- 1Department of Otolaryngology Head and Neck Surgery, Beijing Tongren Hospital, Capital Medical University, Beijing, China
- 2Department of Allergy, Beijing Tongren Hospital, Capital Medical University, Beijing, China
- 3Beijing Laboratory of Allergic Diseases, Beijing Municipal Education Commission and Beijing Key Laboratory of Nasal Diseases, Beijing Institute of Otolaryngology, Beijing, China
- 4Research Unit of Diagnosis and Treatment of Chronic Nasal Diseases, Chinese Academy of Medical Sciences, Beijing, China
Background: Several epidemiological studies have shown that allergic rhinitis (AR) patients are more susceptible to coronavirus disease 2019 (COVID-19).
Objective: We aim to investigate the risk factors for COVID-19 in AR patients.
Methods: A retrospective nationwide cohort study was conducted based on a questionnaire survey in China. The baseline characteristics, region of residence, smoking and drinking status, comorbidities, vaccination status and previous infection information were obtained. Allergen test results, the SARS-CoV-2 nucleic acid test, and antigen detection results were collected. Information on AR and comorbid medication use pre-SARS-CoV-2 infection was also collected. Binary logistic regression and analysis of covariance (different adjusted models) were conducted.
Results: In all, 830 AR patients were included; 627 patients (75.54%) were infected with SARS-CoV-2. AR comorbid with allergic conjunctivitis (AC) was a protective factor [OR: 0.525 (95% CI = 0.296–0.929), P = 0.027] against SARS-CoV-2 infection, while AR comorbid with food allergy was a risk factor [OR: 6.404 (95% CI = 1.349–30.402), P = 0.0195]. Although fewer patients received four doses of the vaccine, the results showed a significant protective effect against SARS-CoV-2 infection in AR patients [OR: 0.093 (95% CI = 0.025–0.348), P = 0.0004]. Underweight was a protective factor against COVID-19 [OR: 0.287 (95% CI = 0.147–0.562), P = 0.0003] after full multivariable adjustment. Overweight was associated with a 2.071-fold higher risk for COVID-19 compared with normal weight [(95% CI = 1.045–4.105), P = 0.0370]. Additionally, house dust mite (HDM)-specific allergies were also protective against COVID-19 [OR: 0.537 (95% CI = 0.290–0.996), P = 0.0484].
Conclusions: This study revealed underlying protective and risk factors, which might be used to improve the management of AR and COVID-19.
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19), has had a huge impact on global population health. According to the Johns Hopkins Coronavirus Resource Centerboard (https://coronavirus.jhu.edu/map.html), there have been more than 600,000,000 COVID-19 cases and 687,000 related deaths globally as of March 1, 2023. More COVID-19 cases are probably not diagnosed due to limited testing availability (1). With the rapid increase in the number of patients with COVID-19, clinical progress and prognosis have received more attention. Allergic disease is associated with complex immune responses (2), and persistent inflammation may affect the structure and abnormalities in their function (3). Allergic rhinitis (AR) is the most common nasal disorder, which contributes to the aggravation of respiratory virus-related disease (4) and affects 10%–40% of the population (5, 6). Interestingly, there was no statistically significant difference between the incidence of AR diagnosed during the pandemic compared with the period before the pandemic (7). The incidence of AR comorbid with COVID-19 was 1.8% in China (8). Whether AR is a risk factor for COVID-19 is controversial; nevertheless, accumulating evidence suggests that AR is a risk factor for COVID-19. In a nationwide Korean cohort study, AR conferred a greater risk of sensitivity to SARS-CoV-2 infection and severe clinical outcomes (9). In a Chinese cohort study, AR was also an independent risk factor [OR: 1.324 (95% CI: 1.049–1.671), P = 0.0183] for susceptibility to COVID-19 based on a propensity-score-matched nationwide cohort (10). However, a separate study in China showed that AR may not have a significant modifying effect on the development of COVID-19 (11). Moreover, AR patients had lower positive SARS-CoV-2 test rates in a cohort study in England (12). Even in a study in Iranian patients, AR disease status was inversely associated with the severity of COVID-19 (13). Based on clinical observations, AR patients have shown diverse clinical manifestations during the SARS-CoV-2 pandemic. Considering the current extent of different effects of AR disease status on COVID-19 and the complex pathological mechanisms of AR, we further investigated the risk factors for COVID-19 in AR patients.
China adjusted the national prevention and control strategy of 2019-nCoV infection and emerge an epidemic peak from early Dec.2022. We immediately and efficiently performed a nationwide survey with large sample size and short collection time (Dec. 28, 2022-Jan. 13, 2023), and the survey time coincided with this epidemic peak. To our knowledge, this is the first nationwide cohort study exploring the risk factors for COVID-19 in AR patients, with the goal of improving the management of AR and COVID-19.
Methods
Study population
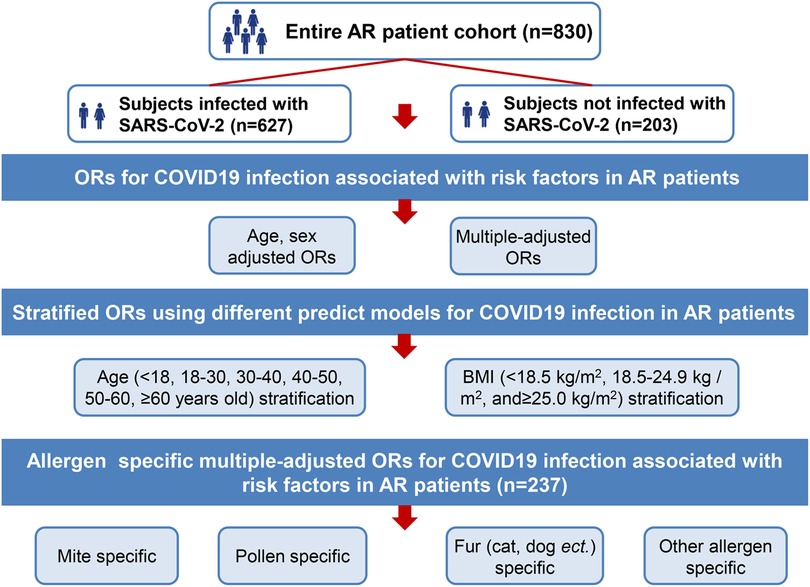
This was a retrospective cohort study. A structured questionnaire encompassing various aspects of AR and COVID-19 results was designed. We conducted a structured questionnaire survey using convenience sampling. An online informed consent form was provided to participants. The questionnaire was self-administered and was required to be completed online (mini app in WeChat on mobile phone) by the participating volunteers. At the start of the survey, all participants were told about the study goals. Since it was an anonymous questionnaire, personal information was not recorded. This study was conducted following the Declaration of Helsinki (1964) guidelines and its later amendments or comparable ethical standards. Participation in the survey was voluntary. A total of 830 participants were involved the questionnaire survey during the period (Dec. 28, 2022∼Jan. 13, 2023) (Figure 1). The online questionnaire was set so that each IP address could only answer once to avoid duplicate answers and questionnaires with missing data cannot be submitted. Our study followed the STROBE guideline, STROBE checklist was provided as Supplementary Material.
