibit elevated levels of TNF-a, leptin, and visfatin, while adiponectin was decreased (24). Maternal obesity during pregnancy is always accompanied by insufficient intake of vitamin D and an imbalance of intestinal flora, which can increase the risk of AR (25).
The mode of delivery was reported to affect the prevalence of AR in the offspring. The cesarean section increases the risk, while vaginal delivery is the opposite (26–29). Children delivered by cesarean section had no opportunity to be inoculated vaginally with beneficial bacteria from their mothers at birth, such as bifidogenic microflora, which makes them hard to build a balance of gut microbiota in early life (29). The imbalance of gut microbiota impairs the natural development of the immune system and increases the risk of allergic disease in the future.
Breastfeeding reduces the risk of AR in offspring after birth (30). Relevant mechanisms were systematically introduced as follows: 1. Breastfeeding promotes the maturation of the gastrointestinal mucosa. 2. Breastfeeding can reduce the occurrence of infectious diseases in infants and regulate their intestinal flora, thereby reducing the possibility of sensitization. These benefit from the secreted antibodies in the breast milk. 3. Human milk contains functional immunomodulatory and anti-inflammatory factors (31).
4 Personal characteristics
Personal situations such as age, gender, ABO blood group, and obesity are all related to the risk of AR.
The prevalence of AR in the population increases steadily with age in the early life period, reaches the peak at about 20 years old, and then gradually decreases, reaching the lowest at 65–85 years old. This trend happened both in male and female (86). IgE against specific allergens decreases with age in elderly AR patients (36). Rhinorrhea and nasal congestion are the most common symptoms in elderly and adult AR patients, respectively. Compared to adult patients, elderly patients had a lower response to 4 weeks of AR treatment recommended by existing guidelines (87).
There is a “gender shift” in AR prevalence: from a male predominance before puberty and finally to a female predominance after puberty, in which sex hormone levels may play a key role (32, 33). Studies have shown that androgen can reduce allergic airway inflammation by increasing the T-reg/Th2 ratio in the lung (34). On the contrary, estrogen increases the risk of AR by mechanisms such as promoting the polarization of Th2, priming B cells with the potential for IgE expression, and increasing the degranulation of mast cells and basophils (35). The dynamic changes of sex hormones make the “gender shift” in AR prevalence. ABO blood groups were also found to be associated with allergic diseases. Blood group O had greater susceptibility to AR and asthma, while non-O blood groups were related to AD (88, 89). This is possibly related to the diverse glycoconjugate expression profile and content induced by the ABO gene (37).
The risk of AR is significantly increased in obese people, which may be related to the endocrine function of adipose tissue and the change in immune response in obese people (38, 39). Studies indicated that leptin secreted by adipose tissue in obese AR patients reduced immune tolerance to allergens by induction of proinflammatory cytokines, such as IL-6 and TNF-a, enhancement of Th2 and Th17 inflammation, and inhibition of T-reg cell function (90, 41).
5 Nutrition and food intake
Adequate vitamin supplementation is helpful for AR. Specifically, vitamin D mitigates allergic inflammation through augmentation of IL-10 production, suppression of TNF, IL-2, and IL-5, and inhibition of excessive macrophage activation (42, 43). Vitamin C functions as an important antioxidant to diminish airway inflammation (44). Vitamin E may alleviate AR symptoms by managing IL-4, IL-5, and other Th2-type cytokines, serum IgE, and histamine levels (45).
Dietary fiber demonstrates a protective role for AR (46). A plant-based, high-fiber regimen notably invigorated favorable gut bacteria, reducing circulation levels of systemic inflammatory markers including IL-6 (47). The intake of trace elements is also associated with AR. Lower serum concentrations of selenium (Se) and zinc (Zn) were found in AR patients, while copper (Cu) and nickel (Ni) were increased on the contrary (49, 91). Selenium triggers the human body's expression of glutathione peroxidase, further participating in inflammation regulation and affecting the Th1/Th2 balance (45). Zinc supplementation diminished serum IgE levels and downregulated IL-6 and TNF-α secretion in mice (92). Cadmium influences immune cell subset activation and IgE production, while blood plumbum positively correlates with eosinophil IgE levels and T cell dysregulation (48).
Probiotics supplementation was reported to lessen AR risk, which can help to keep the balance of intestinal flora and inhibit inflammation by immune protection and T-reg regulation (50, 51). A high abundance of Prevotella may have a protective effect on the development of AR (93). Lactis BB12 and E. faecium L3 supplementation mitigate AR symptoms indicating the benefit of probiotics for allergic diseases (94).
High-fat foods have demonstrated an enhancement of neutrophilic airway inflammation (52). While polyunsaturated fatty acids (PUFAs) such as eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and arachidonic acid have protective effects on AR (53, 54). Fast food, which typically includes lots of saturated fatty acids, trans fatty acids, carbohydrates, and preservatives, is linked to an augmented risk of AR by potentially influencing the body's inflammatory and immune responses (95, 96). In addition, unhealthy dietary habits such as snacking between meals and eating dinner less than 1 h before bedtime both increased the risk of AR (96).
6 Personal behavior and habits
Individual behavior, habits, and lifestyle patterns such as smoking, physical activity, and life rhythm can affect the development of allergic rhinitis.
Smoking and exposure to second-hand smoke are risk factors for AR, and the types of cigarettes are not limited to traditional cigarettes but also include heated tobacco products, electronic cigarettes, and so on (55, 56). Tobacco smoke contains many toxic chemicals and carcinogens such as acrolein, acetaldehyde, and formaldehyde, which directly irritate the respiratory tract, and may also affect inflammatory processes in human and animal studies. It leads to increased eosinophil and affects the recruitment of inflammatory cells through the secretion of cytokines and chemokines, leading to airway remodeling (56). It has also been shown that cigarette smoke exposure leads to a significant increase in nasal levels of various cytokines such as IL-4, IL-5, and IL-13, which can lead to Th2 polarization (56, 97).
Physical activity correlates with the risk of AR. Moderate exercise mitigates, whilst intense exercise and sedentary conditions precipitate the opposite effect (57–59). Moderate exercise attenuates the oxidative stress response and regulates the immune cell expression profile, including reducing IL-4, IL-5, and IgE antibody production, consequently lessening AR severity and diminishing AR incidence (98). Intense exercise elevates the probability of allergic rhinitis. Intense exercise heightens Th2 cytokine production and elevates the count of nasal mucosal neutrophils, leading to the stimulation of T cells and promoting eosinophil aggregation (58).
Disturbances or disruptions in circadian clock activity will enhance the susceptibility and severity of allergic diseases (60, 61). Any disruption in the biological clock results in alterations in the metabolic rhythm and hormone levels such as glucocorticoids, affecting the metabolism, expression, and distribution of several immune cells like mast cells and eosinophils. These alterations in immune cell behavior heighten allergic risks.
7 Acquired environmental and chemical exposure
Living and occupational environment as well as chemical exposure are closely related to the risk of AR. High humidity that benefits mold growth, an environment with severe air pollution or with a high concentration of allergens, ambient temperature changes, and extremely hot weather are all the risks of AR (62–67). However, household use of temperature and humidity control machines and air purifiers all have a protective effect (99, 100).
High humidity environment fosters mold growth. Specifically, Curvularia lunata, Aspergillus spp., Streptomyces, and Aspergillus versicolor in the atmosphere can augment the probability of AR (101). Microbial volatile organic compounds (MVOCs) such as extracellular polysaccharides, and mycotoxins in the fungal cell wall architecture may instigate detrimental immune responses in humans. Mold exposure may lead to repeated irritation of the respiratory tract and immune activation, thereby leading to long-term inflammatory responses (62).
