- 1Epithelial Therapeutics Unit, National Institute of Allergy and Infectious Disease, National Institutes of Health, Bethesda, MD, United States
- 2Global Parents for Eczema Research, Santa Barbara, CA, United States
- 3Allergy and Asthma Network, Vienna, VA, United States
Background: Despite the recent expansion of treatment options in atopic dermatitis (AD), most management responsibilities fall on the patient and/or caregivers. Disease control often requires vigilance about and avoidance of common exposures, however the concerns for patients and caregivers living with AD have not been well enumerated.
Methods: An IRB approved survey was distributed to the public to evaluate the patient and caregiver concerns for topical exposures and potential triggers.
Results: 323 people accessed the link to the survey with 259 providing response to at least one section of questions (response rate 80.2%). Results indicated that temperature and other weather related changes were the most common trigger. Nearly all respondents avoided at least one topical ingredient, with fragrances being the most common. Steroid exposure was common, however respondents expressed concerns about overall steroid exposure.
Conclusions: Our results attempt to enumerate the daily topical exposure concerns for patients and caregivers living with AD. While our online survey is both limited and without mechanistic insights, our results provide insight to providers by highlighting the role of temperature in AD symptoms; identifying commonly perceived triggers; indicating the value of provider insight for topical product selection; and indicating that no specific aspect of topical corticosteroid exposure may alleviate the general steroid concerns for patients or caregivers.
Introduction
Despite the recent expansion of treatment options in atopic dermatitis (AD) (1), most management responsibilities fall on the patient and/or caregivers. Disease control often requires vigilance about and avoidance of common exposures, including environmental triggers, skin care products, topical medications, as well as cleansers and detergents used in the home (2). However, patients' and caregivers' considerations and concerns about trigger avoidance have not been well enumerated (1).
Materials and methods
Survey
An IRB approved survey was distributed to the public through Research Match, Global Parents for Eczema Research, and the Coalition of Skin Diseases. 323 people accessed the link to the survey with 259 providing response to at least one section of questions (response rate 80.2%).
Statistics
Body surface area (BSA) was calculated using SCORAD. Statistical analyses and visualization were conducted in R using the packages: ggplot2, epitools, pheatmap, sf, and corrplot.
Results
Consistent with the patient and caregiver population, a female predominance was seen (shown in Figure 1A) (2). Our results reflected the US population but not the established racial disparities in AD (3) (shown in Figure 1B). The median age was 29 (shown in Figure 1C), mean age was 15.1 (standard deviation 13.6), and results extended across the US (shown in Figure 1D). Average itch severity was 4/10 (shown in Figure 1E) while during flares it was 8/10 (shown in Figure 1F). Temperature, seasonal weather, and humidity were the most common triggers (shown in Figure 1G) although the correlations between these similar factors were low (shown in Figure 1H). On average, respondents indicated being in an active flare 18.3 weeks of the year (shown in Figure 1I). For those indicating food as a potential trigger, “dairy” was the most common answer (N = 23) (shown in Figure 1G).

Figure 1. Demographics and triggers in respondents. (A) Self-identified gender (A, N = 227) and ethnicity (B, N = 228) of respondents. (C) Reponses to “how old is the person you are filling this out for?” (N = 237). (D) Zip codes for those identifying place of residence (N = 201). (E,F) Reponses to “how severe is your itch on an average day?” (E) or severity “during a flare?” (F) (N = 162). (G) % indicating the listed variable can induce a flare (N = 177). (H) Collinearity assessment for responses in G. (I) Mean ± SD of weeks per year the respondent indicated they or their child are in an active flare, symptom free, or are at baseline (N = 123). N values indicate the number of respondents that completed the indicated question.
The most avoided ingredient in topical cleaners were fragrances (35.0%) followed by parabens (18.0%) (Figure 2A). 5 respondents wrote in “sodium dodecyl sulfate”, which has been recently linked to allergic disease (4). A fake ingredient intended as a negative control (Zirodidem) ranked last with only 8 of 186 indicating avoidance (shown in Figure 2A). In total, 98.9% of respondents avoid at least one topical ingredient (N = 179). Physician recommendation was ranked as the most influential endorsement for topical products selection (shown in Figure 2B).