Measurement
The characteristics of age, sex, weight, height, BMI, region of residence, smoking and drinking habits, comorbidity, vaccination status, previous infection with SARS-CoV-2 or not, and so on were collected. Information on allergen test results [mite, pollen, fur allergy (cat, dog, etc.) and other allergens] was collected. The questionnaire also included items related to the SARS-CoV-2 nucleic acid test and antigen detection results. Information on AR and comorbidity medication use pre-SARS-CoV-2 infection was also collected (Figure 1).
Statistical analysis
The sample size of 609 subjects was calculated based on the estimated prevalence of COVID-19 infection prevalence of 72.49%, to attain a significant level (alpha) of 0.05 and error tolerance 0.10 p (or 10%) using PASS15 software (NCSS, USA).
Baseline characteristics are presented as the mean ± (SD)/median (min, max) and the number (%) for continuous and categorical variables, respectively. The t test/Satterthwaite's test (equal variances not assumed)/paired t-test, chi-square (χ2) test/Fisher's exact test, or Wilcoxon rank-sum test was conducted to compare participant characteristics between two groups.
We performed multivariable logistic regression analyses to investigate risk factors for COVID-19 in AR patients. Five logistic regression models (OR1–5) including different confounding factors were involved in our analysis. We also calculated BMI stratification (underweight, normal weight, and overweight) and age stratification and allergen specific ORs for COVID-19 in AR patients as sensitive analysis.
All statistical analyses were performed with SAS (version 9.4). A p value < 0.05 (two-sided) was set as statistically significant.
Analysis overview
The following analyses were performed:
(1) Demographic and clinical characteristics and SARS-CoV-2 infection results of all study subjects (n = 830) were compared (infected with SARS-CoV-2 v.s. not infected with SARS-CoV-2).
(2) We conducted a primary analysis using binary logistic regression and analysis of covariance (model adjusted for age and sex; fully adjusted for all covariance) to control potential confounding factors and calculate the odds ratio (OR) and 95% confidence interval (95% CI) of outcomes.