Air contaminants such as sulfur dioxide, PM2.5, assorted dust, and diesel exhaust particulates are exceedingly concentrated in the atmosphere adjacent to traffic thoroughfares with intense traffic volumes. These airborne pollutants not only induce direct harm and irritation to the respiratory apparatus but also impact the body's oxidative stress and inflammatory reaction. Air pollutants yield reactive oxygen species (ROS) and free radicals damage airway epithelial cells leading to airway inflammation and airway hyperresponsiveness. Inhalation of air pollutants may also affect the function of macrophages, neutrophils, T cells, and other immune cells in the body, affecting the production and secretion of a variety of cytokines, adhesion factors, and proteases (64, 102, 103).
Living on a farm and maintaining domesticated animals are vulnerability factors for AR, which may be related to increased exposure to endotoxin, pollen, animal dander, and other allergens (104, 105). However, this pattern can be reversed during one's early life. According to the hygiene hypothesis, preschool exposure to these substances in a “dirty” environment can facilitate the development and maturation of children's immune systems, which protect them from future allergies and sensitivity (106). On the contrary, deficient exposure could escalate the susceptibility to allergic diseases (107).
Environmental temperature changes and extreme heat events are associated with increased AR. Researchers propose that extreme heat events potentially increase human exposure to high concentrations of pollen allergens by affecting the plant flowering period (66, 67).
Some special occupational personnel, such as hairdressers, bakers, animal breeders who frequently contact with animals, cleaning workers, farmers, pulp mill workers, and automobile repair workers have a high incidence of AR (68–75). Occupational exposure to various prevalent allergens, including dust mites, dust, dander, and pollen will increase the risk of sensitivity, which can explain the higher risk of AR among workers engaged in animal management as well as bakers (2). Hairdressers and auto repair workers have a high frequency of exposure to chemical reagents such as perfume, cosmetics, bleach, and synthetic metalworking fluid. Cleaning workers and farmers have an increased exposure to cleaning agents and pesticides, respectively. Workers in pulp manufacturing plants are exposed to ozone more frequently, and ozone may promote the polarization of Th2 cells in the body (108).
Chemical exposure is similar to environmental exposure. The usage of cleaning products, pesticides, passive intake of phthalates, or taking some special medicine such as antibiotics, antacids, and acetaminophen are all risks of AR (72, 73, 76–82). Chemical cleaners, such as diluted bleach, not only cause strong irritation to the respiratory tract but also may act as adjuvants to affect the immune response (72, 109). Certain pesticides, especially organophosphorus pesticides, inhibit acetylcholinesterase activity, leading to the accumulation of acetylcholine, which induced the abnormal of nasal glands (77, 110). Phthalates, pervasive plasticizers in everyday life, particularly di-2-ethylhexyl phthalate (DEHP) could potentially heighten the risk of allergic reactions (111). The use of antibiotics such as cephalosporins and macrolides especially in childhood caused gut flora imbalance and maturation disorders of the immune system, which in turn facilitated the Th2 immune response and development of allergic diseases (80, 112, 113). Antacids not only cause intestinal dysbiosis but also increase sensitivity to ingested antigens by reducing protein breakdown in the stomach (79). Acetaminophen may affect cyclooxygenase-2 activity by promoting prostaglandin E2 synthesis, which in turn promotes the Th2 response (82).
8 Diseases and health status
8.1 Psychiatric diseases and AR
Research demonstrates that psychiatric diseases such as anxiety, depression, attention deficit hyperactivity disorder (ADHD), and post-traumatic stress disorder (PTSD) augment the vulnerability to AR (114–117). The paraventricular nucleus of the hypothalamus is commonly activated and the secretion of corticotropin-releasing hormone (CRH) is increased in patients with anxiety and depression. CRH stimulates the secretion of corticosteroids in a series of pathways, which in turn promotes the production of Th2 cytokines. CRH also increased epinephrine and norepinephrine production, thereby inhibiting IL-12 production (115). ADHD may be a high inflammation and immune-associated disease (118). A study found that the positive rate of skin prick tests for a variety of allergens in ADHD patients was greater than that in the control group (119). PTSD is not only involved in promoting low-grade systemic inflammation but also may lead to the methylation of immune-related genes, which increases the risk of allergy (117). Diseases and health status associated with AR are shown in Table 3.
8.2 Atopic march and AR
The theory of atopic march indicates that allergic diseases occur in a time order. Atopic dermatitis and food allergy in infancy would gradually develop into allergic rhinitis and allergic asthma when they grow up (120). Previous studies also certified that atopic dermatitis and eczema increased the risk of AR (121). These patients demonstrate compromised epithelial functionality, augmented epidermal sensitivity, and IgE production (122). Post-sensitization, T cells can infiltrate the respiratory tract and instigate hypersensitive reactions in the upper airway (123). Although the mechanisms underlying the atopic march are not fully clear. It still provides a perspective for the prediction and prevention of allergic diseases.
8.3 Autoimmune diseases and AR
Autoimmune diseases such as Kawasaki disease, alopecia areata, psoriasis, and ankylosing spondylitis are associated with the risk of AR (124–127). Kawasaki disease instigates cytokine storm, highly activating the immune system. Levels of IgE antibodies, Th2 cytokines IL-4, IL-5, and eosinophil cationic protein in patients are all elevated, thereby triggering Th2 immune-related responses in vivo (124, 144). Immunological dysregulations with overexpressed of both TH1 and TH2-related markers existed in patients with alopecia areata. The prevalence of AR and asthma is higher in the population with alopecia areata than in the control (125, 145). Psoriasis with high Th17 responses was also found to be associated with allergic rhinitis and asthma (126). Whereas ankylosing spondylitis augments Th2 responses, thereby escalating AR risk (127).
8.4 Microbial pathogens infection and AR
Virus infections were associated with AR susceptibility. One important virus is Rhinovirus. Rhinovirus (RV) infection may destroy the integrity of the nasal mucosal barrier and facilitate the allergens to penetrate the tissue (128, 129). HIV infection could foster IgE synthesis and augment the Th2 response, which increases the likelihood of developing allergic diseases (130, 131). AR prevalence also escalates in hepatitis B virus (HBV) carriers. It might be attributed to the absence of HBV surface antigen-specific Th1-type immune responses among carriers, which potentially leads to an enhanced Th2 response (132). However, infection of Human T-lymphotropic virus type I (HTLV-I) and measles virus exhibit beneficial effects against AR by enhancing the Th1 response and inhibiting the Th2 response in humans (133, 134).
Specific bacterial infection influence AR diversely. Concurrent research revealed that individuals with AR possess elevated S. aureus in their noses. S. aureus-generated enterotoxin can function as a superantigen to elevate IL-8, and IL-13 levels and diminish IFN-γ secretion that intensifies the Th2 response in humans (135). Mycobacterium tuberculosis and Salmonella, Helicobacter pylori, and Chlamydia pneumoniae infections are reported to lessen the risk of AR (139–146). Upon host infection, the Th1 response heightens to facilitate pathogen elimination, thereby reducing the Th2 response.
Parasitic infections such as Schistosoma mansoni, Schistosoma japonicum, Angiostrongylus cantonensis, and Schistosoma haematobium mostly are “protective” for allergic diseases (140, 141). Studies of the inverse association between parasitic infections and allergy indicated that parasitic infections may “protect” allergy by inducing suppressive cytokines released from regulatory lymphocytes, ablating the eosinophil response, making effector T helper 2 (Th2) cells enter anergic state and switching antibody production from IgE to the non-inflammatory isotype IgG4 (142, 143).
9 Prevention strategies for AR based on risk factors
Practice of AR prevention is mostly stay in the concept until now. With a deepening understanding of the pathogenesis and risk factors of AR, several corresponding prevention strategies for AR are proposed as follows. Although some strategies seem avant-garde and need further certification by evidence-based medicine.
