
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Allergy, 04 May 2023
Sec. Therapies and Therapeutic Targets
Volume 4 - 2023 | https://doi.org/10.3389/falgy.2023.1159387
This article is part of the Research TopicAllergies and Anti-allergy Drug Discovery in AfricaView all 5 articles
Photosensitive dermatoses are seen in 5% of HIV-infected persons. These include drug- and chemical-induced photoallergic and phototoxic reactions, chronic actinic dermatitis of HIV, photo lichenoid drug eruptions, and porphyria. Data on photodermatitis in HIV are limited to case reports and series. The pathogenesis is not completely understood and includes a th2 phenotype in HIV which results in impaired barrier function and resultant allergen sensitisation as well as immune dysregulation. The objective of this manuscript is to review the literature on the clinical phenotype, pathogenesis, role of photo and patch testing, outcomes, and treatment of photodermatitis in HIV in an African population.
Photosensitivity is defined as a pathologic response to non-ionising radiation after normal exposures. On the other hand, photodermatoses refers to a varied group of skin disorders that are caused or exacerbated by an abnormal or excessive reaction to sunlight (1). A good understanding of the pathogenesis of photodermatoses is important as it not only helps establish the diagnosis but guides the management and prevention of complications. Ultraviolet (UV) radiation is the major driver of photosensitivity reactions, although some reactions are seen in response to visible light (2).
Based on the current understanding of pathogenesis, there are varying classifications used for photodermatoses. Some authors include four types, these being immunologically mediated; drug- and chemical-induced, photoaggravated, and photosensitivity associated with defective DNA repair mechanisms (1). Others group them into five types, namely primary; exogenous; photo-exacerbated; metabolic, and genetic photodermatoses (3). There is considerable overlap between these classifications as well as between the individual types themselves as highlighted below.
i) Immunologically mediated photodermatoses (IMP) are sometimes referred to as idiopathic or primary photodermatoses. However, as the pathogenesis of these disorders is understood, the term idiopathic continues to lose relevance. The main pathomechanism of IMP is thought to be UV-driven autoimmunity. Exposure of normal endogenous components of the body to ionizing radiation alters them structurally to become antigenic and trigger an immune response. Genetic predisposition and environmental factors, beyond exposure to ionizing radiation, are thought to play a significant role. Photodermatoses that are thought to be immunologically mediated include polymorphic light eruption (PMLE), actinic prurigo, hydroa vacciniforme, chronic actinic dermatitis (CAD), and solar urticaria (1, 3).
ii) In drug- and-chemical induced photodermatoses, the causative chemical is known and is usually exogenous. The resultant reactions are either immunologically mediated (photoallergic) or non-immunologic (phototoxic). Some of the photosensitizing compounds have found a therapeutic role in the management of skin disorders. Examples include psoralen which is combined with UVA to treat a number of skin conditions and methyl aminolevulic acid (ALA) which is used in photodynamic therapy to treat cancerous and precancerous skin conditions (4). Exposure to sunlight is not essential for the development of
iii) Photoaggravated dermatoses. However, sun exposure can trigger and/or exacerbate these conditions. Photoaggravated dermatoses include lupus erythematosus, dermatomyositis, Darier's disease, rosacea, and melasma (5–9).
iv) Genetic photodermatoses include many due to defective DNA repair mechanisms and metabolic abnormalities. The classic example of defective repair mechanisms is xeroderma pigmentosum, an autosomally recessive inherited disease associated with photosensitivity, premature ageing, and early development of skin cancers. Included in this group are Bloom, Rothmund-Thomson, and Cockayne syndromes. Porphyrias, also genetic photodermatoses, are the best-known
v) Metabolic photodermatoses. In porphyria, there is an accumulation to phototoxic porphyrins in the skin amongst other organs. This is due to genetic defects in various enzymes resulting in the accumulation of phototoxic precursors. Porphyria cutanea tarda on the other hand is an acquired form of the disease (10). Porphyrias may be activated by exposure to certain medications or toxins. Hartnup disease also referred to as “pellagra-like dermatosis” is an autosomal recessive metabolic disorder associated with the malabsorption of nonpolar amino acids including tryptophan. Tryptophan is a precursor to nicotinamide, the deficiency of which also manifests as nutritional pellagra, which can occur independent of the genetic defect (11).
Classically photodermatitis is characterised by the involvement of sun-exposed areas, such as the face, ears, scalp, posterior neck, upper back, “V” area of the chest, extensor arms, and dorsum of hands. It spares the nasolabial fold, supraorbital fold, post auricular and submental area (2). However, there are subtle differences between the different types depending largely on pathogenesis.
i) Phototoxic reactions result from direct tissue or cellular injury. It may occur in any individual exposed to a high enough dosage or the wavelength of radiation and does not require previous sensitisation (12). Clinically, phototoxicity presents as an exaggerated sunburn reaction with burning and stinging in the sun-exposed sites within minutes or hours of exposure. Histologically it is characterised by apoptic keratinocytes (sunburn cells) with dermal lymphocytic and neutrophilic infiltrate (13).
ii) Photoallergic reactions are less common than phototoxic reactions and usually require a minimal exposure to the photosensitizing drug and prior sensitization (14). A photoproduct acts as a hapten or as a complete antigen to generate a type-IV hypersensitivity reaction. The reaction develops 24 h or more after the initial exposure and is clinically eczematous. If extensive it may extend to include non-sun-exposed skin. Prior contact may not be necessary if the patient has been sensitised to a similar molecule (12, 14). Photoallergic reactions are similar to ordinary allergic contact reactions on histology, the main features being spongiosis and a dermal lymphohistiocytic infiltrate (15).
iii) Lichen planus and lichenoid reactions as a whole are a poorly characterised group as is the photolichenoid subset. The unifying feature of these “lichen planus-like” reactions and lichen planus is the presence of an inflammatory pattern histologically characterized by damage to the basal keratinocytes, usually by lymphocytes (16).
Data on photodermatitis in HIV are limited to case reports and small case series and on photodermatitis in HIV-infected Africans it is even more limited. HIV infection is associated with higher odds of developing photosensitivity, and photosensitivity can be a presenting feature of HIV (2, 17, 18). The spectrum of photodermatoses that have been reported in HIV includes PMLE, CAD, photodistributed drug eruptions, photoaggravated granuloma annulare, pellagra, porphyria cutanea tarda, and actinic prurigo (19–24). Actinic lichenoid leukomelanoderma of HIV, a photosensitive eruption observed in South African patients, in the authors' opinion most likely represents CAD in HIV-infected persons (25).
CD4+ T-cell depletion is the hallmark of HIV infection systemically and on the skin. This is due to the active destruction of CD4+ cells by the virus, increased percentage of CD4+ T-cells undergoing apoptosis, reduction in the proliferative capacity of CD4+ T-cells, and an increase in the expression of CD4+ T-cell inhibitory molecules like CTLA-4 and PD-1. The reduction in CD4+ T-cells drives a switch from Th1 to Th2 cytokine polarization resulting in a progressive decline of IFN-γ and cytotoxic T-lymphocyte functioning and an incline in IL-4, IL-5, and IgE (26). The Th2 polarization results in impaired barrier function and resultant predisposition to allergen sensitization. Th17 cells that contribute to epithelial barrier integrity are also targets for HIV infection (27, 28). CD4 expressing cells affected by HIV infection include T regulatory cells (Treg) and Langerhan cells. Tregs serve an important role as guardians of immunological self-tolerance and prevention of autoimmune diseases. Langerhan cells are the major cutaneous antigen-presenting cells and lead off immune responses during initial antigen exposure by activating resident immune cells as well as linking innate and adaptive immune systems. In HIV infection, there is a compensatory expansion of CD8+ T-cells, terminal effector T cells in the skin in an effort to control ongoing retroviral infection and mediate tissue damage. The overall effect of this immune dysregulation sometimes has a consequence of inducing new skin disorders, including photodermatoses (26, 29).
Persistent T-cell activation is another hallmark of HIV infection. This has been attributed to the persistence of HIV viral reservoirs, even after successful virological control; intestinal microbial translocation into circulation as a result of impaired gut barrier; depletion and functional impairment of Treg cells; and coinfection with or reactivation of other viruses (30, 31). It has recently been established that human T cells are independently photosensitive. Under the influence of light, T cells were triggered to produce hydrogen peroxide (H2O2), a major source of reactive oxygen species and effector of oxidative stress. Blue light was also found to potently enhance T cell motility, stimulating random cell movement and chemotaxis. Significantly, photosensitivity was greater in activated compared to naïve T cells (32).
