- 1Department of Otorhinolaryngology-Head & Neck Surgery, Mayo Clinic in Arizona, Phoenix, AZ, United States
- 2Department of Immunology and Medicine, Mayo Clinic in Arizona, Scottsdale, AZ, United States
- 3Library Services, Mayo Clinic Libraries-Arizona, Scottsdale, AZ, United States
Background: Unlike acute rhinosinusitis (ARS) which is mostly viral in etiology, the role of viruses in chronic rhinosinusitis (CRS) remains unclear. Viruses may play a role in initiation, exacerbations or perpetuate chronic inflammatory responses in the sinonasal mucosa. Research needs to characterize whether viruses are part of the normal sinonasal microbiome, colonizers or pathogenic.
Methods: Systematic review of the English literature was conducted. Following databases were searched with an initial search conducted in November 2021 and then updated through June 2023: Ovid Medline (1946 to present), Ovid Embase (1988 to present), Scopus (2004 to present) and Web of Science (1975 to present). MeSH (Medical Subject Headings) terms included: viruses, virus diseases, sinusitis, and rhinovirus. Keywords: virus, viral infection*, sinusitis, rhinovirus, chronic rhinosinusitis, CRS, respiratory virus, respiratory infection*, and exacerbat*. A supplementary search was conducted through September 2023: Ovid Medline (1946 to present), Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) Daily. Keywords used were: virus, viral infection*, sinusitis, chronic rhinosinusitis, CRS, respiratory virus, respiratory infection*, and exacerbat*.
Results: Thirty studies on viruses in CRS met inclusion criteria for full review. These included 17 studies on prevalence of virus in CRS, 5 examining probable causes of host susceptibility to viral infections in CRS, and 8 studies examining pathological pathways in viral association of CRS. The prevalence of viruses in nasal specimens of CRS subjects was higher as compared to controls in most studies, though a few studies showed otherwise. Rhinovirus was the most common virus detected. Studies showed that viruses may be associated with persistent hyper-responsiveness in the sinonasal mucosa, susceptibility to bacterial infections, upregulation of genes involved in the immune response and airway remodeling as well as CRS exacerbations. Presence of viruses was also associated with worse symptom severity scores in CRS subjects.
Conclusion: Most data show higher presence of viruses in nasal and serum samples of CRS subjects as compared to controls but their exact role in CRS pathophysiology in unclear. Large studies with longitudinal sampling at all disease phases (i.e., prior to disease initiation, during disease initiation, during disease persistence, and during exacerbations) using standardized sampling techniques are needed to definitively elucidate the role of virus in CRS.
1. Introduction
Chronic Rhinosinusitis (CRS) is a significant public health problem afflicting 5%–12% of the global population (1). Historically, CRS was assumed to be an “infection”, but contemporary studies have moved away from this dogma. A complex interplay between host and environmental factors likely results in chronic inflammation of the sinonasal mucosa (2). The initial trigger has been hypothesized to be disruption of the sinonasal mucosal barrier by infection (bacteria, fungi, viruses), mechanical trauma, allergies, etc. The initial insult triggers a cascade of immunological responses that get dysregulated, ultimately resulting in a chronically inflamed sinonasal epithelium that is independent of the cessation of the initial insult.
Microbiome dysbiosis has been characterized as a hallmark in CRS, with CRS patients demonstrated to have microbial community collapse and loss of diversity compared to healthy controls (3, 4). In health, the normal sinonasal mucosa acts as an immuno-mechanical barrier against pathogens. In targeting pathogens, the sinonasal mucosa deploys Type 1 immune responses against intracellular pathogens, most commonly against viruses. Type 1 inflammation is associated with IL (interleukin)-2, IFN (interferon)-gamma, with canonical effector cells being M1 macrophages, Natural killer (NK) cells, CD8+ T cells, Th1 cells and ILC (Innate Lymphoid Cell) 1 cells. Type 2 immune response is directed against large extracellular pathogens such as parasites, and associated with IL-4, IL-5, IL-13, IL-25, IL-33 and IgE with effector cells that include M2 macrophages, eosinophils, Th2 cells, ILC2 cells and mast cells. Type 3 immune responses are directed towards extracellular bacteria and fungi, and is associated with cytokines IL-17A, IL-17F and IL-22, with the effector cells being neutrophils, Th17 cells and ILC3 (5). In the United States and the Western hemisphere, CRS with nasal polyps (CRSwNP) is traditionally characterized by a predominantly Type 2 inflammatory profile response, whereas CRS sine (without) nasal polyps (CRSsNP) has been classically associated with type 1 and type 3 responses, however there is heterogeneity within endotype and phenotype correlation (6). In the United States, data shows that a significant number of CRSsNP may have Type-2 features (7, 8).
Given the inflammatory profile in CRS, microbes may have a significant role in causing initial immuno-mechanical insult to the respiratory and sinonasal lining that results in a chronic inflammatory condition. Unlike acute rhinosinusitis (ARS) which is mostly viral in etiology (9–11) and self-limiting, chronic rhinosinusitis is characterized by unremitting inflammation of the sinonasal mucosa. In addition to disease initiation, virus may play a role in the acute exacerbations of the chronically inflamed state in addition to potentially perpetuating chronic inflammatory responses in the sinonasal mucosa. Though certain respiratory viruses such as rhinovirus have been found more commonly in nasal brushings/tissue samples in patients with CRS vs. healthy controls (12–15), the role of viruses in CRS remains unclear (16) and further research is necessary to elucidate and characterize viruses in the sinonasal cavity as part of the normal microbiome, colonizers or pathogens.
Until a few years ago, viral detection was time consuming as it was culture based; however, with the use of molecular—Polymerase chain reaction (PCR) based techniques, detection is more sensitive, samples can be rapidly analyzed, and new serotypes of viruses identified. Presented in Figure 1 are viral detection techniques that are currently in use, with their advantages and disadvantages.
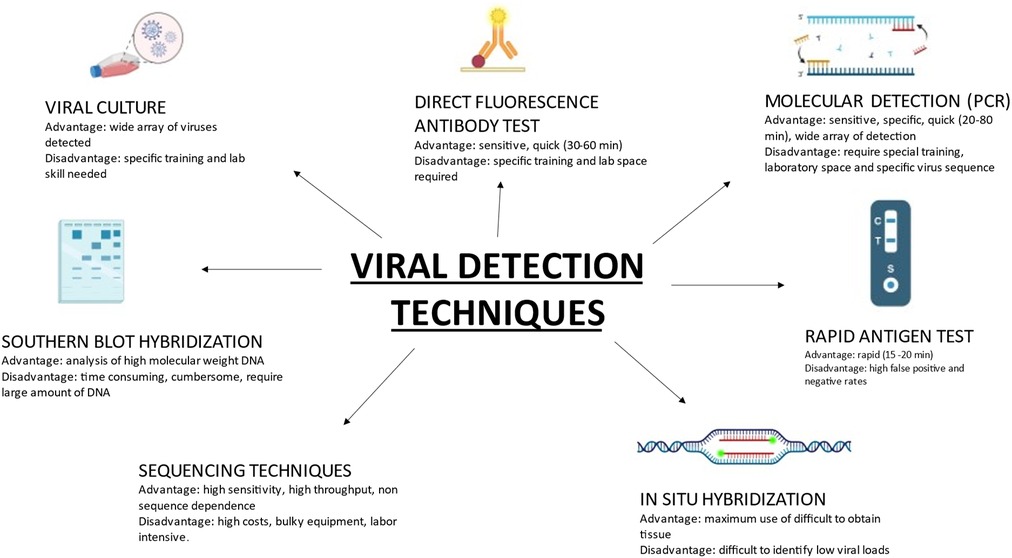
Figure 1. Contemporary viral detection techniques in common use, with specific advantages and disadvantages of each.
This systematic review scrutinizes the contemporary literature for studies on viruses in CRS initiation, exacerbation and persistence.
2. Materials and methods
2.1. Study design
A systematic review of the English literature was conducted. The following databases were searched initially in November 2021 then updated though June 2023: Ovid Medline (1946 to present), Ovid Embase (1988 to present), Scopus (2004 to present) and Web of Science (1975 to present). A combination of MeSH (Medical Subject Headings) and keywords were used. The MeSH terms included: viruses, virus diseases, sinusitis, and rhinovirus. Keywords used: virus, viral infection*, sinusitis, rhinovirus, chronic rhinosinusitis, CRS, respiratory virus, respiratory infection*, and exacerbat*. The MeSH terms and counterpart keywords were combined using the Boolean operator “OR” then OR'd concepts were combined using the Boolean operator “AND”. Finally, the MeSH term and keyword of asthma was eliminated from the search strategy using the Boolean operator of “NOT”. (* indicates truncation of word or phrase).
The following databases were searched again through September 2023 to supplement the previously searched data: Ovid Medline (1946 to present), Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) Daily. A combination of MeSH (Medical Subject Headings) and keywords were used. The MeSH terms included: viruses, virus diseases, sinusitis, and rhinovirus. Keywords used: virus, viral infection*, sinusitis, chronic rhinosinusitis, CRS, respiratory virus, respiratory infection*, and exacerbat*. The MeSH terms and counterpart keywords were combined using the Boolean operator “OR” then OR' concepts were combined using the Boolean operator “AND”. Finally, the MeSH term and keyword of asthma was eliminated from the search strategy using the Boolean operator of “NOT”.
