- 1Department of Translational Medical Science, University of Naples “Federico II,” Naples, Italy
- 2ImmunoNutritionLab at the CEINGE Advanced Biotechnologies Research Center, University of Naples “Federico II,” Naples, Italy
- 3Pediatric Unit, Department of Clinical, Surgical, Diagnostic and Pediatric Sciences, University of Pavia, Pavia, Italy
- 4Pediatric Clinic, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy
- 5European Laboratory for the Investigation of Food-Induced Diseases, University of Naples Federico II, Naples, Italy
- 6Task Force for Microbiome Studies, University of Naples Federico II, Naples, Italy
Eosinophilic esophagitis (EoE) is a chronic, immune-mediated disease characterized by eosinophilic infiltration, leading to esophageal dysfunction, inflammation, and fibrotic remodeling. In the last few decades, there has been an increased prevalence of EoE at an alarming rate in the pediatric age. The pathogenesis of EoE is still largely undefined, and this limits the definition of effective strategies for the prevention and management of this condition. EoE is considered a multifactorial disease arising from a negative interaction between environmental factors and genetic background, causing an impaired esophageal epithelial barrier with subsequent abnormal allergen exposure activating type 2 (Th2) inflammation. Food antigens have been suggested as key players in Th2 inflammation in pediatric patients with EoE, but emerging evidence suggests a potential role of other dietary factors, including ultraprocessed foods, as possible triggers for the occurrence of EoE. In this paper, we discuss the potential role of these dietary factors in the development of the disease, and we propose a new approach for the management of pediatric patients with EoE.
Introduction
Food allergy (FA) in children is a major health concern, with an increased prevalence in the past two decades (1–4). Different clinical phenotypes of FA have been described, all deriving from the alteration of the mechanisms of immune tolerance to dietary antigens (5). Concomitantly, a similar increase in the prevalence of eosinophilic esophagitis (EoE) has been observed in the pediatric age (6–8). Children affected by FA present an increased risk of developing EoE later in life, and now EoE is considered as a component of the allergic march (9). EoE is a chronic disease characterized by an eosinophilic inflammation of the esophagus and symptoms of esophageal dysfunction (10, 11). Like FA, EoE is considered a condition deriving from a negative interaction between genetic background and environmental factors, leading to esophageal barrier dysfunction. The esophageal barrier alteration facilitates an abnormal exposure to dietary antigens and the consequent activation of type 2 (Th2) inflammatory response (6, 12). EoE has evolved from a rare condition to a commonly encountered disease in pediatric clinical practice and a significant cause of upper gastrointestinal morbidity (13). The global prevalence of EoE is 0.5–1 cases/1,000 persons (13). In children, the pooled incidence of EoE is 6.6 cases/100,000 person years, whereas the pooled prevalence is 34 cases/100,000 children (14). During the last few years, several studies reported a dramatic increase in EoE prevalence, especially in children in Western Countries (7, 14–16). Although this evidence might be related to improved medical awareness and knowledge, it could also be related to the global increase in allergic disorders. Despite some genetic factors have been associated with an increased risk of developing EoE, environmental factors seem to be the most relevant players facilitating the occurrence of the disease (13). In the last few years, one of the most impressive changes in the exposure to environmental factors concerns dietary habits. The consumption of ultraprocessed foods (UPFs) rapidly spread in the last few decades among children living in Westernized countries (17, 18). Increased exposure to UPFs is considered a facilitating factor for the occurrence of several chronic non-communicable diseases, including FA (19, 20).
In this paper, we discuss the potential role of UPFs and FA in the development of the disease, and we propose a new approach for the management of pediatric patients with EoE.
Genetic and environmental factors: an intriguing interplay in the pathogenesis of EoE
The pathogenesis of EoE is still largely undefined. It is commonly considered a multifactorial disease in which genetic and environmental factors may play a role. These factors, through intricate and bidirectional interactions, are responsible for esophageal barrier impairment, with loss of cell-to-cell adhesion mechanisms (desmosomes, tight, and adherence junctions), increased permeability, and consequent abnormal exposure to dietary antigens (21, 22). Alteration of the esophageal barrier leads to the epithelial release of inflammatory molecules such as thymic stromal lymphopoietin (TSLP) and interleukin (IL)-33, also called alarmins. These mediators drive the differentiation of Th2 effector cells, with the consequent production of several Th2 cytokines (IL-4, IL-5, IL-9, and IL-13) and massive recruitment of eosinophils (23). Simultaneously, luminal antigens encountering antigen-presenting cells (APC), activate specific antigen Th2 differentiation, induce additional release of inflammatory cytokines, eosinophils recruitment, and plasma cell activation with specific IgE production (23).
Lessons from genetic findings
The role of genetic factors in EoE pathogenesis was postulated with the observation that disease prevalence varies among sex and ethnicity. Epidemiological studies show that EoE is most common in white males, in children, and in adults (24–26). Genetic susceptibility is also supported by the evidence that having a first-degree family member affected by EoE increases the risk for disease occurrence (OR, 16.3; 95% CI, 9.4–28.3) (27). The relevance of the genetic background has also been supported by the results of candidate-gene and genome-wide association studies (GWAS), highlighting the role of different loci involved in the Th2 inflammatory response, and in the regulation of epithelial barrier structure and function in patients with EoE (12, 28, 29). The integrity of the esophageal epithelial barrier is ensured by desmosomes, tight and adherence junctions, as well as by several genes involved in epithelial cell differentiation, including filaggrin (FLG) and desmoglein 1 (DSG1). A genetic variation in these genes was detected in patients with EoE (24). The most powerful association has been found in the alteration of calpain 14 (CAPN14) production, an enzyme involved in esophageal barrier regulation via the IL-13 pathway (30, 31). Lastly, two other variations in serine peptidase inhibitors, kazal type 5 and 7 (SPINK5 and SPINK7), were also detected in barrier integrity maintenance (12, 28, 29).
EoE is characterized by a Th2 inflammatory response and a high prevalence of other atopic comorbidities (32, 33). Several genetic alterations were detected in patients with EoE, mainly related to the Th2 response, resulting in the upregulation (up to 53-fold) of eotaxin-3 (CCL26), TGF-β, and Periostin (POSTN), respectively, involved in eosinophil chemotaxis and adhesion, with the consequent production of TSLP (12, 34). TSLP is considered a crucial mediator involved in the EoE inflammatory cascade. Although TSLP is also expressed in other atopic disorders, TSLP production seems unrelated to other concomitant allergic diseases in patients with EoE (28, 29).