Some of the medications used to treat HIV, HIV-associated opportunistic infections, and complications such as trimethoprim-sulfamethoxazole, non-steroidal anti-inflammatories (NSAIDs), and antiretrovirals (ART) can be photosensitizing and increase the risk of photodermatitis. A dose-response association with increasing UV exposure and photosensitivity in HIV-infected individuals has been reported. This suggests that there is variation in the intensity and duration of exposure to initiate and maintain different clinical phenotypes of photodermatitis (2, 17). However, multiple studies have failed to find an association or a change in MED in HIV-infected populations. (17, 23, 33)
There is an increasing awareness of the underrepresentation of pigmented skin in both undergraduate and postgraduate teaching, textbooks, journal articles, and search engines (34). Photodermatoses are no exception and there is a need to correct this anomaly. Photosensitivity is up to seven times more common in HIV-infected individuals with pigmented skin (2). Globally, Africa carries the heaviest burden of HIV, thus the largest proportion of HIV-infected persons are melanin rich (35). There are numerous factors that may potentially impact the clinical presentation of photodermatoses as well as severity in African populations with higher Fitzpatrick skin types (34, 36). These include (i) detection of erythema and purpura, both being more difficult in melanin-rich skin, (ii) susceptibility to dyspigmentation based on the chromatic tendency theory which seems to be genetically pre-determined and inherited in an autosomal dominant pattern, (iii) contrast between normal skin and dyspigmented skin which has a more detrimental cosmetic outlook for darker skin, (iv) the generally higher preponderance of darker skin to pruritus, a major feature in many photodermatoses (v) pigmented skin has larger mast cell granules and increased activity in some disorders like keloids. Mast cells play a significant role in HIV by promoting infection of CD4+ as well as being associated with increased antigenic responses in HIV-infected patients (34, 37–40).
Diltiazem, a calcium channel blocker is associated with photodistributed hyperpigmentation that seems to be confined to darker skin tones. The exact pathomechansim is unclear but seems to be due to impaired melanogenesis and aberrant transfer of immature melanosomes from melanocytes to keratinocytes (41, 42).
Table 1 summarises the clinical and histological features of photodermatoses relevant to HIV-infected persons.
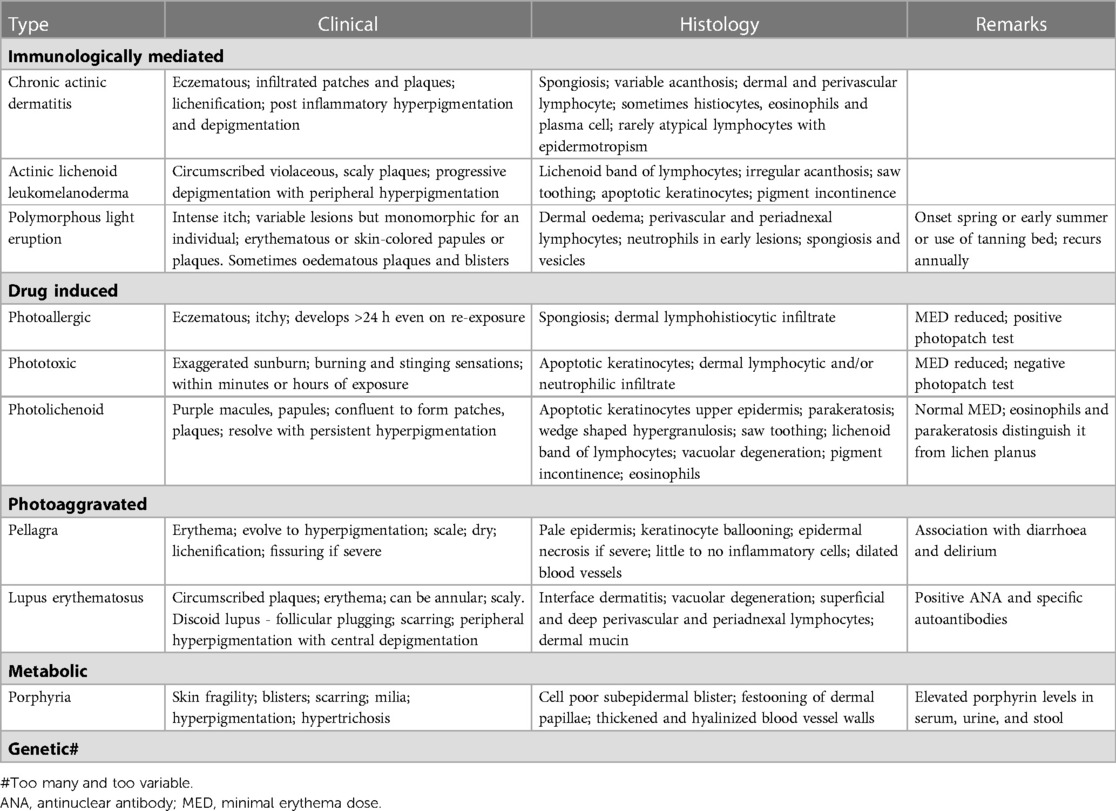
Table 1. Summarises the clinical and histological features of photodermatoses relevant to HIV-infected persons.
Chronic actinic dermatitis (CAD) is a rare persistent and disfiguring photodermatosis, encompassing a spectrum of disorders including actinic reticuloid, photosensitive eczema, and persistent light reactivity. It represents 4.8 to 17% of photodermatoses seen in photobiology clinics (43). Individuals of all skin types can be affected, but it has been reported more commonly in individuals with Fitzpatrick V and VI. There is no data supporting familial inheritance. CAD has a predilection for men over the age of 60 who spend time outdoors, and a history of allergic or photoallergic contact dermatitis to multiple allergens. CAD has also been reported in younger patients with atopic dermatitis. The disease also has a strong association with contact or photoallergy to Compositae, a group of plant-based aero allergens (44, 45).
The pathogenesis of CAD is poorly understood. It is postulated to be a delayed-type hypersensitivity reaction to an altered endogenous protein that has been made antigenic by photoinduced reaction (46). Its clinical and histopathological characteristics, and predominance of CD8+ T cells, resembles allergic contact dermatitis and hence a similar pathogenesis can be inferred. The antigenic molecules in CAD have been postulated to be DNA, RNA, or molecules related to these (46, 47). Thus, CAD may be a delayed hypersensitivity response to UV-damaged nucleic acids, triggered by various forms of immunosuppression including ultraviolet (UV) radiation and HIV. Contact dermatitis-induced enhanced immunoreactivity has also been postulated to play a role (46).
The diagnostic criteria of CAD as proposed by Hawk and Magnus are:
• chronic photodermatitis, characterized by a persistent eczematous eruption of infiltrated papules and plaques, in predominantly sun-exposed sites, in the absence of continued exposure to photosensitizers.
• abnormal photo test responses namely reduced minimal erythema dose (MED) to UVA, UVB and/or visible light.
• and histological changes consistent with photodermatosis, with or without lymphoma-like changes (48).
Clinically CAD is characterized by pruritic eczematous or lichenified or even infiltrated patches and plaques that are initially limited to sun-exposed areas. Figure 1 Sparing of the nasolabial folds; post auricular, submental, and periorbital areas; skin folds; and interdigital areas is typical (43, 48, 49). In severe cases, CAD may involve sun-protected areas and rarely it can become erythrodermic (50). CAD in darker skin types (Fitzpatrick IV-VI) sometimes results in marked hyperpigmentation that is followed by vitiligo-like depigmentation (49). Other presentations include palmar-plantar hyperkeratosis as well as loss of eyebrows and scalp hair, presumably due to scratching, and (46, 48). Lymphadenopathy and peripheral atypical lymphocytes may be detected in a limited number of patients, reminiscent of Sezary syndrome and cutaneous T-cell lymphoma, creating diagnostic confusion (51–55). On histology CAD is characterized by spongiosis, variable acanthosis with dermal and perivascular lymphocytes (56). There may be atypical lymphocytes with large, hyperchromatic, and convoluted nuclei and epidermotropism. In severe cases, the histological picture may not be distinguishable from cutaneous T cell lymphoma. Histiocytes, eosinophils, and plasma cells may also be present (44, 54, 55, 57). Immunohistochemical studies of CAD biopsies show that most of the skin-infiltrating cells are CD8+ T lymphocytes, with less than 10% showing CD4+ dominant T cell infiltrate in the dermis (55, 56, 58).

Figure 1. Chronic actinic dermatitis of HIV in Fitzpatrick skin type IV showing a photodistributed eczematous eruption.
Chronic actinic dermatitis with eczematous features has been described as the presenting illness in patients with HIV infection (18, 19). The clinical features, including distribution and morphology are indistinguishable from CAD in HIV-uninfected persons. In severe cases, HIV-associated late-stage CAD may present with hypopigmented or vitiligo-like depigmentation (19,20). Figure 2 CAD in the setting of HIV seems to predominantly affect men of Fitzpatrick skin types V–VI, which is consistent with observed studies of CAD in HIV-uninfected patients. HIV-associated CAD has also been described in Fitzpatrick skin types III and IV, although less commonly. However, CAD cases in HIV tend to be younger than those who are HIV-uninfected individuals (<50 years vs. >60 years), and usually have significant immunosuppression at presentation (CD4 counts of <200 cells/mm (19, 22). This suggests that HIV, especially advanced disease, hastens the onset of CAD in predisposed individuals and plays a role in its pathogenesis (22).
