- 1NIHR Leicester Biomedical Research Centre, Department of Respiratory Sciences, Glenfield Hospital, Leicester, United Kingdom
- 2Leicester School of Allied Health Sciences, Faculty of Health and Life Sciences, De Montfort University, Leicester, United Kingdom
Sputum induction is a technique that covers the induction and the subsequent processing of the expectorate primarily for the analysis of cells and different inflammatory biomarkers present in the airways to further understand the pathophysiology of different inflammatory respiratory disorders such as asthma and chronic obstructive pulmonary disease (COPD) as well as the diagnosis of lung diseases such as lung cancer, tuberculosis, and Pneumocystis jirovecii pneumonia. It is a non-invasive, safe, cost-effective, and reliable technique reported to exhibit a high success rate. However, due to being technically demanding and time-consuming and having the need of employing trained staff, this technique is only used in restricted research centres and in limited centres of clinical use. When the sputum is collected after induction, the primary goal is to obtain a differential cell count and evaluate the molecular biomarkers of airway inflammation such as eosinophil cationic protein, eosinophil-derived neurotoxin, major basic protein, tryptase, cytokine production [e.g., interleukin (IL)-5], albumin, and fibrinogen. In addition, cytospins from the processed sputum are used for immunocytochemical staining of cellular products such as EG-2 reactive protein, granulocyte-macrophage colony-stimulating factor, tumour necrosis factor alpha, and IL-8 that play significant roles in understanding the pathophysiology of inflammatory airway diseases. Nowadays, this technique can be further used by performing an additional analysis such as flow cytometry and in situ hybridisation on the sputum supernatant to investigate more the immune response and pathophysiological process of such various respiratory diseases. In addition, the application of sputum fluid phase to assess the biomarkers could be used more routinely in pathological laboratories for diagnosing lung cancer, COPD, and asthma as well as for monitoring lung cancer progression and asthma and COPD treatment, allowing for early detection and a better treatment provided by the clinicians.
Introduction
Airway diseases are a major problem in today's society that are significantly increasing due to climate change and affecting the lives of many individuals around the world and the healthcare services (1). The main airway diseases include asthma and chronic obstructive pulmonary disease (COPD). Both diseases are characterised with exacerbations and airway remodelling. Most importantly, in both pathologies, airway inflammation is considered their primary cause. It has been shown that improved understanding, management, and treatment of these respiratory diseases lie with the measurement of airway inflammation and, in some cases, the associated microbial infections (1–3).
Several methods to study airway inflammation, namely, direct methods such as bronchoalveolar lavage (BAL) and bronchial biopsies and indirect methods such as blood analysis, lung function tests, and even symptom assessment, were identified (4). On the one hand, the direct methods are most commonly used compared with the indirect ones as these techniques generate more reliable results. However, they are invasive, expensive procedures, and they cannot be repeatedly performed due to its invasive nature; therefore, it is not feasible to be performed in large-scale clinical studies (5). In addition, both methods sample different parts of the airways. Bronchoscopy samples cells and mediators that are present in the lumen of the airways and therefore enable the mucosal tissue to be biopsied. BAL mainly allows the distal part of the bronchus to be sampled. However, mixing of the different lung compartments can also occur, which do not allow an accurate result of the sample obtained. Furthermore, the mediators and cells obtained in the samples are most often diluted in large amounts of saline, and blood contamination in this procedure can also occur. On the other hand, the indirect methods are inexpensive and not invasive. However, the results obtained using these techniques do not correlate well with the direct assessment of airway inflammation (4, 5).
Due to the limitations of these techniques, another direct measurement of airway inflammation has been developed, called sputum collection. Sputum collection involves sputum production either spontaneously or by induction and subsequent processing of the sputum. Spontaneous sputum was routinely used in the past. However, research showed that most of the samples obtained were of poor quality and not every patient was able to produce sputum (6–9). In 1958, Bickerman et al. (10) first used sputum induction for diagnosing lung cancer by making patients inhale hypertonic saline to produce sputum in order to overcome this limitation. Then, later in 1986, Pitchenik et al. (11) used the same technique to diagnose Pneumocystis carinii (now Pneumocystis jirovecii) pneumonia in patients that were infected with HIV and in patients with AIDS.
Pin et al. (12) published the first study of sputum induction using hypertonic saline as a method to study airway inflammation in patients with asthma, and since this first successful attempt, different researchers have reported using sputum induction to study airway inflammation not only in asthma but also in other respiratory disorders such as COPD and chronic cough (9, 13). For the wide use of these techniques and for the comparison of the results of the different published papers, this method became standardised in 1999 by the task force that was approved by the European Respiratory Society (13). Standardisation of this technique not only enabled data to be globally compared but also allowed the quality and the reproducibility of sputum samples to be improved (14). Consequently, allowing sputum induction and its successive processing to become an important non-invasive research and clinical tool for the assessment of airway inflammation and for the discovery of novel new therapeutics.
Sputum induction is non-invasive and less costly compared with the other techniques (15). In addition, it can be performed as required and repeatedly in patients regardless of the severity of their disease and even during exacerbations, making this technique appropriate for large studies and clinical trials with multiple visits. Although it can cause bronchospasm in patients with hyperresponsive airway, this can be overcome if patients are given a short-acting beta-agonist before the technique. It has been demonstrated that sputum induction is a safe, successful, reproducible, and reliable method, making it a useful tool in the assessment of airway inflammation (6–9, 14, 16).
Generally, sputum expectoration is induced; therefore, it can be collected and processed to obtain a differential cell count and subsequently measure the type of inflammation present in the lumen of the airways. Different methods currently used in literature include the following: plug selection method, whole sputum method, phosphate-buffered saline (PBS)-treated method, and traditional sputum processing method using dithiothreitol (DTT). This paper reviews the different methodology for sputum induction and laboratory processing in literature and the different diagnostic applications of sputum induction.