Despite this evidence, twin studies reporting a low disease concordance in both monozygotic (41%) and dizygotic (22%) twins suggest the greater importance of environmental factors as a major driving force for the occurrence of EoE in genetically predisposed children (35, 36).
The potential role of environmental factors in facilitating the occurrence of EoE
Growing evidence underlines that early life exposure to several detrimental factors, as already reported for FA pathogenesis, could promote esophageal barrier dysfunction and Th2 inflammatory response in EoE (37–39). In contrast, several beneficial environmental factors, such as breastfeeding and the Mediterranean diet, showed a protective role against these conditions (40–42).
Several environmental agents could induce esophageal barrier dysfunction. This could be the case of the detergents that altering the epithelial barrier, induced mucosal inflammation and the typical histological features of EoE in a preclinical model (43). Immortalized esophageal epithelial cells (EPC2) exposed to sodium dodecyl sulfate (SDS), a widely used detergent contained in domestic cleaning, cosmetic, pharmaceutical, and food products, showed a significant decrease in transepithelial electrical resistance and a significant increase of FITC-dextran flux. In addition, a proinflammatory IL-33 mRNA expression and a reduction of DSG1 expression were detected, with consequent alteration of epithelial barrier integrity. It was also observed that mice exposed to SDS showed a marked activation of proinflammatory cytokine pathways and esophageal eosinophilia compared with not-exposed controls (43).
Like FA, infections have also been proposed as potential risk factors for the occurrence of EoE. Some case series showed a direct association between herpes simplex virus esophageal infection and the development of EoE (44).
Data regarding different living areas are discordant. Some studies showed a positive association between EoE occurrence and suburban areas (36, 45, 46). It is well known that rural vs. urban or suburban areas are characterized by a considerable difference in pollution exposure, aeroallergen content, and climate temperature, which can modify the allergen air concentration. Living in a cold climate zone seems related to a higher risk of EoE occurrence, but more studies are needed to support this hypothesis (47). Aeroallergens have long been proposed as a trigger or worsening factor for EoE (48, 49), but their role in the pathogenesis of EoE is still controversial. Indeed, if it is well known that aeroallergens induce a Th2-orientated immune response in other allergic diseases (i.e., allergic rhinitis or asthma), their role in EoE occurrence or exacerbation needs to be better investigated (50–53). Recent studies on the role of seasonality were unable to demonstrate significant differences in EoE occurrence and disease course (54, 55).
New studies are now exploring the potential role of the Western diet as a trigger for non-communicable disease occurrence, including FA (19, 20, 56). Western diet is low in fibers and polyunsaturated fats and rich in UPFs (57). During the last few decades, the consumption of UPFs significantly increased in children living in Western countries. It was estimated that 65% of the total daily energy intake derives from UPF consumption in children in the US and EU (18, 19).
Smith and colleagues highlighted how dietary patterns could be related to FA occurrence in children (20, 56). They linked different types of foods consumed by US children and fast-food consumption by Australian pediatric subjects, with the increase in FA prevalence (58, 59).
Furthermore, countries with a huge increase in the EoE, FA, and anaphylaxis rates were also the countries where Western diet rapidly spread among the child population in the same period (7, 14–16, 60–65).
One of the main UPF-derived compounds are the advanced glycation end-products (AGEs), deriving from the non-enzymatic reaction between proteins and sugars via the Maillard reaction (66).
Dietary AGEs activate several inflammatory pathways, including the Th2 inflammatory response, through interaction with specific receptor (RAGE) expressed by epithelial cells, peripheral blood mononuclear cells, human esophageal mucosal cells, and by human eosinophils (17, 67–69). The activation of RAGE induces several intracellular pathways that activate the alarmins signal with increased production of TSLP, IL-33, and IL-25 (70). These inflammatory cytokines exert a pivotal role in EoE and FA pathogenesis, and they induce differentiation of innate lymphoid cells 2 in Th2 effector cells with a consequent production of IL-4, IL-5, IL-9, and IL-13 (12). AGEs also activate mast cells, via RAGE activation, with a consequent release of proinflammatory cytokines, and may induce the production of specific IgE against dietary antigens (71, 72). In addition, dietary AGEs increase oxidative stress levels, and may also act at the gastrointestinal (GI) level by impairing gut microbiome structure and function and tight junction protein expression (56, 73). These proteins are crucial in maintaining the esophageal and gut barrier integrity; thus, an increased epithelial permeability allows an abnormal antigen passage (56, 74). In summary, the alteration of the gut and esophageal barrier integrity, the abnormal antigen translocation, and the alarmin activation with a consequent Th2-orientated response, may allow an altered antigen presentation, resulting in a potentially harmful condition for the maintenance of immune tolerance to dietary antigens (75, 76).
Lastly, it has been demonstrated that proton pump inhibitors (PPIs) could modulate both esophageal barrier integrity and alarmin signal (77). This could be an additional mechanism of action of PPIs in EoE treatment.
Altogether, these data, from the epidemiological and immunological points of view, add plausibility to the potential role of UPFs in facilitating the occurrence of EoE and FA.
Food allergy-EoE links
As EoE is characterized by Th2 inflammatory response, most pediatric patients have other coexisting atopic comorbidities, such as FA, allergic oculorhinitis, and asthma (32, 33). This clinical picture demands multidisciplinary management involving pediatric allergy, gastroenterology, and nutrition expertise (78). Observational studies have demonstrated that the risk of developing EoE increases in allergic children, especially in those with ≥1 allergic disease, and to date, EoE has been proposed as a component of the allergic march (9). Moreover, allergic sensitization has been reported in most pediatric patients with EoE (79). According to recent FA classification, EoE can also be considered a mixed (IgE- and non-IgE-mediated) FA, where food antigens have been proposed as triggers for esophageal Th2 inflammation in genetically susceptible patients (80). In 2017, a systematic review with the meta-analysis by Gonzalez-Cervera et al. reported that the frequency of FA in patients with EoE, compared with healthy controls, ranged from 0% to 44%, with a relevant clinical heterogeneity in FA definition (81). Thereafter, Capucilli and Hill, assessing the prevalence of allergic diseases in patients with EoE, reported a 24%–68% prevalence from 2015 to 2019 (33). A more recent literature revision confirmed that the prevalence of IgE-mediated FA varies between 25% and 70% (31).
