- 1Department of Respiratory Medicine, Copenhagen University Hospital-Hvidovre, Hvidovre, Denmark
- 2Institute of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark
Introduction: Add-on magnesium sulfate (MgSO4) for refractory asthma exacerbation has been much debated. The aim of this review and meta-analysis is, therefore, to provide an update on the current evidence for the efficacy of MgSO4 in exacerbations of asthma in adults refractory to standard of care treatment.
Methods: A systematic review was performed in accordance with the PRISMA guidelines. The search was performed in the PubMed database (updated April 2023). For the meta-analysis, a random-effects model was applied using the metaphor package for RStudio (RStudio, Inc.).
Results: A total of 17 randomized controlled trials were included. Three of the nine studies addressing treatment with intravenous (IV) MgSO4 found a significant effect on lung function compared to placebo. Of the eight studies investigating hospital admission rate, only two found a significant effect of MgSO4. Six of the nine studies investigating treatment with nebulized MgSO4 compared to placebo found a favorable effect on forced expiratory volume in 1. second (FEV1) and peak expiratory flow rate (PEF). Only two of the five studies investigating the effect on hospital admission rate found an effect of MgSO4. Comparing effect sizes in a meta-analysis revealed a greater effect on PEF in asthma patients treated with nebulized MgSO4 (MD, 23.57; 95% CI, −2.48 to 49.62, p < 0.01) compared to placebo. The analysis of patients treated with i.v. MgSO4 compared to placebo showed no statistically significant difference (MD, 5.49; 95% CI, −18.67 to 29.65, p = 0.10).
Conclusion: Up to two out of three studies revealed an effect of MgSO4 treatment for asthma exacerbation when assessed by FEV1/PEF, but fewer studies were positive for the outcome of hospital admissions.
Introduction
Asthma is a chronic inflammatory airway disease that even in patients with mild disease is associated with periodically severe worsening, also referred to as exacerbations (1). While mild-to-moderate exacerbations may be managed in primary care, more severe exacerbations often require management in the ER and/or hospital admission.
In Denmark, a country of 5.8 million inhabitants, asthma exacerbations result in approximately 1,400 ER visits and 6,300 hospital admissions each year (2). The standard of care for patients with severe acute exacerbations managed in hospitals comprises supplemental oxygen, high doses of nebulized short-acting β2 agonists (SABA) in combination with a short-acting muscarinic antagonist (SAMA), and systemic corticosteroids (3, 4). Despite the initial treatment in the ER, some patients do not improve sufficiently and, therefore, require admission for further treatment. In very severe acute exacerbations of asthma refractory to standard treatment, the patient may need to be transferred to the intensive care unit (ICU), where intubation and mechanical ventilation may be needed. In the United States, about 10% of patients admitted with asthma exacerbations were reported to require transferal to the ICU in the year 2,000 (5), but the proportion differs between countries at least partly due to differences in referral criteria.
In severe refractory acute asthma, a number of guidelines recommend add-on intravenous infusion and/or nebulized magnesium sulfate (MgSO4) (3, 4), but in contrast to the standard treatment of asthma exacerbations, the clinical effect of MgSO4 has been much debated.
The aim of this review and meta-analysis is to provide an update on the present evidence for the efficacy of MgSO4 in the treatment of acute refractory asthma in adults.
Methods
This review and meta-analysis were carried out in accordance with the PRISMA statement (6).
A systematic search was performed in the PubMed, Medline, and Embase databases and updated in April 2023. The strategy was to identify all RCTs addressing the treatment of acute asthma with MgSO4. The search algorithm consisted of whole words (acute asthma AND magnesium sulfate) combined with the MeSH terms (“asthma” AND “magnesium sulfate”). All of the records were systematically reviewed by all the authors using Covidence; first on the title/abstract level, then on the full-text level. All conflicts were handled by at least two of the authors, who discussed why or why not to include the study of conflict.
Publications were included in the present review provided they fulfilled the following criteria: (1) addressed treatment of acute asthma in adults (≥15 years) with magnesium sulfate (i.v./nebulized), (2) RCT, (3) published in 1990 or later, and that they did not fulfil the following criteria: (1) non-RCT, (2) addressing treatment of asthma in children, and (3) non-English publication.
The main outcomes of focus were FEV1, PEF, and hospital admission/discharge.
To avoid missing any relevant studies, all reference lists of the included studies and previous reviews were scanned for additional studies potentially fulfilling the criteria for inclusion in the present review.
After including all relevant studies, a risk-of-bias analysis was performed using the Cochrane risk-of-bias tool for randomized trials (7).
A meta-analysis of the studies fulfilling the criteria was conducted. Studies were included in the meta-analysis if they provided relevant data on peak expiratory flow (PEF). Most studies provided PEF at the baseline and at the end of the study period, whereas studies had missing data on FEV1 and variation in definition of hospital admissions, which made PEF the preferred variable for inclusion in a meta-analysis of the study findings. Duration of treatment with either nebulized or intravenous magnesium or placebo until the end of the study period differed between studies. Some studies provided treatment durations ranging from one to four hours, while others only provided a final PEF before discharge. We calculated the mean difference (MD) with 95% confidence intervals to assess the final difference in PEF between patients and placebo. We assessed heterogeneity in the included trials and considered a p-value threshold of 0.1 or less for the test of heterogeneity or less as statistically significant. Random-effects models were used for the meta-analysis if statistical significance was present. A random-effects model analysis was carried out using the metafor package of RStudio Version 1.2.5001 2009–2019 RStudio, Inc.