Sputum induction
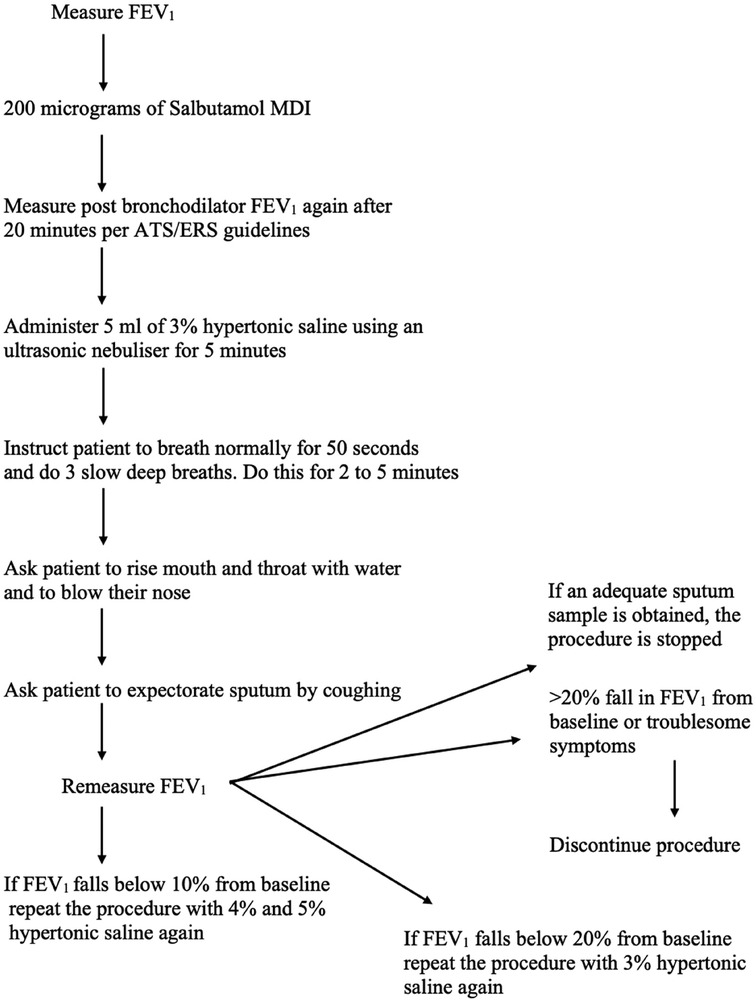
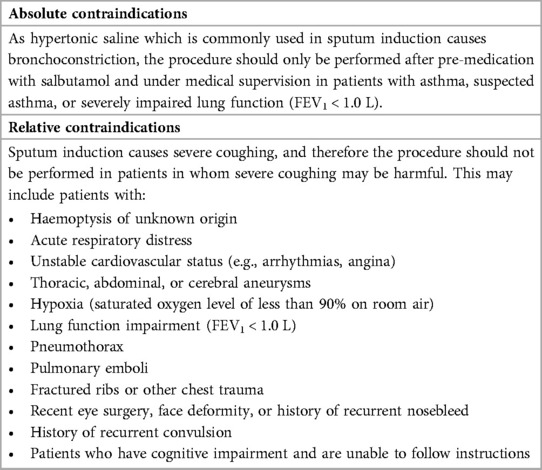
Sputum induction is performed by inhalation of isotonic or hypertonic saline solution using ultrasonic nebulizers which is followed by the expectoration of airway secretions by coughing (Table 1). Before this technique is performed, the lung function of the patient needs to be measured using a spirometry. This needs to be conducted due to the fact that hypertonic saline inhalation by asthmatic patients causes bronchoconstriction (17). Spirometry is used over measuring peak flow because they offer greater sensitivity of forced expiratory volume in 1 s (FEV1) to detect bronchoconstriction. It is recommended that short-acting beta-2-agonist should always be administered as a standard protocol before treatment (18). The induction must be conducted under medical supervision, performed by trained technicians, and a physician needs to be always present during the process in case any adverse events occur during the procedure. Approximately 200–400 µg of salbutamol is given using a pressurised metered-dose inhaler (pMDI) as pre-treatment to all the patients as broncho-protection against saline causing no affect in cell counts and inflammatory markers (19).
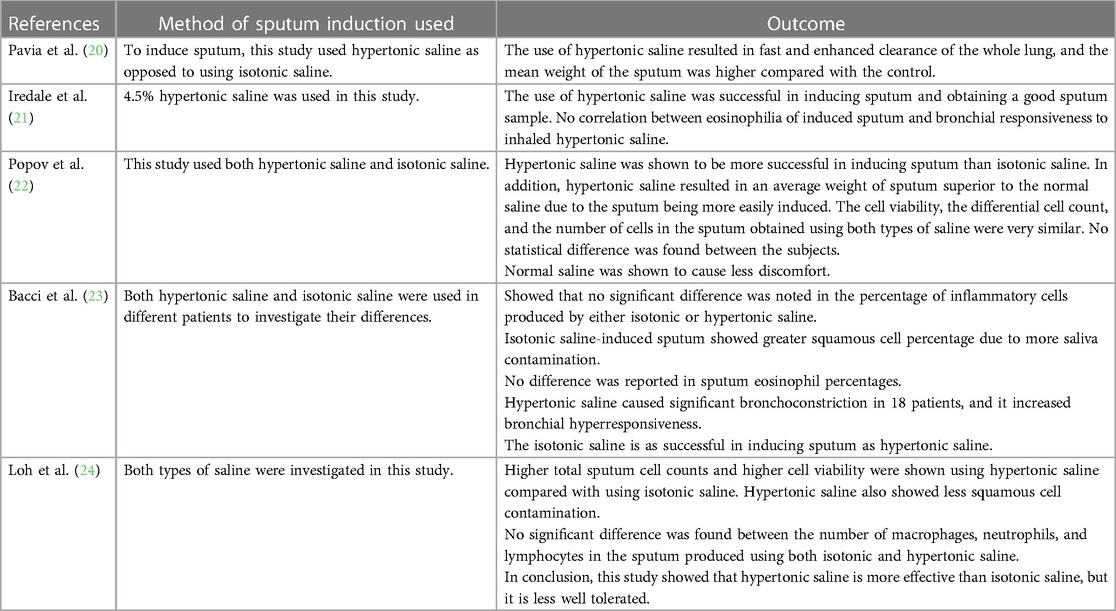
Saline concentrations may vary from 0.9% to 7% in different studies. However, 4.5% of sodium chloride concentration is the recommended value for general use (Figure 1). One study showed that no significant difference was found in the cellular composition and in the differential cell count of the induced sputum when using isotonic or hypertonic saline for the induction (25). However, different research showed that hypertonic saline is more effective in inducing sputum (26). During the procedure, different parts of the airways are sampled; firstly, the central airway, then the peripheral airway, and lastly the alveoli are sampled, and this occurs during different points of the induction. Shorter durations (15–20 min) and longer periods (about 30 min) of inhalation seem to exhibit the same success rate and feasibility in sputum production (26). However, Chanez et al. stated that the consensus is to use a cumulative duration of 15–20 min of nebulisation with the patient being asked to cough and expectorate every 5 min and in each period the lung function being measured for detection of bronchoconstriction (27).