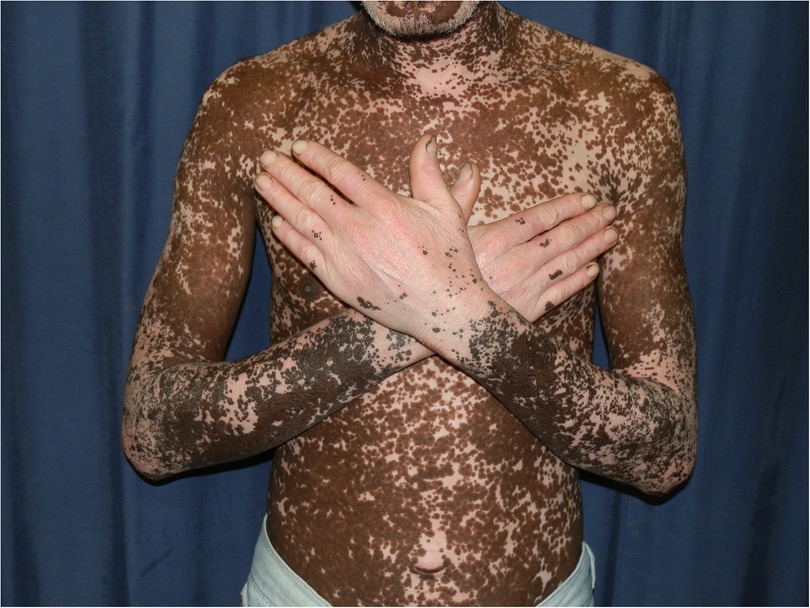
Figure 2. Chronic actinic dermatitis of HIV in Fitzpatrick skin type V with extension to non-sun exposed areas demonstrating hyperpigmentation and extensive depigmentation.
Like CAD in general, the pathogenesis of HIV-associated CAD has not been defined. CD8+ T lymphocytes are thought to play a central role. A decrease in CD4:CD8 ratio in lesional skin has been reported in all forms of CAD (18, 46, 55, 56). On treating the skin, with associated clinical improvement, there is a reversal of CD4:CD8 ratio in both the skin and blood (18, 56). Pappert et. al. speculated that the decreased CD4:CD8 ratio in HIV results in immune dysregulation and loss of control of anti-self-responses and proliferation of photo-induced self-antigens. The shift towards CD8+ T cell phenotype enhances cytotoxic activity against these self-antigens (18). However, a recent study has shown that these infiltrating CD8+ T cells rarely express cytotoxic molecules like TIA-1, granzyme B, or granulysin (56). The implications then of the diminished cytotoxic markers in CD8+ lesional skin on this hypothesis is not clear. Additionally, there is evidence to suggest that in CAD there may be an acquired quantitative or qualitative defect in Tregs resulting in unchecked cytotoxic T cell responses (59).
Actinic lichenoid leukomelanoderma of HIV is a photosensitive eruption anecdotally observed in the South African clinical setting (25). The condition presents in the setting of advanced HIV with low CD4 counts and absence of other photosensitising medication. Clinically it is characterised by well-circumscribed, photodistributed scaly plaques resembling discoid lupus erythematosus. The lesions characteristically start as violaceous macules, which become progressively depigmented with peripheral hyperpigmentation, sometimes with scale (25). While it remains to be established whether this condition is a distinct entity, it is also possible that this condition falls along the spectrum of HIV photodermatitis with vitiligo-like depigmentation or CAD in HIV-infected persons (60, 61). Further studies are needed to characterise this condition.
HIV photodermatitis with widespread vitiligo-like depigmentation is rarely reported (60, 61). We reported a photodistributed lichenoid drug eruption with depigmentation in an HIV-infected man on treatment for a second episode of tuberculosis. The rash initially developed during the first course of tuberculosis treatment and resulted in areas of depigmentation and hyperpigmentation. On reexposure to the same regimen to treat the second episode of tuberculosis, there was recurrence with violaceous patches within the depigmented areas from the first episode. On completion of the second regimen, repigmentation was considerably better than after the first episode. Phillips et al. reported a 60-year-old man ART naïve man with advanced HIV (CD4 count of 7 cells/µl) who presented with a photodistributed, pruritic eruption associated with extensive depigmentation surrounded by hyperpigmentation. Histology revealed a spongiotic dermatitis with abundant eosinophils. A similar case was described involving a Ugandan woman with advanced HIV (CD4 count of 2 cells/µl) occurring one month after initiation of ART with stavudine, lamivudine, and nevirapine (60, 61). Most areas repigmented after the regimen was changed to zidovudine, lamivudine, and efavirenz. The authors postulated that the photodistributed depigmentation was due to a photosensitizing effect of either the ART or cotrimoxazole; with the ART being more likely. Histology was not reported (60). All three cases were of Fitzpatrick skin phototype V or greater (62).
Depigmentation has also been reported in chronic lichenoid photo eruptions in HIV patients with a low CD4 T-lymphocyte count <50 cells/µl (63, 64). We reported an HIV-infected man with low CD4 count and multidrug-resistant tuberculosis developed a generalised lichenoid eruption that progressively worsened and depigmented after initiating tuberculosis treatment. Treatment was continued under the cover of sun protection, potent topical corticosteroids, and PUVA to induce hardening. He completed nine months of treatment and soon afterwards the depigmentation reversed gradually (65).
Photosensitivity with depigmentation prior to the diagnosis of HIV, has also been reported. The presentation was marked by scaling, erythema, and concurrent vitiligo-like depigmentation of the face, arms, and hands. The condition improved with the use of sunscreen and topical steroids (2).
The differential diagnosis of photodermatitis with vitiligo-like depigmentation includes discoid lupus erythematosus (DLE), vitiligo, and lichen simplex chronicus. To differentiate from DLE there is an absence of scarring and histology does not demonstrate an interface dermatitis. The immunodeficiency, photo distribution, and histology demonstrating spongiotic dermatitis with eosinophils distinguish HIV photodermatitis from vitiligo. Likewise, the photo distribution and absence of lichenification exclude lichen simplex chronicus.
Actinic lichen planus is a morphologic variant of LP that predominantly affects sun-exposed areas and has a high prevalence in darker skin. Three variants of actinic LP have been described, namely annular, pigmented, and dyschromic, in that order of their frequency. The annular variant presents as annular erythematous brownish patches or plaques with or without atrophy. The major feature of the pigmented variant is the presence of melasma-like pigmentation. Dyschromic-type actinic lichen planus presents as whitish pinhead-sized and coalescent papules (66, 67). HIV seems to have a stronger association with photolichenoid eruptions. In a series of 32 patients with histologically confirmed lichenoid eruption or photodermatitis, 12/32 were HIV infected, and in all 12, the lesions were photodistributed (63).
Photosensitive drugs are chromophores that absorb photons and undergo chemical reactions. The chemical structure of the chromophore determines the wavelengths of radiation it absorbs, with most reactions being caused by UVA rather than UVB. Drug-induced photodermatoses in the setting of HIV include phototoxic, photoallergic, and photolichenoid reactions. Drugs most commonly associated with photosensitization include amiodarone; chlorpromazine; thiazide diuretics; tetracyclines; quinolones, particularly nalidixic acid; nonsteroidal anti-inflammatory drugs derived from propionic acid, voriconazole and vemurafenib, a B-Raf enzyme inhibitor used in the management of late-stage melanoma (68).
ART, antituberculosis drugs, sulphonamides, and antifungals, all drugs used to treat HIV and associated opportunistic infections, have at least one drug or a class of drugs that has been reported to be a photosensitizer. Efavirenz, an ART drug, has been reported to cause a photodistributed transient eruption (69–71). A small series of five cases in South Africa reported photodistributed annular plaques with an indurated erythematous edge as the most common presentation of the efavirenz reaction. Figures 3, 4 Histology findings were non-specific. Systemic features were mild and included a mild elevation in alanine and/or aspartate transaminases. Despite the continuation of efavirenz, the majority resolved (72, 73). Cotrimoxazole has been implicated in vitro as a photosensitizer, and the sulfamethoxazole component is believed to be responsible (74). Dapsone photoallergic dermatitis has been described in case reports (75–77). Despite its use as an alternative to cotrimoxazole for Pneumocystis jirovecii prophylaxis, there are no reports of dapsone photoallergic dermatitis in African people or HIV patients.

Figure 3. Efavirenz-associated reaction demonstrating in photodistributed indurated erythema with sharp cut off and annularity.
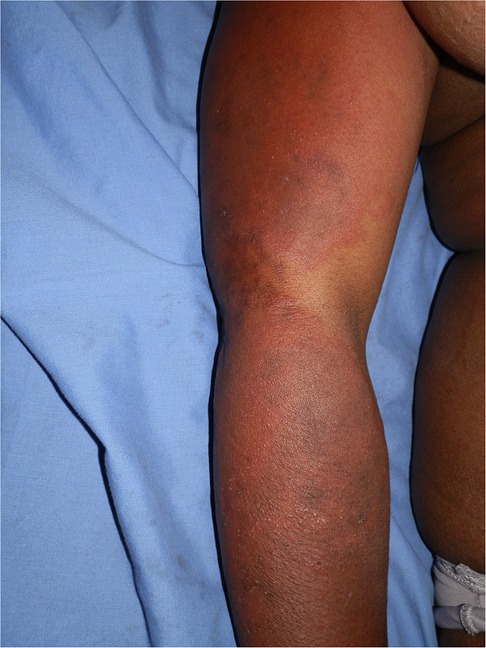
Figure 4. Efavirenz-associated reaction demonstrating in photodistributed indurated erythema with annularity of plaques and dusky centres.