The present study was a systematic review and meta-analysis and, therefore, approval from the scientific ethical committee and the Danish Medicines Agency was not required.
Results
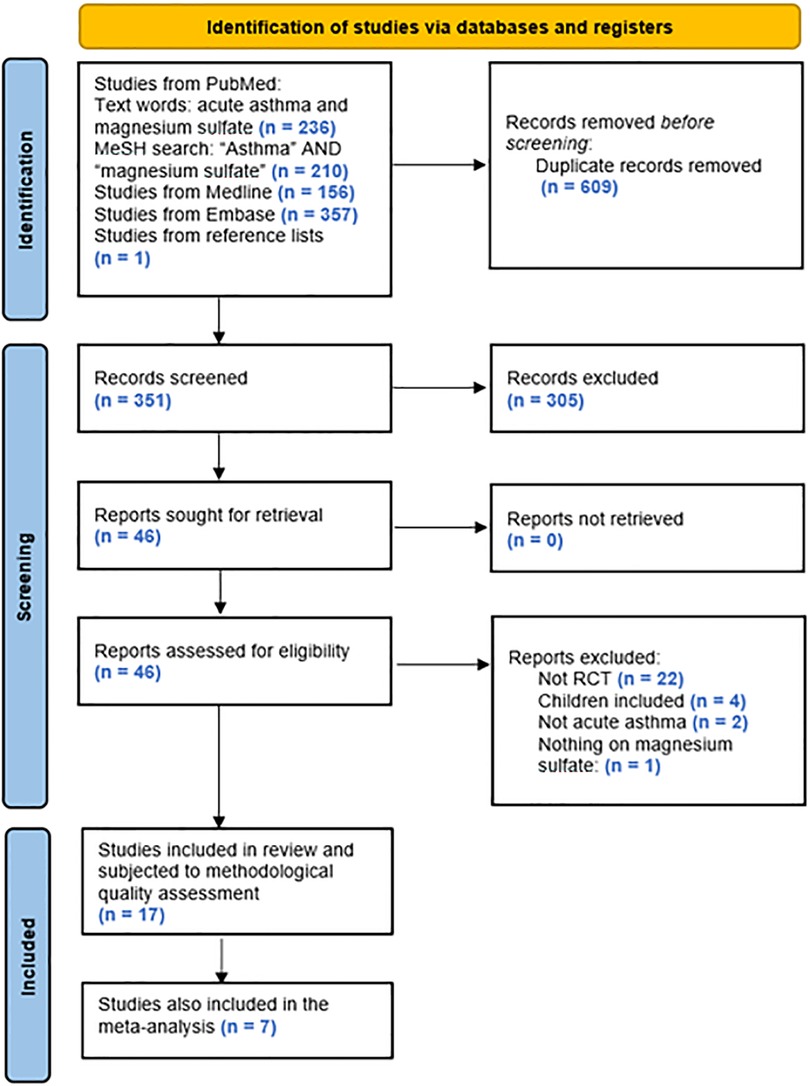
The search algorithm provided 236, 210, 156, and 357 hits, respectively, of which 16 studies fulfilled the predefined criteria and were included in the present review. Based on the additional search described, 1 further study fulfilling the criteria was identified and added, leading to a total of 17 studies included (Figure 1).
Of the 17 studies included, 8 assessed i.v. MgSO4 treatment, 8 investigated treatment with nebulized MgSO4, and 1 assessed both i.v. and nebulized MgSO4 treatment.
Apart from one study, all studies investigating hospital admission provided exact criteria for hospital admission (8). In the former study, however, the clinician responsible for the decision was blinded to treatment allocation and may therefore not be considered biased (8).
Only one study mentioned that asthma diagnosis had previously been objectively verified (bronchodilator reversibility) (9). The largest study included did not find any significant difference between placebo and either i.v. or nebulized MgSO4 with regard to hospital admission rate or improvement in PEF%predicted (10).
Table 1 summarizes the main characteristics of the included studies.