Induced sputum processing
An expert consensus-based recommendation was published in 2002 by the European Respiratory Task Force (ERTF) in which they formulate the basic rules for the use of sputum processing technique in adults and in children (13). Two main protocols are used in literature for processing the expectorate: the whole sputum method (Figure 2) and the selected plug method (Figure 3); both of these methods are shown to be valid and reproducible by various studies (22, 28). However, a deviation of these methods not related to how the sputum is selected (whole or plugs) but on how the sputum is treated after selection has been developed: the PBS method and the non-PBS method.
The whole sputum processing method has been first described by Fahy et al. (28), and it comprises the selection of all sputum samples, such as selection of the mucus plugs that are opaque and dense and the clear surrounding saliva. On the other hand, the plug selection method, described by Popov et al. (22), is the selection of only the opaque or coloured mucus plugs for the whole sputum sample to reduce saliva contamination. The methods may differ on how the sputum sample is selected; however, the rest of the protocol is the same for both if the non-PBS method is performed. The sputum needs to be processed for no longer than 3 h of the sample being collected to ensure that the cell viability is maintained. DTT is used to treat the sample to allow complete homogenisation and to ensure that cells are released and dispersed (Figure 2). In the non-PBS method, DTT is used first, but in the PBS method, the PBS is used first to wash the cells to obtain a supernatant that is further employed for the analysis of various inflammatory mediators such as interleukins present in the supernatant (29). The cellular and biochemical analysis of the sputum is conducted in research using the PBS method; however, in the clinical setting, the non-PBS method that does not collect any supernatants is used (13).
Centrifugation is needed in both methods to obtain the supernatant, PBS and DTT, for the analysis of the sputum; different studies used different centrifugal forces, but they all range from 300 ×g to 1,500 ×g and last for about 10 min (13). Different studies use different stains for differential cell counts, but all have in common in the determination of non-squamous cells, meaning the counting of eosinophils, neutrophils, macrophages, and lymphocytes. Spanevello et al. (30) showed that the method of selecting sputum plugs is a more advantageous technique over the whole sputum sample as the cell viability percentage, total cell count percentage, is higher than the whole sputum method. However, it was also demonstrated that both methods have the same ability to distinguish individuals with asthma from healthy individuals (30, 31). The whole sputum method provides a faster processing compared with the selected method, but the saliva contamination in the whole sputum samples causes an increase in squamous cell contamination that decreases the quality of the sample of the cytospins (12, 31). A limitation of the selected sputum method is that not all samples obtained will contain sputum plugs that cause a limitation in the sample processing, and the plugs are not representative of all the samples.
Applications of induced sputum
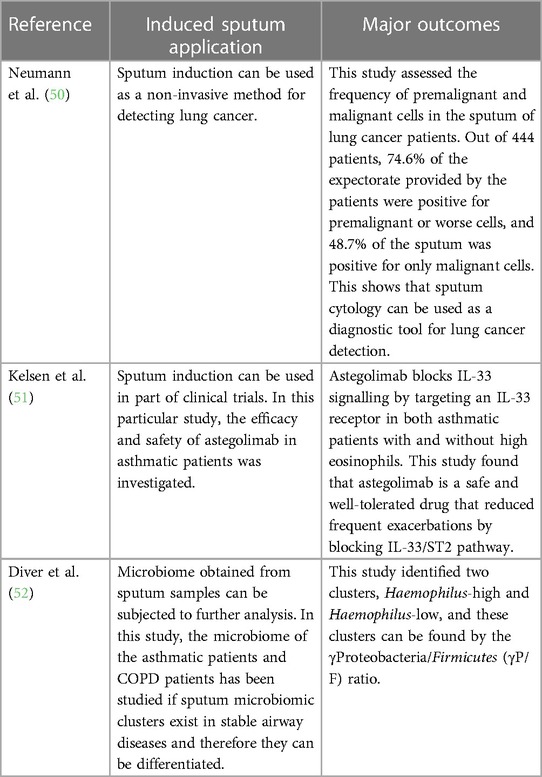
Induced sputum has been clinically used in a variety of different ways, such as the detection of lung cancer, management of asthma in asthmatic patients by observing changes in sputum eosinophils, phenotyping of different types of asthma, and assessment of airway inflammation in COPD patients (Table 2). In asthma, this technique has been used as a diagnostic tool by performing sputum eosinophil count as asthma is an airway disease associated with sputum eosinophilia. In addition, induced sputum has enabled the assessment of short-term and long-term response of patients with asthma to inhaled corticosteroids providing evidence that corticosteroids have a moderate impact on improving sputum eosinophils and symptoms (8, 32). This finding allowed the use of induced sputum for the assessment of airway inflammation in response to different drugs and therefore allowed for a more personalised treatment as different phenotypes of asthma respond differently to different medications. Cytospins from processed sputum are used for immunocytochemical staining of cellular products such as EG-2 reactive protein, granulocyte-macrophage colony-stimulating factor (GM-CSF), tumour necrosis factor alpha (TNF-α), and interleukin (IL)-8 (8, 33). Similarly, the supernatants obtained from the processed sputum are used in evaluating molecular markers of airway inflammation such as eosinophil cationic protein (ECP), eosinophil-derived neurotoxin, and major basic protein for eosinophil activation, tryptase for mast cell activation, and cytokine production (e.g., IL-5) as well as albumin and fibrinogen that are useful markers of microvascular leakage (6, 8). The levels of these molecular markers were raised in the processed sputum from asthmatic patients than from control subjects (3, 6, 33–35).