1. AR exhibits inheritance patterns. Several susceptibility genes with AR were found by GWAS. Adenovirus-mediated gene therapy was proposed to be a promising therapy for allergy treatment. Gene therapy against these abnormal genes before AR onset based on a personal gene map provides a feasible way not only for AR treatment but also for prevention if the therapy is safe and permitted by medical ethics.
2. Pregnant parents especially mothers have a great influence on the physical condition of their offspring. Maintaining maternal health during this period was very important. Pregnant women must adopt a nutritious and balanced diet, including the consumption of more fresh fruits, vegetables, fish, and yogurt, while diminishing foods with high sugar and fat intake, which is helpful to supplement beneficial ingredients as well as to reduce the harmful ingredients. Moreover, regular exercise, ideal body weight, low stress, and a good mood during pregnancy will foster a favorable environment for the development of the fetal immune system. Avoidance of smoke or other pollutants during pregnancy is beneficial due to the detrimental impact of these substances on both inflammatory responses and fetal growth via the placenta.
3. Vaginal delivery and postnatal breastfeeding are instrumental in reducing allergic disease risks. Because balanced vaginal microbiota supports immune system development and breast milk provides nutrients and postpones allergen exposure, they offer additional protection. It is necessary to encourage mother to choose vaginal delivery and postnatal breastfeeding for their offspring.
4. Nasal recurrent infection by microbial pathogens may destroy the integrity of the nasal mucosal barrier, making humans much more susceptible to environmental allergens. In addition, some ingredients of these pathogens are potentially allergens which can cause sensitization (147). Therefore, prevention of nasal infections through vaccination against certain pathogens in early life may be benefit for AR prevention. It's worthy of further study.
5. The phenomenon of atopic march existed and the theory was reasonable. According to these, early good control of allergic diseases such as atopic dermatitis, eczema, and food allergy in infancy is helpful to prevent the possibility of allergic rhinitis and allergic asthma in one's childhood.
6. A healthy lifestyle for an individual such as a normal sleep and rest schedule, moderate physical exercise, balanced nutritional intake and healthy eating habits, a positive attitude towards life, and maintaining a good mental state are all crucial for keeping healthy. Keeping in a good environment, no smoking, less exposure to toxic and harmful substances, safety protection for some special occupational exposure, early exposure to allergens during early life (especially the immune establishment period), avoiding excessive hygiene, and maintaining the diversity and homeostasis of gut flora is helpful for people to keep away from AR and other allergic diseases.
10 Summary
The risk factors of AR were clarified systemically in this review. Hereditary factors and family history, maternal situation & mode of delivery and feeding, personal characteristics, nutrition and food intake, personal behavior and habits, acquired environmental and chemical exposure, diseases and health status are all involved in disease susceptibility of AR. It seems that we cannot do too much about our genes, sex, and blood group, but we can choose a healthy lifestyle, no matter whether we are pregnant parents or not. We should intake balanced nutrition and develop good living habits. We can improve our living and working environment which are very important to us. All of these can help us to keep good immunity and healthy status. These can help us to keep away from AR and other diseases to our best. We hope this review helped improve people's awareness of the risk factors of AR and put forward AR prevention steps. It's a positive response to advocacy of European Forum for Research and Education in Allergy and Airways diseases group (EUFOREA) that aim to reduce the prevalence and burden of chronic allergic and respiratory diseases via research, education, and advocacy (148).
Author contributions
RC: Writing – original draft. WA: Writing – original draft. XL: Funding acquisition, Writing – original draft, Writing – review & editing. JY: Funding acquisition, Writing – original draft, Writing – review & editing. YH: Conceptualization, Funding acquisition, Writing – review & editing. JZ: Conceptualization, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82370037, 82100025, 82371797), General Guidance Project of Guangzhou Municipal Health Commission (No. 20241A010065), and the National Science and Technology Innovation 2030 Major Project (2023ZD0406303).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Meng Y, Wang C, Zhang L. Recent developments and highlights in allergic rhinitis. Allergy. (2019) 74(12):2320–8. doi: 10.1111/all.14067
2. Bousquet J, Anto JM, Bachert C, Baiardini I, Bosnic-Anticevich S, Walter Canonica G, et al. Allergic rhinitis. Nat Rev Dis Primers. (2020) 6(1):95. doi: 10.1038/s41572-020-00227-0
3. Mims JW. Epidemiology of allergic rhinitis. Int Forum Allergy Rhinol. (2014) 4(Suppl 2):S18–20. doi: 10.1002/alr.21385
4. Wise SK, Damask C, Roland LT, Ebert C, Levy JM, Lin S, et al. International consensus statement on allergy and rhinology: allergic rhinitis—2023. Int Forum Allergy Rhinol. (2023) 13(4):293–859. doi: 10.1002/alr.23090
5. Yii ACA, Tay TR, Choo XN, Koh MSY, Tee AKH, Wang DY. Precision medicine in united airways disease: a “treatable traits” approach. Allergy. (2018) 73(10):1964–78. doi: 10.1111/all.13496
6. Meltzer EO. Allergic rhinitis. Immunol Allergy Clin North Am. (2016) 36(2):235–48. doi: 10.1016/j.iac.2015.12.002
7. Scadding GK, Smith PK, Blaiss M, Roberts G, Hellings PW, Gevaert P, et al. Allergic rhinitis in childhood and the new EUFOREA algorithm. Front Allergy. (2021) 2:706589. doi: 10.3389/falgy.2021.706589
8. Colas C, Brosa M, Anton E, Montoro J, Navarro A, Dordal MT, et al. Estimate of the total costs of allergic rhinitis in specialized care based on real-world data: the FERIN study. Allergy. (2017) 72(6):959–66. doi: 10.1111/all.13099
9. Greiner AN, Hellings PW, Rotiroti G, Scadding GK. Allergic rhinitis. Lancet. (2011) 378(9809):2112–22. doi: 10.1016/S0140-6736(11)60130-X
10. Scheire S, Germonpre S, Mehuys E, Van Tongelen I, De Sutter A, Steurbaut S, et al. Rhinitis control and medication use in a real-world sample of patients with persistent rhinitis or rhinosinusitis: a community pharmacy study. J Allergy Clin Immunol Pract. (2024) 12(7):1865–76.e6. doi: 10.1016/j.jaip.2024.04.031