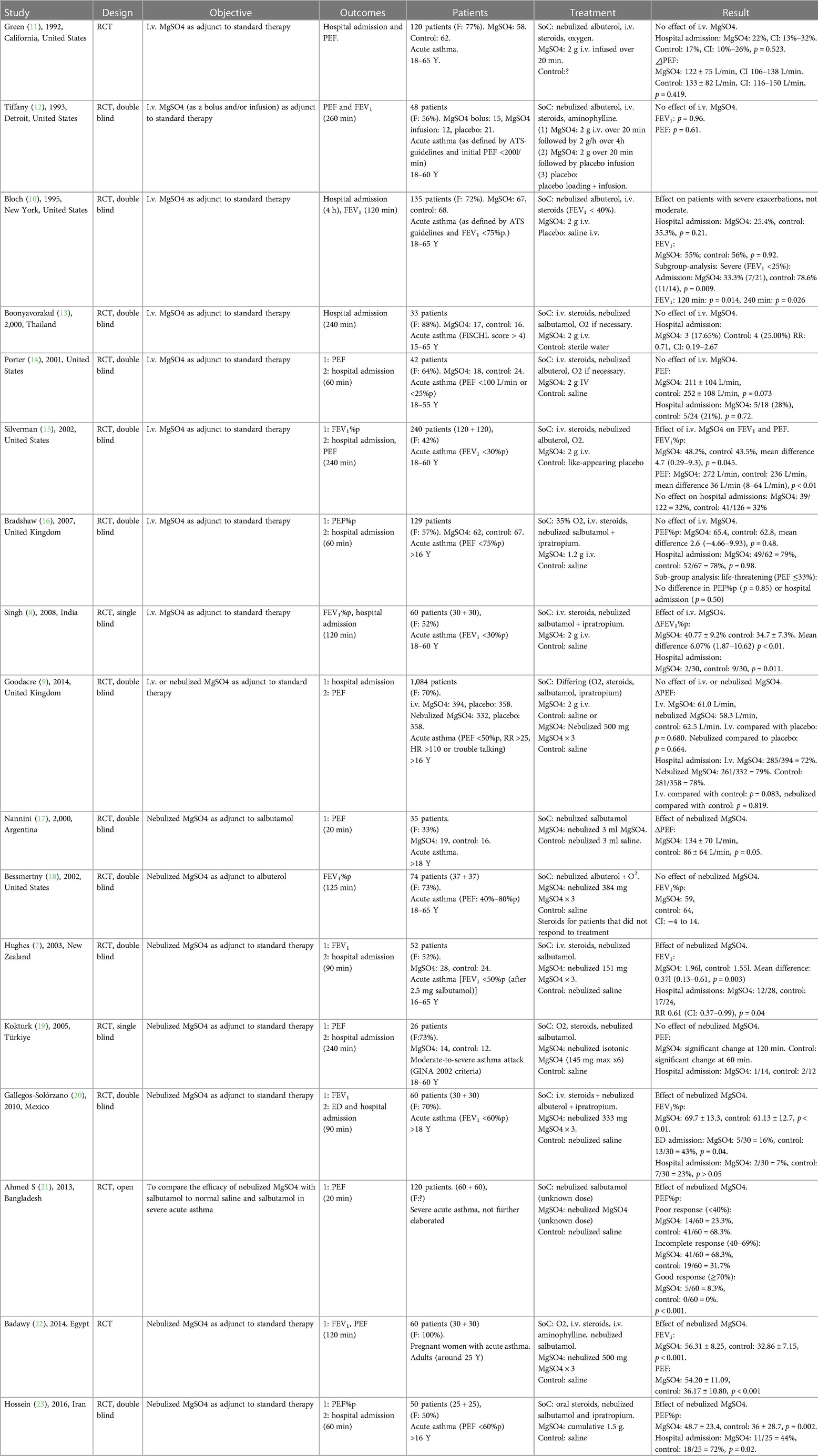
Table 1. Overview of the studies on MgSO4, comparing designs, outcome measurements, study populations, different treatments, and results.
Intravenous magnesium sulfate in acute refractory asthma exacerbation
Only three of the nine studies investigating treatment with i.v. MgSO4 for acute asthma exacerbation found a significant effect on PEF and/or FEV1 compared to placebo. Bloch et al. did not find a significant difference between groups when comparing all the patients included (135 patients, FEV1 <75%predicted), but when analyzing data for a subgroup of patients presenting with exacerbation and FEV1 <40%predicted (35 patients), they did find a significant effect of MgSO4 in this subgroup of patients (only p-values given: after 120 min: p = 0.014, 240 min: p = 0.026) (15). Singh et al. found that the group treated with i.v. MgSO4 had a higher FEV1%predicted (62.8%pred ± 10.0% vs. 56.7%pred ± 6.2%) and significantly greater %predicted improvement from baseline (40.7%pred ± 9.2% vs. 34.7%pred ± 7.3%, p < 0.01) compared to the placebo group (9). At the final assessment, Silverman et al. found that mean FEV1 in the i.v. MgSO4 group was 48.2%predicted compared to 43.5%predicted in the placebo group (mean difference 4.7%predicted; p = 0.045), and there was also a statistically significant improvement in PEF (mean difference 36 L/min; p = 0.01) (11).
In conclusion, three studies comprising a total of 335 patients found an effect of i.v. MgSO4 on improvement in PEF/FEV1 (although one of these studies only found an effect in a subgroup analysis) (9, 11, 15), whereas six studies with a total of 1,124 patients did not find any effect (10, 12–14, 16, 17).
Only two of the eight studies, which also investigated hospital admission, found that i.v. MgSO4 significantly increased the hospital discharge rate compared to placebo. Singh et al. showed that the discharge rate after two hours was significantly higher in the MgSO4 group (placebo: 21/30 and MgSO4: 28/30, p < 0.05) (9). Bloch et al. did not find an effect on the entire patient cohort, but in the subgroup analysis with patients suffering from severe exacerbations (FEV1 <25%predicted), they found a significant difference in hospital admission rates between the patients treated with MgSO4 (7/21 = 33.3%) compared with the placebo group (11/14 = 78.6%, p = 0.009) (15).
All the studies except one (13) also described side effects of i.v. MgSO4. The most common side effects were fatigue (14, 15) and flushing (14, 15, 17), but none of the studies observed severe adverse effects.
