- 1Department of Psychology, University of Calgary, Calgary, AB, Canada
- 2Department of Educational and Counselling Psychology and Special Education, University of British Columbia, Vancouver, BC, Canada
- 3Department of Psychiatry and Addiction, University of Montréal, Montréal, QC, Canada
- 4CHU Sainte-Justine Research Center, Montréal, QC, Canada
- 5Alberta Children’s Hospital Research Institute, Calgary, AB, Canada
- 6Department of Paediatrics, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada
- 7Community Health Sciences, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada
Following up on previous findings from the All Our Families (AOF) cohort, the current study investigated the relationship between birthing parent history of adverse childhood experiences (ACEs) and child atopy, including asthma, allergy, and eczema, at five years of age. Potential indirect effects were explored. Participants completed the ACEs scale, validated questionnaires of anxiety and depression symptoms, and reported on their and their children's atopic disease history. Archival analyses of AOF data (N = 3,387) was conducted using logistic regression and path analysis with counterfactually based indirect effects. Birthing parent history of ACEs was associated with an 18% increased risk of child allergy at five years (OR = 1.18, 95% CI: 1.09, 1.20). Exploratory path analyses indicated a significant indirect effect of ACEs through birthing parent history of atopy on child asthma, allergy, and eczema at five years. There were no significant indirect effects through birthing parent symptoms of anxiety or depression during pregnancy, at two or five years postpartum. Birthing parent history of ACEs, combined with birthing parent history of atopy, may elevate the risk of child atopy. This presents an opportunity for early intervention for children at risk of atopic disease.
Introduction
Atopic diseases, including asthma, allergy, and eczema are the most commonly reported chronic conditions in childhood, with prevalence estimates ranging from 5%–25% between 0 and 10 years old, depending on disease type (1–5). Unfortunately, atopic diseases in childhood present significant health concerns that impact quality of life and increase risk for other poor health outcomes and chronic diseases [e.g., anxiety; (6–10)]. Improving understanding of the complex interplay of processes influencing the development of atopic diseases in childhood could improve prevention, detection, and early intervention efforts. There are some known genetic, obstetric, and environmental risk factors for child atopic disease, including family history, preterm birth, and exposure to irritants/allergens. There is also emerging evidence demonstrating an intergenerational impact of birthing parents' adverse childhood experiences on their children's risk for developing atopic disease (11–14).
Adverse childhood experiences (ACEs) refer to events that may be highly distressing or traumatic (e.g., abuse, neglect, exposure to domestic violence and significant household dysfunction) occurring prior to 18 years of age. ACEs are associated with a range of negative physical (e.g., cardiovascular disease, obesity, asthma) and mental (e.g., mood and anxiety disorders) health outcomes in adulthood (15–21). Research suggests that the risk conferred by ACEs may extend across generations, leading to increased risk for birth complications, developmental delays, as well as internalizing and externalizing problems (22–25). Data from the All Our Families prospective cohort study demonstrated an association between birthing parents' history of experiencing childhood abuse and diagnoses of asthma and allergies in children at the age of 2, as well as indirect effects through perinatal depression and postpartum anxiety (14, 26, 27). More longitudinal evidence is required to clarify the relationship between birthing parent ACEs with the onset and progression of child atopic disease.
Several explanations have been proposed for the intergenerational transmission of the risks conferred by birthing parent history of ACEs. Potential pathways include both genetic factors (e.g., birthing parent history of atopic disease) and environmental factors (e.g., birthing parent depression and anxiety) during the prenatal and early developmental periods, which have been linked with increased risk of child atopic disease (28, 39). Pregnancy represents a sensitive period in which fetal immune system development may be impacted by alterations to the birthing parent neuroendocrine system, nervous system reactivity, and/or Hypothalamic Pituitary Adrenal Axis (HPA) function resulting from ACEs, in addition to genetic inheritance (28–37, 39–43). Additionally, immune system development continues into the postpartum period in which environmental factors, such as birthing parent mental health, impact risk of atopic disease (13, 14, 44–46). Thus, in utero programming and early environmental exposures impact susceptibility to the development of atopic disease.
The current study aimed to investigate whether the relationship between birthing parent ACEs and child atopic diseases persisted when children were five years old, given more definitive diagnoses are possible at this age (47, 48). Based on previous findings, birthing parent ACEs were expected to be associated with an increased risk of diagnoses of child atopic diseases, including asthma, allergy, and eczema, at five years. Potential indirect effects through birthing parent history of atopy as well as birthing parent mental health, including symptoms of depression and anxiety, were also explored (see Figure 1).
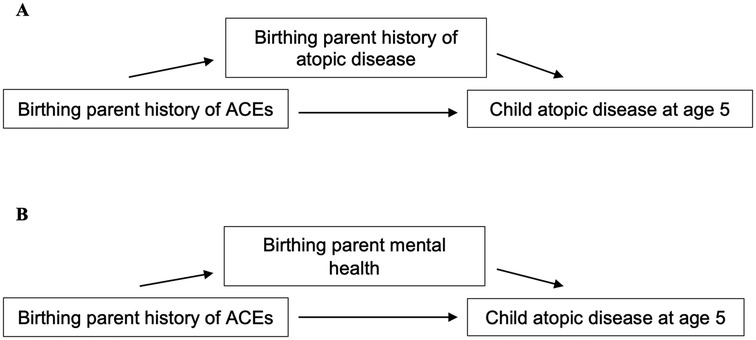
Figure 1. Model of relationship between birthing parent ACEs and child atopic disease at 5 years. (A) Model of indirect effects through birthing parent history of atopic disease. (B) Model of indirect effects through birthing parent mental health, including symptoms of depression and anxiety.
Method
Participants and procedures
The current investigation utilizes longitudinal data from the All Our Families (AOF) cohort study and was approved by the Conjoint Health Research Ethics Board (CHREB) at the University of Calgary (REB19-1646) (26, 27). Given the All Our Families (previously All Our Babies) study was designed to be an epidemiological prospective cohort study, the largest sample possible was recruited from all “women” accessing prenatal care in Calgary, Canada between 2008 and 2011, to enable longitudinal analyses (26). A total of 4,011 individuals responded to community advertisements or researchers at primary health care offices and laboratory services recruiting “pregnant women” (hereafter referred to as birthing parents, as gender identity information was not collected), of which 3,387 met inclusion criteria and were enrolled in the study (26). Eligibility criteria included being less than 25 weeks gestation, at least 18 years of age, able to complete questionnaires in English, and receiving prenatal care in Calgary, Alberta. Participants completed a battery of questionnaires before 25 weeks gestation (early pregnancy), at 34–36 weeks gestation (late pregnancy), at 4 and 12 months postpartum, and at 2, 3, and 5 years postpartum.
