- College of Nursing and Health Sciences, Texas A&M University—Corpus Christi, Corpus Christi, TX, United States
Children around the world are continuing to develop and suffer from chronic lung diseases such as asthma. Childhood asthma commonly presents with recurrent episodes of cough, shortness of breath, and wheezing, all of which can lead to missed school days and hospitalization admissions. The role of environmental pollutants and aeroallergens has been increasingly recognized in relation to asthma etiology. We showcase the impacts of air pollution and pollen exposures in early life on childhood asthma and allergies through an epidemiologic perspective. We also examine the effects of indoor microbial exposures such as endotoxin and glucan on allergic diseases in schoolchildren as many spend most of their time in a household or classroom setting. Findings of this work can assist in the identification of key environmental factors in critical life periods and improve clinicians’ diagnoses of asthma during early childhood.
Introduction
Asthma is a chronic respiratory disease caused by a combination of genetic, environmental, and lifestyle factors (1–3). Globally, asthma is the 28th leading cause of disease burden and 16th leading cause of years lived with disability, based on disability-adjusted life years. In the next year, the prevalence of asthma is expected to reach 400 million worldwide (4). Children represent a large fraction of asthma incidence and prevalence (5). Asthma development begins during early childhood and can progress throughout life, with some adults experiencing a first-time occurrence (4). Because of their underdeveloped organs and weakened immune systems, children are more susceptible to asthma symptoms (i.e., shortness of breath, cough, wheezing) and hospitalization admissions compared to their adult counterparts (6). However, asthma morbidity and mortality remain higher in adults (4) who have an increased risk of fixed airflow obstruction (7), alongside a greater potential for rapid lung function decline (4, 7, 8). Despite these differences, children often possess several risk factors for poor health outcomes and thus depend on others daily to meet their basic needs (9, 10).
Various environmental exposures are established risk factors for asthma including air pollution, tobacco smoke, and farm animals (11). The degree and timing of environmental exposures in early life (i.e., pregnancy and infancy) is crucial and may either serve as risk factors or protective factors for asthma. Maternal risk factors during pregnancy including antibiotic exposure, atopic disease, smoking, and stress have been associated with asthma (12, 13). Postnatal factors such as respiratory viral infections and indoor microbial exposures have shown to potentially safeguard against the development of asthma (12, 14). Climate change on the other hand is indirectly correlated with the increasing incidence of allergic diseases with recent focus on the role of air pollution and pollen exposures in asthma development and exacerbation (15). Studies provide compelling evidence that air pollution and pollen exposures result in asthma exacerbations and related allergy symptoms, as well as hospitalizations and emergency department presentations, respectively (16–20). The impact of prenatal and postnatal exposures to air pollution and pollen on childhood asthma and allergic disease risk is paramount and still uncertain in epidemiology.
As such, the primary objective of this perspective is to reveal the latest epidemiological evidence on early life exposures to air pollution and pollen exposures and their associations with childhood allergic diseases. In a secondary objective, we examine the role of microbial exposures on the risk of asthma and allergic conditions in schoolchildren. Enhanced knowledge of environmental exposures in early life can improve asthma prevention efforts, thereby improving the health of the pediatric population.
Air pollution
Particulate matter
Particulate matter (PM) consists of microscopic solid and liquid particles whose diameters are less than 10 (PM10) or 2.5 (PM2.5) micrometers. The impact of early life exposures of PM on childhood allergic diseases are well-studied (21–27) (Table 1). In a sex-stratified analysis, maternal daily exposure to elevated PM2.5 levels at midgestation (16–25 weeks) was found to associate with an increased risk of asthma development in urban boys by age 6 (21). Furthermore, a population-based study of four European birth cohorts reported an increased risk of asthma incidence after age 4 from early life exposure to PM2.5 (Odds Ratio [OR] 1.29, 95% Confidence Interval [CI] (1.00–1.66). The authors also examined the effect of PM2.5 on allergic rhinoconjunctivitis but did not identify a positive association (22). A Danish study on COPSAC10 birth cohort observed significant associations between postnatal PM2.5 [OR 1.51, 95% CI (1.08–2.07)] and PM10 [OR 1.56, 95% CI (1.14–2.09)] in relation to childhood asthma at age 6, supporting the impact of PM exposures during early life.