Nebulized magnesium sulfate in acute refractory asthma exacerbation
Six of the nine studies investigating treatment with nebulized MgSO4 compared to placebo in acute asthma exacerbations found a significant effect on FEV1 and/or PEF compared to placebo. The study by Nannini et al. showed a greater increase in PEF in the MgSO4 group at 10 min after baseline (difference 30%; 95% CI: 3% to 56%; p = 0.03) and again at 20 min after baseline (difference 57%; 95% CI: 4% to 110%; p = 0.04) compared to the placebo group. The absolute increase, however, did not statistically differ significantly at any time point (10 min difference: 23 L/min, p = 0.18; 20 min difference: 48 L/min, p = 0.05) (20). Hughes et al. found a significant difference in the mean FEV1 between the two groups (0.37 L; p = 0.003) in favor of MgSO4 (8). Gallegos-Solórzano et al. showed that adding nebulized MgSO4 to the treatment resulted in statistically significant increases in FEV1%predicted (placebo: 61.13%pred ± 12.7 vs. MgSO4: 69.7%pred ± 13.3; p < 0.01) (21). Ahmed et al. found that the % increase in PEF after 20 min was significantly greater in the MgSO4 group (35% ± 7%) than in the placebo group (24% ± 6%, p < 0.001) (22). The study by Badawy et al. similarly found significant improvement of FEV1 after 120 min in the MgSO4 group compared to placebo (MgSO4: 56.31 ± 8.25, control: 32.86 ± 7.15, p < 0.001, measuring unit not given) (23). Hossein et al. showed that PEF%predicted was significantly higher in the MgSO4 group (48.7%pred ± 23.4) than in the placebo group (36%pred ± 28.7; p = 0.002 and p < 0.001) after 60 min (18).
Even though six studies found an effect of nebulized MgSO4 on PEF/FEV1, the studies only included a total number of 377 patients, compared to the 790 patients in the three studies that did not find an effect (10, 19, 24).
Only two of five studies, which also investigated hospital admission, found that nebulized MgSO4 increased the hospital discharge rate compared to placebo. Hughes et al. showed that the hospital admission rate was significantly higher in the placebo group (MgSO4: 12/28, placebo: 17/24; p = 0.04) (8). Hossein et al. found that the hospital admission rate after 60 min was lower in the MgSO4 group compared to placebo (MgSO4: 44%, placebo: 72%, p = 0.02) (18).
All studies besides that of Badawy (23) also described the difference in adverse effects between groups. The most common adverse effects of MgSO4 described are transient hypotension (10, 24) and bitter taste (19, 21), but none of the adverse effects resulted in withdrawal from the studies. Three studies did not report any adverse effects (8, 18, 20).
Pooled estimate analysis of PEF measurements
A total of eight studies were included in the meta-analyses for a pooled estimate analysis of mean differences in PEF measurements. Four studies in one meta-analysis illustrated an effect of i.v. MgSO4 compared to placebo, and four studies in another meta-analysis illustrated nebulized MgSO4 compared to placebo. Excluded studies provided measurements of FEV1 and/or PEF%predicted and were hence not included in the meta-analyses. In total, 793 patients were treated with i.v. MgSO4, and 575 patients were treated with nebulized MgSO4. In all, 592 of those receiving i.v. MgSO4 and 441 of those receiving nebulized MgSO4 were included in the meta-analyses.
Through a pooled estimate analysis of mean differences, the weighted mean difference in PEF measurements using a random-effects model was 5.49 (95% CI, −18.67 to 29.65, p = 0.10) in patients receiving i.v. MgSO4 compared to placebo, while the weighted mean differences using a random-effects model was 23.57 (95% CI, −2.48 to 49.62, p < 0.01) in patients receiving nebulized MgSO4 compared to placebo.
The analysis of patients receiving i.v. MgSO4 compared to placebo was not significant, while the analysis of nebulized MgSO4 compared to placebo showed a statistically significant difference.
More details are provided in Figures 2, 3.
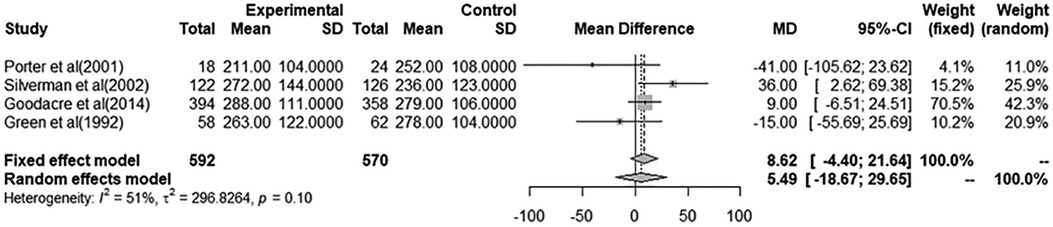
Figure 2. Meta-analysis using a random effects model to determine mean peak expiratory flow (L/min) difference (95% confidence interval) between placebo/controls and severe asthma patients receiving intravenous magnesium sulfate.
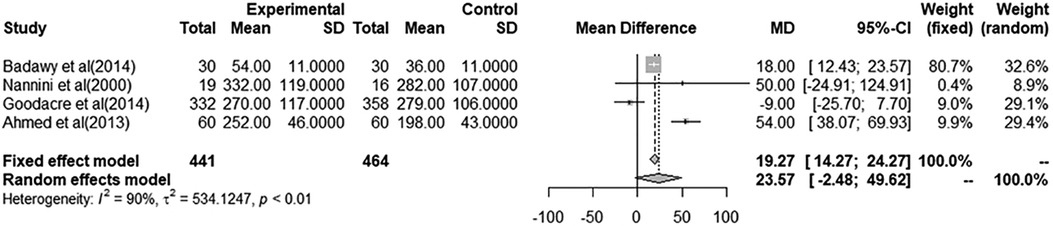
Figure 3. Meta-analysis using a random effects model to determine mean peak expiratory flow (L/min) difference (95% confidence interval) between placebo/controls and severe asthma patients receiving nebulized magnesium sulfate.