The primum movens in allergic diseases is the epithelial barrier alteration, as in the case of FA (21). After this, the loss of immune tolerance against allergens is crucial for FA development (33). In the context of IgE-mediated disease, specific IgG4 are generally increased and considered a marker of immune tolerance. Evidence shows that patients with EoE may also present high levels of IgG4, but their role in EoE pathogenesis and diagnosis is unclear. In fact, EoE shares some clinical features not only with IgE-mediated FA but also with IgG4-related disease, characterized by progressive fibrosis (82). In 2014, Clayton et al. showed increased IgG4-positive plasma cells (IgG4-PC) in the lamina propria and granular extracellular IgG4 deposits in adults with EoE. In addition, the authors reported high IgG4 serum levels against milk, wheat, egg, and nuts in these patients, demonstrating that the esophageal deposition of IgG4 was associated with food-specific IgG4 antibodies (83). Recent studies confirmed the presence of total specific IgG4 high serum level in pediatric patients with EoE compared with healthy controls (84, 85). Unfortunately, despite this evidence, the pathogenetic role of IgG4 in EoE is still unclear and requires further research.
FA and EoE have also been linked by the response to the elimination diet (32, 86–88). However, despite the fact that a complete clinical response to the elimination diet is observed in all children with FA, as this is mandatory for making a definite diagnosis of FA (89), the response to the elimination diet has not been reported in all children with EoE (90). The first evidence that foods were the triggers of esophageal inflammation were reported by Kelly et al. (91). The authors highlighted the link between FA and EoE by showing that children treated with an exclusive elemental (amino acid–based) formula completely recovered from GI symptoms and showed a drastic decrease in esophageal eosinophilia (91). The elemental diet is effective in up to 90% of pediatric patients with EoE (90).
The most frequently implied foods in pediatric patients with EoE are cow's milk, wheat, soy and/or legumes, egg, tree nuts, and shellfish. The elimination of these food allergens showed different efficacy rates, depending on the number of foods removed and the rationale used to eliminate them from the diet (empirical vs. targeted) (92, 93). The empirical elimination of all these six food antigens produced effective results in approximately 72% of patients, and the targeted one could induce a similar remission rate in patients with EoE when a combination allergy screening tests is performed [skin-prick tests (SPT), atopy patch tests (APT), and/or specific IgE] (90, 94). The four-food elimination diet (cow's milk, wheat, soy, and egg) induces histological remission in above 53% of patients, with higher efficacy in children than in adults (60% vs. 46%) (90). Kagalwalla et al. performed a prospective observational study in children with EoE treated with a four (cow's milk, wheat, egg, and soy)-food elimination diet finding that after food reintroduction, the most common food triggers that induced histologic inflammation were cow's milk (85%), egg (35%), wheat (33%), and soy (19%) (93). Therefore, since milk and wheat are the most allergenic foods, Molina-Infante et al. proposed starting with an empirical 2-food elimination diet, finding that this approach was effective in 43% of treated patients (95). The authors thus proposed a step-up approach that avoids unnecessary dietary restrictions and spare GI endoscopies to assess histologic remission (96). A recent prospective study in children with EoE found that the single milk elimination diet was effective in more than 50% of patients, suggesting that this dietary intervention may be proposed as first-line treatment because of the ease of implementation and adherence (96). More recently, de Rooij et al. proposed a mixed dietary treatment in adults with active EoE, combining the empirical four-food elimination diet with an amino acid–based formula. The authors found that, although the combined dietary treatment significantly improved the quality of life in adult patients with EoE, it did not lead to a more considerable decrease in the peak of eosinophil count at 6-week follow-up (97). As already reported in patients with atopic dermatitis, children with EoE may develop IgE-mediated hypersensitivity to food antigens (98, 99). On the other hand, children who outgrow IgE-mediated FA and reintroduce the culprit food(s) in their diet, can later develop EoE for the same food (100).
Unfortunately, the response to the food-elimination diet is not complete or sustained over time in many children with EoE (90). Several factors impacting clinical or histologic response should be considered in patients with EoE who are unresponsive to the elimination diet (Table 1) (101).
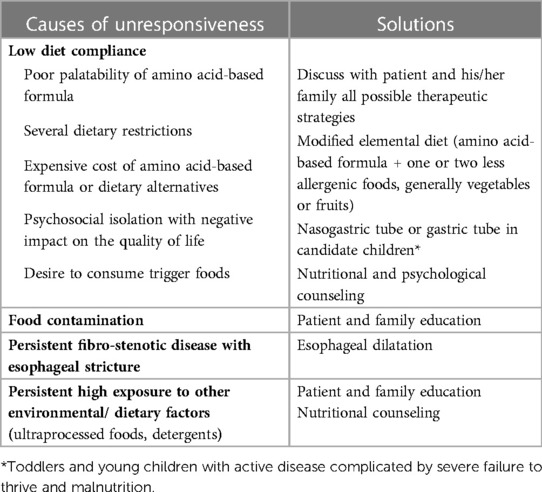
Table 1. Established and possible causes of unresponsiveness to food elimination diets and suggested solutions.
Discussion
In the last few decades, the increased incidence and prevalence of pediatric EoE paralleled with the increased incidence, prevalence, and severity of the clinical manifestations of FA, in the pediatric age. The origin of these parallel epidemiologic patterns is still largely undefined, but it could be the target for innovative preventive and therapeutic strategies against both conditions.
The role of dietary factors in EoE pathogenesis has been long considered only from the FA point of view, in which food antigens are considered triggers for the esophageal barrier dysfunction, for the occurrence of Th2 inflammatory response and the consequent clinical and histological features of EoE (10). It is now time to speculate that the abnormal food antigen exposure could be just the consequence of a first hit, which could be mainly responsible for the occurrence of EoE in genetically predisposed individuals (21). Thus, defining which environmental factor could elicit the first hit could be paramount for designing disrupting strategies against EoE.
The activation of alarmins is one of the initial signals in EoE pathogenesis, driving a Th2 inflammatory response and esophageal barrier alteration (79, 102). Recent data suggest that selected environmental factors could induce alarmins signal and esophageal barrier dysfunction (20, 103). Among these factors, the UPF detrimental compounds, AGEs, seem to be relevant candidates able to directly “switch on” EoE inflammation (20). AGEs directly activate the production of alarmins. Then, esophageal barrier impairment could be responsible for increased epithelial permeability and abnormal exposure to food allergens, with subsequent sensitization of food antigens (24). This could explain why sensitization of food antigens is commonly observed in pediatric patients with EoE. In the light of this, sensitization of FA and food antigens cannot be the trigger but just an epiphenomenon in several pediatric patients with EoE. This could justify why the response to the food-elimination diet may be ineffective in a number of children with EoE.
