- 1Department of Allergy, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing Key Laboratory of Precision Medicine for Diagnosis and Treatment of Allergic Diseases, Beijing, China
- 2National Clinical Research Center for Dermatologic and Immunologic Disease (NCRC-DID), Beijing, China
Background: The clinical efficacy of allergen-specific immunotherapy (AIT) for Alternaria alternata (A. alt) and Dermatophagoides farinae (Der f) extracts remains largely unknown in China. We sought to retrospectively evaluate the efficacy caused by AIT agents manufactured in China of patients who are sensitized to A. alt and Der f.
Methods: Patients aged 5–27 years with asthma and perennial allergic rhinitis (AR), and AIT with A. alt and Der f were recruited, and then classified into two groups: A. alt-AIT (n = 31) and A. alt + Der f-AIT group (n = 39). All data were gathered retrospectively, including biological parameters, pulmonary function, and symptom and medication scores.
Results: 70 patients who underwent A. alt and Der f AIT were enrolled. A significant improvement was observed in the values of FEV1% (P < 0.0001) and MEF 25 (P = 0.023) of lung function. Both the rhinitis symptoms and combined symptoms and medication scores for asthma decreased after AIT (by 45.3% and 80.3%, respectively, P < 0.0001 for each). Nearly 67% improvement rate (P < 0.0001) occurred in rhinoconjunctivitis quality of life, and a great increase existed in Asthma Control Test (ACT) score (P < 0.0001) after at least 1 year AIT, although there were no significant changes between these two groups. Besides, no significance was displayed in specific IgE to different allergens.
Conclusion: AIT with A. alt and Der f extracts had clinical efficacy for many patients in China, with a reduction of symptom and medication scores, and great improvement in spirometry function.
1 Introduction
Sensitization to Alternaria alternata and Dermatophagoides farinae is a common cause of rhinitis and asthma, Alternaria is also strongly associated with the persistence and exacerbation of asthma in children (1). Chinese research has reported that Alternaria alternata is the most abundant fungal genera in the environment among 667 fungal strains (2). Additionally, the sensitization rate of Alternaria reaches 11.9% in several European countries (3), and more than 100 million patients suffer from allergic diseases due to dust mites around the world (4).
In patients with allergen-induced respiratory allergies, AIT is considered effective in preventing the development and progression of new sensitization and is the only approach that maintains long-lasting effects. However, few studies focused on immunotherapy of Alternaria allergy (5), and there are few studies evaluating the efficacy of AIT with Alternaria alternata extracts in China until now since the clinical efficacy controversy still existed in mold immunotherapy. Besides, numerous studies have confirmed the efficacy of AIT with house dust mite (6), but little is known about the immunotherapy response to A. alt and Der f extracts in Chinese patients, although a high prevalence of Alternaria alternata and Dermatophagoides farinae in the grassland of China among pediatrics.
Consequently, We conducted a retrospective study to evaluate clinical efficacy in terms of symptoms, life quality, asthma control, and lung function in patients who underwent AIT with A. alt and Der f extracts.
2 Materials and methods
2.1 Study design and patients
All data were collected retrospectively from the outpatient allergy department of Peking Union Medical College Hospital (PUMCH) between January 2022 and January 2023. This study was approved by the IRB of PUMCH (No. S-K1672). All informed consent was completed.
A total of 70 patients aged 5–27 years with a diagnosis of mild or moderate asthma and perennial AR associated with fungal exposure were recruited. All patients presented both positive skin tests (wheal size ≥5 mm) with whole extract of A. alternata (PUMCH, Beijing, China) and sIgE (≥0.7 kUA/L) to Alternaria (Phadia ImmunoCAP, Uppsala, Sweden). The skin test grading system (1 + to 4+) was used according to our previous study (7). Besides, they all received only A. alt extracts AIT or A. alt extracts combined with Der f extracts AIT for at least 1 year. Subjects were classified into 2 major groups: A. alt-AIT group, referred to patients who were individually allergic to A. alt and also only accepted AIT with A. alt extracts (n = 31); A. alt + Der f-AIT group, referred to patients who were only allergic to A. alt + Der f and without any other allergens, and subcutaneous injection with A. alt + Der f extracts at the same time in different arms (n = 39). The reasons for the selection of the AIT group of Alternaria combined with dust mites are as follows: (1) Both are perennial allergens, seasonal factors will not be considered before the comparison, and further reduce the mixture factors; (2) The two groups of patients are similar in age and are more comparable. Patients who received AIT previously, AIT with pollens or animal dander, suffered from unstable asthma, any nasal disease except for AR, autoimmune or chronic diseases, and incomplete data were excluded from the study.
