
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Allergy, 27 June 2023
Sec. Allergens
Volume 4 - 2023 | https://doi.org/10.3389/falgy.2023.1173540
This article is part of the Research TopicMolecular Allergology in Daily Practice: Detecting Allergen Component Specific IgE Profiles and Determining their Clinical RelevanceView all articles
 Lonneke J. Landzaat1*
Lonneke J. Landzaat1* Joyce A. M. Emons1
Joyce A. M. Emons1 Laura J. H. Sonneveld1
Laura J. H. Sonneveld1 Marco W. J. Schreurs2
Marco W. J. Schreurs2 Nicolette J. T. Arends1
Nicolette J. T. Arends1
Background: Allergic rhinitis is a common respiratory disease in children and sensitization to inhalant allergens plays a significant role in its development. However, limited knowledge exists regarding sensitization profiles of inhalant allergen components in atopic children, particularly in the very young individuals. Understanding these profiles could provide insights into the early development of allergic rhinitis. The objective of this cross-sectional retrospective study was to evaluate the IgE-sensitization profiles to multiple inhalant allergen components and their clinical relevance in Dutch atopic children, with specific focus on children under the age of 4 years.
Methods: A total of 243 atopic children were included in the study and sensitization profiles were analyzed using multiplex microarray analysis (ISAC). Clinical information was obtained from records of a pediatric allergy outpatient clinic between 2011 and 2020. Specific IgE responses to inhalation allergen components from five allergen sources (grass pollen, tree pollen, house dust mite, cat and dog), were examined. The study encompassed children of different age groups and compared those with and without symptoms.
Results: The results demonstrated that sensitization to inhalant allergen components was present in 92% of the cohort. Sensitization was already evident at a young age (87%), including infancy, with a rapid increase in prevalence after 1 year of age. House dust mite emerged as the most predominant sensitizing allergen in early childhood, followed by tree pollen in later years. Sensitization patterns were similar between symptomatic and asymptomatic children, although symptomatic children exhibited higher frequencies and values. The sensitization profiles in very young children were comparable to those of children across all age groups.
Conclusion: These findings highlight the presence of sensitization to inhalant allergen components and the early onset of allergic rhinitis before the age of 4, including infancy, in Dutch atopic children. Notable allergen molecules in Dutch atopic children under the age of 4 years include Bet v 1, Fel d 1, Der f 1, Der p 1, Der p 10 and Phl p 4, with house dust mite sensitization being the most common among Dutch infants. Moreover, the prevalence of sensitization to inhalant allergens in this Dutch cohort surpassed that of general European populations, emphasizing the importance of early assessment and management of allergic rhinitis in young atopic children.
Allergic rhinitis is a common respiratory disease in children, with its prevalence increasing over time, ranging from 1 to 30% (1). However, limited data are available on the prevalence of allergic rhinitis in Dutch children. A Dutch population-based prospective cohort study, the Generation R Study, reported a prevalence of 12.4% for allergic rhinitis and 32.2% for inhalant sensitization at the age of 10 (2).
Atopic diseases often follow a characteristic sequence known as the “atopic march”, where atopic eczema and food allergy manifest in the early years of life, followed by respiratory allergies later on (3, 4) Previous studies have shown that sensitization to inhalant allergens typically occurs after infancy and increases thereafter (5–7). Sensitization to seasonal allergens (i.e., tree and grass pollen) tends to appear later than sensitization to perennial allergens (i.e., house dust mite) (5, 6).
In most studies, IgE antibody reactivity has been analyzed using singleplex assays with a limited number of allergen extracts. To gain a better understanding of allergic disease development, it is crucial to map the sensitization profiles of inhalant allergen components using advanced molecular allergology techniques, especially in very young children. Sensitization to different allergen components was found to be predictive of allergic disease development and treatment outcomes in children (8–11). Additionally, studies have shown similar sensitization profiles to inhalant allergen components in symptomatic and asymptomatic children (12). Therefore, obtaining more data on sensitization profiles in allergic populations, particularly in very young children, could provide valuable insights into the development and management of allergic rhinitis in early childhood.
The aim of this study was to assess the IgE sensitization profiles to multiple inhalant allergen components and their clinical relevance in Dutch atopic children, with a focus on the children under the age of 4 years.