Altogether, it is possible to hypothesize that UPFs, and in particular dietary AGEs, could act as the primum movens for the esophageal barrier dysfunction, mimicking the innate alarmin pathways and facilitating the occurrence of EoE in genetically predisposed children. This hypothesis could drive innovative preventive measures to limit UPFs/AGEs exposure in the pediatric age and provide a new strategy for EoE management. This could be a reasonable, affordable, and easily applicable strategy against EoE.
Thus, we propose a new approach for pediatric EoE management, in which nutritional counseling aimed to reduce exposure to UPFs/AGEs could facilitate better therapeutic outcomes in pediatric patients with EoE (Figure 1). Future preclinical and clinical studies are advocated to explore the potential of this approach.
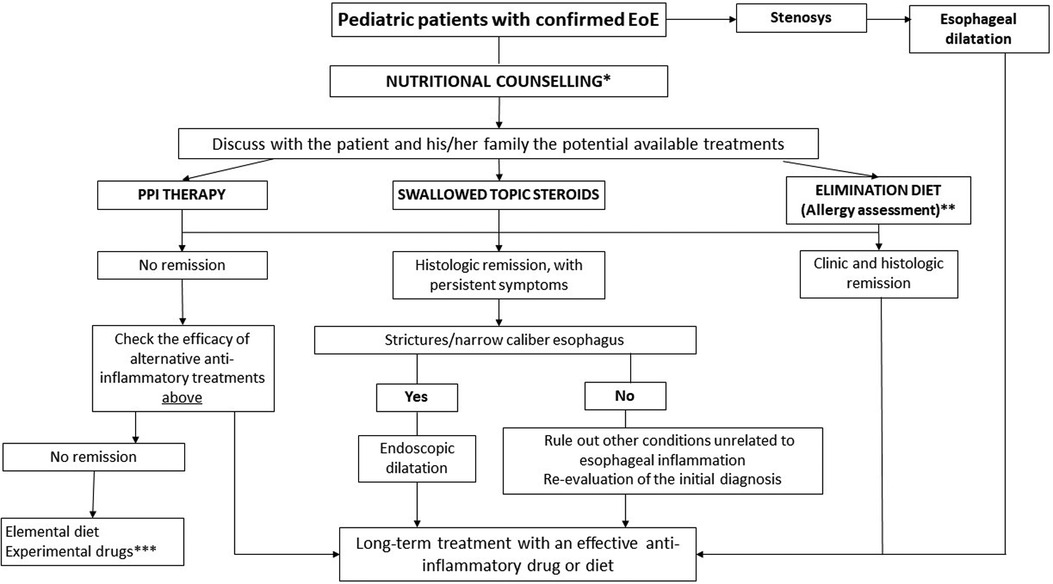
Figure 1. Toward an innovative strategy for the management of pediatric EoE. EoE, eosinophilic esophagitis; PPI, proton pump inhibitors. *Nutritional counseling aims to assess the patient's diet, eating habits, potential nutritional deficiencies, and reduces exposure to ultraprocessed foods. **Skin prick test, specific IgE, and atopy patch test + nutritional counseling. ***Consider biological treatment (dupilumab was approved by the FDA in patients >12 years with EoE).
Author contributions
LC and MV analyzed the literature, wrote the manuscript, and read the manuscript. RC designed and structured the review, wrote the manuscript, and read the manuscript. AL and GM analyzed the literature and read the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Renz H, Allen KJ, Sicherer SH, Sampson HA, Lack G, Beyer K, et al. Food allergy. Nat Rev Dis Primers. (2018) 4:17098. doi: 10.1038/nrdp.2017.98
2. Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ, Matheson MC, et al. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol. (2011) 127(3):668–76.e1-2. doi: 10.1016/j.jaci.2011.01.039
3. Nocerino R, Leone L, Cosenza L, Berni Canani R. Increasing rate of hospitalizations for food-induced anaphylaxis in Italian children: an analysis of the Italian Ministry of Health database. J Allergy Clin Immunol. (2015) 135(3):833–5.e3. doi: 10.1016/j.jaci.2014.12.1912
4. De Filippo M, Votto M, Albini M, Castagnoli R, De Amici M, Marseglia A, et al. Pediatric anaphylaxis: a 20-year retrospective analysis. J Clin Med. (2022) 11(18):5285. doi: 10.3390/jcm11185285
5. Azouz NP, Rothenberg ME. Mechanisms of gastrointestinal allergic disorders. J Clin Invest. (2019) 129(4):1419–30. doi: 10.1172/JCI124604
6. Votto M, Marseglia GL, De Filippo M, Brambilla I, Caimmi SME, Licari A. Early life risk factors in pediatric EoE: could we prevent this modern disease? Front Pediatr. (2020) 8:263. doi: 10.3389/fped.2020.00263
7. Votto M, Raffaele A, De Filippo M, Caimmi S, Brunero M, Riccipetitoni G, et al. Eosinophilic gastrointestinal disorders in children and adolescents: a single-center experience. Dig Liver Dis. (2022) 54(2):214–20. doi: 10.1016/j.dld.2021.06.027
8. Arias Á, Pérez-Martínez I, Tenías JM, Lucendo AJ. Systematic review with meta-analysis: the incidence and prevalence of eosinophilic esophagitis in children and adults in population-based studies. Aliment Pharmacol Ther. (2016) 43(1):3–15. doi: 10.1111/apt.13441
9. Hill DA, Grundmeier RW, Ramos M, Spergel JM. Eosinophilic esophagitis is a late manifestation of the allergic march. J Allergy Clin Immunol Pract. (2018) 6(5):1528–33. doi: 10.1016/j.jaip.2018.05.010
10. Muir A, Falk GW. Eosinophilic esophagitis: a review. J Am Med Assoc. (2021) 326(13):1310–8. doi: 10.1001/jama.2021.14920
11. Licari A, Votto M, D'Auria E, Castagnoli R, Caimmi SME, Marseglia GL. Eosinophilic gastrointestinal diseases in children: a practical review. Curr Pediatr Rev. (2020) 16(2):106–14. doi: 10.2174/1573396315666191022154432
12. O’Shea KM, Aceves SS, Dellon ES, Gupta SK, Spergel JM, Furuta GT, et al. Pathophysiology of eosinophilic esophagitis. Gastroenterology. (2018) 154(2):333–45. doi: 10.1053/j.gastro.2017.06.065
13. Dellon ES, Hirano I. Epidemiology and natural history of eosinophilic esophagitis. Gastroenterology. (2018) 154(2):319–332.e3. doi: 10.1053/j.gastro.2017.06.067
14. Navarro P, Arias Á, Arias-González L, Laserna-Mendieta EJ, Ruiz-Ponce M, Lucendo AJ. Systematic review with meta-analysis: the growing incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment Pharmacol Ther. (2019) 49(9):1116–25. doi: 10.1111/apt.15231
15. Allin KH, Poulsen G, Melgaard D, Frandsen LT, Jess T, Krarup AL. Eosinophilic oesophagitis in Denmark: population-based incidence and prevalence in a nationwide study from 2008 to 2018. United European Gastroenterol J. (2022) 10(7):640–50. doi: 10.1002/ueg2.12273
16. Arias Á, Lucendo AJ. Incidence and prevalence of eosinophilic oesophagitis increase continuously in adults and children in central Spain: a 12-year population-based study. Dig Liver Dis. (2019) 51(1):55–62. doi: 10.1016/j.dld.2018.07.016
17. Khandpur N, Neri DA, Monteiro C, Mazur A, Frelut ML, Boyland E, et al. Ultra-processed food consumption among the paediatric population: an overview and call to action from the European Childhood Obesity Group. Ann Nutr Metab. (2020) 76(2):109–13. doi: 10.1159/000507840
