- 1Pediatric Unit, NESMOS Department, Sant’Andrea University Hospital, Sapienza University of Rome, Rome, Italy
- 2Pediatric Clinic, Department of Surgical and Biomedical Sciences, University of Perugia, Perugia, Italy
- 3Department of Woman, Child and General and Specialist Surgery, University of Campania “Luigi Vanvitelli”, Naples, Italy
- 4Department of Maternal Infantile and Urological Sciences, AOU Policlinico Umberto I, Rome, Italy
- 5Department of Clinical, Surgical, Diagnostic and Pediatric Sciences, University of Pavia, Pavia, Italy
- 6Section of Pediatrics, Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy
- 7Pediatric Unit, Sant'Eugenio Hospital, Rome, Italy
This review delves into the potential of manipulating the microbiome to enhance oral tolerance in food allergy, focusing on food allergen-specific immunotherapy (FA-AIT) and the use of adjuvants, with a significant emphasis on probiotics. FA-AIT, including oral (OIT), sublingual (SLIT), and epicutaneous (EPIT) immunotherapy, has shown efficacy in desensitizing patients and achieving sustained unresponsiveness (SU). However, the long-term effectiveness and safety of FA-AIT are still under investigation. Probiotics, particularly strains of Lactobacillus, play a crucial role in enhancing immune tolerance by promoting regulatory T cells (Tregs) and modulating cytokine profiles. These probiotics can induce semi-mature dendritic cells, enhance CD40 expression, inhibit IL-4 and IL-5, and promote IL-10 and TGF-β, thus contributing to mucosal defense and immunological tolerance. Clinical trials combining probiotics with FA-AIT have demonstrated improved desensitization rates and immune tolerance in food-allergic patients. For example, the combination of Lactobacillus rhamnosus with peanut OIT resulted in a significantly higher rate of SU compared to the placebo group, along with notable immune changes such as reduced peanut-specific IgE and increased IgG4 levels. The review also explores other adjuvants in FA-AIT, such as biologic drugs, which target specific immune pathways to improve treatment outcomes. Additionally, nanoparticles and herbal therapies like food allergy herbal formula 2 (FAHF-2) are discussed for their potential to enhance allergen delivery and immunogenicity, reduce adverse events, and improve desensitization. In conclusion, integrating probiotics and other adjuvants into FA-AIT protocols could significantly enhance the safety and efficacy of FA-AIT, leading to better patient outcomes and quality of life.
Introduction to food allergy landscape
Food allergy is a global pediatric health issue whose prevalence has risen in the past two decades. The prevalence of food allergy is currently estimated to be around 4% of children worldwide and 25% in Western countries. However, when accurately diagnosed by testing and oral food challenge, its true prevalence appears closer to 6%–8% in children, with 2.4% having multiple food allergies and 3% having experienced severe reactions (1). Standard management includes strict food avoidance, patient education, and provision of emergency medication. However, this approach is perceived as restrictive by patients, and the risk of accidental exposure still exists (1). In this scenario, allergen-specific immunotherapy (AIT) has emerged as the only treatment with disease-modifying effects (2). Allergen immunotherapy for food allergy (FA-AIT) is considered an immunomodulatory intervention for IgE-mediated food allergy based on recurrent exposure to increasing doses of food at regular intervals. This desensitization process was conceived to improve the patient's threshold to elicit an allergic reaction, reducing the risks of accidental food ingestion (3). Indeed, with promising results, FA-AIT trials started focusing on developing oral immunotherapy (OIT) for peanut, cow's milk, and hen egg allergy (4). However, in real life, FA-AIT is still not widely available for all children with food allergies, and its use is limited due to the absence of formally OIT-approved protocols in most countries. OIT usually requires months or years, and some treated patients lose tolerance once they stop taking the maintenance amount of the culprit food. In addition, it remains unclear if OIT would produce long-lasting tolerance similar to the natural tolerance acquired by previously allergic children or if it would only induce transient desensitization (5). Thus, some authors consider OIT an additional risk rather than a therapy (6).
Managing food allergies: dietary restrictions, symptom alleviation, allergen immunotherapy and drugs
Managing food allergies has traditionally relied on dietary restrictions and symptom alleviation, but recent advancements have introduced new promising approaches (7).
Dietary restriction
The cornerstone of managing food allergies has been strict avoidance of allergenic food. This approach is straightforward but requires meticulous effort to identify and avoid all allergen sources (8). For many individuals, particularly children, this can be challenging and significantly impact their quality of life. Accidental exposure is a constant risk, leading to anxiety and necessitating the need for emergency medication such as epinephrine auto-injectors.
It was reported that the mean number of accidental reactions is 2.10 (SD 2.0) per person per year in children managing food allergies solely through dietary restrictions. Patients who reported reactions were significantly more often women, had a significantly longer duration of food allergy and had significantly more often a confirmed allergy for peanut, sesame and vegetables compared with patients who did not report reactions (9).
While effective in preventing allergic reactions, dietary restrictions do not address the underlying immune response.
Symptom alleviation
Symptom alleviation in food allergy management primarily involves using antihistamines and corticosteroids to manage mild to moderate allergic reactions. For severe reactions, epinephrine is the treatment of choice. These medications do not prevent allergic reactions but help manage symptoms.
While symptom alleviation is critical in acute management, it does not provide a long-term solution, and patients remain at risk of accidental exposure and subsequent reactions.
Allergen immunotherapy
FA-AIT has emerged as a promising method for inducing tolerance to food allergens. It involves regularly administering gradually increasing amounts of the allergen to modify the immune response. There are three primary forms of FA-AIT: oral immunotherapy (OIT), sublingual immunotherapy (SLIT), and epicutaneous immunotherapy (EPIT) (10).
Oral immunotherapy (OIT)
OIT involves ingesting small, gradually increasing amounts of the allergen, typically in food or capsules. The goal is to desensitize the immune system to the allergen, allowing the patient to tolerate more significant amounts without experiencing severe reactions.
OIT had a significant positive effect in achieving tolerance, with a relative risk (RR) of 11.94 for peanut allergy compared to avoidance or placebo (7).
While OIT has shown promise in inducing desensitization and, in some cases, sustained unresponsiveness (SU), it is associated with a high incidence of adverse reactions, particularly gastrointestinal symptoms. These adverse events are usually mild to moderate but can impact the patient's quality of life and adherence to the therapy (11).
Sublingual immunotherapy (SLIT)
SLIT involves placing small doses of the allergen under the tongue, where it is absorbed into the bloodstream. This method is less invasive than OIT and generally has a better safety profile, with fewer and milder adverse reactions (12).