2.2. Eligibility criteria
EndNote X9 software (Clarivate Analytics, Philadelphia, PA) was used to compile the studies. A preliminary screen was conducted by three reviewers (N.K., T.B., D.L) in which the titles and abstracts were reviewed. Criteria for inclusion was any mention of virus and sinusitis, nasal epithelial cells, and upper airway. Exclusion criteria were acute sinusitis, cystic fibrosis, chronic obstructive pulmonary disease, immunocompromised or any other non-CRS condition. Additional exclusion criteria were review articles, book chapters and abstracts without full text. A secondary screen was then conducted by all the reviewers in which full text articles were evaluated by each reviewer.
2.3. Data extraction
Three authors (N.K., T.B., D.L) evaluated each full text article. The authors then looked at the bibliography and found additional articles that offered insight into the role of viruses in CRS and included them in the review and discussion. Data that were extracted included subjects (animal/human), number of subjects, brief study description (including site of sampling), viruses studied and findings of the study.
3. Results
The results of the literature search and subsequent screenings are shown in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) diagram in Figure 2. The search resulted in 549 (517 + 32) studies. After review of the abstracts, 6 (2 + 4) articles were included for full review of the manuscript. Search was conducted multiple times with change in keywords but did not reveal any significant difference in the quality of results. This could indicate a problem in indexing of the articles. Reference lists of the shortlisted articles were studied to identify missing articles, not identified in literature search, satisfying the inclusion criteria. 24 articles were identified during this manual search and after full review, total 30 articles included in the study.
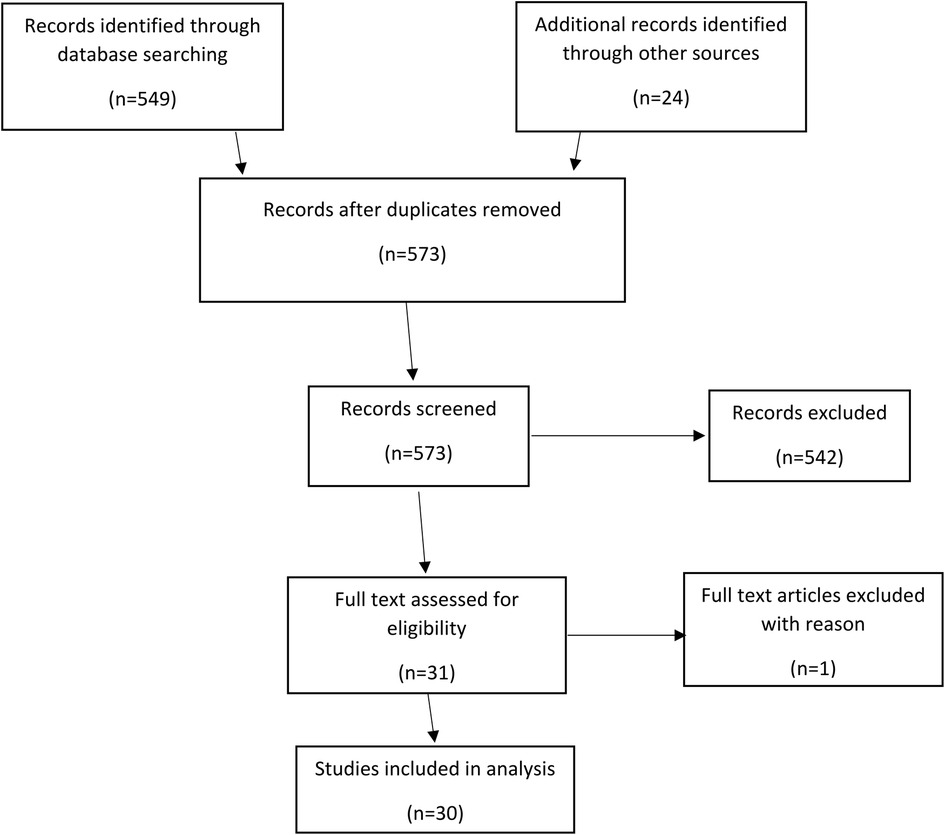
Figure 2. PRISMA diagram showing methodology of systematic review. (PRISMA, preferred reporting items for systematic reviews and meta-analyses).
Some studies further subclassified CRS based on phenotype (CRSwNP and CRSsNP) or endotype (eosinophilic CRSwNP and noneosinophilic CRSwNP). The number of subjects within subgroups ranged from as 2 to 133. The studies were further categorized into studies regarding the prevalence of virus in CRS subjects (17), studies investigating the causes of host susceptibility to viral infections in CRS (5) and those investigating role of virus in CRS immunopathogenesis (8).
3.1. Samples studied
The samples studied in human studies were variable and included nasal mucosa, uncinate tissue, brush swabs from middle meatus or ethmoidal sinus, inferior turbinate tissue and nasal lavage fluid. One study investigated serum antibody levels to 47 viral antigens (17).
3.2. Prevalence of virus in CRS patients
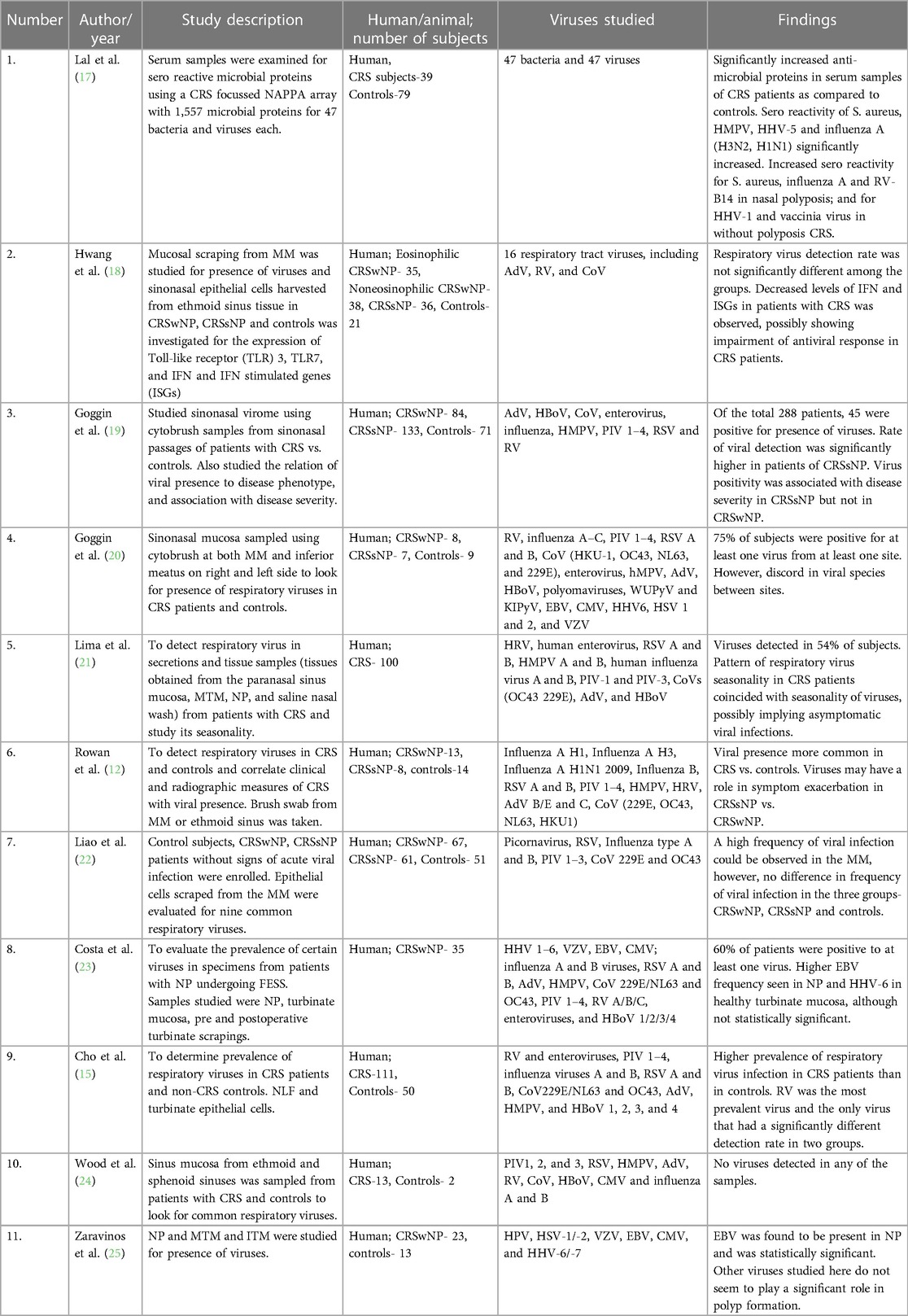
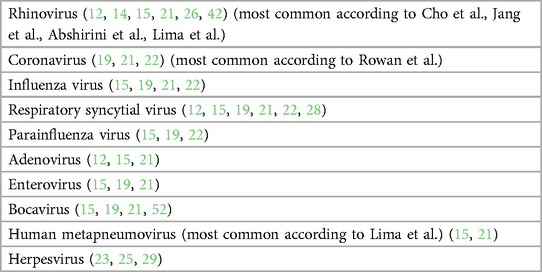
Seventeen studies investigated the prevalence of various airway viruses in sinonasal samples of CRS subjects. Some studies investigated an array of respiratory viruses (Table 1A) whereas others studied 1 or 2 specific viruses (Table 1B). Polymerase Chain Reaction (PCR) testing was the most common mechanism used for study, followed by Enzyme Linked Immuno Sorbent Assay (ELISA). One study did not sample the sinonasal mucosa at all but looked at serum antibody profiles to 47 viral antigens (17). The viruses studied included subtypes of Rhinovirus (RV), Influenza virus, Parainfluenza virus (PIV), respiratory syncytial virus (RSV), adenovirus (AdV), coronavirus (CoV), Bocavirus (BoV), human metapneumovirus (HMPV), human papilloma virus (HPV), herpes simplex virus (HSV-1/-2), varicella zoster virus (VZV), Epstein Barr virus (EBV), cytomegalovirus (CMV), and human herpes virus (HHV-6/-7). Eleven studies showed a higher prevalence of one or more viruses in patients of CRS vs. controls (12–15, 17, 19–21, 25, 26, 29). Most commonly identified viruses include HRV (13–15, 17, 19, 26), EBV (20, 25, 29), CoV (12, 19), HMPV (21). Six showed either no viral detection, or no significant difference in presence of viruses between CRS subjects and controls (18, 22–24, 27, 28).