Sensitivity analysis
The pooled effect size estimate analysis was repeated in series after stepwise omission of each included RCT in a sensitivity analysis, which revealed that no individual study had an impact of the mean difference estimate of more than 8.86 L/min in studies focusing on difference in PEF between i.v. and placebo (variation of estimates was −3.37 [−28.18–21.43] to 11.30 [−11.23–33.83]) and 13.91 L/min in studies focusing on difference in PEF between nebulized and placebo (variation of estimates was 9.66 [−14.72–34.03] to 37.06 [5.29–68.83]) (Table 2).
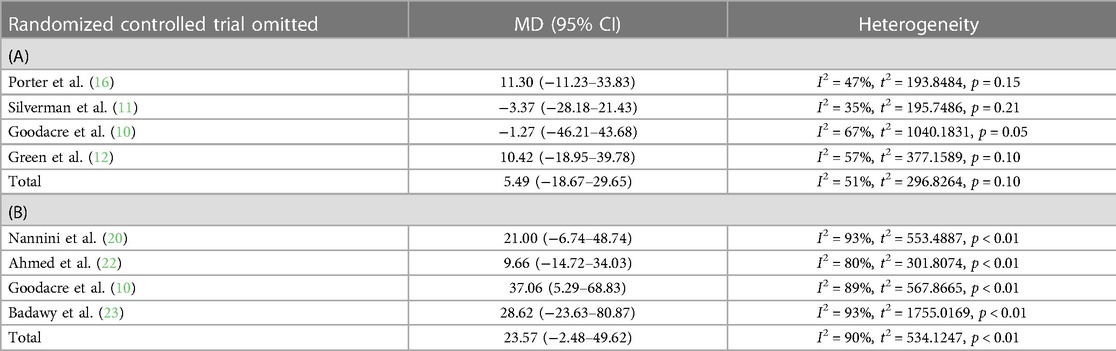
Table 2. Sensitivity analysis of included studies focusing on either (A) intravenous or (B) nebulized magnesium sulfate.
Quality and bias risk assessment
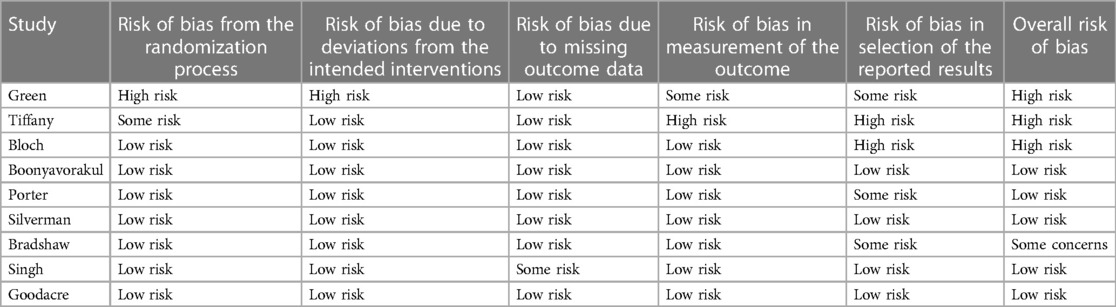
Overall, the studies on i.v. magnesium sulfate for acute asthma had a low risk of bias. Boonyavorakul, Silverman, and Goodacre were classified as having low risk of bias in all categories. See Table 3 for the total assessment.
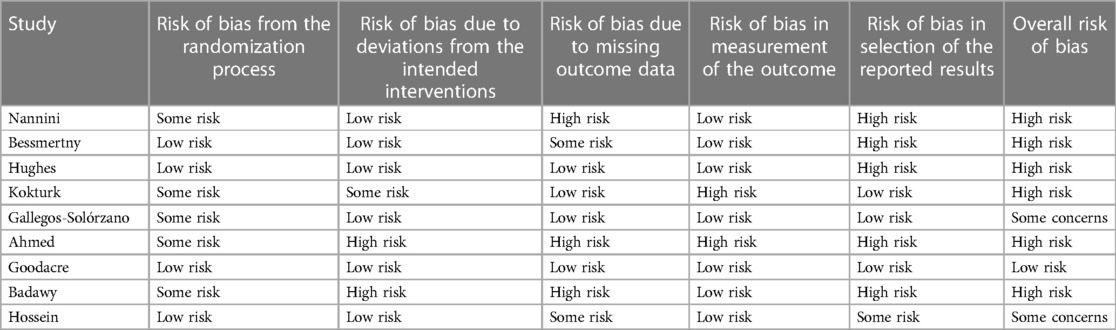
The studies on nebulized magnesium sulfate for the treatment of acute asthma generally had a higher risk of bias. Of the nine studies included, only Goodacre was low in risk of bias in all categories. Many of the studies had a high risk of bias in their reporting of results, mostly because data were not reported on all the outcomes of interest (see Table 4).
Discussion
The evidence concerning treatment of acute asthma with MgSO4 continues to be, as shown above, rather conflicting, irrespective of route of administration of MgSO4.
Nine RCTs investigate the effect of i.v. MgSO4 on acute asthma, three studies demonstrate an effect of i.v. MgSO4 (9, 11, 15) (335 patients), and six studies do not find a significant effect of i.v. MgSO4 as an add-on to standard therapy (10, 12–17) (1,124 patients).