This cross-sectional retrospective study included 243 atopic children who were analyzed for presence of allergen specific IgE. The analysis was conducted using a multiplex microarray with 112 allergen components [Immuno-solid-phase Allergen Chip (ISAC), Thermo Fisher Scientific, Uppsala, Sweden]. The data collection period was between 2011 and 2020. Clinical information was obtained from our pediatric allergy outpatient clinic at the Erasmus MC Sophia Children's Hospital in Rotterdam, the Netherlands. The study included children who had been referred or were being treated by a pediatric allergist for a food allergy.
The study focused on 37 inhalant allergen components present on the ISAC. These components were derived from the five most significant sources of inhalation allergen: grass pollen, tree pollen, house dust mite, cat and dog. Table 1 provides an overview of the studied inhalation allergen components. Additionally, four allergen components (Pla a 2, Der p 23, Can f 4 and Can f 6) were added to the ISAC in 2017 and included in the analysis. Sensitization was considered present when the ISAC Standardized Units (ISU) value was equal or greater than 0.3.
For each patient, the medical records were retrospectively reviewed to identify symptoms of food allergy, rhinitis, asthma and/or eczema. Atopy was defined as having one or more of these diseases, accompanied by sensitization indicated by a skin prick test result larger than 4 mm or a specific IgE lever higher than 0.35 kU/liter. The diagnosis of food allergy required confirmation through a positive oral food challenge or a clear history of IgE-mediated symptoms such as angioedema, urticaria, dyspnea, rhinitis, gastro-intestinal and/or neurological symptoms after ingestion of the allergen. Symptoms related solely to oral allergy or sensitization to a food allergen without known clinical relevance were not classified as food allergies. Rhinitis was confimed when one or more symptoms of rhinorrhea, sneezing, nasal obstruction or nasal itching were present, with or without itchy, red or watery eyes. Asthma, including pre-school wheeze, was considered present if the patient was prescribed salbutamol and/or inhaled corticosteroids. Eczema was defined based on the use of topical corticosteroids. The presence of food allergy, rhinitis, asthma and eczema was also determined based on diagnoses mentioned in the medical chart by a pediatric allergist, pediatric pulmonologist and/or dermatologist.
The statistical analysis involved examining sensitization to inhalant components and symptoms of atopy across different age groups using an Analysis of Variance (ANOVA). The level of specific IgE for each positive allergen component was compared between symptomatic and asymptomatic children using the nonparametric U test. Statistical significance was defined as a P-value of ≤0.05. IBM SPSS Statistics was used for data analysis.
A total of 243 children (63% male, mean age 9.0 years) were included in this study. Table 2 presents the baseline characteristics for each age group. The majority of the children (88%) exhibited two or more atopic features, with food allergy being present in most cases (84%). Rhinitis was the most common symptom among older children, but it was already present in 55% of children under the age of 4. Asthma was diagnosed in 63% of the children, while eczema was present in the majority of children under 12 years (ranging from 84% to 90%). Among the cohort, 92% of the children, were sensitized to at least one of the 37 inhalant allergen components. Sensitization rates varied by age, with 87% in the youngest age group, increasing to 96% around the age of 12 to 15 years and then declining to 88%.
Figure 1 provides an overview of sensitization to the five inhalant allergen sources divided by age category. Sensitization was already present in young children and increased rapidly after the age of 1 year (Figure 1B). In the first year of life, 50% of the casus exhibited sensitization to at least one of the 37 inhalation allergens, while in the subsequent three years these percentages were 91%, 88% and 95% respectively. House dust mite dominated sensitization in the first three years, after which tree pollen became the most common source. Figure 1A demonstrates that sensitization continues to increase during primary school, remaining stable or slightly decreasing afterwards. Among all sensitized children (n = 223), 80.7% had relevant sensitization with rhinitis symptoms, ranging from 57% in the youngest age group (n = 26) to 100% in the oldest age group (n = 28). In the 2 to 4 age group, the percentages of relevant sensitization with rhinitis were 67%, 89% and 98%, respectively, and these differences were statistically significantly (F = 12.41, p = 0.00); The likelihood of experiencing rhinitis symptoms when sensitized increased with age.