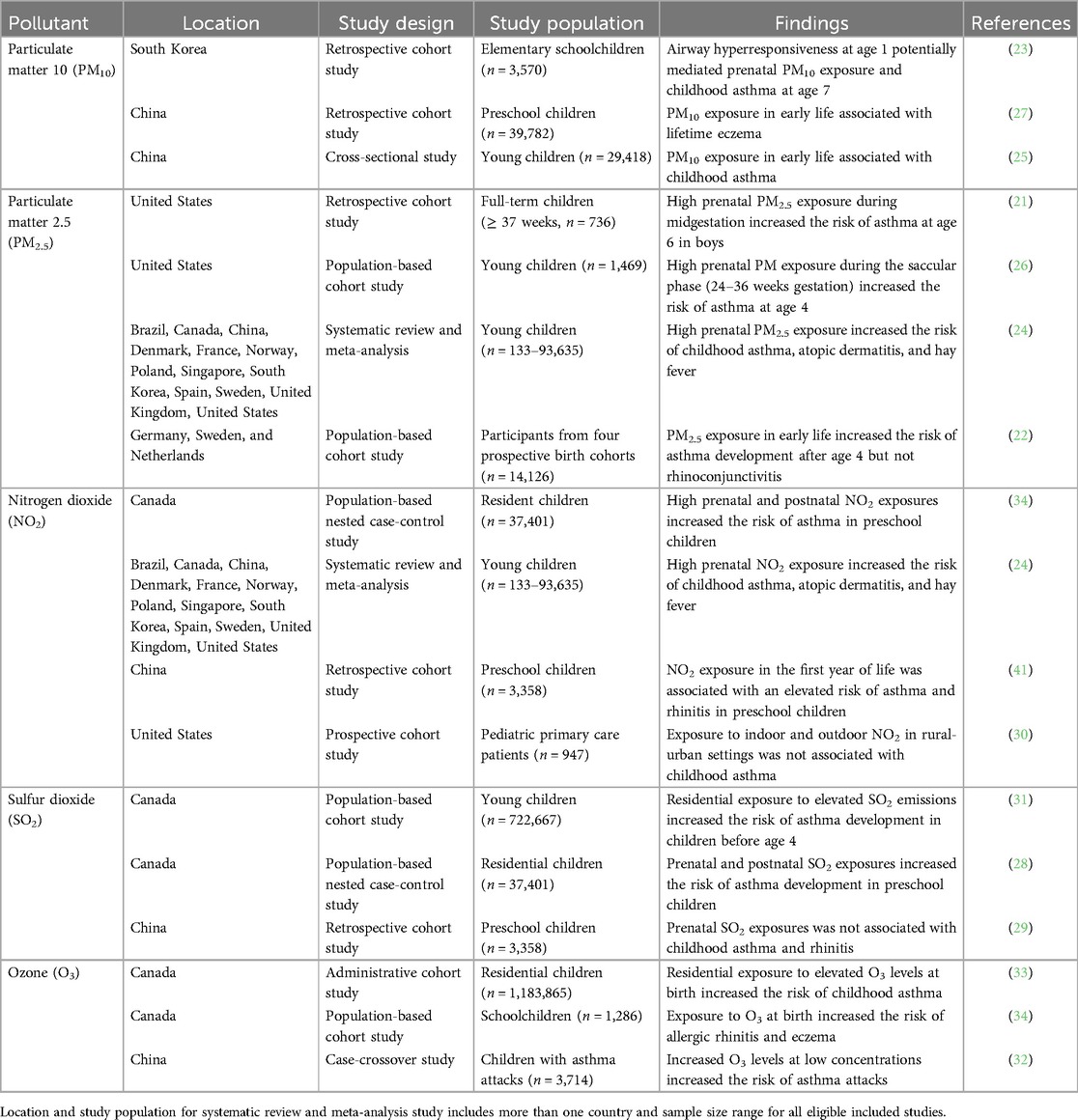
Table 1. Epidemiological studies on air pollution exposures in early life and childhood allergic diseases.
In terms of childhood allergic diseases, there are fewer studies addressing the effect of prenatal PM exposures, particularly on asthma. In the United States, a multicity sample of two pregnancy cohorts demonstrated the saccular phase (24–36 weeks gestation) to be a critical window for PM2.5 exposure and subsequent development of asthma at age 4 (27). A mediation analysis on prenatal PM10 exposure and asthma incidence showed that airway hyperresponsiveness at age 1 potentially mediates the association between exposure to PM10 during the second trimester and asthma incidence at age 7 in schoolchildren (23). For early life exposure, outdoor PM10 was found to associate with lifetime eczema [OR 1.17, 95% CI (1.06–1.28)], but not asthma, wheeze, or rhinitis among preschool children (26). A different study observed an increased risk of childhood asthma from early life exposure to PM10 [OR 1.11, 95% CI (1.02–1.20)]. Compared to asthma, more attention has shifted towards allergic rhinitis as of late given its increased prevalence alongside the compounding effects of climate change and ambient particle pollution in Westernized countries.
Nitrogen dioxide
Nitrogen dioxide (NO2) is a gaseous oxide commonly produced by combustion processes, fossil fuel emissions, industrial activities, and transportation (35). Indoor sources of NO2 include building heat, natural gas stoves, and tobacco smoke (36, 37). Because children spend ∼70% of their time inside, they may be exposed to higher levels of indoor NO2 than outdoor NO2 (38). Epidemiological studies have identified associations between outdoor NO2 exposure and risk of childhood asthma and wheezing (39, 40). The effect of prenatal and postnatal NO2 exposures on childhood asthma remains less investigated, with few studies highlighting an increased risk of asthma in preschool-aged children (28, 29). More recently, a prospective cohort study explored the relationship of indoor and outdoor NO2 in mixed rural-urban settings with childhood asthma; however, no significant association was detected (30). The feasibility study used a small sample (n = 947), which may have contributed to a lack of an association.