The MgSO4 dose administered ranges from 2 g (9, 11, 14, 16) to 1.2 g (17), and no obvious relationship between the size of dose and effect on outcome is seen.
If we look at the RCTs' different measurements used to determine the effect of MgSO4, there is no clear association either. Objective measurements of lung function as FEV1 and PEF are used by studies that find an effect of MgSO4 (9, 11, 15) and by studies that do not find an effect (10, 13, 16, 17).
A more subjective measure used is hospital admission rate, which is very relevant because it is a measure that directly affects the patient (who will either spend days in the hospital or go home) and the economics of the healthcare system. Two studies (9, 15) (only sub-analysis)) find a decrease in hospital admission in the MgSO4 group compared to placebo, and six studies (10–12, 14, 16, 17) do not find any difference between the two groups.
Another possible explanation for the conflicting results could be differences in severity of asthma exacerbations, but if we assume that FEV1 and PEF are comparable measurements, the results concerning severe exacerbations are conflicting and not exclusively pointing towards an effect of MgSO4 as implied (9, 11, 15).
When only looking at the studies with low risk of bias, the results are still conflicting. When excluding Green (12), Bradshaw (17), Bloch (15), and Tiffany (13) from the analysis, two studies find an effect of MgSO4 on lung function (n = 200) and three do not (n = 827).
In the nine RCTs investigating the effect of nebulized MgSO4 in acute asthma, six studies find a significant effect of MgSO4 (n = 377), while three studies do not (n = 790). The conflicting results regarding nebulization cannot be explained by the size of the MgSO4 dose given or by the different outcome measurements either. The treatment dose of MgSO4 differs between the different studies. Three studies give the highest cumulative dose of 1,5 g of nebulized MgSO4 (10, 18, 23). Of these three studies, two find an effect, and one does not find an effect of MgSO4, which indicates that the difference in the given dose is unlikely to explain the different results.
The study with the second highest MgSO4 dosage is that of Bessmertny et al. (384 mg × 3) (19), who do not find an effect of MgSO4 either.
When delivering medication through a nebulizer compared to i.v., the delivered dose also depends on, among other factors, particle size and device technique. Most of the studies provide details on administering isotonic MgSO4, and some also describe the specific nebulizer used (jet nebulizer (8, 20, 24) or circulaire nebulizer (19)), but it is not possible to assess the impact of the more precise impact on particle sizes and delivered dose in the included studies.
Four studies use FEV1 (8, 19, 21, 23) as the outcome measure, of which three find a significant effect of MgSO4, and one study does not find any effect. The five other studies use PEF as a primary outcome; three find an effect (18, 20, 22) and two do not (10, 24).
Two studies find a significant decrease in hospital admission rate using MgSO4 (8, 18); three studies find no difference in admission rate (10, 21, 24).
Only Goodacre (10), who does not find an effect of nebulized MgSO4, has a low risk of bias in all categories. Gallegos-Solórzano (21) and Hossein (18) have some concerns in the analysis, while the rest of the studies reach high risk in at least one category. Gallegos-Solórzano and Hossein both find an effect of nebulized MgSO4, which results in conflicting results, even when taking the risk of bias analysis into account.
The Danish Society for Respiratory Medicine recommends treatment with i.v. MgSO4 in severe asthma exacerbation (3), with reference to MgSO4 having a proven effect on the length of hospital stay but not on the risk of need for intubation. This review, however, does not provide evidence that MgSO4 shortens hospital stays of asthmatic patients. Only two of the studies on i.v. MgSO4 find a significant effect of MgSO4 on discharge rate. Six studies do not find an effect of i.v. MgSO4 on hospital admission rate/length of stay.
The GINA guidelines from 2023 do not recommend the routine use of MgSO4 for asthma exacerbations but mention the possible effect in some patients suffering from severe exacerbations not responding well to standard treatment (4). Again, they recommend i.v. and not nebulized, MgSO4.
This recommendation fits better with the finding in our study, namely, not promising an evidential effect but using MgSO4 when proven treatments have been given without satisfying effect. The recommended pathway of delivering MgSO4 is, however, questionable.
The recommendation to give MgSO4 for patients not responding to standard treatment makes sense, considering that none of the studies included in this review report serious side effects to the treatment with MgSO4.
The findings reported for i.v. MgSO4 are not totally aligned with the latest Cochrane review performed on the same subject (1). Kew et al.'s review concludes that i.v. MgSO4 given to patients with status asthmaticus lowers hospital admission rate and improves lung function. Even though the Cochrane review was performed in 2014, no new RCTs have been included in this study that was not included in that review. The different conclusion may be caused by Kew et al. having included studies on children and studies where only abstracts were available. We decided to concentrate on adults since the way treatment works on children and adults is not always the same. We decided not to include studies that only published an abstract since we do not think an abstract provides enough information about the study for us to decide if the results are reliable.
Our conclusions on the doubtful effect of nebulized MgSO4 are very similar to those of the Cochrane review by Knightly et al. (25), even though they included studies on children and studies in which only abstracts were available.
The comparison of the different RCT's on the subject has not been easy. The SoC differs a lot between the studies; only five studies give nebulized SAMA; three studies do not use steroids at all, while two studies only include steroids if needed; and one study does not even add SABA to all the patients' treatments. This means that a lot of the included RCTs do not follow standard treatment guidelines for patients presenting with acute asthma. The RCTs not following standard treatment guidelines are represented in cases both for and against MgSO4. Bradshaw (17) and Goodacre (10) find no effect of i.v. MgSO4, while Singh (9) does find an effect; meanwhile, Gallegos-Solórzano (21) and Hossein (18) find an effect of nebulized MgSO4, while Goodacre (10) does not.