(3) Age stratification (younger than 18 years old, 18–30, 30–40, 40–50, 50–60, and older than 60 years old) and BMI stratification (<18.5 kg/m2, 18.5–24.9 kg/m2, and ≥25.0 kg/m2) ORs for COVID-19 in AR patients (n = 830) were calculated based on different adjusted models (model 1, adjusted for age, sex; model 2, adjusted for age, sex, weight, height; model 3, adjusted for age, sex, weight, height, BMI, region of residence, education, occupation, smoking and drinking; model 4, adjusted for age, sex, weight, height, BMI, region of residence, education, occupation, smoking and drinking, comorbidity, treatment; model 5 (fully adjusted), adjusted for age, sex, weight, height, BMI, region of residence, education, occupation, smoking and drinking, comorbidity, treatment, vaccination and previous infection).
(4) Allergen [mite, pollen, fur allergy (cat, dog etc.), and other allergen]-specific multiple-adjusted ORs for COVID-19 (n = 237) were calculated in AR patients (patients who had allergen testing results).
(5) We redefined the cohort by using a stricter definition of COVID-19 status as self-reported COVID-19 and PCR-test positivity or antigen detection positivity or both (Supplementary Materials). Then, we performed the primary analysis again to calculate the ORs for COVID-19.
Results
Demographics and clinical characteristics
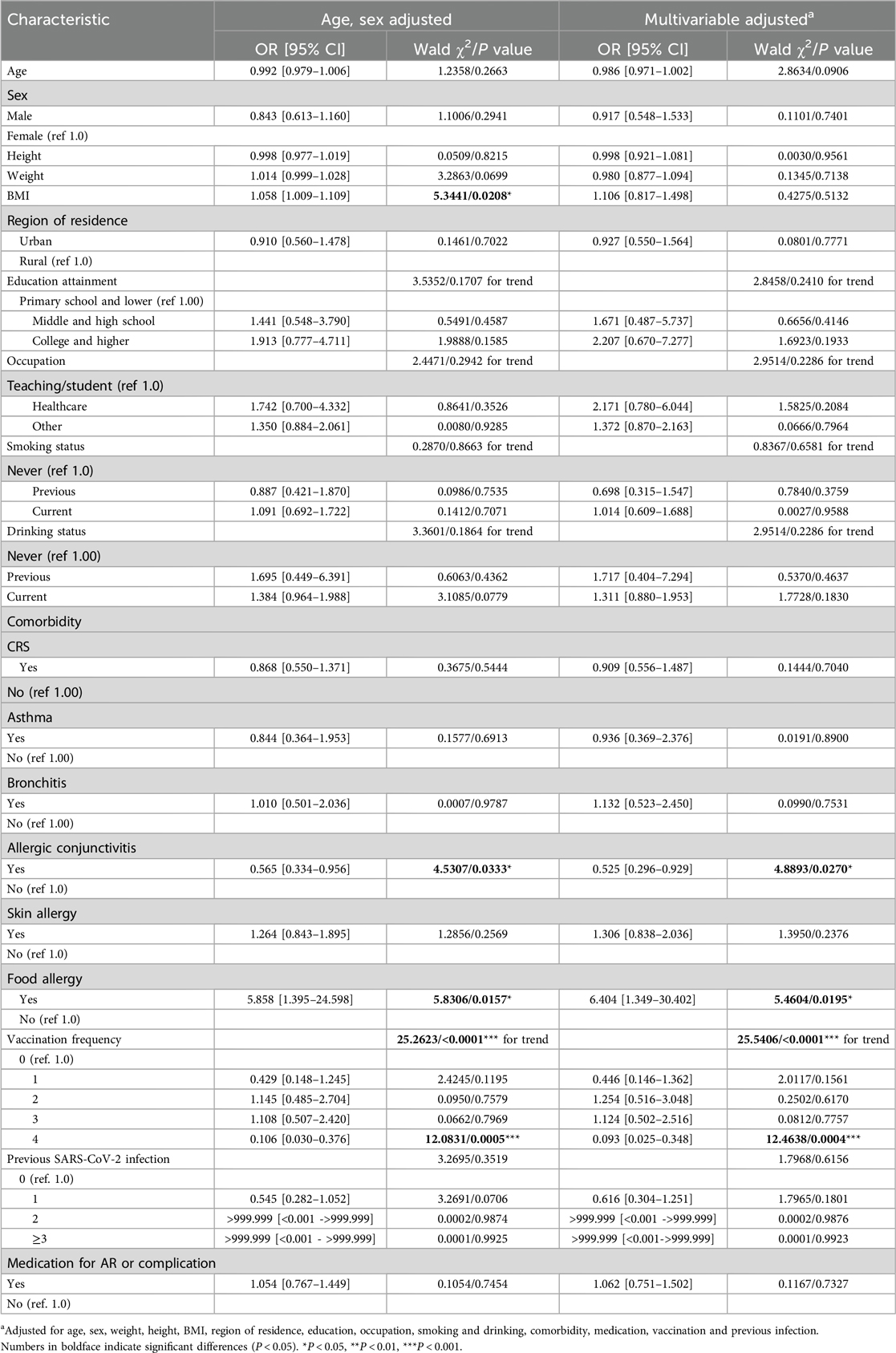
A total of 830 patients with AR and available data were included in this study, and the demographic and clinical characteristics are summarized in Table 1. In this cohort, 627 patients (75.54%) had AR comorbid with SARS-CoV-2 infection, and 203 patients (24.46%) did not. In these two groups, patients' age, sex, BMI, region of residence, education attainment, occupation, and smoking or drinking habits were not significantly different. Considering allergic disease comorbidities, the number of patients with AR comorbid with allergic conjunctivitis (AC) and AR comorbid with food allergy was relatively lower than the number of AR patients without these comorbidities (P = 0.0300 and P = 0.0048, respectively). Other comorbidities, including chronic rhinosinusitis (CRS), asthma, bronchitis, and skin allergy, did not show variation. Patients received vaccines at different frequencies, with the majority of patients receiving 3 doses of vaccine (P < 0.0001). Most patients had no history of previous SARS-CoV-2 infection. Patients who received medication for AR or other comorbidities also did not show differences.
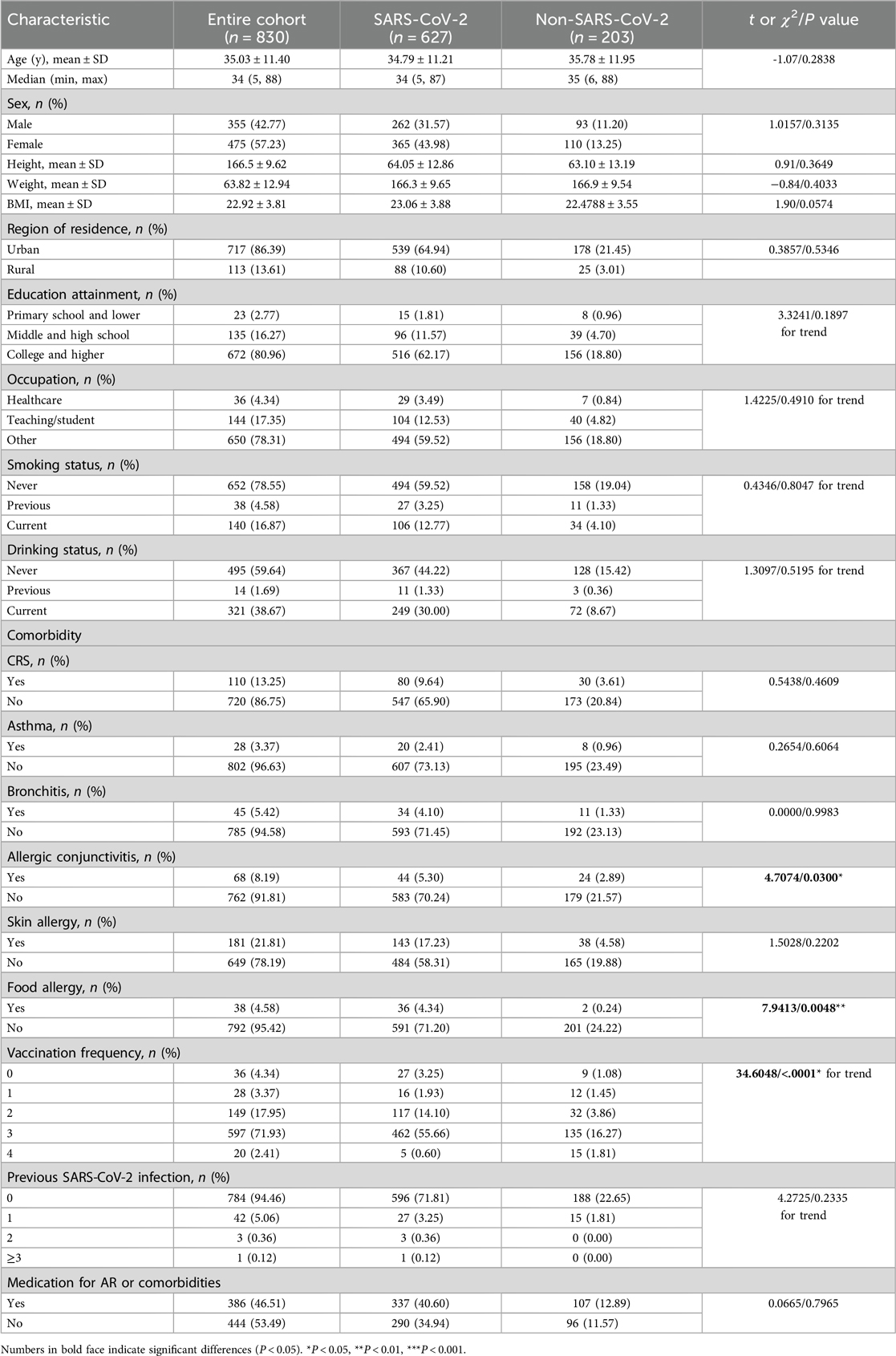
Table 1. Demographic and clinical characteristics and SARS-CoV-2 infection results of all study subjects.