Moreover, a study in Canada explored the role of NO2 exposure in the first year of life and risk of allergic diseases including asthma, allergic rhinitis, and eczema. Early life exposures to NO2 were associated with an increased risk of incident asthma [OR 1.06, 95% CI (0.96–1.16)] and eczema [OR 1.05, 95% CI (0.99–1.11)] in comparison to allergic rhinitis [OR 0.94, 95% CI (0.87–1.02)] (34). In China, a group of researchers analyzed the association between prenatal and postnatal NO2 exposures and childhood allergic rhinitis prevalence. No significant association was observed for prenatal NO2, while an increased odds of allergic rhinitis resulted from postnatal exposure in the first year of life [OR 1.013, 95% CI (1.002–1.025)] (41). Considering these findings, the association between early life exposure to NO2 and childhood allergic diseases are inconsistent and necessitate longitudinal cohort studies.
Sulfur dioxide
Sulfur dioxide (SO2) is a noxious gas primarily generated from fossil fuel combustion or industrial processes. Epidemiological studies revealed an increased risk of asthma exacerbations in children exposed to high levels of SO2 in the short term (42–44). Continued interest in this arena may be due to the toxic effects of SO2 in sensitive asthmatics, although the exact mechanisms are not completely understood (45). However, researchers report inconsistent findings on early life SO2 exposures and risk of asthma and allergic rhinitis. For example, residential exposure to industrial SO2 emissions in Quebec, Canada was shown to contribute to childhood asthma development mostly before 4 years of age (31). In an earlier study, children exposed to high levels of SO2 in pregnancy and in infancy had an elevated risk of asthma onset [OR 1.03, 95% CI (1.02–1.05) for both periods] (28). No significant relationship was observed between prenatal SO2 exposure and risk of childhood asthma and allergic rhinitis in China (29). A follow-up study among Korean schoolchildren discovered a high risk of allergic rhinitis associated with high atmospheric SO2 concentrations [OR 1.056, 95% CI (1.006–1.109)] (46). With continued investigations on SO2 exposures and childhood allergic diseases associations may become clearer.
Ozone
Ozone (O3) is a reactive gas existing in both the stratosphere and troposphere of Earth's atmosphere. Stratospheric O3 prevents harmful health effects by absorbing ultraviolet rays from the sun. Inhalation of tropospheric O3 at high concentrations can cause cardiovascular and respiratory diseases in children (47). There is increasing evidence supporting the relationship of O3 exposure with asthma exacerbations or development in childhood. A case-cross over study found that O3 exposure >80 µg/m3 increased the risk for asthma attacks on each day of lag, with a significant effect observed for levels >100 µg/m3 (32). Conversely, findings from an administrative cohort study in Québec, Canada indicated an increased risk of asthma development with respect to residential exposure to O3 at birth [Hazard Ratio (HR) 1.11 95% CI (1.10–1.12)] (33). Associations have similarly been reported for O3 exposure at birth and childhood allergic rhinitis [HR 1.15, 95% CI (1.00–1.31)] and eczema [HR 1.05, 95% CI (0.95–1.16)] (34).
Pollen
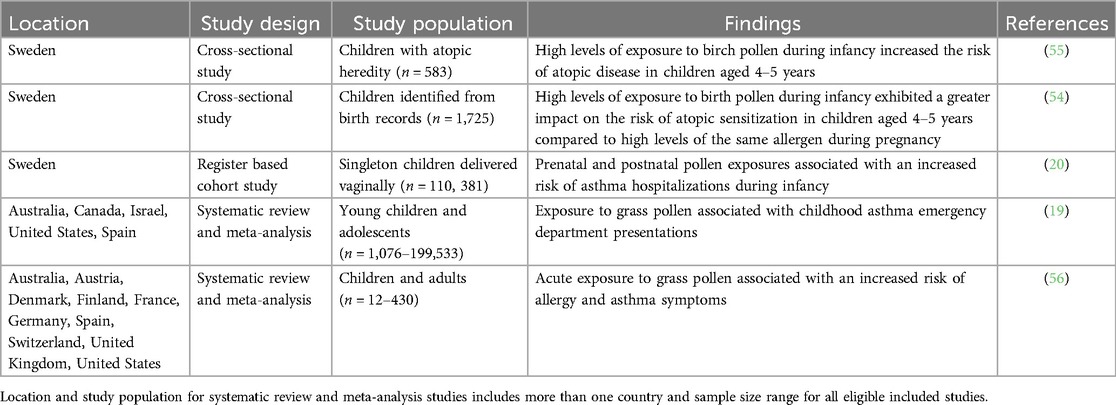
With global climate change extending the pollen season and distribution of airborne pollen, the adverse effects of early life pollen exposures on young children are becoming more pronounced. Several factors contribute to variable pollen levels in the atmosphere including vegetation source (i.e., trees, grasses, and weeds), seasonality, and weather conditions (48). Seasonal pollen exposure stimulates IgE-mediated inflammatory responses, resulting in itchy eyes, nasal congestion, rhinorrhea, and persistent sneezing (49, 50). Many epidemiological studies have thus investigated associations between pollen season of birth and childhood allergic diseases (51–53). Given the increasing trends in global climatic patterns some studies are seeking to understand the impact of pollen exposures on asthma and allergic disease outcomes (Table 2). For instance, one study reported an increased risk of sensitization to atopic disease from high pollen exposure during pregnancy and in infancy, with the latter showing a greater tendency towards sensitization (55). An earlier cross-sectional study by the same authors revealed an increased odds of sensitization [OR 2.4, 95% CI (1.2–4.6)] and allergic asthma [OR 2.6, 95% CI (1.2–5.6)] from high pollen exposure in infancy.