The hierarchy of inhalant allergen components based on frequencies of specific IgE reactivity per inhalation group was determined for all children. The children were categorized into those with and without rhinitis symptoms (Figures 2A–C). The most frequently represented allergen components corresponded to the major allergens: Phl p 1 (52%) in grass pollen, Bet v 1 (62%) in tree pollen, Der f 1 (51%) and Der p 1 (51%) in house dust mite, Fel d 1 (58%) in cat and Can f 1 (33%) in dog. Sensitization to all 37 inhalation allergen components was observed in children with rhinitis (Figure 2B). In children without rhinitis, no sensitization was seen for Phl p 7, Bet v 4, Pla a 1 and Can f 4. The hierarchy of inhalant allergen components in symptomatic children was similar to that of asymptomatic children (Figures 2B,C). However, symptomatic children demonstrated higher frequencies of sensitization to Phl p 1 (59%), Bet v 1 (71%), Der f 1 (58%), Fel d 1 (65%) and Can f 1 (37%) compared to Phl p 1 (30%), Bet v 1 (34%), Der p 1 (29%), Fel d 1 (36%) and Can f 3 (21%) in asymptomatic children. As expected, the frequencies of sensitization were higher in symptomatic. Additionally, the levels of specific IgE were significantly higher for Phl p 1, Cyn d 1, Phl p 5, Bet v 1 and Can f 3 in symptomatic children (Supplementary Table S1A).
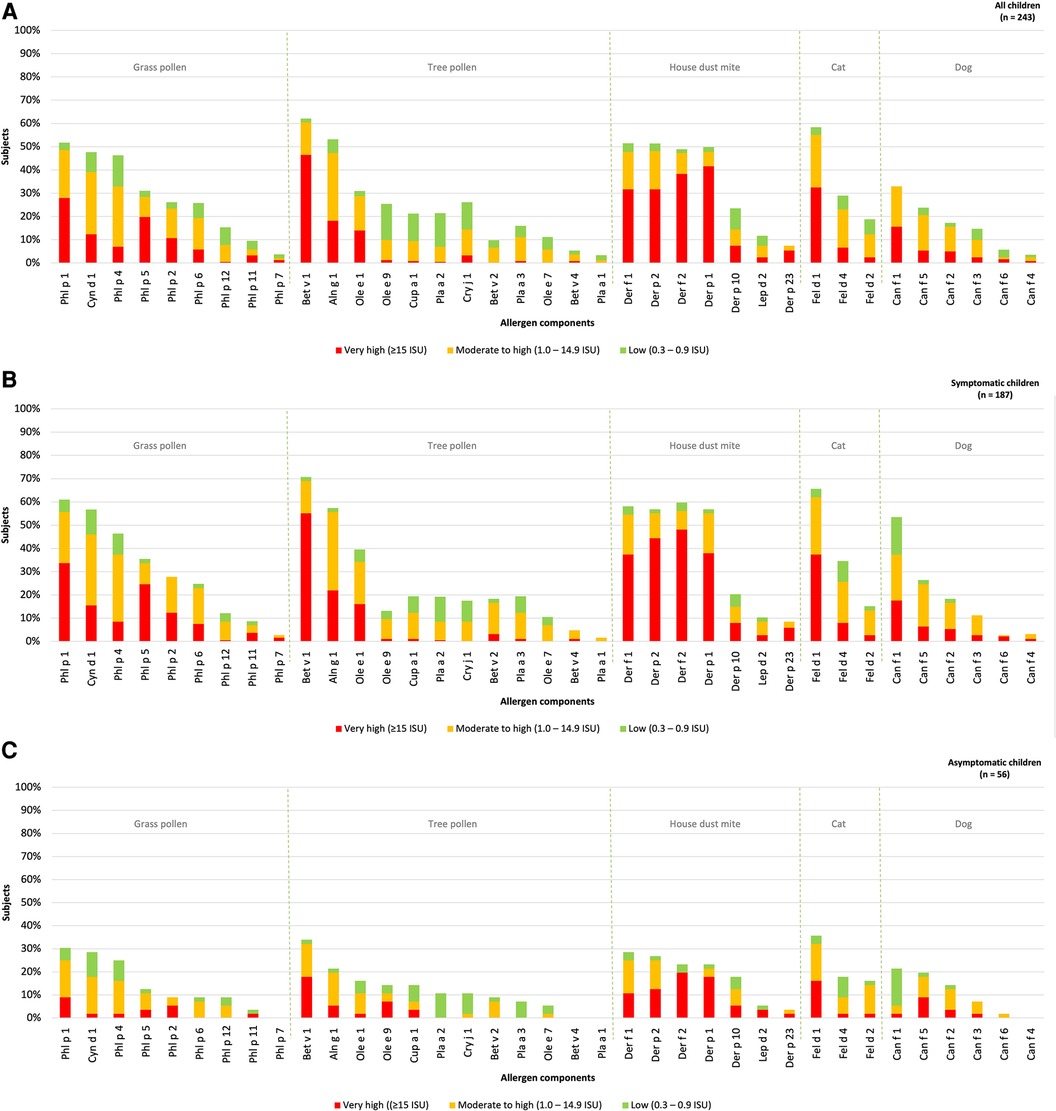
Figure 2. Sensitization patterns of inhalant allergen components in all (A), symptomatic (B) and asymptomatic (C) children.