Rowan et al. used nasal brush swab samples in 13 CRSwNP, 8 CRSsNP and 14 controls and found 24% prevalence of viruses in CRS cases, in which 50% prevalence as associated with CRSsNP and only 8% with CRSwNP; highest prevalence was found of CoV using real time polymerase chain reaction (rt-PCR) technique for viral detection (12). Lee et al. used nasal lavage samples for detection of only RV in 111 CRS patients and 51 controls and found a higher prevalence of 36% in CRS as compared to 20% in controls using rt-PCR technique. Further subtyping reveled maximum prevalence of RV-A serotype (13). Cho et al. also used nasal lavage samples along with inferior turbinate scrapings and found a higher rate of viral detection in nasal scrapings (64%) as compared to nasal lavage (50.5%). Prevalence was significantly high in cases as compared to controls and RV was the most common virus identified. They also noticed a higher rate of co-infection of viruses in cases (24.3%) as compared to controls (4.0%) (15). Jang et al. used similar technique in a smaller sample size of 39 cases and 27 controls where all nasal lavage samples were negative for viruses whereas inferior turbinate scrapings had a 21% viral detection rate in cases and none in controls (14). Lima et al. attempted to identify the seasonal variation of viral prevalence in CRS where she studied 100 CRS patients over a period of 2 years and found a viral detection rate of 54% in CRS subjects. Most common identified viruses were HMPV followed by HRV. Co-infection was found in 44% of subjects and maximum seasonal correlation was demonstrated by HRV followed by HMPV (21). Abshirini et al. did a case only study with 76 CRS cases undergoing endoscopic sinus surgery where they collected mucus specimen from ethmoidal sinus during surgery for viral detection using rt-PCR and found an overall prevalence of 32.89%, 28.94% for RV and 11.84% for RSV (26). Zaravinos et al. sampled polyp tissue for detection of viruses using rt-PCR where they found maximum prevalence of EBV followed by HPV (25). Goggin et al, in an attempt to find the most effective way of sampling to obtain highest viral yield, detected a prevalence of 75% using a cytology brush for nasal mucosal sampling and found maximum prevalence of EBV in CRS patients (20). In another study for detecting viral prevalence in 288 subjects, they found maximum viral positivity associated with CRSsNP—20.3%, compared to CRSwNP—15.4%, and controls—7%. In this study the most common identified viruses were RV and CoV. Also, to find the effect of viral association on disease severity, they found higher Lund-Mackay and Lund-Kennedy scores only in patients of CRSsNP with viral association (19). Tao et al. used southern blot hybridization, PCR and in situ hybridization for EBV encoded small nuclear RNA (snRNA) for detection of EBV in 13 patients of nasal polyposis and found the detection rates of 15%, 69% and 85% respectively (29).
The studies that showed no significant difference in the prevalence of viruses in CRS patient include that by Divekar et al. where they detected no difference of prevalence of RV and enterovirus (EV) between CRS patients and controls, but detected a significantly higher levels of IL-6, IL-5, VEGF (Vascular endothelial growth factor), GM-CSF (Granulocyte monocyte colony stimulating factor) and eosinophilic major basic protein (EMBP) in CRSwNP patients (27). Wood et al. used PCR for detection of common airway viruses in sinonasal mucosal samples but didn't detect any virus in the 13 subjects as well as 2 controls (24). Costa et al. used polyp tissue, turbinate mucosa and pre and post operative scrapings for detection of community acquired respiratory viruses and found no significant difference in the viral prevalence among cases and controls (23). Liao et al. and Hwang et al. also attempted to detect the viral prevalence using nasal swabs and middle meatal scrapings using rt-PCR but didn't find any significant difference in prevalence amongst cases and controls (18, 22).
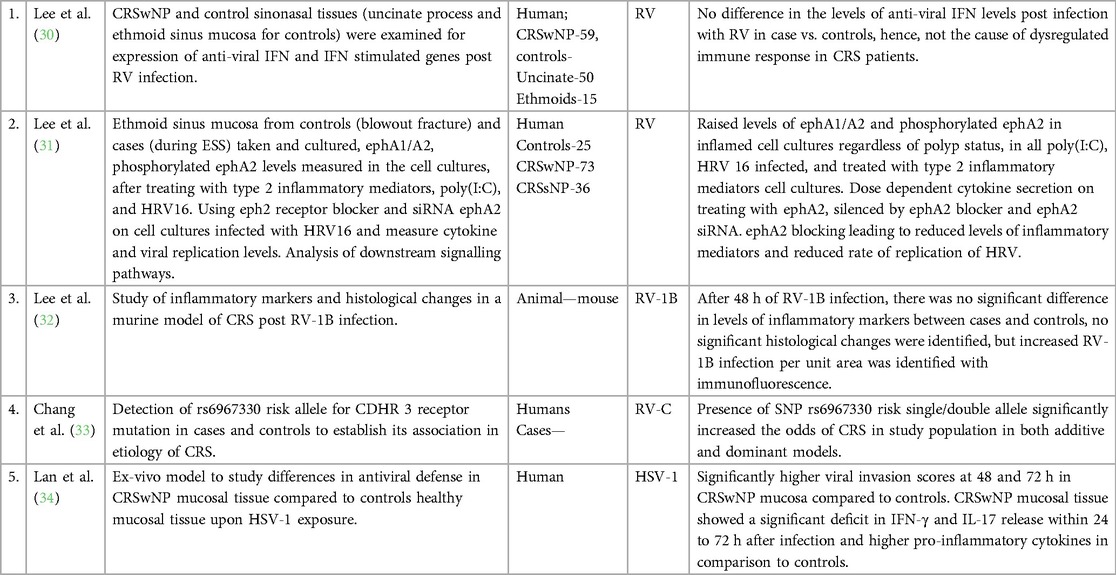
3.3. Host susceptibility to viral infection
Five studies investigated host factors in CRS patients that might be responsible for increased susceptibility to viral infection and pathogenicity (Table 2). These studies found reduced anti-viral cytokine (IFN-γ, IL-17) levels indicating compromised antiviral defense mechanisms (34), increased permeability of the inflamed mucosa to viruses leading to increased viral invasion (34), mutations in CDHR3 viral receptor leading to increased RV-C binding (33), increased viral binding per unit area in inflamed nasal tissue in murine model (32), ephrin A1/A2 receptor mediated dysfunctional innate immune response, again indicating compromised antiviral defense mechanisms (31). Lee et al. found that in CRS subjects, air liquid interface (ALI) culture of cells obtained from ethmoidal sinus did not show any significant difference in the levels of IFN-β or IFN stimulated genes (ISGs) like viperin vs. controls after RV-16 infection, which was in contrast to findings of above mentioned studies and denies role of compromised anti-viral IFN response in virus mediated pathogenesis of CRS (30).