We also analyzed AR patients under a stricter definition of COVID-19, where in these patients had PCR test positivity, antigen detection positivity, or both of them. Under this definition, there were 460 (55.42%) patients with AR comorbid with COVID-19 and 370 (44.58%) patients with AR without COVID-19 comorbidity (More details are given in Supplementary Table S1).
Effect of demographics and clinical characteristics on COVID-19
BMI was a risk factor for COVID-19 (OR: 1.058 (95% CI = 1.009–1.109, P = 0.0208) after adjusting for age and sex in the AR cohort. After full multivariable adjustment, BMI was not significantly associated with COVID-19 (P = 0.4275). The region of residence, educational attainment, occupation, smoking habits, and drinking habits of AR patients were not related to COVID-19 after age and sex adjustment or full multivariable adjustment (all P values > 0.05) (Figure 2 and Table 2).
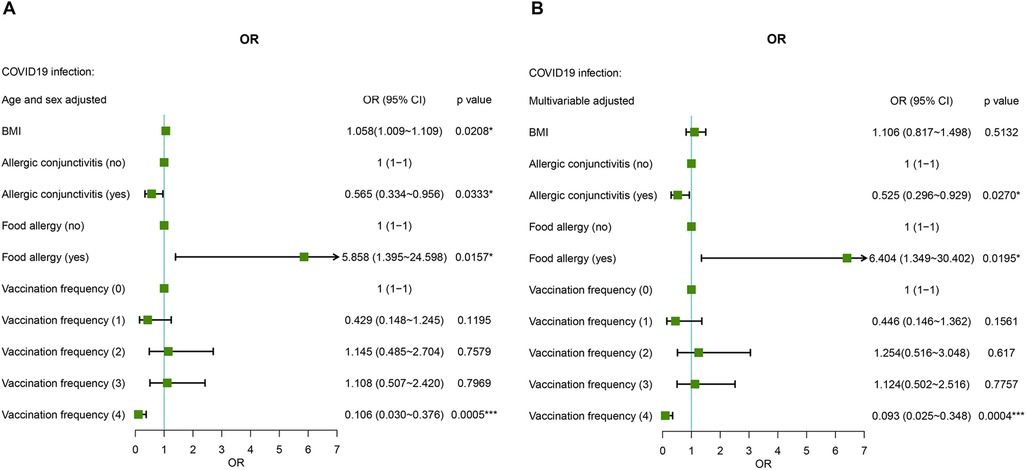
Figure 2. Age-, sex- (A) and multiple-adjusted (B) ORs for COVID-19 associated with risk factors in AR patients.
AR comorbid without AC was used as the reference, and AR comorbid with AC was a protective factor against SARS-CoV-2 infection both after sex and age adjustment and fully multivariable adjustment conditions (Figure 2 and Table 2). AR not comorbid without food allergy was used as the reference, and AR comorbid with food allergy was a risk factor for SARS-CoV-2 infection. The risk fold was 5.858 (95% CI = 1.395–24.598, P = 0.0157) and 6.404 (95% CI = 1.349–30.402, P = 0.0195) under the two methods of adjustment, respectively (Figure 2 and Table 2). In addition, vaccination significantly influenced the prevalence of COVID-19. Fewer patients received four doses of the vaccine, which was a significant protective factor against SARS-CoV-2 infection in AR cohort patients under both methods of adjustment [OR: 0.106 (95% CI = 0.030–0.376), P = 0.0005; OR: 0.093 (95% CI = 0.025–0.348), P = 0.0004, respectively] (Figure 2 and Table 2).
We also analyzed the factors influencing the AR cohort under the stricter definition of COVID-19. AR comorbid with food allergy is still a risk factor, and receiving four doses of the vaccine has protective effects against COVID-19. In addition, age, weight, BMI, residence in urban areas, and occupation in healthcare may be risk factors for SARS-CoV-2 infection (Supplementary Table S1 and Figure S1).
Effect of BMI, age, and allergy-specific AR on COVID-19
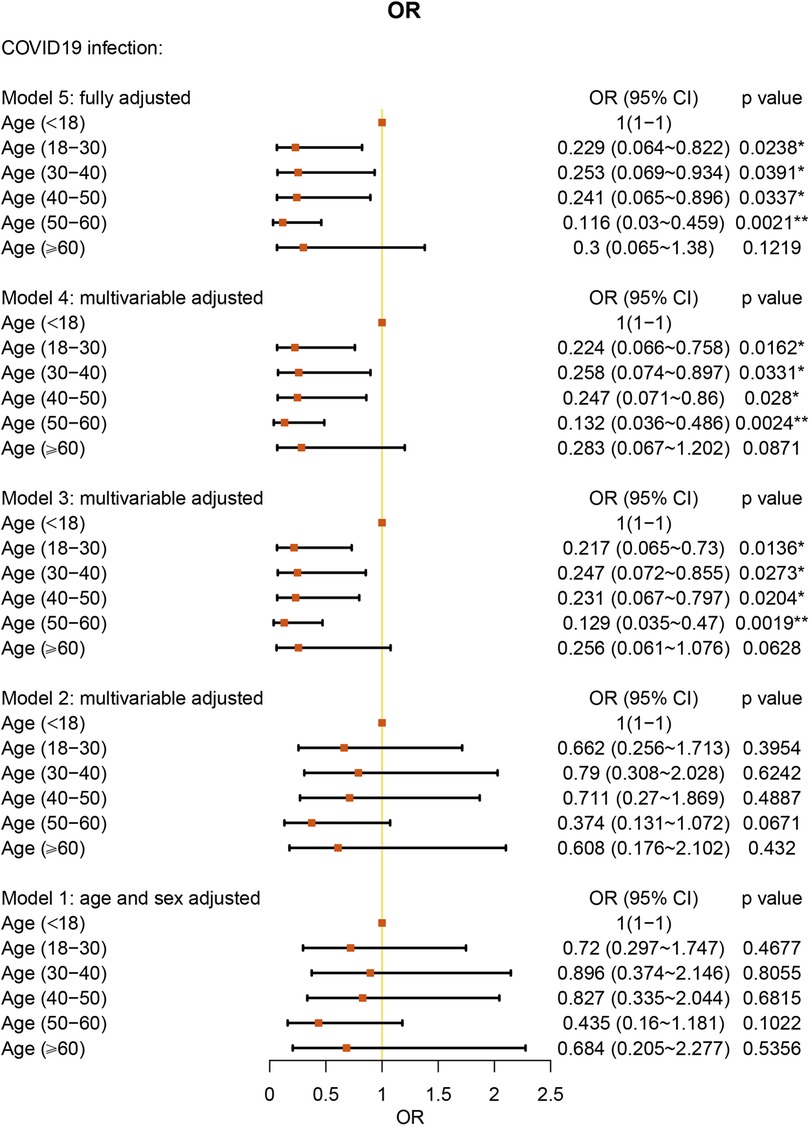
We analyzed the age of the patients by categories: younger than 18 years old, 18–30 years old, 30–40 years old, 40–50 years old, 50–60 years old, and older than 60 years old. With age younger than 18 years old as the reference, under the fully multivariable adjusted model, age under 60 years old showed a significant protective factor against COVID-19 in AR patients (all P values < 0.05) (Figure 3 and Supplementary Table S2).