Another study noted an increased risk of asthma hospitalization from pollen exposure during late pregnancy and in the first year of life (20). Interestingly, the same study showed that high pollen levels in early pregnancy had a protective effect on asthma hospitalization by age 1. Limitations within the study included a lack of allergic disease phenotypes and potential misclassification of pollen exposures (20).
Microbial allergens
Bacterial endotoxin
Bacterial endotoxins are lipopolysaccharide (LPS) molecules found in Gram-negative bacteria. Multiple studies from the early 2000 s assessed the relationship between household endotoxin exposures and allergic diseases among schoolchildren (57–61). Various associations on endotoxin exposure occurred from these studies including an increased or a decreased risk of asthma or allergies to no association with asthma. In a longitudinal study of children with a parental history of atopy, high endotoxin exposure at ages 2–3 months associated with a decreased odds of atopy [OR 0.6, 95% CI (0.3–1.4)] and rhinitis [OR 0.3, 95% CI (0.1–0.9)] in schoolchildren, but an increased risk of wheeze from ages 1 to 7 years [HR 1.23, 95% CI (1.07–1.43)]. No significant association occurred between endotoxin exposure and asthma at 7 years (62). A case-control study in Canada suggested an increased risk of asthma or wheeze at age 12 in children whose parents reported a history of allergic disease to endotoxin exposure, but not for non-allergic children (63). These findings demonstrate that parental history of atopic disease could play a part in the protective effect of high postnatal endotoxin exposure and development of allergic diseases at earlier ages, and thus be treated as a potential confounder in epidemiological analyses.
Glucan
Fungi are heterotrophic microorganisms composed of chitinous cell walls and release spores for dispersal and colonization. Few exposure assessments on fungal species or glucan in house dust have been conducted (64–66). High glucan exposure has been associated with a decreased risk of atopic eczema by school age [OR 0.73, 95% CI (0.51–1.05)] (67). Similarly, a decreased risk of allergic sensitization in children ages 2–4 years resulted from glucan exposure [OR 0.67, 95% CI (0.56–0.81)] (68). In Puerto Rico, an increased odds of atopy in control subjects [OR 2.46, 95% CI (0.37–4.55)] and emergency department/urgent care visits for asthma [OR 8.76, 95% CI (2.70–28.4)] was reported in schoolchildren exposed to high glucan levels (69). The study was cross-sectional in design, meaning it only captured the link between glucan exposure and risk of atopy and emergency department visits at a single time point. Collectively, epidemiological studies on glucan exposure are scarce, suggesting mixed findings on the risk of atopic and allergic phenotypes. Longitudinal cohort studies are therefore needed to assess the temporal sequence of indoor fungal exposures and allergic diseases in schoolchildren.