Antifungals known to cause photosensitivity include voriconazole, itraconazole, ketoconazole, and griseofulvin (78–81). Itraconazole reactions include a sunburn-like reaction, most likely phototoxic with decreased MED and negative photopatch tests (78). Voriconazole photosensitivity includes sunburn-like erythema, hand hyperpigmentation, linear papulo-vesicular lesions, erythroderma, discoid lupus erythematosus, actinic cheilitis and pseudoporphyria (78, 82). Most reactions occurred in patients receiving long-term immunotherapy, however, there is no data reported in the HIV infected population. There are reports of the development of squamous cell carcinoma and melanoma in voriconazole-induced sites of photosensitivity (83). Amongst the drugs used to treat tuberculosis, photodistributed eczematous eruption due to isoniazid and pyrazinamide has been described (84). On discontinuing the drug, the photosensitivity usually resolves. However, persistent photosensitivity and progression to CAD have been reported (12, 14).
Photolichenoid drug reactions seem to be more common in HIV-infected people than in the general population and in this setting often pose major management challenges. In a case series of 32 patients in San Francisco, with a histologic diagnosis of lichenoid eruption or photodermatitis, 12/32 were HIV infected, and all 12 were photodistributed. Exposure to known photosensitizing drugs preceded the eruption in 10/12. Patients with pigmented skin and advanced HIV disease (CD4 < 50 cells/µl), were disproportionately affected. Histologically 9/12 showed lichenoid reaction, 2/12 had features of lichen nitidus, and 1/12 was eczematous. Two of those with lichenoid reactions on histology developed marked depigmentation. No cases of lichen planus were found in the HIV-infected group. The authors suggested that previous cases of classic lichen planus in HIV may represent lichenoid photodermatitis (63).
Anti-tuberculosis medications, including isoniazid and pyrazinamide, have been reported to cause a lichenoid and/or photo lichenoid eruption (65, 85, 86). The pathogenesis is unclear but may be due to delayed hypersensitivity. LDR presents as purple itchy papules becoming confluent and hyperpigmented with continuing exposure to the offending drug. It is often photodistributed and lacks mucosal involvement Figures 5, 6. The interval between drug initiation and the rash ranges from days to years, with most cases occurring within months. On drug withdrawal, the lesions resolve with persistent hyperpigmentation, often lasting for many years. Although photolichenoid eruptions may be related to photosensitizing agents, there seems to be no association or changes in minimal erythemal dose (17, 23, 33). Photolichenoid reactions have been reported for isoniazid, confirmed by positive photopatch testing and oral rechallenge (86). Due to the lack of acute markers and delayed resolution of symptoms and signs of the reaction it is often difficult to identify the offending drug. Due to the relative urgency of treating tuberculosis in HIV and the limited number of effective safe drugs, all the drugs are sometimes continued with supportive care until completion of treatment in those with active tuberculosis (62, 65).
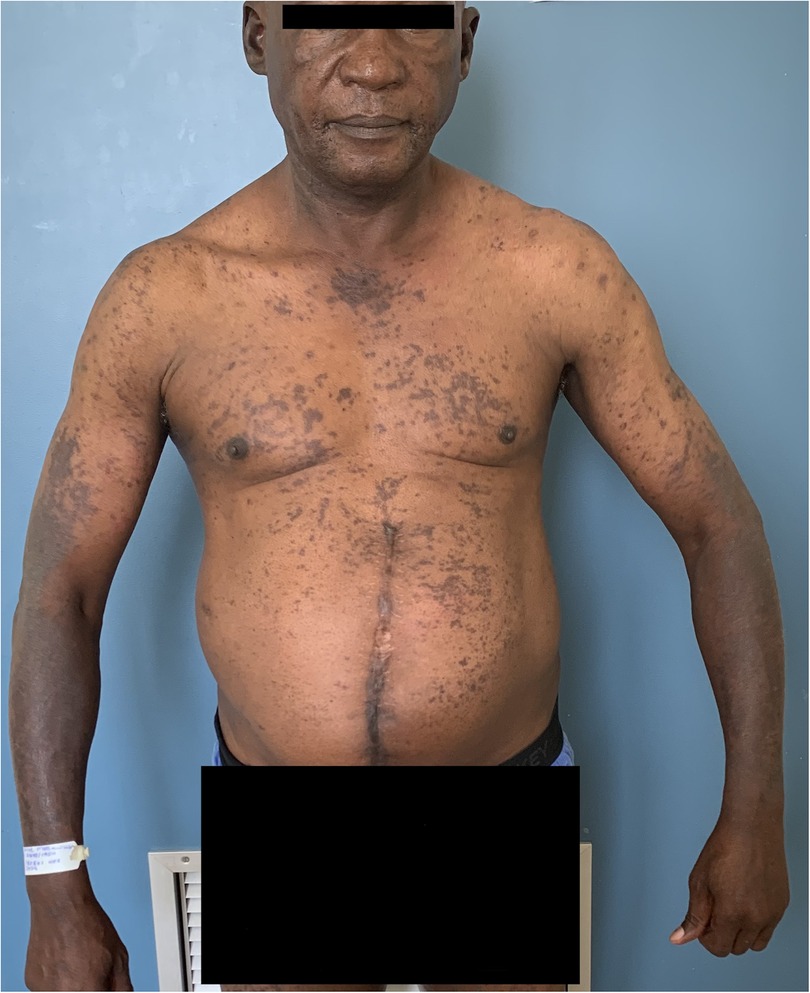
Figure 5. Photolichenoid drug eruption to first line antituberculosis treatment demonstrating violaceous patches, plaques.
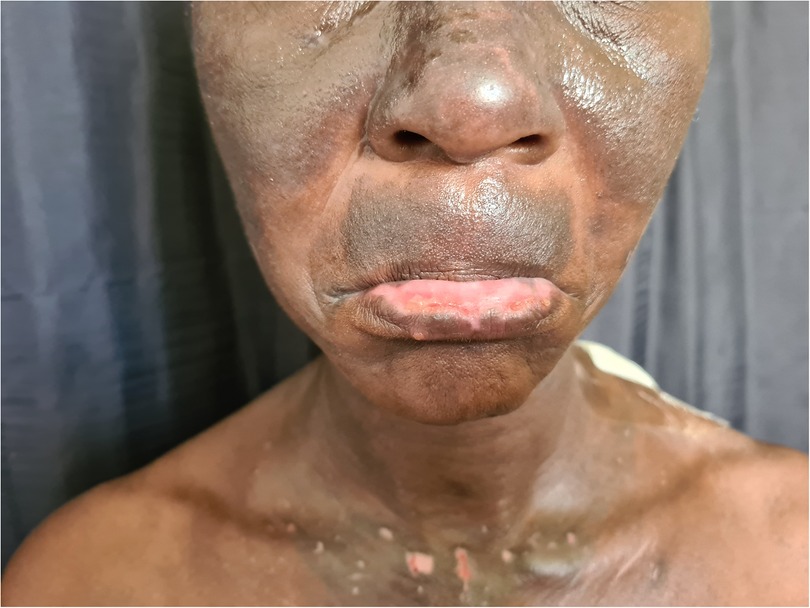
Figure 6. Photolichenoid drug eruption to first line antituberculosis treatment demonstrating violaceous patches and plaques with depigmentation.
Porphyria cutanea tarda (PCT), the most common form of porphyria, is caused by a deficiency of the fifth enzyme of the heme biosynthesis pathway, uroporphyrinogen decarboxylase (UROD). Decreased UROD activity leads to the overproduction of porphyrins in the liver. PCT is a cause of chemical-induced phototoxicity caused by sunlight interacting with endogenous porphyrins within the body. Clinically, these manifest in the third to fourth decade of life with photosensitivity, skin fragility, and blisters. The blisters occur in photodistributed sites particularly the face, V of the neck, and dorsa of the hands, and are associated with scarring and milia Figures 7, 8. Other features include hyperpigmentation, hypertrichosis, and rarely sclerodermoid changes. Earlier studies suggested a causal association between HIV and PCT (87, 88). While the mechanism of PCT in HIV is still not fully understood, it is thought to be due to changes in porphyrin metabolism and liverinjury in the setting of co-infection with hepatitis C. It is now postulated that in most HIV-infected patients with PCT, hepatitis C and not HIV may induce a decrease in UROD activity (89). Drug exposure influences PCT. Amongst first-line antituberculosis drugs, rifampicin is the most likely to be associated with PCT (90, 91). We recently encountered an HIV-infected man who presented with PCT after initiating rifampicin, isoniazid, pyrazinamide, and ethambutol. Based on published reports, we decided to replace rifampicin with rifabutin, a similarly effective rifamycin with less effect on the liver (92). The photosensitivity and blistering improved until he completed the remaining four months of his six-month course of treatment.