Demographic and clinical information including sex, eosinophils, T-IgE, spirometry testing, and symptoms and medication scores were acquired.
2.2 Specific immunotherapy
Allergen extracts of A. alt (PUMCH, China, batch number: S20120006, total protein content 0.5 mg/5 ml) and Der f (PUMCH, China, batch number: S20130002, total protein content 1.75 mg/5 ml) were used for AIT with subcutaneous injection following an “up dosing” schedule, and two treatment stages were included. For the initial phase, patients were injected of 0.1 ml as a minimal dose, and increased step by step to achieve the maximum dose of 1.0 ml. The maintenance phase always kept an injection with 1.0 ml until the end of AIT. All the patients sustained an injection twice a week.
2.3 Symptom and medication assessment
Symptoms and medication assessment were evaluated by a series of questionnaires that were delivered to patients or their parents. Symptoms related to nose and ocular were recorded using Rhinoconjunctivitis Total Symptom Score (RTSS), including pruritus, nasal congestion, mucus production, sneezing, tearing, and itching. Pulmonary symptoms included cough, wheezing, dyspnea, and exercise-induced asthma (1, no symptoms; 2, mild; 3, moderate; 4, severe). The Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ) and ACT were used to clarify patients' nasal, ocular, and pulmonary control status. Medication score was applied and graded according to Tabar et al. (8) as follows: 0, no rescue medication; 1, topical and/or systemic antihistamines; 2, intranasal corticosteroids; 3, oral corticosteroids.
2.4 Statistics analysis
All data were analyzed using Prism 9.0 (GraphPad, California, USA). Descriptive data were performed and presented in tables. Continuous variables were expressed as mean ± SD. The differences between the two groups were analyzed by using an unpaired t-test. Within-group comparisons, the paired t-test was used. For categorical data, percentages were summarized and the χ2 test was performed. Statistical significance was set at P < 0.05.
3 Results
3.1 Demographic characteristics
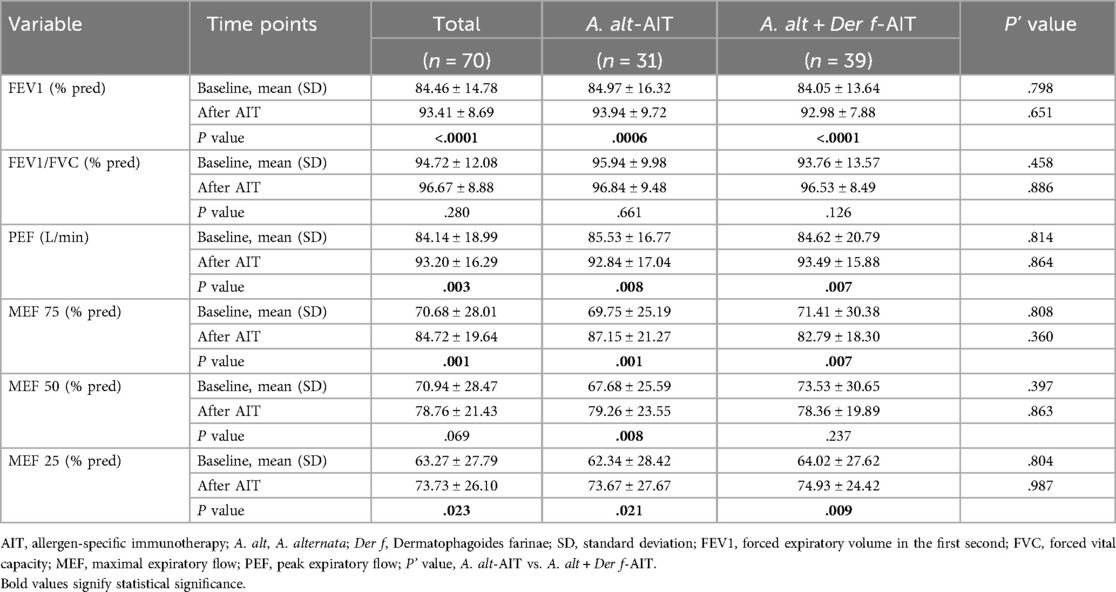
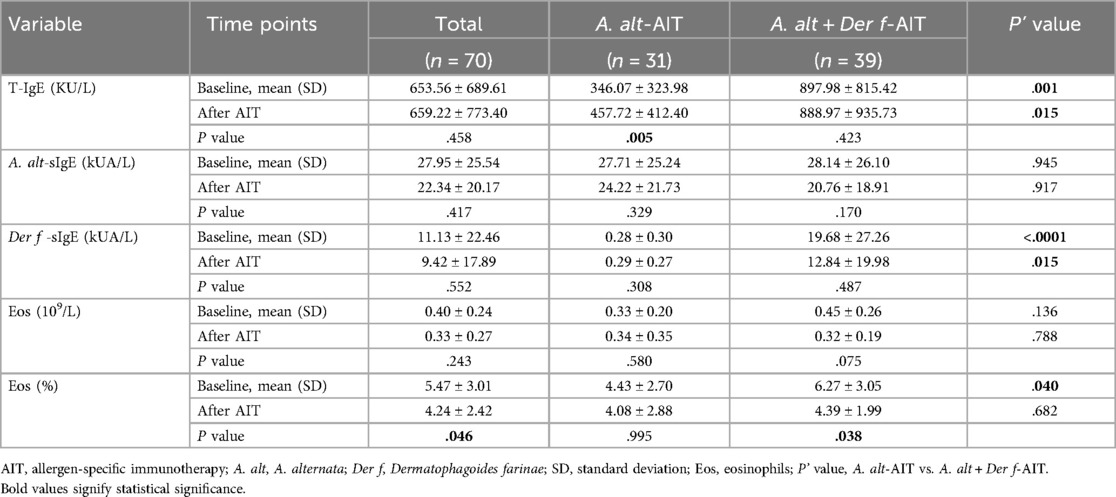