Conclusion
This perspective sheds light on air pollution and pollen exposures as key environmental determinants in early life with respect to childhood allergic diseases. Findings on prenatal and postnatal air pollution and pollen exposures were analyzed and presented to indicate the potential risk of developing asthma and allergy in early childhood. As climate change continues to influence environmental changes, the interactive effects of air pollution and pollen exposures on allergic diseases may receive increasing attention. We even evaluated the role of indoor microbial exposures in connection with allergic diseases in schoolchildren. Despite conflicting findings on postnatal endotoxin and glucan exposures, future studies should explore maternal exposure to indoor pollutants and aeroallergens as well as potential mediators (i.e., viral infection and DNA methylation) with childhood allergic diseases.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
RM: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Johnson CC, Ownby DR, Zoratti EM, Alford SH, Williams LK, Joseph CLM. Environmental epidemiology of pediatric asthma and allergy. Epidemiol Rev. (2002) 24(2):154–75. doi: 10.1093/epirev/mxf013
2. Salam MT, Li YF, Langholz B, Gilliland FD, Children's Health Study. Early-life environmental risk factors for asthma: findings from the children’s health study. Environ Health Perspect. (2004) 112(6):760–5. doi: 10.1289/ehp.6662
3. Thomsen SF. Genetics of asthma: an introduction for the clinician. Eur Clin Respir J. (2015) 2:24643. doi: 10.3402/ecrj.v2.24643
4. Dharmage SC, Perret JL, Custovic A. Epidemiology of asthma in children and adults. Front Pediatr. (2019) 7:246. doi: 10.3389/fped.2019.00246
5. Toskala E, Kennedy DW. Asthma risk factors. Int Forum Allergy Rhinol. (2015) 5(Suppl 1):S11–6. doi: 10.1002/alr.21557
6. Searing DA, Rabinovitch N. Environmental pollution and lung effects in children. Curr Opin Pediatr. (2011) 23(3):314–8. doi: 10.1097/MOP.0b013e3283461926
7. Aanerud M, Carsin AE, Sunyer J, Dratva J, Gislason T, Jarvis D, et al. Interaction between asthma and smoking increases the risk of adult airway obstruction. Eur Respir J. (2015) 45(3):635–43. doi: 10.1183/09031936.00055514
8. Tai A, Tran H, Roberts M, Clarke N, Gibson AM, Vidmar S, Wilson J, Robertson CF. Outcomes of childhood asthma to the age of 50 years. J Allergy Clin Immunol. (2014) 133(6):1572–8.e3. doi: 10.1016/j.jaci.2013.12.1033
9. Stevens GD, Seid M, Mistry R, Halfon N. Disparities in primary care for vulnerable children: the influence of multiple risk factors. Health Serv Res. (2006) 41(2):507–31. doi: 10.1111/j.1475-6773.2005.00498.x
10. Bagattini A. Children’s well-being and vulnerability. Ethics and Soc Welf. (2019) 13(3):211–5. doi: 10.1080/17496535.2019.1647973
11. Kothalawala DM, Weiss VBN, Kadalayil L, Granell R, Curtin JA, Murray CS, et al. Nonlinear effects of environment on childhood asthma susceptibility. Pediatr Allergy Immunol. (2022) 33(4):e13777. doi: 10.1111/pai.13777
12. Subbarao P, Mandhane PJ, Sears MR. Asthma: epidemiology, etiology and risk factors. CMAJ. (2009) 181(9):E181–90. doi: 10.1503/cmaj.080612
13. Wang CM, Yang ST, Yang CC, Chiu HY, Lin HY, Tsai ML, et al. Maternal and neonatal risk factors of asthma in children: nationwide population based study. J Microbiol Immunol Infect. (2023) 56(1):182–91. doi: 10.1016/j.jmii.2022.10.005
14. Castro-Rodriguez JA, Forno E, Rodriguez-Martinez CE, Celedón JC. Risk and protective factors for childhood asthma: what is the evidence? J Allergy Clin Immunol Pract. (2016) 4(6):1111–22. doi: 10.1016/j.jaip.2016.05.003
15. D'Amato G, Akdis CA. Global warming, climate change, air pollution and allergies. Allergy. (2020) 75(9):2158–60. doi: 10.1111/all.14527
16. Baldacci S, Maio S, Cerrai S, Sarno G, Baïz N, Simoni M, et al. Allergy and asthma: effects of the exposure to particulate matter and biological allergens. Respir Med. (2015) 109(9):1089–104. doi: 10.1016/j.rmed.2015.05.017
17. Hassoun Y, James C, Bernstein DI. The effects of air pollution on the development of atopic disease. Clin Rev Allergy Immunol. (2019) 57(3):403–14. doi: 10.1007/s12016-019-08730-3