11. Baumann LM, Romero KM, Robinson CL, Hansel NN, Gilman RH, Hamilton RG, et al. Prevalence and risk factors for allergic rhinitis in two resource-limited settings in Peru with disparate degrees of urbanization. Clin Exp Allergy. (2015) 45(1):192–9. doi: 10.1111/cea.12379
12. Choi BY, Han M, Kwak JW, Kim TH. Genetics and epigenetics in allergic rhinitis. Genes (Basel). (2021) 12(12):2004. doi: 10.3390/genes12122004
13. Waage J, Standl M, Curtin JA, Jessen LE, Thorsen J, Tian C, et al. Genome-wide association and HLA fine-mapping studies identify risk loci and genetic pathways underlying allergic rhinitis. Nat Genet. (2018) 50(8):1072–80. doi: 10.1038/s41588-018-0157-1
14. North ML, Jones MJ, MacIsaac JL, Morin AM, Steacy LM, Gregor A, et al. Blood and nasal epigenetics correlate with allergic rhinitis symptom development in the environmental exposure unit. Allergy. (2018) 73(1):196–205. doi: 10.1111/all.13263
15. Norback D, Zhang X, Tian L, Zhang Y, Zhang Z, Yang L, et al. Prenatal and perinatal home environment and reported onset of wheeze, rhinitis and eczema symptoms in preschool children in northern China. Sci Total Environ. (2021) 774:145700. doi: 10.1016/j.scitotenv.2021.145700
16. Baiz N, Just J, Chastang J, Forhan A, de Lauzon-Guillain B, Magnier AM, et al. Maternal diet before and during pregnancy and risk of asthma and allergic rhinitis in children. Allergy Asthma Clin Immunol. (2019) 15(1):40. doi: 10.1186/s13223-019-0353-2
17. Li X, Jing R, Feng S, Zhang H, Zhang J, Li J, et al. Association between prenatal or postpartum exposure to tobacco smoking and allergic rhinitis in the offspring: an updated meta-analysis of nine cohort studies. Tob Induc Dis. (2022) 20:37. doi: 10.18332/tid/146905
18. Chen Y, Lyu J, Xia Y, Zhu J, Tong S, Ying Y, et al. Effect of maternal sleep, physical activity and screen time during pregnancy on the risk of childhood respiratory allergies: a sex-specific study. Respir Res. (2020) 21(1):230. doi: 10.1186/s12931-020-01497-8
19. Keski-Nisula L, Pekkanen J, Xu B, Putus T, Koskela P. Does the pill make a difference? Previous maternal use of contraceptive pills and allergic diseases among offspring. Allergy. (2006) 61(12):1467–72. doi: 10.1111/j.1398-9995.2006.01201.x
20. Wang JQ, Li ZJ, Gao H, Sheng J, Liang CM, Hu YB, et al. Mediation effects of placental inflammatory transcriptional biomarkers on the sex-dependent associations between maternal phthalate exposure and infant allergic rhinitis: a population-based cohort study. Biomed Environ Sci. (2022) 35(8):711–21. doi: 10.3967/bes2022.093
21. Pedersen C-ET, Eliasen AU, Ketzel M, Brandt J, Loft S, Frohn LM, et al. Prenatal exposure to ambient air pollution is associated with early life immune perturbations. J Allergy Clin Immunol. (2023) 151(1):212–21. doi: 10.1016/j.jaci.2022.08.020
22. Douwes J, Cheng S, Travier N, Cohet C, Niesink A, McKenzie J, et al. Farm exposure in utero may protect against asthma, hay fever and eczema. Eur Respir J. (2008) 32(3):603–11. doi: 10.1183/09031936.00033707
23. Rosa MJ, Lee AG, Wright RJ. Evidence establishing a link between prenatal and early-life stress and asthma development. Curr Opin Allergy Clin Immunol. (2018) 18(2):148–58. doi: 10.1097/ACI.0000000000000421
24. Kumar R, Ouyang F, Story RE, Pongracic JA, Hong X, Wang G, et al. Gestational diabetes, atopic dermatitis, and allergen sensitization in early childhood. J Allergy Clin Immunol. (2009) 124(5):1031–8.e1–4. doi: 10.1016/j.jaci.2009.06.052
25. Chen Y, Zhu J, Lyu J, Xia Y, Ying Y, Hu Y, et al. Association of maternal prepregnancy weight and gestational weight gain with children’s allergic diseases. JAMA Netw Open. (2020) 3(9):e2015643. doi: 10.1001/jamanetworkopen.2020.15643
26. Pistiner M, Gold DR, Abdulkerim H, Hoffman E, Celedon JC. Birth by cesarean section, allergic rhinitis, and allergic sensitization among children with a parental history of atopy. J Allergy Clin Immunol. (2008) 122(2):274–9. doi: 10.1016/j.jaci.2008.05.007
27. Sigurdardottir ST, Jonasson K, Clausen M, Lilja Bjornsdottir K, Sigurdardottir SE, Roberts G, et al. Prevalence and early-life risk factors of school-age allergic multimorbidity: The EuroPrevall-iFAAM birth cohort. Allergy. (2021) 76(9):2855–65. doi: 10.1111/all.14857
28. Krzych-Falta E, Furmanczyk K, Lisiecka-Bielanowicz M, Sybilski A, Tomaszewska A, Raciborski F, et al. The effect of selected risk factors, including the mode of delivery, on the development of allergic rhinitis and bronchial asthma. Postepy Dermatol Alergol. (2018) 35(3):267–73. doi: 10.5114/ada.2018.76222
29. Han DH, Shin JM, An S, Kim JS, Kim DY, Moon S, et al. Long-term breastfeeding in the prevention of allergic rhinitis: allergic rhinitis cohort study for kids (ARCO-kids study). Clin Exp Otorhinolaryngol. (2019) 12(3):301–7. doi: 10.21053/ceo.2018.01781
30. Bion V, Lockett GA, Soto-Ramirez N, Zhang H, Venter C, Karmaus W, et al. Evaluating the efficacy of breastfeeding guidelines on long-term outcomes for allergic disease. Allergy. (2016) 71(5):661–70. doi: 10.1111/all.12833
31. Oddy WH. A review of the effects of breastfeeding on respiratory infections, atopy, and childhood asthma. J Asthma. (2004) 41(6):605–21. doi: 10.1081/JAS-200026402
32. Keller T, Hohmann C, Standl M, Wijga AH, Gehring U, Melen E, et al. The sex-shift in single disease and multimorbid asthma and rhinitis during puberty—a study by MeDALL. Allergy. (2018) 73(3):602–14. doi: 10.1111/all.13312
33. Hohmann C, Keller T, Gehring U, Wijga A, Standl M, Kull I, et al. Sex-specific incidence of asthma, rhinitis and respiratory multimorbidity before and after puberty onset: individual participant meta-analysis of five birth cohorts collaborating in MeDALL. BMJ Open Respir Res. (2019) 6(1):e000460. doi: 10.1136/bmjresp-2019-000460
34. Gandhi VD, Cephus JY, Norlander AE, Chowdhury NU, Zhang J, Ceneviva ZJ, et al. Androgen receptor signaling promotes treg suppressive function during allergic airway inflammation. J Clin Invest. (2022) 132(4):e153397. doi: 10.1172/JCI153397
35. Bonds RS, Midoro-Horiuti T. Estrogen effects in allergy and asthma. Curr Opin Allergy Clin Immunol. (2013) 13(1):92–9. doi: 10.1097/ACI.0b013e32835a6dd6
36. Slavin RG. Allergic rhinitis: managing the adult spectrum. Allergy Asthma Proc. (2006) 27(1):9–11.16598986
37. Falsarella N, Ferreira AI, Nakashima F, de Cássia Brandão de Mattos C, de Mattos LC. Evidence of an association between the O blood group and allergic rhinitis. Rev Bras Hematol Hemoter. (2011) 33(6):444–8. doi: 10.5581/1516-8484.20110120