3.4. Role of viruses in pathophysiology of CRS
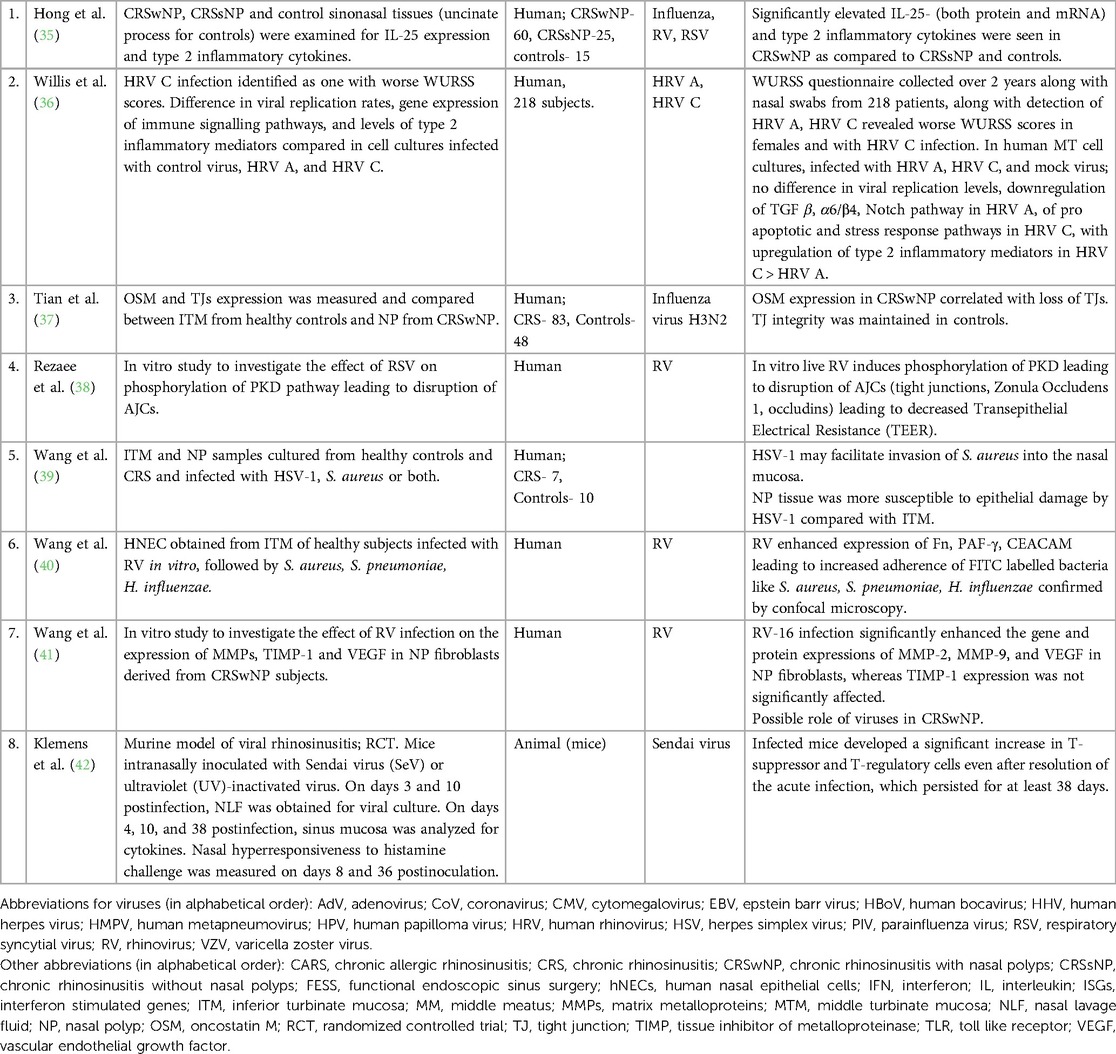
Eight studies investigated the role of viruses in persistent chronic inflammation of CRS as well as in periodic exacerbations (Table 3). Of them, 4 focused on the immuno-epithelial barrier disruption caused by viral infection as a mechanism leading to persistent inflammation and increased susceptibility to bacterial infections (35, 39, 42, 43). These studies were conducted on cultured epithelial cells and found disruption of the epithelial barrier via increased expression of Oncostatin M (OSM) post H3N2 virus infection (37), increased phosphorylation of protein kinase D (PKD) leading to destabilization of actin cytoskeleton and disruption of apical junctional complexes (AJCs) confirmed via immunofluorescence and confocal microscopy (38). Increased adherence of bacteria was found via upregulated expression of cellular adhesion molecules (CAM) in cultured epithelial cells post RV-16 infection and increased adhesion of bacteria like S. aureus, S. pneumoniae and H. influenzae compared to controls was visualized on confocal microscopy and immunofluorescence (40). Wang et al. also noticed increased mucosal invasion and S. aureus infection in cultured nasal epithelial cells of patient with CRSwNP as compared to controls on infection with HSV-1 (39). Higher levels of inflammatory mediators like IL-25, IL-1β, IL-10, IL-5 and tumor necrosis factor (TNF) -α after viral infection were found in human nasal epithelial cell (HNEC) culture compared to controls (34, 35). Persistent hyper responsiveness of nasal mucosa post viral infection was demonstrated in a mouse model after infection with sendai virus (SeV) where elevated levels of CD8+, CD-4+ and CD-25+ cells were found after resolution of acute phase of viral infection along with more severe symptoms on histamine challenge test (42).
4. Discussion
4.1. What is the role of viruses in rhinosinusitis?
Viruses have an established role in the etiopathogenesis of acute rhinosinusitis (ARS), defined as rhinosinusitis lasting less than 12 weeks (12–14). ARS is usually a self-limiting condition, but sometimes viral ARS leads to secondary bacterial infection due to epithelial changes, microbial dysbiosis, immune suppression and changes in the local environment favoring the growth of bacteria (38–40, 43, 44). Rhinovirus is the most commonly implicated virus in ARS (45, 46).
In patients with CRS, respiratory viruses are often found in nasal samples, but their role is not fully understood and yet to be firmly elucidated (13, 15, 26). Virus may cause sinonasal mucosal inflammation, disruption of the immuno-mechanical barrier at the sinonasal epithelium, increased susceptibility to bacterial adherence and resultant immuno pathogenic mechanisms at the cellular and tissue levels that result in persistent sinonasal inflammation characteristic of CRS (31, 47). Viruses may alter host gene expression, leading to altered patterns of immune response and pathogenic changes in levels of cytokines/chemokines (35, 36, 48, 49). Additionally, viral infections may lead to alteration of the normal sinonasal microbiome, which could lead to a cascade of events causing inflammation of the upper airway (47).
In vitro studies have shown that after a viral infection, host antiviral response genes may inhibit ciliogenesis and ciliary function of nasal epithelial cells, ultimately leading to chronic inflammation of the airway (50). Disruption of AJCs have been seen in response to RSV infection that led disruption of the barrier function of the epithelium predisposing to chronic inflammation (38). Some studies identified difference in the cytokine levels post viral infection in HNEC precipitating an inflammatory cascade e.g., IL 25 level escalation post influenza A infection (35), increased ephrin A1/A2 levels leading to type 2 inflammatory reaction (31), and elevated levels of CXCL-10 post RV infection (36). Although pretreatment with IFN-α was correlated with decrease in levels of inflammatory markers (35), Lee et al. found conflicting evidence as similar levels of antiviral IFN-β and IFN stimulated genes (ISGs) were found in subjects with CRSwNP and control group, post viral infection in cultured sinonasal epithelial cells, thus refuting their role in immune dysregulation seen in CRS patients (30).
In a mouse model that compared the rhinovirus infected area of nasal epithelium, cytokines and histology between a control group and induced chronic allergic rhinosinusitis group, there was a significant difference of concentration of viral particles per unit area of infected epithelium as measured by immunofluorescence in the study group and the levels of cytokines and the histology did not differ significantly between the groups (32).
In a study that compared atopic with non-atopic individuals, virus induced inflammatory response as measured by cytokine levels, differed in both groups during the acute as well as the convalescent phase. Thus, certain individuals may be more predisposed to developing inflammatory changes of the airway because of an acute viral infection (51). Interpersonal variation of inflammatory response to viral infection in CRS patients was also supported by the identification of rs6967330 risk allele associated with CDHR3 receptor gene, which is the receptor for RV-C and increased the odds of CRS in the patients harboring it (33).
Figure 3 illustrates the various effects viruses may have on the airway epithelium. Several viruses that have been detected in patients of CRS are listed in Table 4 below.
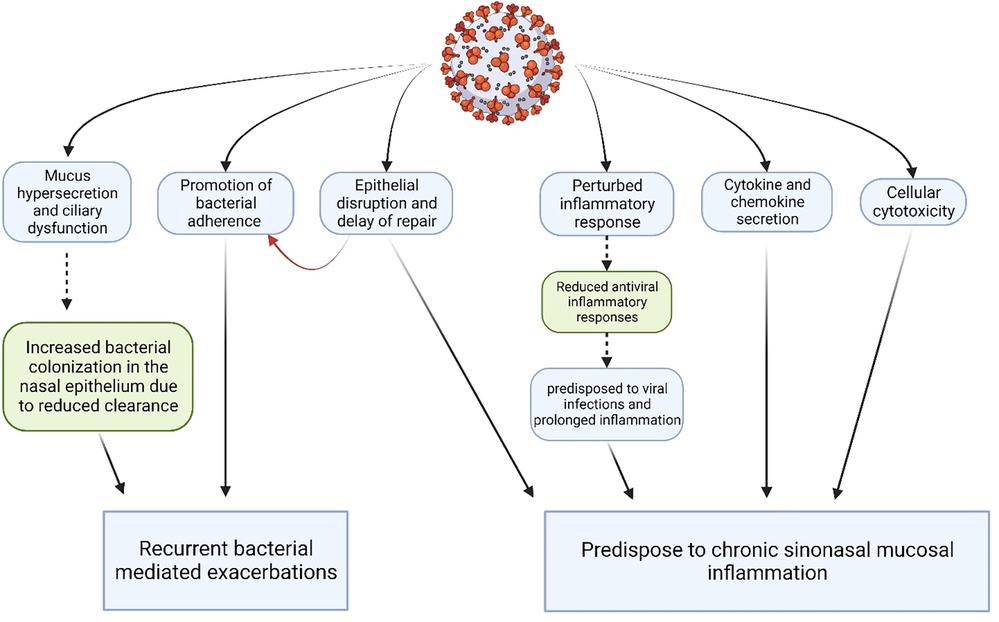
Figure 3. Synopsis of major pathogenic effects of viruses on the sinonasal epithelium. (Created with Biorender.com).
4.2. Possible role of viruses in CRS etiopathogenesis
Viruses may have a role in CRS disease initiation, exacerbation, and persistence.