Despite its safer profile, SLIT may be less effective than OIT in inducing desensitization and SU.
Epicutaneous immunotherapy (EPIT)
EPIT involves the application of a patch containing the allergen to the skin. The allergen is absorbed through the skin, which has a high density of immune cells that can promote tolerance. EPIT is associated with mild local skin reactions, including itching and redness (13).
EPIT's non-invasive nature and favorable safety profile make it an attractive option, particularly for young children who may not tolerate OIT or SLIT well. However, its efficacy in inducing long-term tolerance is still under investigation.
Biologics
Biologics, such as monoclonal antibodies targeting IgE, offer another avenue for managing food allergies. Omalizumab, an anti-IgE antibody, has shown promise in increasing the threshold of allergens required to trigger a reaction (14).
Biologics can be adjuncts to FA-AIT to enhance its efficacy and reduce adverse reactions. They work by lowering the levels of free IgE, thereby decreasing the immune system's sensitivity to the allergen. This approach can be particularly beneficial for patients who experience severe reactions during FA-AIT.
The efficacy of FA-AIT and biologics in achieving desensitization and SU is well-supported by meta-analysis, which shows significant positive effects compared to avoidance or placebo (7). However, the long-term efficacy and safety profiles remain areas of concern. Most adverse reactions reported during FA-AIT are mild to moderate, but there is limited data on severe or life-threatening adverse reactions.
Studies have reported significant improvements in quality of life (QoL) measures for patients undergoing FA-AIT, reflecting reduced fear of accidental exposure and, more importantly, social participation.
However, the burden of frequent clinic visits, the potential for adverse reactions, and the requirement for long-term adherence to treatment protocols can negatively impact QoL. Balancing the benefits and challenges of FA-AIT is essential to ensure that patients derive maximum benefit from these therapies (15).
Future research should focus on long-term follow-up studies to assess the durability of tolerance induced by FA-AIT and biologics.
Potential role of adjuvants to Fa-AIT
Adjuvants, derived from the Latin word adjuvant—meaning “to help” -are usually added to vaccines to enhance their antigen-specific immune response and reduce some undesirable reactions (16). In allergy, an adjuvant is a substance or compound co-administered with the allergen extract and can increase allergen immunogenicity and modulate the immune response (17). Their role has, therefore, been investigated in OIT to improve the duration of tolerance, allow the administration of lower doses, and reduce treatment duration and side effects (18, 19). The ideal adjuvant should be biodegradable, stable, sustainable, non-toxic, and cost-effective, able to promote an appropriate immune response and combine optimal physiochemical properties with biological activity properties (17).
Several types of adjuvants have been evaluated for FA-AIT. However, in allergy, the preexisting TH2 immune response is very robust, and establishing a protective immune reaction requires application schemes over 3–5 years to induce an immunomodulatory process. Although rates of successful desensitization are generally much high for SLIT and OIT, the effects are commonly lost after treatment cessation, and patients need to continue immunotherapy indefinitely to ensure ongoing tolerance (20).
Furthermore, safety concerns exist for food immunotherapy related to adverse allergic events (AEs). For EPIT and SLIT, AEs are generally limited, typically involving the treatment site, whereas OIT may provoke systemic reactions, including anaphylaxis requiring epinephrine use.
The optimal adjuvant should improve the safety and efficacy of FA-AIT by leading to faster relief of clinical symptoms and resulting in better patient adherence (21).
Rational of bacterial therapies
Studies have investigated bacterial therapies, such as probiotics, in treating allergic disorders, although challenges still need to be addressed due to study variability and inconsistent outcome measures. Probiotics significantly contribute to the production of Th1 cytokines, induce regulatory T cells (Tregs), and suppress Th2 pathways. Specifically, Lactobacillus strains facilitate the generation of semi-mature dendritic cells (DCs), enhance CD40 expression, inhibit IL-4 and IL-5, and promote IL-10 and TGF-β. They also boost local IgA production, which is essential for mucosal defenses (22, 23).
Research has explored the immunomodulatory effects of probiotics in promoting tolerance, showcasing their anti-inflammatory properties and ability to reduce reactive oxygen species (ROS) (24). Probiotics help balance Th1 and Th2 responses by regulating pro-inflammatory and anti-inflammatory cytokines and influencing gene expression during inflammation, which affects cell morphology and targeting. DCs, as antigen-presenting cells (APCs), are crucial for distinguishing between commensal bacteria and probiotics, with toll-like receptors (TLRs) playing a vital role in this process.
In food allergy treatment, the induction of Tregs has garnered significant interest. B regulatory cells (Bregs) exhibit immunosuppressive functions in various inflammatory diseases, including food allergies. Bregs produce cytokines such as IL-10, TGF-β, and TSP1, which repress T-cell mediated inflammation, enhance Treg function, induce tolerogenic DCs, and alter the phenotype of other local B cells. Bacterial signals can influence Breg phenotypes through interactions with short-chain fatty acids (SCFAs) like butyrate. Butyrate increases the production of the serotonin-derived metabolite 5-hydroxyindole-3-acetic acid (5-HIAA), which binds to the Aryl Hydrocarbon receptor (AhR). AhR acts as a transcriptional regulator for Bregs, boosting IL-10 production and suppressing pro-inflammatory cytokines such as TNFα and IL-6 (25).
Key findings from clinical trials exploring probiotic supplementation alongside Fa-AIT for managing food allergies
Recent studies have explored the efficacy of combining probiotics with OIT to treat food allergies. One significant study investigated the effects of a probiotic, Lactobacillus rhamnosus CGMCC 1.3724, combined with peanut OIT (PPOIT) in peanut allergic children. This double-masked, placebo-controlled, randomized trial aimed to induce SU and found that 82.1% of participants receiving PPOIT achieved SU compared to just 3.6% in the placebo group. Furthermore, 89.7% of the PPOIT group were desensitized to peanuts, demonstrating significant immune changes, including reduced peanut-specific IgE levels and increased peanut-specific IgG4 levels. However, PPOIT-treated participants reported more adverse events, mainly during home dosing.
It is necessary to underline that the mentioned study compares only the PPOIT group with the placebo, without including a comparison arm for those who underwent peanut OIT alone. It is important to highlight that this represents a significant limitation of the study, as it does not allow for an assessment of whether the benefits of PPOIT are solely due to the presence of probiotics or also to the effects of OIT itself (26).
Further research assessed the impact of PPOIT on health-related quality of life (HRQoL). In a study, those who received PPOIT showed significant improvements in HRQoL measures, such as the Food Allergy Quality of Life Questionnaire (FAQLQ-PF) and Food Allergy Independent Measure (FAIM). Improvements were noted at three months and sustained up to 12 months post-treatment, indicating that PPOIT helped achieve SU and enhanced participants’ psychosocial well-being (27).