18. Boulet LP, Cartier A, Thomson NC, Roberts RS, Dolovich J, Hargreave FE. Asthma and increases in nonallergic bronchial responsiveness from seasonal pollen exposure. J Allergy Clin Immunol. (1983) 71(4):399–406. doi: 10.1016/0091-6749(83)90069-6
19. Erbas B, Jazayeri M, Lambert KA, Katelaris CH, Prendergast LA, Tham R, et al. Outdoor pollen is a trigger of child and adolescent asthma emergency department presentations: a systematic review and meta-analysis. Allergy. (2018) 73(8):1632–41. doi: 10.1111/all.13407
20. Lowe AJ, Olsson D, Bråbäck L, Forsberg B. Pollen exposure in pregnancy and infancy and risk of asthma hospitalisation - a register based cohort study. Allergy Asthma Clin Immunol. (2012) 8(1):17. doi: 10.1186/1710-1492-8-17
21. Hsu HH, Chiu YH, Coull BA, Kloog I, Schwartz J, Lee A, et al. Prenatal particulate air pollution and asthma onset in urban children. Identifying sensitive windows and sex differences. Am J Respir Crit Care Med. (2015) 192(9):1052–9. doi: 10.1164/rccm.201504-0658OC
22. Gehring U, Wijga AH, Hoek G, Bellander T, Berdel D, Brüske I, et al. Exposure to air pollution and development of asthma and rhinoconjunctivitis throughout childhood and adolescence: a population-based birth cohort study. Lancet Respir Med. (2015) 3(12):933–42. doi: 10.1016/S2213-2600(15)00426-9
23. Yang SI, Lee SY, Kim HB, Kim HC, Leem JH, Yang HJ, et al. Prenatal particulate matter affects new asthma via airway hyperresponsiveness in schoolchildren. Allergy. (2019) 74(4):675–84. doi: 10.1111/all.13649
24. Ai S, Liu L, Xue Y, Cheng X, Li M, Deng Q. Prenatal exposure to air pollutants associated with allergic diseases in children: which pollutant, when exposure, and what disease? A systematic review and meta-analysis. Clin Rev Allergy Immunol. (2024) 66(2):149–63. doi: 10.1007/s12016-024-08987-3
25. Wu C, Zhang Y, Wei J, Zhao Z, Norbäck D, Zhang X, et al. Associations of early-life exposure to submicron particulate matter with childhood asthma and wheeze in China. JAMA Netw Open. (2022) 5(10):e2236003. doi: 10.1001/jamanetworkopen.2022.36003
26. Norbäck D, Lu C, Zhang Y, Li B, Zhao Z, Huang C, et al. Sources of indoor particulate matter (PM) and outdoor air pollution in China in relation to asthma, wheeze, rhinitis and eczema among pre-school children: synergistic effects between antibiotics use and PM(10) and second hand smoke. Environ Int. (2019) 125:252–60. doi: 10.1016/j.envint.2019.01.036
27. Hazlehurst MF, Carroll KN, Loftus CT, Szpiro AA, Moore PE, Kaufman JD, et al. Maternal exposure to PM(2.5) during pregnancy and asthma risk in early childhood: consideration of phases of fetal lung development. Environ Epidemiol. (2021) 5(2):e130. doi: 10.1097/EE9.0000000000000130
28. Clark NA, Demers PA, Karr CJ, Koehoorn M, Lencar C, Tamburic L, et al. Effect of early life exposure to air pollution on development of childhood asthma. Environ Health Perspect. (2010) 118(2):284–90. doi: 10.1289/ehp.0900916
29. Liu W, Huang C, Hu Y, Fu Q, Zou Z, Sun C, et al. Associations of gestational and early life exposures to ambient air pollution with childhood respiratory diseases in Shanghai, China: a retrospective cohort study. Environ Int. (2016) 92-93:284–93. doi: 10.1016/j.envint.2016.04.019
30. Wi CI, Gent JF, Bublitz JT, King KS, Ryu E, Sorrentino K, et al. Paired indoor and outdoor nitrogen dioxide associated with childhood asthma outcomes in a mixed rural-urban setting: a feasibility study. J Prim Care Community Health. (2023) 14:21501319231173813. doi: 10.1177/21501319231173813
31. Buteau S, Shekarrizfard M, Hatzopolou M, Gamache P, Liu L, Smargiassi A. Air pollution from industries and asthma onset in childhood: a population-based birth cohort study using dispersion modeling. Environ Res. (2020) 185:109180. doi: 10.1016/j.envres.2020.109180
32. Huang W, Wu J, Lin X. Ozone exposure and asthma attack in children. Front Pediatr. (2022) 10:830897. doi: 10.3389/fped.2022.830897
33. Tétreault LF, Doucet M, Gamache P, Fournier M, Brand S, Kosatsky T, et al. Childhood exposure to ambient air pollutants and the onset of asthma: an administrative cohort study in Quebec. Environ Health Perspect. (2016) 124(8):1276–82. doi: 10.1289/ehp.1509838
34. To T, Zhu J, Stieb D, Gray N, Fong I, Pinault L, et al. Early life exposure to air pollution and incidence of childhood asthma, allergic rhinitis and eczema. Eur Respir J. (2020) 55(2):1900913. doi: 10.1183/13993003.00913-2019