Correspondingly, Eltboli and Brightling (36) showed that in COPD patients, induced sputum can be used to assess airway inflammation for individuals suffering from the disease by performing differential cell counts and therefore allowing for a more tailored treatment and management and in diagnosing chronic lung diseases. A raised sputum neutrophilia combined with high sputum levels of TNF-α and IL-8 has been linked with COPD and is suggested as a potential marker for the diagnosis of COPD (2, 37). Kirsch et al. (38) showed that the analysis of induced sputum can be used in diagnosing Pneumocystis carinii (currently named Pneumocystis jirovecii) pneumonia (38, 39). In addition, induced sputum can be used as a diagnostic tool in detecting pulmonary tuberculosis (40–43), pulmonary sarcoidosis (44, 45), and lung cancer (46–49). Therefore, the introduction of this method in pathological laboratories will provide an additional tool for diagnosing different pulmonary diseases (53).
In research, several techniques can be applied on induced sputum such as DNA extraction on microbiome obtained for the sputum sample, enzyme-linked immunosorbent assay (ELISA), flow cytometry, and in situ hybridisation to further understand the underlying airway inflammation and the role of different cells and cytokines in different airway diseases for better treatment and management. Furthermore, induced sputum has been used in various clinical trials to test new drugs for the treatment of asthma. In a study by Russell and Brightling (54), sputum induction was used as a method to support in the development of a new therapy (mepolizumab) that targets IL-5 to help with asthma exacerbations. The emergence of proteomics, lipidomics, metabolomics, exosomics, exposomics, transcriptomics, functional assays, whole genome sequencing, genome-wide sequencing, microRNA assays, and bioinformatics tools (55) as well as data science, artificial intelligence, and machine learning (52) will further revolutionalise the usefulness of induced sputum as both a diagnostic and research tool in understanding the pathophysiology and diagnosis and for monitoring the treatment progress of asthma, COPD, lung cancer, and pulmonary tuberculosis.
Concluding remarks
As discussed in the previous sections, sputum induction and processing is a well-tolerated, safe, and non-invasive method for the collection and analysis of cells from the airways making it an important procedure for the diagnosis of various respiratory diseases such as asthma, COPD, chronic cough, or idiopathic pulmonary fibrosis. However, the technique is currently limited to research services and specialised centres in clinical practice because it is technically demanding and time-consuming and requires trained staff. In the laboratory in which BG performed her placement, the procedure is generally used for the administration of research and clinical trials. A patient is initially prepared, and spirometry [forced expiratory volume in 1 s (FEV1)] is performed according to the standard criteria formulated by the American Thoracic Society (ATS)/European Respiratory Society (ERS) (13). Sputum is induced by inhalation of 3% hypertonic saline solution (for patients with post-bronchodilator FEV1 of >65% predicted) or 0.9% isotonic saline (for patients with post-bronchodilator FEV1 of ≤65% predicted) using an ultrasonic nebuliser after the administration of 400 µg of inhaled salbutamol from a metered-dose inhaler (MDI) via a spacer or an equivalent dose of other inhaled short-acting β2-agonist. The nebulisation and sputum collection are performed under medical supervision. Sputum samples are collected on ice and processed following the procedure in Figure 3 within 2 h of expectoration to ensure optimum cell viability. However, at the peak of the COVID-19 pandemic, spontaneously expectorated sputum was mainly used during a stable clinical state for the health and safety of the research administrators and other patients since sputum induction posed a major risk of increasing aerosol transmission.
In a recent prospective internally controlled interventional trial carried out at the Children's Hospital for Wales (Cardiff, UK) in children with cystic fibrosis, it was reported that sputum induction is superior to cough swab for pathogen detection, is effective at sampling the lower airway, and is a credible surrogate for bronchoalveolar lavage in symptomatic children (56). In the study, 124 patients were prospectively recruited, and 84% of the sputum induction were successful; the sputum induction procedures was well tolerated by the patients (56). In a study by Guiot et al. (5), the success rate of sputum induction and a readable cytospin was 75% in healthy subjects, 82% in patients with asthma, and 82% in COPD patients, with an overall success rate of 82%. In addition, Pin et al. (12) reported a success rate of 77% in the first attempts and 84% in the second attempts among 17 asthmatic and 17 healthy patients. Other researchers have reported success rates of 80%–91% in adults and children (57–59), 81% in healthy adult subjects (60), and 80% in patients with asthma (61). However, Vieira et al. (62) reported that sputum induction in patients with severe exacerbations of asthma using a modified method was successful in 93% of subjects. In their study, Vieira et al. (62) performed sputum induction in 45 patients after pre-treatment with 400 mg salbutamol by inhalation, for repeated periods of 1–2 min, of an aerosol of isotonic saline only or followed by hypertonic (3%–4%) saline. They recommended that sputum induction can be successful and safe even in severe exacerbations of asthma if this modified method is carefully followed.
Although sputum induction is generally regarded as safe and successful in both children and adults (63–70), recommended contraindications that must be taken into account to preclude or delay induced sputum collection are noted (Table 3). After exclusion or resolution of the contraindicated conditions, sputum induction can be considered (71). Similarly, at the peak of the COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a multidisciplinary consensus on sputum induction biosafety was issued since sputum induction is associated with the generation of aerosols, cough manoeuvres, and the handling of sputum samples (73). The conditions shown in Table 4 were regarded as contraindications for sputum induction during the COVID-19 pandemic.
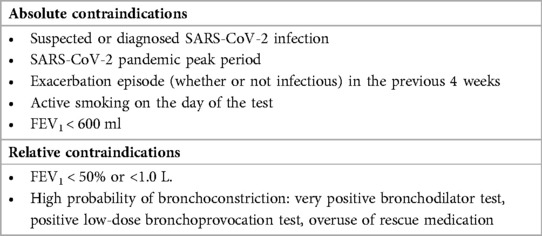
Table 4. Contraindications for sputum induction during the COVID-19 pandemic (73).