Another limitation worth mentioning is that the proportion of patients admitted differs between the included studies. In some studies, relatively few participants were included. This may be due to regional differences in hospital practices and varied clinical assessments of exacerbation severity. In addition, the included studies may have included individuals with a variety of differences in terms of gender, age, and ethnicity. These variations are usually adjusted for in the randomization process in each study. However, in our meta-analysis, it caused a high variation in our summary of mean differences. This is indicated by the higher I2 statistic in both the analysis of IV MgSO4 compared to placebo and nebulized MgSO4 compared to placebo, suggesting substantial heterogeneity.
Conclusion
In conclusion, the reported findings regarding the treatment of acute asthma with intravenous/nebulized MgSO4 are conflicting. Overall, the evidence points against MgSO4 having a beneficial effect on lung function and decreasing admission rate in patients presenting with acute asthma. On the other hand, none of the included studies demonstrate severe side effects of MgSO4; thus, considering the low risk, treatment with MgSO4 can be attempted as a last resort in patients with refractory symptoms after standard treatment.
Key messages
• Of the studies investigating the effect of intravenous magnesium sulfate, more than half found no effect on lung function.
• Only two of the five studies investigating the effect of nebulized magnesium sulfate on hospital admission found a positive effect.
• The effect of intravenous or nebulized magnesium sulfate for acute asthma exacerbation refractory to standard of care treatment is inconclusive when assessed by lung function and hospital admission.
• The meta-analysis revealed a significant effect on PEF in asthma patients receiving nebulized MgSO4 compared to placebo, rather than an effect of intravenous MgSO4 compared to placebo, which was insignificant.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
All authors contributed equally to this work. All authors contributed to the article and approved the submitted version.
Conflict of interest
AHR has received travel grant from GSK outside the submitted work. OS has received personal fees for advisory board meeting from GSK outside the submitted work. CSU has received personal fees for oral presentations, advisory board meetings etc. from AstraZeneca, Novartis, GSK, Boehringer-Ingelheim, ALK-Abello, Chiesi, Sanofi Genzyme, MundiPharma, Actelion, Covis Pharma, Orion Pharma and TEVA outside the submitted work.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kew KM, Kirtchuk L, Michell CI. Intravenous magnesium sulfate for treating adults with acute asthma in the emergency department. Cochrane Database Syst Rev. (2014) 5:Cd010909. doi: 10.1002/14651858.CD010909.pub2
2. Sundhedsstyrelsen. Sygdomsbyrden i danmark sygdomsbyrden i danmark. (2015) (2):297–310. Available at: https://wwwsstdk/da/sygdom-og-behandling/∼/media/00C6825B11BD46F9B064536C6E7DFBA0ashx (Accessed March 4, 2022).
3. Dansk lungemedicinsk selskab. Guidelines concerning acute asthma. Available at: https://wwwlungemedicindk/fagligt/33-akut-astmahtml (Accessed March 4, 2022).
4. Global initiative for asthma. Global strategy for asthma management and prevention, 2023. (Updated May 2023). Available at: https://ginasthma.org/ (Accessed June 7, 2023).
5. Pendergraft TB, Stanford RH, Beasley R, Stempel DA, Roberts C, McLaughlin T, et al. Rates and characteristics of intensive care unit admissions and intubations among asthma-related hospitalizations. Ann Allergy Asthma Immunol. (2004) 93(1):29–35. doi: 10.1016/S1081-1206(10)61444-5