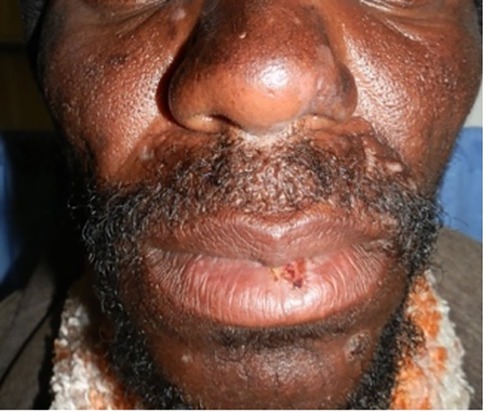
Figure 7. Blisters, erosions, scarring and hyperpigmentation on the face of a patient with porphyria cutanea tarda.
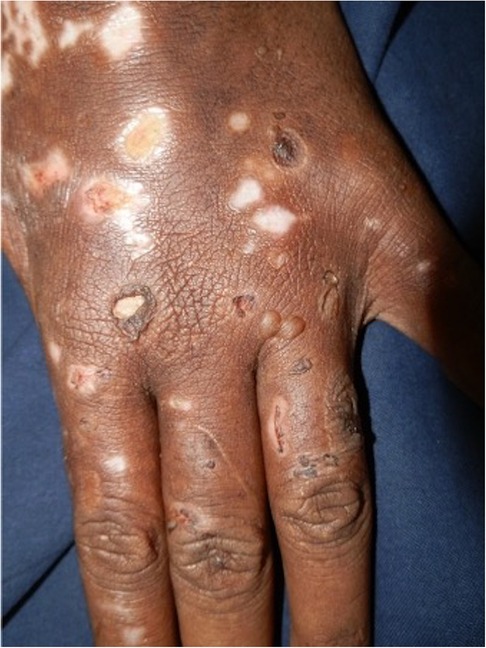
Figure 8. Blisters, erosions, and scarring on the dorsa of the hand of a patient with porphyria cutanea tarda.
Diagnosis of PCT can be confirmed by histology and porphyrin levels. Histologically, PCT is characterized by a cell-poor subepidermal blister, festooning of the dermal papillae, and thickening and hyalinization of the dermal blood vessel walls. Additional features include caterpillar bodies and dermal sclerosis and a mild infiltrate of perivascular mononuclear cells in the upper dermis. These features are not specific to porphyria and can also occur in pseudoporphyria syndromes. The most important diagnostic test to diagnose porphyria remains porphyrin levels in serum, urine, and stool (93).
Pellagra, as mentioned earlier is a nutritional disease caused by the deficiency of niacin (also known as vitamin B3). Clinically pellagra is characterized by photodermatitis with gastrointestinal symptoms and neuropsychiatric ailments. Pellagra can be fatal if not timeously recognized and treated. Erythema is the initial clinical feature, and this evolves into hyperpigmentation, dryness, scale, lichenification, and fissuring. On the dorsum of the hands and forearms this is referred to as the gauntlet or glove sign. Asimilar feature on the lower extremities is called the boot sign. The development of fissures on the hands and feet surface is called goose skin. The eruption surrounding the base of the neck is called the Casal's collar or necklace Figure 9. In severe cases blistering occurs within the lesions and this is referred to as wet pellagra which tends to heal with scarring. Additionally, lesions may develop over bony prominences, perineum, and scrotum. The eruption usually presents as erosions with burning pain that intensifies on palpation (94). HIV as well as isoniazid, pyrazinamide, and ethionamide have been shown to cause pellagra or pellagra-like disease (95, 96). It is thus even more important to recognize and prevent pellagra in HIV-infected people on treatment for tuberculosis, particularly breast-feeding women and young children. Breastfed infants receiving isoniazid should also receive pyridoxine 1 mg/kg daily (97).
There is a general consensus that chronic photosensitization is associated with an increase in the development skin cancer. This risk is determined by the Fitzpatrick skin type, age at which photosensitizer was used, duration and severity of photosensitization, immunosuppression, the photosensitizing agent/drug, and spectrum of UV absorption amongst others (98). Azathioprine, a photosensitizing immunosuppressant, used after solid organ transplant and inflammatory disorders is associated with an increased risk of squamous cell carcinoma (SCC) by additionally producing mutagenic reactive oxygen species on the skin and an additional risk of SCC on the skin. In transplant patients, the risk is estimated to be higher by the magnitude of 65–250 times (99). This is still applicable when comparing immunosuppressive regimens that incorporate azathioprine and those that do not (100, 101). On the other hand, mycophenolate mofetil, a non-photosensitizing immunosuppressant of similar potency, reduces the incidence of SCC in this setting (99). Other photosensitizing drugs that have been associated with an increased risk of skin cancer include thiazides, angiotensin-converting-enzyme inhibitors, cotrimoxazole, tetracyclines, and azoles (98, 102). On the other hand, long-term use of NSAIDs including those that are photosensitisers, seems to reduce the risk of developing cutaneous SCC (102). Photosensitivity also has a direct relationship with photoaging (103, 104). Concerns about the adverse effects of using light therapies like lasers are not supported by any published reports. This further supports the view that photosensitivity as described is wavelength specific (105).
A recent systematic review that included 19 studies found that between 31% and 39% of photosensitive patients suffered a very large impact on their quality of life. Employment, education, social and leisure activities, and clothing choices were most affected. The study also confirmed that the levels of anxiety and depression were twice those of the general population. Involvement of the face, being female, and an earlier age of onset were associated with significantly more severe psychological morbidity. This study confirmed findings from multiple previous studies and highlights the hidden toll of photosensitivity on sufferers (106, 107). There are limited data on the psychosocial impact of photosensitivity focusing on pigmented skin. The additional impact of more severe dyspigmentation is likely to exacerbate the psychosocial impact of photosensitivity in this population, more so if HIV-infected as both these are independent predictors of poorer quality of life and psychosocial morbidity (108–111).
A good history, clinical examination, a skin biopsy, and photo testing are helpful in identifying photodermatitis and in distinguishing phototoxic from photoallergic reactions. However, this distinction may be difficult as there is sometimes an overlap. Photo testing and photopatch testing may be helpful in assessing patients with photodermatitis and have proven useful to differentiate the photosensitivity mechanism (112). Photo testing, the aim of which is to determine whether the minimal erythema dose (MED) is reduced in the presence of the drug, can be conducted with the use of a solar simulator or in resource-limited settings with the crude method of exposure to midday sunlight. It is done in a number of shielded and unshielded areas on the upper back of the patient while taking and then not taking the suspected drug (13). A reduced MED indicates photosensitivity.
Photo patch testing is an important tool in diagnosing photoallergic drug reactions. It involves the application of drugs on the patient's back and then occlusion. Individual Finn chambers used in standard patch testing may be used. The patches are uncovered after 24 h and irradiated with UVR below the pre-determined MED. The patch is then read 24 h post UV irradiation. A positive photo patch test implies a photoallergic reaction. Although used in the clinical setting, its use for the diagnosis of photo drug reactions due to systemic medication has not yet been validated (13).
Most photosensitivity drug reactions resolve with sun avoidance and drug discontinuation. Patients who are unable to discontinue the offending agent, need multiple photoprotective measures including sun avoidance, protective clothing, broad protection sunscreen, and potent topical steroids. Treatment is often difficult. Phototherapy has been used with success to induce hardening (65). Treatment with thalidomide has been successful in refractory cases (113, 114). However, it is important to note that thalidomide has been reported to increase the HIV viral load, although the clinical significance of this phenomenon is unknown. Therefore, the HIV RNA levels should be monitored after the first and third months of thalidomide treatment and should subsequently be repeated every three months (115).
HIV photodermatitis is a presenting feature of a myriad of clinical conditions, which should be considered in patients with HIV/AIDS who present with a pruritic, photodistributed eruption. Drug-induced photosensitivity remains a frequent clinical concern. Among the photosensitizers include such drugs as efavirenz, cotrimoxazole, INH, PZA, and antifungal agents. Photo testing may be useful in establishing photosensitivity in both drug-induced and non-drug-induced photodermatitis. Additionally, photo patch testing may be useful to establish the causative agent. Photodermatitis has a profound impact on quality of life. Treatment includes stopping potential photosensitisers, sun avoidance, photoprotective clothing, broad-spectrum sunscreens, and potent topical steroids.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
TI and RL: contributed to the conception, writing, and literature review of the manuscript. Both authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bylaite M, Grigaitiene J, Lapinskaite GS. Photodermatoses: classification, evaluation and management. Br J Dermatol. 2009;161 Suppl 3:61–8. doi: 10.1111/j.1365-2133.2009.09451.x
2. Bilu D, Mamelak AJ, Nguyen RH, Queiroz PC, Kowalski J, Morison WL, et al. Clinical and epidemiologic characterization of photosensitivity in HIV-positive individuals. Photodermatol Photoimmunol Photomed. 2004;20(4):175–83. doi: 10.1111/j.1600-0781.2004.00101.x
5. Foering K, Chang AY, Piette EW, Cucchiara A, Okawa J, Werth VP. Characterization of clinical photosensitivity in cutaneous lupus erythematosus. J Am Acad Dermatol. 2013;69(2):205–13. doi: 10.1016/j.jaad.2013.03.015
7. Auriemma M, Capo A, Meogrossi G, Amerio P. Cutaneous signs of classical dermatomyositis. G Ital Dermatol Venereol. 2014;149(5):505–17.25014587
8. Plantin P, Le Noac’h E, Leroy JP, Gourcuff H. Localized recurrent light-induced darier disease following blaschko lines. Ann Dermatol Venereol. 1994;121(5):393–5.7702264
9. Ott A. Persistent acantholytic dermatosis with increased light sensitivity. Z Hautkr. 1987;62(5):369–70, 75–8.3590911
10. Singal AK. Porphyria cutanea tarda: recent update. Mol Genet Metab. 2019;128(3):271–81. doi: 10.1016/j.ymgme.2019.01.004
12. Palmer RA, White IR. Phototoxic and photoallergic reactions. In: Frosch PJ, Menné T, Lepoittevin J-P, editors. Contact Dermatitis. Berlin, Heidelberg: Springer Berlin Heidelberg; 2006. p. 309–17.