However, it has been recommended that sputum induction by the inhalation of lower concentration of the isotonic saline, hypertonic saline, or dry mannitol powder using an ultrasonic nebulizer are safe for patients with relative contraindications (73). In a comparative assessment of the use of hypertonic (4.5%) saline and a mannitol in 55 subjects with stable asthma, induced sputum was successfully obtained from 49 (89%) subjects using the hypertonic saline solution and 42 (76%) subjects challenged with mannitol solution (74). In a different clinical trial consisting of 592 participants, it was demonstrated that mannitol dry powder had a sensitivity of 81% and a specificity of 87% with respect to a cut-off of 15% fall in FEV1 for 4.5% hypertonic saline (75). It was further demonstrated that inhalation of dry mannitol powder had a sensitivity of up to 89% identifying the presence of asthma and a specificity of 95% for clinical diagnosis of asthma (75). A previous study had also shown a correlation between 15% fall in FEV1 for mannitol and hypertonic saline (76). This further corroborates the safety of sputum induction using the inhaled mannitol powder and hypertonic saline in detecting airway hyperresponsiveness, in obtaining good-quality sputum for the analysis of inflammatory cells and inflammatory mediators, and for predicting the inflammatory phenotype in individual patients with asthma (73–77). It is worth noting that well-trained staff in hospitals can successfully perform sputum induction with good-quality sputum for diagnostic applications; therefore, readers are encouraged to conduct sputum induction as this is now generally considered safe and useful results obtained for the management of patients with airway disorders.
Sputum induction and the subsequent processing of sputum are useful methods to assess the airways and have various applications from diagnosis to developing more target therapies compared with other methods. Even with limitations and with only few laboratories that are able to use this technique due to being time-consuming and having the need of employing trained workforce, it should be made more accessible and be put more into a clinical setting by all medical centres. In the future, sputum induction can be used more widely in research to provide further information on the mechanisms, both cellular and molecular, of the different airway diseases, so that the treatment and management is even more specific towards different patients and therefore more effective than the treatments currently available. In addition, the use of sputum fluid phase in the assessment of biomarkers could be used more routinely in pathological laboratories for the diagnosis of lung cancer and for monitoring cancer progression, allowing for early detection and a better treatment provided by the clinicians.
Author contributions
BG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, and Writing – original draft. UE: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, and Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors are grateful to Dr. William Monteiro for all the support he rendered to BG during her placement period at the NIHR Leicester Biomedical Research Centre, Department of Respiratory Sciences, in Glenfield Hospital, Leicester, UK.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Athanazio R. Airway disease: similarities and differences between asthma, COPD and bronchiectasis. Clinics. (2012) 67:1335–43. doi: 10.6061/clinics/2012(11)19
2. Venegas C, Zhao N, Ho T, Nair P. Sputum inflammometry to manage chronic obstructive pulmonary disease exacerbations: beyond guidelines. Tuberc Respir Dis (Seoul). (2020) 83(3):175. doi: 10.4046/trd.2020.0033
3. Pandey R, Parkash V, Kant S, Verma AK, Sankhwar SN, Agrawal A, et al. An update on the diagnostic biomarkers for asthma. J Family Med Prim Care. (2021) 10(3):1139–48. doi: 10.4103/jfmpc.jfmpc_2037_20
4. Jayaram L, Parameswaran K, Sears MR, Hargreave FE. Induced sputum cell counts: their usefulness in clinical practice. Eur Respir J. (2000) 16(1):150–8. doi: 10.1034/j.1399-3003.2000.16a27.x
5. Guiot J, Demarche S, Henket M, Paulus V, Graff S, Schleich F, et al. Methodology for sputum induction and laboratory processing. J Vis Exp. (2017) 130:e56612. doi: 10.3791/56612
6. Pizzichini E, Pizzichini MM, Efthimiadis A, Evans S, Morris MM, Squillace D, et al. Indices of airway inflammation in induced sputum: reproducibility and validity of cell and fluid-phase measurements. Am J Respir Crit Care Med. (1996) 154(2):308–17. doi: 10.1164/ajrccm.154.2.8756799
7. Spanevello A, Migliori GB, Sharara A, Ballardlni L, Bridge P, Pisatt P, et al. Induced sputum to assess airway inflammation: a study of reproducibility. Clin Exp Allergy. (1997) 27(10):1138–44. doi: 10.1111/j.1365-2222.1997.tb01150.x
8. Pavord ID, Pizzichini MM, Pizzichini E, Hargreave FE. The use of induced sputum to investigate airway inflammation. Thorax. (1997) 52(6):498–501. doi: 10.1136/thx.52.6.498
9. Kelly MG, Ennis M, Elborn JS. Safety and success rate of sputum induction and repeatability of sputum characteristics in patients with COPD. Thorax. (1999) 54:A71.