18. Wang L, Martínez Steele E, Du M, Pomeranz JL, O'Connor LE, Herrick KA, et al. Trends in consumption of ultraprocessed foods among US youths aged 2–19 years, 1999–2018. J Am Med Assoc. (2021) 326(6):519–30. doi: 10.1001/jama.2021.10238
19. Zhang Q, Wang Y, Fu L. Dietary advanced glycation end-products: perspectives linking food processing with health implications. Compr Rev Food Sci Food Saf. (2020) 19(5):2559–87. doi: 10.1111/1541-4337.12593
20. Smith PK, Masilamani M, Li XM, Sampson HA. The false alarm hypothesis: food allergy is associated with high dietary advanced glycation end-products and proglycating dietary sugars that mimic alarmins. J Allergy Clin Immunol. (2017) 139(2):429–37. doi: 10.1016/j.jaci.2016.05.040
21. Yazici D, Ogulur I, Kucukkase O, Li M, Rinaldi OA, Pat Y. Epithelial barrier hypothesis and the development of allergic and autoimmune diseases. Allergo J. (2022) 31:18–31. doi: 10.1007/s15007-022-5033-8
22. Cianferoni A, Jensen E, Davis CM. The role of the environment in eosinophilic esophagitis. J Allergy Clin Immunol Pract. (2021) 9(9):3268–74. doi: 10.1016/j.jaip.2021.07.032
23. Ruffner MA, Kennedy K, Cianferoni A. Pathophysiology of eosinophilic esophagitis: recent advances and their clinical implications. Expert Rev Clin Immunol. (2019) 15(1):83–95. doi: 10.1080/1744666X.2019.1544893
24. Chang JW, Jensen ET, Dellon ES. Nature with nurture: the role of intrinsic genetic and extrinsic environmental factors on eosinophilic esophagitis. Curr Allergy Asthma Rep. (2022) 22(12):163–70. doi: 10.1007/s11882-022-01042-1
25. Liacouras CA, Spergel JM, Ruchelli E, Verma R, Mascarenhas M, Semeao E. Eosinophilic esophagitis: a 10-year experience in 381 children. Clin Gastroenterol Hepatol. (2005) 3(12):1198–206. doi: 10.1016/s1542-3565(05)00885-2
26. Mansoor E, Cooper GS. The 2010–2015 prevalence of eosinophilic esophagitis in the USA: a population-based study. Dig Dis Sci. (2016) 61(10):2928–34. doi: 10.1007/s10620-016-4204-4
27. Allen-Brady K, Firszt R, Fang JC, Wong J, Smith KR, Peterson KA. Population-based familial aggregation of eosinophilic esophagitis suggests a genetic contribution. J Allergy Clin Immunol. (2017) 140(4):1138–43. doi: 10.1016/j.jaci.2016.12.979
28. Kottyan LE, Rothenberg ME. Genetics of eosinophilic esophagitis. Mucosal Immunol. (2017) 10(3):580–8. doi: 10.1038/mi.2017.4
29. Chang X, March M, Mentch F, Nguyen K, Glessner J, Qu H, et al. A genome-wide association meta-analysis identifies new eosinophilic esophagitis loci. J Allergy Clin Immunol. (2022) 149(3):988–98. doi: 10.1016/j.jaci.2021.08.018
30. Sleiman PM, Wang ML, Cianferoni A, Aceves S, Gonsalves N, Nadeau K, et al. GWAS identifies four novel eosinophilic esophagitis loci. Nat Commun. (2014) 5:5593. doi: 10.1038/ncomms6593
31. Davis BP, Stucke EM, Khorki KM, Litosh VA, Rymer JK, Rochman M, et al. Eosinophilic esophagitisir-linked calpain 14 is an IL-13-induced protease that mediates esophageal epithelial barrier impairment. JCI Insight. (2016) 1:1–11. doi: 10.1172/jci.insight.86355
32. Rossi CM, Lenti MV, Merli S, Licari A, Votto M, Marseglia GL, et al. Primary eosinophilic gastrointestinal disorders and allergy: clinical and therapeutic implications. Clin Transl Allergy. (2022) 12(5):e12146. doi: 10.1002/clt2.12146
33. Capucilli P, Hill DA. Allergic comorbidity in eosinophilic esophagitis: mechanistic relevance and clinical implications. Clin Rev Allergy Immunol. (2019) 57(1):111–27. doi: 10.1007/s12016-019-08733-0
34. Blanchard C, Mingler MK, McBride M, Putnam PE, Collins MH, Chang G, et al. Periostin facilitates eosinophil tissue infiltration in allergic lung and esophageal responses. Mucosal Immunol. (2008) 1(4):289–96. doi: 10.1038/mi.2008.15
35. Alexander ES, Martin LJ, Collins MH, Kottyan LC, Sucharew H, He H, et al. Twin and family studies reveal strong environmental and weaker genetic cues explaining heritability of eosinophilic esophagitis. J Allergy Clin Immunol. (2014) 134(5):1084–1092.e1. doi: 10.1016/j.jaci.2014.07.021
36. Jensen ET, Dellon ES. Environmental factors and eosinophilic esophagitis. J Allergy Clin Immunol. (2018) 142(1):32–40. doi: 10.1016/j.jaci.2018.04.015
37. Berni Canani R, Di Costanzo M. Gut microbiota as potential therapeutic target for the treatment of cow’s milk allergy. Nutrients. (2013) 5(3):651–62. doi: 10.3390/nu5030651
38. Jensen ET, Kappelman MD, Kim HP, Ringel-Kulka T, Dellon ES. Early life exposures as risk factors for pediatric eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. (2013) 57:67–71. doi: 10.1097/MPG.0b013e318290d15a
39. Radano MC, Yuan Q, Katz A, Fleming JT, Kubala S, Shreffler W, et al. Cesarean section and antibiotic use found to be associated with eosinophilic esophagitis. J Allergy Clin Immunol Pract. (2014) 2014(2):475–7.e1. doi: 10.1016/j.jaip.2014.02.018
40. Slae M, Persad R, Leung A-T, Gabr R, Brocks D, Huynh H. Role of environmental factors in the development of pediatric eosinophilic esophagitis. Dig Dis Sci. (2015) 60(11):3364–72. doi: 10.1007/s10620-015-3740-7
41. Jensen ET, Kuhl JT, Martin LJ, Rothenberg ME, Dellon ES. Prenatal, intrapartum, and postnatal factors are associated with pediatric eosinophilic esophagitis. J Allergy Clin Immunol. (2017) 141(1):214–22. doi: 10.1016/j.jaci.2017.05.018