Measures
Demographics
Relevant sociodemographic information was collected via self-report in early pregnancy, including birthing parent ethnicity (coded as European-Canadian or not), education (coded as ≥ post-secondary), household income (coded as ≥$80,000 CAD) (14, 49), marital status (coded as partnered or not), and parity (coded as primiparous or not). At four months postpartum, participants reported on gestational age at birth (preterm <37 weeks) and infant sex (coded as male or not). Participants reported on breastfeeding duration (weeks) at 12 months postpartum.
Birthing parent history of adverse childhood experiences
At 3 years postpartum, the Adverse Childhood Experiences (ACE) scale was administered. The ACE scale is an 11-item retrospective self-report questionnaire that measures eight categories of child abuse and household dysfunction before the age of 18 (15, 50). Some items (e.g., “Were your parents separated or divorced?”) are rated dichotomously (0 = no, 1 = yes) and others (e.g., “How often did anyone at least 5 years older than you or an adult ever touch you sexually?”) are rated on a 3-point frequency scale (1 = never, 2 = once, 3 = more than once). Participants were coded as having experienced zero to four or more categories of ACEs (continuous variable of 0 to ≥4) in accordance with previous research that notes a dose-response relationship between ACEs and mental health outcomes (15, 50, 51). The ACE questionnaire is widely used and has demonstrated satisfactory consistency and test-retest reliability (52).
Birthing parent mental health
Symptoms of anxiety and depression measured using the Spielberger State Anxiety Inventory (STAI) and the Edinburgh Postnatal Depression Scale (EPDS), respectively, during late pregnancy and at 2 and 5 years postpartum were included in the current investigation (53, 54). The EPDS is a 10 item self-report questionnaire used to measure perinatal and postnatal depression. Items are rated on a 4-point scale and summed to produce a total score ranging from 0–30, wherein higher scores indicating more depressive symptoms (53, 55). The STAI is a 20-item self-report measure of state anxiety, with items rated on a 4-point scale (ranging from “Almost Never” to “Almost Always”) based on “how you feel right now” (54). Responses are summed to calculate a total score ranging from 20–80, wherein higher scores indicate higher state anxiety. Both the STAI and the EPDS have demonstrated satisfactory validity and reliability during the perinatal period (53, 56–61). Total continuous scores for the STAI and the EPDS were used for all analyses.
Atopic disease
At 5 years postpartum, participants reported on their own history of atopic disease, including asthma and allergy (coded as 0 = none, 1 = either or both). Participants were also asked if their child had experienced asthma, allergies (environmental or food), or eczema (dermatitis/psoriasis) within the past year (between 4 and 5 years old) to ensure the measure did not capture experiences of childhood wheeze (38, 47). Participants responded no or yes (coded as 0 or 1, respectively) to each disease outcome.
Statistical analysis
Descriptive statistics were conducted using IBM SPSS Statistics [Version 27; (62)]. Differences in demographic and birthing parent characteristics among children with and without atopy at 5 years old were tested for descriptive purposes using independent t-tests for continuous variables (birthing parent age, breastfeeding duration, symptoms of anxiety and depression) and χ2 tests for dichotomous variables (birthing parent ACEs and history of atopy, birthing parent ethnicity, education, household income, marital status, parity, preterm birth, and infant sex). Logistic regressions and path analyses were conducted with Mplus 8, as this program can handle binary outcomes and mediators (63). Logistic regressions [using the Categorical option to specify the dependent variable; (64)] tested if birthing parent ACEs were associated with risk for atopy (asthma, allergies, and eczema, respectively) in children at 5 years of age. Odds ratios were estimated, where significance is indicated by a 95% confidence interval (CI) that does not include one. Birthing parent history of atopy, ethnicity, education, household income, parity, gestational age, and child sex were included as covariates in adjusted models since they have been associated with risk for child atopic disease (34, 38, 65–72). Exploratory path analyses (using the Model Indirect command (64); tested indirect effects of birthing parents' history of ACEs on their children's risk for atopic disease through birthing parent history of atopy and birthing parent mental health, including anxiety and depression during late pregnancy and at 2 and 5 years postpartum, respectively. Total natural indirect effects (TNIE) were derived using counterfactuals (i.e., the contrast between the effect of the mediator on the outcome at different levels of the exposure), which is the Mplus default for logistic regression (72). Models were estimated using 1,000 bootstrapped resamples, where significance is indicated by a 95% confidence interval (CI) that does not cross zero (74). Missing data was handled using full information maximum likelihood (FIML), which produces unbiased model parameters (75).
Results
Sample description
The AOF cohort is representative of the pregnant population in an urban centre in (1, 27, 76). Of the 3,387 participants who reported demographics in early pregnancy, most (81%) completed post-secondary schooling, most (78.6%) identified as European-Canadian (with 4.4% identifying as Chinese, 3.5% Mixed/Other, 3.0% South Asian, 2.3% Latin American, 1.9% Filipino, 1.5% Southeast Asian, 1.5% Black/African North American, 1.3% Arab, 0.9% First Nations/Metis, 0.1% Korean, 0.4% West Asian, and 0.3% Japanese), almost all (98.6%) reported having a partner, and half (50.0%) were pregnant with their first child.