A long-term follow-up study, conducted four years after the cessation of treatment, evaluated the persistence of PPOIT's benefits. The study found that participants receiving PPOIT were significantly more likely to continue consuming peanuts and had fewer allergic reactions than the placebo group. These findings suggest that PPOIT provides long-lasting clinical benefits and persistent suppression of the allergic immune response (28).
An economic analysis of PPOIT demonstrated its cost-effectiveness, estimating the cost per quality-adjusted life year (QALY) gained to be approximately $A20,000, below the conventional value judgment threshold, suggesting it is a good value for money (29).
Another study explored the combined effects of Lactobacillus casei variety rhamnosus (Lcr35) and egg OIT in a mouse model of egg allergy. The results indicated that co-administration of Lcr35 and OIT significantly reduced the severity of anaphylaxis and decreased ovomucoid-specific IgE levels, effects that were sustained even after ceasing treatment. This combination also reduced mucin production in the small intestine, highlighting a synergistic effect in enhancing protection against anaphylaxis (30).
A phase IIb multicentre, double-blind, randomized, controlled trial (PPOIT-003) randomly assigned to receive probiotic (Lactobacillus rhamnosus, ATCC 53103) and peanut oral immunotherapy, placebo probiotic and peanut OIT, or placebo probiotic and placebo OIT for 18 months, and were followed up until 12 months after completion of treatment. During the 12-month post-treatment period, 60 (85%) of 71 participants in the PPOIT group, 60 (86%) of 70 participants in the OIT group, and six (18%) of 34 participants in the placebo group were eating peanut; rescue epinephrine use was infrequent (two [3%] of 71 in the PPOIT group, four [6%] of 70 in the OIT group, and none in the placebo group). The authors concluded that adding a probiotic did not improve the efficacy of OIT but might offer a safety benefit compared with OIT alone, particularly in preschool children (31).
In another study, heat-killed Lactiplantibacillus plantarum YIT 0132 (LP0132) combined with low-dose cow milk (CM) OIT was tested in children with cow milk allergy. This randomized, double-masked, placebo-controlled trial found that LP0132 with OIT improved tolerance to CM and was associated with favorable immunological changes, such as increased specific IgG4 levels and decreased IL-5 and IL-9 levels (32).
These studies collectively demonstrate the potential of combining probiotics with OIT in treating food allergies, highlighting various strains such as Lactobacillus rhamnosus CGMCC 1.3724, Lactobacillus rhamnosus ATCC 53103, Lactobacillus casei variety rhamnosus (Lcr35), and Lactiplantibacillus plantarum YIT 0132. This combination approach improves immunological outcomes, induces sustained unresponsiveness, and enhances the quality of life for individuals with food allergies.
As noted, FA-AIT represents the primary therapeutic strategy. However, it appears to be insufficient and requires some adjuvants. Probiotics, particularly strains of Lactobacillus, could play a crucial role in this context (Table 1).
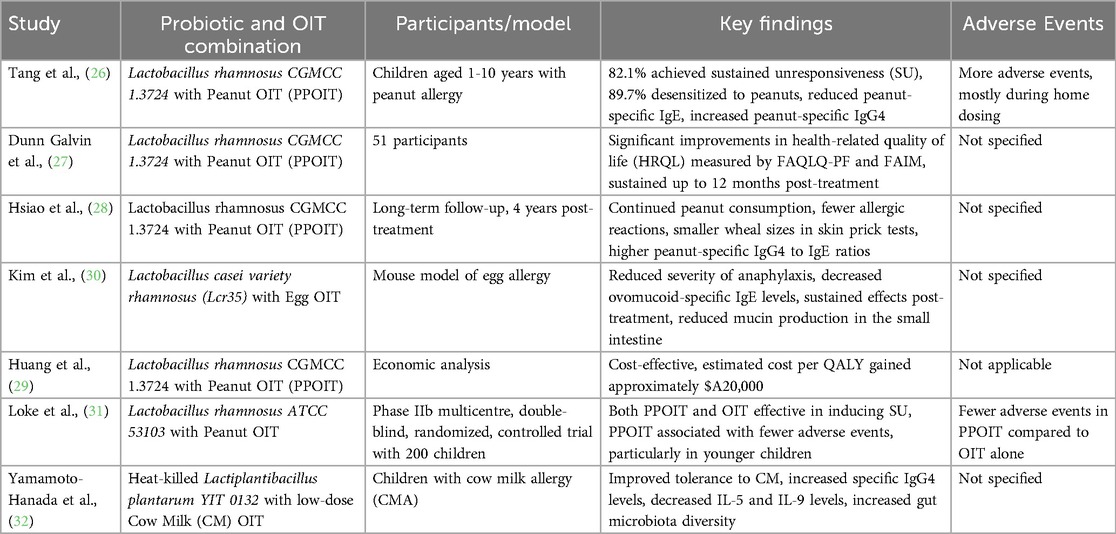
Table 1. Evidence of using probiotics in combination with allergen-specific immunotherapy for food allergy.
Mechanisms, strategies, and future research directions for adjuvants in Fa-AIT
Food allergen immunotherapeutic research tried to focus on other adjuvants, including biologics, novel delivery vehicles, adjuvants designed to target toll-like receptor pathways, and other innovative alternatives. Furthermore, different routes of administration exist for adjuvants in FA-AIT: oral, sublingual, and subcutaneous (33).
Oral immunotherapy adjuvants
Omalizumab
Anti-immunoglobulin E (IgE) monoclonal antibody (mAb) was introduced as an adjunctive therapy to reduce OIT-related allergic reactions. Omalizumab, a humanized monoclonal antibody targeting the fragment crystallizable (Fc) portion of IgE antibodies, thereby preventing mast cell and basophil activation, has shown promising results in the treatment of food allergy, both as monotherapy and as an adjuvant to OIT. Numerous studies showed an increase in threshold dose for various food allergens after omalizumab treatment, limiting AEs such as urticaria and anaphylaxis (34, 35), laying the foundations for more rapid escalation of OIT dosing. Therefore, studies about omalizumab facilitating OIT confirm that omalizumab allows safer, more rapid desensitization from milk, although without sustained unresponsiveness (SU) (33, 35–38). Similarly, studies about omalizumab-facilitated peanut OIT demonstrated a more rapid desensitization with low rates of adverse reactions, providing sustained unresponsiveness after discontinuation (39–41). In the United States, 30% of children with food allergies react to multiple foods, and the worldwide prevalence of numerous food allergies reaches 2.4% (42). In this scenario, the ability to simultaneously treat multiple food allergies makes omalizumab attractive as an adjuvant for FA-AIT. Three studies demonstrated the efficacy of omalizumab in achieving multifood desensitization, which persisted even after discontinuation, although the durability of the effect varied depending on the food. Furthermore, biologics may enable some patients to undergo OIT by allowing better management of relative contraindications, such as uncontrolled asthma, eczema, urticaria, or eosinophilic esophagitis (43–45).