6. PRISMA Guidelines. Available at: http://wwwprisma-statementorg/PRISMAStatement/Checklist (Accessed March 4, 2022).
7. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. (2019) 366:l4898. doi: 10.1136/bmj.l4898
8. Hughes R, Goldkorn A, Masoli M, Weatherall M, Burgess C, Beasley R, et al. Use of isotonic nebulised magnesium sulphate as an adjuvant to salbutamol in treatment of severe asthma in adults: randomised placebo-controlled trial. Lancet. (2003) 361(9375):2114–7. doi: 10.1016/S0140-6736(03)13721-X
9. Singh AK, Gaur S, Kumar R. A randomized controlled trial of intravenous magnesium sulphate as an adjunct to standard therapy in acute severe asthma. Iran J Allergy Asthma Immunol. (2008) 7(4):221–9.19052352
10. Goodacre S, Cohen J, Bradburn M, Stevens J, Gray A, Benger J, et al. The 3Mg trial: a randomised controlled trial of intravenous or nebulised magnesium sulphate versus placebo in adults with acute severe asthma. Health Technol Assess. (2014) 18(22):1–168. doi: 10.3310/hta18220
11. Silverman RA, Osborn H, Runge J, Gallagher EJ, Chiang W, Feldman J, et al. IV Magnesium sulfate in the treatment of acute severe asthma: a multicenter randomized controlled trial. Chest. (2002) 122(2):489–97. doi: 10.1378/chest.122.2.489
12. Green SM, Rothrock SG. Intravenous magnesium for acute asthma: failure to decrease emergency treatment duration or need for hospitalization. Ann Emerg Med. (1992) 21(3):260–5. doi: 10.1016/S0196-0644(05)80885-6
13. Tiffany BR, Berk WA, Todd IK, White SR, et al. Magnesium bolus or infusion fails to improve expiratory flow in acute asthma exacerbations. Chest. (1993) 104(3):831–4. doi: 10.1378/chest.104.3.831
14. Boonyavorakul C, Thakkinstian A, Charoenpan P. Intravenous magnesium sulfate in acute severe asthma. Respirology. (2000) 5(3):221–5. doi: 10.1046/j.1440-1843.2000.00252.x
15. Bloch H, Silverman R, Mancherje N, Grant S, Jagminas L, Scharf SM, et al. Intravenous magnesium sulfate as an adjunct in the treatment of acute asthma. Chest. (1995) 107(6):1576–81. doi: 10.1378/chest.107.6.1576
16. Porter RS, Nester BA, Braitman LE, Geary U, Dalsey WC, et al. Intravenous magnesium is ineffective in adult asthma, a randomized trial. Eur J Emerg Med. (2001) 8(1):9–15. doi: 10.1097/00063110-200103000-00003
17. Bradshaw TA, Matusiewicz SP, Crompton GK, Innes JA, Greening AP, et al. Intravenous magnesium sulphate provides no additive benefit to standard management in acute asthma. Respir Med. (2008) 102(1):143–9. doi: 10.1016/j.rmed.2007.07.022
18. Hossein S, Pegah A, Davood F, Said A, Babak M, Mani M, et al. The effect of nebulized magnesium sulfate in the treatment of moderate to severe asthma attacks: a randomized clinical trial. Am J Emerg Med. (2016) 34(5):883–6. doi: 10.1016/j.ajem.2016.01.024
19. Bessmertny O, DiGregorio RV, Cohen H, Becker E, Looney D, Golden J, et al. A randomized clinical trial of nebulized magnesium sulfate in addition to albuterol in the treatment of acute mild-to-moderate asthma exacerbations in adults. Ann Emerg Med. (2002) 39(6):585–91. doi: 10.1067/mem.2002.123300
20. Nannini LJ Jr, Pendino JC, Corna RA, Mannarino S, Quispe R, et al. Magnesium sulfate as a vehicle for nebulized salbutamol in acute asthma. Am J Med. (2000) 108(3):193–7. doi: 10.1016/S0002-9343(99)00463-5
21. Gallegos-Solórzano MC, Pérez-Padilla R, Hernández-Zenteno RJ. Usefulness of inhaled magnesium sulfate in the coadjuvant management of severe asthma crisis in an emergency department. Pulm Pharmacol Ther. (2010) 23(5):432–7. doi: 10.1016/j.pupt.2010.04.006
22. Ahmed S, Sutradhar SR, Miah AH, Bari MA, Hasan MJ, Alam MK, et al. Comparison of salbutamol with normal saline and salbutamol with magnesium sulphate in the treatment of severe acute asthma. Mymensingh Med J. (2013) 22(1):1–7.23416800
23. Badawy M, Hasannen I. The value of magnesium sulfate nebulization in treatment of acute bronchial asthma during pregnancy. Eur Respir J. (2012) 40(Suppl 56):1789. doi: 10.1016/j.ejcdt.2013.12.011
24. Kokturk N, Turktas H, Kara P, Mullaoglu S, Yilmaz F, Karamercan A, et al. A randomized clinical trial of magnesium sulphate as a vehicle for nebulized salbutamol in the treatment of moderate to severe asthma attacks. Pulm Pharmacol Ther. (2005) 18(6):416–21. doi: 10.1016/j.pupt.2005.03.003
Keywords: MgSO4, intravenous, nebulized, acute asthma, systematic review, meta-analysis, PEF, FEV1
Citation: Rovsing AH, Savran O and Ulrik CS (2023) Magnesium sulfate treatment for acute severe asthma in adults—a systematic review and meta-analysis. Front. Allergy 4:1211949. doi: 10.3389/falgy.2023.1211949
Received: 25 April 2023; Accepted: 11 July 2023;
Published: 28 July 2023.
Edited by:
Hayley Scott, The University of Newcastle, AustraliaReviewed by:
Tomoya Harada, Tottori University Hospital, JapanKateryna Gashynova, Dnipro State Medical University, Ukraine
© 2023 Rovsing, Savran and Ulrik. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alma Holm Rovsing YWxtYS5ob2xtLnJvdnNpbmdAcmVnaW9uaC5kaw==
†These authors have contributed equally to this work
Abbreviations BP, blood pressure; CI, confidence interval; ER, emergency room; FEV1, forced expiratory volume in 1. Second; FISCHL index, Scale used to measure the severity of an asthma attack. The index ranges from 0 to 7, with score 7 representing the most severe asthma exacerbation. The index includes HR, RR, PP, PEF, subjective dyspnea, accessory muscle use, and wheezing; HDU, high-dependency unit; HR, heart rate; ICU, intensive care unit; IV, intravenous; MeSH, medical subject headings; MgSO4, magnesium sulfate; PaO2, arterial oxygen tension; PEF, peak expiratory flow rate; PP, pulsus paradoxus; PRISMA, preferred reporting items for systematic reviews and meta-analyses; QoL, quality of life; RCT, randomized controlled trial; RR, respiratory rate; SAMA, short-acting muscarinic antagonist; SoC, standard of care; SpO2, oxygen saturation; VAS, visual analogue scale.
 Alma Holm Rovsing
Alma Holm Rovsing Osman Savran1,†
Osman Savran1,†