The hierarchy of inhalant allergen components based on the frequencies of specific IgE reactivity per inhalation group was determined for children under the age of 4 year (n = 53) and divided into those with or without rhinitis (Figure 3). Sensitization patterns in young children were similar to those observed in all children and were dominated by Phl p 4 (26%), Cyn d 1 (23%), Phl P 1 (17%), Bet v 1 (30%), Bet v 2 (21%), Def f 1, Derp 1, Der p 10 (all 28%), Fel d 1 (38%), Can f 1 and Can f 3 (both 17%). Differences were observed in the order of grass pollen components, with Phl p 4 and Cyn d 1 being more frequently represented in young, compared to Phl p 1 in older children. No sensitization was observed for Bet v 4, Pla a 1, Can f 4 and Can f 6, which aligns with the sensitization patterns in all children without symptoms.
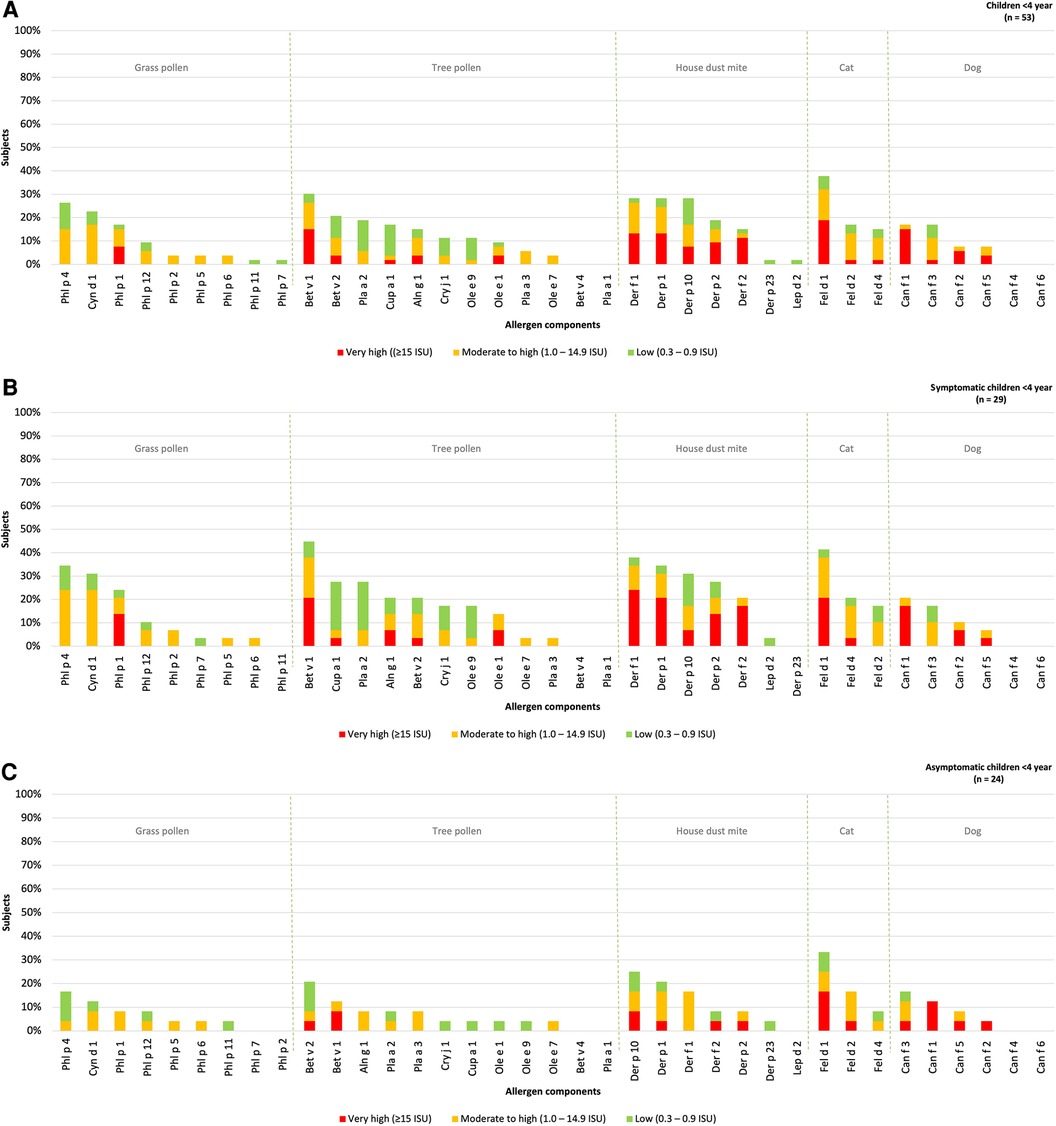
Figure 3. Sensitization patterns of inhalant allergen components in all (A), symptomatic (B) and asymptomatic (C) young children.