Dupilumab
Dupilumab is a monoclonal antibody targeting the IL-4 receptor-α, thus inhibiting both IL-4 and IL-13 production. As omalizumab, dupilumab can succeed as an emerging adjuvant for OIT, and it is currently being studied for peanut allergy OIT (46).
Herbal therapy
Another novel approach to food allergy treatment is herbal therapy based on traditional Chinese medicine, which is thought to be effective in treating many diseases, including food allergies. Studies on peanut-allergic mice demonstrated that treatment with food allergy herbal formula 2 (FAHF-2) can reduce the frequency of adverse events, including anaphylaxis, down-regulating TH2 responses, thus enhancing OIT desensitization (47, 48). However, the only study on humans revealed no clinical benefit, probably due to poor adherence (49).
Interferon-γ
Interferon-γ (IFN-γ) is a pro-inflammatory cytokine that usually protects cells from viral infections, that can counterbalance TH2 responses while promoting TH1 reactions, reducing the production of IL-4 and IgE and inhibiting allergic sensitization (50, 51). Two studies evidenced successful sustained unresponsiveness or desensitization in food-allergic children treated with IFN-γ as an adjuvant to OIT (52, 53). Although the addition of IFN-γ seems to improve tolerability, studies are limited, and several side effects have been described related to IFN-γ administration to allergic patients.
Nanoparticles
Particle delivery systems are adjuvants that aim to facilitate the work of antigen-presenting cells by increasing the length of contact between the allergen and the patient's mucosa. Nanoparticles are delivery systems currently under research as new adjuvants in FA-AIT. They can provide an extra layer of protection for the allergen from degradation, therefore achieving high concentration at the site of action and increasing immunogenic properties. They can also prevent allergen recognition by IgE from basophils or mast cells, reducing allergenicity and, thus, the risk of adverse events (54). Given the promising immunomodulatory effects of CpG, two more recent studies evaluated the use of CpG-coated nanoparticles loaded with peanuts. CpG/peanut nanoparticles prevented anaphylaxis to oral peanut challenge, reduced Th2 cytokines, and increased IFN-γ levels in peanut-allergic mice (55). Recent studies have also investigated nanoparticles containing rapamycin, also known as sirolimus, an inhibitor of the mammalian target of the rapamycin (mTOR) pathway, known to induce antigen-specific immune tolerance. These studies proved the efficacy of rapamycin in attenuating allergic responses to food allergy (56, 57). Despite the potential intrinsic benefits of these systems, further studies to evaluate the safety profile of these compounds are still required to warrant the use of these emerging delivery systems as potential adjuvants in FA-AIT.
Sublingual immunotherapy adjuvants
Purified microbial macromolecules
Common pathogens infecting the gastrointestinal (GI) tract have developed mechanisms to evade host immunity and cause infections. Vaccine vectors generated from those pathogens have been engineered to express different antigens, including food allergens. These vectors contain pathogen-associated molecular patterns (PAMPs), such as unmethylated CpG DNA, lipoproteins, and lipopolysaccharides that can activate the host immune system (58). However, creating modified heat-killed bacteria may be time-consuming and induce adverse events (gastroenteritis, sore throat, severe abdominal pain, and even anaphylaxis) while modifying pro-allergic TH2 responses.
Purified microbial macromolecules, such as DNA, lipopeptides, and proteins, may induce similar beneficial immune responses as whole-cell bacteria without the associated disadvantages. Bacteria and virus express toll-like receptor ligands that are PAMPS, which activate the host immune system and may modulate pre-existing immune responses. Microbial macromolecules expressing Toll-like receptor-9 (TLR9) ligands, such as unmethylated CpG oligodeoxynucleotides (ODN), are potent inducers of TH1 (59) and Treg (60) immunity and may determine a sustained decrease in IgE and IgG1, as well as an increase in allergen-specific IgG2a. Although TLR ligands (TLRL) can induce similar TH1-associated immune responses as bacteria, such as Escherichia coli and Listeria monocytogenes, it is suggested that TLRL adjuvants only direct immune responses to the co-administered allergen, inducing persistent protection and enhancing safety. Despite these promising results, host immunity varies with age in response to TLR stimulation (61, 62), with neonates and infants being less responsive (63).
Subcutaneous and epicutaneous immunotherapy adjuvants
Lysosomal-associated membrane proteins (LAMPs) are integral membrane proteins specific to lysosomes, thought to play an essential role in the degradation of extracellular material and phagocytosis (64). A novel approach currently under investigation involves the insertion of DNA encoding the allergen in a plasmid containing the coding sequence for LAMP, thus inducing the APCs to the synthesis of an allergen-LAMP fusion protein hypothesized to elicit TH1 responses (65). One study is currently evaluating the tolerability, safety, and immune responses in peanut-allergic adolescents receiving intradermal injections of ARA-LAMP-vax [NCT03755713 (66)].
Aluminum salts (alum) remain the most used form of adjuvant in FA-AIT formulations in Europe, given their ability to enhance safety through a limited rate of systemic exposure (67). Alum's precise mechanism of action has not been clarified yet. However, it is believed to adsorb proteins via electrostatic interaction with the protein's hydroxyl groups (depot effect), reducing allergen diffusion, thus lowering the chance of anaphylactic reactions and prolonging the exposure of immune cells to these antigens at the injection site (68). It is a robust inducer of a TH2-mediated response, promoting antigen-specific Immunoglobulin (Ig) E and IgG1 and Interleukin-4 (IL-4), although arguably counter-intuitive for AIT (19, 69). In food allergy, aluminum hydroxide adsorbed modified peanut extract (HAL-MPE1) administered subcutaneously reduces allergic responses while retaining peanut extract's immunogenicity (PE) (70).
Protamines are arginine-rich proteins that can spontaneously assemble into nanoparticles with CpG-ODNs, which drive the immune response toward TH1 responses. Findings suggest that protamine-based nanoparticles with CpG-ODN counteract the allergen-induced IgE, inducing a favorable increase in allergen-specific IgG2a and may be considered a novel allergen immunotherapy delivery system (71).