38. Luo X, Xiang J, Dong X, Cai F, Suo J, Wang Z, et al. Association between obesity and atopic disorders in Chinese adults: an individually matched case-control study. BMC Public Health. (2013) 13(12). doi: 10.1186/1471-2458-13-12
39. Zhou J, Luo F, Han Y, Lou H, Tang X, Zhang L. Obesity/overweight and risk of allergic rhinitis: a meta-analysis of observational studies. Allergy. (2020) 75(5):1272–5. doi: 10.1111/all.14143
40. Liu W, Zeng Q, Zhou L, Luo R, Dong H. Association of leptin with disease severity and inflammation indicators in Chinese obese children with allergic rhinitis. Pediatr Allergy Immunol. (2018) 29(2):186–93. doi: 10.1111/pai.12856
41. Zeng Q, Luo X, Han M, Liu W, Li H. Leptin/osteopontin axis regulated type 2T helper cell response in allergic rhinitis with obesity. EBioMedicine. (2018) 32:43–9. doi: 10.1016/j.ebiom.2018.05.037
42. Hollams EM, Hart PH, Holt BJ, Serralha M, Parsons F, de Klerk NH, et al. Vitamin D and atopy and asthma phenotypes in children: a longitudinal cohort study. Eur Respir J. (2011) 38(6):1320–7. doi: 10.1183/09031936.00029011
43. Searing DA, Leung DY. Vitamin D in atopic dermatitis, asthma and allergic diseases. Immunol Allergy Clin North Am. (2010) 30(3):397–409. doi: 10.1016/j.iac.2010.05.005
44. Farchi S, Forastiere F, Agabiti N, Corbo G, Pistelli R, Fortes C, et al. Dietary factors associated with wheezing and allergic rhinitis in children. Eur Respir J. (2003) 22(5):772–80. doi: 10.1183/09031936.03.00006703
45. Jiang J, Mehrabi Nasab E, Athari SM, Athari SS. Effects of vitamin E and selenium on allergic rhinitis and asthma pathophysiology. Respir Physiol Neurobiol. (2021) 286:103614. doi: 10.1016/j.resp.2020.103614
46. Sdona E, Georgakou AV, Ekstrom S, Bergstrom A. Dietary fibre intake in relation to asthma, rhinitis and lung function impairment—a systematic review of observational studies. Nutrients. (2021) 13(10):3594. doi: 10.3390/nu13103594
47. Sdona E, Ekstrom S, Andersson N, Hakansson N, Wolk A, Westman M, et al. Dietary fibre in relation to asthma, allergic rhinitis and sensitization from childhood up to adulthood. Clin Transl Allergy. (2022) 12(8):e12188. doi: 10.1002/clt2.12188
48. Pesce G, Sese L, Calciano L, Travert B, Dessimond B, Maesano CN, et al. Foetal exposure to heavy metals and risk of atopic diseases in early childhood. Pediatr Allergy Immunol. (2021) 32(2):242–50. doi: 10.1111/pai.13397
49. Ma R, Shen Y, Hou L, Yang Z, Feng N, Yan X, et al. The correlation of allergic rhinitis and trace elements in Ningxia region. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2014) 49(12):1017–20. doi: 10.3760/cma.j.issn.1673-0860.2014.12.010
50. Lopez-Santamarina A, Gonzalez EG, Lamas A, Mondragon ADC, Regal P, Miranda JM. Probiotics as a possible strategy for the prevention and treatment of allergies. A narrative review. Foods. (2021) 10(4):701. doi: 10.3390/foods10040701
51. Savilahti E. Probiotics in the treatment and prevention of allergies in children. Biosci Microflora. (2011) 30(4):119–28. doi: 10.12938/bifidus.30.119
52. Zhou H, Urso CJ, Jadeja V. Saturated fatty acids in obesity-associated inflammation. J Inflamm Res. (2020) 13:1–14. doi: 10.2147/JIR.S229691
53. Miles EA, Calder PC. Can early omega-3 fatty acid exposure reduce risk of childhood allergic disease? Nutrients. (2017) 9(7):784. doi: 10.3390/nu9070784
54. Magnusson J, Ekstrom S, Kull I, Hakansson N, Nilsson S, Wickman M, et al. Polyunsaturated fatty acids in plasma at 8 years and subsequent allergic disease. J Allergy Clin Immunol. (2018) 142(2):510–6.e6. doi: 10.1016/j.jaci.2017.09.023
55. Lee A, Lee SY, Lee KS. The use of heated tobacco products is associated with asthma, allergic rhinitis, and atopic dermatitis in Korean adolescents. Sci Rep. (2019) 9(1):17699. doi: 10.1038/s41598-019-54102-4
56. Grillo C, La Mantia I, Grillo CM, Ciprandi G, Ragusa M, Andaloro C. Influence of cigarette smoking on allergic rhinitis: a comparative study on smokers and non-smokers. Acta Biomed. (2019) 90(7-S):45–51. doi: 10.23750/abm.v90i7-S.8658
57. Antonogeorgos G, Priftis KN, Panagiotakos DB, Ellwood P, Garcia-Marcos L, Liakou E, et al. Exploring the relation between atopic diseases and lifestyle patterns among adolescents living in Greece: evidence from the Greek global asthma network (GAN) cross-sectional study. Children (Basel). (2021) 8(10):932. doi: 10.3390/children8100932
58. Park J, Park JH, Park J, Choi J, Kim TH. Association between allergic rhinitis and regular physical activity in adults: a nationwide cross-sectional study. Int J Environ Res Public Health. (2020) 17(16):5662. doi: 10.3390/ijerph17165662
59. Lim MS, Lee CH, Sim S, Hong SK, Choi HG. Physical activity, sedentary habits, sleep, and obesity are associated with asthma, allergic rhinitis, and atopic dermatitis in Korean adolescents. Yonsei Med J. (2017) 58(5):1040–6. doi: 10.3349/ymj.2017.58.5.1040
60. Nakao A. Clockwork allergy: how the circadian clock underpins allergic reactions. J Allergy Clin Immunol. (2018) 142(4):1021–31. doi: 10.1016/j.jaci.2018.08.007
61. Nakao A, Nakamura Y, Shibata S. The circadian clock functions as a potent regulator of allergic reaction. Allergy. (2015) 70(5):467–73. doi: 10.1111/all.12596
62. Thacher JD, Gruzieva O, Pershagen G, Melen E, Lorentzen JC, Kull I, et al. Mold and dampness exposure and allergic outcomes from birth to adolescence: data from the BAMSE cohort. Allergy. (2017) 72(6):967–74. doi: 10.1111/all.13102
63. Li S, Cao S, Duan X, Zhang Y, Gong J, Xu X, et al. Household mold exposure in association with childhood asthma and allergic rhinitis in a northwestern city and a southern city of China. J Thorac Dis. (2022) 14(5):1725–37. doi: 10.21037/jtd-21-1380
64. Jung DY, Leem JH, Kim HC, Kim JH, Hwang SS, Lee JY, et al. Effect of traffic-related air pollution on allergic disease: results of the children’s health and environmental research. Allergy Asthma Immunol Res. (2015) 7(4):359–66. doi: 10.4168/aair.2015.7.4.359
65. Wang W, Huang X, Chen Z, Zheng R, Chen Y, Zhang G, et al. Prevalence and trends of sensitisation to aeroallergens in patients with allergic rhinitis in Guangzhou. China: a 10-year retrospective study. BMJ Open. (2016) 6(5):e011085. doi: 10.1136/bmjopen-2016-011085
66. Gao J, Lu M, Sun Y, Wang J, An Z, Liu Y, et al. Changes in ambient temperature increase hospital outpatient visits for allergic rhinitis in Xinxiang, China. BMC Public Health. (2021) 21(1):600. doi: 10.1186/s12889-021-10671-6