The hierarchy inhalant allergen components in symptomatic children was found to be similar to that of asymptomatic children. The most dominant components in symptomatic children were Phl p 4 (34%), Bet v 1 (45%), Der f 1 (38%), Fel d 1 (41%) and Can f 1 (21%). These components were also observed as the most frequently recognized in asymptomatic children with Phl p 4 (17%), Bet v 2 (21%), Der p 10 (25%), Fel d 1 (33%), Can f 3 (17%) being the prominent ones (Figures 3B,C). Although there was no significant difference in the the levels of specific IgE for Phl p 1 and 4 between symptomatic and asymptomatic children, it was observed that 14% of the symptomatic children demonstrated a “very high” sensitization level to Phl p 1 compared to none of the asymptomatic children. Additionally, 24% of the symptomatic children showed a “moderate to high” sensitization level to Phl p 4, while only to 4% of asymptomatic children exhibited such sensitization. Bet v 2 and Can f 3 were predominantly present in asymptomatic children, but these components were also present at similar percentages in children with rhinitis (21% and 17% respectively). Overall, the frequency of sensitization was higher in symptomatic children, however the levels of specific IgE (measured in ISU) are not significantly higher in children with symptoms (Supplementary Table S1B).
In this Dutch cohort of atopic pediatric patients, the prevalence of sensitization inhalant allergen components is 92%. This prevalence is higher compared to general European pediatric populations (25%–31%) (2, 13, 14) and more similar to other atopic cohorts (30%–100%) (5, 12, 15). Our findings indicate that IgE-reactivity to inhalant allergens is frequently present at very young age, with sensitization reported in half of the children under 1 year of age. The prevalence of sensitization rapidly increases prevalence from the year onwards and is clinically relevant in the majority of cases. Most studies on sensitization assess children in larger age groups or from the age of 4 years onwards, with limited data available on sensitization in atopic infants, which generally reports a lower prevalence (11%–30%) (5, 16, 17). The factors that predict allergic sensitization in the first year of life are still poorly understood. One possible explanation for the high number of sensitized young children in our cohort is the presence of eczema, as recent studies suggest that impaired skin barrier function in early infancy can lead to sensitization (4, 17, 18).
Although sensitization does not directly correspond to allergic symptoms, inhalant allergen sensitization is a significant factor in the development of allergic respiratory diseases. Early onset of inhalant sensitization (8, 9, 13, 14, 19), poly-sensitization (5, 20–22) and higher levels of specific IgE at young age (8, 14, 21, 22) have been associated with an increased risk of developing allergic rhinitis. Furthermore, sensitization to inhalant allergens during the first year of life appears to be a strong predictor for the development of allergic rhinitis later in life (19). This suggest that the young atopic sensitized children in our study who are asymptomatic will likely develop allergic rhinitis later in life, which aligns with the 100% prevalence of allergic rhinitis observed in our oldest children. Our results also indicate that allergic rhinitis can manifest earlier in life than previously thought. However, data on the prevalence of allergic rhinitis in infancy is limited and exhibits high variability due to the lack of universal criteria (17, 23, 24). Allergic rhinitis, generally considered as a mild disease, carries a significant burden that is often underestimated. Studies have revealed that allergic rhinitis adversely affects children's quality of life, sleep patterns, school and physical and emotional well-being (25). Therefore, it is crucial to consider and recognize allergic rhinitis in atopic children from a very young age.