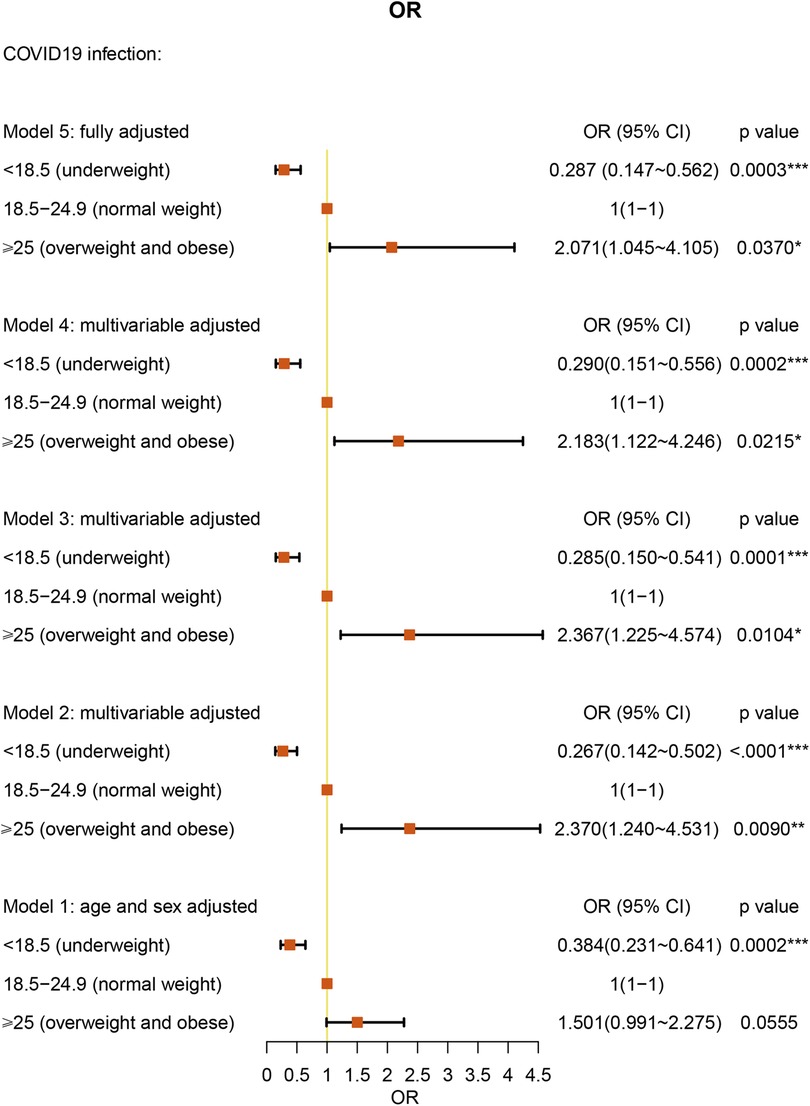
We analyzed the BMI of the patients by categories: underweight (BMI < 18.5 kg/m2), normal weight (BMI = 18.5–24.9 kg/m2), and overweight (BMI > 25.0 kg/m2). Interestingly, underweight BMI had a protective effect against COVID-19 [OR: 0.287 (95% CI = 0.147–0.562), P = 0.0003] under full multivariable adjustment (model 5). Overweight BMI was associated with a 2.071-fold higher risk for COVID-19 compared with normal weight AR patients [(95% CI = 1.045–4.105), P = 0.0370] (Figure 4 and Supplementary Table S3).
We analyzed 237 AR patients with specific allergens. Only HDM-specific AR patients showed protective factors against COVID-19 [OR: 0.537 (95% CI = 0.290–0.996), P = 0.0484] after adjusting for age, sex, weight, and height (Figure 5 and Supplementary Table S4). For specific allergies to mites, pollen, mold, fur, or other allergens, we did not find a significant association with COVID-19 (Supplementary Table S4 and Figure S2). The geographical information, the STROBE checklist and blank questionnaire template were provided as Supplementary Figure S3, Table S5 and S6.
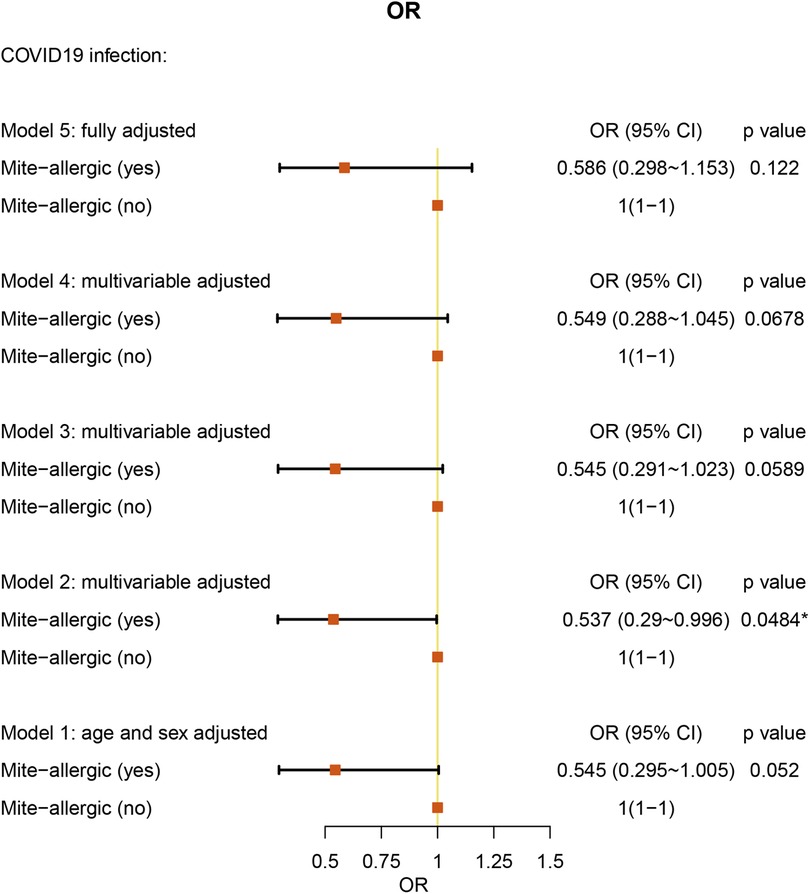
Figure 5. Allergen (mite)-specific multiple-adjusted ORs for COVID-19 associated with risk factors in AR patients (with allergen testing results).