We analyzed and compared the differences in age, gender, IgE titers, pulmonary function, the medication used, another atopic status, and family allergic history between these two groups (Table 1). The mean age of patients was 11.80 ± 3.83 years, and there were far more males than females in both groups. At the baseline, patients in the A. alt + Der f-AIT group showed higher concentrations of T-IgE (P = 0.001) and Der f-sIgE (P < 0.0001), larger wheals of Der f, and increased percentages of eosinophils than the A. alt-AIT group (P = 0.04). However, there were no statistical differences in respiratory function, medication assessment, and other clinical characteristics between the two treatment groups at the baseline level.
3.2 A. alt or A. alt combined with Der f-AIT influenced the spirometry function
Spirometry values are displayed in Table 2. In the total of 70 patients, FEV1% of predicted increased after AIT (93.41 ± 8.69) compared to baseline levels (84.46 ± 14.78), these results were similar existed in both the A. alt-AIT (P = 0.0006) and A. alt + Der f-AIT group (P < 0.0001). In addition, changes in the small airway function (MEF 25) also displayed an upregulation rather in the A. alt-AIT patients (P = 0.021) or in the A. alt + Der f-AIT patients (P = 0.009), since it was a reliable parameter of respiratory function. Mean PEF increased from 84 to 93 after administering AIT in all patients. However, the values of FEV1/FVC% did not present significant changes between assessments. It was also worth noting that no significance of respiratory function was shown between A. alt-AIT and A. alt + Der f-AIT group at baseline or after treatment.
Besides, we also analyzed the changes in FEV1% levels of individual patients. Patients who had an FEV1% value lower than 70% before immunotherapy, showed not much improvement after AIT treatment, which only recovered to about 80%–90% of predicted FEV1%, whether in A. alt-AIT or A. alt + Der f-AIT patients.
3.3 Changes in biologic parameters after A. alt or A. alt combined with Der f-AIT
In the A. alt-AIT group, the level of T-IgE demonstrated a higher tendency after AIT (P = 0.005) while there were no significant differences in the A. alt + Der f-AIT group (Table 3). However, the changes that occurred similarly before AIT, and T-IgE values still showed nearly double the level in the patients who accepted A. alt and Der f mixture extracts than in the A. alt-AIT patients (P = 0.015). Besides, specific IgE to A. alt and the numbers of eosinophils with no obvious difference between before and after AIT, and also without significance between group-analysis. However, the Der f-sIgE titers reached a high concentration in the A. alt + Der f-AIT group all the time. Meanwhile, the proportions of eosinophils reduced from 6.27 to 4.39 after the A. alt combined with Der f immunotherapy compared with the baseline (P = 0.038).