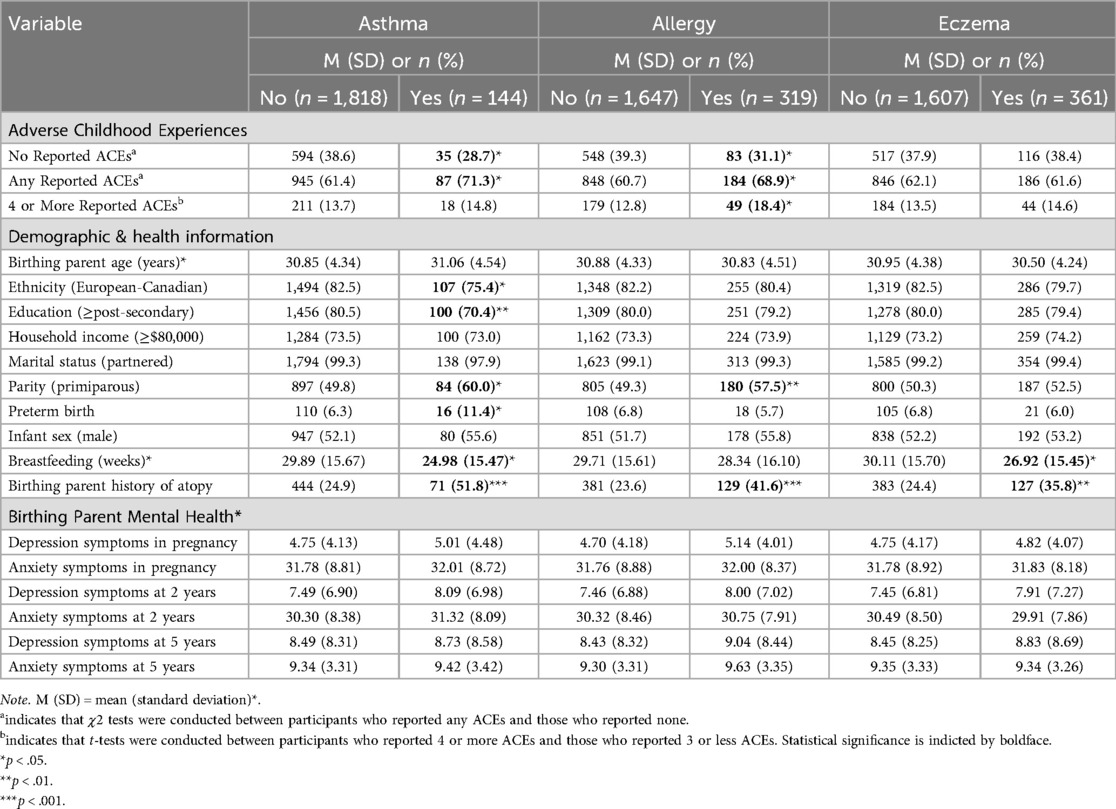
Of the participants who reported on history of ACEs (n = 1,984), 62.3% (n = 1,237) reported experiencing at least one ACE and 14.8% (n = 294) reported four or more ACEs. Of the participants who reported on children's atopic disease at 5 years (n = 1,960), 31.4% (n = 616) reported having a child with an atopic disease: 7.3% (n = 144) with asthma, 9.4% (n = 319) with allergies, and 18.3% (361) with eczema, respectively. Birthing parents of children with asthma reported less education, were less likely to identify as European-Canadian, and were more likely to report one or more ACEs. Birthing parents of children with allergies reported more ACEs. Birthing parents of children with asthma, allergy, or eczema were also more likely to report a history of asthma or allergy themselves. Characteristics of the sample according to child atopic disease are presented in Table 1.
Logistic regression
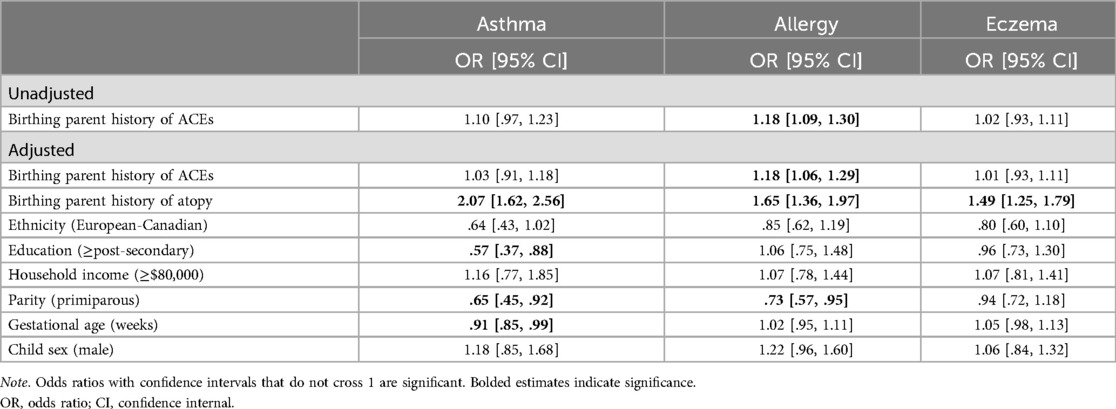
Birthing parent ACEs were not associated with child asthma in either unadjusted (OR = 1.10, 95% CI: .97, 1.23) or adjusted models (OR = 1.03, 95% CI: .91, 1.18). Similarly, birthing parent history of ACEs was not significantly associated with child eczema in unadjusted (OR = 1.02, 95% CI: .93, 1.11) or adjusted models (OR = 1.01, 95% CI: .92, 1.10). However, birthing parent ACEs were significantly associated with child allergies at 5 years old in both unadjusted (OR = 1.18, 95% CI: 1.09, 1.30) and adjusted models (OR = 1.18, 95% CI: 1.06, 1.29). See Table 2 for complete presentation of the binary logistic regression results.
Exploratory path analysis
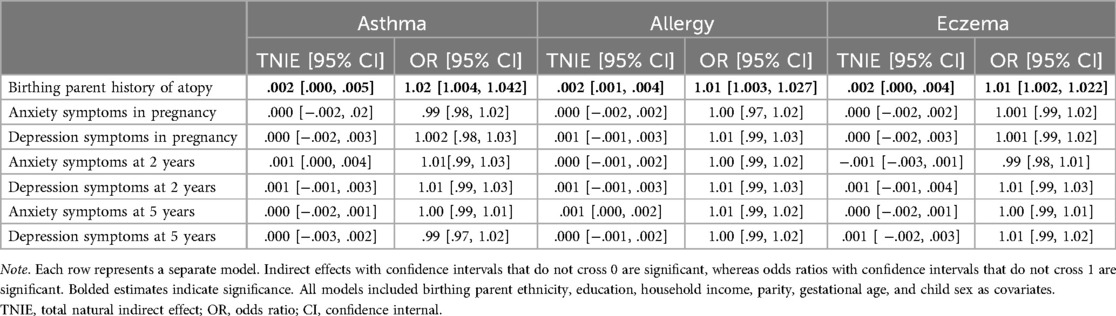
Indirect effects are presented in Table 3. After adjusting for birthing parent ethnicity and education, household income, parity, gestational age, and child sex, there were significant indirect effects of birthing parents' ACEs through birthing parent history of atopy on increased risk of children's asthma, allergies, and eczema at 5 years old. There were no significant indirect effects through birthing parent mental health during pregnancy, at 2 or 5 years postpartum for children's asthma, allergies, or eczema.
Discussion
This follow-up study examined the relationship between birthing parents' ACEs and their children's risk of atopic disease at 5 years old, in the All Our Families cohort, and explored potential genetic and environmental pathways of intergenerational transmission. Results indicated that birthing parent ACEs were directly associated with an increased risk of allergies at 5 years, but not asthma or eczema, beyond other known correlates of atopic disease in children. There was an indirect effect of ACEs on children's asthma, allergy, and eczema at 5 years, with indirect effects observed through birthing parent history of atopy, but not symptoms of birthing parent mental health.