Discussion
In the present nationwide retrospective cohort study, we investigated the development of COVID-19 among 830 AR patients. Furthermore, we studied the association between the clinical outcomes of COVID-19 and the characteristics of AR. The results showed complex associations between age, BMI, comorbidity with allergic diseases, type of allergen, and frequency of vaccination and COVID-19 in patients with AR.
During the study period, China experienced the peak of the epidemic after the adjustment of the national prevention and control strategy of COVID-19 infection. The present study shows that during this period, the overall infection rate (75.54%) was much higher than before (1.8% in Wuhan and Zhuhai SARS-CoV-2 infection hospitalized patients (8) and 9.8% in Wuhan COVID-19-positive patients (11)). Of note, our infection rate (75.54%) was similar to the SARS-CoV-2 infection rate of 77.89% in Beijing city (n = 873) (Dec. 20∼23, 2023) in the same study period (14).
In a large cohort of patients with AR, we performed a category analysis based on BMI (<18.5 kg/m2, 18.5–24.9 kg/m2, and > 25.0 kg/m2). Similar to the studies in all patients (15), using normal BMI as the reference, overweight BMI was associated with a 2.038-fold higher risk of COVID-19 in the AR cohort. As other studies have reported, obesity (BMI ≥ 30 kg/m2) was associated with a significantly increased risk of critical COVID-19 and in-hospital mortality due to COVID-19 (16, 17). In addition, we found that underweight BMI was a protective factor against COVID-19 in AR patients. However, one study reported that underweight patients were more likely than normal-weight patients to develop secondary infections (18). However, the number of individuals in this study was only 11 patients and is not large enough to explain the relationship between underweight and COVID-19. Further research is warranted to clarify this interesting association. We also found a correlation between age and COVID-19. Under fully multivariable adjusted conditions, age under 60 years old was a protective factor against COVID-19 in AR patients. The result was similar to the advocation that age under 65 years old acts as a protective factor in asthma patients (12). Our study did not present old age as a significant risk factor for COVID-19. However, one study has already shown that older patients have a higher percentage of hospitalizations, ICU admissions, and deaths (19).
Comorbidities, including asthma, atopic dermatitis, allergic rhinitis, allergic conjunctivitis, chronic rhinosinusitis, and food allergy, represent a collection of disorders mediated by innate and adaptive immune responses. After evaluating 830 patients for AR comorbid with other types of allergic diseases and prevalence of COVID-19 positivity, our results showed that AR comorbid with AC was a major protective factor against COVID-19 and that AR comorbid with food allergy was a major risk factor for COVID-19 under the fully multivariable adjusted condition. In our cohort, 5.3% of patients with AR comorbid with AC were COVID-19 positive, which was lower than 10.8% in an Italian study (20). We consider the reason why AC has a protective effect. According to previous results, thirty-eight genes involved in the defense response to virus were overexpressed, while ACE II had low expression in conjunctivitis (21). In addition, the typical Th2 phenotype and eosinophilia may be important protective factors against COVID-19. Although there is no direct explanation for the protective effect of AR comorbid with AC against COVID-19, AR patients have a strengthened type 2 immune response to some extent. This may explain our results. The main clinical symptoms of food allergy are impaired skin barrier or gastrointestinal tract accompanied by basophil and mast cell activation. Seibold et al. found food allergies associated with lower risk of COVID-19 infection (22). In asthma cohort, there were more food allergy comorbid in COVID-19 infection than non-COVID-19 infection patients by Francisco's study (23). This study was similar to our study, food allergy comorbid with AR was a risk factor for COVID-19 infection. We consider that both food allergy and AR are IgE-mediated immune responses. Patients with AR comorbid with food allergy are in a state of prolonged B-cell activation. In addition, SARS-CoV-2 infection elicits an IgE response, and the serum levels positively correlate with the severity of COVID-19 (24). Therefore, we suggest that the immune responses resulting from B cell activation could explain why AR comorbid with food allergy is a risk factor for COVID-19.
During the period of our study, the predominant variant of SARS-CoV-2 was omicron. According to previous study, two doses of vaccine only offered limited protection against the omicron variant, and the third dose could be effective in preventing patients' symptoms and severe disease (25). More importantly, a fourth dose is more effective in preventing COVID-19 compared with three standard doses of vaccine (26). In our study, it was also clear that receiving four doses of the vaccine had a significant protective effect in patients with AR, while three doses did not show protection against COVID-19 in AR patients (cohort with large sample size verification is required). In addition, even after full multivariable adjustment, four doses showed a protective factor.
House dust mite (HDM)-specific AR is prevalent due to the ubiquitous and perennial presence of HDMs in indoor environments worldwide (27). From an immune characteristic perspective, HDM-allergic AR patients show increased group 2 innate lymphoid cells (ILC-2s) and high levels of secretion of IL-5 and IL-13 (28). In COVID-19 immunopathology, the interferon (IFN) response results in exacerbated proinflammatory cytokine production in the respiratory tract and lung pathology (29). In the nasal tissue and epithelial cells of COVID-19 patients, type 2 cytokines are downregulated, while IFNs are unregulated. In addition, allergen stimulation of lower airways induces a reduction in ACE II expression, suggesting that allergic inflammation may play a significant role in lowering the risk of COVID-19. In addition, patients with HDM-specific AR are permanently exposed to an immune environment mediated by type 2 inflammation response (30). This may explain why HDM-specific AR was found to be a protective factor in COVID-19 patients.