10. Bickerman HA, Sproul EE, Barach AL. An aerosol method of producing bronchial secretions in human subjects: a clinical technic for the detection of lung cancer. Dis Chest. (1958) 33(4):347–62. doi: 10.1378/chest.33.4.347
11. Pitchenik AE, Ganjei P, Torres A, Evans DA, Rubin E, Baier H. Sputum examination for the diagnosis of Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. Am Rev Respir Dis. (1986) 133(2):226–9. doi: 10.1164/arrd.1986.133.2.226
12. Pin I, Gibson PG, Kolendowicz R, Girgis-Gabardo A, Denburg JA, Hargreave FE, et al. Use of induced sputum cell counts to investigate airway inflammation in asthma. Thorax. (1992) 47(1):25–9. doi: 10.1136/thx.47.1.25
13. Djukanović R, Sterk PJ, Fahy JV, Hargreave FE. Standardised methodology of sputum induction and processing. Eur Respir J. (2002) 20(37 Suppl):1s–2s. doi: 10.1183/09031936.02.00000102
14. Brightling CE, Monterio W, Green RH, Parker D, Morgan MD, Wardlaw AJ, et al. Induced sputum and other outcome measures in chronic obstructive pulmonary disease: safety and repeatability. Respir Med. (2001) 95(12):999–1002. doi: 10.1053/rmed.2001.1195
15. Pizzichini MM, Leigh R, Djukanović R, Sterk PJ. Safety of sputum induction. Eur Respir J. (2002) 20(37 suppl):9s–18s. doi: 10.1183/09031936.02.00000902
16. Belda J, Parameswaran K, Hargreave FE. Comparison of two methods of processing induced sputum: selected versus entire sputum. Am J Respir Crit Care Med. (1998) 158(2):680–2. doi: 10.1164/ajrccm.158.2.15824
17. Smith CM, Anderson SD. Inhalation provocation tests using non-isotonic aerosols. J Allergy Clin Immunol. (1989) 84(5):781–90. doi: 10.1016/0091-6749(89)90309-6
18. Shimoda T, Obase Y, Kishikawa R, Shoji S, Nishima S. Comparison of central and peripheral airway inflammation between cough variant asthma and mild asthma by examination of hypertonic saline inhalation-induced sputum. J Allergy Clin Immunol. (2007) 119(1):S130. doi: 10.1016/j.jaci.2006.11.493
19. Delvaux M, Henket M, Lau L, Kange P, Bartsch P, Djukanovic R, et al. Nebulised salbutamol administered during sputum induction improves bronchoprotection in patients with asthma. Thorax. (2004) 59(2):111–5. doi: 10.1136/thorax.2003.011130
20. Pavia D, Thomson ML, Clarke SW. Enhanced clearance of secretions from the human lung after the administration of hypertonic saline aerosol. Am Rev Respir Dis. (1978) 117(2):199–203. doi: 10.1164/arrd.1978.117.2.199
21. Iredale MJ, Wanklyn SA, Phillips IP, Krausz T, Ind PW. Non-invasive assessment of bronchial inflammation in asthma: no correlation between eosinophilia of induced sputum and bronchial responsiveness to inhaled hypertonic saline. Clin Exp Allergy. (1994) 24(10):940–5. doi: 10.1111/j.1365-2222.1994.tb02725.x
22. Popov TA, Pizzichini MM, Pizzichini E, Kolendowicz R, Punthakee Z, Dolovich J, et al. Some technical factors influencing the induction of sputum for cell analysis. Eur Respir J. (1995) 8(4):559–65. doi: 10.1183/09031936.95.08040559
23. Bacci E, Cianchetti S, Paggiaro PL, Carnevali S, Bancalari L, Dente FL, et al. Comparison between hypertonic and isotonic saline-induced sputum in the evaluation of airway inflammation in subjects with moderate asthma. Clin Exp Allergy. (1996) 26(12):1395–400. doi: 10.1111/j.1365-2222.1996.tb00541.x
24. Loh LC, Eg KP, Puspanathan P, Tang SP, Yip KS, Vijayasingham P, et al. A comparison of sputum induction methods: ultrasonic vs. compressed-air nebulizer and hypertonic vs. isotonic saline inhalation. Asian Pac J Allergy Immunol. (2004) 22(1):11.15366653
25. Cataldo D, Foidart JM, Lau L, Bartsch P, Djukanovic R, Louis R. Induced sputum: comparison between isotonic and hypertonic saline solution inhalation in patients with asthma. Chest. (2001) 120(6):1815–21. doi: 10.1378/chest.120.6.1815
26. Bartoli ML, Bacci E, Carnevali S, Cianchetti S, Dente FL, Di Franco A, et al. Quality evaluation of samples obtained by spontaneous or induced sputum: comparison between two methods of processing and relationship with clinical and functional findings. J Asthma. (2002) 39(6):479–86. doi: 10.1081/JAS-120004907
27. Chanez P, Holz O, Ind PW, Djukanović R, Maestrelli P, Sterk PJ. Sputum induction. Eur Respir J. (2002) 20(37 suppl):3s–8s. doi: 10.1183/09031936.02.00000302
28. Fahy JV, Liu J, Wong H, Boushey HA. Cellular and biochemical analysis of induced sputum from asthmatic and from healthy subjects. Am Rev Respir Dis. (1993) 147:1126. doi: 10.1164/ajrccm/147.5.1126
29. Keatings V, Leigh R, Peterson C, Shute J, Venge P, Djukanović R. Analysis of fluid-phase mediators. Eur Respir J. (2002) 20(37 suppl):24s–39s. doi: 10.1183/09031936.02.00002402
30. Spanevello A, Beghe B, Bianchi A, Migliori GB, Ambrosetti M, Neri M, et al. Comparison of two methods of processing induced sputum: selected versus entire sputum. Am J Respir Crit Care Med. (1998) 157(2):665–8. doi: 10.1164/ajrccm.157.2.9705095
31. Spanevello A, Confalonieri M, Sulotto F, Romano F, Balzano G, Migliori GB, et al. Induced sputum cellularity: reference values and distribution in normal volunteers. Am J Respir Crit Care Med. (2000) 162(3):1172–4. doi: 10.1164/ajrccm.162.3.9908057
32. Green RH, Brightling CE, Woltmann G, Parker D, Wardlaw AJ, Pavord ID. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax. (2002) 57(10):875–9. doi: 10.1136/thorax.57.10.875
33. Girgis-Gabardo A, Kanai N, Denburg JA, Hargreave FE, Jordana M, Dolovich J. Immunocytochemical detection of granulocyte-macrophage colony-stimulating factor and eosinophil cationic protein in sputum cells. J Allergy Clin Immunol. (1994) 93(5):945–7. doi: 10.1016/0091-6749(94)90390-5