4.2.1. Initiation-trigger
Viruses may cause loss of epithelial integrity by viral induced cytotoxicity, epithelial barrier disruption, delayed and abnormal epithelial repair (38, 47, 53). Rezaee et al. demonstrated the early and late phase phosphorylation of PKD leading to cortactin phosphorylation destabilizing the actin cytoskeleton and disruption of AJCs like occludins, Zonula Occludens (ZO) −1 and tight junctions, confirmed by confocal microscopy and loss of TEES (transepithelial electrical resistance) in epithelial cell culture post infection with live RSV, and not with ultraviolet (UV) inactivated RSV (38).
Additionally, they may affect the immune system leading to the disruption of immune response towards the virus by subversion of IFN signaling (inhibition of IFN synthesis, inhibition of IFN downstream signaling) (18), expression of a Th2 dominated inflammatory pattern instead of Th1, and they may cause deleterious effects due to an exaggerated response (34, 47). Anti-viral IFN are the first line of defense against viral invasion in the sinonasal epithelium and have been hypothesized as the primary factor responsible for the dysregulated innate immune response leading to viral induction and exacerbation in CRS. Hong et al. confirmed the reduced viral invasion in IFN-α pre-treated airway epithelial cells (35), but Lee et al. found no difference in anti-viral IFN-β levels and ISGs between CRS patients' cultured sinonasal epithelial cells and control groups (30). In the search for factors responsible for immune dysregulation, ephrin A1/A2 was identified as phosphorylated (activated) and upregulated in viral infected CRS ethmoid mucosal cells; ephrin A1/A2 is speculated to increase levels of inflammatory mediators and downregulate the protective PI3K-AkT-NFκβ pathway which activates antiviral immune responses. Additionally, pretreatment with ephrin A1/A2 led to reduced antiviral IFN levels in the cell culture medium. These findings were absent when viral infected cells were treated with ephrin A2 blocker or ephrin A2 silencing RNA (siRNA), thus, confirming the role of ephrin A1/A2 in dysregulated immune response observed in CRS patients (31).
Viral infections also lead to increased mucus production, mucostasis and ciliary impairment (47, 50). They may promote bacterial infection through synergy between respiratory viruses and bacteria, decreased clearance, facilitated bacterial penetration and viral induced bacterial adherence (39, 47). Wang et al. showed in an in vitro study using 7 CRS patients and 10 controls that HSV-1 may facilitate invasion of S. aureus into the nasal mucosa (39). In another study using RV-16, Wang et al. demonstrated increased expression of CAMs like Fn (fibronectin), platelet activated factor (PAF) -γ, CEACAM (carcinoembryonic antigen associated CAM) determined by messenger RNA (mRNA) levels of their respective genes and via confocal microscopy. This was followed by increased adherence of bacteria like S. aureus, S. pneumoniae, H. influenzae to the HNEC (40). This study provides insights into how viral infections may predispose to secondary bacterial infections, as well as alter the microbiome which could be an important pathological mechanism behind periodic exacerbations of CRS.
While viruses may provide the trigger, disease initiation also depends on the host response. Proud et al. demonstrated in an in vivo study with experimental rhinovirus infection that expression of many genes including those associated with immune response, chemokines and antivirals is significantly altered (43, 48, 54). Identification of the rs6967330 genetic single nucleotide polymorphism (SNP) associated with the CDHR3 gene, which is a receptor for RV-C also led to the hypothecation of its role in viral associated CRS pathogenesis. Chang et al. found that the odds of CRS increased significantly when RV-C infection was found along with CDHR3 gene mutation, irrespective of asthma status (33). These groups of genes are likely to be the host factors in the virus associated pathogenesis of rhinosinusitis.
In a mice model, authors showed persistent hyperresponsiveness in nasal mucosa for at least 38 days, even after clearance of acute viral infection (42). This may suggest that viruses may provide an initial trigger that leads to an altered inflammatory response.
4.2.2. Persistence and ongoing stimulus of chronic inflammation
4.2.2.1. Data refuting role of virus in CRS persistence
There is a seasonal variation in the detection of respiratory viruses in the airway (55), with viral infections being more common in the winter months. Lima et al. analyzed this variation in CRS patients undergoing surgery and found that the same seasonal variation existed. The most frequent viruses detected by real time PCR (rt-PCR) were HRV and HMPV, and were the only viruses maintaining seasonal variation despite the detection during several periods of study. They studied this in nasal washes and sinus mucosa of 100 patients with CRS and thus, observed that this is a consequence of probable asymptomatic infection, and not of persistence of viruses in these patients, as the seasonal detection of viruses in CRS patients correlated with seasonal pattern of detection in other acute rhinitis patients. However, no controls were used in this study (21). In a study by Wood et al, tissue samples from 15 patients undergoing sinonasal surgery were analyzed by PCR assays for the presence of common respiratory viruses. All samples were negative for viruses. Out of these 15 patients, 2 were controls, 8 had CRSsNP and 5 had CRSwNP. They did further assays to look for Human herpes virus-6 (HHV-6) and Epstein Barr virus (EBV). Low titer HHV-6 was found in 3 of 8 CRSsNP, 4 of 5 CRSwNP and 1 of 2 normal subjects. Low titer EBV was found in 1 of 8 CRSsNP, 4 of 5 CRSwNP and 0 of 2 normal subjects. They concluded that persistence of respiratory viruses is not responsible for CRS. However, they collected the specimens only during the summer months, and the sample size was small (24). Liao et al. looked for the presence of viruses in the nasal mucosa from the middle meatus of 67 patients with CRSwNP, 61 CRSsNP and 53 controls. The viruses were detected using rt-PCR and the samples were equally distributed throughout the study period of almost 3 years. None of them had acute respiratory tract symptoms in the 4 weeks prior. They studied nine common respiratory viruses and did not find any significant difference in the overall viral detection rates, or individual viruses, across the three study groups. Also, they did not find any difference in disease severity within patients of CRS with and without viral detection. While their study spanned across all seasons, they suggest that they did not quantify the viral copies between the groups, which may be the cause of not finding a significant difference (22).
4.2.2.2. Data supporting role of virus in CRS persistence
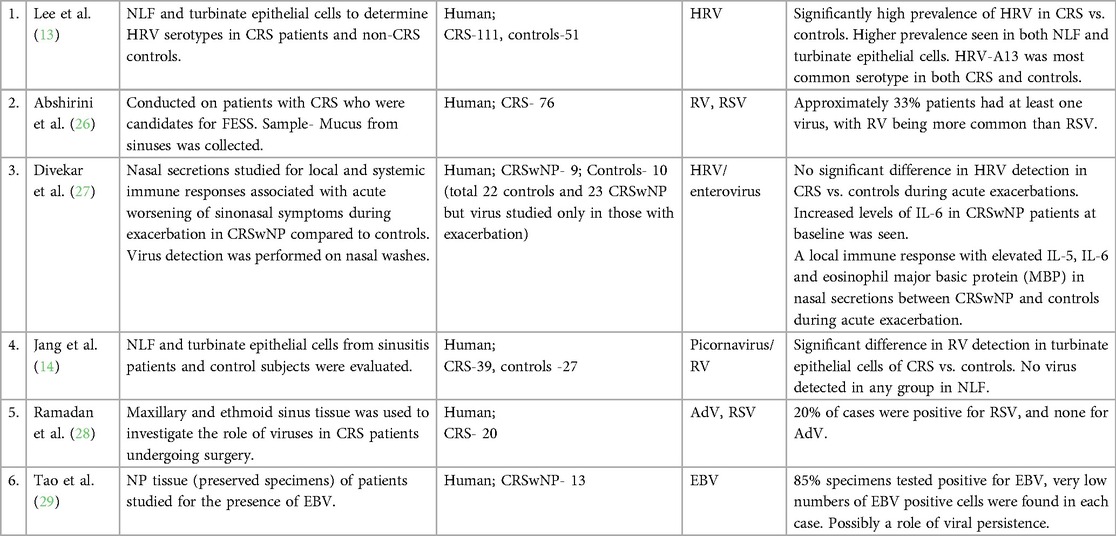
In an ex-vivo study that compared tissue from nasal polyps of patients with CRSwNP vs. inferior turbinate from healthy controls, a significant difference was seen in cytokine response on infection with HSV1. CRSwNP showed significantly higher IL-1β, IL-10 and TNF (tumor necrosis factor)-α, and significantly lower IFN-γ and IL-17 response (34). This deficient antiviral response as demonstrated by lower IFN-γ may be responsible for persistence of viruses in CRSwNP as compared to controls. Hwang et al. did not find a significantly different virus detection rate in mucosal scrapings from middle meatus in 35 eosinophilic CRSwNP, 38 non-eosinophilic CRSwNP, 36 CRSsNP and 21 controls but they also showed decreased levels of IFNs and IFN stimulated genes (ISGs) in patients of CRS, possibly showing an impaired antiviral response (18). In a study by Cho et al, the presence of respiratory viruses in 111 patients of CRS and 50 controls was studied using the PCR technique using two methods—nasal lavage samples and scrapings from inferior turbinate. A significant difference was seen in the overall viral detection rate and in the detection of Rhinovirus in CRS vs. controls. For parainfluenza, influenza, and RSV though there was a higher detection rate in CRS vs. controls, but it was not statistically significant. Overall, 64% of CRS samples detected positive for the presence of a respiratory virus. These patients had been selected after excluding those that had acute viral upper respiratory tract symptoms in the 4 weeks prior. Therefore, the presence of viruses, in the absence of symptoms may be due to asymptomatic infection, the incubation period before the onset of symptoms, or the persistence of viruses in CRS patients. However, they did not study the sinus mucosa (15). Jang et al. conducted a study on 39 patients with CRS and 29 controls who did not have any acute upper respiratory tract symptoms in the prior 4 weeks. 21 percent of CRS patients were positive for rhinovirus, and none of the controls were positive. They used nasal lavage samples and inferior turbinate scrapings. Whether the presence of virus was due to a new subclinical infection due to increased susceptibility to rhinovirus infections, or persistence from a previous infection, is difficult to establish (14). They did not study the sinus mucosa, however, it's persistence in the absence of acute symptoms of upper respiratory tract infection (URI) may suggest possibilities like in asthma, where rhinovirus RNA has been found in 32.4% of children with asthma, and is a possible important factor in the pathogenesis of the disease (56). Lee et al. to attempted determine HRV serotypes in 111 CRS patients and 51 non-CRS controls. No participant had an upper respiratory tract infection in the prior 4 weeks. HRV was detected using PCR technique in 40 CRS subjects (36%) and 11 non-CRS controls (21%). The overall detection rates of HRV in CRS patients from nasal lavage fluid and inferior turbinate epithelial cells were significantly higher than in non-CRS controls (13). Abshirini et al. compared for the presence of rhinovirus and RSV in the sinus mucosa of patients undergoing functional endoscopic sinus surgery (FESS). They found that the prevalence of at least one virus was 32.89% (26). Cho et al. detected a higher rate of viruses, probably also because they studied for the presence of 11 viruses, as compared to 2 viruses by Abshirini et al. (15, 26).