There existed some limitations in our study. First, our study was not a clinical study, and all patients included in the study provided information by filling in online questionnaires using mobile phones. Second, some history of comorbidity data in our study was taken from the patient's self-report only. Third, we only recorded the frequency of vaccinations given to participants, without carefully distinguishing the type of vaccines. Fourth, the subjects of our study were all Chinese, and there was a lack of inclusion of other ethnic groups. Although this is a nationwide survey, the sample size is not big. Study with large sample size verification is required. Other limitations including most of the participants who were able to successfully complete the online survey were young people, with a median age of 34, which may be subject to confounding bias, etc. Despite these limitations, to our knowledge, this is the first time that a questionnaire has been used to explore risk factors in AR patients during the COVID-19 peak in China. The advantages of our cohort study are that the collection time was short, and the survey time coincided with the epidemic peak.
Of note, the SARS-CoV-2 variants at the peak of the Chinese epidemic in 2022–2023 are quite different from the main prevalent variants now. Moreover, the prevalence of SARS-CoV-2 variants varies globally, which may be one of the reasons for the conflicting results of several previous studies. Cohort study involved different countries with large sample size verification are required.
In summary, the different clinical manifestations of AR patients are closely associated with COVID-19. We analyzed the demographic characteristics of the patients, and BMI and age had a significant impact on COVID-19. Underweight BMI and age lower than 60 years were protective factors, and overweight BMI was a risk factor for COVID-19 after full multivariable adjustment. We present for the first time the effect of different allergic disease comorbidities on COVID-19 in AR patients. AR comorbid with AC and AR comorbid with food allergy have the opposite effect on COVID-19. HDM-specific AR patients, compared to other AR patients, are better protected against COVID-19.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Beijing Tongren Hospital Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. An online informed consent form was provided to participants.
Author contributions
XY: Data curation, Formal Analysis, Funding acquisition, Investigation, Writing – original draft, Writing – review & editing. YS: Data curation, Writing – original draft, Writing – review & editing. JZhao: Investigation, Writing – original draft. JZhang: Data curation, Writing – review & editing. XW: Conceptualization, Formal Analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. LZ: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Key R&D Program of China (2022YFC2504100), the program for the Changjiang Scholars and Innovative Research Team (IRT13082), the National Natural Science Foundation of China (82171110), the CAMS Innovation Fund for Medical Sciences (2019-I2M-5-022), Special Funds for the Construction of High-level Public Health Technical Talents (Lingjunrencai-01-08, Lingjunrencai-02-09), Beijing Municipal Science & Technology Commission (Z211100002921057), the Capital's Funds for Health Improvement and Research (CFH 2022-1-1091, CFH 2024-4-1093), the Beijing Natural Science Foundation (7222024), the Beijing New-star Plan of Science and Technology (20220484226), Beijing Municipal Public Welfare Development and Reform Pilot Project for Medical Research Institutes (JYY2021-2), Beijing Hospitals Authority Innovation Studio of Young Staff Funding Support (202306).
Acknowledgments
We thank all participants for their work which made this study possible.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/falgy.2024.1479493/full#supplementary-material
References
1. Wu SL, Mertens AN, Crider YS, Nguyen A, Pokpongkiat NN, Djajadi S, et al. Substantial underestimation of Sars-Cov-2 infection in the United States. Nat Commun. (2020) 11(1):4507. doi: 10.1038/s41467-020-18272-4
2. Zhang Y, Lan F, Zhang L. Advances and highlights in allergic rhinitis. Allergy. (2021) 76(11):3383–9. doi: 10.1111/all.15044
3. Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. (2008) 454(7203):445–54. doi: 10.1038/nature07204
4. Juhn YJ. Risks for infection in Patients with asthma (or other atopic conditions): is asthma more than a chronic airway disease? J Allergy Clin Immunol. (2014) 134(2):247–57, quiz 58-9. doi: 10.1016/j.jaci.2014.04.024
5. Brożek JL, Bousquet J, Agache I, Agarwal A, Bachert C, Bosnic-Anticevich S, et al. Allergic rhinitis and its impact on asthma (Aria) guidelines-2016 revision. J Allergy Clin Immunol. (2017) 140(4):950–8. doi: 10.1016/j.jaci.2017.03.050
6. Wang XD, Zheng M, Lou HF, Wang CS, Zhang Y, Bo MY, et al. An increased prevalence of self-reported allergic rhinitis in Major Chinese cities from 2005 to 2011. Allergy. (2016) 71(8):1170–80. doi: 10.1111/all.12874
7. Choi HG, Kim SY, Joo YH, Cho HJ, Kim SW, Jeon YJ. Incidence of asthma, atopic dermatitis, and allergic rhinitis in Korean adults before and during the COVID-19 pandemic using data from the Korea National Health and nutrition examination survey. Int J Environ Res Public Health. (2022) 19(21):14274. doi: 10.3390/ijerph192114274
8. Jian L, Yi W, Zhang N, Wen W, Krysko O, Song WJ, et al. Perspective: COVID-19, Implications of nasal diseases and consequences for their management. J Allergy Clin Immunol. (2020) 146(1):67–9. doi: 10.1016/j.jaci.2020.04.030
9. Yang JM, Koh HY, Moon SY, Yoo IK, Ha EK, You S, et al. Allergic disorders and susceptibility to and severity of COVID-19: a nationwide cohort study. J Allergy Clin Immunol. (2020) 146(4):790–8. doi: 10.1016/j.jaci.2020.08.008