34. Dawood HN, Alwan JB, Khalaf SA. The eosinophilic and neutrophilic counts in sputum of asthmatic Iraqi patients and its correlation with asthma control. Mustansiriya Med J. (2020) 19(1):11. doi: 10.4103/MJ.MJ_3_20
35. Caballero ML, Dominguez-Ortega J, Nin-Valencia AR, Sánchez-Ocando H, Barranco P. Eosinophil count in induced sputum could be more sensitive than in peripheral blood to phenotype patients with severe eosinophilic asthma. J Investig Allergol Clin Immunol. (2021) 31(4):360–61. doi: 10.18176/jiaci.0647
36. Eltboli O, Brightling CE. Eosinophils as diagnostic tools in chronic lung disease. Expert Rev Respir Med. (2013) 7(1):33–42. doi: 10.1586/ers.12.81
37. Keatings VM, Collins PD, Scott DM, Barnes PJ. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med. (1996) 153(2):530–4. doi: 10.1164/ajrccm.153.2.8564092
38. Kirsch CM, Azzi RL, Yenokida GG, Jensen WA. Analysis of induced sputum in the diagnosis of Pneumocystis carinii pneumonia. Am J Med Sci. (1990) 299(6):386–91. doi: 10.1097/00000441-199006000-00006
39. LaRocque RC, Katz JT, Perruzzi P, Baden LR. The utility of sputum induction for diagnosis of Pneumocystis pneumonia in immunocompromised patients without human immunodeficiency virus. Clin Infect Dis. (2003) 37(10):1380–3. doi: 10.1086/379071
40. Bell D, Leckie V, McKendrick M. The role of induced sputum in the diagnosis of pulmonary tuberculosis. J Infect. (2003) 47(4):317–21. doi: 10.1016/S0163-4453(03)00093-8
41. Bothamley GH, Ditiu L, Migliori GB, Lange C. Active case finding of tuberculosis in Europe: a Tuberculosis Network European Trials Group (TBNET) survey. Eur Respir J. (2008) 32(4):1023–30. doi: 10.1183/09031936.00011708
42. Biswas S, Das A, Sinha A, Das SK, Bairagya TD. The role of induced sputum in the diagnosis of pulmonary tuberculosis. Lung India. (2013) 30(3):199. doi: 10.4103/0970-2113.116259
43. Gopathi NR, Mandava V, Namballa UR, Makala S. A comparative study of induced sputum and bronchial washings in diagnosing sputum smear negative pulmonary tuberculosis. J Clin Diagn Res. (2016) 10(3):OC07. doi: 10.7860/JCDR/2016/18767.7474
44. Porzezińska M, Knopińska-Posłuszny W, Cynowska B, Słomiński JM. Cellular composition of induced sputum in sarcoidosis. Adv Respir Med. (2013) 81(3):192–9. doi: 10.5603/ARM.34105
45. Baha A, Yıldırım F, Stark M, Kalkancı A, Fireman E, Köktürk N. Is induced sputum a useful non-invasive tool in the diagnosis of pulmonary sarcoidosis? Turk Thorac J. (2019) 20(4):248. doi: 10.5152/TurkThoracJ.2018.180147
46. Olivieri D, D’Ippolito R, Chetta A. Induced sputum: diagnostic value in interstitial lung disease. Curr Opin Pulm Med. (2000) 6(5):411–4. doi: 10.1097/00063198-200009000-00004
47. Fernández AA, Río FG, Alises SM, Fraga SS, San Andrés OM, León JV. Utilidad de la citología de esputo inducido en el estudio de masas centrales en ancianos. Rev Clín Esp. (2001) 201(8):444–7. doi: 10.1016/S0014-2565(01)70876-4
48. Thunnissen FB. Sputum examination for early detection of lung cancer. J Clin Pathol. (2003) 56(11):805–10. doi: 10.1136/jcp.56.11.805
49. D’Urso V, Doneddu V, Marchesi I, Collodoro A, Pirina P, Giordano A, et al. Sputum analysis: non-invasive early lung cancer detection. J Cell Physiol. (2013) 228(5):945–51. doi: 10.1002/jcp.24263
50. Neumann T, Meyer M, Patten FW, Johnson FL, Erozan YS, Frable WJ, et al. Premalignant and malignant cells in sputum from lung cancer patients. Cancer Cytopathol J Am Cancer Soc. (2009) 117(6):473–81. doi: 10.1002/cncy.20052
51. Kelsen SG, Agache IO, Soong W, Israel E, Chupp GL, Cheung DS, et al. Astegolimab (anti-ST2) efficacy and safety in adults with severe asthma: a randomized clinical trial. J Allergy Clin Immunol. (2021) 148(3):790–8. doi: 10.1016/j.jaci.2021.03.044
52. Diver S, Richardson M, Haldar K, Ghebre MA, Ramsheh MY, Bafadhel M, et al. Sputum microbiomic clustering in asthma and chronic obstructive pulmonary disease reveals a Haemophilus-predominant subgroup. Allergy. (2020) 75(4):808–17. doi: 10.1111/all.14058
53. Weiszhar Z, Horvath I. Induced sputum analysis: step by step. Induced sputum analysis: step by step. Breathe. (2013) 9(4):301–6. doi: 10.1183/20734735.042912
54. Russell R, Brightling C. Mepolizumab for the reduction of exacerbations in severe eosinophilic asthma. Expert Rev Respir Med. (2016) 10(6):607–17. doi: 10.1080/17476348.2016.1176532
55. Wheelock CE, Goss VM, Balgoma D, Nicholas B, Brandsma J, Skipp PJ, et al. Application of -omics technologies to biomarker discovery in inflammatory lung diseases. Eur Respir J. (2013) 42(3):802–25. doi: 10.1183/09031936.00078812
56. Ronchetti K, Tame JD, Paisey C, Thia LP, Doull I, Howe R, et al. The CF-sputum induction trial (CF-SpIT) to assess lower airway bacterial sampling in young children with cystic fibrosis: a prospective internally controlled interventional trial. Lancet Respir Med. (2018) 6(6):461–71. doi: 10.1016/S2213-2600(18)30171-1
57. Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. (2009) 180(1):59–99. doi: 10.1164/rccm.200801-060ST
58. Covar RA, Spahn JD, Martin RJ, Silkoff PE, Sundstrom DA, Murphy J, et al. Safety and application of induced sputum analysis in childhood asthma. J Allergy Clin Immunol. (2004) 114:575–82. doi: 10.1016/j.jaci.2004.06.036
59. Duncan CJA, Lawrie A, Blaylock MG, Douglas JG, Walsh GM. Reduced eosinophil apoptosis in induced sputum correlates with asthma severity. Eur Respir J. (2003) 22(3):484–90. doi: 10.1183/09031936.03.00109803a