Although house dust mite sensitization dominates in the first three years of life, tree pollen sensitization becomes more prevalent in adulthood. This confirms previous findings that identify house dust mite as the main sensitizing inhalant allergens in early life. House dust mite sensitization has been reported to be most prevalent in atopic infants from Italy (40%), Sweden (11%), Belgium (11%) and Japan (31%) (5, 16, 17, 24) and is associated with presence or development of allergic respiratory disease (17, 19, 23, 24). Other studies have reported sensitization to grass and tree pollen in Dutch children at rates of 16% and 11% respectively, with house dust mite sensitization (20%) including allergic symptoms being the most dominant at 10 years of age (2). Similar results have been found in the Swedish BAMSE-cohort and German MAS-cohort were tree and grass pollen sensitization become more common in later life (13, 14).
This is the first study to establish specific molecular IgE sensitization profiles in Dutch atopic children. While the rates of sensitization to inhalant allergen components may vary between regions due to differences in allergen exposure, our study reveals a profile characterized by dominant sensitization of Bet v 1, Fel d 1, Phl p 1 and various house dust mite components, which is consistent with findings in other European children (9, 12). Notably, major allergens associated with house dust mites include Der p 1, Der p 2, Der f 1, and Der f 2. Nevertheless, there exists heterogeneity in reported sensitization to Der p 10 across Europe, with children showing relatively high levels of sensitization, similar to our findings (26). Symptomatic and asymptomatic children exhibited similar sensitization patterns to allergen molecules and symptoms were associated with higher frequencies and IgE-levels of major allergen components such as Bet v 1 and Phl p 1. In young children, Fel d 1 and Bet v 1 were also prominent allergen components, along with Der f 1, Der p 1, Der p 10 and Phl p 4. The presence of specific allergen components during early childhood has been identified as a predictive factor for the development of respiratory diseases later in life. Previous studies in several Northern European countries have demonstrated the association between sensitization to allergens such as Fel d 1, Phl p 1, Bet v 1, Der p 1 and Der f 2 and the subsequent onset of respiratory conditions (9, 20, 27). Additionally, elevated Bet v 1-specific IgE during early age have been linked to more severe rhinitis symptoms in adolescence (8). Moreover, Phl p 4 has been identified as an early indicator of grass pollen allergy, along with Phl p 1 (10), implying young children in our cohort will likely develop grass pollen allergy later in life.
This study has some limitations. It is a cross-sectional analysis, lacking longitudinal follow-up of patients and the analysis is conducted and compared across different age groups at a single time point. Therefore, the progression and potential remission of sensitization cannot be determined from this analysis alone. It is important to note that the study cohort consists of allergic patients who were primarily diagnosed and treated at the pediatric allergy outpatient clinic. Thus, caution must be exercised when extrapolating these findings to the general pediatric population.
In conclusion, this study is first to provide insights into sensitization patterns at allergen molecular level in Dutch atopic children, highlighting the presence of sensitization to inhalant allergen components and associated symptoms before the age of 4 year, including infancy. It may indicate that sensitization to inhalant allergen components is shifting towards an earlier onset in atopic children, challenging the notion that the prevalence of allergic rhinitis primarily increases during primary school years as commonly indicated in literature but can also manifest during infancy. Furthermore, Bet v 1, Fel d 1, Der f 1, Der p 1, Der p 10 and Phl p 4 are identified as prominent allergen molecules in Dutch atopic children under the age of 4 year, while and house dust mite sensitization is most prevalent among Dutch infants. Early diagnosis of allergic rhinitis is crucial to initiate timely treatment and alleviate the associated burden. This necessitates early determination of specific IgE to inhalant allergens, which can be performed as early as infancy. Additionally, gaining further understanding of sensitization prevention strategies, such as improving skin barrier function, and exploring treatment opportunities, such as immunotherapy in sensitized atopic children under the age of 4 would be of great interest. Consequently, more studies focusing on young atopic children are necessary to expend our knowledge in this aera.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval and written informed consent was not required for this retrospective study in accordance with the local legislation and institutional requirements.