The current findings indicated that the association between birthing parent history of childhood abuse and child asthma at 2 years of age, observed in a previous report with this cohort (14), did not persist at five years. However, asthma diagnoses at two years could have been inflated due to the prevalence of childhood wheeze (36, 38). There is a reduced probability of childhood wheeze being captured by the measure of atopic disease in the current study, as a more definitive diagnosis of asthma is possible at 5 years of age (47, 48). Birthing parent ACEs was also not directly associated with child eczema at 5 years. However, the relationship may be better explained by indirect effects, such as birthing parent history of atopy as examined in the exploratory path analyses. It is also possible that other risk factors not included in the current study, such as environmental exposures (e.g., climate, pollution, microbial exposure), allergen exposure (e.g., animal dander), and family history of atopic disease (beyond the birthing parent), play role a bigger role as children age and should be investigated (3, 5, 77, 78). Birthing parent history of ACEs was directly associated with birthing parents' reports of child allergies at 5 years, which corresponds with the previous findings from this cohort (14), and the observed increases in effect size were aligned with what has been previously published in studies of child atopic disease outcomes (12, 19, 21). It is possible that the measurement of birthing parent childhood abuse in the previous study and ACEs in the current investigation are differentially related to child atopic diseases; the ACEs questionnaire was administered at 3 years postpartum, as compared to the childhood abuse questionnaire administered during pregnancy, and captures experiences of neglect and household dysfunction in addition to childhood abuse (79).
The exploratory path analyses suggest that birthing parent history of atopy may represent a genetic pathway through which birthing parent exposure to ACEs confers risk for children's development of atopic disease. These findings align with research that demonstrates that ACEs are associated with an increased risk of atopic disease onset and that family history of atopic disease is a risk factor for the development of child atopic disease (19, 28, 29, 31, 38, 80). Due to the high heritability of atopic disease (5, 29, 38), it is possible that a genetic predisposition to atopy is animated by exposure to ACEs, which then increases intergenerational risk of transmission through pathways such as disrupted maternal cortisol production and/or immune disruption during pregnancy, which impact fetal immune-system development (30, 32). It is also possible that greater exposure to ACEs increases the likelihood of individuals developing atopy, which in turn increases the risk that their children will develop asthma, allergies, or eczema by 5 years of age (19, 28, 29, 31, 38, 80). As the relationship between genetic and environmental factors in the development of atopic disease is bidirectional, future studies should conduct cross-lagged panel analyses to disentangle the longitudinal direction of the relationship between birthing parent atopic disease and exposure to ACEs.
Despite prior findings that birthing parent mental health was significantly associated with risk of child atopic disease at 2 years of age (14), we found no significant associations or indirect effects through birthing parent mental health and child atopic disease at 5 years old. It may be that birthing parent mental health has less influence on children's atopy at 5 years than other environmental factors not included in the current study, such as airborne pollutants (77, 81–83). Other parental factors, such as parenting, may exert greater influence at age 5 and interact with environmental factors not included in the current study to impact child risk of atopy (84, 85). In addition, the current sample has been found to have relatively high and stable levels of social support, which may impact risk for symptoms of depression and anxiety (79, 86, 87). Future investigations should consider clinical samples to ensure that findings are generalizable to subpopulations with higher rates of depression and anxiety (27, 76). Additionally, future studies should examine the interactions between parental factors, such as mental health and parenting, and environmental factors to better understand the joint contributions of social and physical environmental characteristics to child risk of atopic disease.
The findings of the current study have important implications for paediatric and family health care. Having a better understanding of birthing parents' psychosocial and medical history may help to identify children at higher risk of developing atopic diseases and provide an opportunity for early intervention. While there are multiple factors that impact risk for child atopic disease not included in this study, the current investigation suggests that birthing parent history of ACEs and atopic disease are important factors. Identifying children at higher risk of atopic disease may be useful for future research on prevention strategies and guidelines, as targeting high risk populations presents benefits to clinical trials (i.e., smaller sample size, participants motivated to adhere to intervention (88, 89). Providing information on the prevention and management of atopic disease to birthing parents with a history of ACEs, asthma, or allergy could decrease pediatric exposure to known risk factors for child atopy and mitigate intergenerational transmission of risk (36, 85, 90–92). Other findings from the AOF cohort suggest that interventions aimed to build social support may further reduce the impact of birthing parent ACEs on infant health outcomes (85). Lastly, ensuring trauma-informed care is available to patients with a history of ACEs is a critical part of reducing the inequities that may result from ACEs exposure and its sequelae (93–95). The current findings highlight an opportunity to reduce intergenerational health inequities resulting from childhood adversity by providing early intervention to children at higher risk of developing atopic diseases.
Strengths and limitations
The large sample size and prospective design of the All Our Families cohort study enabled a robust test of the unique impact of birthing parent ACEs on child atopy, beyond several known risk factors, as well as exploration of potential indirect effects using an advanced statistical approach. However, the findings from the current investigation should be interpreted with consideration of some limitations. Participants only reported on atopic diseases that children had experienced between the ages of four and five years old. It is possible that parents of children with persistent atopic disease that had presented before the age of four may not have reported the diagnosis due to the phrasing of the measure, resulting in an underestimation of the rates of child atopic disease in our sample. While parent-report of child atopic disease is commonly used in epidemiological research and has been found to be a valid measure (96–98), physician-reported diagnosis would provide a more reliable measure of atopic disease diagnosis. While the rates of child atopic disease in our sample was representative of national prevalence rates (1–5), future studies should consider including data obtained from health records and ensure that measures include all current and active diagnoses of child atopic disease at the time of data collection. The AOF sample is representative of a pregnant urban population of parents in Canada with access to public health care and results may not be generalized to rural populations or those with limited health care access (14, 35). Future studies should ensure representation from socioeconomically and ethnically diverse families to address inequities in health care access and other social determinants of health. Lastly, other important factors that are known correlates of child atopic disease, including parenting, children's ACEs, and environmental factors such as allergen exposure, airborne pollutants, diet, climate-related factors, which were not included in the current study should be considered in future studies to offer a more holistic view of the various interacting contributors to disease risk (19, 77, 81, 85, 99, 100). Further research, addressing these limitations, will enable a more nuanced and complete understanding of the relationship between parent ACEs and child atopic disease.
Conclusions
Birthing parents' own exposure to adverse childhood experiences may elevate the risk of their children developing allergy at 5 years. Birthing parent's exposure to adverse childhood experiences may also elevate the risk of their children developing atopic disease, including asthma, allergy, and eczema, through their own history of atopy. Identification of birthing parent history of ACEs and atopic disease during the perinatal period presents an opportunity for early intervention among children at risk of developing atopic disease.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.maelstrom-research.org/study/aof.
Ethics statement
The studies involving humans were approved by Conjoint Health Research Ethics Board (CHREB), University of Calgary (REB19-1646). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MF: Conceptualization, Formal Analysis, Writing – original draft, Writing – review & editing. AM: Conceptualization, Formal Analysis, Writing – original draft, Writing – review & editing. MA: Writing – review & editing. ST: Funding acquisition, Project administration, Writing – review & editing. LT: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by funding from the Canadian Child Health Clinician Scientist Program (LTM), the Canadian Institutes of Health Research Early Career Investigator Award in Maternal, Reproductive, Child and Youth Health (LTM), and a Social Sciences and Humanities Research Council fellowship (ALM). All Our Babies is funded through Alberta Innovates Interdisciplinary Team Grant #200700595. Additional funding for the cohort was provided by the Alberta Children's Hospital Foundation and The Max Bell Foundation.