13. Drucker AM, Rosen CF. Drug-induced photosensitivity: culprit drugs, management and prevention. Drug Saf. 2011;34(10):821–37. doi: 10.2165/11592780-000000000-00000
14. Moore DE. Drug-induced cutaneous photosensitivity: incidence, mechanism, prevention and management. Drug Saf. 2002;25(5):345–72. doi: 10.2165/00002018-200225050-00004
15. Kutlubay Z, Sevim A, Engin B, Tuzun Y. Photodermatoses, including phototoxic and photoallergic reactions (internal and external). Clin Dermatol. 2014;32(1):73–9. doi: 10.1016/j.clindermatol.2013.05.027
16. Gru AA, Salavaggione AL. Lichenoid and interface dermatoses. Semin Diagn Pathol. 2017;34(3):237–49. doi: 10.1053/j.semdp.2017.03.001
17. Vin-Christian K, Epstein JH, Maurer TA, McCalmont TH, Berger TG. Photosensitivity in HIV-infected individuals. J Dermatol. 2000;27(6):361–9. doi: 10.1111/j.1346-8138.2000.tb02185.x
18. Pappert A, Grossman M, DeLeo V. Photosensitivity as the presenting illness in four patients with human immunodeficiency viral infection. Arch Dermatol. 1994;130(5):618–23. doi: 10.1001/archderm.1994.01690050086015
19. Meola T, Sanchez M, Lim H, Buchness M, Soter N. Chronic actinic dermatitis associated with human immunodeficiency virus infection. Br J Dermatol. 1997;137(3):431–6. doi: 10.1046/j.1365-2133.1997.18641956.x
20. Mercer JJ, Yin NC, Lee ES, Mosam A, Dlova NC. Photodermatitis with subsequent vitiligo-like leukoderma in HIV infection. Int J Dermatol. 2015;55(5):306–7. doi: doi: 10.1111/ijd.13075
21. Wong S, Khoo L. Analysis of photodermatoses seen in a predominantly Asian population at a photodermatology clinic in Singapore. Photodermatol Photoimmunol Photomed. 2005;21(1):40–4. doi: 10.1111/j.1600-0781.2005.00137.x
22. Wong SN, Khoo LS. Chronic actinic dermatitis as the presenting feature of HIV infection in three Chinese males. Clin Exp Dermatol. 2003;28(3):265–8. doi: 10.1046/j.1365-2230.2003.01249.x
23. Gregory N, DeLeo VA. Clinical manifestations of photosensitivity in patients with human immunodeficiency virus infection. Arch Dermatol. 1994;130(5):630–3. doi: 10.1001/archderm.1994.01690050098018
24. Cohen PR, Grossman ME, Silvers DN, DeLeo VA. Generalized granuloma annulare located on sun-exposed areas in a human immunodeficiency virus-seropositive man with ultraviolet B photosensitivity. Arch Dermatol. 1990;126(6):830–1. doi: 10.1001/archderm.1990.01670300130029
25. Koch K. Photosensitive disorders in HIV. South Afr J HIV Med. 2017;18(1):676. doi: 10.4102/sajhivmed.v18i1.676
26. Chimbetete T, Buck C, Choshi P, Selim R, Pedretti S, Divito SJ, et al. HIV-associated immune dysregulation in the skin: a crucible for exaggerated inflammation and hypersensitivity. J Invest Dermatol. 2022;143(3):362–73. doi: 10.1016/j.jid.2022.07.035
27. Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112(7):2826–35. doi: 10.1182/blood-2008-05-159301
28. Klatt NR, Brenchley JM. Th17 cell dynamics in HIV infection. Curr Opin HIV AIDS. 2010;5(2):135–40. doi: 10.1097/COH.0b013e3283364846
29. Hirahara K, Nakayama T. CD4+ T-cell subsets in inflammatory diseases: beyond the Th1/Th2 paradigm. Int Immunol. 2016;28(4):163–71. doi: 10.1093/intimm/dxw006
30. Lv T, Cao W, Li T. HIV-related immune activation and inflammation: current understanding and strategies. J Immunol Res. 2021;2021:7316456. doi: 10.1155/2021/7316456
31. Lopez-Abente J, Correa-Rocha R, Pion M. Functional mechanisms of treg in the context of HIV infection and the Janus face of immune suppression. Front Immunol. 2016;7:192. doi: 10.3389/fimmu.2016.00192
32. Phan TX, Jaruga B, Pingle SC, Bandyopadhyay BC, Ahern GP. Intrinsic photosensitivity enhances motility of T lymphocytes. Sci Rep. 2016;6:39479. doi: 10.1038/srep39479
33. Aubin F, Parriaux N, Robert C, Blanc D, Drobacheff C, Laurent R, et al. Cutaneous reaction to ultraviolet irradiation in human-immunodeficiency-virus-infected patients. A case-control study. Dermatology. 1999;198(3):256–60. doi: 10.1159/000018125
34. Lehloenya RJ, Phillips EJ, Pasieka HB, Peter J. Recognizing drug hypersensitivity in pigmented skin. Immunol Allergy Clin North Am. 2022;42(2):219–38. doi: 10.1016/j.iac.2022.01.005
35. UNAIDS D, Sheet AF. (2021). Available at: https://www. unaids. org/sites/default/files/media_asset. JC3032_AIDS_Data_book_2021_En pdf (Accessed January 23, 2023).