67. Upperman CR, Parker JD, Akinbami LJ, Jiang C, He X, Murtugudde R, et al. Exposure to extreme heat events is associated with increased hay fever prevalence among nationally representative sample of US adults: 1997–2013. J Allergy Clin Immunol Pract. (2017) 5(2):435–41.e2. doi: 10.1016/j.jaip.2016.09.016
68. Macan J, Babic Z, Hallmann S, Havmose MS, Johansen JD, John SM, et al. Respiratory toxicity of persulphate salts and their adverse effects on airways in hairdressers: a systematic review. Int Arch Occup Environ Health. (2022) 95(8):1679–702. doi: 10.1007/s00420-022-01852-w
69. Hiller J, Greiner A, Drexler H. Respiratory afflictions during hairdressing jobs: case history and clinical evaluation of a large symptomatic case series. J Occup Med Toxicol. (2022) 17(1):10. doi: 10.1186/s12995-022-00351-5
70. Mbatchou Ngahane BH, Afane Ze E, Nde F, Ngomo E, Mapoure Njankouo Y, Njock LR. Prevalence and risk factors for allergic rhinitis in bakers in Douala, Cameroon. BMJ Open. (2014) 4(8):e005329. doi: 10.1136/bmjopen-2014-005329
71. Swiderska-Kielbik S, Krakowiak A, Wiszniewska M, Nowakowska-Swirta E, Walusiak-Skorupa J, Sliwkiewicz K, et al. Occupational allergy to birds within the population of Polish bird keepers employed in zoo gardens. Int J Occup Med Environ Health. (2011) 24(3):292–303. doi: 10.2478/s13382-011-0027-x
72. Wang YH, Su HH, Hsu L, Wang CY, Wu PH. Correlation between novel potential indoor risk factors and frequency of doctor’s visit for respiratory problem in Taiwan’s tropical environment. Int J Occup Environ Med. (2018) 9(1):10–22. doi: 10.15171/ijoem.2018.1143
73. Chatzi L, Alegakis A, Tzanakis N, Siafakas N, Kogevinas M, Lionis C. Association of allergic rhinitis with pesticide use among grape farmers in Crete, Greece. Occup Environ Med. (2007) 64(6):417–21. doi: 10.1136/oem.2006.029835
74. Hoffman CD, Henneberger PK, Olin AC, Mehta A, Toren K. Exposure to ozone gases in pulp mills and the onset of rhinitis. Scand J Work Environ Health. (2004) 30(6):445–9. doi: 10.5271/sjweh.833
75. Park DU, Jin KW, Koh DH, Kim BK, Kim KS, Park DY. Association between use of synthetic metalworking fluid and risk of developing rhinitis-related symptoms in an automotive ring manufacturing plant. J Occup Health. (2008) 50(2):212–20. doi: 10.1539/joh.O7006
76. De Troeyer K, De Man J, Vandebroek E, Vanoirbeek JA, Hoet PH, Nemery B, et al. Identifying cleaning products associated with short-term work-related respiratory symptoms: a workforce-based study in domestic cleaners. Environ Int. (2022) 162:107170. doi: 10.1016/j.envint.2022.107170
77. Koureas M, Rachiotis G, Tsakalof A, Hadjichristodoulou C. Increased frequency of rheumatoid arthritis and allergic rhinitis among pesticide sprayers and associations with pesticide use. Int J Environ Res Public Health. (2017) 14(8):865. doi: 10.3390/ijerph14080865
78. Hsu NY, Wu PC, Bornehag CG, Sundell J, Su HJ. Feeding bottles usage and the prevalence of childhood allergy and asthma. Clin Dev Immunol. (2012) 2012:158248. doi: 10.1155/2012/158248
79. Mitre E, Susi A, Kropp LE, Schwartz DJ, Gorman GH, Nylund CM. Association between use of acid-suppressive medications and antibiotics during infancy and allergic diseases in early childhood. JAMA Pediatr. (2018) 172(6):e180315. doi: 10.1001/jamapediatrics.2018.0315
80. Ni J, Friedman H, Boyd BC, McGurn A, Babinski P, Markossian T, et al. Early antibiotic exposure and development of asthma and allergic rhinitis in childhood. BMC Pediatr. (2019) 19(1):225. doi: 10.1186/s12887-019-1594-4
81. Gabryszewski SJ, Dudley J, Grundmeier RW, Hill DA. Early-life environmental exposures associate with individual and cumulative allergic morbidity. Pediatr Allergy Immunol. (2021) 32(5):1089–93. doi: 10.1111/pai.13486
82. Zeng Y, Song B, Gao Y, Cao W, Li J, Liu Q, et al. Cumulative evidence for association of acetaminophen exposure and allergic rhinitis. Int Arch Allergy Immunol. (2020) 181(6):422–33. doi: 10.1159/000506807
83. Zhou Y, Chen J, Dong Y, Shen J, Tian M, Yang Y, et al. Maternal tobacco exposure during pregnancy and allergic rhinitis in offspring: a systematic review and meta-analysis. Medicine (Baltimore). (2021) 100(34):e26986. doi: 10.1097/MD.0000000000026986
84. Ai S, Liu L, Xue Y, Cheng X, Li M, Deng Q. Prenatal exposure to air pollutants associated with allergic diseases in children: which pollutant, when exposure, and what disease? A systematic review and meta-analysis. Clin Rev Allergy Immunol. (2024) 66(2):149–63. doi: 10.1007/s12016-024-08987-3
85. Magnusson LL, Wennborg H, Bonde JP, Olsen J. Wheezing, asthma, hay fever, and atopic eczema in relation to maternal occupations in pregnancy. Occup Environ Med. (2006) 63(9):640–6. doi: 10.1136/oem.2005.024422
86. Cazzoletti L, Ferrari M, Olivieri M, Verlato G, Antonicelli L, Bono R, et al. The gender, age and risk factor distribution differs in self-reported allergic and non-allergic rhinitis: a cross-sectional population-based study. Allergy Asthma Clin Immunol. (2015) 11(36). doi: 10.1186/s13223-015-0101-1
87. Xu H, Zhang Y, Gu M, Shan Y, Zhang Q. A prospective study on the difference of clinical outcomes between elderly and adult patients with allergic rhinitis. Am J Otolaryngol. (2022) 43(4):103509. doi: 10.1016/j.amjoto.2022.103509
88. Uwaezuoke SN, Eze JN, Ayuk AC, Ndu IK. ABO histo-blood group and risk of respiratory atopy in children: a review of published evidence. Pediatric Health Med Ther. (2018) 9:73–9. doi: 10.2147/PHMT.S162570
89. Dahalan NH, Tuan Din SA, Mohamad SMB. Association of ABO blood groups with allergic diseases: a scoping review. BMJ Open. (2020) 10(2):e029559. doi: 10.1136/bmjopen-2019-029559
90. Ciprandi G, Pistorio A, Tosca M, Ferraro MR, Cirillo I. Body mass index, respiratory function and bronchial hyperreactivity in allergic rhinitis and asthma. Respir Med. (2009) 103(2):289–95. doi: 10.1016/j.rmed.2008.08.008
91. Peroni DG, Hufnagl K, Comberiati P, Roth-Walter F. Lack of iron, zinc, and vitamins as a contributor to the etiology of atopic diseases. Front Nutr. (2023) 9:1032481. doi: 10.3389/fnut.2022.1032481
92. Shi Q, Shen X, Long C, Mi Z, Li Y, Ma R. Zinc supplement reduces allergic responses through modulating the p38 MAPK pathway activation in an allergic rhinitis mouse model. J Trace Elem Med Biol. (2023) 75:127094. doi: 10.1016/j.jtemb.2022.127094
93. Sahoyama Y, Hamazato F, Shiozawa M, Nakagawa T, Suda W, Ogata Y, et al. Multiple nutritional and gut microbial factors associated with allergic rhinitis: the Hitachi health study. Sci Rep. (2022) 12(1):3359. doi: 10.1038/s41598-022-07398-8
94. Di Pierro F, Basile I, Danza ML, Venturelli L, Contini R, Risso P, et al. Use of a probiotic mixture containing bifidobacterium animalis subsp. lactis BB12 and enterococcus faecium L3 in atopic children. Minerva Pediatr. (2018) 70(5):418–24. doi: 10.23736/S0026-4946.18.05203-9