Future perspectives
Synthetic peptides representing T-cell epitope sequences of food are theorized to target allergen-specific T cells without causing IgE-mediated inflammatory cell activation (72). A novel product, PVX108, conceived for intradermal immunotherapy, demonstrated a favorable safety profile in a phase 1 trial (ACTRN12617000692336) (73).
The Bruton tyrosine kinase (BTK) is a critical component in B-cell receptor signaling and the activation of mast cells and basophils via FcεRI signaling. Ibrutinib is a BTK inhibitor currently used as an anti-neoplastic treatment and is being investigated for its potential role in food allergy immunotherapy. One study demonstrated the efficacy of Ibrutinib in decreasing skin test reactivity and IgE-mediated basophil activation test (BAT) responses to peanut and tree nuts, though without a sustained response (74). Ibrutinib was considered safe and well tolerated with no severe adverse reaction; however, only a limited sample size was involved in the study. Thus, further investigation is necessary to evaluate its potential use in preventing allergic reactions.
Finally, there is preliminary evidence for the potential utility of ketotifen, an H1 anti-histamine and mast cell stabilizer that has been used to treat a variety of allergic diseases, and leukotriene receptor antagonists (LTRAs), inhibitors of the pro-inflammatory leukotrienes’ action which have proved to be effective in asthma and in reducing side effects of OIT (75). However, more extensive randomized controlled trials, with more prolonged treatment and follow-up periods, are required to explore these compounds’ concrete effectiveness and safety in food AIT.
Research gaps and future directions
Current research on probiotics as adjuvants to FA-AIT highlights significant gaps and suggests future research directions. While existing studies provide promising insights into the efficacy of probiotics in enhancing FA-AIT outcomes, several critical gaps remain to be addressed (16). Firstly, there is a need for large-scale randomized clinical trials to validate the efficacy and safety of probiotic supplementation in diverse patient populations. Such trials require large sample sizes and precise inclusion and exclusion criteria for this therapy, specifying the individual characteristics of patients, including age, microbiota composition, and allergy profile, which are essential to optimize the synergistic effects of probiotic-AIT combinations. Additionally, the variability in probiotic strains used across studies necessitates standardization to ensure consistent results and facilitate meaningful comparisons, which needs improvement. Furthermore, determining the optimal probiotic doses for use in combination with FA-AIT is essential to maximize therapeutic benefits, minimize potential adverse effects, and achieve therapy standardization. Long-term safety evaluations are necessary to verify probiotic-AIT combinations’ durability and sustained efficacy in managing food allergies.
Future research should focus on elucidating the mechanisms by which probiotics modulate immune responses. Investigating new formulations and delivery methods to optimize clinical outcomes, considering factors such as bioavailability and stability in the gastrointestinal tract, is necessary. Furthermore, understanding the interactions between probiotics, gut microbiota composition, and host immune response dynamics is crucial, as it could provide valuable insights for personalized treatment strategies similar to those employed in other diseases.
In conclusion, probiotic supplementation offers significant promise as an adjuvant therapy in managing food allergies. Addressing current research gaps through rigorous clinical trials and mechanistic studies allows the scientific community to establish robust evidence-based guidelines for using probiotics in combination with FA-AIT. This approach enhances treatment efficacy and advances personalized medicine strategies, benefiting patients by mitigating allergic symptoms and improving long-term outcomes.
Author contributions
MM: Conceptualization, Project administration, Writing – original draft, Writing – review & editing. MP: Conceptualization, Project administration, Writing – original draft, Writing – review & editing. SF: Methodology, Resources, Writing – original draft, Writing – review & editing. FV: Methodology, Resources, Writing – original draft, Writing – review & editing. AF: Methodology, Resources, Writing – original draft, Writing – review & editing. CS: Supervision, Visualization, Writing – original draft, Writing – review & editing. MD: Supervision, Visualization, Writing – original draft, Writing – review & editing. PP: Supervision, Visualization, Writing – original draft, Writing – review & editing. DP: Supervision, Visualization, Writing – original draft, Writing – review & editing. GD: Supervision, Validation, Writing – original draft, Writing – review & editing. FF: Data curation, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Feuille E, Nowak-Wegrzyn A. Allergen-specific immunotherapies for food allergy. Allergy Asthma Immunol Res. (2018) 10(3):189–206. doi: 10.4168/aair.2018.10.3.189
2. De Filippo M, Votto M, Caminiti L, Panasiti I, Carella F, De Castro G, et al. Safety of allergen-specific immunotherapy in children. Pediatr Allergy Immunol. (2022) 33(Suppl 27):27–30. doi: 10.1111/pai.13622
3. Muraro A, Tropeano A, Giovannini M. Allergen immunotherapy for food allergy: evidence and outlook. Allergol Select. (2022) 6:285–92. doi: 10.5414/ALX02319E
4. Anagnostou K, Stiefel G, Brough H, du Toit G, Lack G, Fox AT. Active management of food allergy: an emerging concept. Arch Dis Child. (2015) 100(4):386–90. doi: 10.1136/archdischild-2014-306278