LJL, JAME, MWJS, and MWJS: contributed to conception and design of the study. LJL, JAME, LJHS, and NJTA: collected the data. LJL: organized the database, reviewed the data, performed the statistical analysis and wrote the first draft of the manuscript. JAME, MWJS, LJHS, NJTA, and LJL: wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/falgy.2023.1173540/full#supplementary-material
1. Strachan DP, Rutter CE, Innes Asher M, Bissel K, Chiang CY, El Sony A, et al. Worldwide time trends in prevalence of symptoms of rhinoconjunctivitis in children: global asthma network phase I. Pediatr Allergy Immunol. (2022) 33:e13656. doi: 10.1111/pai.13656
2. De Jong NW, Elbert NJ, Mensink-Bout SM, Van der Valk JPM, Pasmans SGMA, Jaddoe VWV, et al. Parental and child factors associated with inhalant and food allergy in a population-based prospective cohort study: the generation R study. Eur J Pediatr. (2019) 178:1507–17. doi: 10.1007/s33431-019-03441-5
3. Hahn EL, Bacharier LB. The atopic march: the pattern of allergic disease development in childhood. Immunol Allergy Clin North Am. (2005) 25:231–46. doi: 10.1016/j.iac.2005.02.004
4. Dharmage SC, Lowe AJ, Matheson MC, Burgess JA, Allen KJ, Abramson MJ. Atopic dermatitis and the atopic march revisited. Allergy. (2014) 69:17–27. doi: 10.1111/all.12268
5. Melioli G, Marcomini L, Agazzi A, Bazurro G, Tosca M, Rossi GA, et al. The IgE repertoire in children and adolescents resolved at component level: a cross-sectional study. Pediatr Allergy Immunol. (2012) 23:433–40. doi: 10.1111/j.1399-3038.2011.01228.x
6. Kulig M, Bergmann R, Klettke U, Wahn V, Tacke U, Wahn U. Natural course of sensitization to food and inhalant allergens during the first 6 years of life. J Allergy Clin Immunol. (1999) 103:1173–9. doi: 10.1016/s0091-6749(99)70195-8
7. Sigurs N, Hattevig G, Kjellman B, Nilsson L, Björkstén B. Appearance of atopic disease in relation to serum IgE antibodies in children followed up from birth for 4 to 15 years. J Allergy Clin Immunol. (1994) 94:757–63. doi: 10.1016/0091-6749(94)90184-8
8. Westman M, Lupinek C, Bousquet J, Andersson N, Pahr S, Baar A, et al. Early childhood IgE reactivity to pathogenesis-related class 10 proteins predicts allergic rhinitis in adolescence. J Allergy Clin Immunol. (2014) 135:1199–206. doi: 10.1016/j.jaci.2014.10.042
9. Wickman M, Lupinek C, Andersson N, Belgrave D, Asarnoj A, Benet M, et al. Detection of IgE reactivity to a handful of allergen molecules in early childhood predicts respiratory allergy in adolescence. EBioMedicine. (2017) 26:91–9. doi: 10.1016/j.ebiom.2017.11.009
10. Westman M, Åberg K, Apostolovic D, Lupinek C, Gattinger P, Mittermann I, et al. Sensitization to grass pollen allergen molecules in a birth cohort—natural phl p 4 as an early indicator of grass pollen allergy. J Allergy Clin Immunol. (2020) 145:1174–81. doi: 10.1016/j.jaci.2020.01.006
11. Tripodi S, Frediani T, Lucarelli S, Macrì F, Pingitore G, Di Rienzo Businco A, et al. Molecular profiles of IgE to Phleum pratense in children with grass pollen allergy: implications for specific immunotherapy. J Allergy Clin Immunol. (2012) 129:834–9. doi: 10.1016/j.jaci.2011.10.045
12. Elisyutina O, Lupinek C, Fedenko E, Litovkina A, Smolnikov E, Ilina N, et al. IgE-reacitvity profiles to allergen molecules in Russian children with and without symptoms of allergy revealed by micro-array analysis. Pediatr Allergy Immunol. (2021) 32:251–63. doi: 10.1111/pai.13354
13. Asarnoj A, Ostblom E, Kull I, Lilja G, Pershagen G, Hedlin G, et al. Sensitization to inhalant allergens between 4 and 8 years of age is a dynamic process: results from the BAMSE birth cohort. Clin Exp Allergy. (2008) 38:1507–13. doi: 10.1111/j.1365-2222.2008.03046.x
14. Matricardi PM, Bockelbrink A, Keil T, Grüber C, Niggeman B, Hamelmann E, et al. Dynamic evolution of serum immunoglobuline E to airborne allergens throughout childhood: results from the multi-centre allergy study birth cohort. Clin Exp Allergy. (2009) 39:1551–7. doi: 10.1111/j.1365-2222.2009.03348.x