3.4 Symptom and medication scores
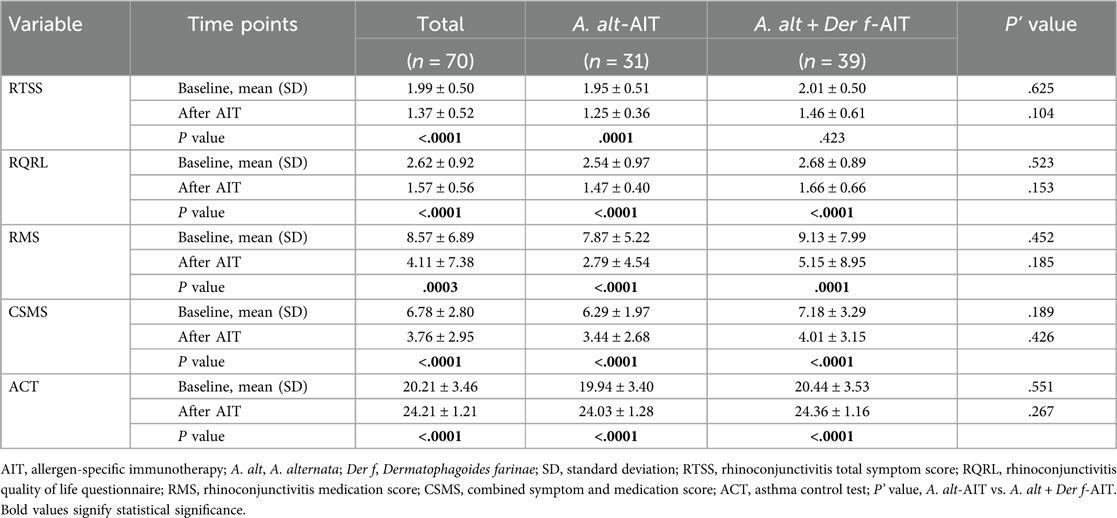
Symptom and medication scores in patients according to the condition were recorded in Table 4. For all patients with AR and asthma, there was a greater improvement (45.3%) in RTSS after immunotherapy (1.37 ± 0.52) than baseline (1.99 ± 0.50), as well as the better RQLQ acquired when patients administrated AIT at least one year (improvement rate: 66.9%, 2.62 ± 0.92 vs. 1.57 ± 0.56). Meanwhile, a significant reduction was observed in the RMS at the baseline level relative to the immunotherapy level (8.57 ± 6.89 vs. 4.11 ± 7.38, P = 0.0003). Differences in the CSMS and ACT scores between baseline and at least one year of AIT were statistically significant (P < 0.0001 in both cases). When analyzing the different allergen extracts of AIT separately, we found better improvement in all symptom and medication scores in the A. alt-AIT population, rather than patients in the A. alt + Der f-AIT group. However, we did not find the differences that existed in A. alt-AIT and A. alt + Der f-AIT groups whether before or after immunotherapy.
We further analyzed the differences in symptom and medication scores according to the age of all patients (Figure 1). The age of patients receiving AIT focused on 5–15 years old, and the course of AR combined with asthma ranged from 5 to 10 years (Figure 1A). After classifying the age into two different scopes (5–11 years vs. ≥12 years) (Figure 1B), results demonstrated that a statistical significance only occurred in the evaluation of CSMS. Patients in 5–11 years showed a better response in CSMS compared to the older after immunotherapy (P = 0.0025).
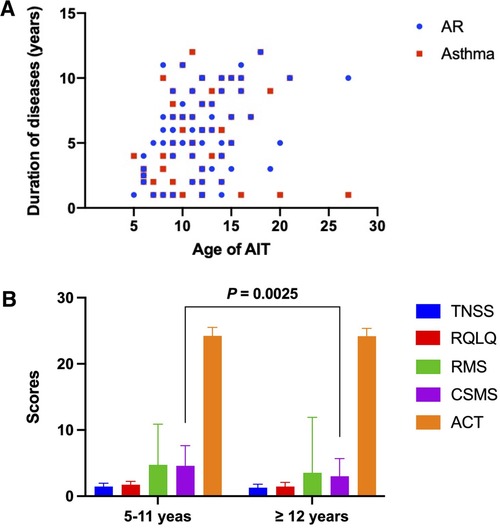
Figure 1. The differences in administrating AIT and scoring symptoms and medication according to the age of all patients. (A) The age of patients who received AIT. (B) Analysis of symptom and medication scores in two different age groups. AIT, allergen-specific immunotherapy.