5. Elghoudi A, Narchi H. Food allergy in children-the current status and the way forward. World J Clin Pediatr. (2022) 11(3):253–69. doi: 10.5409/wjcp.v11.i3.253
6. Nowak-Węgrzyn A, Sampson HA. Future therapies for food allergies. J Allergy Clin Immunol. (2011) 127(3):558–73. quiz 74-5. doi: 10.1016/j.jaci.2010.12.1098
7. Riggioni C, Oton T, Carmona L, Du Toit G, Skypala I, Santos AF. Immunotherapy and biologics in the management of IgE-mediated food allergy: systematic review and meta-analyses of efficacy and safety. Allergy. (2024) 79(8):2097–127. doi: 10.1111/all.16129
8. Meyer R. Nutritional disorders resulting from food allergy in children. Pediatr Allergy Immunol. (2018) 29(7):689–704. doi: 10.1111/pai.12960
9. Michelsen-Huisman AD, van Os-Medendorp H, Blom WM, Versluis A, Castenmiller JJM, Noteborn H, et al. Accidental allergic reactions in food allergy: causes related to products and patient’s management. Allergy. (2018) 73(12):2377–81. doi: 10.1111/all.13560
10. Rodríguez Del Río P, Alvarez-Perea A, Blumchen K, Caimmi D, Caubet JC, Konstantinopoulos AP, et al. Food immunotherapy practice: nation differences across Europe, the FIND project. Allergy. (2022) 77(3):920–32. doi: 10.1111/all.15016
11. Todoric K, Merrill S. Oral immunotherapy: an overview. Prim Care. (2023) 50(2):269–81. doi: 10.1016/j.pop.2022.11.006
12. Soller L, Williams BA, Mak R, Wong T, Erdle SC, Chomyn A, et al. Safety and effectiveness of bypassing oral immunotherapy buildup with an initial phase of sublingual immunotherapy for higher-risk food allergy. J Allergy Clin Immunol Pract. (2024) 12(5):1283–96.e2. doi: 10.1016/j.jaip.2024.02.024
13. Hervé PL, Dioszeghy V, Matthews K, Bee KJ, Campbell DE, Sampson HA. Recent advances in epicutaneous immunotherapy and potential applications in food allergy. Front Allergy. (2023) 4:1290003. doi: 10.3389/falgy.2023.1290003
14. Ghouri H, Habib A, Nazir Z, Lohana N, Akilimali A. Omalizumab for the reduction of allergic reactions to foods: a narrative review. Front Allergy. (2024) 5:1409342. doi: 10.3389/falgy.2024.1409342
15. Shin S, Jang S, Kim J, Song J, Park S, Kim Y, et al. Initial updosing phase of oral immunotherapy improves quality of life and psychological burden in parents of children with food allergy. Allergy Asthma Proc. (2024) 45(2):128–36. doi: 10.2500/aap.2024.45.240001
16. Peroni DG, Morelli L. Probiotics as adjuvants in vaccine strategy: is there more room for improvement? Vaccines (Basel). (2021) 9(8):811. doi: 10.3390/vaccines9080811
17. Gamazo C, D'Amelio C, Gastaminza G, Ferrer M, Irache JM. Adjuvants for allergy immunotherapeutics. Hum Vaccin Immunother. (2017) 13(10):2416–27. doi: 10.1080/21645515.2017.1348447
18. Awate S, Babiuk LA, Mutwiri G. Mechanisms of action of adjuvants. Front Immunol. (2013) 4:114. doi: 10.3389/fimmu.2013.00114
19. Alvaro-Lozano M, Akdis CA, Akdis M, Alviani C, Angier E, Arasi S, et al. EAACI allergen immunotherapy user’s guide. Pediatr Allergy Immunol. (2020) 31(Suppl 25):1–101. doi: 10.1111/pai.13189
20. Gernez Y, Nowak-Węgrzyn A. Immunotherapy for food allergy: are we there yet? J Allergy Clin Immunol Pract. (2017) 5(2):250–72. doi: 10.1016/j.jaip.2016.12.004
21. Jensen-Jarolim E, Roth-Walter F, Jordakieva G, Pali-Schöll I. Allergens and adjuvants in allergen immunotherapy for immune activation, tolerance, and resilience. J Allergy Clin Immunol Pract. (2021) 9(5):1780–9. doi: 10.1016/j.jaip.2020.12.008
22. Kim HJ, Kim HY, Lee SY, Seo JH, Lee E, Hong SJ. Clinical efficacy and mechanism of probiotics in allergic diseases. Korean J Pediatr. (2013) 56(9):369–76. doi: 10.3345/kjp.2013.56.9.369
23. Fiocchi A, Cabana MD, Mennini M. Current use of probiotics and prebiotics in allergy. J Allergy Clin Immunol Pract. (2022) 10(9):2219–42. doi: 10.1016/j.jaip.2022.06.038
24. Virk MS, Virk MA, He Y, Tufail T, Gul M, Qayum A, et al. The anti-inflammatory and curative exponent of probiotics: a comprehensive and authentic ingredient for the sustained functioning of major human organs. Nutrients (2024) 16(4):546. doi: 10.3390/nu16040546
25. Noh G, Lee JH. Regulatory B cells and allergic diseases. Allergy Asthma Immunol Res. (2011) 3(3):168–77. doi: 10.4168/aair.2011.3.3.168
26. Tang ML, Ponsonby AL, Orsini F, Tey D, Robinson M, Su EL, et al. Administration of a probiotic with peanut oral immunotherapy: a randomized trial. J Allergy Clin Immunol. (2015) 135(3):737–44.e8. doi: 10.1016/j.jaci.2014.11.034
27. Galvin A D, McMahon S, Ponsonby AL, Hsiao KC, Tang MLK. The longitudinal impact of probiotic and peanut oral immunotherapy on health-related quality of life. Allergy. (2018) 73(3):560–8. doi: 10.1111/all.13330
28. Hsiao K-C, Ponsonby A-L, Axelrad C, Pitkin S, Tang MLK, Burks W, et al. Long-term clinical and immunological effects of probiotic and peanut oral immunotherapy after treatment cessation: 4-year follow-up of a randomised, double-blind, placebo-controlled trial. Lancet Child Adolesc Health. (2017) 1(2):97–105. doi: 10.1016/S2352-4642(17)30041-X
29. Huang L, Dalziel K, Lloyd M, Loke P, Lozinsky AC, Tang M. Cost-effectiveness analysis of probiotic peanut oral immunotherapy (PPOIT) versus placebo in Australian children with peanut allergy alongside a randomised trial. BMJ Open. (2023) 13(12):e075521. doi: 10.1136/bmjopen-2023-075521
30. Kim BG, Kim JN, Jang AS, Shin M. Combined effects of lactobacillus rhamnosus and egg oral immunotherapy in a mouse model of egg allergy. Allergy Asthma Immunol Res. (2020) 12(4):701–11. doi: 10.4168/aair.2020.12.4.701