95. Ellwood P, Asher MI, Garcia-Marcos L, Williams H, Keil U, Robertson C, et al. Do fast foods cause asthma, rhinoconjunctivitis and eczema? Global findings from the international study of asthma and allergies in childhood (ISAAC) phase three. Thorax. (2013) 68(4):351–60. doi: 10.1136/thoraxjnl-2012-202285
96. Wasilewska E, Malgorzewicz S, Gruchala-Niedoszytko M, Skotnicka M, Jassem E. Dietary habits in children with respiratory allergies: a single-center polish pilot study. Nutrients. (2020) 12(5):1521. doi: 10.3390/nu12051521
97. Polosa R, Russo C, Caponnetto P, Bertino G, Sarva M, Antic T, et al. Greater severity of new onset asthma in allergic subjects who smoke: a 10-year longitudinal study. Respir Res. (2011) 12(1):16. doi: 10.1186/1465-9921-12-16
98. Tongtako W, Klaewsongkram J, Mickleborough TD, Suksom D. Effects of aerobic exercise and vitamin C supplementation on rhinitis symptoms in allergic rhinitis patients. Asian Pac J Allergy Immunol. (2018) 36(4):222–31. doi: 10.12932/AP-040417-0066
99. Park KH, Sim DW, Lee SC, Moon S, Choe E, Shin H, et al. Effects of air purifiers on patients with allergic rhinitis: a multicenter, randomized, double-blind, and placebo-controlled study. Yonsei Med J. (2020) 61(8):689–97. doi: 10.3349/ymj.2020.61.8.689
100. Manuyakorn W, Padungpak S, Luecha O, Kamchaisatian W, Sasisakulporn C, Vilaiyuk S, et al. Assessing the efficacy of a novel temperature and humidity control machine to minimize house dust mite allergen exposure and clinical symptoms in allergic rhinitis children sensitized to dust mites: a pilot study. Asian Pac J Allergy Immunol. (2015) 33(2):129–35. doi: 10.12932/AP0524.33.2.2015
101. Sio YY, Pang SL, Say YH, Teh KF, Wong YR, Shah SMR, et al. Sensitization to airborne fungal allergens associates with asthma and allergic rhinitis presentation and severity in the Singaporean/Malaysian population. Mycopathologia. (2021) 186(5):583–8. doi: 10.1007/s11046-021-00532-6
102. Takizawa H. Impact of air pollution on allergic diseases. Korean J Intern Med. (2011) 26(3):262–73. doi: 10.3904/kjim.2011.26.3.262
103. Cesaroni G, Badaloni C, Porta D, Forastiere F, Perucci CA. Comparison between various indices of exposure to traffic-related air pollution and their impact on respiratory health in adults. Occup Environ Med. (2008) 65(10):683–90. doi: 10.1136/oem.2007.037846
104. Arcangeli G, Traversini V, Tomasini E, Baldassarre A, Lecca LI, Galea RP, et al. Allergic anaphylactic risk in farming activities: a systematic review. Int J Environ Res Public Health. (2020) 17(14). doi: 10.3390/ijerph17144921
105. Brunekreef B, Von Mutius E, Wong GK, Odhiambo JA, Clayton TO, Group IPTS. Early life exposure to farm animals and symptoms of asthma, rhinoconjunctivitis and eczema: an ISAAC phase three study. Int J Epidemiol. (2012) 41(3):753–61. doi: 10.1093/ije/dyr216
106. Haspeslagh E, Heyndrickx I, Hammad H, Lambrecht BN. The hygiene hypothesis: immunological mechanisms of airway tolerance. Curr Opin Immunol. (2018) 54(6710):102–8. doi: 10.1016/j.coi.2018.06.007
107. Hyytiainen H, Kirjavainen PV, Taubel M, Tuoresmaki P, Casas L, Heinrich J, et al. Microbial diversity in homes and the risk of allergic rhinitis and inhalant atopy in two European birth cohorts. Environ Res. (2021) 196:110835. doi: 10.1016/j.envres.2021.110835
108. Zhou PE, Qian ZM, McMillin SE, Vaughn MG, Xie ZY, Xu YJ, et al. Relationships between long-term ozone exposure and allergic rhinitis and bronchitic symptoms in Chinese children. Toxics. (2021) 9(9):221. doi: 10.3390/toxics9090221
109. De Matteis S, Ronsmans S, Nemery B. Respiratory health effects of exposure to cleaning products. Clin Chest Med. (2020) 41(4):641–50. doi: 10.1016/j.ccm.2020.08.010
110. Slager RE, Simpson SL, Levan TD, Poole JA, Sandler DP, Hoppin JA. Rhinitis associated with pesticide use among private pesticide applicators in the agricultural health study. J Toxicol Environ Health A. (2010) 73(20):1382–93. doi: 10.1080/15287394.2010.497443
111. Kimber I, Dearman RJ. An assessment of the ability of phthalates to influence immune and allergic responses. Toxicology. (2010) 271(3):73–82. doi: 10.1016/j.tox.2010.03.020
112. Yamamoto-Hanada K, Yang L, Narita M, Saito H, Ohya Y. Influence of antibiotic use in early childhood on asthma and allergic diseases at age 5. Ann Allergy Asthma Immunol. (2017) 119(1):54–8. doi: 10.1016/j.anai.2017.05.013
113. Oyama N, Sudo N, Sogawa H, Kubo C. Antibiotic use during infancy promotes a shift in the T(H)1/T(H)2 balance toward T(H)2-dominant immunity in mice. J Allergy Clin Immunol. (2001) 107(1):153–9. doi: 10.1067/mai.2001.111142
114. Tas HI, Caglar O. The role of anxious temperament in patients with allergic rhinitis. Saudi Med J. (2019) 40(1):45–51. doi: 10.15537/smj.2019.1.23754
115. Lee MR, Son BS, Park YR, Kim HM, Moon JY, Lee YJ, et al. The relationship between psychosocial stress and allergic disease among children and adolescents in Gwangyang Bay, Korea. J Prev Med Public Health. (2012) 45(6):374–80. doi: 10.3961/jpmph.2012.45.6.374
116. Miyazaki C, Koyama M, Ota E, Swa T, Mlunde LB, Amiya RM, et al. Allergic diseases in children with attention deficit hyperactivity disorder: a systematic review and meta-analysis. BMC Psychiatry. (2017) 17(1):120. doi: 10.1186/s12888-017-1281-7
117. Ryklief Z, Suliman S, Hemmings SMJ, van den Heuvel LL, Seedat S. Rates of and factors associated with atopy and allergies in posttraumatic stress disorder as compared to controls. J Psychosom Res. (2022) 158:110938. doi: 10.1016/j.jpsychores.2022.110938
118. Zhou RY, Wang JJ, Sun JC, You Y, Ying JN, Han XM. Attention deficit hyperactivity disorder may be a highly inflammation and immune-associated disease (review). Mol Med Rep. (2017) 16(4):5071–7. doi: 10.3892/mmr.2017.7228
119. Suwan P, Akaramethathip D, Noipayak P. Association between allergic sensitization and attention deficit hyperactivity disorder (ADHD). Asian Pac J Allergy Immunol. (2011) 29(1):57–65.21560489
120. Yang L, Fu J, Zhou Y. Research progress in atopic march. Front Immunol. (2020) 11:1907. doi: 10.3389/fimmu.2020.01907
121. Chiu CY, Yang CH, Su KW, Tsai MH, Hua MC, Liao SL, et al. Early-onset eczema is associated with increased milk sensitization and risk of rhinitis and asthma in early childhood. J Microbiol Immunol Infect. (2020) 53(6):1008–13. doi: 10.1016/j.jmii.2019.04.007
122. von Kobyletzki LB, Bornehag CG, Hasselgren M, Larsson M, Lindstrom CB, Svensson A. Eczema in early childhood is strongly associated with the development of asthma and rhinitis in a prospective cohort. BMC Dermatol. (2012) 12(11). doi: 10.1186/1471-5945-12-11