42. Jensen ET, Shaheen O, Koutlas NT, Chang AO, Martin LJ, Rothenberg ME, et al. Early life factors are associated with risk for eosinophilic esophagitis diagnosed in adulthood. Gastroenterology. (2021) 152:S861. doi: 10.1093/dote/doaa074
43. Doyle AD, Masuda MY, Pyon GC, Luo H, Putikova A, LeSuer EW. Detergent exposure induces epithelial barrier dysfunction and eosinophilic inflammation in the esophagus. Allergy. (2022) 78(1):192–201. doi: 10.1111/all.15457
44. Squires KA, Cameron DJ, Oliver M, da Fonseca Junqueira JC. Herpes simplex and eosinophilic oesophagitis: the chicken or the egg? J Pediatr Gastroenterol Nutr. (2009) 49:246–50. doi: 10.1097/MPG.0b013e31817b5b73
45. Spergel JM, Book WM, Mays E, Song L, Shah SS, Talley NJ, et al. Variation in prevalence, diagnostic criteria, and initial management options for eosinophilic gastrointestinal diseases in the United States. J Pediatr Gastroenterol Nutr. (2021) 52:300–6. doi: 10.1097/MPG.0b013e3181eb5a9f
46. Lee YJ, Redd M, Bayman L, Frederickson N, Valestin J, Schey R. Comparison of clinical features in patients with eosinophilic esophagitis living in an urban and rural environment. Dis Esophagus. (2015) 28:19–24. doi: 10.1111/dote.12164
47. Hurrell JM, Genta RM, Dellon ES. Prevalence of esophageal eosinophilia varies by climate zone in the United States. Am J Gastroenterol. (2012) 107:698–706. doi: 10.1038/ajg.2012.6
48. Fogg I, Ruchelli E, Spergel JM. Pollen and eosinophilic esophagitis. J Allergy Clin Immunol. (2003) 112:796–7. doi: 10.1016/s0091-6749(03)01715-9
49. Moawad FJ, Veerappan GR, Lake JM, Maydonovitch CL, Haymore BR, Kosisky SE. Correlation between eosinophilic oesophagitis and aeroallergens. Aliment Pharmacol Ther. (2010) 31:509–15. doi: 10.1111/j.1365-2036.2009.04199.x
50. Almansa C, Krishna M, Buchner AM, Ghabril MS, Talley N, DeVault KR, et al. Seasonal distribution in newly diagnosed cases of eosinophilic esophagitis in adults. Am J Gastroenterol. (2009) 104:828–33. doi: 10.1038/ajg.2008.169
51. Elitsur Y, Aswani R, Lund V, Dementieva Y. Seasonal distribution and eosinophilic esophagitis: the experience in children living in rural communities. J Clin Gastroenterol. (2013) 47:287–8. doi: 10.1097/MCG.0b013e31826df861
52. Iwanczak B, Janczyk W, Ryzko J, Banaszkiewicz A, Radzikowski A, Jarocka-Cyrta E, et al. Eosinophilic esophagitis in children: frequency, clinical manifestations, endoscopic findings, and seasonal distribution. Adv Med Sci. (2011) 56:151–7. doi: 10.2478/v10039-011-0038-7
53. Larsson H, Bergquist H, Bove M. The incidence of esophageal bolus impaction: is there a seasonal variation? Otolaryngol Head Neck Surg. (2011) 144:186–90. doi: 10.1177/0194599810392655
54. Lucendo AJ, Arias A, Redondo-Gonzalez O, González-Cervera J. Seasonal distribution of initial diagnosis and clinical recrudescence of eosinophilic esophagitis: a systematic review and meta-analysis. Allergy. (2015) 70:1640–50. doi: 10.1111/all.12767
55. Guajardo JR, Zegarra-Bustamante MA, Brooks EG. Does aeroallergen sensitization cause or contribute to eosinophilic esophagitis? Clin Rev Allergy Immunol. (2018) 55:65–9. doi: 10.1007/s12016-018-8671-6
56. Smith PK, Venter C, O'Mahony L, Canani RB, Lesslar OJL. Do advanced glycation end products contribute to food allergy? Front Allergy. (2023) 4:1148181. doi: 10.3389/falgy.2023.1148181
57. Elizabeth L, Machado P, Zinöcker M, Baker P, Lawrence M. Ultra-processed foods and health outcomes: a narrative review. Nutrients. (2020) 12(7):1955. doi: 10.3390/nu12071955
58. Saavedra JM, Deming D, Dattilo A, Reidy K. Lessons from the feeding infants and toddlers study in North America: what children eat, and implications for obesity prevention. Ann Nutr Metab. (2013) 62:27–36. doi: 10.1159/000351538
59. Venter C, Maslin K, Holloway JW, Silveira LJ, Fleischer DM, Dean T, et al. Different measures of diet diversity during infancy and the association with childhood food allergy in a UK birth cohort study. J Allergy Clin Immunol. (2020) 8:2017–26. doi: 10.1016/j.jaip.2020.01.029
60. Newens KJ, Walton J. A review of sugar consumption from nationally representative dietary surveys across the world. J Hum Nutr Diet. (2015) 29:225–40. doi: 10.1111/jhn.12338
61. Baseggio Conrado A, Ierodiakonou D, Gowland MH, Boyle RJ, Turner PJ. Food anaphylaxis in the United Kingdom: analysis of national data, 1998–2018. Br Med J. (2021) 372:n251. doi: 10.1136/bmj.n251
62. Mullins RJ, Wainstein BK, Barnes EH, Liew WK, Campbell DE. Increases in anaphylaxis fatalities in Australia from 1997 to 2013. Clin Exp Allergy. (2016) 46:1099–110. doi: 10.1111/cea.12748
63. Keet CA, Savage JH, Seopaul S, Peng RD, Wood RA, Matsui EC. Temporal trends and racial/ethnic disparity in self-reported pediatric food allergy in the United States. Ann Allergy Asthma Immunol. (2014) 112:222–9.e3. doi: 10.1016/j.anai.2013.12.007
64. Loh W, Tang M. The epidemiology of food allergy in the global context. Int J Environ Res Public Health. (2018) 15:2043. doi: 10.3390/ijerph15092043
65. Nwaru BI, Hickstein L, Panesar SS, Muraro A, Werfel T, Cardona V, et al. The epidemiology of food allergy in Europe: a systematic review and meta-analysis. Allergy. (2013) 69:62–75. doi: 10.1111/all.12305
66. Nursten HE. The Maillard reaction: chemistry, biochemistry, and implications. Royal society of chemistry. J Am Chem Soc. (2005) 41:14527–8.