60. Demarche SF, Schleich FN, Paulus VA, Henket MA, Van Hees TJ, Louis RE. Asthma control and sputum eosinophils: a longitudinal study in daily practice. J Allergy Clin Immunol Pract. (2017) 5(5):1335–43. doi: 10.1016/j.jaip.2017.01.026
61. Belda J, Leigh R, Parameswaran K, O’Byrne PM, Sears MR, Hargreave FE. Induced sputum cell counts in healthy adults. Am J Respir Crit Care Med. (2000) 161(2):475–8. doi: 10.1164/ajrccm.161.2.9903097
62. Vieira MO, Pizzichini E, Steidle LM, da Silva JK, Pizzichini MM. Sputum induction in severe exacerbations of asthma: safety of a modified method. Eur Respir J. (2011) 38(4):979–80. doi: 10.1183/09031936.00029511
63. Rueda ZV, Bermúdez M, Restrepo A, Garcés C, Morales O, Roya-Pabón C, et al. Induced sputum as an adequate clinical specimen for the etiological diagnosis of community-acquired pneumonia (CAP) in children and adolescents. Int J Infect Dis. (2022) 116:348–54. doi: 10.1016/j.ijid.2022.01.026
64. Jasin MR, Setyanto DB, Hadinegoro SR, Lisnawati L, Gayatri P, Kurniati N. Efficacy of sputum induction from lower respiratory tract in children. Paediatr Indones. (2015) 55(2):101–8. doi: 10.14238/pi55.2.2015.101-8
65. D’Sylva P, Caudri D, Shaw N, Turkovic L, Douglas T, Bew J, et al. Induced sputum to detect lung pathogens in young children with cystic fibrosis. Pediatr Pulmonol. (2017) 52(2):182–9. doi: 10.1002/ppul.23636
66. Licari A, Manti S, Castagnoli R, Leonardi S, Marseglia GL. Measuring inflammation in paediatric severe asthma: biomarkers in clinical practice. Breathe. (2020) 16:190301. doi: 10.1183/20734735.0301-2019
67. Nyangulu W, Thole H, Chikhoza A, Msakwiza M, Nyirenda J, Chisala M, et al. Performance and safety of the induced sputum procedure in young children in Malawi: a prospective study. Trans R Soc Trop Med Hyg. (2021) 115(11):1247–50. doi: 10.1093/trstmh/trab151
68. Ognibene FP, Gill VJ, Pizzo PA, Kovacs JA, Godwin C, Suffredini AF, et al. Induced sputum to diagnose Pneumocystis carinii pneumonia in immunosuppressed pediatric patients. J Pediatr. (1989) 115(3):430–3. doi: 10.1016/S0022-3476(89)80848-0
69. Zar HJ, Tannenbaum E, Hanslo D, Hussey G. Sputum induction as a diagnostic tool for community-acquired pneumonia in infants and young children from a high HIV prevalence area. Pediatr Pulmonol. (2003) 36(1):58–62. doi: 10.1002/ppul.10302
70. Wark PA, Simpson JL, Hensley MJ, Gibson PG. Safety of sputum induction with isotonic saline in adults with acute severe asthma. Clin Exp Allergy. (2001) 31(11):1745–53. doi: 10.1046/j.1365-2222.2001.01230.x
71. Mohamed NR, Ghany EA, Othman KM. Analysis of induced sputum in patients with bronchial asthma. Egypt J Chest Dis Tuberc. (2014) 63(1):21–7. doi: 10.1016/j.ejcdt.2013.11.008
72. New South Wales Health. Sputum induction guidelines. North Sydney: NSW Department of Health. (2018) Available at: https://www.health.nsw.gov.au/Infectious/tuberculosis/Pages/tb-sputum-induction-guidelines.aspx (Accessed 13 September 2023).
73. Crespo-Lessmann A, Plaza V, Consensus Group, Almonacid C, Caballero ML, Antonio Cañas J, et al. Multidisciplinary consensus on sputum induction biosafety during the COVID-19 pandemic. Allergy. (2021) 76(8):2407–19. doi: 10.1111/all.14697
74. Wood LG, Powell H, Gibson PG. Mannitol challenge for assessment of airway responsiveness, airway inflammation and inflammatory phenotype in asthma. Clin Exp Allergy. (2010) 40(2):232–41. doi: 10.1111/j.1365-2222.2009.03371.x
75. Brannan JD, Anderson SD, Perry CP, Freed-Martens R, Lassig AR, Charlton B. The safety and efficacy of inhaled dry powder mannitol as a bronchial provocation test for airway hyperresponsiveness: a phase 3 comparison study with hypertonic (4.5%) saline. Respir Res. (2005) 6(1):1–2. doi: 10.1186/1465-9921-6-144
76. Anderson SD, Brannan J, Spring J, Spalding N, Rodwell LT, Chan KI, et al. A new method for bronchial-provocation testing in asthmatic subjects using a dry powder of mannitol. Am J Respir Crit Care Med. (1997) 156(3):758–65. doi: 10.1164/ajrccm.156.3.9701113
Keywords: sputum induction, spirometry, asthma, chronic obstructive pulmonary disease, dithiothreitol sputum processing, airway inflammatory disorders, induced sputum cytospins, sputum eosinophil differential cell count
Citation: Goncalves B and Eze UA (2023) Sputum induction and its diagnostic applications in inflammatory airway disorders: a review. Front. Allergy 4:1282782. doi: 10.3389/falgy.2023.1282782
Received: 24 August 2023; Accepted: 26 September 2023;
Published: 12 October 2023.
Edited by:
Akira Yamasaki, Tottori University, JapanReviewed by:
Makoto Kudo, Yokohama City University, Japan© 2023 Goncalves and Eze. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ukpai A. Eze dWtwYWkuZXplQGRtdS5hYy51aw==
Abbreviations BAL, bronchoalveolar lavage; COPD, chronic obstructive pulmonary disease; DTT, dithiothreitol; TB, tuberculosis; PBS, phosphate-buffered saline; HIV, human immunodeficiency virus; AIDS, acquired immunodeficiency syndrome; FEV1, forced expiratory volume in 1 s; pMDI, pressurised metered-dose inhaler; ATS, American Thoracic Society; ERS, European Respiratory Society; MDI, metered-dose inhaler; ERTF, European Respiratory Task Force; GM-CSF, granulocyte-macrophage colony-stimulating factor; TNF-α, tumour necrosis factor alpha; IL, interleukin; ECP, eosinophil cationic protein; ELISA, enzyme-linked immunosorbent assay; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
†ORCID Ukpai A. Eze orcid.org/0000-0002-9960-4714
 Beatriz Goncalves
Beatriz Goncalves Ukpai A. Eze
Ukpai A. Eze