31. Loke P, Orsini F, Lozinsky AC, Gold M, O’Sullivan MD, Quinn P, et al. Probiotic peanut oral immunotherapy versus oral immunotherapy and placebo in children with peanut allergy in Australia (PPOIT-003): a multicentre, randomised, phase 2b trial. Lancet Child Adolesc Health. (2022) 6(3):171–84. doi: 10.1016/S2352-4642(22)00006-2
32. Yamamoto-Hanada K, Sato M, Toyokuni K, Irahara M, Hiraide-Kotaki E, Harima-Mizusawa N, et al. Combination of heat-killed lactiplantibacillus plantarum YIT 0132 (LP0132) and oral immunotherapy in cow’s milk allergy: a randomised controlled trial. Benefic Microbes. (2023) 14(1):17–30. doi: 10.3920/BM2022.0064
33. Nicolaides RE, Parrish CP, Bird JA. Food allergy immunotherapy with adjuvants. Immunol Allergy Clin North Am. (2020) 40(1):149–73. doi: 10.1016/j.iac.2019.09.004
34. Savage JH, Courneya JP, Sterba PM, Macglashan DW, Saini SS, Wood RA. Kinetics of mast cell, basophil, and oral food challenge responses in omalizumab-treated adults with peanut allergy. J Allergy Clin Immunol. (2012) 130(5):1123–9.e2. doi: 10.1016/j.jaci.2012.05.039
35. Fiocchi A, Artesani MC, Riccardi C, Mennini M, Pecora V, Fierro V, et al. Impact of omalizumab on food allergy in patients treated for asthma: a real-life study. J Allergy Clin Immunol Pract. (2019) 7(6):1901–9.e5. doi: 10.1016/j.jaip.2019.01.023
36. Nadeau KC, Schneider LC, Hoyte L, Borras I, Umetsu DT. Rapid oral desensitization in combination with omalizumab therapy in patients with cow’s milk allergy. J Allergy Clin Immunol. (2011) 127(6):1622–4. doi: 10.1016/j.jaci.2011.04.009
37. Martorell-Calatayud C, Michavila-Gómez A, Martorell-Aragonés A, Molini-Menchón N, Cerdá-Mir JC, Félix-Toledo R, et al. Anti-IgE-assisted desensitization to egg and cow’s milk in patients refractory to conventional oral immunotherapy. Pediatr Allergy Immunol. (2016) 27(5):544–6. doi: 10.1111/pai.12567
38. Wood RA, Kim JS, Lindblad R, Nadeau K, Henning AK, Dawson P, et al. A randomized, double-blind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow’s milk allergy. J Allergy Clin Immunol. (2016) 137(4):1103–10.e11. doi: 10.1016/j.jaci.2015.10.005
39. Yee CSK, Albuhairi S, Noh E, El-Khoury K, Rezaei S, Abdel-Gadir A, et al. Long-Term outcome of peanut oral immunotherapy facilitated initially by omalizumab. J Allergy Clin Immunol Pract. (2019) 7(2):451–61.e7. doi: 10.1016/j.jaip.2018.09.015
40. MacGinnitie AJ, Rachid R, Gragg H, Little SV, Lakin P, Cianferoni A, et al. Omalizumab facilitates rapid oral desensitization for peanut allergy. J Allergy Clin Immunol. (2017) 139(3):873–81.e8. doi: 10.1016/j.jaci.2016.08.010
41. Schneider LC, Rachid R, Lebovidge J, Blood E, Mittal M, Umetsu DT. A pilot study of omalizumab to facilitate rapid oral desensitization in high-risk peanut-allergic patients. J Allergy Clin Immunol. (2013) 132(6):1368–74. doi: 10.1016/j.jaci.2013.09.046
42. Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. (2011) 128(1):e9–e17. doi: 10.1542/peds.2011-0204
43. Andorf S, Purington N, Kumar D, Long A, O'Laughlin KL, Sicherer S, et al. A phase 2 randomized controlled multisite study using omalizumab-facilitated rapid desensitization to test continued vs discontinued dosing in multifood allergic individuals. eClinicalMedicine. (2019) 7:27–38. doi: 10.1016/j.eclinm.2018.12.006
44. Andorf S, Manohar M, Dominguez T, Block W, Tupa D, Kshirsagar RA, et al. Observational long-term follow-up study of rapid food oral immunotherapy with omalizumab. Allergy Asthma Clin Immunol. (2017) 13(1):51. doi: 10.1186/s13223-017-0223-8
45. Bégin P, Dominguez T, Wilson SP, Bacal L, Mehrotra A, Kausch B, et al. Phase 1 results of safety and tolerability in a rush oral immunotherapy protocol to multiple foods using omalizumab. Allergy Asthma Clin Immunol. (2014) 10(1):7. doi: 10.1186/1710-1492-10-7
46. Campbell RL, Bashore CJ, Lee S, Bellamkonda VR, Li JTC, Hagan JB, et al. Predictors of repeat epinephrine administration for emergency department patients with anaphylaxis. J Allergy Clin Immunol Pract. (2015) 3(4):576–84. doi: 10.1016/j.jaip.2015.04.009
47. Srivastava KD, Song Y, Yang N, Liu C, Goldberg IE, Nowak-Węgrzyn A, et al. B-FAHF-2 plus oral immunotherapy (OIT) is safer and more effective than OIT alone in a murine model of concurrent peanut/tree nut allergy. Clin Exp Allergy. (2017) 47(8):1038–49. doi: 10.1111/cea.12936
48. Srivastava KD, Kattan JD, Zou ZM, Li JH, Zhang L, Wallenstein S, et al. The Chinese herbal medicine formula FAHF-2 completely blocks anaphylactic reactions in a murine model of peanut allergy. J Allergy Clin Immunol. (2005) 115(1):171–8. doi: 10.1016/j.jaci.2004.10.003
49. Wang J, Jones SM, Pongracic JA, Song Y, Yang N, Sicherer SH, et al. Safety, clinical, and immunologic efficacy of a Chinese herbal medicine (food allergy herbal formula-2) for food allergy. J Allergy Clin Immunol. (2015) 136(4):962–70.e1. doi: 10.1016/j.jaci.2015.04.029
50. Romagnani S. The role of lymphocytes in allergic disease. J Allergy Clin Immunol. (2000) 105(3):399–408. doi: 10.1067/mai.2000.104575
51. Maggi E. The TH1/TH2 paradigm in allergy. Immunotechnology. (1998) 3(4):233–44. doi: 10.1016/S1380-2933(97)10005-7
52. Noh G, Jang EH. Dual specific oral tolerance induction using interferon gamma for IgE-mediated anaphylactic food allergy and the dissociation of local skin allergy and systemic oral allergy: tolerance or desensitization? J Investig Allergol Clin Immunol. (2014) 24(2):87–97.24834771
53. Noh G, Lee SS. A pilot study of interferon-γ-induced specific oral tolerance induction (ISOTI) for immunoglobulin E-mediated anaphylactic food allergy. J Interferon Cytokine Res. (2009) 29(10):667–75. doi: 10.1089/jir.2009.0001
54. Pohlit H, Bellinghausen I, Frey H, Saloga J. Recent advances in the use of nanoparticles for allergen-specific immunotherapy. Allergy. (2017) 72(10):1461–74. doi: 10.1111/all.13199