123. Hon KL, Wang SS, Leung TF. The atopic march: from skin to the airways. Iran J Allergy Asthma Immunol. (2012) 11(1):73–7.22427479
124. Lei WT, Hsu CW, Chen PC, Tseng PT, Kuo HC, Guo MM, et al. Increased risk of asthma and allergic rhinitis in patients with a past history of Kawasaki disease: a systematic review and meta-analyses. Front Pediatr. (2021) 9:746856. doi: 10.3389/fped.2021.746856
125. Ghaffari J, Rokni GR, Kazeminejad A, Abedi H. Association among thyroid dysfunction, asthma. Allergic rhinitis and eczema in children with alopecia areata. Open Access Maced J Med Sci. (2017) 5(3):305–9. doi: 10.3889/oamjms.2017.050
126. Galili E, Barzilai A, Twig G, Caspi T, Daniely D, Shreberk-Hassidim R, et al. Allergic rhinitis and asthma among adolescents with psoriasis: a population-based cross-sectional study. Acta Derm Venereol. (2020) 100(10):adv00133. doi: 10.2340/00015555-3485
127. Chang WP, Kuo CN, Kuo LN, Wang YT, Perng WT, Kuo HC, et al. Increase risk of allergic diseases in patients with ankylosing spondylitis. Medicine (Baltimore). (2016) 95(45):e5172. doi: 10.1097/MD.0000000000005172
128. Yeo NK, Jang YJ. Rhinovirus infection-induced alteration of tight junction and adherens junction components in human nasal epithelial cells. Laryngoscope. (2010) 120(2):346–52. doi: 10.1002/lary.20764
129. Gangl K, Waltl EE, Vetr H, Cabauatan CR, Niespodziana K, Valenta R, et al. Infection with rhinovirus facilitates allergen penetration across a respiratory epithelial cell layer. Int Arch Allergy Immunol. (2015) 166(4):291–6. doi: 10.1159/000430441
130. Stokes SC, Tankersley MS. HIV: practical implications for the practicing allergist-immunologist. Ann Allergy Asthma Immunol. (2011) 107(1):1–9; quiz-11. doi: 10.1016/j.anai.2011.05.004
131. Linhar LS, Traebert J, Galato D, da Silva RM, Schuelter-Trevisol F, Rovaris NS, et al. Allergic diseases in subjects under: 18, years living with HIV. Allergy Asthma Clin Immunol. (2014) 10(1):35. doi: 10.1186/1710-1492-10-35
132. Cakir M, Karakas T, Orhan F, Okten A, Gedik Y. Atopy in children with chronic hepatitis B virus infection. Acta Paediatr. (2007) 96(9):1343–6. doi: 10.1111/j.1651-2227.2007.00405.x
133. Souza-Machado A, Galvao TS, Porto A, Figueiredo J, Cruz AA. Skin reactivity to aeroallergens is reduced in human T-lymphotropic virus type I-infected healthy blood-donors (asymptomatic carriers). Allergy. (2005) 60(3):379–84. doi: 10.1111/j.1398-9995.2005.00709.x
134. Kucukosmanoglu E, Cetinkaya F, Akcay F, Pekun F. Frequency of allergic diseases following measles. Allergol Immunopathol (Madr). (2006) 34(4):146–9. doi: 10.1157/13091040
135. Riechelmann H, Essig A, Deutschle T, Rau A, Rothermel B, Weschta M. Nasal carriage of staphylococcus aureus in house dust mite allergic patients and healthy controls. Allergy. (2005) 60(11):1418–23. doi: 10.1111/j.1398-9995.2005.00902.x
136. Pelosi U, Porcedda G, Tiddia F, Tripodi S, Tozzi AE, Panetta V, et al. The inverse association of salmonellosis in infancy with allergic rhinoconjunctivitis and asthma at school-age: a longitudinal study. Allergy. (2005) 60(5):626–30. doi: 10.1111/j.1398-9995.2005.00747.x
137. Imamura S, Sugimoto M, Kanemasa K, Sumida Y, Okanoue T, Yoshikawa T, et al. Inverse association between helicobacter pylori infection and allergic rhinitis in young Japanese. J Gastroenterol Hepatol. (2010) 25(7):1244–9. doi: 10.1111/j.1440-1746.2010.06307.x
138. Obihara CC, Kimpen JL, Gie RP, Lill SW, Hoekstra MO, Marais BJ, et al. Mycobacterium tuberculosis infection may protect against allergy in a tuberculosis endemic area. Clin Exp Allergy. (2006) 36(1):70–6. doi: 10.1111/j.1365-2222.2005.02408.x
139. Schmidt SM, Muller CE, Wiersbitzky SK. Inverse association between chlamydia pneumoniae respiratory tract infection and initiation of asthma or allergic rhinitis in children. Pediatr Allergy Immunol. (2005) 16(2):137–44. doi: 10.1111/j.1399-3038.2005.00229.x
140. Wuhao L, Ran C, Xujin H, Zhongdao W, Dekumyoy P, Zhiyue L. Parasites and asthma. Parasitol Res. (2017) 116(9):2373–83. doi: 10.1007/s00436-017-5548-1
141. van den Biggelaar AH, van Ree R, Rodrigues LC, Lell B, Deelder AM, Kremsner PG, et al. Decreased atopy in children infected with schistosoma haematobium: a role for parasite-induced interleukin-10. Lancet. (2000) 356(9243):1723–7. doi: 10.1016/S0140-6736(00)03206-2
142. Allen JE, Maizels RM. Diversity and dialogue in immunity to helminths. Nat Rev Immunol. (2011) 11(6):375–88. doi: 10.1038/nri2992
143. Cruz AA, Cooper PJ, Figueiredo CA, Alcantara-Neves NM, Rodrigues LC, Barreto ML. Global issues in allergy and immunology: parasitic infections and allergy. J Allergy Clin Immunol. (2017) 140(5):1217–28. doi: 10.1016/j.jaci.2017.09.005
144. Huang PY, Huang YH, Guo MM, Chang LS, Kuo HC. Kawasaki disease and allergic diseases. Front Pediatr. (2021) 8:614386. doi: 10.3389/fped.2020.614386
145. Kridin K, Renert-Yuval Y, Guttman-Yassky E, Cohen AD. Alopecia areata is associated with atopic diathesis: results from a population-based study of 51,561 patients. J Allergy Clin Immunol Pract. (2020) 8(4):1323–8.e1. doi: 10.1016/j.jaip.2020.01.052
146. Obihara CC, Beyers N, Gie RP, Potter PC, Marais BJ, Lombard CJ, et al. Inverse association between mycobacterium tuberculosis infection and atopic rhinitis in children. Allergy. (2005) 60(9):1121–5. doi: 10.1111/j.1398-9995.2005.00834.x
147. Song WJ, Chang YS, Lim MK, Yun EH, Kim SH, Kang HR, et al. Staphylococcal enterotoxin sensitization in a community-based population: a potential role in adult-onset asthma. Clin Exp Allergy. (2014) 44(4):553–62. doi: 10.1111/cea.12239
Keywords: allergic rhinitis, risk factor, prevention strategy, hereditary factors, maternal situation, health status
Citation: Chen R, An W, Liu X, Yan J, Huang Y and Zhang J (2024) Risk factors of allergic rhinitis and its prevention strategies. Front. Allergy 5:1509552. doi: 10.3389/falgy.2024.1509552
Received: 11 October 2024; Accepted: 6 November 2024;
Published: 27 November 2024.
Edited by:
Pongsakorn Tantilipikorn, Mahidol University, ThailandReviewed by:
Diego Marcelo Conti, KU Leuven, BelgiumEduardo Javier Correa, Nuevo Hospital Comarcal de La Linea de La Concepción, Spain
Copyright: © 2024 Chen, An, Liu, Yan, Huang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junyan Zhang, emp5MjAxOEBnemhtdS5lZHUuY24=; Yuyi Huang, aHVhbmd5dXlpQGd6aG11LmVkdS5jbg==
†These authors have contributed equally to this work
 Ruzhi Chen1,†
Ruzhi Chen1,† Junyan Zhang
Junyan Zhang