In another study, Goggin et al. investigated on 24 patients with CRS, and established a discord between the middle meatus and inferior meatus with respect to virus sampling (20). This may be an important factor in different studies leading to such varying results.
Goggin et al. next published their study of 288 patients, with 71 controls, 133 subjects with CRSsNP, and 84 CRSwNP. They used cytobrush samples from sinus mucosa and performed PCR for detection of common respiratory viruses. RV and coronavirus were the most common detected viruses. The maximum association with viral detection was found with CRSsNP and only CRSsNP subjects showed worsened sino-nasal outcome questionnaire test (SNOT) 22, endoscopic and radiological report, correlating with viral detection (19). Zaravinos et al. looked for the presence of HHV, HPV, EBV and CMV in 23 CRSwNP vs. 13 controls and found that statistically significant higher levels of EBV were found in CRSwNP. They sampled the polyp tissue and nasal mucosa from middle and inferior turbinates (25). Costa et al. in a case-only study on CRSwNP found that 60% of patients were positive to at least one virus, and EBV was seen in higher frequency in polyps and HHV-6 in healthy turbinate mucosa, though no statistically significant association was seen (23). Tao et al. also found that 85% of the 13 CRSwNP patients tested positive for EBV in polyp tissue, though very low numbers of EBV positive cells were found in each case, implying a possible role of viral persistence (29). However, these both studies were without controls. In a study that looked at differences in sero-reactivity between CRS and controls, Lal et al. found significantly elevated sero-reactivity in CRS patients against HMPV, HHV-4 and HHV-5 (17). In another case-only study to detect for the presence of adenovirus and RSV in 20 CRS patients undergoing surgery, 20% of the cases were positive for RSV, and none for adenovirus (28).
In summary, the association with viruses has been limited by incidental detection of virus in patient with CRS vs. controls. Only a few studies investigated viral detection with correlating measures of symptoms, radiological, endoscopic findings, or with inflammatory markers.
4.2.3. Exacerbation
Demonstration of increased bacterial adherence following viral infection by HSV-1 (39) and RV (40) may indicate a mechanism of periodic exacerbation in CRS involving bacterial mediated inflammatory response. While the role of virus in exacerbation has been speculated, ascertaining their role has been challenging.
In a longitudinal study by Hardjojo et al., infants (205 samples from 32 subjects with prolonged/ recurrent rhinitis and 215 samples from 32 matched controls—healthy infants) were followed up to 18 months of age and presence of virus in anterior nasal swab was detected by PCR at regular quarterly visits, which revealed that presence of virus in the swabs obtained in 1 month pre and post rhinitis period was significantly higher in recurrent rhinitis group, indicating the possible role of viruses in periodic exacerbations (57).
Tacon et al., in an in vitro and in vivo study demonstrated that the destruction of the epithelial layer and macrophage recruitment due to RV infection induces production of MMP (matrix metalloproteinase) −9 (58). Wang et al. performed an in vitro study and found that rhinoviral infection enhanced the gene and protein expressions of MMP-2, MMP-9, and VEGF in nasal polyp fibroblasts, derived from polypoidal nasal tissue, implying the possible role of viruses in exacerbation of CRSwNP (41). Matrix metalloproteinases (MMPs) comprise a family of Ca2+-activated, Zn2+-dependent endopeptidases that participate in the degradation of extracellular matrix (ECM) (59). Vascular endothelial growth factor (VEGF) induces endothelial cell proliferation and vascular hyperpermeability (60). Both have been suspected to play an important role in pathogenesis of nasal polyposis.
Oncostatin M (OSM), a cytokine belonging to IL-6 family, is found to increase significantly following influenza A infection. In the study by Tian et al., OSM levels in nasal polyps of CRSwNP vs. inferior turbinate tissue from controls were found to be higher in the former, by analysis of the mRNA levels of OSM gene. Higher levels of OSM have been associated with epithelial barrier dysfunction. Their findings suggested that OSM could be expressed by both ciliated and goblet cells, disrupting the tight junctions following viral infections, and possibly exposing the subepithelia to invading pathogens to elicit inflammatory responses, causing exacerbations in CRSwNP (37). Alho et al. looked for the presence of virus during a “natural cold” in those with recurrent sinusitis vs. healthy controls. They used nasal mucosal biopsies with viral culture, antigen detection and PCR methods to maximize viral detection rates. They found 14 of 19 recurrent sinusitis patients detected positive for a respiratory virus and 12 of 20 healthy controls were positive. No significant difference could be found denying the viral associated exacerbation of rhinitis (45).
Rowan et al. used brush swabs from middle meatus or ethmoid sinus to collect samples from 13 CRSwNP, 8 CRSsNP and 14 controls to detect a panel of respiratory viruses and found viral presence more commonly in CRS patients. They further used sinonasal questionnaire, modified Lund-Mackay and modified Lund-Kennedy scores to determine the severity of symptoms in these patients. The results indicated predominant association of viruses with CRSsNP group (50% incidence) and only 8% in CRSwNP group; and non-significant symptomatic score, radiographic, and endoscopic differences between viral and non-viral associated CRS. Hence, they were unable to display an exacerbated symptomatology in viral associated CRS patients, although establishing their association with CRSsNP (12).
Divekar et al. conducted a prospective study in which CRSwNP cases and controls were asked to self-report immediately during exacerbation. Viral detection was done to find any difference in viral association in exacerbation of cases and controls. No significant difference in RV detection rates using PCR on nasal secretions was found during acute exacerbations in 9 CRSwNP vs. 10 controls (27).
In summary, study of viral association in CRS exacerbations was attempted using immunological markers or identification of virus itself. Of the 6 studies referenced, 3 supported the association whereas 3 couldn't identify viruses with CRS exacerbations.
5. Conclusions
A systematic review of the published data provides insufficient evidence regarding the conclusive role of viruses in CRS pathogenesis and exacerbations. Evidence suggests some probable higher prevalence of virus in the CRS subjects. CRS hosts may also possess immune characteristics that make them susceptible to virus infection and vulnerable to persistent sinonasal infections. Further studies on causation vs. association, possible mechanisms like subsequent immune dysregulation and epithelial instability with viral infections are necessary to provide further clarity to results from the current literature. To comprehensively evaluate the role of viruses in CRS with certainty, large studies with longitudinal sampling at all disease phases (i.e., prior to disease initiation, during disease initiation, during disease persistence, and during exacerbations) using standardized sampling techniques may be required. While such studies may be expensive to conduct, ascertaining the role of viruses may have important implications in the treatment and prevention of chronic rhinosinusitis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
NK: Conceptualization, Methodology, Data curation, Investigation, Writing – original draft preparation. TB: Conceptualization, Methodology, Data curation, Investigation, Writing – original draft preparation. HK: Conceptualization, Supervision. LM: Data curation, Software, Resources. AM: Supervision. MM: Review, Editing, Supervision. DL: Conceptualization, Methodology, Writing – review and editing, Supervision. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. (2021) 103:2. doi: 10.4193/rhin20.600. Dieudonné Nyenbue Tshipukane.