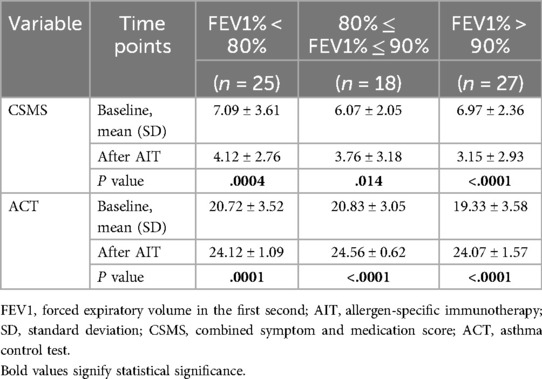
When analyzing the improvement of CSMS and ACT in different FEV1% grades separately (Table 5, Figure 2), we classified patients into three subgroups according to the values of FEV1%. Results displayed that patients in the FEV1% >90% group (n = 27) had the greatest response of CSMS (P < 0.0001) and ACT (P < 0.0001) among the three groups. Followed by the group of FEV1% <80% (n = 25), which also showed a 72% reduction in the mean CSMS (P = 0.0004) and a significant elevation in ACT (P = 0.0001). Although the group of 80% ≤FEV1% ≤90% (n = 18) shared similar improvements in these two scores, CSMS only changed 61.4% (P = 0.014).

Figure 2. The improvement of CSMS and ACT in different FEV1% grades. CSMS, combined symptom and medication score; ACT, Asthma Control Test; FEV1%, forced expiratory volume in the first second.
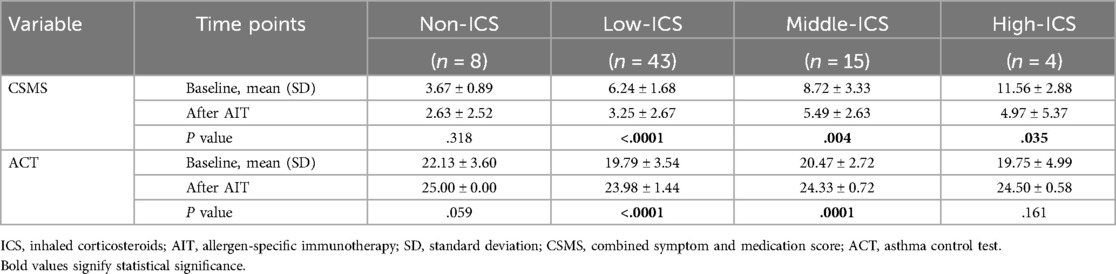
To determine whether individuals receiving inhaled corticosteroids before immunotherapy had different degrees of change in symptom and medication scores after AIT, patients were initially grouped into those who had never been given ICS (non-ICS, n = 8), those who had previously been on low (low-ICS, n = 43), middle (middle-ICS, n = 15), or high ICS (high-ICS, n = 4) therapy (Table 6). Our results showed that CSMS and ACT scores were comparable between baseline and receiving AIT at least 1 year in non-ICS patients. In comparison, followed by AIT, low ICS therapy in individuals presented the most statistical significance in CSMS and ACT improvement (P < 0.0001). Individuals in the middle ICS-taken group revealed a reduction of 58.8% in CSMS evaluation (P = 0.004), and also a significant difference between before and after AIT was observed for middle ICS treatment in the ACT (P = 0.0001). In addition, this same reduction trend of CSMS occurred in the high ICS treatment patients (P = 0.035), although only a few patients inhaled high doses of corticosteroids. For further comparison of ACT scores in this group, the difference between before and after AIT was not significant.
4 Discussion
Few studies focused on the efficacy of AIT with fungal extracts worldwide, especially for Alternaria alternata (9–11), although AIT has been concluded effective for mold allergy by the American Academy of Allergy, Asthma & Immunology (12). More importantly, little research on immunotherapy with A. alt and Der f extracts has been published in China, except for a real-world analysis of a small sample published by us (13). Here, we retrospectively studied the efficacy of A. alternata and Dermatophagoides farinae which was produced by PUMCH in the course of clinical usage. Results in our study displayed that perennial AIT for A. alt and Der f were significantly improving the symptom and medication scores for AR and asthma, along with the quality of life, and asthma control, and had a large increase in respiratory function.