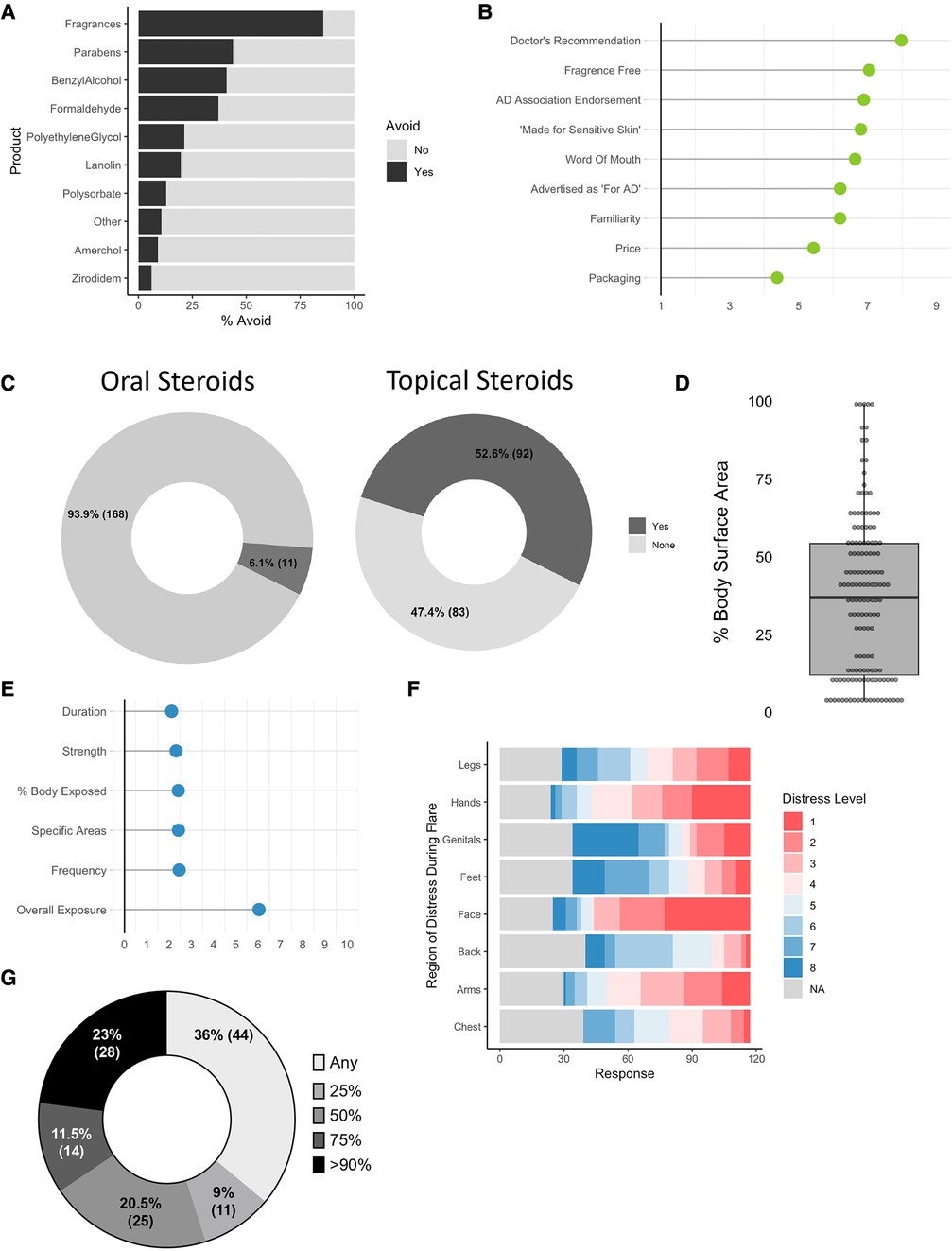
Figure 2. Enumerating concerns in patients and caregivers with AD. (A) For those affirming that “there are skin care products that you avoid because they worsen your or your child”s eczema”, % indicating the specified ingredient is avoided (N = 186). (B) Average rank score (1–9) for “most important factor in selecting a skin care product” (N = 182). (C) % of respondents indicating they have previously been prescribed oral (N = 179) or topical (N = 175) steroids for atopic dermatitis. (D) Participants were asked “when you apply steroids, which sites do you typically apply them to?”; derivation of body surface area (BSA) exposed to topical steroids (N = 144). (E) Mean response for level of concern (0–10) for overall and parameters of steroid exposure (N = 130). (F) Body site ranks for areas of most (1) to least (8) concern for application of steroids (N = 117). (G) Responses to “A new drug is announced that ONLY reduced the amount of topical steroids patients needed to use. How much of a reduction would make taking the new drug 'worth it'?” (N = 122). N values indicate the number of respondents that completed the indicated question.
6.1% of respondents indicated they were currently taking oral steroids for AD (shown in Figure 2C). 52.6% indicated they used topical steroids (18.1% over the counter, 81.9% prescription). The median BSA coverage for topical corticosteroids (TCS) when used was 26.5% (shown in Figure 2D). When given a Likert scale for how concerned they were about TCS use, overall use ranked 6.5/10 with each of the specific aspects of use (such as potency, duration, or total coverage) scoring below 3 each (shown in Figure 2E). Concerns for exposure varied by body site with the face having the highest concerns of use (shown in Figure 2F). A plurality of patients (36%) reported that a novel drug might be “worth using” if it provided “any” reduction in steroid exposure (shown in Figure 2G). Some respondents indicated a concern for “topical steroid withdrawal”, an emerging but recently appreciated syndrome linked to discontinuation of TCS after prolonged use of mid-to-high potency formulations (5).