15. Christiansen ES, Kjaer HF, Eller E, Bindslev-Jensen C, Høst A, Gotthard Mortz C, et al. The prevalence of atopic disease and the patterns of sensitization in adolescence. Pediatr Allergy Immunol. (2016) 27:847–53. doi: 10.1111/pai.12650
16. De Bilderling G, Mathot M, Augustsson S, Tuerlinckx D, Jamart J, Bodart E. Early skin sensitization to aeroallergens. Clin Exp Allergy. (2008) 38:643–8. doi: 10.1111/j.1365-2222.2008.02938.x
17. Almqvist C, Li Q, Britton WJ, Kemp AS, Xuan W, Tovey ER, et al. Early predictors for developing allergic disease and asthma: examining separate steps in the ‘allergic march’. Clin Exp Allergy. (2007) 37:1296–302. doi: 10.1111/j.1365-2222.2007.02796.x
18. Wärnberg Gerdin S, Lie A, Asarnoj A, Borres MP, Lødrup Carlsen KC, Färdig M, et al. Impaired skin barrier and allergic sensitization in early infancy. Allergy. (2022) 77:1464–76. doi: 10.1111/all.15170
19. Brockow I, Zutavern A, Hoffmann U, Grübl A, von Berg A, Koletzko S, et al. Early allergic sensitizations and their relevance to atopic diseases in children aged 6 years: results of the GINI study. J Investig Allergol Clin Immunol. (2009) 19:180–7.19610260
20. Asarnoj A, Hamsten C, Wadén K, Lupinek C, Andersson N, Kull I, et al. Sensitization to cat and dog allergen molecules in childhood and prediction of symptoms of cat and dog allergy in adolescence: a BAMSE/MeDALL study. J Allergy Clin Immunol. (2016) 137:813–21. doi: 10.1016/j.jaci.2015.09.052
21. Wickman M, Lilja G, Söderström L, Hage-Hamsten M, Ahlstedt S. Quantitative analysis of IgE antibodies to food and inhalant allergens in 4-year-old children reflects their likelihood of allergic disease. Allergy. (2005) 60:650–7. doi: 10.1111/j.1398-9995.2004.00764.x
22. Bousquet J, Anto JM, Bachert C, Bousquet PJ, Colombo P, Crameri R, et al. Factors responsible for differences between asymptomatic subjects and patients presenting an IgE sensitization to allergens. A GA2LEN project. Allergy. (2006) 61:671–80. doi: 10.1111/j.1398-9995.2006.01048.x
23. Herr M, Clarisse B, Nikasinovic L, Foucault C, Le Marec AM, Giordanella JP, et al. Does allergic rhinitis exist in infancy? Findings from the PARIS birth cohort. Allergy. (2011) 66:214–21. doi: 10.1111/j.1398-9995.2010.02467.x
24. Masuda S, Nagao M, Usui S, Nogami K, Tohda Y, Fujisawa T, et al. Development of allergic rhinitis in early life: a prospective cohort study in high-risk infants. Pediatr Allergy Immunol. (2022) 33:e13733. doi: 10.1111/pai.13733
25. Meltzer EO, Blaiss MS, Derebery MJ, Mahr TA, Gordon BR, Sheth KK, et al. Burden of allergic rhinitis: results from the pediatric allergies in America survey. J Allergy Clin Immunol. (2009) 124:43–70. doi: 10.1016/j.jaci.2009.05.013
26. Bronnert M, Mancini J, Birnbaum J, Agabriel C, Liabeuf V, Porri F, et al. Component-resolved diagnosis with commercially available D. Pteronyssinus der p 1, der p 2 and der p 10: relevant markers for house dust mite allergy. Clin Exp Allergy. (2012) 42:1406–15. doi: 10.1111/j.1365-2222.2012.04035.x
Keywords: allergen, allergy, inhalant allergen, age group, sensitization, microarray analysis, rhinitis, allergen component.
Citation: Landzaat LJ, Emons JAM, Sonneveld LJH, Schreurs MWJ and Arends NJT (2023) Early inhalant allergen sensitization at component level: an analysis in atopic Dutch children. Front. Allergy 4:1173540. doi: 10.3389/falgy.2023.1173540
Received: 24 February 2023; Accepted: 7 June 2023;
Published: 27 June 2023.
Edited by:
Kirsi Jarvinen-Seppo, University of Rochester, United StatesReviewed by:
Wayne Robert Thomas, University of Western Australia, Australia© 2023 Landzaat, Emons, Sonneveld, Schreurs and Arends. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lonneke J. Landzaat bC5sYW5kemFhdEBldHoubmw=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.