35. Demirel G, Özden O, Döğeroğlu T, Gaga EO. Personal exposure of primary school children to BTEX, NO(2) and ozone in eskisehir, Turkey: relationship with indoor/outdoor concentrations and risk assessment. Sci Total Environ. (2014) 473-474:537–48. doi: 10.1016/j.scitotenv.2013.12.034
36. Salonen H, Salthammer T, Morawska L. Human exposure to NO2 in school and office indoor environments. Environ Int. (2019) 130:104887. doi: 10.1016/j.envint.2019.05.081
37. Spengler JD, Schwab M, McDermott A, Lambert WE, Samet JM. Nitrogen dioxide and respiratory illness in children. Part IV: effects of housing and meteorologic factors on indoor nitrogen dioxide concentrations. Res Rep Health Eff Inst. (1996) (58):1–29. discussion 31-6.9063844
38. Gent JF, Holford TR, Bracken MB, Plano JM, McKay LA, Sorrentino KM, et al. Childhood asthma and household exposures to nitrogen dioxide and fine particles: a triple-crossover randomized intervention trial. J Asthma. (2023) 60(4):744–53. doi: 10.1080/02770903.2022.2093219
39. Garcia E, Berhane KT, Islam T, McConnell R, Urman R, Chen Z, et al. Association of changes in air quality with incident asthma in children in California, 1993–2014. JAMA. (2019) 321(19):1906–15. doi: 10.1001/jama.2019.5357
40. Takenoue Y, Kaneko T, Miyamae T, Mori M, Yokota S. Influence of outdoor NO2 exposure on asthma in childhood: meta-analysis. Pediatr Int. (2012) 54(6):762–9. doi: 10.1111/j.1442-200X.2012.03674.x
41. Huang Q, Ren Y, Liu Y, Liu S, Liu F, Li X, et al. Associations of gestational and early life exposure to air pollution with childhood allergic rhinitis. Atmos Environ. (2019) 200:190–6. doi: 10.1016/j.atmosenv.2018.11.055
42. Smargiassi A, Kosatsky T, Hicks J, Plante C, Armstrong B, Villeneuve PJ, et al. Risk of asthmatic episodes in children exposed to sulfur dioxide stack emissions from a refinery point source in Montreal, Canada. Environ Health Perspect. (2009) 117(4):653–9. doi: 10.1289/ehp.0800010
43. Orellano P, Quaranta N, Reynoso J, Balbi B, Vasquez J. Effect of outdoor air pollution on asthma exacerbations in children and adults: systematic review and multilevel meta-analysis. PLoS One. (2017) 12(3):e0174050. doi: 10.1371/journal.pone.0174050
44. Schildcrout JS, Sheppard L, Lumley T, Slaughter JC, Koenig JQ, Shapiro GG. Ambient air pollution and asthma exacerbations in children: an eight-city analysis. Am J Epidemiol. (2006) 164(6):505–17. doi: 10.1093/aje/kwj225
45. Reno AL, Brooks EG, Ameredes BT. Mechanisms of heightened airway sensitivity and responses to inhaled SO2 in asthmatics. Environ Health Insights. (2015) 9(Suppl 1):13–25. doi: 10.4137/EHI.S15671
46. Kim SH, Lee J, Oh I, Oh Y, Sim CS, Bang JH, et al. Allergic rhinitis is associated with atmospheric SO2: follow-up study of children from elementary schools in Ulsan, Korea. PLoS One. (2021) 16:e0248624. doi: 10.1371/journal.pone.0248624
47. Manisalidis I, Stavropoulou E, Stavropoulos A, Bezirtzoglou E. Environmental and health impacts of air pollution: a review. Front Public Health. (2020) 8:14. doi: 10.3389/fpubh.2020.00014
48. Zhang Y, Steiner AL. Projected climate-driven changes in pollen emission season length and magnitude over the continental United States. Nat Commun. (2022) 13(1):1234. doi: 10.1038/s41467-022-28764-0
49. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the world health organization, GA(2)LEN and AllerGen). Allergy. (2008) 63(Suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x
50. Schober A, Tizek L, Johansson EK, Ekebom A, Wallin JE, Buters J, et al. Monitoring disease activity of pollen allergies: what crowdsourced data are telling US. World Allergy Organ J. (2022) 15(12):100718. doi: 10.1016/j.waojou.2022.100718
51. Graf N, Johansen P, Schindler C, Wüthrich B, Ackermann-Liebrich U, Gassner M, et al. Analysis of the relationship between pollinosis and date of birth in Switzerland. Int Arch Allergy Immunol. (2007) 143(4):269–75. doi: 10.1159/000100572
52. Kuwabara Y, Nii R, Tanaka K, Ishii E, Nagao M, Fujisawa T. Season of birth and risk of atopic disease among children and adolescents. J Asthma. (2007) 44(4):257–60. doi: 10.1080/02770900701246832