67. Ramasamy R, Yan SF, Herold K, Clynes R, Schmidt AM. Receptor for advanced glycation end products: fundamental roles in the inflammatory response: winding the way to the pathogenesis of endothelial dysfunction and atherosclerosis. Ann N Y Acad Sci. (2008) 1126:7–13. doi: 10.1196/annals.1433.056
68. Zen K, Chen CX, Chen YT, Wilton R, Liu Y. Receptor for advanced glycation end products mediates neutrophil migration across intestinal epithelium. J Immunol. (2007) 178(4):2483–90. doi: 10.4049/jimmunol.178.4.2483
69. Curran CS, Bertics PJ. Human eosinophils express RAGE, produce RAGE ligands, exhibit PKC-delta phosphorylation and enhanced viability in response to the RAGE ligand, S100B. Int Immunol. (2011) 23(12):713–28. doi: 10.1093/intimm/dxr083
70. Roan F, Obata-Ninomiya K, Ziegler SF. Epithelial cell-derived cytokines: more than just signaling the alarm. J Clin Invest. (2019) 129(4):1441–51. doi: 10.1172/JCI124606
71. Sick E, Brehin S, André P, Coupin G, Landry Y, Takeda K, et al. Advanced glycation end products (ages) activate mast cells. Br J Pharmacol. (2010) 161:442–55. doi: 10.1111/j.1476-5381.2010.00905.x
72. Mueller GA, Maleki SJ, Johnson K, Hurlburt BK, Cheng H, Ruan S, et al. Identification of Maillard reaction products on peanut allergens that influence binding to the receptor for advanced glycation end products. Allergy. (2013) 68:1546–54. doi: 10.1111/all.12261
73. Cohen-Or I, Katz C, Ron EZ. AGEs secreted by bacteria are involved in the inflammatory response. PLoS One. (2011) 6:e17974. doi: 10.1371/journal.pone.0017974
74. Qu W, Yuan X, Zhao J, Zhang Y, Hu J, Wang J, et al. Dietary advanced glycation end products modify gut microbial composition and partially increase colon permeability in rats. Mol Nutr Food Res. (2017) 61:10. doi: 10.1002/mnfr.201700118
75. Sappington PL, Yang R, Yang H, Tracey KJ, Delude RL, Fink MP. HMGB1 B box increases the permeability of caco-2 enterocytic monolayers and impairs intestinal barrier function in mice. Gastroenterology. (2002) 123:790–802. doi: 10.1053/gast.2002.35391
76. Shi J, Zhao X-H. Influence of the Maillard-type caseinate glycation with lactose on the intestinal barrier activity of the caseinate digest in IEC-6 cells. Food Funct. (2019) 10:2010–21. doi: 10.1039/c8fo02607f
77. Franciosi JP, Mougey EB, Dellon ES, Gutierrez-Junquera C, Fernandez-Fernandez S, Venkatesh RD, et al. Proton pump inhibitor therapy for eosinophilic esophagitis: history, mechanisms, efficacy, and future directions. J Asthma Allergy. (2022) 15:281–302. doi: 10.2147/JAA.S274524
78. Votto M, De Filippo M, Olivero F, Raffaele A, Cereda E, De Amici M, et al. Malnutrition in eosinophilic gastrointestinal disorders. Nutrients. (2020) 13(1):128. doi: 10.3390/nu13010128
79. Spergel J, Aceves SS. Allergic components of eosinophilic esophagitis. J Allergy Clin Immunol. (2018) 142(1):1–8. doi: 10.1016/j.jaci.2018.05.001
80. Berni Canani R, Caffarelli C, Calvani M, Martelli A, Carucci L, Cozzolino T, et al., diagnostic therapeutic care pathway for pediatric food allergies and intolerances in Italy: a joint position paper by the Italian Society for Pediatric Gastroenterology Hepatology and Nutrition (SIGENP) and the Italian Society for Pediatric Allergy and Immunology (SIAIP). Ital J Pediatr. (2022) 48(1):87. doi: 10.1186/s13052-022-01277-8
81. González-Cervera J, Arias Á, Redondo-González O, Cano-Mollinedo MM, Terreehorst I, Lucendo AJ. Association between atopic manifestations and eosinophilic esophagitis: a systematic review and meta-analysis. Ann Allergy Asthma Immunol. (2017) 118(5):582–90.e2. doi: 10.1016/j.anai.2017.02.006
82. McGowan EC, Platts-Mills TAE, Wilson JM. Food allergy, eosinophilic esophagitis, and the enigma of IgG4. Ann Allergy Asthma Immunol. (2019) 122(6):563–4. doi: 10.1016/j.anai.2019.03.024
83. Clayton F, Fang JC, Gleich GJ, Lucendo AJ, Olalla JM, Vinson LA, et al. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology. (2014) 147(3):602–9. doi: 10.1053/j.gastro.2014.05.036
84. Rosenberg CE, Mingler MK, Caldwell JM, Collins MH, Fulkerson PC, Morris DW, et al. Esophageal IgG4 levels correlate with histopathologic and transcriptomic features in eosinophilic esophagitis. Allergy. (2018) 73(9):1892–901. doi: 10.1111/all.13486
85. Schuyler AJ, Wilson JM, Tripathi A, Commins SP, Ogbogu PU, Kruzsewski PG, et al. Specific IgG4 antibodies to cow’s milk proteins in pediatric. Patients with eosinophilic esophagitis. J Allergy Clin Immunol. (2018) 142(1):139–48.e12. doi: 10.1016/j.jaci.2018.02.049
86. Votto M, De Filippo M, Caminiti L, Carella F, de Castro G, Landi M. Eosinophilic gastrointestinal disorders and allergen immunotherapy: lights and shadows. Pediatr Allergy Immunol. (2021) 32(5):814–23. doi: 10.1111/pai.13458