Acknowledgments
The authors thank the All Our Families study team and the study participants and their families for their valuable contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ACEs, adverse childhood experiences; AOF, all our families; CI, confidence interval; OR, odds ratio.
References
1. Canada S. Table 13-10-0763-01 Health Characteristics of Children and Youth Aged 1 to 17 Years, Canadian Health Survey on Children and Youth 2019.
2. Clarke AE, Elliott SJ, St. Pierre Y, Soller L, La Vieille S, Ben-Shoshan M. Temporal trends in prevalence of food allergy in Canada. J Aller Clin Immunol Pract. (2020) 8(4):1428–1430.e5. doi: 10.1016/j.jaip.2019.10.021
3. Laughter MR, Maymone MBC, Mashayekhi S, Arents BWM, Karimkhani C, Langan SM, et al. The global burden of atopic dermatitis: lessons from the global burden of disease study 1990–2017*. Br J Dermatol. (2021) 184(2):304–9. doi: 10.1111/bjd.19580
4. Silverberg JI, Barbarot S, Gadkari A, Simpson EL, Weidinger S, Mina-Osorio P, et al. Atopic dermatitis in the pediatric population: a cross-sectional, international epidemiologic study. Ann Allergy Asthma Immunol. (2021) 126(4):417–428.e2. doi: 10.1016/j.anai.2020.12.020
5. Thyssen JP, Rinnov MR, Vestergaard C. Disease mechanisms in atopic dermatitis: a review of aetiological factors. Acta Derm Venereol. (2020) 100(12):adv00162. doi: 10.2340/00015555-3512
6. Geraldo José Cunha Â, Zbonik Mendes A, Dias Wanderley de Carvalho F, Aparecida Ribeiro de Paula M, Gonçalves Brasil T. The impact of asthma on quality of life and anxiety: a pilot study. J Asthma. (2019) 56(6):680–5. doi: 10.1080/02770903.2018.1486854
7. Chipps BE, Haselkorn T, Paknis B, Ortiz B, Bleecker ER, Kianifard F, et al. More than a decade follow-up in patients with severe or difficult-to-treat asthma: the epidemiology and natural history of asthma: outcomes and treatment regimens (TENOR) II. J Allergy Clin Immunol. (2018) 141(5):1590–1597.e9. doi: 10.1016/j.jaci.2017.07.014
8. Hossny E, Caraballo L, Casale T, El-Gamal Y, Rosenwasser L. Severe asthma and quality of life. World Allergy Organ J. (2017) 10(1):28–28. doi: 10.1186/s40413-017-0159-y
9. Silverberg JI, Garg NK, Paller AS, Fishbein AB, Zee PC. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. (2015) 135(1):56–66. doi: 10.1038/jid.2014.325
10. Wise SK, Lin SY, Toskala E, Orlandi RR, Akdis CA, Alt JA, et al. International consensus statement on allergy and rhinology: allergic rhinitis. Int Forum Allergy Rhinol. (2018) 8(2):108–352. doi: 10.1002/alr.22073
11. Jones CW, Esteves KC, Gray SAO, Clarke TN, Callerame K, Theall KP, et al. The transgenerational transmission of maternal adverse childhood experiences (ACEs): insights from placental aging and infant autonomic nervous system reactivity. Psychoneuroendocrino. (2019) 106:20–7. doi: 10.1016/j.psyneuen.2019.03.022
12. Lê-Scherban F, Wang X, Boyle-Steed K, Pachter L. Intergenerational associations of parent adverse childhood experiences and child health outcomes. Pediatrics. (2018) 141(6):e20174274. doi: 10.1542/peds.2017-4274
13. McDonnell CG, Valentino K. Intergenerational effects of childhood trauma: evaluating pathways among maternal ACEs, perinatal depressive symptoms, and infant outcomes. Child Maltreat. (2016) 21(4):317–26. doi: 10.1177/1077559516659556
14. Tomfohr-Madsen LM, Bayrampour H, Tough S. Maternal history of childhood abuse and risk of asthma and allergy in 2-year-old children. Psychosom Med. (2016) 78(9):1031–42. doi: 10.1097/PSY.0000000000000419
15. Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the adverse childhood experiences (ACE) study. Am J Prev Med. (1998) 14(4):245–58. doi: 10.1016/S0749-3797(98)00017-8
16. Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, et al. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health. (2017) 2(8):e356–66. doi: 10.1016/S2468-2667(17)30118-4
17. Kalmakis KA, Chandler GE. Health consequences of adverse childhood experiences: a systematic review. J Am Assoc Nurse Pra. (2015) 27(8):457–65. doi: 10.1002/2327-6924.12215
18. Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull. (2011) 137(6):959–97. doi: 10.1037/a0024768
19. Pape K, Cowell W, Sejbaek CS, Andersson NW, Svanes C, Kolstad HA, et al. Adverse childhood experiences and asthma: trajectories in a national cohort. Thorax. (2021) 76(6):547–53. doi: 10.1136/thoraxjnl-2020-214528
20. Suglia SF, Koenen KC, Boynton-Jarrett R, Chan PS, Clark CJ, Danese A, et al. Childhood and adolescent adversity and cardiometabolic outcomes: a scientific statement from the American heart association. Circulation. (2018) 137(5):e15–28. doi: 10.1161/CIR.0000000000000536
21. Wing RMD, Gjelsvik AP, Nocera MMD, McQuaid ELP. Association between adverse childhood experiences in the home and pediatric asthma. Ann Allerg Asthma Imnunol. (2015) 114(5):379–84. doi: 10.1016/j.anai.2015.02.019
22. Möhler E, Matheis V, Marysko M, Finke P, Kaufmann C, Cierpka M, et al. Complications during pregnancy, peri- and postnatal period in a sample of women with a history of child abuse. J Psychosom Obst Gyn. (2008) 29(3):197–202. doi: 10.1080/01674820801934252
23. Rijlaarsdam J, Stevens G, Jansen P, Ringoot AP, Jaddoe VWV, Hofman A, et al. Maternal childhood maltreatment and offspring emotional and behavioral problems: maternal and paternal mechanisms of risk transmission. Child Maltreat. (2014) 19(2):67–78. doi: 10.1177/1077559514527639
24. Stein AP, Pearson RMP, Goodman SHP, Rapa ED, Rahman AP, McCallum MMA, et al. Effects of perinatal mental disorders on the fetus and child. The Lancet. (2014) 384(9956):1800–19. doi: 10.1016/S0140-6736(14)61277-0