2. Takahashi T, Schleimer RP. Epithelial-cell-derived extracellular vesicles in pathophysiology of epithelial injury and repair in chronic rhinosinusitis: connecting immunology in research lab to biomarkers in clinics. Int J Mol Sci. (2021) 22:2. doi: 10.3390/ijms222111709
3. Lee K, Pletcher SD, Lynch SV, Goldberg AN, Cope EK. Heterogeneity of microbiota dysbiosis in chronic rhinosinusitis: potential clinical implications and microbial community mechanisms contributing to sinonasal inflammation. Front Cell Infect Microbiol. (2018) 8(MAY):2. doi: 10.3389/fcimb.2018.00168
4. Lal D, Keim P, Delisle J, Barker B, Rank MA, Chia N, et al. Mapping and comparing bacterial microbiota in the sinonasal cavity of healthy, allergic rhinitis, and chronic rhinosinusitis subjects. Int Forum Allergy Rhinol. (2017) 7(6):561–9. doi: 10.1002/alr.21934
5. Wang M, Wang C, Zhang L. Inflammatory endotypes of CRSwNP and responses to COVID-19. Curr Opin Allergy Clin Immunol. (2021) 21:8–15. doi: 10.1097/ACI.0000000000000700
6. Tomassen P, Vandeplas G, Van Zele T, Cardell LO, Arebro J, Olze H, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. (2016) 137(5):1449–1456.e4. doi: 10.1016/j.jaci.2015.12.1324
7. Stevens WW, Peters AT, Tan BK, Klingler AI, Poposki JA, Hulse KE, et al. Associations between inflammatory endotypes and clinical presentations in chronic rhinosinusitis. J Allergy Clin Immunol Pract. (2019) 7(8):2812–2820.e3. doi: 10.1016/j.jaip.2019.05.009
8. Klingler AI, Stevens WW, Tan BK, Peters AT, Poposki JA, Grammer LC, et al. Mechanisms and biomarkers of inflammatory endotypes in chronic rhinosinusitis without nasal polyps. J Allergy Clin Immunol. (2021) 147(4):1306–17. doi: 10.1016/j.jaci.2020.11.037
9. Brook I. Microbiology of chronic rhinosinusitis. Eur J Clin Microbiol Infect Dis. (2016) 35:1059–68. doi: 10.1007/s10096-016-2640-x
10. Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S, et al. Executive summary of EPOS 2020 including integrated care pathways. Rhinology. (2020) 58(2):82–111. doi: 10.4193/Rhin20.601
11. Wald ER. Microbiology of acute and chronic sinusitis in children. Am J Med Sci. (1981) 316:2. doi: 10.1097/00000441-199807000-00003
12. Rowan NR, Lee S, Sahu N, Kanaan A, Cox S, Phillips CD, et al. The role of viruses in the clinical presentation of chronic rhinosinusitis. Am J Rhinol Allergy. (2015) 29(6):e197–200. doi: 10.2500/ajra.2015.29.4242
13. Lee SB, Yi JS, Lee BJ, Gong CH, Kim NH, Joo CH, et al. Human rhinovirus serotypes in the nasal washes and mucosa of patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. (2015) 5(3):197–203. doi: 10.1002/alr.21472
14. Jang YJ, Kwon HJ, Park HW, Lee BJ. Detection of rhinovirus in turbinate epithelial cells of chronic sinusitis. Am J Rhinol. (2006) 20(6):634–6. doi: 10.2500/ajr.2006.20.2899
15. Cho GS, Moon BJ, Lee BJ, Gong CH, Kim NH, Kim YS, et al. High rates of detection of respiratory viruses in the nasal washes and mucosae of patients with chronic rhinosinusitis. J Clin Microbiol. (2013) 51(3):979–84. doi: 10.1128/JCM.02806-12
16. Basharat U, Aiche MM, Kim MM, Sohal M, Chang EH. Are rhinoviruses implicated in the pathogenesis of sinusitis and chronic rhinosinusitis exacerbations? A comprehensive review. Int Forum Allergy Rhinol. (2019) 9:1159–88. doi: 10.1002/alr.22403
17. Lal D, Song L, Brar T, Cope EK, Keim P, Williams S, et al. Antibody responses to the host microbiome in chronic rhinosinusitis. Int Forum Allergy Rhinol. (2023) 13(8):1503–10. doi: 10.1002/alr.23107
18. Hwang JW, Lee KJ, Choi IH, Han HM, Kim TH, Lee SH. Decreased expression of type I (IFN-β) and type III (IFN-λ) interferons and interferon-stimulated genes in patients with chronic rhinosinusitis with and without nasal polyps. J Allergy Clin Immunol. (2019) 144(6):1551–1565.e2. doi: 10.1016/j.jaci.2019.08.010
19. Goggin RK, Bennett CA, Bialasiewicz S, Vediappan RS, Vreugde S, Wormald PJ, et al. The presence of virus significantly associates with chronic rhinosinusitis disease severity. Allergy. (2019) 74:1569–72. doi: 10.1111/all.13772
20. Goggin RK, Bennett CA, Bassiouni A, Bialasiewicz S, Vreugde S, Wormald PJ, et al. Comparative viral sampling in the sinonasal passages; different viruses at different sites. Front Cell Infect Microbiol. (2018) 8(SEP):4–11. doi: 10.3389/fcimb.2018.00334
21. Lima JT, Paula FE, Proença-Modena JL, Demarco RC, Buzatto GP, Saturno TH, et al. The seasonality of respiratory viruses in patients with chronic rhinosinusitis. Am J Rhinol Allergy. (2015) 29(1):19–22. doi: 10.2500/ajra.2015.29.4129
22. Liao B, Hu CY, Liu T, Liu Z. Respiratory viral infection in the chronic persistent phase of chronic rhinosinusitis. Laryngoscope. (2014) 124(4):832–7. doi: 10.1002/lary.24348
23. Costa C, Garzaro M, Boggio V, Sidoti F, Simeone S, Raimondo L, et al. Detection of herpesviruses 1-6 and community-acquired respiratory viruses in patients with chronic rhinosinusitis with nasal polyposis. Intervirology. (2014) 57(2):101–5. doi: 10.1159/000358880
24. Wood AJ, Antoszewska H, Fraser J, Douglas RG. Is chronic rhinosinusitis caused by persistent respiratory virus infection? Int Forum Allergy Rhinol. (2011) 1(2):95–100. doi: 10.1002/alr.20030
25. Zaravinos A, Bizakis J, Spandidos DA. Prevalence of human papilloma virus and human herpes virus types 1–7 in human nasal polyposis. J Med Virol. (2009) 81(9):1613–9. doi: 10.1002/jmv.21534
26. Abshirini H, Makvandi M, Ashrafi MS, Hamidifard M, Saki N. Prevalence of rhinovirus and respiratory syncytial virus among patients with chronic rhinosinusitis. Jundishapur J Microbiol. (2015) 8(3):4–11. doi: 10.5812/jjm.20068
27. Divekar RD, Samant S, Rank MA, Hagan J, Lal D, O’Brien EK, et al. Immunological profiling in chronic rhinosinusitis with nasal polyps reveals distinct VEGF and GM-CSF signatures during symptomatic exacerbations. Clin Exp Allergy. (2015) 45(4):767–78. doi: 10.1111/cea.12463
28. Ramadan HH, Farr HW, Wetmore SJ. Adenovirus and respiratory syncytial virus in chronic sinusitis using polymerase chain reaction. Laryngoscope. (1997) 107(7):923–5. doi: 10.1097/00005537-199707000-00017
29. Tao Q, Srivastava G, Dickens P, Ho FC, Kong H. Communication detection of epstein-barr virus-infected mucosal lymphocytes in nasal polyps. Am J Pathol. (1996) 149:4–11
30. Lee SH, Han MS, Lee TH, Bin LD, Park JH, Lee SH, et al. Rhinovirus-induced anti-viral interferon secretion is not deficient and not delayed in sinonasal epithelial cells of patients with chronic rhinosinusitis with nasal polyp. Front Immunol. (2022) 13:7–9. doi: 10.3389/fimmu.2022.1025796
31. Lee SH, Kang SH, Han MS, Kwak JW, Kim HG, Lee TH, et al. The expression of ephrinA1/ephA2 receptor increases in chronic rhinosinusitis and ephrinA1/ephA2 signaling affects rhinovirus-induced innate immunity in human sinonasal epithelial cells. Front Immunol. (2021) 12:7–9. doi: 10.3389/fimmu.2021.793517
32. Lee SB, Song JA, Choi GE, Kim HS, Jang YJ. Rhinovirus infection in murine chronic allergic rhinosinusitis model. Int Forum Allergy Rhinol. (2016) 6(11):1131–8. doi: 10.1002/alr.21805
33. Chang EH, Willis AL, McCrary HC, Noutsios GT, Le CH, Chiu AG, et al. Association between the CDHR3 rs6967330 risk allele and chronic rhinosinusitis. J Allergy Clin Immunol. (2017) 139(6):1990–1992.e2. doi: 10.1016/j.jaci.2016.10.027
34. Lan F, Wang XD, Nauwynck HJ, Holtappels G, Zhang L, Johnston SL, et al. Th2 biased upper airway inflammation is associated with an impaired response to viral infection with herpes simplex virus. Rhinology. (2016) 54(2):141–9. doi: 10.4193/rhino15.213
35. Hong H, Tan KS, Yan Y, Chen F, Ong HH, Oo Y, et al. Induction of il-25 expression in human nasal polyp epithelium by influenza virus infection is abated by interferon-alpha pretreatment. J Inflamm Res. (2021) 14:2769–80. doi: 10.2147/JIR.S304320