In our data analysis, the mean age of the patients was nearly 12 years old and the proportion of males was higher than females. This young age reflects that sensitization to mold and Der f had higher incidence at early ages (14, 15), as well as the limitation of inclusion criteria only to A. alt immunotherapy and A. alt combined with Der f immunotherapy. A higher prevalence of A. alternata sensitization related to males was confirmed by several studies (16, 17). Furthermore, we did not find a significance of a family allergic history and pets in both groups at the baseline level, since the progress of asthma was influenced by genetic and environmental factors (18).
A significant increase in pulmonary function was demonstrated as a result of AIT with both Alternaria alternata and Dermatophagoides farinae extracts. A study performed by Fielding et al. (19) showed that falling FEV1% might be related to the exacerbation of asthma and the change in FEV1% can be a valuable predictor for the risk of asthma. Therefore, these results demonstrated the important role of FEV1% in asthma assessment. However, many immunotherapy studies of A. alternata and Dermatophagoides farinae did not evaluate the response of pulmonary, because they mainly highlight the role of symptom and medication scores. Our study concluded a remarkable improvement of FEV1%, PEF, and MEF 25 after accepting immunotherapy, which is similar to the results of Kim et al. (20). It is also worth noting that the lower the value of FEV1% sustained before immunotherapy, the harder to recover to a higher level followed by AIT, although we did not know the reasons. Nevertheless, the variations of respiratory function remained similar change between A. alt-AIT and A. alt + Der f-AIT patients, which indicated the improvement of lung function after immunotherapy was not related to the type of allergen, but the mechanisms also might be explored further.
Regarding the biological parameters, including IgE and eosinophils in serum, we did not find prominent variations in both groups either before or after immunotherapy. Studies have proved that changes in IgE values are probably not a vital mechanism of the AIT (21), and the study conducted by Uriarte et al. (22) also did not report remarkable variations at one year of AIT. Additionally, a reduction of sIgE levels might be observed after long AIT over several years (23). Patients in the A. alt combined Der f immunotherapy group presented a higher level of T-IgE may be attributed to the sensitization to Der f excepted for A. alternata. Researchers found decreases in nasal and sputum eosinophilia predicted the improvement of asthma control (22). Meanwhile, The activation and recruitment of eosinophils to target organs could be reduced during AIT (24). This same result can be found in our study.
The clinical improvements were significantly followed by at least one year of AIT in our study analysis, these improvements included RTSS, RMS, RQLQ, CSMS, and ACT. The majority of clinical trials of A. alternata have emphasized and confirmed a significant improvement in symptom and medication scores compared with placebo (8, 10). However, there was no clinical data shown in China, although both of the extracts had been administrated in patients for a long history. Our study displayed that both nasal and respiratory symptoms largely decreased after AIT, and we found no association between clinical efficacy and a unique pattern of sensitization to different allergens, which is similar to the study of cat and dog immunotherapy (22). Furthermore, our findings about the symptom and medication scores in different ages of all patients were interesting, younger patients indicated better response to CSMS than the older, two possible reasons might explain it. Firstly, the earlier immunotherapy for asthma patients, the better the therapeutic effect acquired. Secondly, younger children were mainly supervised by their parents and showed good compliance. At the same time, our results for the relationship between diverse FEV1% grades and CSMS and ACT were meaningful. The higher predicted values of FEV1% among patients, the probably greater improvement of CSMS and ACT scores were obtained, which suggested that patients with AR combined with asthma could administrate immunotherapy as soon as possible without damage to lung function. More importantly, most of the patients still inhaled corticosteroids during AIT, we found patients with a low dose of ICS before AIT acquired the best improvement in CSMS and ACT. This finding may be due to the mild symptoms of these patients relative to other middle or high-ICS subjects. In overall terms, the parameters we applied to estimate clinical efficacy suggested that immunotherapy with Alternaria alternata and Dermatophagoides farinae extracts produced in China were beneficial to AR and asthma patients.
A lack of safety assessment was the major limitation in our study since AIT shows a risk of local and systemic side effects. Another issue was the lack of placebo control in immunotherapy, we only compared clinical efficacy before and after AIT, and a further prospective study is required to perform with a large cohort. Detecting the variations of the fractional exhaled nitric oxide (FeNO) value in all patients through immunotherapy is significant since it is recommended by the international guideline for monitoring asthma (25). Furthermore, we did not record the change in wheel size after immunotherapy because of the partial lack of data.