55. Srivastava KD, Siefert A, Fahmy TM, Caplan MJ, Li XM, Sampson HA. Investigation of peanut oral immunotherapy with CpG/peanut nanoparticles in a murine model of peanut allergy. J Allergy Clin Immunol. (2016) 138(2):536–43.e4. doi: 10.1016/j.jaci.2016.01.047
56. Maldonado RA, LaMothe RA, Ferrari JD, Zhang AH, Rossi RJ, Kolte PN, et al. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc Natl Acad Sci U S A. (2015) 112(2):E156–65. doi: 10.1073/pnas.1408686111
57. Yamaki K, Yoshino S. Preventive and therapeutic effects of rapamycin, a mammalian target of rapamycin inhibitor, on food allergy in mice. Allergy. (2012) 67(10):1259–70. doi: 10.1111/all.12000
58. Le Gouëllec A, Chauchet X, Polack B, Buffat L, Toussaint B. Bacterial vectors for active immunotherapy reach clinical and industrial stages. Hum Vaccin Immunother. (2012) 8(10):1454–8. doi: 10.4161/hv.21429
59. Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. Cpg oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. (1997) 186(10):1623–31. doi: 10.1084/jem.186.10.1623
60. Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu Y-J, et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+ CD25+regulatory T cells. J Immunol. (2004) 173(7):4433–42. doi: 10.4049/jimmunol.173.7.4433
61. van Haren SD, Dowling DJ, Foppen W, Christensen D, Andersen P, Reed SG, et al. Age-specific adjuvant synergy: dual TLR7/8 and mincle activation of human newborn dendritic cells enables Th1 polarization. J Immunol. (2016) 197(11):4413–24. doi: 10.4049/jimmunol.1600282
62. van Haren SD, Ganapathi L, Bergelson I, Dowling DJ, Banks M, Samuels RC, et al. In vitro cytokine induction by TLR-activating vaccine adjuvants in human blood varies by age and adjuvant. Cytokine. (2016) 83:99–109. doi: 10.1016/j.cyto.2016.04.001
63. De Wit D, Olislagers V, Goriely S, Vermeulen F, Wagner H, Goldman M, et al. Blood plasmacytoid dendritic cell responses to CpG oligodeoxynucleotides are impaired in human newborns. Blood. (2004) 103(3):1030–2. doi: 10.1182/blood-2003-04-1216
64. Schwake M, Schröder B, Saftig P. Lysosomal membrane proteins and their central role in physiology. Traffic. (2013) 14(7):739–48. doi: 10.1111/tra.12056
65. Vickery BP, Ebisawa M, Shreffler WG, Wood RA. Current and future treatment of peanut allergy. J Allergy Clin Immunol Pract. (2019) 7(2):357–65. doi: 10.1016/j.jaip.2018.11.049
66. Mertes PM, Tajima K, Regnier-Kimmoun MA, Lambert M, Iohom G, Guéant-Rodriguez RM, et al. Perioperative anaphylaxis. Med Clin N Am. (2010) 94(4):761–89. doi: 10.1016/j.mcna.2010.04.002
67. Hogenesch H. Mechanism of immunopotentiation and safety of aluminum adjuvants. Front Immunol. (2013) 3:406. doi: 10.3389/fimmu.2012.00406
68. Lin YJ, Zimmermann J, Schülke S. Novel adjuvants in allergen-specific immunotherapy: where do we stand? Front Immunol. (2024) 15:1348305. doi: 10.3389/fimmu.2024.1348305
69. Mannhalter JW, Neychev HO, Zlabinger GJ, Ahmad R, Eibl MM. Modulation of the human immune response by the non-toxic and non-pyrogenic adjuvant aluminium hydroxide: effect on antigen uptake and antigen presentation. Clin Exp Immunol. (1985) Jul;61(1):143–51.3876178
70. van der Kleij HPM, Warmenhoven HJM, van Ree R, Versteeg SA, Pieters RHH, Dreskin SC, et al. Chemically modified peanut extract shows increased safety while maintaining immunogenicity. Allergy. (2019) 74(5):986–95. doi: 10.1111/all.13687
71. Pali-Schöll I, Szöllösi H, Starkl P, Scheicher B, Stremnitzer C, Hofmeister A, et al. Protamine nanoparticles with CpG-oligodeoxynucleotide prevent an allergen-induced Th2-response in BALB/c mice. Eur J Pharm Biopharm. (2013) 85(3 Pt A):656–64. doi: 10.1016/j.ejpb.2013.03.003
72. Prickett SR, Voskamp AL, Phan T, Dacumos-Hill A, Mannering SI, Rolland JM, et al. Ara h 1 CD4+ T cell epitope-based peptides: candidates for a peanut allergy therapeutic. Clin Exp Allergy. (2013) 43(6):684–97. doi: 10.1111/cea.12113
73. Prickett SR, Hickey PL, Bingham J, Phan T, Abramovitch J, Rolland JM, et al. Safety and tolerability of a novel peptide-based immunotherapy for peanut allergy. J Allergy Clin Immunol. (2019) 143(2):AB431. doi: 10.1016/j.jaci.2018.12.975
74. Dispenza MC, Pongracic JA, Singh AM, Bochner BS. Short-term ibrutinib therapy suppresses skin test responses and eliminates IgE-mediated basophil activation in adults with peanut or tree nut allergy. J Allergy Clin Immunol. (2018) 141(5):1914–6.e7. doi: 10.1016/j.jaci.2017.12.987
Keywords: microbiome, oral tolerance, food allergy, probiotics, immunotherapy
Citation: Mennini M, Piccirillo M, Furio S, Valitutti F, Ferretti A, Strisciuglio C, De Filippo M, Parisi P, Peroni DG, Di Nardo G and Ferrari F (2024) Probiotics and other adjuvants in allergen-specific immunotherapy for food allergy: a comprehensive review. Front. Allergy 5:1473352. doi: 10.3389/falgy.2024.1473352
Received: 30 July 2024; Accepted: 23 September 2024;
Published: 10 October 2024.
Edited by:
Lucie Mondoulet, Independent Researcher, Kremlin Bicêtre, FranceReviewed by:
Douglas Jones, Tanner Clinic, United StatesCopyright: © 2024 Mennini, Piccirillo, Furio, Valitutti, Ferretti, Strisciuglio, De Filippo, Parisi, Peroni, Di Nardo and Ferrari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maurizio Mennini, bWF1cml6aW8ubWVubmluaUBnbWFpbC5jb20=
†These authors have contributed equally to this work
 Maurizio Mennini
Maurizio Mennini Marisa Piccirillo
Marisa Piccirillo Silvia Furio1
Silvia Furio1 Francesco Valitutti
Francesco Valitutti Alessandro Ferretti
Alessandro Ferretti Caterina Strisciuglio
Caterina Strisciuglio Maria De Filippo
Maria De Filippo Pasquale Parisi
Pasquale Parisi Diego Giampietro Peroni
Diego Giampietro Peroni Giovanni Di Nardo
Giovanni Di Nardo Federica Ferrari
Federica Ferrari