10. Yang X, Zhao J, Sima Y, Zhao Y, Zhang J, Wang X, et al. The association between allergic rhinitis and the risk of coronavirus disease 2019 (COVID-19): a national survey in China. Allergy. (2023) 78(10):2783–6. doi: 10.1111/all.15823
11. Wang H, Song J, Yao Y, Deng YK, Wang ZC, Liao B, et al. Angiotensin-Converting enzyme ii expression and its implication in the association between COVID-19 and allergic rhinitis. Allergy. (2021) 76(3):906–10. doi: 10.1111/all.14569
12. Ren J, Pang W, Luo Y, Cheng D, Qiu K, Rao Y, et al. Impact of allergic rhinitis and asthma on COVID-19 infection, hospitalization, and mortality. J Allergy Clin Immunol Pract. (2022) 10(1):124–33. doi: 10.1016/j.jaip.2021.10.049
13. Darabi A, Dehghanfard M, Jozan S, Tahmasebi R, Movahed A, Zamani M, et al. Investigating the association between allergic diseases and COVID-19 in 400 Iranian patients. Allergol Immunopathol (Madr). (2021) 49(5):9–15. doi: 10.15586/aei.v49i5.105
14. Wei YY, Gao WJ, Zhang LY, Wang SG, Zhang SY, Ren T, et al. Epidemiological survey of 2019-nCoV infection in staff and students in some public health schools in China. Chin J Epidemiol. (2023) 44(2):175–83. doi: 10.3760/cma.j.cn112338-20221231-01092
15. Du Y, Lv Y, Zha W, Zhou N, Hong X. Association of body mass Index (bmi) with critical COVID-19 and in-hospital mortality: a dose-response meta-analysis. Metab Clin Exp. (2021) 117:154373. doi: 10.1016/j.metabol.2020.154373
16. Hendren NS, de Lemos JA, Ayers C, Das SR, Rao A, Carter S, et al. Association of body mass Index and age with morbidity and mortality in patients hospitalized with COVID-19: results from the American Heart Association COVID-19 cardiovascular disease registry. Circulation. (2021) 143(2):135–44. doi: 10.1161/CIRCULATIONAHA.120.051936
17. Sanchis-Gomar F, Lavie CJ, Mehra MR, Henry BM, Lippi G. Obesity and outcomes in COVID-19: when an epidemic and pandemic collide. Mayo Clin Proc. (2020) 95(7):1445–53. doi: 10.1016/j.mayocp.2020.05.006
18. Ye P, Pang R, Li L, Li HR, Liu SL, Zhao L. Both underweight and obesity are associated with an increased risk of coronavirus disease 2019 (COVID-19) severity. Front Nutr. (2021) 8:649422. doi: 10.3389/fnut.2021.649422
19. Chen Y, Klein SL, Garibaldi BT, Li H, Wu C, Osevala NM, et al. Aging in COVID-19: vulnerability, immunity and intervention. Ageing Res Rev. (2021) 65:101205. doi: 10.1016/j.arr.2020.101205
20. Ferreli F, Gaino F, Russo E, Di Bari M, Pirola F, Costantino A, et al. Clinical presentation at the onset of COVID-19 and allergic rhinoconjunctivitis. J Allergy Clin Immunol Pract. (2020) 8(10):3587–9. doi: 10.1016/j.jaip.2020.08.009
21. Leonardi A, Rosani U, Cavarzeran F, Daull P, Garrigue JS, Paola B. Antiviral response in Vernal Keratoconjunctivitis may be protective against COVID-19. Allergy. (2022) 77(1):298–300. doi: 10.1111/all.15048
22. Seibold MA, Moore CM, Everman JL, Williams BJM, Nolin JD, Fairbanks-Mahnke A, et al. Risk factors for Sars-Cov-2 infection and transmission in households with children with asthma and allergy: a prospective surveillance study. J Allergy Clin Immunol. (2022) 150(2):302–11. doi: 10.1016/j.jaci.2022.05.014
23. Ruano FJ, Somoza Álvarez ML, Haroun-Díaz E, de la Torre M V, González P L, Prieto-Moreno A, et al. Impact of the COVID-19 pandemic in children with allergic asthma. J Allergy Clin Immunol Pract. (2020) 8(9):3172–4.e1. doi: 10.1016/j.jaip.2020.07.019
24. Plūme J, Galvanovskis A, Šmite S, Romanchikova N, Zayakin P, Linē A. Early and strong antibody responses to Sars-Cov-2 predict disease severity in COVID-19 patients. J Transl Med. (2022) 20(1):176. doi: 10.1186/s12967-022-03382-y
25. Magen O, Waxman JG, Makov-Assif M, Vered R, Dicker D, Hernán MA, et al. Fourth dose of Bnt162b2 Mrna COVID-19 vaccine in a nationwide setting. N Engl J Med. (2022) 386(17):1603–14. doi: 10.1056/NEJMoa2201688
26. Regev-Yochay G, Gonen T, Gilboa M, Mandelboim M, Indenbaum V, Amit S, et al. Efficacy of a fourth dose of COVID-19 Mrna vaccine against Omicron. N Engl J Med. (2022) 386(14):1377–80. doi: 10.1056/NEJMc2202542
27. Zock JP, Heinrich J, Jarvis D, Verlato G, Norbäck D, Plana E, et al. Distribution and determinants of house dust Mite allergens in Europe: The European Community Respiratory Health Survey II. J Allergy Clin Immunol. (2006) 118(3):682–90. doi: 10.1016/j.jaci.2006.04.060
28. Zhong H, Fan XL, Yu QN, Qin ZL, Chen D, Xu R, et al. Increased innate type 2 immune response in house dust mite-allergic patients with allergic rhinitis. Clin Immunol. (2017) 183:293–9. doi: 10.1016/j.clim.2017.09.008
29. Lowery SA, Sariol A, Perlman S. Innate immune and inflammatory responses to Sars-Cov-2: implications for COVID-19. Cell Host Microbe. (2021) 29(7):1052–62. doi: 10.1016/j.chom.2021.05.004
Keywords: allergic rhinitis, COVID-19, risk factor, protective factor, infection
Citation: Yang X, Sima Y, Zhao J, Zhang J, Wang X and Zhang L (2024) Risk and protective factors for coronavirus disease 2019 (COVID-19) in allergic rhinitis patients: a national survey in China. Front. Allergy 5:1479493. doi: 10.3389/falgy.2024.1479493
Received: 12 August 2024; Accepted: 18 November 2024;
Published: 10 December 2024.
Edited by:
Yuxiang Zhi, Peking Union Medical College Hospital (CAMS), ChinaReviewed by:
Wan Yi Fu, First Affiliated Hospital of Guangzhou Medical University, ChinaHaiyu Hong, Sun Yat-sen University, China
Copyright: © 2024 Yang, Sima, Zhao, Zhang, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangdong Wang, ZW50d3hkQHZpcC5zaW5hLmNvbQ==; Luo Zhang, ZHIubHVvemhhbmdAMTM5LmNvbQ==
†These authors have contributed equally to this work
 Xiaozhe Yang1,2,3,†
Xiaozhe Yang1,2,3,† Jinming Zhao
Jinming Zhao Xiangdong Wang
Xiangdong Wang Luo Zhang
Luo Zhang