5 Conclusion
Our results proved that immunotherapy with Alternaria alternata and Dermatophagoides farinae extracts could significantly improve the pulmonary function, and symptom and medication scores of asthma patients. There was no difference between the immunotherapy of Der f combined with A. alt and the immunotherapy of A. alt alone. In addition, we evaluated the improvement of patients' symptoms and medication by grading different age groups, pulmonary function, and the use of ICS, which was of clinical significance. Therefore, AIT with A. alt and Der f extracts showed great clinical efficacy for patients in China.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by IRB of Peking Union Medical College Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
JL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. JY: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This study was supported by the CAMS Initiative for Innovative Medicine (CAMS-I2M), Grant/Award Number: 2021-I2M-1-017.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AIT, allergen-specific immunotherapy; A. alt, Alternaria alternata; Der f, Dermatophagoides farinae; AR, allergic rhinitis; FEV1%, forced expiratory volume in the first second; MEF, maximal expiratory flow; FVC, forced vital capacity; PEF, peak expiratory flow; ACT, asthma control test; HDM, house dust mites; RTSS, rhinoconjunctivitis total symptom score; RQLQ, rhinoconjunctivitis quality of life questionnaire; CSMS, combined symptom and medication score; RMS, rhinoconjunctivitis medication score.
References
1. Bush RK, Prochnau JJ. Alternaria-induced asthma. J Allergy Clin Immunol. (2004) 113:227–34. doi: 10.1016/j.jaci.2003.11.023
2. Lu Y, Wang X, Almeida L, Pecoraro L. Environmental factors affecting diversity, structure, and temporal variation of airborne fungal communities in a research and teaching building of Tianjin university, China. J Fungi (Basel). (2022) 8:1–24. doi: 10.3390/jof8050431
3. Heinzerling L, Frew AJ, Bindslev-Jensen C, Bonini S, Bousquet J, Bresciani M, et al. Standard skin prick testing and sensitization to inhalant allergens across Europe–a survey from the GALEN network. Allergy. (2005) 60:1287–300. doi: 10.1111/j.1398-9995.2005.00895.x
4. Caraballo L, Valenta R, Puerta L, Pomés A, Zakzuk J, Fernandez-Caldas E, et al. The allergenic activity and clinical impact of individual IgE-antibody binding molecules from indoor allergen sources. World Allergy Organ J. (2020) 13:100118. doi: 10.1016/j.waojou.2020.100118
5. Di Bona D, Albanesi M, Macchia L. Is immunotherapy with fungal vaccines effective? Curr Opin Allergy Clin Immunol. (2019) 19:646–53. doi: 10.1097/aci.0000000000000582
6. Agache I, Lau S, Akdis CA, Smolinska S, Bonini M, Cavkaytar O, et al. EAACI guidelines on allergen immunotherapy: house dust mite-driven allergic asthma. Allergy. (2019) 74:855–73. doi: 10.1111/all.13749
7. Liu J, Li J, Yin J. Clinical relevance of alternaria alternata sensitization in patients within type 2-high and type 2-low asthma. Int Immunopharmacol. (2021) 101:108333. doi: 10.1016/j.intimp.2021.108333
8. Tabar AI, Prieto L, Alba P, Nieto A, Rodríguez M, Torrecillas M, et al. Double-blind, randomized, placebo-controlled trial of allergen-specific immunotherapy with the major allergen alt a 1. J Allergy Clin Immunol. (2019) 144:216–223.e213. doi: 10.1016/j.jaci.2019.02.029
9. Tabar AI, Lizaso MT, García BE, Gómez B, Echechipía S, Aldunate MT, et al. Double-blind, placebo-controlled study of alternaria alternata immunotherapy: clinical efficacy and safety. Pediatr Allergy Immunol. (2008) 19:67–75. doi: 10.1111/j.1399-3038.2007.00589.x
10. Kuna P, Kaczmarek J, Kupczyk M. Efficacy and safety of immunotherapy for allergies to alternaria alternata in children. J Allergy Clin Immunol. (2011) 127:502–508.e1–6. doi: 10.1016/j.jaci.2010.11.036
11. Zapatero L, Martínez-Cañavate A, Lucas JM, Guallar I, Torres J, Guardia P, et al. Clinical evolution of patients with respiratory allergic disease due to sensitisation to alternaria alternata being treated with subcutaneous immunotherapy. Allergol Immunopathol (Madr). (2011) 39:79–84. doi: 10.1016/j.aller.2010.03.011
12. Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Allergen immunotherapy: a practice parameter second update. J Allergy Clin Immunol. (2007) 120:S25–85. doi: 10.1016/j.jaci.2007.06.019
13. Gao X, Yin J. Efficacy and safety of specific immunotherapy for treatment of allergic asthma by using fungal allergen extract. Chin J Allergy Clin Immunol. (2017) 11:216–27.