87. Cafone J, Capucilli P, Hill DA, Spergel JM. Eosinophilic esophagitis during sublingual and oral allergen immunotherapy. Curr Opin Allergy Clin Immunol. (2019) 19(4):350–7. doi: 10.1097/ACI.0000000000000537
88. Petroni D, Spergel JM. Eosinophilic esophagitis and symptoms possibly related to eosinophilic esophagitis in oral immunotherapy. Ann Allergy Asthma Immunol. (2018) 120(3):237–240.e4. doi: 10.1016/j.anai.2017.11.016
89. Muraro A, Werfel T, Hoffmann-Sommergruber K, Roberts G, Beyer K, Bindslev-Jensen C, et al. EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy. (2014) 69(8):1008–25. doi: 10.1111/all.12429
90. Arias A, González-Cervera J, Tenias JM, Lucendo AJ. Efficacy of dietary interventions for inducing histologic remission in patients with eosinophilic esophagitis: a systematic review and meta-analysis. Gastroenterology. (2014) 146(7):1639–48. doi: 10.1053/j.gastro.2014.02.006
91. Kelly KJ, Lazenby AJ, Rowe PC, Yardley JH, Perman JA, Sampson HA. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology. (1995) 109(5):1503–12. doi: 10.1016/0016-5085(95)90637-1
92. Votto M, De Filippo M, Lenti MV, Rossi CM, Di Sabatino A, Marseglia GL, et al. Diet therapy in eosinophilic esophagitis. Focus on a personalized approach. Front Pediatr. (2022) 9:820192. doi: 10.3389/fped.2021.820192
93. Kagalwalla AF, Wechsler JB, Amsden K, Schwartz S, Makhija M, Olive A, et al. Efficacy of a 4-food elimination diet for children with eosinophilic esophagitis. Clin Gastroenterol Hepatol. (2017) 15(11):1698–707.e7. doi: 10.1016/j.cgh.2017.05.048
94. Pitsios C, Vassilopoulou E, Pantavou K, Terreehorst I, Nowak-Wegzryn A, Cianferoni A, et al. Allergy-test-based elimination diets for the treatment of eosinophilic esophagitis: a systematic review of their efficacy. J Clin Med. (2022) 11(19):5631. doi: 10.3390/jcm11195631
95. Molina-Infante J, Arias Á, Alcedo J, Garcia-Romero R, Casabona-Frances S, Prieto-Garcia A, et al. Step-up empiric elimination diet for pediatric and adult eosinophilic esophagitis: the 2-4-6 study. J Allergy Clin Immunol. (2018) 141(4):1365–72. doi: 10.1016/j.jaci.2017.08.038
96. Wechsler JB, Schwartz S, Arva NC, Kim KA, Chen L, Makhija M, et al. A single-food milk elimination diet is effective for treatment of eosinophilic esophagitis in children. Clin Gastroenterol Hepatol. (2022) 20(8):1748–56.e11. doi: 10.1016/j.cgh.2021.03.049
97. De Rooij WE, Vlieg-Boerstra B, Warners MJ, Van Ampting MTJ, van Esch BCAM, Eussen SRBM, et al. Effect of amino acid-based formula added to four-food elimination in adult eosinophilic esophagitis patients: a randomized clinical trial. Neurogastroenterol Motil. (2022) 34(7):e14291. doi: 10.1111/nmo.14291
98. Hill DA, Shuker M, Cianferoni A, Wong T, Ruchelli E, Spergel JM, et al. The development of IgE-mediated immediate hypersensitivity after the diagnosis of eosinophilic esophagitis to the same food. J Allergy Clin Immunol Pract. (2015) 3:123–4. doi: 10.1016/j.jaip.2014.08.005
99. Chang A, Robison R, Cai M, Singh AM. Natural history of food-triggered atopic dermatitis and development of immediate reactions in children. J Allergy Clin Immunol Pract. (2016) 4:229–36. doi: 10.1016/j.jaip.2015.08.006
100. Maggadottir SM, Hill DA, Ruymann K, Brown-Whitehorn TF, Cianferoni A, Shuker M, et al. Resolution of acute IgE-mediated allergy with development of eosinophilic esophagitis triggered by the same food. J Allergy Clin Immunol. (2014) 133:1487–9. doi: 10.1016/j.jaci.2014.02.004
101. Chang JW, Haller E, Dellon ES. Dietary management of eosinophilic esophagitis: man versus food or food versus man? Gastroenterol Clin North Am. (2021) 50(1):59–75. doi: 10.1016/j.gtc.2020.10.009
102. Rochman M, Azouz NP, Rothenberg ME. Epithelial origin of eosinophilic esophagitis. J Allergy Clin Immunol. (2018) 142(1):10–23. doi: 10.1016/j.jaci.2018.05.008
Keywords: Th2 inflammation, esophageal barrier, advanced glycation end products, alarmins, ultraprocessed foods
Citation: Carucci L, Votto M, Licari A, Marseglia GL and Berni Canani R (2023) Food allergy: cause or consequence of pediatric eosinophilic esophagitis? Potential implications of ultraprocessed foods in prevention and management. Front. Allergy 4:1138400. doi: 10.3389/falgy.2023.1138400
Received: 5 January 2023; Accepted: 12 June 2023;
Published: 29 June 2023.
Edited by:
Silvia Salvatore, University of Insubria, ItalyReviewed by:
Dan Atkins, University of Colorado Denver, United StatesJeffrey M. Wilson, University of Virginia, United States
Vivian Hernandez-Trujillo, Nicklaus Children’s Health System, United States
© 2023 Carucci, Votto, Licari, Marseglia and Berni Canani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roberto Berni Canani YmVybmlAdW5pbmEuaXQ=
†These authors have contributed equally to this work
 Laura Carucci
Laura Carucci Martina Votto3,†
Martina Votto3,† Roberto Berni Canani
Roberto Berni Canani