36. Willis AL, Calton JB, Calton J, Kim AS, Lee R, Torabzadeh E, et al. RV-C infections result in greater clinical symptoms and epithelial responses compared to RV-A infections in patients with CRS. Allergy. (2020) 75:3264–7. doi: 10.1111/all.14435
37. Tian T, Zi X, Peng Y, Wang Z, Hong H, Yan Y, et al. H3n2 influenza virus infection enhances oncostatin M expression in human nasal epithelium. Exp Cell Res. (2018) 371(2):322–9. doi: 10.1016/j.yexcr.2018.08.022
38. Rezaee F, DeSando SA, Ivanov AI, Chapman TJ, Knowlden SA, Beck LA, et al. Sustained protein kinase D activation mediates respiratory syncytial virus-induced airway barrier disruption. J Virol. (2013) 87(20):11088–95. doi: 10.1128/JVI.01573-13
39. Wang XD, Zhang N, Glorieux S, Holtappels G, Vaneechoutte M, Krysko O, et al. Herpes simplex virus type 1 infection facilitates invasion of Staphylococcus aureus into the nasal mucosa and nasal polyp tissue. PLoS One. (2012) 7(6):5–11. doi: 10.1371/journal.pone.0039875
40. Wang JH, Kwon HJ, Jang YJ. Rhinovirus enhances various bacterial adhesions to nasal epithelial cells simultaneously. Laryngoscope. (2009) 119(7):1406–11. doi: 10.1002/lary.20498
41. Wang JH, Kwon HJ, Jang YJ. Rhinovirus upregulates matrix metalloproteinase-2, matrix metalloproteinase-9, and vascular endothelial growth factor expression in nasal polyp fibroblasts. Laryngoscope. (2009) 119(9):1834–8. doi: 10.1002/lary.20574
42. Klemens JJ, Thompson K, Langerman A, Naclerio RM. Persistent inflammation and hyperresponsiveness following viral rhinosinusitis. The laryngoscope. (2006) 116:7–10. doi: 10.1097/01.mlg.0000224526.43698.52
43. Das S, Palmer OP, Leight WD, Surowitz JB, Pickles RJ, Randell SH, et al. Cytokine amplification by respiratory syncytial virus infection in human nasal epithelial cells. Laryngoscope. (2005) 115(5):764–8. doi: 10.1097/01.MLG.0000159527.76949.93
44. Wu D, Bleier BS, Wei Y. Current understanding of the acute exacerbation of chronic rhinosinusitis. Front Cell Infect Microbiol. (2019) 9:8. doi: 10.3389/fcimb.2019.00415
45. Alho OP, Karttunen R, Karttunen TJ. Nasal mucosa in natural colds: effects of allergic rhinitis and susceptibility to recurrent sinusitis. Clin Exp Immunol. (2004) 137(2):366–72. doi: 10.1111/j.1365-2249.2004.02530.x
46. Mackay IM. Human rhinoviruses: the cold wars resume. J Clin Virol. (2008) 42:297–320. doi: 10.1016/j.jcv.2008.04.002
47. Vareille M, Kieninger E, Edwards MR, Regamey N. The airway epithelium: soldier in the fight against respiratory viruses. Clin Microbiol Rev. (2011) 24(1):210–29. doi: 10.1128/CMR.00014-10
48. Proud D, Turner RB, Winther B, Wiehler S, Tiesman JP, Reichling TD, et al. Gene expression profiles during in vivo human rhinovirus infection insights into the host response. Am J Respir Crit Care Med. (2008) 178(9):962–8. doi: 10.1164/rccm.200805-670OC
49. Roger T, Bresser P, Snoek M, van der Sluijs K, van den Berg A, Nijhuis M, et al. Exaggerated IL-8 and IL-6 responses to TNF-α by parainfluenza virus type 4-infected NCI-H292 cells. Am J Physiol Lung Cell Mol Physiol. (2004) 287:1048–55. doi: 10.1152/ajplung.00396.2003
50. Chen Q, Tan KS, Liu J, Ong HH, Zhou S, Huang H, et al. Host antiviral response suppresses ciliogenesis and motile ciliary functions in the nasal epithelium. Front Cell Dev Biol. (2020) 8:9. doi: 10.3389/fcell.2020.581340
51. Corne JM, Lau L, Scott SJ, Davies R, Johnston SL, Howarth PH. The relationship between atopic status and IL-10 nasal lavage levels in the acute and persistent inflammatory response to upper respiratory tract infection. Am J Respir Crit Care Med. (2001) 163:9. doi: 10.1164/ajrccm.163.5.9902047
52. Chuang CY, Kao CL, Huang LM, Lu CY, Shao PL, Lee PI, et al. Human bocavirus as an important cause of respiratory tract infection in Taiwanese children. J Microbiol Immunol Infect. (2011) 44(5):323–7. doi: 10.1016/j.jmii.2011.01.036
53. Jiao J, Wang C, Zhang L. Epithelial physical barrier defects in chronic rhinosinusitis. Expert Rev Clin Immunol. (2019) 15:679–88. doi: 10.1080/1744666X.2019.1601556
54. Savlevich EL, Kurbacheva OM, Egorov VI, Dyneva ME, Shilovskiy IP, Khaitov MR. Gene expression levels of cytokines in different phenotypes of CRSwNP. Vestn Otorinolaringol. (2019) 84(6):42–7. doi: 10.17116/otorino20198406142
55. Monto AS. The seasonality of rhinovirus infections and its implications for clinical recognition. Clin Ther. (2002) 24:10. doi: 10.1016/s0149-2918(02)80093-5
56. Marin J, Jeler-Kačar D, Levstek V, Maček V. Persistence of viruses in upper respiratory tract of children with asthma. Journal of Infection. (2000) 41(1):69–72. doi: 10.1053/jinf.2000.0688
57. Hardjojo A, Goh A, Shek LPC, Van Bever HPS, Teoh OH, Soh JY, et al. Rhinitis in the first 18 months of life: exploring the role of respiratory viruses. Pediatr Allergy Immunol. (2015) 26(1):25–33. doi: 10.1111/pai.12330
58. Tacon CE, Wiehler S, Holden NS, Newton R, Proud D, Leigh R. Human rhinovirus infection up-regulates MMP-9 production in airway epithelial cells via NF-κB. Am J Respir Cell Mol Biol. (2010) 43(2):201–9. doi: 10.1165/rcmb.2009-0216OC
59. Chen YS, Langhammer T, Westhofen M, Lorenzen J. Relationship between matrix metalloproteinases MMP-2, MMP-9, tissue inhibitor of matrix metalloproteinases-1 and IL-5, IL-8 in nasal polyps. Allergy. (2007) 62(1):66–72. doi: 10.1111/j.1398-9995.2006.01255.x
Keywords: sinusitis, respiratory virus, chronic rhinosinusitis, viral infection, exacerbation, inflammation
Citation: Kumar N, Brar T, Kita H, Marks LA, Miglani A, Marino MJ and Lal D (2023) Viruses in chronic rhinosinusitis: a systematic review. Front. Allergy 4:1237068. doi: 10.3389/falgy.2023.1237068
Received: 8 June 2023; Accepted: 20 November 2023;
Published: 5 December 2023.
Edited by:
Pongsakorn Tantilipikorn, Mahidol University, ThailandReviewed by:
Nathachit Limjunyawong, Mahidol University, ThailandYu Xu, Renmin Hospital of Wuhan University, China
© 2023 Kumar, Brar, Kita, Marks, Miglani, Marino and Lal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Devyani Lal bGFsLmRldnlhbmlAbWF5by5lZHU=
Abbreviations AdV, adenovirus; AJC, apical junctional complex; ALI, air liquid interface; ARS, acute rhinosinusitis; BoV, bocavirus; CAM, cell adhesion molecule; CEACAM, carcinoembryonic antigen related cell adhesion molecule; CMV, cytomegalovirus; CoV, corona virus; CRS, chronic rhinosinusitis; CRSwNP, chronic rhinosinusitis with nasal polyps; CRSsNP, chronic rhinosinusitis sine (without) nasal polyps; EBV, epstein-barr virus; ECM, extra cellular matrix; ELISA, enzyme linked immuno sorbent assay; EMBP, eosinophilic major basic protien; EV, enterovirus; Fn, fibronectin; GM-CSF, granulocyte monocyte colony stimulating factor; HHV, human herpes virus; HMPV, human metapneumovirus; HNEC, human nasal epithelial cell; HPV, human papilloma virus; HRV, human rhinovirus; HSV, herpes simplex virus; ILC, innate lymphoid cell; IFN, interferon; Ig, immunoglobulin; IL, interleukin; ISG, interferon stimulated genes; MeSH, medical subject headings; MMP, matrix metallo proteinase; mRNA, messenger ribonucleic acid; siRNA, silencing ribonucleic acid; snRNA, small nuclear RNA; NK, natural killer cells; OSM, oncostatin M; PAF, platelet activated factor; PCR, polymerase chain reaction; PIV, para influenza virus; PKD, protein kinase D; PRISMA, preferred reporting items for systematic reviews and meta-analyses; rt-PCR, real time PCR; RSV, respiratory syncytial virus; RV, rhinovirus; SeV, sendai virus; SNOT, sino nasal outcome questionnaire test; SNP, single nucleotide polymorphism; TEER, trans epithelial electrical resistance; Th, T helper cell; TNF, tumor necrosis factor; UV, ultraviolet; VEGF, vascular endothelial growth factor; VZV, varicella zoster virus; ZO, zonula occludens.
 Nitish Kumar
Nitish Kumar Tripti Brar
Tripti Brar Hirohito Kita
Hirohito Kita Lisa A. Marks
Lisa A. Marks Amar Miglani1
Amar Miglani1 Devyani Lal
Devyani Lal