14. Andersson M, Downs S, Mitakakis T, Leuppi J, Marks G. Natural exposure to alternaria spores induces allergic rhinitis symptoms in sensitized children. Pediatr Allergy Immunol. (2003) 14:100–5. doi: 10.1034/j.1399-3038.2003.00031.x
15. Brzozowska A, Woicka-Kolejwa K, Jerzynska J, Majak P, Stelmach I. Allergic rhinitis and house dust mite sensitization determine persistence of asthma in children. Indian J Pediatr. (2022) 89:673–81. doi: 10.1007/s12098-021-04052-5
16. Katotomichelakis M, Anastassakis K, Gouveris H, Tripsianis G, Paraskakis E, Maroudias N, et al. Clinical significance of alternaria alternata sensitization in patients with allergic rhinitis. Am J Otolaryngol. (2012) 33:232–8. doi: 10.1016/j.amjoto.2011.07.004
17. Cantani A, Ciaschi V. Epidemiology of alternaria alternata allergy: a prospective study in 6840 Italian asthmatic children. Eur Rev Med Pharmacol Sci. (2004) 8:289–94.15745389
18. Liu T, Valdez R, Yoon PW, Crocker D, Moonesinghe R, Khoury MJ. The association between family history of asthma and the prevalence of asthma among US adults: national health and nutrition examination survey, 1999–2004. Genet Med. (2009) 11:323–8. doi: 10.1097/GIM.0b013e31819d3015
19. Fielding S, Pijnenburg M, de Jongste JC, Pike KC, Roberts G, Petsky H, et al. Change in FEV(1) and Feno measurements as predictors of future asthma outcomes in children. Chest. (2019) 155:331–41. doi: 10.1016/j.chest.2018.10.009
20. Kim CK, Callaway Z, Park JS, Kwon E. Efficacy of subcutaneous immunotherapy for patients with asthma and allergic rhinitis in Korea: effect on eosinophilic inflammation. Asia Pac Allergy. (2021) 11:e43. doi: 10.5415/apallergy.2021.11.e43
21. Lizaso MT, Tabar AI, García BE, Gómez B, Algorta J, Asturias JA, et al. Double-blind, placebo-controlled alternaria alternata immunotherapy: in vivo and in vitro parameters. Pediatr Allergy Immunol. (2008) 19:76–81. doi: 10.1111/j.1399-3038.2007.00587.x
22. Uriarte SA, Grönlund H, Wintersand A, Bronge J, Sastre J. Clinical and immunologic changes due to subcutaneous immunotherapy with cat and dog extracts using an ultrarush up-dosing phase: a real-life study. J Investig Allergol Clin Immunol. (2022) 32:133–40. doi: 10.18176/jiaci.0656
23. Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy: multiple suppressor factors at work in immune tolerance to allergens. J Allergy Clin Immunol. (2014) 133:621–31. doi: 10.1016/j.jaci.2013.12.1088
24. Shamji MH, Sharif H, Layhadi JA, Zhu R, Kishore U, Renz H. Diverse immune mechanisms of allergen immunotherapy for allergic rhinitis with and without asthma. J Allergy Clin Immunol. (2022) 149:621–31. doi: 10.1016/j.jaci.2022.01.016
Keywords: efficacy, allergen immunotherapy, Alternaria alternata, Dermatophagoides farinae, allergic rhinitis, allergic asthma, retrospective
Citation: Liu J and Yin J (2024) A retrospective analysis of the clinical efficacy in patients treated with Alternaria alternata and Dermatophagoides farinae immunotherapy. Front. Allergy 5:1453446. doi: 10.3389/falgy.2024.1453446
Received: 23 June 2024; Accepted: 7 August 2024;
Published: 22 August 2024.
Edited by:
Rongfei Zhu, Huazhong University of Science and Technology, ChinaReviewed by:
Enrique Fernandez-Caldas, Inmunotek SL, SpainHontian Wang, Capital Medical University, China
Copyright: © 2024 Liu and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia Yin, ZG9jdG9yeWluamlhQDE2My5jb20=
 Juan Liu
Juan Liu Jia Yin
Jia Yin