25. Sun J, Patel F, Rose-Jacobs R, Frank DA, Black MM, Chilton M. Mothers’ adverse childhood experiences and their young children’s development. Am J Prev Med. (2017) 53(6):882–91. doi: 10.1016/j.amepre.2017.07.015
26. McDonald SW, Lyon AW, Benzies KM, McNeil DA, Lye SJ, Dolan SM, et al. The all our babies pregnancy cohort: design, methods, and participant characteristics. BMC Pregnancy Childbirth. (2013) 13(Suppl 1):S2–S2. doi: 10.1186/1471-2393-13-S1-S2
27. Tough SC, McDonald SW, Collisson BA, Graham SA, Kehler H, Kingston D, et al. Cohort profile: the all our babies pregnancy cohort (AOB). Int J Epidemiol. (2017) 46(5):1389–90. doi: 10.1093/ije/dyw363
28. Alford SH, Zoratti E, Peterson EL, Maliarik M, Ownby DR, Johnson CC. Parental history of atopic disease: disease pattern and risk of pediatric atopy in offspring. J Allergy Clin Immunol. (2004) 114(5):1046–50. doi: 10.1016/j.jaci.2004.08.036
29. Bohme M, Wickman M, Lennart Nordvall S, Svartengren M, Wahlgren CF. Family history and risk of atopic dermatitis in children up to 4 years. Clin Exp Allergy. (2003) 33(9):1226–31. doi: 10.1046/j.1365-2222.2003.01749.x
30. Brunst KJ, Rosa MJ, Jara C, Lipton LR, Lee A, Coull BA, et al. Impact of maternal lifetime interpersonal trauma on children’s asthma: mediation through maternal active asthma during pregnancy. Psychosom Med. (2017) 79(1):91–100. doi: 10.1097/PSY.0000000000000354
31. Burke W, Fesinmeyer M, Reed K, Hampson L, Carlsten C. Family history as a predictor of asthma risk. Am J Prev Med. (2003) 24(2):160–9. doi: 10.1016/S0749-3797(02)00589-5
32. Chida Y, Hamer M, Steptoe A. A bidirectional relationship between psychosocial factors and atopic disorders: a systematic review and meta-analysis. Psychosom Med. (2008) 70(1):102–16. doi: 10.1097/PSY.0b013e31815c1b71
33. Fujimura T, Lum SZC, Nagata Y, Kawamoto S, Oyoshi MK. Influences of maternal factors over offspring allergies and the application for food allergy [review]. Front Immunol. (2019) 10:1933. doi: 10.3389/fimmu.2019.01933
34. Gilliland FD, Li Y-F, Peters JM. Effects of maternal smoking during pregnancy and environmental tobacco smoke on asthma and wheezing in children. Am J Resp Crit Care. (2001) 163(2):429–36. doi: 10.1164/ajrccm.163.2.2006009
35. Guxens M, Sonnenschein–van der Voort AM, Tiemeier H, Hofman A, Sunyer J, de Jongste JC, et al. Parental psychological distress during pregnancy and wheezing in preschool children: the generation R study. J Allergy Clin Immunol. (2013) 133(1):59–67.e12. doi: 10.1016/j.jaci.2013.04.044
36. Lim RH, Kobzik L, Dahl M. Risk for asthma in offspring of asthmatic mothers versus fathers: a meta-analysis. PLoS One. (2010) 5(4):e10134–e10134. doi: 10.1371/journal.pone.0010134
37. Suh DI, Chang HY, Lee E, Yang SI, Hong SJ. Prenatal maternal distress and allergic diseases in offspring: review of evidence and possible pathways. Allergy Asthma Immunol Res. (2017) 9(3):200–11. doi: 10.4168/aair.2017.9.3.200
38. Thomsen SF. Epidemiology and natural history of atopic diseases. Eur Clin Respir J. (2015) 2(1):24642. doi: 10.3402/ecrj.v2.24642
39. Vehmeijer FOL, Guxens M, Duijts L, El Marroun H. Maternal psychological distress during pregnancy and childhood health outcomes: a narrative review. J Dev Origins Health Dis. (2019) 10(3):274–85. doi: 10.1017/S2040174418000557
40. Grieger JA, Clifton VL, Tuck AR, Wooldridge AL, Robertson SA, Gatford KL. In utero programming of allergic susceptibility. Int Arch Allergy Immunol. (2016) 169(2):80–92. doi: 10.1159/000443961
41. Thomas JC, Letourneau N, Campbell TS, Giesbrecht GF. Social buffering of the maternal and infant HPA axes: mediation and moderation in the intergenerational transmission of adverse childhood experiences. Dev Psychopathol. (2018a) 30(3):921–39. doi: 10.1017/S0954579418000512
42. Thomas JC, Magel C, Tomfohr-Madsen L, Madigan S, Letourneau N, Campbell TS, et al. Adverse childhood experiences and HPA axis function in pregnant women. Horm Behav. (2018b) 102:10–22. doi: 10.1016/j.yhbeh.2018.04.004
43. Wright RJ. Stress and childhood asthma risk: overlapping evidence from animal studies and epidemiologic research. All Asthma Clin Immunol. (2008) 4:29. doi: 10.1186/1710-1492-4-1-29
44. Kawaguchi C, Murakami K, Obara T, Ishikuro M, Ueno F, Noda A, et al. Maternal psychological distress during and after pregnancy and atopic dermatitis in children. Eur J Public Health. (2020) 30:ckaa166–972. doi: 10.1093/eurpub/ckaa166.972
45. van der Leek AP, Bahreinian S, Chartier M, Dahl ME, Azad MB, Brownell MD, et al. Maternal distress during pregnancy and recurrence in early childhood predicts atopic dermatitis and asthma in childhood. Chest. (2020) 158(1):57–67. doi: 10.1016/j.chest.2020.01.052
46. Wang IJ, Wen HJ, Chiang TL, Lin SJ, Guo YL. Maternal psychologic problems increased the risk of childhood atopic dermatitis. Pediatr Allergy Immunol. (2016) 27(2):169–76. doi: 10.1111/pai.12518
47. Oluwole O, Rennie DC, Goodridge D, Blackburn D, Litzenberger T, Penz E, et al. The course of asthma: a population-based 10-year study examining asthma remission in children diagnosed with asthma in preschool. Pediatr Pulmonol. (2020) 55(8):1924–35. doi: 10.1002/ppul.24881
48. Bacharier LB, Boner A, Carlsen KH, Eigenmann PA, Frischer T, Götz M, et al. Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report: diagnosis and treatment of asthma in childhood. Allergy. (2008) 63(1):5–34. doi: 10.1111/j.1398-9995.2007.01586.x
49. Canada S. Calgary, CMA, Alberta (Code 825) (Table). National Household Survey (NHS) Profile. Statistics Canada (2013). Available online at: https://www12.statcan.gc.ca/nhs-enm/2011/dp-pd/prof/details/page.cfm?Lang=E&Geo1=CMA&Code1=825&Data=Count&SearchText=Calgary&SearchType=Begins&SearchPR=48&A1=Income%20of%20households&B1=All&Custom=& (Accessed November 15, 2019).
50. Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, et al. The enduring effects of abuse and related adverse experiences in childhood: a convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. (2006) 256(3):174–86. doi: 10.1007/s00406-005-0624-4
51. Racine N, Zumwalt K, McDonald S, Tough S, Madigan S. Perinatal depression: the role of maternal adverse childhood experiences and social support. J Affect Disorders. (2020b) 263:576–81. doi: 10.1016/j.jad.2019.11.030
52. Dube SR, Williamson DF, Thompson T, Felitti VJ, Anda RF. Assessing the reliability of retrospective reports of adverse childhood experiences among adult HMO members attending a primary care clinic. Child Abuse Neglect. (2004) 28(7):729–37. doi: 10.1016/j.chiabu.2003.08.009
53. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh postnatal depression scale. Br J Psychiatry. (1987) 150(6):782–6. doi: 10.1192/bjp.150.6.782
54. Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press (1983).
55. Beck CT. Predictors of postpartum depression: an update. Nurs Res. (2001) 50(5):275–85. doi: 10.1097/00006199-200109000-00004
56. Tendais I, Costa R, Conde A, Figueiredo B. Screening for depression and anxiety disorders from pregnancy to postpartum with the EPDS and STAI. Span J Psychol. (2014) 17(2):E7. doi: 10.1017/sjp.2014.7
57. Affonso DD, De AK, Horowitz JA, Mayberry LJ. An international study exploring levels of postpartum depressive symptomatology. J Psychosom Res. (2000) 49(3):207–16. doi: 10.1016/S0022-3999(00)00176-8
58. Benediktsson I, McDonald S, Tough S. Examining the psychometric properties of three standardized screening tools in a pregnant and parenting population. Matern Child Health J. (2017) 21(2):253–9. doi: 10.1007/s10995-016-2128-4
59. Bergink V, Kooistra L, Lambregtse-van den Berg MP, Wijnen H, Bunevicius R, van Baar A, et al. Validation of the Edinburgh depression scale during pregnancy. J Psychosom Res. (2011) 70(4):385–9. doi: 10.1016/j.jpsychores.2010.07.008
60. Grant K-A, McMahon C, Austin M-P. Maternal anxiety during the transition to parenthood: a prospective study. J Affect Disorders. (2007) 108(1):101–11. doi: 10.1016/j.jad.2007.10.002
61. Loughnan SA, Wallace M, Joubert AE, Haskelberg H, Andrews G, Newby JM. A systematic review of psychological treatments for clinical anxiety during the perinatal period. Arch Womens Mental Health. (2018) 21(5):481–90. doi: 10.1007/s00737-018-0812-7
63. Valente MJ, Rijnhart JJM, Smyth HL, Muniz FB, MacKinnon DP. Causal mediation programs in R, M plus, SAS, SPSS, and stata. Struct Equ Model. (2020) 27(6):975–84. doi: 10.1080/10705511.2020.1777133
64. Muthén LK, Muthén BO. Mplus User’s Guide (8th ed.). Los Angeles, CA: Muthén & Muthén (1998-2017).
65. Almqvist C, Worm M, Leynaert B. Impact of gender on asthma in childhood and adolescence: a GA(2)LEN review. Allergy. (2008) 63(1):47–57. doi: 10.1111/j.1398-9995.2007.01524.x
66. Hafkamp-De Groen E, Sonnenschein-Voort A, Mackenbach J, Duijts L, Jaddoe V, Moll H, et al. Socioeconomic and sociodemographic factors associated with asthma related outcomes in early childhood: the generation R study. PLoS One. (2013) 8(11):e78266–e78266. doi: 10.1371/journal.pone.0078266
67. Karmaus W, Botezan C. Does a higher number of siblings protect against the development of allergy and asthma? A review. J Epidemiol Commun Health. (2002) 56(3):209–17. doi: 10.1136/jech.56.3.209
68. Leeners B, Stiller R, Block E, Görres G, Rath W. Pregnancy complications in women with childhood sexual abuse experiences. J Psychosom Res. (2010) 69(5):503–10. doi: 10.1016/j.jpsychores.2010.04.017
69. Spencer N. Maternal education, lone parenthood, material hardship, maternal smoking, and longstanding respiratory problems in childhood: testing a hierarchical conceptual framework. J Epidemiol Commun Health. (2005) 59(10):842–6. doi: 10.1136/jech.2005.036301
70. Tough SC, Siever JE, Leew S, Johnston DW, Benzies K, Clark D. Maternal mental health predicts risk of developmental problems at 3 years of age: follow up of a community based trial. BMC Pregnancy Childbirth. (2008) 8(1):16. doi: 10.1186/1471-2393-8-16
71. Zacharasiewicz A. Maternal smoking in pregnancy and its influence on childhood asthma. ERJ Open Res. (2016) 2(3):42. doi: 10.1183/23120541.00042-2016
72. Trønnes H, Wilcox AJ, Lie RT, Markestad T, Moster D. The association of preterm birth with severe asthma and atopic dermatitis: a national cohort study. Pediatr Allergy Immunol. (2013) 24(8):782–7. doi: 10.1111/pai.12170
73. Muthén BO, Muthén LK, Asparouhov T. Regression and Mediation Analysis Using Mplus. Los Angeles, CA: Muthén & Muthén (2017).
74. Hayes AF. Beyond baron and kenny: statistical mediation analysis in the new millennium. Commun Monogr. (2009) 76(4):408–20. doi: 10.1080/03637750903310360
75. Enders CK. Applied Missing Data Analysis. Methodology in the Social Sciences Series. New York, NY: Guilford Press (2010).