36. Pandey A, Galvani AP. The global burden of HIV and prospects for control. Lancet HIV. 2019;6(12):e809–11. doi: 10.1016/S2352-3018(19)30230-9
37. Espindola MS, Soares LS, Galvao-Lima LJ, Zambuzi FA, Cacemiro MC, Brauer VS, et al. HIV infection: focus on the innate immune cells. Immunol Res. 2016;64(5-6):1118–32. doi: 10.1007/s12026-016-8862-2
38. Coopman SA, Johnson RA, Platt R, Stern RS. Cutaneous disease and drug reactions in HIV infection. N Engl J Med. 1993;328(23):1670–4. doi: 10.1056/NEJM199306103282304
39. Marone G, Varricchi G, Loffredo S, Galdiero MR, Rivellese F, de Paulis A. Are basophils and mast cells masters in HIV infection? Int Arch Allergy Immunol. 2016;171(3-4):158–65. doi: 10.1159/000452889
40. Craig SS, DeBlois G, Schwartz LB. Mast cells in human keloid, small intestine, and lung by an immunoperoxidase technique using a murine monoclonal antibody against tryptase. Am J Pathol. 1986;124(3):427–35.3532813
41. Oiso N, Yanagihara S, Nakagawa K, Kawada A. Diltiazem-associated photodistributed hyperpigmentation with abundant immature melanosome leakage into the dermis. J Dermatol. 2021;48(8):e390–2. doi: 10.1111/1346-8138.15960
42. Siegel JD, Ko CJ. Diltiazem-associated photodistributed hyperpigmentation. Yale J Biol Med. 2020;93(1):45–7.32226335
44. Lim HW, Cohen D, Soter NA. Chronic actinic dermatitis: results of patch and photopatch tests with compositae, fragrances, and pesticides. J Am Acad Dermatol. 1998;38(1):108–11. doi: 10.1016/S0190-9622(98)70549-3
45. Russell S, Dawe R, Collins P, Man I, Ferguson J. The photosensitivity dermatitis and actinic reticuloid syndrome (chronic actinic dermatitis) occurring in seven young atopic dermatitis patients. Br J Dermatol. 1998;138(3):496–501. doi: 10.1046/j.1365-2133.1998.02132.x
46. Hawk JL. Chronic actinic dermatitis. Photodermatol Photoimmunol Photomed. 2004;20(6):312–4. doi: 10.1111/j.1600-0781.2004.00129.x
47. Paek SY, Lim HW. Chronic actinic dermatitis. Dermatol Clin. 2014;32(3):355–61. doi: 10.1016/j.det.2014.03.007
48. Hawk JL, Magnus IA. Chronic actinic dermatitis–an idiopathic photosensitivity syndrome including actinic reticuloid and photosensitive eczema [proceedings]. Br J Dermatol. 1979;101 Suppl 17:24.465324
49. Millard TP, Hawk JL. Photosensitivity disorders. Am J Clin Dermatol. 2002;3(4):239–46. doi: 10.2165/00128071-200203040-00002
50. Somani VK. Chronic actinic dermatitis–a study of clinical features. Indian J Dermatol Venereol Leprol. 2005;71(6):409–13. doi: 10.4103/0378-6323.18946
51. Chu AC, Robinson D, Hawk JL, Meacham R, Spittle MF, Smith NP. Immunologic differentiation of the Sezary syndrome due to cutaneous T-cell lymphoma and chronic actinic dermatitis. J Invest Dermatol. 1986;86(2):134–7. doi: 10.1111/1523-1747.ep12284160
52. Pacheco D, Fraga A, Travassos AR, Antunes J, Freitas J, Soares de Almeida L, et al. Actinic reticuloid imitating Sezary syndrome. Acta Dermatovenerol Alp Pannonica Adriat. 2012;21(3):55–7.23267873
53. Preesman AH, Schrooyen SJ, Toonstra J, van der Putte SC, Rademakers LH, Willemze R, et al. The diagnostic value of morphometry on blood lymphocytes in erythrodermic actinic reticuloid. Arch Dermatol. 1995;131(11):1298–303. doi: 10.1001/archderm.1995.01690230078012
54. Agar N, Morris S, Russell-Jones R, Hawk J, Whittaker S. Case report of four patients with erythrodermic cutaneous T-cell lymphoma and severe photosensitivity mimicking chronic actinic dermatitis. Br J Dermatol. 2009;160(3):698–703. doi: 10.1111/j.1365-2133.2008.08955.x
55. Bakels V, van Oostveen JW, Preesman AH, Meijer CJ, Willemze R. Differentiation between actinic reticuloid and cutaneous T cell lymphoma by T cell receptor gamma gene rearrangement analysis and immunophenotyping. J Clin Pathol. 1998;51(2):154–8. doi: 10.1136/jcp.51.2.154
56. Hamada T, Aoyama Y, Shirafuji Y, Iwatsuki K. Phenotypic analysis of circulating T-cell subset and its association with burden of skin disease in patients with chronic actinic dermatitis: a hematologic and clinicopathologic study of 20 subjects. Int J Dermatol. 2017;56(5):540–6. doi: 10.1111/ijd.13486
57. Lim HW, Buchness MR, Ashinoff R, Soter NA. Chronic actinic dermatitis: study of the spectrum of chronic photosensitivity in 12 patients. Arch Dermatol. 1990;126(3):317–23. doi: 10.1001/archderm.1990.01670270049008
58. Sidiropoulos M, Deonizio J, Martinez-Escala ME, Gerami P, Guitart J. Chronic actinic dermatitis/actinic reticuloid: a clinicopathologic and immunohistochemical analysis of 37 cases. Am J Dermatopathol. 2014;36(11):875–81. doi: 10.1097/DAD.0000000000000076
59. Chevalier MF, Weiss L. The split personality of regulatory T cells in HIV infection. Blood. 2013;121(1):29–37. doi: 10.1182/blood-2012-07-409755
60. Kigonya C, Lutwama F, Colebunders R. Extensive hypopigmentation after starting antiretroviral treatment in an human immunodeficiency virus (HIV)-seropositive African woman. Int J Dermatol. 2008;47(1):102–3. doi: 10.1111/j.1365-4632.2007.03353.x
61. Philips RC, Motaparthi K, Krishnan B, Hsu S. HIV Photodermatitis presenting with widespread vitiligo-like depigmentation. Dermatol Online J. 2012;18(1):6. doi: 10.5070/D374H8T36W
62. Lehloenya RJ, Kgokolo M. Clinical presentations of severe cutaneous drug reactions in HIV-infected Africans. Dermatol Clin. 2014;32(2):227–35. doi: 10.1016/j.det.2013.11.004
63. Berger TG, Dhar A. Lichenoid photoeruptions in human immunodeficiency virus infection. Arch Dermatol. 1994;130(5):609–13. doi: 10.1001/archderm.1994.01690050077013
64. Smith KJ, Skelton HG, Yeager J, Tuur S, Angritt P, Wagner KF. Histopathologic features seen in cutaneous photoeruptions in HIV-positive patients. Military Medical Consortium for the Advancement of Retroviral Research (MMCARR). Int J Dermatol. 1997;36(10):745–53. doi: 10.1046/j.1365-4362.1997.00285.x
65. Lehloenya RJ, Todd G, Mogotlane L, Gantsho N, Hlela C, Dheda K. Lichenoid drug reaction to antituberculosis drugs treated through with topical steroids and phototherapy. J Antimicrob Chemother. 2012;67(10):2535–7. doi: 10.1093/jac/dks225
66. Mebazaa A, Denguezli M, Ghariani N, Sriha B, Belajouza C, Nouira R. Actinic lichen planus of unusual presentation. Acta Dermatovenerol Alp Pannonica Adriat. 2010;19(2):31–3.20664919
67. Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1(3):140–9. doi: 10.1016/j.ijwd.2015.04.001
68. Montgomery S, Worswick S. Photosensitizing drug reactions. Clin Dermatol. 2022;40(1):57–63. doi: 10.1016/j.clindermatol.2021.08.014
69. Bossi P, Colin D, Bricaire F, Caumes E. Hypersensitivity syndrome associated with efavirenz therapy. Clin Infect Dis. 2000;30(1):227–8. doi: 10.1086/313629
70. Treudler R, Husak R, Raisova M, Orfanos CE, Tebbe B. Efavirenz-induced photoallergic dermatitis in HIV. AIDS. 2001;15(8):1085–6. doi: 10.1097/00002030-200105250-00029
71. Newell A, Avila C, Rodgers ME. Photosensitivity reaction of efavirenz. Sex Transm Infect. 2000;76(3):221. doi: 10.1136/sti.76.3.221
72. Isaacs T, Ngwanya RM, Dlamini S, Peter J, Lehloenya R. Treatment can be continued for mild cutaneous reactions associated with efavirenz. J Allergy Clin Immunol Pract. 2019;7(5):1676–8. doi: 10.1016/j.jaip.2018.11.041
73. Isaacs T, Ngwanya MR, Dlamini S, Lehloenya RJ. Annular erythema and photosensitivity as manifestations of efavirenz-induced cutaneous reactions: a review of five consecutive cases. J Antimicrob Chemother. 2013;68(12):2871–4. doi: 10.1093/jac/dkt287
74. Zhou W, Moore DE. Photosensitizing activity of the anti-bacterial drugs sulfamethoxazole and trimethoprim. J Photochem Photobiol B. 1997;39(1):63–72. doi: 10.1016/S1011-1344(96)07468-4
75. De D, Dogra S, Kaur I. Dapsone induced acute photosensitivity dermatitis; a case report and review of literature. Lepr Rev. 2007;78(4):401–4. doi: 10.47276/llr.78.4.401
76. Kar BR. Dapsone-induced photosensitivity: a rare clinical presentation. Photodermatol Photoimmunol Photomed. 2008;24(5):270–1. doi: 10.1111/j.1600-0781.2008.00372.x
77. Karjigi S, Murthy SC, Kallappa H, Kusuma MR, Reddy YN. Early onset dapsone-induced photosensitive dermatitis: a rare Side effect of a common drug. Indian J Lepr. 2015;87(3):161–4.26999988
78. Alvarez-Fernandez JG, Castano-Suarez E, Cornejo-Navarro P, de la Fuente EG, Ortiz de Frutos FJ, Iglesias-Diez L. Photosensitivity induced by oral itraconazole. J Eur Acad Dermatol Venereol. 2000;14(6):501–3. doi: 10.1046/j.1468-3083.2000.00164.x