53. Kemp A, Ponsonby AL, Dwyer T, Cochrane J, Pezic A, Carmichael A, et al. The interaction between early life upper respiratory tract infection and birth during the pollen season on rye-sensitized hay fever and ryegrass sensitization – a birth cohort study. Pediatr Allergy Immunol. (2009) 20(6):536–44. doi: 10.1111/j.1399-3038.2008.00817.x
54. Kihlström A, Lilja G, Pershagen G, Hedlin G. Exposure to birch pollen in infancy and development of atopic disease in childhood. J Allergy Clin Immunol. (2002) 110(1):78–84. doi: 10.1067/mai.2002.125829
55. Kihlström A, Lilja G, Pershagen G, Hedlin G. Exposure to high doses of birch pollen during pregnancy, and risk of sensitization and atopic disease in the child. Allergy. (2003) 58(9):871–7. doi: 10.1034/j.1398-9995.2003.00232.x
56. Kitinoja MA, Hugg TT, Siddika N, Yanez DR, Jaakkola MS, Jaakkola JJK. Short-term exposure to pollen and the risk of allergic and asthmatic manifestations: a systematic review and meta-analysis. BMJ Open. (2020) 10(1):e029069. doi: 10.1136/bmjopen-2019-029069
57. Rabinovitch N, Liu AH, Zhang L, Rodes CE, Foarde K, Dutton SJ, et al. Importance of the personal endotoxin cloud in school-age children with asthma. J Allergy Clin Immunol. (2005) 116(5):1053–7. doi: 10.1016/j.jaci.2005.08.045
58. Hoopmann M, Hehl O, Neisel F, Werfel T. Associations between bioaerosols coming from livestock facilities and asthmatic symptoms in children. Gesundheitswesen. (2006) 68(8-9):575–84. doi: 10.1055/s-2006-926987
59. Matsui EC, Hansel NN, Aloe C, Schiltz AM, Peng RD, Rabinovitch N, et al. Indoor pollutant exposures modify the effect of airborne endotoxin on asthma in urban children. Am J Respir Crit Care Med. (2013) 188(10):1210–5. doi: 10.1164/rccm.201305-0889OC
60. Delfino RJ, Staimer N, Tjoa T, Gillen DL. Relations of exhaled nitric oxide and FEV1 to personal endotoxin exposure in schoolchildren with asthma. Occup Environ Med. (2015) 72(12):830–6. doi: 10.1136/oemed-2014-102651
61. Lai PS, Sheehan WJ, Gaffin JM, Petty CR, Coull BA, Gold DR, et al. School endotoxin exposure and asthma morbidity in inner-city children. Chest. (2015) 148(5):1251–8. doi: 10.1378/chest.15-0098
62. Celedón JC, Milton DK, Ramsey CD, Litonjua AA, Ryan L, Platts-Mills TAE, et al. Exposure to dust mite allergen and endotoxin in early life and asthma and atopy in childhood. J Allergy Clin Immunol. (2007) 120(1):144–9. doi: 10.1016/j.jaci.2007.03.037
63. Lawson JA, Dosman JA, Rennie DC, Beach JR, Newman SC, Crowe T, et al. Endotoxin as a determinant of asthma and wheeze among rural dwelling children and adolescents: a case–control study. BMC Pulm Med. (2012) 12:1–10. doi: 10.1186/1471-2466-12-56
64. Rylander R, Lin RH. (1–>3)-beta-D-glucan - relationship to indoor air-related symptoms, allergy and asthma. Toxicology. (2000) 152(1-3):47–52. doi: 10.1016/S0300-483X(00)00291-2
65. Chew GL, Douwes J, Doekes G, Higgins KM, van Strien R, Spithoven J, et al. Fungal extracellular polysaccharides, beta (1–>3)-glucans and culturable fungi in repeated sampling of house dust. Indoor Air. (2001) 11(3):171–8. doi: 10.1034/j.1600-0668.2001.011003171.x
66. Iossifova Y, Reponen T, Sucharew H, Succop P, Vesper S. Use of (1-3)-beta-d-glucan concentrations in dust as a surrogate method for estimating specific fungal exposures. Indoor Air. (2008) 18(3):225–32. doi: 10.1111/j.1600-0668.2008.00526.x
67. Karadag B, Ege MJ, Scheynius A, Waser M, Schram-Bijkerk D, van Hage M, et al. Environmental determinants of atopic eczema phenotypes in relation to asthma and atopic sensitization. Allergy. (2007) 62(12):1387–93. doi: 10.1111/j.1398-9995.2007.01505.x
68. Gehring U, Heinrich J, Hoek G, Giovannangelo M, Nordling E, Bellander T, et al. Bacteria and mould components in house dust and children’s allergic sensitisation. Eur Respir J. (2007) 29(6):1144–53. doi: 10.1183/09031936.00118806
Keywords: early life, air pollution, pollen, asthma, allergies
Citation: Melaram R (2024) Early life exposures of childhood asthma and allergies—an epidemiologic perspective. Front. Allergy 5:1445207. doi: 10.3389/falgy.2024.1445207
Received: 6 June 2024; Accepted: 2 August 2024;
Published: 23 August 2024.
Edited by:
João Cavaleiro Rufo, University Porto, PortugalReviewed by:
Inês Paciência, University of Oulu, FinlandCopyright: © 2024 Melaram. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rajesh Melaram, cmFqZXNoLm1lbGFyYW1AdGFtdWNjLmVkdQ==
 Rajesh Melaram
Rajesh Melaram