76. McDonald SW, Madigan S, Racine N, Benzies K, Tomfohr L, Tough S. Maternal adverse childhood experiences, mental health, and child behaviour at age 3: the all our families community cohort study. Prev Med. (2019) 118:286–94. doi: 10.1016/j.ypmed.2018.11.013
77. Pfirrman S, Devonshire A, Winslow A. Environmental interventions for preventing atopic diseases. Curr Allergy Asthma Rep. (2024) 24(5):233–51. doi: 10.1007/s11882-024-01141-1
78. Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. (2015) 66(1):8–16. doi: 10.1159/000370220
79. Racine N, Plamondon A, Mcdonald S, Tough S, Madigan S. The consistency of maternal childhood abuse reporting in pregnancy and the postpartum period. J Women’s Health. (2020) 29(4):561–9. doi: 10.1089/jwh.2019.7795
80. Exley D, Norman A, Hyland M. Adverse childhood experience and asthma onset: a systematic review. Eur Respir Rev. (2015) 24(136):299–305. doi: 10.1183/16000617.00004114
81. Gold DR, Adamkiewicz G, Arshad SH, Celedón JC, Chapman MD, Chew GL, et al. NIAID, NIEHS, NHLBI, and MCAN workshop report: the indoor environment and childhood asthma—implications for home environmental intervention in asthma prevention and management. J Allergy Clin Immunol. (2017) 140(4):933–49. doi: 10.1016/j.jaci.2017.04.024
82. Kantor R, Silverberg JI. Environmental risk factors and their role in the management of atopic dermatitis. Expert Rev Clin Immunol. (2017) 13(1):15–26. doi: 10.1080/1744666X.2016.1212660
83. Koh HY, Kim TH, Sheen YH, Lee SW, An J, Kim MA, et al. Serum heavy metal levels are associated with asthma, allergic rhinitis, atopic dermatitis, allergic multimorbidity, and airflow obstruction. J Allergy Clin Immunol Pract. (2019) 7(8):2912–2915.e2. doi: 10.1016/j.jaip.2019.05.015
84. Chen E, Miller GE, Shalowitz MU, Story RE, Levine CS, Hayen R, et al. Difficult family relationships, residential greenspace, and childhood asthma. Pediatrics. (2017) 139(4):e20163056. doi: 10.1542/peds.2016-3056
85. Letourneau NL, Kozyrskyj AL, Cosic N, Ntanda HN, Anis L, Hart MJ, et al. Maternal sensitivity and social support protect against childhood atopic dermatitis. Allergy Asthma Clin Immunol. (2017) 13(1):26–26. doi: 10.1186/s13223-017-0199-4
86. Hentges RF, Graham SA, Fearon P, Tough S, Madigan S. The chronicity and timing of prenatal and antenatal maternal depression and anxiety on child outcomes at age 5. Depress Anxiety. (2020) 37(6):576–86. doi: 10.1002/da.23039
87. Hetherington E, McDonald S, Williamson T, Patten SB, Tough SC. Social support and maternal mental health at 4 months and 1 year postpartum: analysis from the all our families cohort. J Epidemiol Commun Health. (2018) 72(10):933–9. doi: 10.1136/jech-2017-210274
88. Chu DK, Koplin JJ, Ahmed T, Islam N, Chang C, Lowe AJ. How to prevent atopic dermatitis (eczema) in 2024: theory and evidence. J Allergy Clin Immunol Pract. (2024) 12(7):1695–704. doi: 10.1016/j.jaip.2024.04.048
89. Ravn NH, Halling A, Berkowitz AG, Rinnov MR, Silverberg JI, Egeberg A, et al. How does parental history of atopic disease predict the risk of atopic dermatitis in a child? A systematic review and meta-analysis. J Allergy Clin Immunol. (2020) 145(4):1182–93. doi: 10.1016/j.jaci.2019.12.899
90. Abreo A, Gebretsadik T, Stone CA, Hartert TV. The impact of modifiable risk factor reduction on childhood asthma development. Clin Trans Med. (2018) 7(1):1–12. doi: 10.1186/s40169-018-0195-4
91. Fleischer DM, Chan ES, Venter C, Spergel JM, Abrams EM, Stukus D, et al. A consensus approach to the primary prevention of food allergy through nutrition: guidance from the American academy of allergy, asthma, and immunology; American college of allergy, asthma, and immunology; and the Canadian society for allergy and clinical immunology. J Allergy Clin Immunol Pract. (2021) 9(1):22–43.e24. doi: 10.1016/j.jaip.2020.11.002
92. Trambusti I, Nuzzi G, Costagliola G, Verduci E, D'Auria E, Peroni DG, et al. Dietary interventions and nutritional factors in the prevention of pediatric asthma. Front Pediatr. (2020) 8:480–480. doi: 10.3389/fped.2020.00480
93. Muzik M, Ads M, Bonham C, Lisa Rosenblum K, Broderick A, Kirk R. Perspectives on trauma-informed care from mothers with a history of childhood maltreatment: a qualitative study. Child Abuse Neglect. (2013) 37(12):1215–24. doi: 10.1016/j.chiabu.2013.07.014
94. Searle J, Goldberg L, Aston M, Burrow S. Accessing new understandings of trauma-informed care with queer birthing women in a rural context. J Clin Nurs. (2017) 26(21-22):3576–87. doi: 10.1111/jocn.13727
95. Ward LG. Trauma-informed perinatal healthcare for survivors of sexual violence. J Perinat Neonat Nurs. (2020) 34(3):199–202. doi: 10.1097/JPN.0000000000000501
96. Furu K, Karlstad Ø, Skurtveit S, Håberg SE, Nafstad P, London SJ, et al. High validity of mother-reported use of antiasthmatics among children: a comparison with a population-based prescription database. J Clin Epidemiol. (2011) 64(8):878–84. doi: 10.1016/j.jclinepi.2010.10.014
97. Hedman AM, Gong T, Lundholm C, Dahlén E, Ullemar V, Brew BK, et al. Agreement between asthma questionnaire and health care register data. Pharmacoepidem Drug Safety. (2018) 27(10):1139–46. doi: 10.1002/pds.4566
98. Wogelius P, Poulsen S, Toft S rensen H. Validity of parental-reported questionnaire data on Danish children? use of asthma-drugs: a comparison with a population-based prescription database. Eur J Epidemiol. (2005) 20(1):17–22. doi: 10.1007/s10654-004-1501-6
99. Oh DL, Jerman P, Silvério Marques S, Koita K, Purewal Boparai SK, Burke Harris N, et al. Systematic review of pediatric health outcomes associated with childhood adversity. BMC Pediatr. (2018) 18(1):83–19. doi: 10.1186/s12887-018-1037-7
Keywords: adverse childhood experiences, atopic diseases, intergenerational health outcomes, pregnancy, asthma, allergy
Citation: Freeman M, MacKinnon AL, Anselmo M, Tough S and Tomfohr-Madsen L (2025) Birthing parent adverse childhood experiences and risk of atopic diseases in 5-year-old children. Front. Allergy 5:1483911. doi: 10.3389/falgy.2024.1483911
Received: 20 August 2024; Accepted: 16 December 2024;
Published: 7 January 2025.
Edited by:
Agata Wypych-Ślusarska, Medical University of Silesia, PolandReviewed by:
Karolina Sobczyk, Medical University of Silesia, PolandMateusz Krystian Grajek, Medical University of Silesia in Katowice, Poland
Karolina Krupa-Kotara, Medical University of Silesia, Poland
Copyright: © 2025 Freeman, MacKinnon, Anselmo, Tough and Tomfohr-Madsen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lianne Tomfohr-Madsen, bGlhbm5lLnRvbWZvaHJtYWRzZW5AdWJjLmNh
†These authors have contributed equally to this work and share senior authorship
 Makayla Freeman
Makayla Freeman Anna L. MacKinnon
Anna L. MacKinnon Mark Anselmo5,6
Mark Anselmo5,6 Suzanne Tough
Suzanne Tough Lianne Tomfohr-Madsen
Lianne Tomfohr-Madsen