79. Mohamed KN. Severe photodermatitis during ketoconazole therapy. Clin Exp Dermatol. 1988;13(1):54. doi: 10.1111/j.1365-2230.1988.tb00654.x
80. Kojima T, Hasegawa T, Ishida H, Fujita M, Okamoto S. Griseofulvin-induced photodermatitis–report of six cases. J Dermatol. 1988;15(1):76–82. doi: 10.1111/j.1346-8138.1988.tb03654.x
81. Auffret N, Janssen F, Chevalier P, Guillemain R, Amrein C, Le Beller C. Voriconazole photosensitivity: 7 cases. Ann Dermatol Venereol. 2006;133(4):330–2. doi: 10.1016/S0151-9638(06)70910-3
82. Tolland JP, McKeown PP, Corbett JR. Voriconazole-induced pseudoporphyria. Photodermatol Photoimmunol Photomed. 2007;23(1):29–31. doi: 10.1111/j.1600-0781.2007.00263.x
83. Williams K, Mansh M, Chin-Hong P, Singer J, Arron ST. Voriconazole-associated cutaneous malignancy: a literature review on photocarcinogenesis in organ transplant recipients. Clin Infect Dis. 2014;58(7):997–1002. doi: 10.1093/cid/cit940
84. Katiyar SK, Bihari S, Prakash S. Pyrazinamide-induced phototoxicity: a case report and review of literature. Indian J Dermatol. 2010;55(1):113–5. doi: 10.4103/0019-5154.60368
85. Jin HJ, Kang DY, Nam YH, Ye YM, Koh YI, Hur GY, et al. Severe cutaneous adverse reactions to anti-tuberculosis drugs in Korean patients. Allergy Asthma Immunol Res. 2021;13(2):245–55. doi: 10.4168/aair.2021.13.2.245
86. Lee AY, Jung SY. Two patients with isoniazid-induced photosensitive lichenoid eruptions confirmed by photopatch test. Photodermatol Photoimmunol Photomed. 1998;14(2):77–8. doi: 10.1111/j.1600-0781.1998.tb00017.x
87. Wissel PS, Sordillo P, Anderson KE, Sassa S, Savillo RL, Kappas A. Porphyria cutanea tarda associated with the acquired immune deficiency syndrome. Am J Hematol. 1987;25(1):107–13. doi: 10.1002/ajh.2830250112
88. Lafeuillade A, Dhiver C, Martin I, Quilichini R, Gastaut JA. Porphyria cutanea tarda associated with HIV infection. AIDS. 1990;4(9):924. doi: 10.1097/00002030-199009000-00017
89. Aguilera P, Laguno M, To-Figueras J. Human immunodeficiency virus and risk of porphyria cutanea tarda: a possible association examined in a large hospital. Photodermatol Photoimmunol Photomed. 2016;32(2):93–7. doi: 10.1111/phpp.12222
90. Lee CH, Yuen MM, Chow WS, Tso AW, Yeung CK, Chan JC, et al. A man with a blistering eruption and tuberculosis. Br Med J. 2012;344:d8351. doi: 10.1136/bmj.d8351
91. Millar JW. Rifampicin-induced porphyria cutanea tarda. Br J Dis Chest. 1980;74(4):405–8. doi: 10.1016/0007-0971(80)90079-0
92. Chien JY, Chien ST, Huang SY, Yu CJ. Safety of rifabutin replacing rifampicin in the treatment of tuberculosis: a single-centre retrospective cohort study. J Antimicrob Chemother. 2014;69(3):790–6. doi: 10.1093/jac/dkt446
93. Maynard B, Peters MS. Histologic and immunofluorescence study of cutaneous porphyrias. J Cutan Pathol. 1992;19(1):40–7. doi: 10.1111/j.1600-0560.1992.tb01557.x
94. Holubiec P, Leonczyk M, Staszewski F, Lazarczyk A, Jaworek AK, Rojas-Pelc A. Pathophysiology and clinical management of pellagra - a review. Folia Med Cracov. 2021;61(3):125–37. doi: 10.24425/fmc.2021.138956
95. Isoniazid. Drugs and Lactation Database (LactMed®). Bethesda (MD): National institute of child health and human development 2006.
96. Kipsang JK, Choge JK, Marinda PA, Khayeka-Wandabwa C. Pellagra in isoniazid preventive and antiretroviral therapy. IDCases. 2019;17:e00550. doi: 10.1016/j.idcr.2019.e00550
97. Di Comite A, Esposito S, Villani A, Stronati M, Italian Pediatric TBSG. How to manage neonatal tuberculosis. J Perinatol. 2016;36(2):80–5. doi: 10.1038/jp.2015.99
98. O’Gorman SM, Murphy GM. Photosensitizing medications and photocarcinogenesis. Photodermatol Photoimmunol Photomed. 2014;30(1):8–14. doi: 10.1111/phpp.12085
99. Na R, Laaksonen MA, Grulich AE, Meagher NS, McCaughan GW, Keogh AM, et al. High azathioprine dose and lip cancer risk in liver, heart, and lung transplant recipients: a population-based cohort study. J Am Acad Dermatol. 2016;74(6):1144–52 e6. doi: 10.1016/j.jaad.2015.12.044
100. Molina BD, Leiro MG, Pulpon LA, Mirabet S, Yanez JF, Bonet LA, et al. Incidence and risk factors for nonmelanoma skin cancer after heart transplantation. Transplant Proc. 2010;42(8):3001–5. doi: 10.1016/j.transproceed.2010.08.003
101. Lindelof B, Granath F, Dal H, Brandberg Y, Adami J, Ullen H. Sun habits in kidney transplant recipients with skin cancer: a case-control study of possible causative factors. Acta Derm Venereol. 2003;83(3):189–93. doi: 10.1080/00015550310007193
102. Damps T, Czuwara J, Warszawik-Hendzel O, Misicka A, Rudnicka L. The role of drugs and selected dietary factors in cutaneous squamous cell carcinogenesis. Postepy Dermatol Alergol. 2021;38(2):198–204. doi: 10.5114/ada.2021.106196
103. Taylor CR, Stern RS, Leyden JJ, Gilchrest BA. Photoaging/photodamage and photoprotection. J Am Acad Dermatol. 1990;22(1):1–15. doi: 10.1016/0190-9622(90)70001-X
104. Bosch R, Philips N, Suarez-Perez JA, Juarranz A, Devmurari A, Chalensouk-Khaosaat J, et al. Mechanisms of photoaging and cutaneous photocarcinogenesis, and photoprotective strategies with phytochemicals. Antioxidants. 2015;4(2):248–68. doi: 10.3390/antiox4020248
105. Kerstein RL, Lister T, Cole R. Laser therapy and photosensitive medication: a review of the evidence. Lasers Med Sci. 2014;29(4):1449–52. doi: 10.1007/s10103-014-1553-0
106. Rutter KJ, Ashraf I, Cordingley L, Rhodes LE. Quality of life and psychological impact in the photodermatoses: a systematic review. Br J Dermatol. 2020;182(5):1092–102. doi: 10.1111/bjd.18326
107. Walburn J, Sarkany RPE. Quality of life in the photodermatoses: the hidden toll of photosensitivity. Br J Dermatol. 2020;182(5):1077. doi: 10.1111/bjd.18804
108. Shittu RO, Odeigah LO, Mahmoud AO, Sani MA, Bolarinwa OA. Dermatology quality of life impairments among newly diagnosed HIV/AIDS-infected patients in the university of Ilorin teaching hospital (Uith), Ilorin, Nigeria. J Int Assoc Provid AIDS Care. 2013. doi: 10.1177/2325957413488207. [Epub ahead of print].
109. Owolabi RS, Araoye MO, Osagbemi GK, Odeigah L, Ogundiran A, Hussain NA. Assessment of stigma and discrimination experienced by people living with HIV and AIDS receiving care/treatment in university of Ilorin teaching hospital (UITH), Ilorin, Nigeria. J Int Assoc Physicians AIDS Care (Chic). 2012;11(2):121–7. doi: 10.1177/1545109711399443
110. Abasiubong F, Ekott JU, Bassey EA, Etukumana EA, Edyang-Ekpa M. Quality of life in people living with HIV/AIDS in Niger Delta region, Nigeria. J Ment Health. 2010;19(2):211–8. doi: 10.3109/09638230903469210
111. Yang TT, Lan CE. Impacts of skin disorders associated with facial discoloration on quality of life: novel insights explaining discordance between life quality scores and willingness to pay. J Cosmet Dermatol. 2022;21(7):3053–8. doi: 10.1111/jocd.14546
112. Monteiro AF, Rato M, Martins C. Drug-induced photosensitivity: photoallergic and phototoxic reactions. Clin Dermatol. 2016;34(5):571–81. doi: 10.1016/j.clindermatol.2016.05.006
113. Rodwell GE, Berger TG. Pruritus and cutaneous inflammatory conditions in HIV disease. Clin Dermatol. 2000;18(4):479–84. doi: 10.1016/S0738-081X(99)00143-1
114. James WD, Berger TG, Elston DM. Dermatoses Resulting from Physical Factors. Andrews’ Diseases of the Skin, Clinical Dermatology. I11th ed. London: Saunders Elsevier; 2011. p. 34–5.
115. Jacobson JM, Greenspan JS, Spritzler J, Ketter N, Fahey JL, Jackson JB, et al. Thalidomide for the treatment of oral aphthous ulcers in patients with human immunodeficiency virus infection. National institute of allergy and infectious diseases AIDS clinical trials group. N Engl J Med. 1997;336(21):1487–93. doi: 10.1056/NEJM199705223362103
Keywords: photodermatitis, HIV, photosensitivity, actinic dermatitis, photodermatoses, pigmented skin
Citation: Isaacs T and Lehloenya R (2023) HIV-associated photodermatitis in African populations. Front. Allergy 4:1159387. doi: 10.3389/falgy.2023.1159387
Received: 5 February 2023; Accepted: 14 April 2023;
Published: 4 May 2023.
Edited by:
Andrew Glory Mtewa, Malawi University of Science and Technology, MalawiReviewed by:
Paulo Ricardo Criado, Faculdade de Medicina do ABC, Brazil© 2023 Isaacs and Lehloenya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thuraya Isaacs VGh1cmF5YS5pc2FhY3NAdWN0LmFjLnph
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.