Discussion/conclusion
Our results attempt to enumerate the daily topical exposure concerns for patients and caregivers living with AD. Our findings are limited by low N value and inablility to offer mechanistic insights. The reported avoidance of dairy (6), fragrances, and parabens (7) may reflect genuine allergic reactions or concerns without biologic ties. In addition, reports have shown that barrier function (as measured by transepidermal water loss) in nonlesional skin of patients treated with dupilumab also improves with EASI reduction (8). Thus, patients with worse symptom control may be more sensitive to exposures due to reduced barrier function. While our survey cannot separate these possibile mechanisms, our results do provide insights for providers to probe with their patients during clinical visits. The results are also limited by potential selection bias inherent to online surveys and the inability to obtain provider-level verification for the diagnosis and thus did not ask about infectious triggers of AD that have been reported, such as COVID-19 (9, 10). However, our results provide insight to providers by highlighting the role of temperature in AD symptoms; identifying commonly perceived triggers; and indicating the value of provider insight for topical product selection. The work also highlights the ability to use shared decision making in improving dermatologic conditions (11). While there are no specific aspects of TCS exposure which may alleviate the general steroid concerns for patients or caregivers, our results also indicate that oral steroids are used more than advised by the guidelines (1) and TCS are applied onto body surface areas above what has been indicated as safe (12).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by IRB of NIH. The patients/participants provided their written informed consent to participate in this study.
Author contributions
GR performed all statical assessments and visualizations, AS, KC, and KB provided administrative support for conducting and recruiting for the survey, IM oversaw the project and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases (NIAID).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Schneider L, Tilles S, Lio P, Boguniewicz M, Beck L, LeBovidge J, et al. Atopic dermatitis: a practice parameter update 2012. J Allergy Clin Immunol. (2013) 131(2):295–9. e291–227. doi: 10.1016/j.jaci.2012.12.672
2. Kim RW, Barta K, Begolka WS, Capozza K, Eftekhari S, Tullos K, et al. Qualitative analysis of the impact of atopic dermatitis on caregivers. Br J Dermatol. (2022) 187(6):1038–41. doi: 10.1111/bjd.21828
3. Croce EA, Levy ML, Adamson AS, Matsui EC. Reframing racial and ethnic disparities in atopic dermatitis in black and latinx populations. J Allergy Clin Immunol. (2021) 148(5):1104–11. doi: 10.1016/j.jaci.2021.09.015
4. Akdis CA. The epithelial barrier hypothesis proposes a comprehensive understanding of the origins of allergic and other chronic noncommunicable diseases. J Allergy Clin Immunol. (2022) 149(1):41–4. doi: 10.1016/j.jaci.2021.11.010
5. Hajar T, Leshem YA, Hanifin JM, Nedorost ST, Lio PA, Paller AS, et al. A systematic review of topical corticosteroid withdrawal (“steroid addiction”) in patients with atopic dermatitis and other dermatoses. J Am Acad Dermatol. (2015) 72(3):541–9. doi: 10.1016/j.jaad.2014.11.024
6. Dominguez O, Plaza AM, Alvaro M. Relationship between atopic dermatitis and food allergy. Curr Pediatr Rev. (2020) 16(2):115–22. doi: 10.2174/1573396315666191111122436
7. Thurmann L, Herberth G, Seiwert B, Schlittenbauer L, Rolle-Kampczyk U, Roder S, et al. Prenatal paraben exposure and atopic dermatitis-related outcomes among children. Allergy. (2021) 76(10):3122–32. doi: 10.1111/all.14890
8. Ferrucci S, Romagnuolo M, Maronese CA, Germiniasi F, Tavecchio S, Angileri L, et al. Skin barrier status during dupilumab treatment in patients with severe atopic dermatitis. Ther Adv Chronic Dis. (2021) 12:20406223211058332. doi: 10.1177/20406223211058332
9. Marasca C, Ruggiero A, Napolitano M, Fabbrocini G, Megna M. May COVID-19 outbreaks lead to a worsening of skin chronic inflammatory conditions? Med Hypotheses. (2020) 143:109853. doi: 10.1016/j.mehy.2020.109853
10. Bawane J, Potestio L, Genco L, Villani A, Marasca C, Fabbrocini G, et al. Cutaneous adverse effects of the available COVID-19 vaccines in India: a questionnaire-based study. J Eur Acad Dermatol Venereol. (2022) 36(11):e863–4. doi: 10.1111/jdv.18150
Keywords: atopic dermatatis, steroids, patient survey, triggers, eczema
Citation: Ratley G, Sun AA, Capozza K, Barta K and Myles IA (2023) Survey of topical exposure concerns for patients and caregivers dealing with atopic dermatitis. Front. Allergy 4:1210973. doi: 10.3389/falgy.2023.1210973
Received: 23 April 2023; Accepted: 2 August 2023;
Published: 10 August 2023.
Edited by:
Andaç Salman, Acibadem University, TürkiyeReviewed by:
Angelo Ruggiero, University of Naples Federico II, ItalyCarlo Alberto Maronese, IRCCS Ca 'Granda Foundation Maggiore Policlinico Hospital, Italy
Angelo Valerio Marzano, University of Milan, Italy
© 2023 Ratley, Sun, Capozza, Barta and Myles. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ian A. Myles bXlsZXNpQG5paC5nb3Y=
 Grace Ratley1
Grace Ratley1 Korey Capozza
Korey Capozza Kelly Barta
Kelly Barta Ian A. Myles
Ian A. Myles